Translate this page into:
Chemical, technological, and rheological properties of hydrocolloids from sesame (Sesamum indicum) with potential food applications
⁎Corresponding author at: Departamento de Operaciones Unitarias, Facultad de Ingeniería. Grupo de Investigación Ingeniería de Fluidos Complejos y Reología de Alimentos (IFCRA), Indias, Colombia. lgarciaz@unicartagena.edu.co (Luis A. García-Zapateiro)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Hydrocolloids are hydrophilic biopolymers which are widely used in the food industry due to their functional properties. In the present study, sesame hydrocolloids (Sesamum indicum) were obtained and, consequently, their physicochemical, proximal composition, functional, and rheological properties were evaluated to establish their potential applications in the food industry. Methods: Hydrocolloids were obtained from sesame seeds at 80 °C on evaluating the pH at 3, 7, and 10 and specific flour: water ratios during the solubilization process. Results: The hydrocolloids obtained had a good relationship between carbohydrates and proteins, which increased their potential use in the development of colloidal systems. The samples had high water holding capacity, solubility, and appropriate emulsifying and foaming properties. The hydrocolloids showed non-Newtonian shear-thinning behavior, adjusted to the Carreau-Yasuda model. Based on the dynamic viscoelastic rheological test, samples were characterized as a gel-like state when storage modulus values were higher than the loss modulus in the frequency and temperature ranges investigated. Conclusion: The findings revealed that sesame seeds can be considered appropriate raw material for extracting hydrocolloids as an alternative for obtaining natural food ingredients with interesting functional and rheological properties, with further applications in the development and formulation of micro-structured products.
Keywords
Sesame
Hydrocolloids
Rheology
Carreau-Yasuda
Functional properties
1 Introduction
Hydrocolloids are a heterogeneous group of long-chain polymers, mainly proteins and polysaccharides (Dickinson, 2003), employed in food as modifiers of the physical properties of solutions due to their ability to form gels or as a thickening, emulsification, coating, and stabilization agent (Williams and Phillips, 2000). Polysaccharide-based hydrocolloids exhibit functional and textural properties such as viscosity control, stabilization, and gel formation (Rascón-Chu et al., 2016), while protein-based hydrocolloids exhibit properties such as water or fat absorption capacity, protein solubility, foaming capacity, and emulsifying activity (Bessada et al., 2019), then, polysaccharide-protein hydrocolloids interaction is an interesting alternative for producing hydrocolloids that improve the properties of food products.
Different sources of hydrocolloids have been explored to take advantage of natural resources with renewable raw materials, such as algae (Borah et al., 2020), seeds, fruits, and plant exudates (Naji-Tabasi and Razavi, 2017), employing simpler and safer processes to obtain hydrocolloids with low cost, environmental sustainability, biosecurity, biodegradability, and superior physical and chemical properties (Liu et al., 2018).
Sesame (Sesamum indicum) is an annual plant belonging to the Pedaliaceae family. It is well known as the queen of oilseeds due to its excellent oil quality, which presents greater resistance to oxidation and rancidity (Mailer, 2016), and the meal. Sesame seeds are characterized by a high oil (56.56 %) and protein (18–23.5 %) content (Mailer, 2016), followed by carbohydrate (18.44 %), fiber (8.22 %), and low moisture (3.49 %) (Dravie et al., 2020). In comparison, the oil-free sesame content contains approximately 42 % of proteins (Sharma et al., 2016), which possess the ability to stabilize colloidal systems such as foam and emulsions as an alternative to develop food ingredients. In the present study, the objective was to extract hydrocolloids from sesame waste obtained during the oil extraction process and to determine their proximal composition, physicochemical, functional, and rheological properties, as well as their potential applications in the food industry.
2 Materials and methods
2.1 Chemical reagents
Ethanol (99.5 % purity) and hexane were obtained from Panreac (Barcelona, Spain). NaOH, acetic acid, and phenolphthalein were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of analytical grade and used with further processing.
2.2 Materials
Sesame seeds (Sesamum indicum) were purchased from a local food market in the city of Cartagena, Colombia. A defatted sesame flour was obtained following the procedures described by Rafe et al., (2014), with some modifications. Briefly, the seeds were washed, disinfected, and defatted using an oil extractor (Dulong model DL-ZYJ05); then, the defatted seeds were ground using a basic microfine grinder drive MF 10 (IKA, Germany) to obtain a flour (extraction yield 45.06 ± 1.89 %) with a particle size < 200 m.
2.3 Hydrocolloids extraction
Sesame hydrocolloid (HS) extractions were carried out following the methods described by Orgulloso-Bautista et al., (2021) and Ibañez and Ferrero, (2003) with some modifications, evaluating the effect of solubilization pH (3, 7, and 10) and the sesame flour: water ratio (1:4, 1:6, and 1:9) obtaining nine experiments (Table 1). Initially, solid–liquid extraction was carried out by mixing different sesame flour: distilled water ratios; for 4 h at 80 °C for the solubilization.; after that, the pH was adjusted using acetic acid and NaOH. The mixture was separated by centrifugation for 15 min at 4000 rpm, and the supernatant was collected. Then, the viscous solution was mixed with ethanol in a 1:1 ratio at 4.0 ± 0.5 °C for 2 h to precipitate the hydrocolloid-based extract. The mixture was centrifuged, and the precipitate was collected, lyophilized at − 45 °C and 0.1 mbar for 48 h, and milled. The extraction yield of hydrocolloids (g of hydrocolloid obtained/g of sesame flour × 100) was determined for the different assays performed.
Code Sample
Ratio
flour: water
pH
HS1
1:4
3
HS2
1:6
3
HS3
1:8
3
HS4
1:4
7
HS5
1:6
7
HS6
1:8
7
HS7
1:4
10
HS8
1:6
10
HS9
1:8
10
2.4 Chemical analysis
The physicochemical and proximal composition of the hydrocolloids was determined following the method described by the Association of Official Analytical Chemists, (AOAC, 2000). The pH values were determined using a digital pH meter (Model HANNA HI 9124). The titratable acidity (TTA) was determined by titration. The moisture content was determined by dehydration in an oven at 105 °C for 4 h. The ash content was determined by incineration at 550 °C until constant weight. The total fat content was assessed by Soxhlet using hexane as a solvent. The total protein content was determined using the Kjeldahl method. The total carbohydrate content was analyzed per difference.
The functional groups were identified by Fourier transform infrared spectroscopy (FTIR). FTIR spectra were obtained with an infrared spectrophotometer (Shimadzu IR-Affinity model instrument, Japan). The samples were placed between two KBr discs (dimensions 32 mm × 3 mm) in a suitable sample holder. Spectra were acquired in an absorbance mode with a resolution of 4 cm−1 in the wavelength range 500 – 4000 cm−1. Analyses were performed in duplicate, and average spectra were used.
2.5 Technological properties
2.5.1 Water holding capacity (WHC)
WHC ( ) expresses the amount of water retained by hydrocolloids. For quantification, 0.5 g of hydrocolloids were placed in a centrifuge tube, 3 mL of water was added, followed by shaking for 1 min. The mixture was centrifuged at 3200 rpm at 24 °C for 30 min to measure the volume of water held (Modercay and Bermudez, 2010).
2.5.2 Solubility
The solubility ( ) was carried out following the procedure described by Betancur-Ancona et al., (2003). It was determined by dispersion of 1 % w/v of hydrocolloids in 30 mL of distilled water at 25, 45, and 65 °C for 30 min with continuous stirring. The 10 mL aliquots were centrifuged, and the supernatant was dried in a muffle at 125° C until a constant weight was obtained.
2.5.3 Emulsifying ability (EA) and emulsifying stability (ES)
EA ( ) and ES ( ) were analyzed following the method described by Sciarini et al., (2009). The emulsions were prepared by mixing 6 mL of commercial oil in 60 mL of distilled water, along with hydrocolloids at 0.5, 0.25, and 0.1 % w/w. The dispersions were mixed with a magnetic stirrer and subsequently homogenized for 1 min at 3000 rpm (Ultra Turrax T-25, IKA, Germany). In order to determine ES, the emulsions were heated in a water bath at 80° C for 30 min and then centrifuged for 10 min and the volume was taken.
2.5.4 Foaming capacity (FC) and foam stability (FS)
FC ( ) and FS ( ) were determined as described by Jahanbin et al., (2012). 1 g of hydrocolloids was added to 100 mL of distilled water and vigorously agitated for 5 min.
2.6 Rheological measurements
The rheological characterization of hydrocolloids was performed using a controlled stress rheometer (Modular Advanced Rheometer System Haake Mars 60, Thermo-Scientific, Germany) using a rough plate geometry of 35 mm diameter and a 1 mm gap to prevent wall slip effects. The procedures were done following the method described by Quintana et al., (2018). The temperature was maintained using a Peltier system, and each sample was equilibrated for 600 s before the rheological test to ensure the same thermal and mechanical history for each sample.
Viscous flow tests were performed at steady-state, analyzing variations in apparent viscosity at shearing rates ranging between 10-3 and 103 s−1. The viscoelastic properties were evaluated with small-amplitude oscillatory shear (SAOS) tests. Stress sweeps were carried out at a frequency of 1 Hz applying an ascending series of stress values from 10-3 and 103 Pa to determine the linear viscoelasticity range. Frequency sweeps were performed to obtain the mechanical spectrum applying a stress value within the linear viscoelastic range at 10 Pa, in the frequency range of 10-2 - 102 rad·s−1. All analyses were performed at 10, 25, 40, and 80 °C.
2.7 Statistical analysis
All experiments were done per triplicate. The data obtained were analyzed by ANOVA using Statgraphics software (Centurion version 16.1) to determine statistically significant differences (p < 0.05) between samples.
3 Results and discussion
3.1 Extraction Yield, physicochemical and proximal composition
The extraction yield of hydrocolloids from sesame flour in acid (pH 3), neutral (pH 7), and alkaline (pH 10) medium, with various mass ratios of sesame flour: water (1:4, 1:6, and 1:9) at 80 °C are shown in Fig. 1. The pH of solubilization exhibits significant differences (p < 0.05); hydrocolloids obtained at pH 3 demonstrated the highest extraction yield, followed by pH10, whereas the extraction condition at pH 7 was the lowest. Then, the extraction yield at pH 10 favored the removal of the remaining fatty component of the defatted sesame flour to form a paste, in this case the employee of a second solubilization step was performed to remove part of the compounds solubilized in the aqueous medium; this is in accordance with the results reported by Karazhiyan et al., (2011) as the yields were higher in the acid and alkaline medium than those obtained at neutral pH.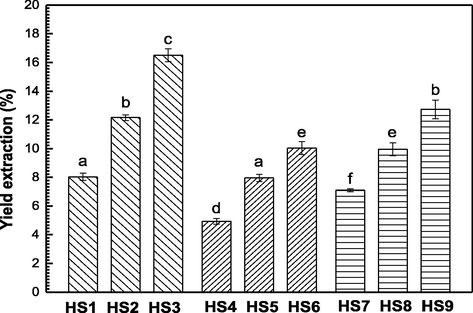
Yield extraction of each hydrocolloid from sesame (HS1, HS2 and HS3 Acid pH extraction); (HS4, HS5 and HS6 Neutral pH extraction) and (HS7, HS8 and HS9 Alkaline pH extraction). Means with different letters are significantly different (p > 0.05). Error bars show the standard deviation of experimental values (n = 3).
The flour:water ratio presented a linear increase in yield extraction (p < 0.05) which was attributed to the greater mass and energy transfer induced by the increase in the proportion of water used for extraction attributed to the formation of hydrogen bonds of polysaccharide molecules of hydroxyl also the pH of solubilization that causes electrostatic repulsion and hydration of charged residues, thus promoting protein solubilization at the isoelectric point (Petrucci et al., 2011). Similar findings have been observed with hydrocolloids obtained from seeds of Lepidium sativum (Karazhiyan et al., 2011), and gums of basil seed (Razavi et al., 2009) and the roots of Acanthophyllum bracteatum (Ghosh et al., 2005). Based on these findings, samples obtained at pH 3 (HS-3) and pH 10 (HS-9), in a 1:8 flour to water ratio, were characterized to analyze their proximal composition, functional and rheological properties.
The physicochemical and proximal compositions of the hydrocolloids obtained at pH 3 (HS-3) and pH 10 (HS-9) are shown in Table 2. The sample presents different pH values (p < 0.05) depending on the conditions of solubilization. HS-3 had a pH of 4.21 ± 0.03, while HS-9 had a pH of 8.85 ± 0.04. The marginal increase or decrease in the pH value of the extraction is related to the addition of a neutral solvent, such as ethanol, in the precipitation step; the pH will attempt to balance the pH of the two components based on its ability to transfer H + ions in the case of an acidic solution, or OH– ions when the solution is basic (Petrucci et al., 2011). Similarly, the TTA was consistent with the pH (p < 0.05), indicating that HS-3 contained a higher percentage of acid than HS-9, although it has a certain amount of acid attributable to sesame. Results are expressed as mean ± standard deviation. Different letters within each row are significantly different (p < 0.05). TTA, titratable acidity; WHC, water holding capacity; EA, emulsifying ability; ES, emulsifying stability; FC, foaming capacity; FS, foam stability.
Parameter
HS-3
HS-9
Proximal and Physicochemical Properties
TTA (mg Citric acid/100 mL)
0.48 ± 0.01a
0.08 ± 0.00b
pH
4.21 ± 0.03a
8.85 ± 0.04b
Moisture (%)
5.46 ± 0.04a
7.75 ± 0.11b
Protein (%)
29.55 ± 0.48a
23.31 ± 0.76b
Fat (%)
8.65 ± 0.13a
12.47 ± 0.59b
Ash (%)
8.33 ± 0.11a
7.48 ± 0.72a
Carbohydrate (%)
47.98 ± 0.53a
48.92 ± 1.13a
Technological Properties
%WHC
486.67 ± 23.09a
400.00 ± 34.64a
% Solubility 25 °C
35.00 ± 1.73a
32.00 ± 3.46a
% Solubility 45 °C
40.00 ± 9.64a
38.00 ± 4.58a
% Solubility 65 °C
47.00 ± 11.35a
67.00 ± 3.46b
% EA
100.01 ± 0.01a
100.00 ± 0.01a
% ES
94.69 ± 2.37a
96.43 ± 0.62a
% FC
49.67 ± 3.51a
60.33 ± 1.53b
% FS
21.65 ± 3.88a
68.48 ± 2.23b
The moisture content of the hydrocolloids showed significant differences (p < 0.05), with HS-9 showing a higher content than HS-3. Furthermore, the fat content of the extracted HS demonstrated significant differences (p < 0.05), with samples at alkaline pH 10 presenting the highest values, with a difference of 3.82 %. The ash content did not differ significantly between the extracted HS (p > 0.05). Moreover, the carbohydrate content did not significantly differ among samples (p < 0.05), but it was the major macronutrient in the proportion of the hydrocolloids; this can be attributed to the extraction process, which results in high proportions of starches and other polysaccharides, as it has been reported in guar gum and locust bean gum (Martínez et al., 2015). The similar carbohydrate content of the samples can be attributed to the degradation of sesame polymeric chains in alkaline and acidic media, extracting mainly glucose, arabinose, xylose, galactose, mannose, and rhamnose present in sesame flour (Ghosh et al., 2005). Protein percentage also differed significantly between samples (p < 0.05), with values of 29.55 ± 0.48 % and 23.31 ± 0.76 % for HS-3 and HS-9, respectively; this finding could be attributed to the co-precipitation of proteins and polysaccharides on mixing the supernatant with ethanol.
Furthermore, sesame proteins could be isolated using water at pH 10 or precipitation at acid pH, which can facilitate the extraction of globular proteins such as albumins, globulins, prolamins and glutelins (Hassan et al., 2018; Onsaard, 2012), which are primarily composed of leucine, valine, phenylalanine, threonine and other amino acids (Kotecka-Majchrzak et al., 2020) and presenting predominantly hydrophobic components compared to amino acids that possess hydrophilic groups (Gromiha, 2010). Based on the composition presented by bromatological characterization of hydrocolloids and previously reported components of sesame, the relationship between carbohydrate and proteins mainly improves the emulsifying properties and functions as a molecular linker (Kačuráková and Wilson, 2001). Furthermore, isolated hydrocolloids that demonstrate a close relationship between polysaccharides and proteins have shown amphiphilic characteristics capable of easily stabilizing colloidal systems (Manzoor et al., 2020).
The analysis of chemical groups of samples was done by FTIR. Most of the investigated components usually have chemical bonds that exhibit vibrational motion in mid-infrared spectroscopy (4000–650 cm−1), where proteins and carbohydrates can be identified as the main components of hydrocolloids. FTIR spectra for HS-3 and HS-9 were determined in the range of 4000 to 550 cm−1, as can be observed in Fig. 2. The band between 3800 and 3100 cm−1 belonged to the –OH stretching. The band in the region of 3000–2800 cm−1 was related to the stretching vibration of –CH. A substantial peak was recorded between about 2700 cm−1 and 2950 cm−1, corresponding to C—H stretching vibrations, and the peak bands at wavenumbers 1157, and 1022 cm−1 were attributed to the stretching vibration of the C—O bond. Then hydrocolloids present a typical polysaccharide profile, with multiple peaks between the abovementioned regions 1160–1130 cm−1. The profile of peaks with vibrations between 1100 cm−1 and 980 cm−1 has been attributed to ring and side ring vibrations of the individual sugar components (C—O—C, C–OH) (Kacuráková et al., 2000). The peak at 1663 cm−1 can be attributed to adsorbed water (Kac-Ura, et al., 1998; Kačuráková and Wilson, 2001), the protein component of the extract can also account for the adsorption of the amide band in this area, while the typical in these wavenumbers is also the adsorption of carboxylic groups (Haris and Severcan, 1999).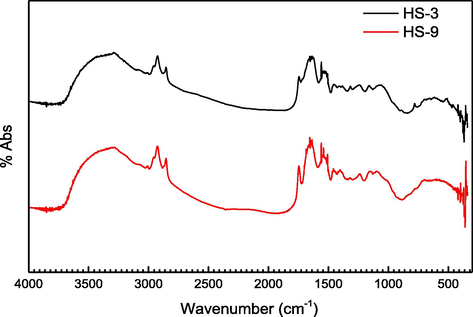
FTIR of sesame hydrocolloids in acid (HS-3) and alkaline (HS-9) medium.
3.2 Technological properties
The functional properties of HS are presented in Table 2. The WHC of sample HS-3 was slightly greater than that of sample HS-9, as the presence of carbohydrates, the number of hydration positions, the configuration of the protein and fiber, and the biopolymer's protein content (Ragab et al., 2004), allowed higher retention between hydrocolloids and water favoring retention (Alpizar-Reyes et al., 2017). This can also be explained by the structure, pH, temperature, and density of the total charge, as well as the hydrophilic nature of molecules (Bayar et al., 2016).
The HS solubility was evaluated at different temperatures and revealed a linear increase with significant differences (p < 0.05); this difference could be attributed to the isoelectric point of proteins (Badui, 2006) since the solubility was markedly enhanced under alkaline conditions and was considerably improved by protein-carbohydrate conjugates formed through glycosylation reactions, as indicated by the amount of these components determined in the hydrocolloid composition. The formation of these conjugates markedly helps mitigate the drop in hydrocolloid solubilization at pH values close to the isoelectric point and can even affect solubility at basic pH values (Saatchi et al., 2019). This functional property presents a high potential for the dispersion of these compounds, mainly in aqueous colloidal systems, to exploit their other functional properties as stabilizers or thickeners.
The EA% in all cases was 100 % of the mixture volume, and St% was 94.96 2.37 for HS-3 and 96.43 0.62 for HS-9, respectively, presenting a high emulsifying capacity and showing enhanced stability throughout the time, without significant differences (p > 0.05). This behavior could be attributed to the high protein content of HS, as the presence of proteins decreases the surface tension as a result of electrostatic repulsion on molecule surfaces; therefore, the stability of the emulsion is higher (Dickinson, 1994).
Significant differences (p < 0.05) were observed in terms of FE% and FS%, HS-3 presenting the lowest values of FC% and FS%, while 3-fold increased values of HS-9; these results depend on various factors, including protein and carbohydrate content, structure, molecular weight, and the presence of additional agents in hydrocolloids (Mahfoudhi et al., 2014). The high protein content of hydrocolloids may be directly related to interfacial properties (Fedeniuk and Biliaderis, 1994). Then, the polysaccharide content facilitates foaming, as it has been previously confirmed that polysaccharides can increase the foaming properties of protein systems by increasing the viscosity of the continuous phase, creating a network of biopolymers that trap air bubbles, thus preventing their rupture or coalescence (Makri and Doxastakis, 2007).
3.3 Rheological properties
3.3.1 Steady-state viscous-flow curves
The apparent viscosity (η) of HS-3 and HS-9 was analyzed against the deformation rate (
) at 10, 25, 40, and 80 °C (Fig. 3). The samples showed a decrease in η as a function of
typical behavior of non-Newtonian fluid-type shear thinning (Piñeiro-Lago et al., 2020), which presents three regions: an initially Newtonian plateau at low shear rates, followed by a power drop, and an infinite Newtonian region at high shear rates. This behavior can be explained by a break in the reticular structure of polysaccharide molecules of hydrocolloids during shear, with their chains aligned along the flow direction, leading to an exponential reduction in viscosity (Mir et al., 2016). Experimental data were adjusted to the Carreau-Yasuda model (Carreau, 1972) (R2 > 0.98) (Equation (1)):
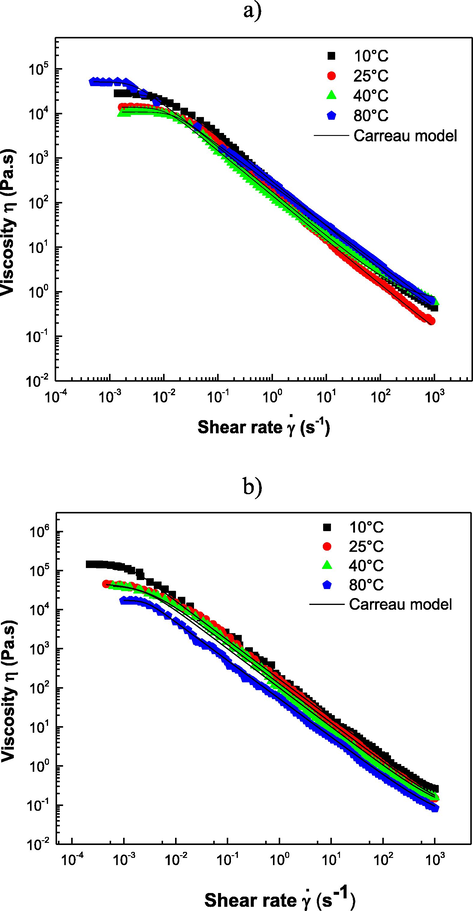
Fitting the flow curves with the Carreau-Yasuda model for sesame hydrocolloids at 10, 25, 40, and 80 °C in a) acid (HS-3) and b) alkaline (HS-9) medium.
The parameters for adjusting the Carreau-Yasuda equation are shown in Table 3, where the effect of the temperature on the flow parameters was observed. In all cases,
< 1 confirmed a shear-thinning behavior. The hydrocolloids presented relatively high viscosities at different temperatures. When comparing each parameter of the Carreau-Yasuda model at the different temperatures of hydrocolloids, significant differences were obtained (p < 0.05). For HS-3, the parameter
increased at 80 °C, which can be related to an increase in the rigidity and viscosity owing to apparent gelatinization, forming viscous agglomerates that cause particles to coalesce and form networks that trap the internal fluids, inducing the formation of higher viscosity aggregates (Ramírez-Navas, 2006). The decrease in viscosity in HS-9 with the increase in temperature even at 80 °C is related to their fat content, considering that the gelation temperature depends on the water proportion and the concentration of gelling agent (Badui, 2006). However, it appears to enter a pre-gelatinization phase, as the viscosity did not decrease with the same potential from 10 to 25 °C. Results are expressed as mean ± standard deviation. Different letters within each column are significantly different (p < 0.05).
Code Sample
Temp.
(°C)
(Pa·s)
(Pa·s)
(s)
α
n
R2
HS-3
10
29999.99 ± 1.12a
0.42 ± 0.03a
108.95 ± 4.20ad
1.52 ± 0.04a
0.01 ± 0.01a
0.99
25
14516.32 ± 14.07b
0.22 ± 0.01b
57.21 ± 4.98a
1.37 ± 0.08a
0.01 ± 0.01a
0.99
40
10826.92 ± 70.43c
0.59 ± 0.01c
61.09 ± 3.58a
2.79 ± 0.27b
0.01 ± 0.01a
0.99
80
620616.67 ± 24.64d
0.30 ± 0.01d
2216.64 ± 40.80b
8.00 ± 1.12c
0.10 ± 0.01a
0.99
HS-9
10
145132.24 ± 1.17e
0.15 ± 0.06e
873.73 ± 7.04c
2.69 ± 0.03b
0.01 ± 0.01a
0.99
25
50298.83 ± 80.17f
0.04 ± 0.01f
185.11 ± 5.79d
0.92 ± 0.03a
0.08 ± 0.01b
0.98
40
40232.02 ± 54.40 g
0.11 ± 0.04e
503.81 ± 3.07e
2.81 ± 0.17b
0.01 ± 0.01a
0.99
80
17340.21 ± 94.41 h
0.03 ± 0.01f
370.21 ± 6.30f
3.75 ± 0.22d
0.01 ± 0.01a
0.99
3.3.2 Viscoelastic oscillatory test
The frequency sweep (Fig. 4) revealed that the samples present a gel-type solution in the mechanical spectrum of the studied frequency by a network of entangled macromolecules. The magnitudes of the storage module
was greater than the loss modulus
for hydrocolloids within the frequency range analyzed, indicating that the elastic behavior predominates against the viscous component (Ibañez and Ferrero, 2003) showing an increment of solid properties than liquid ones, influenced by the organization and structure (Quintana et al., 2018). The structure of hydrocolloids with protein-carbohydrate conjugates is a complex matrix, and the links between molecules determine their mechanical properties. Hence, the protein and carbohydrate content define the structure's firmness by combining double helices to form links between molecules in a three-dimensional network, thus promoting stable gel formation (Drapala et al., 2019). Consequently, protein-carbohydrate complexes have been developed to improve the elastic properties of gels; this was not required to improve the viscoelastic properties of the prepared HS. Moreover, these complexes can be used in gelled products that require this kind of hydrocolloids (Moreno-Santander et al., 2020).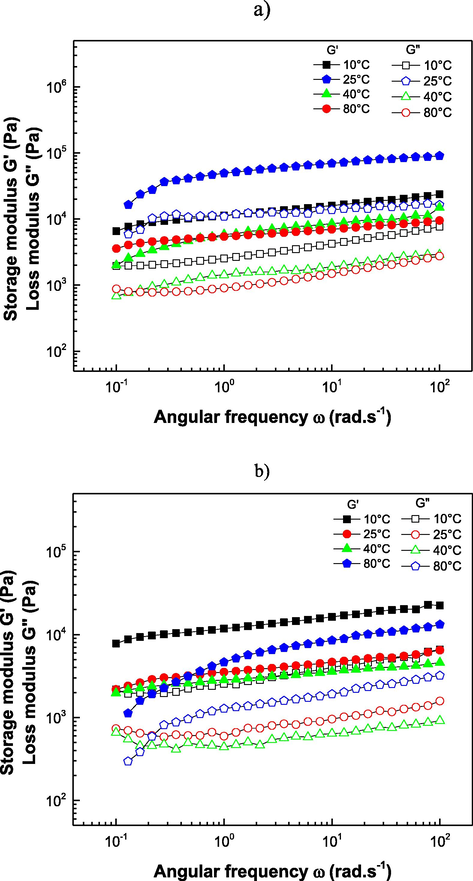
Frequency tests. Elastic modulus (
) and Viscous modulus (
) for sesame hydrocolloids at 10, 25, 40, and 80 °C in a) acid (HS-3) and b) alkaline (HS-9) medium.
HS-3 exhibits higher G′ and G″ values than HS-9 at 80 °C indicating a higher gel strength, which can be attributed to the links between proteins and carbohydrates due to decreased pH, mediated by the Maillard reaction and crosslinking of protein molecules (Drapala et al., 2019), which can increase the viscosity of different matrices. The viscoelastic properties of hydrocolloids are shown in Table 4, where G′ at 1 Hz frequency decreases from 10 to 40 °C, which then changes the value depending on the pH of solubilization. Hydrocolloids depended on temperature variation; at 80 °C G′ increased at 80 °C for HS-3 describing particle entanglement due to gel formation (Larrosa, 2014), whereas for HS-9, G′ did not increase significantly at the gelling temperature owing to the melted fat content at 40 °C; both HS-3 and HS-9 showed thermal disruption of physical bonds (Piñeiro-Lago et al., 2020). The standard deviation of the samples was < 0.01. Different letters within each column are significantly different (p < 0.05).
Code Sample
Temp
(°C)
G*
(kPa)
Tan δ
peak
(kPa)
Tan δpeak
HS-3
10
15.29c
0.25b
23.64e
0.33a
25
6.74b
0.20a
9.48c
0.29a
40
8.06b
0.21ab
14.89d
0.28a
80
66.48d
0.18a
90.38f
0.50b
HS-9
10
15.59c
0.23a
22.79e
0.28a
25
4.43a
0.20a
6.48b
0.33a
40
3.45a
0.17a
4.58a
0.33a
80
7.95b
0.22b
13.21d
0.29a
The loss factor (tan δ) did not show significant differences (p > 0.05), indicating that the elastic character of the internal hydrocolloid networks was maintained. Fig. 5 shows the values of Tan δ as the angular frequency increases at different temperatures, in which the hydrocolloids show values closer to 0, confirming that storage modulus (G′) was persistently greater than the loss modulus (G″), presenting a clear predominance of elastic properties over viscous properties, as mentioned earlier.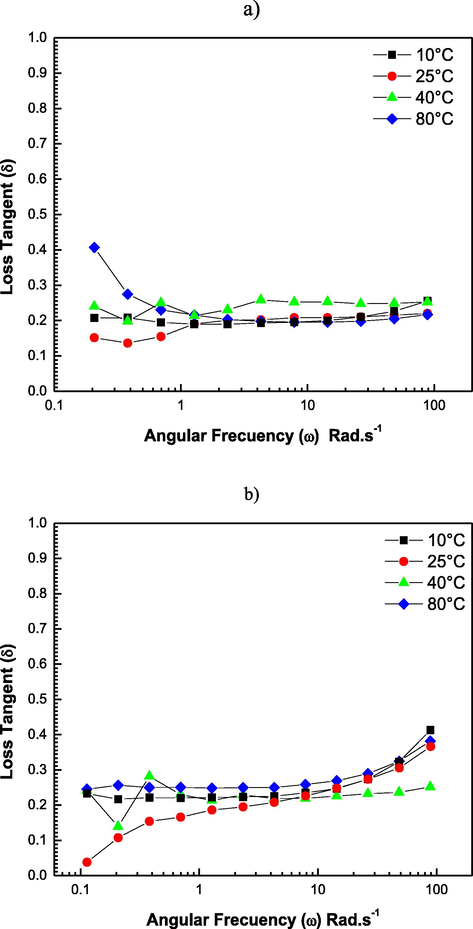
Phase angle tangent (tan δ) as a function of angular frequency for sesame hydrocolloids at 10, 25, 40, and 80 °C in a) acid (HS-3) and b) alkaline (HS-9) medium.
3.4 Potential use of hydrocolloids as food ingredients
Hydrocolloids have different technological properties which make them suitable ingredients for the development of food products. Different applications have been investigated, such as hydrocolloids for improving food structures (Mir et al., 2016), or as a functional ingredient to encapsulate, protect, and release nutrients (Taheri & Jafari, 2019) associated to their technological and rheological properties.
The retention of HS, along with a good protein-carbohydrate ratio and adequate properties at different pH values, can facilitate its development as additives/substituents with distinct physicochemical properties, increasing the range of product applications, as the final pH of the samples influences the interactions that affect techno-functional properties in a specific matrix (Yousefi and Jafari, 2019). For example, the water retention capacity of HS-3 and HS-9 could be utilized in meat emulsions as an alternative to improve the textural properties, stability, and yield of this food matrix,(Yao et al., 2018) or to modify the texture of beef patties (Pematilleke et al., 2020).
The solubility afforded by these hydrocolloids presents a high potential for dispersion, mainly in aqueous colloidal systems, to exploit their additional functional properties as stabilizers or thickeners, as demonstrated in a wide range of dairy products (Yousefi and Jafari, 2019). The obtained HS, owing to emulsification properties, could be used as an emulsifying agent or to deliver an active compound; these properties have been investigated in other gums possessing this important polysaccharide (Singh and Kumar, 2020) or emulsion gels (Shi et al., 2020), edible films, or coatings,(Trinetta, 2016) among others. The superior foaming capacity of HS-9 can be used to highlight specific and vital products in the.
industry, including whipped cream, mousses, soufflés, ice cream, bread, cakes, and carbonated beverages, as these colloidal systems provide texture, color, novelty, and excitement to the consumer (Murray, 2020). HS showed different rheological properties, which could improve.
rheological properties if used in food products.
4 Conclusions
Hydrocolloids from sesame seeds were obtained in alkaline (pH 10) and acidic (pH 3) media, employing a 1:8 ratio at 80 °C. The pH of the samples was determined according to the pH used for extraction; the proximal composition and functional properties of hydrocolloids revealed a high percentage of protein and carbohydrates contributing to the improvement of technological properties. HS presented a high WHC, solubility, and emulsion formation. Hydrocolloids obtained in an alkaline medium presented a better foaming ability. The samples presented a non-Newtonian flow behavior type shear-thinning, which was adjusted to the Carreau-Yasuda model (R2 > 0.98) when the pH of the extraction process induced a change in viscosity related to the bromatological composition. The viscoelastic properties revealed a were higher than , with higher strength. The suitable protein-carbohydrate ratio of hydrocolloids can be exploited for developing products with different physicochemical properties. HS demonstrated different technological and rheological properties that can be employed to improve the physical stability, as well as the textural and sensorial properties of microstructured food products, including emulsions, salads, coatings, or dressings, as well as for the development of carriers in the encapsulation process owing to their rheological properties.
Acknowledgements
The authors acknowledge the financial support from the Ministry of Science, Technology, and Innovation - MinCiencias (Contract: 368-2019, research project No. 110780864755.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng.. 2017;209:68-75.
- [CrossRef] [Google Scholar]
- Official Methods of Analysis (17th Edit. ed.). MD, USA: Gaithersburg; 2000.
- Food chemistry, 4th (editio. ed.). Mexico: Pearson education; 2006.
- Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol.. 2016;92:441-450.
- [CrossRef] [Google Scholar]
- Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol.. 2019;93:53-68.
- [CrossRef] [Google Scholar]
- Comparison of the chemical composition and functional properties of Phaseolus lunatus prime and tailing starches. Food Chem.. 2003;82:217-225.
- [CrossRef] [Google Scholar]
- Ozone enhanced production of potentially useful exopolymers from the cyanobacterium Nostoc muscorum. Polym. Test.. 2020;84:106385
- [CrossRef] [Google Scholar]
- Rheological Equations From Molecular Network Theories. Trans Soc Rheol. 1972;16:99-127.
- [CrossRef] [Google Scholar]
- Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids 2003
- [CrossRef] [Google Scholar]
- Protein-Carbohydrate Conjugation 2019:1-7.
- Antioxidant, phytochemical and physicochemical properties of sesame seed (Sesamum indicum L) Sci Afr. 2020;8:e00349.
- [Google Scholar]
- Composition and Physicochemical Properties of Linseed (Linum usitatissimum L.) Mucilage. J. Agric. Food. Chem.. 1994;42:240-247.
- [CrossRef] [Google Scholar]
- Food Chemistry Polysaccharides from Sesamum indicum meal : Isolation and structural features. 2005;90:719-726.
- [CrossRef]
- Gromiha, M.M., 2010. Chapter 1 - Proteins, in: Gromiha, M.M.B.T.-P.B. (Ed.), . Academic Press, Singapore, pp. 1–27. https://doi.org/https://doi.org/10.1016/B978-8-1312-2297-3.50001-1.
- FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal. B Enzym.. 1999;7:207-221.
- [CrossRef] [Google Scholar]
- Effects of gamma irradiation on the protein characteristics and functional properties of sesame (Sesamum indicum L.) seeds. Radiat. Phys. Chem.. 2018;144:85-91.
- [CrossRef] [Google Scholar]
- Extraction and characterization of the hydrocolloid from Prosopis flexuosa DC seeds. Food Res. Int.. 2003;36:455-460.
- [CrossRef] [Google Scholar]
- Isolation, purification and characterization of a new gum from Acanthophyllum bracteatum roots. Food Hydrocolloids. 2012;27:14-21.
- [CrossRef] [Google Scholar]
- FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr. Polym.. 2000;43:195-203.
- [CrossRef] [Google Scholar]
- Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym.. 2001;44:291-303.
- [CrossRef] [Google Scholar]
- Hydration Properties of Xylan-Type Structures: an Study of Xylooligosaccharides FTIR. J Sci Food Agric. 1998;77:38-44.
- [CrossRef] [Google Scholar]
- Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocolloids. 2011;25:915-920.
- [Google Scholar]
- Oilseed proteins – Properties and application as a food ingredient. Trends Food Sci. Technol.. 2020;106:160-170.
- [CrossRef] [Google Scholar]
- Efectos de los hidrocoloides en las caracteristicas fisicoquimicas y reológicas de pastas kibres de gluten aptas para indiciduos celíacos. Boletín de Ciencias Agropecuarias del ICAP: Universidad Nacional de la Plata; 2014. https://doi.org/https://doi.org/10.35537/10915/35442
- Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol.. 2018;75:146-157.
- [CrossRef] [Google Scholar]
- Assessment of emulsifying ability of almond gum in comparison with gum arabic using response surface methodology. Food Hydrocolloids. 2014;37:49-59.
- [CrossRef] [Google Scholar]
- Oilseeds: Overview. Oxford: Academic Press; 2016. p. :221-227.
- Surface tension of Phaseolus vulgaris and coccineus proteins and effect of polysaccharides on their foaming properties. Food Chem.. 2007;101:37-48.
- [CrossRef] [Google Scholar]
- Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol.. 2020;165:554-567.
- [CrossRef] [Google Scholar]
- New structural features of Acacia tortuosa gum exudate. Food Chem.. 2015;182:105-110.
- [CrossRef] [Google Scholar]
- Influence of hydrocolloids on dough handling and technological properties of gluten-free breads. Trends Food Sci. Technol. 2016
- [CrossRef] [Google Scholar]
- Preparación y determinación de propiedades funcionales de Concentrados proteicos de haba (Vicia faba) Revista Colombiana de Química. 2010;23:73-86.
- [Google Scholar]
- Contenido rheological characterization of gums-gel obtained from the proteic isolate of sesame (Sesamum indicum) Revista Mexicana de Ingeniera Quimica. 2020;19:21-31.
- [CrossRef] [Google Scholar]
- Recent developments in food foams. Curr. Opin. Colloid Interface Sci.. 2020;50:101394
- [CrossRef] [Google Scholar]
- Functional properties and applications of basil seed gum: An overview. Food Hydrocolloids. 2017;73:313-325.
- [CrossRef] [Google Scholar]
- Design and Application of Hydrocolloids from Butternut Squash (Cucurbita moschata) Epidermis as a Food Additive in Mayonnaise-type Sauces. ACS Omega 2021
- [CrossRef] [Google Scholar]
- Effect of the addition of hydrocolloids on beef texture: Targeted to the needs of people with dysphagia. Food Hydrocolloids 2020106413
- [CrossRef] [Google Scholar]
- general chemistry. Madrir: Pearson education; 2011.
- Temperature dependence of the viscoelastic properties of an acid-curd Spanish cheese: Afuega’l Pitu atroncau roxu variety (PDO) Lwt. 2020;126:109304
- [CrossRef] [Google Scholar]
- Steady and shear dynamic rheological properties of squash (Cucurbita moschata) pulp. Contemporary Engineering Sciences. 2018;11:1013-1024.
- [CrossRef] [Google Scholar]
- Dynamic rheological behavior of rice bran protein (RBP): Effects of concentration and temperature. J. Cereal Sci.. 2014;60:514-519.
- [CrossRef] [Google Scholar]
- Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem.. 2004;84:207-212.
- [CrossRef] [Google Scholar]
- Ionic gelation of low-esterification degree pectins from immature thinned apples. Revista Fitotecnia Mexicana. 2016;39:17-24.
- [CrossRef] [Google Scholar]
- Optimisation study of gum extraction from Basil seeds (Ocimum basilicum L.) Int. J. Food Sci. Technol.. 2009;44:1755-1762.
- [CrossRef] [Google Scholar]
- A new functional protein–polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol. Macromol. 2019
- [CrossRef] [Google Scholar]
- Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocolloids. 2009;23:306-313.
- [CrossRef] [Google Scholar]
- Assessment of functionality of sesame meal and sesame protein isolate from Indian cultivar. J. Food Meas. Charact.. 2016;10:520-526.
- [CrossRef] [Google Scholar]
- Fabrication of emulsion gel based on polymer sanxan and its potential as a sustained-release delivery system for β-carotene. Int. J. Biol. Macromol.. 2020;164:597-605.
- [CrossRef] [Google Scholar]
- International Journal of Biological Macromolecules Exploration of arabinogalactan of gum polysaccharide potential in hydrogel formation and controlled drug delivery applications. 2020;147:482-492.
- Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019
- [CrossRef] [Google Scholar]
- Handbook of Hydrocolloids. Boca Raton: CRC Press; 2000.
- Effect of sodium alginate with three molecular weight forms on the water holding capacity of chicken breast myosin gel. Food Chem.. 2018;239:1134-1142.
- [CrossRef] [Google Scholar]
- Recent advances in application of different hydrocolloids in dairy products to improve their techno-functional properties. Trends Food Sci. Technol.. 2019;88:468-483.
- [CrossRef] [Google Scholar]







