Translate this page into:
Rutin induces endoplasmic reticulum stress-associated apoptosis in human triple-negative breast carcinoma MDA-MB-231 cells – In vitro and in silico docking studies
⁎Corresponding author. sumathi_bc@avinuty.ac.in (Sundaravadivelu Sumathi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Rutin is a bioactive compound that possesses anti-tumor activities through triggering apoptosis. Triple-negative breast cancer (TNBC) is insensitive to targeted anti-tumoral drugs, and drug resistance in TNBC poses a challenge for a successful cure. The accumulation of misfolded proteins in the lumen of the endoplasmic reticulum (ER) results in cellular stress that initiates a specialized response designated as the unfolded protein response. This study aimed to find potential ER stress targets in triple-negative breast cancer. The viability of cells was evaluated using an MTT assay. Cell migration and proliferation were done by wound scratch and colony formation assay. Cell cycle detection, measurement of ER stress, mitochondrial membrane potential disruption, and cell death identification was performed using flow cytometry. The interaction of rutin with ER stress proteins is predicted using in silico docking. The pattern of gene expression was determined by qRT-PCR. The elevated rate of cell viability, cell cycle arrest, ER stress, MMP, and apoptotic induction was observed in combination treatment. Rutin exhibited the highest glide score with ASK1 and JNK. The results of qRT-PCR showed that rutin induced apoptosis through upregulation of ASK1 and JNK. The present study provides strong evidence supporting an important role of the ER stress response in mediating rutin-induced apoptosis in triple-negative breast cancer.
Keywords
Triple-negative breast cancer
Endoplasmic reticulum stress
Unfolded protein response
Rutin
Chemotherapeutic drug
- TNBC
-
Triple-negative breast cancer
- ER
-
Endoplasmic reticulum
- MTT
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- MMP
-
Mitochondrial membrane potential
- HER2
-
Human epidermal growth factor receptor 2
- UPR
-
Unfolded protein response
- DMEM
-
Dulbecco's Modified Eagle Medium
- FBS
-
Fetal bovine serum
- EDTA
-
Ethylenediaminetetraacetic acid
- PBS
-
Phosphate Buffered Saline
- DMSO
-
Dimethyl sulfoxide
- PI
-
Propidium iodide
- IRE1
-
Inositol-requiring enzyme 1
- PERK
-
Protein kinase R-like endoplasmic reticulum kinase
- TRAF2
-
TNF Receptor Associated Factor 2
- BCL-XL
-
B-cell lymphoma-extra large
- DGC4
-
Dystrophin-associated glycoprotein complex 4
- BCHE
-
Butyrylcholinesterase
- VCP
-
Valosin-containing protein
- HMOX1
-
Heme oxygenase 1
- ASK1
-
Apoptosis signal-regulating kinase 1
- JNK
-
c-Jun N-terminal kinase
- SD
-
Standard deviation
Abbreviations
1 Introduction
Cancer is a disease that develops when alterations in a group of healthy cells cause them to proliferate excessively, resulting in a lump known as a tumor. Tumors can form and spread through the blood and lymphatic systems, affecting healthy cells and other areas of the body if left untreated (Sun et al., 2017). Breast cancer growth is a multi-stage process involving a wide range of cell types, and prevention remains a challenge around the world. Breast cancer is a type of metastatic cancer that spreads to specific organs such as the lungs, stomach, bones, and brain. Early detection of the illness will result in a positive diagnosis and a high survival rate (Tong et al., 2018). Patients now have more options for breast cancer chemoprevention medications, while biological protection has recently been introduced to enhance the quality of life for patients. Triple-negative breast cancer (TNBCs) is characterized by a lack of receptors for estrogen and progesterone and the lack of HER2 overexpression. These cancers are a heterogeneous, poorly prognosed subtype of breast cancer. These types of cancers are very aggressive when compared to other types. Because of the lack of receptors, many treatments, including hormonal therapy, are not possible (Shindikar et al., 2016). So patients with TNBC do not benefit from chemotherapy-based treatments. Progress in managing TNBC appears to be a major obstacle (Collignon et al., 2016).
Protein folding and maturation, lipid or steroid production, calcium accumulation, and detoxification are all carried out by the endoplasmic reticulum (ER). ER stress is caused by the activation of various signaling pathways known as the unfolded protein response (UPR). The activated UPR controls downstream effectors with modification mechanism, frequency modulation, and cell fate regulation functions (Almanza et al., 2019). Therefore, studying ER stress and UPR using mammalian cells is necessary to understand the stress-related diseases of the UPR and ER. ER stress has altered metabolism in a cancer cell in comparison with normal cells. Eventually, the UPR pathways cause oxidative damage and cell death. Tumor cells have not only developed the ability to inhibit death-inducing pathways, trigger angiogenesis and reprogram their metabolism, but they have also demonstrated increased demand for protein synthesis and folding efficiency (Schmitz et al.,2018). Intrinsic stress and strain in the tumor, such as oncogenic activity, as well as external pressures imposed by tumor cells, enhance the creation of misfolded proteins in the ER, triggering UPR pathway development (McGrath et al., 2018). Furthermore, recent studies suggest that UPR activation could also decrease the effectiveness of chemotherapy by leading to the production of chemoresistance. ER stress-induced apoptosis helps to better explain ER stress-related cancer pathogenesis (Jin et al., 2016).
Bioactive compounds with anticancer properties through different mechanisms of action that influence the various stages of carcinogenesis, so their usage may be useful for chemoprevention and complementary care (Tong et al., 2018). The use of biologically active substances or metabolites extracted from plants in sufficient amounts and in a suitable form may be an effective technique for cancer prevention and treatment. Many active compounds are isolated from plants and used directly as patented medicine like taxol and, artemisinin. Due to the multi-targeting effects, low cost, and safety, there is a great need to explore the potential of medicine from bioactive compounds (Tuasha et al.,2018).
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucosideis) is a flavonoid that has been found in a variety of plants and it was first discovered in Ruta graveolens. Citrus fruits such as lemon, orange, lime, and grapefruit, as well as their components, contain rutin. Buckwheat is thought to be one of the best sources of rutin in the daily diet. Rutin is one of the most common dietary flavonoids in the form of human daily intake of vegetables, fruits, and plant-derived products, such as wine and tea (Ghițu et al., 2019). Plant nutritional supplements contain flavonoids that contribute to cancer suppression by inhibiting cell development, inducing cell cycle detection, apoptosis, inhibiting proliferation, angiogenesis, anti-inflammatory, and antioxidant effects. In recent years, numerous studies have shown that rutin acts as an important chemopreventive agent (Sharma et al., 2013).
The present research aims to identify the mechanism of action of rutin that can be exploited to find triple-negative breast cancer targets.
2 Materials and methods
Commercial rutin is a flavonoid typically found in various plant types. Rutin is used as a compound for the present study. The compound was purchased from HiMedia. Different concentrations of rutin were prepared by dissolving the compound in distilled water. Five separate concentrations were taken for the present analysis (10 μM, 20 μM, 40 μM, 80 μM, and 160 μM).
2.1 Culturing and maintenance of cell lines
The TNBC cell line MDA-MB-231 was purchased at the National Center for Cell Science (NCCS) in Pune, India. The cells were maintained at 1X final concentration from a 100X stock in an incubator with 5% CO2 and 95% humidity and supplemented with DMEM, 10% FBS, non-essential amino acids, penicillin, and streptomycin. When the cells accomplished confluent growth, cells were trypsinized using Trypsin-EDTA and seeded the cells into sterile 6-well and 96-well plates to carry out various assays. The cytotoxicity tests were done in 96-well plates, and staining was done in 6-well plates.
2.2 Evaluation of the anticancer activity of rutin
TNBC cells were treated for 6 h, 12 h, 24 h, 48 h, 60 h, and 72 h with various rutin concentrations ranging from 10 to 160 μM, and the dose and time of treatment were standardized in the MDA-MB-231 cell line using the MTT method. Further testing was conducted using this optimal dosage and period. The MDA-MB-231 has been treated with and without rutin. The cytotoxicity was determined using the MTT assay (Igarashi and Miyazawa, 2001). With 50 μl of MTT, 100 μl of the treated cells were incubated at 37° C for 3 h. After incubation, 200 μl of PBS was implemented to all samples, and carefully aspired to remove the excess MTT. 200 μl of acid propanol has been added and left overnight in the dark for solubilization. The absorbance was measured at 650 nm using a microtitre plate reader. The control cell's optical density was set to 100 % viable, and the percentage viability of the other treatment group's cells was calculated using the formula,
2.3 Cytotoxicity assay
Further through the MTT dye reduction test, the level of rutin cytotoxicity in the cancer cells was determined following the method described above (Igarashi and Miyazawa, 2001). Doxorubicin is a drug for chemotherapy against cancer. For the present study, doxorubicin has been used as a standard drug at a concentration of 0.5 μM. The following groups were set for the assay.
-
Cells alone
-
Cells + Rutin
-
Cells + Doxorubicin
-
Cells + Rutin + Doxorubicin
2.4 Colony-forming assay
A colony formation assay (Patrawala et al., 2007) was performed to test the ability of cancer cells to develop proliferative and long-term survival capability. An assay of colony formation was performed on a 6-well plate. 1 × 103 MDA-MB-231 cells were seeded on 6-well sterile plates. 0.1% DMSO was used to treat control wells. The cells were treated with various groups and incubated in a 37 °C incubator for 7 days. The colonies developed were fixed with glutaraldehyde, and stained with violet crystal. The stained clones were examined under an inverted microscope.
2.5 Cell migration assay
The cell migration assay was used to determine basic cell migration properties such as speed, persistence, and polarity (Walter et al., 2010). A total of 100,000 cells were seeded into each well of a 24-well tissue culture plate, and these cells were kept at 37 °C and 5% CO2 for 24 h to facilitate cell adhesion and the creation of a confluent monolayer. Then all these confluent monolayers have been scored with an aseptic pipette tip to leave a scratch in width of ∼ 0.4 - 0.5 mm. To remove dislodged cells, the cell surface was washed three times with a serum-free culture medium. Wound closure was observed at 0, and 24 hrs after the scratch was completed by collecting digitalized images under an inverted microscope.
2.6 Cell cycle arrest analysis
Flow cytometry is used to examine the cell cycle. Cells were trypsinized and centrifuged after treatment with rutin, doxorubicin, and a combination of rutin and doxorubicin. The cells were then stained in the dark at room temperature for 30 min with 1 ml of the prepared PI reagent mixture. Flow cytometry was used to determine the cell cycle stages of G0/G1, S, and G2/M populations after incubation. The findings were analyzed using FACSuite (BD Bioscience, USA) software.
2.7 Measurement of endoplasmic reticulum stress – Thioflavin staining
Measuring ER stress-mediated apoptosis will aid in understanding the pathogenesis of ER stress-related cancer. The cells were trypsinized and centrifuged at 5000 rpm for 10 min, and the supernatant was removed. 100 μl 1X binding buffer was implemented to the pellet and shook vigorously. The cells were then stained with 5 μl thioflavin for 15 min at room temperature in the dark. Following the incubation period, 400 μl of binding buffer was added and thoroughly mixed. The cells were measured using the BD FACSverse flow cytometer.
2.8 Detection of mitochondrial membrane potential (ΔΨ) by – JC-1 staining
The mitochondrial membrane potential (ΔΨm) for the whole cells was evaluated using a BD Mitoscreen kit (BD Biosciences, USA). The cells were trypsinized and centrifuged at 400 × g for 5 min, after which the supernatant was discarded. Incubate the cells at 37 °C for 10–15 min after adding 0.5 ml of JC-1 working solution. The cells were then rinsed with 2 ml 1 X assay buffer and centrifuged for 5 min at 400 × g. The pellet was resuspended in 0.5 ml of 1X assay buffer after the above step was repeated with 1 ml of 1X assay buffer. BD FACSverse was used to analyze the samples within one hour.
2.9 Mechanism of cell death by Annexin V/FITC-PI apoptosis staining
Cell death was detected using Annexin V/FITC-PI apoptosis staining (BD Biosciences, USA). The treated cells have been trypsinized and separated by centrifugation at 5000 rpm for 10 min, and the supernatant was removed. 100 μl 1X binding buffer has been applied to the pellet and started shaking vigorously. The cells then were stained with 5 μl of Annexin V/FITC and 5 μl of Propidium iodide for 15 min at room temperature in dark. After the incubation time, 400 μl of binding buffer was implemented and combined well. Use of the BD FACS verse flow cytometer to observe the cells.
2.10 Molecular docking (Schrodinger –Maestro version 11.8)
Maestro is the graphical user interface (GUI) for almost all computational programs within Schrodinger. It contains resources for viewing, constructing, and manipulating chemical structures; for preparing, organizing, and storing structures and related data; and for setting up, uploading, tracking, and visualizing the effects of structural calculations.
2.11 Modulation of ER stress protein expression by rutin by qRT-PCR
The method used to calculate the gene expression is the real-time polymerase chain reaction (Guo et al., 2015). The qRT-PCR reaction is the conversion of RNA to complementary DNA (cDNA), known as the reverse transcription process. To amplify and identify different genes it uses fluorescent reporters and a PCR reaction. Using the trizol method qRT-PCR was done. A trizol kit was used to extract total RNA from cells. Using the 12–18 primer oligo(dT) and the reverse transcriptase Superscript II, cDNA was synthesized from 2 μg total RNA. The mRNA levels of ASK1, JNK, and β-actin were determined using the SYBR green master mix and the primers listed in Table 1. The PCR reactions were conducted with a mixture of 20 μl containing 100 ng of cDNA, 10 μl of SYBR green master mix, and 1 μl of each particular primer. The PCR reactions were run on the Bio-Rad iCycler. The ASK1 and JNK mRNA expression levels were normalized with endogeneous control (β-actin) and expressed relative to control.
S.No
Gene
Forward primer
Reverse primer
1.
ASK1
CACTGAATGTACAGCTTGGA
CGATGAAGGAGTGCTTGTAA
2.
JNK
CCAGGACTGCAGGAACGAGT
CCACGTTTTCCTTGTAGCCC
3.
β-actin
GATGGATGATGATATCGCCGCG
GCTAAGGTGTGCACTTTTATTCAAC
2.12 Statistical analysis
GraphPad Prism 6.0 was used to complete the data analysis. To determine significance, t-tests were employed; *p < 0.05, **p < 0.01, ***p < 0.001 were considered to be statistically significant. All experiments were carried out in triplicate. The data is presented as mean ± SD.
3 Results
3.1 Rutin inhibits the growth of MDA-MB-231 cells in a dose and time-dependent manner
TNBC cell lines were treated with different concentrations of rutin and exposed to different time intervals. The MTT cell proliferation assay tests the rate of cell proliferation and conversely, cell viability reduction when metabolic events lead to apoptosis or necrosis. MDA-MB 231 cells were exposed to different concentrations of rutin ranging from 10 µM, 20 µM, 40 µM, 80 µM, and 160 µM for different periods namely 6 h, 12 h, 24 h, 48 h, 60 h, and 72 h to optimize the dose and time of rutin in triple-negative breast cancer cell line. Cell viability was assessed using an MTT assay. The IC50 value was found to be 40 µM for rutin treated group with an optimal exposure period of 24 h. The dose and time optimization in MDA-MB 231 was depicted in Fig. 1(a). The MTT experiment revealed that rutin administration dramatically reduced MDA-MB-231 cell viability in a dose-dependent manner. The MTT experiment indicated that rutin inhibited MDA-MB-231 cell growth and viability in a concentration-dependent manner.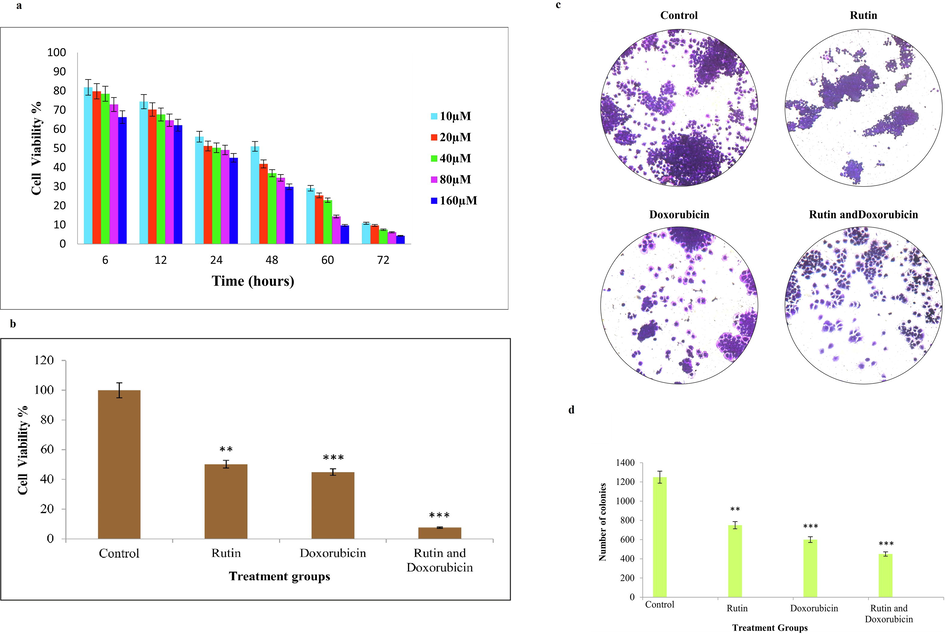
(a). MTT assay shows rutin suppresses the growth of MDA-MB-231 cells in a dose and time-dependent manner. The IC50 values of rutin to MDA-MB-231 cells were 40 ± 1.0 µM for 24 h respectively; Fig. 1(b). Cell viability of MDA-MB-231 cells after exposure to 40 µM/ml rutin and 0.5 µM/ml doxorubicin alone and in combination. The combination of rutin and doxorubicin showed enhanced cytotoxic activity. Data are presented as ± SD normalized to untreated control. Statistical significance was assessed by student t-test (*P < 0.01, *** P < 0.001); Fig. 1(c). Representative images of colonies formed by MDA-MB-231 cells after treatment with 40 µM/ml rutin and 0.5 µM/ml doxorubicin alone and in combination. Fig. 1(d). The graphical representation of MDA-MB-231 cells after treatment with rutin, doxorubicin, and combined group. Each bar represents the mean ± SD of triplicate samples from three independent experiments (*P < 0.01, *** P < 0.001 compared with untreated control).
3.2 Combination treatment of rutin with doxorubicin-induced cell death in MDA-MB-231 cells
The cytotoxic activity was measured with an MTT assay. The combination of rutin and doxorubicin treatment significantly inhibited the viability of TNBC cells than the rutin and doxorubicin alone treated group as shown in Fig. 1(b). The results revealed that combined exposure of rutin and doxorubicin decreased the cell viability drastically in TNBC cells. When treated with rutin alone the decrease in viability was on par with doxorubicin - the standard chemotherapeutic agent.
3.3 Rutin and doxorubicin inhibit breast cancer cells proliferation
Colony formation, also known as clonogenic assay, is an in-vitro quantitative approach for determining a single cell's potential to clonally expand into a large colony. Clonogenic activity is a sensitive indicator of undifferentiated cancer cells. This method demonstrates that cancer cells can survive and produce colonies in an anchorage-free culture medium. We analyzed if the different treatment groups could impair colony formation. The treatment group decreased the cell population as shown in Fig. 1(c-d). This indicates treatment group has an antiproliferative effect.
3.4 Rutin and doxorubicin decrease the migratory capability of MDA-MB-231 cells
Migration is an important function of living cells that is required for normal growth, immune response, and disease processes such as cancer spread and inflammation. Cell migration and invasion are processes providing rich targets for intervention in the cancer metastasis of key physiological and pathological phenomena. It allows for more effective detection of lead compounds and faster recognition of harmful effects in the screening phase of drug discovery. Based on the results of cytotoxicity and clonogenic assays, it was evident that rutin exerted inhibitory effects on breast cancer cells. The cells were treated with rutin, doxorubicin, and the combination of rutin and doxorubicin for 24 h, and screened for their migratory capability. Treatment with rutin and doxorubicin decreased their migratory capability more effectively. Treatment with either rutin or doxorubicin decreased the migratory capacity of the cells to some extent as shown in Fig. 2(a-e). Quantification of migration rate of MDA‐MB‐231 cells after treatment with rutin and doxorubicin was shown in Fig. 2(f). The results indicate that rutin is involved in the migration capability of MDA-MB-321cells.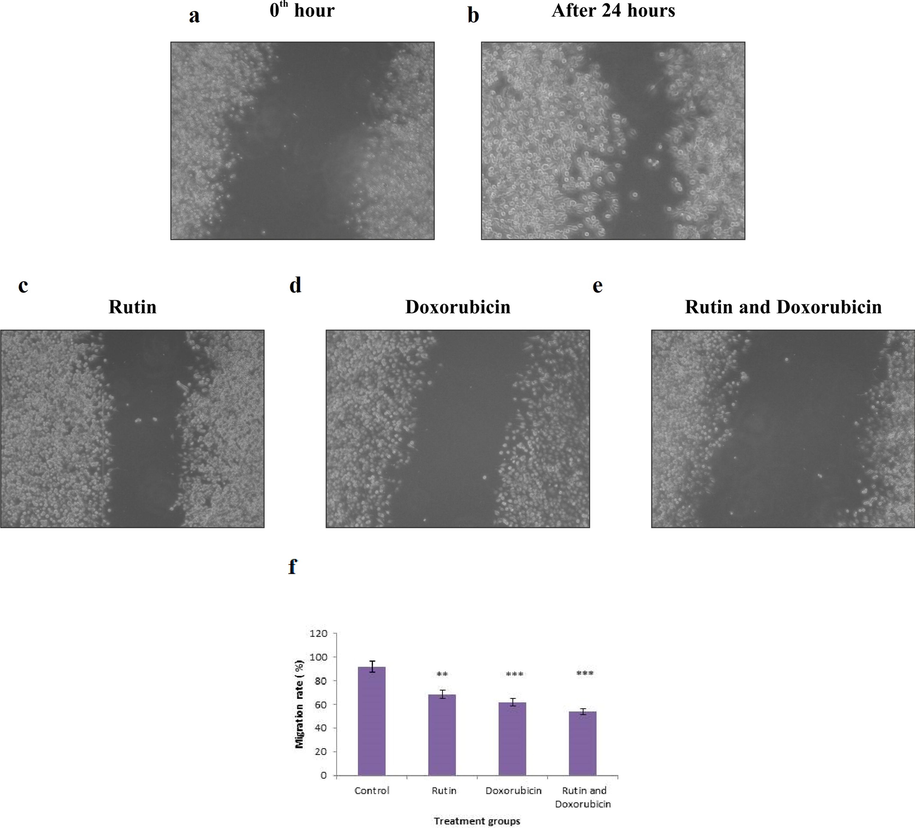
(a-e). MDA-MB-231 cells were cultured to confluence and scratched for the wound. Combinational treatment shows a greater influence on cell migration. The results are presented as the gap area in the initial scratch and 24 h of observation. (f). Quantification of migration rate of MDA‐MB‐231 cells after treatment with rutin and doxorubicin. Each bar represents the mean ± SD of triplicate samples from three independent experiments (*P < 0.01, *** P < 0.001 compared with untreated control).
3.5 Rutin and doxorubicin arrest TNBC cells at the G2/M phase of the cell cycle
Cancer is triggered by the activation of all signaling pathways, and the phases of the cell cycle, as well as the timing of events that modulate cell proliferation and division, have been studied. The effect of rutin and doxorubicin on cell cycle distribution was studied to determine the mechanism of their antiproliferative activity against cancerous cells. Cell cycle analyses were used to see if cell cycle arrest was occurring in a triple-negative breast cancer cell line caused by rutin and doxorubicin treatment. So we attempted to check the effect of rutin in stages of the cell cycle using flow cytometry. Untreated TNBC cells were uniformly dispersed in all phases of the cell cycle, indicating that no cell cycle arrest had occurred. TNBC cells that had only been treated with rutin were arrested in the G2/M phase. This showed that rutin can prevent cells from going through mitosis. In, doxorubicin alone treated cells showed that they were arrested at the G2/M phase of the cell cycle, which indicated that doxorubicin can arrest the cells at the mitotic phase. On combined treatment rutin and with doxorubicin we found that cells were arrested again at the G2/M phase of the cell cycle. Fig. 3(a-b) shows the distribution of different phases of the cell cycle. The distribution of cells after exposure to rutin to analyze cell death showed it was mediated by apoptosis and to assess the stage of cell cycle arrest, the result showed the cells were arrested at the G2M phase which is a mitotic phase of the cell cycle.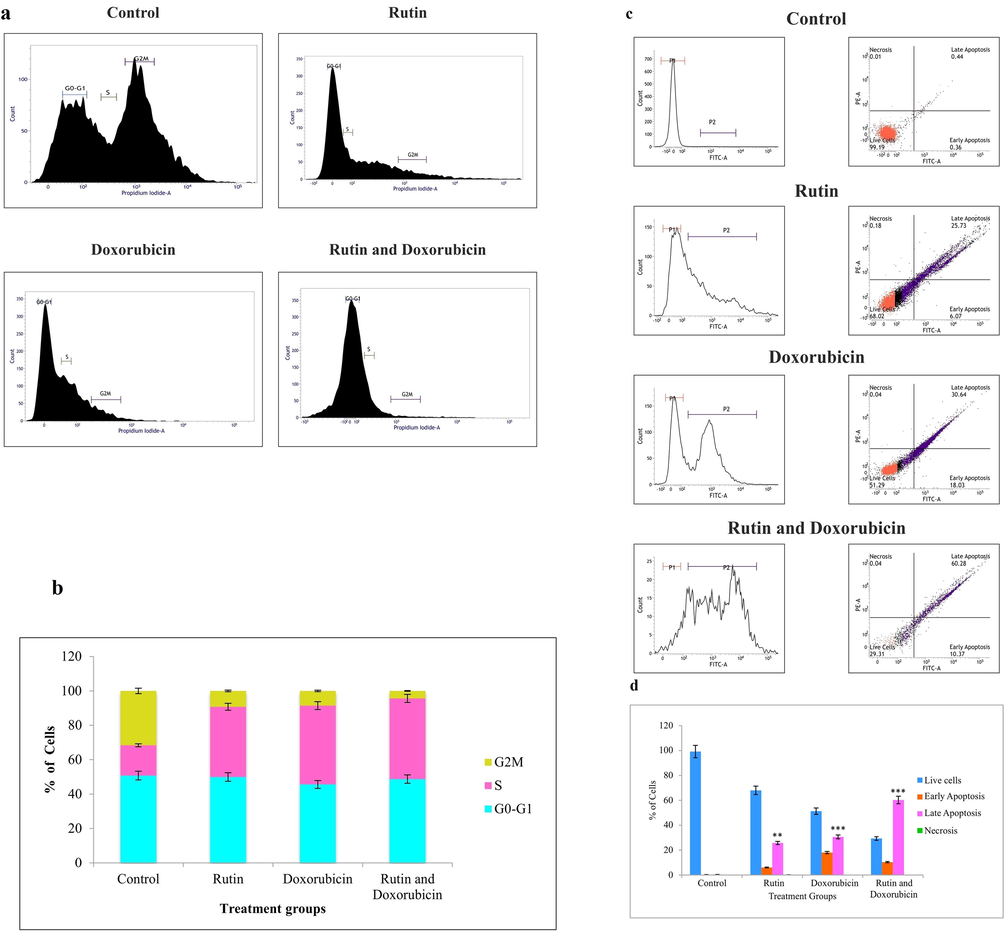
(a). After 24 h of treatment with40 µM rutin, 0.5 µM doxorubicin, and in combination group, flow cytometry analysis shows all the treated groups arrested the G2/M phase of the cell cycle. (b). Distribution of cells in various phases of the cell cycle. Each bar represents the mean ± SD of triplicate samples from three independent experiments (**P < 0.01, ***P < 0.001 versus untreated control). (c). Rutin + doxorubicin-induced significant ER stress mediated apoptotic cell death in MDA-MB-231 cells and synergistically increased the protein aggregate formation. (d). The bar chart shows the proportion of apoptotic cell death. Each bar represents the mean ± SD from three independent experiments (**P < 0.01, ***P < 0.001 compared to untreated control).
3.6 Rutin and doxorubicin mediate cell death through apoptosis by endoplasmic reticulum stress in MDA-MB-231 cells
Our research aimed primarily towards targeting the endoplasmic reticulum. The endoplasmic reticulum is a multi-functional organelle important for secretory and transmembrane protein synthesis, folding, and processing. Physiological and pathological stimuli may cause the ER homeostasis to disrupt, resulting in misfolded or unfolded proteins known as ER stress. Change in ER functions mediated by ER stress plays a crucial role in apoptotic cell death. Thioflavin dye is commonly used to image and measure misfolded protein aggregates that are present. The induction of ER stress-induced apoptotic effect by rutin was further confirmed by the flow cytometry study of the number of cells with thioflavin. The results showed that in the control group, a higher percentage of cells were in the viable stage and when the MDA-MB-231 cells were treated with rutin alone it showed 31.8% of cells were in an apoptotic stage. Among those 6.07% were in the early apoptotic stage and 25.73% were in the late apoptotic stage. Whereas, the cells treated with doxorubicin alone showed that 48.67% of cells were in the apoptotic stage. Among them, 18.03% were in the early apoptotic stage and 30.64% were in the late apoptotic stage. In the case of the rutin and doxorubicin treated group, 70.65% of cells were in the apoptotic stage. Among which 60.28% were late apoptotic and 10.37% were in an early apoptotic stage. The results clearly showed that rutin + doxorubicin-induced significant ER stress-mediated apoptotic cell death in TNBC cells can be exploited for combinational treatment. Fig. 3(c-d) shows the measurement of endoplasmic reticulum stress by flow cytometry.
3.7 Rutin and doxorubicin induce loss of mitochondrial membrane potential (ΔΨm) in TNBC cells
Mitochondria play a significant role in ATP production. An essential organelle involved in apoptosis is the mitochondrion. The loss of mitochondrial membrane potential (ΔΨ) is putatively the initial event leading to apoptosis. The untreated group existed several cells in monomer form. Whereas, in the rutin and doxorubicin alone treated group lesser amount of cells formed J-aggregates. However, in combined treatment with rutin and doxorubicin, there was a significant increase of cells containing J-aggregates when compared to rutin and doxorubicin alone treated groups. The detection of mitochondrial membrane potential by flow cytometry is shown in Fig. 4(a-b). The reaction of mitochondrial membrane potential is a classical phenomenon of apoptosis. Changes in mitochondrial permeability have been demonstrated to cause the depolarization of transmembrane potential (ΔΨm) and our study demonstrated an increase in mitochondrial membrane potential when treated in a combination with rutin and doxorubicin.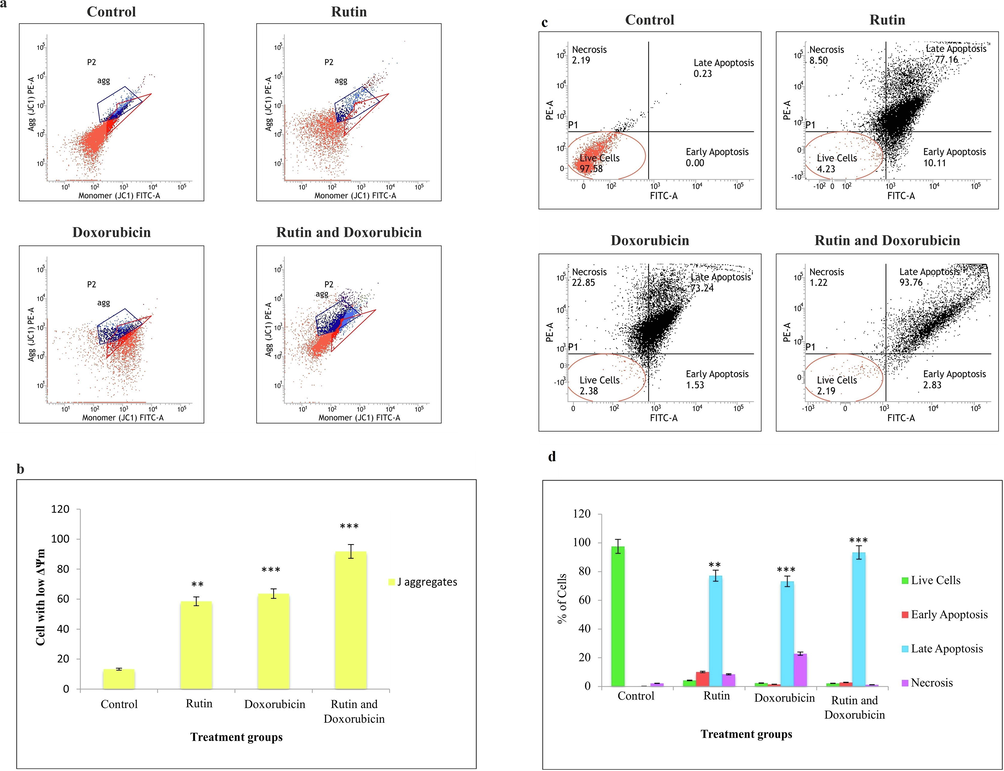
(a). Evaluation of mitochondrial membrane potential in MDA-MB-231 cells. The treatment group showed a loss of mitochondrial membrane potential. (b). The graph shows the TNBC cells with low ΔΨm. Each bar represents the mean ± SD of triplicate samples from three independent experiments (**P < 0.01, ***P < 0.001). (c). Apoptosis rates of MDA-MB-231 cells incubated with different treatment groups, the percentage of apoptotic cells were measured by flow cytometry. (d). The graph shows the distribution of cells in the apoptotic phase. Each bar represents the mean ± SD of triplicate samples from three independent experiments (**P < 0.01, ***P < 0.001).
3.8 Apoptotic cell death is induced by the combination of rutin with doxorubicin in TNBC cells
The key cellular event that happened during the development of the anticancer activity of an agent will comprise a large effect on the extent of DNA damage and tumor growth. Furthermore, this will disrupt cell cycle activity and finally result in cell death–either by apoptosis or other forms of cell death. Flow cytometry was used to examine apoptotic events using Annexin-V / FITC-PI. The fraction of cells in the life, early and late apoptotic, and necrotic states is determined using Annexin-V / FITC-PI.
The induction of apoptotic effect by rutin was further confirmed by evaluation of several cells by flow cytometry analysis with Annexin V-FITC/ PI. The results of Annexin-V/FITC-PI showed that, in the control group, a higher percentage of cells were in the viable stage and when the MDA-MB-231 cells were treated with rutin alone it showed 87.27% of cells in the apoptotic stage. Among those 77.16% were in the late apoptotic and 10.11% were in the early apoptotic stages. Whereas, the cells treated with doxorubicin alone showed that 74.77% of cells were in the apoptotic stage. Among which, 73.24% were late apoptotic and 1.53% were in the early apoptotic stage. In the case of the rutin and doxorubicin treated group, 96.18% of cells were in the apoptotic stage. Among which 93.35% were late apoptotic and 2.83% were in an early apoptotic stage. The results clearly showed that rutin + doxorubicin-induced significant apoptotic cell death in TNBC cells can be exploited for combinational treatment. The detection of cell death by apoptosis was shown in Fig. 4(c-d). The study revealed that MDA-MB-231 cells on treatment with rutin induced cell death mediated by apoptosis, especially in the late apoptotic stage.
3.9 Interaction analysis of rutin with ER stress proteins
Specific computational approaches are used to test the drugs or chemicals' capacity for anticancer. In silico docking was a commonly used method in drug designing for cancer. Binding mode studies are important for elucidating crucial structural properties and interactions, as well as providing useful information for the development of effective inhibitors. Molecular docking studies were done with the selected ER stress proteins, namely IRE1, PERK, TRAF2, GRP78, ASK1, JNK, BCL-XL, DGC4, BCHE, VCP, and HMOX1. The results obtained in the molecular docking studies of the target proteins with active phytocompound rutin are shown in Table 2. The results of the docking studies showed that among the several ER Stress proteins interaction with rutin showed a high glide score of −8.953 and −8.248 with ASK1 and JNK compared to other proteins. Apoptosis signal-regulating kinase1, also known as mitogen-activated protein kinase kinase kinase 5 (MAP3K5), is a protein that regulates apoptosis. It activates c-Jun N-terminal kinase and p38 in response to oxidative damage, endoplasmic reticulum stress, and calcium influx. ASK family plays important role in neurodegenerative diseases and cancer. JNK (c-Jun N-terminal kinase) is one of the mitogen-activated protein kinase (MAPK) key signaling cassettes. JNK activation is a common response to a variety of stressors, and it is known to regulate cell death machinery by directing the BCL2 family of proteins. Fig. 5 (a-f) shows the interaction of rutin with ASK1 and JNK.
S.No
Protein
Glide score
Glide energy
H Bond
1.
IRE1
−7.932
−67.693
7
2.
PERK
−5.213
−49.506
7
3.
TRAF2
−7.049
−64.769
5
4.
GRP78
−4.687
−46.170
2
5.
ASK1
−8.953
−67.337
6
6.
JNK
−8.248
−65.342
6
7.
BCL – XL
−6.148
−62.488
6
8.
BCHE
−8.182
−64.899
3
9.
VCP
−6.092
−57.247
6
10.
HMOX1
−6.227
−56.693
4
11.
DGC4
−6.112
−50.355
6
Rutin Pubchem Id – 5,280,805
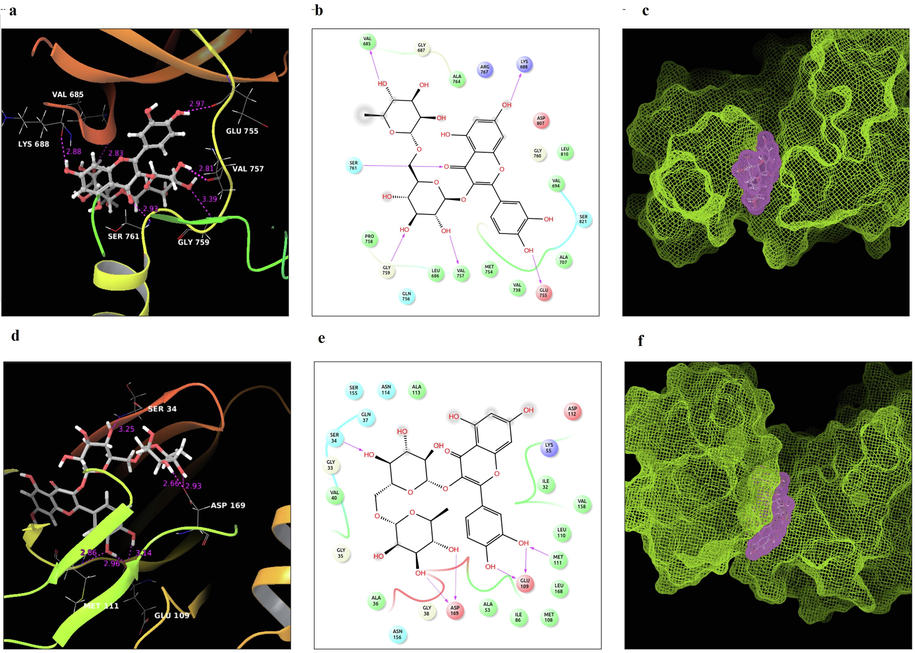
(a). H-bond interaction between active compound rutin and ASK1; (b). Ligand interaction of active compound rutin and ASK1; (c). Surface view of rutin with the active site of target ASK1; (d). H-bond interaction between active compound rutin and JNK; (e). Ligand interaction of active compound rutin and JNK; (e). Surface view of rutin with the active site of target JNK.
3.10 Up-regulation of ASK1 and JNK by rutin induces MDA-MB-231 cells apoptosis
The study of gene expression is becoming more necessary in biological fields. Understanding patterns of expressed genes is expected to provide insight into complicated regulatory networks and contribute to the discovery of genes involved in certain biological processes, such as cancer. Among different approaches, qRT-PCR is now considered the gold standard for accurate, sensitive, and rapid gene expression assessment. We attempted to see the expression of two ER stress-mediated cell death genes ASK1 and JNK which we got the highest glide score. β-Actin was used as a control. Once amplification was done the results were obtained. Total RNA was extracted from MDA-MB-231 cells by Trizol extraction method. Our results showed rutin alone and in combination with doxorubicin increased the expression of both ASK1 and JNK. The expression levels of the two genes were tested in the treated and untreated samples. The efficiency of amplification was tested by monitoring the slope of amplification curves produced during the amplification in real-time as shown in Fig. 6A.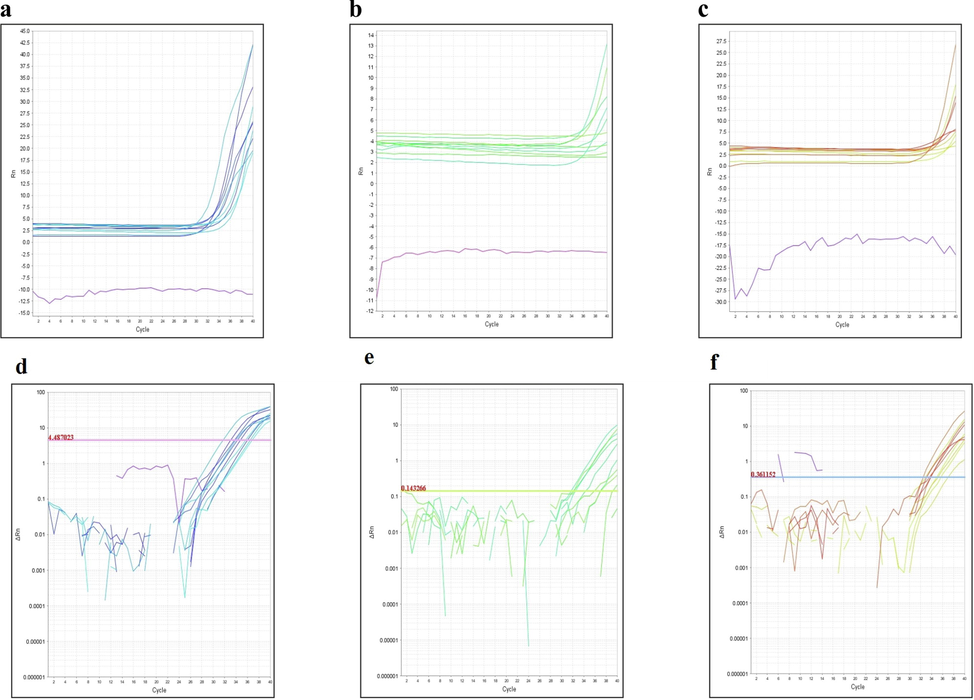
(A). The efficiency of amplification was tested by monitoring the slope of amplification curves produced during the amplification in real-time. (a) ASK1; (b) JNK; (c) β-actin. (B). The target's Ct values were incorporated into the standard curve, as calculated by qRT-PCR. The Ct values plotted for each dilution are the mean of triplicates. (a) ASK1; (b) JNK; (c) β-actin.
The target's Ct values were incorporated into the standard curve, as calculated by qRT-PCR assayed in the experiment as shown in Fig. 6B. The Ct values plotted for each dilution are the mean of triplicates. The expression of ASK1 and JNK in MDA-MB-231 cells was measured by qRT- PCR assay; MDA-MB-231 cells were used as the normal control. ASK1 is an apoptosis signal-regulating kinase and plays a key role in ER-stress-triggered cell death. ER-stress activates ASK1 by forming the complex IRE1-TRAF2-ASK1, and thus mediates cell death. Hence upregulation of ASK1 will trigger ER stress-mediated cell death. JNK known as C-Jun-N terminal kinase activates cell death through the regulation of Bcl-2 family proteins. In the present study, ER stress mediates the overexpression of JNK and hence it triggers ER stress-JNK mediated apoptosis in the breast cancer cells.
Relative quantification normally expressed as 2−ΔΔCt is usually expressed as fold change was compared with calibrator β-actin. The relative expression of genes was shown in Fig. 7. The fold change values indicate that there is less expression of ASK1 and JNK. When treated with rutin the expression of both ASK1 and JNK was found to be higher. The standard drug doxorubicin showed an RQ value of −0.5 indicating it was less expressed. A combination of rutin and doxorubicin showed a minor increase in the expression level which may be because of rutin. The results clearly showed that rutin by itself can modulate the expression of ASK and JNK, a set of genes implicated in apoptosis mediated by the ER stress pathway. Our findings reveal that rutin causes apoptosis in breast cancer cells, which is linked to the ER stress response, via up-regulation of ASK1 and JNK.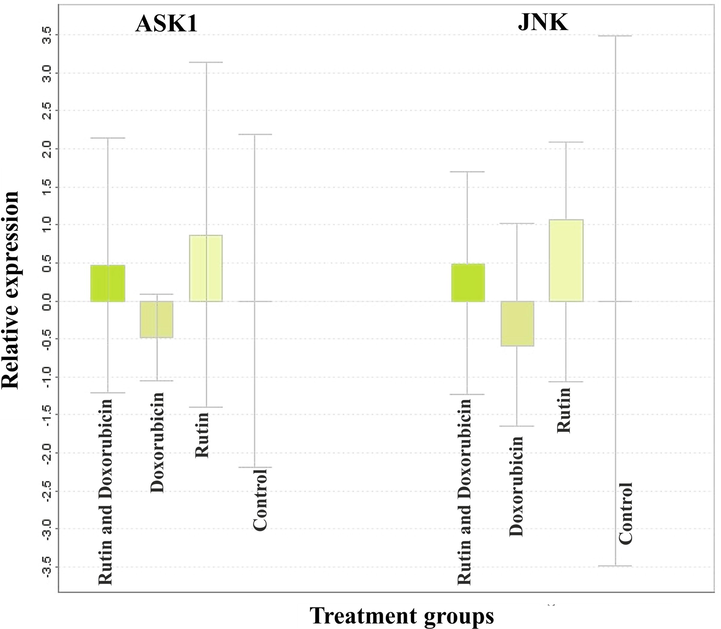
Relative quantification normally expressed as 2−ΔΔCt is usually expressed as fold change was compared with calibrator β-actin. Rutin by itself can modulate the expression of ASK and JNK, the genes involved in ER stress pathway-mediated apoptosis.
4 Discussion
One of the key drawbacks of treating cancer is the lack of treatments that can effectively prevent metastasis from developing. Metastatic breast cancer is largely untreatable, which accounts for most breast cancer deaths. TNBC patients are considered to have a greater risk for metastatic disease compared to patients with other subtypes of breast cancer (García-Alonso et al., 2018). The unfolded protein response (UPR), a response mechanism for cell stress, is considered a prominent target for novel therapies for breast cancer (McGrath et al., 2018). Phytocompounds have been shown to improve treatment efficacy, reduce toxic chemotherapy side effects, prevent metastasis, and improve the overall quality of life. Rutin, a polyphenolic bioflavonoid, offers a wide range of therapeutic applications due to its high antioxidant capabilities. The current study has shown that it has pharmacological benefits in the treatment of chronic diseases such as triple-negative breast cancer (Ghițu et al., 2019). In the present study, we observed that rutin and doxorubicin (standard chemotherapeutic drug) suppressed the proliferation of cells by inducing the arrest of the G2 / M cell cycle and induced apoptosis mediated by ER stress in MDA-MB-231 cells.
Rutin could induce substantial cytotoxic effects in MDA-MB-231 cells in a dose and time-dependent manner, according to the findings. Uncontrolled proliferation and a greater ability to migrate are two essential basic properties of tumor cells (García-Alonso et al., 2018). The MTT assay indicated that MDA-MB-231 cell proliferation and viability had been substantially inhibited by rutin and doxorubicin. This research has shown that in MDA-MB-231 cells, the combination treatment of rutin and doxorubicin has a decreased cell proliferation and migratory ability.
The cell cycle is the series of events occurring in a cell that leads to a cell's survival through its division and replication process. Previous research has shown that flavonoids are a significant aspect of cancer treatment, promoting cell cycle arrest at different stages (Majewska-Wierzbicka and Czeczot, 2012). Our results indicated that all the treatment groups induced G2/M phase arrest in MDA-MB-231 cells which is an indicator of ER stress. The endoplasmic reticulum is a key organelle involved in protein production and folding (Wang et al., 2014). Several natural compounds and pharmacological reagents cause ER stress-induced G2/M cell cycle arrest and cell death in diverse cell lines. Hence, the induction of ER stress-mediated by rutin and doxorubicin was evaluated by flow cytometric analysis with thioflavin. The results showed that in the untreated group a higher percentage of cells were in the viable stage and when the MDA-MB-231 cells were treated with rutin alone it showed more aggregates and decreased viability. Whereas, the cells treated with the combination of rutin and doxorubicin showed significantly more aggregates than either alone. The results clearly showed that rutin and doxorubicin caused a considerable amount of apoptosis mediated by ER stress and hence ER stress and were also able to arrest the cells at the mitotic phase of the cell cycle. The outcome of our study showed that ER stress can cause apoptosis by inducing changes in mitochondrial membrane potential.
Mitochondria also play a key function in activating apoptosis (Yeap et al., 2017). The intrusion of mitochondrial function is significantly an early incident in cell commitment to apoptosis. The inconsistency in the levels of mitochondrial membrane potential was measured using flow cytometry using JC-1 stain and the fluorescent dye was used to observe mitochondrial membrane potential to calculate the viability and thus the function of mitochondria. The number of cells in the cell alone (MDA-MB-231) group illustrated more number of J-aggregates whereas in the cells exposed to rutin and doxorubicin there was a decrease in the number of J-aggregates. These results were indicative of the fact that mitochondria are naturally involved in the process of apoptosis and there was a drastic change in the mitochondrial membrane potential expressed in the form of well-dispersed monomers when treated with combination treatment when compared to rutin and doxorubicin alone treatment. In addition, it also suggested that ER stress when exposed to rutin and doxorubicin in MDA-MB-231 cells. It demonstrates that rutin inhibits mitochondrial membrane potential and causes apoptosis in MDA-MB-231 cells. Apoptosis is a significant form of programmed cell death (Gao et al., 2016). Apoptosis suppresses cell proliferation and the ER-stress proteins play an important role in the response to apoptosis of cancer cells (Czabotar et al., 2014). This study revealed that rutin with doxorubicin showed a drastic effect on MDA-MB-231 cells and cell death has occurred in late apoptosis. This finding suggested that the combinatorial effect of rutin and doxorubicin can be exploited for the programmed cell death of triple-negative breast cancer. Our results too confirmed that the major pathway of cell death was mediated by apoptosis. These results further support the fact that rutin can be used as an alternative or in combination with chemotherapeutic drugs because it was effective against triple-negative breast cancer cells. The outcome of our study showed that treatment with rutin and doxorubicin together induced apoptosis at the late apoptotic stage. The observed cytotoxicity and cell migratory capacity of anethole can be exploited along with doxorubicin as an anti-cancer agent which can act by mediating apoptosis, inhibiting cell proliferation in breast cancer cells.
Our investigation explains that rutin has good interactive properties with ASK1 and JNK oncoprotein expressed stress conditions. The spontaneous interactions of rutin with ASK1 and JNK indicated that rutin had the potential to disrupt the natural integrity of ASK1 and JNK of any confirmation (Kim and Roberts, 2016), and our study too authenticated the fact that rutin promotes ER stress-mediated apoptosis in cancer. qRT-PCR array design and validation enable accurate analysis of gene expression regulation that occurs in biological models subjected to drug treatment(Amatori et al., 2017). This can be used for the detailed investigation of gene expression involving regulation of the cell cycle, DNA damage, and apoptosis (Poloni et al., 2014). It gives an overview of the molecular pathways that are triggered in human cancer cells by drug treatment (Guerzoni et al., 2015). In our study, rutin alone treatment was able to up-regulate growth arrest and DNA damage response, and ER-stress genes ASK1 and JNK. Our results provoked that ER stress-associated apoptosis was induced upon rutin treatment, which may be a promising therapeutic target for triple-negative breast cancer.
In all the experiments rutin showed promising results and its activity was on par with the standard drug doxorubicin. Our findings validate the fact that rutin may trigger cell death through endoplasmic reticulum stress-mediated cell death. Further other genes involved in ER stress-related pathways need to be probed to understand the mechanism of action of rutin in TNBC.
5 Conclusion
Rutin is a flavonoid abundantly found in plants, and attributed with a lot of pharmaceutical properties, and is being exploited to treat a variety of ailments. This is a potent anticancer agent that exhibits its anticancer activity by various mechanisms, including inhibiting cancer cell proliferation and inhibiting the protein synthesis that is responsible for tumor growth. Whereas the results suggest that rutin can be effectively used to target TNBC. The findings clearly showed that ER stress plays a major role in causing apoptosis induced by cell death. Gene expression studies also clearly justified these findings. Hence rutin can be exploited to target TNBC and can be used alone even without doxorubicin because the results of our study in many assays revealed rutin alone is also more effective than doxorubicin. This fact can be exploited to use rutin as a drug for TNBC to avoid the toxic side effects of chemical-based drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. The FEBS J.. 2019;286:241-278.
- [CrossRef] [Google Scholar]
- Real-time quantitative PCR array to study drug-induced changes of gene expression in tumor cell lines. J. Cancer Metastasis Treat.. 2017;3:90.
- [CrossRef] [Google Scholar]
- Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer: Targets Therapy.. 2016;8:93-107.
- [CrossRef] [Google Scholar]
- Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol.. 2014;15:49-63.
- [CrossRef] [Google Scholar]
- Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet.. 2016;48:1119-1130.
- [CrossRef] [Google Scholar]
- A comprehensive assessment of apigenin as an antiproliferative, proapoptotic, antiangiogenic and immunomodulatory phytocompound. Nutrients.. 2019;11:858.
- [CrossRef] [Google Scholar]
- CD99 triggering in Ewing sarcoma delivers a lethal signal through p53 pathway reactivation and cooperates with doxorubicin. Clin. Cancer Res.. 2015;21:146-156.
- [CrossRef] [Google Scholar]
- LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci.. 2015;22:1-9.
- [CrossRef] [Google Scholar]
- The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochimica et Biophysica Acta (BBA)-Mol. Cell Biol. Lipids. 2001;1530:162-171.
- [CrossRef] [Google Scholar]
- Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619-3625.
- [CrossRef] [Google Scholar]
- Anticancer activity of flavonoids. PolskiMerkuriuszLekarski: Organ PolskiegoTowarzystwaLekarskiego. 2012;33:364-369.
- [Google Scholar]
- Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+ α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res.. 2007;67:6796-6805.
- [CrossRef] [Google Scholar]
- Overexpression of CDKN2B (p15INK4B) and altered global DNA methylation status in mesenchymal stem cells of high-risk myelodysplastic syndromes. Leukemia. 2014;28:2241-2244.
- [CrossRef] [Google Scholar]
- Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages. BMC Cancer.. 2018;18:1197.
- [CrossRef] [Google Scholar]
- Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin. Invest. Drugs. 2013;22:1063-1079.
- [CrossRef] [Google Scholar]
- Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J. Oncol. 2016
- [CrossRef] [Google Scholar]
- Risk factors and preventions of breast cancer. Int. J. Biol. Sci.. 2017;13:1387-1397.
- [CrossRef] [Google Scholar]
- Recent advances in the treatment of breast cancer. Front. Oncol.. 2018;8:227.
- [CrossRef] [Google Scholar]
- Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomedicine.. 2018;14:15.
- [CrossRef] [Google Scholar]
- Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Experimental Cell. 2010;research.316:1271-1281.
- [CrossRef] [Google Scholar]
- Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490
- [CrossRef] [Google Scholar]
- Automatic contour propagation using deformable image registration to determine delivered dose to spinal cord in head-and-neck cancer radiotherapy. Phys. Med. Biol.. 2017;62:6062.
- [CrossRef] [Google Scholar]







