Translate this page into:
On valency based topological properties of the starphene and fenestrene
⁎Corresponding author. attari_ab092@yahoo.com (Abdul Rauf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In theoretical chemistry the quantitative parameters which are used to describe the atomic topology of graphs are termed as topological indices. Through these topological indices many physical and chemical characteristics such as melting point, entropy, energy generation and vaporisation enthalpy of chemical compounds can be predicted. The theory of graphs has a significant use in measuring the relationship of certain associated graphs with various topological indices. In this paper, we compute novel topological indices based on eV- and ve-degrees for starphene and fenestrene . A Maple-based algorithm is proposed for the calculation of ve and eV-degree based topological indices from the graph adjacency matrix.
Keywords
Valency based topological indices
Starphene and fenestrene structures
Algorithm for calculating valency based topological indices
05C09
05C92
92E10

1 Introduction
Chemical graph theory is a branch of mathematical chemistry that applies graph theory to mathematical modeling of chemical phenomena. The topological index is a graph invariant that describes the molecular structure’s topology and transforms it to a real number that predicts certain of its physico-chemical characteristics such as freezing point, infrared spectrum, boiling point, viscosity, melting point, electronic parameters, and density (Zhong et al., 2021; Süleyman, 2017; Rauf et al., 2021). The reader may access to some of the previously published works on topological indices (Rauf et al., 2022; Sarkar et al., 2018; Sarkar and Pal, 2020; Naeem and Rauf, 2021; Rauf et al., 2021).
Starphene is a single ring of benzene that was surrounded by three identical substituents of arene (Rüdiger et al., 2019). The derivatives of benzene are manufactured on large scale for use in high-quality octane gasoline and production of polymer. Ortho-bromotetracene and ortho-dibromopentacene are suitable and undergoes the trimerization of Ni-catalyzed for the formation of starphene. The red starphene is made up of three hexacenes, with one centre ring shared with the orange star. Decastarphene-(3,3,3) is the largest unsubstituted derivatives of starphene which is formulated in 1968 by Clar and Mullen (1968). It is very complicated to prove that this is a complete aromatic conjugation that does not exist in starphene substituent. Starphene is an attractive compound due to its physicochemical properties. They inherited their character from arenes, which make them an interesting compound for the study of organic electronics and optics. It makes it very challenging for the synthesis of larger starphene due to their instability (Holec et al., 2021). The general fenestrenes uses the following formulas: This is the ZZ polynomial of fenestrenes. This formula is helpful to find the alternative formula for the ZZ polynomial of fenestrenes . The structure of fenestrenes contains zigzag chains that forms four disconnected parts (Chou and Witek, 2015). Generally, fenestrenes are used for the testing of pro-apoptotic activities on the two human cancer cell line (THP-1 and SW620) (Hulot et al., 2010).
Let be a simple graph, where is a non-empty set of elements referred to as vertices or points and is a set of unordered pairings of different members of referred to as edges or lines. The sets and are referred to as the vertex set and edge set respectively. The degree of a vertex , represented by the symbol , is the number of edges that are incident to v. Two vertices u and v of G are adjacent if they are connected by an edge . The open neighbourhood of v is defined as the collection of vertices that are connected to u and is represented by the symbol . The closed neighbourhood of u denoted by is obtained by adding the vertex u to .
Topological indices (TI) plays an important role in explaining and quantifying the molecular structure of hydrocarbons. The first topological index was defined by Wiener (1947) while he was working on the boiling point of paraffin. Randic index was introduced by Randic (1975) and for any graph G, it is defined as Gutman and Trinajstic (1972) proposed the first and second Zagreb index in 1972 and applied them to the branching problem (Gutman et al., 1975). The first and second Zagreb indices are defined as The sum connectivity index was proposed by Zhou and Trinajstic (2009). It is denoted and defined as: Zhong (2012) proposed the harmonic index which is defined as follows: Estrada et al. (1998) introduced the notion of Atom Bond Connectivity (ABC) index which is defined as Graovac and Pisanski (1991) proposed Geometric Arithmetic (GA) index which is defined as For more details on the study and computation of topological indices, the reader may refer to (Aslam et al., 2018; Aslam et al., 2017; Bashir et al., 2017; Chu et al., 2021).
Recently, two variants of degree based topological indices were proposed by Randic (1975). These indices are based on ve-degree and eV degree. Some mathematical properties of these indices were discussed by Bollobs and Erdos (1998). Ediz (Amic et al., 1998; Graovac and Pisanski, 1991; Estrada et al., 1998) translated the classical degree based topological indices into eV-degree and ve-degree based topological indices. It was observed that ve-degree based zagreb index has strong predictability power than the classical Zagreb index. The eV-degree of any edge
is the total number of the vertices of closed neighbourhoods of the end vertices of an edge e. The eV degree of an edge e is denoted by
. The ve-degree of any vertex
is the total number of different edges which are adjacent to v and the first neighbor of v, and is denoted by
. The Mathematical formulas for some of the eV-degree and ve-degree based indices are presented in Table 1.
Ev-degree based topological indices
Notation
Mathematical Formula
Randić index
Zagreb index
Ve-degree based topological indices
Notation
Mathematical Formula
First Zagreb
-index
Second Zagreb
-index
Harmonic index
Sum Connectivity index
Geometric Arithmetic index
Atom Bond Connectivity index
Randić index
First Zagreb
-index
2 Structure of starphene
In this section, we discuss a class of benzenoid system named starphene. Starphene is colorless and its melting point is 198°C. If three hexagons are attached by a common vertex, the benzenoid structure is called peri-condensed, otherwise, we say catacondensed. We denote the structure of starphene by
, where
denotes the number of hexagons in each linear chain attached with the central hexagon respectively. The molecular structure of starphene is depicted in Fig. 1. The structure of
has total number of
vertices and
edges. Let
denotes the vertex set of
containing the vertices of degree i. Then the vertex set
can be partitioned into two sets
and
with
and
. Let
denotes the edge set containing the edges of
having degree of end vertices as i and j. The edge set of
can be partitioned as follows:
containing 9 edge,
containing
edges and
containing
edges.![Structure of starphene St [ n , m , l ] .](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104054-fig1.png)
Structure of starphene
.
2.1 Main results
Let H denoted the graph of starphene . Let , then eV-degree Zagreb and Randic index are
(a) .
(b) .
By using the edge partition given in Table 2, we can compute the eV-degree Zagreb and Randić index as follows
| Frequency | ||
|---|---|---|
| 4 | 9 | |
| 5 | ||
| 6 |
(a) Ev-degree Zagreb index
(b) Ev-degree Randić index
Fig. 2 and Numerical computation depicted in Table 5 and 6 shows an increasing behavior of eV-degree based indices with the increase in the value of and l. Zhong et al. (2021) developed the quantitative structure–property relationship (QSPR) between physical properties (Docking Score, Binding Affinity, Molecular Weight and Topological Polar Surface) and ve- and eV-degree based topological indices. They examined that among eV-degree based indices, the first eV-degree Zagreb index ( ) predict the molecular weight better than eV-degree Randic index ( ). Hence, the above results of eV-degree based indices are helpful to measure the physical properties of starphene.
Let H be a structure of starphene, then
The partition of vertex set of starphene based on ve degree of each vertex is depicted in Table 3. By using Table 3, we have first ve-degree Zagreb -index
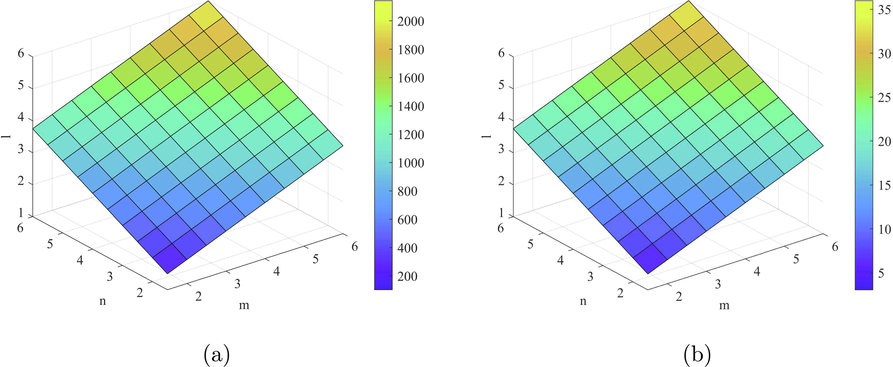
- Plots of the indices (a)
and (b)
.
| Frequency | ||
|---|---|---|
| 2 | 4 | 6 |
| 2 | 5 | 6 |
| 2 | 6 | |
| 3 | 7 | |
| 3 | 8 | 6 |
Fig. 3 shows an increasing behavior of first Zagreb alpha index ( ) with an increase in the values of and l. Süleyman (2017) developed QSPR between physical properties (Enthalpy of vaporization (HVAP), Entropy, Standard enthalpy of vaporization (DHVAP), and Acentric factor) and ve and eV-degree based Zagreb and Randic type indices(Süleyman, 2017). He examines that can be helpful to predict the physical property Acentric Factor. Zhong et al. (2021) analyzed that can predict the molecular weight better than eV-degree Randic index ( ). Hence, the above result of ve-degree based index can be helpful to estimate the physical properties of starphene.
Let H be a structure of starphene structure and , then
(a) .
(b) .
(c) .
(d) .
(e) .
(f) .
(g) .
By using the edge partition given in Table 4, we can compute the topological indices based on the ve degree of end vertices of each edge as follows
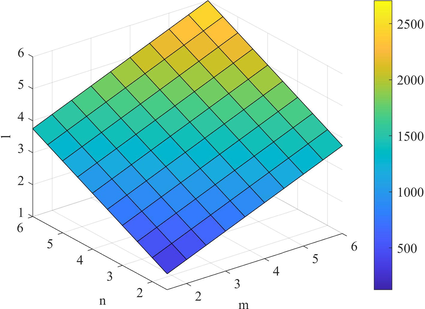
- Plot of
.
| Edge | Frequency | |
|---|---|---|
| (4, 4) | 3 | |
| (4, 5) | 6 | |
| (5, 7) | 6 | |
| (6, 7) | ||
| (6, 8) | 6 | |
| (7, 7) | ||
| (8, 8) | 6 |
| ) | |||||
|---|---|---|---|---|---|
| 102 | 3.0415 | 126 | 54 | 147 | |
| 510 | 9.6328 | 642 | 252 | 798 | |
| 918 | 16.2241 | 1158 | 450 | 1449 | |
| 1326 | 22.8154 | 1674 | 648 | 2100 | |
| 1734 | 29.4067 | 2190 | 846 | 2751 | |
| 2142 | 35.9980 | 2706 | 1044 | 3402 | |
| 2550 | 42.5893 | 3222 | 1242 | 4053 | |
| 2958 | 49.1806 | 3738 | 1440 | 4704 | |
| 3366 | 55.7719 | 4254 | 1638 | 5355 | |
| 3774 | 62.3632 | 4770 | 1836 | 6006 |
(b) Second Zagreb -index
(c) Atom-bond connectivity index
(d) Geometric-arithmetic index
(e) Harmonic index
(f) Sum-connectivity index
(g) Randic index
Figs. 4–7 shows the behavior of the computed topological indices based on ve degree. The values of all these topological indices increases with the increase in the value of
and l. Süleyman (2017) examines that the second Zagreb beta index can be used to predicts the entropy and the Randic index can be helpful to predicts the Enthalpy of vaporization and Standard enthalpy of vaporization. Zhong et al. (2021) investigated that the first Zagreb beta index (
) predicts the molecular weight and Topological Polar Surface better than other ve-degree based topological indices. Overall,
is the best predictor of the molecular weight and Topological Polar Surface in all ve- and eV-degree based indices. Hence, the above results are helpful to measure the physical properties of starphene.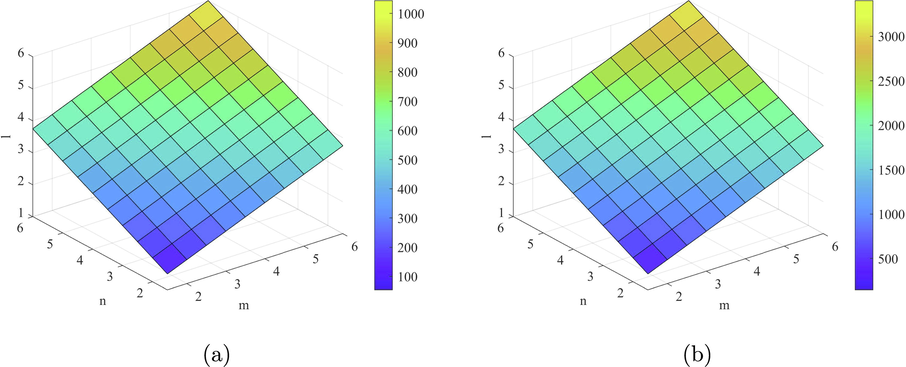
plots of the indices (a)
and (b)
.
plots of the indices (a)
and (b)
.
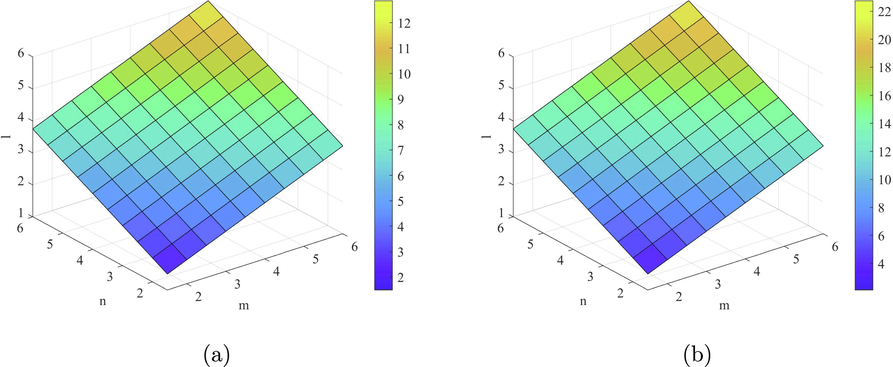
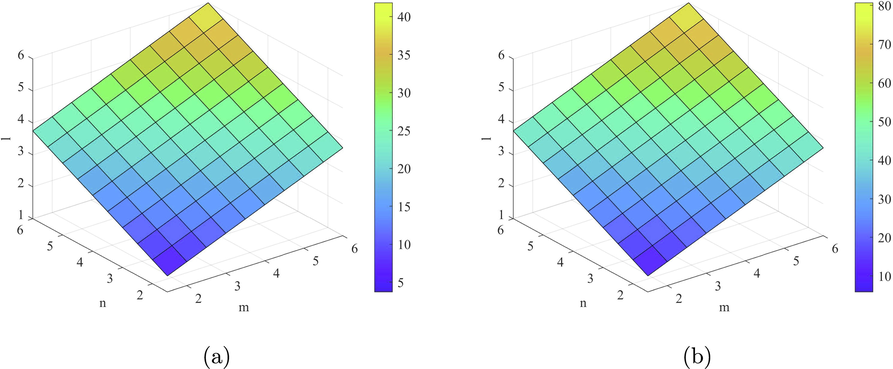
Plots of the indices (a)
and (b)
.
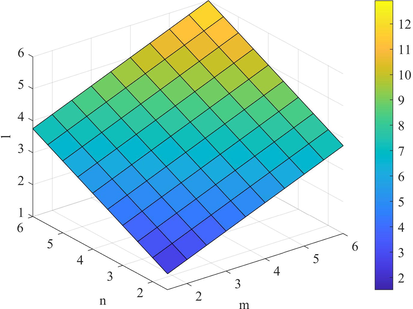
Plot of the indices
.
3 Structure of fenestrene
In this section, we discuss about the fenestrene structure which belongs to the class of benzenoid systems. Acenes are benzene rings fused linearly. We denote the structure of fenestrene by
, where m and n denoted the number of hexagons in each row and column respectively. The molecular structure of fenestrene is depicted in Fig. 8. The structure of
has
vertices. For
the graph of
has
edges and for
it has
edges. Let
denotes the edge set containing the edges of
with end vertices of degree i and j respectively. The edge set of
can be partitioned based on the degree of end vertices as follows:
containing
edge,
containing
edges and
containing
edges.![Fenestrene structure F [ n , m ] .](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104054-fig8.png)
Fenestrene structure
.
3.1 Main results
Let H denotes the structure of fenestrene . Let and , then eV-degree Zagreb and Randic index of fenestrene are
(a) .
(b) .
By using the edge partition given in Table 7, we can compute the eV-degree Zagreb and Randic index as follows
| ) | ) | ) | ) | ) | |
|---|---|---|---|---|---|
| 3.7037 | 5.8707 | −0.0073 | 2.1022 | 1.5158 | |
| 11.3295 | 20.8352 | 2.2674 | 6.2322 | 3.7960 | |
| 18.9554 | 35.7996 | 4.5421 | 10.3622 | 6.0762 | |
| 26.5812 | 50.7641 | 6.8168 | 14.4921 | 8.3565 | |
| 34.2070 | 65.7285 | 9.0916 | 18.6221 | 10.6367 | |
| 41.8328 | 80.6929 | 11.3663 | 22.7521 | 12.9169 | |
| 49.4586 | 95.6574 | 13.6410 | 26.8821 | 15.1971 | |
| 57.0844 | 110.6218 | 15.9158 | 31.0121 | 17.4773 | |
| 64.7102 | 125.5863 | 18.1905 | 35.1421 | 19.7575 | |
| 72.3360 | 140.5507 | 20.4652 | 39.2721 | 22.0377 |
| Frequency | ||
|---|---|---|
| 4 | ||
| 5 | ||
| 6 |
(b) Ev-degree Randić index
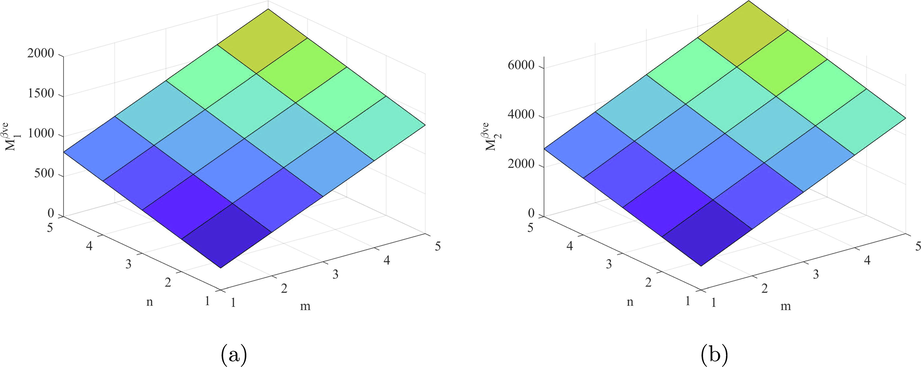
Let H denotes the structure of fenestrene , then
The vertex partition of H based on ve degree is depicted in Table 8. Using the definition of the topological index we get
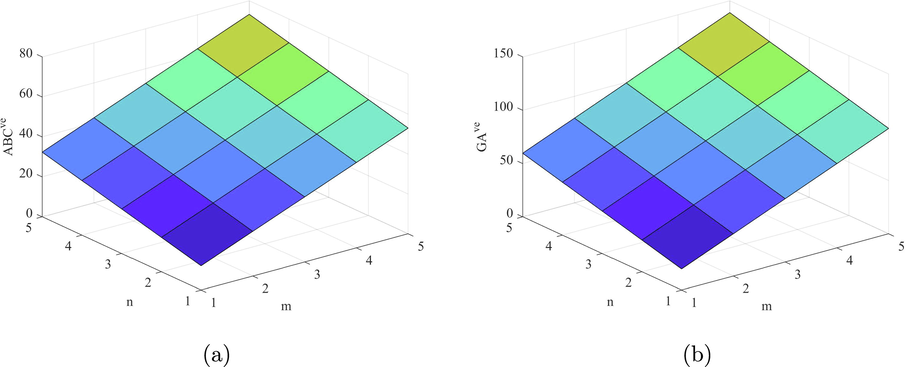
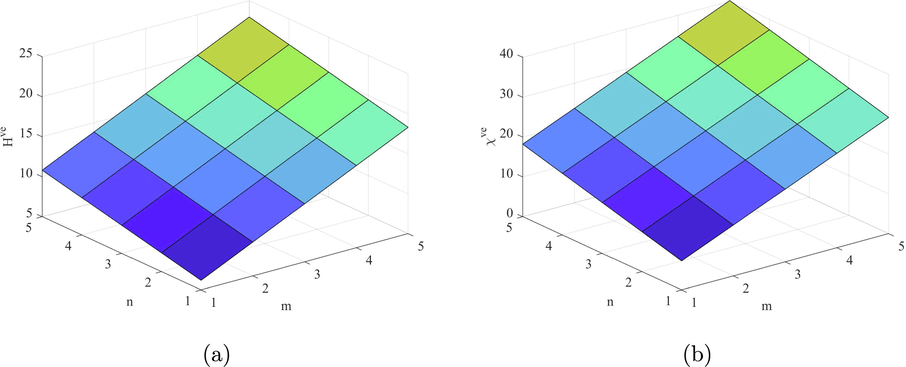
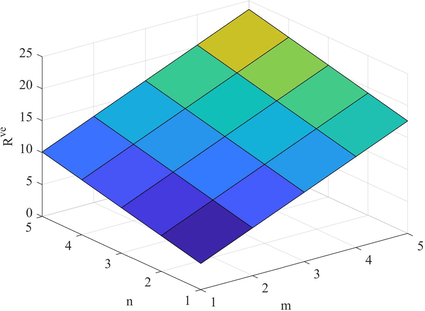
Let H be a structure of fenestrene structure and and , then
(a) .
(b) .
(c) . .
(d) . .
(e) .
(f) . .
(g) . .
By using the edge partition given in Table 9, we can compute the indices based on the ve degree of end vertices of each edge as follows
| Frequency | ||
|---|---|---|
| 2 | 5 | |
| 2 | 6 | |
| 3 | 7 | |
| 3 | 8 | |
| 3 | 9 | 4 |
| Edge | Frequency | |
|---|---|---|
| (5, 5) | ||
| (5, 7) | 12 | |
| (5, 8) | ||
| (6, 7) | ||
| (6, 8) | 4 | |
| (7, 8) | ||
| (7, 9) | 4 | |
| (8, 8) | ||
| (8, 9) | 8 |
(a) First Zagreb -index
(b) Second Zagreb -index
(c) Atom-bond connectivity index
(d) Geometric-arithmetic index
(e) Harmonic index
(f) Sum-connectivity index
(g) Randić index
Figs. 9–14 and Numerical computations depicted in Tables 10 and 11 shows that an increase in the value of n and m increases the value of these topological indices. Zhong et al. (2021) examined that the first eV-degree Zagreb index (
) predict the molecular weight better than eV-degree Randic index (
). The first ve-degree Zagreb beta index (
) predicts the molecular weight and Topological Polar Surface better than other ve-degree of end vertices based indices. Overall,
is the best predictor of the molecular weight and Topological Polar Surface in all ve- and eV-degree based indices. Süleyman (2017) examines that the
can be helpful to predicts the physical property Acentric Factor. Also, the second Zagreb beta index predicts the entropy and the Randic index predicts the Enthalpy of vaporization and Standard enthalpy of vaporization, respectively. Hence, the above results are helpful to measure the physical properties of fenestrene.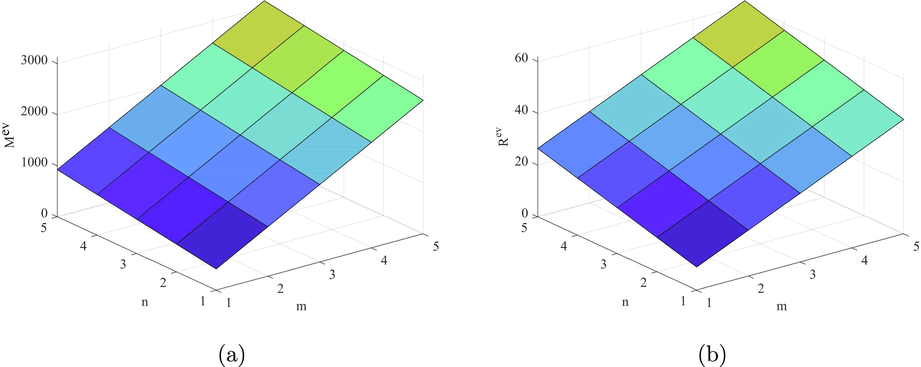
Plots of the indices, (a)
and (b)
.
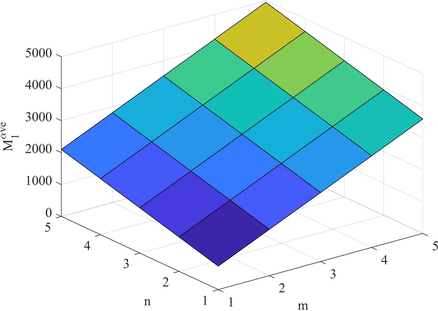
Plot of
.
plots of the indices (a)
and (b)
.
plots of the indices (a)
and (b)
.
Plots of the indices (a)
and (b)
.
Plot of the indices
.
)
556
14.2379
736
272
958
1380
27.4758
1788
678
2338
2204
40.7137
2840
1084
3718
3028
53.9516
3892
1490
5098
3852
67.1895
4944
1896
6478
4676
80.4274
5996
2302
7858
5500
93.6653
7048
2708
9238
6324
106.9032
8100
3114
10618
7148
120.1411
9152
3520
11998
7972
133.3790
10204
3926
13378
)
)
)
)
)
12.1459
19.7815
6.1934
7.2746
4.0880
27.4033
49.5374
10.3797
15.4936
8.9708
42.6607
79.2933
14.5660
23.7125
13.8536
57.9181
109.0492
18.7523
31.9314
18.7364
73.1755
138.8051
22.9387
40.1503
23.6193
88.4329
168.5610
27.1250
48.3692
28.5021
103.6903
198.3170
31.3113
56.5881
33.3849
118.9477
228.0729
35.4976
64.8070
38.2678
134.2051
257.8288
39.6840
73.0259
43.1506
149.4624
287.5847
43.8703
81.2448
48.0334
4 Numerical results and discussions
There are numerous applications of experimental research in chem-informatics and biomedicine, where different graph topological evaluations are utilized to handle many complex schemes. In the book ”Methods of Chemometrics”, Xu and Shao (2004) incorporated the QSAR/QSPR applications systematically. We have calculated the numerical results based on the topological indices of starphene and fenestrene . Graphs and tables assist visualize the physical meaning of computed indices. The results are shown in the Tables 5 and 6 for the molecular structure of starphene and in Tables 10 and 11 for the molecular structure of fenestrene . It can been seen from Figs. 2,3,4,5,6,7,9,10,11,12,13,14 that all the computed topological indices increase with the increase in the values of , and l respectively. Rauf et al. (2021) developed the QSPR between ve and eV-degree based indices and physical properties of benzene derivatives. They examined that enthalpy, boiling point, molecular weight and -electron energy can be predicted by and , respectively. Hence, the computed results can be helpful to predict certain physical/chemical properties of the considered structures.
5 Special case for calculations in starphene molecular structure: Application of maple algorithm
We can compute the degree matrix, eV-degree and ve-degree for the given graph through Maple software. Consider the molecular graph of starphene with and having 30 vertices and 36 edges. First we get an adjacency matrix M of a graph whose size is 30-by-30. The resultant adjacency matrix M is obtained by using newGraph software whose calculation procedure is given in Hayat and Khan (2021).
Now, from the adjacency matrix we calculated ve-degree and eV-degree of vertices using Maple software. For the degree of vertices, we add the row entries of M. After adding the row entries we get a matrix MI of order 30-by-1.
MI =[2, 2, 2, 3, 2, 3, 3, 2, 3, 2, 2, 2, 2, 3, 2, 3, 3, 2, 3, 2, 2, 2, 2, 3, 2, 3, 3, 2, 3, 2].
The above matrix gives the degree of vertices according to vertices labelled in Fig. 15. For ve-degree of vertices, we will multiply the adjacency matrix M and MI. We get the required column matrix KI for ve-degree of vertices.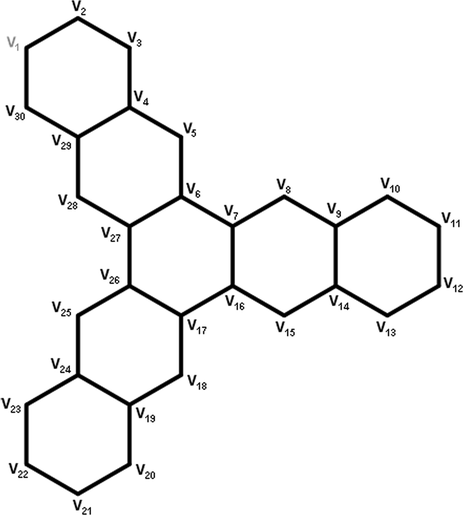
Starphene structure for
and
.
KI= [4, 4, 5, 7, 6, 8, 8, 6, 7, 5, 4, 4, 5, 7, 6, 8, 8, 6, 7, 5, 4, 4, 5, 7, 6, 8, 8, 6, 7, 5] Let i and j denotes the rows and columns of adjacency matrix. For eV-degree we will multiply the th entry of the adjacency matrix with the sum of and . We get a matrix (namely N) of order 30-by-30. Finally we consider the upper triangular matrix and use the commands to compute each topological index. The values of the computed indices are presented below Similarly, we can calculate all the above indices through an adjacency matrix for fenestrene. Our results can be verified of fenestrene for case and .
6 Conclusion
In this paper, we assessed the eV and ve degree indices and their graphical representations for the molecular structures of starphene and fenestrene . These indices may be helpful to predict the physicochemical characteristics of starphene and fenestrene (Süleyman, 2017; Rauf et al., 2021). Moreover, a special case for starphene is presented in Section 6 by using software (Maple) to calculate the eV and ve-degree based topological indices. All the results obtained here can be verified by using our proposed algorithm.
Funding
This work was funded in part by the National Natural Science Foundation of China (grant no. 62002079).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The vertex-connectivity index revisited. J. Chem. Informat. Comput. Sci.. 1998;38(5):819-822.
- [Google Scholar]
- On topological indices of certain dendrimer structures. Z. Naturforsch.. 2017;72:559-566.
- [Google Scholar]
- On topological ascpects of some dendrimer structures. Nanotechnol. Rev.. 2018;7:123-129.
- [Google Scholar]
- On forgotten topological indices of some dendrimers structure. Molecules. 2017;22:867-874.
- [Google Scholar]
- Chou, Chien-Pin, Witek, Henryk A. 2015. Two examples for the application of the ZZDecomposer: Zigzag-edge coronoids and fenestrenes. MATCH Commun. Mathe. Comput. Chem. 73(2).
- Irregular topological indices of certain metal organic frameworks. Main Group Metal Chem.. 2021;44(1):73-81.
- [Google Scholar]
- The non-existence of a threefold aromatic conjugation in linear benzologues of triphenylene (starphenes) Tetrahedron. 1968;24(23):6719-6724.
- [Google Scholar]
- An atom-bond connectivity index: Modelling the enthalpy of formation of alkanes. Indian J. Chem.. 1998;37A(10):849-855.
- [Google Scholar]
- Chem. Phys. Lett.. 1972;17:535-538.
- J. Chem. Phys.. 1975;62(9):3399-3405.
- Quality testing of spectrum-based valency descriptors for polycyclic aromatic hydrocarbons with applications. J. Mol. Struct.. 2021;1228:129789.
- [Google Scholar]
- Holec, Jan, Beatrice Cogliati, James Lawrence, Alejandro Berdonces-Layunta, Pablo Herrero, Yuuya Nagata, Marzena Banasiewicz et al. 2021. A Large Starphene Comprising Pentacene Branches. Angew. Chem. Int. Ed. 60(14), 7752–7758.
- Synthesis of exotic polycycles such as cyclooctatrienes and fenestrenes with differential pro-apoptotic activities on human TRAIL-resistant metastatic cell lines. Bioorganic Medicinal Chem. Lett.. 2010;20(22):6836-6839.
- [Google Scholar]
- Degree Based Weighted Entropy Indices of Hyaluronic Acid-Curcumin Conjugates: an Anti-Tumor Drug. Polycyclic Aromat. Compd. 2021:1-18.
- [Google Scholar]
- Quantitative structure–property relationship of Ev-degree and Ve-degree based topological indices with physico-chemical properties of benzene derivatives and application. Int. J. Quantum Chem. 2021:e26851.
- [Google Scholar]
- Irregularity indices for certain anti-tumor and anti-covid drugs. Polycyclic Aromat. Compd. 2021:1-14.
- [Google Scholar]
- Quantitative structure–property relationship of edge weighted and degree-based entropy of benzene derivatives. Int. J. Quantum Chem.. 2022;122(3):e26839.
- [Google Scholar]
- Starphenes and Phenes: Structures and Properties. Organic Mater.. 2019;1(01):001-018.
- [Google Scholar]
- General fifth M-Zagreb polynomials of benzene ring implanted in the p-type-surface in 2D network. Biointerface Res. Appl. Chem.. 2020;10(6):6881-6892.
- [Google Scholar]
- The (a, b)-Zagreb index of nanostar dendrimers. U.P.B. Sci. Bull. Series B. 2018;80(4):67-82.
- [Google Scholar]
- Predicting some physicochemical properties of octane isomers: A topological approach using ev-degree and ve-degree Zagreb indices. Int. J. Syst. Sci. Appl. Mathe.. 2017;2(5):87-92.
- [Google Scholar]
- Structural determination of paraffin boiling points. J. Am. Chem. Soc.. 1947;69(1):17-20.
- [Google Scholar]
- Xu, Lu, Shao, X.G. 2004. Methods of chemometrics. Academic Press, Beijing.
- Quantitative Structure-Property Relationships (QSPR) of Valency Based Topological Indices with Covid-19 drugs and Application. Arabian J. Chem. 2021:103240.
- [Google Scholar]






