Translate this page into:
The latest research progress on the prevention of storage pests by natural products: Species, mechanisms, and sources of inspiration
⁎Corresponding authors at: Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, PR China (Chuan Zheng). Chengdu University of TCM, No.1166 Liutai Avenue Chengdu 611137, China (Li Han, Ding-kun Zhang). zhengchuan@cdutcm.edu.cn (Chuan Zheng), hanliyx@163.com (Li Han), 465790643@qq.com (Dingkun Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The quality of grains is influenced by storage pests, which are not only spoilers of stored grain, but also vectors of human and animal diseases. Chemical pesticides play an essential role in the cultivation and storage of cereals, however, due to the low degradability and residual toxicity of synthetic pesticides on the environment and non-target organisms, as well as the increasing resistance of target organisms to them, consideration should be given to the development of alternative pest control agents. Compounds isolated from natural sources have emerged as preferred targets for the development of novel insecticidal agents because of their eco-friendliness, safety, and effectiveness. In this review, we primarily focus on the natural product (NPs) control of storage pests. The effective monomer components of NPs and their anti-insect mechanisms were discussed, and natural sources of inspiration and models for insect repellents are described. This review aimed to provide guidelines for the exploitation and utilization of green and efficient natural insecticides.
Keywords
Natural products
Storage pests
The insecticidal mechanism
Novel insecticide
- NPs
-
Natural products
- RASFF
-
A Rapid Alert System for Food and Feed
- LD50
-
lethal dose, 50%
- EC50
-
50% effective concentration
- AChE
-
Acetylcholinesterase
- α-AIS
-
α-amylase inhibitors
- AhAI2
-
Amaranthus hypochondriacus
- AsAI
-
Alternanthera sessilis
- CqAI
-
Chenopodium quinoa
- GABA
-
Glutamate and gamma-aminobutyric acid
- ALT
-
Alanine aminotransferase
- GAD
-
Glutamate decarboxylase
- EO
-
Essential oil
- Ach
-
Acetylcholine
- BC
-
Bacopa caroliniana;JH, Juvenile hormone
- JHDs
-
Juvenile hormone disruptors
- Met
-
Methoprene-tolerant
- SRC
-
Steroid receptor coactivator
- VOCs
-
volatile organic compounds
- EβF
-
(E) -β-Farnesene
Abbreviations
1 Introduction
Well-preserved cereals are essential to provide a sufficient amount of food for the people. However, stored-grain pests pose a serious threat to the quantity and quality of global grains. Stored grain losses caused by storage pests have been estimated to be 5 %–10 % worldwide, and up to 40 % in developing countries (Cao et al., 2019). The chemicals and accompanying microorganisms such as bacteria and fungi from storage pests can cause allergic reactions in humans (Hubert et al., 2018). In addition, t of fungi or other microorganisms, promoting the spread of harmful organisms and increasing the opportunities for food contamination. This increases the risk of acute toxicity, sensitization, and cancer in humans (Hubert et al., 2018).
Currently, the application of chemical pesticides is one of the most effective and extensive methods to control storage pests. Chemical insecticides have been proven to be indispensable tools in global agriculture and public health with high work efficiency, low labor intensity, and increased crop yield (Mpumi et al., 2016). However, it cannot be ignored that studies have shown that some synthetic insecticides can cause damage to the central nervous system, reproductive system, and immune system of mammals, leading to neurodegenerative disease, decreased reproductive capacity, and immune dysfunction (Garg et al., 2004, Madhubabu and Yenugu 2014, Magby and Richardson 2017, Wang et al., 2017). Furthermore, with the widespread application of chemical pesticides, the issue of selective resistance of pests has become increasingly prominent. It has been widely documented that pesticide-resistant bacteria and insects, which acquire resistance through single, continuous or spontaneous mutations, are present in numerous agricultural products worldwide. At present, numerous genes conferring resistance have emerged and more than 7,00 kinds of pests are resistant to chemical insecticides, leading to more pesticide application and financial investment (Rangasamy et al., 2018). Therefore, the toxicity and pest resistance of chemical insecticides are the major obstacles to their widespread use.
People often use natural products (NPs) such as Allium sativum and Zanthoxylum bungeanum as insect repellants to protect food from pests. Therefore, it gives us great inspiration to seek safe and effective insect repellents in nature. With further research on NPs, a growing number of NPs are seen as effective alternatives to traditional synthetic pesticides by affecting the chemical receptors, digestive system, and nervous system of pests. This review provides an overview of the common monomer components of NPs and their insect control mechanism, as well as natural product structure as inspiration sources for insect repellant development, and summarizes the utilization and future applications of NPs. This study will be of great value to provide new ideas and methods for storage pest control.
2 Hazards associated with storage pests
Storage pests can survive and reproduce on dry or wet food, so they can infect food throughout the storage process (Hubert et al., 2018). On the one hand, the consequence of feeding, excretion, molting and heat production of storage pests ultimately results in a decline in the quantity and quality of grain. On the other hand, storage pests can actively or passively promote the spread of microorganisms, increasing the chance of food contamination. “A Rapid Alert System for Food and Feed” (RASFF) database established by the European Union contains internationally significant data regarding food safety and contamination violations, which reveals that arthropod pests, glass fragments, and metals investigated between 1992 and 2015 are the top three foreign bodies, accounting for 54.6 %, 17.4 %, and 11.5 %, respectively. Two arthropods, Psocoptera and Acari, have a growing influence on global food (Hubert et al., 2018). Storage pests are the highest source of product contamination, as they infest a large number of unprocessed food commodities throughout the food production chain (Aulicky et al., 2019).
3 Natural insecticide
Plants often suffer from biotic and abiotic stresses during their entire life, including heavy metals, ultraviolet radiation, heat and cold stress, and insect pests, resulting in nutrient loss and pathogen invasion (Chang et al., 2017). To cope with such adverse conditions, plants have perfected their sophisticated and robust defense mechanisms to specifically respond to stresses, one of which is the formation of secondary metabolites such as alkaloids, terpenoids, organic acids, saponins, phenols, and phenylpropanoids (Francisco et al., 2016). These secondary metabolites are structurally and functionally diverse groups of chemicals and respond to different environmental stimuli individually or in combination, playing an extremely important role in pest control, wound healing, resistance to microbial infection, and response to environmental stresses. Studies have shown that garlic and chili pepper oils have a high repellency to Blattella germanica (L.), with a strong time-dependent toxic effect on common storage pests, such as Tribolium castaneum (Herbst) (Wang et al., 2019). Compared to chemical pesticides, NPs are not bioaccumulated in organisms due to their edible properties, low initial toxicity, and residual toxicity (Tripathi et al., 2003). Moreover, the opportunities to generate drug-resistant individuals are quite low because of the multi-target mechanism of NPs, such as inhibition of a carbonic anhydrase, two sodium-dependent cation-chloride co-transporters, and a histone deacetylase (Tripathi et al., 2003). In addition to the direct application of NPs as insect repellents, they can also serve as an inspiration and model for the development of novel pest control agents, to greatly improve the insecticidal characteristics, toxicological properties, and environmental impact of pesticides (Gerwick and Sparks 2014). Therefore, NPs provide a spectrum of valuable tools for effectively preventing the invasion of storage pests.
3.1 Terpenoids
Essential oils (EOs) have broad application prospects in the fields of pest control, of which terpenes account for a large proportion. Terpenes are widely present in plants and could act as allelochemicals to attract pollinators and herbivorous predators while deterring herbivores. Terpenoids have high chemical diversity, likely reflecting a natural history characterized by herbivorous stress and other selective pressures imposed by animals (Bergman et al., 2019). Researchers reported the biological activity of terpenes with different structural groups against stored grain pests, with ketones being the most biologically active (Pizzolitto et al., 2015). Pizzolitto et al. tested (S)-(+)-carvone, (R)-(+)-Pulegone and other nine kinds of ketone terpenoids on the repellency of Sitophlilus zeamais, and the results showed that except S-carvone and camphor, all tested ketones showed repellent effects on S. zeamais at the dose of 4 µL/L. Thymoquinone and α-thujone were strong repellents, dihydrochalcone, α-thujone and camphor showed significant repellent effects, while pulegone and verbenone were significant attractants, and verbenone showed significant attraction effects at 0.05 µL/L (Pizzolitto et al., 2015). Terpenoids with activity against storage pests are listed in Fig. 1 and Table 1.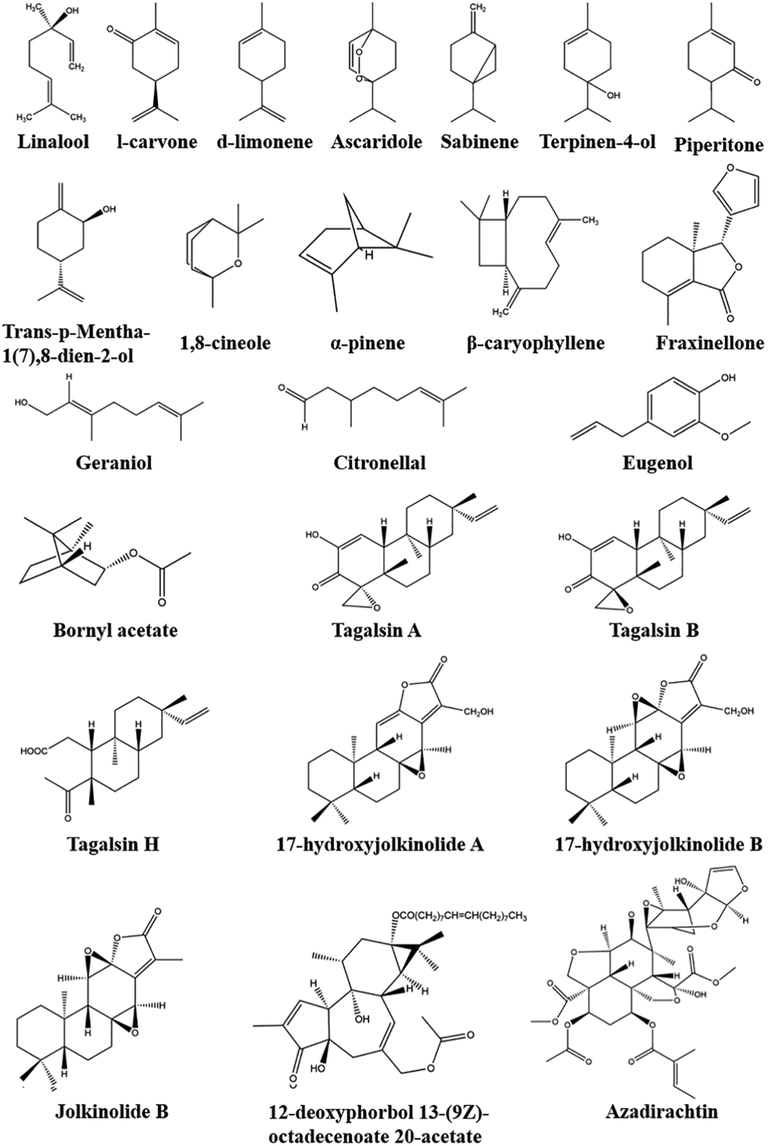
Structures of terpenoids with insecticidal activity.
Molecular name
Source of natural plants
Insect-resistant species and activity
Pharmacological potential
References
Linalool
Cinnamomum camphora, Zanthoxylum planispinum
The LC50 value for Tribolium castaneum (Herbst) is 2.5 × 104 ppm; The LC50 values for each species were: Zabrotes subfasciatus-429.3 ug/cm2; Acanthoscelides obtectus-412.1 ug/cm2; Rhyzopertha dominica-430.2 ug/cm2; Sitophilus oryzae − 426.7 ug/cm2
anti-inflammatory, anticancer, antihyperlipidemic, antimicrobial, etc.
(Pereira et al., 2018, Wang et al., 2019)
l-carvone
Anethum graveolens L.
It completely suppressed egg hatching at the concentration of 7.72 mg/cm2 and above.
Antitumor, anesthetic
(Du et al., 2011, Patel and Thakkar 2014, Brosnan et al., 2022)
d-limonene
Citrus reticulata Blanco, Amomum tsaoko, Zanthoxylum armatum
It exhibited contact toxicity against S. zeamais and T. castaneum adults, with LD50 (lethal dose, 50 %) values of 29.86 μg/adult and 20.14 μg/adult, and showed fumigant toxicity with LD50 values of 33.71 mg/L and 21.24 mg/L
Anticancer, antioxidant, antidiabetic, anti-inflammatory, cardioprotective, etc
(Du et al., 2011, Anandakumar et al., 2021, Chebet et al., 2021)
Ascaridole
Chenopodium ambrosioides L.
It showed strong fumigant toxicity against S. zeamais adults with the LC₅₀ values of 0.84 mg/L air and exhibited contact toxicity with the LD₅₀ values of 0.86 µg g − 1 body weight
Sedative hypnotic effect
(Chu et al., 2011, Dougnon and Ito 2021)
Sabinene
Zingiber purpureum
It exhibited contact toxicity against L. serricorne with LD₅₀ values of 15.7 µg per adult and showed fumigant toxicity against T. castaneum with LD₅₀ values of 18.2 mg/liter of air
Flavorings, flavor additives, fine chemicals, and advanced biofuels
(Wang et al., 2015, Cao et al., 2018)
Terpinen-4-ol
Melaleuca alternifolia, Zanthoxylum planispinum
It showed the strongest contact toxicity against T. castaneum and L. serricorne (LD50 = 19.7 and 5.4 µg per adult, respectively) and also the strongest fumigant toxicity against T. castaneum and L. serricorne (LC 50 = 3.7 and 1.3 mg/liter of air, respectively)
Antimicrobial, anti-arthritic, anti-cancer
(Wang et al., 2019, Liao et al., 2020, Aslam et al., 2022)
Piperitone
Zanthoxylum armatum, Clinopodium chinense
It gave 90 % mortality against C. maculatus after 24 h at a concentration of 6.7 μL/L and had LC50 values of 311.12 ug/liter against booklice
modulate cholesterol metabolism
(Li et al., 2015, Wang et al., 2015, Sut et al., 2021)
Trans-p-Mentha-1(7),8-dien-2-ol
Illicium pachyphyllum
It exhibited contact toxicity against S. zeamais and T. castaneum adults, with LD50 values of 8.66 μg/adult and 13.66 μg/adult, and showed fumigant toxicity with LD50 values of 6.01 mg/L and 8.14 mg/L
_-
(Liu et al., 2012)
1,8-cineole
Artemisia annua L., Morinda lucida, Zanthoxylum armatum
It gave 100 % mortality in closed cups but no mortality in open ones
hypnotic-sedative and antipsychotic-like effects
(Tripathi et al., 2003, Owolabi et al., 2014, Sobreira Dantas Nóbrega de Figuêiredo et al., 2019)
α-pinene
Callistemon citrinus, Artemisia mongolica
It showed fumigant toxicity with LD50 values of 1.402 ppm and exhibited contact toxicity with LD50 values of 4.133 ppm
Antibacterial, antifungal,anti-leishmania, neuroprotective
(Geng et al., 2011, Allenspach and Steuer 2021)
β-caryophyllene
Plectranthus zeylanicus, Clinopodium chinense
It exhibited contact toxicity against L. bostrychophila with an LC50 value of 275.00 μg/cm2
Anti-inflammatory and anti-metabolic disease
(S et al., 2017, Mantzoukas et al., 2020, Franco-Arroyo et al., 2022)
Fraxinellone
Dictamnus dasycarpus, Fagaropsis glabra, Melia azedarach
It possessed feeding deterrent activity against adults and larvae of T. castaneum as well as S. zeamais adults with EC50 values of 36.4, 29.1, and 71.2 ppm, respectively.
Alleviate kidney fibrosis and secondary brain damage.
(Liu et al., 2002, Guo et al., 2019, Lu et al., 2021, Zheng et al., 2021)
Geraniol
Cinnamomum tenuipilum, Valeriana officinalis
The LD50 value for C. maculatusis is 0.7140 µL
neuroprotective effects as well as ameliorating influence in memory impairment; anti-diabetic
(Reis et al., 2016, Lei et al., 2019, El Azab and Mostafa 2022, Liu et al., 2022)
Citronellal
Cymbopogon nardus
The LD50 value for C. maculatusis is 2.261 µL
Antifungal; inhibit Staphylococcus aureus growth and enterotoxin production
(Reis et al., 2016, Zhang et al., 2022)
Eugenol
Syringa oblata Lindl.
The LD50 value for C. maculatusis is 0.9473 µL
antioxidant, anti-inflammatory, and antibacterial effects
(Reis et al., 2016, Zhao et al., 2022)
Bornyl acetate
Clinopodium chinense
It exhibited acute toxicity against Liposcelis bostrychophila with LC50 values of 321.42 µg/cm2
anti-inflammatory, antioxidant activities
(Chen et al., 2014, Li et al., 2015)
Tagalsin A
Ceriops tagal
It exhibited strong feeding deterrent activity against T. castaneum adults with EC50 (50 % effective concentration) values of 375.3 ppm
_
(Lei et al., 2019)
Tagalsin B
Ceriops tagal
It exhibited strong feeding deterrent activity against T. castaneum adults with EC50 values of 277.3 ppm
_
(Lei et al., 2019)
Tagalsin H
Ceriops tagal
It exhibited strong feeding deterrent activity against T. castaneum adults with EC50 values of 285.45 ppm
_
(Lei et al., 2019)
17-hydroxyjolkinolide A
Euphorbia fischeriana
It exhibited feeding deterrent activity with EC50 values of 631.9 ppm for S. zeamais and 656.5 ppm for T. castaneum adults.
_
(Geng et al., 2011)
17-hydroxyjolkinolide B
Euphorbia fischeriana
It possessed strong feeding deterrent activities against S. zeamais and T. castaneum adults with EC50 of 543.9 and 551.5 ppm, respectively
Anticancer
(Wang et al., 2009, Geng et al., 2011)
Jolkinolide B
Euphorbia fischeriana
It possessed strong feeding deterrent activities against S. zeamais and T. castaneum adults with EC50 of 342.1 and 361.4 ppm, respectively
Anticancer, reduce renal fibrosis
(Geng et al., 2011, Li et al., 2022, Wang et al., 2022)
12-deoxyphorbol 13-(9Z)-octadecenoate 20-acetate
Euphorbia fischeriana
It exhibited feeding deterrent activity against the two-grain storage insects with EC50 values of 884.3 ppm for S. zeamais and 1058.4 ppm for T. castaneum adults.
_-
(Geng et al., 2011)
Azadirachtin
Azadirachta indica A. Juss.
The longer the treatment time, the lower the lethal concentration of azadirachtin to Corcyra cephalonica, and the difference between them was significant
antimalarial, and anticancer
(Tripathi et al., 2002, Zandi-Sohani et al., 2013, Fernandes et al., 2019)
3.2 Alkaloids
Alkaloids are a large group of naturally occurring organic nitrogen-containing compounds found in 300 plant families as well as bacteria, fungi, and animals. So far, more than 18,000 different alkaloids have been identified (Casciaro et al., 2020). Alkaloids have a variety of biological activities such as poisoning, antifeeding, and inhibiting the growth and development of insects. For example, Matrine-type alkaloids such as marine, sophocarp and oxymatrine, etc. are present in Sophora popecuroides (Fabaceae) and exhibit insecticidal, antifungal, antibacterial, and antiviral activities. Among these, the toxicological effects of matrine and sophocarpine are related to the regulation of glutamate and gamma-aminobutyric acid systems (Ma et al., 2020). As shown in Fig. 2 and Table 2, several alkaloids have potential application prospects for pest control.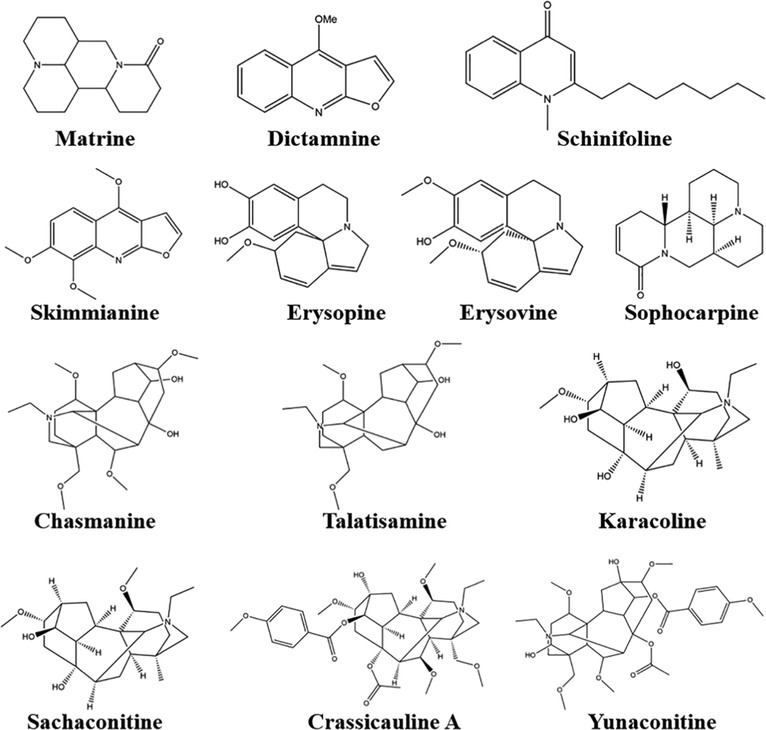
Structures of alkaloids with insecticidal activity.
Molecular name
Source of natural plants
Insect-resistant species and activity
Pharmacological potential
References
Matrine
Sophora flavescens
The LD50 value for Acyrthosiphon pisum is 68.88 ng/aphid
anti-oxidative stress, anti-inflammation, and anti-apoptosis
(Ma et al., 2020, Yuan et al., 2022)
Dictamnine
Dictamnus dasycarpus
It had feeding deterrent activity against adults and larvae of T. castaneum as well as S. zeamais adults with EC50 values of 57.6, 47.9, and 91.7 ppm, respectively
anti-bacterial, anti-fungal, anti-cancer, and hepatoxicity
(Liu et al., 2002, Lin et al., 2021)
Schinifoline
Zanthoxylum schinifolium
Schinifoline has feeding deterrent activity against T. castaneum and S. zeamais adults with EC50 values of 47.8 and 85.6 ppm respectively
tubulin polymerization inhibitors, heterogeneous enzyme inhibitors, and antiplatelet agents
(Liu et al., 2009, Wang et al., 2014)
Skimmianine
Zanthoxylum schinifolium
Skimmianine possesses feeding deterrent activity against T. castaneum and S. zeamais adults with EC50 values of 75.7 and 129.7 ppm respectively
Antitumor, anti-inflammatory
(Liu et al., 2009, Ratheesh et al., 2013)
Erysopine
Erythrina variegata
It possessed antifeedant activity against S. zeamais adults with EC50 values of 108.5 ppm.
_
(Liu et al., 2012)
Erysovine
Erythrina variegata
It possessed antifeedant activity against S. zeamais adults with EC50 values of 89.7 ppm.
_
(Liu et al., 2012)
Sophocarpine
Sophora alopecuroides
the LD50 value for Acyrthosiphon pisum is 83.44 ng/aphid
anti-oxidation, anti-inflammation, anti-tumor, antivirus, and immune regulation
(Ma et al., 2020, Yang et al., 2021)
Chasmanine
Aconitum episcopale
It exhibited feeding deterrent activity against T. castaneum adults with EC50 values of 297.0 ppm
activating blood circulation and removing blood stasis.
(Liu et al., 2011, Yang et al., 2019)
Talatisamine
Aconitum episcopale
It exhibited feeding deterrent activity against T. castaneum adults, with EC50 values of 342.8 ppm
Antitumor; neuroprotection.
(Liu et al., 2011, Wang et al., 2012)
Karacoline
Aconitum episcopale
It exhibited feeding deterrent activity against T. castaneum adults with EC50 values of 395.3 ppm
analgesic effect
(Liu et al., 2011, Zhou et al., 2020)
Sachaconitine
Aconitum episcopale
It exhibited feeding deterrent activity against T. castaneum adults with EC50 values of 427.8 ppm
_
(Liu et al., 2011)
Crassicauline A
Aconitum episcopale
It possessed feeding deterrent activity against T. castaneum adults with EC50 values of 1134.5 ppm
_
(Liu et al., 2011)
Yunaconitine
Aconitum episcopale
It possessed feeding deterrent activity against T. castaneum adults, with EC50 values of 653.4 respectively.
Anti-inflammatory, analgesic, sedative, antipyretic, and antineoplastic effection
(Liu et al., 2011, Zhang et al., 2020)
3.3 Phenylpropanoids
Phenylpropanols are a large class of secondary metabolites that protect plants from biotic and abiotic stresses. The effects of cuminaldehyde and other compounds on adults of Sitophilus zeamais were assessed by acute toxicity and repellence (Rosa et al., 2020). The results showed that cuminaldehyde had an LD50 value of 484.8 mg L−1 in fumigant toxicity and an LD50 value of 96.5 µg per adult in contact toxicity. In the area preference bioassays, cuminaldehyde, (S)-carvone, and estragole are the most repellent with an RD50 less than 4.9 µg m−2, which is significantly different from the others. Cuminaldehyde not only changed the nutritional parameters relative growth rate but also affected the efficiency conversion index of ingested food and antifeeding effect, showing an antinutritional effect on S. zeamais. Jun Pan et al. (Pan et al., 2009) explored the insecticidal effects of osthol powder against Rhizopertha dominica, Sitophilus zeamais, and Tribolium castaneum. It was found that when the spraying ratio was 0.5 mg/kg (osthole: grain), the lethality rate of R.dominica, S.zeamais, and T.castaneum were 97.78 %, 100 %, and 86.70 % respectively after 7 days. Four months after the treatment of the osthol powder, the control effect of the grains on R.dominica and S.zeamais can still reach 100 %, which meets the requirements for the control of storage pests. In addition, cinnamaldehyde and (E)-anethole have also been found to have activity against storage pests (Tripathi et al., 2003, Wang et al., 2015).
3.4 Organic acids
Organic acids are widely distributed in the roots, leaves, stems, and fruits of plants. They are not only the intermediate products of carbon metabolism but are also key components in the mechanisms that some plants use to cope with nutrient deficiencies, metal tolerance, and plant-soil microbial interactions at the plant root-soil interface (Herrera-Estrella 2000). Gokhan Abay et al. (Abay et al., 2013) studied the composition of fatty acids and insecticidal effects of plants such as Turkish mosses Dicranum scoparium, Polytrichastrum formosum, the Turkish liverwort Conocephalum conicum, etc. The structures of fatty acids were determined by gas chromatography and gas chromatography-mass spectrometry techniques, and the contact toxicity activities of lauric, myristic, and palmitic acids were performed. Myristic acid exhibited the highest mortality rate of 53.34 % among the tested pure fatty acids, and the activities of palmitic and lauric acids were 17.75 % and 4.32 %, respectively. Fig. 3 and Table 3 exhibit the organic acid compounds with insect-resistant activity.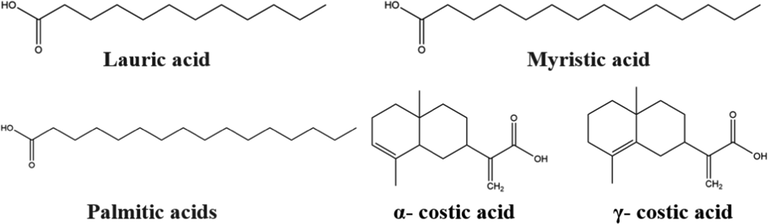
Structures of organic acids with insecticidal activity.
Moleculer
Source of natural plants
Insect-resistant species and activity
Pharmacological potential
References
Lauric acid
Cocos nucifera L.;Litsea cubeba
It showed a mortality rate of 4.32 % against Sitophilus granarius
Antibacterial
(Abay et al., 2013, Zhang et al., 2017)
Myristic acid
Myristica fragrans
Myristic acid showed a mortality rate of 53.34 % against Sitophilus granarius
reduces skin inflammation and nociception; antibacterial
(Abay et al., 2013, Alonso-Castro et al., 2022)
Palmitic acids
Trachycarpus fortunei
It showed a mortality rate of 17.75 % against Sitophilus granarius
Anti-Inflammatory
(Abay et al., 2013, de Souza et al., 2018)
α- costic acid
Dittrichia viscosa (L.)
The calculated dose of α-costic acid was 3.40 μg/adult in contact toxicity assay
Acaricidal activity
(Rotundo et al., 2019)
γ- costic acid
Dittrichia viscosa (L.)
The calculated dose ofγ- costic acid was 9.57 μg/adult in the contact toxicity assay
_
(Rotundo et al., 2019)
3.5 Other compounds
Some flavonoids and sulfides, such as rotenone and methyl allyl disulfide isolated from natural products, have also been found to be effective insect repellents. Jan Nawrot et al. tested the antifeedant activity of rotenone and its five derivatives against Sitophilus granarius and Tribolium confusum adults and found that rotenone showed the strongest deterrent effect on all species and was the best antifeedant tested in their laboratory so far, while rotenone derivatives had lower antifeedant activity and showed a certain selectivity. Some studies have shown that garlic extract has a repellent effect on arthropod pests (Nchu et al., 2016). Yan Huang et al. (Huang et al., 2000) studied the contact toxicity, fumigation toxicity, and antifeedant effects of Methyl allyl disulfide and Diallyl trisulfide in garlic essential oil with corn weevil and Tribolium castaneum as experimental materials. Both compounds showed the inhibition of egg hatching of Tribolium castaneum and the subsequent emergence of progeny. Diallyl trisulfide completely inhibited egg hatching at 0.32 mg /cm2 and the emergence of larvae and adults at 0.08 mg/cm2. Methyl allyl disulfide reduced the growth rate and food utilization rate of adults of the two insects, with a deterrent index of 6.08 mg/g for S. Zeamais and 1.52 mg/g for T. castaneum. In addition, 2-tridecanone isolated from the essential oil of Zanthoxylum bungeanum has contact toxicity to Lasioderma serricorne, with LD50 of 5.74 μg/adult (Wang et al., 2015).
4 Insecticidal mechanisms of natural products
NPs have various complex pest control mechanisms, as these have effects on insect chemoreceptors, the digestive system, the nervous system, growth and development, energy metabolism, and alarm pheromone generation.
4.1 Effects on chemoreceptors of insects
NPs that act as pheromones have important potential for monitoring, luring, repelling, confusing, and trapping pests by manipulating specific olfactory behaviors to resist pests (Sharma et al., 2019). NPs have antifeedant activity against insects, one of the mechanisms is to block the chemoreceptors of insects (Mitchell and Harrison 1985). The feeding behavior in insects is dependent on the neural inputs received from the chemical sensors in their mouthparts, tarsi, and oral cavity, which integrate a “sensory code” that is transmitted to the central nervous system. Azadirachtin, a common insect antifeedant, blocks the feeding stimulation by stimulating deterrent cells in insect chemoreceptors and “sugar” receptor cells(Chaudhary et al., 2017). In addition, steroid glycoalkaloids have significant damaging effects on insect chemoreceptors, and their effects may be independent of any specific receptor. The steroidal glycoalkaloids found in species of the Solanaceae can induce bursting activity in galeal and tarsal chemosensilla in Colorado potato beetle adults, with an average latency of 6–12 s, depending on the receptor/alkaloid combination (Mitchell and Harrison 1985). Moreover, nine alkaloids, including lycopine, solanine, and papaverine, were used to study their effects on the taste of adult Colorado potato beetles. Although there was no evidence of a common “deterrent receptor” in these insects, some alkaloids still inhibited normal chemical reactions (Mitchell 1987).
4.2 Effects on the digestive system of insects
α-amylase is an essential digestive enzyme required for the growth and development of insects, which can catalyze the hydrolysis of α-d-(1,4)-glucan chains in starch, glycogen, and other related carbohydrates, thus improving the digestive capacity of insects (Kaur et al., 2014). α-amylase inhibitors (α-AIS) target α-amylases and interfere with carbohydrate digestion in insects, resulting in starvation and death (Channale et al., 2016). In a previous study, three knottin-type α-AIs from several Amaranthaceae plants, namely Amaranthus hypochondriacus (AhAI2), Alternanthera sessilis (AsAI) and Chenopodium quinoa (CqAI), were found to be specific against coleopteran storage pests. The highest inhibition potency on Tribolium castaneum α-amylase was observed for AhAI2, followed by AsAI and CqAI (Fig. 4) (Rane et al., 2020). α-amylases from different plants exhibited a distinct specificity and binding affinity to amylases from different sources, which probably contributed to the conformation of α-AIs and the sequence and/or structural changes in and around the substrate-binding pocket of α-amylases from different sources, giving it a great potential to control pests as it avoids the possibility of cross-reaction with α -amylases in mammals (Rane et al., 2020).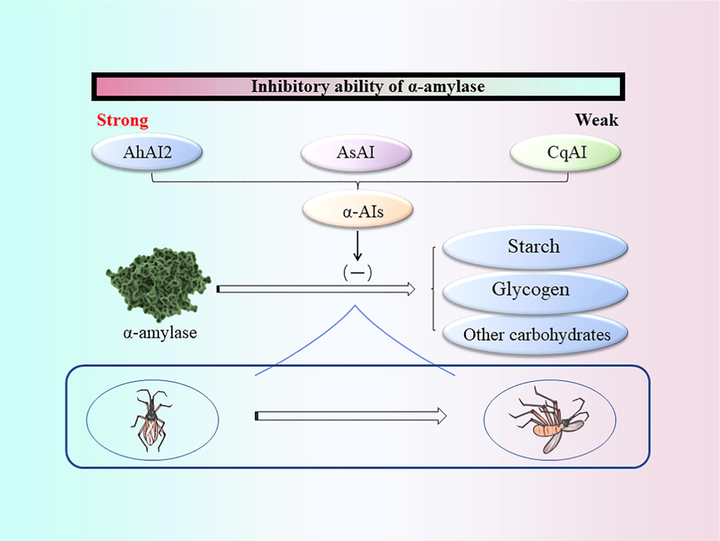
Inhibition mechanism of α-amylase (α-AIs: α-amylase inhibitors α-amylase inhibitors; AhAI2: Amaranthus hypochondriacus α-Ais; AsAI: Alternanthera sessilis α-Ais; CqAI: Chenopodium quinoa α-Ais).
4.3 Effects on the nervous system of insects
The neurotoxic targets of NPs on storage pests are reflected in the following aspects: AChE (Seo et al., 2014), glutamate and gamma-aminobutyric acid (GABA) systems (Ma et al., 2020), metabotropic octopamine receptors, and Na+, K+, Ca2+, Mg2+-ATPase. Xiaoling Shi et al. studied the effects of matrine and Sophocarpine on pea aphid and found that these two alkaloids increased the content of Glu and GABA, inhibited the activity of Alanine aminotransferase (ALT), and activated the activity of Glutamate decarboxylase (GAD). They hypothesized that the inhibition of ALT by matine led to a relative increase of Glu, which further activated the accumulation of GABA in GAD, thus enhancing the influx of Cl-, triggering the inhibitory postsynaptic potential, and thus the conduction of action potential was interrupted. In addition, they found that these two alkaloids inhibited Na+, K+, Ca2+, and Mg2+-ATPase activities in a dose-dependent manner, with the highest inhibition rates reaching 45.23 % and 36.05 %, respectively (Ma et al., 2020). Octopamine exists in the nervous system, neuroendocrine cells, and hemolymph, and plays an important role in insect stress behavior as a neurotransmitter, neurohormone, and neuromodulator in the invertebrate system (Kostyukovsky et al., 2002, Zhou et al., 2008). The influence of plant essential oils (EOs) on acute and sublethal behaviors of insects, as well as their low toxicity to mammals and other vertebrate could be partly explained by the effects on octopamine receptors in insects. The effects of two purified EOs, SEM-76 and ZP-51, on the levels of intracellular cyclic AMP in abdominal segments of the model insect were studied in vitro. It was found that both SEM-76 and ZP-51 significantly caused a remarkable increase in cAMP levels at 10-7M and 10-8M, which was comparable to those induced by octopamine at the same doses. In addition, phentolamine, an octopamine antagonist significantly inhibited the reaction between octopamine and SEM-76, suggesting that EOs may target octopamine receptors (Kostyukovsky et al., 2002).
NPs may also repel pests by reacting on acetylcholinesterase (AChE). Acetylcholine (ACh) is one of the key enzymes needed to maintain cholinergic transmission in the insects’ central nervous system (Chang et al., 2017). AChE inhibition leads to the accumulation of Ach in the postsynaptic membrane, subsequent permanent stimulation of the postsynaptic membrane, and eventual lack of coordination of the neuromuscular system and death. The composition of the essential oil (EO) of Bacopa caroliniana (BC) has been shown to inhibit AChE. BC is a perennial creeping herb that contains 18 volatile constituents, among which α-pinene, limonene, and α-terpinolene exhibit good AChE inhibitory activity against Sitophilus oryzae L, with IC50 values less than 10 µL mL−1. In addition, 1,8-cineole and linalool exhibited a relatively moderate activity, with IC50 values of 30-–45 µL mL−1 (Liu et al., 2019). However, the inhibitory strength of the same compound on AChE varies among literatres, which may be attributed to differences in enzyme sources and sample preparation methods (Lee et al., 2001, Dohi et al., 2009, Liu et al., 2019). Furthermore, different compounds with similar activities may have synergistic effects on AChE inhibitory activity (Liu et al., 2019). Liu et al. (Liu et al., 2019) reported that interactions tend to be synergistic if individual compounds are sufficiently active, such as α-terpinolene or linalool with all test compounds. Furthermore, the interaction is additive if the insecticidal activity is moderate and comparable, such as sabinene and 1,8-cineole.
4.4 Effects on the growth and development of insects
Juvenile hormone (JH) exerts versatile functions in insect life, including the control of larval development by preventing premature metamorphosis, regulation of molting function, pheromone production, and cast differentiation (Hartfelder 2000, Shin et al., 2018). Many plant-derived natural products have the function of interfering with the activity of JH, secreting juvenile hormone disruptors (JHDs) that disrupt endocrine regulation in insects. Hyun-Woo Oh et al. (Oh et al., 2017) constructed an insect species-specific JHAN assay system and tested the interference activity of 3,704 plant extracts against the binding of Methoprene-tolerant (Met) and steroid receptor coactivator (SRC) mediated by JH-III. It was found that substances extracted from conifers, especially members of Pinaceae, exhibit potent interfering activity. Four compounds were obtained by further separation: 7α-dehydroabietic acid, 7-oxodehydroabietic acid, dehydroabietic acid, and sandaracopimaric acid (Fig. 5). In addition, with the evolution of JHDs and JH receptors, reciprocal diversification has occurred between plants and insects, allowing JHDs to be developed as effective insecticides with limited effects on non-target species and high lethality against insects (Shin et al., 2018).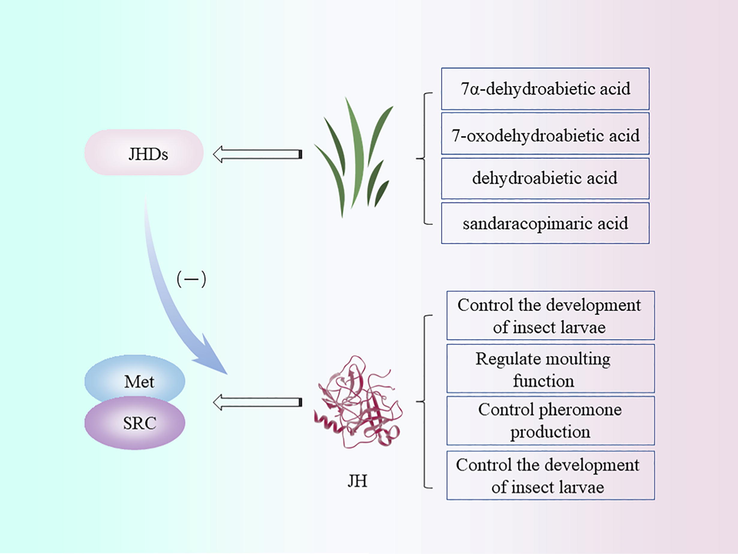
Mechanism of juvenile hormone interference (JHDs: Juvenile hormone disruptors; Met: Methoprene-tolerant; SRC: Steroid receptor coactivator; JH: Juvenile hormone).
4.5 Effects on energy metabolism of insects
NPs can exert insecticidal effects by inducing structural changes and mitochondrial dysfunction in insects. Mitochondria are involved in various important cellular activities, among which energy conversion is the most critical (Greber and Ban 2016). Plant metabolites with fumigant-like insecticidal activities have the potential to induce mitochondrial dysfunction, leading to a decrease in ATP levels(de Carvalho et al., 2017). Rotenone is a well-known broad-spectrum insecticide and is widely used as an inhibitor of electron transfer, which prevents the transfer of electrons from nicotinamide adenine dinucleotide to coenzyme Q and limits the generation of cell energy by delaying the electron transfer chain in the tricarboxylic acid cycle of insect mitochondria, thus having a control effect on the pests of 137 families in 15 orders (Han et al., 2020). Min Liao et al. (Liao et al., 2018) evaluated the action of the M. alternifolia EO in degrading the mitochondria of T. confusum and the inhibitory effects of the EO on NAD+/NADH dehydrogenase. Results showed that the mitochondria of untreated T. confusum larvae had highly electron-dense cristae, membranes, and matrix. However, when compared to non-fumigated adults, the mitochondria of columnar and regenerated cells from the thorax of T. confusum larvae treated with EOs had undergone ultra-structural changes. Mitochondrial vacuolation after EOs treatment increased with time, and this may lead to mitochondrial fragmentation in severe cases. In addition, the biochemical analysis found that treatment with EOs significantly inhibited the level of NADH but increased NAD+ levels at 12–48 h, with an opposite trend after 60 h. This may be partly because T. confusum coverts NADH excessively to the oxidized NAD+ to increase energy production in response to oil treatment, resulting in increased levels of NAD+. The regulatory mechanism of T. confusum is destroyed with time, however, resulting in a significantly lower level of NAD+. Therefore, it was concluded that NAD+/NADH dehydrogenase might be a major target of insects, leading to energy system dysfunction, mitochondrial damage, and death (Mpumi et al., 2016).
4.6 Alarm pheromone effect
In addition to direct insecticidal effects through the above mechanisms, NPs release certain volatile organic compounds (VOCs) based on mutualistic relationships with predators and parasitoids to achieve the avoidance of pests. (E)-β-Farnesene (EβF) is the main component of the alarm pheromones of pest species. The natural enemies of pests utilize EβF to orient their prey, and pests often avoid these induced VOCs during host choosing (Li et al., 2019). In addition to direct repellent effects on pests and their function as triggers of plant defense against pests, terpenoids can also serve as indirect defenses against insect predators or parasites. For example, terpenoids act as chemical attractors to lure herbivores into traps (Li et al., 2019).
5 Natural products as models and inspirations for insecticides
In addition to their direct use as insecticides, NPs can also be regarded as the basis of a large number of commercial pesticides on the market for the development of novel insect repellants (Gerwick and Sparks 2014) (Fig. 7 and Table 4). Changes in pest species and their resistance to synthetic pesticides, as well as regulatory requirements for environmental protection call for new insecticidal tools. Therefore, eco-friendly NPs are important source templates for novel pesticides. Novel insecticides with stronger efficacy and higher bioavailability could be achieved by parsing effective insecticidal ingredient formulas of biological extracts, coupled with chemical modification to improve them. One of the most successful examples in the history of structural modification and commercialization of NPs is the development of pyrethroid insecticides. Pyrethrin is a domestic pesticide with low toxicity to mammals and high efficacy to target animals. However, they have not been widely used for plant protection since they degrade rapidly in the environment. Structural modification of pyrethrin began in the early 20th century. After years of research, synthetic pyrethroid insecticides such as biological allethrin, permethrin, permethrin, cypermethrin, fenvalerate, etc. were successively developed (Cycoń and Piotrowska-Seget 2016). More than 30 pyrethroids have been commercialized in the past few years (Matsuo 2019). Compared with natural pyrethroids, synthetic pyrethroids have stronger photostability and insecticidal activity (Bao et al., 2020).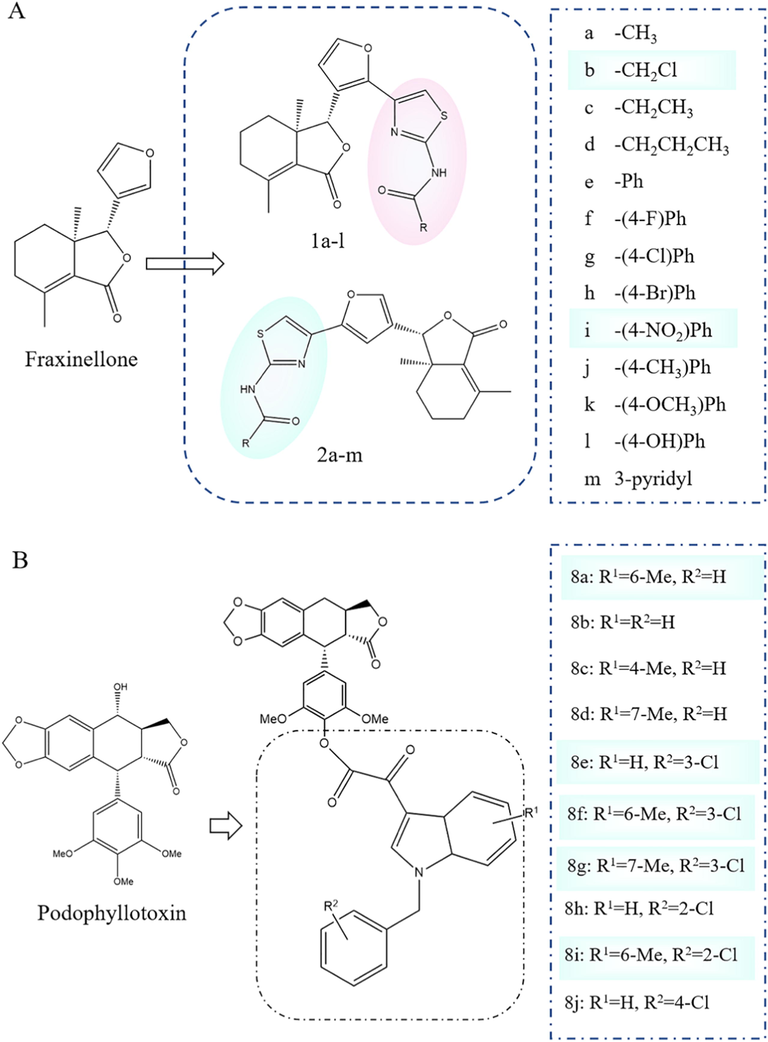
Examples of structural modifications of natural products as model products (A. Fraxinellone and its derivatives B. Podophyllotoxin and its derivatives).
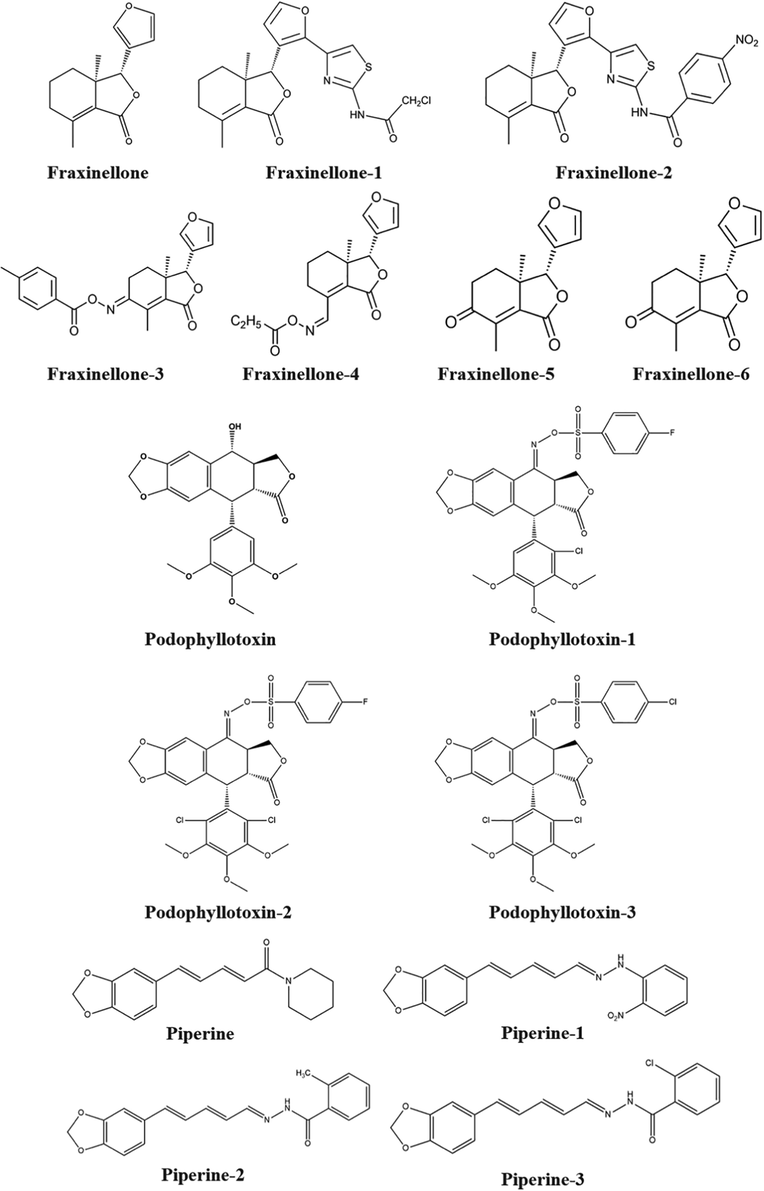
Examples of structural modifications of natural products as model products (a. Fraxinellone and its derivatives b. Podophyllotoxin and its derivatives).
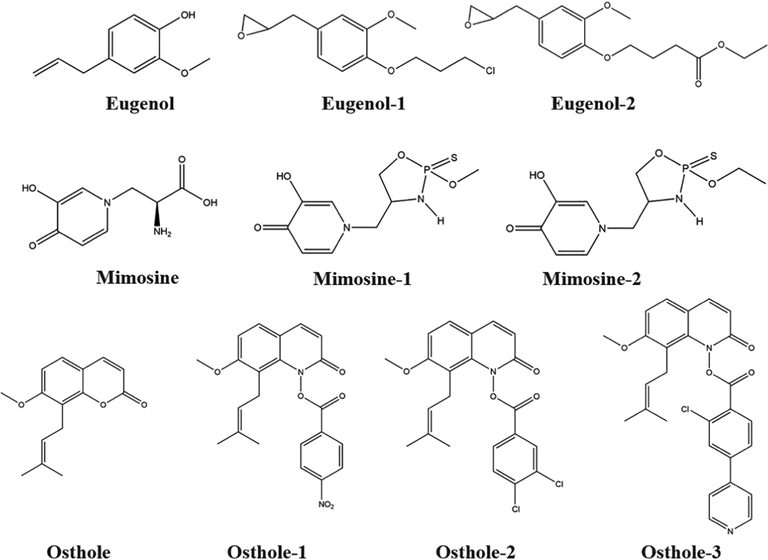
Examples of structural modifications of natural products as model products (a. Fraxinellone and its derivatives b. Podophyllotoxin and its derivatives).
The name of the natural compound
The structural formula of new insect-resistant compounds
Structure-activity relationship
Characteristics and mechanism of insect resistance
references
Fraxinellone
Fraxinellone-1
Fraxinellone-2when R was alkyl groups, the long length of the alkyl chain could decrease the growth inhibitory activity
They affected the development, ecdysis, and emergence of pests
(Guo et al., 2019)
Fraxinellone-3
Fraxinellone-4
Fraxinellone-5
Fraxinellone-6Derivatives obtained by introducing the C-4 carbonyl or oxime substituent are more effective than those bearing a C-10
They showed growth inhibitory activity against pests and affected the insect molting hormone
(Li et al., 2016)
Podophyllotoxin
Podophyllotoxin −1
Podophyllotoxin-2
Podophyllotoxin-3The introduction of the chlorine atom at the C-2′ or C-2′,6′ position on the E-ring of picropodophyllotoxin and oxime sulfonate derivatives of picropodophyllotoxin was essential for the insecticidal activity.
The podophyllotoxin derivatives showed insecticidal activity equal to, or higher than, that of the positive control toosendanin and exhibited the anti-molting hormone effect
(Wang et al., 2015)
Piperine
Piperine-1
Piperine-2
Piperine-3The introduction of the substituents at the C-2 position on the phenyl ring of the hydrazone derivatives afforded the potent compounds.
They exhibited the anti-molting hormone effect
(Qu et al., 2013)
Eugenol
Eugenol-1
Eugenol-2—
Trigger a process of programmed cell death
(Fernandes et al., 2020)
Mimosine
Mimosine-1
Mimosine-2The length of the alkyl groups and the functional substituents at the C-5 position of phosphoramidothionates derived from mimosinol are important for their insecticidal activities
Acetylcholinesterase inhibitory activity
(Nguyen et al., 2015)
Osthole
Osthole-1
Osthole-2
Osthole-3
Osthole-4The conversion of the carbonyl group into a thiocarbonyl group does not improve its insecticidal activity, and the introduction of electron-withdrawing groups on the phenyl ring of intermediate afford more potent derivatives.
Growth inhibitory activity
(Guo et al., 2020)
The discovery of new nicotine insecticides is another important milestone in agricultural chemistry research. Neonicotinoids are a product of a series of structural modifications of nicotine, which is an alkaloid extracted from the tobacco plant Nicotiana tobacum. From the introduction of the neonicotinoid insecticide imidacloprid in 1991, nitenpyram in 1995, thiamethoxam in 1997, thiacloprid in 1999, and clothianidin in 2002, neonicotinoid insecticides have become the fastest growing category of insecticides entering the market since the commercialization of pyrethroids (Jeschke and Nauen 2008, Mörtl et al., 2020). The active group of nicotine involves the 3-pyridyl methylamine portion, in which an amino nitrogen atom is its basic structural requirement. The lead compound was optimized in the 1970 s, and the first nitromethylene insecticide called nitrothiazine was produced. However, due to its light instability, 6-chloropyridine-3-methyl was introduced as a substituent for nitromethylene heterocycles, and nitroguanidine or cyanamide were used to partly replace the nitromethylene group to improve the photostability and activity of insecticides (Thany 2010). Diamide insecticides acting on the ryanodine receptor of insects have also been the research hotspots in the field of insecticides in recent years. Leonidine, which is an alkaloid extracted from Ryania speciosa, can be used as a natural insecticide against lepidopteran pests such as apple lepidoptera and European corn borer. However, it is toxic to mammals (Jeanguenat 2013). In the 1990 s, a Japanese company cooperated with Bayer to develop flubendiamide, a new class of insecticide with high activity against lepidopteran pests. DuPont made major modifications to its active structure and groups, finally launched chlorantraniliprole in 2000. Subsequently, various polar groups in the benzene ring were changed, and cyantraniliprole was successfully listed in 2012. This series of structural modifications resulted in highly efficient insecticides suitable for a wider range of crops (Jeanguenat 2013, Zhao et al., 2015).
Fraxinellone is a degraded limonoid with extensive insecticidal activities, which is extracted from Dictamnus dasycarpus and Melia azedarach. 1,3- thiazole, an important scaffold, has also made great contributions to the improvement of crop yield. However, its development was hampered by more troubling problems, such as the difficulty in its degradation, high toxicity to non-target organisms, and resistance of pests. To improve that, taking fraxinellone as the lead compound, N-(1,3-thiazole-2-yl) formamide was successfully introduced into the C-2′/5′site of fraxinellone by reacting it with acetyl chloride, thiourea and different carboxylic acids, forming two series of 25 novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides. The bioassay showed that compounds with -(4-NO2) Ph and -CH2Cl groups displayed more insecticidal and growth inhibitory activities, and low cytotoxicity to noncancerous mammalian cells. In addition, the structure–activity relationships of all target compounds indicated that the introduction of N-(1,3-thiazol-2-yl) carboxamides on C-2′ position of fraxinellone could acquire more effective fraxinellone derivatives than that on C-5′site. The length of alkyl groups of N-(1,3-thiazol-2-yl) carboxamides of fraxinellone has also important impact on its insecticidal activity (Fig. 6A) (Guo et al., 2019).
Yi Wang et al.(Xu and Wang 2012) semi-synthesized novel 4′-substituted indolyl glyoxal derivatives of 4-deoxypodophyllotoxin using podophyllotoxin as the lead material. The insecticidal activity test showed that compounds 8a, 8e-g, and 8i exhibited more potent insecticidal activities compared to toosendanin at 1 mg/mL (Fig. 6B). Their structure–activity relationships indicated that the introduction of a methyl group at the 6-position on the indolyl ring, chlorine atom at the 3-position on the benzyl moiety, and 4′-demethyl-4-deoxypodophyllotoxin on the C- 4′ hydroxyl group exhibited significant effects on insecticidal activities.
6 Application prospect of natural products for insect control
There are now some NPs that can easily be produced on a large scale at a low cost. NPs can offer a good alternative to conventional synthetic pesticides with the following advantages, enabling safer and more effective control of storage pests. First, the multi-target mode of action of NPs against pests endows them with multiple insecticidal mechanisms (Araújo et al., 2020), so they are relatively easy to fight against pest resistance. Second, most NPs extracted from plants are relatively easy to degrade and are environmentally friendly (Yuan et al., 2016). Moreover, in addition to terrestrial products, NPs in the marine environment are largely a huge potential resource for the development of agrochemicals (Peng et al., 2003). Therefore, obtaining effective insect repellent materials from nature has always been a trend and hot topic of research.
Secondary metabolites in plants are key components for the defense and adaptation of plants to biological and abiotic stress conditions. However, the synthesis of secondary metabolites requires specific environmental conditions and time, and various factors, such as genetics, ontogeny, morphogenesis and environment, can affect the synthesis and accumulation of secondary metabolites. Therefore, various novel methods such as plant cell and tissue culture (Sekh et al., 2010), plant metabolic engineering (R. et al., 2002), and transgenic culture production (Hussain et al., 2012) can be applied for better production of secondary metabolites independent of climatic and soil conditions, which can provide methods and approaches to produce commercially acceptable sustainable products. Furthermore, the diversity of pests and best standards for pest management will change over time and, therefore, new pest-control strategies or moth repellants will be needed.
Author contributions
Ding-kun Zhang, Chuan Zheng, Li Han put forward the idea; Shengjie Huang, Haozhou Huang, gathered the materials, and wrote the paper; Jin Xie, Fang Wang, Sanhu Fan and Ming Yang contributed to the revisions.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No 82074026), Open Fund Projects of Key Laboratory of Modern Preparation of TCM, Ministry of Education, Jiangxi University of Chinese Medicine (grant numbers TCM-201904), Sanajion Pharmaceutical-Chengdu University of Traditional Chinese Medicine Industry-University-Research Associated Project (grant numbers 2019-YF04-00086-JH), Project of Sichuan Administration of traditional Chinese Medicine (grant numbers 2021MS018).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Insecticidal activity of fatty acid-rich Turkish bryophyte extracts against Sitophilus granarius (Coleoptera: Curculionidae) Comb. Chem. High Throughput Screen. 2013;16:806-816.
- [CrossRef] [Google Scholar]
- Myristic acid reduces skin inflammation and nociception. J. Food Biochem.. 2022;46:e14013.
- [Google Scholar]
- D-limonene: a multifunctional compound with potent therapeutic effects. J. Food Biochem.. 2021;45:e13566.
- [Google Scholar]
- Larvicidal activity of cinnamic acid derivatives: investigating alternative products for Aedes aegypti L. Control. Mol.. 2020;26
- [CrossRef] [Google Scholar]
- Pharmacological evaluation of anti-arthritic potential of terpinen-4-ol using in vitro and in vivo assays. Inflammopharmacology. 2022;30:945-959.
- [CrossRef] [Google Scholar]
- Evaluation of contamination of packages containing cereal-fruit bars by eggs of the pest Indian meal moth (Plodia interpunctella, Lepidoptera) due to perforations in their polypropylene foil packaging. J. Food Sci. Technol.. 2019;56:3293-3299.
- [CrossRef] [Google Scholar]
- Geraniol ameliorates the progression of high fat-diet/streptozotocin-induced type 2 diabetes mellitus in rats via regulation of caspase-3, Bcl-2, and Bax expression. J. Food Biochem.. 2022;46:e14142.
- [Google Scholar]
- Association between exposure to pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general US adult population. JAMA Intern. Med.. 2020;180:367-374.
- [CrossRef] [Google Scholar]
- Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action. Molecules. 2019;24:3961.
- [CrossRef] [Google Scholar]
- Anesthetic pharmacology of the mint extracts L-carvone and methyl salicylate. Pharmacology. 2022;107:167-178.
- [CrossRef] [Google Scholar]
- Biosynthesis and production of sabinene: current state and perspectives. Appl. Microbiol. Biotechnol.. 2018;102:1535-1544.
- [CrossRef] [Google Scholar]
- Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol.. 2019;10:206.
- [CrossRef] [Google Scholar]
- Eugenia uniflora leaf essential oil promotes mitochondrial dysfunction in Drosophila melanogaster through the inhibition of oxidative phosphorylation. Toxicol. Res. (Camb).. 2017;6:526-534.
- [CrossRef] [Google Scholar]
- Naturally-occurring alkaloids of plant origin as potential antimicrobials against antibiotic-resistant infections. Molecules. 2020;25:3619.
- [CrossRef] [Google Scholar]
- Protection of grain products from Sitophilus oryzae (L.) contamination by anti-insect pest repellent sachet containing allyl mercaptan microcapsule. J. Food Sci.. 2017;82:2634-2642.
- [CrossRef] [Google Scholar]
- Characterization of two coleopteran α-amylases and molecular insights into their differential inhibition by synthetic α-amylase inhibitor, acarbose. Insect Biochem. Mol. Biol.. 2016;74:1-11.
- [CrossRef] [Google Scholar]
- Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci.. 2017;8:610.
- [CrossRef] [Google Scholar]
- Effect of d-limonene and its derivatives on breast cancer in human trials: a scoping review and narrative synthesis. BMC Cancer. 2021;21:902.
- [CrossRef] [Google Scholar]
- Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J. Surg. Res.. 2014;186:436-445.
- [CrossRef] [Google Scholar]
- Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest. Manag. Sci.. 2011;67:714-718.
- [CrossRef] [Google Scholar]
- Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microbiol.. 2016;7:1463.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem.. 2009;57:4313-4318.
- [CrossRef] [Google Scholar]
- Role of ascaridole and p-cymene in the sleep-promoting effects of Dysphania ambrosioides essential oil via the GABAergic system in a ddY mouse inhalation model. J. Nat. Prod.. 2021;84:91-100.
- [CrossRef] [Google Scholar]
- Antifeedant diterpenoids against Tribolium castaneum from the stems and twigs of Ceriops tagal (Rhizophoraceae) Molecules. 2011;16:6060-6067.
- [CrossRef] [Google Scholar]
- Chemistry, bioactivities, extraction and analysis of azadirachtin: state-of-the-art. Fitoterapia. 2019;134:141-150.
- [CrossRef] [Google Scholar]
- New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci.. 2020;21:9257.
- [CrossRef] [Google Scholar]
- Figuêiredo, F. R., B. Monteiro, Á. I. R. Alencar, M., et al., 2019. Effects of the Hyptis martiusii Benth. leaf essential oil and 1,8-cineole (eucalyptol) on the central nervous system of mice. Food. Chem. Toxicol. 133, 110802. https://doi.org/10.1016/j.fct.2019.110802
- Genome wide association mapping in Arabidopsis thaliana identifies novel genes involved in linking allyl glucosinolate to altered biomass and defense. Front. Plant Sci.. 2016;7:1010.
- [CrossRef] [Google Scholar]
- β-caryophyllene, a dietary cannabinoid, protects against metabolic and immune dysregulation in a diet-induced obesity mouse model. J. Med. Food 2022
- [CrossRef] [Google Scholar]
- Haemato-biochemical and immuno-pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. Int. Immunopharmacol.. 2004;4:1709-1722.
- [CrossRef] [Google Scholar]
- Feeding deterrents against two grain storage insects from Euphorbia fischeriana. Molecules. 2011;16:466-476.
- [CrossRef] [Google Scholar]
- Natural products for pest control: an analysis of their role, value and future. Pest. Manag. Sci.. 2014;70:1169-1185.
- [CrossRef] [Google Scholar]
- Structure and function of the mitochondrial ribosome. Annu. Rev. Biochem.. 2016;85:103-132.
- [CrossRef] [Google Scholar]
- Turning natural products into insecticide candidates: design and semisynthesis of novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides against two crop-threatening insect pests. Bioorg. Med. Chem. Lett.. 2019;29:179-184.
- [CrossRef] [Google Scholar]
- Application of natural products as insecticide candidates: semisynthesis and biological evaluation of some novel osthole-based esters. Bioorg. Med. Chem. Lett.. 2020;30:127260
- [CrossRef] [Google Scholar]
- Effect of rotenone-induced stress on physiologically active substances in adult Aphis glycines. PLoS One. 2020;15:e0234137.
- [CrossRef] [Google Scholar]
- Insect juvenile hormone: from “status quo” to high society. Braz. J. Med. Biol. Res.. 2000;33:157-177.
- [CrossRef] [Google Scholar]
- Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci.. 2000;160:1-13.
- [CrossRef] [Google Scholar]
- Bioactivities of methyl allyl disulfide and diallyl trisulfide from essential oil of garlic to two species of stored-product pests, Sitophilus zeamais (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) J. Econ. Entomol.. 2000;93:537-543.
- [CrossRef] [Google Scholar]
- Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol.. 2018;63:553-573.
- [CrossRef] [Google Scholar]
- Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci.. 2012;4:10-20.
- [CrossRef] [Google Scholar]
- The story of a new insecticidal chemistry class: the diamides. Pest. Manag. Sci.. 2013;69:7-14.
- [CrossRef] [Google Scholar]
- Neonicotinoids-from zero to hero in insecticide chemistry. Pest. Manag. Sci.. 2008;64:1084-1098.
- [CrossRef] [Google Scholar]
- Structural features, substrate specificity, kinetic properties of insect α-amylase and specificity of plant α-amylase inhibitors. Pestic. Biochem. Physiol.. 2014;116:83-93.
- [CrossRef] [Google Scholar]
- Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest. Manag. Sci.. 2002;58:1101-1106.
- [CrossRef] [Google Scholar]
- Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L) Pest. Manag. Sci.. 2001;57:548-553.
- [CrossRef] [Google Scholar]
- Pharmacological properties of geraniol - a review. Planta Med.. 2019;85:48-55.
- [CrossRef] [Google Scholar]
- Defense of pyrethrum flowers: repelling herbivores and recruiting carnivores by producing aphid alarm pheromone. New Phytol.. 2019;223:1607-1620.
- [CrossRef] [Google Scholar]
- Semisynthesis of esters of fraxinellone C4/10-oxime and their pesticidal activities. J. Agric. Food Chem.. 2016;64:5472-5478.
- [CrossRef] [Google Scholar]
- Chemical composition and insecticidal activities of the essential oil of Clinopodium chinense (Benth.) Kuntze Aerial Parts against Liposcelis bostrychophila Badonnel. J. Food Prot.. 2015;78:1870-1874.
- [CrossRef] [Google Scholar]
- Jolkinolide B alleviates renal fibrosis via anti-inflammation and inhibition of epithelial-mesenchymal transition in unilateral ureteral obstruction mice. J. Asian Nat. Prod. Res.. 2022;24:76-87.
- [CrossRef] [Google Scholar]
- Toxicity of Melaleuca alternifolia essential oil to the mitochondrion and NAD(+)/NADH dehydrogenase in Tribolium confusum. PeerJ.. 2018;6:e5693.
- [Google Scholar]
- Effects of terpinen-4-ol fumigation on protein levels of detoxification enzymes in Tribolium confusum. Arch. Insect Biochem. Physiol.. 2020;103:e21653.
- [Google Scholar]
- Biotransformation patterns of dictamnine in vitro/in vivo and its relative molecular mechanism of dictamnine-induced acute liver injury in mice. Environ. Toxicol. Pharmacol.. 2021;85:103628
- [CrossRef] [Google Scholar]
- Composition and insecticidal activity of essential oil of Bacopa caroliniana and interactive effects of individual compounds on the activity. Insects. 2019;11:23.
- [CrossRef] [Google Scholar]
- Chemical composition and insecticidal activity of the essential oil of Illicium pachyphyllum fruits against two grain storage insects. Molecules. 2012;17:14870-14881.
- [CrossRef] [Google Scholar]
- Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J. Agric. Food Chem.. 2002;50:1447-1450.
- [CrossRef] [Google Scholar]
- Feeding deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agric. Food Chem.. 2009;57:10130-10133.
- [CrossRef] [Google Scholar]
- Feeding deterrents from Aconitum episcopale roots against the red flour beetle, Tribolium castaneum. J. Agric. Food Chem.. 2011;59:3701-3706.
- [CrossRef] [Google Scholar]
- Antifeedants from Chinese medicinal herb, Erythrina variegata var. orientalis, against maize weevil Sitophilus zeamais. Nat. Prod. Commun.. 2012;7:171-172.
- [CrossRef] [Google Scholar]
- Mechanistic insight of the potential of geraniol against Alzheimer's disease. Eur. J. Med. Res.. 2022;27:93.
- [CrossRef] [Google Scholar]
- Fraxinellone ameliorates intracerebral hemorrhage-induced secondary brain injury by regulating Krüppel-like transcription factor 2 expression in rats. Brain Res. Bull.. 2021;177:340-351.
- [CrossRef] [Google Scholar]
- Evaluation of physiological and biochemical effects of two Sophora alopecuroides alkaloids on pea aphids Acyrthosiphon pisum. Pest. Manag. Sci.. 2020;76:4000-4008.
- [CrossRef] [Google Scholar]
- Allethrin induced toxicity in the male reproductive tract of rats contributes to disruption in the transcription of genes involved in germ cell production. Environ. Toxicol.. 2014;29:1330-1345.
- [CrossRef] [Google Scholar]
- Developmental pyrethroid exposure causes long-term decreases of neuronal sodium channel expression. Neurotoxicology. 2017;60:274-279.
- [CrossRef] [Google Scholar]
- Larvicidal action of cannabidiol oil and neem oil against three stored product insect pests: effect on survival time and in progeny. Biology. 2020;9:321.
- [CrossRef] [Google Scholar]
- Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci.. 2019;95:378-400.
- [CrossRef] [Google Scholar]
- Interactions of alkaloids with galeal chemosensory cells of Colorado potato beetle. J. Chem. Ecol.. 1987;13:2009-2022.
- [CrossRef] [Google Scholar]
- Effects ofSolanum glycoalkaloids on chemosensilla in the Colorado potato beetle : a mechanism of feeding deterrence? J. Chem. Ecol.. 1985;11:73-83.
- [CrossRef] [Google Scholar]
- Mörtl, M., Vehovszky, Á., Klátyik, S., et al., 2020. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public. Health. 17, 2006 https://doi.org/10.3390/ijerph17062006.
- The toxicity, persistence and mode of actions of selected botanical pesticides in Africa against insect pests in common beans, P. vulgaris: a review. Am. J. Sci.. 2016;7:138-151.
- [CrossRef] [Google Scholar]
- Repellent activities of dichloromethane extract of Allium sativum (garlic) (Liliaceae) against Hyalomma rufipes (Acari) J. S. Afr. Vet. Assoc.. 2016;87:e1-e5.
- [CrossRef] [Google Scholar]
- Insecticidal and nematicidal activities of novel mimosine derivatives. Molecules. 2015;20:16741-16756.
- [CrossRef] [Google Scholar]
- Conifer diterpene resin acids disrupt juvenile hormone-mediated endocrine regulation in the Indian meal moth Plodia interpunctella. J. Chem. Ecol.. 2017;43:703-711.
- [CrossRef] [Google Scholar]
- Insecticidal activity and chemical composition of the Morinda lucida essential oil against pulse beetle Callosobruchus maculatus. Sci. World J.. 2014;2014:784613
- [CrossRef] [Google Scholar]
- Control effect of 1% Osthol powder to three stored-grain pests. Bull. Entomol. Res.. 2009;587–591
- [CrossRef] [Google Scholar]
- L-carvone induces p53, caspase 3 mediated apoptosis and inhibits the migration of breast cancer cell lines. Nutr. Cancer. 2014;66:453-462.
- [CrossRef] [Google Scholar]
- Marine natural products as prototype agrochemical agents. J. Agric. Food Chem.. 2003;51:2246-2252.
- [CrossRef] [Google Scholar]
- Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces. 2018;171:566-578.
- [CrossRef] [Google Scholar]
- Bioactivities of ketones terpenes: antifungal effect on F. verticillioides and repellents to control insect fungal vector, S. zeamais. Microorganisms. 2015;3:851-865.
- [CrossRef] [Google Scholar]
- Natural-product-based insecticidal agents 14. semisynthesis and insecticidal activity of new piperine-based hydrazone derivatives against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett.. 2013;23:5552-5557.
- [CrossRef] [Google Scholar]
- Molecular investigation of Coleopteran specific α-Amylase inhibitors from Amaranthaceae members. Int. J. Biol Macromol.. 2020;163:1444-1450.
- [CrossRef] [Google Scholar]
- Pesticide degrading natural multidrug resistance bacterial flora. Microb. Pathog.. 2018;114:304-310.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res.. 2013;62:367-376.
- [CrossRef] [Google Scholar]
- Typical monoterpenes as insecticides and repellents against stored grain pests. Molecules. 2016;21:258.
- [CrossRef] [Google Scholar]
- Bioactivity of some Apiaceae essential oils and their constituents against Sitophilus zeamais (Coleoptera: Curculionidae) Bull. Entomol. Res.. 2020;110:406-416.
- [CrossRef] [Google Scholar]
- Biological activity of Dittrichia viscosa (L.) Greuter extracts against adult Sitophilus granarius (L.) (Coleoptera, Curculionidae) and identification of active compounds. Sci. Rep.. 2019;9:6429.
- [CrossRef] [Google Scholar]
- S, K., Kujur, A., Patel, L., et al., 2017. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 139, 17-23. https://doi.org/10.1016/j.pestbp.2017.04.004
- Sekh, A., Nasim, Aslam, J., et al., 2010. Secondary metabolites production through biotechnological intervention: A Review. Emir. J. Food. Agric. 22, 147-161. https://doi.org/10.9755/ejfa.v22i3.4886
- Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe) Pestic. Biochem. Physiol.. 2014;113:55-61.
- [CrossRef] [Google Scholar]
- A review of interactions between insect biological control agents and semiochemicals. Insects. 2019;10:439.
- [CrossRef] [Google Scholar]
- Species-specific interactions between plant metabolites and insect juvenile hormone receptors. J. Chem. Ecol.. 2018;44:1022-1029.
- [CrossRef] [Google Scholar]
- Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res.. 2018;62:e1800322.
- [Google Scholar]
- The modulation of PCSK9 and LDLR by supercritical CO(2) extracts of Mentha longifolia and isolated piperitone oxide, an in vitro study. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Neonicotinoid insecticides: historical evolution and resistance mechanisms. Adv. Exp. Med. Biol.. 2010;683:75-83.
- [CrossRef] [Google Scholar]
- Bioactivities of the leaf essential oil of Curcuma longa (var. ch-66) on three species of stored-product beetles (Coleoptera) J. Econ. Entomol.. 2002;95:183-189.
- [CrossRef] [Google Scholar]
- Effect of d-limonene on three stored-product beetles. J. Econ. Entomol.. 2003;96:990-995.
- [CrossRef] [Google Scholar]
- Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol.. 2003;96:1594-1601.
- [CrossRef] [Google Scholar]
- Radiosensitizing effect of schinifoline from Zanthoxylum schinifolium Sieb et Zucc on human non-small cell lung cancer A549 cells: a preliminary in vitro investigation. Molecules. 2014;19:20128-20138.
- [CrossRef] [Google Scholar]
- Cis-bifenthrin causes immunotoxicity in murine macrophages. Chemosphere. 2017;168:1375-1382.
- [CrossRef] [Google Scholar]
- 17-hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus kinases and induces apoptosis of human cancer cells. Cancer. Res.. 2009;69:7302-7310.
- [CrossRef] [Google Scholar]
- The newly identified K+ channel blocker talatisamine attenuates beta-amyloid oligomers induced neurotoxicity in cultured cortical neurons. Neurosci. Lett.. 2012;518:122-127.
- [CrossRef] [Google Scholar]
- Bioactivity of essential oil of zingiber purpureum rhizomes and its main compounds against two stored product insects. J. Econ. Entomol.. 2015;108:925-932.
- [CrossRef] [Google Scholar]
- Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from Zanthoxylum planispinum var. dintanensis. Environ. Sci. Pollut. Res. Int.. 2019;26:24988-24997.
- [CrossRef] [Google Scholar]
- Jolkinolide B inhibits proliferation or migration and promotes apoptosis of MCF-7 or BT-474 breast cancer cells by downregulating the PI3K-Akt pathway. J. Ethnopharmacol.. 2022;282:114581
- [CrossRef] [Google Scholar]
- Insecticidal constituents of essential oil derived from Zanthoxylum armatum against two stored-product insects. J. Oleo. Sci.. 2015;64:861-868.
- [CrossRef] [Google Scholar]
- Synthesis of novel oxime sulfonate derivatives of 2'(2',6')-(Di)chloropicropodophyllotoxins as insecticidal agents. J. Agric. Food Chem.. 2015;63:6668-6674.
- [CrossRef] [Google Scholar]
- Natural products-based insecticidal agents 8. design, semisynthesis and insecticidal activity of novel O-(Deoxypodophyllotoxin-4'-yl)-(N-((un)substituted benzyl)indol-3-yl)glyoxylesters against Mythimna separata Walker. Heterocycles. 2012;84:505.
- [CrossRef] [Google Scholar]
- Study on absorbed components of Aconitum kusnezoffii under Yunnan Baiyao compatibility in effect of activating blood circulation and removing blood stasis. China J. Chin. Mater. Med.. 2019;44:3349-3357.
- [CrossRef] [Google Scholar]
- Sophocarpine alleviates injury-induced intima hyperplasia of carotid arteries by suppressing inflammation in a rat model. J. Clin. Med.. 2021;10
- [CrossRef] [Google Scholar]
- The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559.
- [CrossRef] [Google Scholar]
- Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J. Cell. Mol. Med.. 2022;26:3702-3715.
- [CrossRef] [Google Scholar]
- Insecticidal and repellent activities of the essential oil of Callistemon citrinus (Myrtaceae) against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) Neotrop. Entomol.. 2013;42:89-94.
- [CrossRef] [Google Scholar]
- Effects of citronellal on growth and enterotoxins production in Staphylococcus aureus ATCC 29213. Toxicon.. 2022;213:92-98.
- [CrossRef] [Google Scholar]
- Partial synthesis of Crassicauline A from Yunaconitine. Nat. Prod. Bioprospect.. 2020;10:105-108.
- [CrossRef] [Google Scholar]
- Insecticidal activities of constituents of Litsea cubeba fruit extracts effective against the maize weevil (Coleoptera: Curculionidae) J. Insect Sci.. 2017;17:103.
- [CrossRef] [Google Scholar]
- Current status and prospects of bisamide insecticides pesticide science and administration. Plants. 2015;36:23-29.
- [CrossRef] [Google Scholar]
- The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro. Poult. Sci.. 2022;101:101801
- [CrossRef] [Google Scholar]
- Fraxinellone alleviates kidney fibrosis by inhibiting CUG-binding protein 1-mediated fibroblast activation. Toxicol. Appl. Pharmacol.. 2021;420:115530
- [CrossRef] [Google Scholar]
- Karacoline, identified by network pharmacology, reduces degradation of the extracellular matrix in intervertebral disc degeneration via the NF-κB signaling pathway. J. Pharm. Anal.. 2020;10:13-22.
- [CrossRef] [Google Scholar]
- A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci.. 2008;11:1059-1067.
- [CrossRef] [Google Scholar]







