Translate this page into:
Antimicrobial activities of polyethylene glycol and citric acid coated graphene oxide-NPs synthesized via Hummer’s method
⁎Corresponding author. arslan4physics@gmail.com (Arslan Mahmood), sulman.shafeeq@ki.se (Sulman Shafeeq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Graphene oxide (GO)-NPs possess excellent physicochemical and biological properties and could have prominent antimicrobial activities. We used solution-based Hummer’s method to synthesize pure, CA coated and PEG coated GO-NPs and the XRD revealed hexagonal lattice structure and the crystallite size of pure and coated GO-NPs calculated in the range of 7–16 nm. In addition, a spongy like surface in pure GO-NPs and randomly crumpled like surface by using the CA and PEG coating agents was also identified and SEM images irregular, non-uniform and rod shape morphology of the graphite powder. The GO in the UV region and the band gap decreased from 2.30 to 2.03 eV while different modes (C⚌C and C⚌O) on the surface of pure and coated GO-NPs were evident as indicated by Raman spectra. Finally, in-vitro antimicrobial activity was assessed against two-gram negative bacterial strains viz. A. hydrophilaand E. coli and one fungal stain F. avenaceum at 10 and 20 mg/mL concentrations. Our results indicated enhancement in the inhibition zones of pure GO-NPs from 8 to 17 mm, PEG coated GO-NPs from 8 to 20 mm and CA coated GO-NPs from 8 to 17 mm respectively. Overall the PEG coated GO-NPs had prominent antimicrobial activities and we recommend application of polymer coated GO-NPs for wound healing process.
Keywords
PEG
Graphene oxide (GO)
Antimicrobial
A. hydrophila
E. coli
F. avenaceum
1 Introduction
The potential threat of bacterial infectious diseases which include Pneumonia, Anthrax, Cholera, Tuberculosis, Botulism, and Tetanus cause of distressing in millions of people. The treatment of pathogenic bacterial infection possible with drugs createsa lot of side effects on human health (Ahmad et al., 2021; Muhammad et al., 2020). These side effects are reduced by using the non-traditional antibacterial nanomaterials (Munir et al., 2021; Munir et al., 2020; Robkhob et al., 2020). The carbon base nanomaterials/ nanosheet (Graphene and graphene oxide) have excellent physicochemical and biological properties and are preferred for biomedical applications which include antibacterial, anticancer, antifungal, antioxidant, and antiviral infections (Bilal et al., 2021; Chung et al., 2013; Priyadarsini et al., 2018). Recent studies are provided detailed information about synthetic, miscible, and biodegradable polymers-coated nanomaterials that improve the stability, biocompatible and nontoxic for cells and tissues (Sunderrajan et al., 2018). The PEG is a hydrophilic polymer and CA is an organic acid that reacts with Graphene oxide nanoparticles (GO-NPs) to increase its physical and chemical characteristics.
Graphene is the most prominent material in lab research and industrial applications (Kamenska et al., 2021). The chemical addition of functional groups (OH, COOH) in graphene materialthen it was changed into graphene oxide. Nowadays, the main challenge for researchers is the synthesis of graphene-based nanomaterials with control physical properties (Al Mogbel et al., 2021). The organic/polymer coating agents increase the agglomeration level in graphene base nanomaterial (Chowdhury et al., 2014). The GO materials show excellent biocompatibility, tissue repair and enhance the proliferation level of stem cells. The GO-NPs have antifungal, anticancer, and antibacterial activities against gram-negative and positive strains of bacteria (Placha and Jampilek, 2019). Likewise, GO-NPs can damage the bacterial membrane and cause oxidative stress the bacteria was dead. A huge number of coating agents were used to enhance antimicrobial activity (Sengupta et al., 2019). Al Mogbel et al (2021) reported that the PVP-coated GO-NPs provided significant results in the field of biophysics but they have weak mechanical properties as compared to GO-NPs. After coating the GO-NPs it was identified that the hydrogen bonding takes place between hydroxyl and carboxylic groups in GO with pyrrolidone rings in PVP. The PVP-coated GO-NPs enhance tissue compatibility, limited absorption of water, and high mechanical strength and could have substantial biological activities.
The linear synthetic Polyethylene glycol (PEG) and biodegradable Citric acid (CA) coated Graphene oxide (GO)-NPs was synthesized by using Hummer’s method. The prepared samples were characterized with different techniques like X-ray powder diffraction (XRD), Scanning electron microscope (SEM), Ultraviolet–visible spectroscopy (UV–VIS) and Raman spectroscopy. The bio assay was completed with the help of well diffusion method against two gram negative strains such as Aeromonas hydrophila (A. hydrophila), Escherichia coli (E. coli), and one fungi Fusarium avenaceum (F. avenaceum).
2 Experiment
2.1 Chemicals
To investigate the quantitative analysis of pure and coated GO-NPs were synthesized by using various chemicals such as graphite powder, hydrogen peroxide (H2O2), sulphuric acid (H2SO4), and potassium permanganate (KMnO4), de-ionize water, citric acid, and polyethylene glycol. The prepared pure and coated GO-NPswere used for antimicrobial activities.
2.2 Synthesis of GO-NPs
Hummer’s method was used to synthesize of GO-NPs in which 3 g of graphite powder was dissolved in 75 mL of H2SO4. After that, the materials were stored in an ice bath and continuous stirring on a magnetic stirrer for three hours. During this process, the 9 g of KMnO4 was added slowly to the graphite powder solution and the temperature was adjusted at 15 °C to control the reaction rate. Furthermore, the 300 mL of de-ionized water was added dropwise to dilute the solution and then remove from the ice bath to increase the temperature upto 50 °C. In the end, the reaction was terminated by using 15 mL of H2O2, and the final product was filtered using filter paper. The materials were dried in an oven at 100 °C for 3 days and the materials were ground by using mortar and pestle.
2.3 Citric acid and Polyethylene glycol coated GO-NPs
During the synthesis process, the 0.5 g polyethylene glycol was added to 10 mL of de-ionized water. The prepared PEG solution was added to the GO solution to obtain the PEG-coated GO materials. After that, the same process was repeated for citric acid 0.2 g to obtain the CA-coated GO materials.
2.4 Material characterization techniques
The prepared pure and coated GO-NPs were characterized by using various characterization techniques such as XRD, SEM, UV–VIS, and Raman spectroscopy. The structural analysis was identified by XRD (Cu-Kα radiation, D8 Advance, Bruker, X'Pert3 MRD XL), and the surface morphology was investigated via SEM (tabletop) and UV–VIS (Lambda 25, Perkin Elmer) was used to identifyabsorbance bands. Finally, confocal Raman spectroscopy (MN STEX_PR1100) was used to calculate the different modes attached to the surface of pure and coated GO-NPs.
2.5 Antimicrobial assay
2.5.1 Culturing of Aeromonas hydrophila, Escherichia coli, and Fusarium avenaceum
The well diffusion method was used to culture bacteria and fungi{A. hydrophila (CECT 839), E. coli (MTCC 443) and F. avenaceum (PDA)}to investigate the antimicrobial activity. The initial step was preferred to solidifying media and then cultured samples were implanted in Petri dishesfor 24 h at 37 °C. After that the different concentrations (10 and 20 mg/mL) of pure, CA coated and PEG coated GO-NPs and then again incubated for 24 h to investigate the changes in the inhibition zone.
3 Results and discussion
3.1 XRD analysis
The XRD analysis was performed to investigate the crystalline nature of pure and coated GO-NPs. Fig. 1 shows the multiple diffracted peaks representing the different miller indices planes such as (0 0 1) and (0 0 2) compared with previously published data (Jabbar et al., 2017; Johra et al., 2014). The hexagonal lattice structureidentified the most prominent peaks of pure and coated GO-NPs. The analysis indicated that coating agents enhance the intensity level which depends upon the orientation of the peaks and no extra peak appeared. Moreover, the CA coating agent shifted the peak toward shorter (2θ) as compared to pure and PEG-coated GO-NPs (Jihad et al., 2021). In the case of graphite powder, the d-spacing (0.3395 nm) and crystallite sizeslightly increased as compared to pure and coated GO-NPs. The crystallite size, FWHM, and d-spacing value of pure and coated GO-NPswere calculated by using equations ((1) and (2)). Table 1 indicated that PEG-coated GO-NPscrystallite size is much greater as compared to pure andCA-coated GO-NPs.
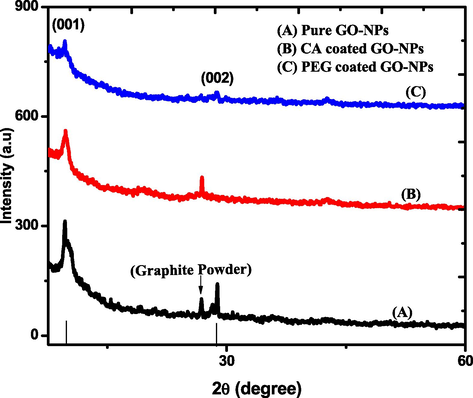
XRD spectrum of pure and coated GO-NPs.
Nanoparticles
d-spacing Value (nm)
FWHM (0 0 1)
(rad)Crystallite size
(nm)
Pure GO-NPs
0.9069
0.020
7
CA coated GO-NPs
0.8923
0.019
7.4
PEG coated GO-NPs
0.8926
0.009
15.4
3.2 SEM analysis
The SEM analysis was used to identify the surface morphology of the pure and coated GO-NPs. Fig. 2 (A) Graphite power (B) Pure GO-NPs (C) CA-coated GO-NPs (D) PEG-coated GO-NPs. Fig. 2 (A) shows the irregular, non-uniform, and rod-like shape attachedto the surface of the sheet. Fig. 1(B) represents the wrinkles and spongy-like surface of pure GO-NPs. It means that the GO-NPs have a large surface area due to oxidation as compared to Graphite powder (Samadian et al., 2020; Hayes et al., 2015). Likewise, Fig. 2 (C) indicatesthe surface morphology of GO-NPs changes with CA and also shows that the agglomeration increases between GO-NPs. Fig. 2(D) shows the strong interaction between the PEG and GO-NPs. Due to this interaction, the randomly crumpled-like morphology appeared in PEG-coated GO-nanomaterial.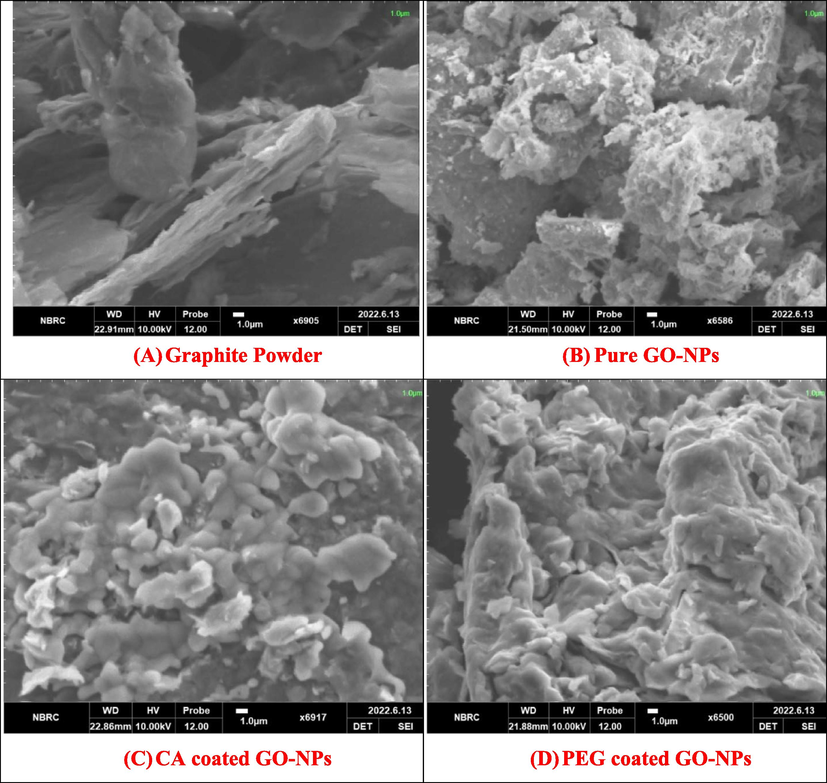
SEM analysis of Graphite powder, Pure and coated GO-NPs.
3.3 UV–VIS analysis
Theoptical absorbance spectrum of pure and coated GO-NPs was investigated via UV–VIS spectroscopy. Fig. 3 indicates the spectrums of pure, CA-coated, and PEG-coated GO-NPs in which the 1st peak at 247 nm and 2nd peak at 323 nm. Furthermore, the coating agents CA and PEG enhance the intensity level of the spectrums (Georgieva et al., 2021). The peaks at 247 nm show the conformation of GO-NPsandit represented the π-π* transition of the C—C bond. Furthermore, the 2nd peak at 323 nm expressed the n-π* transition of the C⚌O bond (Bhargava and khan, 2018). The Tauc relationexpressed in equation (III) was used to calculatethe band gap of pure, CA-coated, and PEG-coated GO-NPs (Gospodinova et al., 2021). Fig. 4 shows that band gap decreases (2.30–2.01 eV) due toan increase in the particle size of CA-coated and PEG-coated GO-NPs.
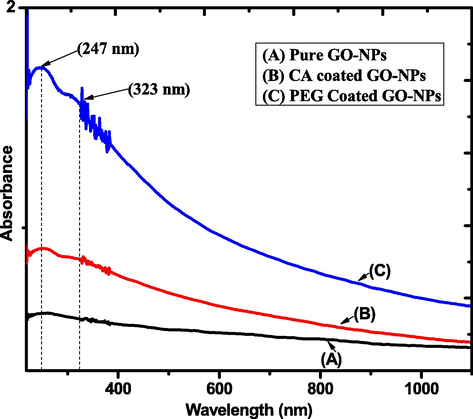
UV–VIS spectrum of pure and coated GO-NPs.
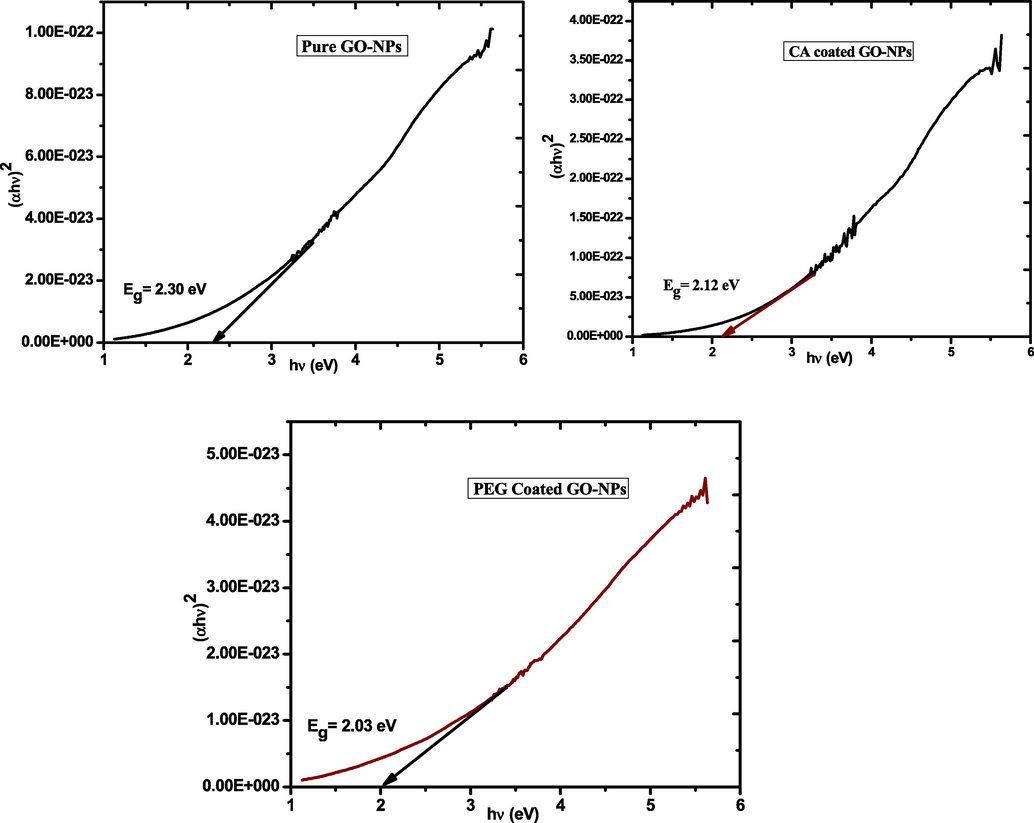
Band gap of pure and coated GO-NPs.
3.4 Raman spectroscopy analysis
The Raman spectroscopy was used to collect structural information such as disorder, defect, and doping level. Itis an essential tool to provide details informationabout carbon-based nanomaterials. Fig. 5 indicates the spectrum of pure, CA-coated, and PEG-coated GO-NPs which appear in D and G bands (Li et al., 2018). The D band at 1336 cm−1 indicate the presence of a C⚌C ring and the G band that appeared at 1586 cm−1 shows that sp2 hybridization and also expressed the scattering E2g phonon. After coating the GO-NPs were expressed G-band broader due to a reduction in the size of the sp2 plan which cause the excess oxidation (Orth et al., 2013). The D band was expressed more intense beakswhich means that the more reduction due to coated GO-NPs. Likewise, the intensity level increase of coatedGO-NPs due to surface activity and the relative intensity of D and G indicates the cluster size increase. Furthermore, the previous study provided the detailed information about the intensity level increase at every stage of modification (Usman et al., 2018).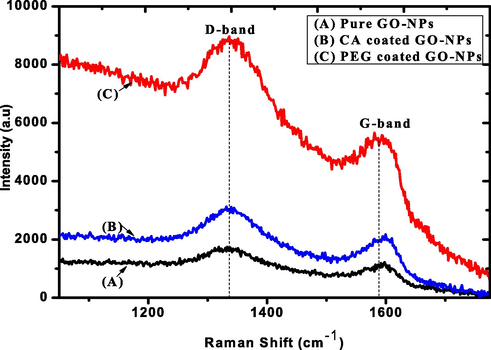
Raman spectrum of pure and coated GO-NPs.
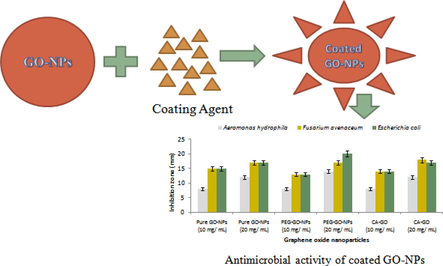
3.5 Antimicrobial assay
Fig. 6 shows thatin-vitro antimicrobial activity was assessed by using the well diffusion method. The reactive oxygen species was responsible to increase the inhibition zone by using the different doses of pure and coated GO-NPs. The free radical played a central role to damage the cell wall cause the bacteria dead. This study was done with the help of two different concentrations (10 mg/mL and 20 mg/mL) of pure and surface-coated GO-NPs against two gram-negative bacteria such as Aeromonas hydrophila and Escherichia coli, as well as one filamentous fungi Fusarium avenaceum (Olborska et al., 2020; Valentini et al., 2019). The inhibition zone change by using 10 mg/mL of pure, PEG and CA GO-NPs was investigated for A. hydrophila (8,8, and 8 mm), E. coli (15, 13, and 14 mm), and F. avenaceum (15, 13, and 14 mm). Furthermore, by increasing the concentration upto 20 mg/mL then inhibition zone change was investigated forA. hydrophila (12, 14, and 12 mm), E. coli (17, 20 and 17 mm) and F. avenaceum (17, 17, and 18 mm). The antimicrobial analysis shows that the PEG-coated GO-NPs are suitable for antimicrobial activity.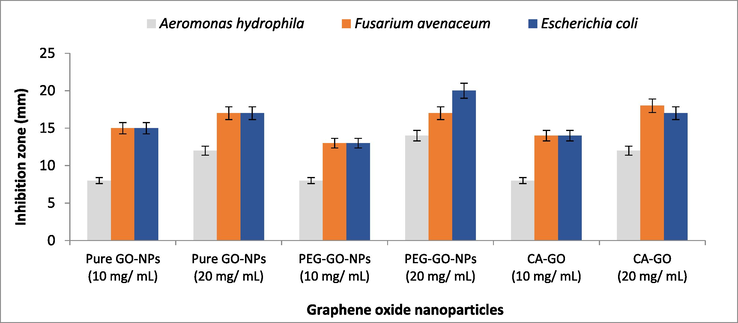
Antimicrobial activity of pure and coated GO-NPs.
4 Conclusion
The hummer’s method was used to synthesize pure and coated GO-NPs for antimicrobial activity. The hexagonal lattice structureand crystallite size in the range of 7–16 nm were investigated with the help of XRD analysis. The irregular, non-uniform, rod shape, the spongy and randomly crumpled-like surface of graphite powder, pure, CA-coated, and PEG-coated GO-NPswere identified via SEM analysis. Moreover, the optical absorbance in the range of the UV region and the band gap decrease (2.30–2.03 eV) with CA and PEG-coated GO-NPs were calculated with UV–VIS analysis. The presence of different bands on the surface of the spectrum was measured by Raman spectroscopy analysis. After that, the antimicrobial activity against A. hydrophila, E. coli and F. avenaceum were done by using 10 and 20 mg/mL of pure and (PEG and CA) coated GO-NPs. The PEG-coated GO-NPs enhance the antimicrobial activity upto a significant level of inhibition zone of 20 mm. In future the polymer-coated GO-NPs are preferred for antioxidant and wound healing purposes.
Acknowledgement
Researchers Supporting Project number (RSP-2021/397), King Saud University, Riyadh, Saudi Arabia.
References
- Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist.. 2021;27:101-111.
- [Google Scholar]
- Improvement in antibacterial activity of Poly Vinyl Pyrrolidone/Chitosan incorporated by graphene oxide NPs via laser ablation. J. Polym. Res.. 2021;28(12):1-8.
- [Google Scholar]
- Bhargava, R., Khan, S., 2018. Structural, optical and dielectric properties of graphene oxide. In: AIP Conference Proceedings, vol. 1953, no. 1, AIP Publishing LLC, p. 030011.
- Biocompatible graphene oxide (GO) nanobiosensor used for quantitative analysis of glucose. Digest J. Nanomater. Biostruct. (DJNB). 2021;16(1)
- [Google Scholar]
- Interactions of graphene oxide nanomaterials with natural organic matter and metal oxide surfaces. Environ. Sci. Technol.. 2014;48(16):9382-9390.
- [Google Scholar]
- Biomedical applications of graphene and graphene oxide. Acc. Chem. Res.. 2013;46(10):2211-2224.
- [Google Scholar]
- PEGylated Nanographene Oxide in Combination with Near-Infrared Laser Irradiation as a Smart Nanocarrier in Colon Cancer Targeted Therapy. Pharmaceutics. 2021;13(3):424.
- [Google Scholar]
- Production of reduced graphene oxide via hydrothermal reduction in an aqueous sulphuric acid suspension and its electrochemical behaviour. J. Solid State Electrochem.. 2015;19(2):361-380.
- [Google Scholar]
- Gospodinova, Z., Kamenska, T., Gencheva, G., Georgieva, M., Krasteva, N., 2021. PEGylation of graphene oxide nanosheets modulate cancer cell motility and proliferative ability. In: Journal of Physics: Conference Series, vol. 1762, no. 1. IOP Publishing, p. 012001.
- Electrochemical deposition of nickel graphene composite coatings: effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv.. 2017;7(49):31100-31109.
- [Google Scholar]
- Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules. 2021;26(11):3067.
- [Google Scholar]
- Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem.. 2014;20(5):2883-2887.
- [Google Scholar]
- Impact of Polyethylene Glycol Functionalization of Graphene Oxide on Anticoagulation and Haemolytic Properties of Human Blood. Materials. 2021;14(17):4853.
- [Google Scholar]
- 3D silver nanoparticles with multilayer graphene oxide as a spacer for surface enhanced Raman spectroscopy analysis. Nanoscale. 2018;10(13):5897-5905.
- [Google Scholar]
- Beyond risk: bacterial biofilms and their regulating approaches. Front. Microbiol.. 2020;11:928.
- [Google Scholar]
- Influence of IP-injected ZnO-nanoparticles in Catlacatla fish: hematological and serological profile. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2020;393(12):2453-2461.
- [Google Scholar]
- Experimental and theoretical analyses of nano-silver for antibacterial activity based on differential crystal growth temperatures. Saudi J. Biol. Sci.. 2021;28(12):7561-7566.
- [Google Scholar]
- Antibacterial Effect of Graphene and Graphene Oxide as a Potential Material for Fiber Finishes. Autex Res. J.. 2020;1(ahead-of-print)
- [Google Scholar]
- Targeted thiolation of graphene oxide and its utilization as precursor for graphene/silver nanoparticles composites. Carbon. 2013;61:543-550.
- [Google Scholar]
- Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem.. 2018;8(2):123-137.
- [Google Scholar]
- Effect of silver doping on antidiabetic and antioxidant potential of ZnO nanorods. J. Trace Elem. Med. Biol.. 2020;58:126448
- [Google Scholar]
- A de novo theranostic nanomedicine composed of PEGylated graphene oxide and gold nanoparticles for cancer therapy. J. Mater. Res.. 2020;35(4):430-441.
- [Google Scholar]
- Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun.. 2019;28:60-68.
- [Google Scholar]
- Improved stability and catalytic activity of graphene oxide/chitosan hybrid beads loaded with porcine liver esterase. Prep. Biochem. Biotech.. 2018;48(4):343-351.
- [Google Scholar]
- Graphene oxide as a nanocarrier for a theranostics delivery system of protocatechuic acid and gadolinium/gold nanoparticles. Molecules. 2018;23(2):500.
- [Google Scholar]
- Functionalized graphene derivatives: Antibacterial properties and cytotoxicity. J. Nanomater. 2019
- [Google Scholar]







