Translate this page into:
Chemical characterization and antioxidant, anti-inflammatory, and anti-septic activities of the essential oil from the aerial parts of Atractylodes macrocephala Koidz
⁎Corresponding authors. lm159@sina.com (Chang-yu Li), jfs1020@163.com (Fu-sheng Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Atractylodes macrocephala Koidz. (AM) is a rhizome plant traditionally used as an herbal medicine to normalize gastrointestinal function and treat inflammation-related diseases. However, there are no comprehensive studies that have examined the chemical composition or biological activity of the aerial parts of AM, which may be a promising raw material for the pharmaceutical industry. The aim of this study was to investigate the constituents, anti-oxidant, anti-inflammatory and anti-septic activities of the essential oil of the aerial parts of Atractylodes macrocephala (EOAPA). The chemical composition analysis of EOAPA by gas chromatography-quadrupole-time of flight-mass spectrometry (GC-Q/TOF-MS) led to the identification of 73 components, and the dominant compounds were hinesol (12.61%), β-eudesmol (11.51%), and atractylone (5.8%). EOAPA significantly inhibited lipopolysaccharide (LPS)-induced RAW264.7 cell expression of monocyte chemokine-1 (MCP-1), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in a dose-dependent manner via regulating the nuclear transcription factor-κB (NF-κB) pathway, which may be related to its strong reactive oxygen species (ROS)-scavenging ability against LPS challenge at cellular level. While, its antioxidant activity in vitro was weak. Finally, LPS-induced septic shock in mice confirmed that EOAPA can effectively prevent mouse death by downregulating the levels of inflammatory factors in serum, such as IL-6, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). The above results showed that there is satisfactory potential for EOAPA to be developed and applied as a natural pharmaceutical and cosmetic additive.
Keywords
Essential oil
Atractylodes macrocephala Koidz.
Chemical composition
Antioxidant
Anti-inflammatory
LPS-induced septic shock
1 Introduction
Inflammation refers to the immune system’s responses that defends against harmful stimuli, containing tissue infection, tissue injury, and toxins (Nathan, 2002). Normal inflammation plays a protective role in innate immune responses against interference from external microbes, while nonresolution of inflammation has been proven to lead to various diseases, including diabetes, cardiovascular disease, atherosclerosis, rheumatoid arthritis and so on (Nathan and Ding, 2010). One crucial regulator of inflammatory response is nuclear transcription factor-κB (NF-κB), which activates target genes including inducible enzymes, chemokines, as well as proinflammatory cytokines, and leads to high secretion of interleukin-6 (IL-6), monocyte chemokine-1 (MCP-1), and tumor necrosis factor-α (TNF-α), belonging to proinflammatory cytokines (Dunkhunthod et al., 2021).
Inflammation is tightly intertwined with oxidative stress (Liu et al., 2020), which can activate NF-κB signaling (Wang et al., 2014), leading by imbalance between the release of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidants. Therefore, oxidative stress has been showed a potential adverse effect on the organism attributed to its damage of crucial biomolecules and cells (Tu et al., 2019). ROS not only plays an important role in numerous physiological functions, but also leads to various inflammatory diseases including cardiovascular disease, COVID-19, diabetes, and cancer (Barciszewska, 2021). Studies have indicated that scavenging ROS can effectively prevent NF-κB activation and inhibit the inflammatory response (Guo et al., 2014).
Chinese herbs, used in preventing and treating diseases with thousands of years, have gained more attention from world, and numerous of which have been developed into natural additives of antioxidant and anti-inflammatory functional foods with low adverse side effects. Atractylodes macrocephala Koidz. (AM) belonging to the genus Atractylodes in the largest Compositae family, has been distributed throughout the tropical and subtropical regions for more than 700 years (Zhu et al., 2018). The rhizome of AM, called bai-zhu, clinically used for thousands of years, has been reported that it possesses a variety of pharmacological activities, such as improving gastrointestinal function (Xiang et al., 2020), anti-tumor, anti-inflammatory (Bailly, 2021), and anti-oxidative activities. AM has been not only approved as a functional food in China, but also broadly used in Japanese and Korean traditional medicine with long history.
Essential oil is a natural volatile produced by plants with important application value in the food, cosmetics, perfume, and pharmaceutical industries. Essential oil derived from the rhizome of AM has proved its ability in significant anti-inflammatory, anti-oxidant, and neuroprotective activities, which mainly contains atractylone, β-eudesmol, thymol, hinesol, atractylenolide I-IV (Wu et al., 2020). Therefore, there is significant medicinal value in the essential oil from the rhizome of AM.
In China, it is estimated that the demand for the AM rhizome on an annual basis is approximately 7,000 tonnes. However, the aerial (aboveground) parts of AM, which account for approximately 60% of the total plant mass, has not been reasonably developed and utilized (Peng et al., 2010). Active natural compounds in the aerial parts of AM, including lupeol, β-amyrin, and (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid, have been proven to possess bioactivities containing antibacterial and antitumor activities (Peng et al., 2010). Therefore, as a promising choice, there is still great potential in utilization of the remaining aerial parts of the AM, and if this occurred, it would greatly support sustainable agricultural practices. However, there are no comprehensive studies have been performed based on the biological activity or constituents of the essential oil of the aerial parts of AM (EOAPA), which restrict its development. Therefore, to promote the application of EOAPA as the antioxidant and anti-inflammatory raw material, the study was conducted to characterize the constituents, investigate the antioxidant and anti-inflammatory effects, and explore the potential mechanisms of anti-inflammatory action of EOAPA.
2 Materials and methods
2.1 Reagents
Ferric chloride, gallic acid and butylated hydroxytoluene (BHT) were purchased from Bide Pharmatech ltd. (Shanghai, China). Cell counting kit-8 (CCK-8, Biosharp China), bicinchoninic acid assay kit (BCA, Beyotime, Shanghai, China), 2,7-dichlorodi-hydrofluorescein diacetate (DCFH-DA), Folin-Ciocalteu phenol reagent, 4′,6-diamidino-2-phenylindole (DAPI) and 2,3,5-triphenyltetrazolium chloride (TPTZ) were obtained from Macklin Biochemical Co., ltd. (Shanghai, China). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and lipopolysaccharide (LPS) were derived from Sigma-Aldrich (St. Louis, MO, USA). Anti-p65 (ab32536), anti-iNOS (ab178945), anti-COX2 (ab179800) antibodies and Alexa Fluor® 488-labeled secondary antibody (ab150073) were purchased from Abcam (Cambridge, MA, USA). Anti-β-actin (4970S) antibody (CST, Danvers, MA, USA) and BD Cytometric Bead Array (CBA) mouse inflammation kit (BD Biosciences, San Diego, EE. UU).
2.2 Plant materials and essential oil preparation
In November 2021, fresh aerial parts of cultivated AM were collected at Lishui (Zhejiang, China) (27°61′N, 119°06′E). After being authenticated by Prof. Zhishan Ding of Zhejiang Chinese Medical University in China, the plant materials were air-dried in the shade. A voucher specimen (No. AM20211112) was deposited at the Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University.
First, 600 g of dried powdered aerial parts of AM was suspended in 3 L water, and submitted to simultaneous distillation extraction (SDE) using a classical Likens-Nickerson apparatus for 4 h with 100 mL of dichloromethane. Then, the extract was concentrated at 25°C under reduced pressure, after dried with anhydrous magnesium sulfate, and a canary yellow EOAPA was then obtained.
2.3 Animals
Male C57BL/6 mice (aged 9–12 weeks, 19–22 g) and commercial pelleted feed were supplied by the Laboratory Animal Research Center of Zhejiang Chinese Medical University (Hangzhou, Zhejiang Province, China). The animals were maintained on a 12-hour light/dark cycle, at 25 °C and 40%–60% humidity, and fed with normal diet. The experiments carried on were approved by the Animal Ethical Committee of Zhejiang Chinese Medical University (IACUC-20220314–17).
2.4 Gas chromatography-quadrupole-time of flight-mass spectrometry (GC-Q/TOF-MS) analysis of essential oil components
The EOAPA components were characterized by an Agilent Technologies 7890B GC system (Agilent Technologies, MA, USA) equipped with an HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm), combined with an Agilent 7250 time-of-flight mass spectrometer (Agilent Technologies, MA, USA) using helium as carrier gas (flow rate, 1.0 mL/min). The initial temperature was kept at 50°C during 3 min, increased by 5°C/min to 280°C, and then held for 5 min. The ion source and injector temperature were maintained at 250°C. The mass spectra were operated using 70 eV of electron energy in the scan range of m/z 40–450. Then, 1 μL of the sample (diluted with ethyl acetate) was injected at a split ratio of 10:1. The retention indices (RI) of the EOAPA components were characterized using a C7–C40 n-alkane series. The identification of the components was performed by the comparison of the retention indices and mass spectra from the NIST library (NIST 14).
2.5 Assay of total phenolic content
The Folin-Ciocalteu method was conducted to characterize the total phenolic content in EOAPA (Hazrati et al., 2020). In brief, 100 μL of EOAPA (1.25 mg/mL in 50% ethanol) was added to 60 μL of Folin-Ciocalteu reagent. After 3 min of incubation, 60 μL of 10% sodium carbonate was added. After 1 h of incubation in dark, the absorbance was recorded at the wavelength of 765 nm using a standard curve obtained with a series of concentrations of gallic acid.
2.6 Assay of antioxidants
2.6.1 DPPH• radical scavenging activity
The DPPH• test was conducted following the procedure of Hazrati et al. (Hazrati et al., 2019) with slight modification. Briefly, 100 μL of diluted EOAPA was added to 100 μL of DPPH• solution in ethanol (0.1 mM) and incubated in dark for 30 min before measuring the absorbance at 517 nm. BHT was used as a positive control.
2.6.2 ABTS• + radical scavenging activity
The ABTS• + test of EOAPA was adapted from Srećković et al. (Srećković et al., 2020) compared with a positive control (BHT). In short, 180 μL of newly prepared ABTS• + solution was mixed with 20 μL of different concentrations of EOAPA. After 6 min, the values of absorbance were determined at 734 nm.
2.6.3 Ferric reducing antioxidant power (FRAP) assay
FRAP assay was carried out as reported by Yosr et al. (Yosr et al., 2018). The reagent of FRAP was freshly prepared by mixing 1 mL of TPTZ (10 mM) solution in HCl (40 mM), 1 mL of ferric chloride solution (20 mM), and 10 mL of acetate buffer (300 mM, pH = 3.6). Then, 180 μL of FRAP solution was reacted with 20 μL of different concentrations of EOAPA for 6 min. The absorbance value of 593 nm was measured on a Multimode Plate Reader (EnSpire, PerkinElmer, USA).
2.7 Anti-inflammatory effects of EOAPA on LPS-induced RAW264.7 cells
2.7.1 Cytotoxicity analysis
The cytotoxic effect of EOAPA in vitro against RAW264.7 cells (American Type Culture Collection) was evaluated using CCK-8 assay. EOAPA was dissolved in DMSO and diluted into a series of concentrations with culture medium (the final concentration of DMSO was 0.2%). RAW264.7 cells were inoculated into 96-well plates at a density of 8,000 cells per well, and then treated with various concentrations of EOAPA or vehicle (medium containing 0.2% DMSO) for 1 h, followed by the presence or absence of LPS (100 ng/mL) for the next 24 h. Furtherly, 10 μL of CCK-8 reagent was added and incubated for another 3 h, and the absorbance values of solution at 450 nm were determined using a Multimode Plate Reader (EnSpire, PerkinElmer, USA).
2.7.2 Cytokine and nitric oxide (NO) levels in supernatants
RAW264.7 cells were seeded into 96-well plates at a density of 8 × 104 cells/mL. After 12 h incubation, various concentrations of EOAPA (0–40 μg/mL) were added and incubated for 1 h. Afterwards, LPS (100 ng/mL) was added and incubated for another 24 h. The supernatants of cells were collected for measurement of inflammatory cytokines and NO levels. The IL-6 and MCP-1 levels in the supernatants were analyzed by the cytometric bead array (CBA) method (Jiang et al., 2019). The NO concentrations were determined using the Griess reagent method (Brindisi et al., 2020).
2.7.3 Determination of intracellular ROS
RAW264.7 cells (8 × 104 cells/mL) were seeded into 96-well (100 μL/well) and 6-well (2 mL/well) plates, and treated according to the above assay. After a 24 h treatment, cells were stained with DCFH-DA (10 Μm) for 30 min at 37°C. The cells were gently rinsed thrice with serum-free Dulbecco’s modified Eagle’s medium (DMEM) to remove free EOAPA. The cells in 96-well plates were photographed using the ImageXpress Micro XLS system (Molecular Devices LLC, CA, USA). Cells in 6-well plates were collected, resuspended in phosphate-buffered saline (PBS), and then immediately analyzed using a Beckman Cytoflex S flow cytometer (Beckman Coulter, Brea, CA, USA).
2.8 Immunofluorescent analysis
The immunofluorescence method was performed to analyze the nuclear translocation of NF-Κb p65 as we previously reported (Jiang et al., 2019) with modification. Briefly, RAW264.7 cells were treated with various concentrations of EOAPA. After 1 h, 100 ng/mL LPS was added into cell culture for another 45 min. Fixed cells were incubated with a p65-specific antibody (1:100 dilution in PBS) at 4°C overnight firstly and further incubated with an Alexa Fluor® 488-labeled secondary antibody (1:600 dilution in PBS, ab150073, Abcam) for 2 h. After re-stained with DAPI (10 μg/mL) for 10 min, cells were photographed using an ImageXpress Micro XLS system (Molecular Devices LLC, CA, USA).
2.9 RNA extraction and quantitative Real-Time polymerase chain reaction (qRT-PCR)
Total RNA from the cells was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol and then quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, United States). One microgram of RNA was reversely transcribed into complementary DNA (cDNA) using PrimeScript reverse transcription (RT) reagent kit with genomic DNA Eraser (Cwbio, China). qRT-PCR reactions were performed to detect iNOS and COX2 mRNA expression using SYBR Green I mix reagents (TaKaRa, China) on a 7500 Real-Time PCR System (Applied Biosystems). β-Actin was used as the internal control. Each reaction was run in triplicate. The change in gene expression was calculated with the 2−ΔΔCt method. The details of PCR primers were described in Table 1.
Gene
Forward sequence (5′-3′)
Reverse sequence (5′-3′)
iNOS
CTTGGAGCGAGTTGTGGATTGTC
TAGGTGAGGGCTTGGCTGAGTGA
COX2
AGAAGGAAATGGCTGCAGAA
GCTCGGCTTCCAGTATTGAG
β-actin
CGTGGGCCGCCCTAGGCACCA
TTGGCCTTAGGGTTCAGGGGGG
2.10 Western blotting analysis
The total proteins of RAW264.7 cells were extracted according to the instructions of the M−PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA), and protein concentrations were calculated using the CBA assay. The levels of inflammation-related proteins were determined by immunoblotting with specific antibodies and performed on a simple western system (ProteinSimple, San Jose, CA, USA) according to the manufacturer's instructions (Jiang et al., 2019). The immunoblotting bands were quantified by Compass Software V6.0 (ProteinSimple, San Jose, CA, USA).
2.11 Model of septic shock in mice and drug administration
Forty male mice were randomly divided into PBS, LPS, EOAPA (40 mg/kg) + LPS, and EOAPA (120 mg/kg) + LPS groups, n = 10. The mice in the control group and LPS group received an injection of the same volume of PBS (containing 1% Tween-80) for three days. In the EOAPA group, EOAPA was dissolved in PBS (containing 1% Tween-80) for intraperitoneal injection at a dose of 40 mg/kg/d and 120 mg/kg/d for 3 consecutive days. The septic shock model was induced by intraperitoneal injection of LPS (25 mg/kg) 1 h after the last administration. Survival rate was monitored for 72 h. Mice were bled by tail clipping at 24 h after LPS injection.
2.12 Plasma analysis
After centrifugated at 3,000 rpm for 10 min at 4°C, the levels of serum cytokines were analyzed by the CBA method (Jiang et al., 2019).
2.13 Statistical analysis
The obtained data in the current study are defined as arithmetic means ± standard deviation (SD) from at least three independent experiments. One-way ANOVA was performed to calculate the significance of the differences between the groups, and significance level was considered at p < 0.05.
3 Results
3.1 GC-Q/TOF-MS analysis of EOAPA
The chemical composition of EOAPA was analyzed by GC-Q/TOF-MS, as given in Table 2 and Fig. 1. Totally, 73 constituents were identified in the EOAPA, representing 94.75% of the total oil composition. The qualitative and quantitative determination of EOAPA showed that it was primarily composed of terpenes, accounting for 62.32%, of which 44.62% were oxygenated bicyclic sesquiterpenes, followed by bicyclic sesquiterpenes (13.82%), monocyclic sesquiterpenes (12.95%), oxygenated tricyclic sesquiterpenes (10.96%), tricyclic sesquiterpenes (8.42%), oxygenated monocyclic sesquiterpenes (6.71%), and a very small amount of tetracyclic sesquiterpenes (1.75%) and monoterpenes (0.77%). Aliphatic hydrocarbons were also important compounds, accounting for 24.39% of the EOAPA, and there were a very small proportion of aromatic compounds (8.04%). Hinesol (12.61%) was the dominant constituent of EOAPA, followed by β-eudesmol (11.51%), atractylone (5.8%), germacrene B (4.86%), valencene (3.57%), and α-oxobisabolene (2.71%). (a) Retention indices experimentally calculated based on the C7-C40 n-alkanes standard, (b) Retention indices were taken from NIST GC-RI database.
Class
Component
Molecular formula
RIa
RI(NIST)b
Area%
Aliphatic compounds
24.39
Aliphatic acids
6.89
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester
C16H22O4
1870
1870 ± 4
0.33
n-Hexadecanoic acid
C16H32O2
1961
1968 ± 7
4.26
Phytol
C20H40O
2114
2114 ± 5
0.61
9,12-Octadecadienoic acid (Z,Z)-
C18H32O2
2132
2133 ± 12
0.64
9,12,15-Octadecatrienoic acid, (Z,Z,Z)-
C18H30O2
2138
2139 ± 20
0.57
Octadecanoic acid
C18H36O2
2160
2172 ± 7
0.48
Aliphatic alcohols
5.52
3-Hexen-1-ol, (E)-
C6H12O
854
856 ± 1
1.42
1-Hexanol
C6H14O
867
868 ± 4
2.81
Heptanal
C7H14O
903
901 ± 2
0.28
1-Octen-3-ol
C8H16O
979
980 ± 2
0.25
Pentadecanal-
C15H30O
1714
1715 ± 3
0.52
1-Heneicosanol
C21H44O
2394
2380 ± 15
0.24
Aliphatic aldehydes
6.94
Hexanal
C6H12O
802
800 ± 2
3.06
2-Hexenal
C6H10O
851
851 ± 5
0.76
2-Heptenal, (Z)-
C7H12O
956
958 ± 6
0.21
Nonanal
C9H18O
1104
1104 ± 2
0.62
2-Nonenal, (E)-
C9H16O
1160
1162 ± 3
0.32
Decanal
C10H20O
1206
1206 ± 2
0.53
2,4-Decadienal, (E,E)-
C10H16O
1316
1317 ± 3
1.03
Cyclohexanebutanal, 2-methyl-3-oxo-, cis-
C11H18O2
1521
1515 ± 6
0.41
Aliphatic ethers
2.91
3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran
C10H16O
1195
1192
0.32
Dihydroedulan IIA
C13H22O
1296
1293
0.35
Theaspirane A
C13H22O
1301
1302 ± 4
0.32
Tau-Cadinol acetate
C17H28O2
1798
1805
1.56
Hexadecanoic acid, ethyl ester
C18H36O2
1993
1993 ± 3
0.36
Aliphatic ketones
1.57
5,9-Undecadien-2-one, 6,10-dimethyl-, (E)-
C13H22O
1454
1453 ± 2
0.33
trans-β-Ionone
C13H20O
1489
1486 ± 4
0.36
2-Pentadecanone, 6,10,14-trimethyl-
C18H36O
1846
1844 ± 4
0.88
Aliphatic hydrocarbons
0.56
1-Tridecyne
C13H24
1294
1297 ± 1
0.18
Cetene
C16H32
1592
1592 ± 1
0.19
3-Octadecene, (E)-
C18H36
1792
1785 ± 1
0.19
Aromatic compounds
8.04
Aromatic alcohols
1.6
Benzyl alcohol
C7H8O
1034
1036 ± 4
0.33
Phenylethyl Alcohol
C8H10O
1113
1116 ± 5
0.86
Phenol, 2,5-bis(1,1-dimethylethyl)-
C14H22O
1513
1514
0.41
Aromatic aldehydes
1.04
Benzaldehyde
C7H6O
960
962 ± 3
0.24
Benzeneacetaldehyde
C8H8O
1043
1045 ± 4
0.8
Aromatic ethers
3.15
3-Furaldehyde
C5H4O2
832
833 ± 4
1.13
Furan, 2-pentyl-
C9H14O
992
993 ± 2
1.42
Benzofuran, 2,3-dihydro-
C8H8O
1219
1224 ± 3
0.6
Aromatic ketones
1.14
Ethanone, 1-(2-hydroxy-5-methylphenyl)-
C9H10O2
1314
1316
1.14
Aromatic hydrocarbons
1.11
Ethylbenzene
C8H10
860
855 ± 10
0.3
p-Xylene
C8H10
868
865 ± 7
0.4
α-Curcumene
C15H22
1485
1483 ± 3
0.41
Terpenes
62.32
monoterpenes
0.48
Oxygenated acyclic monoterpenes
0.48
Linalool
C10H18O
1100
1099 ± 2
0.48
Sesquiterpenes
61.84
Monocyclic sesquiterpenes
12.25
Humulene
C15H24
1458
1454 ± 3
0.85
γ-Curcumene
C15H24
1482
1480 ± 2
0.66
β-Sesquiphellandrene
C15H24
1527
1524 ± 2
1.7
Elemol
C15H26O
1553
1549 ± 2
1.27
Germacrene B
C15H24
1562
1557 ± 3
4.86
Zingiberenol
C15H26O
1617
1616 ± 5
0.2
α-Oxobisabolene
C15H24O
1750
1750
2.71
Bicyclic sesquiterpenes
36.42
Caryophyllene
C15H24
1424
1419 ± 3
1.76
Alloaromadendrene
C15H24
1467
1461 ± 2
0.29
β-Selinene
C15H24
1491
1486 ± 3
1.53
7-epi-Sesquithujene
C15H24
1497
1391 ± 2
0.93
Cashmeran
C15H22O
1513
1508 ± 5
0.59
γ-Cadinene
C15H24
1518
1513 ± 2
0.53
(4aR,8aS)-4a-Methyl-1-methylene-7-(propan-2-ylidene) decahydronaphthalene
C15H24
1540
1544
3.57
4a,5-Dimethyl-3-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-ol
C15H24O
1550
1553
0.45
γ-Eudesmol
C15H26O
1635
1631 ± 1
1.86
Hinesol
C15H26O
1645
1645 ± 3
12.61
β-Eudesmol
C15H26O
1656
1649 ± 2
11.51
Guai-1(10)-en-11-ol
C15H26O
1671
1667 ± 2
0.79
Tricyclic sesquiterpenes
12.08
7-epi-Silphiperfol-5-ene
C15H24
1347
1348
0.55
Modephene
C15H24
1384
1385 ± 0
1.03
Berkheyaradulene
C15H24
1390
1386 ± 6
1.77
β-Maaliene
C15H24
1404
1405 ± 16
0.48
β-Isocomene
C15H24
1411
1412 ± 0
1.42
Caryophyllene oxide
C15H24O
1589
1581 ± 2
0.26
Epiglobulol
C15H26O
1597
1585 ± 5
0.51
Atractylone
C15H20O
1663
1662 ± 9
5.8
Cedr-8-en-13-ol
C15H24O
1691
1688 ± 1
0.26
Tetracyclic sesquiterpenes
1.09
Ledene-oxide-Ⅱ
C15H24O
1632
1631
1.09
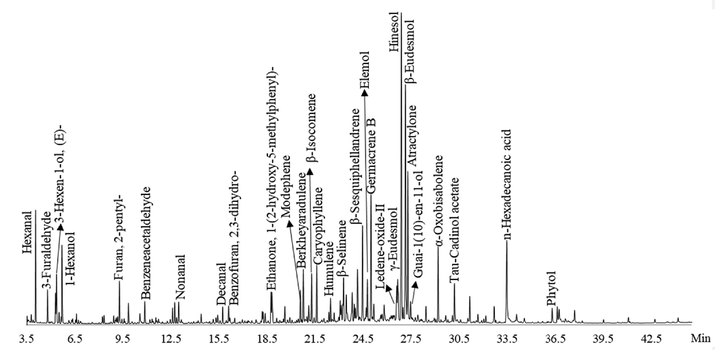
Total ion current chromatogram of EOAPA by GC-Q/TOF-MS.
3.2 Total phenol
The total phenol amount of EOAPA was 3.01 ± 0.06 mg gallic acid/100 g oil with a calibration curve equation of Y = 13.077 X + 0.0113, R2 = 0.9 989 (gallic acid), which was consistent with the above data showing that the content of terpenes was much higher than that of aromatic compounds (62.32% vs 8.04%). To our knowledge, there is no available data regarding the phenolic content of EOAPA.
3.3 Antioxidant activity
DPPH•, ABTS•+, and FRAP assays were conducted to assess the antioxidant activity of EOAPA considering different mechanisms. As depicted in Fig. 2, EOAPA characterized significant antioxidant properties in a dose-dependent manner in the concentration range of 0.625 mg/mL to 10 mg/mL. However, its IC50 value for DPPH• and ABTS•+ radicals and OD0.5 for iron ion reduction (3.113 ± 0.037 mg/mL, 4.837 ± 0.169 mg/mL, and 3.978 ± 0.258 mg/mL, respectively) were significantly higher than those of positive control BHT (12.257 ± 0.136 μg/mL, 35.697 ± 0.860 μg/mL, and 35.397 ± 0.580 μg/mL, respectively), indicating that the direct antioxidant capacity of EOAPA was weak. Reports have shown that the total phenol was closely related to the antioxidant capacity (Akgün et al., 2021), while the EOAPA was abundant in non-phenolic components, which were proved to be poor antioxidant.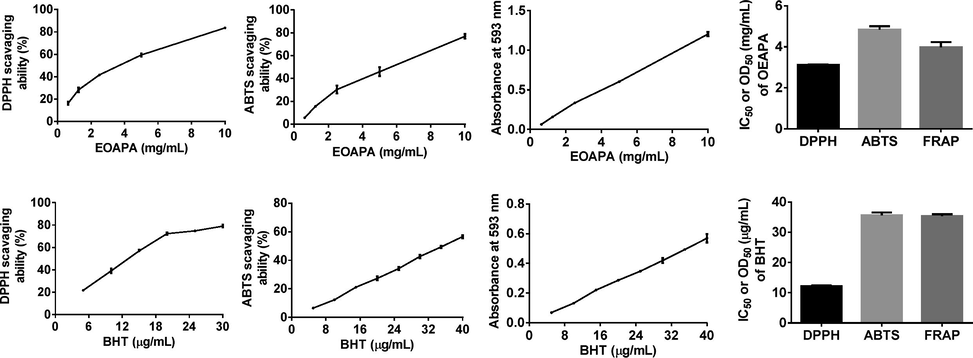
Antioxidant activity of the EOAPA in DPPH•, ABTS• + and FRAP assay.
3.4 Cytotoxic effect of EOAPA on RAW264.7 cells
The cytotoxicity of EOAPA on RAW264.7 cells was evaluated by CCK-8 assay. The results revealed that treatments with 5–40 μg/mL of EOAPA did not cause distinct cytotoxicity, regardless of the presence or absence of LPS (100 ng/mL) (Fig. 3A). Therefore, concentrations within 40 μg/mL of EOAPA were chosen for further exploration of anti-inflammatory and anti-oxidant effects and potential mechanism.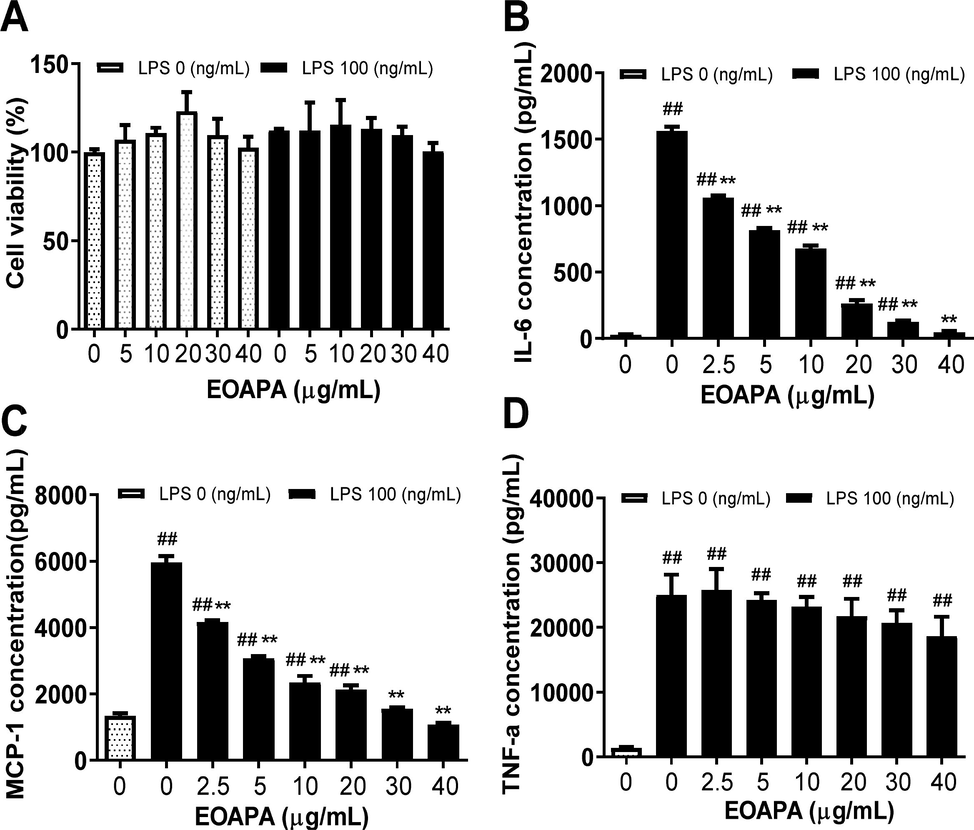
Evaluation of anti-inflammatory of EOAPA in LPS-stimulated RAW264.7 cells. (A) Effect of EOAPA on cell viability of RAW264.7 cells was estimated by CCK-8 assay. (B) IL-6 and (C) MCP-1 levels in culture supernatant pre-treated by different concentrations of EOAPA. Data represent Mean ± SD (n = 3). ## p < 0.01 versus control group (treated by reagent); ** p < 0.01 versus LPS treated group.
3.5 Anti-inflammatory effects on LPS-induced RAW264.7 cells
3.5.1 EOAPA inhibits the secretion of IL-6 and MCP-1 in a dose-dependent manner
Molecular components of pathogens such as LPS can activate macrophages to secrete cytokines and mediate the inflammatory response. As shown in Fig. 3, LPS stimulation dramatically increased the cytokine levels of IL-6, TNF-α and MCP-1, while pretreatment with different concentrations of EOAPA significantly decreased the secretion of MCP-1 and IL-6 in a dose-dependent manner (Fig. 3B, C). As displayed in Fig. 3, there was significant inhibition of MCP-1 and IL-6 by EOAPA, with inhibition rates reaching 38.997 ± 1.339% and 32.822 ± 1.766%, respectively, at 2.5 μg/mL of EOAPA treatment, and IC50 values of 3.52 ± 0.56 μg/mL and 5.35 ± 0.82 μg/mL, respectively. However, EOAPA could not effectively downregulate TNF-α levels (Fig. 3D).
3.5.2 EOAPA attenuates inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression
LPS stimulated macrophages to secrete TNF-α, IL-6, and MCP-1, and also promoted the expression of iNOS and COX-2 genes to release inflammatory mediators including prostaglandin E2 (PGE2) and nitric oxide (NO), and aggravate the inflammatory response (Yao et al., 2018). To investigate whether EOAPA can inhibit the expression of iNOS and COX-2, quantitative Real-time PCR (qRT-PCR) and western blot analysis were performed.
The data revealed dramatic overexpression of iNOS and COX-2 mRNA and proteins induced by LPS in RAW264.7 macrophages compared to unchallenged cells (Fig. 4). As expected, EOAPA at all pretreated concentrations significantly downregulated LPS-induced iNOS and COX-2 mRNA levels compared to LPS-treated cells (Fig. 4A, B). For protein levels, compared with the LPS-stimulated group, there was no significant downregulation of iNOS and COX-2 by EOAPA at 5 μg/mL, and in other treatment groups, there was a remarkable decrease in the protein levels of iNOS and COX-2 (Fig. 4C, D).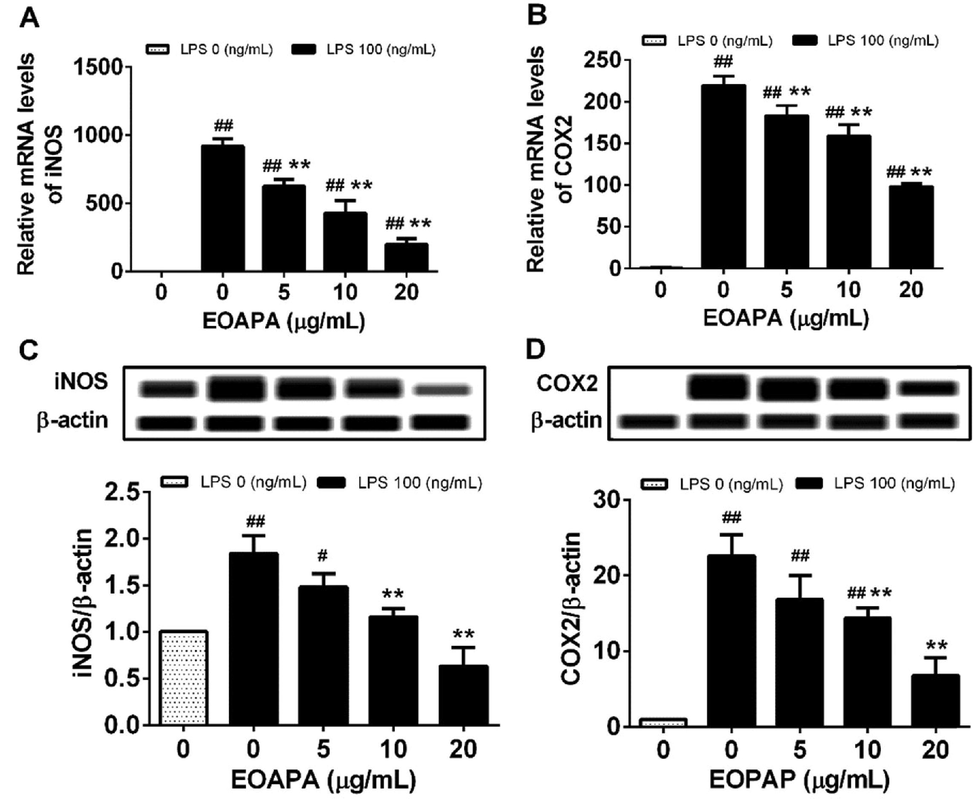
Effects of EOAPA on overexpression of (A) iNOS and (B) COX-2 genes and (C) iNOS and (D) COX-2 proteins. Cells were treated with increasing concentrations of EOAPA for 1 h and with LPS (100 ng/mL). The expression of iNOS and COX-2 genes were determined by qRT-PCR after treated with LPS for 6 h. While, the expression of iNOS and COX-2 proteins were analyzed using simple western immunoblotting after treated with LPS for 24 h. Data represent Mean ± SD (n = 3). # p < 0.05, ## p < 0.01 versus control group (treated by reagent); ** p < 0.01 versus LPS treated group.
3.5.3 EOAPA exerts anti-inflammatory activity by inhibiting NF-κB p65 nuclear translocation
The activated NF-κB p65 protein can control the expression of numerous inflammatory genes, such as TNF-α, IL-6, MCP-1, iNOS, and COX-2. Therefore, western blotting was conducted to determine the level of p65 protein in nuclei. As shown in Fig. 5A, LPS significantly increased the level of p65 in the nucleus, while EOAPA pretreatment dramatically attenuated LPS-induced p65 nuclear translocation in a dose-dependent manner, which was further reconfirmed by immunofluorescence assay, as shown in Fig. 5B. Based on these data, it was observed that EOAPA inhibits NF-κB p65 nuclear translocation, which further inhibits the expression of target inflammatory genes.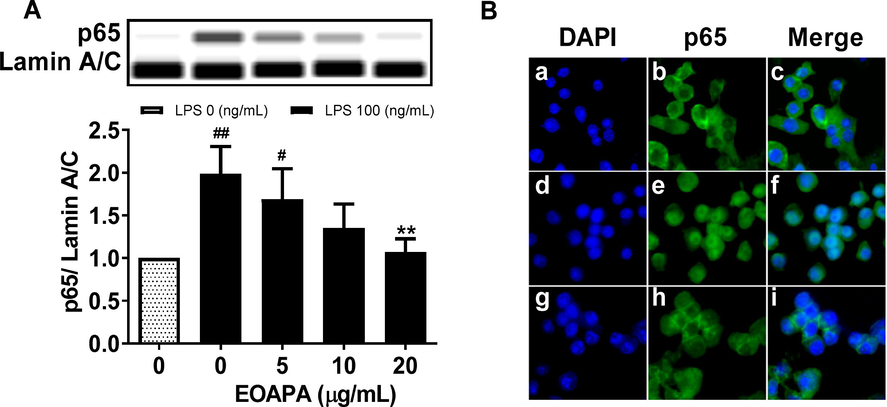
Effects of EOAPA on NF-κB p65 nuclear translocation. RAW264.7 cells were pre-treated with various concentrations of EOAPA for 1 h, and then LPS-stimulated during 1 h. (A) The content of p65 in the nucleus was determined by simple western immunoblotting. (B) Cells were fixed, permeabilized and conducted immunofluorescence assay. a–c control group, d–f LPS induced group, g–i 20 μg/mL EOAPA pre-treated and then LPS induced group. Data represent Mean ± SD (n = 3). # p < 0.05, ## p < 0.01 versus control group; ** p < 0.01 versus LPS group.
3.6 ROS production inhibition by EOAPA in LPS-induced RAW264.7 cells
Based on the inhibitory effect of EOAPA on NF-κB and related inflammatory genes, we speculated that EOAPA may exert its anti-inflammatory activity by inhibiting the production of ROS induced by LPS. To verify the speculation, the levels of ROS were measured. As expected, flow cytometric analysis indicated that LPS exposure dramatically increased intracellular ROS. On the contrary, EOAPA pretreatments effectively downregulated the production of ROS in a dose-dependent manner (Fig. 6A), which was confirmed by fluorescence microscopy analysis (Fig. 6B).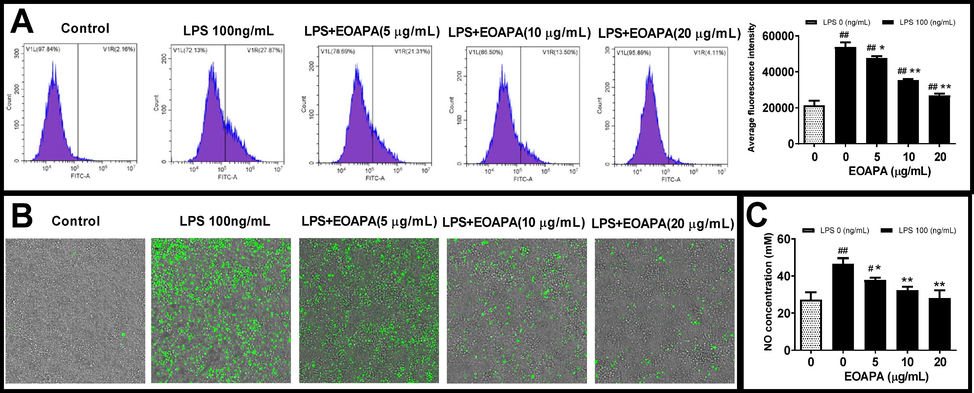
Evaluation of EOAPA on LPS induced cell oxidative stress. RAW264.7 cells were pre-treated with various concentrations of EOAPA for 1 h, and then LPS-stimulated during 24 h. Intracellular ROS was (A) recorded by flow cytometer and (B) photographed on an ImageXpress Micro Confocal High-Content Imaging System. (C) NO concentrations were measured using Griess reagent method. Data represent Mean ± SD (n = 3). # p < 0.05, ## p < 0.01 versus control group; * p < 0.05, ** p < 0.01 versus LPS group.
In addition to ROS, RNS is also a vital factor leading to cellular oxidative stress. NO, as a multi-effector molecule, can also be rapidly oxidized by ROS to RNS, which is closely related to inflammation. It is well known that LPS can induce macrophages to overexpress iNOS, resulting in a large amount of NO release (Reuter et al., 2010). The above data showed that EOAPA significantly inhibited the expression of iNOS induced by LPS. Therefore, the level of NO in the cell culture supernatant was determined. Consistently, compared to LPS-exposed group, EOAPA pretreatment dose-dependently decreased the NO level (Fig. 6C). Compared with the weak antioxidant activity based on chemical reaction in vitro, EOAPA exhibited a more significant free radical scavenging activity against LPS-induced excess ROS and RNS at the cellular level. Therefore, further research is needed to uncover the potential mechanism of EOAPA.
3.7 EOAPA reduced septic death and inflammatory cytokine production in an LPS-induced septic model
Based on the above findings that EOAPA blocked LPS-induced macrophage activation, we then hypothesized that EOAPA possessed the ability to inhibit LPS-induced immune responses in vivo. To verify this, a model of septic shock was established in male C57BL/6 mice by intraperitoneal injection of LPS (Fig. 7A). As shown in Fig. 7B, the survival rate of LPS group was 40%, whereas EOAPA increased the survival rate to 60% at 40 mg/kg and 80% at 120 mg/kg.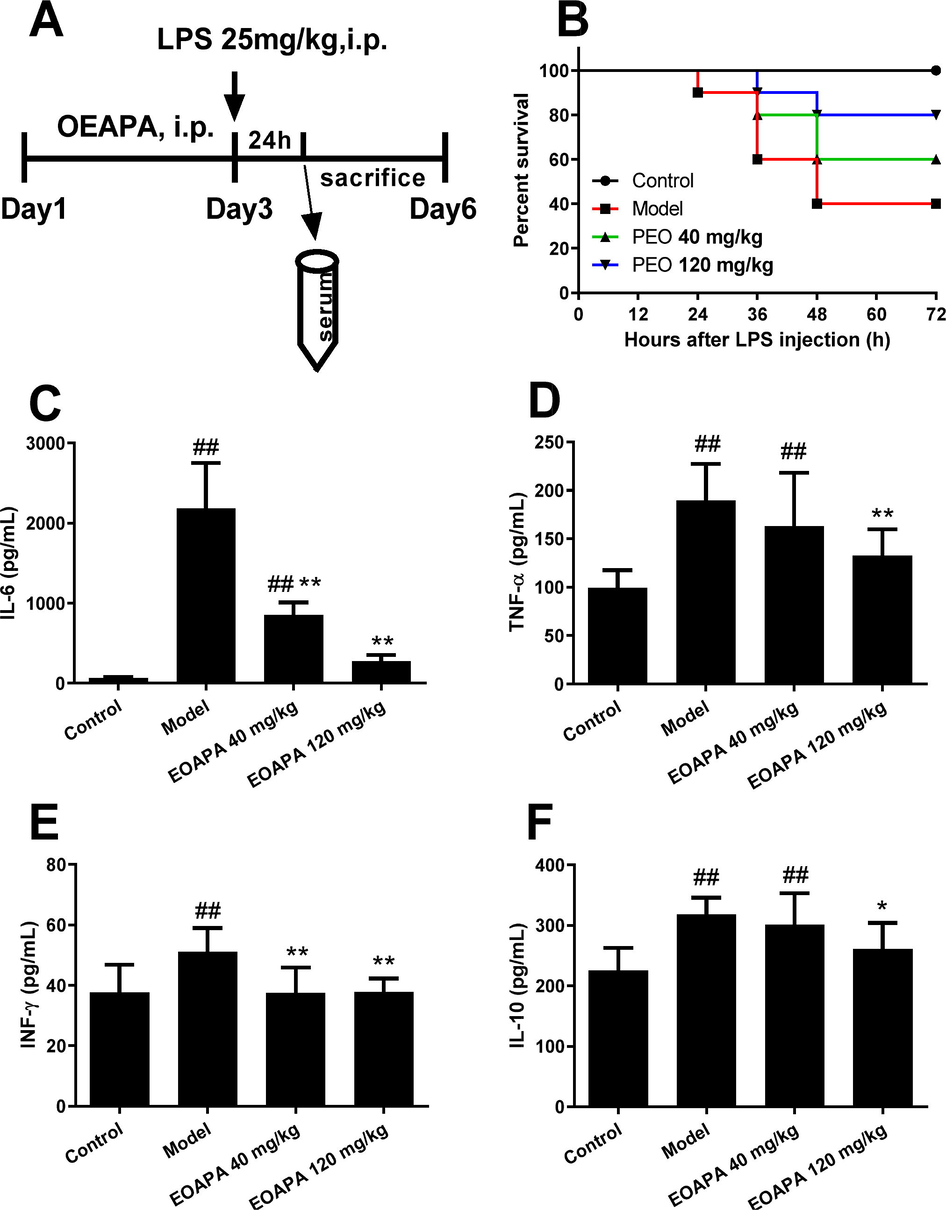
EOAPA prevented septic death and downregulated inflammatory cytokines level in LPS-induced septic shock mouse model. (A) Timetable of the LPS-induced septic shock mouse model. (B) Mice survival rate after 72-h post-LPS challenge (i.p., 25 mg/kg) with or without pre-administration of EOAPA (40 or 120 mg/kg) (n = 10/group). Serum levels of (C) IL-6, (D) TNF-α, (E) IFN-γ and (F) IL-10 24 h after the administration of LPS with or without pre-administration of EOAPA. Data represent Mean ± SD (n = 10). # p < 0.05, ## p < 0.01 versus control group; * p < 0.05, ** p < 0.01 versus model group.
Next, to examine the effect of EOAPA on the production of inflammatory cytokines in an LPS-induced septic model, the CBA method was performed. As expected, the levels of IL-6 dramatically increased in response to LPS stimulation, which was remarkably downregulated by EOAPA pretreatment (Fig. 7C). Consistently, LPS stimulation promoted the production of TNF-α, IFN-γ and IL-10, while pre-administration of EOAPA (120 mg/kg) reduced the corresponding inflammatory cytokines (Fig. 7D–F). However, EOAPA at 40 mg/kg could not effectively downregulate TNF-α or IL-10 levels. These results confirmed that EOAPA prevented LPS-induced septic shock by alleviation of inflammatory responses in vivo.
4 Discussion
Previous studies have described that the rhizome oil of AM included atractylone, β-eudesmol, germacrene B and valencene. Although the constituents in rhizome oil vary with different origins (Guo et al., 2021), the rhizome oil component present in the highest quantity was atractylone, accounting for 40–70%, which is more than 7 times the amount found in the aerial parts oil (Gu et al., 2019). Compared with rhizome oil, there were more abundant components in the aerial parts oil in addition to the main common components of hinesol, β-eudesmol, atractylone, and germacrene B. Hinesol is the dominant component of the aerial parts oil, but the amount reported in the rhizome oil of AM is low (Wu et al., 2020). Hinesol exerts the most promising anticancer activity by downregulating NF-κB and MEK/ERK pathways to induce apoptosis and reduce cell growth (Guo et al., 2019). β-Eudesmol, main constituents of EOAPA, has been developed into a novel agent of cosmetics as well as medicine and pharmacy attributed to the function of anti-inflammatory and antioxidant via NF-κB signaling pathway (Han et al., 2017). Similarly, atractylone could block NF-κB signaling pathway and mitigate inflammatory conditions (Kim et al., 2016). Based on these, we surmised EOAPA had advantageous potential in antioxidation and anti-inflammation and furtherly, would develop into a natural additive of antioxidant and anti-inflammatory functional food.
LPS, a glycan, consisted of O-antigen, core sugars and lipid A, which is termed as pathogen associated molecular patterns (PAMP), prompts innate immunity by engagement of Toll-like receptor 4 (TLR4) /myeloid differentiation-2 (MD-2) /LPS complexes (Zamyatina and Heine, 2020). With cooperation of transfer to cluster of differentiation-14 (CD14) and LPS-binding protein (LBP), LPS is presented to MD-2 and associated to TLR4. Subsequently, adapter molecules such as myeloid differentiation factor (MyD)88, TIR-containing adapter molecule (TRIF) and TRIF-related adapter are recruited, resulting in the activation of different communication signals such as NF-κB and MAPK (Neuder et al., 2009) and increasing transcription of a series of chemokines and cytokines containing MCP-1, IL-6, IL-1β and TNF-α for the mediation of the inflammatory response (Zamyatina and Heine, 2020). Excessive stimulation of LPS can lead to various disease including septic shock (Oh et al., 2019), chronic obstructive pulmonary, etc. NF-κB is a proinflammatory transcription factor acting importantly to regulate inflammatory response (Hayden and Ghosh, 2008), which is divided into five subunits: RelA/p65, p50, p52, c-Rel and RelB, sharing their Rel homology domain (RHD) responsible for DNA binding (Hayden and Ghosh, 2008). Activated NF-κB increases the expression of numerous inflammatory genes containing iNOS, COX-2, IL-1, IL-6 and TNF-α (Kumar et al., 2021).
The study of EOAPA was performed to develop a new LPS immune adjuvant or antagonists. EOAPA research into the function and regulation of the LPS-induced inflammatory provided evidence that EOAPA inhibited NF-κB p65 nuclear translocation used western blotting, further confirmed by immunofluorescence assay, and subsequently attenuated expression of iNOS and COX-2. Results from vitro and vivo studies using LPS induced Raw264.7 and septic shock mouse model, showed that EOAPA inhibited dramatically the secretion of IL-6 in vitro and in vivo, respectively, and decreased TNF-α levels in vivo but not effectively in vitro. In conclusion, the suppression of the NF-κB pathway of EOAPA played primary role in the inhibition on the inflammatory response and septic shock by LPS. Therefore, advances in understanding how the EOAPA inhibited NF-κB p65 nuclear translocation piqued our interests.
It is well known that the production of ROS constitutively increases in LPS-induced RAW264.7 cells (More and Makola, 2020). Numerous considerable studies have been carried out to show NF-κB activation not only relies on the classical pathway induced by immunity mediators also ROS, such as H2O2, singlet oxygen and hypochlorous acid (Gloire et al., 2006). Studies have indicated that scavenging ROS can effectively prevent NF-κB activation (Nishanth et al., 2011), which subsequently inhibits the inflammatory response. EOAPA showed significant free radical scavenging activity against LPS-induced excess ROS at the cellular level measured by flow cytometer and photographed. Overall, the EOAPA inhibition of NF-κB p65 nuclear translocation may be related to its strong ROS-scavenging ability, and the molecular mechanism of EOAPA inhibitory action on ROS-mediated NF-κB activation requires further investigation in the future.
5 Conclusions
Seventy-three compounds from EOAPA were identified by GC–MS, and the main compounds were hinesol, β-eudesmol, atractylone, germacrene B, and valencene. EOAPA inhibited NF-κB p65 nuclear translocation, which may be related to its strong ROS-scavenging ability against LPS challenge, thereby downregulating the expression of pro-inflammatory enzymes (iNOS and COX-2) and cytokines (MCP-1 and IL-6). Most importantly, in vivo experiments, it was confirmed that EOAPA also effectively decreased LPS-induced septic shock death by decreasing the levels of serum inflammatory cytokines such as IL-6, TNF-α, and INF-γ. These results indicated that EOAPA might be used as a natural antioxidant and anti-inflammatory agent, and it exhibited potential value for application in pharmaceutical industries.
Funding
The present research was carried out with the financial support of the Natural Science Foundation of Zhejiang Province (No. LQ20H270008) and Science Foundation of Zhejiang Chinese Medical University (Project NOs. 2020ZG31 and 2020ZG35).
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physicochemical properties, total phenolic content, and antioxidant activity of chestnut, rhododendron, acacia and multifloral honey. J. Food Meas. Charact.. 2021;15:3501-3508.
- [CrossRef] [Google Scholar]
- Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol.. 2021;891:173735
- [CrossRef] [Google Scholar]
- Elucidating of oxidative distress in COVID-19 and methods of its prevention. Chem. Biol. Interact.. 2021;344:109501
- [CrossRef] [Google Scholar]
- Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants (Basel). 2020;9:826-846.
- [CrossRef] [Google Scholar]
- Dunkhunthod, B., Talabnin, C., Murphy, M., et al., 2021. Gymnema inodorum (Lour.) Decne. Extract Alleviates Oxidative Stress and Inflammatory Mediators Produced by RAW264.7 Macrophages. Oxid. Med. Cell. Longev. 2021, 8658314. https://doi.org/10.1155/2021/8658314.
- NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol.. 2006;72:1493-1505.
- [CrossRef] [Google Scholar]
- Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. produced with different processing methods. Molecules. 2019;24:2956-2969.
- [CrossRef] [Google Scholar]
- The antitumor effect of hinesol, extract from Atractylodes lancea (Thunb.) DC. by proliferation, inhibition, and apoptosis induction via MEK/ERK and NF-kappaB pathway in non-small cell lung cancer cell lines A549 and NCI-H1299. J. Cell. Biochem.. 2019;120:18600-18607.
- [CrossRef] [Google Scholar]
- Drying kinetics and the variation of volatile components of Atractylodes macrocephala during hot-air drying process. China. J. Chin. Mater. Med.. 2021;47:922-930.
- [CrossRef] [Google Scholar]
- AMPK inhibition blocks ROS-NFkappaB signaling and attenuates endotoxemia-induced liver injury. PLoS One.. 2014;9:e86881
- [CrossRef] [Google Scholar]
- beta-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin. Exp. Pharmacol. Physiol.. 2017;44:257-265.
- [CrossRef] [Google Scholar]
- Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss. Ind. Crop. Prod.. 2019;140
- [CrossRef] [Google Scholar]
- A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior) Ind. Crop. Prod.. 2020;157
- [CrossRef] [Google Scholar]
- Coelonin, an anti-inflammation active component of Bletilla striata and its potential mechanism. Int. J. Mol. Sci.. 2019;20:4422.
- [CrossRef] [Google Scholar]
- Atractylone, an active constituent of KMP6, attenuates allergic inflammation on allergic rhinitis in vitro and in vivo models. Mol. Immunol.. 2016;78:121-132.
- [CrossRef] [Google Scholar]
- Melatonin ameliorates LPS-induced testicular nitro-oxidative stress (iNOS/TNFα) and inflammation (NF-kB/COX-2) via modulation of SIRT-1. Reprod. Sci.. 2021;28:3417-3430.
- [CrossRef] [Google Scholar]
- Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun.. 2020;11:2788-2803.
- [CrossRef] [Google Scholar]
- In-vitro analysis of free radical scavenging activities and suppression of LPS-induced ROS production in macrophage cells by Solanum sisymbriifolium extracts. Sci. Rep.. 2020;10:6493.
- [CrossRef] [Google Scholar]
- Role of p38 MAPK in LPS induced pro-inflammatory cytokine and chemokine gene expression in equine leukocytes. Vet. Immunol. Immunopathol.. 2009;129:192-199.
- [CrossRef] [Google Scholar]
- Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: role of ROS-NFkappaB signaling pathway. Nanotoxicology. 2011;5:502-516.
- [CrossRef] [Google Scholar]
- Erastin inhibits septic shock and inflammatory gene expression via suppression of the NF-kappaB pathway. J. Clin. Med.. 2019;8:2210-2222.
- [CrossRef] [Google Scholar]
- Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med. Chem. Res.. 2010;20:146-151.
- [CrossRef] [Google Scholar]
- Oxidative stress, inflammation, and cancer: how are they linked? Free. Radic. Biol. Med.. 2010;49:1603-1616.
- [CrossRef] [Google Scholar]
- Lythrum salicaria L. (Lythraceae) as a promising source of phenolic compounds in the modulation of oxidative stress: comparison between aerial parts and root extracts. Ind. Crop. Prod.. 2020;155
- [CrossRef] [Google Scholar]
- The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis.. 2019;10:637-651.
- [CrossRef] [Google Scholar]
- Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ. Res.. 2014;114:421-433.
- [CrossRef] [Google Scholar]
- Antioxidant, antimicrobial and anti-inflammatory activities of essential oil derived from the wild rhizome of Atractylodes macrocephala. Chem. Biodivers.. 2020;17:e2000268
- [CrossRef] [Google Scholar]
- Polysaccharide of Atractylodes macrocephala Koidz (PAMK) alleviates cyclophosphamide-induced immunosuppression in mice by upregulating CD28/IP3R/PLCgamma-1/AP-1/NFAT signal pathway. Front. Pharmacol.. 2020;11:529657
- [CrossRef] [Google Scholar]
- Yao, Y., Liu, K. H., Zhao, Y., et al., 2018. Pterostilbene and 4'-Methoxyresveratrol Inhibited Lipopolysaccharide-Induced Inflammatory Response in RAW264.7 Macrophages. Molecules. 23, https://doi.org/10.3390/molecules23051148.
- Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crop. Prod.. 2018;121:151-159.
- [CrossRef] [Google Scholar]
- Lipopolysaccharide recognition in the crossroads of TLR4 and caspase-4/11 mediated inflammatory pathways. Front. Immunol.. 2020;11:585146
- [CrossRef] [Google Scholar]
- The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: a review. J. Ethnopharmacol.. 2018;226:143-167.
- [CrossRef] [Google Scholar]







