Translate this page into:
Immobilized sulfonic acid functionalized ionic liquid on magnetic cellulose as a novel catalyst for the synthesis of triazolo[4,3-a]pyrimidines
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Brønsted acidic ionic liquid 1-(propyl-3-sulfonate) vinyl imidazolium hydrogen sulfate [IL] was supported on modified magnetic cellulose. The physical structure, composition, and functional groups of the novel supported ionic liquid catalyst were characterized via XRD, FT-IR, EDS, SEM, VSM, TGA, TEM, and BET techniques. Owing to the combination of nano-support features and flexible imidazolium linkers, it acted as a “quasi-homogeneous” catalyst to catalyze the preparation of triazolo[4,3-a]pyrimidine derivatives by a one-pot three-component reaction of active methylene compounds (ethyl cyanoacetate or malononitrile), aminotriazole and aryl aldehydes. The catalyst shows good catalytic activity for the synthesis of triazolo pyrimidines after six times of recycling.
Keywords
Cellulose
Fe3O4
Immobilized catalyst
Ionic liquid
Triazolo[4,3-a]pyrimidine
1 Introduction
From significant important aspects of green chemistry are the expansion and usage of green solvents. Nowadays, ILs is introduced as green solvents in alternative to conventional volatile organic solvents. ILs, liquid salts, were formed wholly of cations and anions and are mainly defined as being liquid beneath an ideal amount, such as 100 °C or at room temperature (Morais et al., 2022; Minea and Sohel Murshed, 2021; Greer et al., 2020). Ionic liquids (ILs) as eco-friendly reaction media have attracted increasing attention due to their particular properties, such as tunable acidity, selective dissolvability, very low viscosity, negligible vapor pressure, wide liquid range, high thermal stability, and easiness of product separation (Han et al., 2022; Singh and Savoy, 2020; Tajbakhsh et al., 2013; Miao et al., 2011a, 2011b). The strongly acidic ionic liquids (Brønsted-type and Lewis-type) have been exploited as efficient catalysts for many reactions and generally can afford reusability, higher yields, and selectivity against traditional acid catalysts (Azizi and Shirdel, 2016; Cole et al., 2002; Tayebee et al., 2015). Among them, SO3H-functionalized ILs with a hydrogen sulfate counteranion have been intensively studied as a class of dual acidic functionalized ILs during the last years since the existence of both SO3H-functional groups and the hydrogen sulfate counteranion can increase their acidities. Ionic liquids based on imidazolium cation, because of their high stability, are also widely used in organic reactions in laboratories and industries (Tayebee et al., 2017; Elavarasan et al., 2020; Miao et al., 2011a, 2011b). However, immobilizing ionic liquids on various solid supports is one of the efficient ways to overcome these problems. An exciting combining feature of ionic liquid with those of supporting material will develop novel performances when the synergistic effects appear (Li et al., 2014a, 2014b). Therefore, stabilized ionic liquid phase catalyst is an emerging concept in heterogeneous catalysis. Mainly, polymer-supported ionic liquids facilitate easy catalyst recovery, high selectivity, recyclability, and less contamination of products with a catalyst (Patil et al., 2022; Pei et al., 2022). The heterogeneous catalysts, due to easy segregation from the products, recyclability and reusability, and much stability, received remarkable attention (Saeedi and Rahmati, 2022). The heterogenization of ILs on appropriate porous carriers (Oliveira et al., 2019; Sun et al., 2017; Wang et al., 2020), suitable magnetic nanoparticles (Veisi et al., 2021; Zolfigol et al., 2016; Sheykhan et al., 2011; Zhang et al., 2012a, 2012b), immobilized on solid supports by either a physical coating of ionic liquids on Al2O3 (Li et al., 2017; Khoshnevis et al., 2013), SiO2 (Jamshidi et al., 2018; Testa et al., 2010; Niknam et al., 2011) and TiO2 (Atghia and Sarvi-Beigbaghlou, 2013; Tabrizian et al., 2016) or covalent attachment of ionic liquids to the support surface, would be a viable and appealing approach to fabricate an efficient solid catalyst with superior activity and stability (Safari and Ahmadzadeh, 2017). In this sense, cellulose, one of the most critical natural organic polymers, can be used as nanocomposite, support materials, and emulsions because of various advantages such as biocompatible, renewability, flexibility, dimensional stability, and ability to modify its surface chemistry and hydrophilicity (Kamel and Khattab, 2021; Hamdy et al., 2017; Sabaqian et al., 2017; Abdelhameed et al., 2020). The hydroxyl groups are reactive on the surface of cellulose, which helps to modify their surface with organic and inorganic groups chemically and increase cellulose-based substances (Marghaki et al., 2022; Hasan et al., 2021; Niu et al., 2021).
In recent years, green chemistry has been one of the essential aspects of chemists' experimental and industrial efforts (Ruijter and Orru, 2013; Wu et al., 2014; Cioc et al., 2014). Due to their atom efficiency and significant diversity, multi-component reactions (MCRs) have occupied an essential part of the green chemistry world (Jarrahi et al., 2021; Zhi et al., 2019; Sunderhaus and Martin, 2009; Biggs-Houck et al., 2010; Zareai et al., 2012). The multi-component reaction is a powerful synthetic strategy in modern chemistry in which three or more simple components as starting reagents are involved in a one-pot system to achieve new complex molecules at a less processing procedure in comparison to the step-by-step approach with usually few side-products (Váradi et al., 2016; Liu et al., 2019; Abdollahi-Basir et al., 2019; Jalili et al., 2021). Multi-component reactions have been widely used to synthesize critical heterocyclic compounds, which have many biological activities (Slobbe et al., 2012; Maleki et al., 2015; Wei et al., 2021). The pyrimidine family is one of the essential nitrogen-containing heterocycles due to their presence in many natural and biologically active products (Amin et al., 2009; Rashad et al., 2010; Singh et al., 2019). It is well known that the condensation of triazole and pyrimidine gives rise to the formation of bicyclic heterocycles known as triazolo pyrimidines, which exhibit a wide range of biological properties. Triazolo pyrimidines can be applied in various synthetic pharmacophores (Kolos et al., 2011; Gladkov et al., 2012; El-Gendy et al., 2008). Furthermore, they are valuable building blocks in the structure of many herbicidal drugs, such as penoxsulam, diclosulam, flumetsulam, azafenidin, and floransulan. Triazolopyrimidines are synthetic analogs of purines and nucleosides (Navarro et al., 1998; Magan et al., 2004; Magan et al., 2005). [1,2,4]Triazolo[1,5-a]pyrimidines, a subtype of purine bioisosteric analogs, were also reported to possess potential anti-tumor activities, especially those bearing functional groups at C-5, C-6, or C-7 positions (Traxler et al., 1996; Rusinov et al., 1986). Several synthetic strategies have been reported for the preparation of triazolo pyrimidine derivatives, most of which are based on the modification of the classical Biginelli reaction (Bhatt et al., 2016). Although some of these procedures are efficient, a number of them have limiting factors, including long reaction time, side reactions, rigid workup, high-temperature conditions, and non-recyclable reagents (Lauria et al., 2002).
In continuation of our efforts to synthesize a new heterogeneous nanocatalyst, in this study (keshavarz et al., 2022), we have reported the preparation and characterization of Fe3O4/MPC-[IL] as a supported Bronsted acidic ionic liquid catalyst. Moreover, the catalytic activity and reusability of the ionic liquid catalyst were examined for the synthesis of triazolo[4,3-a]pyrimidine derivatives.
2 Experimental
2.1 Methods and materials
All chemicals, as well as all solvents, were purchased from Merck, Aldrich-Sigma, and Fluka chemical companies and were used without further purification. Fourier-transform infrared spectrum was carried out in the region 400–4000 cm−1 by an FT-IR JASCO 6300D instrument. NMR spectra were taken with a Bruker 400 MHz Ultrashield spectrometer at 400 MHz (1H) and 100 MHz (13C) using DMSO‑d6 as solvent. Melting points were determined in open capillaries using an electrothermal KSB1N-apparatus (Kruss, Germany). X-ray diffraction analysis was studied using a Rigaku Ultima IV, Japan diffractometer operated. Scanning electron microscopy was carried out by SEM: KYKY-EM3200 instrument operated at 26 kV. EDS was determined using the TESCAN vega model instrument. Thermo gravimetric (TGA) analyses were regulated using a Perkin Elmer STA 6000 instrument. The vibration sample-magnetometry (VSM) was monitored by the Kavir Magnet VSM. The surface area was investigated using the Brunauer-Emmett-Teller (BET) technique.
2.2 Synthesis of Fe3O4 NPs
FeCl3·6H2O (2.3 g, 8.7 mmol) and FeCl2·4H2O (0.86 g, 4.3 mmol) were mixed in distilled water (30 ml) under stirring and N2 atmosphere at 80 °C for 30 min. Afterward, NaOH solution (10 ml, 25 %) was added dropwise to the mixture until the brown color solution turned out to the black and then was stirred for one h. The resulting product was collected using an external magnet and was washed with distilled water, and dried in an oven (Farahi et al., 2017).
2.3 Synthesis of 3-mercaptopropylcellulose (MPC)
The mixture of cellulose (1 g) and 3-mercaptopropyltrimethoxysilane (3 ml) in anhydrous toluene (40 ml) was stirred for 15 min at room temperature and then refluxed for 24 h. After this period, the mixture was filtered and repeatedly washed with anhydrous toluene and dried to obtain 3-mercaptopropylcellulose (MPC) (Carvalho et al., 2021).
2.4 Preparation of Fe3O4/MPC
In a round-bottomed flask (100 ml), the prepared Fe3O4 (1 g) was well dispersed in an aqueous solution (40 ml) containing NaOH (7 wt%) and urea (12 wt%) (ultrasonication for 10 min). Then 3-mercaptopropylcellulose (MPC) (1 g) was added to the flask, and the mixture was stirred for 1.5 h at −12 °C. After freezing for one h, the 3-mercaptopropylcellulose was dissolved fully. Ultimately, Fe3O4/MPC was collected with an external magnet, washed with deionized water, and dried in a vacuum oven for 24 h.
2.5 Preparation of acidic Bronsted ionic liquid [IL]
First, 1,4-butanesultone (12.2 g) was added to 1-vinyl imidazole (9.4 g) slowly at 0 °C. Then, the mixture was stirred at room temperature for about 24 h until it turned solid. The obtained solid was washed with diethyl ether and dried in a vacuum at 50 °C. Second, the prepared solid salt (2 g) was dissolved in H2O (5 ml) in a 100 ml round bottom flask, and equal molar sulfuric acid was slowly dropped into the flask at 0 °C. Then the mixture was heated up to 60 °C gradually and then stirred for 12 h. Finally, the formed liquid was washed with diethyl ether and dried in a vacuum at 50 °C for six h.
2.6 Preparation of Fe3O4/MPC-[IL] (1)
For the synthesis of supported ionic liquid, the mixture of Fe3O4/MPC (1 g), [IL] (5 mmol), anhydrous toluene (100 ml), and azodiisobutyronitrile (AIBN) (5 mol %) were refluxed under N2 atmosphere for 30 h. Finally, the product was washed with diethyl ether and dried under a vacuum.
2.7 General procedure for the synthesis of 5
A mixture of aldehyde (1 mmol), ethyl cyanoacetate or malononitrile (1 mmol), 3-amine-1H-1,2,4-triazole (1 mmol), and catalyst 1 (0.003 g) were stirred under solvent-free conditions at 100 °C. After completion of the process, monitored by TLC, hot ethanol (10 ml) was added, and the Fe3O4/MPC-[IL] was separated using an external magnet. Then, the pure product was obtained by recrystallization from EtOH. The recycled catalyst was washed with distilled water (10 ml) and ethanol (10 ml) and then dried at 100 °C. Finally, it was reused in subsequent runs.
2.8 Spectral data
Ethyl 5-amino-7-phenyl-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5a). FT-IR (KBr) ( max, cm−1): 3396, 3375, 3326, 2746, 1687, 1565, 1490, 1110. 1H NMR (400 MHz, DMSO‑d6): δ = 9.25 (s, 1H), 8.08 (d, 1H, J = 8 Hz), 7.66 (s, 2H), 7.57–7.64 (m, 5H) ppm, 5.37 (s, 1H), 3.93 (q, 2H, J = 8 Hz), 0.84 (t, 3H, J = 8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ = 162.28, 155.60, 133.90, 131.84, 131.28, 129.82, 116.09, 103.10, 24.96, 14.46.
Ethyl 5-amino-7-(4-chlorophenyl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5b). FT-IR (KBr) ( max, cm−1): 3412, 3253, 3115, 2872, 1692, 1532, 1485, 1203, 1H NMR (400 MHz, DMSO‑d6): δ = 9.25 (s, 1H), 8.08 (d, 1H, J = 8 Hz), 7.65 (s, 2H), 7.45–7.58 (m, 5H) ppm, 5.41 (s, 1H), 4.38 (q, 2H, J = 7 Hz), 0.92 (t, 3H, J = 7 Hz).13C NMR (100 MHz, DMSO‑d6): δ = 163.83, 160.63, 154.82, 134.82, 130.96, 129.87, 129.04, 110.16, 61.09, 57.01, 14.02 ppm.
Ethyl 5-amino-7-(4-bromophenyl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5c). FT-IR (KBr) ( max, cm−1): 3427, 3389, 3098, 2923, 1677, 1587, 1488, 1178. 1H NMR (400 MHz, DMSO‑d6): δ = 9.16 (s, 1H), 8.39 (s, 2H), 7.41–7.43 (m, 5H) ppm, 7.08 (d, 1H, J = 3.6 Hz), 5.21 (s, 1H), 3.98 (q, 2H, J = 6.4 Hz), 1.33 (t, 3H, J = 6.6 Hz). 13C NMR (100 MHz, DMSO‑d6): δ = 169.6, 156, 146, 141.99, 132.5, 129, 126.8, 62.1, 40.6, 27.5 ppm.
Ethyl 5-amino-7-(4-nitrophenyl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5d). FT-IR (KBr) ( max, cm−1): 3480, 3430, 3198, 2917, 1680, 1604, 1504, 1054. 1H NMR (400 MHz, DMSO‑d6) δ = 9.09 (s, 1H), 8.10 (d, 1H, J = 8 Hz), 7.83–7.91 (m, 4H), 5.99 (s, 1H), 4.43 (q, 2H, J = 7.2 Hz), 1.03 (t, 1H, J = 3.2 Hz) ppm. 13C NMR (100 MHz, DMSO‑d6) δ = 163.62, 154.95, 149.60, 144.21, 134.01, 131.06, 130.42, 124.86, 124.46, 114.11, 63.57, 59.84, 14.00 ppm.
Ethyl 5-Amino-7-(3-nitrophenyl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5e). FT-IR (KBr) ( max, cm−1): 3444, 3328, 3097, 2888, 1697, 1623, 1531, 1272. 1H NMR (400 MHz, DMSO‑d6): δ = 8.09 (d, 1H, J = 8 Hz), 7.80–7.83 (q, 4H), 6.41 (s, 1H), 5.69 (s, 1H), 4.03 (q, 2H, J = 8 Hz), 0.92 (t, 3H, J = 8 Hz) ppm. 13C NMR (100 MHz, DMSO‑d6): δ = 163.63, 154.55, 150.36, 147.93, 134.84, 131.17, 130.83, 125.30, 123.78, 123.13, 113.63, 63.19, 59.06, 13.86 ppm.
Ethyl 5-amino-7-(2,4dichlorophenyl)-7,8-dihydro-[1,2,4]triazolo[4,3-a]pyrimidine-6-carboxylate (5f). FT-IR (KBr) ( max, cm−1): 3489, 3421, 3085, 2878, 1699, 1586, 1474, 1106. 1H NMR (400 MHz, DMSO‑d6): δ = 11.67 (s, 1H), 8.11 (d, 1H, J = 8 Hz), 7.87 (s, 2H), 7.26–7.77 (t, 3H) ppm, 4.16 (s, 1H), 2.48 (q, 2H, J = 8 Hz), 1.25 (t, 3H, J = 7 Hz). 13C NMR (100 MHz, DMSO‑d6): δ = 160.6, 153.6, 137.6, 130.2, 120.8, 127, 67.1, 40.4, 21.6 ppm.
Ethyl 5-amino-7-(4-methylphenyl)-7,8-dihydro-[1,2,4]-triazolo[4,3-a]pyrimidine-6-carbonitrile (5 g). FT-IR (KBr) ( max, cm−1): 3347, 3262, 3185, 3118, 2921, 2192, 1660, 1633, 1531, 1482, 1363, 1286, 1214, 1157. 1H NMR (400 MHz, DMSO‑d6) δ = 8.75 (d, 1H, J = 1.6 Hz) ppm, 7.71 (s, 1H), 7.21 (s, 2H), 7.18 (s, 4H), 5.29 (d, 1H, J = 2.4 Hz), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO‑d6) δ = 153.92, 151.83, 146.93, 140.24, 137.26, 129.18, 126.00, 119.06, 56.06, 53.70, 20.64 ppm.
Ethyl 5-amino-7-(4-isopropylphenyl)-7,8-dihydro-[1,2,4]-triazolo[4,3-a]pyrimidine-6-carbonitrile (5 h). FT-IR (KBr) ( max, cm−1): 3378, 3295, 3181, 3118, 2964, 2186, 1656, 1627, 1523, 1479, 1367, 1284, 1211, 1151. 1H NMR (400 MHz, DMSO‑d6) δ = 8.80 (d, 1H, J = 1.6 Hz) ppm, 7.76 (s, 1H), 7.24 (s, 4H), 7.31 (s, 2H), 5.33 (d, 1H, J = 2 Hz), 2.92 (s, 1H), 1.23 (d, 6H, J = 6.8 Hz). 13C NMR (100 MHz, DMSO‑d6) δ = 153.91, 151.83, 148.21, 146.95, 140.71, 126.59, 125.99, 119.11, 55.97, 53.68, 33.11, 23.81 ppm.
3 Results and discussion
In this work, we report the preparation of Fe3O4/MPC-[IL] with high catalytic activity and its application in the synthesis of triazolo [4,3-a]-pyrimidines. The details of the preparation of magnetic nanoparticles supported by an acidic ionic liquid are presented in Scheme 1. Initially, the cellulose surface was functionalized with commercially available 3-mercaptopropyltrimethoxysilane (MPTMS) through siloxane linkages (MPC). Then, Fe3O4/MPC was fabricated through chemical modification of Fe3O4 nanoparticles with MPC in the presence of urea/NaOH. Afterward, the precursor ionic liquid was prepared using the reaction of 1-vinyl imidazole with 1,4-butane sultone, followed by treatment with sulfuric acid [IL]. Finally, Fe3O4/MPC-[IL] was synthesized by a radical grafting copolymerization using the reaction of [IL] with Fe3O4/MPC. The prepared Fe3O4/MPC-[IL] was characterized using XRD, FT-IR, SEM map, EDS, SEM, VSM, TGA, and BET techniques.![Preparation of Fe3O4/MPC-[IL] (1).](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig1.png)
Preparation of Fe3O4/MPC-[IL] (1).
Characterization by X-ray diffraction (XRD) was performed to investigate the structure of cellulose, Fe3O4, and Fe3O4/MPC-[IL] in a range of 2θ = 10-80° (Fig. 1). The analysis of the XRD pattern of cellulose (Fig. 1a) shows three characterization peaks at 2θ = 16.2°, 18° and 24° assigned to the (1 1 0), (1 1 1) and (2 0 0) planes of crystalline cellulose (Jokar et al., 2021; Zhang et al., 2019; Karami et al., 2018). In XRD pattern of Fe3O4, six characteristic peaks at 2θ = 30.26°, 35.7°, 43.5°, 53.59°, 57.5° and 63.26° were corresponding to the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) crystal planes of a pure Fe3O4 with a spinal structure (Fig. 1b) (Dang et al., 2018; Ghanbari et al., 2018; Sadeghzadeh et al., 2014). XRD diffraction patterns were presented in Fig. 1c for the new catalyst Fe3O4/MPC-[IL]. As shown, on the path to the new catalyst synthesis, the diffraction pattern of cellulose was changed after functionalization with different layers and Fe3O4. In the pattern XRD, the Fe3O4/MPC-[IL] nanocatalyst show that severities of peaks declined; however, comparing Fig. 1c with Fig. 1a and 1b confirms the stabilization of the Fe3O4 nanoparticles with IL on cellulose and Fe3O4/MPC-[IL] nanocatalyst was successfully synthesized.![XRD patterns of a) cellulose, b) Fe3O4, and c) Fe3O4/MPC-[IL].](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig2.png)
XRD patterns of a) cellulose, b) Fe3O4, and c) Fe3O4/MPC-[IL].
The FT-IR analyses of cellulose, MPC, Fe3O4, Fe3O4/MPC, [IL], and Fe3O4/MPC-[IL] were performed to prove their synthesis at each step. In Fig. 2a, the broad peak at 3350 cm−1 is attributed to the stretching vibration of the hydroxy groups of cellulose. The band at 1462 cm−1 is related to the bending vibrations of H—C—H and O—C—H (Fig. 2a) (Liu et al., 2021; Heidari and Aliramezani, 2021). The peaks of symmetric and asymmetric vibrations of the Si-O bond were seen at 973 and 1140 cm−1. The typical peak of the thiol group (S—H) was observed at 2550 cm−1 (Fig. 2b) (Jankauskaite et al., 2020; Shang et al., 2018; Loof et al., 2016). The spectra of the blank Fe3O4 show that the peak at 580 cm−1 could be due to the Fe–O vibration (Fig. 2c) (Maleki et al., 2017; Zhang et al., 2019). The presence of the characteristic absorption peak of Fe-O in all compared spectra is a confirmation of how nanoparticles of Fe3O4 have remained during the process (Fig. 2d and Fig. 2f). The IR curve of IL shows typical bands at 1563 cm−1 can be assigned to the imidazolium ring (C⚌C, C⚌N). The peaks at around 3143 cm−1 correspond to the C—H stretching vibration of imidazole moiety (sp2) (Zhang et al., 2012a, 2012b; Zhang et al., 2018). The bands around 1039 cm−1 and 1165 cm−1 were associated with the signals of C—S and S⚌O bonds, indicating the existence of the -SO3H group (Fig. 2e) (Qiao et al., 2006). However, the peak of S—H at 2565 cm−1 and end-group C⚌C totally at 1647 cm−1 disappeared, while the peak of the imidazole ring at 1563 cm−1 remained after a radical chain transfer reaction between modified cellulose and ionic liquid occurred (Fig. 2f), which convinced us that the [(CH2)3SO3HVIm]HSO4 ionic liquid was successfully grafted on functionalized cellulose via the route shown in Scheme 1.![FT-IR spectra of a) cellulose, b) MPC, c) Fe3O4, d) Fe3O4/MPC, e) [IL] and f) Fe3O4/MPC-[IL].](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig3.png)
FT-IR spectra of a) cellulose, b) MPC, c) Fe3O4, d) Fe3O4/MPC, e) [IL] and f) Fe3O4/MPC-[IL].
The element mapping analysis of the Fe3O4/MPC-[IL] nanocatalyst was shown in Fig. 3. As can be seen, all elements (C, Si, Fe, O, S, and N) are uniformly distributed on the surface of the catalyst, which indicates the successful immobilization of expected elements on cellulose.![Elemental mapping analysis of Fe3O4/MPC-[IL] nanocatalyst.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig4.png)
Elemental mapping analysis of Fe3O4/MPC-[IL] nanocatalyst.
The EDX analysis confirmed the presence of the desired elements in the Fe3O4/MPC-[IL] nanocatalyst. As shown, the peaks of C, Fe, Si, O, N and S are observed in the EDX spectrum, and these elements confirm that the magnetite NPs and ionic liquid are successfully immobilized onto functionalized cellulose surface and the desired catalyst has been synthesized (Fig. 4).![Energy dispersive X-ray spectroscopy (EDS) result for Fe3O4/MPC-[IL] nanocatalyst.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig5.png)
Energy dispersive X-ray spectroscopy (EDS) result for Fe3O4/MPC-[IL] nanocatalyst.
The morphology and size of the Fe3O4 and Fe3O4/MPC-[IL] were investigated using scanning electron microscopy (SEM) (Fig. 5). These images confirm the formation of the desired nanoparticles with spherical morphology. The average nanoparticles diameter of Fe3O4/MPC-[IL] is around 56–73 nm.![FE-SEM images of a) Fe3O4/MPC-[IL] and b) Fe3O4.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig6.png)
FE-SEM images of a) Fe3O4/MPC-[IL] and b) Fe3O4.
The transmission electronic microscopy (TEM) images of Fe3O4/MPC-[IL] nanocatalyst are shown in Fig. 6. These images indicate magnetite NPs black cores surrounded by a gray shell of modified cellulose. Furthermore, the TEM images showed that nanoparticles were composed of relatively small and almost spherical particles.![TEM images of the Fe3O4/MPC-[IL] nanocatalyst.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig7.png)
TEM images of the Fe3O4/MPC-[IL] nanocatalyst.
The magnetic properties of Fe3O4 and Fe3O4/MPC-[IL] nanoparticles were investigated using the VSM technique. As shown in Fig. 7, VSM measurements for Fe3O4 nanoparticles show that the saturation magnetization is 53.03 emu/g, while the saturation magnetization of Fe3O4/MPC-[IL] is decreased to 15.2 emu/g. The free MNPs show a higher magnetic valence in comparison with functionalized Fe3O4, which this result is due to the coated cellulose and the ionic liquid that joined to support.![VSM analysis of Fe3O4 and Fe3O4/MPC-[IL].](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig8.png)
VSM analysis of Fe3O4 and Fe3O4/MPC-[IL].
In the next step, the thermal stability of the catalyst was studied using TGA (Fig. 8). These were performed from 30 to 900 °C, showing the TGA curve of Fe3O4/MPC-[IL], which this analysis confirms the stability and presence of fixed groups on nanostructures. As can be seen in the TGA curve of the prepared Fe3O4/MPC-[IL], it has an initial weight loss of about 3.5 % up to 180 °C, which was attributed to desorption of physically adsorbed solvents, surface hydroxyl groups, and structural water. The second and significant weight loss occurs between 180 and 550 °C, showing a weight loss of 30 %, which may be related to the decomposition of organic groups, amine groups, and removal of the sulfuric acid group on the surface of the nanocatalyst. The last weight loss between 550 and 900 °C, with an observed weight loss of 4 %, is related to the immobilized organic groups grafting to the cellulose surface and confirms the stability of the synthesized nanocatalyst.![TGA curve of Fe3O4/MPC-[IL] 1.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig9.png)
TGA curve of Fe3O4/MPC-[IL] 1.
In the following, the surface area of the solid acid catalyst Fe3O4/MPC-[IL] was investigated using the N2 adsorption–desorption curve (Fig. 9). According to the IUPAC classification, the Fe3O4/MPC-[IL] nanocomposite reveals a type IV isotherm with an H1 hysteresis loop. Based on this analysis, the area of the surface 23.92 m2g−1, the total volume of the pores 5.49 cm3g−1 and the mean pores diameter 6.79 nm were obtained.![BET of Fe3O4/MPC-[IL] catalyst.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig10.png)
BET of Fe3O4/MPC-[IL] catalyst.
After successful preparation and characterization, Fe3O4/MPC-[IL] was applied as an effective nanocatalyst in the synthesis of triazolo[4,3-a]-pyrimidines derivatives 5 (Scheme 2).![Synthesis of triazolo [4,3-a]-pyrimidines derivatives 5 in the presence of nanocatalyst 1.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig11.png)
Synthesis of triazolo [4,3-a]-pyrimidines derivatives 5 in the presence of nanocatalyst 1.
To optimize the reaction conditions, the effect of catalyst, solvent and temperature was investigated in the reaction of benzaldehyde (1 mmol), ethyl cyanoacetate (1 mmol), and 3-amine-1H-1,2,4-triazole (1 mmol) as the model reaction. In the first step, the effect of catalyst loading in the reaction progress was studied under solvent-free conditions, and the desired product was not produced without a catalyst. The best result was conducted in the presence of 0.003 g of catalyst 1. Furthermore, the model reaction was performed by 0.003 g of Fe3O4/MPC-[IL] in some solvents such as EtOH, EtOH: H2O, MeOH, H2O, DMF, DMSO, and toluene. As can be seen, considerable acceleration is observed chiefly in reactions performed under solvent-free conditions. Next, the effect of the temperature was studied. We performed the reaction at different temperatures, and the reaction at 100 °C gave the best result. The results of this study are summarized in Table 1. According to these results, using Fe3O4/MPC-[IL] (0.003 g) as a catalyst under solvent-free conditions at 100 °C would be the best choice.
Entry
Catalyst 1 (g)
Solvent
Temp. (°C)
Yield (%)b
1
–
–
25
–
2
–
–
80
5
3
–
–
90
8
4
–
–
100
9
5
0.001
–
100
60
6
0.003
–
100
90
7
0.005
–
100
85
8
0.007
–
100
85
9
0.003
Ethanol
reflux
25
10
0.003
EtOH:H2O
reflux
34
11
0.003
Methanol
reflux
38
12
0.003
H2O
100
43
13
0.003
DMF
100
20
14
0.003
DMSO
100
35
15
0.003
Toluene
100
23
16
0.003
–
80
75
17
0.003
–
110
90
18
0.003
–
120
85
To investigate the generality of this protocol, different aryl aldehydes containing both electron-donating and electron-withdrawing groups were employed in the reaction. The reaction proceeded smoothly to afford the desired products 5 in good to excellent yields. Furthermore, under similar conditions, aryl aldehydes and 3-amine-1H-1,2,4-triazole were reacted with malononitrile in the presence of catalyst 1. The obtained results are summarized in Table 2.
Entry
Aldehyde
Product 5
Yield (%)b
M. p. (°C)
References
5a
C6H4CHO
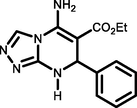
96189-191c
–
5b
4-Cl C6H4CHO
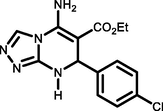
92
190-191c
–
5c
4-Br C6H4CHO
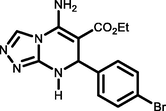
93184-184c
–
5d
4-NO2 C6H4CHO
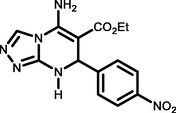
92194-196c
–
5e
3-NO2 C6H4CHO
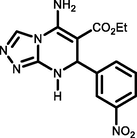
95190-192c
–
5f
2,4-Cl2 C6H3CHO
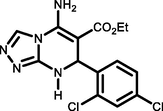
96243-245c
–
5 g
4-CH3 C6H4CHO
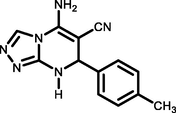
92243–245
Ablajan et al., (2012)
5 h
4-i-Pr C6H4CHO
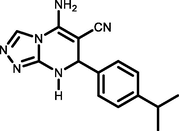
93218-220c
–
5i
4-NO2 C6H4CHO
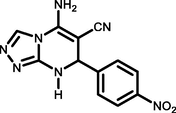
92245–247
Ablajan et al., (2012)
5j
2-Cl C6H4CHO
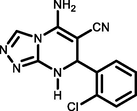
94263–266
Ablajan et al., (2012)
5 k
4-Br C6H4CHO
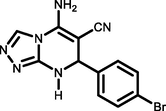
95264–266
Ablajan et al., (2012)
5 l
4-Cl C6H4CHO
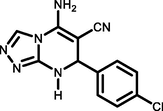
97257–258
Ablajan et al., (2012)
According to the reported mechanisms in the literature (Ablajan et al., 2012), we proposed a plausible mechanism for the synthesis of [1,2,4]-triazolo[4,3-a]-pyrimidines (5) (Scheme 3). Initially, aldehyde (2) is activated by the acidic surface sites of the catalyst. Next, the catalyst-activated aldehyde is attacked by 3-amine-1H-1,2,4-triazole (3), followed by the elimination of water to form intermediate I. Then, compound 4 performs a Michael-type addition to intermediate I to give intermediate II. The desired product 5 was obtained after the intramolecular cyclization and tautomerization intermediate III (Scheme 3).![Proposed mechanism for synthesizing 5 in the presence of Fe3O4/MPC-[IL] as a catalyst.](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig12.png)
Proposed mechanism for synthesizing 5 in the presence of Fe3O4/MPC-[IL] as a catalyst.
In the subsequent study, a leaching experiment was performed in the model reaction. When the reaction progress reached 50 %, hot EtOH (5 ml) was added, and the catalyst was magnetically separated and removed. After removing the solvent, the reaction of residue was screened under optimal conditions. Interestingly, no remarkable product was obtained in the reaction progress, indicating that the catalyst operates heterogeneously. The most important advantage of the applied catalyst is recoverability and reusability. It is important to note that the magnetic property of this catalyst facilitates its efficient recovery from the final products. Furthermore, we examined the recyclability of Fe3O4/MPC-[IL] in the model reaction. After completion of the reaction, EtOH (5 ml) was added to the mixture, and the catalyst was filtered and washed with distilled water three times by ethanol, followed by drying at 100 °C. Applying the recovered catalyst for six successive runs in the model reaction generated the product, having a low reduction in yield (Fig. 10). These experiments indicate the high stability and durability of this nanocatalyst under the applied conditions.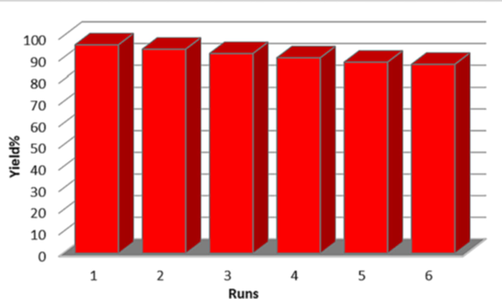
Reusability of the heterogeneous nanocatalyst 1 for the synthesis of 5a.
FT-IR spectrums of the Fe3O4/MPC-[IL] and recycled catalyst are shown in Fig. 11. This spectrum confirms good stability of the structure of Fe3O4/MPC-[IL] after recycling. The structural properties of the Fe3O4/MPC-[IL] and recycled catalyst were analyzed by XRD. This spectrum indicates structural stability; as shown in Fig. 12, the position and relative intensities of all peaks confirm this well.![FT-IR spectrum of a) Fe3O4/MPC-[IL] and b) recycled Fe3O4/MPC-[IL].](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig14.png)
FT-IR spectrum of a) Fe3O4/MPC-[IL] and b) recycled Fe3O4/MPC-[IL].
![The XRD pattern of a) Fe3O4/MPC[IL] and b) recycled Fe3O4/MPC[IL].](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104311-fig15.png)
The XRD pattern of a) Fe3O4/MPC[IL] and b) recycled Fe3O4/MPC[IL].
Next, we compared the efficiency of Fe3O4/MPC[IL] nanocatalyst with previous catalysts utilized recently in the synthesis of triazolo[4,3-a]pyrimidine derivatives (Table 3). As shown, Fe3O4/MPC[IL] is a catalyst with high durability, stability, recycling times, reaction time, and increased product yield better than the other catalysts.
Catalyst
Conditions
Time (min)
Yield (%)
Recovery times
References
Bi2O3/FAp
Cat. (30 mg), EtOH, r. t.
25–35
92–96
5
Kerru et al., 2020
TMDP
Cat. (10.5 mg), Solvent-free, 50 °C
35–70
71–93
–
Zaharani et al., 2020
TMDPS
Cat. (1 ml), Neat, r. t.
105–150
73–94
–
Zaharani et al., 2020
NaOH
Cat. (20 mol %), Ultrasonic, 25–35 °C EtOH
60
35–88
–
Ablajan et al., 2012
mPMF
Cat. (20 mg), 600 rpm, Solvent-free, r. t.
90
76–94
5
Khaligh and Mihankhah, 2022
3-vinyl-1-(4-sulfobutyl) imidazolium
Cat. (0.01 g), Solvent-free, 100 °C
20
–
–
This work
IL
Cat. (1 ml), Solvent-free, 100 °C
20
–
–
This work
Fe3O4/MPC[IL]
Cat.(0.003 g), Solvent-free, 100 °C
15–20
90–97
6
This work
4 Conclusions
Herein, given one of the bases of green chemistry, we introduced Fe3O4/MPC[IL] as a novel Fe3O4-cellulose-supported ionic liquid. Structural characterization was performed using XRD, FT-IR, EDX, SEM, VSM, TGA, and BET. Finally, this new nanocatalyst as an efficient heterogeneous catalyst with the ability to be recyclable and reusable, biocompatible, easy separation, wide substrate tolerance, high atom economy, mild reaction conditions, good to excellent yields, and short reaction times for the synthesis of triazolo[4,3-a]pyrimidine derivatives are the major important features of this new catalytic system. Also, the catalyst can be quickly recovered using a simple external magnet and reused several times without significantly losing its catalytic activity. The results of this study give us hope that it will be used in the future to synthesize other reactions and organic compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Employable metal (Ag & Pd)@MIL-125-NH2@cellulose acetate film for visible-light driven photocatalysis for reduction of nitro-aromatics. Carbohydr. Polym.. 2020;247:116695-116721.
- [CrossRef] [Google Scholar]
- MIL-53(Fe): Introduction of a new catalyst for the synthesis of Pyrimido[4,5-d]pyrimidine derivatives under solvent-free conditions. J. Mol. Struct.. 2019;1197:318-325.
- [CrossRef] [Google Scholar]
- An efficient three component one-pot synthesis of 5-amino-7-aryl-7,8-dihydro-[1,2,4] triazolo[4,3-a]-pyrimidine-6-carbonitriles. Molecules. 2012;17:1860-1869.
- [CrossRef] [Google Scholar]
- Synthesis, analgesic and anti-inflammatory activities evaluation of some bi-, tri- and tetracyclic condensed pyrimidines. Eur. J. Med. Chem.. 2009;44:4572-4584.
- [CrossRef] [Google Scholar]
- Nanocrystalline titania-based sulfonic acid (TiO2-Pr SO3H) as a new, highly efficient and recyclable solid acid catalyst for the N-Boc protection of amines at room temperature. J. Appl. Organomet. Chem.. 2013;745:42-49.
- [CrossRef] [Google Scholar]
- Task specific dicationic acidic ionic liquids catalyzed efficient and rapid synthesis of benzoxanthenones derivatives. J. Mol. Liq.. 2016;222:783-787.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis of pyrimidines in ionic liquid and their potency as non-classical malarial antifolates. Arch. Pharm.. 2016;349:791-800.
- [CrossRef] [Google Scholar]
- Recent advances in multicomponent reactions for diversity-oriented synthesis. Curr. Opin. Chem. Bio.. 2010;14:371-382.
- [CrossRef] [Google Scholar]
- Silylated thiol-containing cellulose nanofibers as a bio-based flocculation agent for ultrafine mineral particles of chalcopyrite and pyrite. J. Sustain. Metall.. 2021;7:1506-1522.
- [CrossRef] [Google Scholar]
- Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem.. 2014;16:2958-2975.
- [CrossRef] [Google Scholar]
- Novel Brønsted acidic ionic liquids and their use as dual solvent-catalysts. J. Am. Chem. Soc.. 2002;124:5962-5963.
- [CrossRef] [Google Scholar]
- Preparation of high mechanical performance nano-Fe3O4/wood fiber binderless composite boards for electromagnetic absorption via a facile and green method. Nanomater.. 2018;8:52-68.
- [CrossRef] [Google Scholar]
- Kinetics of phenol alkylation with tert-butyl alcohol using supported ionic liquid catalyst. Adv. Chem. Eng.. 2020;4:100045-100053.
- [CrossRef] [Google Scholar]
- Essramycin: A first triazolopyrimidine antibiotic isolated from nature. J. Antibiot.. 2008;61:149-157.
- [CrossRef] [Google Scholar]
- Nano-Fe3O4@SiO2-supported boron sulfonic acid as a novel magnetically heterogeneous catalyst for the synthesis of pyrano coumarins. RSC Adv.. 2017;7:46644-46650.
- [CrossRef] [Google Scholar]
- Fe3O4@SiO2@ADMPT/H6P2W18O62: A novel Wells-Dawson heteropolyacid-based magnetic inorganic–organic nanohybrid material as potent lewis acid catalyst for the efficient synthesis of 1,4-dihydopyridines. Green Chem. Lett. Rev.. 2018;11:111-124.
- [CrossRef] [Google Scholar]
- Features of the behavior of 4-amino-5-carboxamido-1,2,3-triazole in multicomponent heterocyclizations with carbonyl compounds. Beilstein J. Org. Chem.. 2012;8:2100-2105.
- [CrossRef] [Google Scholar]
- New catalyst with multiple active sites for selective hydrogenolysis of cellulose to ethylene glycol. Green Chem.. 2017;19:5144-5151.
- [CrossRef] [Google Scholar]
- Novel self-solidifying double-site acidic ionic liquid as efficient and reusable catalyst for green biodiesel synthesis. Fuel. 2022;315:122815-122824.
- [CrossRef] [Google Scholar]
- Magnetically induced demulsification of water and castor oil dispersions stabilized by Fe3O4-coated cellulose nanocrystals. Cellulose. 2021;28:4807-4823.
- [CrossRef] [Google Scholar]
- Reductant-free and in-situ green synthesis of Ag nanoparticles on Fe3O4@nanocellulose and their catalytic activity for the reduction of dyes. Chemistry Select. 2021;6:1223-1229.
- [CrossRef] [Google Scholar]
- Eco-friendly synthesis of chromeno[4,3-b]chromenes with a new photosensitized WO3/ZnO@NH2-EY nanocatalyst. RSC Adv.. 2021;11:18026-18039.
- [CrossRef] [Google Scholar]
- HPA-dendrimer functionalized magnetic nanoparticles (Fe3O4@D-NH2-HPA) as a novel inorganic-organic hybrid and recyclable catalyst for the one-pot synthesis of highly substituted pyran derivatives. Mater. Chem. Phys.. 2018;209:46-59.
- [CrossRef] [Google Scholar]
- Effect of cellulose microfiber silylation procedures on the properties and antibacterial activity of polydimethylsiloxane. Coatings. 2020;10:567-590.
- [CrossRef] [Google Scholar]
- One-pot multicomponent green LED photoinduced synthesis of chromeno[4,3-b]chromenes catalyzed by a new nanophotocatalyst histaminium tetrachlorozincate. RSC Adv.. 2021;11:19723-19736.
- [CrossRef] [Google Scholar]
- Preparation and characterization of cellulose sulfate/Pd nanocatalsysts with remarkable efficiency for Suzuki-Miyaura reaction. J. Appl. Organomet. Chem.. 2021;35:6266-6275.
- [CrossRef] [Google Scholar]
- Recent advances in cellulose supported metal nanoparticles as green and sustainable catalysis for organic synthesis. Cellulose. 2021;28:4545-4574.
- [CrossRef] [Google Scholar]
- Cellulose supported bimetallic Fe-Cu nanoparticles: a magnetically recoverable nanocatalyst for quick reduction of nitroarenes to amines in water. Cellulose. 2018;25:3295-3305.
- [CrossRef] [Google Scholar]
- Bi2O3/FAp, a sustainable catalyst for synthesis of dihydro-[1,2,4] triazolo [1,5-a] pyrimidine derivatives through green strategy. Appl. Organomet. Chem.. 2020;34:5590-5599.
- [CrossRef] [Google Scholar]
- TiO2-coated graphene oxide-molybdate complex as a new separable nanocatalyst for the synthesis of pyrrole derivatives by Paal-Knorr reaction. Arab. J. Chem.. 2022;15:103736
- [CrossRef] [Google Scholar]
- Green and solid-phase synthesis of new dihydro-[1, 2, 4] triazolo [1, 5-a] pyrimidine scaffolds by using poly-melamine-formaldehyde as a nitrogen-rich porous organocatalyst. Polycycl. Aromat. Compd.. 2022;42:942-950.
- [CrossRef] [Google Scholar]
- Alumina supported acidic ionic liquid: Preparation, characterization, and its application as catalyst in the synthesis of 1,8-dioxo-octahydroxanthenes. Synth. React. Inorg. Met.. 2013;43:1154-1161.
- [CrossRef] [Google Scholar]
- Reactions of 3-aroylacrylates with α-aminoazoles. Chem. Heterocycl. Comp.. 2011;47:983-988.
- [CrossRef] [Google Scholar]
- New tricyclic systems of biological interest. Annelated 1,2,3-triazolo[1,5-a]pyrimidines through domino reaction of 3-azidopyrroles and methylene active nitriles. Tetrahedron. 2002;58:9723-9727.
- [CrossRef] [Google Scholar]
- Development of a Brønsted acid Al–MIL-53 metal–organic framework catalyst and its application in [4+2] cycloadditions. RSC. Adv.. 2017;7:34591-34597.
- [CrossRef] [Google Scholar]
- Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J. Chem.. 2014;38:2435-2442.
- [CrossRef] [Google Scholar]
- MOR zeolite supported Brønsted acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for ketalization. RSC Adv.. 2014;4:12160-12167.
- [CrossRef] [Google Scholar]
- Sandwich-structural Ni/Fe3O4/Ni/cellulose paper with a honeycomb surface for improved absorption performance of electromagnetic interference. Carbohydr. Polym.. 2021;260:117840-117847.
- [CrossRef] [Google Scholar]
- A gold (i)-catalysed three-component reaction via trapping oxonium ylides with allenamides. Chem. Commun.. 2019;55:12675-12678.
- [CrossRef] [Google Scholar]
- Quantitative and qualitative analysis of surface modified cellulose utilizing TGA-MS. Materials. 2016;9:415-428.
- [CrossRef] [Google Scholar]
- Cytotoxicity of three new triazolo-pyrimidine derivatives against the plant trypanosomatid: phytomonas sp. isolated from euphorbia characias. Men Instaswaldo Cruz, Rio de Janeiro. 2004;99:651-656.
- [CrossRef] [Google Scholar]
- Therapeutic potential of new Pt (II) and Ru (III) triazole-pyrimidine complexes against Leishmania donovani. Pharmacology. 2005;73:41-48.
- [CrossRef] [Google Scholar]
- Preparation and application of a magnetic organic-inorganic hybrid nanocatalyst for the synthesis of α-aminonitriles. J. Chem. Sci.. 2017;129:457-462.
- [CrossRef] [Google Scholar]
- One-pot synthesis of polysubstituted imidazoles catalyzed by an ionic liquid. Org. Prep. Proced. Int.. 2015;47:461-472.
- [CrossRef] [Google Scholar]
- Chromium (VI) removal using microbial cellulose/nano-Fe3O4@polypyrrole: Isotherm, kinetic and thermodynamic studies. Mater. Chem. Phys.. 2022;278:125696-125705.
- [CrossRef] [Google Scholar]
- Synthesis of immobilized Brønsted acidic ionic liquid on silica gel as heterogeneous catalyst for esterification. Catal. Commun.. 2011;12:353-356.
- [CrossRef] [Google Scholar]
- Acetalization of carbonyl compounds catalyzed by acidic ionic liquid immobilized on silica gel. J. Mol. Catal. A Chem.. 2011;348:77-82.
- [CrossRef] [Google Scholar]
- Ionic liquids-based nanocolloids-A review of progress and prospects in convective heat transfer applications. Nanomaterials. 2021;11:1039-1062.
- [CrossRef] [Google Scholar]
- Solvent-free synthesis of protic ionic liquids. synthesis, characterization and computational studies of triazolium based ionic liquids. J. Mol. Liq.. 2022;360:119358-119391.
- [CrossRef] [Google Scholar]
- cis-[PtCl2(4,7-H-5-methyl-7-oxo[1,2,4]triazolo[1,5-a]pyrimidine)2]: A sterically restrictive new cisplatin analogue. reaction kinetics with model nucleobases, DNA interaction studies, antitumor activity, and structure-activity relationships. J. Med. Chem.. 1998;41:332-338.
- [CrossRef] [Google Scholar]
- Synthesis of 1,2,4,5-tetrasubstituted imidazoles using silica-bonded propylpiperazine N-sulfamic acid as a recyclable solid acid catalyst. Tetrahedron Lett.. 2011;52:4642-4645.
- [CrossRef] [Google Scholar]
- Recent advances in cellulose-based flexible triboelectric nanogenerators. Nano Energy. 2021;87:106175-106197.
- [CrossRef] [Google Scholar]
- Superior performance of mesoporous MOF MIL-100 (Fe) impregnated with ionic liquids for CO2 adsorption. J. Chem. Eng. Data.. 2019;64:2221-2228.
- [CrossRef] [Google Scholar]
- [MerDABCO-BSA][HSO4]2: A novel polymer supported Brønsted acidic ionic liquid catalyst for the synthesis of biscoumarins and ortho-aminocarbonitriles. J. Mol. Struct.. 2022;1259:132622-132631.
- [CrossRef] [Google Scholar]
- Ionic liquids for advanced materials. Mater. Today Nano.. 2022;17:100159-100182.
- [CrossRef] [Google Scholar]
- Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem.. 2006;246:65-69.
- [CrossRef] [Google Scholar]
- Synthesis and antitumor evaluation of some newly synthesized pyrazolopyrimidine and pyrazolotriazolopyrimidine derivatives. Phosphorus Sulfur Silicon Relat. Elem.. 2010;185:74-83.
- [CrossRef] [Google Scholar]
- Multicomponent reactions opportunities for the pharmaceutical industry. Drug Discov. Today Tech.. 2013;10:15-20.
- [CrossRef] [Google Scholar]
- Synthesis and study of cardiovascular activity of 6-nitro-7-oxo-4,7-dihydroazolo[1,5-a]pyrimidine derivatives. Pharm. Chem. J.. 1986;20:113-117.
- [CrossRef] [Google Scholar]
- Silver (I) dithiocarbamate on modified magnetic cellulose: Synthesis, density functional theory study and application. J. Carbohydr. Polym.. 2017;184:221-230.
- [CrossRef] [Google Scholar]
- Manganese (III) salen complex immobilized on Fe3O4 magnetic nanoparticles: the efficient, green and reusable nanocatalyst. Chin. J. Chem.. 2014;32:349-355.
- [CrossRef] [Google Scholar]
- MNP-cellulose-OSO3H as an efficient and biodegradable heterogeneous catalyst for green synthesis of trisubstituted imidazoles. RSC Adv.. 2022;12:11740-11749.
- [CrossRef] [Google Scholar]
- Zwitterionic sulfamic acid functionalized nanoclay: A novel nanocatalyst for the synthesis of dihydropyrano[2,3-c]pyrazoles and spiro[indoline-3,4́-pyrano[2,3-c]pyrazole] derivatives. J. Taiwan Inst. Chem. Eng.. 2017;74:14-24.
- [CrossRef] [Google Scholar]
- Bio-inspired hydrophobic modification of cellulose nanocrystals with castor oil. Carbohydr. Polym.. 2018;191:168-175.
- [CrossRef] [Google Scholar]
- Sulfamic acid heterogenized on hydroxyapatite-encapsulated γ-Fe2O3 nanoparticles as a magnetic green interphase catalyst. J. Mol. Catal. A: Chem.. 2011;335:253-261.
- [CrossRef] [Google Scholar]
- Ionic liquids synthesis and applications: An overview. J. Mol. Liq.. 2020;297:112038-112101.
- [CrossRef] [Google Scholar]
- An exhaustive compilation on chemistry of triazolopyrimidine: A journey through decades. Bioorg. Chem.. 2019;88:102919-102942.
- [CrossRef] [Google Scholar]
- Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun.. 2012;3:1189-1218.
- [CrossRef] [Google Scholar]
- Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc.. 2017;139:2786-2793.
- [CrossRef] [Google Scholar]
- Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chemistry. 2009;15:1300-1308.
- [CrossRef] [Google Scholar]
- Sulfamic acid-functionalized nano-titanium dioxide as an efficient, mild and highly recyclable solid acid nanocatalyst for chemoselective oxidation of sulfides and thiols. RSC Adv.. 2016;6:21854-21864.
- [CrossRef] [Google Scholar]
- Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J. Mol. Liq.. 2013;177:44-48.
- [CrossRef] [Google Scholar]
- A new natural based ionic liquid 3-sulfonic acid 1-imidazolopyridinium hydrogen sulfate as an efficient catalyst for the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones. J. Mol. Liq.. 2015;206:119-128.
- [CrossRef] [Google Scholar]
- Heteropolyacid-based ionic liquid [Simp]3PW12O40 nanoparticle as a productive catalyst for the one-pot synthesis of 2H-indazolo [2, 1-b] phthalazine-triones under solvent-free conditions. J. Mol. Liq.. 2017;241:447-455.
- [CrossRef] [Google Scholar]
- Esterification of acetic acid with butanol over sulfonic acid-functionalized hybrid silicas. Catal. Today. 2010;158:109-113.
- [CrossRef] [Google Scholar]
- 4-(Phenylamino)pyrrolopyrimidines: Potent and Selective, ATP Site Directed Inhibitors of the EGF-Receptor Protein Tyrosine Kinase. J. Med. Chem.. 1996;39:2285-2292.
- [CrossRef] [Google Scholar]
- Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules. 2016;21:19-40.
- [CrossRef] [Google Scholar]
- Bio-inspired synthesis of palladium nanoparticles fabricated magnetic Fe3O4 nanocomposite over Fritillaria imperialis flower extract as an efficient recyclable catalyst for the reduction of nitroarenes. Scientific Reports. 2021;11:1-15.
- [CrossRef] [Google Scholar]
- High-performance of plasma-enhanced Zn/MCM-41 catalyst for acetylene hydration. Catal. Commun.. 2020;147:106122-106142.
- [CrossRef] [Google Scholar]
- Nickel nanoparticles originated from cressa leafextract in the preparation of a novel Melem@Ni-HPA photocatalyst for the synthesis of some chromenes and a preliminary MTT assay on the anticancer activity of the nanocomposite. Polycycl. Aromat. Compd. 2021;1–20
- [CrossRef] [Google Scholar]
- Mechanism-guided design of flow systems for multicomponent reactions: conversion of CO2 and olefins to cyclic carbonates. Chem. Sci.. 2014;5:1227-1231.
- [CrossRef] [Google Scholar]
- Facile and green synthesis of a series of dihydro-[1,2,4]triazolo[1,5-a]pyrimidine scaffolds. Canad. J. Chem.. 2020;98:630-634.
- [CrossRef] [Google Scholar]
- Synthesis of functionalized furo[3,2-c]coumarins via a one-pot oxidative pseudo three-component reaction in poly(ethylene glycol) Tetrahedron. 2012;68:6721-6726.
- [CrossRef] [Google Scholar]
- Magnetically recyclable acidic polymeric ionic liquids decorated with hydrophobic regulators as highly efficient and stable catalysts for biodiesel production. Appl. Energy. 2018;223:416-429.
- [CrossRef] [Google Scholar]
- Processing cellulose@Fe3O4 into mechanical, magnetic and biodegradable synapse-like material. Compos. B.. 2019;177:107432-107440.
- [CrossRef] [Google Scholar]
- A magnetic nanoparticle supported dual acidic ionic liquid: a quasi-homogeneous catalyst for the one-pot synthesis of benzoxanthenes. Green Chem.. 2012;14:201-208.
- [CrossRef] [Google Scholar]
- A magnetic nanoparticle supported dual acidic ionic liquid: a quasi-homogeneous catalyst for the one-pot synthesis of benzoxanthenes. Green Chem.. 2012;14:201-208.
- [CrossRef] [Google Scholar]
- Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem.. 2019;17:7632-7650.
- [CrossRef] [Google Scholar]
- Applications of a novel nano magnetic catalyst in the synthesis of 1,8-dioxo-octahydroxanthene and dihydropyrano[2,3-c]pyrazole derivatives. J. Mol. Catal. A: Chem.. 2016;418:54-67.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104311.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







