Translate this page into:
Anti-pathogenic, anti-diabetic, anti-inflammatory, antioxidant, and wound healing efficacy of Datura metel L. leaves
⁎Corresponding authors. armankhan0301@gmail.com (Ameer Khusro), sadhaon@gmail.com (Subramaniam Sadhasivam), sadhaofficial@buc.edu.in (Subramaniam Sadhasivam), umar.sahibzada@gmail.com (Muhammad Umar Khayam Sahibzada)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Datura metel L. is an important medicinal plant of Solanaceae family which has extensive pharmacological properties. The present investigation was aimed to identify the presence of phytoconstituents and assess in vitro antibacterial, anti-biofilm, anti-diabetic, anti-inflammatory, antioxidant, cytotoxicity, and wound healing efficacy of D. metel leaves extract. Among different solvent extracts, methanolic extract showed higher amount of phenolic (124.61 ± 0.68 mg GAE/g), alkaloid (88.77 ± 1.01 mg AE/g), flavonoids (42.24 ± 0.18 mg QE/g), and tannins contents (38.72 ± 0.51 mg GAE/g). The extract exhibited not only significantly (P < 0.05) different antibacterial activities against pathogens tested but also showed maximum biofilm inhibition of 94, 88, and 92% against B. subtilis, MRSA, and E. coli, respectively. Anti-diabetic assay depicted 22.55 ± 0.62–79.41 ± 1.13% and 24.31 ± 1.47–72.59 ± 0.22% of α-amylase and α-glucosidase inhibition abilities of methanolic extract, respectively at varied concentrations. The methanolic extract showed potential anti-inflammatory effect (P < 0.05) by showing 28.11 ± 0.13, 34.94 ± 1.11, 55.73 ± 0.42, 73.28 ± 0.72, and 92.62 ± 1.33% of inhibition of protein denaturation at different concentrations with an IC50 value of 52.45 µg/mL. The extract revealed significant (P < 0.05) rate of ABTS scavenging, DPPH degradation, and reducing power assay in a concentration dependent manner. The cytotoxicity assay was demonstrated on L929 mouse fibroblast cell line and found > 90% of cell viability in the presence of methanolic extract, thereby indicating its non-toxicity effect. Wound healing assay indicated that methanolic extract at 50 µg/mL closed 100% of wound gap after 24 h with high rate of migration and proliferation. Furthermore, GC–MS chromatogram revealed the presence of several components in methanolic extract, including neophytadiene, hexadecanoic acid, and hentriacontane as principal phytoconstituents. In conclusion, methanolic extract of D. metel leaves could be used as potent therapeutic agent not only for treating metabolic diseases but also superficial chronic diabetic wounds.
Keywords
Bioactive properties
Datura metel L.
Methanolic extract
Therapeutics
Wound healing
1 Introduction
The practice of herbal plants and its medicinal applications varies geographically, as they are influenced by several factors viz. abiotic parameters, culture, history, personal attitudes, and philosophy (Fitzgerald et al., 2020; Faheem et al., 2022). About 80% of the world population is dependent on herbal medicines for good health maintenance. Plants contain a number of chemical compounds that can be used to cure chronic and infectious diseases (Ingle et al., 2017; Chakraborty et al., 2022; Ahmed et al., 2022). Plant metabolites such as alkaloids, tannins, and flavonoids display microbial defence characteristics, while phenolic compounds have been associated with antioxidant activities due to their free radical scavenging features. All plant parts, i.e., leaves, flowers, roots, stems, barks, rhizomes, bulbs, and/or seeds are frequently used to prepare herbal products (Matotoka and Masoko, 2018; Barbabosa-Pliego et al., 2022).
Wound healing is a dynamic, regulated, and highly complex process that is typically classified into acute and chronic. Acute wounds are surgical or traumatic injury wounds occurring in healthy people that generally heal without any difficulties. Chronic wounds are generally non-healing or take prolonged time to heal, namely pressure sores, leg ulcers, and diabetic foot ulcers, which largely do not occur in healthy people and they are clinically associated with old age, obesity, and diabetes (Han and Ceilley, 2017). Some common phytoconstituents including 13-docosenamide, (Z)-, quercetin, phytol, 1-hexadecanol, and 1-hexadecene exhibited antimicrobial, anti-inflammatory, and antioxidant effects which are required attributes for acute and chronic wound healing process (Beldal et al., 2017; Kabir et al., 2016).
A diabetic patient who has hypoglycemia will be most likely exposed to neuropathy and microvascular complication, thus the development of neuropathy may increase the risk of diabetic foot ulcers due to enlarged shearing forces and pressure load (Mariam et al., 2017). The bacteriology of diabetic foot ulcers is extremely complicated and they are frequently infected with Gram-positive and Gram-negative bacterial pathogens (Banu et al., 2015). Hence, diabetic foot ulcers require broad-spectrum antimicrobial drugs with considerable anti-diabetic and wound healing efficacies to avoid major and minor amputations. Many herbal plant formulations whether monoherbal or polyherbal with potential antimicrobial and antioxidant properties have been studied to hasten the wound healing process (Nagoba and Davane, 2019). For instance, the monoherbal Aloe vera is a first-line therapy for surgical wounds, ulcers, and burn wounds as it exhibits stronger antimicrobial activity due to the presence of acemannan, saponins, and phytol bioactive compounds. Polyherbal root extracts formulation of Rehmannia glutinosa and Astragalus propinquus were reported to be effective for diabetic ulcer treatment (Shedoeva et al., 2019).
Datura metel L. (Family: Solanaceae) is considered one of the most important medicinal plants due to its well-known antimicrobial and anti-inflammatory properties. It contains several bioactive phytoconstituents such as alkaloids, triterpenoids, flavonoids, steroids, tannins, and saponins to treat human diseases like asthma, bronchitis, diabetes, heart diseases, skin disorders, fever, diarrhea, epilepsy (Tijani et al., 2015; Dhawan and Gupta, 2017), and psychological problems (Tijani et al., 2015). The Ayurvedic preparations of D. metel and its phytoconstituents are widely used to treat hair fall, skin disorders, hysteria, and convulsions in India. Traditionally, it was also used to treat burns and wounds. The seeds of D. metel have been found to be effective in treating bleeding disorders. In Africa and Indo-China, the powdered seeds or leaves are often mixed with cannabis and smoked to relieve rheumatism and asthma (Dhawan and Gupta, 2017). D. metel leaves contain hyoscyamine and atropine, which is immensely useful for mind-altering drug development (Tijani et al., 2015).
In view of the enormous benefits of D. metel, the present study was intended not only to determine the phytochemical composition (qualitative and quantitative) of different solvent extracts of D. metel leaves but also assess crucial in vitro biological activities (antimicrobial activity, antibiofilm, anti-diabetic, anti-inflammatory, antioxidant, cytotoxicity, and wound healing efficacy) of potential solvent extract for suggesting its therapeutic roles.
2 Materials and methods
2.1 Plant collection and identification
Leaves of D. metel were collected in the autumn season from Bharathiar University premises, Coimbatore, India. The plant specimen was identified at the Botanical Survey of India, Coimbatore, India by referring to the deposited plant specimen (BSI/SRC/5/23/2017/Tech./1780).
2.2 Extract preparation
Fresh harvested leaves were washed with water and kept in shade to dry for 5 days, and ground into a fine powder. Powdered leaves (30 g/300 mL) were subjected to the successive extraction at 40 °C for 6–8 h with petroleum ether, chloroform, ethyl acetate, and methanol by using soxhlet apparatus. To extract compounds (polar and non polar), distilled water was used for maceration and subsequently extracted with different solvents (Senguttuvan et al., 2014). Following the extraction, the crude extracts were kept at 60 °C in a water bath for evaporating excess solvent. For further analysis, the extracted samples were stored in a tight container.
2.3 Screening of phytochemicals
The phytochemical components in D. metel leaves extracts were determined using both qualitative and quantitative techniques. By using standard methodology, the extracts were analyzed to ascertain the presence of flavonoids, alkaloids, saponins, tannins, phenols, proteins, cardiac glycosides, terpenoids, and carbohydrates (Prabhavathi et al., 2016).
2.4 Quantitative estimation of phytochemicals
Total contents of alkaloid, flavonoids, tannin, and phenols in the extracts were estimated using the methodology of Prasathkumar et al. (2021).
2.5 In vitro antibacterial activity
Based on the preliminary screening of phytochemical characterization, major phytochemicals were found in the methanolic extract. Hence, methanolic extract of D. metel leaves was utilized subsequently for further experiments.
Anti-bacterial activity of the methanolic extract was investigated against Bacillus subtilis, Methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa using agar well diffusion method (Kanmani and Lim, 2013). These human pathogens were obtained from Department of Microbial Biotechnology, Bharathiar University, Coimbatore, India. Bacteria were sub-cultured at 37 °C in Mueller-Hinton agar slants. After 16 h, the bacterial cultures were centrifuged at 6000 rpm for 15 min and then dissolved in sterile water. Mueller-Hinton agar medium was transferred into sterilized Petri dishes and set it aside to cool. The bacterial cultures were swabbed onto the plates and agar wells were prepared. Then, varied concentrations (20, 50, and 80 µL) of methanolic extract were poured into the respective wells, allowed to incubate at 37 °C, and the zone of inhibition was recorded after 24 h of incubation.
2.6 In vitro anti-biofilm activity
Bacterial biofilm inhibitory activity of methanolic extract was investigated against B. subtilis, MRSA, and E. coli (OD545 = 0.2). Bacteria were treated with two different doses (50 and 100 µg/mL) of methanolic extract in a 24-well titer plate. Afterwards, a clean glass slide was placed separately on top of each well. The plate (control and treated samples) was allowed to incubate at 37 °C for 3 days. Then, the dunked glass slides were gently lifted and washed thrice with sterile distilled water, followed by staining with 1 mL of crystal violet solution (0.5%) for 20 min. To remove the stain, slides were washed with distilled water and the inhibition of biofilm formation was observed under light microscope (Optika, X-LED 8 W). The absorbance was measured at 570 nm using a UV– Vis spectrophotometer (JASCO UV VIs model v − 750) to determine the quantitative measurement of anti-biofilm activity (Al Mahmud et al., 2016).
2.7 In vitro anti-diabetic activities
Alpha (α)-amylase inhibitory effect of methanolic extract was quantified based on the previous protocol (Wan et al., 2013) with minor modifications. In brief, 20 to 100 µg/mL of methanolic extract and Acarbose (standard) were diluted with α-amylase (10 µL) and allowed to incubate for 5 min at 37 °C. To produce a total volume of 2 mL, 0.2% starch solution was mixed into the solution and allowed to incubate for 5 min at 37 °C. Further, dinitrosalicylic acid (1 mL) reagent was added and placed in a water bath for 5 min. Later, 6 mL of deionized water was mixed with the reaction solution. The absorbance was recorded by UV–Vis spectrophotometer (JASCO UV VIs model v − 750) at 540 nm.
Alpha (α)-glucosidase inhibitory effect was measured based on the previous study (Wan et al., 2013) with slight modifications. In brief, 20–100 µg/mL of methanolic extract and Acarbose were mixed with 75 µL of α-glucosidase and allowed to incubate at 37 °C for 15 min. After incubation, 20 μL of 5 mM para-nitrophenyl-α-D-glucopyranoside in 0.1 M potassium phosphate buffer was added to start the reaction. The reaction was stopped by adding 80 μL of 0.2 M Na2CO3 prepared in 0.1 M potassium phosphate buffer (pH 6.8) after incubation. The absorbance of α-glucosidase inhibition activity was recorded using UV–Vis spectrophotometer (JASCO UV VIs model v − 750) at 405 nm. α-amylase and α-glucosidase inhibition (%) were calculated as per the equation mentioned below: where, Ac is the absorbance of control and As is the absorbance of methanolic extract.
2.8 In vitro anti-inflammatory activity
The protein denaturation inhibition was assessed based on the previous methodology of Gunathilake et al. (2018) with minor changes. In brief, the reaction mixture contained methanolic extract (20–100 µg/mL), 1% of bovine albumin, and phosphate-buffered saline or PBS (4.78 mL, pH 6.4). The mixture was allowed to incubate for 20 min at 37 °C and further transferred to water bath for 4 min at 70 °C. The turbidity of the reaction mixture was recorded at 660 nm after cooling using PBS as a control and aspirin as a reference. Finally, inhibition of protein denaturation was calculated as per the equation mentioned below: where, A2 is the absorbance of methanolic extract and A1 is the absorbance of control.
2.9 In vitro antioxidant activities
2.9.1 ABTS assay
The antioxidant activity of methanolic extract was performed based on ABTS scavenging method (Ismail et al., 2017). In short, ABTS diammonium salt (2.0 mM) and potassium persulfate (3.5 mM) were dissolved in distilled water and incubated under dark condition for 16 h. Then, the various concentrations of methanolic extract (100–500 µg/mL) were dissolved in ABTS+ solution (290 µL) and kept under incubation for 10 min. After that, the absorbance of reaction mixture was observed at 750 nm, using ascorbic acid as a standard. ABTS scavenging was estimated according to the equation shown below: where, Ac is the absorbance of control and As is the absorbance of methanolic extract.
2.9.2 DPPH assay
The antioxidant activity of methanolic extract was performed based on DPPH scavenging method (Tshibangu et al., 2017) with slight modifications. In short, 1 mL of various concentrations of methanolic extracts (100–500 µg/mL) was dissolved in 5.0 mL of DPPH (0.1 mM) solution and the samples were kept under dark condition for 30 min. The absorbance of the sample was measured at 517 nm and ascorbic acid was employed as a standard. DPPH radical scavenging activity was calculated by the equation given below: where, Ac is the absorbance of control and As is the absorbance of methanolic extract.
2.9.3 Reducing power assay
The reducing power assay of the methanolic extract was carried out according to Forootanfar et al. (2014). Based on the assay, the antioxidant activity was measured by the reduction of ferric ions (Fe3+ to Fe2+). Different concentrations of methanolic extracts (100 to 500 µg/mL) were dissolved with 0.2 M sodium phosphate buffer (0.5 mL, pH 6.6) and 30 mM potassium ferricyanide (0.5 mL). The reaction mixture was then allowed to centrifuge at 100 rpm for 20 min at 50 °C. Further, 2.0 mL of trichloroacetic acid (TCA, 0.6 M) was dissolved into the above mixture and again centrifuged at 3000 rpm for 10 min. 0.5 mL of supernatant was taken and diluted with deionized water and FeCl3 solution (6 mM). Then the absorbance was recorded at 700 nm. The highest absorbance indicated the highest reducing power. Ascorbic acid was used as a standard.
2.10 In vitro cytotoxicity analysis
2.10.1 Cell line
Mouse fibroblast cell line (L929 cells) was obtained from the National Centre for Cell Science (NCCS) in Pune, India. The cell line was fed with 1% antibiotic–antimycotic and 10% fetal bovine serum (FBS) combination. It was then allowed to incubate at 37 °C in a CO2 incubator (5% CO2, 20% O2) in MEM medium with high glucose.
2.10.2 MTT assay
The MTT assay was performed to investigate the cytotoxic effect of methanolic extract on L929 mouse fibroblast cell lines. Briefly, for cell adhesion, a density of 2 × 104 cells were seeded in a 96-well plate with MEM culture medium and 10% FBS was added and allowed to grow overnight. Later, the medium was replaced with fresh MEM medium (200 µL) and treated with 5–100 µg/mL of methanolic extracts and incubated for 24 h. From the MTT stock solution (5 mg/mL prepared in PBS), 50 μL was taken and poured into each well and incubated for 2–4 h in a CO2 incubator at 37 °C. After incubation, MTT-containing medium was discarded and replaced with 200 µL of DMSO. The samples were incubated for 20 min under shaking condition and the absorbance was recorded at 570 nm using ELISA reader (Biotech USA, microplate reader Elx 800) (Prasathkumar et al., 2021). The cell viability (%) was calculated by the equation given below. where, Ac is the absorbance of control and As is the absorbance of methanolic extract.
2.10.3 Trypan blue assay
For cell adhesion, a density of 5 × 105 cells were seeded in a six-well plate with MEM culture medium and 10% FBS. After overnight incubation, the medium was then discarded and normal MEM medium was added. Then, the cells were treated with 5–100 µg/mL of methanolic extracts and allowed to incubate for 24 h. Followed by incubation, the medium was removed and 0.4% of trypan blue stain was added into each well (Prasathkumar et al., 2021). The cell viability was observed using an inverted microscopy (Radical, RTC-7).
2.11 In vitro wound healing (scratch assay)
The density of 2 × 105 cells were cultured in a 24-well plate and grown to 80% confluency. At this point, on the surface of the monolayer, a tiny linear wound was observed by gently scraping with a sterile tip. To remove debris, the cells were washed with PBS and then treated with different concentrations of methanolic extract (5, 25, and 50 µg/mL) for 24 h (Prasathkumar et al., 2021). The proliferation of cells was noted, and the contraction of wound was observed under inverted phase-contrast microscopy (Radical, RTC-7).
2.12 Cells proliferation assay
The attachment and migration of L929 mouse fibroblasts in methanolic extracts treated cells were assessed using fluorescent microscope (EVOS M7000) (Jiji et al., 2019). After 8, 16, and 24 h of incubation, the cell culture medium was discarded and sterile PBS was added to the specimens. Further, the specimens were stained with 5 µL of Acridine orange (AO)/ ethidium bromide (EtBr) (1 mg/mL prepared in PBS) and the proliferation was observed immediately under the fluorescent microscope. Acridine orange penetrates inside the cell without causing cell damage and binds to the DNA inside the nucleus to produce green color fluorescence. Similarly, ethidium bromide (EtBr) enters via the damaged cell membrane and binds with fragmented nucleic acid to form red color fluorescence of the dead cells.
2.13 GC–MS analysis
Thermo ScientificTM GC–MS was used to determine the presence of varied phytoconstituents in the methanolic extract. The analysis was carried out in Elite–MS fused silica column with a helium transporter gas (steady flow rate of 1 mL/min). The injector temperature was kept constant at 260 °C. The spectra of the methanolic extract components were compared with standard components contained in the GC–MS NIST (2008) library.
2.14 Statistical analysis
All the experiments were conducted in triplicate and data were given as mean ± standard deviation (mean ± SD). The statistical analysis was done in SPSS version 12.0 using one-way ANOVA. When the P-value was < 0.05, the differences between all experimental groups were considered as significant.
3 Results and discussion
3.1 Extraction
Among the solvents tested, methanol showed maximum extraction yield (20.93%), followed by chloroform (12.8%), distilled water (4.53%), petroleum ether (3.9%), and ethyl acetate (1.36%) (Fig. 1).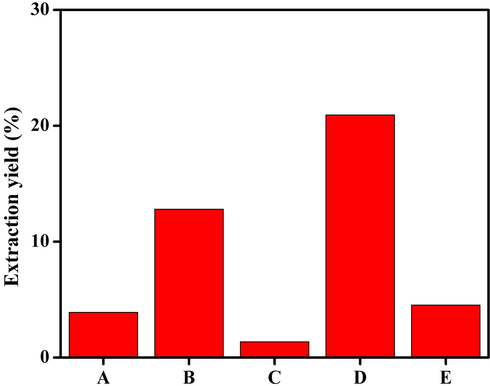
Total yield (%) of D. metel leaves extracts using different solvents. A) Petroleum ether, B) chloroform, C) ethyl acetate, D) methanol, and E) distilled water. Methanol showed highest yield of extract as compared to the other solvents.
3.2 Qualitative and quantitative screening of phytochemicals
The qualitative screening showed the presence of distinct bioactive phytochemicals including flavonoids, alkaloids, saponins, tannins, phenols, proteins, cardiac glycosides, terpenoids, and carbohydrates in different solvent extracts (Table 1). However, the methanolic extract accounted for the presence of maximum phytoconstituents. Ethyl acetate and chloroform extraction reported 5 to 6 types of the major phytocompounds. The petroleum ether and aqueous extracts showed relatively lesser phytochemicals as compared to other solvents. Note. -. Absent, +. Present, ++. Highly present.
Tests
Solvent extracts
Petroleum ether
Chloroform
Ethyl acetate
Methanol
Distilled water
Flavonoids
+
+
++
+
+
Alkaloids
+
–
–
++
+
Saponins
–
++
+
+
–
Tannins
–
+
+
+
+
Phenols
–
++
+
++
–
Proteins
+
+
–
–
–
Glycosides
–
–
–
+
–
Terpenoids
–
–
++
+
–
Carbohydrates
–
–
+
–
–
Similarly, among different solvent extracts, methanolic extract showed higher amount of phytochemicals. In methanolic extracts, total phenol, alkaloid, flavonoid, and tannin content were estimated as 124.61 ± 0.68 mg GAE/g, 88.77 ± 1.01 mg AE/g, 42.24 ± 0.18 mg QE/g, and 38.72 ± 0.51 mg GAE/g, respectively (Table 2). The relevance of natural phytoconstituents for the treatment of several illnesses is widely documented. Hence, the screening of phytochemicals is valued in the field of phytomedicine, and the identification of bioactive compounds may subsequently have a benefit in drug discovery and development (Farooq et al., 2022). Major secondary metabolites like flavonoids are of low molecular weight which possesses antibacterial, antioxidant, and anti-aging activities. Flavonoids can also inhibit carcinogenesis at various stages and can be used as potential chemotherapeutic agents for cancer treatment. Tannins are water-soluble polyphenols which exhibit antimicrobial activity (Abraham et al., 2018). Plant-based secondary metabolites including flavonoids, phenols, tannins, essential oils, and saponins are found to have free radical scavenging activity and protect from pathogen infections due to their antimicrobial action (Prabu et al., 2018; Eftekhari et al., 2021). Note. abcdDifferent letters in each row indicate that values are significantly different (P < 0.05). Each value was expressed as mean ± SD; n = 3 independent experiments.
Tests
Extracts
Petroleum ether
Chloroform
Ethyl acetate
Methanol
Distilled water
TAC (mg AE/g)
30.93 ± 0.21d
36.78 ± 0.06c
46.32 ± 0.11b
88.77 ± 1.01a
31.39 ± 0.14d
TFC (mg QE/g)
34.59 ± 0.24d
45.50 ± 0.23b
118.09 ± 0.37a
42.24 ± 0.18c
31.47 ± 0.08e
TPC (mg GAE/g)
35.31 ± 0.15d
86.22 ± 0.09b
55.54 ± 0.19c
124.61 ± 0.68a
34.43 ± 0.06d
TTC (mg GAE/g)
31.29 ± 0.47d
35.35 ± 0.16b
33.31 ± 0.26c
38.72 ± 0.51a
31.02 ± 0.11d
3.3 In vitro antibacterial activity
The methanolic extract showed significantly different antibacterial properties against all the tested bacterial pathogens. However, maximum activity was observed against E. coli with zone of inhibition of 5.33 ± 0.52, 8.33 ± 1.21, 12.66 ± 0.13 mm at 20–80 µg/mL of methanolic extract, respectively. Vancomycin (30 µg) showed zone of inhibition of 13.33 ± 0.65, 8.33 ± 0.42, 11.66 ± 0.65, and 9.33 ± 0.73 mm against B. subtilis, MRSA, E. coli, and P. aeruginosa, respectively (Table 3). Furthermore, the bacterial surface was examined by SEM before and after methanolic extract treatment. The morphological changes observed in the bacterial cell wall by untreated (control) and treated bacteria are illustrated in Fig. 2. Note. abcdDifferent letters in each column indicate that values are significantly different (P < 0.05). Values are expressed as the mean ± SD; n = 3 independent experiments.
Pathogens
Extract and antibiotic
80 μg/mL
50 μg/mL
20 μg/mL
Vancomycin (30 μg)
B. subtilis
9.33 ± 0.34b
6.66 ± 0.21b
4.33 ± 0.17b
13.33 ± 0.65a
MRSA
12.33 ± 0.42a
5.33 ± 0.14c
3.66 ± 0.12c
8.33 ± 0.42d
E. coli
12.66 ± 0.13a
8.33 ± 1.21a
5.33 ± 0.52a
11.66 ± 0.65b
P. aeruginosa
8.33 ± 0.36c
5.33 ± 0.44c
3.66 ± 0.26c
9.33 ± 0.73c
![SEM analysis of untreated and treated [methanolic extract (50 µg/mL) and vancomycin (30 µg)] bacterial pathogens. Arrows indicate the destruction of bacterial cell wall due to the inhibitory effects of methanolic extract and vancomycin.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104112-fig2.png)
SEM analysis of untreated and treated [methanolic extract (50 µg/mL) and vancomycin (30 µg)] bacterial pathogens. Arrows indicate the destruction of bacterial cell wall due to the inhibitory effects of methanolic extract and vancomycin.
The antibacterial activity of methanolic extracts of Punica granatum, Syzygium cumini, Delonix elata, Digera muricata, Jatropha gasipifed, Maerua oblongifolia, Pterocarpus santalinus, Gyrocaspus americana, Acalipha alinifolia, Hygrophilia auriculata, and Euphorbia heterophilla leaves against multidrug-resistant bacterial pathogens (S. aureus, P. aeruginosa, Klebsiella pneumoniae, and E. coli) have been reported in the past (Pallavali et al., 2019). Among all the plants studied, P. granatum and S. cumini extracts only showed potential antibacterial activities against all the tested bacterial strains. Similarly, the ethanolic extract of P. granatum peels inhibited P. aeruginosa, E. coli, S. typhi, B. cereus, and S. aureus (Mostafa et al., 2018). Antibacterial activities of seeds extracts of Portulaca oleracea, Ocimum basilicum, Brassica juncea, Phyllanthus emblica, and Lycium shawii were determined against both S. aureus and MRSA. P. emblica and L. shawii inhibited both S. aureus and MRSA strains. The seed extracts of P. emblica and L. shawii were reported to be rich sources of alkaloids, flavonoids, tannins, and phenolic contents (Tayel et al., 2018).
3.4 In vitro anti-biofilm activity
Two different concentrations of methanolic extract (50 and 100 µg/mL) significantly (P < 0.05) reduced the biofilm formation against all of the tested pathogens when compared to the control group. The bacterial cells incubated with methanolic extract were found to be detached from the surface of the slide and dispersed. On the other hand, the untreated cells adhered and proliferated on the surface of the slide (Fig. 3). Maximum inhibition effects of methanolic extract were found at 100 µg/mL which showed 94.14 ± 0.27, 88.73 ± 1.51, and 92.38 ± 1.81% inhibition of biofilm against B. subtilis, MRSA, and E. coli, respectively. Similarly, the lower concentration of methanolic extract (50 µg/mL) exhibited 61.66 ± 0.36, 52.59 ± 0.24, and 57.12 ± 0.14% inhibition of biofilm against B. subtilis, MRSA, and E. coli, respectively (Fig. 3). Based on prior reports, biofilm formation inhibitory activity exhibited by methanolic extracts was due to the presence of the bioactive compounds such as lucenin 2, 1-hexadecanol, 1-tetradecanol, and quercetin (Swamy et al., 2017), as suggested by our findings too. The anti-biofilm potential of methanolic extracted of D. metel leaves against B. subtilis, MRSA, and E. coli suggested its role in the mitigation of wound infections (Banu et al., 2015). Alam et al. (2020) reported 60% biofilm inhibition of P. aeruginosa by treating with the aqueous extract of Clematis viticella. Similarly, the biofilm formation of MRSA was inhibited by polyphenolic extracts of Opuntia ficus-indica effectively (Blando et al., 2019). Ethyl acetate extract of Frangula alnus bark prevented the biofilm formation ability of clinically isolated S. aureus due to the presence of important phytoconstituents, particularly phenolic acids (vanillic acid, ferulic acid, and gallic acid) and flavonoids (vitexin, diosmetin, and rutin) (Đukanović et al., 2020).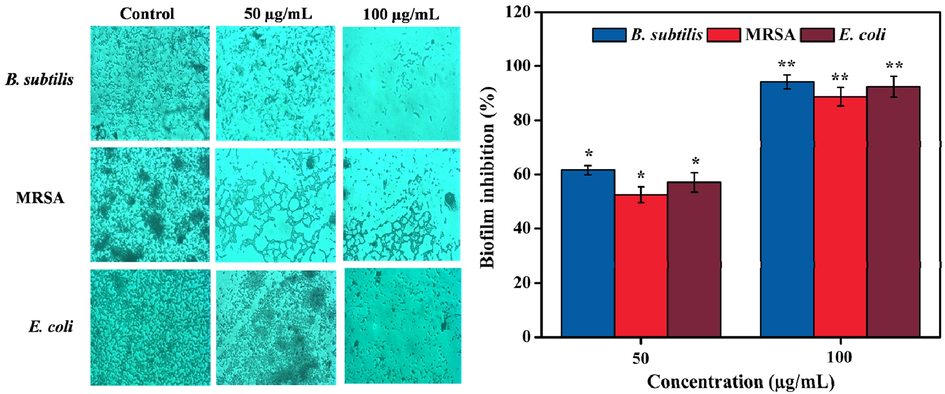
Anti-biofilm effect of methanolic leaves extract against B. subtilis, MRSA, and E. coli on cover glass. The cover glass was not attached with biofilm in the presence of methanolic extract when compared with untreated control group. Values are expressed as the mean ± SD. Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
3.5 In vitro anti-diabetic activities
The inhibition of both α-amylase and α-glucosidase are vital for the regulation of hyperglycemia as well as type 2 diabetes mellitus treatment (Esther Lydia et al., 2019; Monica et al., 2022). The concentration-dependent α-amylase and α-glucosidase inhibitory potentials of methanolic extract are illustrated in Fig. 4. At lower concentration of the methanolic extract, the enzyme inhibition was found to be minimal and the increase in inhibition was observed when the methanolic extract concentrations were increased linearly. Results showed 22.55 ± 0.62–79.41 ± 1.13% of α-amylase inhibition and 24.31 ± 1.47–72.59 ± 0.22% of α-glucosidase inhibition at 20–100 µg/mL concentrations of the extract. The IC50 values of the extract were estimated as 53.14 and 69.7 µg/mL for α-amylase and α-glucosidase, respectively. Previously, α-amylase and α-glucosidase inhibitory efficacy of acetone, ethanol, and aqueous extracts of Morinda lucida leaves with lower IC50 values were reported (Kazeem et al., 2013). Similarly, methanolic extract of Carica papaya seeds showed an IC50 value of 94.63 and 79.35 mg/mL towards α-amylase and α-glucosidase inhibition, respectively (Agada et al., 2020).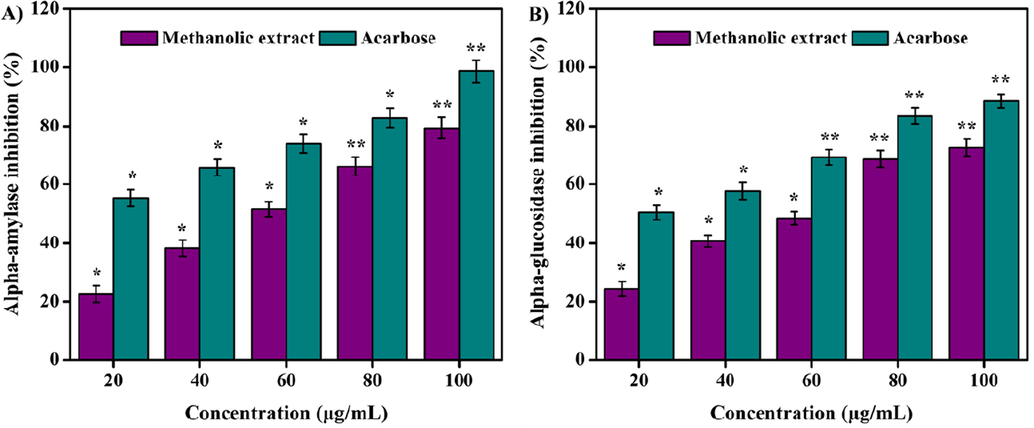
(A) a-amylase and (B) a-glucosidase inhibition activities of methanolic extract. Values are expressed as the mean ± SD. Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
3.6 In vitro anti-inflammatory activity
Typically, tissue injury results in protein denaturation of the intercellular substances or protein constituents of cells. Protein denaturation has been associated with the rate of the inflammatory response and an indicator for the development of different inflammatory diseases including arthritis. Thus, the ability of a material to inhibit the protein denaturation process denotes significant anti-inflammatory activity (Osman et al., 2016). In this study, methanolic extract revealed significant (P < 0.05) anti-inflammatory effect in a concentration dependent manner. The extract showed 28.11 ± 0.13, 34.94 ± 1.11, 55.73 ± 0.42, 73.28 ± 0.72, and 92.62 ± 1.33% of inhibition of protein denaturation at varied concentrations. IC50 value of protein denaturation was estimated as 52.45 µg/mL (Fig. 5). In a previous study, methanolic extract of Sesbania grandiflora, Cassia auriculata, Olax zeylanica, Passiflora edulis, Gymnema lactiferum, and Centella asiatica leaves depicted protein denaturation inhibition in the range of 36–75% at distinct concentrations (Gunathilake et al., 2018). Moreover, the ethanolic extract of Mikania scandens stem and leaves at various concentrations showed anti-inflammatory activities (Banerjee et al., 2014).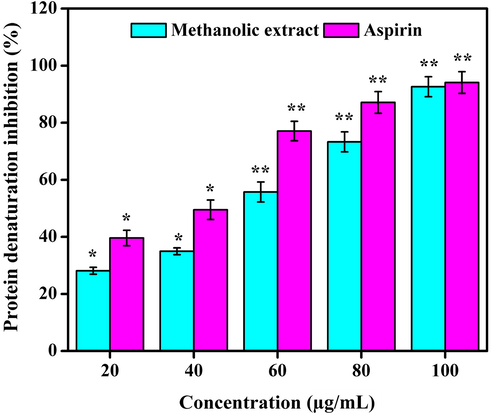
Anti-inflammatory effect of methanolic extract. Values are expressed as the mean ± SD. Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
3.7 In vitro antioxidant activities
Natural antioxidants are able to inhibit or reduce the oxidative damage that occurs due to reactive oxygen species (ROS) or atmospheric oxygen. They play a major role in maintaining good health condition and are known to reduce risks associated with chronic diseases (Saraswathi et al., 2021). Fruits, vegetables, leaves, and whole grains are considered major sources of antioxidants. Antioxidants obtained from plants such as carotenes, phenolic acids, phytoestrogens, vitamin C, and vitamin E have the potential to reduce disease risk (Naji et al., 2020). Various methods of antioxidant assays such as ABTS, DPPH, and reducing power were developed to measure the free radical scavenging properties to compare and grade natural antioxidants present in food and beverages for therapeutic applications (Schaich et al., 2015). Fig. 6 shows the significant antioxidant activity of methanolic extract of D. metel leaves, as estimated by ABTS, DPPH, and reducing power assays. As per ABTS assay, the antioxidant activity was observed from 14.32 ± 0.24 to 75.61 ± 0.31% in a concentration dependent manner with an IC50 value of 293.47 µg/mL. The DPPH assay showed radical scavenging of 48.16 ± 0.43 to 93.55 ± 0.62% at different concentrations with an IC50 value of 146.53 µg/mL. Likewise, the reducing power assay showed potential free radical scavenging activity by reducing Fe3+ to Fe2+ in a concentration dependent manner.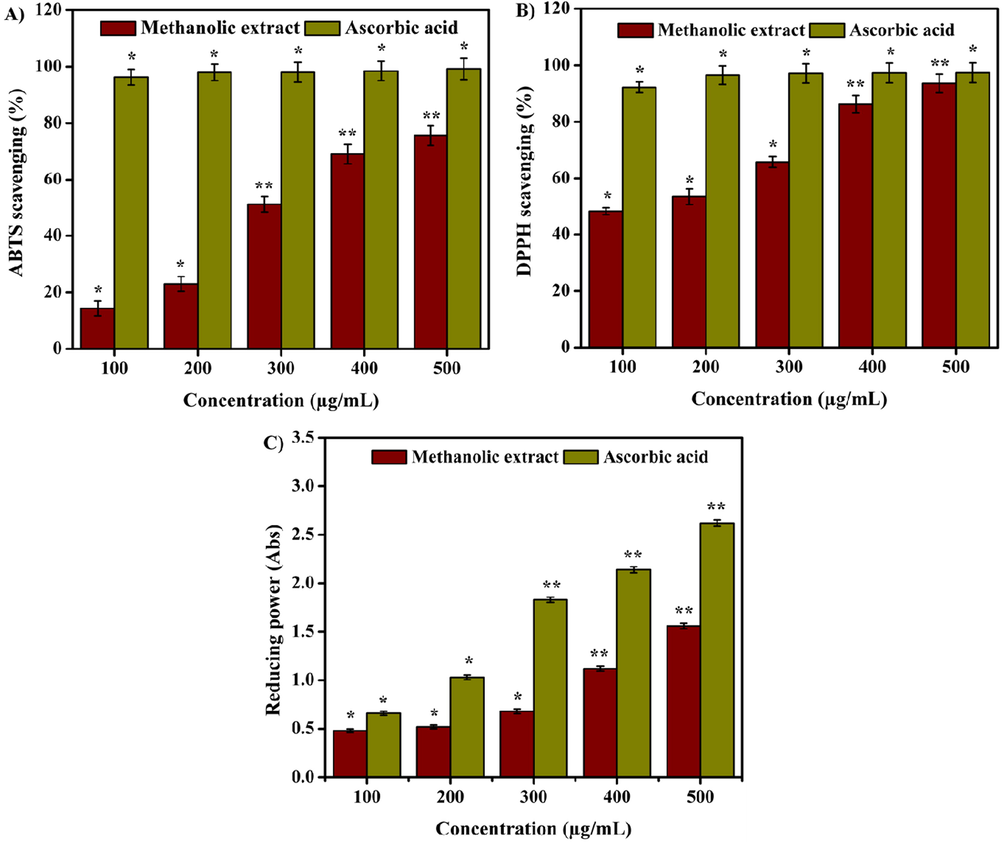
(A) ABTS radical scavenging, (B) DPPH radical scavenging, and (C) reducing power activities of methanolic extract. Values are expressed as the mean ± SD. Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
Herbal plants are rich in natural antioxidants and phenolic compounds, and are used to prevent the oxidative stress by free radical scavenging activity (Murali et al., 2020; Mayasankaravalli et al., 2020). It is presumed that the major phytochemicals, namely phenol and flavonoids are responsible for the enhanced antioxidant activity as reported by other studies. Phenolic compounds are widely utilized as potential antioxidants for various medical applications and are also gaining interest in human health. The aerial parts of Stachys molea extracts showed free radical scavenging activity (Elfalleh et al., 2019). Aerva lanata exhibited significant rate of DPPH scavenging (Al-Ansari et al., 2019). Similarly, acetone, ethanol, and methanol extract of Dendranthema morifolium flower depicted significant antioxidant effects due to the substantial amount of phenolic contents (Ma et al., 2020). In vitro ferrous ion chelating, DPPH radical, and nitric oxide radical scavenging assays also showed potential antioxidant properties of Pimenta dioica ethanolic leaves extract.
3.8 Cell cytotoxicity by MTT and trypan blue assays
The cytotoxicity of methanolic extract of D. metel on L929 mouse fibroblast cell line is presented in Fig. 7. Results showed that 99.33 ± 0.26% of cell viability were recorded at 5–75 µg/mL, while the cell viability was reduced to 93.66 ± 1.14% at 100 µg/mL. Fig. 8 shows the trypan blue assay of live and dead cells. Results revealed that methanolic extract had no significant cytotoxic effect on L929 cell line. Previous report demonstrated no toxic effect of aqueous and ethanolic extracts of Gynura divaricata against L929 cell lines, suggesting the expediency of G. divaricata extract in drug development (Poeaim et al., 2008). Similarly, the cytotoxicity of methanolic extract of Aristolochia saccata leaves was investigated on L929 mouse fibroblasts which showed > 97% of cell viability (Bolla et al., 2019). In contrary, the aqueous extract of Sargassum illicifolium revealed a significant reduction (<50%) in the viability of L929 cells (Premarathna et al., 2019).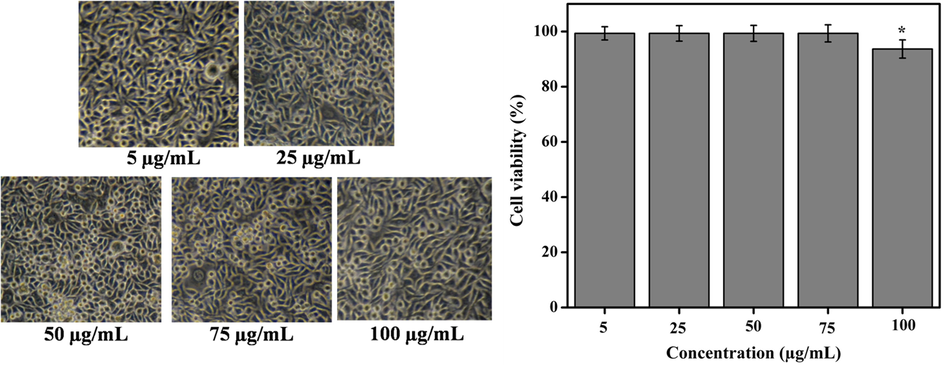
Cytotoxicity effect (morphology and viability) of different concentrations of methanolic extract on L929 mouse fibroblast cell lines. Results showed 99.33 ± 0.26% cell viability at all concentrations of the extract (5–75 µg/mL) while 100 µg/mL of concentration showed 93.66 ± 1.14% cell viability. Values are expressed as the mean ± SD.
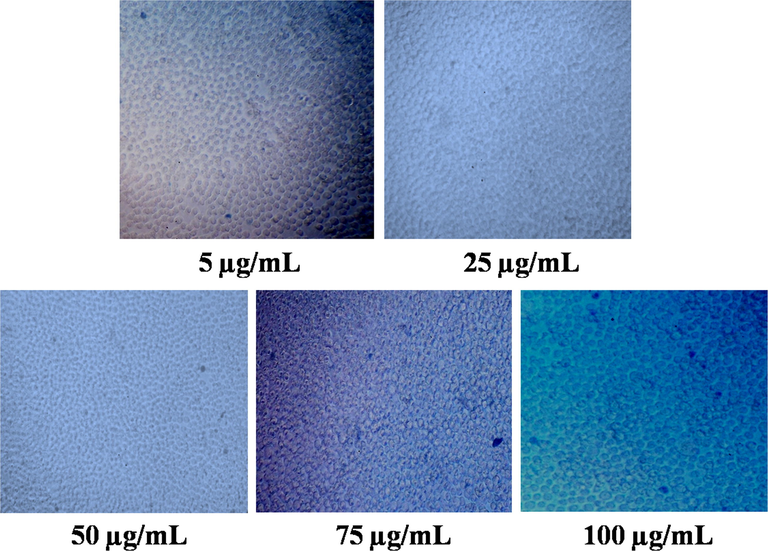
Cytotoxicity assessment of methanolic extract in L929 cell line by trypan blue assay. The extract at different concentrations (5–100 µg/mL) showed no significant toxicity on L929 cell lines.
3.9 In vitro wound healing and cells proliferation
Several existing in vitro and in vivo protocols are developed to evaluate the efficacy of medically important novel drugs and plant extracts (compounds) for wound healing. Among them, fibroblast cells migration/proliferation scratch assay is a promising technique. Scratch assay indicates the primary understanding of wound healing trait of plant extracts (compounds) through stimulating the cells migration/proliferation (Bolla et al., 2019). In the wounded surface of cell monolayer followed by scratching, the stimulating collective cells migration/proliferation towards wound contraction of the resultant gap was observed which is required for tissue regeneration (Javer et al., 2020). In this investigation, the wound healing potency of the methanolic extract (5, 25, and 50 µg/mL) of D. metel leaves was estimated against L929 mouse fibroblasts for 24 h. Results indicated that methanolic extract at 5 µg/mL closed 36.33 ± 0.52, 49.61 ± 0.27, and 57.36 ± 1.89% of wound gap at 8, 16, and 24 h, respectively. The wound closure was even better at 25 µg/mL (77.72 ± 1.19–91.33 ± 0.28%) and 50 µg/mL (79.31 ± 1.55 – 100%) for the same period. On the other hand, the control group showed only 22.83 ± 2.14–41.55 ± 1.62% of wound closure (Fig. 9). In vitro wound healing activity of A. saccata methanolic leaves extract was evaluated in L929 cells and showed 34, 70, and 93.5% of wound closure at 12, 24, and 48 h, respectively (Bolla et al., 2019). Likewise, the chloroform and methanolic extracts of Boerhavia diffusa leaves were tested against HaCaT cell line. The chloroform and methanolic extracts showed 60 and 90% of wound healing, respectively after 60 h (Juneja et al., 2020).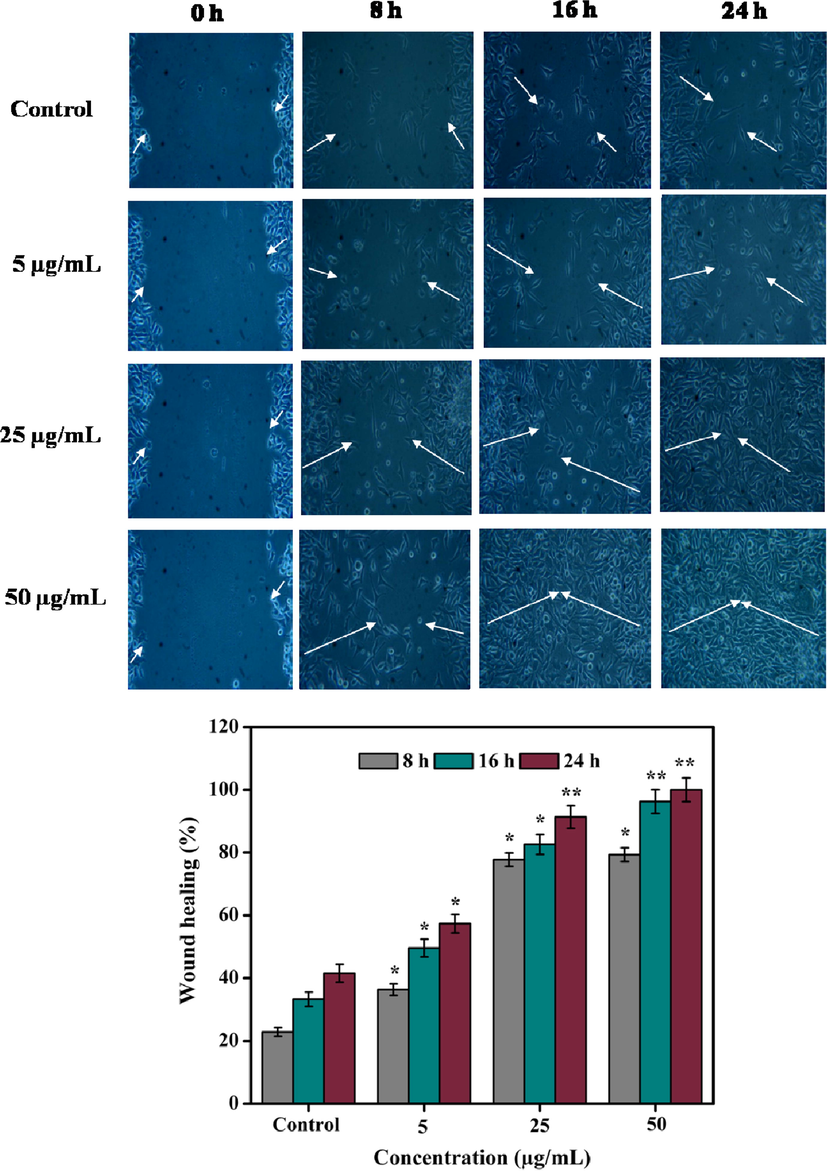
Healing of scratch wounds at different time intervals using three different concentrations (5, 25, and 50 µg/mL) of methanolic extract. White arrow indicates rate of wound closure. Values are expressed as the mean ± SD. Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
The migration of fibroblast cells plays a crucial role in the initial stage of wound healing process. It also promotes the synthesis of new extracellular matrix and thick actin myofibroblasts (Dutta et al., 2020). In this study, the cell proliferation potency of methanolic extract was observed under fluorescent microscopy. The cell density of the methanolic extract was observed considerably higher than the untreated cells (Fig. 10). In an earlier report, the wound healing potential of three different seaweed extracts (Sargassum illicifolium: S.i-SW23, S.i-SW12, and S.i-SW13) was demonstrated using L929 cells (Premarathna et al., 2019). Results showed that S.i-SW23 had a significant proliferative and migratory effect with a wound closure rate of 97.8 ± 0.05% as compared to the control (46.1 ± 0.54%) for 24 h.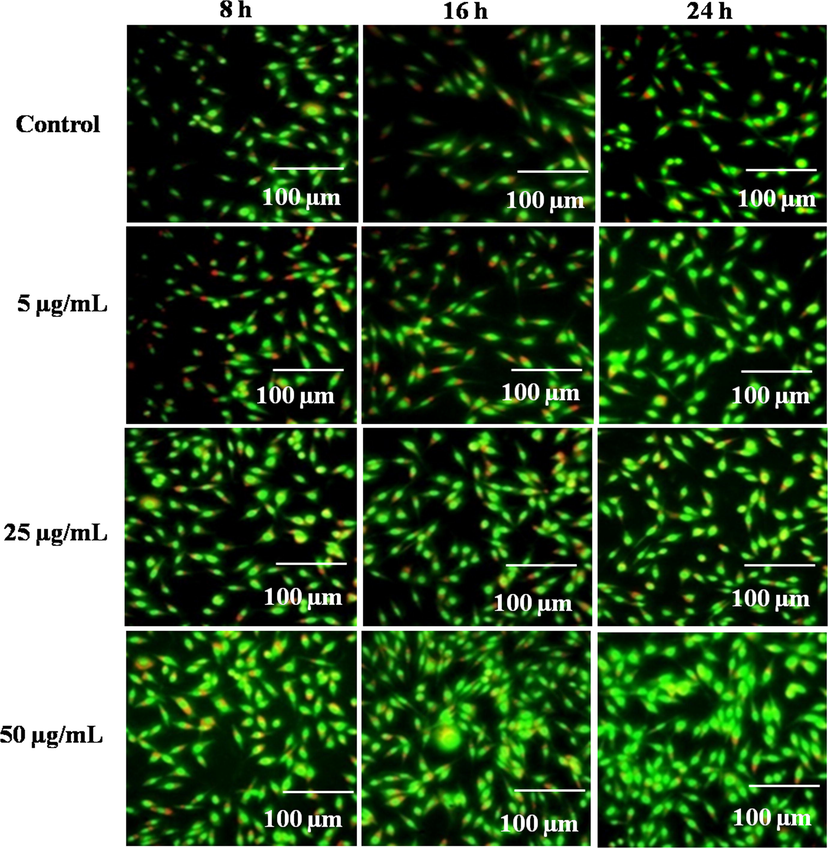
Fluorescent proliferation images of control and methanolic extract (5, 25, and 50 µg/mL) at 8, 16, and 24 h in L929 mouse fibroblast cells.
3.10 GC–MS analysis
GC–MS analysis of the methanolic extract of D. metel leaves is presented in Fig. 11. The presence of the bioactive phytoconstituents, peak area (%), retention time, molecular formula, and molecular weight are shown in Table 4. The major bioactive compounds identified were (–)-Loliolide, neophytadiene, hexadecanoic acid, phytol, 13-docosenamide, (Z)- and hentriacontane. (–)-Loliolide is a commonly available monoterpenoid lactone and it was reported to exhibit significant biological properties including antibacterial, antifungal, antioxidant, and anticancer efficacies (Grabarczyk et al., 2015). Further, it has been used as alternative medicine for diabetes and depression (Dias et al., 2020). Neophytadiene has been reported to reveal antimicrobial, analgesic, antipyretic, antioxidant, and anti-inflammatory properties. It is also used to treat skin diseases, rheumatism, and headache (Raman et al., 2012; Swamy et al., 2017). Phytol is a potent compound to inhibit S. aureus. Additionally, it possesses antinociceptive and antioxidant properties (Santos et al., 2013). Hexadecanoic acid is a well-known anti-inflammatory saturated fatty acid that exhibits antifungal and antibacterial capacity (Aparna et al., 2012). 13-docosenamide, (Z)- has significant antiandrogenic, anti-ulcerogenic, anticancer, antimicrobial, anti-inflammatory, antioxidant, lubricant, nematicide, and hypocholesterolemic activities (Beldal et al., 2017). Hentriacontane stimulated the expression of inflammatory mediators such as TNF-α, iNOS, COX-2, PGE(2), and IL-6 and it has been recommended for development of novel drugs to treat inflammatory diseases (Kim et al., 2011). NA-Not available.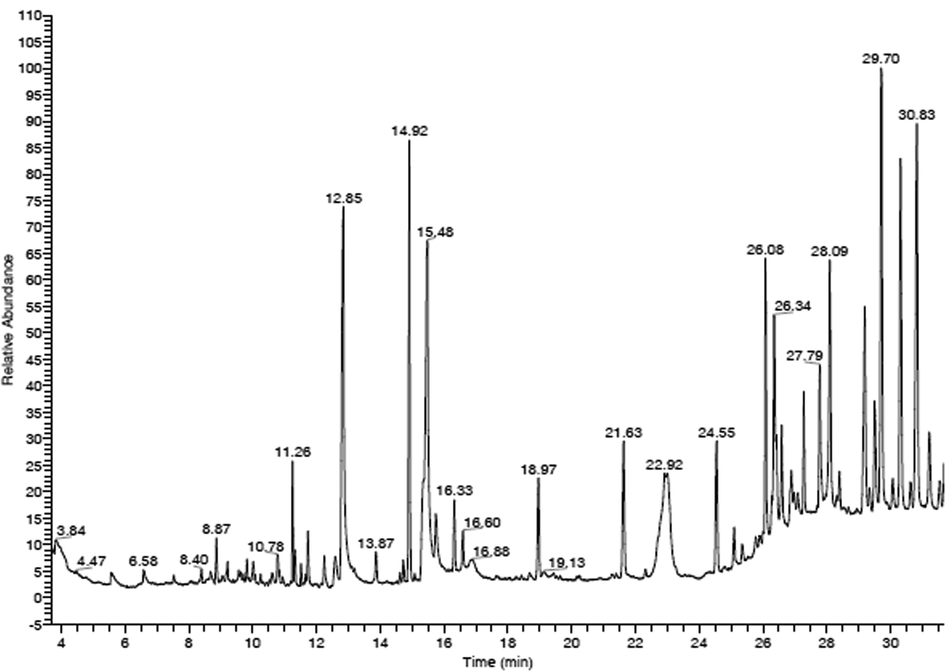
GC–MS chromatogram of methanolic extract. Each peak shows the relative abundance and area of specific compound in the methanolic extract.
S.No
Compounds
Area (%)
Retention Time
Molecular Formula
Molecular Weight
1
N-(1-hydroxy-4-oxo-1-pheny l-decahydro-pyrido[1,2-a]azep in-3-yl)carbamic acid, benzyl ester
0.84
3.84
C24H28N2O4
408
2
2(4H)-benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-tri methyl-
0.67
8.87
C11H16O2
180
3
(-)-Loliolide
0.95
10.78
C11H16O3
196
4
Neophytadiene
1.72
11.26
C20H38
278
5
Hexadecanoic acid
9.23
12.85
C16H32O2
256
6
Silicone oil
0.62
13.87
NA
0
7
Phytol
5.94
14.92
C20H40O
296
8
9,12,15-octadecatrienoic acid, (Z,Z,Z)-
10.40
15.48
C18H30O2
278
9
Silicone oil
1.11
16.33
NA
0
10
Heptasiloxane, hexadecamethyl-
1.77
18.97
C16H48O6Si7
532
11
Silicone oil
2.83
21.63
NA
0
12
1,5-dimethyl-6-(1,5-dimethyl hexyl)-15,16-epoxy-18-oxatet racyclo[9.6.1.0(2,10)0.0(5,9)]o ctdecane-13-one
6.97
22.92
C28H46O2
414
13
Silicone oil
2.63
24.55
NA
0
14
13-docosenamide, (Z)-
4.00
26.08
C22H43NO
337
15
Silicone oil
4.99
26.34
NA
0
16
Silicone oil
2.66
27.79
NA
0
17
Methyl 6-(chloromethyl)-4-(3,4-dimet hoxy-2-(phenylmethoxy)-phe nyl)-3-methyl-2-pyridinecarb oxylate
4.67
28.09
C24H24ClNO5
441
18
Hentriacontane
8.23
29.70
C31H64
436
19
5-amino-2-phenyl-4-(p-meth oxyphenyl)-4-[4′-(N,N-dimet hylamino)phenyl]-7-(pyrrolidi n-1′-yl)-1,6-naphthyridine-8-c arbonitrile
8.35
30.83
C26H23N5O
421
4 Conclusions
This study reports in vitro antibacterial, anti-biofilm, anti-diabetic, anti-inflammatory, and antioxidant activities of methanolic extract of D. metel leaves. Most importantly, the potential wound healing efficiency of methanolic extract was observed at 50 µg/mL and that was further complemented with no cytotoxicity on L929 mouse fibroblasts for 24 h. The collective biological properties of D. metel leaves extract could be useful in treating not only metabolic diseases but also superficial mycoses or chronic diabetic wounds.
Acknowledgement
The author, Subramaniam Sadhasivam acknowledges DBT, India for the financial support provided by the Ramalingaswami Re-entry fellowship (Order No.BT/RLF/Reentry/55/2013). The authors thank the DST FIST (SR/FST/LS-I/2017/10) supported Department of Microbial Biotechnology, Bharathiar University, Coimbatore, India for providing the common instrumentation facilities to carry out this work. This work was supported by the Research Universiti Grant, Geran Universiti Penyelidikan (GUP), code: 2021-074. The authors like to thank Taif University, Taif, Saudi Arabia for their support (Taif University Researcher Supporting Project Number: TURSP-2020/80).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical analysis of Pathyashadangam kwath and its standardization by HPLC and HPTLC. J. Ayurveda Integr. Med.. 2018;11:153-158.
- [CrossRef] [Google Scholar]
- In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon. 2020;6:e03618
- [CrossRef] [Google Scholar]
- Ahmed, M., Sahibzada, M.U.K., Rasheed, H.M., Khan, T., Wahid, F., Farooq, U., et al., 2022. Inhibition of inflammation associated corneal neovascularization by Dalbergia sissoo and Catharanthus roseus leaves extracts in animal model. South Afr. J. Bot. https://doi.org/10.1016/j.sajb.2022.03.047.
- Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb) J. Basic Clin. Physiol. Pharmacol.. 2016;27:63-70.
- [CrossRef] [Google Scholar]
- Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J. Infect. Public Health. 2020;13:1734-1741.
- [CrossRef] [Google Scholar]
- Identification of phytochemical components from Aerva lanata (Linn.) medicinal plants and its in-vitro inhibitory activity against drug resistant microbial pathogens and antioxidant properties. Saudi J. Biol. Sci.. 2019;26:1129-1133.
- [CrossRef] [Google Scholar]
- Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des.. 2012;80:434-439.
- [CrossRef] [Google Scholar]
- Evaluation of phytochemical screening and anti inflammatory activity of leaves and stem of Mikania scandens (l.) wild. Ann. Med. Health Sci. Res.. 2014;4:532-536.
- [Google Scholar]
- Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: a prospective study. Australas Med. J.. 2015;8:280.
- [CrossRef] [Google Scholar]
- Beneficial and adverse effects of medicinal plants as feed supplements in poultry nutrition: a review. Anim. Biotechnol.. 2022;33:369-391.
- [Google Scholar]
- Preliminary phytochemical screening, antibacterial activity and GC-MS analysis of Asparagus racemosus root extract. Int. Res. J. Pharm.. 2017;8:91-94.
- [CrossRef] [Google Scholar]
- Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants. 2019;8:117.
- [CrossRef] [Google Scholar]
- In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon. 2019;5:e01648
- [CrossRef] [Google Scholar]
- Chakraborty, A.J., Uddin, T.M., Zidan, B.M.R.M., Mitra, S., Das, R., Nainu, F., et al., 2022. Allium cepa: A treasure of bioactive phytochemicals with prospective health benefits. Evid. Based Complement. Alternat. Med. 2022, Article ID 4586318. https://doi.org/10.1155/2022/4586318.
- Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int. J. Biol. Chem.. 2017;11:17-22.
- [CrossRef] [Google Scholar]
- (−)-Loliolide isolated from sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants. 2020;9:474.
- [CrossRef] [Google Scholar]
- Antistaphylococcal and biofilm inhibitory activities of Frangula alnus bark ethyl-acetate extract. Ind. Crops Prod.. 2020;158:113013
- [CrossRef] [Google Scholar]
- Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophys. Rep.. 2020;21:100715.
- [CrossRef] [Google Scholar]
- Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: a comprehensive review. Arab. J. Chem.. 2021;14:103106
- [CrossRef] [Google Scholar]
- Antioxidant potential and phenolic composition of extracts from Stachystmolea: An endemic plant from Turkey. Ind. Crops Prod.. 2019;127:212-216.
- [CrossRef] [Google Scholar]
- Susceptibility of poultry associated bacterial pathogens to Momordica charantia fruits and evaluation of in vitro biological properties. Microb. Pathog.. 2019;132:222-229.
- [Google Scholar]
- A comprehensive review on antiepileptic properties of medicinal plants. Arab. J. Chem.. 2022;15:103478
- [CrossRef] [Google Scholar]
- Farooq, U., Sahibzada, M.U.K., Khan, T., Ullah, R., Shahid, M., Khusro, A., et al., 2022. Folecitin isolated from Hypericum oblongifolium exerts neuroprotection against lipopolysaccharide-induced neuronal synapse and memory dysfunction via p-AKT/Nrf-2/HO-1 signalling pathway. Evid. Based Complement. Alternat. Med. 2022, Article ID 9419918. https://doi.org/10.1155/2022/9419918.
- Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol.. 2020;10:1480.
- [CrossRef] [Google Scholar]
- Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol.. 2014;28:75-79.
- [CrossRef] [Google Scholar]
- Loliolide - the most ubiquitous lactone. Acta Universitatis Lodziensis. Folia Biologica et Oecologica. 2015;11:1-8.
- [CrossRef] [Google Scholar]
- In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 2018;6:107.
- [CrossRef] [Google Scholar]
- Chronic wound healing: a review of current management and treatments. Adv. Ther.. 2017;34:599-610.
- [CrossRef] [Google Scholar]
- Phytochemicals: extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem.. 2017;6:32-36.
- [Google Scholar]
- Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med.. 2017;7:452-465.
- [CrossRef] [Google Scholar]
- DeepScratch: Single-cell based topological metrics of scratch wound assays. Comput. Struct. Biotechnol.. 2020;18:2501-2509.
- [CrossRef] [Google Scholar]
- Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol.. 2019;122:452-460.
- [CrossRef] [Google Scholar]
- Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J. Tradit. Complement. Med.. 2020;10:52-59.
- [CrossRef] [Google Scholar]
- Phytochemical screening, Antioxidant, Thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J. Young Pharm.. 2016;8:391.
- [CrossRef] [Google Scholar]
- Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem.. 2013;48:1099-1106.
- [CrossRef] [Google Scholar]
- Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed. Res. Int.. 2013;527570
- [CrossRef] [Google Scholar]
- Antiinflammatory effect of Oldenlandia diffusa and its constituent, hentriacontane, through suppression of caspase-1 activation in mouse peritoneal macrophages. Phytother. Res.. 2011;25:1537-1546.
- [CrossRef] [Google Scholar]
- Solvent effect on phenolics and antioxidant activity of Huangshan Gongju (Dendranthema morifolium (Ramat) Tzvel. cv. Gongju) extract. Food Chem. Toxicol.. 2020;147:111875
- [CrossRef] [Google Scholar]
- Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the University of Gondar Referral Hospital, North West Ethiopia, 2016: institutional-based cross-sectional study. J. Diabetes Res.. 2017;2879249
- [CrossRef] [Google Scholar]
- Phytochemical screening and pharmacological evaluation of herbal concoctions sold at Ga Maja Limpopo Province. S. Afr. J. Bot.. 2018;117:1-10.
- [CrossRef] [Google Scholar]
- Profiling the phyto-constituents of Punica granatum fruits peel extract and accessing its in-vitro antioxidant, anti-diabetic, anti-obesity, and angiotensin-converting enzyme inhibitory properties. Saudi J. Biol. Sci.. 2020;27:3228-3234.
- [Google Scholar]
- Chemical composition of pumpkin (Cucurbita maxima) seeds and its supplemental effect on Indian women with metabolic syndrome. Arab. J. Chem.. 2022;15:103985
- [CrossRef] [Google Scholar]
- Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci.. 2018;25:361-366.
- [CrossRef] [Google Scholar]
- Phytochemical screening, FTIR spectral analysis, antioxidant and antibacterial activity of leaf extract of Pimenta dioica Linn. Mater. Today: Proc.. 2020;2020
- [CrossRef] [Google Scholar]
- Studies on wound healing potential of topical herbal formulations-do we need to strengthen study protocol? J. Ayurveda Integr. Med.. 2019;10:316-318.
- [CrossRef] [Google Scholar]
- Ferric-bipyridine assay: a novel spectrophotometric method for measurement of antioxidant capacity. Heliyon. 2020;6:e03162
- [CrossRef] [Google Scholar]
- In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult. Ethnopharmacol.. 2016;5:343.
- [CrossRef] [Google Scholar]
- Data of antibacterial activity of plant leaves crude extract on bacterial isolates of wound infections. Data Brief. 2019;24:103896
- [CrossRef] [Google Scholar]
- Poeaim, S., Pattamake, A., Praianan, A., Petcharawan, O., 2008. Cytotoxicity of crude extracts from Gynura divaricata on L929 cell lines. In46. Kasetsart University Annual Conference, Bangkok (Thailand), 29 Jan-1 Feb 2008.
- Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Pelagia Res. Libr. Adv. Appl. Sci. Res.. 2016;7:11-17.
- [Google Scholar]
- Anti-oxidant activity, phytochemical screening and HPLC profile of rare endemic Cordia diffusa. J. King Saud Univ. Sci.. 2018;31:724-727.
- [CrossRef] [Google Scholar]
- Phytochemical screening and in vitro antibacterial, antioxidant, anti-inflammatory, anti-diabetic, and wound healing attributes of Senna auriculata (L.) Roxb. leaves. Arab. J. Chem.. 2021;14:103345
- [CrossRef] [Google Scholar]
- Wound healing properties of aqueous extracts of Sargassum illicifolium: an in vitro assay. Wound Med.. 2019;24:1-7.
- [CrossRef] [Google Scholar]
- Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res.. 2012;5:99-106.
- [Google Scholar]
- Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J.. 2013;2013:949452
- [CrossRef] [Google Scholar]
- Assessment on in vitro medicinal properties and chemical composition analysis of Solanum virginianum dried fruits. Arab. J. Chem.. 2021;14:103442
- [CrossRef] [Google Scholar]
- Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods.. 2015;18:782-796.
- [CrossRef] [Google Scholar]
- Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac. J. Trop. Biomed.. 2014;4:S359-S367.
- [CrossRef] [Google Scholar]
- Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med.. 2019;2019
- [CrossRef] [Google Scholar]
- GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid. Based Complement. Altern. Med.. 2017;2017
- [CrossRef] [Google Scholar]
- Bioactivity and application of plant seeds’ extracts to fight resistant strains of Staphylococcus aureus. AOAS. 2018;63:47-53.
- [CrossRef] [Google Scholar]
- Neuro-toxicological impacts of Datura metel Linn. (Family: Solanaceae) leaves extract in mice. J. Neurobehav. Sci.. 2015;2:97-101.
- [CrossRef] [Google Scholar]
- Antiplasmodial activity of Heinsia crinita (Rubiaceae) and identification of new iridoids. J. Ethnopharmacol.. 2017;196:261-266.
- [CrossRef] [Google Scholar]
- In vitro and in vivo anti-diabetic activity of Swertia kouitchensis extract. J. Ethnopharmacol.. 2013;147:622-630.
- [CrossRef] [Google Scholar]







