Translate this page into:
An overview of a sustainable approach to the biosynthesis of AgNPs for electrochemical sensors
⁎Corresponding author. ksanthakumar@vit.ac.in (Santhakumar Kannappan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
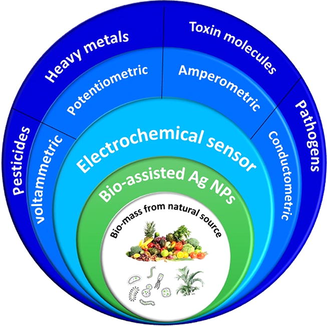
Mother nature furnishes various sources to synthesize nanomaterial’s with different geometry, size, and functionality. In this outline, we aimed to discuss the biological source-mediated fabrication of Ag NPs because of their easy handling, yields, and economical and non-toxicity. The literature reveals that different plant species, fungi, and bacteria can employ biosynthesis, enabling the fabrication of nanoparticles with different features, notably size, geometry, and morphology. The exact mechanisms have not been understood well, even though it is trusted that bio-sourced is responsible for this process. The method of synthesis can be influenced by pH, concentration, time, and biomass. The optimized biosynthesized AgNPs can employ in various domains like sensors, nanomedicine, environmental pollution etc., The main objective of the paper is to elaborate on the biosynthesized AgNPS in electrochemical sensing and its surface modifications. Furthermore, these electroanalytical techniques are to be used for real-time sampling to allow the selective detection of the target analyte.
Keywords
Biosynthesis
Silver nanoparticle
Electrochemical sensor
Bio-reduction
Biosensor
1 Introduction
To attain the expanded applications of today and tomorrow’s lifestyle, nano-biotechnology is one of the cutting-edge techniques with various applications like medicine, food cosmetics, environmental monitoring, soil, and water quality checking(Grieshaber et al., 2008), industrial chemicals, drug discovery, space equipment, photo-electrochemical, optical, electrical and electronic applications(Mansur et al., 1995) (Wilmut, 1997) (Hoffman et al., 1992) (Schmid, 1998). In nanotechnology, the greener method also played a major role to synthesize metal nanoparticles (NPs) by using plants, bacteria, yeast, algae, enzymes, etc., the sustainable plant-based biosynthesized NPs are efficient for various applications and are eco-friendly and valuable substitutes for environmental management (Birla et al., 2013). Using plant parts to synthesize nanoparticles receives more attention due to the mystery of the plant world, still, the plants are surprising us with their unique properties, and the exact mechanisms for plant metabolites are challenging to researchers and not explored well. To improve in the medicinal field, nanomaterial plays a vital role in milestones and now it is the better substitute for biomedical applications (Balantrapu and Goia, 2009). In the current situation, the biggest challenge is to identify and treat new diseases, food adulterations, and environmental monitoring. However, Nano-sized functionalized NPs are an astonishing tool for such kind of identification(Khan, Saeed, and Khan, 2019).
This outline is an attempt to exhibit sustainable biosynthesized silver NPs towards electrochemical biosensors to track target analytes. Before concluding this outline shows the necessity of portable electrochemical biosensors with prospects.
2 Why green synthesis?
Green synthesis is the only way to avert harmful materials as compared to chemical synthetic procedures, toxic side products, and also the current and fascinating research domain (Hasan et al., 2015). Furthermore, it is reliable, sustainable, and eco-friendly. The synthesis procedure is also simple, and lesser time conception moreover the plants and the plant's medicinal properties and bioactive ingredients will be explored by using this method (Yousaf et al., 2019) (Hasan, 2019) (Hasan et al., 2013). To attain better, long-lasting, safe, and cleaner techniques for NPs synthesis, it is crucial to develop such strategies that aid to reduce the cost, boost the yield, and develop better ways that may be used at both industrial and commercial levels. Therefore, it is essential to research environmentally friendly, cost-effective, and high-production processes that use inexpensive reagents without compromising safety or the environment. Some key benefits of biological NPs production methods over physical and chemical methods (Hasan et al., 2018). The chemical composition which is present in the bio-sourced will act as a natural reducing agent without any hazards and environmentally friendly at a low cost.
2.1 The impetus of silver nanoparticles
Metals like Iron (Fe), Copper (Cu), platinum (Pt), Gold (Au), Palladium (Pd), and Silver (Ag) show engrossed in research, it has varying assets due to their physical and chemical properties. For millennia, silver has been used as an antibacterial to lessen microbial contamination and its claim to elevate our immune system. As silver is a valuable antibacterial and antioxidant, our ancestors also used it in vessels and ornaments. Ionic silver is also used to heal wounds in conjunction with this. A mechanism for silvers’ antimicrobial activity remains a mystery. During the last decades, the utilities of Ag NPs are increased in many paths like medical devices, electronic equipment, drug delivery, and other applications (Tashi et al., 2016) (Slane et al., 2015). So silver gained much attention in every field, and also Ag is one of the best natural sources of abundance.
2.2 Importance of sensor
The sensor is one of the basic aspects of daily life just like the five senses of humans. The sensor is used for monitoring, security, maintenance, protection, and many more [12–14]. The accurate and rapid identifications of the target analyte are received more focus and demand (Li et al., 2019) (Kaviya, 2020a). Traditional techniques like atomic absorption spectroscopy, UV–visible spectroscopy, and mass spectroscopy are widely used for the identification of target analytes. The drawbacks of these techniques are the need for trained manpower, skilled source, pre-treatment, expensive, long procedures, etc.,(Akhgari et al., 2015). With this goal in mind, better precision, sensitivity, and budget-friendly sensors are the better technique to identify analytes such as toxic metal ions, dyes, gases, preservatives, drug discovery, water, and soil monitoring, etc.,(Sapsford et al., 2008) (Chen et al., 2018).
2.3 Role of metal NPs in sensor
Noteworthy sensors are based on two basics: (i) analyzing the components for a specific analyte and (ii) a transducer compound for recording the better interaction event. These basics are in terms of selectivity, reproducibility, stability, time of response, noise ratio, and LOD (Saha et al., 2012). Furthermore in this outline, we focused on Ag NPs and their physical and chemical character and varying asset like antimicrobial(Kaviya et al., 2011) bio-imaging agent(Dayanand and Justyna, 2018) photocatalyst (Hashemi et al., 2022), etc., “The special properties of the metal make this asset possible, such as biocompatibility, chemical inertness, massive surface area, simple chemical synthesis, surface adaption, and optical properties as a result of its electron oscillation with the analyte” (Kaviya, 2020b).
2.4 Reason for bio-assisted NPs for sensor
A bio-assisted nanoparticle is eco-friendly, sustainable, cost-effective, and stable. Even though sensors have evolved over the years, the process has lost some of its affordability, biocompatibility, and greenness. Biosynthesis permits the identification of the target analyte with good selectivity without generating unwanted or harmful products. We are focused on revealing the bio-assisted metal NPs turned into the electrochemical sensor.
2.5 Fabrication of nanoparticle
Mainly metallic nanoparticles are fabricated by two methods: (i) Top-down and (ii) Bottom-up methods as shown in Fig. 1. Top-down techniques include processes like pulsed laser ablation (Zakaria et al., 2020), Arc discharge (El-Khatib et al., 2018), Spray pyrolysis (Kaya et al., 2020), Electro-explosion (Singh et al., 2016), Ball milling (Baláž et al., 2017), and lithography (Wang et al., 2019) that reduce large-scale materials into the minute, fine particles. However, these methods are better than chemical methods because of not use solvents or any toxic chemicals. Meanwhile, Bottom-up techniques, in contrast, involve shrinking the size of the material into the nanoscale through the process like micro-emulsion (Rivera-Rangel et al., 2018), photochemical (Gabriel et al., 2017), electrochemical pyrolysis (Chae, Lee, and Joo, 2019), microwave (Iqbal et al., 2014), solvothermal (Wani et al., 2010), Co-precipitation (Minh Dat et al., 2021), and sonochemical (Zhang and Li, 2012) as well as chemical reduction using reducing agents like sodium borohydride(Chouhan, Ameta and Meena, 2017), quinone based compounds (Jacob et al., 2008). The final method is the Bio-reduction process by using plant parts, bacteria, fungi, yeast, and algae. The chemical composition which is present in the bio-sourced will act as a natural reducing agent without any hazards and environmentally friendly at a low cost. The bio-assisted NPs are named Green synthesized NPs with desired metal under optimal conditions.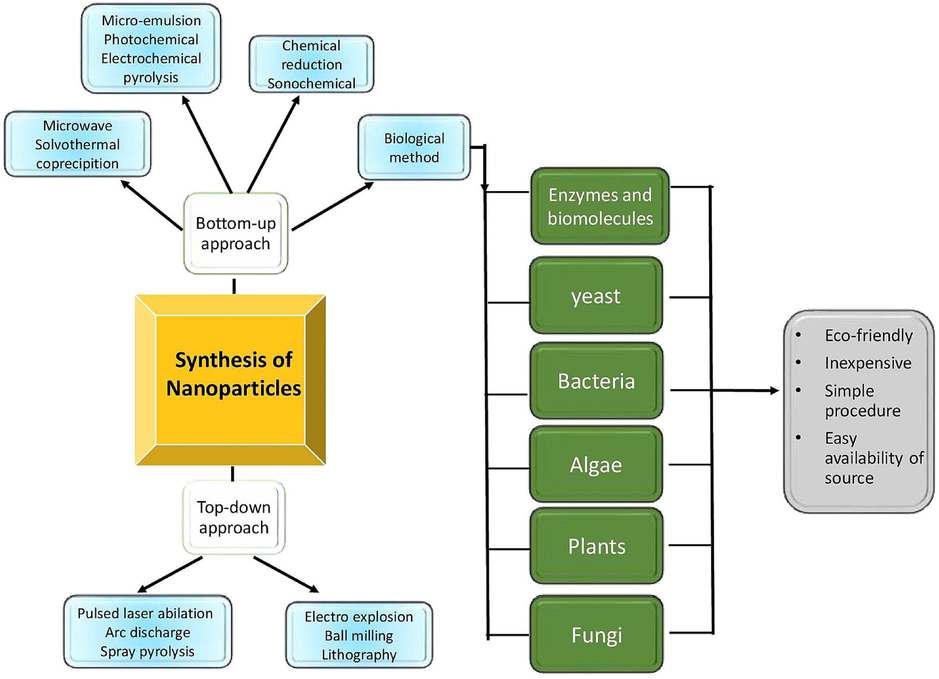
Routes to synthesis nanoparticle.
2.6 Biological methods
The metal NPs can synthesize from various bio-sourced including bacteria, fungi, yeast, algae, and plant parts (Fig. 2). There is no need for an external stabilizing agent because the bio-sourced has the active property that makes it a bio-reducing agent in the fabrication of NPs synthesis. Since the fabrication is a harmless and prominent particle of metal NPs have received more focus from researchers (Iravani, 2011).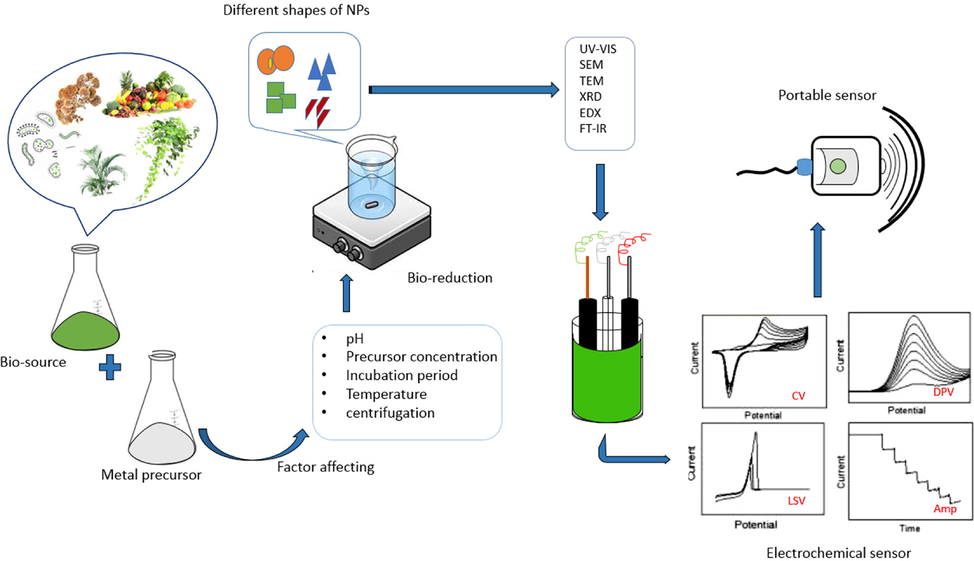
Consequences of biogenic nanoparticle, b. Consequences of biogenic nanoparticle.
Consequences of biogenic nanoparticle, b. Consequences of biogenic nanoparticle.
2.7 Bacteria-assisted synthesis of Ag NPs
Microorganisms are one of the best candidates for the bio-fabrication of NPs but it has contamination of culture medium, long procedure, and are not able to control the size of NPs(Rafique et al., 2017). However, bacteria-assisted synthesis of Ag NPs can able to reduce heavy metal ions, and it receives a better interest in NPs synthesis. Based on the location of synthesis in bacteria it can classify into distinct types (Li et al., 2011). Survey shows that Pseudomonas stutzeri and Pseudomonas aeruginosa have a better ability to overcome the toxicity of heavy metal ions and even can grow in higher heavy metal concentrations (Iravani, 2014). Table 1 provides several studies related to the biosynthesis of Ag NPs by using bacteria. In 1999 Klaus et al. (Klaus et al., 1999) reported the bio-fabrication of Ag NPs by using Pseudomonas stutzeri strain and this is one of the earliest articles on the fabrication of triangular-shaped Ag NPs with 200 nM by using bacteria. Shivaji and Madhu et al., (Shivaji, Madhu and Singh, 2011) purposed the synthesis of Ag NPs by using psychrophilic bacteria. The recent article purposed by Garibo et al. studied the bio-fabrication of Ag NPS by using Escherichia coli (Garibo et al., 2020).
Bacteria species
Size (nm)
Shape
Reference
Escherichia coli
1.2–62
Spherical
(Garibo et al., 2020)
Bacillus siamensis
25–50
spherical
(Ibrahim et al., 2019)
Serratia nematodiphila
65–70
Spherical
(Malarkodi et al., 2013)
Bacillus stearothermophilus
65–70
Spherical
(Mohamed et al., 2009)
Lactobacillus casei
42–92
Spherical
(Iravani et al., 2012)
Nocardiopsis spp.
25–50
Spherical
(Manivasagan et al., 2013)
Streptomyces hygroscopicus
45 ± 0.15
–
(Sadhasivam et al., 2010)
Staphylococcus aureus
20–30
Irregular
(Nanda and Saravanan, 2009)
Rhodococcus spp.
160–180
Spherical
(Otari et al., 2015)
Marine Ochrobactrum spp.
5–50
Spherical
(Thomas et al., 2014)
Escherichia coli
38–85
Spherical
(Ghorbani and Rashidi, 2013)
Lactobacillus strains
1–100
Triangular/
hexagonal(Nair and Pradeep, 2002)
Bacillus methylotrophicus
15–500
Spherical
(Wang et al., 2016)
Vibrio alginolyticus
50–100
Crystalline/spherical
(Garibo et al., 2020)
2.8 Fungi-assisted synthesis of Ag NPs
Fungi are one of the better potential biomass for the production of Ag NPs, nearly 6,400 bioactive ingredients are present in fungi and fungal species (Kim et al., 2017). Moreover, it can use for large-scale production with optimized parameters, and also it has several advantages over other microorganisms to produce a large amount of NPs. The biosynthesis of NPs by fungi can be classified into two parts: intracellular and extracellular(Azmath et al., 2016) (Khan et al., 2017). If the metal precursor is added in mycelial it is called intracellular whereas in extracellular, the metal precursor will be added in aqueous filtrate which contains only fungal biomass (Rose et al., 2019). Table 2 reported some illustrative examples of the bio-fabrication of Ag NPs by fungi-assisted.
Fungus
Size
Shape
Reference
Colleotrichum sp. ALF2-6
5–60 nm
Myriad shapes
(Azmath et al., 2016)
Aspergillus oryzae
7–27 nm
Spherical
(Phanjom and Ahmed, 2017)
Rhizopus stolonifer
2 nm
Spherical
(AbdelRahim et al., 2017)
Aspergillus fumigatus BTCB10
322.8 nm
spherical
(Shahzad et al., 2019)
Fusarium oxysporum
5–13 nm
Spherical
(Husseiny et al., 2015)
Guignardia mangifera
5–30 nm
spherical
(Balakumaran et al., 2015)
Duddingtonia flagans
30–409
spherical
(Costa Silva et al., 2017)
Trichoderma longibrachiatum
24
spherical
(Elamawi et al., 2018)
Penicillium oxalicum
36
spherical
(Du et al., 2015)
Arthroderma fulvum
20
spherical
(Xue et al., 2016)
Sclerotinia sclerotiorum
10–15
spherical
(Saxena et al., 2016)
2.9 Yeast-assisted synthesis of Ag NPs
Yeast is one of the simple processes in the bio-fabrication of NPs with mass production. Yeast is known as the eukaryotic microorganism of fungi, around 1,500 species are present now. Table 3 shows the reported bio-synthesis of Ag NPs by using various yeast.
Yeast
Size (nm)
Shape
Reference
Saccaromyces cerevisiae
6.7
spherical
(Elnagar et al., 2021)
Pichia kudriavzeviiHA‑NY2
12.4
round
(Ammar et al., 2021)
Saccharomyces uvarumHA‑NY3
20.6
cubic
(Ammar et al., 2021)
Crataegus pinnatifida
50–100
spherical
(Liu et al., 2021)
Vitis vinifera
50–100
spherical
(Liu et al., 2021)
Saccharomyces cerevisiae
11–25
Spherical
(Kthiri et al., 2021)
Staphylococcus aureus
13
Spherical
(Shu et al., 2020)
Saccharomyces cerevisiae
16
Oval
(Ibraheim et al., 2016)
Rhodotorula sp. strain
8–21
Oval
(Soliman et al., 2018)
2.10 Plant-assisted synthesis of Ag NPs
In greener synthesis, plant-mediated synthesis (Table 4) is the most believed route as it gives a plethora of merits over the chemical and physical route. The extended research of plant-mediated synthesis is fractionating the crude extract of the plant-like ethanol–water mixture. Murtaza Hasan et al used Fagonia Cretica 50 % fractionation to achieve enhanced anti-oxidant, anti-tyrosinase, and antiurease. Whereas the plant extract's 90 % and 70 % ethanol fractions have greater anti-tyrosinase and anti-urease activities. Plant extract fractions with 50 % ethanol show potential for anti-oxidant and anti-urease properties. When diluted and doubled, the 30 % ethanol fraction of the plant extract gets stronger and exhibits anti-oxidant and antiurease activity. Only when employed in high concentrations do the 50 % and 30 % ethanol fractions of the plant extract exhibit anti-tyrosinase activity. Therefore, among all fractions of plant extract, the 50 % ethanoic fraction contains more active chemicals that are involved in a variety of pharmacological activities (Hasan, 2019). This process is eco-friendly, simple, has no sophisticated equipment, and is non-toxic without any external capping, reducing, or stabilizing agent (Nadaroğlu et al., 2017). Plants have free natural reducing, stabilizing, and capping activity in short it is a method of natural ingredients with modern technology. Moreover, the product formed by using plant extract is more stable with optimized shape and size (Shumin Yang and Yanqing Wu, 2013). The phytonutrients which are present in plant extract contain proteins, amino acids, terpenoids, flavonoids, and polyphenols are the basic metabolites for natural reducing properties(Silva, Pereira and Bonatto, 2019). Krishnaraj et al.,(Krishnaraj et al., 2010) reveal the secret of Acalypha Indica plant nutrients, they reported quercetin is responsible for the Ag NPs formation and will act as a natural reducing agent in the bio-reduction process. Similarly, Ahmad et at.,(Ahmad et al., 2011) synthesized Ag NPs by using Desmodium triflorum here ascorbic acid is the plant metabolite that serves as a natural reducing agent.
plant
Size (nm)
Shape
reference
Salvadora persica
37
spherical
(Arshad et al., 2021)
Parthenium hysterophorus
10
spherical
(Sivakumar et al., 2021)
Populus ciliata
4
Spherical
(Hafeez et al., 2021)
Rosa canina
150
spherical
(Ozlem Saygi and Usta, 2021)
Cuminum cyminum L.
50
spherical
(Chamkouri et al., 2021)
Cleome viscosa
20–50
spherical
(Lakshmanan et al., 2018)
Ficus hispida Linn. f.
20
spherical
(Ramesh et al., 2018)
Acacia cyanophylla
88
spherical
(Jalab et al., 2021)
A. niloticaWilld twig bark
10–50
circular
(Karuppiah et al., 2014)
Azadirachta indica leaves
5
spherical
(Gnana Kumar et al., 2014)
Callicarpa Mainga yi leaf
15
sheet
(Chekin et al., 2014)
Citrus sinensis var. Kozan yerly fruit
4–10
spherical
(Khan et al., 2015)
Plectranthus amboinicus leaf
22
spherical
(Zheng et al., 2016)
Mimosa Pudica root
35–42
spherical
(Sreenivasulu et al., 2016)
Convolvulus pluricaulis leaf
10–11
spherical
(Sandeep et al., 2016)
Piper betel leaves
48
spherical
(Ramachandran et al., 2016)
Onion extract
10
Spherical
(Khalilzadeh and Borzoo, 2016)
Ocimum tenuiflorum leaves
20–25
Spherical
(Dayakar et al., 2018)
Lithodora Hispidula leaves
15
spherical
(Turunc et al., 2017)
Salvia leriifolia leaves
27
spherical
(Baghayeri et al., 2018)
Camellia japonica leaves
12–25
spherical
(Karthik et al., 2017)
Oakleaf
20–25
spherical
(Hemmati et al., 2018)
Allium sativum cloves
19
spherical
(Aravind et al., 2018a)
2.11 Characterization of NPs
Instrumentation of NPs is an important part of the material science domain, various analytical instrumentation will be carried out to confirm the production of NPs. Some basic analytical techniques like spectroscopy and microscopy are very fruitful to study scientifically and from the SEM, XRD, and HRTEM we can confirm the geometry of fabricated NPs with size. The techniques like UV–vis, FT-IR, Zeta potential, VSM, BET, Raman, Fluorescence, photo-luminescence, AFM, EDX, etc., were used to study the nature of synthesized NPs.
2.12 Morphology control of the bio-assisted synthesis of NPs
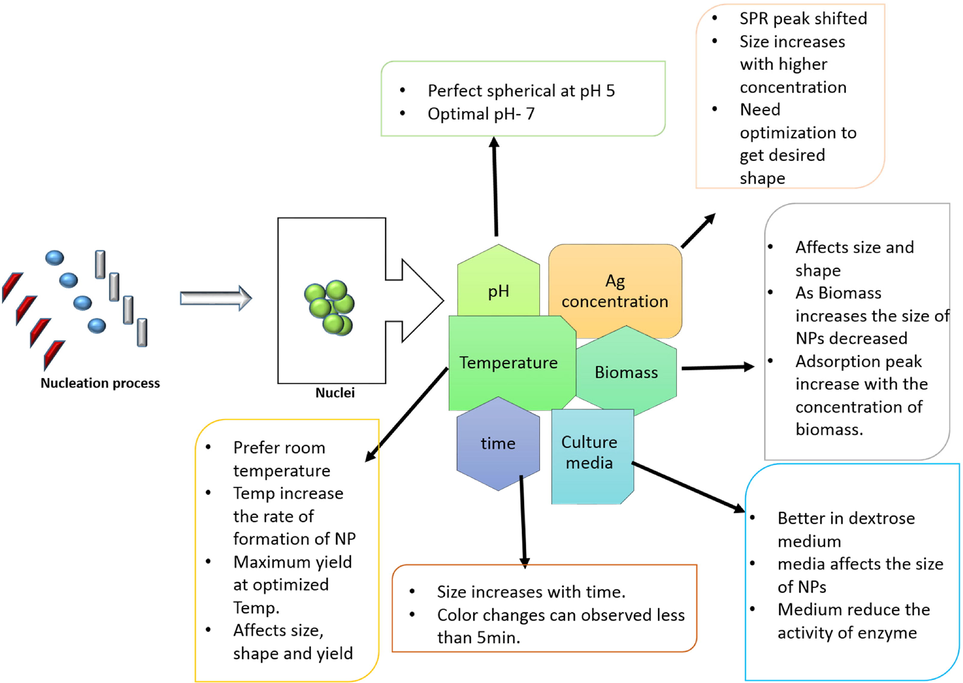
Similar to chemical synthesis, parameters such as temperature, pH, solvent, nature/composition of the natural source present in the extract/bio-source, concentration of the biosource, reaction time/incubation period, gas atmosphere, stirring speed, calcination temperature, concentration/chemistry of the precursor, capping, reducing, and stabilizing agent, allows for fine-tuning of the size and shape of the biosynthesized nanoparticles(Fig. 2b). Due to the dense components in the bio-source, it is challenging to control the shape of the biosynthesized NP. The ratio between the concentration of the precursor and the capping agent typically controls the size and formation of nanoparticles. When the ratio is very high or low, the likelihood of aggregation increases, which can result in polydisperse or irregularly shaped particles. Biosynthesis of Ag NPs can also vary based on optimization including temperature, pH, time of reaction, agitation, metal salts, and plant concentration. With the help of optimized parameters, we can attain the desired shape and size of NPs. Since optimization is one of the important factors to get proper monodispersity, stability, and bio-compatibility of fabricated NPs (Balakumaran et al., 2015). In 2013 Hasan et al. attempted to synthesize AgNPs using dragon’s blood (Dracaena Cochinchinensis, Lour. S.C. Chen) resin extract at various times and temperatures namely 4 h, 2 h, and 30 min of reaction at 30 °C, 60 °C and 90 °C and they achieved the size of nanoparticles varying from 10 nm to 30 nm(Hasan et al., 2013). The same group in 2015 reported the synthesis of AgNPs using dragon's blood with 1 mM of AgNO3 and the main outcome of the results in the synthesis of round shaped AgNPs ranging from 5 to 50 nm in short time and also they revealed the main active ingredients which is present in the dargon’s blood biosource like pterostilbene, cinnabarone, Loureirin A, and Loureirin, etc.,(Hasan et al., 2015). Hence the optimization plays a vital in the synthesis of nanoparticles.
2.12.1 The consequence of reaction time
The effect of time influences the quality of the NPs and also the structure and active properties can change(Darroudi et al., 2011). The duration of reaction time is incubated considerably affects the size, shape, and extent of nanoparticle formation. According to Nazeruddin et al. (Nazeruddin et al., 2014), Coriandrum sativum seed extract produces Ag nanoparticles rapidly, taking only 1 to 2 h as opposed to the 2 to 4 days needed by microorganisms. Similar to this, Noruzi et al. (Noruzi et al., 2011) observed that the Rosa hybrid petal-mediated Au nanoparticle manufacturing reaction was quick and finished in under 5 min. Although compared to microorganisms, plant-mediated biosynthesis of nanoparticles is often faster, some scientists have found that the production rate is still efficient at a fast reaction time (Darroudi et al., 2011). Within 15 min of the reaction beginning, Ag and Au nanoparticle production was detected by Dwivedi and Gopal (Dwivedi and Gopal, 2010a). Also, they discovered that an increase in contact time had a significant impact on how sharp the peaks in both Ag and Au nanoparticles were. A few investigations also suggested that the nanoparticles' size and shape change depending on how long it takes to react, Li et al. (Rousta and Ghasemi, 2019) found that five hours of reaction time resulted in spherical and polycrystalline-shaped nanoparticles (102 nm) when Ag nanoparticles were synthesized using Capsicum annuum L. extract. The nanoparticles' sizes rose with an increase in reaction time to 9 h and 13 h, respectively, to 25 nm and 40 nm. NPs can experience size effects if the incubation time is increased beyond 10 min. (Velgosova et al., 2017).
2.12.2 The consequence of bio-mass
The amount of bio-mass plays a vital role to form desired size and geometry of the bio-fabricated NPs. Researchers also tried various concentrations of bio-mass to optimize the size and geometry of NPs like 0.5, 1, 2.8, and 4.8 mL of Tanacetum vulgare extract to fabricate Ag NPs(Dubey, Lahtinen and Sillanpää, 2010). They reported that the concentration of bio-mass increased with a decrease in the size of the synthesized Nps(Dwivedi and Gopal, 2010b), and the absorption peak of synthesized NPs increased with an increase in the concentration of bio-mass due to the presence of the dense molecule present in the biomass (Korbekandi et al., 2013). It can be stated that an appropriate balance between the amount of organic material, obtained from the fungus, and the amount of metal precursor is required for efficient nanoparticle synthesis, despite variances in the amount of biomass used depending on the species of fungus used(Shahzad et al., 2019).
2.12.3 The consequence of Ag-salt quantity
This factor is one of the important factors to make the synthesis route economical in the biotransformation process. This effect will optimize the level of agglomeration and the surface plasmon resonance(SPR). The SPR peak often shifts towards the higher wavelength area and gets narrower as concentration values rise. We conclude that increasing the concentration of the solution causes the formation of nanoparticles with smaller sizes because, assuming that the plasmon resonance peak shift indicates a change in the size of AgNPs, any shift of the peak towards the higher wavelength is followed by a decrease in the size of the prepared AgNPs. On the other hand, the expansion of the surface plasmon resonance peak suggests that the solution contains a wider range of sizes(Bar et al., 2009). Dubey et al (Dubey, Lahtinen and Sillanpää, 2010)try to optimize the concentration of Ag salt varies from 1 to 3 mM and they observed the size of the NPs increases with increasing the concentration of Ag salt. Similarly, the other group use the Chenopodium album to optimize Ag salt, they also reported that the size of NPs increases with increasing the concentration of Ag salt(Dwivedi and Gopal, 2010b). However the gradual increasing the concentration of Ag salt and the size of NPs increases up to 10 mM, after 10 mM it starts to decrease the production. Hence the optimal concentration of Ag salt is 5 mM (Korbekandi et al., 2013).
2.12.4 The consequence of pH
In the production of nanoparticles by bio-source, pH is crucial. Researchers have found that pH affects the size, shape, and production rate of synthesized NPs (Armendariz et al., 2004). The creation of nucleation centres rises with an increase in pH. The reduction of metallic ions to metal nanoparticles increases along with the nucleation centre. Additionally, the solution pH affects the rate of reduction of a metal salt as well as the activity of the functional groups in the plant extract or bio-source(Bali and Harris, 2010). Furthermore, Jacob et al.(Jacob, Mukherjee and Kapoor, 2012) investigated the Au and Ag ion reduction rates at pH 3.3 and 10.8. According to their research, Ag ions reduced at pH 3.3 more slowly than Au ions, and the resultant nanoparticles precipitated in just two days. Nevertheless, both metal ions underwent simultaneous decreases at pH 10.8, and a single Surface plasma resonance band was seen. The homogeneous electron density visible in the TEM image suggests that alloyed nanoparticles were created. Additionally, they claimed that a high pH value helped create the molecules AgO- and AgO, which bind strongly with the hydroxyl groups in plant extracts and efficiently cover the surface of nanoparticles. pH also depends on the reaction medium. Literature survey shows that the lower the pH favoured increasing the size of NPs (Gericke and Pinches, 2006). Spherical shapes of Ag NPs can get at pH 5 by using Cinnamon zeylanicum (Sathishkumar et al., 2009). Researchers show that the optimal pH value is 7 and also the maximum yield can get at pH 7.
2.12.5 The consequence of culture medium
In the bio-synthesis of Ag NPs by using fungi, the culture medium plays an important role and its different responses are based on the culture medium. Higher concentrations and smaller sizes of the nanoparticles were present in the nanoparticle dispersions made using the filtrate from the fungus growing in the enzyme induction medium. This was explained by the nitrogen source's stimulation of enzymatic activity in the modified medium, which increased nanoparticle production. In trials where various mediums for the growing of fungi mediuwere investigated, various behaviours were seen (Costa Silva et al., 2017). Ashrafi et al. discovered that the same medium prevented the formation of silver nanoparticles using Rhizoctonia solani filtrate, but the synthesis was effective when the fungus was grown in a potato dextrose medium. It was postulated that a component of the medium may have reduced the activity of the enzyme responsible for the reduction process (Ashrafi et al., 2013). Conversely, Birla et al. tried with different media nearly 10 to optimize the better yield of Ag NPs (Birla et al., 2013). It was proposed that a component of the medium may have impeded the activity of the enzyme in charge of the reduction process.
2.12.6 The consequence of temperature
Another significant element that affects the size, shape, and rate of nanoparticles is temperature. Similar to pH, temperature increases cause more nucleation centres to develop, which in turn speeds up the biosynthesis process and gets the maximum yield of product. Most of the optimal temperature was room temperature (25 °C) with this temperature can get maximum yield (Zhang et al., 2013). To find the ideal temperature, Sheny et al.(Sheny, Mathew and Philip, 2011) studied the reduction processes of Au and Ag ions by Anacardium occidentale leaf extract at various values of time and temperature. The authors discovered that producing stable nanoparticles at low reaction temperatures required more leaf extract than when doing so at high temperatures. More specifically, 0.6 mL of leaf extract was all that was needed for biosynthesis to occur at 100 °C, whereas 2.5 mL of leaf extract was needed for synthesis to occur at 27 °C. The authors also noted that high-temperature nanoparticles were not only more stable but also greater in size. Iravani and Zolfaghari (Iravani and Zolfaghari, 2013) heated the reaction mixture to various temperatures to study the impact of temperature and the authors state that with increasing temperature whereas the size of Ag NPs decreases with increasing absorbance. Similar results are also get by using Annona squamosal peel to get a higher yield at higher temperatures (Kumar et al., 2012). Furthermore, temperature affects the absorption peak, at low temperatures the band was sharp whereas at the higher temperature the band became broad (Shahzad et al., 2019). Meanwhile, Raju et al., (Raju, Mehta, and Hazra, 2011) get the perfect geometry of spherical at a higher temperature. The ability of some fungal species to transfer electrons from free amino acids to silver ions at high temperatures is demonstrated. However, the proteins that make up the nanoparticle capping get denaturated at extremely high temperatures, between 80 °C and 100 °C. Ag + ion nucleation is altered by this denaturation, causing the nanoparticles to aggregate and grow in size reported by Birla et al., [7]. Due to the low activity of the synthesis-related enzymes, Husseiny et al. (Husseiny et al., 2015) claim that suboptimal temperatures cause increased nanoparticle size and loss of stability.
In short, the varying factor of synthesis can vary the characteristics of NPs. however, the consequences are still unclear and need further pieces of information for each organism employed. It is important to reveal the optimized physicochemical characteristics of NPs, in the flow to find the parameters used in the fabrication like pH, temperature, the concentration of Ag-salt, and bio-mass. The optimization only can enable achieving rapid large-scale NPs fabrication.
2.13 Plant-assisted synthesis of Ag NPs as sensors
The Ag NPs can be employed in various fields (Fig. 3) listed such as food and agriculture, textiles, electronics, health care, biomedical, environmental, etc. In sensors, there are different types of sensors like SERS, Colorimetric, fluorescence, and electrochemical sensors. Electrochemical sensors are one important technique to analyze quantitatively and qualitatively any target material with good selectivity and sensitivity. The electrochemical sensors are portable and have good sensitivity with better selectivity with economical. It does not require any prolonged procedure, skilled power, pre-treatment for the mediator, etc., the various techniques in electrochemical like amperometry, cyclic-voltammetry, impedimetric, linear sweep voltammetry, potentiometric, etc., can be used to detect the specific target analyte. Table 5 reveals the elaborated bio-assisted Ag Nps utilized for electrochemical detection of the targeted analyte. To create an electrochemical sensor, a working electrode is either glassy carbon or screen printed. Reference electrodes (Ag/AgCl) must be used to maintain a stable potential, and counter electrodes (Au/Pt) will be used to connect to the electrolyte and provide current to the working electrode [70]. CDA: Cellulose Diacetate; GCE: Glassy Carbon electrode; Gr: Graphite rod; CPE-Carbon paste electrode; NS: Nanosphere; Hb: Hemoglobin; SPCNFE: Screen printed carbon nanofibre electode; CS: chitosan; PPy: Pyrrole electropolymerized; PPO: Poly phenol oxidase; Pt: Platinum electrode; GO: Graphene oxide; SPE-Screen printed electrode. MIHP- Mihp n-hexyl-3-methylimidazolium hexafluoro phosphate; PO: Paraflim oil; PGE: Pencil graphite electrode; GnP: Graphite nano platelets; G: graphene.
Applications of biosynthesized Ag NPs.
Bio-source
Size(nm)
Shape
Colour change
Modified electrode
Analyte
Linear range
LOD
Technique
Reference
V.planifolia
9–25
shell
Yellow to brown
CDA/Au-Ag/GCE
Vanillin
0.2–50 µM
40 nM
Amp-It
(Zheng et al., 2010)
Bacillus subtilis
50–100
rod
Yellow to brownish
Bio-Ag NPs/GCE
H2O2
0.05 to
120 nM mmol L − 18 µmol/L
Amp-It
(Liu et al., 2013)
A. niloticaWilld twig bark
10–50
circular
Yellow to brown
Ag-NPs/GCE
4-nitro phenol
100 nM to
350 μM15 nM
DPV
(Karuppiah et al., 2014)
Azadirachta indica leaves
5
spherical
–
Ag–Au–rGO/GCE
H2O2
100–5000 μM
1 μM
Amp-It
(Gnana Kumar et al., 2014)
Callicarpa
Mainga yi leaf15
sheet
Yellowish green to dark brown
AgNPs-GO/GCE
H2O2
5 μM
to 700 μM0.6 μM
Amp-It
(Chekin et al., 2014)
Citrus sinensis var. Kozan yerly fruit
4–10
spherical
Yellow to dark black
Ag NPs/GCE
Catechol
–
–
CV
(Khan et al., 2015)
Plectranthus amboinicus
Leaf22
spherical
Brown yellow to black
RGO-Ag-GCE
H2O2
1 and 800 μM
0.312 μM
Amp-It
(Zheng et al., 2016)
Mimosa Pudica root
35–42spherical
Pale yellow to dark brown
AgNPs/GCE
Dopamine
10–60
μM0.5 μM
DPV
(Sreenivasulu et al., 2016)
Convolvulus pluricaulis leaf
10–11
spherical
Pale yellow to dark brown
Gr-AgNPS/GCE
–
–
–
CV
(Sandeep et al., 2016)
Piper betel leaves
48
spherical
Colourless to olive
Ag/GCE
NO2–
1–6000 μM
0.046 μM
Amp-It
(Ramachandran et al., 2016)
Onion extract
10
Spherical
Watery to brown
AG/CPE/GCE
Ascorbic acid
0.4–450.0 μM
0.1 mM
SWV
(Khalilzadeh and Borzoo, 2016)
Ocimum tenuiflorum leaves
20–25
Spherical
Pale yellow to dark brown
Ag/GCE
glucose
1
to 8.9 mM0.0048
Amp-It
(Dayakar et al., 2018)
Streptomyces sp. BHUMBU-80
11–38
spherical
Light brown to dark brown
AgNPs/GCE
H2O2
50 μM to 1000 μM
50 μM
CV
(Gupta et al., 2017)
Nilgiri wood
30
spherical
Light yellow to reddish amber
Ag-NS/GCE
Nitrite
0.1–8 μM
0.031 μM
Amp-It
(Shivakumar et al., 2017)
Instant dry yeast
–
Core-shell
–
CDA/Au–AgNPs modified GCE
paracetamol
0.01–0.1
mM2.6 μM
Amp-It
(Wei, 2017)
Lithodora
Hispidula leaves15
spherical
Yellowish brown to dark brown
AgPdNPs-GCE
H2O2
20 mM − 5.00 mM
0.52 μM
Amp-It
(Turunc et al., 2017)
Salvia leriifolia leaves
27
spherical
Light yellow to brown
AgNPs/GCE
Nitrite
1 to 3.75 μM
–
CV
(Baghayeri et al., 2018)
Camellia japonica leaves
12–25
spherical
White into yellow
AgNPs/GCE
nitrobenzene
0.05 to 3637 μM
0.012 μM
Amp-It
(Karthik et al., 2017)
Oak leaf
20–25
spherical
Yellow to yellowish red
Hb-AG/GCE
H2O2
–
–
CV
(Hemmati et al., 2018)
Allium sativum cloves
19
spherical
Yellow to brown
AgNP-Pt
Cd
10–90 μM.
0.277 μM
DPV
(Aravind et al., 2018a)
Grape stalk water
27
spherical
Pale yellow to reddish brown
Ag-NPs-SPCNFE
Pb and Cd
3.3–100.4 and 16.0–39.7 μg L-1
4 and 4.8 μg L-1
DPV
(Bastos-Arrieta et al., 2018)
Lycopersicon esculentum
10
spherical
Light yellow to brown
AgNP-Pt
Cr
10 to 90 μM
0.804 μM
DPV
(Aravind et al., 2018b)
mushroom
10
spherical
–
Pd-Ag/CPE
Uric acid
4.6– 273 nM
5.54 nM
DPV
(Mallikarjuna et al., 2018)
Rosa damascena waste
30
irregular
Light brown
AgNPs/CS/GE.
H2O2 and
vanillin–
8.4 μM
Amp-It & DPV
(Dodevska et al., 2019)
Convolvulus pluricaulis leaf
–
–
Pale yellow to dark brown
Gr/PPy/AgNPs/PPO
catechol
0.001 – 0.015 mM
0.47 μM
Amp-It
(Sandeep et al., 2019)
Mangifera indica leaves
–
spherical
Colorless to yellowish brown
Pt/AgNP
Ascorbic acid
–
–
CV
(A. Mamuru et al., 2019)
Achillea millefolium wastes
2.8
spherical
Yellow to brown
AgNPs–CS/Gr
H2O2
–
–
Amp-It
(Lazarova et al., 2019)
Eucalyptus bark
18
spherical
Yellow to brown
AgNPS/GCE
Nitro benzene
5–40 μM
0.027 μM
DPV
(Shivakumar et al., 2020)
Moringa oleifera Extract
18–23
spherical
Yellow to brownish yellow
AgNPs/PE
Cu
10–90 μM
0.530 μM.
CV and DPV
(Sebastian, Aravind and Mathew, 2019)
Pistia stratiotes L. leaves
30
spherical
Yellow to deep brown
AgNPs/GO/SPE
Na
0–100 mM
9.344 mM
CV
(Traiwatcharanon et al., 2020)
Acacia Melanoxylon leaves
10
cubic
Pale yellow to brown
Ag NP/CPE
Dopamine and H2O2
50 to
500 μM and
8 to 28 μM–
CV
(Shashanka and Kumara Swamy, 2020)
Peganum Harmala Extract
10
spherical
–
Ag-NP/PO/MIHP/CPE
Quercetin
0.01–550
μM
0.005
μMAmp-It
(Davarnia et al., 2020)
Tagetes erecta (Marigold) flowers
20–50
spherical
Red to orange
PGE/AgNPs/CS
H2O2
1 μM
−10 μM0.52 μM
CV
(Salve et al., 2020)
Opuntia-ficus-indica stem
10–20
spherical
Colorless to brown
AgNPs/GCE
glucose
0.01–2.2 mM
0.01 mM
SWV
(Khalifa et al., 2020)
Prangos ferulacea roots
79
spherical
Pale yellow to red
AgNPs/GCE
H2O2
0.1 μM − 4 mM
0.036 μM
DPV
(Mavaei et al., 2020)
Rumex roseus
50
round
Pale to yellow
AgNPs-rGO/GCE
H2O2
35 μM to 1.95 mM
1.1μ M
Amp-It
(Chelly et al., 2021)
Fusarium oxysporum
6
spherical
Black to brown
Ag/Ag2O NPs/GCE
glucose
–
–
CV
(Islam et al., 2021)
Araucaria angustifolia
100
Spherical
Brownish yellow
AgNP-xGnP/GCE
paracetamol
4.98 × 10-6to 3.38 × 10-5 M
8.50 × 10-8molL − 1
SWV
(Zamarchi and Vieira, 2021)
Mimosa diplotricha leaf
33
spherical
Yellowish brown
AgNPs/GCE
Hg
5–45 μM)
1.46 μM.
DPV
(Punnoose et al., 2021)
Lycoris longituba
24
Spherical
–
G/Ag NPs/GCE
imatibin
10 nM-
280 μM1.1 nM
DPV
(Wu et al., 2021)
Cupressus sempervirens L.
11
Spherical
Yellow to dark brown
AgNPs-GCE
H2O2
5.0 μM − 2.5 mM
0.23 μM.
SWV Amp-It and DPV
(Ersan Turunc, 2021)(Turunc, 2021)
2.14 Bio-assisted synthesis of Ag NPs for electrochemical sensor
Huge effects have been required to build a sustainable sensor with high sensitivity and selectivity. To overcome this dilemma the utilization of bio-synthesized nanomaterials is one of the promising tools to build biocompatibility, the low-cost sensor which is prepared from natural sources. In this context, the main purpose of biosynthesized NPs is to develop highly sensitive, non-toxic sustainable sensors. The electrochemical sensor has a sophisticated facility and plays a vital role in the food industry, medical, pharmaceutical, etc., due to its inexpensive, rapid, simple procedure with good sensitivity and selectivity, and real-time analysis. This method is one of the promising alternative analyses over spectroscopy and chromatography with various techniques like cyclic voltammetry (CV), amperometry (Amp), differential pulse voltammetry (DPV), square wave voltammetry (SWV), and linear sweep voltammetry (LSV).
An electrochemical sensor design, requires a working electrode either glassy carbon or screen printed electrode it can transduction element for chemical reaction, a reference electrode (Ag/AgCl) for stable potential, counter electrode (Au/Pt) to form a link with electrolyte to give current to the working electrode (Grieshaber et al., 2008).
Biosynthesized AgNPs for H2O2 reduction: Generally, NPs have good conducting and catalytic properties due to their large surface area, the huge number of research groups were involved in the NPs employed electrochemical sensors (Table 5), especially the H2O2 sensor·H2O2 and its derivatives are highly oxidizing agent it cause several issues and is widely used in various domain like industry, food product, clinical, etc., it causes severe environmental issue. Turunc et al., (Turunc, Kahraman, and Binzet, 2021) use pollen extract for the detection of H2O2 by using amperometry and the square wave voltammetry method (Fig. 4). Pollen grains are collected from C. Sempervirens and treated with 1 mM AgNO3 to form Ag NPs at room temperature. The experiment was carried out with a pre-treated glassy carbon electrode that was drop cast by 8 µL of synthesized Ag NPs and 5 µL of Nafion in 0.1 M PBS (pH 7). They show a linear range between 5 µM and 2.5 mM with a limit of detection of 0.23 µM in the detection of H2O2 and the interference was also carried out to find better selectivity. Similarly Mavaei et al (Mavaei et al., 2020) attempt to sensor H2O2 by using Prangos ferulacea roots extract. The isoimperatorin solution was isolated from Prangos ferulacea roots and it was treated with 1 mM AgNO3 to synthesize spherical shape Ag NPs. The GCE was constructed by drop-casting of 5 µL of synthesized Ag NPs and the prepared electrode was characterized in 0.1 M PBS (pH 7) to catalyze H2O2 with a linear range of 0.1 μM − 4 mM and LOD was calculated and found to be 0.036 μM. In another work, Tagetes eracta (Salve et al., 2020) was used to synthesize Ag NPs with a 0.5 mM solution of Silver nitrate salt to catalyze H2O2 and supercapacitor application. They construct Pencil graphite electrode /AgNPs/Chitosan in HCl-KCl solution (pH 2). By adopting cyclic-voltammetry the H2O2 was catalyzed from the lower concentration of 1 μM to a higher concentration of 10 μM with 0.52 μM of the detection limit.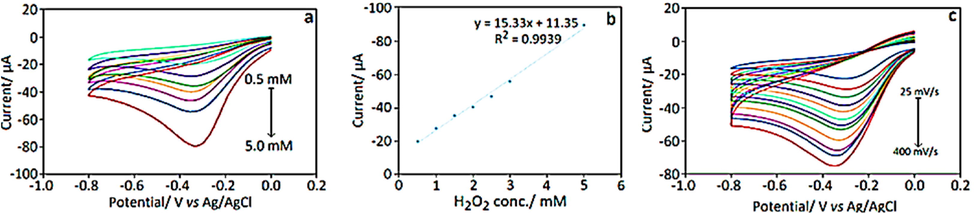
(a) Cyclic voltammograms for various concentrations of H2O2 at AgNPs-GCE in 0.1 M phosphate buffer solution with pH = 7.0 (from top to down 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 and 5.0 mM, respectively), scan rate 50 mV/s, (b) variation of peak current versus concentration, (c) cyclic voltammograms of AgPdNPs-GCE in 0.1 M PBS containing 1.0 mM H2O2 at variable scan rates (from top to down 25, 50, 75, 125, 100, 150, 175, 200, 250, 300, 350 and 400 mV/s, respectively (Ersan Turunc, 2021).
Biosynthesized AgNPs to detect toxic metals and drugs: Imatinib is a cancer drug to treat chronic myeloid leukamia however, it leads to a decrease in the plasma concentrations in inpatients. Hence it is mandatory to monitor the imatinib level in the body. By using lycoris longituba leaf extract, the Ag NPs were synthesized to detect imatinib (Wu et al., 2021) they chose graphene-based modified electrode to sense imatinib by adopting DPV technique with various concentration ranges from 10 nM − 280 μM and the LOD is 1.1 nM. They attempt to show better selectivity using real sample analysis and interfere effect with various analytes like sodium, potassium, and copper but imatinib only shows 10 times higher current value than the rest of all. In the list of toxic metal ions, mercury is one of the toxic heavy metals which cause drastic effects on the environment. Mimosa diplotricha assisted Ag NPs were synthesized for the electrochemical catalyze of mercuric ions (Punnoose et al., 2021) the analysis was carried out in a pH 6 acetate buffer solution. They chose the DPV technique to sense mercuric ions at pulse amplitude of 25 mV and 200 ms pulse width. The Mimosa diplotricha employed Ag NPs modified pt electrode was used to sense mercury ion with the linear range of 5–45 μM and shows excellent selectivity over Ni2+, Cu2+, Mn2+, Co2+, Cd2+, Zn2+, Pb2+.
Araucaria angustifolia mediated Ag NPs was formed for the detection of paracetamol (Zamarchi and Vieira, 2021). Further, Graphite nanoplatelet electrodes were employed to form AgNP-xGnP/GCE as shown in Fig. 5. The modified electrode can detect up to 3.38 × 10-5 M paracetamol in PBS at pH7 conditions and it shows detection of limit (8.50 × 10-8molL−1). The modified electrode was employed to investigate the real sample of the commercially available pharmaceutical product.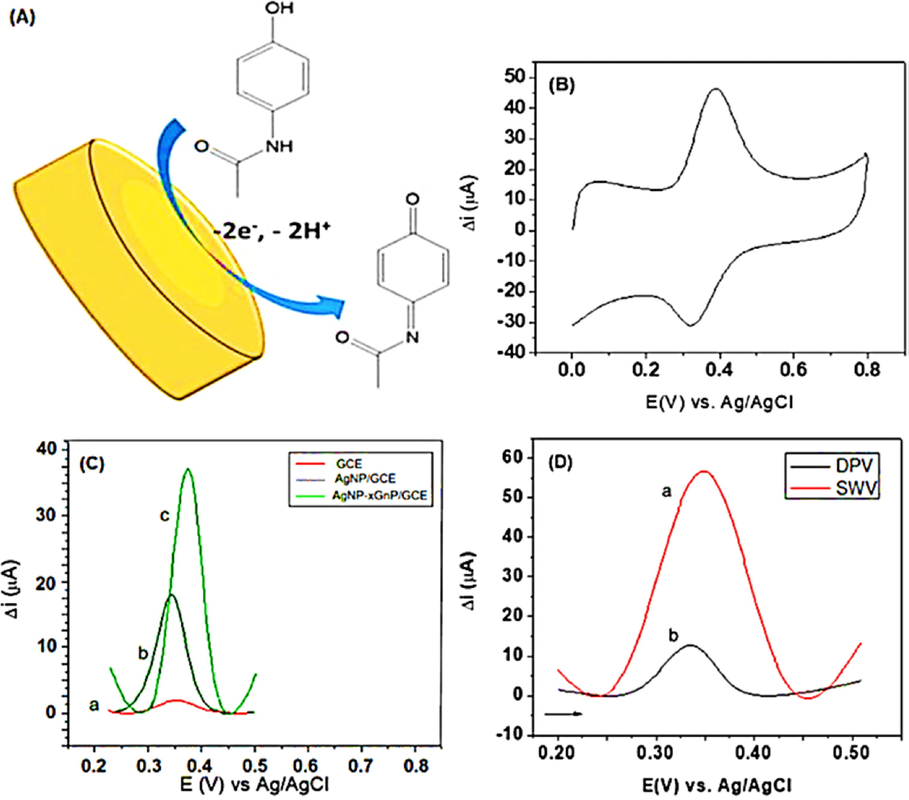
(A) Schematic representation of the reaction involving paracetamol on the surface of the sensor; (B) cyclic voltammogram at 100 mV s−1, (C) SWV using the (a) bareGCE, (b) AgNP/GCE, (c) AgNP-xGnP/GCE and (D) voltammograms obtained applying the electroanalytical techniques: (a) SWV and (b) DPV in PBS solution (0.1 mol L − 1, pH7.4) containing 1.48 × 10-5 mol L − 1paracetamol (Felipe Zamarchi, 2021).
Biosynthesized AgNPs for biosensor: In the other investigation 6 nM-sized round spherical-shaped Ag Nps were fabricated by using the endophytic fungus Fusarium oxysporum. Ag/Ag2O NPs/GCE electrode was designed by bio-transformation of silver oxide micro powder into Ag/Ag2O nanoparticles. The modified electrode was constructed by the process of 50 µL of micron Ag2O and Ag/Ag2O nanoparticles on GCE and 1 M KOH as an electrolyte to catalyze non-enzymatic glucose. They tried various concentrations of glucose ranging from 25 µmol/L to 125 µmol/L at 50 mVs−1. The lack of the purposed work is they do not provide an exact linear range of detection and do not show the LOD value (Islam et al., 2021). The Ag NPs with reduced graphene oxide were immobilized on a glassy carbon electrode. Amperometric techniques were involved in the experiment to detect H2O2, with concentrations ranging from 35 μM to 1.95 mM in pH 7 conditions in presence of 0.1 M PBS electrolyte. They also performed an interference effect to show better selectivity and stability of the modified electrode containing 800 μM H2O2 at 50 mVS-1 checked along with this the relative standard deviation value was found to be 8.2 % (Chelly et al., 2021). In 2020 Bahareh et al., attempted to fabricate Ag NPs from peganum harmala to make Ag-NP/Paraffin oil(PO)/n-hexyl-3-methylimidazolium hexafluoro phosphate(MIHP)/Carbon paste electrode(CPE), which demonstrated excellent performance for the electrocatalytic determination of quercetin with a detection limit of 5.0 nM. Finally, the Ag-NP/PO/MIHP/CPE method was successful in determining quercetin in a variety of dietary samples, including hawthorn and onion (Davarnia et al., 2020). Sreenivasulu et al., (Sreenivasulu et al., 2016) developed an electrochemical sensor for dopamine (DA) based on biosynthesized AgNPs. Dopamine (4-(2-aminoethyl)benzene-1,2-diol) is a neurotransmitter that influences a range of biological processes and has a wide range of functions in the brain. Parkinson's disease, depression, schizophrenia, and certain brain cancers all include dopamine as a biomarker. Because of its electroactive nature, reliable detection of dopamine is vital in research and clinical illness diagnosis, and many types of electrochemical sensors have been developed. For the production of AgNPs, Sreenivasulu et al. employed an aqueous root extract of Mimosa pudica. The UV–Vis spectrophotometer was used to identify AgNP form, and XRD, FTIR, SEM, EDAX, and TEM were used to completely describe AgNPs. The produced AgNPs had a spherical form with average diameters of 35.0 to 42.5 nm, according to the TEM examination. For quantitative detection of DA, amperometric tests demonstrated that the Synthesized nanoparticles electrode has a high sensitivity, a low limit of detection (0.5 µM), and an outstanding dynamic range (10–60 µM). These results, according to the authors, are equivalent to those previously reported in the literature utilizing chemically modified GC electrodes.
Biosynthesized AgNPs to detect toxic chemicals: Aniline, pesticides, herbicides, insecticides, azo colors, explosives, and pharmaceuticals all employ nitrobenzene (NB) as a precursor. Humans' blood, central nervous system, liver, and kidneys are affected by acute (short-term) and chronic (long-term) inhalation, oral, and cutaneous exposure to NB. Long-term exposure can result in headaches, nausea, tiredness, dizziness, cyanosis, and anemia. Unfortunately, companies released a large amount of NB into water, soil, and sediments. As a result, fast and precise detection of NB is critical for public and environmental safety. For the detection of NB, many analytical approaches have been utilized, with electrochemical methods being deemed easier and more sensitive than chromatographic and spectrophotometric methods. Using Eucalyptus extract (Shivakumar et al., 2020) as a reducing and stabilizing agent, Shivakumar et al. reported an environmentally friendly AgNP synthesis. The synthesized nanoparticles were used to modify a GC electrode, and AgNPs/GC was used to test for quantitative detection of NB. Fig. 7. Shows the electrochemical behaviour of AgNPs/GC was investigated using two electrochemical techniques: CV and DPV. The modified electrode had high electrocatalytic activity in the NB electroreduction reaction, with a linear current response in the concentration range of 5 to 40 M, a sensitivity of 2.262 AM−1 cm−2, a detection limit of 0.027 M, and good selectivity. The CV of the modified electrode was conducted in the presence of 1 mM NB on the day of preparation and every other day for the next 20 days to test stability. According to the scientists, the sensor had excellent storage stability, maintaining up to 92.8 percent of the initial current after the test time. The created electrode material's practical usefulness in detecting NB selectively in tap water and lake water was tested, and good results were achieved. Karthik et al. (Karthik et al., 2017)modified a GC electrode with sphere-like AgNPs biosynthesized using Camellia japonica leaves to increase selectivity, sensitivity, and detection limit for determining NB. The constructed electrocatalyst AgNPs/GC has been proven to have high selectivity, a low limit of detection (0.012 M), and a wide linear range for detecting NB (up to 2.593 mM). The AgNPs/GC showed excellent selectivity for NB detection – the results of the selectivity test showed that adding potentially interfering species (common metal ions, some anions, and nitroaromatic containing substances) into the system at a 500-fold concentration relative to the analyte did not affect the electrode signal. This is interesting since, due to their structural similarity, other nitroaromatic chemicals normally have a large impact on the peak current response of NB. In polluted wastewater, the catalyst's practical application for the selective quantitative determination of NB in actual samples was successfully evaluated. The sensor gadget is easy to use, inexpensive, and portable, and it may be used in a variety of industrial and research applications.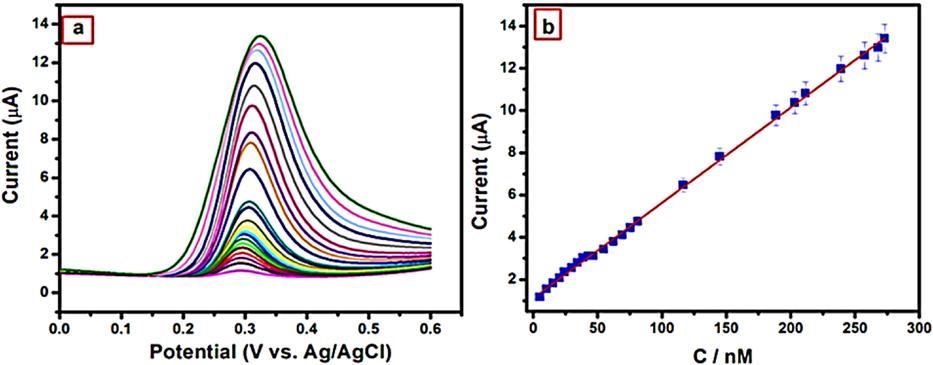
Mechanism for the electrochemical oxidation of UA, (a). DPV obtained for Pd-Ag/CPE due to the addition of 4.69–273 nM UA into 0.1 M PBS (pH 7.0) and (b) Calibration plot of Ipa vs concentration of UA (Malllikarjuna, 2018),
![(A) CVs of 300 μM nitrobenzene at green synthesized Ag-NPs modified GCE in 0.05 M PBS pH ranges from 3 to 11 (Right to left side). (B) Calibration plot of nitrobenzene reduction peak current (Ipc) vs different pH (C) Reduction peak potential (Ep) vs pH, (D) Amperometric (i–t) response at green synthesized Ag-NPs modified RDE upon successive additions of 0.05–3693 μM nitrobenzene into continuously stirred N2 saturated PBS. Applied potential: −0.42 V; Rotation speed: 1200 rpm. Inset (upper) is the plot of response current vs [nitrobenzene]. Inset (bottom) the plot of response current vs [nitrobenzene]. (E) Amperometric (i-t) response at green synthesized Ag-NPs modified RDE for addition of nitrobenzene (a) in the presence of 500 folds excess concentration of (b) Co2+ (c) Ni2+ (d) Na+ (e) Ba2+ (f) Ca2+ (g) Pb2+ (h) Cd2+ (i) Cl- (j) S- (k) Br- (I) I-and nitro aromatic compounds derivatives (m) 4-aminophenol (n) 4-acetamidophenol (o) 2- nitro aniline (p) 4- nitro aniline and (q) 4-nitrophenol in 100 fold excess, respectively (sivakumar, 2020).](/content/184/2022/15/12/img/10.1016_j.arabjc.2022.104324-fig9.png)
(A) CVs of 300 μM nitrobenzene at green synthesized Ag-NPs modified GCE in 0.05 M PBS pH ranges from 3 to 11 (Right to left side). (B) Calibration plot of nitrobenzene reduction peak current (Ipc) vs different pH (C) Reduction peak potential (Ep) vs pH, (D) Amperometric (i–t) response at green synthesized Ag-NPs modified RDE upon successive additions of 0.05–3693 μM nitrobenzene into continuously stirred N2 saturated PBS. Applied potential: −0.42 V; Rotation speed: 1200 rpm. Inset (upper) is the plot of response current vs [nitrobenzene]. Inset (bottom) the plot of response current vs [nitrobenzene]. (E) Amperometric (i-t) response at green synthesized Ag-NPs modified RDE for addition of nitrobenzene (a) in the presence of 500 folds excess concentration of (b) Co2+ (c) Ni2+ (d) Na+ (e) Ba2+ (f) Ca2+ (g) Pb2+ (h) Cd2+ (i) Cl- (j) S- (k) Br- (I) I-and nitro aromatic compounds derivatives (m) 4-aminophenol (n) 4-acetamidophenol (o) 2- nitro aniline (p) 4- nitro aniline and (q) 4-nitrophenol in 100 fold excess, respectively (sivakumar, 2020).
For the electrochemical detection of uric acid, a Pd–Ag bimetallic NP was constructed by using edible mushrooms (Mallikarjuna et al., 2018). The experiment was conducted in phosphate buffer solution (PBS) at various pH levels and scan rates. They compared the performance of carbon past electrodes (CPE) and Pd–Ag modified CPE electrodes and discovered that the Pd–Ag modified electrode had better electrocatalytic activity than the CPE electrode. For example, a cyclic voltammetry (CV) measurement of CPE yielded an oxidation peak current (Ipa) of 50 µA, but a CV measurement of Pd–Ag/CPE yielded an Ipa of 100 µA, owing to the composite's increased electrical conductivity. The probe responded linearly between 4.69 and 273 nM, with a detection limit of 5.543 nM (Fig. 6.) and they used the probe in a real sample analysis of urine and retrieval of UA range of 93 % to 103 %.
For the detection of 40 nM vanillin, biosynthesized Ag–Au alloy NP was used (Zheng et al., 2010). They employed cellulose diacetate as a link material with modified glassy carbon electrodes by Ag–Au alloy NPs. According to the findings, the modified electrode has a performance that is roughly 5 times better than CCE when it comes to detecting vanillin. On the modified electrode, amperometric measurements were made at 1 V and a steady-state response for vanillin was obtained in the range of 0.2–50 μM. They also used 2.5 X10-4 M caffeine, sucrose, l-arginine, hypoxanthine, barbitone, cholesterol, benzoic acid, vitamin B, xanthine, and ethyl vanillin (6.5X10-6 M), and glucose in an interference experiment to detect 6.5X10-6 M vanillin as shown in Fig. 8. Nonetheless, due to their similar structures and simple electrochemical oxidation, xanthine and ethyl vanillin cause substantial interference with vanillin. They tested the probe's stability and repeatability by exposing it to air for a week and obtaining 93 % of its original performance. Furthermore, they investigated the probe's potential use for the real-time measurement of vanillin in vanilla bean and vanilla tea and obtained 99.86 %and 100.09 % responses, respectively.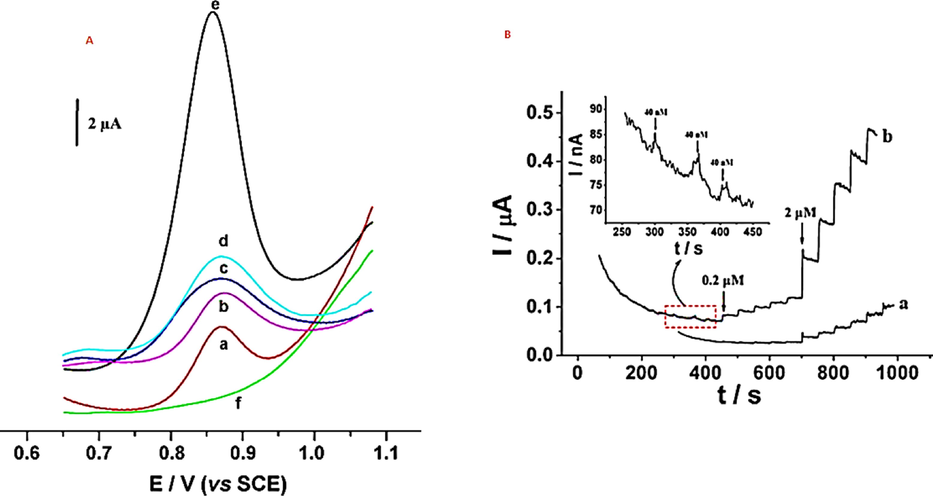
(A) Square wave voltammogramms of bare GCE (a), CDA/GCE (b), CDA/AgNPs/GCE (c), CDA/AuNPs/GCE (d) and CDA/Au–AgNPs/GCE (e and f) in the presence (a, b, c, d and e) and absence (f) of 6.6 × 10–5 M vanillin in phosphate solution (pH 2.0). Amplitude: 20 mV, frequency: 20 Hz. (B) Amperometric response of vanillin at bare GCE (a) and CDA/Au-AgNPs/GCE (b) with successive injections of 0.2Mand 2Mvanillin at the operational potential of 1.0 V. Inset: Amperometric response of 40 nM vanillin at CDA/Au–AgNPs/GCE (Zheng et al., 2010).
An electrochemical sensor for NB has been constructed utilizing reduced graphene oxide (rGO) and AgNPs, which were biosynthesized using Justicia glauca leaf extract(Karuppiah et al., 2015). When compared to other modified electrodes, the modified rGO/AgNPs/GC electrode performed well for the selective quantitative determination of NB: the electrode signal remained linear in the concentration range of 0.5 to 900 M, and the sensitivity was determined to be 0.836 A/M cm−2 with a detection limit of 0.261 M NB. rGO/AgNPs/GC was also kept in phosphate buffer solution (PBS) when not in use, and the decreased peak current response to 100 M NB was investigated by CV for up to 52 days. After 52 days, the modified electrode had kept roughly 90.15 percent of its initial current responsiveness, indicating exceptional storage stability. According to the scientists, the constructed sensor demonstrated good repeatability with an RSD of 3.8 percent when determining NB using five distinct sensors. The developed sensor's good recovery results and RSDs in wastewater samples demonstrated its practical utility for determining NB in actual samples.
AgNPs–GO for electrochemical sensor: Apart from the uses of plants, fungi, and bacteria to fabricate Ag NPs. Researchers used GO to reduce Ag ions by simple, green route synthesis with bio source (Table 6) (Fig. 9). The few reports of biosynthesis of Ag NPs with reduced GO with the simple and economical procedure to enhance the better results of the sensor. The oxidized form of graphene is graphene oxide (GO). It has a 2-D plane with a large number of oxygen-containing functional groups, along with this GO has better dispersibility and film-form capability. The covalent bond which is present in the GO enhances the tremendous mechanical strength of the molecular-level chemical sensor with good selectivity. The combination of nanostructured metal particles or clusters with GO has been used for wide applications due to its functional groups such as –OH, C—O—C, –COOH (Compton and Nguyen, 2010) (Atif and Inam, 2016).
Sensors
Applied potential (V)
Linear range
LOD
Analyte
Method
Reference
AgNPs/GO/GCE
0.38
50–800(µM)
0.002 µM
tryptophan
DPV
(Li et al., 2013)
β-CD/AgNPs-GO
−0.6
13–375(nM)
0.24 (nM)
As(III)
SW-ASV
(Dar et al., 2014)
NG-Ag NPs
−0.47
0.005-47X 10-3 M
0.56 X 10-6 M
H2O2
Amp-It
(Li et al., 2016)
GCE/rGOX/AgNPs
−0.4
0.002–20 (mM)
30–160 (mM)
H2O2
Amp-it
(Salazar et al., 2019)
AgNPs-rGO
−0.4
0.0015–100(mM)
1.90 (µM)
H2O2
Amp-It
(Bhangoji et al., 2021)
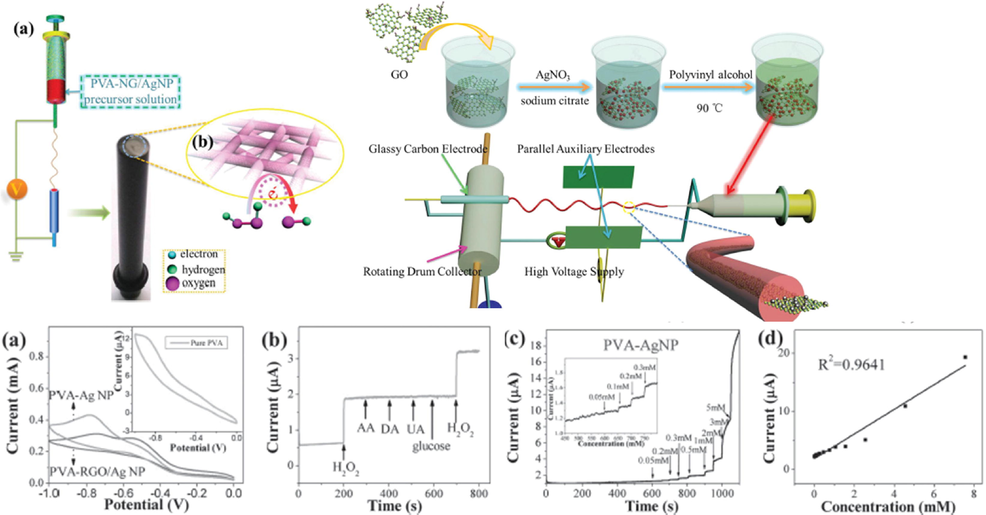
(a) Schematic presentation for the fabrication mechanism of NG/AgNP hybrid membrane and MME. (b) Schematic fabrication of MMEs; b) the possible detection mechanism of H2O2. (c) Electrochemical H2O2 biosensing: a) CVs of PVA, PVA-AgNP, PVA-NG, and PVA-NG/AgNP MMEs; b) selectivity of biosensor: amperometric responses upon successive addition of 1 × 10–3 M of H2O2, 0.10 × 10 −3 M of AA, 0.10 × 10 −3 M of DA, 0.10 × 10 −3 M of UA, 5.0 × 10 −3 M of glucose, and 1 × 10–3 M of H2O 2;(c,d) I–T response and calibrated line of PVA-AgNP MME in 0.1 M PBS with successive addition of H 2O 2 at − 0.47 V versus SCE(Yang li, 2016).
Tryptophan is one of the essential amino acids for human nutrition, woefully it cannot be secreted in the human body and hence it should be taken via food and medicine. And also tryptophan will act as a precursor for the secretion of serotonin and melatonin which give better sleep and relaxation. However, an excessive amount of tryptophan created toxic to the brain, even causing hallucinations and delusion. Junhua Li et al., (Li et al., 2013) proposed Ag NPs/GO nanomaterial synthesized by reduction of Ag ion on graphene oxide surface in presence of glucose as reducing agent and they adopted Hummers’ method to synthesize GO. The AgNPs/GO nanocomposite was first manufactured using a low-cost and environmentally friendly method and then utilized to fabricate a new electrochemical sensor. The nano-hybrid SEM, TEM, EDX, FTIR, and SEM were used to describe the composite film. Electrochemical research has shown that AgNPs/GO had a synergetic catalytic impact on tryptophan oxidation and that the substantial Peak current has increased, whereas peak potential has decreased. The constructed sensor's analytical performance has substantially increased. The kinetic parameters, such as the number of transported electrons, for the oxidation of tryptophan, the electron transfer coefficient, surface coverage, and standard heterogeneous rate constant were measured and attained. Wider linear ranges and a lower detection limit were attained under optimal circumstances. To our knowledge, this is the first time an electrochemical sensor for tryptophan has been built with that kind of a low detection limit (2 nM), and its qualitative properties outperformed the majority of previously reported electrochemical sensors. Furthermore, the nanocomposite enhanced tryptophan and tyrosine oxidation peak separation, and DPV was able to determine tryptophan on-site in the presence of a high quantity of tyrosine.
Furthermore, the AgNPs/GO composite has several intriguing properties, including simplicity of synthesis, low toxicity, surface functions, and outstanding stability, all of which make it a promising candidate for biological and organic molecule electroanalysis and electrocatalysis. Another research group fabricated a new electrochemical sensor for As(III) detection employing Anodic stripping voltammetry using a green and simple technique to synthesize cyclodextrin (CD) stabilized AgNPs–GO composite. Because arsenic pollution of groundwater is a worldwide major concern that affects the safety of drinking water in various nations. Because of its interactions with enzymes in human metabolism, As(III) is regarded to be more hazardous than As(V). Many ailments are caused by this, including skin lesions, keratosis, lung cancer, bladder cancer, and others. In January 2011, the United States Environmental Protection Agency (USEPA) set a new arsenic threshold for drinking water at 10 parts per billion (ppb). The created sensor had a low detection limit, a wide operating range, and analytical properties that exceeded most electrochemical sensors previously described. We were able to detect As(III) in the presence of Cu and other organics using the AgNPs–GO composite sensor. The findings suggest that As(III) and Cu(II) might be determined at the same time. As(III) was measured in-ground and river water samples using the newly designed sensor. The suitability of the material for As (III) measurements in mineral water and other samples containing As (III) was demonstrated by the high recovery of the analysis (Dar et al., 2014).
2.15 Outline and prospects
The creation of superstructures is simple by using a bio-sourced mediated nanomaterial synthesis process. The bio-functionalized NPs' inherent adaptability allows for a wide variety of analyte detection, making them a viable alternative to non-eco-friendly technologies. It also adheres to the principles of sustainability and biocompatibility. The nanoparticles' bio-driven genesis and biocompatibility make them valuable in a variety of biomedical applications.
The lack of the biosynthesis of NPs using biosource is understanding the exact mechanism and the major challenges are to control the shape and size to attain the monodispersity. It seems like various important technical steps must be taken to cross this bio-mediated route for industrial synthesis. We need to level up biosynthesis into large-scale synthesis and provide portable sensors. Even though electroanalytical biosensors are highly effective and economical, it requires enhancing the performance likely step down the electrode surface modification steps and conversion of the portable sensor with gadgets, wireless technology will attain more attraction. Finally, the goal of this outline is to present a summary of the current research of the art in the domain of electrochemical sensors for pesticide, and heavy metals detection. We hope this study will serve as a useful resource, raising understanding and appreciation of the role that electrochemical biosensors may play in protecting our environment.
CRediT authorship contribution statement
Jayaprakash Meena: Writing – original draft, Formal analysis. K. Santhakumar: Supervision, Conceptualization.
Acknowledgment
We thank Ms. IshitaGhai, Harvard Graduate School of Education, US. for the English correction.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci.. 2017;24(1):208-216.
- [CrossRef] [Google Scholar]
- Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A Novel Approach Towards Weed Utilization. Biotechnol. Res. Int.. 2011;2011:1-8.
- [CrossRef] [Google Scholar]
- Recent advances in nanomaterial-based sensors for detection of trace nitroaromatic explosives. Sens. Actuators, B 2015
- [CrossRef] [Google Scholar]
- Extracellular myco-synthesis of nano-silver using the fermentable yeasts Pichia kudriavzeviiHA-NY2 and Saccharomyces uvarumHA-NY3, and their effective biomedical applications. Bioprocess Biosyst. Eng.. 2021;44(4):841-854.
- [CrossRef] [Google Scholar]
- Green silver nanoparticles as a multifunctional sensor for toxic Cd(ii) ions. New J. Chem.. 2018;42(18):15022-15031.
- [CrossRef] [Google Scholar]
- Green synthesized unmodified silver nanoparticles as a multi-sensor for Cr(iii) ions. Environ. Sci. Water Res. Technol.. 2018;4(10):1531-1542.
- [CrossRef] [Google Scholar]
- Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanopart. Res.. 2004;6(4):377-382.
- [CrossRef] [Google Scholar]
- Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep.. 2021;11(1):1-11.
- [CrossRef] [Google Scholar]
- Influence of external factors on the production and morphology of biogenic silver nanocrystallites. J. Nanosci. Nanotechnol.. 2013;13(3):2295-2301.
- [CrossRef] [Google Scholar]
- Modeling and Simulation of Graphene Based Polymer Nanocomposites: Advances in the Last Decade. Graphene. 2016;05(02):96-142.
- [CrossRef] [Google Scholar]
- Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharmaceutical Journal. 2016;24(2):140-146.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem.. 2018;32(2):1-9.
- [CrossRef] [Google Scholar]
- Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res.. 2015;178:9-17.
- [CrossRef] [Google Scholar]
- Silver nanoparticles for printable electronics and biological applications. J. Mater. Res.. 2009;24(9):2828-2836.
- [CrossRef] [Google Scholar]
- Bio-mechanochemical synthesis of silver nanoparticles with antibacterial activity. Adv. Powder Technol.. 2017;28(12):3307-3312.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of Au nanoparticles using vascular plants. Ind. Eng. Chem. Res.. 2010;49(24):12762-12772.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf., A. 2009;339(1–3):134-139.
- [CrossRef] [Google Scholar]
- Green synthesis of Ag nanoparticles using grape stalk waste extract for the modification of screen-printed electrodes. Nanomaterials. 2018;8(11)
- [CrossRef] [Google Scholar]
- Facile and green synthesis of silver nanoparticle-reduced graphene oxide composite and its application as nonenzymatic electrochemical sensor for hydrogen peroxide. Current Chemistry Letters. 2021;10(3):295-308.
- [CrossRef] [Google Scholar]
- (2013) ‘Rapid Synthesis of Silver Nanoparticles from Fusarium oxysporum by Optimizing Physicocultural Conditions’. The Scientific World Journal. 2013;1:1-12.
- [CrossRef] [Google Scholar]
- Directly synthesized silver nanoparticles on gas diffusion layers by electrospray pyrolysis for electrochemical CO 2 reduction. Electrochim. Acta. 2019;303:118-124.
- [CrossRef] [Google Scholar]
- Cuminum cyminum L.-Mediated Synthesis of Silver Nanoparticles: Their Characterization and Effect on Formalin-Induced Nociceptive Response in Male Rats. Biol. Trace Elem. Res.. 2021;199(11):4171-4182.
- [CrossRef] [Google Scholar]
- Green synthesis of Ag nanoparticles by Callicarpa Maingayi: Characterization and its application with graphene oxide for enzymeless hydrogen peroxide detection. J. Chin. Chem. Soc.. 2014;61(6):631-637.
- [CrossRef] [Google Scholar]
- Synthesis of silver and gold nanoparticles from rumex roseus plant extract and their application in electrochemical sensors. Nanomaterials. 2021;11(3):1-18.
- [CrossRef] [Google Scholar]
- Nanostructures confined self-assembled in biomimetic nanochannels for enhancing the sensitivity of biological molecules response. J. Mater. Sci.: Mater. Electron.. 2018;29(23):19757-19767.
- [CrossRef] [Google Scholar]
- Biogenic silver nanoparticles from Trachyspermum ammi (Ajwain) seeds extract for catalytic reduction of p-nitrophenol to p-aminophenol in excess of NaBH4. J. Mol. Liq.. 2017;230:74-84.
- [CrossRef] [Google Scholar]
- Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small. 2010;6(6):711-723.
- [CrossRef] [Google Scholar]
- Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagous fungus Duddingtonia flagrans. Int. J. Nanomed.. 2017;12:6373-6381.
- [CrossRef] [Google Scholar]
- Green synthesis of a silver nanoparticle-graphene oxide composite and its application for As(iii) detection. RSC Adv.. 2014;4(28):14432-14440.
- [CrossRef] [Google Scholar]
- Time-dependent effect in green synthesis of silver nanoparticles. Int. J. Nanomed.. 2011;6(1):677-681.
- [CrossRef] [Google Scholar]
- Biosynthesis of Ag nanoparticle by peganum harmala extract; antimicrobial activity and ability for fabrication of quercetin food electrochemical sensor. Int. J. Electrochem. Sci.. 2020;15(3):2549-2560.
- [CrossRef] [Google Scholar]
- Non-enzymatic biosensing of glucose based on silver nanoparticles synthesized from Ocimum tenuiflorum leaf extract and silver nitrate. Mater. Chem. Phys.. 2018;216:502-507.
- [CrossRef] [Google Scholar]
- Rosa damascena waste mediated synthesis of silver nanoparticles: Characteristics and application for an electrochemical sensing of hydrogen peroxide and vanillin. Mater. Chem. Phys.. 2019;231(April):335-343.
- [CrossRef] [Google Scholar]
- Synthesis of small silver nanoparticles under light radiation by fungus Penicillium oxalicum and its application for the catalytic reduction of methylene blue. Mater. Chem. Phys.. 2015;160:40-47.
- [CrossRef] [Google Scholar]
- Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem.. 2010;45(7):1065-1071.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf., A. 2010;369(1–3):27-33.
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egyptian Journal of Biological Pest Control. 2018;28(1):1-11.
- [CrossRef] [Google Scholar]
- Synthesize of Silver Nanoparticles by Arc Discharge Method Using Two Different Rotational Electrode Shapes. J. Cluster Sci.. 2018;29(6):1169-1175.
- [CrossRef] [Google Scholar]
- Innovative biosynthesis of silver nanoparticles using yeast glucan nanopolymer and their potentiality as antibacterial composite. J. Basic Microbiol.. 2021;61(8):677-685.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using pollen extract: Characterization, assessment of their electrochemical and antioxidant activities. Analytical Biochemistry 2021
- [CrossRef] [Google Scholar]
- Determination of paracetamol using a sensor based on greensynthesis of silver nanoparticles in plant extract. Journal of pharmaceutical and biomedical analysis 2021
- [CrossRef] [Google Scholar]
- Photochemical synthesis of silver nanoparticles on chitosans/montmorillonite nanocomposite films and antibacterial activity. Carbohydr. Polym.. 2017;171:202-210.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep.. 2020;10(1):1-11.
- [CrossRef] [Google Scholar]
- Biological synthesis of metal nanoparticles. Hydrometallurgy. 2006;83(1–4):132-140.
- [CrossRef] [Google Scholar]
- Biosynthesis of gold nanoparticles by Escherichia coli. Minerva Biotecnologica. 2013;25(3):161-164.
- [Google Scholar]
- A facile one-pot green synthesis of reduced graphene oxide and its composites for non-enzymatic hydrogen peroxide sensor applications. RSC Adv.. 2014;4(16):7944-7951.
- [CrossRef] [Google Scholar]
- Electrochemical biosensors - Sensor principles and architectures. Sensors. 2008;8(3):1400-1458.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles from the novel strain of Streptomyces Sp. BHUMBU-80 with highly efficient electroanalytical detection of hydrogen peroxide and antibacterial activity. J. Environ. Chem. Eng.. 2017;5(6):5624-5635.
- [CrossRef] [Google Scholar]
- Populus ciliata mediated synthesis of silver nanoparticles and their antibacterial activity. Microsc. Res. Tech.. 2021;84(3):480-488.
- [CrossRef] [Google Scholar]
- Synthesis of Silver Nanoparticles using Fagonia Cretica and their Antimicrobial Activities. Nanoscale Advance 2019
- [CrossRef] [Google Scholar]
- Assessment of Bioreducing and Stabilizing Potential of Dragon’s Blood (Dracaena Cochinchinensis, Lour. S. C. Chen) Resin Extract in Synthesis of Silver Nanoparticles. Nanoscience and nanotechnology Letters. 2013;5(5):780-784.
- [CrossRef] [Google Scholar]
- Hasan, M., et al., 2015. ‘Mechanistic Study of Silver Nanoparticle’s Synthesis by Dragon’s Blood Resin Ethanol Extract and Antiradiation Activity’. doi:10.1166/jnn.2015.9090.
- Hasan, M. et al., 2018. ‘Biological entities as chemical reactors for synthesis of nanomaterials: Progress, challenges and future perspective’, 8. doi:10.1016/j.mtchem.2018.02.003.
- Sustainable green synthesis of silver nanoparticles using Sambucus ebulus phenolic extract (AgNPs@SEE): Optimization and assessment of photocatalytic degradation of methyl orange and their in vitro antibacterial and anticancer activity. Arabian J. Chem.. 2022;15(1):103525
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using oak leaf extract and their application for electrochemical sensing of hydrogen peroxide. Appl. Organomet. Chem.. 2018;32(11):1-7.
- [CrossRef] [Google Scholar]
- Q-sized CdS: Synthesis, characterization, and efficiency of photoinitiation of polymerization of several vinylic monomers. J. Phys. Chem.. 1992;96(13):5546-5552.
- [CrossRef] [Google Scholar]
- Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef University Journal of Basic and Applied Sciences. 2015;4(3):225-231.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using Pomegranate juice extract and its antibacterial activity. International Journal of Applied Sciences and Biotechnology. 2016;4(3):254-258.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv.. 2019;9(50):29293-29299.
- [CrossRef] [Google Scholar]
- Characterization, antibacterial and in vitro compatibility of zinc-silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int.. 2014;40(3):4507-4513.
- [CrossRef] [Google Scholar]
- Green synthesis of metal nanoparticles using plants. Green Chem.. 2011;13(10):2638-2650.
- [CrossRef] [Google Scholar]
- Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. International Scholarly Research Notices. 2014;2014:1-18.
- [CrossRef] [Google Scholar]
- Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. Journal of Chemical technology and biotechnology 2012
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res. Int.. 2013;2013
- [CrossRef] [Google Scholar]
- ‘Application of mycogenic silver/silver oxide nanoparticles in electrochemical glucose sensing; alongside their catalytic and antimicrobial activity’, 3. Biotech. 2021;11(7):1-11.
- [CrossRef] [Google Scholar]
- Role of phenol derivatives in the formation of silver nanoparticles. Langmuir. 2008;24(2):528-533.
- [CrossRef] [Google Scholar]
- A simple approach for facile synthesis of Ag, anisotropic Au and bimetallic (Ag/Au) nanoparticles using cruciferous vegetable extracts. Mater. Sci. Eng., C. 2012;32(7):1827-1834.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using aqueous extract of Acacia cyanophylla and its antibacterial activity. Heliyon. 2021;7(9):e08033.
- [Google Scholar]
- Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. J. Photochem. Photobiol., B. 2017;170:164-172.
- [CrossRef] [Google Scholar]
- Green biosynthesis of silver nanoparticles and nanomolar detection of p-nitrophenol. J. Solid State Electrochem.. 2014;18(7):1847-1854.
- [CrossRef] [Google Scholar]
- Green synthesized silver nanoparticles decorated on reduced graphene oxide for enhanced electrochemical sensing of nitrobenzene in waste water samples. RSC Adv.. 2015;5(39):31139-31146.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2011;79(3):594-598.
- [CrossRef] [Google Scholar]
- Progression in Fenton Process for the Wastewater Treatment. 2020
- [CrossRef]
- Synthesis, self-assembly, sensing methods and mechanism of bio-source facilitated nanomaterials: A review with future outlook. Nano-Structures and Nano-Objects. 2020;23:100498
- [CrossRef] [Google Scholar]
- New proposal for size and size-distribution evaluation of nanoparticles synthesized via ultrasonic spray pyrolysis using search algorithm based on image-processing technique. Materials. 2020;13(1)
- [CrossRef] [Google Scholar]
- Mucilage-capped silver nanoparticles for glucose electrochemical sensing and fuel cell applications. RSC Adv.. 2020;10(62):37675-37682.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using onion extract and their application for the preparation of a modified electrode for determination of ascorbic acid. J. Food Drug Anal.. 2016;24(4):796-803.
- [CrossRef] [Google Scholar]
- Electrochemical and antioxidant properties of biogenic silver nanoparticles. Int. J. Electrochem. Sci.. 2015;10(10):7905-7916.
- [Google Scholar]
- An Overview: Biological Organisms That Serves as Nanofactories for Metallic Nanoparticles Synthesis and Fungi Being the Most Appropriate. Bioceramics Development and Applications. 2017;07(01):1-4.
- [CrossRef] [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arabian J. Chem.. 2019;12(7):908-931.
- [CrossRef] [Google Scholar]
- Madurahydroxylactone, an inhibitor of Staphylococcus aureus FtsZ from Nonomuraea sp. AN100570. J. Microbiol. Biotechnol.. 2017;27(11):1994-1998.
- [CrossRef] [Google Scholar]
- Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iranian J. Pharm. Res.. 2013;12(3):289-298.
- [Google Scholar]
- Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf., B. 2010;76(1):50-56.
- [CrossRef] [Google Scholar]
- Novel static magnetic field effects on green chemistry biosynthesis of silver nanoparticles in Saccharomyces cerevisiae. Sci. Rep.. 2021;11(1):1-9.
- [CrossRef] [Google Scholar]
- Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2012;90:173-176.
- [CrossRef] [Google Scholar]
- Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: Assessment of their antibacterial and anticancer activity. Karbala Int. J. Mod. Sci.. 2018;4(1):61-68.
- [CrossRef] [Google Scholar]
- Biosynthesized silver nanoparticles: Electrochemical application. Bul. Chem. Commun.. 2019;51(D):192-197.
- [Google Scholar]
- Biosynthesis of nanoparticles by microorganisms and their applications. Journal of Nanomaterials. 2011;2011
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles-graphene oxide nanocomposite and its application in electrochemical sensing of tryptophan. Biosens. Bioelectron.. 2013;42(1):198-206.
- [CrossRef] [Google Scholar]
- Nanoscale Graphene Doped with Highly Dispersed Silver Nanoparticles: Quick Synthesis, Facile Fabrication of 3D Membrane-Modified Electrode, and Super Performance for Electrochemical Sensing. Adv. Funct. Mater.. 2016;26(13):2122-2134.
- [CrossRef] [Google Scholar]
- Nanoparticle-based sensors for food contaminants. TrAC - Trends in Analytical Chemistry. 2019;113:74-83.
- [CrossRef] [Google Scholar]
- A hydrogen peroxide sensor based on silver nanoparticles biosynthesized by bacillus subtilis. Chin. J. Chem .. 2013;31(12):1519-1525.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles with antimicrobial and anticancer properties using two novel yeasts. Sci. Rep.. 2021;11(1):1-12.
- [CrossRef] [Google Scholar]
- Bactericidal activity of bio mediated silver nanoparticles synthesized by Serratia nematodiphila. Drug Invention Today. 2013;5(2):119-125.
- [CrossRef] [Google Scholar]
- Simple synthesis of biogenic Pd–Ag bimetallic nanostructures for an ultra-sensitive electrochemical sensor for sensitive determination of uric acid. J. Electroanal. Chem.. 2018;822(2017):163-170.
- [CrossRef] [Google Scholar]
- Simple synthesis of biogenic Pd-Ag bimetallic nanostructures based an ultra-sensitive electrochemical sensor for the sensing or uric acid. Journal of Electroanalytical Chemistry 2018
- [CrossRef] [Google Scholar]
- Mangifera indica Mediated Synthesis of Silver Nanoparticles as An Efficient Electrochemical Sensor for The Detection of Ascorbic Acid. Journal of Advanced Chemical Sciences. 2019;5(2):643-645.
- [CrossRef] [Google Scholar]
- Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. Biomed Res. Int.. 2013;2013
- [CrossRef] [Google Scholar]
- ‘Arachidic Acid Langmuir-Blodgett Films’. 1995;91(4):665-672.
- One-step Synthesized Silver Nanoparticles Using Isoimperatorin: Evaluation of Photocatalytic, and Electrochemical Activities. Sci. Rep.. 2020;10(1):1-12.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and antibacterial activity investigation of silver nanoparticle-decorated graphene oxide. Mater. Lett.. 2021;285:128993
- [CrossRef] [Google Scholar]
- ‘Studies on Bacterial Synthesis of Silver Nanoparticles Using Gamma Radiation and Their Activity against Some Pathogenic Microbes Studies on Bacterial Synthesis of Silver Nanoparticles Using Gamma Radiation and Their Activity Against Some Pathogenic Microb’, 2013 [Preprint] Available at 2009
- [Google Scholar]
- Synthesis of Nanoparticles by Green Synthesis Method. International Journal of Innovative Research and Reviews. 2017;1(1):6-9. Available at:
- [Google Scholar]
- Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst. Growth Des.. 2002;2(4):293-298.
- [CrossRef] [Google Scholar]
- ‘Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE’, Nanomedicine: Nanotechnology. Biology, and Medicine. 2009;5(4):452-456.
- [CrossRef] [Google Scholar]
- Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crops Prod.. 2014;60:212-216.
- [CrossRef] [Google Scholar]
- Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2011;79(5):1461-1465.
- [CrossRef] [Google Scholar]
- Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2015;136(PB):1175-1180.
- [CrossRef] [Google Scholar]
- Rosa canina waste seed extract-mediated synthesis of silver nanoparticles and the evaluation of its antimutagenic action in Salmonella typhimurium. Mater. Chem. Phys.. 2021;266(March):124537
- [CrossRef] [Google Scholar]
- Phanjom, P., Ahmed, G., 2017. ‘Effect of different physicochemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae (MTCC No. 1846) and their antibacterial effect’, Adv. Natural Sci. Nanosci. Nanotechnol. 8(4), p. aa92bc. doi:10.1088/2043-6254/aa92bc.
- Green Synthesized Unmodified Silver Nanoparticles as Reproducible Dual Sensor for Mercuric Ions and Catalyst to Abate Environmental Pollutants. BioNanoScience. 2021;11(3):739-754.
- [CrossRef] [Google Scholar]
- A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol.. 2017;45(7):1272-1291.
- [CrossRef] [Google Scholar]
- Synthesis of gold nanoparticles by various leaf fractions of Semecarpus anacardium L. tree. Trees - Structure and Function. 2011;25(2):145-151.
- [CrossRef] [Google Scholar]
- A facile green synthesis of silver nanoparticles using Piper betle biomass and its catalytic activity toward sensitive and selective nitrite detection. J. Ind. Eng. Chem.. 2016;35:29-35.
- [CrossRef] [Google Scholar]
- A Facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida Linn. f. for catalytic, antioxidant and antibacterial applications. S. Afr. J. Chem. Eng.. 2018;26(July):25-34.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf., A. 2018;536:60-67.
- [CrossRef] [Google Scholar]
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens. Green Process. Synth,. 2019;8(1):144-156.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using a mountain plant extract. Rev. Roum. Chim.. 2019;64(2):143-152.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf., B. 2010;81(1):358-362.
- [CrossRef] [Google Scholar]
- Gold nanoparticles in chemical and biological sensing. Chem. Rev.. 2012;112(5):2739-2779.
- [CrossRef] [Google Scholar]
- One-step green synthesis of silver nanoparticle-modified reduced graphene oxide nanocomposite for H2O2 sensing applications. J. Electroanal. Chem.. 2019;855:113638
- [CrossRef] [Google Scholar]
- Greenly synthesized silver nanoparticles for supercapacitor and electrochemical sensing applications in a 3D printed microfluidic platform. Microchem. J.. 2020;157(April):104973
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using Convolvulus pluricaulis leaf extract and assessment of their catalytic, electrocatalytic and phenol remediation properties. Advanced Materials Letters. 2016;7(5):383-389.
- [CrossRef] [Google Scholar]
- Detection of catechol using a biosensor based on biosynthesized silver nanoparticles and polyphenol oxidase enzymes. Portugaliae Electrochimica Acta. 2019;37(4):257-270.
- [CrossRef] [Google Scholar]
- Sensors for detecting biological agents. Mater. Today. 2008;11(3):38-49.
- [CrossRef] [Google Scholar]
- Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf., B. 2009;73(2):332-338.
- [CrossRef] [Google Scholar]
- Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. SpringerPlus. 2016;5(1)
- [CrossRef] [Google Scholar]
- Large metal clusters and colloids - Metals in the embryonic state. Prog. Colloid Polym. Sci.. 1998;111:52-57.
- [CrossRef] [Google Scholar]
- Green Silver Nanoparticles Based Multi-Technique Sensor for Environmental Hazardous Cu(II) Ion. BioNanoScience. 2019;9(2):373-385.
- [CrossRef] [Google Scholar]
- Size-Controlled Production of Silver Nanoparticles by Aspergillus fumigatus BTCB10: Likely Antibacterial and Cytotoxic Effects. Journal of Nanomaterials. 2019;2019
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using leaves of Acacia melanoxylon and their application as dopamine and hydrogen peroxide sensors. Physical Chemistry Research. 2020;8(1):1-18.
- [CrossRef] [Google Scholar]
- Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2011;79(1):254-262.
- [CrossRef] [Google Scholar]
- Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem.. 2011;46(9):1800-1807.
- [CrossRef] [Google Scholar]
- Electrochemical Detection of Nitrite Using Glassy Carbon Electrode Modified with Silver Nanospheres (AgNS) Obtained by Green Synthesis Using Pre-hydrolysed Liquor. Electroanalysis. 2017;29(5):1434-1442.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles (SNPs)-modified electrode for electrochemical detection of nitrobenzene. J. Iran. Chem. Soc.. 2020;17(4):893-900.
- [CrossRef] [Google Scholar]
- Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett.. 2020;15(1)
- [CrossRef] [Google Scholar]
- Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. Bacterial Pathogenesis and Antibacterial Control Species 2013:29.
- [CrossRef] [Google Scholar]
- Silva, L.P., Pereira, T.M., Bonatto, C.C., 2019. Frontiers and perspectives in the green synthesis of silver nanoparticles, Green Synthesis, Characterization and Applications of Nanoparticles. Elsevier Inc. doi:10.1016/b978-0-08-102579-6.00007-1.
- Electrochemical sensing of nitro-aromatic explosive compounds using silver nanoparticles modified electrochips. Anal. Methods. 2016;8(39):7158-7169.
- [CrossRef] [Google Scholar]
- Parthenium hysterophorus Mediated Synthesis of Silver Nanoparticles and its Evaluation of Antibacterial and Antineoplastic Activity to Combat Liver Cancer Cells. J. Cluster Sci.. 2021;32(1):167-177.
- [CrossRef] [Google Scholar]
- Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater. Sci. Eng., C. 2015;48:188-196.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egyptian Journal of Basic and Applied Sciences. 2018;5(3):228-233.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using mimosa pudica plant root extract: Characterization, antibacterial activity and electrochemical detection of dopamine. Int. J. Electrochem. Sci.. 2016;11(12):9959-9971.
- [CrossRef] [Google Scholar]
- Silver nanoparticles: Synthesis, mechanism of antimicrobial action, characterization, medical applications, and toxicity effects. Journal of Chemical and Pharmaceutical Research. 2016;8(2):526-537.
- [Google Scholar]
- Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Brazilian Journal of Microbiology. 2014;45(4):1221-1227.
- [CrossRef] [Google Scholar]
- Electrochemical sodium ion sensor based on silver nanoparticles/graphene oxide nanocomposite for food application. Chemosensors. 2020;8(3)
- [CrossRef] [Google Scholar]
- Green synthesis of silver and palladium nanoparticles using Lithodora hispidula (Sm.) Griseb. (Boraginaceae) and application to the electrocatalytic reduction of hydrogen peroxide. Mater. Chem. Phys.. 2017;202:310-319.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using pollen extract: Characterization, assessment of their electrochemical and antioxidant activities. Anal. Biochem.. 2021;621(January):114123
- [CrossRef] [Google Scholar]
- Effect of storage conditions on long-term stability of Ag nanoparticles formed via green synthesis. Int. J. Miner. Metall. Mater.. 2017;24(10):1177-1182.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol.. 2016;44(4):1127-1132.
- [CrossRef] [Google Scholar]
- Large-scale diamond silver nanoparticle arrays as uniform and sensitive SERS substrates fabricated by surface plasmon lithography technology. Opt. Commun.. 2019;444(March):56-62.
- [CrossRef] [Google Scholar]
- Silver nanoparticles: Large scale solvothermal synthesis and optical properties. Mater. Res. Bull.. 2010;45(8):1033-1038.
- [CrossRef] [Google Scholar]
- Biosynthesis of Au-Ag alloy nanoparticles for sensitive electrochemical determination of paracetamol. Int. J. Electrochem. Sci.. 2017;12(10):9131-9140.
- [CrossRef] [Google Scholar]
- Detection of Imatinib Based on Electrochemical Sensor Constructed Using Biosynthesized Graphene-Silver Nanocomposite. Front. Chem.. 2021;9(April):1-7.
- [CrossRef] [Google Scholar]
- ‘Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida. Aspergillus and Fusarium’, International Journal of Nanomedicine. 2016;11:1899-1906.
- [CrossRef] [Google Scholar]
- Yousaf, A., et al., 2019. ‘Intrinsic Bio-Enhancer Entities of Fagonia cretica for Synthesis of Silver Nanoparticles Involves Anti-Urease, Anti-Oxidant and Anti-Tyosinase Activity’, pp. 455–468. doi:10.4236/abb.2019.1012032.
- Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces. 2020;19:100438
- [CrossRef] [Google Scholar]
- Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. J. Pharm. Biomed. Anal.. 2021;196:113912
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties. Colloids Surf., A. 2013;423:63-68.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of silver nanoparticles by a sonochemical method. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Metal Materials and Engineering. 2012;41(10):1700-1705.
- [CrossRef] [Google Scholar]
- Preparation and application of a novel vanillin sensor based on biosynthesis of Au-Ag alloy nanoparticles. Sens. Actuators, B. 2010;148(1):247-252.
- [CrossRef] [Google Scholar]
- Hydrothermal preparation of reduced graphene oxide–silver nanocomposite using Plectranthus amboinicus leaf extract and its electrochemical performance. Enzyme Microb. Technol.. 2016;95:112-117.
- [CrossRef] [Google Scholar]







