Translate this page into:
A molecular insight into deoiled asphalt’s slurry-phase hydrocracking process
⁎Corresponding author. lijiguang.ripp@sinopec.com (Li Jiguang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Qilu vacuum residue with Ni + V content of 120 ppm and Ca content of 40 ppm, is hard to process for fixed bed hydrocracking technology. In this work, solvent deasphalting process was used as a pretreatment for processing Qilu vacuum residue, and the yield of deasphalted oil could be up to 48.3 %, with less metal (Ni + V + Ca < 15 ppm) and asphaltene (<0.1 %) in deasphalted oil. The n-butane solvent in solvent deasphalting (SDA) process had higher selectivity for HC class, and correspondingly the relative abundance of HC class of deasphalted oil (DAO) was much higher than that of deasphalted oil (DAO). The abundance of DAO was higher than that of DOA when double bond equivalent (DBE) < 14 or carbon number (CN) < 46, and the relative abundance of SDA1-DOA was higher than that of SDA2-DOA when DBE < 20 or CN < 46. Deoiled asphalt was taken as the feed of slurry-phase hydrocracking (SHC) process, and the conversion ratio of deoiled asphalt in slurry-phase hydrocracking process could be more than 80 % for 240 min reaction. The conversion ratios of SDA1-DOA for SHC1-120 min and SHC1-240 min reactions were 66.92 % and 81.64 % respectively, and the conversion ratios of SDA2-DOA for SHC2-120 min and SHC2-240 min reactions were 70.19 % and 85.71 % respectively. Sulfur species and asphaltene changing rules at molecular level were determined to evaluate DOA’s slurry-phase hydrocracking process.
Keywords
Vacuum residue
Solvent deasphalting
Slurry-phase hydrocracking
Asphaltene structure
1 Introduction
Vacuum residue (VR) is a distillate with the most complex structure and the largest polarity and the highest boiling point in petroleum, and VR contains a large number of heteroatoms such as sulfur, nitrogen, oxygen and heavy metals with various binding forms (León et al., 2020). In recent years, with the deterioration of vacuum residue quality, increasingly stringent environmental protection requirements and increasing light oil market demands, vacuum residue hydrogenation lightening technology has been paid more and more attention and is becoming a hot topic in the global oil refining industry (León et al., 2020; Sahu et al., 2015; Prajapati et al., 2021).
Slurry-phase hydrocracking technologies have great potential in the field of vacuum residue processing with high asphaltene content and impurities in the feed, and also have high feed conversion ratio and relatively green process (Bellussi et al., 2013; Manek and Haydary, 2019). In the long run, the technologies for deep conversion of inferior vacuum residue have wide application prospects (León et al., 2020; Sahu et al., 2015). It is urgent to develop effective processing technology for inferior vacuum residue. In nowadays, research on slurry-phase hydrocracking processes is very active. Although there are now more than 10 such technologies some of which are in pilot stage and some of which have already developed industrialized application (Zhang et al., 2007), the popularized application of the technology is still challenging for economic and long-term operation. The academic research focuses on catalyst and technology optimization and control.
Improvement of the performance of catalyst was on the top priority to improve the economic benefit and make the process environmental-friendly (Nguyen et al., 2016). An oil-soluble MoS2 catalyst precursor was successfully synthesized by a simple method and the target catalyst exposed more active sites and showed much improved efficiency in VR slurry-phase hydrocracking process (Liu et al., 2019 a,b). It was investigated that Mo, Ni, or Co precursors showed different oxidation state and oil solubility in VR slurry-phase hydrocracking process (Kim et al., 2018). The general trends in operating pressure and corresponding reaction performance implied that reduction of catalyst particle size would lead to the lower operational pressure (Shekarriz et al., 2016). Li et al. studied on the influence of catalyst dosage on coke formation for slurry-phase hydrocracking process (Li et al., 2020).
Lim et al. studied the effect of reaction time and temperature on the products and the asphaltene dispersion stability in a slurry-phase hydrocracking process (Lim et al., 2018), and the effects of the initial hydrogen pressure and temperature on the isomerization reaction in slurry-phase hydrocracking process were systematically studied (Du et al., 2015). Du et al. studied the structure, composition, and colloidal stability of hydrocracking products at various hydrogen pressures to investigate the role of hydrogen pressure in slurry-phase hydrocracking process (Du et al., 2015). Pham et al. studied the kinetic models for slurry-phase hydrocracking process using dispersed catalyst for a range of liquid hourly space velocities of 0.25–1 hr-1 and reaction temperatures of 410–450 °C (Pham et al., 2021). The VR slurry-phase hydrocracking process in the presence of dispersed MoS2 catalyst followed parallel reaction pathways in the formation of lighter oils as main products, which were strongly governed by operating conditions such as reaction time, reaction temperature and initial H2 pressure (Kim et al., 2017). Coke formation is another critical issue, and until now, the knowledge that relates to is still very limited. Feeds containing high molecular weight species and many impurities of heteroatom-containing organic compounds could lead to relatively quick fouling and deactivation of catalysts during conventional ebullated-bed or fixed-bed hydrocracking process, and slurry-phase hydrocracking process in the presence of the oil-dispersed catalyst has the ability to overcome these drawbacks through the enhancement of hydrogenation reactions (Nguyen et al., 2016; Al-Attas et al., 2019). The coke formation behavior of coal tar atmospheric residue during slurry-phase hydrocracking process had been studied, and the results showed that coal tar atmospheric residue with higher asphaltene content could produce little cokesur under the optimized conditions (Deng et al., 2016). Asphaltene dispersion stability and sediment formation for a slurry-phase hydrocracking reaction of vacuum residue was investigated with changes of liquid hourly space velocities (0.20–0.45 hr−1), temperatures (405–435 °C), and pressures (80–180 bar) in a bench-scale continuous reactor (Lim et al., 2020).
Vacuum residue’s slurry-phase hydrocracking process is a hot topic, while academic research and industrial application are still on the way. Qilu vacuum residue (Abbreviation for QLVR, provided by SINOPEC Qilu Petrochemical Corporation) with relatively higher metal (Ca, Ni, V) content, is hard to process for fixed-bed hydrocracking technology. In this work, solvent deasphalting (SDA) process was used as a pretreatment, the corresponding DAO could be treated as the feed of fluid catalytic cracking (FCC) (Zhang et al., 2007). The corresponding DOA could be taken as the feed of slurry-phase hydrocracking (SHC) process, compared to VR as the feed, which can greatly decrease the scale of SHC process. Optimized SDA and SHC test conditions were carried out to ensure QLVR in this process with high conversion ratio, and further molecular level analysis for the feed and products were determined to evaluate the SDA and SHC process. Furthermore, there was no systematic study on DOA’s slurry-phase hydrocracking process, and this work could give a preliminary exploration.
2 Experimental parts
2.1 Chemicals
Toluene and n-heptane (short for heptane) were at least 99 % purity, purchased from Sinopharm Chemical Reagent Co., ltd. n-butane with the purity of at least 99 % was supplied by Zhongwei Fine Chemical Co., ltd.
2.2 Feed properties
It can be seen from Table 1 that QLVR was a typical vacuum residue, the asphaltene (C7-Asphaltene) content was 5.8 % and the residual carbon value was 18.24 %. The proportion of greater than 524 °C fractions was about 94 %, and the metal content of Ca + Ni + V was more than 150 µg/g.
Items
QLVR
Methods
Density(20 °C)/(kg/m3)
1013
GB/T 13,377
Residue carbon/%
18.24
GB/T17144
w(Elements)/%
w (C)/%
84.82
SH/T 0656
w (H)/%
10.77
SH/T 0656
w (S)/%
2.85
GB/T 17,040
w (N)/%
0.84
SH/T 0704
w(C7-Asphaltene)/%
5.8
w (metal)/(µg/g)
SH/T 0715
Ca
40
Ni
60
V
60
Fe
19
greater than524 °C fractions/%
∼94
ASTM D 7169
2.3 Asphaltene
The asphaltenes in this work were determined using the standard ASTM D6560 (IP143/01) method. The samples were mixed with a certain proportion of heptane, and then the mixture were heated under reflux. The precipitated waxy substances, asphaltenes, and inorganic materials were collected on a filter paper. The waxy substances were further removed by washing with hot heptane, and then the asphaltenes were extracted from inorganic materials and asphaltenes mixture by refluxing toluene. After evaporating most toluene from the toluene extracts, the remaining toluene extracts were dried in a vacuum oven until there was no further mass change.
2.4 X-ray photoelectron spectroscopy (XPS) analysis
All the XPS experiments were performed on a Thermo Fisher ESCALAB 250Xi spectrometer using a 150 W monochromatic Al Kα source to provide chemical states and quantitative information on the sample surface. The pass energy of narrow scan was set at 30 eV to obtain high-resolution spectra, and the binding energies were referenced to the C1s line at 284.8 eV from adventitious carbon. Avantage software was used to fit the sulfur spectra. Noteworthy, in the S 2p spectrum, the same type of sulfur appeared as a pair of splitting peaks which were S 2P3/2 of the main peak and S 2p1/2 of the accompanying peak. The double split peak method was used to fit the S 2p spectrum.
2.5 Fourier transform ion cyclotron resonance mass spectroscopy (FT-ICR MS) analysis
The samples were dissolved in toluene (HPLC grade, Fisher Chemical, distilled prior to use) and diluted to 0.2 ∼ 0.5 mg/mL for FT-ICR MS analysis. The sample solutions were analyzed by a 15 Tesla Bruker SolariX XR FT-ICR MS spectrometer with a positive APPI source. The chemical formulas (CcHhOoNnSs) were calculated according to the m/z values, with<1 ppm relative error. The double bond equivalent was calculated by the equation DBE = c + n/2 − h/2 + 1 (c, n, and h were the numbers of the carbon, nitrogen, and hydrogen atoms in the chemical formulas). DBE is a method of calculating unsaturation degree for molecules with known chemical formulas, and it indicates a number of rings plus double bonds to carbon (Mikhaylova et al., 2022; Pinto et al., 2022). DBE method is a better method for determining the unsaturation degree of different species in this work, but this method could give wrong values for complex organic molecules containing multiple elements with different valence states (Mikhaylova et al., 2022; Pinto et al., 2022).
2.6 Solvent deasphalting test
1, feed tank; 2, feed pump; 3, feed preheating furnace; 4, extraction tower; 5, deasphlated oil (DAO) preheating furnace; 6, deoiled asphalt (DOA) preheating furnace; 7, DAO flash column; 8, DOA flash column; 9, DAO collection system; 10, DOA collection system; 11, light oil buffer tank; 12, solvent condenser; 13, solvent tank; 14, solvent pump.
QLVR was firstly pretreated by SDA process, and the schematic diagram of the experimental apparatus presented in Fig. 1. The main steps are as follows: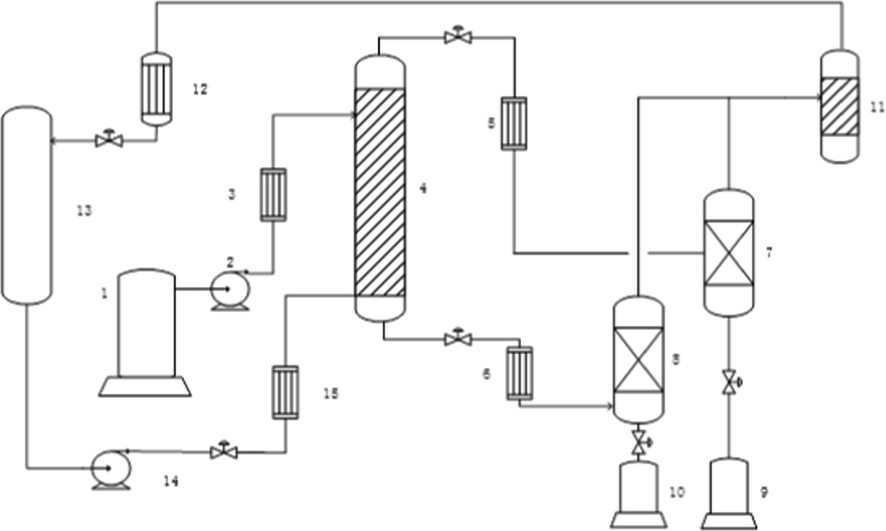
Schematic diagram of solvent deasphalting (SDA) process.
(1) Pressure setting: after gas tightness test and nitrogen replacement, put a certain amount of solvent into the device, and then control the flow by adjusting the solvent pump. When the device pressure reaches the setting value, adjusting the solvent pump to ensure that the flow value was required; (2) Feeding: after the system is heated to the required temperature, adjust the vacuum residue flow through the vacuum residue pump to ensure the solvent to oil ratio; (3) Product collection: the heavy and light components are separated in the extraction tower to obtain DOA and DAO. DOA is discharged from the DOA flash tower, and DAO and solvent is separated in DAO flash column by reducing the pressure; (4) Material balance: The optimized separation conditions (temperature, pressure, solvent/oil ratio) are carried out to ensure the separation efficiency, and the criterion of material balance is there is no DOA and DAO yields change.
The test conditions were shown in Table 2: solvent, n-butane; extraction temperature of SDA1, 125–135 °C; extraction temperature of SDA2, 113–123 °C; pressure, 4.0 MPa; and volume ratio of solvent/oil, 6:1.
The test number
SDA1
SDA2
Processing parameters
Residence time/min
∼ 30
∼ 30
Solvent
n-butane
n-butane
volume of solvent-oil ratio
∼ 6
∼ 6
Extraction temperature/℃
125–135
113 ∼ 123
Extraction pressure/MPa
4.0
4.0
2.7 Slurry-phase hydrocracking test
All the slurry-phase hydrocracking tests were conducted in a batch-type autoclave with a volume of 1.8 L. Slurry-phase hydrocracking reactions were carried out under the following conditions: SDA1-DOA and SDA2-DOA as the feed, 2500 ppm dispersed molybdenum catalyst, initial hydrogen pressure of 9.5 MPa, reaction temperature of 430 °C, stirring speed of 500r/min, and reaction time of 120 min or 240 min respectively.
3 Results and discussion
3.1 Product distribution and properties for solvent deasphalting process of QLVR
3.1.1 Product distribution and upgrading results for solvent deasphalting process
All the property data were analyzed at least two times till the parallel of the assay was quite well. For SDA1 test, it can be seen from the Table 3 that the yield of DAO was 32.1 % and the corresponding yield of DOA was 67.9 %. If decreasing the extraction temperature (Cao et al., 2010), the yield of DAO for SDA2 test was increased to 48.3 % and the corresponding yield of DOA was reduced to 51.7 %. It can be seen from Table 3 that the hydrogen content of SDA1-DAO was slightly higher than that of SDA2-DAO (12.18 % vS 11.96 %), with the opposite trend for S and N content. The hydrogen content of SDA1-DOA was higher than that of SDA2-DOA (10.08 % vS 9.68 %), with the opposite trend for S and N content. DAO has much higher hydrogen and much lower metal and asphaltene content than that of DOA, which could prove the high separation efficiency for SDA process.
SDA results
Items
SDA1
SDA2
DAO yield /%
32.10
48.30
Properties of DAO
Items
SDA1-DAO
SDA2-DAO
Methods
Elements
C/wt%
85.16
85.47
SH/T 0656
H/wt%
12.18
11.96
SH/T 0656
S/wt%
1.45
1.63
GB/T 17,040
N/wt%
0.38
0.44
SH/T 0704
Ni/ppm
7.0
8.9
SH/T 0715
V/ ppm
2.4
3.4
SH/T 0715
Ca/ppm
0.3
0.6
SH/T 0715
w (Asphaltene)/%
< 0.1
< 0.1
Properties of DOA
Items
SDA1-DOA
SDA2-DOA
Elements
C/wt%
84.52
84.21
SH/T 0656
H/wt%
10.08
9.68
SH/T 0656
S/wt%
3.51
4.01
GB/T 17,040
N/wt%
1.06
1.21
SH/T 0704
It can be seen from Table 3, in DAO the metal content for Ni + V + Ca was<15 ppm and the asphaltene content was<0.1 %. DAO could be good feedstock for FCC or hydrocracking process, and the DOA could be taken as the feed of slurry-phase hydrocracking process in this work.
3.1.2 FT-ICR analysis of DAO and DOA derived from solvent deasphalting process
FT-ICR MS is an effective method (Li et al., 2021; Chacón-Patiño et al., 2020) to characterize the heavy oil even for the heaviest fractions rich in asphaltenes. As illustrated in Fig. 2, typical aromatic compounds including HC, S1, S2, S3, N1, O1, et al. classes were detected by FT-ICR MS (Chacón-Patiño et al., 2020; Hsu et al., 2011; Wang et al., 2016). Relative abundance of HC and S1 classes of DAO was much higher than that of DOA, which could prove HC and S1 classes with lower polarity have higher selectivity in solvent extraction for SDA process. Relative abundance of HC and S1 classes of SDA1-DAO was a little higher than that in SDA2-DAO, which could prove SDA1 test have higher selectivity for HC and S1 classes with lower polarity than that of SDA2 test in solvent extraction for process.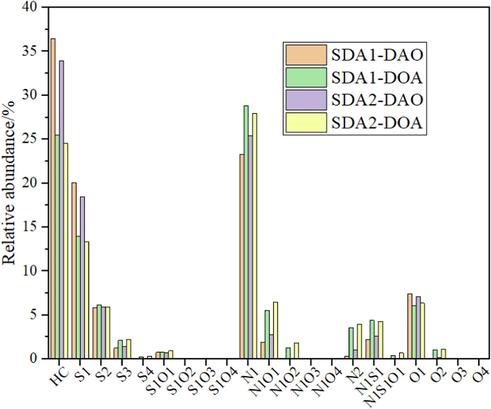
The aromatic species of SDA1-DAO and SDA1-DOA by FT-ICR MS.
The comparisons of DBE distribution of HC class species of DAO and DOA were illustrated in Fig. 3. As seen in Fig. 3, in all DAO and DOA, the DBE of HC class species distributed in a broad range of 4 ∼ 29 for DAO and 4 ∼ 36 for DOA, with corresponding distribution centers at 8–12 for DAO and 10–19 for DOA respectively. The relative abundance of DAO was much higher than that of DOA when DBE < 14, with the opposite trend for DBE greater than 14. The relative abundance of SDA1-DAO was also higher than that of SDA2-DAO when DBE < 14, with the opposite trend for DBE ≥ 14. The relative abundance of SDA1-DOA was higher than that of SDA2-DOA when DBE < 20, with the opposite trend for DBE ≥ 20.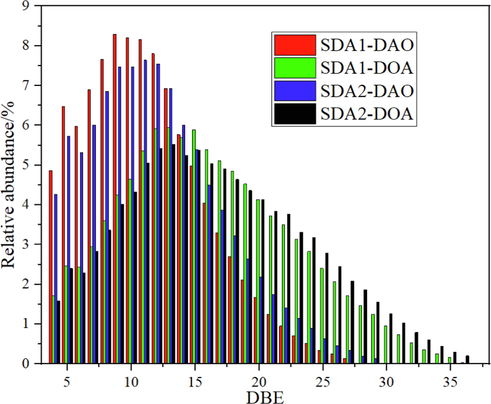
DBE distribution of HC class species of DAO and DOA by FT-ICR MS.
As seen in Fig. 3, the abundance of SDA1-DOA was higher than that of SDA2-DOA when DBE < 20, with the opposite trend for DBE ≥ 20. The most abundance HC classes were located at DBE = 10–19, and the adjusted relative abundance as a function of CN for HC class species with DBE = 10, 13, 16 and 19 was illustrated in Fig. 4. For both SDA1-DOA and SDA2-DOA, the CN distribution was centered at 45 ∼ 46, and the relative abundance of HC class species of SDA1-DOA shows a little higher around the CN center at 45 ∼ 46 than that of SDA2-DOA.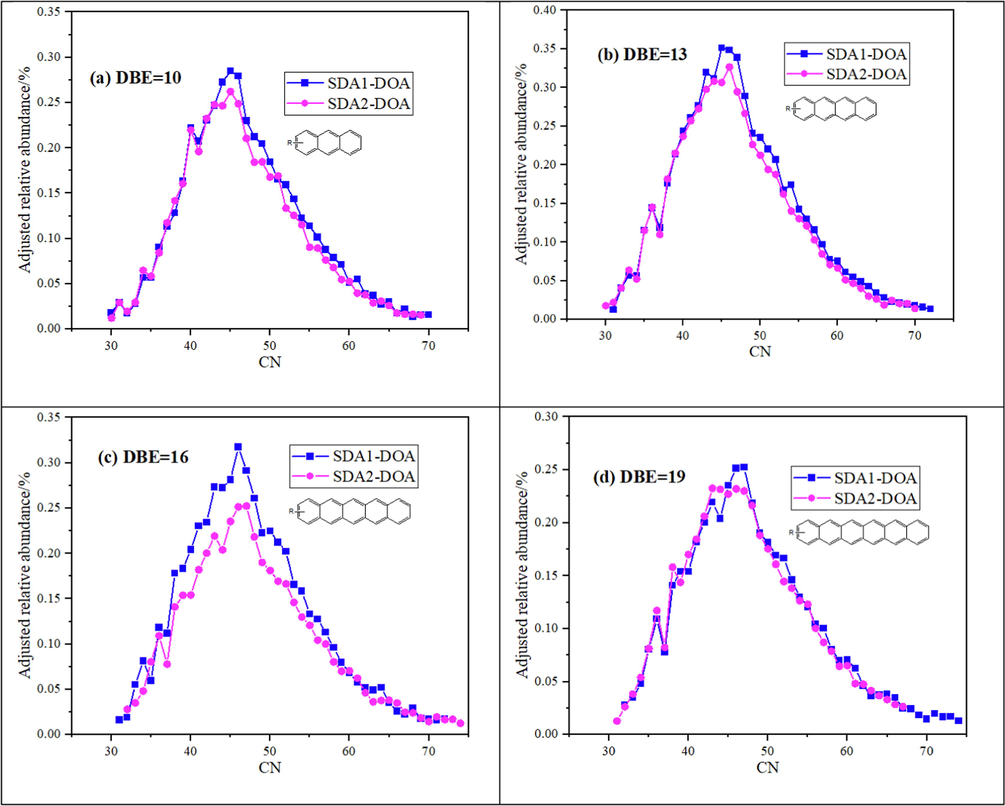
HC compounds of SDA1-DOA and SDA2-DOA with DBE = 10, 13, 16, and 19 distribution. The relative abundance was adjusted by its absolute HC content, and the skeletons are shown only for illustrative purposes.
The comparisons of CN distribution of HC class species of DAO and DOA were illustrated in Fig. 5. As seen in Fig. 5, in all DAO and DOA, the HC class species distributed in a broad range of CN (28 ∼ 73), with corresponding distribution centers at 42–47. The relative abundance of DAO was higher than that of DOA when CN < 46, with the opposite trend for CN ≥ 46. The relative abundance of SDA1-DAO was also higher than that of SDA2-DAO when CN < 45, with the opposite trend for CN ≥ 45. The abundance of SDA1-DOA was also higher than that of SDA2-DOA when CN < 46, with the opposite trend for CN ≥ 46.
CN distribution of HC class species of DAO and DOA by FT-ICR MS.
It can be concluded that the SDA process in this work not only can obtain different DAO and DOA yields but also obtain measurably different DAO and DOA properties. The n-butane solvent in SDA process has higher selectivity for HC class, and correspondingly the relative abundance of HC class of DAO was much higher than that of DOA. The abundance of DAO was higher than that of DOA when DBE < 14 or CN < 46. The relative abundance of SDA1-DAO was higher than that of SDA2-DAO when DBE < 14 or CN < 45. The relative abundance of SDA1-DOA was higher than that of SDA2-DOA when DBE < 20 or CN < 46.
3.2 Slurry-phase hydrocracking process for DOA and VR
3.2.1 Product distribution and upgrading results for DOA and VR in slurry-phase hydrocracking process
Taking the conversion ratio and the stability control into account, the operating parameters listed in Table 4 were taken in SHC process. The distillation range of the products were obtained by ASTM D7169 method. The conversion depth for each experiment could be evaluated by CRSHC in Equation (1).
Items
SHC1-120 min
SHC1-240 min
SHC2-120 min
SHC2-240 min
Reaction time/min
120
240
120
240
Initial pressure/MPa
9.5
9.5
9.5
9.5
Feed
SDA1-DOA
SDA1-DOA
SDA2-DOA
SDA2-DOA
Products/%
Gas
8.66
10.80
9.10
11.40
naphtha(IBP ∼ 180 °C)
7.91
11.02
9.00
12.01
AGO(180–350 °C)
22.10
30.79
23.45
32.79
VGO(350–524 °C)
28.25
29.03
28.64
29.51
Residue( > 524 °C)
33.08
18.36
29.81
14.29
Total/%
100.00
100.00
100.00
100.00
CRSHC /%
66.92
81.64
70.19
85.71
CRSHC represents the conversion ratio of DOA in slurry-phase hydrocracking reaction, %. in this work represents the yield of × in slurry-phase hydrocracking process, %. The CRSHC of SDA1-DOA for SHC1-120 min and SHC1-240 min reactions were 66.92 % and 81.64 % respectively, and the CRSHC of SDA2-DOA for SHC2-120 min and SHC2-240 min reactions were 70.19 % and 85.71 % respectively. Under the same experimental conditions, the reaction time has a great influence on the conversion, and the longer the reaction time was, the higher conversion ratio could be. The conversion ratio of DOA could be more than 80 % for 240 min reaction.
3.2.2 Comparisons of product distribution and upgrading results for different DOA in slurry-phase hydrocracking process
There is an interesting phenomenon that under the same experimental conditions, heavier DOA has higher CRSHC in SHC process. The CRSHC of SDA2-DOA was higher than that of SDA1-DOA for 120 min and 240 min reactions respectively, while SDA2-DOA has lower H content than that of SDA1-DOA. Because dispersed catalysts in slurry-phase hydrocracking process is lack of acidic sites for cracking (Kang et al., 2021; Cui et al., 2021), slurry-phase hydrocracking process still follows the mechanism of free radicals (Du et al., 2015). Hydrocarbons are decomposed through thermal cracking by free radicals because dispersed catalyst shows no catalytic function for C—C cleavage (Kang et al., 2021), so the conversion from heavy oil to light products is driven by thermal cracking. During this reaction, H2 molecule splits to hydrogen free radicals through homolytic and heterolytic ways on dispersed catalysts, and those hydrogen free radicals could quench with macromolecular free radicals (Zhang et al., 2008; Du et al., 2015). This process can greatly inhibit secondary radical reactions that lead to the formation of coke precursors and gases (Prajapati et al., 2021; Kang et al., 2021).
In slurry-phase hydrocracking process, the macromolecules convert to smaller molecules by hydrogenlysis and side-chain breaking (Ortiz-Moreno et al., 2012). The bond energy of C—S is much lower, and sulfur can abstract H from hydrocarbon molecule to form free radicals even at a lower temperature (Nguyen et al., 2021; Chang et al., 2001). Sulfur was an effective free-radical initiator to enhance the reaction (Chang et al., 2001).
As seen in Table 3, S content of SDA2-DOA was higher than that of SDA1-DOA. For further analyzing sulfur species and distribution, XPS spectra were recorded as seen in Fig. 6 and Table 5, and the sulfur functional groups contain alkyl sulfur and thiophene sulfur and sulfur oxide (Wang et al., 2021). SDA2-DOA had higher sulfur content but a little lower thiophene sulfur content than that of SDA1-DOA, which could be the reason that SDA2-DOA had higher conversion ratio under the same reaction conditions. As illustrated in Fig. 2, the sum of S-containing structures (S1, S2, S3, S1O1, S1O2, S1O3, S1O4, N1S1, and N1S1O1) of SDA2-DOA was a little lower than that of SDA1-DOA, which could give an indirect identification of lower thiophene sulfur content for SDA2-DOA.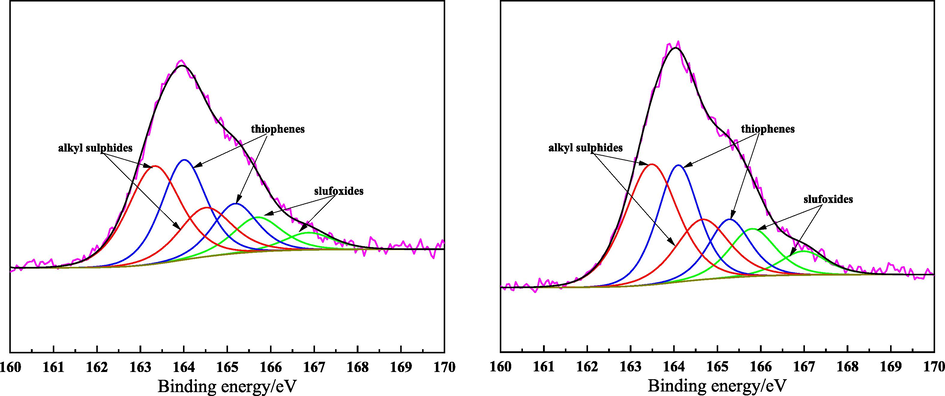
XPS spectra curve-resolution of S in SDA1-DOA (left) and SDA2-DOA (right).
Element peak
functionalities
SDA1-DOA
SDA2-DOA
S 2p
alkyl sulphides
45.88 %
47.59 %
thiophenes
39.20 %
35.99 %
sulfoxides
14.92 %
16.42 %
3.2.3 Stability analysis for DOA in slurry-phase hydrocracking process
mTot means total mass of the feed.
Coking resulting from asphaltene precipitation is still one of the biggest concerns in heavy oil processing industry and causes equipment blocking and catalyst deactivation (Wiehe, et al., 2000; Wiehe, et al., 2001; Xu, 2018; Wang et al., 2009; Liu et al., 2019); and toluene insoluble (TI) content obtained by GB/T2292 method is an important coking index for SHC process. As seen in Table 6 for SDA1-DOA,
increased to 2.39 % from 1.62 % and
decreased to 1.18 % from 4.13 % when reaction time increased to 240 min from 120 min respectively. For SDA2-DOA,
increased to 3.10 % from 2.14 % when reaction time increased to 240 min from 120 min respectively, with opposite trend for
. The overall conversion of asphaltene decomposition including coke formation has been found to follow first-order reaction (Phama et al., 2020), and the quantity and quality of asphaltenes play main factor for coke formation in residue hydroprocessing (Almutairi et al., 2007). The quality of asphaltenes of SDA1-DOA and SDA2-DOA was the same, but the quantity of asphaltenes followed the order SDA2-DOA > SDA1-DOA. SDA2-DOA (C7-Asphaltene% = 11.1 %) had higher asphaltene content than that of SDA1-DOA (C7-Asphaltene% = 8.5 %), which could be the main reason that SHC2-120 min and SHC2-240 min reactions have higher
than that of SHC1-120 min and SHC1-240 min reactions respectively.
Items
/%
/%
SHC1-120 min Reaction
4.13
1.62
SHC1-240 min Reaction
1.18
2.39
SHC2-120 min Reaction
6.68
2.14
SHC2-240 min Reaction
2.04
3.10
Except for the quantity of
and
, it’s important to investigate asphaltene structure changing rules in high conversion process to evaluate the reaction. The comparisons of asphaltenes analyzed by FT-ICR MS were illustrated in Fig. 7. As illustrated in Fig. 7, typical aromatic compounds including HC, S-containing, O-containing, and N-containing structures of secondary asphaltenes were detected, and N1 compounds were the most abundant aromatic compounds and had the most significant increase with increasing the reaction time. Furthermore, the organic nitrogen compounds present in the feed in hydrocracking process, act as gum and coke precursors that cause asphaltene precipitation and catalyst inhibition and deactivation (Sau, et al., 2005; Liu, et al., 2012; Bello et al., 2021) DBE versus CN distributions of aromatic N1 compounds of asphaltenes were shown in Fig. 8, and the size of the bubble indicates the relative abundance of the compound. For those asphaltenes, the carbon number distribution varied from about 30 to 70, and the DBE distribution varied from about 15 to 45. The size of the center got smaller, which could be derived from lighter components or coke formation by asphaltene conversion. For further analyzing the asphaltene structure, DBE distribution of N1 compounds of different asphaltenes was shown in Fig. 9. As a result, in all asphaltenes, the N1 class species distributed in a broad range of DBE (15 ∼ 40), with corresponding distribution centers at 26–31. When DBE greater than 31, the relative abundance of N1 compounds followed the order SHC2-240 min-Asp > SHC1-240 min-Asp, which agreed with the trend for TI yields.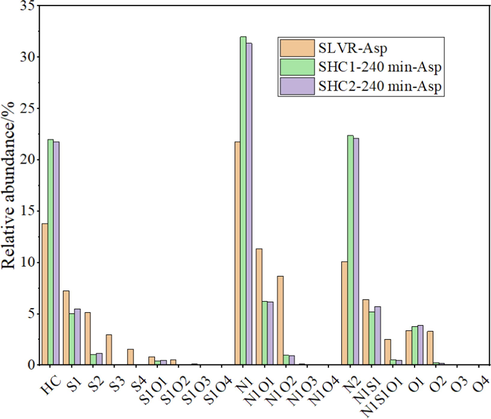
The aromatic species of the different asphaltenes by FT-ICR MS.
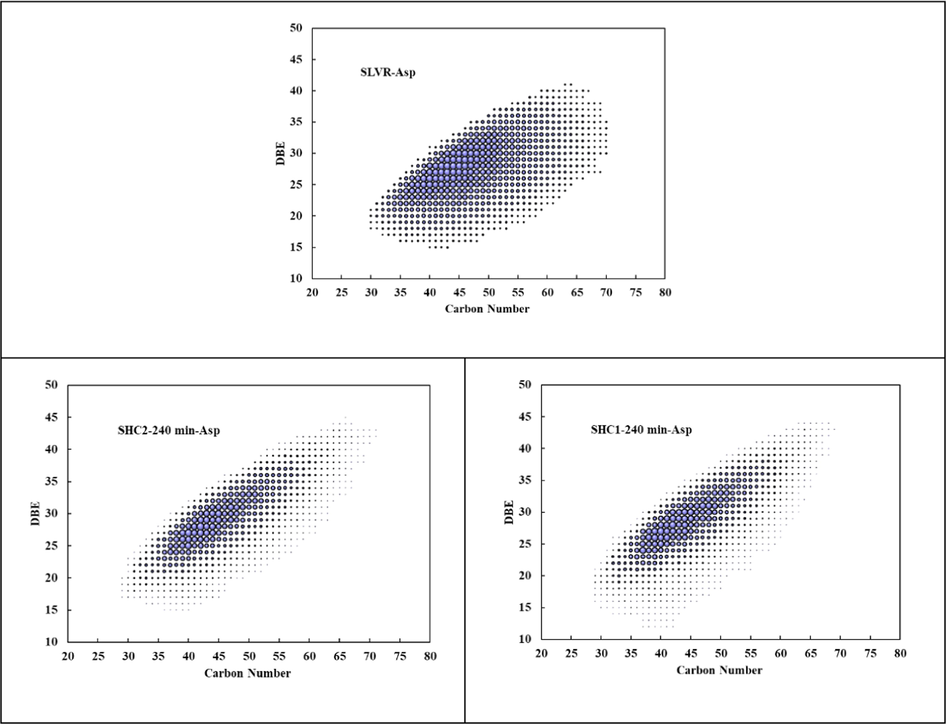
DBE vS carbon number distribution of aromatic N1 compounds of different asphaltenes.
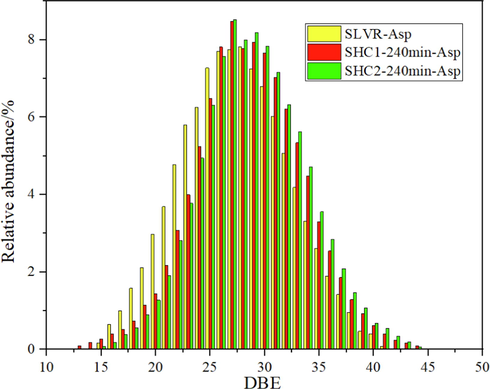
DBE distribution of N1 compounds of different asphaltenes.
4 Conclusions
With optimized operating conditions, the yields of DAO were 32.1 % and 48.3 % for SDA1 and SDA2 tests respectively. The metal content for Ni + V + Ca was<20 ppm and the asphaltene content was<0.1 % in DAO. DAO could be good feedstock for FCC or hydrocracking process, and the DOA could be taken as the feed of slurry-phase hydrocracking process. SDA process for QLVR had great efficiency to obtain different yields and properties of DAO and DOA respectively. The n-butane solvent in SDA process had higher selectivity for HC class, and correspondingly the relative abundance of HC class of DAO was much higher than that of DOA. The abundance of DAO was higher than that of DOA when DBE < 14 or CN < 46, and the relative abundance of SDA1-DOA was higher than that of SDA2-DOA when DBE < 20 or CN < 46.
DOA was taken as the feed of slurry-phase hydrocracking process, and the conversion ratio of deoiled asphalt in slurry-phase hydrocracking process could be more than 80 % for 240 min reaction. The conversion ratios of SDA1-DOA for SHC1-120 min and SHC1-240 min reactions were 66.92 % and 81.64 % respectively, and the conversion ratios of SDA2-DOA for SHC2-120 min and SHC2-240 min reactions were 70.19 % and 85.71 % respectively. SDA2-DOA had higher sulfur content but a little lower thiophene sulfur content than that of SDA1-DOA, which could be the reason that SDA2-DOA had higher conversion ratio under the same reaction conditions. The relative abundance of N1 compounds followed the order SHC2-240 min-Asp > SHC1-240 min-Asp when DBE > 31, which agreed with the trend of TI yields . Those methods in solvent deasphalting and slurry-phase hydrocracking process in this work can play a good guidance role in vacuum residue processing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advances in heavy oil upgrading using dispersed catalysts. Energy Fuels. 2019;33:7917-7949.
- [Google Scholar]
- A comparative study on the performance of a catalyst system for the desulfurization of two kinds of atmospheric residues, Kuwait Export and Eocene residual oils. Catal. Today. 2007;125:203-210.
- [Google Scholar]
- A review on the reaction mechanism of hydrodesulfurization and hydrodenitrogenation in heavy oil upgrading. Energy Fuels. 2021;35:10998-11016.
- [Google Scholar]
- Hydroconversion of heavy residues in slurry reactors: developments and perspectives. J. Catal.. 2013;308:189-200.
- [Google Scholar]
- Process analysis of the extract unit of vacuum residue through mixed C4 solvent for deasphalting. Chem. Eng. Process.. 2010;49:91-96.
- [Google Scholar]
- Advances in asphaltene petroleomics. part 4. compositional trends of solubility subfractions reveal that polyfunctional oxygen-containing compounds drive asphaltene chemistry. Energy Fuels. 2020;34:3013-3030.
- [Google Scholar]
- Elemental sulfur as an effective promoter for the catalytic hydrocracking of Arabian vacuum residue. Fuel. 2001;80:1639-1643.
- [Google Scholar]
- Mo supported on natural rectorite catalyst for slurry-phase hydrocracking of vacuum residue: an effect of calcination. Petrol. Sci.. 2021;18:1867-1876.
- [Google Scholar]
- Exploratory investigation for the coking behavior during slurry-bed hydrocracking of coal tar atmospheric residue. Energy Fuels. 2016;30:8623-8629.
- [Google Scholar]
- Slurry-phase hydrocracking of heavy oil and model reactant: effect of dispersed Mo catalyst. Appl. Petrochem. Res.. 2015;5:89-98.
- [Google Scholar]
- Effects of the temperature and initial hydrogen pressure on the isomerization reaction in heavy oil slurry-phase hydrocracking. Energy Fuels. 2015;29:626-633.
- [Google Scholar]
- Role of hydrogen pressure in slurry-phase hydrocracking of Venezuela heavy oil. Energy Fuels. 2015;29:2104-2110.
- [Google Scholar]
- Ligand structure effect in oil-soluble phosphorus-containing molybdenum precursors for slurry-phase hydrocracking of heavy oil. J. Catal.. 2021;402:194-207.
- [Google Scholar]
- Effects of dispersed MoS2 catalysts and reaction conditions on slurry phase hydrocracking of vacuum residue. J. Catal.. 2017;347:127-137.
- [Google Scholar]
- Structure and activity of dispersed Co, Ni, or Mo sulfides for slurry phase hydrocracking of vacuum residue. J. Catal.. 2018;364:131-140.
- [Google Scholar]
- Reactivity of vacuum residues by thermogravimetric analysis and nuclear magnetic resonance spectroscopy. Energy Fuels. 2020;34:9231-9242.
- [Google Scholar]
- The solubility of asphaltene in organic solvents and its relation to the molecular structure. J. Mol. Liq.. 2021;327:114826
- [Google Scholar]
- Preliminary study on the influence of catalyst dosage on coke formation of heavy oil slurry-bed hydrocracking. Fuel. 2020;270:117489
- [Google Scholar]
- Effect of reaction temperature and time on the products and asphaltene dispersion stability in slurry-phase hydrocracking of vacuum residue. Fuel. 2018;234:305-311.
- [Google Scholar]
- Investigation of asphaltene dispersion stability in slurry-phase hydrocracking reaction. Fuel. 2020;271:117-509.
- [Google Scholar]
- FT-ICR MS Analysis of nitrogen-containing compounds in the products of Liaohe atmospheric residue hydrocracking. Energy Fuels. 2012;26:624-628.
- [Google Scholar]
- Coke yield prediction model for pyrolysis and oxidation processes of low-asphaltene heavy oil. Energy Fuels. 2019;33:6205-6214.
- [Google Scholar]
- Slurry phase hydrocracking of vacuum residue in the presence of presulfided oil-soluble MoS2 catalyst. Fuel. 2019;246:133-140.
- [Google Scholar]
- Investigation of the liquid recycle in the reactor cascade of an industrial scale ebullated bed hydrocracking unit. Chin. J. Chem. Eng.. 2019;27:298-304.
- [Google Scholar]
- Molecular analysis of nitrogen-containing compounds in vacuum gas oils hydrodenitrogenation by (ESI+/-)-FTICR-MS. Energy Fuels. 2022;323:124302
- [Google Scholar]
- A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil. J. Ind. Eng. Chem.. 2016;43:1-12.
- [Google Scholar]
- Recent advances in asphaltene transformation in heavy oil hydroprocessing: Progress, challenges, and future perspectives. Fuel Process. Technol.. 2021;213:106681
- [Google Scholar]
- Heavy oil upgrading at moderate pressure using dispersed catalysts: effects of temperature, pressure and catalytic precursor. Fuel. 2012;100:186-192.
- [Google Scholar]
- Hydrocracking and hydrotreating reaction kinetics of heavy oil in cstr using a dispersed catalyst. J. Petrol. Sci. Eng.. 2021;197:107-997.
- [Google Scholar]
- Kinetic study of thermal and catalytic hydrocracking of asphaltene. Catal. Today. 2020;353:112-118.
- [Google Scholar]
- Pinto, F.E., Fonseca, V.R., Souza, L. M., Terra, L.A., Subramanian, S., Simon, S., Sjöblom, J., Pereira, T.M., Jr, V.L., Romão, W., 2022. Asphaltenes subfractions characterization and calculation of their solubility. Fuel 312, 122864.
- Slurry phase hydrocracking of heavy oil and residue to produce lighter fuels: an experimental review. Fuel. 2021;288:119686
- [Google Scholar]
- A review of recent advances in catalytic hydrocracking of heavy residues. J. Ind. Eng. Chem.. 2015;27:12-24.
- [Google Scholar]
- Effects of organic nitrogen compounds on hydrotreating and hydrocracking reactions. Catal. Today. 2005;109:112-119.
- [Google Scholar]
- Design and preparation of nanoscale catalysts for slurry bed hydrocracking using the microemulsion method. Energy Fuels. 2016;30:10777-10782.
- [Google Scholar]
- Different mechanisms of coke precursor formation in thermal conversion and deep hydroprocessing of vacuum residue. Energy Fuels. 2016;30:8171-8176.
- [Google Scholar]
- Phase separation and colloidal stability change of Karamay residue oil during thermal reaction. Energy Fuels. 2009;23:3002-3007.
- [Google Scholar]
- The chemical structure and thermal evolution of oil Sands bitumen: experimental and molecular simulation study. J. Anal. Appl. Pyrol.. 2021;158:105271
- [Google Scholar]
- Application of the oil compatibility model to refinery streams. Energy Fuels. 2000;14:60-63.
- [Google Scholar]
- Asphaltene precipitation in paraffinic froth treatment: effects of solvent and temperature. Energy Fuels. 2018;32:2801-2810.
- [Google Scholar]
- Slurry-phase residue hydrocracking with dispersed nickel catalyst. Energy Fuels. 2008;22:3583-3586.
- [Google Scholar]
- A review of slurry-phase hydrocracking heavy oil technology. Energy Fuels. 2007;21:3057-3062.
- [Google Scholar]







