Translate this page into:
Synthesis and physicochemical studies of some new quinolinoxazine pentamethine cyanine dyes
⁎Corresponding author. Tel.: +20 643341071; fax: +20 623666420. zahraaessam@yahoo.com (Z.M. Essam)
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University

Abstract
Polymethine cyanine dyes belong to a well-known class of organic compounds, which have been used in photography and as information storage in laser technology. A series of novel cyanine dyes were synthesized through the formylation of quinolinium[b,c]1,4-oxazine-chloride salt 1. Reaction of compound 2-chloro-3-formyl-quinolinium[b,c]1,4-oxazine-chloride salt 2 with different molar ratios of 2(4)-methyl substituted heterocyclic in basic catalysis afforded the corresponding 2-chloroquinolinium[b,c]1,4-oxazine-chloride salt-3[2(4)]-dimethine (3a–c), quinolinium[b,c]-1,4-oxazinechloride salt-2,3[2(4)]-pentamethine (4a–c) and quinolino[b,c]1,4-oxazine-6yl[2(4)]-monomethine-/2,3[2(4)]-pentamethine cyanine dyes (5a–c) respectively. The structure of the dyes was characterized by elemental analysis, visible absorption, fluorescence emission, IR, 1H-NMR and mass spectroscopy. The correlations between the structure and properties of these dyes have been studied. A comparison of the visible absorption maxima between compounds 3b, 4b and 5b showed that asymmetrical mono-pentamethine cyanine dye 5b reveals a bathochromic shift than both dimethine 3b and pentamethine cyanine dyes 4b. This could be attributed to the more extensive π-conjugation and increasing number of methine groups in asymmetric pentamethine. All the observations and analytical spectra in this paper support the syntheses of new di-, penta- and mono-/pentamethine cyanine dyes. The absorption spectra of dyes were investigated in organic solvents. The results indicated that the excitation for their colour is a simple charge-transfer from oxygen atom of oxazine nucleus and/or nitrogen atom of pyridine (quinoline) nucleus to N-quaternary salts in di-, penta- and mono-/pentamethine cyanine dyes respectively. These dyes showed positive solvatochromism with increased solvent polarity, which depends on the structure and the type of dye.
Keywords
Quinolinoxazine
Synthesis
Electronic absorption spectra
Fluorescence emission
Solvatochromic behaviour
1 Introduction
There is growing interest in our group toward the synthesis of N-bridgehead heterocyclic compounds in view of their use in the synthesis of cyanine dyes (Abd El-Aal, 1998, 1999, 2006; Koraiem et al., 2002, 2006). Polymethine cyanine dyes belong to a well-known class of organic compounds, which have been used as data storage materials and organic semiconductor materials (Mustroph and Stollenwerk, 2006; Spitler and Parkinson, 2009), in laser technology (Chatterjee et al., 1988; Yabushita et al., 2004; Shank and Ippen, 1973), and as spectral sensitizers in photography (Tani et al., 1992).
Quinoxazine compounds have a wide spectrum range of biological activities and pharmaceutical actions like kinase inhibitor activity and antibacterial activity (He et al., 2003; Seitz et al., 2002). In addition, quinoxazine compounds are electroluminescent materials (Thomas et al., 2005) and organic semiconductors (Brien et al., 1996).
This paper reported the synthesis of some novel N-bridgehead heterocycles dimethine, pentamethine, and mono/pentamethine cyanine dyes, and evaluated the structure–properties relationship of the new dyes on the basis of their visible absorption spectra/fluorescence emission in ethanol. Also, photophysics studies in different organic solvents are discussed, which might be used as photosensitizer dyes in different optical applications.
2 Experimental
All melting points are uncorrected. Elemental analysis was carried out at the Microanalytical center (Cairo-University). The IR (νKBr) spectra were determined with Perkin Elmer Infrared 127ß spectrophotometer (Cairo-University). 1H-NMR spectra were recorded with a Bruker AMX-250 spectrometer. 1H-NMR, spectra were measured with a Bruker AMX-400 spectrometer, with TMS as an internal standard. Mass spectra were recorded on an HpMs 6988 spectrometer (Cairo University). The electronic absorption spectra were recorded within the wavelength range (350–700) on Shimadzu-1601VC UV/Visible automatic recording spectrophotometer with 1 cm quartz cell used for absorbance and spectra measurements, Faculty of Science, Suez. The fluorescence emission spectra were recorded within the wavelength range (480–600) on JASCO-FB6300 spectrofluorometer with 1 cm quartz cell emission and spectra measurements, Faculty of Science, Suez. The synthesis of quinolinium[b,c]-1,4-oxazine-2-one chloride salt 1, was carried out according to Abd El-Aal (1998).
2.1 Synthesis of 2-chloro-3-formyl-quinolinium[b,c]-1,4-oxazine chloride salt 2
To a solution of compound 2 (3 g, 0.013 mol) in (10 mL) dry dimethylformamide and phosphorous oxychloride (5 mL, 0.033 mol) were added under stirring in an ice-bath. The second step was stirring at room temperature for 15 min. The solution was heated for 30 min, cooled and then poured into 400 mL ice-water. The solid product was collected and crystallized from petroleum ether 60–80 °C; m.p. > 300 °C, Yield = 80%.
Analytical data for C12H7NO2Cl (M.wt = 232.5).
Calcd.%; C = 61.94; H = 3.01; N = 6.02.
Found%; C = 61.79; H = 2.97; N = 5.87.
2.2 Synthesis of 2-chloro-quinolinium[b,c]-1,4-oxazine-3[2(4)]-dimethine cyanine dyes (3a–c)
A mixture of equimolar amounts of compound 2, (0.1 g, 0.0004 mol) and methyl substituted heterocyclic [α-(γ)-picoline and quinaldine ethyl iodide] (0.11 g, 0.0004 mol) were dissolved in ethanol (20 mL) in the presence of piperidine (0.7 mL) as basic catalyst. The reaction mixture was refluxed for 16 h. filtered hot, cooled and neutralized with acetic acid (0.3 mL). The precipitated solid, after dilution with water (5 mL) was collected and recrystallized from ethanol to give the corresponding compounds (3a–c).
Characterization data are listed in Table 1.
Comp. No.
M.P. °C
Yield %
Colour
Mol. formula (Mol. wt.)
Calcd.%
(Found)%Absorption spectra in EtOH
C
H
N
λmax (nm)
ɛmax × 103 (L mol−1 cm−1)
3a
280
75
Reddish violet
C20H17N2OICl2
48.19
3.41
4.81
358
3.33
(498)
(48.00)
(3.30)
(4.70)
3b
>300
89
Intense violet
C24H19N2OICl2
52.55
3.46
4.37
405sh
3.00
(548)
(52.00)
(3.44)
(4.20)
432sh
2.90
519sh
3.30
560
5.80
587
5.60
3c
>300
80
Violet
C20H17N2OICl2
48.19
3.41
4.81
359
3.80
(498)
(48.10)
(3.00)
(4.70)
4a
>300
76
Brownish violet
C28H27N3OICl
57.58
4.26
7.19
478
1.30
(583.50)
(57.50)
(4.00)
(7.15)
4b
>300
90
Intense violet
C36H31N3OICl
63.20
4.53
6.14
485sh
11.00
(683.50)
(63.00)
(4.40)
(6.00)
519
16.00
561
19.00
600sh
10.00
4c
>300
78
Reddish violet
C28H27N3OICl
57.58
4.62
7.19
488
1.50
(583.50)
(57.00)
(4.50)
(7.15)
5a
>300
75
Reddish violet
C36H36N4OI2
54.40
4.53
7.10
467sh
1.55
(794)
(54.00)
(4.40)
(6.96)
5b
>300
89
Intense violet
C48H42N4OI2
61.00
4.45
5.93
518sh
11.00
(944)
59.90
(4.40)
(5.88)
560sh
16.00
597
19.00
696sh
2.70
5c
>300
77
Violet
C36H36N4OI2
54.40
4.53
7.10
387sh
1.30
(794)
(54.00)
(4.40)
(6.96)
485sh
1.60
Compound 3a: IR (νKBr, cm−1), at 3010 cm−1 (Ar-C–H, str.), 2980–2940 cm−1 (C2H5I, str.), 1600–1580 cm−1 (C⚌C ring, str.), 1430 cm−1 (C⚌N), 1090 cm−1 (Ar-Cl), 1050 cm−1 (C–O–C cyclic) and 805–785 cm−1 (⚌C–H bend. benzene ring), 748, 705 cm−1 (pyridine ring).
Compound 3a: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 7.02–8.01 (m, 12H, Ar-H + heter-H + CH⚌CH), 3.9 (q, 2H, CH2–N+), 1.9 (t, 3H, CH3).
Compound 3a: Mass spectroscopy M++4 = 502.
Compound 3b: IR (νKBr, cm−1), at 3030 cm−1 (Ar-C–H, str.), 2970–2920 cm−1 (C2H5I, str.), 1600–1585 cm−1 (C⚌C ring, str.), 1435 cm−1 (C⚌N), 1085 cm−1 (Ar-Cl), 1080 cm−1.
(C–O–C cyclic) and 795–745 cm−1 (⚌C–H bend. quinoline ring).
Compound 3b: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 6.75–7.75 (m, 14H, Ar-H + heter-H + CH⚌CH), 4.1 (q, 2H, CH2–N+), 1.75 (t, 3H, CH3.
Compound 3b: Mass spectroscopy M++4 = 552.
2.3 Synthesis of quinolinium[b,c]-1,4-oxazine-chloride salt-2,3,[2(4)]-pentamethine cyanine dyes (4a–c)
A mixture of compound 2, (0.1 g, 0. 0004 mol) and bi molar amounts of methyl substituted heterocyclic [α-(γ)-picoline and quinaldine ethyl iodide] (0.22 g, 0.0008 mol) were fused in (2–3 mL) piperidine for 15 min. The reaction mixture was dissolved in ethanol and refluxed for 13 h, filtered hot, concentrated and dried to give compounds (4a–c). Characterization data are listed in Table 1.
Compound 4b: IR (νKBr, cm−1), at 3010 cm−1 (Ar-C–H, str.), 2960–2920 cm−1 (C2H5I, str.), 1600–1580 cm−1 (C⚌C ring, str.), 1430 cm−1 (C⚌N), 1050 cm−1 (C–O–C cyclic) and 765–730 cm−1 (⚌C–H bend. quinoline ring).
Compound 4b: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 6.65–7.85 (m, 21H, Ar-H + heter-H + CH⚌CH), 4.3 (q, 2H, CH2–N+), 2.3 (q, CH2–N),1.75 (t, 3H, CH3), 1.02 (t, 3H, CH3).
Compound 4b: Mass spectroscopy M++2 = 685.
Compound 4c: IR (νKBr, cm−1), at 3030 cm−1 (Ar-C–H, str.), 2980–2940 cm−1 (C2H5I, str.), 1600–1585 cm−1 (C⚌C ring, str.), 1430 cm−1 (C⚌N), 1050 cm−1 (C–O–C cyclic) and 775–745 cm−1 (⚌C–H bend. quinoline ring), 745, 705 cm−1 (pyridine ring).
Compound 4c: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 6.75–8.15 (m, 17H, Ar-H + heter-H + CH⚌CH), 4.25 (q, 2H, CH2–N+), 2.1 (q, CH2–N), 1.65 (t, 3H, CH3), 1.10 (t, 3H, CH3).
Compound 4c: Mass spectroscopy M++2 = 635.
2.4 Synthesis of quinolino[b,c]-1,4-oxazine-6yl[2(4)]-monomethine-2,3[2(4)]-pentamethine cyanine dyes (5a–c)
A mixture of compound 2, (0.1 g, 0. 0004 mol) and tri molar amounts of methyl substituted heterocyclic [α-(γ)-picoline and quinaldine ethyl iodide] (0.33 g, 0.001 mol) were dissolved in ethanol (20 mL) in presence of piperidine (0.7 mL) and refluxed for 16 h. The products were similarly obtained using the same method for compounds 4a–c to give compounds 5a–c. Characterization data are listed in Table 1.
Compound 5a: IR (νKBr, cm−1), at 3030 cm−1 (Ar-C–H, str.), 2980–2940 cm−1 (C2H5I, str.), 1600–1585 cm−1 (C⚌C ring, str.), 1430 cm−1 (C⚌N), 1050 cm−1 (C–O–C cyclic) and 785–750 cm−1 (⚌C–H bend. quinoline ring), 745, 705 cm−1 (pyridine ring).
Compound 5a: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 6.55–8.05 (m, 21H, Ar-H + heter-H + CH⚌CH), 4.25 (q, 4H, CH2–N+), 2.1 (q, 2H, CH2–N),1.75 (t, 6H, CH3), 1.10 (t, 3H, CH3).
Compound 5a: Mass spectroscopy M++1 = 795.
Compound 5b: IR (νKBr, cm−1), at 3010 cm−1 (Ar-C–H, str.), 2970–2930 cm−1 (C2H5I, str.), 1600–1585 cm−1 (C⚌C ring, str.), 1435 cm−1 (C⚌N), 1050 cm−1 (C–O–C cyclic) and 785–740 cm−1 (⚌C–H bend. quinoline ring).
Compound 5b: 1H-NMR (DMSO, 300 MHz) spectra reveal signals at δ 6.50–7.95 (m, 27H, Ar-H + heter-H + CH⚌CH), 4.3 (q, 2H, CH2–N+), 2.15 (q, CH2-N),1.65 (t, 3H, CH3), 1.02 (t, 3H, CH3).
Compound 5b: Mass spectroscopy M++1 = 945.
3 Results and discussion
All titled synthesized cyanine dyes (3a–c), (4a–c) and (5a–c), were started by the preparation of 2-chloro-3-formyl quinolinium[b,c]-1,4-oxazine-chloride salt 2 which was prepared via the Vilsmeier reaction through treating the compounds 1 (Abd El-Aal, 1998) with phosphorous oxychloride in dimethylformamide at 0 °C Scheme1.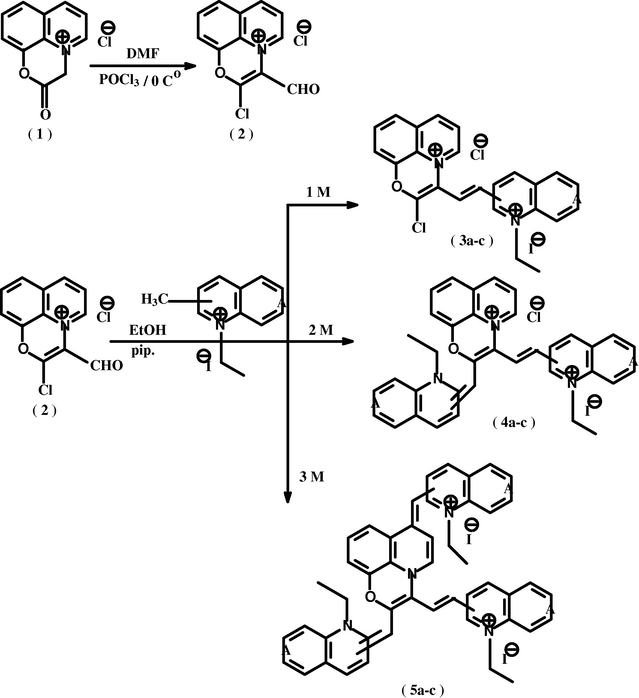
(3a–c), (4a–c), (5a–c): A = 1-ethylpyridin-2-ium iodide (a); A = 1-ethylquinolin-2-ium iodide (b); A = 1-ethylpyridin-4-ium iodide (c).
The structure of compound 2 was established on analytical and spectral data. IR spectra showed characterization absorption bands at 3010 cm−1 (aromatic C–H, str.), 2780 cm−1 (aldehydic C–H, str.), 1700 cm−1 (CHO), 1605 cm−1 (C⚌C ring, str.), 1090 cm−1 (Ar-Cl), 1050 cm−1 (C–O–C cyclic) and 745 cm−1 (⚌C–H bend. For disubst. benzene ring). 1H-NMR (DMSO, 300 MHz) spectra (Wade, 1999) reveal signals at δ = 6.8–7.9 ppm (m, 6H, Ar-H), and at δ = 9.5 ppm (s, 1H, CHO) for compound 2. Mass spectroscopy of the compound 2 M+ = 233.5.
Reaction of 2 with equimolar ratios of 2-(4)-ethyl-substituted heterocyclic quaternary salts afforded the corresponding 2-chloro-quinolinium[b,c]-1,4-oxazine chloride salt-3[2(4)]-dimethine cyanine dyes (3a–c). The reaction sequence is as shown in Scheme1.
The formation of 3[2(4)]-dimethine cyanine dyes (3a–c), was suggested to proceed through nucleophilic addition reaction of the active methyl of 2(4)-ethyl heterocyclic quaternary salt to the carbonyl group of compounds 2 followed by dehydration processes according to the following (Eq. (1):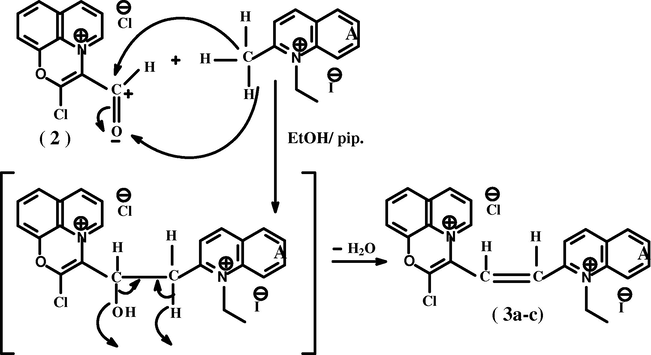
(3a–c): A = 1-ethylpyridin-2-ium iodide; (a); A = 1-ethylquinolin-2-ium iodide (b); A = 1-ethylpyridin-4-ium iodide (c).
The structure of the compounds (3a–c) was confirmed by analytical and spectral data, reported in Table 1.
Reaction of compound 2 with bimolar ratios of 2-(4)-ethyl-substituted heterocyclic quaternary salts afforded the corresponding quinolinium[b,c]-1,4-oxazine-chloride salt-2,3[2(4)]-pentamethine cyanine dyes (4a–c). The reaction sequence is as shown in Scheme 1.
The formation of 2,3[2(4)]-pentamethine cyanine dyes (4a–c), was suggested to proceed through nucleophilic addition reaction of the active methyl group of 2(4)-ethyl heterocyclic quaternary salt to the carbonyl group of compounds 2 followed by dehydration and dehydrohalogenation processes to give compounds (4a–c). The reaction was confirmed chemically by the interaction of compounds (3a–c) with equimolar ratios of 2-(4)-ethyl-substituted heterocyclic quaternary salts which give the same compounds (4a–c).
The structure of compounds (4a–c) was confirmed by analytical and spectral data, reported in Table 1.
Reaction of compound 2 with trimolar ratios of 2-(4)-methyl-substituted heterocyclic quaternary salts afforded the corresponding quinolino[b,c]-1,4-oxazine-6yl [2(4)]-monomethine-2,3[2(4)]-pentamethine cyanine dyes (5a–c). The reaction sequence is as shown in Scheme 1. The reaction was suggested to proceed through nucleophilic addition reaction of the active methyl group of 2(4)-ethyl heterocyclic quaternary salt to the carbonyl group of compound 2 and oxidative elimination followed by dehydrohalogenation process to give compounds (5a–c).
The structure of compounds (5a–c) was confirmed by analytical and spectral data, reported in Table 1.
The 2-chloroquinolinium[b,c]-1,4-oxazine chloride salt-3[2(4)]-dimethine (3a–c), quinolinium[b,c]1,4-oxazine-chloride salt-2,3[2(4)]-pentamethine (4a–c) and quino-linium[b,c]-1,4-oxazine-6yl[2(4)]-monomethine-2,3[2(4)]-pentamethine cyanine dyes (5a–c) cyanine dyes are highly coloured compounds. Their colour in ethanol ranges from brownish-violet to intense violet, easily (partially) soluble in polar and nonpolar organic solvents exhibiting coloured solutions (red/violet) concomitant with slight or intense greenish-red fluorescence depending upon the solvent used. They are soluble in concentrated H2SO4 acid liberating iodine vapour on warming. Their ethanolic solutions give permanent colours in basic media, which reversibly discharged on acidification. They possess interachargable colours solution (brownish-violet/intense violet → yellow) in basic and acidic media.
3.1 Relation between structure and electronic absorption spectra of novel cyanine dyes
3.1.1 Electronic absorption spectra in ethanol
Electronic absorption spectra features (λmax and εmax values) of newly synthesized cyanine dyes (3a–c) Fig. 1, (4a–c) and (5a–c) in ethanol (alchromic behaviour) solution are shown in Table 1. The visible absorption maxima of newly synthesized cyanine dyes in ethanol undergo a bathochromic shift or hypsochromic shift depending on the nature of heterocyclic quaternary residue A and the number of methine groups. Thus, substitution of A = N-ethylpyridin-2-ium in compound 3a by A = N-ethylquinolin-2-ium in compound 3b resulted in a bathochromic shift of 46 nm with increasing number of absorption bands at 519, 559 and 587, which is due to the increasing π-conjugation in quinolin-2-ium moiety. Similarly, increasing the number of methine groups in pentamethine compound 4b with respect to dimethine compound 3b resulted in a bathochromic shift of 80 nm and appearing of shoulder at 600 nm as shown in Fig. 2. This could be attributed to the more extensive π-conjugation in pentamethine than dimethine moieties.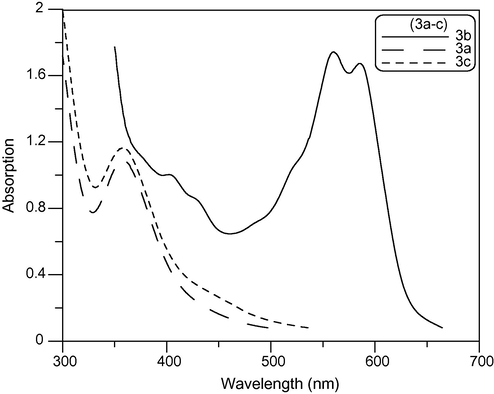
Absorption spectra of dimethine cyanine dyes (3a–c) in ethanol solution in (300–700 nm) wavelength range.
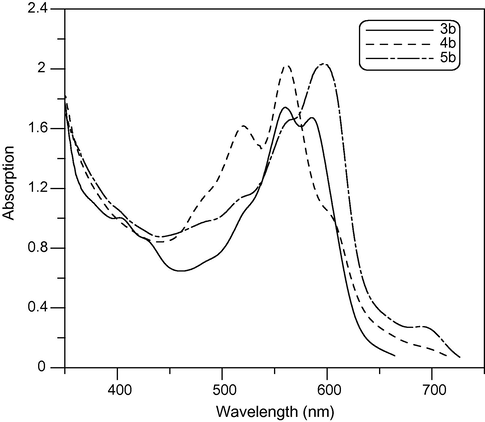
Absorption spectra of 3b, 4b, 5b compounds in ethanol solution in (350–750 nm) wavelength range.
A comparison of the visible absorption maxima between compounds 3b, 4b and 5b showed that asymmetrical mono-pentamethine cyanine dye 5b reveals a bathochromic shift than both dimethine 3b and pentamethine cyanine dyes 4b. This could be attributed to the more extensive π-conjugation and increasing number of methine groups in asymmetric pentamethine Fig. 2.
3.1.2 Solvatochromic behaviour of some selected cyanine dyes 3a, 3b, 3c, 4b, 5b
The UV–Vis spectra of some newly synthesized dyes in several common solvents were determined. All the data were collected at room temperature and measured at a concentration of approximately 10−4 mol/L. The λmax and εmax of the dyes in different solvents are presented in Table 2. As indicated in the table, the λmax ranges from 358 to 591 nm for dyes (3a–c) and from 478 to 605 nm in (4a–c) and from 467 to 700 nm in (5a–c) in different solvents. At the same time, the εmax values were in the range of 1100–5600 L mol−1 cm−1, 1000–22,000 L mol−1 cm−1 and 1100–18,000 L mol−1 cm−1 for (3a–c), (4a–c) and (5a–c) respectively. The order of the λmax for dyes (3a–c) is 3b > 3c > 3a as shown in Figs. 3–5 respectively. This is due to increasing conjugation in the dyes. Where a = λmax/nm and b = εmax × 103/L mol−1 cm−1.
Compd. No.
Water λmaxa (εmax)b
DMF λmax (εmax)
EtOH λmax (εmax)
CH3CN λmax (εmax)
CHCl3 λmax (εmax)
2-PrOH λmax (εmax)
n-ButOH λmax (εmax)
Dioxane λmax (εmax)
CCl4 λmax (εmax)
3a
353(1.43)
363(2.20)
358(3.33)
359(2.10)
358(2.30)
358(2.40)
366(6.20)
361(3.33)
361(3.30)
3b
405sh(2.00)
409sh(2.10)
405sh(3.30)
–
–
–
–
–
–
432sh(1.70)
436sh(1.80)
432sh(2.90)
–
–
–
–
–
–
519sh(1.50)
521 sh(1.60)
519sh(3.30)
560(2.33)
570(2.20)
560(2.20)
563(1.90)
562(2.10)
563(2.00)
559(2.10)
562(2.30)
559(5.80)
583(2.23)
589(2.10)
585(2.20)
585(1.93)
589(2.13)
591(2.13)
587(2.20)
587(2.30)
587(5.60)
3c
355(1.10)
363(1.73)
359(3.80)
362(2.36)
361(2.26)
361(2.20)
364(2.60)
363(3.90)
363(2.26)
4a
463(1.40)
472(1.10)
478(1.30)
470(1.20)
484(1.10)
473(1.10)
476(1.60)
472(1.20)
476(1.20)
4b
480sh(9.40)
481sh(11.00)
485sh(11.00)
477sh(12.00)
481(8.30)
482sh(10.00)
482sh(12.00)
481sh(11.00)
479sh(14.80)
517(12.00)
521(16.00)
519(16.00)
519(17.00)
511(8.30)
519(15.00)
520(17.80)
519(15.00)
517(21.00)
562(13.00)
564(19.80)
561(19.60)
561(21.00)
562(4.70)
562(19.60)
563(20.00)
564(18.00)
566(22.00)
597sh(9.70)
597sh(10.00)
597sh(10.00)
597sh(10.00)
597sh(5.10)
597sh(10.00)
597sh(7.00)
597sh(9.00)
597sh(7.00)
4c
473(1.30)
484(1.30)
488(1.50)
484(1.30)
494(1.10)
486(0.75)
490(0.75)
480(1.00)
468(1.10)
5b
512sh(8.80)
519sh(9.00)
518sh(11.00)
518sh(9.70)
519sh(8.70)
516sh(9.00)
520sh(9.30)
511sh(8.00)
521sh(7.40)
556sh(11.90)
562sh(14.80)
560sh(16.00)
556sh(14.50)
562(12.50)
560sh(13.70)
565sh(13.70)
555sh(10.00)
561sh(7.40)
592(14.50)
599(18.00)
597(19.70)
594(17.00)
597(16.60)
598(17.40)
598(17.40)
592(16.00)
594(12.00)
–
–
–
–
–
–
–
617(13.50)
617sh(15.70)
680sh(1.70)
700sh(2.70)
696sh(2.70)
694sh(2.70)
702sh(2.50)
699sh(2.70)
699sh(2.70)
704sh(2.10)
708sh(1.90)
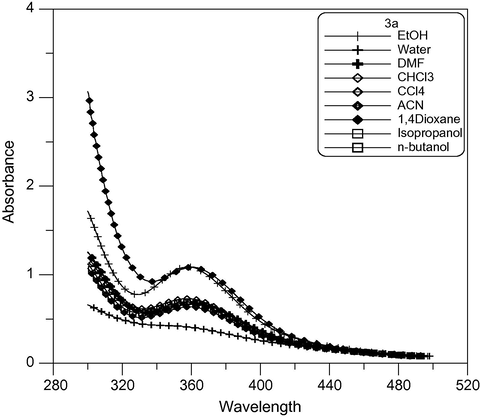
Absorption spectra of dimethine cyanine dye 3a in different solvents.
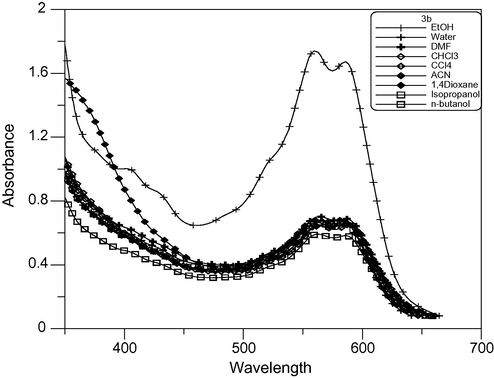
Absorption spectra of dimethine cyanine dye 3b in different solvents.
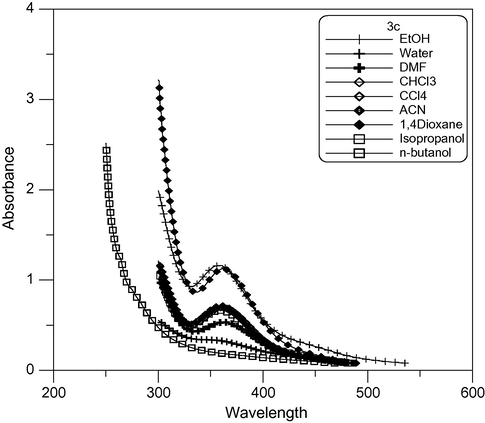
Absorption spectra of dimethine cyanine dye 3c in different solvents.
The electronic absorption spectra of 4a and 4c cyanine dyes showed a positive solvatochromism with increasing solvent polarity, which depends on the structure and the type of dye. The visible absorption spectra of cyanine dyes 3b, 4b and 5b in pure solvents showed a negative solvatochromism with increasing polarity as shown in Figs. 4, 6 and 7 respectively, which depends on the type of dye. Because the ground state is more stabilized than the excited state due to solvation by solvents of higher polarity, absorption bands in the range 591–708 nm in polar solvents decrease to range 587–696 nm in nonpolar solvent. This finding clearly indicates that the absorption bands of these dyes undergo bathochromic or hypsochromic shifts according to increasing or decreasing conjugation in the dye molecules.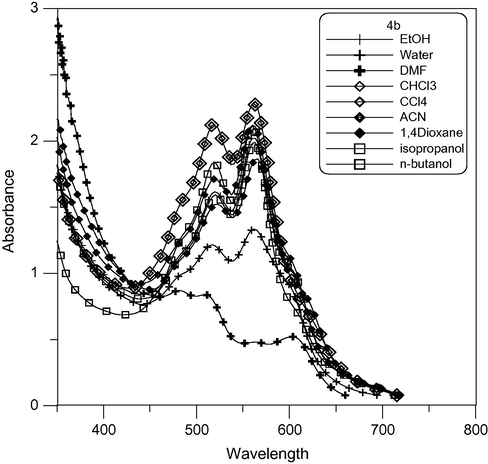
Absorption spectra of pentamethine cyanine dye 4b in different solvents.
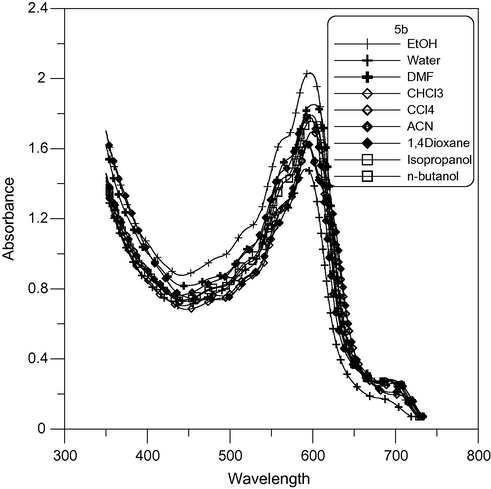
Absorption spectra of mono-pentamethine cyanine dye 5b in different solvents.
3.1.3 Emission spectra of some selected new synthesized dyes 4b,5b
Fluorescence emission spectra of compounds 4b and 5b in EtOH were measured at an excitation wavelength of 600–630 nm and are shown in Figs. 7 and 8. It can be found that compounds 4b and 5b exhibit strong fluorescence intensity. The fluorescence emission wavelengths of these compounds were around 595–630 nm. The absorption maxima of 4b and 5b in EtOH showed a bathochromic shift Fig. 9.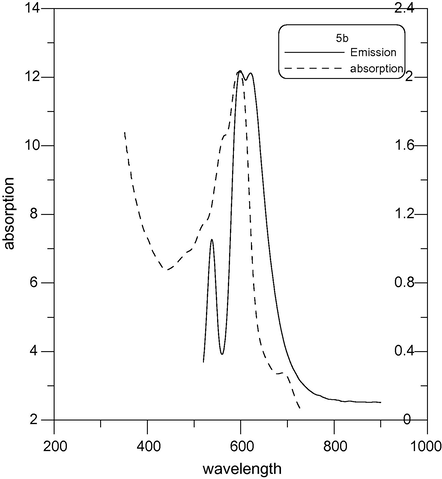
Absorption and emission (λex = 630) spectra of 5b.
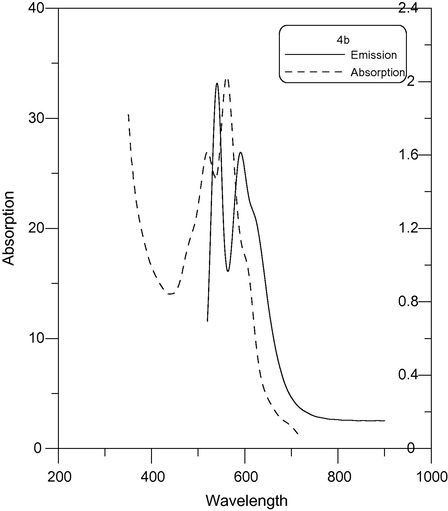
Absorption and emission (λex = 600) spectra of 4b.
3.1.4 Fluoro-solvatochromism
The fluorescence maxima of 4b showed a negative solvatochromism when going from carbon tetrachloride to dimethylformamide for longer wavelength at 630 nm and a positive solvatochromism when going from carbon tetrachloride to dimethylformamide for shorter wavelength at 595 nm Fig. 10. Meanwhile the fluorescence maxima of 5b showed a positive solvatochromism when going from carbon tetrachloride to dimethylformamide Fig. 11. The λabs, λemiss and stock shifts of the dyes in different solvents are listed in Table 3. This maybe attributed to that fluorescence maxima affected by the type of cyanine dye.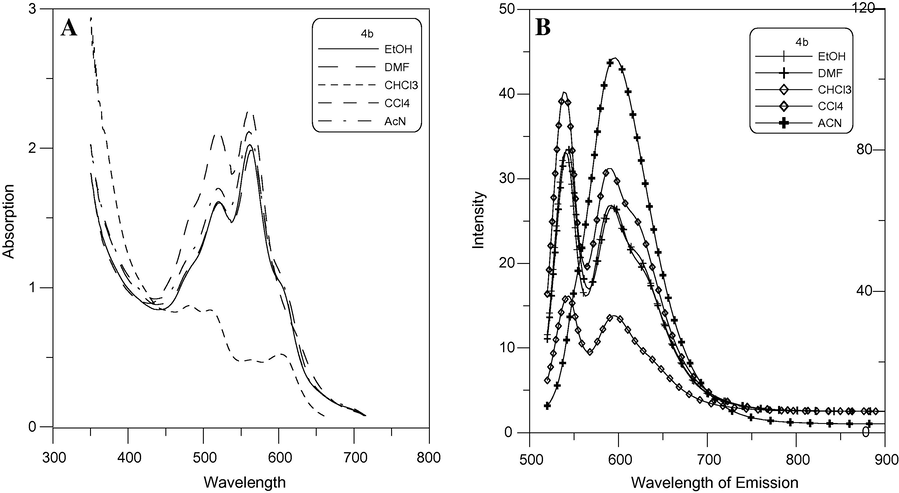
Emission (λex = 600 nm) A and absorption B spectra of 4b (2 × 10−5 mol/L).
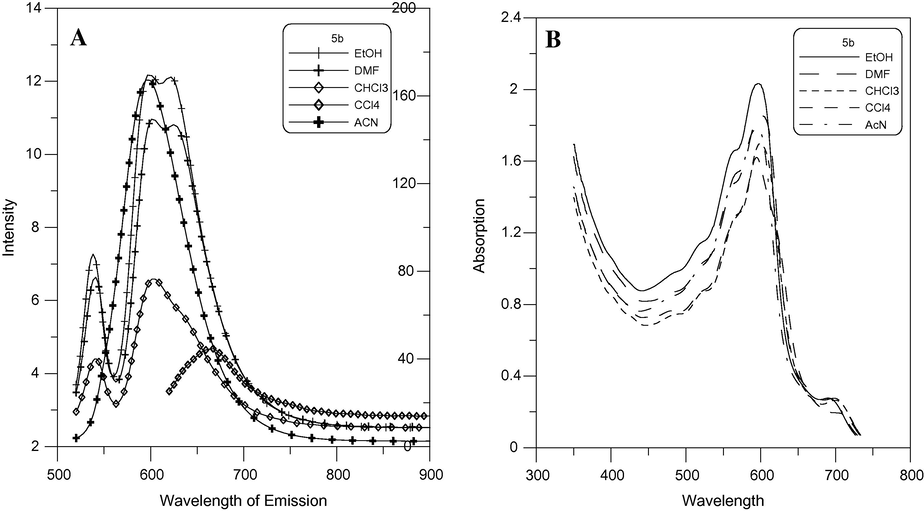
Emission (λex = 630 nm) A and absorption B spectra of 5b (2 × 10−5 mol/L).
Compd.
DMF
EtOH
ACN
CHCl3
CCl4
λabs
λemis
St. Shift
λabs
λemis
St. Shift
λabs
λemis.
St. Shift
λabs
λemis
St. Shift
λabs
λem
St. Shift
4b
481sh
–
–
485sh
–
–
477sh
–
–
481
–
–
479sh
–
–
521
543
22
519
542
23
519
541
22
511
–
–
517
542
25
564
599
35
561
600
39
561
597
36
562sh
601
39
566
595
29
605sh
627sh
22
600sh
624sh
24
600sh
622sh
22
605
–
–
605sh
630
25
5b
519sh
543sh
24
518sh
535sh
17
477sh
–
–
481sh
–
–
521sh
545sh
24
562sh
604sh
42
560sh
601sh
39
519sh
–
–
511sh
–
–
561sh
–
13
599
624
25
597
625
28
591
664
73
595
604
9
594
610sh
16
700sh
–
–
696sh
–
–
600sh
–
–
605sh
–
–
617sh
635sh
18
708sh
–
–
4 Conclusions
All the observations and analytical spectra in this paper support the syntheses of new di-, penta- and mono-/pentamethine cyanine dyes. The absorption spectra of dyes were investigated in organic solvents. The results indicated that the excitation for their colour is a simple charge-transfer from oxygen atom of oxazine nucleus and/or nitrogen atom of pyridine (quinoline) nucleus to N-quaternary salts in di-, penta- and mono-/pentamethine cyanine dyes respectively. These dyes showed a positive solvatochromism with increased solvent polarity, which depends on the structure and the type of dye. Fluorescence emission spectra of some selected compounds in EtOH and the Stock shift were measured.
References
- The synthesis of some bridgehead heterocyclic monomethine cyanine dyes. Dyes Pigm.. 1998;39(4):267-280.
- [Google Scholar]
- Synthesis and characterization of new photosensitizer bridgehead cyanine dyes. Proc. Indian Acad. Sci.. 1999;111(2):343-352.
- [Google Scholar]
- The use of metal chelators in the synthesis of monomethine cyanine dyes. Dyes Pigm.. 2006;61(3):251-261.
- [Google Scholar]
- Use of poly(polyquinoxaline) as an electron transport material in polymer light-emitting diodes. Appl. Phys. Lett.. 1996;69:881.
- [Google Scholar]
- Studies on the near infrared laser induced photopolymerization employing a cyanine dye borate complex as the photoinitiator. J. Am. Chem. Soc.. 1988;110:2326.
- [Google Scholar]
- Potent quinoxaline based inhibitors of PDGF receptor tyrosine kinase activity part 2: the synthesis and biological activities of RPR 127963 an orally bioavailable inhibitor. Bioorg. Med. Chem. Lett.. 2003;13:3097.
- [Google Scholar]
- The use of N-bridgehead heterocyclic indolizinium ylide in the synthesis of aza-cyanine dyes. Dyes Pigm.. 2006;68:235-242.
- [Google Scholar]
- Synthesis and visible spectral behaviour of some new bridgehead heterocyclic cyanine dyes incorporating pyrazole (4,6-b) inolizine (Benzoindolizine) J. Chin. Chem. Soc.. 2002;49(4):571-580.
- [Google Scholar]
- Current developments in optical data storage with organic dyes. Angew. Chem., Int. Ed.. 2006;45:2016-2035.
- [Google Scholar]
- Shapiro S.L., ed. Topics in applied physics. Vol vol. 18. Heidelberg: Springer-Verlag; 1973.
- Dye sensitization of single crystal semiconductor electrodes. Acc. Chem. Res.. 2009;42:2017-2029.
- [Google Scholar]
- Picosecond kinetics of light induced electron transfer from J- aggregated cyanine dyes to silver bromide microcrystals:effect of aggregate size. J. Phys. Chem.. 1992;96:2778.
- [Google Scholar]
- 2,8-Bis(3-phenylquinoxalin-2-yl)-56-dibenzo[b,d]thiophene-5,5-dione. Chem. Mater.. 2005;17:1860.
- [Google Scholar]
- Organic Chemistry. NJ Prentice Hall: Upper Saddle River; 1999.
- Nonlinear propagation of ultrashort pulses in cyanine dye solution investigated by SHG FROG. Chem. Phys. Lett.. 2004;398:495.
- [Google Scholar]







