Translate this page into:
Exploring facile synthesis and cholinesterase inhibiting potential of heteroaryl substituted imidazole derivatives for the treatment of Alzheimer’s disease
⁎Corresponding authors. faryal.chaudhry@kinnaird.edu.pk (Faryal Chaudhry), frylchaudhry@yahoo.com (Faryal Chaudhry), munawarali@ucp.edu.pk (Munawar Ali Munawar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder and cholinesterase (ChE) enzymes are considered as crucial targets for the treatment of AD. Herein, a series of heteroaryl substituted imidazole derivatives (5a-5x) was prepared using amino acid catalyzed, one-pot facile synthetic approach. In this context, the catalytic potentials of different amino acids were investigated and 15 mol% of glutamic acid was identified as the most suitable catalyst to obtain the target products in good yields up to 90 %. These structurally exciting heterocyclic hybrids were screened against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. This series displayed moderate to excellent inhibitory potential against AChE with IC50 values > 25 µM and the most active compound was 3-(4-(1-(3,5-dimethylphenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazol-3-yl)–2H-chromen-2-one (5x) with IC50 value of 25.83 ± 0.25 µM.This inhibitory potential was attributed to hydrophobicity as the major contributory factor. The most potent compound against BChE was 1,3-diphenyl-4-(1,4,5-triphenyl-1H-imidazol-2-yl)-1H-pyrazole (5a) with IC50 value of 0.35 ± 0.02 µM followed by other potent compounds 5p, 5 m, 5x, 5b, 5c, 5e and 5f with IC50 values < 10 µM. SAR studies further revealed that coumarinyl moiety at R1 position in the imidazolylpyrazole skeleton significantly improved the overall cholinesterase inhibitory potential. However, a simple phenyl ring attached at this R1 site was highly effective and selective for BChE inhibition (5a) over AChE. Docking data also demonstrated the interaction of 5x and AChE with a docking score of 7564 and atomic contact energy (ACE) value of –291.90 kcal/mol whereas docking score for 5a against BChE was 7096 with ACE value of –332.95 kcal/mol. The results altogether suggest further investigations of the heteroaryl substituted imidazole core skeleton in search of potential leads towards designing of new anti-cholinesterase drugs for the treatment of AD.
Keywords
Amino acid
Cholinesterase inhibition
Alzheimer’s disease
Heterocycle
Imidazolylpyrazole
1 Introduction
Dementia is a neurodegenerative and a progressive cognitive impairment among the elderly people. This lethal syndrome has physical, psychological as well as social influences (Lott and Head 2019). Dementia is a 7th leading cause of mortality. Globally, more than 55 million people are struggling with the dementia per year. This estimate would be increased up to 132 million by the year of 2050 which is an alarming situation (Athar et al. 2021; Gaugler et al. 2022; Porsteinsson et al. 2021). Some of its universal forms have included: Alzheimer’s disease (AD), vascular, Lewy bodies, frontotemporal dementia and Parkinson’s disease. About 60–70 % of the dementia cases are associated with the AD. For many years, the AD was considered as a simple brain disorder. The generalized atrophy, β-amyloid plaques, and neurofibrillary tangles are some of the major AD pathological hallmarks. Scientists have envisaged etiology for the AD progression and also considered proteins as potential therapeutic targets (Cummings et al. 2022). Two forms of cholinesterases (ChEs); acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8), are engaged in regulating the acetylcholine levels which acts as neurotransmitter in a healthy brain. Sometimes this cholinergic disturbance causes substantial impact on cognition and could lead to neurodegeneration (Kabir et al. 2019). The structures of both enzymes are fairly similar; however, some differences have been observed in the sizes of their active site gorges which most probably affect the working approach of both ChEs (Chatonnet and Lockridge 1989; Jing et al. 2019; Rosenberry et al. 2017). So far, the most commonly prescribed formulations to treat AD are the anticholinesterase drugs such as Rivastigmine (Exelon®), Donepezil (Aricept®), Eserine (Anticholium®), Tacrine (Cognex®) and Galantamine (Razadyne®) (Grutzendler and Morris 2001; Kumari et al. 2022) (Fig. 1). The AChE was mainly targeted as key enzyme during AD treatment for a long time. As BChE activity is also enhanced in AD patients and associated with Aβ deposits, therefore, BChE inhibition is also suggested as a prudent curative approach towards minimizing the AD development (Darvesh 2016; Fernández-Bolaños and López 2022; Li et al. 2017b; Purgatorio et al. 2019).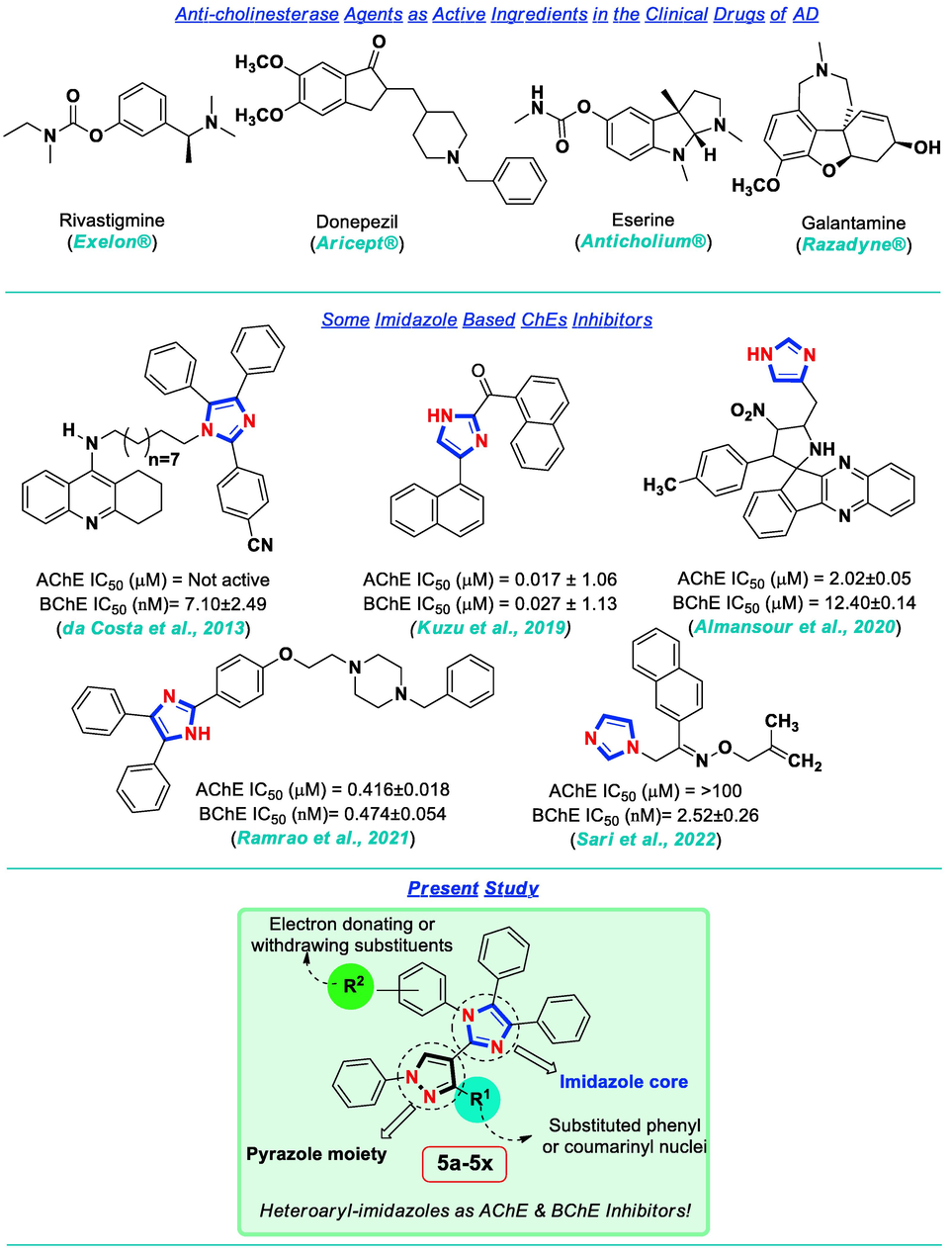
Significant clinical drugs of AD and imidazole based potent anti-cholinesterase agents as rationale of the present study.
Literature survey represents various N-heterocyclic scaffolds as the promising dual or highly selective inhibiting agents of ChE enzymes (Benazzouz-Touami et al. 2022; Derabli et al. 2018; Kumari et al. 2022; Li et al. 2021; Ma et al. 2020; Mlakić et al. 2022; Tariq et al. 2022; Tripathy et al. 2020). Imidazole is an example of N-heterocycle which constitutes many important biomolecules like histamine, purine, nucleic acid, and biotin etc. Medicinal chemists usually employ this easily ionizable N-heterocyclic moiety in improving the pharmacokinetics of lead molecules (Alghamdi et al. 2021). Due to its wide-spread applications, different imidazole and/or benzimidazole analogues are proposed as effective molecules that can deal with AD and other neurodegenerative disorders. A benzimidazole derivative - PQ912 was recently discovered as the most potent glutaminyl cyclase inhibitor and currently under Phase II trials in the patients of AD (Vijverberg et al 2021). Another analogue of benzimidazole has displayed excellent activity against a different target enzyme: C-jun N-terminal kinase (Jun et al. 2021; Qin et al. 2022). A biphenyl linked imidazole derivative was also proposed as a promising inhibitor of glutaminyl cyclase (Li et al. 2017a). Recent discoveries have highlighted the imidazole based templates as ChEs inhibitors also (Almansour et al. 2020; da Costa et al. 2013; Dhingra et al 2022; Karlsson et al. 2012; Kuzu et al. 2019; Obaid et al. 2022; Ramrao et al. 2021; Sari et al. 2022) (Fig. 1).
There are limited clinical options of medications to treat AD. The rapid increase in the drug resistance and different side effects associated with some of the existing clinical drugs underline the need to design and prepare new and more potent ChEs inhibitors for the better treatment of AD. The pivotal role of imidazoles in the prior literature has motivated us to design current research study. In this context, an imidazole based core skeleton of the targeted molecules was prepared through a facile and an efficient synthetic approach. So far, different catalysts have been reported in the literature to prepare imidazole derivatives via one-pot multi-component reaction (MCR) (Nguyen et al. 2019; Rossi et al. 2019; Takeda et al. 2022). Amino acids and their derivatives are among those promising versatile organocatalysts that could promote handy, facile and expedient transformations of precursors in to various heterocycles (Kaur et al. 2022; Vachan et al. 2020). Thus, the utility of amino acids in the preparation of heteroaryl imidazoles was explored in the present study through a model preparation of a derivative 5a. The scope of current strategic methodology is further increased in delivering versatility by preparing other N-substituted imidazoles (5b-5x). Furthermore, all of these synthesized derivatives were evaluated against both ChEs to find out inhibiting potential of these molecules. The most potent compounds of the series could be further investigated as “lead” molecules towards designing of new drugs for better AD treatment.
2 Materials and methods
2.1 Chemicals and physical measurements
Chemicals and reagents of Sigma-Aldrich were purchased from commercial sources. TLC was performed over aluminum supported sheets of DC-Alufolien Silica Gel 60, F254 Merck. For the determination of melting points, Gallen Kamp apparatus was used. FTIR spectra were recorded in Agilent Technologies Cary 630 FTIR spectrophotometer. After dissolving compounds in CDCl3 solvent, their 1H (13C) NMR spectra were acquired either on MHz Bruker DPX Instruments of 300 (75), 500 (1 2 5) or on 700 (1 7 5) MHz with the coupling constant (J) in Hz. Mass spectra of the samples were recorded on GC MS DFS-Thermo and for elemental analyses; Perkin Elmer 2400 Series II CHN/S Analyzer was used. Pyrazole-4-carbaldehydes (1a-1g) were prepared by following conventional formylation protocol (Abdel-Wahab et al. 2011).
2.2 General synthetic protocol of heteroaryl-imidazoles (5a-5x)
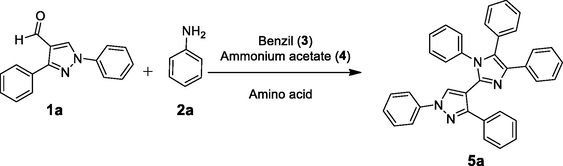
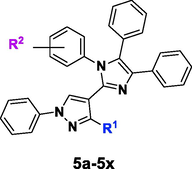
A mixture of the corresponding aldehyde (1a-1g) (2.0 mmol, 1.0 equiv), substituted aniline (2a-2f) (2.0 mmol, 1.0 equiv), benzil (3) (2.0 mmol, 1.0 equiv), ammonium acetate (4) (2.0 mmol, 1.0 equiv) and glutamic acid (15 mol%, 0.3 mmol, 0.15 equiv) in EtOH (10 mL) was heated under reflux till reaction completion. The reaction mixture was allowed to cool down to r.t. The solution was diluted with cold water with vigorous stirring to obtain the precipitates of the desired product (5a-5x) which were filtered off and later purified through recrystallization with EtOH. The formation of compounds 5a, 5b, and 5k-5x was confirmed by comparing with the already reported data (Chaudhry et al. 2017c; Chaudhry et al. 2019; Shirole et al. 2017). Characterization data of the novel derivatives (5c-5j) are given below.
2.2.1 4-(1-(4-Bromophenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1,3-diphenyl-1H-pyrazole (5c)
Yield 72 % as white powder; m.p. 180–182 °C; IR (neat, νmax-cm−1): 3137–3049 (C—H), 1596 (C⚌N), 1578 (C⚌C); 1H NMR (500 MHz, CDCl3) δ/ppm 6.33 (d, 2H, J = 8.5 Hz; Ar3-2H), 6.97 (d, 2H, J = 8.5 Hz; Ar3-2H), 7.09 (d, 2H, J = 6.5 Hz; Ar4-2H), 7.21–7.24 (m, 6H; Ar1-1H, Ar2-3H, Ar4-1H & Ar5-1H), 7.27–7.34 (m, 6H; Ar2-2H, Ar4-2H & Ar5-2H), 7.48 (t, 2H, J = 7.9 Hz; Ar1-2H), 7.64 (d, 2H, J = 7.3 Hz; Ar5-2H), 7.77 (d, 2H, J = 7.8 Hz; Ar1-2H), 8.34 (s, 1H; H-5 Py); 13C NMR (125 MHz, CDCl3) δ/ppm 112.19 (C-4 Py), 119.11, 121.54 (C-Br), 126.94, 126.96, 127.44, 127.80, 128.17, 128.32, 128.33, 128.37, 128.70, 129.16, 129.52, 129.65, 130.09 (CH-5 Py), 130.35 (C-4′ Im), 131.03, 131.41, 132.84, 134.24, 134.93, 138.52 (C-5′ Im), 139.73, 140.55 (C-2′ Im), 152.09 (C-3 Py); MS (EI+); m/z (%), 592.0 (M+, 89.1), 594.1 ([M + 2]+, 90.2); Anal. Calcd. for C36H25BrN4; C, 72.85; H, 4.25; N, 9.44 %. Found: C, 72.69; H, 4.18; N, 9.35 %.
2.2.2 4-(1-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1,3-diphenyl-1H-pyrazole (5d)
Yield 80 % as purple powder; m.p. 156–158 °C; IR (neat, νmax-cm−1): 3053–2844 (C—H), 1601 (C⚌N), 1591 (C⚌C), 1249 & 1026 (Ar3-O-CH3); 1H NMR (500 MHz, CDCl3) δ/ppm 3.68 (s, 3H; –OCH3), 6.43 (d, 2H, J = 8.2 Hz; Ar3-2H), 6.48 (d, 2H, J = 8.2 Hz; Ar3-2H), 7.13–7.16 (m, 2H; Ar4-2H), 7.24–7.34 (m, 10H; Ar1-1H, Ar2-3H, Ar4-3H & Ar5-3H), 7.42–7.45 (m, 2H, Ar2-2H), 7.49 (t, 2H, J = 7.5 Hz; Ar1-2H), 7.69 (d, 2H, J = 7.2 Hz; Ar5-2H), 7.78 (d, 2H, J = 7.5 Hz; Ar1-2H), 8.27 (s, 1H; H-5 Py); 13C NMR (125 MHz, CDCl3) δ/ppm 55.36 (–OCH3), 112.38 (C-4 Py), 113.51, 119.05, 126.72, 126.78, 127.37, 127.81, 127.80, 128.03, 128.06, 128.25, 128.30, 128.48, 128.75, 128.82, 129.46 (CH-5 Py), 129.58, 130.61 (C-4′ Im), 130.75, 131.05, 132.97, 137.93 (C-5′ Im), 139.79, 140.79 (C-2′ Im), 152.19 (C-3 Py), 158.56 (C-OCH3); MS (EI+); m/z (%), 544.5 (M+, 100); Anal. Calcd. for C37H28N4O; C, 81.59; H, 5.18; N, 10.29 %. Found: C, 81.47; H, 5.10; N, 10.32 %.
2.2.3 4-(1-(3,5-Dimethylphenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1,3-diphenyl-1H-pyrazole (5e)
Yield 71 % as white powder; m.p. 102–104 °C; IR (neat, νmax-cm−1): 3064–2918 (C—H), 1598 (C⚌N), 1561 (C⚌C); 1H NMR (500 MHz, CDCl3) δ/ppm 1.94 (s, 6H; 2 × CH3), 6.11 (s, 2H; Ar3-2H), 6.63 (s, 1H; Ar3-1H), 7.11–7.50 (m, 16H; Ar1-3H, Ar2-5H, Ar4-5H & Ar5-3H), 7.64 (dd, 2H, J = 8.2, 1.1 Hz; Ar5-2H), 7.74 (d, 2H, J = 8.5 Hz; Ar1-2H), 8.23 (s, 1H; H-5 Py); 13C NMR (125 MHz, CDCl3) δ/ppm 21.07 (2 × CH3), 112.67 (C-4 Py), 119.15, 125.68, 126.67, 126.77, 127.39, 127.93, 127.96, 127.98, 128.28, 128.29, 128.37, 129.39 (CH-5 Py), 129.44, 129.60, 130.57 (C-4′ Im), 130.87, 131.03, 132.99, 134.67, 135.79 (C-5′ Im), 137.86 (2 × C-CH3), 137.98, 139.91, 140.59 (C-2′ Im), 152.42 (C-3 Py); MS (EI+); m/z (%), 542.5 (M+, 100); Anal. Calcd. for C38H30N4; C, 84.10; H, 5.57; N, 10.32 %. Found: C, 84.33; H, 5.65; N, 10.40 %.
2.2.4 3-([1,1′-Biphenyl]-4-yl)-4-(1-(3-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazole (5f)
Yield 77 % as white powder; m.p. 158–160 °C; IR (neat, νmax-cm−1): 3050–3028 (C—H), 1595 (C⚌N), 1588 (C⚌C), 763 (C-Cl); 1H NMR (500 MHz, CDCl3) δ/ppm 6.42 (s, 1H; Ar3-1H), 6.47 (d, 1H, J = 8.0 Hz; Ar3-1H), 6.82 (t, 1H, J = 8.0 Hz; Ar3-1H), 6.97 (d, 1H, J = 8.0 Hz; Ar3-1H), 7.08 (d, 2H, J = 6.5 Hz; Ar4-2H), 7.18–7.21 (m, 4H; Ar2-1H & Ar4-3H), 7.24–7.28 (t, 2H; Ar2-2H), 7.29–7.33 (m, 2H; Ar1-1H & Ar5-1H), 7.36 (d, 2H, J = 8.0 Hz; Ar2-2H), 7.41 (t, 2H, J = 7.5 Hz; Ar5-2H), 7.45–7.48 (m, 4H; Ar1-2H & Ar2-2H), 7.57 (d, 2H, J = 7.5 Hz; Ar5-2H), 7.62 (d, 2H, J = 7.5 Hz; Ar1-2H), 7.75 (d, 2H, J = 8.0 Hz; Ar2-2H), 8.31 (s, 1H; H-5 Py); 13C NMR (75 MHz, CDCl3) δ/ppm 111.54 (C-4 Py), 119.22, 126.03, 127.05, 127.21, 127.50, 127.98, 128.04, 128.11, 128.44, 128.55, 128.75, 128.88, 129.24, 129.67, 129.81, 129.91, 130.22, 130.97, 131.56 (C-4′ Im), 133.85 (C-Cl), 136.46, 136.72, 137.88 (C-5′ Im), 139.68, 140.43, 140.82, 141.11 (C-2′ Im), 151.78 (C-3 Py); Anal. Calcd. for C42H29ClN4; C, 80.69; H, 4.68; N, 8.96 %. Found: C, 80.78; H, 4.76; N, 9.15 %.
2.2.5 3-([1,1′-Biphenyl]-4-yl)-4-(1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazole (5 g)
Yield 75 % as white powder; m.p. 200–202 °C; IR (neat, νmax-cm−1): 3066–3028 (C—H), 1598 (C⚌N), 1577 (C⚌C), 764 (C-Cl); 1H NMR (700 MHz, CDCl3) δ/ppm 6.45 (d, 2H, J = 8.4 Hz; Ar3-2H), 6.83 (d, 2H, J = 8.4 Hz; Ar3-2H), 7.10 (d, 2H, J = 6.9 Hz; Ar4-2H), 7.21–7.26 (m, 4H; Ar2-1H & Ar4-3H), 7.29 (t, 2H, J = 7.7 Hz; Ar2-2H), 7.33 (t, 1H, J = 7.7 Hz; Ar5-1H), 7.35–7.37 (m, 3H; Ar1-1H & Ar2-2H), 7.45 (t, 2H, J = 7.7 Hz; Ar5-2H), 7.46–7.50 (m, 4H; Ar1-2H & Ar2-2H), 7.59 (d, 2H, J = 7.7 Hz; Ar5-2H), 7.66 (d, 2H, J = 7.6 Hz; Ar1-2H), 7.79 (d, 2H, J = 7.9 Hz; Ar2-2H), 8.38 (s, 1H; H-5 Py); 13C NMR (175 MHz, CDCl3) δ/ppm 112.13 (C-4 Py), 119.09, 126.97, 127.00, 127.09, 127.46, 127.56, 128.22, 128.33, 128.39, 128.41, 128.71, 128.90, 128.99, 129.66, 130.11 (C-4′ Im), 130.22, 131.00, 131.84, 133.50 (C-Cl), 134.08, 134.37, 138.41 (C-5′ Im), 139.69, 140.53, 140.83, 140.95 (C-2′ Im), 151.64 (C-3 Py); MS (EI+); m/z (%), 624.7 (M+, 100), 626.5 ([M + 2]+, 39.8); Anal. Calcd. for C42H29ClN4; C, 80.69; H, 4.68; N, 8.96 %. Found: C, 80.77; H, 4.75; N, 9.01 %.
2.2.6 3-([1,1′-Biphenyl]-4-yl)-4-(1-(4-bromophenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazole (5 h)
Yield 78 % as white powder; m.p. 218–220 °C; IR (neat, νmax-cm−1): 3064–3028 (C—H), 1598 (C⚌N), 1561 (C⚌C); 1H NMR (500 MHz, CDCl3) δ/ppm 6.37 (d, 2H, J = 8.5 Hz; Ar3-2H), 6.97 (d, 2H, J = 8.5 Hz; Ar3-2H), 7.09 (dd, 2H, J = 7.5, 1.2 Hz; Ar4-2H), 7.20–7.26 (m, 4H; Ar2-1H & Ar4-3H), 7.29 (t, 2H, J = 7.5 Hz; Ar2-2H), 7.31–7.34 (m, 3H; Ar2-2H & Ar5-1H), 7.36–7.37 (m, 1H; Ar1-1H), 7.44–7.51 (m, 6H; Ar1-2H, Ar2-2H & Ar5-2H), 7.60 (d, 2H, J = 7.4 Hz; Ar5-2H), 7.65 (d, 2H, J = 7.4 Hz; Ar1-2H), 7.79 (d, 2H, J = 7.9 Hz; Ar2-2H), 8.41 (s, 1H; H-5 Py); 13C NMR (125 MHz, CDCl3) δ/ppm 112.15 (C-4 Py), 119.08, 121.63 (C-Br), 126.97, 127.10, 127.47, 127.56, 128.24, 128.34, 128.29, 128.72, 128.99, 129.19, 129.67, 130.01 (C-4′ Im), 130.19, 131.00, 131.37, 131.83, 134.07, 134.09, 134.86, 138.45 (C-5′ Im), 139.69, 140.48, 140.84, 140.94 (C-2′ Im), 151.64 (C-3 Py); MS (EI+); m/z (%), 668.0 (M+, 84.1), 670.0 ([M + 2]+, 88.2); Anal. Calcd. for C42H29BrN4; C, 75.33; H, 4.37; N, 8.37 %. Found: C, 74.20; H, 4.28; N, 8.46 %.
2.2.7 3-([1,1′-Biphenyl]-4-yl)-4-(1-(4-methoxyphenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazole (5i)
Yield 82 % as purple powder; m.p. 186–187 °C; IR (neat, νmax-cm−1): 3053–2972 (C—H), 1602 (C⚌N), 1578 (C⚌C), 1244 & 1024 (Ar1-O-CH3); 1H NMR (500 MHz, CDCl3) δ/ppm 3.63 (s, 3H; –OCH3), 6.38 (d, 2H, J = 8.8 Hz; Ar3-2H), 6.47 (d, 2H, J = 8.8 Hz; Ar3-2H), 7.10 (dd, 2H, J = 7.8, 2.0 Hz; Ar4-2H), 7.16–7.20 (m, 4H; Ar2-1H & Ar4-3H), 7.24–7.34 (m, 4H; Ar1-1H, Ar2-2H & Ar5-1H), 7.40–7.47 (m, 8H; Ar1-2H, Ar2-4H & Ar5-2H), 7.57 (d, 2H, J = 7.4 Hz; Ar5-2H), 7.62 (d, 2H, J = 7.3 Hz; Ar1-2H), 7.73 (d, 2H, J = 7.8 Hz; Ar2-2H), 8.21 (s, 1H; H-5 Py); 13C NMR (5 MHz, CDCl3) δ/ppm 55.39 (–OCH3), 111.69 (C-4 Py), 113.53, 119.13, 126.92, 127.05, 127.50, 128.21, 128.39, 128.57, 128.80, 128.95, 129.63, 129.94, 130.29, 130.58, 131.05, 131.89 (C-4′ Im), 133.72, 137.35, 137.41 (C-5′ Im), 139.71, 140.65, 140.78, 140.86 (C-2′ Im), 151.75 (C-3 Py), 158.69 (-C-OCH3); MS (EI+); m/z (%), 620.6 (M+, 100); Anal. Calcd. for C43H32N4O; C, 83.20; H, 5.20; N, 9.03 %. Found: C, 83.09; H, 5.11; N, 8.90 %.
2.2.8 3-([1,1′-Biphenyl]-4-yl)-4-(1-(3,5-dimethylphenyl)-4,5-diphenyl-1H-imidazol-2-yl)-1-phenyl-1H-pyrazole (5j)
Yield 73 % as light brown powder; m.p. 164–166 °C; IR (neat, νmax-cm−1): 3127–2919 (C—H), 1598 (C⚌N), 1561 (C⚌C); 1H NMR (700 MHz, CDCl3) δ/ppm 1.93 (s, 6H; 2 × CH3), 6.18 (s, 2H; Ar3-2H), 6.64 (s, 1H; Ar3-1H), 7.14 (dd, 2H, J = 6.6, 1.1 Hz; Ar4-2H), 7.19–7.23 (m, 4H; Ar2-1H & Ar4-3H), 7.29 (t, 2H, J = 7.7 Hz; Ar2-2H), 7.31 (t, 1H, J = 7.7 Hz; Ar5-1H), 7.35 (t, 1H, J = 7.7 Hz; Ar1-1H), 7.44 (t, 2H, J = 7.7 Hz; Ar5-2H), 7.46–7.53 (m, 6H; Ar1-2H & Ar2-4H), 7.61 (d, 2H, J = 7.7 Hz; Ar5-2H), 7.67 (d, 2H, J = 7.7 Hz; Ar1-2H), 7.76 (d, 2H, J = 8.4 Hz; Ar2-2H), 8.27 (s, 1H; H-5 Py); 13C NMR (175 MHz, CDCl3) δ/ppm 21.12 (2 × CH3), 112.52 (C-4 Py), 119.15, 125.69, 126.74, 126.81, 126.91, 127.07, 127.42, 127.44, 128.02, 128.31, 128.32, 128.40, 128.92, 129.36 (CH-5 Py), 129.58, 129.62, 130.58 (C-4′ Im), 130.73, 131.01, 132.07, 134.49, 135.70 (C-5′ Im), 137.91 (2 × C-CH3), 139.87, 140.54, 140.64, 140.87 (C-2′ Im), 151.97 (C-3 Py); MS (EI+); m/z (%), 618.6 (M+, 100); Anal. Calcd. for C44H34N4; C, 85.41; H, 5.54; N, 9.05 %. Found: C, 85.52; H, 5.46; N, 8.95 %.
2.3 Cholinesterase inhibition assay
Ellman’s method with slight modification was used for the inhibition studies against AChE and BChE as reported earlier (Tariq et al. 2022). Total assay volume of 100 µL contained 60 µL of 50 mM phosphate buffer (pH 7.7), 10 µL of 0.5 mM test compounds (5a-5x) and 10 µL of 0.005 units of electric eel AChE enzyme (Sigma) or 0.0005 units equine serum BChE enzyme (Sigma). Reaction contents were pre-incubated at 37 °C for 10 min and pre-read at 405 nm using Synergy HTX BioTek 96-well plate reader. Reaction was started by the addition of 10 µL of substrate (acetylthiocholine iodide or butyrylcholine bromide), 10 µL of coloring reagent DTNB and incubation continued at 37 °C for 30 min. Contents were read at 405 nm and the percentage inhibition was calculated using following formula.
All experiments were performed in triplicates with negative and positive controls. IC50 values of the active compounds were determined by repeating assays with suitable dilutions of the test compounds and computing data using EZ-FIT software of Perrella Int., USA. Data is presented as Mean ± SEM, n = 3.
2.4 Molecular docking calculations
The crystal structures of AChE and BChE proteins were recovered from PDB (ID4M0E and 4BDS). The coordinate files were subjected to the Discovery Studio 4.5 Visualizer for pre-docking receptor formation by eliminating water molecules and incorporating hydrogen atoms. Compounds 5a-5x were docked with both the enzymes by PatchDock which is a molecular docking tool targeted to find docking transformations that produce good molecular shape complementarity (Schneidman-Duhovny et al. 2005). The input files comprised of the receptor protein and ligand in PDB format. PatchDock server afforded multiple solutions and the “solution 1” was selected as it surrounded the most significant amino acid residues as binding pouch for docking analyses assigned in crystal structure of AChE and BChE receptor (Cheung et al., 2013; Nachon et al. 2013). The docked complexes were observed via Discovery Studio 4.5 Visualizer. The binding affinities of the docked complexes were assessed as scores and ACE (atomic contact energy) of the docked structures. The hydrophobic and hydrogen bonding contacts of each ligand were evaluated inside the binding pouch of receptor protein.
3 Results and discussion
3.1 Chemistry
The convenience of MCR synthetic strategy and role of different catalysts in building of imidazole nucleus are acknowledged in the literature (Nguyen et al. 2019; Rossi et al. 2019; Takeda et al. 2022). Our research group has continuously been involved in finding better reaction conditions of imidazole synthesis (Chaudhry et al. 2017a; Chaudhry et al. 2017b; Naureen et al. 2017). Therefore, present work is another effort which showed the expedient preparation of some aryl decorated imidazolylpyrazoles (5a-5x) (Scheme 1).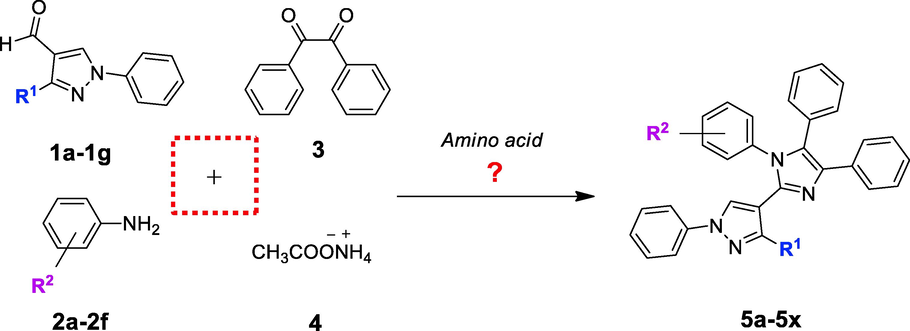
Probing amino acid catalyzed synthesis of heteroaryl substituted imidazoles (5a-5x).
Catalytic utility of some amino acids was explored through a model reaction which was performed between the substrates: 1a, 2a, 3 and 4. For preliminary screening of amino acids, six different amino acids were carefully selected for the investigation. Glycine, a simplest amino acid, was first explored on the basis of our previous findings (Chaudhry et al. 2017a; Naureen et al. 2017). The reaction has involved refluxing of the MCR mixture in EtOH along with glycine (15 mol%) as a catalyst. Proline is one such amino acid which had been used as a catalyst in the green synthesis of different heterocycles (Vachan et al. 2020) and also gave better results in our present research study. Product 5a was obtained in good yield by using aspartic acid or glutamic acid, probably the acidic catalysis enhances the reaction development. However, basic amino acids (lysine and histidine) gave moderate yield of the product even refluxing for 6 hrs. A catalyst-free reaction (Entry-7) did not result in good product yield.
The reaction was afterwards performed by loading different mole percentages (5–20 mol%) of the glutamic acid. The 5 mol% amount of catalyst resulted in 40 % product yield. The most suitable catalyst dose was screened out to be 15 mol% with 83 % product yield with in just 30 min reflux time, while, 20 mol% catalyst dose also gave comparable results. Different solvents were also selected while searching out for the most suitable conditions. Out of six solvents, two solvents MeOH and EtOH were upended to be as the best choice. The reaction completion time was the same with the slight difference in product yield (Table 1).
Entry
Catalyst (mol%)
Solvent (10 mL)/Condition
Time (min)
Yield (%)
1
Glycine (15)
EtOH/Reflux
60
80
2
Proline (15)
EtOH/Reflux
60
75
3
Aspartic acid (15)
EtOH/Reflux
30
80
4
Glutamic acid (15)[a]
EtOH/Reflux
30
83
5
Lysine (15)
EtOH/Reflux
360
33
6
Histidine (15)
EtOH/Reflux
360
30
7
–
EtOH/Reflux
360
< 20
8
Glutamic acid (15)
CH2Cl2/Reflux
60
32
9
Glutamic acid (15)
CH3CN/Reflux
60
45
10
Glutamic acid (15)
MeOH/Reflux
30
78
11
Glutamic acid (15)
DMF/Heating at 100 °C
60
50
12
Glutamic acid (15)
DMSO/Heating at 100 °C
60
54
13
Glutamic acid (15)
H2O/Reflux
120
75
14
Glutamic acid (5)
EtOH/Reflux
30
40
15
Glutamic acid (10)
EtOH/Reflux
30
54
16
Glutamic acid (20)
EtOH/Reflux
30
82
Considering the reaction completion times, calculated yields, and as a simple convenient approach; refluxing of MCR mixture with 15 % glutamic acid in EtOH was preferred for further preparations of 5b-5x. The scope of current reaction approach is evident in Table 2, wherein, the optimized conditions were applied in the MCRs of different substituted anilines and other aldehydic precursors also. The novel products of the series, 5c-5j were characterized with the help of physical and spectral records. Initially FTIR spectra were taken and there was no residual band observed for the precursor’s functional groups which indicated the complete conversion of reactants into their respective products. There was no absorption band detected in N—H region which supported the plausible formation of N-substituted imidazoles. Absorption bands of the finger-print region were also in accordance with the designed molecular structures of 5c-5j.
Sr. No.
R1
R2
Product
Time (min)
Yield[b] (%)
m.p. (°C)
Lit m.p. (°C) (Ref)
1
Ph
H
5a
30
82
200–202
194 (Shirole et al. 2017)
2
Ph
para-Cl
5b
45
75
215–217
214 (Shirole et al. 2017)
3
Ph
para-Br
5c
45
72
180–182
–
4
Ph
para-OCH3
5d
30
80
156–158
–
5
Ph
3,5-(CH3)2
5e
30
71
102–104
–
6
Biph
meta-Cl
5f
60
77
158–160
–
7
Biph
para-Cl
5g
60
75
200–202
–
8
Biph
para-Br
5h
60
78
218–220
–
9
Biph
para-OCH3
5i
45
82
186–187
–
10
Biph
3,5-(CH3)2
5j
45
73
164–166
–
11
para-ClPh
para-Br
5k
30
82
218–220
220 (Chaudhry et al. 2019)
12
para-ClPh
para-OCH3
5l
30
85
200–201
202 (Chaudhry et al. 2019)
13
para-ClPh
3,5-(CH3)2
5m
45
88
170–172
170 (Chaudhry et al. 2019)
14
para-BrPh
para-Cl
5n
45
80
194–196
194 (Chaudhry et al. 2019), 198 (Shirole et al. 2017)
15
para-BrPh
para-OCH3
5o
45
86
200–202
200 (Chaudhry et al. 2019)
16
para-BrPh
3,5-(CH3)2
5p
45
90
168–170
168 (Chaudhry et al. 2019)
17
para-OCH3Ph
para-OCH3
5q
30
85
178–180
180 (Chaudhry et al. 2019)
18
para-OCH3Ph
3,5-(CH3)2
5r
30
88
173–174
172 (Chaudhry et al. 2019)
19
meta-NO2Ph
para-OCH3
5s
45
90
214–216
214 (Chaudhry et al. 2019)
20
meta-NO2Ph
3,5-(CH3)2
5t
45
92
158–160
158 (Chaudhry et al. 2019)
21
Coumarinyl
para-Cl
5u
90
85
204–206
205 (Chaudhry et al. 2017c)
22
Coumarinyl
para-Br
5v
90
82
260–262
260 (Chaudhry et al. 2017c)
23
Coumarinyl
para-OCH3
5w
60
80
238–240
238 (Chaudhry et al. 2017c)
24
Coumarinyl
3,5-(CH3)2
5x
60
86
210–212
210 (Chaudhry et al. 2017c)
All 1H NMR and 13C NMR spectra (5c-5j) had proton and carbon-13 signals at their appropriate positions as well. NMR spectra of the derivative 5c (as a representative analogue) are shown in Fig. 2. The H-5 of pyrazole appeared downfield as a singlet at δ 8.34 ppm. Two doublets were spotted with J value 8.5 Hz for the protons of para-Br substituted phenyl ring (Ar3) at δ 6.33 ppm and δ 6.97 ppm. Other phenyl protons appeared in the aromatic region around δ 7.0 ppm to δ 8.0 ppm. In 13C NMR, two most important signals were observed, one of quaternary C-2 imidazole detected at δ 140.55 ppm while second significant signal of linking C-4 of pyrazole found near to δ 112.19 ppm.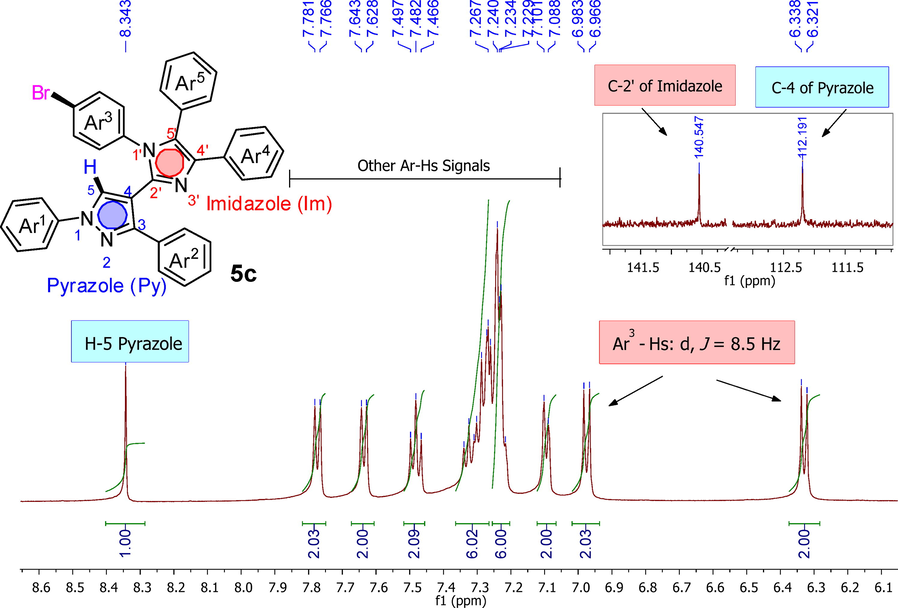
Characteristics signals in 1H NMR and 13C NMR spectra of novel derivative (5c) as a representative analogue.
Considering the expected stability associated with such bulky aryl- and heteroaryl- tethered molecules, the molecular ion peaks (M+) were detected as base peaks in MS data of nearly all derivatives (5c-5j). The expulsion of halogens (M+−Cl and/or M+−Br) was observed in halogenated molecules (5c, 5f, 5g, and 5h). A characteristic stable fragment of m/z 165 was also found in all cases which probably produced through skeletal rearrangement of an unstable intermediate fragment (Chaudhry, et al. 2021; Compernolle and Dekeirel 1971). Identification of probable key fragments in the mass spectrum of representative 5j is presented in Fig. 3. Elemental CHN-analyses also confirmed the synthesis and isolation of pure products.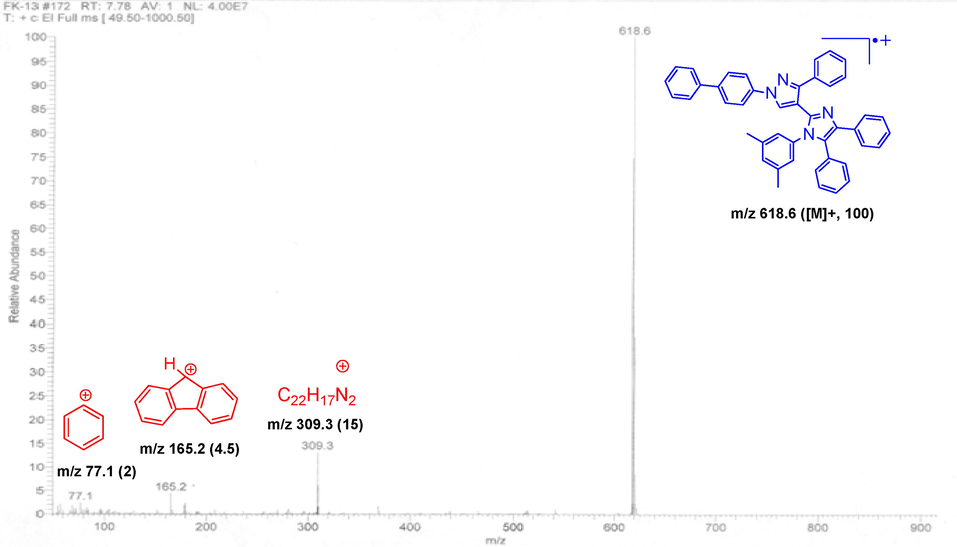
Characteristics mass spectrum of novel derivative (5j) as a representative analogue.
3.2 AChE and BChE inhibitory activities with SAR studies
Herein, these heteroaryl substituted imidazoles (5a-5x) were further investigated for their in vitro AChE and BChE inhibition activity in the quest of new and potent AChE and BChE inhibitors (Table 3). The most potent BChE inhibitory activity was displayed by the simplest molecule 5a of the series with IC50 0.35 ± 0.02 µM in contrast with the reference drug eserine (0.62 ± 0.08 µM) (Fig. 32S). The calculated SI value 117.91 is highlighting the high selectivity of this compound for BChE inhibition over AChE enzyme inhibition (Fig. 4).
SrNo.
Compound
R1
R2
AChE IC50 (µM)
BChE IC50 (µM)
Selectivity Index (SI)
AChE[a]
BChE[b]
1
5a
Ph
H
41.27 ± 0.56
0.35 ± 0.02
0.01
117.91
2
5b
Ph
para-Cl
36.82 ± 0.45
7.82 ± 0.12
0.21
4.71
3
5c
Ph
para-Br
71.46 ± 0.62
8.43 ± 0.11
0.12
8.48
4
5d
Ph
para-OCH3
142.91 ± 0.49
138.21 ± 0.22
0.97
1.03
5
5e
Ph
3,5-(CH3)2
175.28 ± 0.75
9.75 ± 0.13
0.06
17.98
6
5f
Biph
meta-Cl
312.53 ± 0.82
9.84 ± 0.11
0.03
31.76
7
5g
Biph
para-Cl
332.91 ± 0.93
> 500
–
–
8
5h
Biph
para-Br
354.26 ± 0.87
> 500
–
–
9
5i
Biph
para-OCH3
132.68 ± 0.56
136.91 ± 0.25
1.03
0.97
10
5j
Biph
3,5-(CH3)2
116.35 ± 0.54
21.43 ± 0.14
0.18
5.43
11
5k
para-ClPh
para-Br
78.52 ± 0.47
333.44 ± 0.2
4.25
0.24
12
5l
para-ClPh
para-OCH3
135.46 ± 0.49
147.16 ± 0.25
1.09
0.92
13
5m
para-ClPh
3,5-(CH3)2
41.36 ± 0.36
5.73 ± 0.09
0.14
7.22
14
5n
para-BrPh
para-Cl
192.32 ± 0.53
> 500
–
–
15
5o
para-BrPh
para-OCH3
35.19 ± 0.42
235.34 ± 0.15
6.69
0.15
16
5p
para-BrPh
3,5-(CH3)2
63.27 ± 0.56
3.60 ± 0.12
0.06
17.58
17
5q
para-OCH3Ph
para-OCH3
62.75 ± 0.52
> 500
–
–
18
5r
para-OCH3Ph
3,5-(CH3)2
34.12 ± 0.35
> 500
–
–
19
5s
meta-NO2Ph
para-OCH3
342.62 ± 0.85
> 500
–
–
20
5t
meta-NO2Ph
3,5-(CH3)2
36.25 ± 0.37
142.00 ± 0.18
3.92
0.26
21
5u
Coumarinyl
para-Cl
64.12 ± 0.28
24.94 ± 0.21
0.39
2.57
22
5v
Coumarinyl
para-Br
437.52 ± 0.87
19.62 ± 0.15
0.04
22.30
23
5w
Coumarinyl
para-OCH3
35.61 ± 0.36
14.93 ± 0.09
0.42
2.39
24
5x
Coumarinyl
3,5-(CH3)2
25.83 ± 0.25
7.64 ± 0.13
0.30
3.38
25
Eserine
–
–
0.19 ± 0.06
0.62 ± 0.08
3.26
0.31
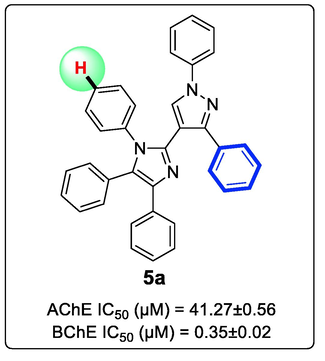
Structure of the most potent and highly selective BChE inhibitor (5a) of the series.
The region A and region B of the main template were further modified with different substitutions to examine the overall SAR (Fig. 7). A para-Cl derivative 5b and para-Br derivative 5c also showed potent BChE inhibition with IC50 7.82 ± 0.12 µM and IC50 8.43 ± 0.11 µM respectively. On the other side, 5b and 5c exhibited moderate AChE inhibitory activity. It is reported that methoxy group substituted structural frameworks are good cholinesterase inhibitors (Gao et al. 2021). However, in the present case, insertion of methoxy group at the para position in compound 5d had no significant improvement in the ChEs inhibition profile (AChE IC50 142.91 ± 0.49 µM and BChE IC50 138.21 ± 0.22 µM). Substitution of two hydrophobic methyl groups in N-aryl ring of imidazole (5e) led to strong in BChE inhibitory activity with IC50 9.75 ± 0.13 µM (SI for BChE = 17.98) (Fig. 4) (Larik et al. 2020).
Wang et al. documented biphenyl derivatives as good ChEs inhibitors (Wang et al. 2017). On the contrary, in the present studies, extending the phenyl to bulky biphenyl group reduced the ChEs inhibitory activity. Substitution of electron withdrawing halogen functionalities did not display much positive influence on inhibitory profile. It has been observed that meta-Cl derivative 5f is 31.76 folds more selective BChE inhibitor with IC50 9.84 ± 0.11 µM. However, the placement of Cl from meta to para position as in 5g (IC50 > 500 µM against BChE, IC50 332.91 ± 0.93 µM against AChE), leads to complete loss of inhibitory activity (Rehuman et al. 2021). It is therefore proposed that the positioning of substituents affects the enzyme inhibitory activity of the compounds and even may result in the loss of activity. A para-Br analogue 5h was also found inactive because of the same reason. On the other hand, replacement of halogen with methoxy group as noticed in 5i, slightly improved the inhibitory activity while the substitution of 3,5-dimethyl in 5j significantly enhanced the inhibition of both enzymes (AChE IC50 116.35 ± 0.54 µM and BChE IC50 21.43 ± 0.14 µM) (Fig. 5).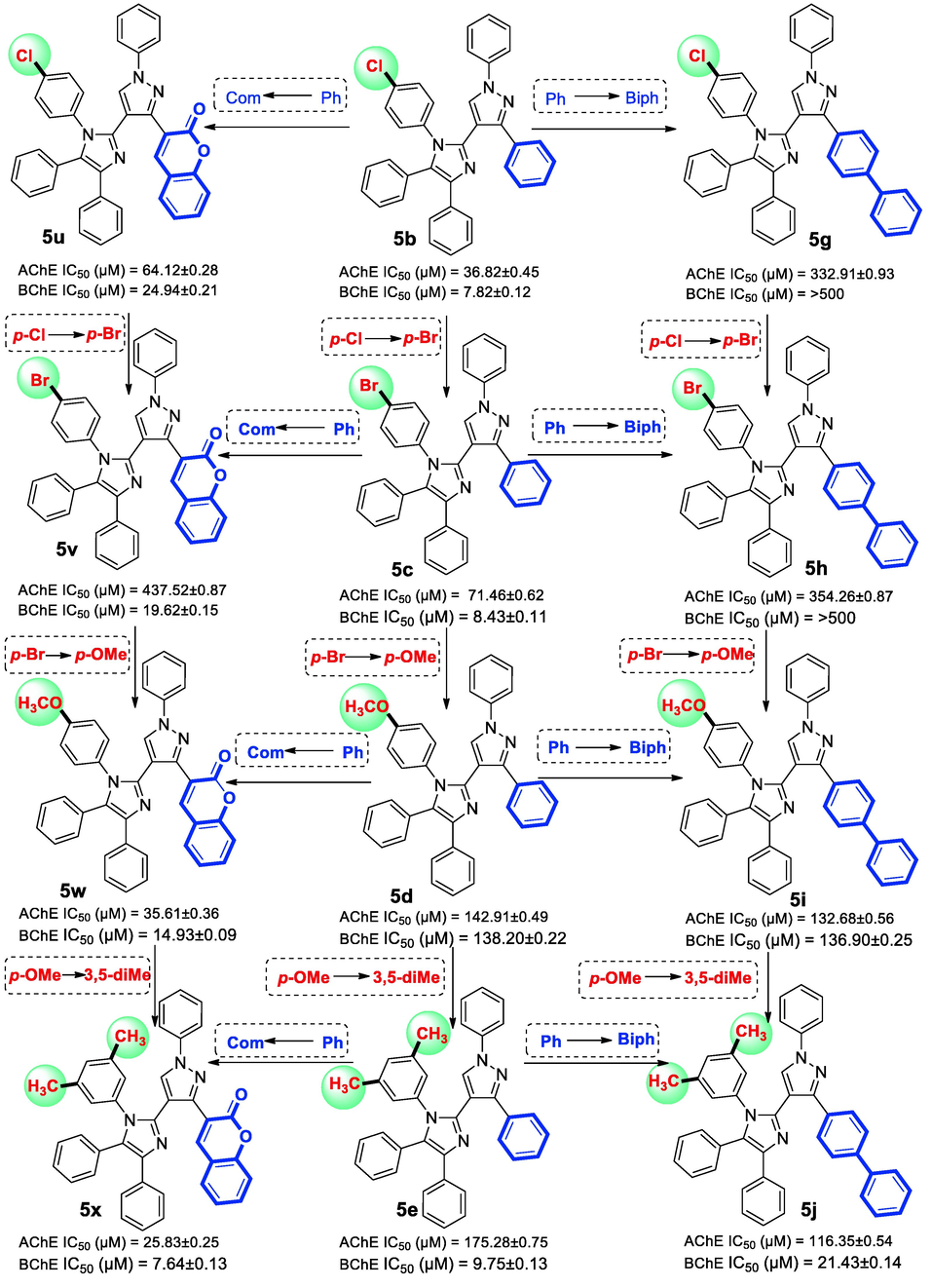
Variation in AChE and BChE inhibitions with structural modifications in the compounds: 5b-5e, 5 g-5j and 5u-5x.
ChEs inhibition was much improved by the introduction of coumarin moiety in the molecular framework (5u-5x) as observed in another study (Rehuman et al. 2021). The AChE and BChE IC50 values of para-Cl derivative 5u are 64.12 ± 0.28 µM and 24.94 ± 0.21 µM respectively. Surprisingly, the AChE inhibition by para-Br derivative 5v was less than of anti-BChE activity with 22.30 folds selectivity for BChE over AChE. Though presence of electron donating methoxy group in compound 5w displayed better inhibition but switching to 3,5-dimethyl substitution has significantly enhanced the ChEs inhibitory potentials, thus, 5x was found out to be the best AChE inhibitor of the series with IC50 25.83 ± 0.25 µM (Fig. 5). A dihalogenated derivative 5k displayed moderate AChE inhibition (IC50 78.52 ± 0.47 µM) but poor BChE inhibitory activity (IC50 333.44 ± 0.2 µM). Swapping the halogens such as para-Cl with para-Br substitutions in 5n did not confer better outcomes. However, some interesting variations were observed with further modifications in the substitutions. When para-Br functionality was replaced with para-OMe group (5l), its BChE inhibiting activity was slightly improved to IC50 147.16 ± 0.25 µM. However, by doing so there was a decline in the AChE inhibition (IC50 135.46 ± 0.49 µM). Comparable trend was found in 5o derivative with BChE (IC50 235.34 ± 0.15 µM), while there was a significant increase in AChE inhibition (IC50 35.19 ± 0.42 µM) with selectivity of 6.69 over BChE. The replacement of halogens with hydrophobic dimethyl substitutions has significantly increased the anti-cholinesterase activity in derivative 5m (AChE IC50 41.36 ± 0.36 µM and BChE IC50 5.73 ± 0.09 µM) and derivative 5p (AChE IC50 63.27 ± 0.56 µM and BChE IC50 3.60 ± 0.12 µM). Three methoxy analogues (5q-5s) of the series were inactive against BChE. On the contrary, derivatives 5q and 5r have displayed good anti-AChE activity IC50 62.75 ± 0.52 µM and IC50 34.12 ± 0.35 µM. A meta-NO2 derivative (5t) possessing dimethyl functionalities is also found to be a good AChE inhibitor (IC50 36.25 ± 0.37 µM) due to possible hydrogen bonding of –NO2 group with amino acids (Fig. 6).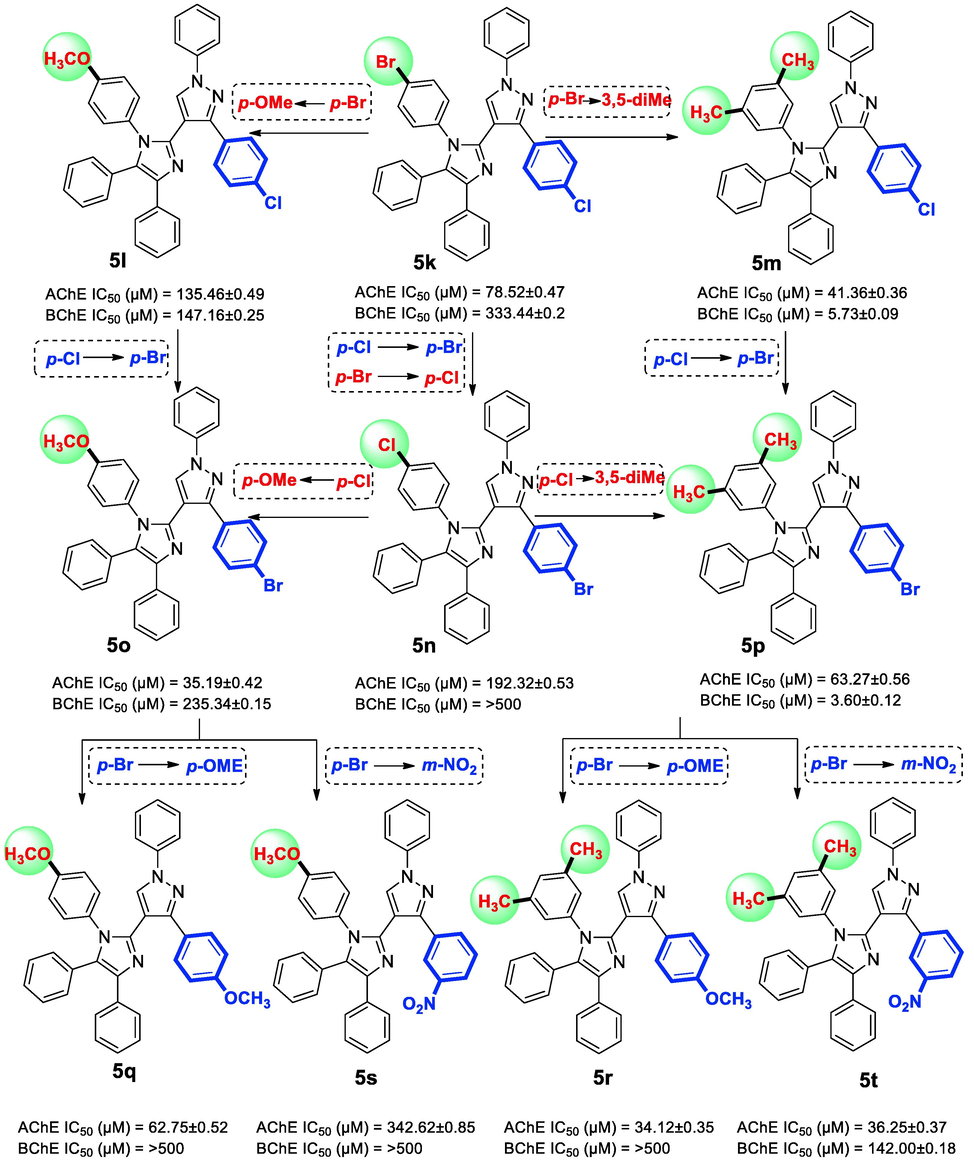
Variation in AChE and BChE inhibitions with structural modifications in the compounds: 5 k-5 t.
This general SAR model concludes that such aryl and/or heteroaryl decorated imidazole derivatives (5a-5x) appear to be a new potent class of ChEs inhibitors wherein the nature and bulkiness of the substituents greatly influenced the binding of the ligand with the target enzyme (Fig. 7). Overall selectivity data facilitates in identifying three selective AChE inhibitors of the series (5k, 5o, and 5t) and five highly selective BChE inhibitors of the series (5a, 5e, 5f, 5p, and 5v) (Table 3). Nevertheless, in silico molecular docking investigations helped to further understand the significant ligand-enzyme interactions in correlation with these in vitro findings.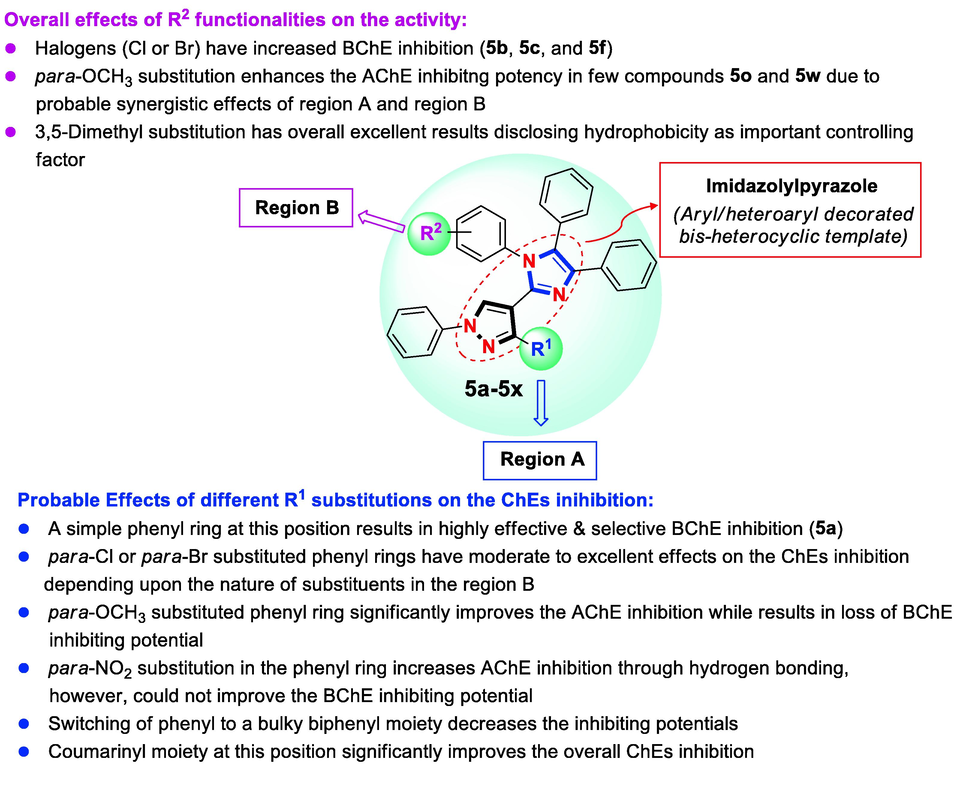
The summarized SAR of heteroaryl substituted imidazole derivatives (5a-5x).
3.3 Molecular docking studies
3.3.1 AChE docking calculations
The docked conformations of compounds (5a-5x) were examined in order to evaluate the qualitative estimation and the recognition of molecular basis of the analyzed biological activities (IC50), as shown in Fig. 8. In the preliminary evaluation of the docked complexes of AChE, it was revealed that all compounds (5a-5x) showed considerable contact patterns (Table S1).
Overlap of bound complexes of compounds (5a-5x) showing overlap in the hotspot 5a (grey), 5b (pink), 5c (yellow magenta), 5d (beige), 5e (mustard), 5f (baby pink), 5g (purple), 5h (brown), 5i (green), 5j (orange), 5k (parrot green), 5l (olive green), 5m (mauve), 5n (reddish brown), 5o (chocolate brown), 5p (pearly purple), 5q (ink blue), 5r (navy blue), 5s (dirty green), 5t (red), 5u (dark purple), 5v (see green), 5w (shocking pink), 5x (sky blue) and eserine (black).
Compound 5x exhibited the most potent contact with AChE with a score of 7564 and an ACE of –291.90 kcal/mol. The interacting residues of this complex are His372, Tyr373, Ala388, Glu387, Glu422, Met511, Tyr500, Pro527, Thr327 and Gly117. Ligand 5x has shown hydrogen bonding with Tyr420. The length of hydrogen bond was 3.24 Å. Compound 5x also exhibited hydrophobic interaction potential with pocket amino acids Thr327, Glu387, Glu422, Met511, Tyr500, His372, Tyr373 and Ala388 residues (Fig. 9).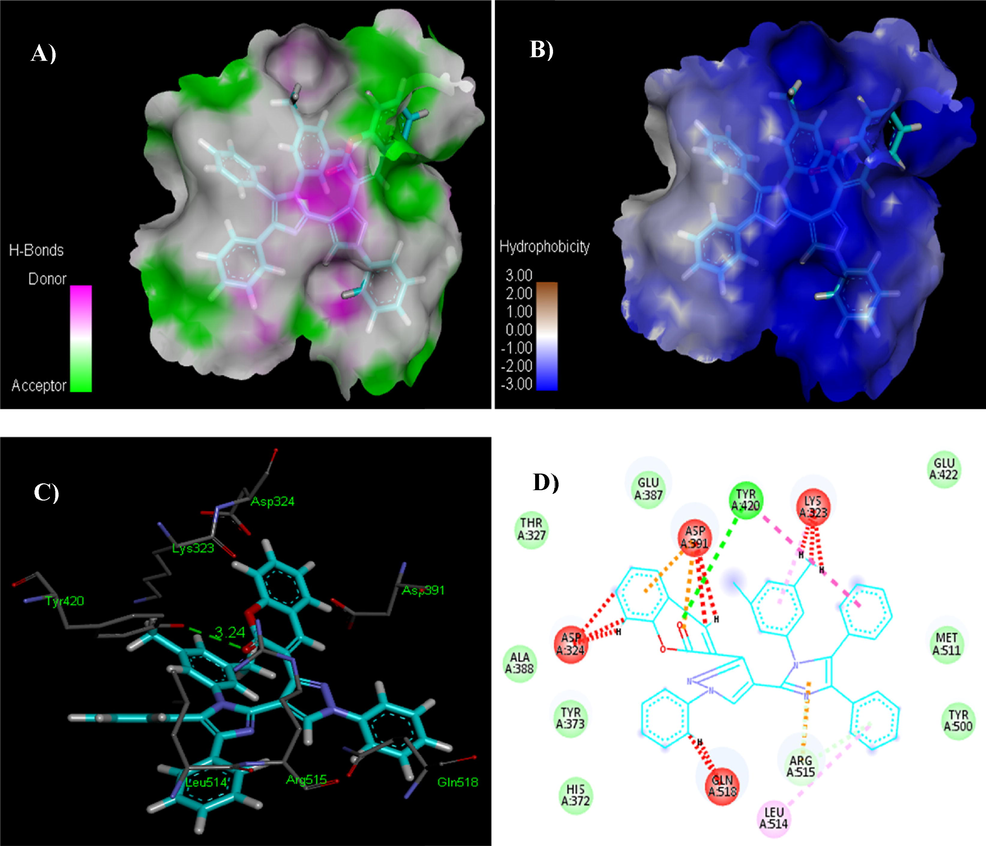
The analyses illustrating H-bonds (A) and hydrophobic contacts (B) of compound 5x. In the hydrophobic interactions (right) the blue color demonstrates favorable structural features (atoms and torsions) donating to the total binding energy within the AChE hotspot (PDB ID: 4m0e), the pink corresponds to unfavorable, and the white is neutral one. Fig. 9C is 3D and Fig. 9D is 2D depictions of docked ligand 5x.
Interestingly, other two best ligands 5o and 5r exhibited no hydrogen bond contact with AChE receptor but instead found considerable geometric fit of these compounds in the receptor and therefore scoring in PatchDock being based on shape complementarity principles, it revealed a score of 7346 and 7411 and ACE values –341.21 and –311.09 kcal/mol respectively in the docked interactions with the AChE enzyme. Compound 5o and 5r have shown π-π T-shaped contacts with Arg424 and Trp231, Gly116 accordingly (Fig. 10). Compound 5t has shown two hydrogen bond contacts with hydroxyl group of Thr59 and oxygen of NO2 functionality with an average distance of 3.25 Å. Similarly, another hydrogen bonding was observed between amino of Lys60 and oxygen of NO2 group (1.34 Å). Compound 5t revealed hydrophobic contact potential with amino acid residues Ser89, Asp91, Tyr94, Trp56, Asn57, Ala58, Gly30 and Leu29 (Table S1). Other potent compounds such as 5a, 5b, 5m and 5w illustrated hydrophobic contact potential with AChE receptor amino acids and compound 5m has exhibited π-π T-shaped contact with Val361 (Table S1). However, our docking evaluations have suggested that compounds 5o, 5r and 5x for AChE revealed the significant inhibitory potential against AChE and these outcomes are consistent with the IC50 values of these ligands. The conformational poses of all structures (5a-5x) are demonstrating the maximal biological activities with their constructive contacts in the binding hotspots (Table 4).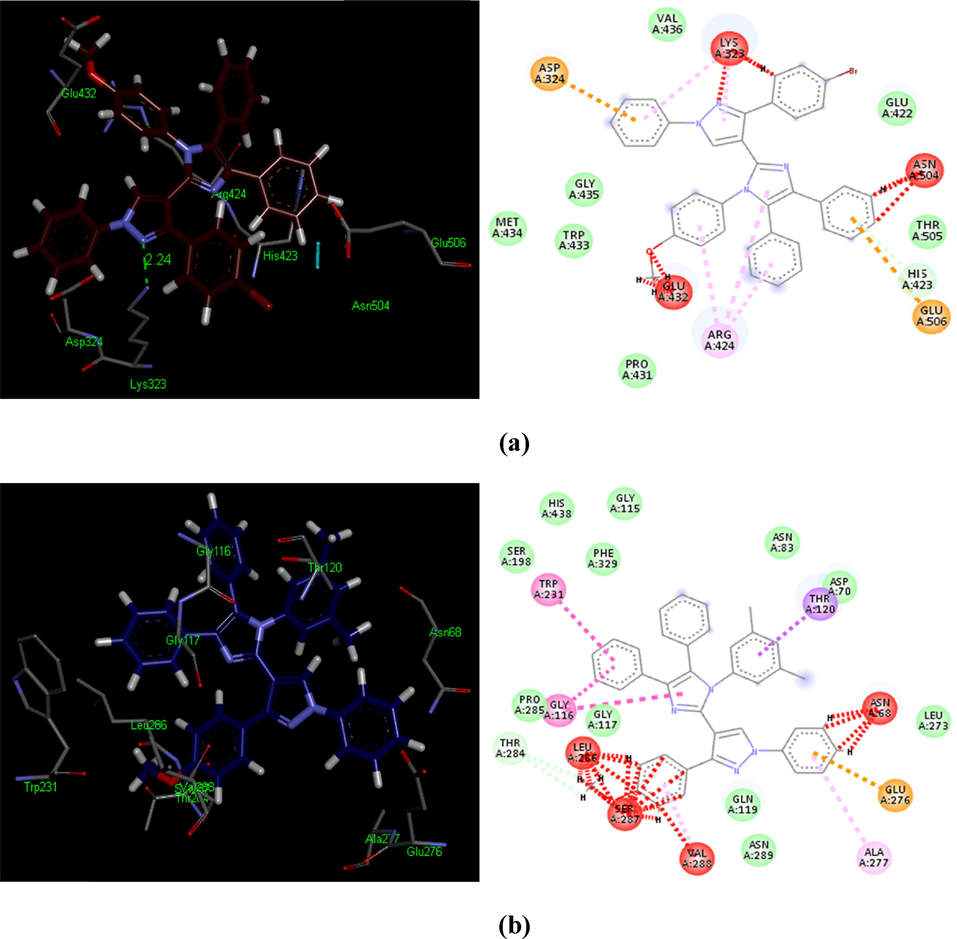
3D and 2D depictions of docked ligands − 5o (a) and 5r (b): demonstrating unfavorable bump (red), π-cation (brown), π-alkyl (light pink), amide π-stack (pink) and π-π T-shaped (shocking pink) contacts inside a hotspot of the AChE (PDB ID: 4M0E).
Compound
Score
ACE (kcal/mol)
Hydrogen bond interactions
Distance (Å)
Hydrophobic interactions
π-π T-shaped interactions
5a
6016
–378.78
–
–
Trp231, Ala232, Ala229, Trp522, Pro527, Pro401, Thr523, Glu404, Asn228, Leu307, Phe227, Pro303, Val294
–
5b
5976
–312.87
–
–
Asp304, Pro303, Met302, Ser235, Thr234, Val361, Val529, Phe526, Phe526, Tyr396, Pro527, Trp522, Thr523, Cys400, Glu404, Asn228
–
5c
6832
–334.87
–
–
Asn384, Gln380, Thr374, Gln518, Ala388
Trp376, Tyr373, Tyr373, His372
5d
6190
–367.34
–
–
Pro285, Asn289, Glu276, Gln119, Asp70, Gly117, His438, Ser198, Ala199, Phe329, Asn397
Trp231, Gly116
5e
5980
–334.56
–
–
Val136, Glu137, Lys476, Tyr477, Asn473, Ser487, Ile462, Ser466
–
5f
6878
–421.54
–
–
Asn289, Val288, Pro359, Tyr396, Phe358, Phe526, Ser362, Tyr237, Asn241
Tyr282
5g
6678
–389.67
–
–
Phe357, Asn397, Pro230, Phe358, Ser362
Tyr396
5h
6679
–355.89
Gln176
2.18
Asn181, Lys9, Trp177, Tyr33, Gln47, Pro46, Asp297, Phe298
–
5i
6490
–400.54
–
–
Val436, Glu422, Met511, Leu514, Tyr500, His372, Tyr373, Ala388, Arg386, Gly390, Thr327, Asp324
Lys323
5j
6567
–367.44
–
–
Thr327, Gly390, Glu387, Thr374, Lys513, Leu514, Tyr500, Glu422
Tyr373
5k
6467
–423.90
–
–
Glu276, Asn289, Gln119, Pro285, Gly117, Phe329, Phe398, Ala199, His438, Gly115, Trp82
Trp231
5l
6467
–378.45
–
–
Asn289, Val288, Gly117, Ser198, Ala199, Gly115, Glu197, Pro84, Ser79, Asn83, Asp70
Trp82
5m
6856
–400.45
–
–
Asn289, Glu238, Asn241, Phe526, Phe358, Tyr396, Phe357, Val288
Val361
5n
6489
–378.56
–
–
Val393, Asn397, Phe357, Tyr396, Phe526, Ser287, Asn289, Thr284
Phe358
5o
7346
–341.21
–
–
Val436, Glu422, Thr505, Pro431, Trp433, Gly435, Met434
Arg424
5p
711.90
–345.90
–
–
Val361, Val529, Phe526, Tyr396, Pro527, Trp522, Cys400, Thr523, Glu404, Asp304, Asn228, Ala232, Ser235, Thr234
–
5q
6367
–378.34
–
–
Ala62, Tyr61, Asn57, Asp54, Asp91, Ser89, Asp87
–
5r
7411
–311.09
–
–
Ser198, His438, Gly115, Phe329, Asn83, Asp70, Leu273, Asn289, Gln119, Gly117, Pro285
Trp231, Gly116
5s
6056
–323.45
Thr59, Lys60
3.25, 1.34
Ser89, Asp91, Tyr94, Trp56, Asn57, Ala58, Gly30, Leu29
–
5t
6589
–300.45
Thr59, Lys60
3.25, 1.34
Ser89, Asp91, Tyr94, Trp56, Asn57, Ala58, Gly30, Leu29
–
5u
6789
–356.23
Tyr420
3.13
His372, Tyr373, Trp376, Ala388, Gly435, Glu387, Tyr500
–
5v
6789
–312.34
–
–
His372, Tyr373, Ala388, Glu387, Thr327, Asp324, Lys323, Glu422, Met511, Tyr500
–
5w
6456
412.67
–
–
Asn342, Asp391, Arg515, Gly435, Glu422, His423, Arg381, Glu383
–
5x
7564
–291.90
Tyr420
3.24
Thr327, Glu387, Glu422, Met511, Tyr500, His372, Tyr373, Ala388
–
Eserine (Std.)
4826
–183.17
Tyr124
2.78
Glu202, Ser203, Phe238, Phe395, Phe297, Arg296, Trp286, Asp74, Trp86, Gly121
Tyr341
3.3.2 BChE docking calculations
The qualitative assessment and the recognition of molecular basis of the calculated BChE inhibitory activity (IC50), the docked conformations of compounds (5a-5x) were examined as shown in Fig. 11.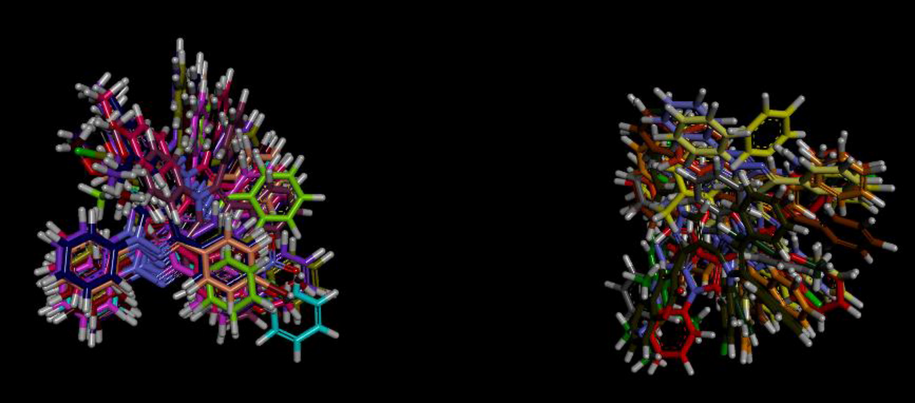
Overlap of bound conformations of compounds 5a-5j showing overlap in two clusters in the binding pocket 5a (grey), 5b (lemon yellow), 5c (magenta), 5d (pearly purple), 5e (parrot green), 5f (brown), 5g (dirty green), 5h (sky blue), 5i (mustard), 5j (purple), 5k (mauve), 5 l (chocolate brown), 5m (navy blue), 5n (orange), 5o (black), 5p (beige), 5q (shocking pink), 5r (reddish brown), 5s (baby pink), 5t (dark purple), 5u (olive green), 5v (red), 5w (dark green), 5x (yellow) and eserine (green).
In the preliminary evaluation of the docked complexes of BChE, it was revealed that all compounds (5a-5x) showed significant interaction patterns (Table S2). Compound 5a exhibited the most potent contact with BChE with a score of 7096 and an ACE of –332.95 kcal/mol. The interacting residues of this complex are Phe398, Gly116, Ala388, Asp391, Gln518, Phe371, Asn384, Arg381, Glu387, Tyr373, Trp82, Trp231 and Trp376 (Fig. 12). Compound 5a exhibited hydrophobic interaction potential with pocket amino acids Tyr440, Asn83, Asp70, Ser287, Thr284, Val288, Gly117, Phe398, Gly116, His438, Glu197 residues and also illustrated π-π T-shaped contact potential with Trp82, Phe329, Trp231 amino acid residues. Interestingly, best two ligands 5a and 5m exhibited no hydrogen bond contact with BChE receptor but instead found considerable geometric fit of these compounds in the receptor and therefore scoring in PatchDock being based on shape complementarity principles. The compound 5m revealed a score of 6998 and ACE value –404.36kcal/mol in the docked interactions with the BChE enzyme. Ligand 5p has shown a hydrogen bond interaction with amino group of Ala388 and bromo of 4–bromophenyl moiety with an average distance of 3.02 Å. Compounds 5m and 5p depicted hydrophobic interaction potential with BChE receptor amino acids Ala388, Glu387, Arg381, Asn384, Gln384, Thr374 and Glu387, Thr374, Gln380, Asp395, Phe371 respectively. Compound 5m and 5p has formed π-π T-shaped interaction with Tyr373, Tyr373, Trp376 and Tyr373, Tyr373, Trp376 correspondingly (Fig. 13).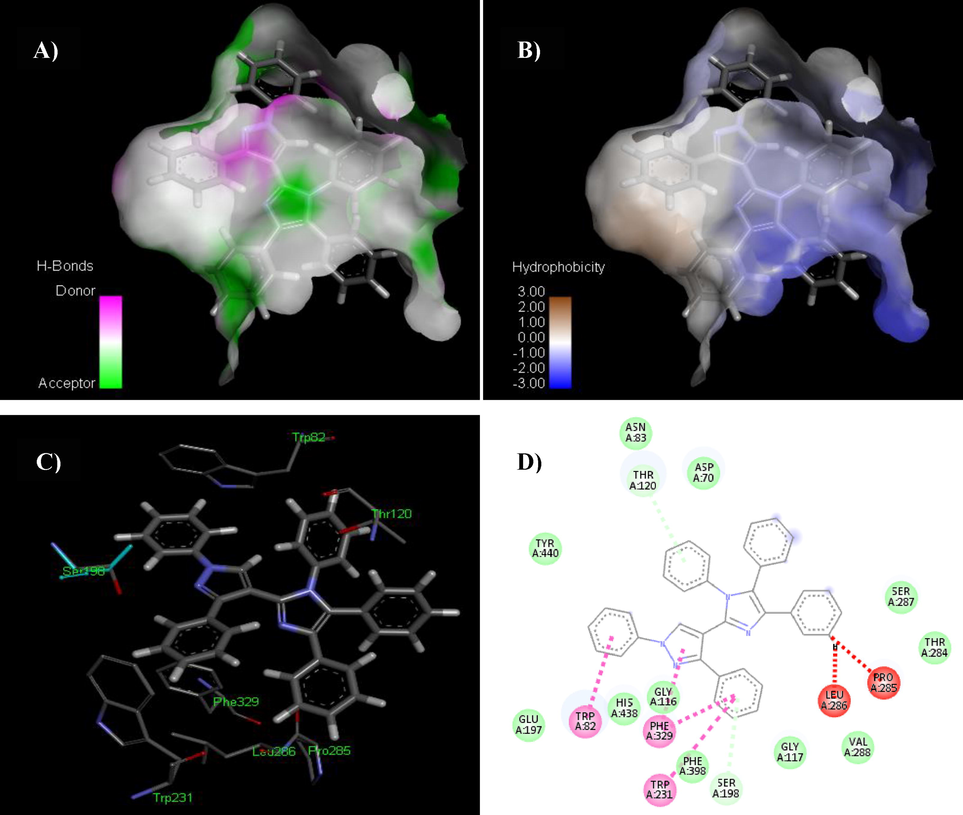
The analyses showing H-bonds (A) and hydrophobic contacts (B) of compound 5a. In the hydrophobic contacts (right) the blue color shows favorable structural features (atoms and torsions) donating to the whole binding energy inside the BChE binding pouch (PDB ID: 4bds), the pink representing unfavorable, and the white is neutral one. Fig. 12-C is 3D and Fig. 12-D is 2D representations of docked ligand 5a.
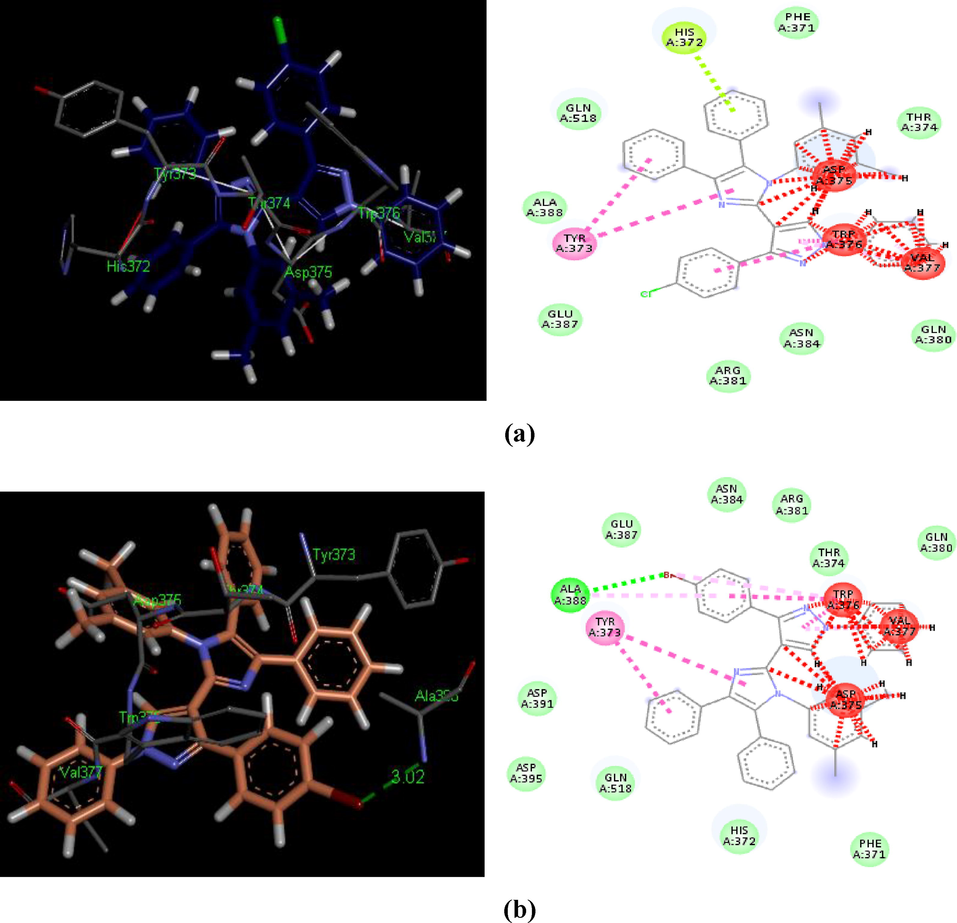
3D and 2D depictions of docked ligands – 5m (a) and 5p (b): demonstrating H-bond (light green), unfavorable bump (red), π-alkyl (light pink), amide π-stack (pink) and π-π T-shaped (shocking pink) contacts inside a binding pouch of the BChE (PDB ID: 4BDS).
Resultantly, our docking calculations have proposed that compounds 5a, 5m and 5p for BChE exhibited the excellent inhibitory potential against BChE and these results are consistent with the IC50 values of these compounds. The conformational orientations of all compounds (5a-5x) are demonstrating the maximal biological activities with their favorable interactions in the binding pouches (Table 5).
Compound
Score
ACE (kcal/mol)
Hydrogen bond interactions
Distance (Å)
Hydrophobic interactions
π-π T-shaped interactions
5a
7096
–332.95
–
–
Tyr440, Asn83, Asp70, Ser287, Thr284, Val288, Gly117, Phe398, Gly116, His438, Glu197
Trp82, Phe329, Trp231
5b
6978
–345.85
–
–
Gly127, Ser198, Phe398, Gly116, Trp82, Asn83, Asp70, Asn68, Glu276, Asn289, Gln119, Val288
–
5c
6940
–356.56
–
–
Ala388, Asp391, Gln518, Phe371, Thr374, Asn384, Arg381, Glu387
Tyr373
5d
5870
–363.06
–
–
Phe371, Thr374, Asn384, Glu387, Ala388, Asp391, Asp395
Tyr332
5e
5862
–311.73
–
–
Gln380, Arg381, ASn384, Glu383, Glu387, Ala388, Asp391, Tyr373, Asp395, Gln518, His372
Trp376
5f
6472
–457.04
–
–
Asp70, Asn83, Gly116, Thr120, Pro285, Thr284, Asn397, Gly117, Phe398, Gly115, Glu197
Trp82, Trp231
5g
6576
–378.61
–
–
Thr374, His372, Gln518, Ala388, Tyr373, Glu387, Arg381
Trp376
5h
6474
–348.11
–
–
Asn384, Arg381, Glu383, Asp391, Ala388, Gln518, Phe371, Thr374
Trp376
5i
6998
–414.34
–
–
Ser79, Asn83, Glu276, Asn289, Ser287, Gln119, Gly117, Val288, Phe398, Asn397, His438, Ile442
Trp231
5j
6462
–362.67
–
–
Gln380, Arg381, Glu387, Tyr373, Ala388, Gln518, His372, Phe371, Thr374
Trp376
5k
6320
–410.48
–
–
Thr284, Ser189, His438, Gly115, Asn83, Glu276
–
5l
6038
–357.18
–
–
Ala388, Thr374, Phe371
Tyr373
5m
6998
–404.36
–
–
Ala388, Glu387, Arg381, Asn384, Gln384, Thr374
Tyr373, Tyr373, Trp376
5n
6386
–369.44
–
–
Thr284, Glu276, Phe398, Ala199, His438, Gly115,
Trp231, Phe329, Gly116, Gly116
5o
6156
–297.92
–
–
Thr374, Phe371, Ala388, Asp391
Tyr373, Trp376
5p
7029
–356.84
Ala388
3.02
Glu387, Thr374, Gln380, Asp395, Phe371
Tyr373, Tyr373, Trp376
5q
6192
–389.06
–
–
Gln380, Ala388, Arg380, Thr374
Tyr373, Tyr373, Trp376
5r
6028
–336.77
–
–
Arg515, Phe371, Thr374, Ala388
Tyr373, Trp376, Trp376
5s
6198
–314.69
–
–
Phe371, Ala388, Thr374
Tyr373, Trp376
5t
6288
–312.13
–
–
Phe371, Ala388, Thr374
Tyr373, Trp376
5u
6408
–388.41
–
–
Phe398, Tyr440, Met437, Ser79
Phe329, Trp231
5v
6198
–336.65
–
–
Gly78, Met437, Tyr440, Ser79, Asn83, Phe398, Phe118
Trp231
5w
6348
400.51
–
–
Glu197, Gly115, Ala199, Asn397, Asn83, Ser79, Tyr332, Glu276
Gly116, Trp82
5x
6252
–520.40
–
–
Glu276, Leu273, Ala199, Thr284, Asn83, Phe389, His438
Gly116, Trp231
Eserine
4462
170.03
Tyr128
3.35
Leu125, Ile442, Glu197, Phe329, Tyr332, Trp430, Ser79, Tyr440, Met437, Gly78
Trp82
4 Conclusion
A series of heteroaryl substituted imidazoles (5a-5x) was synthesized and screened against AChE and BChE to evaluate their inhibitory potential in search of leads in the treatment of AD. At first, the synthetic reaction conditions were optimized by using different amino acids as catalysts, and polar / non-polar solvents. The facile and efficient synthetic methodology was adopted to prepare target series in excellent yields by employing the glutamic acid as a useful catalyst. The novel derivatives were further characterized through FTIR, EI-MS, 1H NMR, 13C NMR and CHN analyses. These compounds were further tested against both AChE and BChE enzymes. It is concluded from SAR results that imidazole is a core moiety and the aryl/heteroaryl rings along with structural modifications in the region-A and region-B are strongly correlated with enzyme inhibitions. A coumarinyl moiety containing imidazolylpyrazole derivative 5x was the most active AChE enzyme inhibitor with IC50 25.83 ± 0.25 µM. While a simple phenyl decorated imidazolylpyrazole scaffold 5a was the most potent BChE inhibitor with IC50 0.35 ± 0.02 µM when compared to standard eserine (BChE IC50 0.62 ± 0.08 µM). This molecule also has SI of 117.91 for BChE. Docking simulation disclosed the strong hydrophobic and π-π T-shaped interactions of 5a with the enzyme though hydrogen bonding was not involved in the enzyme inhibitor complex. Other potent BChE inhibitors of the series are 5p, 5m, 5x, 5b, 5c, 5e and 5f with IC50 values from 3.60 ± 0.12 µM to 9.84 ± 0.11 µM. Moreover, molecular docking studies of all the synthesized compounds have depicted critical interaction patterns with AChE and BChE enzymes and are in complete agreement with the in vitro findings. Hence, this study highlights some hopeful structural features for further studies in detail wherein potent compounds could be employed in new drug discovery.
Acknowledgment
This work was financially supported by the Higher Education Commission of Pakistan. We sincerely acknowledge the School of Chemistry, University of the Punjab, Lahore-Pakistan and the Kinnaird College for Women, Lahore-Pakistan for providing lab facilities for present research study. Inhibitory screening assays were carried out in the Institute of Chemistry, The Islamia University of Bahawalpur, Bahawalpur-Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pyrazole-3(4)-carbaldehyde: synthesis, reactions and biological activity. ARKIVOC. 2011;I:196-245.
- [CrossRef] [Google Scholar]
- Imidazole as a promising medicinal scaffold: current status and future direction. Drug Des. Devel. Ther.. 2021;15:3289-3319.
- [CrossRef] [Google Scholar]
- Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrids. Bioorg. Med. Chem. Lett.. 2020;30(2):126789
- [CrossRef] [Google Scholar]
- Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep.. 2021;48(7):5629-5645.
- [CrossRef] [Google Scholar]
- New coumarin-pyrazole hybrids: synthesis, docking studies and biological evaluation as potential cholinesterase inhibitors. J. Mol. Struct.. 2022;1249:131591
- [CrossRef] [Google Scholar]
- Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J.. 1989;260(3):625-634.
- [CrossRef] [Google Scholar]
- Amino acid catalyzed reactions. a facile route to some heteroarylbispyrazoles. Synth. Commun.. 2017;47(4):310-318.
- [CrossRef] [Google Scholar]
- In search of new α-glucosidase inhibitors: imidazolylpyrazole derivatives. Bioorg. Chem.. 2017;71:102-109.
- [CrossRef] [Google Scholar]
- Hetarylcoumarins: synthesis and biological evaluation as potent α-glucosidase inhibitors. Bioorg. Chem.. 2017;73:1-9.
- [CrossRef] [Google Scholar]
- Imidazole-pyrazole hybrids: synthesis, characterization and in-vitro bioevaluation against α-glucosidase enzyme with molecular docking studies. Bioorg. Chem.. 2019;82:267-273.
- [CrossRef] [Google Scholar]
- Synthesis of imidazole-pyrazole conjugates bearing aryl spacer and exploring their enzyme inhibition potentials. Bioorg. Chem.. 2021;108:104686
- [CrossRef] [Google Scholar]
- Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med. Chem. Lett.. 2013;4(11):1091-1096.
- [CrossRef] [Google Scholar]
- Nitrogen elimination in the mass spectra of diphenyl and triphenyl-v-triazoles. Org. Mass Spectrom.. 1971;5(4):427-436.
- [CrossRef] [Google Scholar]
- Alzheimer's disease drug development pipeline 2022. Alzheimer's Dement. Transl. Res. Clin. Interv.. 2022;8(1):e12295.
- [CrossRef] [Google Scholar]
- Synthesis of tacrine-lophine hybrids via one-pot four component reaction and biological evaluation as acetyl-and butyrylcholinesterase inhibitors. Eur. J. Med. Chem.. 2013;62:556-563.
- [CrossRef] [Google Scholar]
- Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res.. 2016;13(10):1173-1177.
- [CrossRef] [Google Scholar]
- A cascade synthesis, in vitro cholinesterases inhibitory activity and docking studies of novel tacrine-pyranopyrazole derivatives. Bioorg. Med. Chem. Lett.. 2018;28(14):2481-2484.
- [CrossRef] [Google Scholar]
- Imidazole: multi-targeted therapeutic leads for the management of Alzheimer’s disease. Mini-Rev. Med. Chem.. 2022;22(10):1352-1373.
- [CrossRef] [Google Scholar]
- Butyrylcholinesterase inhibitors as potential anti-Alzheimer’s agents: an updated patent review (2018-present) Expert Opin. Ther. Pat.. 2022;32(8):913-932.
- [CrossRef] [Google Scholar]
- Pharmacophore-based drug design of AChE and BChE dual inhibitors as potential anti-Alzheimer’s disease agents. Bioorg. Chem.. 2021;114:105149
- [CrossRef] [Google Scholar]
- 2022 Alzheimer's disease facts and figures. Alzheimer's Dement.. 2022;18(4):700-789.
- [CrossRef] [Google Scholar]
- Cholinesterase inhibitors for Alzheimer’s disease. Drugs. 2001;61(1):41-52.
- [CrossRef] [Google Scholar]
- Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov.. 2019;24(2):629-635.
- [CrossRef] [Google Scholar]
- Discovery of a potent and selective JNK3 inhibitor with neuroprotective effect against amyloid β-induced neurotoxicity in primary rat neurons. Int. J. Mol. Sci.. 2021;22(20):11084.
- [CrossRef] [Google Scholar]
- Cholinesterase inhibitors for Alzheimer's disease: multitargeting strategy based on anti-Alzheimer's drugs repositioning. Curr. Pharm. Des.. 2019;25(33):3519-3535.
- [CrossRef] [Google Scholar]
- Identification and characterization of diarylimidazoles as hybrid inhibitors of butyrylcholinesterase and amyloid beta fibril formation. Eur. J. Pharm. Sci.. 2012;45(1–2):169-183.
- [CrossRef] [Google Scholar]
- Glycine and its derivatives catalyzed one-pot multicomponent synthesis of bioactive heterocycles. Synth. Commun.. 2022;1–22
- [CrossRef] [Google Scholar]
- Pivotal role of nitrogen heterocycles in Alzheimer’s disease drug discovery. Drug Discov.. 2022;27(10):103322
- [CrossRef] [Google Scholar]
- Mono-or di-substituted imidazole derivatives for inhibition of acetylcholine and butyrylcholine esterases. Bioorg. Chem.. 2019;86:187-196.
- [CrossRef] [Google Scholar]
- Synthesis, inhibition studies against AChE and BChE, drug-like profiling, kinetic analysis and molecular docking studies of N-(4-phenyl-3-aroyl-2(3H)-ylidene) substituted acetamides. J. Mol. Struct.. 2020;1203:127459
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of diphenyl conjugated imidazole derivatives as potential glutaminyl cyclase inhibitors for treatment of Alzheimer’s disease. J. Med. Chem.. 2017;60(15):6664-6677.
- [CrossRef] [Google Scholar]
- Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer's disease. Eur. J. Med. Chem.. 2017;132:294-309.
- [CrossRef] [Google Scholar]
- Highly potent and selective butyrylcholinesterase inhibitors for cognitive improvement and neuroprotection. J. Med. Chem.. 2021;64(10):6856-6876.
- [CrossRef] [Google Scholar]
- Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat. Rev. Neurol.. 2019;15(3):135-147.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological evaluation of acridone glycosides as selective BChE inhibitors. Carbohydr. Res.. 2020;491:107977
- [CrossRef] [Google Scholar]
- New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur J. Med. Chem.. 2022;241:114616
- [CrossRef] [Google Scholar]
- Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer's drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J.. 2013;453(3):393-399.
- [CrossRef] [Google Scholar]
- Facile, eco-friendly, one-pot protocol for the synthesis of indole-imidazole derivatives catalyzed by amino acids. Synth. Commun.. 2017;47(16):1478-1484.
- [CrossRef] [Google Scholar]
- An efficient multicomponent synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by a magnetic nanoparticle supported Lewis acidic deep eutectic solvent. RSC Adv.. 2019;9(65):38148-38153.
- [CrossRef] [Google Scholar]
- Inhibitory potential of nitrogen, oxygen and sulfur containing heterocyclic scaffolds against acetylcholinesterase and butyrylcholinesterase. RSC Adv.. 2022;12(31):19764-19855.
- [CrossRef] [Google Scholar]
- Diagnosis of early alzheimer’s disease: clinical practice in 2021. J. Prev. Alzheimer's Dis.. 2021;8(3):371-386.
- [CrossRef] [Google Scholar]
- Investigating 1,2,3, 4,5,6-hexahydroazepino [4,3-b] indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer's disease. Eur. J. Med. Chem.. 2019;177:414-424.
- [CrossRef] [Google Scholar]
- Recent advances of small molecule JNK3 inhibitors for Alzheimer’s disease. Bioorg. Chem.. 2022;128:106090
- [CrossRef] [Google Scholar]
- Design, synthesis, and evaluation of some novel biphenyl imidazole derivatives for the treatment of Alzheimer's disease. J. Mol. Struct.. 2021;1246:131152
- [CrossRef] [Google Scholar]
- Halogenated coumarin–chalcones as multifunctional monoamine oxidase-B and butyrylcholinesterase inhibitors. ACS Omega. 2021;6(42):28182-28193.
- [CrossRef] [Google Scholar]
- Comparison of the binding of reversible inhibitors to human butyrylcholinesterase and acetylcholinesterase: a crystallographic, kinetic and calorimetric study. Molecules. 2017;22(12):2098.
- [CrossRef] [Google Scholar]
- Catalytic synthesis of 1,2,4,5-tetrasubstituted 1H-imidazole derivatives: state of the art. Adv. Synth. Catal.. 2019;361(12):2737-2803.
- [CrossRef] [Google Scholar]
- Sari, S., Akkaya, D., Zengin, M., Sabuncuoğlu, S., Özdemir, Z., Alagöz, M.A., Karakurt, A. and Barut, B., 2022. Antifungal azole derivatives featuring naphthalene prove potent and competitive cholinesterase inhibitors with potential CNS penetration according to the in vitro and in silico studies. Chem. Biodivers. 19 (7), e202200027. https://doi.org/10.1002/cbdv.202200027.
- Schneidman-Duhovny, D., Inbar, Y., Nussinov, R., Wolfson, H.J., 2005. PatchDock and SymmDock: servers for rigid and symmetric docking, Nucleic Acids Res. 33 (suppl_2), W363-W367. https://doi.org/10.1093/nar/gki481.
- Brønsted-acidic ionic liquid: green protocol for synthesis of novel tetrasubstituted imidazole derivatives under microwave irradiation via multicomponent strategy. Res. Chem. Intermed.. 2017;43(2):1089-1098.
- [CrossRef] [Google Scholar]
- Metal-free atom-economical synthesis of tetra-substituted imidazoles via flavin-iodine catalyzed aerobic cross-dehydrogenative coupling of amidines and chalcones. J. Org. Chem.. 2022;87(15):10372-10376.
- [CrossRef] [Google Scholar]
- Synthesis, in vitro cholinesterase inhibition, molecular docking, DFT and ADME studies of novel 1,3,4-oxadiazole-2-thiol derivatives. Chem. Biodiversity. 2022;19:e202200157.
- [Google Scholar]
- Design, synthesis, and biological evaluation of ferulic acid based 1,3,4-oxadiazole hybrids as multifunctional therapeutics for the treatment of Alzheimer’s disease. Bioorg. Chem.. 2020;95:103506
- [CrossRef] [Google Scholar]
- Proline and its derivatives as organocatalysts for multi-component reactions in aqueous media: synergic pathways to the green synthesis of heterocycles. Adv. Synth. Catal.. 2020;362(1):87-110.
- [CrossRef] [Google Scholar]
- Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD. Alzheimer's Res. Ther.. 2021;13(1):1-8.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of a new series of biphenyl/bibenzyl derivatives functioning as dual inhibitors of acetylcholinesterase and butyrylcholinesterase. Molecules. 2017;22(1):172.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104384.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







