Translate this page into:
Green synthesis of plant-stabilized Au nanoparticles for the treatment of gastric carcinoma
⁎Corresponding author. jchai@sdfmu.edu.cn (Jie Chai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, gold nanoparticles (AuNPs) were green synthesized using plant extract. The obtained nanoparticles (Au NPs) were characterized by advanced physical and chemical techniques like TEM, FTIR, UV–vis, SEM, XRD and EDX. SEM image displayed the quasi-spherical shaped nanoparticles of mean diameter 20–50 nm. All the particles were of uniform shape and texture. From the XRD pattern, four distinct diffraction peaks at 38.2°, 44.2°, 64.7° and 77.4° are indexed as (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of fcc metallic gold. The in vitro cytotoxic and anti-gastric carcinoma effects of biologically synthesized Au NPs against cancer cell lines were assessed. The IC50 of the Au NPs were 192, 149, 76 and 85 µg/mL against NCI-N87, MKN45, GC1401 and GC1436 gastric cancer cell lines. The anti-gastric carcinoma properties of the Au NPs could significantly remove the cancer cell lines in a time and concentration-dependent manner. So, the findings of the recent research show that biologically synthesized Au NPs might be used to cure cancer.
Keywords
Au nanoparticles
Chemopreventive and chemotherapeutic agent
Cancer
1 Introduction
Cancer is one of the death factors in most parts of the world. Common methods of cancer treatment such as chemotherapy and radiotherapy lead to drug therapy failure and disease recurrence. The most important problem of cytotoxic drugs is their distribution in healthy and cancer cells, which causes drug toxicity and harmful side effects on healthy cells. It also reduces the dose of drug delivery to cancer cells. In addition, rapid excretion and widespread distribution into the organs and tissues require the administration of drugs in large quantities that are not economically viable (Kanapathipillai et al., 2014; Lerous et al., 1996; Lammers et al., 2012; Panyam et al., 2003). Nanoparticles as pharmaceutical carriers have solved some of the current drug delivery limitations in cancer treatment (Abbasi et al., 2021; Shi et al., 2022; Zarafshani et al., 2022). These structures are known as a very effective drug delivery system due to the protection of the drug molecule, reduction of toxicity and side effects, ability to cross biological barriers to deliver the drug to the target site and increase the shelf life of the drug in the bloodstream, which increases pharmacotherapy efficiency (Zangeneh, 2019; Williams et al., 2003).
The field of synthetic nanotechnology has developed the self-sufficiency of materials with specific sizes and shapes at the nanometer scale environmentally friendly (Ma et al., 2020; Bai et al., 2021; Dou et al., 2021). Nanomaterials can be created from the engineering of multiple or single molecules which are engineered and have several functional groups. Biocompatible nanocarriers include peptides, engineered antibodies, micelles, polymers, and liposomes (Zangeneh et al., 2019a; Mohammadi et al., 2019; Zangeneh et al., 2019b). Nanomaterials can be designed to gain the desired chemical and physical characteristics and purposefully direct drugs to dynamic tumor environment with high therapeutic effects and low toxicity (Abdoli et al. 2020; Han et al., 2020; Jalalvand et al., 2020; Sun et al., 2020; Gholami et al., 2022). Features to be controlled when designing nanoparticles are surface to volume ratio, shape, size, molecular charge, and drug release. For example, nanoparticles, after binding to functional chemical components such as antibodies and ligands, bind to the surface receptors of the cancer cell and prevent its clearance by the blood (Mahdavi et al., 2019; Zangeneh et al., 2019c; Jalalvand et al., 2019).
Drug delivery by nanoparticles using biodegradable polymers solves many problems. In 1975, a drug-conjugated model of the polymer was proposed by Ringdorf, which increased drug delivery to the cancer cell site (Alexis et al., 2010; Zangeneh et al., 2019d; Nie et al., 2007; Ringsdorf et al., 1975). She suggested that the conjugated pharmacological properties of drugs and polymers could be manipulated by altering the chemical and physical properties of the polymer. For example, a water-insoluble drug can be soluble by adding solvent components to a polymer. Therefore, it increases both bioavailability and biodegradability. For cytotoxic drugs to be efficiently delivered to cancer cell tissue, first, cytotoxic agents must be removed from the bloodstream and after leaving the extracellular space, the cancerous tissue must pass through the membrane surface and be targeted inside the cancer cell (Zangeneh et al., 2019e; Rizzo et al., 2013). The ideal properties of nano foundations that should be considered when designing are: 1- Continuous drug release 2- Inactive accumulation of the drug in cancerous tissue 3- Targeting antigens or surface receptors of cancer cells with a controlling effect on endosomal uptake and membrane destruction. 4- Drug release into the cytoplasm and protection from enzymatic degradation (Kroll et al., 1998; Vasir et al., 2005; Singh et al., 2009).
In this work we have proposed the ‘green’ approach for synthesis of stable plant capped gold nanoparticles (Au NPs) with desirable physico-chemical properties. Due to non-toxic effects of plant to human, it is interesting and valuable compounds in pharmaceuticals and biomedical usages. So, we used plant in research work as an ecofriendly natural polymer with minimum side effects and importantly as a natural reducing/stabilizing and solid support agent for fabrication gold nanoparticles without using any toxic and harmful reagent. Then, the prepared Au NPs were characterized by advanced physicochemical techniques. Also, the in vitro cytotoxic and anti-gastric carcinoma effects of biologically synthesized Au NPs against cancer cell lines were evaluated.
2 Experimental
2.1 Materials and methods
The chemicals required for catalyst synthesis and catalyst applications were purchased from Sigma Aldrich.
2.2 Synthesis of Au NPs:
To prepare the aqueous extract of the Citrus aurantium, 6 g of the dried part of the mill was poured into 150 mL of boiling water and boiled for ten minutes. Then the extract was allowed to cool down gradually. After complete cooling, the resulting extract was clarified by a high-speed centrifuge (20,000 rpm) for 15 min. The resulting sample was wrapped in aluminum foil and kept in the refrigerator. To initiate the reaction 20 mL of a freshly prepared aqueous solution of HAuCl4 (10 mM) was added to 100 mL of plant extract solution under ultrasonic conditions and left for 10 min at 60 °C. Then pH of the solution was adjusted to 11.0 (NaOH, 3 wt%) and the reaction mixture was kept with sonication at 60 °C for 60 min. The progress of the reaction could be monitored by change in color from light yellow (Au3+ ion) to reddish-brown (Au0 NP). The prepared Au NPs was collected by centrifugation and washed several times with DI-water.
2.3 Cytotoxicity and anti-cancer properties
The process of the prokaryotic or eukaryotic cells controlled culture in a filtered or unfiltered flask or cell culture plate by a suitable culture medium is called. This term is mostly used for culturing multicellular cells. Special culture media are used to culture cells. The cells are usually cultured at 37° C in equipment such as CO2 incubators. Cell culture should be performed under aseptic (disinfected) conditions because the growth of these cells is much slower than the growth of bacteria and yeasts and there is a possibility of contamination of the culture medium. Antibiotics such as penicillin, streptomycin, or gentamicin are sometimes used to stop the growth of bacteria. In order for cells to proliferate well in culture medium, their density in culture medium must be low. For this purpose, the cells should be passed to the fresh culture medium from time to time. One of the goals of cell culture is to study cells in terms of how they grow, their nutritional needs, and the reasons they stop growing, each of which can have a profound effect on the morphology of the cells we see under a microscope. Therefore, to study the cell growth cycle, develop methods to control the growth of cancer cells and modulate the expression of genes, it is necessary to cultivate these cells in the external environment (Zhang et al., 2021). With the help of cell culture, cells can be prepared that are in different stages of differentiation and can be differentiated into other cells with the help of hormones and growth factors. With the help of cell culture, homogenous cells can be prepared and intracellular activities such as DNA replication, DNA transcription synthesis, RNA and protein synthesis and other details related to metabolism can be studied. It is also possible to examine the subsequent events and intracellular currents, such as the displacement of these complexes, the type of intracellular messages, and how the messages are transmitted, after connecting different molecules to the corresponding membrane receptor. The cultured cells can be stored frozen at very low temperatures. Such conditions will maintain the growth rate or genetic composition of these cells and can be thawed and used again at the appropriate time. This prevents the aging of cells, while it is currently not possible to prevent the aging of animals. When working with laboratory animals, systemic changes due to the effect of the animal's natural homeostasis or the stress of the experiments on the results should be considered. While the use of cell culture eliminates this problem. In addition, standardizing laboratory tests is easier and more practical than tests on living organisms. In laboratory environments, it is much easier to control the physical and chemical factors in the living environment of cells, including acidity, heat, osmotic pressure, and the pressure of gases such as oxygen and carbon dioxide. Cells that are taken directly from the individual are known as primer cells and have a limited lifespan. Most cells have a limited lifespan, except for those taken from a tumor. An immortal cell line can proliferate indefinitely by creating a random or targeted mutation (such as artificial expression of the genus and be established as a representative of specific cell types (Zhang et al., 2021).
In this research, we used Human Umbilical Vein Endothelial Cells (HUVECs) to determine the cytotoxicity potentials of Au NPs using MTT. Also, the in vitro anti-gastric cancer effects of biologically synthesized Au NPs against NCI-N87, MKN45, GC1401 and GC1436 cancer cell lines were evaluated.
The cells were cultured in medium (RPMI1640 = Roswell Park MemoryL Institute1640) with 10 % FBS combined with penicillin and streptomycin antibiotics in an incubator containing 5 % CO2 in a flask (T25). After three passages for purification, the cells were used to perform the next steps. Cell count and the number of viable cells were performed with a homocytometer slide using trypan blue. Evaluation of the cytotoxic effect of the Au NPs was performed by the modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric test. In this method, MTT, which is yellow, is converted to insoluble and formazan purple dye by the dehydrogenase enzymes in the mitochondria of active cells. The adsorption of this compound can be measured after dissolving at 570–540 nm. After two days and covering the flask bottom with cell, the cell layer adhering to the flask bottom was isolated enzymatically using trypsin-EDTA (5 %) (Tetraacetic acid ethylenediamine), after transfer to sterile test tubes, it was centrifuged at 2000 rpm for 10 min. The cells were then resuspended in a fresh culture medium with the help of a pasteur pipette and cell suspension (106 ml/μg) was prepared from them. 40 μl of this cell suspension (equivalent to 104 × 4 cells) was poured into 96-well plate flat-bottomed wells (for cell culture). Then the final volume of each well with 10 % FBS medium reached 200 μl. The first-row containing cell suspension was considered as negative control (control). After incubation for 18–24 h to remove cells from the stress caused by trypsinization, the supernatant was removed slowly and carefully, A new medium was added to all rows with different concentrations of the Au NPs (only a new medium was added to the negative and positive control rows), so that the diluted Au NPs with concentrations of 1–1000 μg/ml was added to the third to sixth rows, respectively, the plate was incubated in CO2 for 48, 24 and 72 h. After the incubation time, the plate was taken out of the incubator and 20 μl of MTT (Sigma) was added to all wells, and incubated for 3 h. The supernatant was then gently removed and 100 μl of DMSO was added to the wells and pipetted to dissolve the formazan crystals. The amount of light absorption (OD) according to the intensity of the blue color of formazan at 540 nm was read by Eliza reader. To convert OD to the percentage of living cells, the following formula was used and the percentage of cell life at each concentration was calculated after 48, 24 and 72 h (Zhang et al., 2021).
The concentration of the tested compounds that reduced the percentage of cell life by half was considered as IC50 (The half maximal inhibitory concentration) (Zhang et al., 2021).
At least three independent replications were performed for each data and the result was presented as mean ± SD. Data statistical analysis was done with SPSS software version 19 and Anova Way One and Duncan’s test. Significance was considered at the level of P ≤ 0.05.
3 Results and discussion
3.1 Structural characterization of Au NPs
The successful biogenic synthesis of Au(0) NP was primarily assured by visual color change from yellow to dark red. The formation of Au NPs was further justified from UV–vis spectroscopy. Fig. 1 displays the typical plasmon resonance band of Au NP, being observed at ∼ 543 nm (λmax).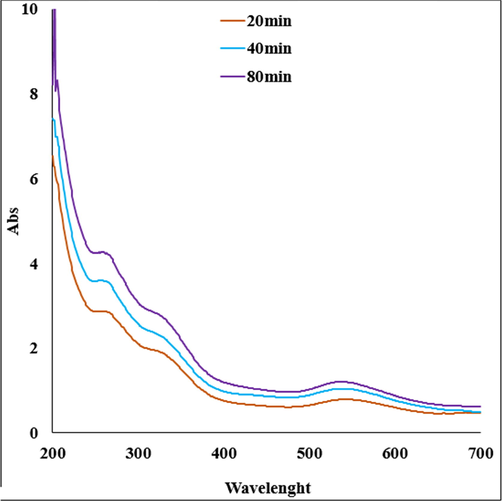
UV–visible absorption spectra of Au NPs.
Fig. 2 exhibits the spectra of Au NPs. The peaks at 471, 587 and 654 cm−1 belong to the bending vibration of Au-O. These peaks for Au nanoparticles have been reported previously with a small difference in the wavenumber (Hofheinz et al., 2005). A broad peak around 3443 relates to O—H, peak at 2968 cm-1shows an aliphatic C—H stretching band. These peaks can be considered for some phytochemical compounds found in extract such as phenolic, flavonoid, triterpenes (Bazak et al., 2014) (see Fig. 3).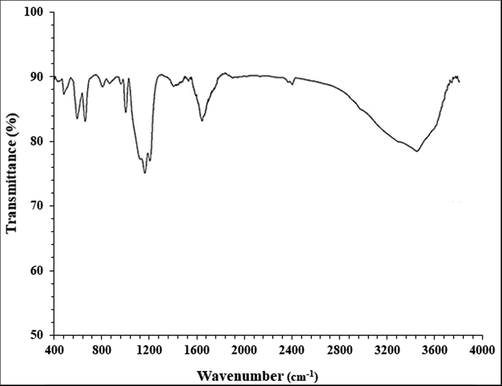
FT-IR analysis of Au NPs.

FE-SEM image of Au NPs.
SEM image displays the quasi-spherical shaped nanoparticles of mean diameter 20–50 nm. All the particles are of uniform shape and texture. For further structural inherence of the Au NPs, TEM analysis was carried out (Fig. 4). The TEM measurements revealed the presence of spherically shaped nanoparticles in the matrix (Fig. 4). The mean diameter of NPs was in the range of 20–50 nm. In the image, the particles were well dispersed without any signs of aggregation, which is consistent with the UV–vis results (see Fig. 5).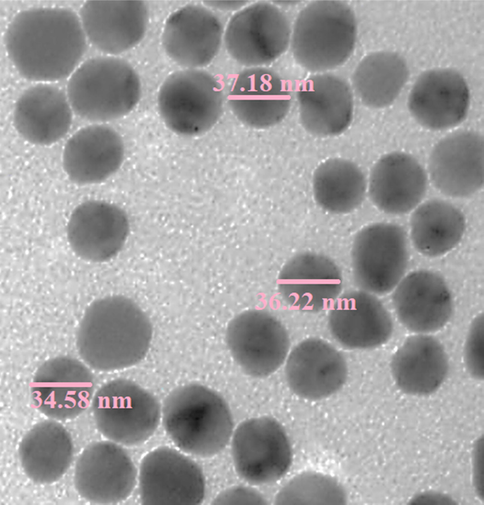
TEM image of Au NPs.
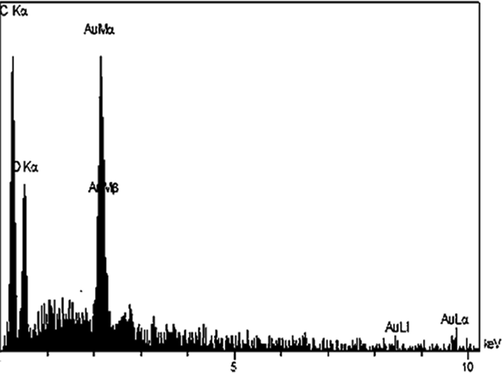
EDX spectrum of the Au NPs.
As depicted in in EDX analysis, the profile displays and confirms the presence of Au in the composite. Again, the signals corresponding to C and O atoms can be ascribed to plant extract molecules thereby assuring the proposed nanostructure.
The typical XRD pattern of Au NPs is exhibited in Fig. 6, illustrating the crystalline nature of synthesized Au NPs. From the XRD pattern, four distinct diffraction peaks at 38.2°, 44.2°, 64.7° and 77.4° are indexed as (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of fcc metallic gold.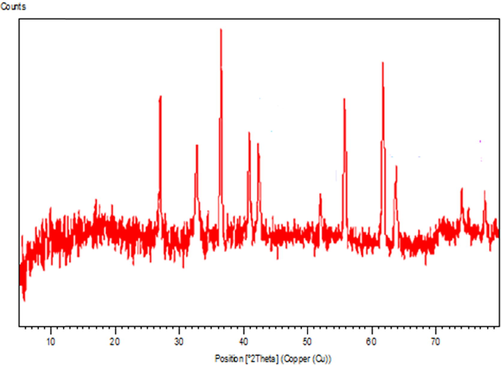
X-ray diffraction analysis of synthesized Au NPs.
3.2 anti-cancer properties analysis
The most important feature of nanoparticles is particle size and particle distribution. Particle size and drug particle distribution play important roles in the biological fate, toxicity, and targeting of drug delivery systems. They affect the loading, release and stability of particle tattoos. Many studies have shown the advantages of nanoparticles compared to microparticles (Sinha et al., 2006). One of the advantages of nanoparticles over micro-particles is their high cellular uptake. Due to their small size, the particles enter various cells and intracellular space. Nanoparticles cross the blood–brain barrier following the opening of hard endothelial junctions by mannitol, which provides stable drug release systems to treat severe brain diseases. Studies have shown that Tween 80-coated nanocomposites are able to cross the barrier of blood–brain (Kreuter et al., 2003). Particles smaller than a micron are removed by a large number of cells (Zauner et al., 2001). The cellular uptake of 100 nm nanoparticles is 2.5 times that of 1 μm particles, and the cellular uptake of 100 nm nanoparticles is 6 times that of 10 μm particles by 2-caco cells (Desai et al., 1997). Drug release is also controlled by particle size. Smaller particles have a lower volume-to-surface ratio. Therefore, if the drugs are placed on or near the surface of the nanoparticles, it will lead to the rapid release of the drug. But, larger particles are annealed at the center of the nanoparticles, which are released more slowly (Redhead et al., 2001). Therefore, particle size control is effective in determining drug release. Smaller particles may form during transport, disrupting their distribution. The decomposition of polymers is affected by particle size. For example, as the particle size of polylactic-co-glycolic acid polymer increases, its decomposition rate increases. Therefore, it is hypothesized that larger particles cause the polymer to decompose faster and the drug to be released faster (Dunne et al., 2000).
In this research, the treated cells with different concentrations of the present Au NPs catalyst were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and human gastric malignancy cell lines (Table 1; Figs. 7-9).
HAuCl4 (µg/mL)
AuNPs (µg/mL)
IC50 against HUVEC
–
–
IC50 against NCI-N87
–
192 ± 0b
IC50 against MKN45
–
149 ± 0ab
IC50 against GC1401
–
76 ± 0a
IC50 against GC1436
–
85 ± 0a
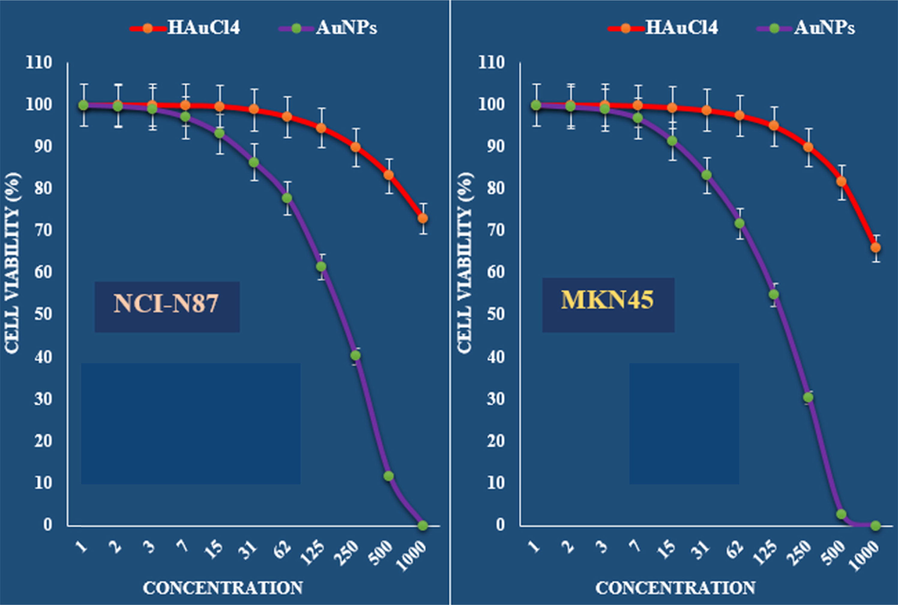
The anti-gastric carcinoma properties (Cell viability (%)) of HAuCl4 and AuNPs (Concentrations of 0–1000 µg/mL) against NCI-N87 and MKN45 cell lines.
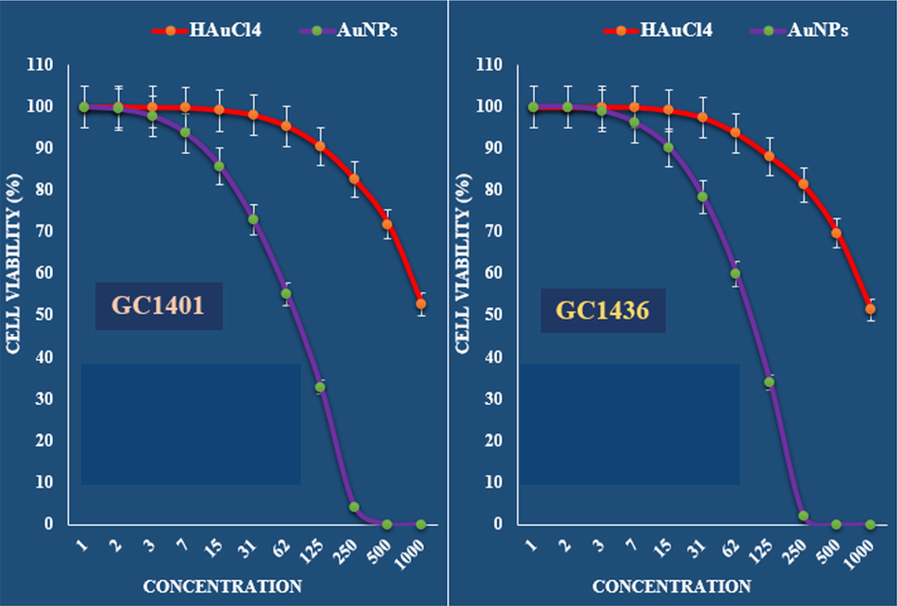
The anti-gastric carcinoma properties (Cell viability (%)) of HAuCl4 and AuNPs (Concentrations of 0–1000 µg/mL) against GC1401 and GC1436 cell lines.
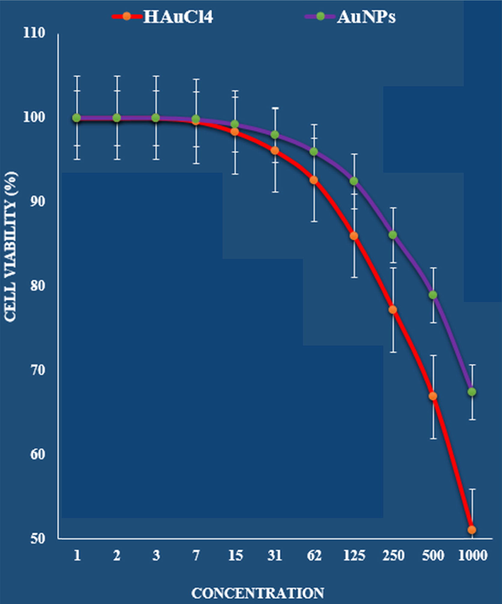
The cytotoxicity effects of HAuCl4 and AuNPs against normal (HUVEC) cell line.
The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Au NPs catalyst (Table 1 and Fig. 9).
The viability of malignant gastric cell lines reduced dose-dependently in the presence of Au NPs catalyst.
The IC50 of Au NPs catalyst were 192, 149, 76 and 85 µg/mL against NCI-N87, MKN45, GC1401 and GC1436 cell lines, respectively (Table 1 and Figs. 7 and 8).
An advantage of gold nanoparticles is their ability to detect microorganisms, cancerous tissues, etc., both in vivo and in vitro. Gold nanoparticles coated with anti-cancer antibodies can effectively bind to cancer cells (Paciotti et al., 2004; Paciotti et al., 2006; Park et al., 2013). Many cancer cells have a protein on their surface called the epidermal growth factor receptor (EFGR). This protein is not found mainly in healthy cells of the body. By attaching gold nanoparticles to the EFGR antibody (known as -Anti EFGR), researchers have attached these nanoparticles to cancer cells. Gold nanoparticles are an excellent carrier for immunotherapy because, like other nanoparticles, they can easily accumulate inside immune cells (Remant Bahadur, 2014; Ansersson et al., 2014). Gold nanoparticles can transport several drug molecules, recombinant proteins, vaccines or nucleotides into the target cell. It can also control the release or release of drugs. Conjugation of gold nanoparticles with drug molecules plays an important role in intracellular diseases. Antibiotics and other drugs can bind directly to gold nanoparticles with the help of ion or covalent bonds. The surface of gold nanoparticles can be easily changed to bind drug molecules. Changing the level of gold nanoparticles with polymers plays an important role in its conjugation with the drug. With this strategy, many therapeutic drugs can be successfully transferred, such as doxorubicin and tamoxifen (Huang et al., 2008; Cruz et al., 2012). An important challenge in cancer is the delivery of water-soluble drugs, which used hydrophobic polymers to coat the nanoparticles; the drug is encapsulated by a hydrophobic coating. Methotrexate is used as an anti-cancer drug. It is a folic acid analog that can inhibit the growth and proliferation of cancer cells (Remant Bahadur, 2014; Ansersson et al., 2014). The carboxylic group on the drug can bind to the surface of gold nanoparticles and exert its effect on cancer cells. Polyethylene glycol increases the adsorption of gold nanoparticles. The hydrophilic properties of PEG prevent the opso and purification of nanoparticles in the body. It can also be used as conjugation of gold nanoparticles and drug molecules (Huang et al., 2008; Cruz et al., 2012; Rita et al., 2017).
4 Conclusion
In summary we report the green synthesis of Au NPs catalyst being synthesized by deposing in situ bioreduced Au NPs over plant extract in alkaline medium. The material was physicochemically characterized using UV–vis and FT-IR spectroscopy, FESEM, TEM and EDX analysis. Average diameters of the particles were ∼ 20–50 nm. The viability of malignant cell lines reduced dose-dependently in the presence of Au NPs. The IC50 of Au NPs were 192, 149, 76 and 85 µg/mL against NCI-N87, MKN45, GC1401 and GC1436 cell lines, respectively. After clinical study, Au NPs can be utilized as an efficient drug in the treatment of gastric carcinoma in humans.
Funding
This work was supported by Key Research and Development Project of the Shandong Province of China (NO.2019JZZY011008) and Shandong Natural Science Foundation (NO.ZR2021MH108).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Formulation and characterization of a novel cutaneous wound healing ointment by silver nanoparticles containing Citrus lemon leaf: A pre-clinical trial study. Arab J Chem. 2021;14:103246
- [Google Scholar]
- Polyvinyl alcohol/Gum tragacanth/graphene oxide composite nanofiber for antibiotic delivery. J Drug Deliv Sci Technol.. 2020;60:102044
- [Google Scholar]
- HSP70 promoterdriven activation of gene expression for immunotherapy using gold nanorods and near infrared light. Vaccines.. 2014;2:216-227.
- [Google Scholar]
- Introducing a modern chemotherapeutic drug formulated by iron nanoparticles for the treatment of human lung cancer. J. Exp. Nanosci.. 2021;16:398-410.
- [Google Scholar]
- Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol Clin Oncol. 2014;2:904-908.
- [Google Scholar]
- Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol.. 2012;509:143-163.
- [Google Scholar]
- The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res.. 1997;14:1568-1573.
- [Google Scholar]
- Efficient biogenesis of Cu2O nanoparticles using extract of Camellia sinensis leaf: Evaluation of catalytic, cytotoxicity, antioxidant, and anti-human ovarian cancer properties. Bioorg. Chem.. 2021;106:104468
- [Google Scholar]
- Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials. 2000;21:16591668.
- [Google Scholar]
- Investigation of biological effects of chitosan magnetic nano-composites hydrogel. Nanotechnology. 2022;33:495603
- [Google Scholar]
- Ag NPs on chitosan-alginate coated magnetite for synthesis of indazolo[2,1-b]phthalazines and human lung protective effects against α-Guttiferin. Int. J. Biol. Macromol.. 2020;164:2974-2986.
- [Google Scholar]
- Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med.. 2008;23:217-228.
- [Google Scholar]
- J. Photochem. Photobiol., B. 2019;192:103-112.
- An elegant technology for ultrasensitive impedimetric and voltammetric determination of cholestanol based on a novel molecularly imprinted electrochemical sensor. Chem. Phys. Lipids. 2020;229:104895
- [Google Scholar]
- Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv. Drug Deliv. Rev.. 2014;79–80:107-118.
- [Google Scholar]
- Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res.. 2003;20:409-416.
- [Google Scholar]
- Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery. 1998;43:879-886.
- [Google Scholar]
- Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161:175-187.
- [Google Scholar]
- Biodegradable nanoparticles From sustained release formulation to improved site specific drug delivery. J. Control. Release. 1996;30:339-350.
- [Google Scholar]
- Immobilized Ag NPs on chitosan-biguanidine coated magnetic nanoparticles for synthesis of Propargylamines and treatment of human lung cancer. Int. J. Biol. Macromol.. 2020;165:767-775.
- [Google Scholar]
- Appl. Organometal. Chem.. 2019;33:e5248.
- Appl. Organometal Chem.. 2019;33:e5136.
- Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv.. 2004;11:169183
- [Google Scholar]
- Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumortargeted drug delivery vectors. Drug Dev. Res.. 2006;67:47-54.
- [Google Scholar]
- Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev.. 2003;55:329-347.
- [Google Scholar]
- Cell penetration and cell-selective drug delivery using alpha-helix peptides conjugated with gold nanoparticles. Biomaterials. 2013;34:4872-4879.
- [Google Scholar]
- Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J. Control. Release. 2001;70:353-363.
- [Google Scholar]
- Gold nanoparticle-based gene delivery: promises and challenges. Nanotechnol Rev. 2014;3:269-280.
- [Google Scholar]
- Structure and properties of pharmacologically active polymers. J Polym Sai Symp. 1975;51:135-153.
- [Google Scholar]
- Recent progress in nanomedicine therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol.. 2013;24:1159-1166.
- [Google Scholar]
- Cu immobilized on chitosan-modified iron oxide magnetic nanoparticles: Preparation, characterization and investigation of its anti-lung cancer effects. Arab J Chem. 2022;14:103224
- [Google Scholar]
- Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther S 2006:1909-1917.
- [Google Scholar]
- Decorated Au NPs on agar modified Fe3O4 NPs: Investigation of its catalytic performance in the degradation of methylene orange, and anti-human breast carcinoma properties. Int. J. Biol. Macromol.. 2020;165:787-795.
- [Google Scholar]
- Targeted drug delivery in cancer therapy. Technol. Cancer Res. Treat.. 2005;4:363-374.
- [Google Scholar]
- Nanoparticle drug delivery system for intravenous delivery of topoisomerase inhibitors. J. Control. Release. 2003;28:167-172.
- [Google Scholar]
- Appl. Organometal. Chem.. 2019;33:e5295.
- Appl. Organometal. Chem.. 2019;33:e4961.
- Appl. Organometal. Chem.. 2019;33:e5016.
- Appl. Organometal. Chem.. 2019;33:e5290.
- Appl. Organometal. Chem.. 2019;33:e5247.
- Appl. Organometal. Chem.. 2019;33:e5246.
- A novel biocompatible and biodegradable electrospun nanofibers containing M. neglectum: Antifungal properties and in vitro investigation. IEEE Trans. Nanobiosci.. 2022;21:520-528.
- [Google Scholar]
- In vitro uptake of polystyrene microspheres: effect of particle size, cell line and cell density. J. Control. Release. 2001;71:39-51.
- [Google Scholar]
- Green formulation, chemical characterization, and antioxidant, cytotoxicity, and anti-human cervical cancer effects of vanadium nanoparticles: A pre-clinical study. Arab J Chem.. 2021;14:103147
- [Google Scholar]







