Translate this page into:
New acetylcholinesterase inhibitors isolated from Delphinium uncinatum
⁎Corresponding authors. akhund83@gmail.com (Hanif Ahmad), manzoorhej@yahoo.com (Manzoor Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
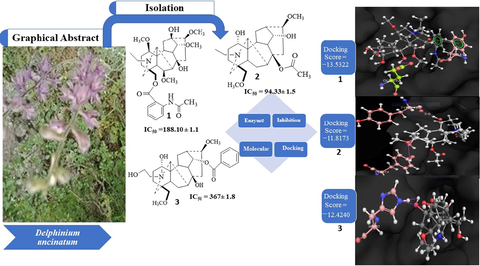
Four (1–4) norditerpenoids have been isolated from D. uncinatum. Structures of (1–4) were deduced based on spectroscopic technique. All the compounds (1–4) were evaluated for their acetylcholinesterase inhibitory potential followed by molecular docking studies. The isolated compounds showed promising acetylcholinesterase inhibition activities.
Abstract
The inhibition of acetylcholinesterase (AChE), the key enzyme in the breakdown of acetylcholine, is presently the most common pharmacological approach available for Alzheimer’s disease (AD). Despite research on the molecular bases of AD, potent therapeutic agent against its expansion is still needed. In searching for natural cholinesterase inhibitors, the present study was focused on the isolation of three new norditerpenoid alkaloids, uncinatine B-D together with known virescenine from Delphinium uncinatum. Chemical structures for all the isolated norditerpenoids (1–4) were established using latest spectroscopic techniques. The isolated undescribed compounds along with known virescenine were testified for their acetylcholinesterase inhibitory activity supported by docking analyses. Molecular docking simulation showed that the isolated compounds (1–4) were observed to adhered in the active site of AChE with docking scores − 13.5322 (1), −11.8173 (2), −12.4240 (3) and − 8.9352 (4) respectively. Overall results demonstrated that these natural norditerpenoids compounds were found as selective inhibitors of AChE. This is the first report regarding the use of bioactive ingredients of Delphinium uncinatum in testing against Alzheimer's disease.
Keywords
Norditerpenoids
Uncinatine B-D
Alzheimer’s disease (AD)
Acetylcholinesterase inhibitory potential
Molecular docking
1 Introduction
Delphinium uncinatum, a renowned species of genus Delphinium, belonging to the family of Ranunculaceae. This species is extensively disseminated in different regions of Pakistan like Dir, Swat, Chitral and Azad (Biosci et al., 2012; Khan et al., 2010, 2015). Genus Delphinium is recognized since long, as a natural source of potent diterpenoid alkaloids, that can be applied as therapeutic remedy for different disorders in human worldwide (Ahmad et al., 2017a, 2016; Liu et al., 2019; Shaheen et al., 2015). Natural products especially diterpenoids and norditerpenoids alkaloids, acting as acetylcholinesterase (AChE) inhibitors were obtained from plant species as reported previously in literature (Ahmad et al., 2017c; Hostettmann et al., 2006; Houghton et al., 2006; Mukherjee et al., 2007a; Ranjan* et al., 2017). Four norditerpenoid alkaloids condelphine, 14-acetylvirescenine, delbrusine and 14-acetylperegrine have been previously investigated and isolated from D. uncinatum (Ulubelen et al., 1998).
AD is an age-related and progressive neurodegenerative disorder of multifactorial nature, a renowned type of dementia that affects a large number of old age (over 65 years) individuals (Konrath et al., 2013; Mughal et al., 2017; Reflexa et al., 2013). The rate of AD immensely increases in the developing countries with rapid increase in ageing of human population. According to WHO (World Health Organization) report, dementia can currently be found among 36 million of the world’s population with a progressive increase in occurrence over the past decades and 71 % dementia cases are expected to occur by 2040 in the inhabitants of developing nations (El-Metwally et al., 2019; Ferri et al., 2005; Kalaria et al., 2008). The basic pathological signs of AD are marked by the growth of senile or amyloid plaques with extracellular deposit of β-amyloid peptide (Aβ) (Kril, 2009; Mughal et al., 2018; Portelius et al., 2006). The collapse of memory with conservation of long-term memory, can lead to cognitive dysfunction is the initial and main behavioral symptom of AD (Schliebs and Arendt, 2006).
The important remedial effect of cholinesterase inhibitors [ChEI] in AD treatment is to fortify cognitive function for at least 1-year period in around 50 % patients. The studies at clinical level illustrates that in a specific ratio of AD patients (about 20 %) cognitive function was stabilized for a period of up to 24 months. Further, those patients of AD who did not show any fruitful response to therapy with one ChEI can be changed into another one with a 50 % success rate. Previous research findings have shown that both AChE and BuChE were found to be associated with the hydrolysis of acetylcholine in brain and the inhibition caused by dual cholinesterase inhibitors might increase the efficiency of treatment (Giacobini, 2004; Mughal et al., 2019).
During extreme condition of AD, the management of ACh depends on BChE. As per clinical examination report, patients using dual cholinesterase inhibitors as medicine revealed minor cortical atrophic changes unlike patients who take selective AChE inhibitor (Ahmad et al., 2017a; Mughal et al., 2021). Cholinesterases, consisting of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) belongs to enzyme family that causes the catalytic hydrolysis of acetylcholine (ACh(Jamila et al., 2015; Obaid et al., 2022). Acetylcholinesterase (AChE) enzyme is the most convenient and attractive target for mechanism-based inhibitors in the rational drug discovery and is useful for neuronic disorder like AD (Choudhary et al., 2006). The acetylcholinesterase (AChE) plays a vital role in AD and hence catalyzes the hydrolysis of ester bond in acetylcholine (ACh) to end the nerve impulse transmitted action of ACh by means of cholinergic synapses (Sultzer et al., 2022). The inhibition of AChE is considered to be the basic therapeutic approach for AD, myasthenia gravis and senile (Atta-ur-Rahman and Choudhary, 2001). Synthetic compounds used for the therapy of memory impairment and cognitive dysfunction had adverse effects and troubles related to bioavailability (Oh et al., 2004; Schulz, 2003) whereas inhibitors extracted from plants were quite safe, having high potency as compared to synthetic inhibitors (Mukherjee et al., 2007a; Owokotomo et al., 2015). Now it is the need of the present era to work for the discovery of excellent AChE inhibitors.
To further discover natural cholinesterase inhibitors, D. uncinatum was investigated as a part of our continuous work, which resulted in the isolation of three unreported norditepenoids 1–3 along with known compound virescenine (4) for the first time.
2 Materials and methods
2.1 General procedures
IR spectrum was acquired on spectrophotometer (Jasco-320-A; Perkinelmer: JASCO, Tokyo, Japan) in KBr and was expressed in cm−1. HR-EIMS [Jeol JMS HX 110] was obtained by mass spectrometer. The 1D and 2D NMR spectrum was obtained on Bruker 500 and 600 MHz for 1H; 125 and 150 MHz for 13C [Bruker; AVANCE III; Karlsruhe; Germany] spectrophotometer with TMS as the internal standard. Chemical shift [δ] was shown in ppm while coupling constant [J] in Hz; deuterated solvents received from Sigma Aldrich was used for NMR analysis. Column chromatography was performed with silica gel (70 230 mesh ASTM, Scharlab S.L, Gato Perez Sentmenat–Spain) whereas TLC (Thin layer chromatography) was run on pre-coated aluminum [silica gel F254] sheets. The developed TLC was then visualized via ultra-violet lamp [254 & 356 nm] or Dragendorff’s reagent or by means of Iodine. Solvents obtained from commercial sources were used for extraction and isolation of pure compounds after proper distillation.
2.2 Plant material
Aerial parts of Delphinium uncinatum were collected at Altitude: 3934 feet, Latitude = 34. 8,778,807 and Longitude = 72.2600655; from Kabal valley district Swat, Khyber Pakhtunkhwa, Pakistan, in May 2014. Taxonomical and Botanical identification was made by Dr. Zahidullah Assistant Professor, Center for Plant Science and Biodiversity University of Swat and voucher specimen (no: H.UOM.BG-162) was deposited in the herbarium of University of Malakand for future reference.
2.3 Isolation
Reported procedure was followed for isolation and purification (Ahmad et al., 2017b). 4 kg shade dried, and powder materials of D. uncinatum were extracted with 80 % methanol four time at room temperature to get crude methanol extract solution. Solvent was evaporated from extracted solution at reduced pressure to obtain crude methanol extract (200 g). The concentrated methanol extract was suspended in water at pH 1–2 (0.5 N H2SO4) and was partitioned with chloroform to get acidic crude extract. Afterwards the acidic aqueous layer basified to pH 8–10 (10 % KOH) and was extracted with chloroform to get crude alkaloid fraction. The crude alkaloid fraction (18 g) loaded onto silica-gel column and was eluted by using n-hexane/ chloroform upto 100 % chloroform and the chloroform/methanol upto 20 % methanol to obtain ten sub-fractions (A-J). According to TLC analysis, sub-fractions (A-J) were condensed to four sub-fractions (N1-N4). Compound 1 (46 mg) and 2 (65 mg) were purified from the sub-fraction N3 by using n-hexane/ acetone/ di-ethyl amine (Et2NH) (85:15 + 3 mL Et2NH per 1 Liter) whereas compound 3 (30 mg) was acquired from sub-fraction N4 by using (80:20 + 3 mL Et2NH per 1 Liter) of n-hexane/ acetone/ Et2NH.
Uncinatine-B (1): White amorphous powder, IR; 3462 (OH); 3355 (NH); 1695 (C⚌O); 1620, 1595 (C⚌C) and 1080 cm−1 (simple ether bonds); 1H NMR (CDCl3, 600 MHz) Table 1; 13C NMR (CDCl3, 150 MHz): Table 2; HR-EIMS (m/z): 628.7580, C34H48N2O9 calcd. 628.7630).
Position
1
(600 MHz)
2
(500 MHz)
3
(500 MHz)
1
3.26 (m, 1H)
3.76 (br s, 1H)
3.98 (br s, 1H)
2
2.21, 2.33, (2H, m)
1.66, 1.91 (2H, m)
2.06 (2H, m)
3
1.84, (t, 2H, 2.3)
1.64, 2.21(2H, m)
1.32 (2H, m)
4
–
–
–
5
2.45, (br s, 1H)
1.88, (m, 1H)
2.44 (m, 1H)
6
3.34, (br s, 1H)
1.69, 1.87 (m, 2H)
1.62, (m, 2H)
7
2.13, dd, (4.7)
2.30, (d, 1H)
1.89, m
8
–
–
–
9
2.19, (br s, 1H)
2.09 (m, 1H)
2.36 (m, 1H)
10
1.71 (m, 1H)
1.94, (m, 1H)
2.48 (m, 1H)
11
–
–
–
12
2.06, 2.43 (br s, 2H)
1.73 (m, 2H)
1.35 (m, 2H)
13
2.66 (m, 1H)
1.91(m, 1H)
14
3.47 (d, 1H,4.3)
4.88 (t, 1H,4.8)
4.25 (d 1H, 5.3)
15
2.42 (d, 2H, 3.5)
2.34 (m, 2H)
2.31, t,2H, 3.3)
16
3.22 (t,1H, 6.9)
3.32, (m, 1H)
3.61 (br s,1H)
17
3.51 (br s,1H)
2.76 (br s, 1H)
5.28 (d, 1H, 4.5)
18
4.24 (m, 2H)
3.18,3.04 (dd,2H,8.8)
3.20,3.15 (d, 2H,8.8)
19
2.58, br s
2.11, 2.37, d(7 & 6.6)
2.26, br s
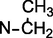 ,
,
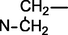
2.53, 2.60(m, 2H)
2.48 and 2.56(m, 2H)
4.17(m, 2H)
1.15(t, 3H, 7.1)
1.14(t, 3H, 7.1)
4.30 (m, 3H)
OCH3
3.43 (s, 3H)
3.29(s, 3H)
OCH3
3.34 (s, 3H)
3.34(s, 3H)
–
OCH3
3.32 (s, 3H)
–
3.33(s, 3H)
OCH3
3.03 (s, 3H)
–
3.39(s, 3H)
1‘
–
–
–
2‘
–
–
7.73 (d,1H, 7.6)
3‘
8.69 (d,1H, 7.9)
–
7.55 (t, 1H, 7.4)
4‘
7.55 (m, 1H)
–
7.15 (m, 1H)
5‘
7.06(m, 1H)
–
7.55, (t, 1H, 7.4)
6‘
7.95 (d, 1H, 6.3)
–
7.73 (d, 1H,7.6)
NH
11.08 (s 1H)
–
–
CH3
2.72 (s, 1H)
–
–
Position
1
(150 MHz)
2
(125 MHz)
3
(125 MHz)
1
84.2
72.0
73.2
2
26.8
26.6
27.8
3
29.6
29.7
31.9
4
31.8
36.5
29.2
5
48.5
41.3
34.0
6
82.9
25.0
24.8
7
49.8
44.8
44.7
8
75.6
74.7
72.4
9
47.6
45.5
45.9
10
38.7
43.3
38.7
11
51.0
48.9
46.3
12
44.8
29.1
29.7
13
78.5
37.2
42.2
14
90.1
76.7
75.2
15
36.3
42.6
34.2
16
84.6
82.0
80.7
17
61.5
63.6
68.9
18
68.1
78.9
78.6
19
56.1
56.0
55.2
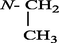

48.9
48.5
62.1
13.5
13.0
60.2
OCH3
59.5
59.4
–
OCH3
57.9
56.5
–
OCH3
56.5
–
59.5
OCH3
55.5
–
56.5
1′
115.8
–
130.0
2′
141.6
–
129.6
3′
120.2
–
128.8
4′
134.3
–
130.0
5′
122.3
–
128.8
6′
131.0
–
129.6
C⚌O
167.4
–
–
NH
–
–
–
C⚌O
169.0
170.4
173.2
CH3
25.5
–
–
Uncinatine-C (2): White amorphous solid. IR; 3492 (OH); 1083 (Ether bonds); 2900 cm-1for C—H stretching. 1H NMR (CDCl3, 500 MHz): Table 1; 13C NMR (CDCl3, 125 MHz) Table 2; HR-EIMS (m/z): 449.2777 observed, C26H43NO6, 449.2783 calculated.
Uncinatine-D (3): White amorphous powder. IR; 3466 (OH); 1722 (ester); 1695 (C⚌O); 1620, 1595 (C⚌C) and 1080 cm−1 (simple ether bonds). HR-EIMS (m/z): C30H41NO7 (m/z 527.6160, calcd. 527.6174. 1H NMR (CDCl3, 500 MHz): Table 1: 13C NMR (CDCl3, 125 MHz): Table 2.
2.4 Acetylcholinesterase inhibition assay
In vitro acetylcholinesterase (AChE) inhibition of compound 1–4 was determined by a modified method of Ellman as described elsewhere (Ellman et al., 1961; Elufioye et al., 2019). Acetylcholinesterase (Electric-eel EC 3.1.1.7), AChI, DTNB and galanthamine were obtained from Sigma Aldrich and used without any further processing. Stock solutions of the test samples (1–4) and the reference standard galanthamine was prepared. Solution was constituted by mixing Ellman reagent i.e DTNB (0.2 mM); sodium phosphate (62 mM) (pH 8.0, 880 μL), solution of the tested compound (40 μL) and AChE solution (40 μL). Reaction contents were incubated for 15 min at room temperature (25 °C). The hydrolysis of acetylcholine iodide (AChI) was marked spectrophotometrically at 412 nm. The experiment was conducted in triplicate by using spectrophotometer (BMS-USA). Compounds concentration that causes inhibition of ACh hydrolysis by 50 % was assessed by observing concentration (62.5–1000 μg/mL) effect of the tested compounds on inhibition values.
2.5 Molecular docking study
The docking simulation of the AChE enzymes and isolated natural products were accomplished though MOE (molecular operating Environment) software, to investigate the binding interaction of compound 1–4 (Bashir et al., 2021). For docking simulation, three-dimensional geometry of 1–4 was launched by means of MOE software tool. Through MOE default parameters (Force Field: MMFF94X, gradient: 0.05), the 3D protonation and energy minimization of 1–4 was conducted and was further saved as mdb file for later evaluation in docking analysis. 3D geometry of target enzyme (PDB ID: 1ACJ) was acquired from Protein Databank (PDB) and was opened, emitting water molecules from each protein. For the stability of protein, 3D protonation and energy minimization of enzyme carried by means of the default MOE parameters (Alam et al., 2016).
3 Results and discussion
3.1 Structure elucidation
Three unreported norditerpenoid alkaloids 1–3 (Fig. 1) together with known compound virescenine (4) were isolated from the chloroform fraction of D. uncinatum (aerial part). Structures of undescribed norditerpenoids 1–3 were established on the basis of spectral data interpretation, whereas structure for the known compound was confirmed by comparing their spectral data available in literature (Chen et al., 1999; Wada et al., 2016; Wangchuk et al., 2007) (Table 1).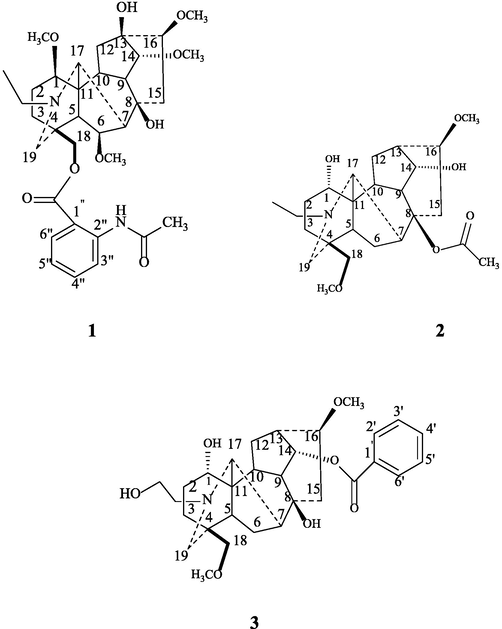
Structures of compounds 1–3.
Uncinatine-B (1) was isolated from basic (pH range = 8–10) chloroform fraction of D. uncinatum as white amorphous powder. Molecular formula C34H48N2O9 (m/z 628.7580, calcd. 628.7630) was established based on HR-EIMS. The IR spectrum of 1 showed bands at 3462 (OH), 3355 (NH), 1695 (C⚌O), 1620, 1595 (C⚌C) and 1080 cm−1 (simple ether bonds). A signal in the downfield region (δH 11.08, s, 1H) of the 1H NMR spectrum (Table 1) was assigned to amide proton of 1. Four other signals (δH = 3.03, 3.32, 3.34, 3.43 3H each, s) were assigned to the –OCH3 at C-1, C-6, C-14, and C-16. The signal at δH = 3.47 (d, J = 4.3 Hz, 1H) was observed for β-oriented H-14 proton. Signal appeared upfield at δH = 1.15 (t, J = 7.1 Hz, 3H) was attributed to methyl of N-CH2CH3, a distinctive group of all norditerpenoids. The 13C NMR spectrum (broad-band decoupled and DEPT]) (Table 2) of compound 1 displayed thirty-four signals for two methyl, four methoxy, seven methylene, thirteen methines and eight quaternary carbons atoms. HMQC spectrum was used to determine 1H–13C correlations whereas the long-range 1H–13C connectivity was confirmed by means of HMBC spectrum. In the 1H–1H COSY 45° spectrum (Fig. 2), H-16 (δ 3.22, m, 1H) displayed correlation with the C-15 methylene protons at [δH 2.42, m] whereas H-14 (δH δ 3.47, d, J = 4.3 Hz) exhibited COSY correlation with H-9 (δH 2.19, br s). The relative positions of the substituents in the basic skeleton of 1 were confirmed by means of HMBC spectrum. In the HMBC spectrum (Fig. 2), H-5 (δC 2.45) showed 2J correlation with C-6 (δC 82.9), C-4 (δC 31.8), C-11 (δC 51.0), as well as 3J correlation with C-7 (δC 49.8) and C-10 (δC 38.7). Similarly, H-6 (δH 3.32) showed interactions with C-7 (δC 49.8), C-5 (δC 48.5) and C-8 (δC 75.6). The structure of compound 1 was established from 1H–1H COSY, 1H–13C, HMBC, and HMQC data as 1β, 6β, 14α, 16β-tetramethoxy-8β, 13β-dihydroxy, 18-acetamidobenzoate-N-ethyl aconitine (uncinatine-B) 1.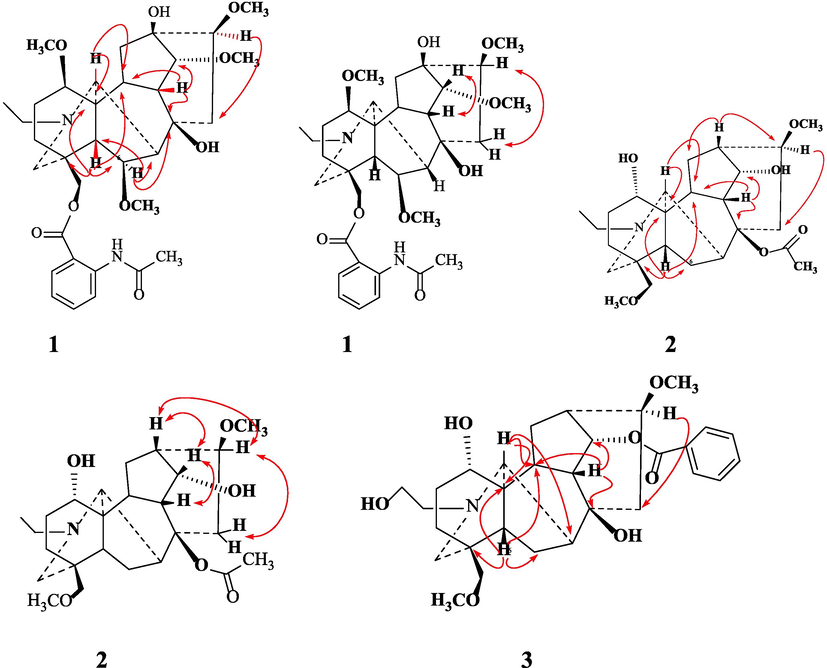
HMBC and COSY correlations of compound 1–3.
Uncinatine-C (2) was isolated as white amorphous powder from basic (pH 8–10.) chloroform fraction of crude extract. The HR-EIMS of 2 showed molecular ion [M]+ at m/z 449.2777 that corresponded to molecular formula C26H43NO6 (calcd. 449.2783), marking the presence of seven degrees of unsaturation. The IR spectral data of 2 showed absorption signals at 3492 (OH groups), 1083 (ether bonds), 2900 cm−1 due to the C—H stretching. The 1H NMR spectrum of 2 displayed signals for N-ethyl, a characteristic group of all norditerpenoid alkaloids, two methoxy groups, eight methylene and several methines protons geminal to oxygen substituted groups. The spectrum exhibited signal at δH = 4.88 (t, J = 4.8, 1H) due to resonance of H-14 methine proton. A broad single at δH 3.76 was assigned to H-1β methine proton and a multiplet at δH 3.32 was due the H-16α methine proton geminal to methoxy substituent. The 13C NMR spectrum of 2 showed twenty-five resonating signals, comprising two –CH3, two –OCH3, eight –CH2-, nine methines and four quaternary carbons. 1H–13C correlations was established by applying HMQC and HMBC (long range) technique. In the 1H–1H COSY 45° spectrum (Fig. 2), H-16 (δH 3.32) showed correlations with the C-15 methylene protons at [δH 2.34 m], as well as H-13 (δH 2.66). The H-9 (δH 2.09, m) proton showed coupling with H-14 (δH 4.88, t, J = 4.8 Hz), and with H-13 (δH 2.66, m). In the HMBC spectrum (Fig. 2), H-5 (δH 1.88, m) showed 2J correlation with C-6 (δC 25.0), C-4 (δC 36.5), C-11 (δC 48.9), as well as 3J correlations with C-7 (δC 44.8) and C-10 (δC 43.3). Similarly, H-14 (δH 4.88) showed interactions with C-16 (δC 82.0), C-14 (δC 76.7) and C-9 (δC 45.5). The structure of 2 was established from 1H–1H COSY, 1H–13C, HMBC and HMQC was deduced as 16β, 18β- dimethoxy-1α, 14α-dihydroxy-8β-acetyl-N-ethyl aconitin (uncinatine-C) (2).
Uncinatine-D (3) was purified as white amorphous powder. The molecular formula C30H41NO7 (m/z 527.6160, calcd. 527.6174) was deduced by means of HR-EIMS. IR spectrum of 3 exhibited absorption bands at 3466 (OH), 1722 (ester), 1695 (C⚌O), 1620, 1595 (C⚌C) and 1080 cm−1 (ether bonds). The 1H NMR spectrum of 3 exhibited signal as triplet at δH 7.55 (J = 7.4 Hz, 1H), 7.15 (1H, t, J = 7.5 Hz) and a doublet at δH 7.73 (1H, d, J = 7.6 Hz) were assigned to aromatic protons. The methoxy protons present at C-18 and C-16 resonated as singlets at δH 3.33 and 3.39. The C-14 methine protons with β-orientation showed a triplet at δH 4.25 (J = 5.3 Hz). In the same spectrum a multiplet signal at δH 3.98 was due to the H-1 methine proton. The 13C NMR spectrum of 3 displayed thirty signals for two –OCH3, nine –CH2-, fourteen methines and five quaternary carbons atoms. In the HMBC spectrum (Fig. 2), H-5 (δH 2.44, m) showed 2J correlation with C-6 (δ 24.8), C-4 (δ 29.2), C-11 (δ 46.3), as well as 3J correlations with C-7 (δ 44.7) and C-10 (δ 38.7). Similarly, H-14 (δH 4.25, t) showed interactions with C-9 (δ 45.9), C-13 (δ 42.2) and C-16 (δ 80.7). Furthermore, H-9 (δH 2.36, m) showed correlation with C-8 (δ 72.4), C-10 (δ 38.7), and C-14 (δ 75.2). While the H-17 (δH 5.28, d) showed interaction with C-10 (δ 38.7), C-7 ((δ 44.7) and C-11 (δ 46.3). The structure of 3 was established from 1H–13C NMR, HMBC and HMQC experiment. Thus, the structure of compound 3 was deduced as 16β, 18β- dimethoxy-1α, 8β-dihydroxy, 14-benzoyl-N-ethanol aconitine (uncinatine-D) (3).
3.2 In vitro acetylcholinesterase inhibitory potential
AD is a neurodegenerative disease which has not been able to be diagnosed and treated so far. AChE inhibitor helps a lot in its treatment and is regarded to be more contributive than BChE but it has been reported that both enzymes boost each other in one or the other (Ahmad et al., 2022; Islam et al., 2022; Luo et al., 2019). The synthetic drugs used for this disease have more negative effects than natural products that are regarded to be safe and low-cost remedy for AD (Mukherjee et al., 2007b; Oh et al., 2004).
To evaluate the potential role of target compounds (1–4) for the treatment of AD, natural products (1–4) were screened for in vitro acetylcholinesterase inhibitory activity, at different concentrations (62.5–1000 μg/mL) using modified spectrophotometric method reported by Ellman et al (Ellman et al., 1961). All the tested natural products were observed to be concentration dependent and specific inhibitor of AChE. The investigated IC50 value of 1, 2, 3, and 4 against AChE was 188.10 ± 1.1, 94.33 ± 1.5, 367 ± 1.8 and 147.63 ± 1.0 respectively (Table 3). It is crystal clear from the experimental results that the isolated compounds (1–4) show promising inhibition against AChE. These inhibitory potential of the isolated natural products are attributed to the smaller active site of AChE enzyme that can fit in smaller group. As all the structural features were found to be actively involved in the inhibitory activity but the variation of various groups on the basic skeleton was really accountable for the change in inhibitory potential. Thus on the basis of aforesaid biological data, the structure–activity relationships (SARs) for the isolated natural compounds (1–4) can be briefly summarized. Among the isolated compounds, uncinatine-C (IC50 = 94.33 ± 1.5), was found to be properly accommodated in the active site of AChE as compared to other compounds. This high potency of compound 2 is attributed to the polar interaction of hydroxyl groups which makes the penetration process facile inside the pocket of AChE. The size and shape uncinatine-C also account for its strong hydrophilic interaction with receptor due to hydrogen bonding whereas other compounds demonstrate least inhibitory activity due to its hydrophobic nature and are unable to established strong hydrogen bonding with the target protein. These considerable results will obviously increase the importance of diterpenoids present in Delphinium species and hence encourage chemists for synthesis of such compounds.
S.No
Compounds
AChE
IC50 [µM] ± SEMa
1
Uncinatine B (1)
188.10 ± 1.1
2
Uncinatine C (2)
94.33 ± 1.5
3
Uncinatine D (3)
367 ± 1.8
4
virescenine (4)
147.63 ± 1.0
5
Galanthamineb
0.50 ± 1.5
3.3 Docking analysis of AChE
The isolated compounds (1–4) were docked into the binding pocket of AChE enzymes to find the interaction of these compounds with protein. On the basis of docking score, the top ranked conformation was chosen for analysis. Among different docking score, the lower scores represent suitable poses. All scoring functions were expressed in the unit of kcal/mol (Alam et al. 2016). All compounds were identified to fit firmly into the binding pocket of the AChE with the docking score − 13.5322, −11.8173, −12.4240 and − 8.9352 respectively. Efficient interactions were observed for all the tested compounds with active site residues of the receptor protein Tyr 341, Glu 292, Leu 76, Trp 286, Ser and Phe 338.
In the skeleton of AChE, aromatic gorge with four loci has an important role in recognition of ligand and catalysis. Ligand interaction in the acyl binding locus is related to the acetyl specificity of AChE (Khalid et al., 2004). Intermolecular interactions like hydrogen bonding and hydrophobic interactions are recognized as basic and key players in the stabilization of energetically-favored ligands, that are usually observed in the open conformational environment of protein structures, but still it is poorly cleared that how binding parameters, relevant to these interactions can facilitate a drug to identify a specific target and enhance drug efficiency(Varma et al., 2010). By this docking study, we inspected the interactions between compounds uncinatine B-D (1–3) and virescenine (4) with the AChE. The interaction type, residues involved and ligand interacting group between enzymes and tested compound 1–4 tabulated in Table 4. In our sequential experiments, 3D docking simulations of the active compound 1–4 were anticipated. Docking scores of AChE-compounds complexes revealed that all the compounds were perfectly adhered in several pocket domains of AChE residues. Docking conformation of uncinatine-B (1) (−13.5322) showed that this compound formed hydrogen bond with Glu 292 and arene-arene or π-π interactions with Tyr 341. Furthermore, uncinatine-B (1) formed hydrophobic interactions with residues Leu 76, Trp 286 and Phe 338 (Fig. 3).
Entry
ligand
enzyme
Binding energy (kcal)
Residue
Type of interaction
Ligand interacting moiety
1
1
TcAChE
−13.5322
Glu 292
Hydrogen
–OH
Tyr 341
π-π interactions
Aromatic moiety
Leu 76,
Hydrophobic
-NHCOR
Trp 286
Hydrophobic
-NHCOR
Phe 338
Hydrophobic
-NHCOR
2
2
TcAChE
−11.8173
Tyr 72
Hydrogen
-O-
Tyr 341
Hydrogen
–OH
3
3
TcAChE
−12.4240
Tyr 341
Hydrogen
–OH
Ser 293
Hydrogen
–OH
Ser 293
Hydrogen
-O--H
Trp 286
Arene-cation
–NHCH2
4
4
TcAChE
−8.9352
His 287
Hydrogen
–OH
Trp 286
Hydrophobic
-OR
Leu 76
Hydrophobic
-OR
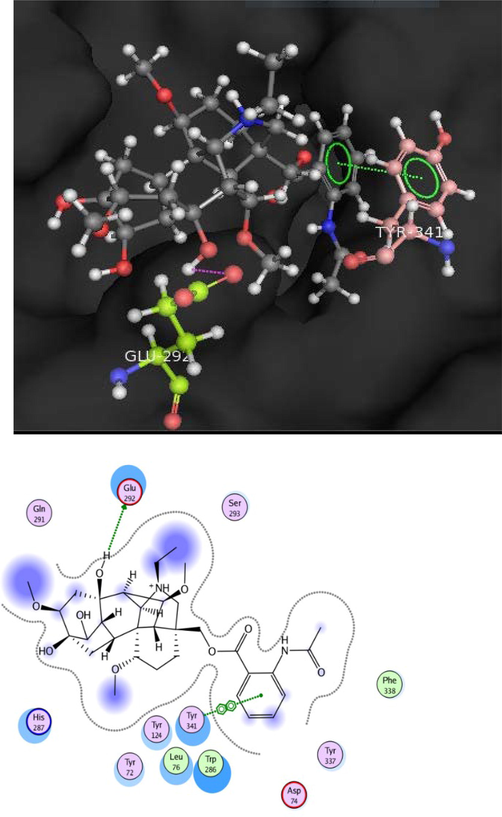
Docking pose of compound 1 within cholinesterase target.
Docking conformation of uncinatine-C (2) (−11.8173), demonstrate that compound 2 exhibited three prominent polar interactions with the Tyr 72 and Tyr 341 residues (Fig. 4). Oxygen of the ether moiety of uncinatine-C (2) established hydrogen bonding with Tyr 72 whereas Tyr 341 also interact through hydrogen bonding with the hydrogen atom of the single bond OH moiety. All the binding interaction of the 2 was detected in the PAS of the AChE. The uncinatine-D (3) (docking score of − 12.4240) have two hydrogen bonds and one arene-cation interaction with the active side residues (Fig. 5). Tyr 341 formed hydrogen bond with OH moiety of this compound. Ser 293 established first hydrogen bond with OH moiety of uncinatine-D (3) and second hydrogen bond involved oxygen of Ser 293 and hydrogen of uncinatine-D (3). Trp 286 involved in arene-cation interaction with this compound. The virescenine (4) (docking score of − 8.9352) showed only two hydrogen bonds with active site residue His 287 and forms hydrophobic interactions with residues Trp 286 and Leu 76 (Fig. 6).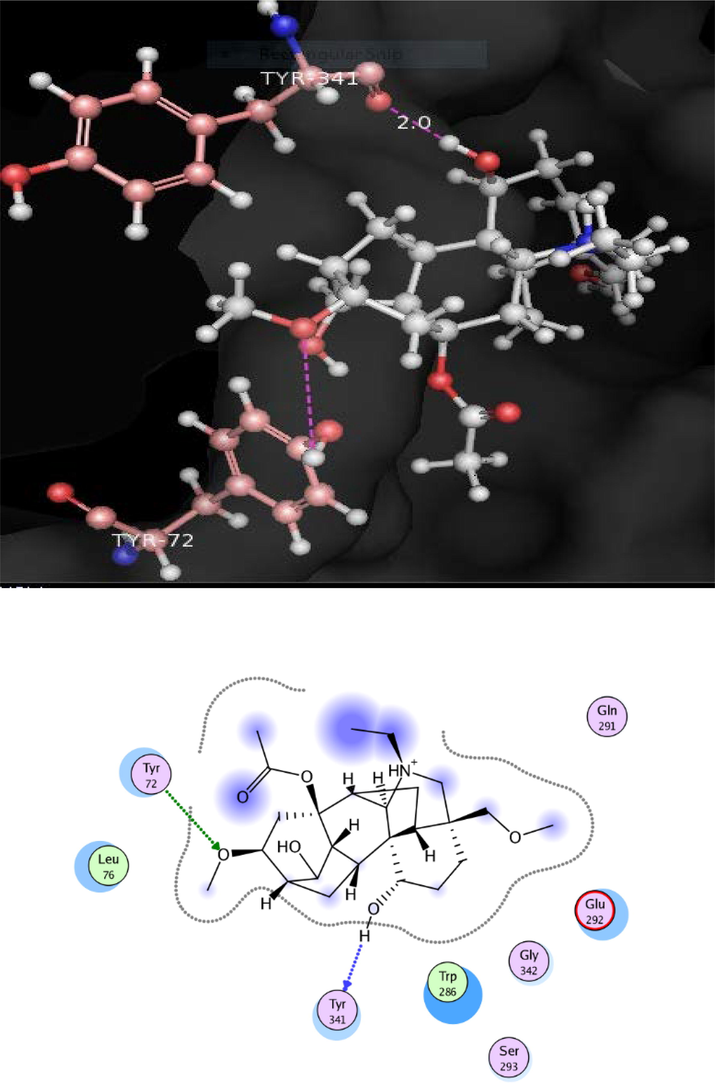
Docking pose of compound 2 within cholinesterase target.
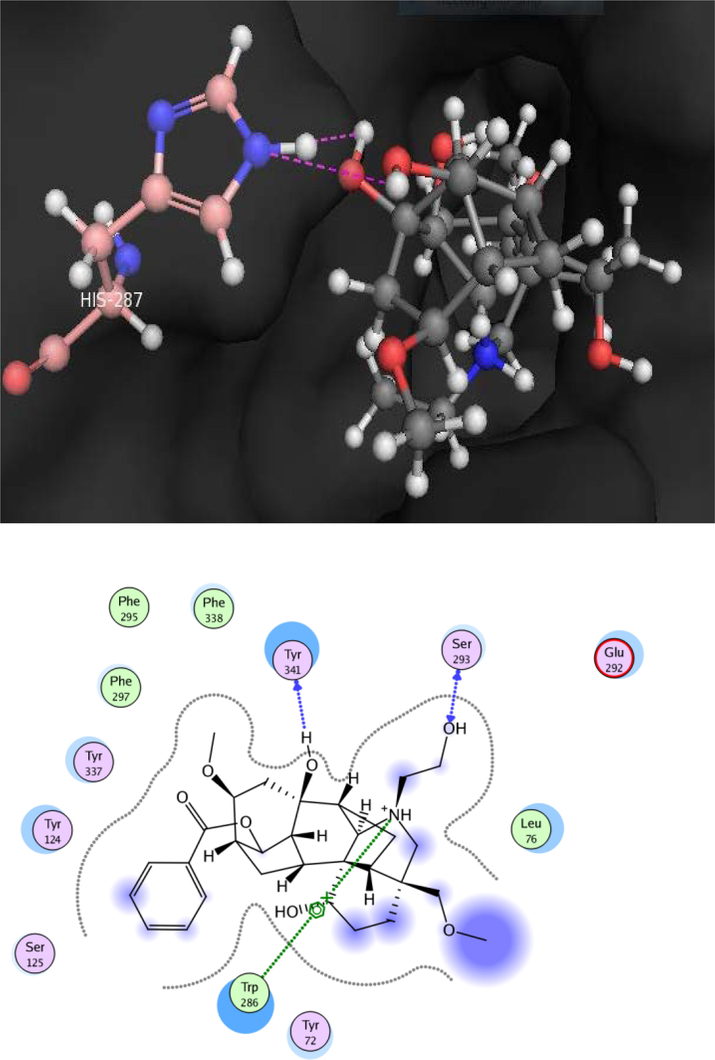
Docking pose of compound 3 within cholinesterase target.
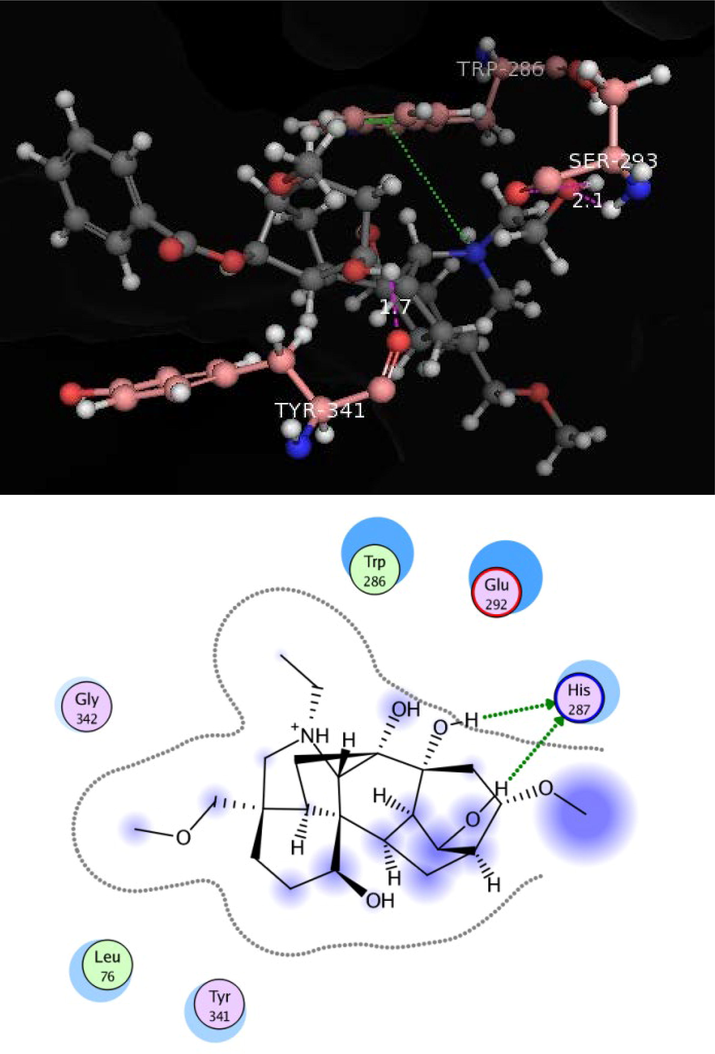
Docking pose of compound 4 within cholinesterase target.
4 Conclusion
The present research work, led to the identification of four natural alkaloids from D. uncinatum. The isolated compounds 1–4 were tested for AChE inhibitory activity. All the isolated natural products were found to possess promising acetylcholinesterase inhibitory potential. In silico assessment of 1–4 revealed binding modes and verified the experimental results. The negative binding energies of natural products 1–4 displayed suitable relationship to AChE enzyme. Therefore, the results obtained in our present study demonstrate the potential role of diterpenoids as possible alternative in drugs formulation. It may be concluded from our current investigation that diterpenoids present in D. uncinatum will be leading drugs for the treatment of AD or dementia-related neurovascular issues.
Acknowledgements
The authors would like to give thanks to Taif University, Taif, Saudi Arabia, for their support (Taif University Researchers Support Project number: TURSP-2020/80).
References
- Crystal structure, phytochemical study and enzyme inhibition activity of Ajaconine and Delectinine. JMoSt. 2016;1123:441-448.
- [CrossRef] [Google Scholar]
- Isolation, crystal structure determination and cholinesterase inhibitory potential of isotalatizidine hydrate from Delphinium denudatum. Pharm. Biol.. 2017;55:680-686.
- [CrossRef] [Google Scholar]
- Ahmad, H., Ahmad, S., Shah, S.A.A., Khan, H.U., Khan, F.A., Ali, M., Latif, A., Shaheen, F., Ahmad, M., 2017b. Selective dual cholinesterase inhibitors from Aconitum laeve. 172–181. Doi: 10.1080/10286020.2017.1319820
- Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum. Bioorg. Med. Chem.. 2017;25:3368-3376.
- [CrossRef] [Google Scholar]
- In vitro and in silico investigation of diterpenoid alkaloids isolated from Delphinium chitralense. Molecules. 2022;27:4348.
- [CrossRef] [Google Scholar]
- Bioassay-guided isolation of sesquiterpene coumarins from Ferula narthex bioss: a new anticancer agent. Front. Pharmacol.. 2016;7:26.
- [CrossRef] [Google Scholar]
- Bioactive natural products as a potential source of new pharmacophores. A theory of memory. Pure Appl. Chem.. 2001;73:555-560.
- [CrossRef] [Google Scholar]
- Assessing the ethnobotanical potential of Carissa opaca berries by merging outcomes from metabolomics profiling, enzyme assays, and in silico docking studies. Food Chem.. 2021;363
- [CrossRef] [Google Scholar]
- Floristic composition of Machiara National Park. District Int. J. Biosci. (IJB). 2012;2:28-45.
- [Google Scholar]
- Isolation of Diterpenoid Alkaloids from Herb and Flowers of Aconitum napellus ssp. vulgare and Electrospray Ion Trap Multiple MS Study of These Alkaloids. J. Nat. Prod.. 1999;62:701-704.
- [CrossRef] [Google Scholar]
- New cholinesterase-inhibiting triterpenoid alkaloids from Buxus hyrcana. Chem. Biodivers.. 2006;3:1039-1052.
- [CrossRef] [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7
- [CrossRef] [Google Scholar]
- El-Metwally, A., Toivola, P., Al-Rashidi, M., Nooruddin, S., Jawed, M., Alkanhal, R., Razzak, H.A., Albawardi, N., 2019. Epidemiology of Alzheimer’s Disease and dementia in Arab countries: a systematic review. Doi: 10.1155/2019/3935943
- Antioxidant and anticholinesterase activities of Macrosphyra Longistyla (DC) hiern relevant in the management of Alzheimer’s disease. Antioxidants. 2019;8
- [CrossRef] [Google Scholar]
- Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112-2117.
- [CrossRef] [Google Scholar]
- Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol. Res.. 2004;50:433-440.
- [CrossRef] [Google Scholar]
- Natural Product Inhibitors of Acetylcholinesterase. Curr. Org. Chem.. 2006;10:825-847.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep.. 2006;23:181-199.
- [CrossRef] [Google Scholar]
- Bioactive compounds and their derivatives: an insight into prospective phytotherapeutic approach against Alzheimer’s Disease. Oxid. Med. Cell. Longev.. 2022;2022
- [CrossRef] [Google Scholar]
- Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzyme Inhib. Med. Chem.. 2015;30:133-139.
- [CrossRef] [Google Scholar]
- Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol.. 2008;7:812-826.
- [CrossRef] [Google Scholar]
- Cholinesterase inhibitory and spasmolytic potential of steroidal alkaloids. J. Steroid Biochem. Mol. Biol.. 2004;92:477-484.
- [CrossRef] [Google Scholar]
- Phytosociology, structure and physiochemical analysis of soil in quercus baloot griff, forest district chitral, pakistan. Pak. J. Bot. 2010;42:2429-2441.
- [Google Scholar]
- Ethnobotanical study of some medicinal plants of Tehsil Kabal. KP, Pakistan. Med Aromat Plants. 2015;4:189.
- [CrossRef] [Google Scholar]
- Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol.. 2013;65:1701-1725.
- [CrossRef] [Google Scholar]
- Alzheimer disease: Alzheimer disease neuropathology in the oldest old. Nat. Rev. Neurol.. 2009;5:411-412.
- [CrossRef] [Google Scholar]
- Anti-inflammation C19-diterpenoid alkaloids from Delphinium giraldii diels. Rec. Nat. Prod.. 2019;13:379-384.
- [CrossRef] [Google Scholar]
- A new diterpenoid alkaloid from Aconitum hemsleyanum. Nat. Prod. Res. 2019:1-6.
- [CrossRef] [Google Scholar]
- Synthesis, structure–activity relationship and molecular docking of 3-oxoaurones and 3-thioaurones as acetylcholinesterase and butyrylcholinesterase inhibitors. Bioorg. Med. Chem.. 2017;25:100-106.
- [CrossRef] [Google Scholar]
- Synthesis, structure-activity relationship and molecular docking studies of 3-O-flavonol glycosides as cholinesterase inhibitors. Bioorganic Med. Chem.. 2018;26:3696-3706.
- [CrossRef] [Google Scholar]
- Flavonols and 4-thioflavonols as potential acetylcholinesterase and butyrylcholinesterase inhibitors: synthesis, structure-activity relationship and molecular docking studies. Bioorg. Chem.. 2019;91:103124
- [CrossRef] [Google Scholar]
- Exploring 3-Benzyloxyflavones as new lead cholinesterase inhibitors: synthesis, structure–activity relationship and molecular modelling simulations. J. Biomol. Struct. Dyn.. 2021;39:6154-6167.
- [CrossRef] [Google Scholar]
- Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phyther. Res.. 2007;21:1142-1145.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289-300.
- [CrossRef] [Google Scholar]
- Inhibitory potential of nitrogen, oxygen and sulfur containing heterocyclic scaffolds against acetylcholinesterase and butyrylcholinesterase. RSC Adv.. 2022;12:19764-19855.
- [CrossRef] [Google Scholar]
- Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine. 2004;11:544-548.
- [CrossRef] [Google Scholar]
- In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. reports. 2015;2:850-857.
- [CrossRef] [Google Scholar]
- An Alzheimer’s disease-specific beta-amyloid fragment signature in cerebrospinal fluid. Neurosci. Lett.. 2006;409:215-219.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibition by medicinal plants: a review. Ann. Plant Sci.. 2017;6:1640-1644.
- [CrossRef] [Google Scholar]
- Reflexa, R., Molecular, T., Study, D., 2013. Cholinesterase enzymes inhibitors from the leaves of Rauvolfia Reflexa and their molecular docking study, 3779–3788. Doi: 10.3390/molecules18043779.
- The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm.. 2006;113:1625-1644.
- [CrossRef] [Google Scholar]
- Ginkgo extract or cholinesterase inhibitors in patients with dementia: what clinical trials and guidelines fail to consider. Phytomedicine. 2003;10(Suppl 4):74-79.
- [CrossRef] [Google Scholar]
- Norditerpenoid alkaloids from Delphinium kohatense Munz. Rec. Nat. Prod.. 2015;9:76-80.
- [Google Scholar]
- Sultzer, D.L., Lim, A.C., Gordon, H.L., Yarns, B.C., Melrose, R.J., 2022. Cholinergic receptor binding in unimpaired older adults, mild cognitive impairment, and Alzheimer’s disease dementia. Alzheimer’s Res. Ther. 2022 141 14, 1–15. Doi: 10.1186/S13195-021-00954-W.
- Diterpenoid alkaloids from Delphinium uncinatum. Phytochemistry. 1998;47:1141-1144.
- [CrossRef] [Google Scholar]
- Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS One. 2010;5
- [CrossRef] [Google Scholar]
- Four new diterpenoid alkaloids from Aconitum japonicum. Planta Med.. 2016;82:P708.
- [CrossRef] [Google Scholar]
- Hetisine-type diterpenoid alkaloids from the Bhutanese medicinal plant Aconitum orochryseum. J. Nat. Prod.. 2007;70:1808-1811.
- [CrossRef] [Google Scholar]







