Translate this page into:
Biodiversity and application prospects of fungal endophytes in the agarwood-producing genera, Aquilaria and Gyrinops (Thymelaeaceae): A review
⁎Corresponding authors at: No. 16, Dongzhimen Southern Street, Beijing 100700, China. huangluqi01@126.com (Luqi Huang), juanliu126@126.com (Juan Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Agarwood is originated from the resinous part of Aquilaria and Gyrinops plants and has been a precious biomaterial for applications in traditional medicine, perfumery, cosmetics, and religious purposes all over the world. In the wild, the formation of agarwood is related to the defense mechanism of the tree in response to physical damage that allows further microbial infestation into its wood, while having the whole tree covered with agarwood would take up a long time, and it rarely happens. For Aquilaria and Gyrinops, the presence of endophytes is mainly found derived from the tree. The isolated endophytes could be important sources of natural products, while some could contribute to the formation of agarwood in the tree, which is safe for the environment and human health. This review summarized the biodiversity of fungal endophytes recorded in Aquilaria and Gyrinops and their potential effects on host trees. Till now, 67 endophytic genera have been isolated from Aquilaria and Gyrinops, and 18 ones were found responsible for the promotion of agarwood formation. Additionally, 92 compounds have been reported to be produced by the agarwood endophytes, and 52 ones displayed biological activities, most of which have anti-inflammatory, anti-bacterial, and anti-cancer activities. Nevertheless, fungal endophytes are promising agents that deserved to be further studied and scaled up to a commercial level for the production of agarwood oil, but the role of endophytes in the agarwood host trees needs to be furtherly investigated in future studies.
Keywords
Agarwood
Fungal endophytes
Aquilaria
Gyrinops
1 Introduction
Members of the agarwood-producing genera, Aquilaria and Gyrinops, are tropical evergreen trees commonly grown in the lowland forest regions, which are mainly distributed in southeast Asia. Agarwood is a highly valuable fragrance derived from the resinous wood of Aquilaria and Gyrinops trees. The intriguing aroma of agarwood makes it a valuable ingredient that has a long history record in the production of traditional medicines as well as used in religious activities, while at present, it is regarded as a luxury biomaterial in perfumery and cosmeceutical industries (Huang et al., 2013; Sun et al., 2020; Xie et al., 2020). Although all 30 species (21 Aquilaria species and 9 Gyrinops species) are believed to be able to produce agarwood, to date, evidence of agarwood formation was only reported for 14 species of Aquilaria and eight species of Gyrinops (Auri et al., 2021; Compton and Zich, 2002; Hou 1964; Kiet et al., 2005; Lee and Mohamed, 2016; Ng et al., 1997; Subasinghe et al., 2012; Turjaman et al., 2016) (Table 1). The high demand but rare occurrence of agarwood in the wild has led to the over-exploitation of these valuable species in the past, causing the decline in the population size of these agarwood-producing species in the wild. As a consequence, some of these species are classified as “Vulnerable”, “Endangered”, and “Critically Endangered” (IUCN 2022), as well as to aid in conserving the resources, all members of the agarwood-producing genera, Aquilaria and Gyrinops, are currently placed under strict monitoring by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in Appendix II (UNEP-WCMC, 2022).
Species Name
Distribution*
Basionyms and synonyms
Agarwood production report
Aquilaria apiculata Merr.
Philippines
–
Aquilaria baillonii Pierre ex Lecomte
Cambodia; Viet Nam; Lao People's Democratic Republic
–
Ng et al., 1997
Aquilaria banaensis P.H. Hô
Viet Nam
Aquilaria banaensae
Aquilaria beccariana Tiegh.
Brunei Darussalam; Indonesia; Malaysia
Aquilaria cumingiana var parvifolia; Aquilaria grandifolia; Gyrinops brachyantha; Gyrinopsis grandifolia
Faridah et al., 2009Compton and Zich, 2002Turjaman et al., 2016
Aquilaria brachyantha (Merr.) Hallier f.
Phillipines
Gyrinopsis brachyantha
Aquilaria citrinicarpa (Elmer) Hallier f.
Phillipines
Gyrinopsis citrinicarpa
Aquilaria crassna Pierre ex Lecomte
Cambodia; Viet Nam; Thailand; China; Malaysia
–
Yoswathana, 2013Ng et al., 1997
Aquilaria cumingiana (Decne.) Ridl.
Phillipines; Indonesia; United State; Malaysia
Aquilaria pubescens; Decaisnella cumingiana; Gyrinopsis cumingiana; Gyrinopsis cumingiana var. pubescens; Gyrinopsis decemcostata; Gyrinopsis pubifolia
Turjaman et al., 2016
Aquilaria decemcostata Hallier f.
Phillippines
–
Aquilaria filaria (Oken) Merr.
Indonesia; Phillipines; Papua New Guinea
Aquilaria cuminate; Aquilaria tomentosa; Gyrinopsis acuminate; Pittosporum filarium
Compton and Zich, 2002Turjaman et al., 2016
Aquilaria hirta Ridl.
Indonesia; Malaysia; Singapore; Thailand
Aquilaria moszkowski
Faridah et al., 2009Compton and Zich, 2002Turjaman et al., 2016
Aquilaria khasiana Hallier f.
India
–
Hallier, 1992
Aquilaria malaccensis Lam.
Bangladesh; Bhutan; China; France; India; Indonesia; Lao People's Democratic Republic; Mauritius; Malaysia; Philippines; Viet Nam; Thailand; Sri Lanka
Agallochum malaccense; Aloexylum agallochum; Aquilaria agallocha; Aquilaria ovate; Aquilaria moluccensis; Aquilaria secundaria; Aquilariella malaccense; Aquilariella malaccensis
Chowdhury et al., 2003Broad, 1995Rahayu and Putridan Juliarni, 2007Turjaman et al. 2016
Aquilaria microcarpa Baill.
Brunei Darussalam; Indonesia; Malaysia; Italy
Aquilaria borneensis; Aquilariella borneensis; Aquilariella microcarpa
Santoso et al., 2011Faridah et al., 2009Compton and Zich, 2002Turjaman et al. 2016
Aquilaria parvifolia (Quisumb.) Ding Hou
Philippines
Gyrinopsis parvifolia
Aquilaria rostrata Ridl.
Malaysia; Thailand
–
Faridah et al., 2009
Aquilaria rugosa K.Le-Cong & Kessler
Thailand; Viet Nam
–
Kiet et al., 2005
Aquilaria sinensis (Lour.) Spreng.
China; Thailand; Malaysia; Viet Nam
Agallochum grandiflorum; Agallochum sinense; Aquilaria chinensis; Aquilaria grandiflora; Aquilaria ophispermum; Ophispermum sinense
Liu et al., 2013Liu et al., 2022Ng et al., 1997Zhang et al., 2014
Aquilaria subintegra Ding Hou
Thailand; Malaysia
–
Hou, 1964
Aquilaria urdanetensis (Elmer) Hallier f.
Philippines
Gyrinopsis urdanetense; Gyrinopsis urdanetensis
Aquilaria yunnanensis S.C.Huang
China
–
Sun et al., 2019Zhang et al. 2019
Gyrinops caudata (Gilg) Domke
Indonesia; Papua New Guinea
Aquilaria caudata; Brachythalamus caudatus; Gyrinops audate
Auri et al., 2021
Gyrinops decipiens Ding Hou
Indonesia
–
Turjaman et al. 2016
Gyrinops ledermannii Domke
Indonesia; Papua New Guinea
–
Compton and Zich 2002Turjaman et al. 2016
Gyrinops moluccana (Miq.) Baill.
Indonesia
Aquilaria moluccana; Lachnolepsis moluccana
Turjaman et al. 2016
Gyrinops podocarpa (Gilg) Domke
Indonesia; Papua New Guinea
Aquilaria podocarpus; Brachythalamus podocarpus; Gyrinops ledermannii; Gyrinops podocarpus
Turjaman et al. 2016
Gyrinops salicifolia Ridl.
Indonesia; Papua New Guinea
Gyrinopsis salicifolia
Shao et al., 2016Dong et al., 2019Turjaman et al., 2016
Gyrinops versteegii (Gilg) Domke
Indonesia; Papua New Guinea
Aquilaria versteegii; Brachythalamus versteegii
Faizal et al., 2020Faizal et al., 2022Turjaman et al., 2016Nasution et al., 2020
Gyrinops vidalii P.H.Hô
Thailand; Lao People's Democratic Republic
–
Gyrinops walla Gaertn.
Indonesia; Papua New Guinea; India; Sri Lanka
Aquilaria walla
Subasinghe et al., 2012
Due to the conservation status and low yield of agarwood production in the wild, relying on the natural population as a source of agarwood to meet the increasing market demand is rather unviable (Deng et al., 2020; Wang et al., 2018). Therefore, the domestication of agarwood-producing trees was introduced and mass cultivation coupled with good agriculture practices was promoted (Liu et al., 2013; Persoon and Beek, 2008). In general, agarwood is naturally formed in Aquilaria and Gyrinops trees as a result of self-defense against physical damage or microbial infection on the trees (Soehartono and Newton, 2000; Xu et al., 2013). While physical damage to the tree is to introduce an opening; fungal infection has always been considered to be a key factor in agarwood formation (Rasool and Mohamed, 2016).
Endophytic fungi can live inside plant tissues without any disease symptoms. The evidence of the use of endophytic fungi to induce agarwood formation has been present for a long time, i.e. 1934 (Turjaman et al., 2016; Yoswathana, 2013), while the fungi that show promising results in the production of agarwood are developed into fungal inoculum. To mimic the mechanism of agarwood formation via microbial infestation, endophytic fungi are introduced into the cultivated stands via inoculation technique in hope that the time to form agarwood will be reduced and the yield could be increased at the same time. It is believed that the quality of the agarwood formed relies heavily on the species or strains selected among the different endophytic fungi; thus, research and exploration to discover additional useful endophytic fungi that could warrant better yield and quality of agarwood are still ongoing (Liu et al., 2022). Additionally, the secondary metabolites from endophytic fungi of agarwood lead the way as sources for various pharmacological properties.
In order to provide a comprehensive review of the information on endophytic fungi involved in the formation of agarwood, we mined various published scientific reports and summarized the distribution and biodiversity of fungal endophytes present in Aquilaria and Gyrinops. In addition, the secondary metabolites produced as well as the pharmacological values of these agarwood-related fungal endophytes in Aquilaria and Gyrinops were documented and discussed too. Finally, the effects on the host trees of Aquilaria and Gyrinops by fungal endophytes were discussed. We believe that our review could be useful for researchers to find innovative directions in the research field of agarwood endophytes.
2 Ecological distribution of Aquilaria and Gyrinops species
Aquilaria and Gyrinops species are widely distributed in southeast Asia, especially Indomalesia region (Lee and Mohamed, 2016). Recently, nine from the total of 21 species in the genus Aquilaria genus are known to grow in Malaysia (data from GBIF-Global Biodiversity Information Facility; Lee and Mohamed, 2016; Lee et al., 2018; Lee et al., 2022), including A. beccariana, A. crassna, A. cumingiana, A. hirta, A. malaccensis, A. microcarpa, A. rostrate, A. sinensis, and A. subintegra (Table 1), which is the country with the most species of Aquilaria. And five species of Aquilaria are naturally distributed in Malaysia, including A. beccariana, A. hirta, A. malaccensis, A. microcarpa, and A. rostrate (Lee et al., 2016). The other four are transplanted from China, Indonesia, Thailand, and Vietnam. Meanwhile, eight of the total of nine species in the Gyrinops genus are known to naturally grow in Indonesia, except for G. vidalii which is only distributed in Thailand and Lao People’s Democratic Republic (Table 1).
At present, the wild resources of Aquilaria and Gyrinops are rather limited. Thus, the reports on agarwood production are confined to 14 species of Aquilaria and eight species of Gyrinops (Table 1). More and more plantation areas are of larger scale, and A. sinensis occupies more than 5245 ha in China, which has the largest plantation size of all the species reported now (Turjaman et al., 2016; Yin et al., 2016). Additionally, A. malaccensis is the most widespread and cultivated species, including 13 countries (Table 1). Thus, A. malaccensis and A. sinensis are the most studied species recently. However, Gyrinops is less planted and studied for its slow-growing features (Lee et al., 2018). Among all the Gyrinops species, G. versteegii is the most popular species in eastern Indonesia, but it is less favored compared to A. malaccensis when it comes to agarwood cultivation in Indonesia (Faizal et al., 2022; Nasution et al., 2020; Turjaman et al., 2016).
3 Biodiversity of fungal endophytes in Aquilaria and Gyrinops
Endophytes are microorganisms that maintain endosymbiotic relationship within plants (Turjaman et al., 2016). At present, studies on endophytic biodiversity on Aquilaria received more attention than that of Gyrinops. Eventually, species that are involved in such studies are mainly those selected as cultivation species, including A. crassna, A. malaccensis, A. microcarpa, A. sinensis, A. subintegra, G. caudata, G. verstegii, and G. walla. However, the biodiversity of fungal endophytes has not been investigated in the other species of Aquilaria and Gyrinops due to the limited plantations, slow-growing features, and difficult species-identification (Hidayat et al., 2021; Lee et al., 2018; Turjaman et al., 2016). Based on our knowledge, a total of 42 fungal families and 67 fungal genera were isolated and identified in these eight agarwood-producing taxa, and 82.8 % of fungal species belonged to Ascomycota (Table 2). Among all the above endophytic genera, Fusarium is the most encountered species recorded in all studied taxa, except for A. microcarpa and G. caudata. a. Distribution percentage = host species number of an isolated fungal genus × 100 % / number of all the reported host species (nine species which include A. crassna, A. malaccensis, A. microcarpa, A. sinensis, A. subintegra, G. walla, G. caudata, and G. verstegii).
Fungal taxa
Host species
Distribution percentagesa
Agarwood-inducing methods
Inducing time
Inducing effects
References
Ascomycota
1. Apiosporaceae
1. Arthrinium
A. subintegra
12.5 %
---
---
---
Monggoot et al., 2017
2. Nigrospora
A. sinensis
12.5 %
---
---
---
Li et al., 2014
2. Ascomycota incertae sedis
3. Gonytrichum
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
3. Aspergillaceae
4. Aspergillus *
G. walla
25.0 %
---
---
---
Vidurangi et al., 2018
A. crassna
Solid inoculation
1 year
Tissue discoloration and resin content improved
(Subasinghe et al., 2019)
---
---
Inducing agarwood formation
Bose, 1938
5. Penicillium *
A. sinensis
25.0 %
Solid inoculation
30 days
Promoting sesquiterpene accumulation
Liu et al., 2022
Infusion
10 months
Promoting the accumulation of active ingredients
Wang et al., 2016
---
---
---
Gong and Guo, 2009
A. malaccensis
---
---
---
(Chhipa et al., 2017)
4. Botryosphaeriaceae
6. Endomelanconiopsis
A. sinensis
12.5 %
---
---
---
Chen et al., 2018
7. Diplodia *
Aquilaria sp.
---
---
---
Inducing agar formation
Bose, 1938
8. Lasiodiplodia *
A. sinensis
37.5 %
Inoculating the fermentation broth
2 months
Promoting the agarwood formation
Chen et al., 2017a
---
---
---
Cui et al., 2011
Inoculation of solid strains
6 months
Promotion of 34 sesquiterpenes and 4 aromatic compounds
Zhang et al., 2014
Infusion
10 months
Promoting the accumulation of active ingredients
Wang et al., 2016
A. malaccensis
---
---
---
Mohamed et al., 2010
A. crassna
---
---
---
Chi et al., 2016
---
---
---
Wang et al., 2019
9. Botryosphaeria *
A. sinensis
37.5 %
Formic acid and pinhole-infusion
1 ∼ 2 years
Producing high yield and high quality artificial agarwood in a relatively short time
Tian et al., 2013
Infusion
10 months
Promoting the accumulation of active ingredients
Wang et al., 2016
Liquid injection
160 days
Promote the formation of the main components of agarwood and incenses
Feng, 2008
A. crassna
---
---
Inducing agar formation
Bose, 1938
G. walla
---
---
---
Vidurangi et al., 2018
5. Chaetomiaceae
10. Chaetomium *
A. malaccensis
25.0 %
Artificial boring onto the plants
30 days
It is different between the oils obtained from naturally infected and healthy plants with regards to their quality.
Tamuli et al., 2005
A. sinensis
---
---
---
Tian et al., 2013
6. Chaetothyriales incertae sedis
11. Sarcinomyces
G. walla
12.5 %
---
---
---
Vidurangi et al., 2018
7. Cladosporiaceae
12. Cladosporium *
A. sinensis
37.5 %
---
---
---
Cui et al., 2011
---
---
---
Gong and Guo, 2009
Solid inoculation
30 days
C. cladosporioides promotes the accumulation of agarwood sesquiterpenes and chromones, while C. parahalotolerans could not promote agarwood formation.
Liu et al., 2022
A. malaccensis
---
---
---
Premalatha and Kalra, 2013
A. subintegra
---
---
---
Monggoot et al., 2017
8. Coniothyriaceae
13. Coniothyrium
A. sinensis
12.5 %
---
---
---
Cui et al., 2011
9. Diaporthaceae
14. Diaporthe
A. sinensis
50.0 %
---
---
---
Chen et al, 2018
A. microcarpa
---
---
---
(Vidurangi et al., 2018)
A. subintegra
---
---
---
Monggoot et al.,2017
G. verstegii
---
---
---
Mega et al., 2016
10. Didymellaceae
15. Epicoccum
A. malaccensis
25.0 %
---
---
---
Bhattacharyya et al, 1952
A. sinensis
---
---
---
Gong and Guo, 2009Cui et al., 2011
16. Leptosphaerulina
A. sinensis
12.5 %
---
---
---
Cui et al., 2011
11. Didymosphaeriaceae
17. Paraconiothyrium
A. sinensis
12.5 %
---
---
---
Cui et al., 2011
18. Montagnulaceae
A. sinensis
12.5 %
---
---
---
Wang et al., 2016
12. Dipodascaceae
19. Geotrichum
A. crassna
25.0 %
---
---
---
Chi et al., 2016
A. sinensis
---
---
---
Gong and Guo, 2009
20. Galactomyces
A. crassna
12.5 %
---
---
---
Chi et al., 2016
13. Dissoconiaceae
21. Ramichloridium
A. sinensis
12.5 %
---
---
---
Tian et al., 2013
14. Erysiphaceae
22. Ovulariopsis
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
15. Glomerellaceae
23. Colletotrichum
A. crassna
50.0 %
---
---
---
Chi et al, 2016
A. sinensis
---
---
---
Tian et al. 2013
A. subintegra
---
---
---
Monggoot et al., 2017
G. walla
---
---
---
Vidurangi et al., 2018
16. Herpotrichiellaceae
24. Rhinocladiella
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
25. Cladophialophora
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
17. Hypocreaceae
26. Trichoderma *
A. sinensis
50.0 %
---
---
---
Li et al., 2012
Infusion
10 months
Promoting the accumulation of active ingredients
Wang et al., 2016
A. malaccensis
---
---
---
Mohamed et al.,2010
G. versteegii
---
---
Contributing to the formation of agarwood sapwood
Mega et al., 2020
G. walla
---
---
---
Vidurangi et al., 2018
27. Hypocrea *
A. malaccensis
25.0 %
---
---
---
Mohamed et al., 2010
A. sinensis
---
---
---
Cui et al., 2011
Liquid injection
160 days
Promoting the transformation of agarwood and speeding up the process of making incense
Feng, 2008
18. Hypocreales incertae sedis
28. Acremonium *
A. microcarpa
37.5 %
---
---
The wood color and terpenoid compounds were changed.
Rahayu and Putridan Juliarni, 2007
G. verstegii
---
---
---
Mega et al., 2016
G. caudata
Fungal-induction with pruning
3–6 months
Showing a considerable effect in wood internal tissue and fragrance.
Auri et al., 2021
29. Cephalosporium
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
30. Verticillium
A. sinensis
12.5 %
---
---
---
Wang et al., 2016
19. Hypocreaceae
31. Diplocladium
G. walla
12.5 %
---
---
---
Subasinghe et al., 2019
20. Lasiosphaeriaceae
32. Fimetariella
A. sinensis
12.5 %
---
---
---
Tao et al., 2011aTao et al., 2011b
21. Mycosphaerellaceae
33. Mycosphaerella (Synonym: Davidiella)
A. sinensis
25.0 %
---
---
---
Tian et al., 2013
A. malaccensis
---
---
---
Premalatha and Kalra, 2013
34. Botryodyplodis * (Synonym: Physalospora)
Aquilaria sp.
---
---
---
Inducing agar formation
Bose, 1938
22. Nectriaceae
35. Cylindrocladium
A. sinensis
12.5 %
---
---
---
Tian et al., 2013
36. Fusarium *
A. crassna
75.0 %
---
---
---
Chi et al., 2016
---
0.5 ∼ 1.5 years
Inducing the formation of agarwood
Nobuchi and Siripatanadilok, 1991
A. sinensis
Formic acid and pinhole-infusion
1 ∼ 2 years
Producing high yield and high quality artificial agarwood in a relatively short time
Tian et al., 2013
---
---
---
Cui et al., 2011
Infusion
10 months
Promoting the accumulation of active ingredients
Wang et al., 2016
Inoculating the fermentation broth
2 months
Promoting the agarwood formation at the initial stage
Chen et al., 2017a
A. malaccensis
---
---
---
Mohamed et al., 2010
A. subintegra
---
---
---
Monggoot et al., 2017
G. walla
---
---
---
Vidurangi et al., 2018
Solid inoculation method
1 year
Promoting the tissue discoloration and resin content
Subasinghe et al., 2019
G. versteegii
---
---
Contributing to the formation of agarwood sapwood
Mega et al., 2020
Fungal inoculant formulation
16 months
Producing the agarwood with good quality.
Mega et al., 2016
37. Nectria
A. sinensis
12.5 %
---
---
---
Wang et al., 2016
23. Phyllostictaceae
38. Guignardia
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
24. Pichiaceae
39. Pichia
A. malaccensis
25.0 %
---
---
---
Premalatha and Kalra, 2013
A. sinensis
---
---
---
Cui et al., 2011
25. Pleosporaceae
40. Curvularia
A. crassna
37.5 %
---
---
---
Chi et al., 2016
A. malaccensis
---
---
---
Mohamed et al., 2010
G. verstegii
---
---
---
Mega et al., 2016
41. Alternaria
A. malaccensis
25.0 %
---
---
---
Premalatha and Kalra, 2013
A. sinensis
---
---
---
Tian et al., 2013
42. Pleospora
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
43. Cochliobolus
A. malaccensis
12.5 %
---
---
---
Mohamed et al., 2010
44. Phoma
A. sinensis
12.5 %
---
---
---
Cui et al., 2011Tian et al., 2013
26. Saccharomycetaceae
45. Lodderomycetes
A. malaccensis
12.5 %
---
---
---
Premalatha and Kalra, 2013
27. Sclerotiniaceae
46. Monilia
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
28. Sporocadaceae
47. Pestalotiopsis *
A. sinensis
25.0 %
Infusion method
6 months
Promoting the agarwood production
Chen et al., 2014Tian et al., 2013
A. subintegra
---
---
---
Monggoot et al., 2017
48. Preussia
A. malaccensis
12.5 %
---
---
---
Premalatha and Kalra, 2013
29. Togniniaceae
49. Phaeoacremonium *
A. malaccensis
25.0 %
---
---
---
Premalatha and Kalra, 2013
A. sinensis
---
---
---
Cui et al., 2011
Solid inoculation
30 days
Promoting the agarwood sesquiterpene accumulation
Liu et al., 2022
30. Trichocomaceae
50. Sagenomella
A. sinensis
12.5 %
---
---
---
Tian et al., 2013
31. Valsaceae
51. Phomopsis
A. sinensis
12.5 %
---
---
---
Tian et al., 2013
32. Xylariaceae
52. Xylaria *
A. sinensis
12.5 %
---
---
---
Cui et al., 2011
---
---
---
Tian et al., 2013
Solid inoculation
2 ∼ 8 months
Promoting agilawood accumulation
Cui et al., 2013
53. Nemania
A. sinensis
12.5 %
---
---
---
Tibpromma et al, 2021
54. Nodulisporium
A. sinensis
12.5 %
---
---
---
Tian et al., 2013Wu et al., 2010
33. Massarinaceae
55. Massarina
A. malaccensis
12.5 %
---
---
---
Premalatha and Kalra, 2013
Mucoromycota
34. Cunninghamellaceae
56. Cunninghamella
A. malaccensis
12.5 %
---
---
---
Mohamed et al., 2010
35. Lichtheimiaceae
57. Rhizomucor
A. sinensis
12.5 %
---
---
---
Cui et al., 2011
36. Mortierellaceae
58. Mortierella
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
37. Mucoraceae
59. Mucor
G. walla
12.5 %
---
---
---
Subasinghe et al., 2019
60. Rhizopus *
G. versteegii
12.5 %
---
---
Promoting the formation of agarwood sapwood
Mega et al., 2020
Mixture of fungal liquid with Fusarium solani
---
Promoting the production of agarwood with best quality
Mega et al., 2016
Basidiomycota
38. Exobasidiaceae
61. Glomerularia
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
39. Fomitopsidaceae
62. Fomitopsis *
A. sinensis
12.5 %
Infusion method
6 months
Promoting the agarwood production
Chen et al., 2017b
40. Meripilaceae
63. Rigidoporus *
A. sinensis
12.5 %
Trunk surface agarwood-inducing technique
2 months
Promoting the agarwood formation
Chen et al., 2018
41. Psathyrellaceae
64. Coprinopsis
A. sinensis
12.5 %
---
---
---
Chen et al., 2018
42. Russulaceae
65. Russula
A. subintegra
12.5 %
---
---
---
Monggoot et al., 2017
Unclassified
66. Mycelia sterilia
A. sinensis
12.5 %
---
---
---
Gong and Guo, 2009
67. Pleosporales
A. sinensis
12.5 %
---
---
---
Chen et al., 2018
It is worth mentioning that there is a discrepancy in the endophytic fungal diversity pattern in Aquilaria trees based on their growing regions, such as A. sinensis, an agarwood-producing species endemic to China that is naturally distributed in three provinces, including Guangdong, Guangxi, and Hainan. It was proposed that the biodiversity of fungal endophytes in A. sinensis might be due to the various geographical locations containing different levels of the atmosphere, light, soil moisture, and nutrient contents. To date, endophytic fungal studies on A. sinensis are abundant and well-documented. It was revealed that the dominant fungal genus from the population in Hainan was Penicillium (Zhang et al., 2009a), while the endophytic fungal diversity for A. sinensis growing in Guangxi was dominated by Fusarium (Huang et al., 2017). The fungal diversity could also be regionally specific, in which two genera, Chaetomium and Pichia, were only identified in A. sinensis of Hainan, but not from those in Guangdong and Guangxi (Chen et al., 2019). Also, the fungal diversity of agarwood derived from A. sinensis showed regional specificity. Lignosphaeria is the dominant fungal genus in agarwood samples produced from Haikou and Wanning in Hainan province. Perenniporia and Pyrigemmula are the dominant fungal genera in agarwood products from Danzhou and Ledong in Hainan province. Phaeoacremonium is the dominant fungal genus in agarwood products collected from Huazhou and Dongguan in Guangdong province.
Furtherly, the variation in microbiome composition is represented by multiple plant host organs, and tissue types (Cregger et al., 2018; Jia et al., 2016). In general, most of the fungal endophytes reside in the root, stem, and leaf at the same time; yet, the leaf tissue contains more fungal species when compared to the root and stem parts. So far, a total of 16 fungal endophytes were discovered commonly present in all three vegetative parts of A. sinensis, including Botryosphaeria, Cephalosporium, Cladophialophora, Epicoccum, Fusarium, Geotrichum, Glomerularia, Gonytrichum, Guignardia, Monilia, Mortierella, Mycelia sterilia, Ovulariopsis, Penicillium, Pleospora, and Rhinocladiella (Gong and Guo, 2009; Tian et al., 2013). On the other hand, four were reported specific to the leaf tissue of A. sinensis, including Alternaria, Cylindrocladium, Phoma, and Phomopsis (Gong and Guo, 2009; Tian et al., 2013), suggesting that these fungal endophytes are only able to survive under certain habitat, which in this event, the requirement for survival was fulfilled in the leaf tissue, but not in the root and stem parts.
At the stem and branch part, fungal endophytes not only colonize the healthy part (white wood), but also can be found in the resinous part (agarwood). Additionally, the diversity of fungal endophytes in resinous wood is much higher than that in the healthy wood of Aquilaria (Chen et al., 2017a); Liu et al., 2022). Based on the studies on A. malaccensis and A. sinensis, it was deduced that the resinous wood of the trees not only contained some of the fungal endophytes which were recorded to be present in both the healthy and resinous wood of the tree, such as Alternaria sp., Hypocrea sp., Lasidiplodia sp., and Trichoderma sp., but also included some of the fungal endophytes which were enriched in the resinous wood, i.e. Cladosporium sp., Curvularia sp., Fusarium sp., Phaeoacremonium sp., and Preussia sp. (Liu et al., 2022; Mohamed et al., 2010; Tian et al., 2013). Endophytic fungi isolated from resinous parts were proven to be good candidates in developing fungal inoculum that could promote agarwood production; however, only a few were evaluated on their efficacies. To date, 18 fungal genera were reported on their capability to promote the formation of agarwood, including Acremonium sp., Aspergillus sp., Botryodyplodias sp., Botryosphaeria sp., Chaetomium sp., Cladosporium sp., Diplodia sp., Fomitopsis sp., Fusarium sp., Hypocrea sp., Lasiodiplodia sp., Penicillium sp., Pestalotiopsis sp., Phaeoacremonium sp., Rhizopus sp., Rigidoporus sp., Trichoderma sp., and Xylaria sp. (Table 2).
Given that the diversity of endophytic fungi in Aquilaria trees could be varied in different regions, little was reported for other agarwood-producing species, especially those from Gyrinops. While the diversity of endophytic fungi in different Aquilaria host parts might be related to the environmental filtering or biotic interaction at the species level which was similar to Populus trees (Cregger et al., 2018). The fungal endophytes that can be identified in the while wood and resinous wood of Aquilaria and Gyrinops could be varied largely. Therefore, it is suggested that studies on endophytic fungi in other agarwood-producing species need to be hastened to uncover the uncertainties.
4 Effects on the host trees of Aquilaria and Gyrinops by endophytes
4.1 Use of fungal endophytes in the promotion of agarwood
In nature, endophytes could maintain endosymbiotic relationship within plants without any harm. However, after the trees of Aquilaria and Gyrinops were wounded, the micro-ecosystem balance was broken, and some of the fungal endophytes might grow fast and could trigger the self-defense reaction of the tree and thus, stimulate the formation of secondary metabolites that protect the host trees against invasions and diseases (Cui et al., 2013; Faizal et al., 2020; Liu et al., 2022; Xu et al., 2013). Similarly, the inoculating the isolated endophytes with agarwood promoting ability to the holed trees could quickly induce the phosphorylation of the plant immune reaction and promote agarwood accumulation (Liu et al., 2022; Xu et al., 2013). Therefore, the agarwood produced in the tree is recognized as the product of the tree’s defense response (Xu et al., 2013). Since the work to investigate the relationship between fungi and agarwood-producing trees in the process of agarwood formation started off in the early nineteenth century, for the past two decades, a considerable number of studies provided evidence on the crucial roles of fungi in enhancing agarwood formation (Huang et al., 2013; Mega et al., 2016; Zhang et al., 2014). It is believed that endophytes secrete signals that will initiate the defense mechanism of the tree; thus, endophytes promote the formation of agarwood (Chen et al., 2017; Sen et al., 2017). Chemometric analysis revealed that aroma (e.g. dodecane, 4-methyl-, tetracosane) in Fusarium sp. played a direct role in the activation of A. malaccensis tree’s defense and secondary metabolism (Sen et al., 2017). Fungal infection often leads immediately to the increased formation of free fatty acids that trigger oxidative burst and fatty acid oxidation cascades leading to the production of oxylipins such as jasmonates (Sen et al., 2017). And the endophytic strains of Lasiodiplodia theobromae were found to produce jasmonic acid (JA) (Chen et al., 2017). JA is known to be one of the crucial signal transducers that is responsible to induce sesquiterpene and chromone derivative formation in A. sinensis and A. malaccensis (Faizal et al., 2021; Xu et al., 2016). Furthermore, agarwood sesquiterpene accumulation can also be achieved by having Phaeoacremonium rubrigenum to induce phosphorylation of the transcription factors (TFs)-mevalonate (MVA) network in A. sinensis (Liu et al., 2022). Despite studies on the molecular interaction between agarwood-producing trees and fungal endophytes have been constantly reported, the findings are still limited and in-depth research ought to be fostered.
To date, six fungal taxa, i.e. Fusarium sp., Trichoderma sp., Acremonium sp., Curvularia sp., Cunninghamella sp., and Phaeoacremonium sp., were commonly known to be potential agents in promoting agarwood formation in Aquilaria and Gyrinops trees (Blanchette 2003; Hidayat et al., 2021; Liu et al., 2022; Mohamed et al., 2010). For the endophytes, Fusarium was most reported when compared to other fungal taxa; while for the host plant, studies on A. sinensis were most abundant (Table 2). In A. sinensis, it is believed that agarwood formed with the aid of Cladorrhinum bulbillosum, Fusarium solani, Gongronella butleri, Humicola grisea, Lasiodiplodia theobromae, Phaeoacremonium rubrigenum, Rigidoporus vinctus, Saitozyma podzolica, and Tetracladium marchalianum was able to produce high-quality raw material for essential oil production (Chen et al., 2017; Chen et al., 2018; Liu et al., 2022; Ma et al., 2021; Zhang et al., 2014). So far, a total of 12 fungal taxa were identified to induce agarwood formation in A. sinensis, including Botryosphaeria, Cladosporium, Fusarium, Fomitopsis, Hypocrea, Lasiodiplodia, Phaeoacremonium, Pestalotiopsis, Penicillium, Rigidoporus, Trichoderma, and Xylaria (Table 2). Three fungal taxa, Aspergillus sp., Botryosphaeria sp., and Fusarium sp. were also reported useful in promoting agarwood formation in A. crassna (Chi et al., 2016), while a mixture of fungi Phialophora sp. and Fusarium sp. applied to A. crassna could result in higher sesquiterpene content compared to the chemical and mechanical treatments (Thanh et al., 2015). Similar to Aquilaria, endophytes in Gyrinops also play an active role in the agarwood development of trees. In Gyrinops walla, the endemic species of Sri Lanka, Aspergillus niger and Fusarium solani have been described to be contributing to agarwood formation; Aspergillus niger is more effective than Fusarium solani in the tree host tissue discoloration and resin content (Subasinghe et al., 2019). On the other hand, three fungal taxa, including Fusarium sp., Rhizopus sp., and Trichoderma sp., were proven effective in the promotion of agarwood in Gyrinops versteegii, a plantation species that is mass cultivated in the western region of Indonesia (Mega et al., 2020; (Faizal et al., 2020)).
4.2 Endophytes improve the ecological adaptability of Aquilaria and Gyrinops
Another function of fungal endophytes of Aquilaria and Gyrinops is improving the ecological adaptability of hosts. Different types of fungi strains could induce the different compounds of Aquilaria and Gyrinops (Monggoot et al., 2017; Mega et al., 2020), which could explain the diversity of agarwood components to increase the resistance to environmental stresses. Similarly, either Aquilaria or Gyrinops grows in certain places with different geographic and climate conditions with certain kinds of fungi, which could promote the ecological adaptability of the host. Consistent with the roles of fungi in the plant defense system, they may be a contributory role in increasing antimicrobial activity, because the resinous site of agarwood tree has a less fungal abundance. When A. malaccensis was infected by Lasiodiplodia theobromae, Cunninghamella bainieri, and Fusarium solani, the abundance of fungi decreased after wounding and the number of target DNA molecules also declined, especially at 6–12 months of post-injury (Mohamed et al., 2014). The lower level of fungal species may be due to the high level of terpenes which are the major components of agarwood and can prevent or control pathogen attacks (Tamuli et al., 2005; Naef, 2011; Zulak and Bohlmann, 2010). Similarly, the agarwood derived from the infected A. sinensis and decayed Gyrinops spp. could show antifungal activity against Candida albicans, Fusarium oxysporum, Fusarium solani, and Lasiodiplodia theobromae (Hidayat et al., 2021; Zhang et al. 2014). So it is believed that the fungi can trigger the plant defense system to protect plants from invasions. And the metabolites produced by fungi also can provide protection for the host, which gives plants the ability to be resistant to abiotic and biotic stresses (Chowdhary et al., 2012; Jong 2012; Kharwar et al., 2011; Kumar and Kaushik, 2013; Suryannaryanan et al., 2009). And agarwood endophytes can also produce chemical compositions such as 2-phenylethyl-1H-indol-3-yl-acetate, (2R)-(3-indolyl)-propionic acid, and 9,11-dehyroergosterol peroxide, which displayed phytotoxic activity, cytotoxic activity, and anti-fungi and anti-bacterial activities, resulting in the enhancement of plant ecological adaptability. However, there are also a plenty of compounds, such as benzylacetone, benzaldehyde, palustrol, anisylacetone and chromone derivatives, the ecological functions of which have not been explored. It is possible that the fungal endophyte is one of the factors to resist the invasion of exogenous pathogens and to keep the plant growing well. Thus, we summarized the effects of fungal endophytes on the host trees of Aquilaria and Gyrinops on two sides: inducing the plant defense system and improving their ecological adaptability (Fig. 1).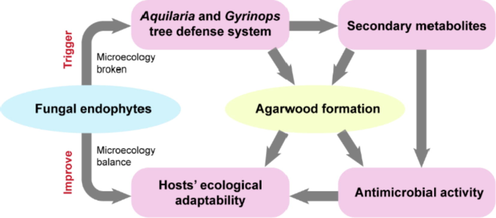
Effects of endophytes on their agarwood host trees, Aquilaria and Gyrinops.
5 Pharmacological effects of metabolites produced by fungal endophytes derived from Aquilaria and Gyrinops
Endophytic fungi can be one of the best-known sources of natural products, while the endophytic fungi present in Aquilaria and Gyrinops tree, in the process of agarwood formation, were also recognized as the new sources of secondary metabolites, which hold pharmaceutical and ecological significance. A total of 92 compounds were recorded from the endophytes of Aquilaria and Gyrinops trees, including terpenoids (40.22 %: containing monoterpenes, sesquiterpenes, and steroids), aromatics (26.09 %), alkaloids (5.44 %), chromones (2.17 %), and others (Fig. 2, Table 3). Interestingly, some endophytes of Aquilaria and Gyrinops could produce sesquiterpenes which were the important compounds of agarwood (Fig. 3, Table 3). Acremonium sp., Arthrinium sp., Collectotrichum sp., Diaporthe sp., Fimetariella rabenhorstii, Nemania sp., Nigrospora oryzae, and Nodulisporium sp. are responsible for the production of sesquiterpenes (Li et al., 2014; Monggoot et al., 2017; Tao et al., 2011a; Tibpromma et al., 2021; Wu et al., 2010; Zhang et al., 2009b). Thus, those fungal stains were considered to be the potential materials for fermenting agarwood compounds. Bioactivity: the effects of the fungal endophytes; Pharmacological values: the functions of the compounds produced by the fungi.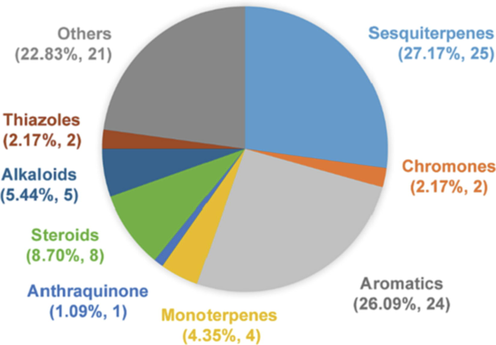
The categories of 92 compounds produced by the endophytes of Aquilaria and Gyrinops.
No.
Compounds
Molecular Formula
Pharmacological Values
Fungal Endophytes
Host Plant
References
Monoterpenes
1
γ-Terpinene
C10H16
Trypanocidal effect
Acaricidal activity
Acremonium sp.
A. sinensis
Tibpromma et al., 2021;Zhang et al., 2009b
2
Terpinen-4-ol
C10H18O
---
Arthrinium sp.
Collectotrichum sp.A. subintegra
Monggoot et al., 2017
3
1,8-Cineole
C10H18O
Antibacterial activity
Acremonium sp.
A. sinensis
Wang et al., 2007;Zhang et al., 2009b
4
Bicyclo[3.1.1]hept-3-ene-2-acetaldehyde, 4,6,6-trimethyl,
C12H18O
---
Nemania aquilariae
A. sinensis
Tibpromma et al., 2021
Sesquiterpenes
5
β-Agarofuran
C15H24O
---
Collectotrichum sp.
Diaporthe sp.A. subintegra
Monggoot et al., 2017
6
Alloaromadendrene
C15H24
Anti-oxidant activity
Cytotoxic activity
Anti-feedant activity
Anti-proliferative activityNemania aquilariae
A. sinensis
Baldissera et al.,2016Jesionek et al, 2018Sawant et al., 2007Yu et al.,2014
7
1,2,3,4,4α,5,6,7-Octahydro-4α,8-dimethyl-2-(1-methylethenyl)-naphthalene
C15H24
---
Nemania aquilariae
A. sinensis
Baldissera et al., 2016
8
Z-Eudesma-6,11-diene
C15H24
---
Arthrinium sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017
9
α-Selinene
C15H24
Anti-cancer activity
Repellent activityNemania aquilariae
A. sinensis
Alakanse et al., 2019Baldissera et al., 2016Mauti et al.,2019
10
α-Agarofuran
C15H24O
Antianxiety activity
Arthrinium sp.
Collectotrichum sp.
Diaporthe sp.A. subintegra
Monggoot et al., 2017Peeraphong et al., 2021Zhang et al., 2004
11
Oxo-agarospirol
C15H24O2
Antioxidant activity
Arthrinium sp.
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017
12
Ar-Curcumene
C15H22
Mosquito larvicides
Anti-inflammatory activity
Anti-ulcer activity
Acremonium sp.
A. sinensis
Duarte et al., 2007Podlogar et al., 2012Yamahara et al.,1992Zhang et al., 2009b
13
Zingiberene
C15H24
Anti-inflammatory activity
Anti-apoptotic effect
Anti-oxidant activity
Anti-cancer activity
Cytotoxicity, Genotoxicity
Acremonium sp.
A. sinensis
Duarte et al., 2007Li et al., 2021Togar et al., 2015Türkez et al., 2014Zhang et al., 2009b
14
10-epi-γ-Eudesmol
C15H26O
Prevention of mosquito-related disease
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015(Kracht et al., 2019)
15
cis-Dihydroagarofuran
C15H26O
Antimicrobial activity
Diaporthe sp.
A. subintegra
Capello et al., 2015Sadgrove et al., 2015
16
β-Dihydroagarofuran
C15H26O
---
Arthrinium sp.
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017
17
Valencen
C15H24
---
Nemania aquilariae
A. sinensis
Baldissera et al., 2016
18
Z-Caryophyllene
C15H24
---
Arthrinium sp.
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017
19
β-Elemene
C15H24
High cytotoxic activity
Diaporthe sp.
A. subintegra
Capello et al., 2015Monggoot et al., 2017
20
δ-Elemene
C15H24
---
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017
21
Agarospirol
C15H26O
Anti-nociceptive activitiy
Anti-oxidant activity
Collectotrichum sp.
Diaporthe sp.A. subintegra
Capello et al., 2015Monggoot et al., 2017Okugawa et al., 1996
22
rel-(1S,4S,5R,7R,10R)-10-Desmethyl-1-methyl-11-eudesmene
C15H26O
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Li et al., 2011
23
Capitulatin B
C15H26O2
---
Nigrospora oryzae
A. sinensis
Zhang et al., 2004
24
6α-Hydroxycyclonerolidol
C15H26O2
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Li et al., 2011
25
Frabenol
C15H26O2
---
Fimetariella rabenhorstii
A. sinensis
Tao et al., 2011a
26
6-Methyl-2-(5-methyl-5-vinyltetrahydrofuran-2-yl) hept-5-en-2-ol
C15H26O2
---
Nodulisporium sp.
A. sinensis
Li et al., 2011
27
11-Hydroxycapitulation B
C15H26O3
---
Nigrospora oryzae
A. sinensis
Zhang et al., 2004
28
δ-Eudesmol
C15H28O
Prevention of mosquito-related disease
Arthrinium sp.
Diaporthe sp.A. subintegra
Capello et al., 2015(Kracht et al., 2019)
29
(3R,6E,10S)-2,6,10-Trimethyl-3-hydroxydodeca-6,11-diene-
C15H28O3
---
Colletotrichum gloeosporioides
A. sinensis
Liu et al., 2018
Chromones
30
2,3-Dihydro-5-hydroxy-2-methylchromen-4-one
C10H10O3
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al., 2010
31
Mellein
C10H10O3
Antibacterial activity
Aspergillus sp.
A. sinensis
Peng et al., 2011
Anthraquinones
32
1,7-Dihydroxy-3-methoxyanthraquinone
C15H10O5
Anti-bacterial activity
Unknown fungal strain AL-2
A. malaccensis
Blakeney et al., 2019Shoeb et al., 2010
Steroids
33
Ergosterol
C28H44O
Anti-inflammatory activity
Nodulisporium sp.
A. sinensis
Kobori et al., 2007Li et al., 2011
34
Ergosterol peroxide
C28H44O3
Induced apoptosis of cells
Anti-inflammatory activity
Cytotoxic activity
Anti-oxidant activities
Anti-complementary activity
Trypanocidal activity
Antibacterial activity
Anti-proliferative activity
Nodulisporium sp.
A. sinensis
Li et al., 2011Takei et al.,2005Kobori et al., 2007Nam et al., 2001Kim et al., 1999Kim et al., 1997Ramos-Ligonio et al., 2012Duarte et al., 2007Nowak et al., 2016
35
5α,8α-Epidioxy-(22E,24R)-ergosta-6,22-dien-3β-ol
C28H44O3
Anti-tumor activity
Fimetariella rabenhorstii
A. sinensis
Li, 2016Plotnikov et al., 2021Tanapichatsakul et al., 2020Nam et al., 2001Kim et al., 1999
36
3β,5α,9α-Trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one
C28H44O3
Anti-tumor activity
Fimetariella rabenhorstii
A. sinensis
Li, 2016Plotnikov et al., 2021Takei et al., 2005
37
Cerevisterol
C28H46O3
Anti-microbial activity
Resistance modifying activity
Nodulisporium sp.
A. sinensis
Li et al., 2011Appiah et al., 2020
38
(3β,5α,6β,22E)-Ergosta-7,22-diene-3,5,6-triol
C28H46O3
---
Phaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007
39
3β,6β,7α-Trihydroxy-(24R)-ergosta-8(14),22-diene
C28H46O3
---
Fimetariella rabenhorstii
A. sinensis
Li, 2016Plotnikov et al., 2021
40
3β,5α,6β-Trihydroxy-(22E,24R)-ergosta-7,22-diene
C28H46O3
Anti-tumor activity
Fimetariella rabenhorstii
A. sinensis
Li, 2016Plotnikov et al., 2021Kobori et al., 2007Kim et al.,1997
Aromatics
41
Methylphenol
C7H8O
Antibacterial activity
Fimetariella rabenhorstii
Phaeoacremonium rubrigenum
A. sinensis
Li, 2016Wei et al., 2011
42
p-Hydroxybenzaldehyde
C7H6O2
Anti-oxidative activity
Blood-brain barrier
ToxicityPhaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007Kinjo et al., 2020Borneman et al., 1986Wei et al., 2011
43
Phenylethyl alcohol
C8H10O
Antibacterial
Sedative effects
Antifungal activity
Acremonium sp.
A. sinensis
Duarte et al.,2007Lilley and Brewer, 1953Oshima and Ito, 2021Boukaew and Prasertsan, 2018Zhang et al., 2009b
44
4-Hydroxyphenyl ethyl alcohol
(Tyrosol; 4-Hydroxy-benzeneethanol)C8H10O2
Nematicide
Antioxidant activity
Anti-inflammatory activity
Fimetariella rabenhorstii
Acremonium sp.
Nodulisporium sp.A. sinensis
Tao et al., 2011bDuarte et al.,2007Li et al.,2018Zhang et al., 2009bLi et al., 2011Wei and Liu, 2007Tao et al., 2011b
45
6-Methoxy-7-O-(p-methoxyphenyl)-coumarin
C17H14O5
---
Unkonw fungal strain AL-2
A. malaccensis
Blakeney et al., 2019
46
3,4-Dihydroxybenzoic acid
C7H6O4
Antibacterial activity
Phaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007
47
Phthalic acid diisobutyl ester
C16H22O4
Phytotoxicity
Testicular atrophyColletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020Huang et al.,2021Oishi et al.,1980
48
Decyl butyl phthalate
C22H34O4
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
49
1-(2,6-Dihydroxyphenyl) ethanone
C8H8O3
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al., 2010
50
8-Methoxynaphthalen-1-ol
C11H10O2
Antifungal activity
Nodulisporium sp.
A. sinensis
Li et al, 2011;Takei et al., 2005
51
p-Hydroxyphenethyl alcohol
C8H10O2
Antibacterial activity
Phaeoacremonium rubrigenum
A. sinensis
Wei et al., 2011
52
Ethyl benzoate
C9H10O2
---
Arthrinium sp.
Collectotrichum sp.
Diaporthe sp.A. subintegra
Monggoot et al., 2017
53
Phenyl butanone
C10H12O
---
Collectotrichum sp.
Diaporthe sp.A. subintegra
Monggoot et al., 2017
54
1-(2,6-Dihydroxyphenyl) butan-1-one
C10H12O3
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al., 2010
55
1-(2,6-Dihydroxyphenyl)-3-hydroxybutan-1-one
C10H12O4
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al., 2010
56
Benzeneacetic acid
C8H8O2
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
57
(2R*,4R*)-3,4-Dihydro-4-methoxy-2-methyl-2H-1-benzopyran-5-ol
C11H14O3
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al., 2010
58
Phenethyl 2-hydroxypropanoate
C11H14O3
---
Colletotrichum gloeosporioides
A. sinensis
Liu et al., 2018
59
Benzyl benzoate
C14H12O2
Treatment of scabies
Arthrinium sp.
Diaporthe sp.A. subintegra
Monggoot et al., 2017;Li et al., 2011
60
1,8-Dimethoxynaphthalene
C12H12O2
---
Nodulisporium sp.
A. sinensis
Li et al., 2011
61
(7R*,8S*)-3,6,7,8-Tetrahydro-4,7,8-trihydroxynaphtho [2,3-
C12H12O5
Cytotoxic activity
Nodulisporium sp.
A. sinensis
Wu et al, 2010
62
Propyl p-methoxy phenyl ether
C10H14O2
---
Unkonw fungal strain AL-2
A. malaccensis
Shoeb et al., 2010
63
p-Hydroxyphenylacetic acid
C8H8O3
---
Phaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007
64
4-Pentylbenzoic acid
C12H16O2
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
Alkaloids
65
Nicotinic acid
C6H5NO2
Prevention of atherosclerosis and reduce the risk of cardiovascular events
Fimetariella rabenhorstii
A. sinensis
Okugawa et al., 1996(Gille et al., 2008)
66
Thymidine
C10H14N2O5
Anti-cancer activity
Anti-metabolites activityPhaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007Stokes and Lacey, 1978Martin et al., 1980O'Dwyer et al, 1987
67
N-Phenylacetamide
C8H9NO
Cytotoxic activity
Fimetariella rabenhorstii
A. sinensis
Okugawa et al.,1996
68
N-(6-Hydroxyhexyl)-acetamide
C8H17O2N
Antibacterial activity
Phaeoacremonium rubrigenum
A. sinensis
Ribeiro et al., 2007
69
2-Anilino-1,4-naphthoquinone
C16H11NO2
Anti-fungal activity
Fimetariella rabenhorstii
A. sinensis
Okugawa et al.,1996(Leyva et al., 2017)
Thiazoles
70
Colletotricole A
C9H13NO3S
---
Colletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020
71
2-(4-Methylthiazol-5-yl)ethyl 2-hydroxypropanoate
C9H13O3NS
---
Colletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020
Others
72
Colletotricone A
C14H20O4
Anti-tumour activity
Cytotoxic activityColletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020Kim et al., 2019Liu et al., 2018
73
Colletotricone B
C14H20O4
---
Colletotrichum gloeosporioides
A. sinensis
Liu et al., 2018
74
Nigrosporanene A
C14H20O4
Cytotoxicity
Radical scavenging activityColletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020Liu et al., 2018Ma and Qi, 2019
75
Nigrosporanene B
C14H22O4
Radical scavenging activity
Colletotrichum gloeosporioides
A. sinensis
Appiah et al., 2020Liu et al., 2018Ma and Qi, 2019
76
d-Galacitol
C6H14O6
---
Fimetariella rabenhorstii
A. sinensis
Tao et al., 2011b
77
2,3-Dihydroxybutane
C4H8O3
---
Acremonium sp.
A. sinensis
Zhang et al., 2009b
78
5-Hydroxymethylfurfural
C6H6O3
Antibacterial activity
Phaeoacremonium rubrigenum
A. sinensis
Wei et al., 2011
79
Cyclohexanone
C6H10O
---
Acremonium sp.
A. sinensis
Zhang et al., 2009b
80
4-Hydroxy-4-methyl-2-phentanone
C6H12O2
---
Acremonium sp.
A. sinensis
Zhang et al., 2009b
81
3,5-Dimethyl cyclopentenolone
C7H11O2
---
Acremonium sp.
A. sinensis
Zhang et al., 2009b
82
5-Methyl-2-vinyltetrahydrofuran-3-ol
C7H12O2
---
Nodulisporium sp.
A. sinensis
Li et al., 2011
83
Octanoic acid
C8H16O2
Toxicity
Reducing the magnitude of tremor
Anti-tumor activity
Acremonium sp.
A. sinensis
Duarte et al., 2007Viegas et al., 1995Lowell et al., 2019Altinoz et al., 2020
84
(Z)-9,17-Octadecadienal
C18H32O
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
85
Sorbic acid
C6H8O2
Anti-fungal activity
Anti-microbial activity
Acremonium sp.
A. sinensis
Duarte et al., 2007Razavi‐Rohani and Griffiths, 1999Eklund et al., 1983
86
Linoleic acid
C18H32O2
Pro-inflammatory activity
Anti-cancer activity
Cholesterol and blood pressure lowering effects
Epidermal permeability barrier
Anaerobic degradability
Inhibitory effects
Acremonium sp.
A. sinensis
Duarte et al., 2007Young et al., 1998Burns et al., 2018Lalman et al., 2000Elias et al., 1980
87
Acetic acid
C2H4O2
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
88
Oleic acid
C18H34O2
Anti-tumor
Anti-inflammatory
Anti-bactericidal
Vasculoprotective effects
Pro-inflammatory
Acremonium sp.
A. sinensis
Duarte et al., 2007(Carrillo Pérez et al., 2012)
Sales-Campos et al., 2013Speert et al., 1979Massaro et al., 2002Young et al., 1998
89
Isovaleric acid
C5H10O2
Reduces Na+, K+-ATPase activity
Causes colonic smooth muscle relaxation
Acremonium sp.
A. sinensis
Duarte et al., 2007Ribeiro et al., 2007Blakeney et al., 2019
90
Methyl jasmonate
C12H18O3
Against pathogens
Salt stress
Drought stress
Low temperature
Heavy metal stress and toxicities of other elementsLasiodiplodia theobromae
A. sinensis
Han et al., 2014Yu et al., 2018
91
Octadecanoic acid
C18H36O2
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
92
Butanoic acid
C10H22O2Si
---
Acremonium sp.
A. sinensis
Duarte et al., 2007
The structures of 92 compounds produced by the endophytes of Aquilaria and Gyrinops.
Endophytic fungi in agarwood-producing trees can also be a rich source of medicinal agents with various pharmacological properties (Chhipa et al., 2017). The majority of endophytes derived from Aquilaria showed both antimicrobial and antitumor activities simultaneously, including Cladosporium tenuissimum, Coniothyrium nitidae, Epicoccum nigrum, Fusarium equiseti, Fusarium oxysporum, Fusarium solani, Hypocrea lixii, Lasiodiplodia theobromae, Leptosphaerulina chartarum, Paraconiothyrium variabile, Phaeoacremonium rubrigenum, Rhizomucor variabilis, and Xylaria mali (Cui et al., 2011). However, few of them were reported to display only antimicrobial activities, i.e. Phoma herbarum, Geotrichum candium, and Fusarium verticillioides (Chi et al., 2016; Cui et al., 2011). The endophytic fungi, Diaporthe sp. and Colletotrichum sp., which were believed to be responsible for the production of sesquiterpene compounds in Aquilaria trees, were claimed to have antioxidant properties; while the latter taxa also came with anti-inflammatory activities (Monggoot et al., 2017; Wang et al., 2016). Although a total of 92 compounds produced by fungal endophytes in Aquilaria were identified so far (Fig. 3), only 52 of them were proven to come with their related biological properties; in general, most of the identified compounds contained anti-inflammatory, anti-bacterial, and anti-cancer properties (Table 3). Compounds produced by all the isolated endophytes from Aquilaria and Gyrinops and their pharmacological values have been shown in Table 3.
6 Conclusion
Endophytes could maintain endosymbiotic relationship within plants at least in one stage of their life cycle (Turjaman et al., 2016). Compared with physical and chemical methods, the use of endophytic fungi has been recognized as a safe method to promote agarwood production for the environment and human health (Tan et al., 2019). Additionally, the endophyte inducing method could be seemed as a prioritized approach to enhance agarwood formation due to its ability to produce the signals associated with continuous agarwood formation and compounds with bioactivity. And the advantages of induced agarwood by endophytes are more pharmaceutical values, higher environmental adaptability, and faster speed of agarwood formation.
To our knowledge, 14 species of Aquilaria and eight of Gyrinops were known to produce agarwood, and different fungal species and abundances were detected because of different planting regions and various species. Fusarium sp. accounted for the largest proportion of Aquilaria and Gyrinops, followed by Colletotrichum sp., Diaporthe sp., and Trichoderma sp. The endophytic fungi spread over various host species with high biodiversity, however, the biodiversity of fungal endophytes distributed in various planting areas is seldom reported, which needs more detailed research. Furtherly, the variation in microbiome composition is represented by multiple A. sinensis tree host organs, and tissue types. The high diversity of fungal endophytes in resinous wood could give some good candidates for developing fungal inoculum that could promote agarwood production.
Various endophytic fungi have been reported to produce metabolites containing sesquiterpenoids and aromatic groups, which are a rich source of medicinal agents to improve the quality and quantity of agarwood. Most of the endophytes from Aquilaria and Gyrinops showed antimicrobial and antitumor activities, and a few fungi that have special abilities, such as the antioxidant activity of Diaporthe sp., and anti-inflammatory activities of Colletotrichum sp. Besides that, some of the secondary metabolites are formed when the fungal endophytes trigger the self-defense reaction of Aquilaria and Gyrinops. In this way, the agarwood fungal endophytes not only protect host trees from microbe invasions and diseases but also activate the accumulation of agarwood. And these compounds produced by the fungal strains are various due to the different species or strains, which might enhance the resistance abilities to various environmental stresses. Conversely, both Aquilaria and Gyrinops trees grow in different places with various geographic and climate conditions and need different sorts of fungi, which could promote the host’s ecological adaptability. In summary, the fungal endophytes on the host trees of Aquilaria and Gyrinops are responsible for activating the plant defense system, strengthening the hosts’ ecological adaptability, and enhancing agarwood production, which may be the reasons why agarwood artificial induction by endophytes has become popular. The mechanism of aroma accumulation and the crucial role of endophytes in the agarwood host trees need to be furtherly explored in the future.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (grant no. ZZ13-YQ-093-C1, ZZXT202112), and the CACMS Innovation Fund (Grant No. CI2021A04101).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- α-Selinene from Syzygium aqueum against aromatase P450 in breast carcinoma of postmenopausal women: in silico study. J. Biomed. Pharm. Sci.. 2019;2:1-7.
- [Google Scholar]
- Caprylic (Octanoic) acid as a potential fatty acid chemotherapeutic for glioblastoma. Prostaglandins Leukot. Essent. Fat. Acids. 2020;159:102142
- [Google Scholar]
- Antimicrobial and resistance modifying activities of cerevisterol isolated from Trametes species. Curre. Bioact. Compd.. 2020;16:115-123.
- [Google Scholar]
- The effect of crown pruning and induction of Acremonium sp. on agarwood formation in Gyrinops caudata in West Papua, Indonesia. Biodivers. J. Biol. Divers.. 2021;22:2604-2611.
- [Google Scholar]
- In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol.. 2016;162:43-48.
- [Google Scholar]
- On the formation and development of agaru in Aquilaria agallocha. Sci. Cult.. 1952;18:240-243.
- [Google Scholar]
- Branched short-chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig. Dis. Sci.. 2019;64:1171-1181.
- [Google Scholar]
- Blanchette, R.A., 2003. Agarwood formation in Aquilaria trees: resin production in nature and how it can be induced in plantation grown trees. In: Notes from presentation at first international agarwood conference, Ho Chi Minh City, Vietnam, 10–15.
- Effect of phenolic monomers on ruminal bacteria. Appl. Environ. Microbiol.. 1986;52:1331-1339.
- [Google Scholar]
- Inhibitory effects of acetophenone or phenylethyl alcohol as fumigant to protect soybean seeds against two aflatoxin-producing fungi. J. Food Sci. Technol.. 2018;55:5123-5132.
- [Google Scholar]
- Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostaglandins Leukot. Essent. Fat. Acids. 2018;135:1-4.
- [Google Scholar]
- Chemical composition and in vitro cytotoxic and antileishmanial activities of extract and essential oil from leaves of Piper cernuum. Nat. Prod. Commun.. 2015;10:285-288.
- [Google Scholar]
- Antitumor effect of oleic acid; mechanisms of action. a review. Nutr. Hosp.. 2012;27:1860-1865.
- [Google Scholar]
- Trunk surface agarwood-inducing technique with Rigidoporus vinctus: an efficient novel method for agarwood production. PLoS One. 2018;13:0198111.
- [Google Scholar]
- Isolation and identification of endophytic fungi which promote agarwood formation in Aquilaria sinensis. Chin. Pharm. J.. 2014;49(13):1118-1120.
- [Google Scholar]
- Agarwood formation induced by fermentation liquid of Lasiodiplodia theobromae, the dominating fungus in wounded wood of Aquilaria sinensis. Curr. Microbiol.. 2017;74:460-468.
- [Google Scholar]
- Effect of Fomitopsis sp. on promoting agarwood formation and its biological characteristics. Mod. Chin. Med.. 2017;19(8):1097-1101.
- [Google Scholar]
- Analysis of fungi diversity in agarwood wood from Hainan province and Guangdong province. Chin. Pharm. J.. 2019;54:1933-1938.
- [Google Scholar]
- Artificial production of agarwood oil in Aquilaria sp. by fungi: a review. Phytochem. Rev.. 2017;16:835-860.
- [Google Scholar]
- Biological characterization of fungi endophytes isolated from agarwood tree Aquilaria crassna Pierre ex Lecomte. Tạp chí Công nghệ Sinh học. 2016;14:149-156.
- [Google Scholar]
- Endophytic fungi and their metabolites isolated from Indian medicinal plant. Phytochem. Rev.. 2012;11:467-485.
- [Google Scholar]
- The Status of agar (Aquilaria agallocha Roxb.) based small-scale cottage industries in Sylhet region of Bangladesh. Bangladesh J. Resour. 2003:1-22.
- [Google Scholar]
- Gyrinops ledermannii (Thymelaeaceae), being an agarwood-producing species prompts call for further examination of taxonomic implications in the generic delimitation between Aquilaria and Gyrinops. Flora Males. Bull.. 2002;13:61-65.
- [Google Scholar]
- The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018:6-31.
- [Google Scholar]
- Antitumor and antimicrobial activities of endophytic fungi from medicinal parts of Aquilaria sinensis. Zhejiang Univ. Sci. B. 2011;12(5):385-392.
- [Google Scholar]
- Effects of inoculating fungi on agarwood formation in Aquilaria sinensis. Chin. Sci. Bull.. 2013;58:3280-3287.
- [Google Scholar]
- Characterization of the complete chloroplast genome of Aquilaria sinensis, an endangered agarwood-producing tree. Mitochondrial DNA B Resour.. 2020;5:422-423.
- [Google Scholar]
- Three new 2-(2-phenylethyl) chromone derivatives of agarwood originated from Gyrinops salicifolia. Molecules. 2019;24(3):576.
- [Google Scholar]
- Antibacterial activity of ergosterol peroxide against Mycobacterium tuberculosis: dependence upon system and medium employed. Phytother. Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Derivatives. 2007;21:601-604.
- [Google Scholar]
- The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol.. 1983;54:383-389.
- [Google Scholar]
- The permeability barrier in essential fatty acid deficiency: evidence for a direct role for linoleic acid in barrier function. J. Invest. Dermatol.. 1980;74:230-233.
- [Google Scholar]
- Fusarium solani induces the formation of agarwood in Gyrinops versteegii (Gilg.) Domke branches. Symbiosis. 2020;81:15-23.
- [Google Scholar]
- Faizal, A., Esyanti, R.R., Adn′ain, N., Rahmani, S., Azar, A.W.P., Iriawati, Turjaman, M., 2021. Methyl jasmonate and crude extracts of Fusarium solani elicit agarwood compounds in shoot culture of Aquilaria malaccensis Lamk. Heliyon, e06725
- Evaluation of biotic and abiotic stressors to artificially induce agarwood production in Gyrinops versteegii (Gilg.) Domke seedlings. Symbiosis. 2022;86:229-239.
- [Google Scholar]
- Notes on the distribution and ecology of Aquilaria lam. (Thymelaeaceae) in Malaysia. Malaysian Fores.. 2009;72(2):247-259.
- [Google Scholar]
- Preliminary study on endophytic fungi of Aquilaria sinensis. Nanchang: Jiangxi University; 2008.
- Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol.. 2008;48:79-106.
- [Google Scholar]
- Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Biotechnol.. 2009;8(5):731-736.
- [Google Scholar]
- Beiträge zur Kenntnis der Thymelaeacean under ihrer natürlichen Umgrenzung. Meded. Rijks-Herb. 1992;44:1-31.
- [Google Scholar]
- Study of production of sesquiterpenes of Aquilaria sinensis stimulated by Lasiodiplodia theobromae. Chin. J. Chin. Mater. Med.. 2014;39:192-196.
- [Google Scholar]
- Bioactive composition, antifungal, antioxidant, and anticancer potential of agarwood essential oil from decaying logs (Gyrinops spp.) of Papua Island (Indonesia). Journal of Applied Pharmaceutical. Science. 2021;11(10):070-078.
- [Google Scholar]
- Notes on some Asiatic species of Aquilaria (Thymelaceae) Blumea. 1964;12(2):285-288.
- [Google Scholar]
- Isolation and screening of endophytic fungi from the resin part of Aquilaria sinensis. Jiangsu Agric. Sci.. 2017;45(20):285-289.
- [Google Scholar]
- Historical records and modern studies on agarwood production method and overall agarwood production method. China J. Chin. Mater. Med.. 2013;38(3):302-306.
- [Google Scholar]
- Phthalic acid esters: Natural sources and biological activities. Toxins. 2021;13(7):495.
- [Google Scholar]
- IUCN. 2022. The IUCN Red List of Threatened Species. Version 2021-3.
- Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J. Chromatogr. B. 2018;1086:63-72.
- [Google Scholar]
- A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol.. 2016;7:906.
- [Google Scholar]
- Jong, P.L., 2012. Effects of mechanical wounding and infection patterns of Fusarium solani on gaharu formation in Aquilaria malaccensis Lam. Master Dissertation, Faculty of Forestry, Universiti Putra Malaysia.Kalra, R., Kaushik, N., 2017. A review of chemistry, quality and analysis of infected agarwood tree (Aquilaria sp.). Phytochem. Rev. 16, 1045–1079
- Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat. Prod. Rep.. 2011;28(7):1208-1228.
- [Google Scholar]
- A New Species of Aquilaria (Thymelaeaceae) from Vietnam. Blumea-Biodivers. Evol. Biogeogr. Plants. 2005;50(1):135-141.
- [Google Scholar]
- Anticomplementary activity of ergosterol peroxide from Naematoloma fasciculare and reassignment of NMR data. Arch. Pharm. Res.. 1997;20:201-205.
- [Google Scholar]
- Antioxidant activity of ergosterol peroxide (5,8-epidioxy-5α,8α-ergosta-6,22E-dien-3β-ol) in Armillariella mellea. Bull. Kor. Chem. Soc.. 1999;20:819-823.
- [Google Scholar]
- The fungus Colletotrichum as a source for bioactive secondary metabolites. Arch. Pharm. Res.. 2019;42:735-753.
- [Google Scholar]
- Effects of p-hydroxybenzaldehyde and p-hydroxyacetophenone from non-centrifuged canesugar, kokuto, on serum corticosterone, and liver conditions in chronically stressed mice fed with a high-fat diet. Food Sci. Technol. Res.. 2020;26:501-507.
- [Google Scholar]
- Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264. 7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol.. 2007;150:209-219.
- [Google Scholar]
- Discovery of three novel sesquiterpene synthases from Streptomyces chartreusis NRRL 3882 and crystal structure of an α-eudesmol synthase. Journal of biotechnology. 2019;297:71-77.
- [Google Scholar]
- Discovery of three novel sesquiterpene synthases from Streptomyces chartreusis NRRL 3882 and crystal structure of an α-eudesmol synthase. Journal of biotechnology. 2019;297:71-77.
- [Google Scholar]
- Endophytic fungi isolated from oilseed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One. 2013;8(2):e56202.
- [Google Scholar]
- Anaerobic degradation and inhibitory effects of linoleic acid. Water Res.. 2000;34:4220-4228.
- [Google Scholar]
- The origin and domestication of Aquilaria, an important agarwood-producing genus. In: Book: Agarwood. Springer Singapore: Tropical Forestry; 2016. p. :1-20.
- [Google Scholar]
- Phylogenetic relatedness of several agarwood-producing taxa (Thymelaeaceae) from Indonesia. Trop. Life Sci. Res.. 2018;29(2):13-28.
- [Google Scholar]
- Phylogenetic relationships of Aquilaria and Gyrinops (Thymelaeaceae) revisited: evidence from complete plastid genomes. Biol. J. Linn. Soc. Lond. 2022:boac014.
- [Google Scholar]
- Synthesis and studies of the antifungal activity of 2-anilino-/2, 3-dianilino-/2-phenoxy-and 2, 3-diphenoxy-1, 4-naphthoquinones. Research on Chemical Intermediates. 2017;43(3):1813-1827.
- [Google Scholar]
- Study on secondary metabolites of endophytic fungus strain Fusarium solani A2 and Fimetariella rabenhorstii A20 from Aquilariae Lignum Resinatum. Guangdong Pharmaceutical University; 2016. p. :41-45. MS thesis
- Two new octahydro naphthalene derivatives from Trichoderma spirale, an endophytic fungus derived from Aquilaria sinensis. Helv. Chim. Acta. 2012;95(5):805-809.
- [Google Scholar]
- Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum. Exp. Toxicol.. 2021;40:915-927.
- [Google Scholar]
- Inhibitory effects of components from root exudates of Welsh onion against root knot nematodes. PLoS One. 2018;13(7):e0201471.
- [Google Scholar]
- Chemical constituents of endophytic fungus Nodulisporium sp. A4 from Aquilaria sinensis. Chin. J. Chin. Mat. Med.. 2011;36:3276-3280.
- [Google Scholar]
- A new eudesmane sesquiterpene from Nigrospora oryzae, an endophytic fungus of Aquilaria sinensis. Rec. Nat. Prod.. 2014;8:330-333.
- [Google Scholar]
- The selective antibacterial action of phenylethyl alcohol. J. Am. Pharm. Assoc.. 1953;42(1):6-8.
- [Google Scholar]
- Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules. 2013;18:3086-3106.
- [Google Scholar]
- Integrating multiple omics identifies Phaeoacremonium rubrigenum acting as Aquilaria sinensis marker fungus to promote agarwood sesquiterpene accumulation by inducing plant host phosphorylation. Spectr: Microbiol; 2022. p. :e0272221.
- Secondary metabolites from the Colletotrichum gloeosporioides A12, an endophytic fungus derived from Aquilaria sinensis. Nat. Prod. Res.. 2018;32:2360-2365.
- [Google Scholar]
- The effect of octanoic acid on essential voice tremor: a double-blind, placebo-controlled study. Laryngoscope. 2019;129(8):1882-1890.
- [Google Scholar]
- The formation and quality evaluation of agarwood induced by the fungi in Aquilaria sinensis. Ind. Crop. Prod.. 2021;173:114129
- [Google Scholar]
- Marine natural products from marine coral-derived microorganisms. In: Symbiotic Microbiomes of Coral Reefs Sponges and Corals. Dordrecht.: Springer; 2019. p. :311-328.
- [Google Scholar]
- Vasculoprotective effects of oleic acid: epidemiological background and direct vascular antiatherogenic properties. Nutr. Metab. Cardiovasc. Dis.. 2002;12:42-51.
- [Google Scholar]
- Evaluation of the repellent effect of Dioscorea sansibarensis Pax (Dioscoreaceae) leaf essential oil against Bruchus chinensis Linnaeus, 1758 (Coleoptera: Bruchidae) Pol. J. Entomol.. 2019;88:119-128.
- [Google Scholar]
- Identification of three isolate fungal to produce agarwood sapwood on Gyrinops versteegii (Gilg.) Domke’. In: Plant By Molecular Analysis international Journal of Research in Engineering and Science (IJRES) ISSN (Online). 2020. p. :2320-9364.
- [Google Scholar]
- Agarwood producing fungal inoculant formulation in ketimunan tree (Gyrinops versteegii DOMKE) Int. J. Biosci. Biotechnol. 2016:1-7.
- [Google Scholar]
- Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers.. 2010;43(1):67-74.
- [Google Scholar]
- Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. For. Res.. 2014;25(1):201-204.
- [Google Scholar]
- Fungal endophytes: an alternative source for production of volatile compounds from agarwood oil of Aquilaria subintegra. Microb. Ecol.. 2017;74(1):54-61.
- [Google Scholar]
- The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: a review. Flavour. Fragr.. 2011;26(2):73-87.
- [Google Scholar]
- Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci.. 2001;69(2):229-237.
- [Google Scholar]
- Nasution, A.A., Siregar, U.J., Miftahudin, Turjaman, M., 2020. Identification of chemical compounds in agarwood-producing species Aquilaria malaccensis and Gyrinops versteegii. J. For. Res. 31, 1371-1380
- A review on agar (gaharu) producing Aquilaria species. Trop. Forest. Prod.. 1997;2(2):272-285.
- [Google Scholar]
- Preliminary observation of Aquilaria crassna wood associated with the formation of aloeswood. Bull. Kyoto Univ. For.. 1991;63:226-235.
- [Google Scholar]
- A new method for the isolation of ergosterol and peroxyergosterol as active compounds of Hygrophoropsis aurantiaca and in vitro antiproliferative activity of isolated ergosterol peroxide. Molecules. 2016;21:946.
- [Google Scholar]
- Role of thymidine in biochemical modulation: a review. Cancer Res.. 1987;47:3911-3919.
- [Google Scholar]
- Testicular atrophy induced by phthalic acid esters: effect on testosterone and zinc concentrations. Toxicol. Appl. Pharmacol.. 1980;53:35-41.
- [Google Scholar]
- Effect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Planta Med.. 1996;62:2-6.
- [Google Scholar]
- Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. J. Nat. Med.. 2021;75:319-325.
- [Google Scholar]
- Volatile constituents and their antimicrobial activities of endophytic fungus Aspergillus sp. A14 from Aquilaria sinensis. Nat. Prod. Res. Dev.. 2011;23:85-88.
- [Google Scholar]
- Growing ‘the wood of the gods’: agarwood production in southeast Asia. Smallholder Tree Growing Rural Develop. Environ. Services 2008:245-262.
- [Google Scholar]
- Tyrosol as a neuroprotector: strong effects of a “weak” antioxidant. Curr. Neuropharmacol.. 2021;19:434-448.
- [Google Scholar]
- Antiinflammatory effects of ginger and some of its components in human bronchial epithelial (BEAS-2B) cells. Phytother. Res.. 2012;26:333-336.
- [Google Scholar]
- Molecular phylogenetic identification of endophytic fungi isolated from resinous and healthy wood of Aquilaria malaccensis, a red listed and highly exploited medicinal tree. Fungal Ecol.. 2013;6(3):205-211.
- [Google Scholar]
- Rahayu, G., Putridan Juliarni, A.L., 2007. Acremonium and methyl-jasmonate induce terpenoid formation in agarwood tree (Aquilaria crassna)’. In: Makalahdi presenta sikandalam 3rd Asian conference on crop protection, Jogyakarta, 22-24.
- Trypanocidal activity of ergosterol peroxide from Pleurotus ostreatus. Phytother. Res.. 2012;26:938-943.
- [Google Scholar]
- Understanding agarwood formation and its challenges. Singapore: Agarwood. Springer; 2016. p. :39-56.
- Antifungal effects of sorbic acid and propionic acid at different pH and NaCl conditions. J. Food Saf.. 1999;19:109-120.
- [Google Scholar]
- Isovaleric acid reduces Na+, K+-ATPase activity in synaptic membranes from cerebral cortex of young rats. Cell. Mol. Neurobiol.. 2007;27:529-540.
- [Google Scholar]
- α-Cyclodextrin encapsulation enhances antimicrobial activity of cineole-rich essential oils from Australian species of Prostanthera (Lamiaceae) Nat. Volatiles Essent. Oils. 2015;2:30-38.
- [Google Scholar]
- An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem.. 2013;13:201-210.
- [Google Scholar]
- Gaharu producing tree induction technology. Develop. Gaharu Prod. Technol.. 2011;31
- [Google Scholar]
- Antiproliferative sesquiterpenes from the red sea soft coral Sarcophyton glaucum. Nat. Prod. Commun.. 2007;2:117.
- [Google Scholar]
- Chemometric analysis reveals links in the formation of fragrant bio-molecules during agarwood (Aquilaria malaccensis) and fungal interactions. Sci. Rep.. 2017;7:44406.
- [Google Scholar]
- Sesquiterpenes of agarwood from Gyrinops salicifolia. Fitoterapia. 2016;113:182-187.
- [Google Scholar]
- Study of an endophytic fungus from Aquilaria malaccensis Lamk. Bangladesh J. Pharmacol.. 2010;5:21-24.
- [Google Scholar]
- Conservation and sustainable use of tropical trees in the genus Aquilaria I. Status and distribution in Indonesia. Biol. Conserv.. 2000;96:83-94.
- [Google Scholar]
- Bactericidal effect of oleic acid on group A streptococci: mechanism of action. Infect. Immun.. 1979;26:1202-1210.
- [Google Scholar]
- Effect of thymidine on activity of trimethoprim and sulphamethoxazole. J. Clin. Pathol.. 1978;31:165-171.
- [Google Scholar]
- Agarwood-type resin from Gyrinops walla Gaertn: a new discovery. J. Trop. For. Environ.. 2012;2:43-48.
- [Google Scholar]
- Use of two fungal species to induce agarwood resin formation in Gyrinops walla. For. Res.. 2019;30:721-726.
- [Google Scholar]
- Sun, H., Zhang, Y.F., Huo, H.X., Guan, P.W., Wang, C.C., Yao, H.N., Zhao, Y.F., Tu, P.F., Li, J., 2019. Benzophenone glycosides from the pericarps of Aquilaria yunnanensis S. C. Huang. Natural Product Research, 1-8
- Determination and comparison of agarwood from different origins by comprehensive two-dimensional gas chromatography-quadrupole time-of-flight mass spectrometry. J. Sep. Sci.. 2020;43:1284-1296.
- [Google Scholar]
- Ergosterol peroxide, an apoptosis-inducing component isolated from Sarcodon aspratus (Berk.) S. Ito. Biosci. Biotech. Bioch.. 2005;69:212-215.
- [Google Scholar]
- Essential oil of eaglewood tree: a product of pathogenesis. Essent. Oil Res.. 2005;17:601-604.
- [Google Scholar]
- Agarwood induction: current developments and future perspectives. Front. Plant Sci.. 2019;10:122.
- [Google Scholar]
- Antifungal activity of 8-methoxynaphthalen-1-ol isolated from the endophytic fungus Diatrype palmicola MFLUCC 17–0313 against the plant pathogenic fungus Athelia rolfsii on tomatoes. PeerJ. 2020;8:e9103.
- [Google Scholar]
- A novel sesquiterpene alcohol from Fimetariella rabenhorstii, an endophytic fungus of Aquilaria sinensis. Nat. Prod. Commun.. 2011;6:763-766.
- [Google Scholar]
- Steroidal metabolites of Fimetariella rabenhorstii endophytic fungus from Aquilaria sinensis. J. Trop. Subtrop. Bot.. 2011;19:75-78.
- [Google Scholar]
- Impacts of biological, chemical and mechanical treatments on sesquiterpene content in stems of planted Aquilaria crassna trees. Agrofor. Syst.. 2015;89:973-981.
- [Google Scholar]
- Molecular identification of endophytic fungi from Aquilaria sinensis and artificial agarwood induced by pinholes-infusion technique. Biotechnol.. 2013;12(21):3115-3131.
- [Google Scholar]
- Volatile constituents of endophytic fungi isolated from Aquilaria sinensis with descriptions of two new species of Nemania. Life (Basel). 2021;11:363.
- [Google Scholar]
- Cytotoxicity and genotoxicity of zingiberene on different neuron cell lines in vitro. Cytotechnology. 2015;67:939-946.
- [Google Scholar]
- Development of agarwood induction technology using endophytic fungi. In: Mohamed R., ed. Agarwood: Science Behind the Fragrance. Singapore: Springer; 2016. p. :57-71.
- [Google Scholar]
- In vitro study of human lymphocytes cytological and biochemical effects by zingiberene. J. Essent. Oil Res.. 2014;26:367-371.
- [Google Scholar]
- UNEP-WCMC., 2022. The Checklist of CITES Species Website. CITES Secretariat, Geneva, Switzerland. Compiled by UNEP-WCMC, Cambridge, UK. Available at: http://checklist.cites.org
- Vidurangi, A.N.G.C.K., Subasinghe, S.M.C.U.P., Fernando, K.M.E.P., 2018. Isolation of associated fungal species in Aquilaria and Gyrinops species of family Thymelaeaceae. Proceedings of International Forestry and Environment Symposium, 22.
- Toxicity of octanoic acid in Saccharomyces cerevisiae at temperatures between 8.5 and 30 C. Enzyme Microb. Technol.. 1995;17:826-831.
- [Google Scholar]
- Lasiodiplodia spp. associated with Aquilaria crassna in Laos. Mycol. Prog.. 2019;18:683-701.
- [Google Scholar]
- Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules. 2018;23:342.
- [Google Scholar]
- Effects of 20 fungal species on compositions of essential oils from Aquilaria sinensis trees. J. South China Agric. Univ.. 2016;37:77-83.
- [Google Scholar]
- Antibacterial metabolites from the endophytic fungus R7 of Aquilaria sinensis. Chin. J. Antibiot.. 2011;36:576-580.
- [Google Scholar]
- β-sitosterol, one of functional component in phytosterols. J. Putian Univ.. 2007;2:38-40.
- [Google Scholar]
- A new isofuranonaphthalenone and benzopyrans from the endophytic fungus Nodulisporium sp. A4 from Aquilaria sinensis. Helv. Chim. Acta. 2010;93:920-924.
- [Google Scholar]
- Rapid authentication of agarwood by using liquid extraction surface analysis mass spectrometry (LESA-MS) Phytochem. Anal.. 2020;31:801-808.
- [Google Scholar]
- Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep.. 2016;6:21843.
- [Google Scholar]
- Identification of genes related to agarwood formation: transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genomics. 2013;14:227-242.
- [Google Scholar]
- Stomachic principles in ginger. II. Pungent and anti-ulcer effects of low polar constituents isolated from ginger, the dried rhizoma of Zingiber officinale Roscoe cultivated in Taiwan. the absolute stereostructure of a new diarylheptanoid. Yakugaku Zasshi: J. Pharma. Soc. Japan. 1992;112:645-655.
- [Google Scholar]
- Wood resources, identification, and utilization of agarwood in China. In: Mohamed R., ed. Agarwood: Science Behind the Fragrance. Singapore: Springer; 2016. p. :21-38.
- [Google Scholar]
- Extraction of agarwood (Aquilaria crassna) oil by using supercritical carbon dioxide extraction and enzyme pretreatment on hydrodistillation. Food Agric. Environ.. 2013;11:1055-1059.
- [Google Scholar]
- Effect of linoleic acid on endothelial cell inflammatory mediators. Metabolism. 1998;47:566-572.
- [Google Scholar]
- Essential oil alloaromadendrene from mixed-type Cinnamomum osmophloeum leaves prolongs the lifespan in Caenorhabditis elegans. J. Agricul. Food Chem.. 2014;62:6159-6165.
- [Google Scholar]
- The roles of methyl jasmonate to stress in plants. Func. Plant Biol.. 2018;46:197-212.
- [Google Scholar]
- Compositions and antifungal activities of essential oils from agarwood of Aquilaria sinensis (Lour.) Gilg induced by Lasiodiplodia theobromae (Pat.) Griffon & Maubl. J. Braz. Chem. Soc.. 2014;25:20-26.
- [Google Scholar]
- Characterization of the complete chloroplast genome of the vulnerable agarwood tree, Aquilaria yunnanensis (Thymelaeaceae) Conserv. Genet. Resour.. 2019;11:161-164.
- [Google Scholar]
- Isolation, identification and antimicrobial activity of endophytic fungi in Aquilaria sinensis (Lour.) Gilg. J. Microbiol.. 2009;29:6-10.
- [Google Scholar]
- Volatile oil constituents of two Acremonium endophyte isolates from Aquilaria sinensis. Microbiol.. 2009;36:37-40.
- [Google Scholar]
- Effects of novel anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine neurotransmitters in rats. Europ. J. Pharmacol.. 2004;504:39-44.
- [Google Scholar]
- Terpenoid biosynthesis and specialized vascular cells of conifer defense. Integr. Plant Biol.. 2010;52:86-97.
- [Google Scholar]







