Translate this page into:
Synthesis of ZnTiO3/tourmaline/Ni foam catalyst and enhanced photocatalytic performance
⁎Corresponding author. hsdhjaq@126.com (Jinlong Zuo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
ZnTiO3/tourmaline loaded on the nickel foam (ZnTiO3/tourmaline/Ni-foam) is prepared by a facile coating method. Morphology and structure of the photocatalyst were characterized by X-ray diffraction (XRD), scanning electrons microscopy (SEM), raman spectroscopy, UV–vis diffuse reflectance spectrum (UV–vis DRS) and photoluminescence spectroscopy (PL). The photocatalytic properties of the materials were tested by using the Rhodamine B (RhB) solution as the target pollutant. The results indicates that the ZnTiO3/tourmaline/Ni foam exhibited higher photocatalytic activity than that of ZnTiO3 and ZnTiO3/Ni foam under ultraviolet (UV) light irradiation, and its degradation rate was up to 99.2%. Moreover, the degradation rate remained at 91.3% after eight consecutive photocatalytic reaction cycles. The outstanding photocatalytic performances of ZnTiO3/tourmaline/Ni foam was mainly attributed to the existence of tourmaline, which can help to inhibit the recombination of electron-hole paris, and the proper pore structure of the carrier. Meanwhile, the trapping experiments indicated that ·O2– was the main active species in the photocatalytic degradation of RhB.
Keywords
ZnTiO3
Tourmaline
Ni foam
Photocatalysis
1 Introduction
Since Fujishima and Honda (Fujishima and Honda, 1972) reported TiO2 photoelectrode decomposed water into hydrogen and oxygen under ultraviolet light source, photocatalytic technology was considered to be one of the most promising methods for solving the two major problems of environmental pollution and energy shortage. The photocatalysis has the advantages of simple preparation process, low energy consumption, high photochemical stability and no secondary pollution. At present, various semiconductors based on photocatalysts (TiO2 (Al-Mamun et al., 2019), ZnO (Guan et al., 2019), CdS (Cui et al., 2017), Ag3PO4 (Shi et al., 2019), g-C3N4 (Prasad et al., 2020), etc.) have been widely used in environmental protection.
ZnTiO3 is a perovskite composite oxide of ABO3 type, which has the advantages of stable crystal structure, excellent electromagnetic properties and higher photocatalytic activity. It is a potential application object of photoluminescent materials (Li and Jiao, 2015), solar thermal reflective pigments (Lv et al., 2019), microwave dielectric materials (Yu et al., 2010), solar cells (Sahu et al., 2018), desulfurization adsorbents (Behl et al., 2012) and photocatalytic materials (Abirami et al., 2020; Li et al., 2019). Because of its good catalytic performance, ZnTiO3 has become one of the research hotspots in the photocatalytic field. However, there are some difficulties in the practical application of ZnTiO3 photocatalyst. First, it is difficult to separate photocatalytic powder material from suspension liquid after the reaction. Second, the high recombination rate of photogenerated electron-hole pairs of ZnTiO3 impacts the photocatalytic activity.
Ni foam has the characteristics of electronic conductivity, three-dimensional network structure, large specific surface area and strong adsorption capacity (Kamyabi and Moharramnezhad, 2020). It can be used as an excellent carrier to solve the difficult recovery of the catalyst. Wang et al. (Wang et al., 2018) fixed the 2D/2D structure of g-C3N4/rGO on the nickel foam by simple dip-coating and hydrazine hydrate reduction methods, which exhibited good photocatalytic activity for the degradation of tetracycline and methyl orange. Jia et al. (Jia et al., 2016) synthesized graphene/TiO2 loaded nickel foam using coating method; which showed excellent photocatalytic activity (85.1 %) and photo-electrocatalytic activity (99.8 %) under the irradiation of xenon lamp.
Photocatalytic performance of ZnTiO3 was improved by means of transition element doping, compounding and other modification methods (Eskandarloo et al., 2016; Acosta-Silva et al., 2016; Chen et al., 2019). Tourmaline, one extremely complicated complicated compositions, are acentric borosilicate crystallographically materials with the following chemical formula:XY3Z6[T6O18][BO3]3V3W; where X = Na, K, Ca, PB2+;Y = Li, Mg, Al, Fe2+, Fe3+, Mn2+, Mn3+, Cu2+, V3+, Cr3+, Ti4+; Z = Mg, Al, Fe2+, Fe3+, V3+, Cr3+; T = Si, B, Al; V = OH, O; W = OH–, F-,O2– (Guan et al., 2019).Tourmaline has unique characteristics of permanent spontaneous polarization effect, thermoelectricity, infrared radiation and good adsorption, and it is widely used in the fields of environment, health, and materials (Chen et al., 2019; Meng et al., 2021; Shi et al., 2018; Li et al., 2020). Under the irradiation of visible light, Tzeng (Tzeng et al., 2019) prepared tourmaline-nitrogen doped-TiO2 composite catalyst (S—N—TiO2) containing 4 wt% tourmaline, which had higher photocatalytic activity for ethylene oxidation than pure TiO2 and N-doped TiO2 (N-TiO2). In the photocatalytic degradation of 2-propanol (IPA), Yin (Yin et al., 2017) manufactured ternary composite material, the G0.5/T5/TiO2 with 0.5 wt% graphene and 5 wt% tourmaline had the highest acetone evolution rate (223 μmol/h). Yu (Yu et al., 2019) synthesized ZnO/tourmaline composite material. Compared with pure ZnO, the photocatalytic degradation activity of the composite material for methylene blue (MB) was significantly improved, and after five consecutive photocatalytic reaction cycles, the degradation rate remained at 90.8 %.
Nowadays, composite photocatalyst water purification is a common treatment method (Zhang et al., 2022; Chen et al., 2021; Zhang et al., 2015). Based on the above analysis, to improve the photocatalytic activity and solve the reclamation difficulty of ZnTiO3, we developed a novel ZnTiO3/tourmaline/Ni foam system. ZnTiO3/Ni foam/ tourmaline material as a new type of photocatalytic material has rarely been reported. In this study, ZnTiO3/Ni foam/ tourmaline material was prepared by adding tourmaline and template SiO2 on the basis of ZnTiO3/Ni foam prepared in the previous stage. According to the degradation rate of RhB to study the photocatalytic performance of the material. we mixed ZnTiO3, tourmaline, and pore-forming agent (SiO2) evenly, and then immobilized them onto the Ni foam through a simple coating approach to design and synthesize ZnTiO3/tourmaline/Ni foam photocatalytic material. So far, there have been few reports on this material. Subsequently, the morphology, structure, optical performance of the resulting materials were characterized by SEM, XRD, Raman, DRS and PL. ZnTiO3/tourmaline/Ni foam catalyst exhibited a superior photocatalytic activity and stability on photocatalytic degradation of RhB in aqueous solution. In addition, the photocatalysis mechanism were also investigated in detail.
2 Material and method
2.1 Reagent
Nickel foam (Kunshan Jiayisheng Electronics Co, ltd); Polytetrafluoroethylene(PTFE, 60 %, Cyber electrochemical material network); Silica (SiO2, Analysis Pure, Tianjin Zhiyuan Chemical Reagent Co, ltd); Tourmaline(10000 mesh, Lingshou Jingwei mineral products processing plant); Zinc dihydrate acetate(C4H6O4Zn·2H2O, Analysis Pure Tianjin BASF Chemical Co, ltd); Butyl titanate (Ti(OC4H9)4 Chemical purity Tianjin Guangfu Fine Chemical Research Institute); The experimental water was deionized water.
2.2 Experimental facility
The photocatalytic reaction device in the experiment is shown in Fig. 1.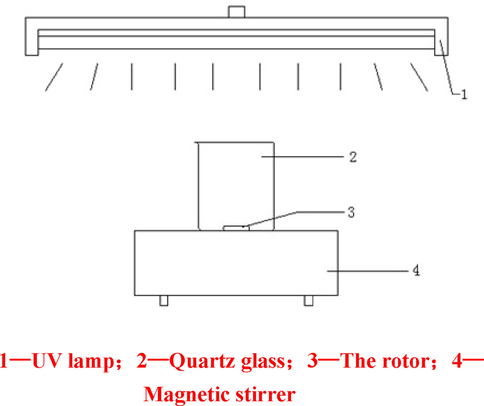
Schematic diagram of photocatalytic degradation.
2.3 Synthesis of ZnTiO3 powder
ZnTiO3 powder was prepared by the hydrothermal-assisted sol–gel method. Typically, 3.7044 g citric acid was dissolved in 20 mL anhydrous ethanol; 20 wt% Cetyltrimethylammonium bromide (CTAB) was added to the above solution and stirred magnetically until completely dissolved; 3 mL tetrabutyl titanate was added slowly under the stirring condition to obtain solution A. Then, 1.9347 g zinc acetate dihydrate was dissolved in 10 mL anhydrous ethanol, which was called as solution B. Under continuous magnetic stirring, solution B was added into solution A dropwise with a separating funnel, and controlled the dropping rate to 5 s/drop. The stirring rate was controlled to ensure that the solution did not generate bubbles or splash droplets. After about 1 h, the dripping was completed. The pH of the solution was adjusted to 3 with nitric acid (HNO3), and the mixed solution continued to be stirred for 3 h to obtain the transparent gel. It was transferred to the high-pressure reaction kettle and kept in the oven at 100 ℃ for 12 h. At last, the obtained white solid was washed with deionized water, calcined at 350 °C for 2 h then ground, and calcined at 800 ℃ for 5 h to obtain nano-ZnTiO3 powder. In addition, tetrabutyl titanate is chemically pure, the remaining drugs were of analytical grade.
2.4 Synthesis of ZnTiO3/tourmaline/Ni foam
Firstly, the Ni foam (2 cm × 5 cm) was pretreated, by soaking in anhydrous ethanol for 20 min. Secondly, the Ni foam was washed with deionized water and soaked in 0.1 mol/L hydrochloric acid solution for 10 min. Finally, ultrasonic cleaning with deionized water was used for 20 min and then dried at 80 ℃.
ZnTiO3, tourmaline and SiO2 prepared in the previous experiment were evenly mixed according to the mass ratio: 1:0.03:1.2, and then put into the agate mortar grinding for 10 min; Polytetrafluoron (PTFE) and anhydrous ethanol were mixed according to the volume ratio of 1:5, and then magnetic stirring for 4 min to form A uniform suspension A; Under the condition of magnetic stirring, put the ground ZnTiO3/tourmaline /SiO2 mixed powder into the above suspension A, and continue to stir for 5 min to form a uniform suspension B; The suspension B was evenly coated on the nickel foam (2 cm × 5 cm) sheet, and then placed in the drying box, drying at 80 ℃ for 4 h; After drying, the crude product was soaked in lmol/L NaOH solution, then removed, rinsed with deionized water to neutral (pH = 7), and then dried at 80℃ for 6 h, finally the foam supported ZnTiO3/tourmaline photocatalytic material. The synthetic route of samples was shown in Fig. 2.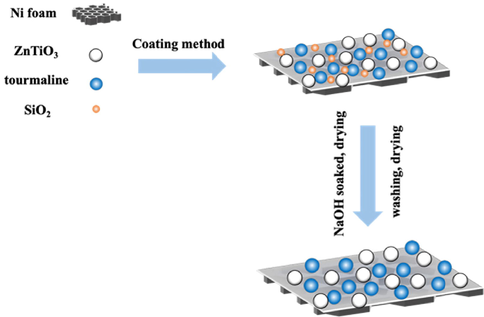
Schematic illustration of the ZnTiO3/tourmaline/Ni foam.
2.5 Characterization
The crystal structure identification of the samples was investigated by XRD with Cu-K α radiation (λ = 0.15418 nm). The morphology of the sample was observed through Supra55 scanning electron microscope (EHT = 15 KV). The HR800 confocal microscopic Raman spectrometer was used to characterize the samples’ structure with the laser wavelength of 785 nm. The Raman displacement range was 50–4000 cm−1; the wavenumber accuracy less than 0.1 cm−1 and the spectral resolution 1 cm−1. The absorbance of the sample was measured by UV-2550 ultraviolet/visible spectrophotometer and BaSO4 was used as the background ranging from 220 nm to 850 nm. The photoluminescencent properties were measured by the photoluminescence spectrometer (F-7000) with the excitation wavelength of 249 nm at room temperature.
2.6 Photocatalytic activity measurement
The photocatalytic experiments were carried out in a home-made reactor under the UV light source with 30 W. The above prepared catalysts were put in 100 mL aqueous solution of 5 mg/L RhB. Then the reaction was stirred for 30 min in the dark to reach the establishment of adsorption equilibrium. During the radiation process, 5 mL aliquots were alternately extracted and centrifuged per 30 min, and then analyzed by UV–vis spectrophotometer under 554 nm. The load amount of ZnTiO3 on nickel foam was 0.1g.
To explore the free radical groups that play a role in the photocatalytic reaction of the catalyst, 1 mmol/L ethylenediamine tetraacetic acid disodium salt (Na2EDTA), p-benzoquinone (C6H4O2, 99 %), and isopropanol [(CH3)2CHOH] were respectively added into the reaction solution as the trapping agents of hole (h+), superoxide free radical (·O2–), hydroxyl groups (·OH) capture agent, and the other experimental conditions were consistent with the above.
3 Results and discussion
3.1 Structure characterizations
Fig. 3(a) showed XRD patterns of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam. It was obvious to see the diffraction peak of ZnTiO3 (hexagonal phase, PDF 26–1500) and Ni foam (JCPDS 04–0850) (Zhang et al., 2022; Chen et al., 2021). Meanwhile, the diffraction peaks at 2θ of 22.4°, 26.6°, 30.3°, and 34.9° are corresponding to the diffraction facets of the tourmaline (Zhang et al., 2015). Because SiO2 was dissolved by NaOH, the addition of SiO2 had no influenced on the phase composition and crystal structure of catalysts. Futhermore, the addition of the nickel foam and tourmaline did not change the crystalline phase of ZnTiO3. And no other impurity peaks can be observed, it can be concluded that ZnTiO3/tourmaline/Ni foam was successfully prepared by the present synthesis conditions.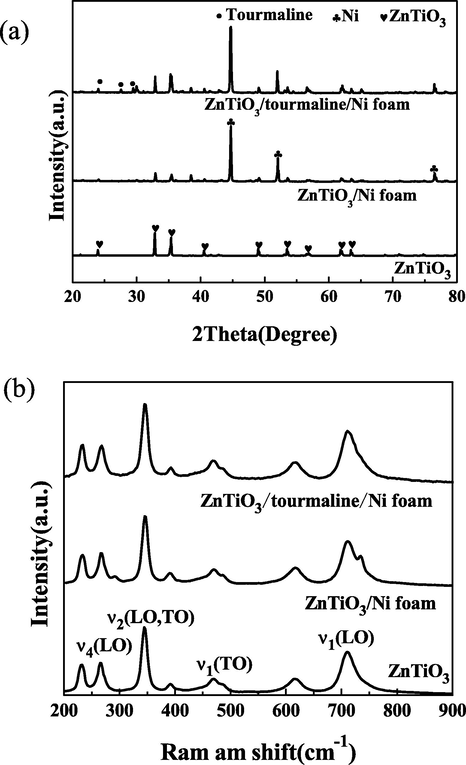
(a) XRD patterns, (b) Raman spectra of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam catalysts.
As seen in Fig. 3(b), the structure of samples was also investigated by Raman spectra. It can be observed that the Raman peaks of ZnTiO3 at 265 cm−1, 343 cm−1, 465 cm−1 and 710 cm−1 appear in the pure ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam, which correspond to four vibration modes of ν4(LO), ν2(LO, TO), ν1(TO), ν1(LO), respectively. And the characteristic peaks are consistent with the spectrum of ZnTiO3 materials synthesized by Surendar et al. (Surendar et al., 2014). The intensity of these characteristic peaks was slightly different, but the position did not change at all. It was further confirmed that the ZnTiO3 has been successfully loaded on the Ni foam. Besides, the addition of tourmaline did not change the characteristic peak of the catalyst, indicating that tourmaline had little influence on the crystal structure of ZnTiO3, which was consistent with the XRD analysis results.
The morphology of samples was observed by SEM, as shown in Fig. 4(a) displayed distinctly that ZnTiO3 powder was granular with similar spherical morphology and slight agglomeration, and the average particle size was about 50 nm. From Fig. 4(b), it was clearly evident that the Ni foam had interconnected three-dimensional porous network structure similar to a sponge and the skeleton is relatively smooth. Fig. 4(c) showed that the pores of the Ni foam network structure were covered by ZnTiO3 and tourmaline with a slight agglomeration. In Fig. 4(d), we can see the ZnTiO3 particles and tourmaline were well dispersed on the surface of nickel foam. Compared with Fig. 4(c), in the presence of the silicon dioxide, the particles were not tightly connected, but form accumulated pore structure (Chong et al., 2017). Fig. 4(e) showed the SEM image of ZnTiO3 supported by nickel foam at 500 times. It can be seen that most of the pores of nickel foam network structure were covered. Fig. 4(f) showed the SEM image of ZnTiO3/Ni after repeated utilization for 8 times at 50,000 times. It can be seen that ZnTiO3 particles and tourmaline were still well dispersed on the surface of nickel foam, with high stability.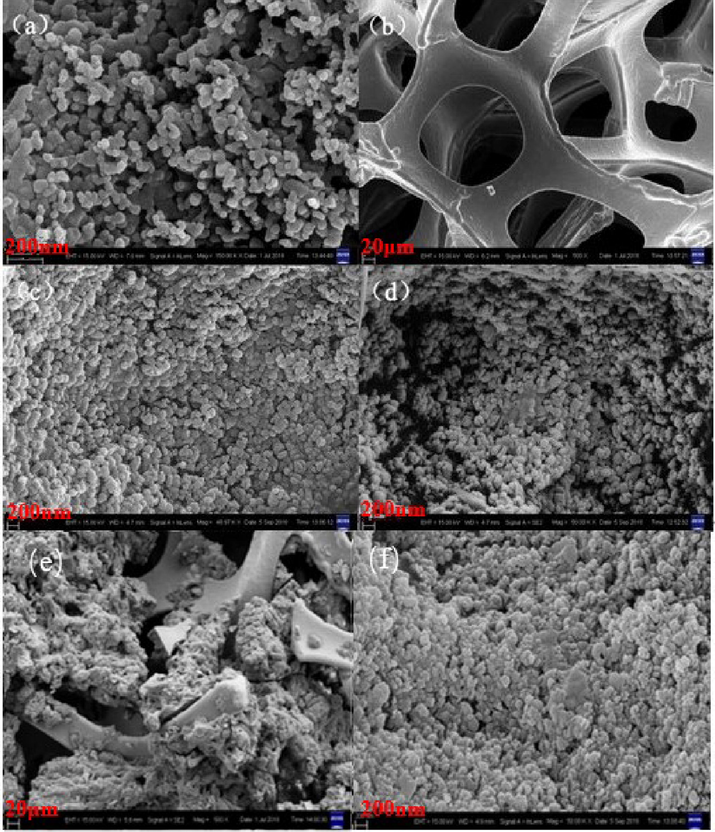
SEM images of (a) ZnTiO3 powder (b) Ni foam (c) ZnTiO3/tourmaline/Ni foam (without additon of SiO2) (d) ZnTiO3/tourmaline/Ni-foam (addition of SiO2 and after removal of SiO2 with NaOH) (e) ZnTiO3/Ni foam (f) ZnTiO3/tourmaline/Ni-foam (addition of SiO2 and after removal of SiO2 with NaOH) after eight cycles of use.
The optical properties of the samples were analyzed. As depicted in Fig. 5(a), ZnTiO3/tourmaline/Ni foam showed an obvious red-shift for an absorbance edge and stronger absorbance in the visible light range compared with ZnTiO3 and ZnTiO3/Ni foam, which could be ascribed to the introduction of tourmaline (Gayathri et al., 2014; Liu et al., 2013). To obtain the band-gap energy of the samples, the spectrum was replotted in Fig. 5(b). We plotted the plotting (αhυ)2 versus hυ, where α was the absorption coefficient and hυ was the photon energy. The band-gap energy of the samples can be estimated by extrapolating the linear portion of the plot at (αhυ)2 = 0. The estimated band-gap energy was 3.55 eV for ZnTiO3, the introduction of nickel foam and tourmaline can reduce the band gap of the composite to a certain extent. The reduction of the band-gap energy can enhance the light absorption, which was a crucial factor to improve the photocatalytic performance (Yin et al., 2017). Therefore, it could be concluded that ZnTiO3/tourmaline/Ni foam should reveal high photocatalytic activity.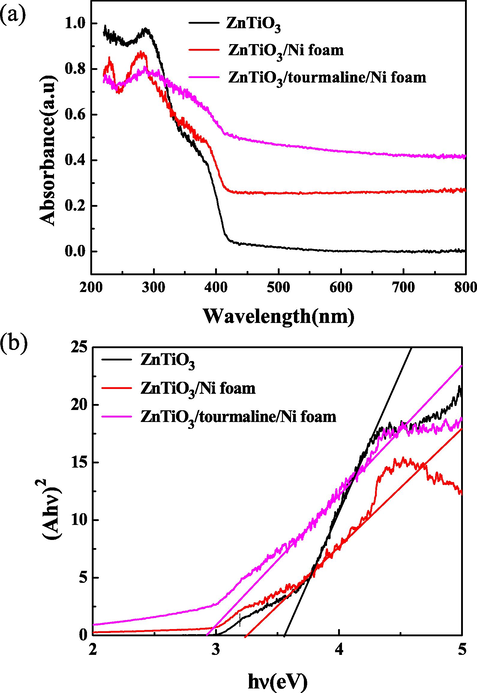
(a) UV–vis diffuse reflectance spectra, (b) Tauc plots of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam.
As can be seen from Fig. 6, the full XPS spectrum has the characteristic peaks of Zn, C, Ti, O, F, Ni and Si, among which the characteristic response peak of F comes from PTFE, the characteristic peak of Ni came from nickel foam and the characteristic peak of Si came from tourmaline. It indicated that the sample does not contain other impurities.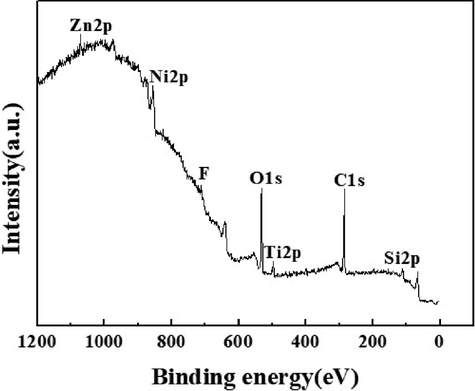
XPS spectra of ZnTiO3/Ni foam.
As known that, the peak intensity of PL spectroscopy reflects the recombination degree of the supports, and the weaker the peak is, the less the recombination degree does. Fig. 7 represents PL spectra under the excitation wavelength of 249 nm at room temperature. All synthesized products displayed the near band edge emission at 365 nm, which demonstrated the excitonic recombination of charge carriers. As shown in Fig. 7, the PL intensity was lower for ZnTiO3/Ni foam than that for ZnTiO3, demonstrating that the Ni foam could transfer electrons to decrease the recombination rate of the electrons and holes. More importantly, compared to that of ZnTiO3/Ni foam, the PL intensity of ZnTiO3/tourmaline/Ni foam was further decreased. This is mainly due to the fact that tourmaline had a spontaneous polarization electric field, which can inhibit the recombination of electrons-holes, causing electrons and holes to move directionally and reducing their recombination rate (Liu et al., 2022; Zhang et al., 2022).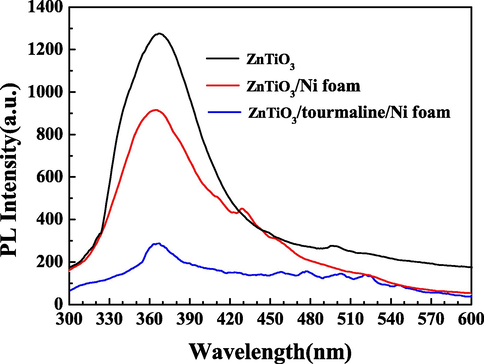
PL spectra of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam.
3.2 Photocatalytic activity
As can be seen from Fig. 8 the maximum degradation rate of tourmaline without doping is 94.1 %, and that of tourmaline with doping of 3 % was 99.2 %. The reaction rate increases when tourmaline is doped. After 1 h, 1.5 h and 2 h degradation, the degradation rates of RhB without tourmaline were respectively 66.0 %, 79.9 % and 88.2 %, while the degradation rates of RhB with 3 % tourmaline were respectively 88.1 %, 95.3 % and 97.7 %. The incorporation of tourmaline can improve the reaction rate because tourmaline had spontaneous permanent magnetism, which can inhibit the electron-hole recombination of the catalyst, thus improving the photocatalytic performance of the supported material (Jia et al., 2016).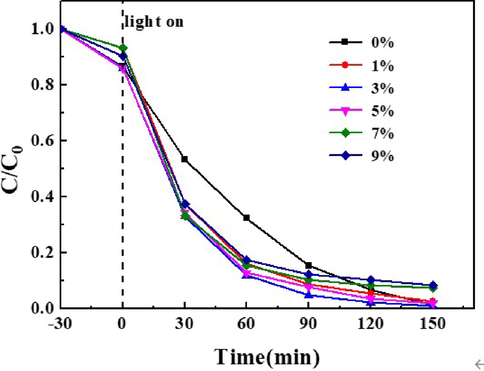
Effect of Hard Template Addition on Degradation of RhB.
The photocatalytic performance of ZnTiO3, ZnTiO3/Ni foam, and ZnTiO3/tourmaline/Ni foam on the degradation of RhB had been studied, as shown in Fig. 9. As expected, Ni foam/tourmaline did not degrade RhB. ZnTiO3/tourmaline/Ni foam exhibited the highest photocatalytic performance compared with ZnTiO3 and ZnTiO3/Ni foam. After 150 min irradiation, the photodegradation rate of ZnTiO3/tourmaline/Ni foam can achieve 99.2 %. The enhanced photocatalytic activity of ZnTiO3/tourmaline/Ni foam can be mainly attributed to the effect of tourmaline, which prevented the recombination of electron-hole pairs photogenerated in ZnTiO3 (Meng et al., 2006). Besides, Ni foam with good adsorption performance can absorb pollutants to its surface, which increased the reactive area between photocatalyst and organic pollutants (Hu et al., 2017). Therefore, the ZnTiO3/tourmaline/ Ni foam catalyst had the best photocatalytic performance.
Degradation of RhB under UV-light irradiation of ZnTiO3, ZnTiO3/Ni foam, ZnTiO3/tourmaline/Ni foam and tourmaline/Ni foam.
As can be seen from Fig. 10, the degradation rate of RhB under the photocatalysis of ZnTiO3/tourmaline/Ni foam was relatively high, and the relationship between -ln(C/C0) and the reaction time was basically linear, and the reaction process was combined with the quasi first-order kinetic equation. The rate constant k of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam on degradation rate and its regression correlation coefficient R2 are shown in Table 1. According to the kinetic rate constant k, the existence of nickel foam only plays the role of carrier, and does not even improve the dispersion, while the addition of tourmaline greatly improves the reaction rate (Chen et al., 2021).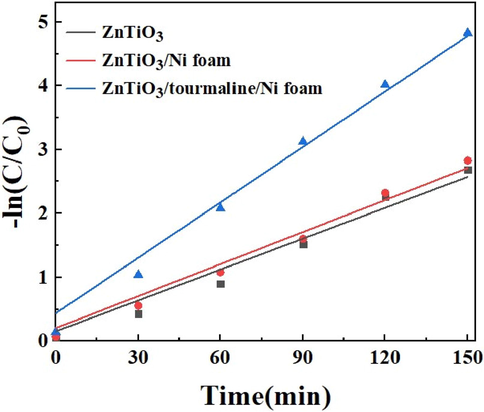
Effect of ZnTiO3, ZnTiO3/Ni foam and ZnTiO3/tourmaline/Ni foam on degradation rate.
material
k(min−1)
R2
ZnTiO3
0.0161
0.953
ZnTiO3/Ni foam
0.0167
0.970
ZnTiO3/tourmaline/Ni foam
0.0290
0.979
The stability of the ZnTiO3/tourmaline/Ni foam was also investigated, as shown in Fig. 11. It was observed that its degradation rate can still reach 91.3 % after eight cycles, suggesting the good stability of the ZnTiO3/tourmaline/Ni foam.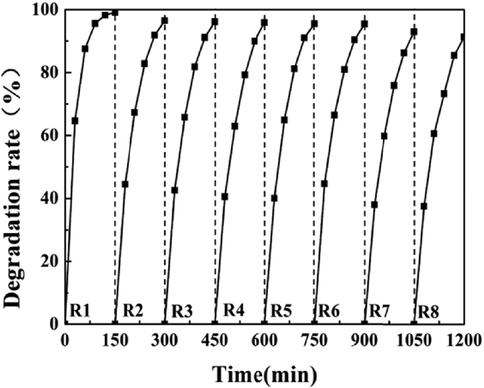
Recycling test on the ZnTiO3/tourmaline/Ni foam for the degradation of RhB.
To investigate the active species during the photocatalytic process, the radicals and holes trapping experiments of the ZnTiO3/tourmaline/Ni foam were further researched, as observed in Fig. 12(a). (CH3)2CHOH, used as ·OH scavenger. Na2EDTA, used as h + trapping. To investigate the role of ·O2–, C6H4O2 was added to the reaction system. The degradation efficiency of RhB decreased significantly when C6H4O2 was added, confirming that the ·O2– was the main reactive species. On the contrary, the photocatalytic degradation of RhB had a little affected by addition of Na2EDTA and (CH3)2CHOH. It can be concluded that ·O2– played an important role, followed by ·OH and h+ in the photocatalytic process of ZnTiO3/tourmaline/Ni foam in RhB solution under UV-light irradiation. Moreover, it can be seen from Fig. 12(b) that free radical degradation masking rates of ZnTiO3/tourmaline/Ni foam for (CH3)2CHOH, Na2EDTA, and C6H4O2 were respectively 14.1 %, 45.9 % and 70.5 %. Compared with ZnTiO3/Ni foam, the effect of h+ and ·O2– was obviously enhanced. After ZnTiO3 powder and tourmaline were loaded on the Ni foam, the band gap of the catalytic material was changed. The conduction band potential was greater than that of the required for generating ·O2–, which made more ·O2– generated, thus increasing the proportion of ·O2– in the photocatalytic reaction. In addition, due to the spontaneous polarization effect of tourmaline, it can reduce the recombination rate of electron-hole pairs, and electrons react with dissolved oxygen in the water to generate ·O2– (Huang et al., 2017).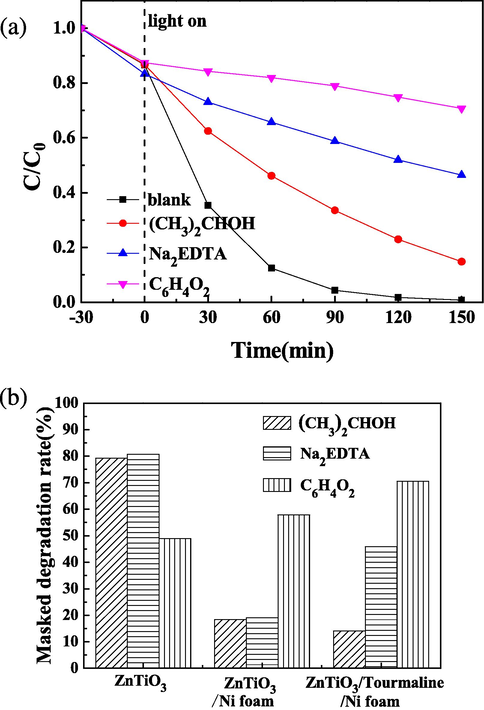
(a) trapping experiment of the active species, (b) free radical degradation masking rate of catalysts for the degradation of RhB.
Based on the above results, it was evident that the tourmaline had a significant influence on the ZnTiO3/tourmaline/Ni foam photocatalytic process. The possible reaction mechanism was proposed, as shown in Fig. 13. ZnTiO3 had narrow band gap energy (3.55 eV), Based on the Butler and Ginley model (Huang et al., 2017), the valence band (VB) and conduction band (CB) energies of ZnO were respectively −0.47 eV and + 3.08 eV, the valence band potential of ZnTiO3 materials (+3.08 eV) was higher than that of H2O/·OH (+2.72 eV), and the conduction band potential of ZnO materials (+2.98 eV) was higher than that of O2/·O2– (+2.72 eV). Therefore, h+ in the valence band of ZnTiO3 materials reacted with H2O to produce a large amount of ·OH. And the e- in the conduction band reacted with O2 to form ·O2–. Under UV-light irradiation, electrons in the valance band of ZnTiO3 could be excited up to higher potential edge and leave the holes on the ZnTiO3 valence band. Photogenerated electron-hole pairs could react with water to form strong oxidizing ·OH and ·O2– (Zhu and Zhou, 2019); and strong oxidizing ·OH and ·O2– can completely degrade the pollutants adsorbed on the catalyst surface into CO2 and H2O (Dutta et al., 2012). Due to the permanent electrical polarity of tourmaline, the photogenerated electrons and holes could be easily to move directionally (Yeredla and Xu, 2008; Yu et al., 2016). This process could effectively prevent the recombination between photogenerated electron-hole pairs and enhance the photocatalytic performance of catalyst.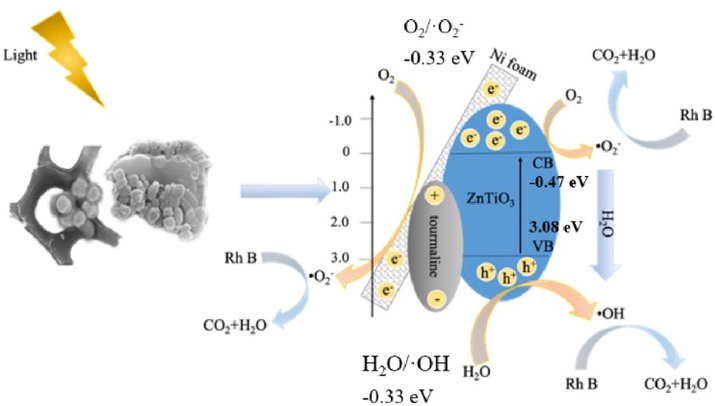
The possible photocatalytic mechanism of ZnTiO3/tourmaline/Ni foam.
4 Conclusions
In summary, ZnTiO3/tourmaline/Ni foam catalyst had been successfully prepared by a facile coating strategy, and applied to photocatalytic degradation of RhB. ZnTiO3/tourmaline/Ni foam exhibited higher photocatalytic performance compared with that of the ZnTiO3 or ZnTiO3/Ni foam. The enhanced photocatalytic activity of the ZnTiO3/tourmaline/Ni foam can be mainly ascribed to the effect of tourmaline, which prevented the recombination of electrons and holes photogenerated in ZnTiO3. A mechanistic study showed that ·O2– was the main reactive species in photocataytic degradation of RhB. As a result, ZnTiO3/tourmaline/Ni foam represented a new class of ZnTiO3 photocatalyst with improved photocatalytic performance and should be further investigated for wider applications. The results of this work also showed that the photocatalytic performance and difficult recovery of the catalyst powder can be effectively modified by introducing an external field and proper support via material design.
CRediT authorship contribution statement
Junsheng Li: Conceptualization, Data curation, Methodology, Writing – original draft, Resources, Validation, Project administration, Funding acquisition. Jialun Xu: Data curation, Methodology, Writing – review & editing. Meiyan Xu: Software. Tianyu Guan: Conceptualization. Zhi Xia: Software. Liming Jiang: Investigation. Chong Tan: Formal analysis. Jinlong Zuo: Supervision.
Acknowledgments
The authors would like to thank the support program for Young Innovative Talents in Colleges and Universities of Heilongjiang Province, grant number UNPYSCT- 2018136.
References
- J. Solid State Chem.. 2020;281:121019
- Superlattice. Microst.. 2016;100:148.
- J. Environ. Chem. Eng.. 2019;7(5):103248
- Nat. Nanotechnol.. 2012;7:810.
- Constr. Build. Mater.. 2019;205:137.
- Sci. Total Environ.. 2019;706:136026
- J Green Energy & Environment 2021:2468.
- Int. J. Hydrogen Energy. 2017;42:20703.
- Chem. Res. Chinese Universities. 2017;33:436.
- Dalton Trans.. 2012;41(34):10238.
- Ultrason. Sonochem.. 2016;29:258.
- Nature. 1972;238(5358):37.
- J. Appl. Phys.. 2014;115(17):173504
- Chem. Res. Chinese Universities. 2019;35:271.
- chem. Eng. J.. 2017;321:608.
- Solid State Sci.. 2017;64:62.
- Appl. Catal. A. 2016;525:128.
- Microchem. J.. 2020;154:104540
- New J. Chem.. 2019;43:3374.
- J. Rare Earths. 2015;33(3):23.
- Ceram. Int.. 2020;46:12637.
- Adv. Mater. Res.. 2013;800:464.
- Colloids Surf A Physicochem Eng Asp. 2022;641:128577
- Ceram. Int.. 2019;45(12):15768.
- Trans. Nonferrous Met. Soc. China 2006:16.
- Sustainable Energy Fuels. 2021;5:6460-6469.
- Int. J. Hydrogen Energy. 2020;45(1):337.
- Sol. Energy. 2018;163:338.
- J. Colloid Interface Sci.. 2018;519:1-10.
- Solid State Sci.. 2019;96:105967
- PCCP. 2014;16:728.
- J. Ind. Eng. Chem.. 2019;80:376.
- Mater. Res. Bull.. 2018;97:306.
- J. Phys. Chem. C. 2008;112(2):532.
- Catal.. 2017;38(8):1307.
- Mater. Lett.. 2019;240:161.
- J. Mol. Catal. A Chem.. 2016;411:1.
- Chem. Res. Chinese Universities 2010:26.
- RSC Adv.. 2015;5:55704.
- J. Taiwan Inst. Chem. Eng.. 2022;132:10411.
- Appl. Surf. Sci.. 2022;592:153337
- Environmental Nanotechnology, Monitoring & Management. 2019;12:100255







