Translate this page into:
Silicotungstic acid (H4SiW12O40): An efficient Keggin heteropoly acid catalyst for the synthesis of oxindole derivatives
⁎Tel./fax: +98 21 88041344. knikoofar@yahoo.com (Kobra Nikoofar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Condensation of various isatins with heteroaromatics (indoles and pyrrole) has been carried out in the presence of catalytic amount of silicotungstic acid (STA) at room temperature to form their corresponding 3,3-bis(indolyl)- and 3,3-bis(2-pyrrolyl)oxindoles. The mechanistic aspects of the reaction have also been discussed.
Keywords
Isatin
Indole
Oxindole
Keggin heteropoly acid
Silicotungstic acid
1 Introduction
Isatin (2,3-dioxindole) identified as an endogenous compound in humans that has a range of biological properties including actions in the brain and offering protection against certain type of infections (Pandeya et al., 2005). Various derivatives of isatin are key motifs of natural product structures for example Convolutamydine A is an alkaloid which is isolated from marine bryozoan amathia convoluta has exhibited potent activity in differentiation of HL-60 human promyelocytic leukemic cells (Kamano et al., 1995). A well-known category of isatins are oxindoles which possess anti-convulsant natures including antibacterial, anti-inflammatory, antiprotozoal and mechanism-specific antiproliferative properties and also patented as PR (progesterone receptors) agonists (Pajouhesh et al., 1983). They can also be used as laxatives (Garrido et al., 1975). Much attention has been devoted to the preparation of oxindole rings because their systems are the core structures of many pharmacological agents. For example, Spiro[indole-thiazolidinones] possess antifungal activities against pathogens (Dandia et al., 2006) and Spirotryprostatine B as a natural alkaloid, showed anti-mitotic properties to get it in great interest as an anti-cancer drug (Sebahar and Williams, 2000). Isatins possess a reactive carbonyl group at 3-position that readily made them good electrophilic candidates to undergo condensation reactions with indoles to form 3,3-bis(indolyl)oxindole derivatives. The synthesis of natural 3,3-bis(indolyl)oxindole (3a) was first reported by Seidel in 1950. 3,3-Bis(indolyl)indoline-2-ones (3,3-bis(indolyl)oxindoles) showed anti-cancer properties (Kamal et al., 2010). During recent years some methods have been reported for the condensation of some electron-rich heteroaromatics such as indoles and pyrroles with isatins using catalysts such as silica sulfuric acid (SSA) (Azizian et al., 2006), KAl(SO4)2.12H2O (Azizian et al., 2004), ceric ammonium nitrate (CAN) (Wang and Ji, 2006), phosphotungstic acid (H6P2W18O62) (Alimohammadi et al., 2008), montmorillonite K10 clay (Chakrabarty et al., 2005), KSF (Nikpasand et al., 2010), ionic liquids (Karimi et al., 2011 and Rad-Moghadam et al., 2010), FeCl3 (Kamal et al., 2010) and I2 (Paira et al., 2009) to prepare the corresponding, 3,3-bis(3-indolyl)- and, 3,3-bis(2-pyrrolyl)oxindoles. Some of these strategies have shortcomings such as cost, reaction time, operational parameters and yield. Due to these deficiencies and also the significance of this class of compounds, offering new and efficient methods for their synthesis is still in demand. Heteropoly acids (HPAs) have been pointed out lately as versatile, environmentally benign, water tolerate, stable and green heterogeneous and homogenous catalysts for a variety of organic reactions (Okuhara, 2002). They contain lewis and strong bronsted acidity (Nandhini et al., 2004). Solid HPAs have discrete ionic structures that contain heteropolyanion (polyoxometalate) units and countercations that are H+ (Kozhevnikov, 1998). This special structure exhibits high proton mobility while heteropolyanions stabilize cationic intermediates (Izumi et al., 1992). The best known HPAs are the Keggin type H8−nXM12O40, where X is he central atom, n is the oxidation state of X and M is the metal ion. In the Keggin type HPAs, protons take part in the formation of the crystal structure by linking the neighboring polyoxometalate units (Kozhevnikov, 1998). Also HPAs possess bronsted acidity, but found to be efficient in catalyzing some reactions that conventionally use lewis acids such as Friedel–Crafts alkylation (Kamakshi and Reddy, 2007). Silicotungstic acid H4W12SiO40 is one of the well-known Keggin types of HPAs. It has been used as an acid catalyst in some transformations such as bis(indolyl)methane synthesis (Rafiee et al., 2011), alkylation of benzene with olefins (Sawant and Halligudi, 2005), production of acrolein from glycerol (Tsukuda et al., 2007), indole Michael addition (Murugan et al., 2005) and 1,2-dihydroquinones (Kamakshi and Reddy, 2007). We wish now to report a new usage of silicotungstic acid as an impressive, inexpensive and easily handling acid catalyst for the synthesis of 3,3-bisoxindole derivatives via the condensation reaction of various isatins with indoles and pyrrole at room temperature.
2 Materials and methods
Isatins, indoles, pyrrole, STA and solvents were purchased from Merck, Aldrich and Alfa Aesar and used without further purification. N-Benzylisatin is synthesized from isatin according to the reported procedure (Azizian et al., 2003). Melting points were determined using a Stuart Scientific SMP2 capillary apparatus and are uncorrected. IR spectra were recorded from KBr disks on a Shimadzu IR-435. 1H NMR spectra were recorded with a Bruker drx 500 (500 MHz) machine. Mass spectra were obtained on a Platform II spectrometer from Micromass; EI mode at 70 eV. Preparative layer chromatography (PLC) was carried out on 20 × 20 cm2 plates, coated with a 1 mm layer of Merck silica gel PF254, prepared by applying the silica as slurry and drying in air.
2.1 General synthetic procedure for the synthesis of 3a–l, 6 and 7
To a solution of isatins 1a–e (1 mmol) and indoles 2a–d or 4 (2 mmol) or pyrrole 5 (3 mmol) in CH3OH (5 ml) silicotungstic acid (0.1 mmol) was added. The mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion, the solvent was evaporated and the resulting crude residue was purified by chromatography on silica gel (eluent: n-hexane–ethyl acetate, 1:1) to afford the pure products (Table 2 and Scheme 2).
Some selected data are as follows:
2.2 3,3-Bis(3-methylindolyl)-oxindole (6)
m.p. 255–258 °C, IR (KBr): υ = 3325 (NH), 1712 (CO), 1618, 1475, 1198, 676 (N-H) cm−1. 1H NMR (CDCl3): δ = 2.05 (s, 6H, 2CH3), 6.97 (d, J = 7.35 Hz, 1H, Ar-H), 7.07–7.35 (m, 9H, Ar-H), 7.55 (d, J = 7.55 Hz, 2H, Ar-H), 8.13 (br s, 2H, NH), 8.21 (br s, 1H, NH) ppm. MS m/z (%) 391 (M+, 18), 376 (M+-CH3, 13), 348 (M+-CO and NH, 15), 332 (M+-HCO and NH and CH3, 4), 261 (M+-3-methylindolyl, 20), 232 (M+-3-methylindolyl and CHO, 20), 130 (3-methylindolyl+, 100).
2.3 3,3-Bis(2-pyrrolyl)-oxindole (7)
m.p. 173–175 °C, IR (KBr): υ = 3351 (NH), 3261 (NH), 1710 (CO), 1618, 1462, 1078, 738 (N-H) cm−1. 1H NMR (CDCl3): δ = 6.02 (br s, 2H, Ar-H), 6.13–6.14 (m, 2H, Ar-H), 6.75 (s, 2H, NH), 6.94 (d, J = 7.74 Hz, 1H, Ar-H), 7.14 (t, J = 7.56 Hz, 1H, Ar-H), 7.28 (br s, 1H, NH), 7.29 (t, J = 7.88 Hz, 1H, Ar-H), 7.49 (d, J = 7.45 Hz, 1H, Ar-H), 8.67 (br s, 2H, NH) ppm. MS m/z (%) 263 (M+, 53), 220 (M+-CO and NH, 25), 153 (M+-HCO and NH and pyrrolyl, 17), 65 (pyrrolyl+, 100).
3 Results and discussion
To investigate the solvent nature on the reaction rate, the model condensation of indole and isatin was performed in various solvents and conditions. According to the data presented in Table 1, it seems that the polarity of the solvent is necessary, as the reaction in n-Hexane, a non-polar solvent candidate, did not progress (entry 1). Using polar solvents like CHCl3, EtOAc, MeOH and EtOH, the model reaction promoted obviously. These observations lead that the solubility of the substrates is essential in reaction promotion. The polarity, dipole moment, and hydrogen bonding of a solvent are determining factors for the solvation of a substrate. Methanol as a polar protic solvent with the highest dielectric constant and dipole moment can solve indole and isatin almost completely. So the best result is gained in MeOH (entry 4). Temperature did not affect the results (entry 6). STA is also solvable in almost polar protic solvents. But this factor is not as important as a substrate solvation .This assumption is confirmed by adding H2O to EtOH that did not promote the reaction more than as utilizing the polar organic solvent lonely (entry 7). It is parallel to this fact that STA as a heteropolyacid can apply its catalytic influence both heterogeneously and homogeneously. So, methanol at room temperature has been chosen as the best condition for this purpose.
Entry
Solvent
Condition
Yield (%)a (Time (min)b)
1
n-Hexane
RT
10 (70)
2
CHCl3
RT
35 (70)
3
EtOAc
RT
40 (70)
4
MeOH
RT
96 (10)
5
–
70 °C
70 (50)
6
MeOH
50 °C
96 (10)
7
EtOH/H2O (4:1)
RT
80 (30)
8
CH3CN
RT
85 (30)
Using the reaction of isatin with indole in methanol as test experiment, the consumption of catalyst amount has been optimized. Due to Fig. 1 using 10 mol% (0.1 mmol) of STA showed the best result.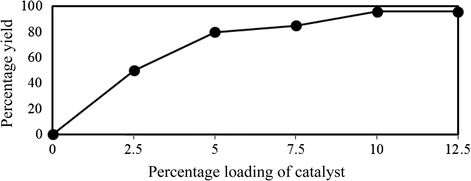
Optimization of catalyst amount.
Under optimized reaction conditions, the reactions of various isatins 1(a–e) with indoles 2(a–d) were carried out until maximum progression of the reactions (Scheme 1). The results are given in Table 2.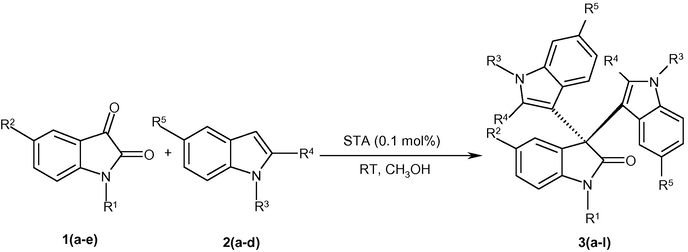
Synthesis of 3,3-bis(indolyl)oxindoles.
Producta
R1
R2
R3
R4
R5
Yield (%)b (Time (min))
M.P. (°C)
Found
Reported
3a
H
H
H
H
H
96 (10)
313–315
312–314 (Bergman and Eklund, 1980)
3b
H
H
CH3
H
H
94 (70)
329–331
330–332 (Bergman and Eklund, 1980)
3c
H
H
H
CH3
H
96 (10)
295–297
300–303 (Karimi et al., 2011)
3d
H
H
H
H
Br
90 (45)
300–302
298–300 (Nikpasand et al., 2010)
3e
CH3
H
H
H
H
95 (40)
287–289
291–293 (Alimohammadi et al., 2008)
3f
CH3
H
H
CH3
H
95 (10)
266–268
271–273 (Karimi et al., 2011)
3g
PhCH2
H
H
H
H
85 (50)
282–284
288–289 (Alimohammadi et al., 2008)
3h
PhCH2
H
H
CH3
H
95 (15)
208–210
211–213 (Alimohammadi et al., 2008)
3i
H
Br
H
H
H
90 (45)
312–314
310–311 (Azizian et al., 2006)
3j
H
Br
CH3
H
H
95 (15)
297–299
>300 (Nikpasand et al., 2010)
3k
H
NO2
H
H
H
95 (15)
300–301
298–299 (Azizian et al., 2006)
3l
H
NO2
CH3
H
H
96 (5)
312–314
>300 (Rad-Moghadam et al., 2010)
As can be seen a wide range of electron-rich and electron-deficient indoles and isatins performed the reaction. The summarized results confirmed the faster reaction of 2-methylindole rather than 1-methylindole with isatin (entries 3b and 3c). The study also revealed the faster reaction of 2-methylindole with electron-donating isatins in comparison with indole (entries 3e–3h). All the products were characterized by recording IR, 1HNMR and MS spectra and also by comparison of their spectral data with those of authentic samples. Under the reaction conditions, skatole (3-methylindole) 4 and pyrrole 5 underwent the condensation with isatin to form their corresponding 3,3-bis(2-indolyl)oxindole (6) and 3,3-bis(2-pyrrolyl)oxindole (7) respectively (Scheme 2). These results revealed the efficacy of the catalyst, as it can condense other heterocycles rather than indoles with isatin. The condensation of 3-methylindole at its 2-position, where its 3-moeity is blocked, with isatin, is another highlighted point in protocol feasibility.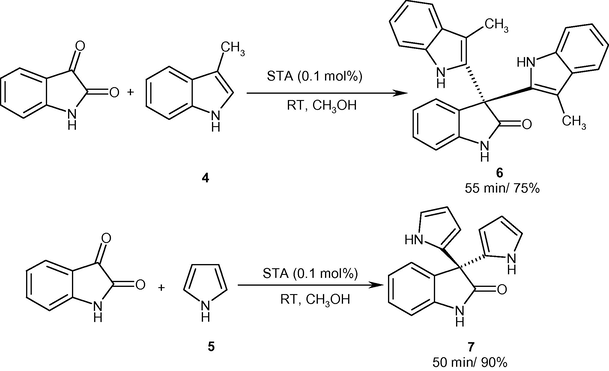
Synthesis of 3,3-bis(2-indolyl)oxindole and 3,3-bis(2-pyrrolyl)oxinole.
On the basis of forgoing results we reported a proposed mechanism for this condensation with the involvement of H4SiW12O40 in the rate determining step (Scheme 3):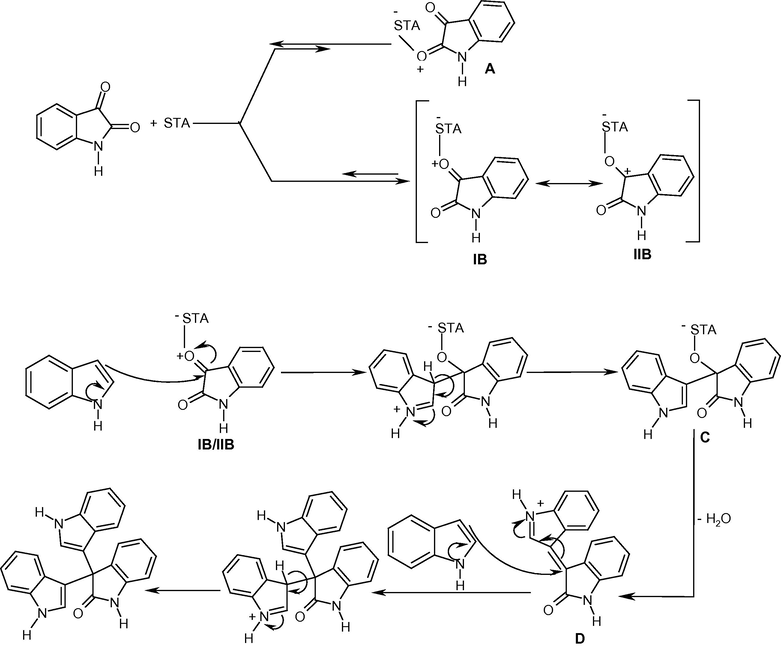
Proposed mechanism of the reaction.
Due to conjugation of the nitrogen lone pair with the carbonyl group at 2-position, this moiety is uncreative and the complexation of STA returns to the substrates (Scheme 3, A). The attack of STA as a solid acid to the reactive ketonic carbonyl group of isatin at 3-position, forms B which is stabilized by resonance structures IB–IIB. Nucleophilic attack of indole to this structure gave the intermediate C that dehydrated to D. Subsequent nucleophilic addition of another indole to D forms 3,3-bis(indolyl)oxindole as the main product.
4 Conclusion
In this report we have demonstrated the application of silicotungstic acid (STA) as a very effective, eco-friendly and inexpensive commercial-available catalyst in the synthesis of symmetrical 3,3-bis(heteroaryl)oxindoles at room temperature. Simple experimental procedure associated with high yield, short reaction time makes this protocol interesting for organic chemists.
Acknowledgment
The author acknowledges the financial support from the Research Council of Alzahra University.
References
- Monatsh. Chem.. 2008;139:1037.
- Synth. Commun. 2003:789.
- J. Chem. Res. 2004:424.
- Catal. Commun.. 2006;7:752.
- Tetrahedron. 1980;36:1445.
- J. Chem. Res. 2005:540.
- Bioorg. Med. Chem.. 2006;14:2409.
- Eur. J. Med. Chem.. 1975;10:143.
- Zeolite, Clay and Heteropolyacid in Organic Reactions. Tokyo: Kodansha/VCH; 1992. p. 99
- Catal. Commun.. 2007;8:825.
- Bioorg. Med. Chem.. 2010;20:5229.
- Tetrahedron Lett.. 1995;36:2783.
- Chin. J. Chem.. 2011;29:321.
- Chem. Rev. 1998:171.
- Tetrahedron. 2005;61:12275.
- J. Mol. Catal. A: Chem.. 2004;223:201.
- Synth. Commun.. 2010;40:5352.
- Chem. Rev.. 2002;102:3641.
- Bioorg. Med. Chem.. 2009;19:4786.
- J. Pharm. Sci.. 1983;72:318.
- Acta Pharm.. 2005;55:27.
- Tetrahedron. 2010;66:2316.
- Synth. Commun.. 2011;41:459.
- J. Mol. Catal. A: Chem.. 2005;237:137.
- J. Am. Chem. Soc.. 2000;122:5666.
- Chem. Ber.. 1950;83:20.
- Catal. Commun.. 2007;8:1349.
- Tetrahedron. 2006;62:1527.







