Translate this page into:
Effect of copper source on the structure–activity of CuAl2O4 spinel catalysts for CO hydrogenation
⁎Corresponding author. gaozhihua@tyut.edu.cn (Zhihua Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Due to the complexity of the structure–activity relationship of the CuAl2O4 spinel catalyst, optimization of the catalyst structure is a great challenge. In this paper, three different CuAl2O4 spinel catalysts were prepared by the solid-phase method using copper hydroxide, copper nitrate, and copper oxide as the copper source, respectively, to study the difference in the structure of CuAl2O4 spinel catalysts induced by the raw materials and the catalytic behavior for CO hydrogenation. The structure of CuAl2O4 spinel catalyst was characterized by XRD, BET, SEM, TEM, H2-TPR and XPS. The activity of CO hydrogenation over the CuAl2O4 spinel catalyst without pre-reduction was evaluated in the slurry reactor. The results demonstrated that different copper sources had obvious influence on the CuAl2O4 spinel texture properties, surface enrichment degree, as well as decomposition and reduction ability, which further regulated the ratio of Cu+/Cu0 and thus affected the catalytic performance, especially the alcohol distribution. The CuAl2O4 spinel, employing copper hydroxide as the copper source, showed better selectivity of C2+OH, which was assigned to a higher ratio of Cu+/Cu0, along with larger pore size and pore volume. Moreover, the synergistic effect between Cu0 and γ-Al2O3 improved the selectivity of dimethyl ether.
Keywords
CuAl2O4
CO hydrogenation
Slurry reactor
Catalyst
Higher alcohols
Dimethyl ether
1 Introduction
CuAl2O4 spinel has a face-centered cubic crystal structure, in which Cu2+ is occupied a tetrahedral position and is stable in the oxide lattice structure (Sickafus et al., 1999, Yong et al., 2013, Wang et al., 2017). The active Cu species with high dispersion were gradually released from CuAl2O4 spinel during the reaction, thereby avoiding the agglomeration and rapid sintering of Cu species (Maiti et al., 2016, Wan et al., 2016, Li et al., 2018). Catalytic conversion of C1 molecules is a vitally important process because of its close correlation to energy and environmental implications (Liu et al., 2020a, Altass et al., 2021, Salama et al., 2021a, 2021b, Altass et al., 2022). It was reported that CuAl2O4 spinel catalyst showed excellent catalytic performance in C1 catalysis, such as methanol steam reforming (Xi et al., 2014, Liu et al., 2022a, 2022b), reverse water gas shift (Bahmanpour et al., 2019), and CO oxidation reaction (Severino et al., 1998).
Our group (Huang et al., 2011, Liu et al., 2020b, Liu et al., 2022a, 2022b) had been engaged in the research of CO hydrogenation in the slurry reactor over the Cu-Zn-Al slurry catalyst for more than two decades, which was prepared by our independently developed catalyst preparation technology-complete liquid-phase method. The novelty of this method was that the catalyst precursor was directly heat-treated in an inert medium to obtain the slurry without conventional drying and calcination procedures. So-prepared Cu-Zn-Al slurry catalyst showed excellent activity and stability for CO hydrogenation to higher alcohols (C2+OH) or dimethyl ether. In our previous work, Deng et al. (Deng et al., 2019) explored the influence of heat treatment pressure on the catalytic performance in CO hydrogenation over Cu-Zn-Al slurry catalyst. Compared with the constant pressure, the catalyst heat-treated under 1.0 MPa exhibited excellent catalytic performance with the higher alcohols mass fraction in total alcohols, increased from 25.8 % to 65.9 %, which was ascribed to the formation of CuAl2O4 spinel phase. But it was difficult to control the CuAl2O4 spinel structure in Cu-Zn-Al slurry catalysts through the complete liquid-phase method. To further investigate the structure change and catalytic performance of CuAl2O4 spinel, Yan et al. (Yan et al., 2021) in our group designed to fabricate CuAl2O4 spinel via traditional preparation methods (such as solid-phase method, sol–gel method, citric acid method, and co-precipitation method), and the spinel was directly added into the slurry reactor for CO hydrogenation without reduction. The results disclosed that the CuAl2O4 spinel obtained by the solid-phase method favored the production of higher alcohols.
Our former study found that the preparation method had a remark influence on the structure and catalytic performance of CuAl2O4 spinel. But the specific relationship between the CuAl2O4 structure and the activity for CO hydrogenation remained unclear. It was therefore of supreme importance to tune the structure of CuAl2O4 spinel. As we known, the synthetic method was one factor to affect catalyst structure (Alshorifi et al., 2021, El-Hakam et al., 2022). Besides, CuAl2O4 spinel synthesis parameters, including Cu/Al molar ratio, calcination atmosphere, and introducing the third components, showed significant influence on its structure (Liu et al., 2020c, Liu et al., 2022c). So, in this paper, the CuAl2O4 spinel catalysts were synthesized by the solid-phase method employing copper hydroxide, copper nitrate, and copper oxide as the copper source, respectively. We attempted to explore the difference in the structure of CuAl2O4 spinel induced by the copper sources and the catalytic behavior for CO hydrogenation and to develop the structure–activity relationship.
2 Experimental
2.1 Materials
Cu(NO3)2·3H2O, liquid paraffin and petroleum ether were obtained from Tianjin Kemiou Chemical Reagent Co., ltd., China. Pseudo-boehmite, CuO and Cu(OH)2 were purchased from Shanghai Aladdin Biochemical Technology Co., ltd., China. All chemical reagents were analytical grade and used without further purification. CO, H2, N2, Ar and He, with purities of 99.99 %, were provided by the Product of Taiyuan Fujiang Special Gas Co., ltd., China.
2.2 Catalyst preparation
The Cu and Al raw materials with Cu/Al = 1/2(molar ratio) were well mixed and ball-milled by a planetary ball mill for 6 h. The powder was calcined in the air atmosphere at 900 ℃ for 3 h. The aluminum source was pseudo-boehmite. Copper hydroxide, copper nitrate, and copper oxide were selected as the copper source, respectively. The corresponding CuAl2O4 catalysts were denoted as So-OH, So-NO, and So-O, respectively.
2.3 Catalyst characterization
Before characterization, the CuAl2O4 spinel slurry catalyst after the reaction was centrifuged, extracted with petroleum ether for 4 days to remove the residual liquid paraffin, and then naturally dried to obtain a solid sample.
The crystal phase structure was characterized by DX-2700 X-ray diffraction apparatus (Dandong Fangyuan Instrument Co., ltd.) with Cu Kα source (λ = 0.1546 nm) with voltage and current at 40 kV and 30 mA, respectively. The sample was scanned over the range of 5–85° at a scanning rate of 8°/min to identify the crystalline structure. The crystallite size of CuAl2O4 spinel catalyst was calculated using the Scherrer formula.
The texture parameter was measured by a QDS-30 physical adsorption instrument (Quantachrome Company, USA) using N2 as adsorbate at −196 ℃. Prior to the test, the sample was pretreated under vacuum at 200 ℃ for 4 h to remove the adsorbed impurities. The specific surface area was determined by the BET (Brunauer-Emmett-Teller) method, and the most probable pore size and pore volume were obtained by the BJH (Barret-Joyner-Halenda) method.
Scanning electron microscopy (SEM) was performed using JSM-6010PLUS/LV emission electron microscope. The samples were observed at an accelerating voltage of 20 kV and sputter-coated for 120 s in a sputter coated fitted with Au before testing.
The transmission electron microscope (TEM) image was determined by the JEM-2100 instrument (Japan JEOL company) with an accelerating voltage of 300 kV. Samples were dispersed in alcohol in an ultrasonic bath, and a drop of the supernatant suspension was poured onto a holey carbon-coated grid and dried completely before the measurements.
Hydrogen temperature-programmed reduction (H2-TPR) was measured in a chemisorption instrument (TP-5080, Tianjin Xianquan Corporation). 50 mg catalyst was pre-heated at 150 ℃ for 0.5 h under He flow and then cooled to 50 ℃. Afterwards, the reduction process was recorded with the temperature ramping up from 50 ℃ to 800 ℃ at 10 ℃·min−1 under a 5 % H2/N2 flow of 30 mL·min−1. The hydrogen consumption signal was recorded using a thermal conductivity detector (TCD).
X-ray photoelectron spectroscopy (XPS) data was performed on an ESCALAB 250Xi spectrometer (American Thermo Fischer Company) equipped with Al Kα radiation (hν = 1486.6 eV). The binding energies of Cu 2p and CuLMM were calibrated by the C 1 s peak (BE = 284.8 eV).
2.4 Catalyst activity evaluation
CO hydrogenation over different CuAl2O4 spinel catalysts was evaluated in a 500 mL slurry reactor with a continuous mechanical agitator. Firstly, the prepared CuAl2O4 spinel was uniformly dispersed in 300 mL of liquid paraffin (solid holdup 10 %), and did not need to be reduced. Then, the reaction was carried out by feeding syngas (H2/CO = 2) at a flow rate of 150 mL·min−1. The reaction pressure and temperature were 4 MPa and 280 ℃, respectively. The steady-state activity measurement was taken after at least 24 h on the stream, and the reaction was continuously evaluated for six days. The H2, CO and CO2 in the gas phase products were detected by thermal conductivity detectors (TCD, TDX-01). Hydrocarbons, alcohols and dimethyl ether were detected by a flame ionization detector (FID, HP-PLOT/Q). The liquid phase product was condensed and collected daily, and analyzed by the FID detector. The carbon balance (
) was determined according to Eq. (1). The CO conversion (XCO) and product selectivity (Si) were calculated after carbon balance according to Eqs. (2) and (3), respectively.
Where ( ) was the carbon balance; XCO was the conversion of CO (C-mol%); Si was the selectivity of component i (C-mol%); i was all C-containing compounds except CO in the gas and liquid products; n(CO)in was the amount of CO in the inlet gas(mol); n(CO)out was the amount of CO in the outlet gas(mol); ni(g) and ni(l) was the carbon atom of component i in the outlet gas(mol) and liquid phase product(mol), respectively.
3 Results and discussion
3.1 XRD characterization
Fig. 1 showed the XRD patterns of different CuAl2O4 spinels. Before the reaction (Fig. 1a), the apparent diffraction peaks were observed in all catalysts at 2θ = 31.29°, 36.87°, 44.84°, 55.70°, 59.40°, 65.29°, 74.18° and 77.41°, which were attributed to CuAl2O4 spinel (PDF No.71–0967). Moreover, So-NO and So-O catalysts showed CuO diffraction peaks at 2θ = 35.72° and 38.96° (PDF No.48–1548). The appearance of CuO was ascribed to the fact that part of Cu2+ did not enter into the interstices of spinel and existed as free CuO. As for the So-OH catalyst, no CuO diffraction peaks appeared, and the diffraction peaks intensity of CuAl2O4 spinel was the weakest among the three catalysts. It was indicated that copper hydroxide as the copper source was conducive to the oxidation of copper hydroxide and pseudo-boehmite during the process of calcination, and then the corresponding oxides formed CuAl2O4 spinel with higher purity through the solid–solid interaction (Luo et al., 2005, Xi et al., 2013). The average crystallite sizes of CuAl2O4 spinel catalysts before the reaction, calculated by the Scherrer equation, were listed in Table 1. It can be founded that the change of copper source had no obvious effect on the crystallite size of CuAl2O4 spinel catalysts.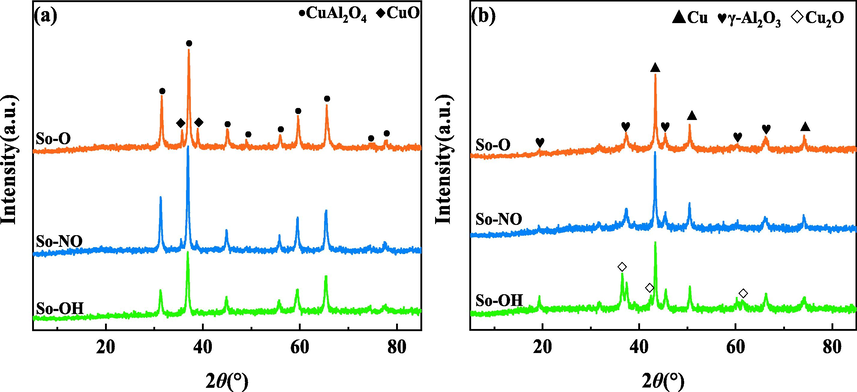
XRD patterns of different CuAl2O4 spinel before (a) and after (b) the reaction.
Sample
Before reactiona
After reactiona
dCuAl2O4(nm)b
SBET
VP
DP
SBET
VP
DP
So-OH
19.2
0.15
30.3
20.8
0.12
30.2
20.6
So-NO
18.7
0.07
9.5
19.2
0.06
9.6
23.6
So-O
31.0
0.10
9.5
33.0
0.09
7.8
22.6
After the reaction (Fig. 1b), the characteristic diffraction peaks of CuAl2O4 disappeared, whereas that of Cu0 and γ-Al2O3 appeared, indicating that the CuAl2O4 was decomposed and reduced to low valence copper species and γ-Al2O3 during the CO hydrogenation reaction at 280 ℃ and 4 MPa (Feng et al., 2009, Arjmand et al., 2012). It was worth noting that only the So-OH catalyst showed obvious Cu2O diffraction peaks at 2θ = 36.52°, 42.42°, and 61.55° (PDF No.05–0667), suggesting that the different copper sources influenced the decomposition and reduction properties of CuAl2O4 spinel catalyst.
3.2 N2 adsorption–desorption characterization
Fig. 2 displayed the N2 adsorption–desorption curves and pore size distribution of CuAl2O4 spinel before and after the reaction. The existence of a typical IV isotherm indicated that the catalysts preserved a uniform mesoporous structure (Salama et al., 2021a, 2021b). However, the shapes of hysteresis loops were somewhat different, implying various porous structures. The isotherm of the So-OH catalyst displayed H3-type hysteresis loop, indicating the existence of slit pores. In contrast, hysteresis loops for So-NO and So-O catalysts were H4-type, which was attributed to the narrow fissure hole. As presented in Table 1, The specific surface area of the catalysts decreased in the following order: So—O > So—OH > So—NO. The pore volume and the most probable pore size of the So-OH catalyst were maximum, reaching 0.15 cm3·g−1 and 30.3 nm, respectively, whereas those of So-NO and So-O catalysts were similar, and their most probable pore size was only 1/3 of So-OH. The results showed that copper sources had a significant impact on the texture properties. After the reaction, the texture properties of all catalysts remained almost unchanged, indicating that the pore structure of catalysts was steady during the reaction.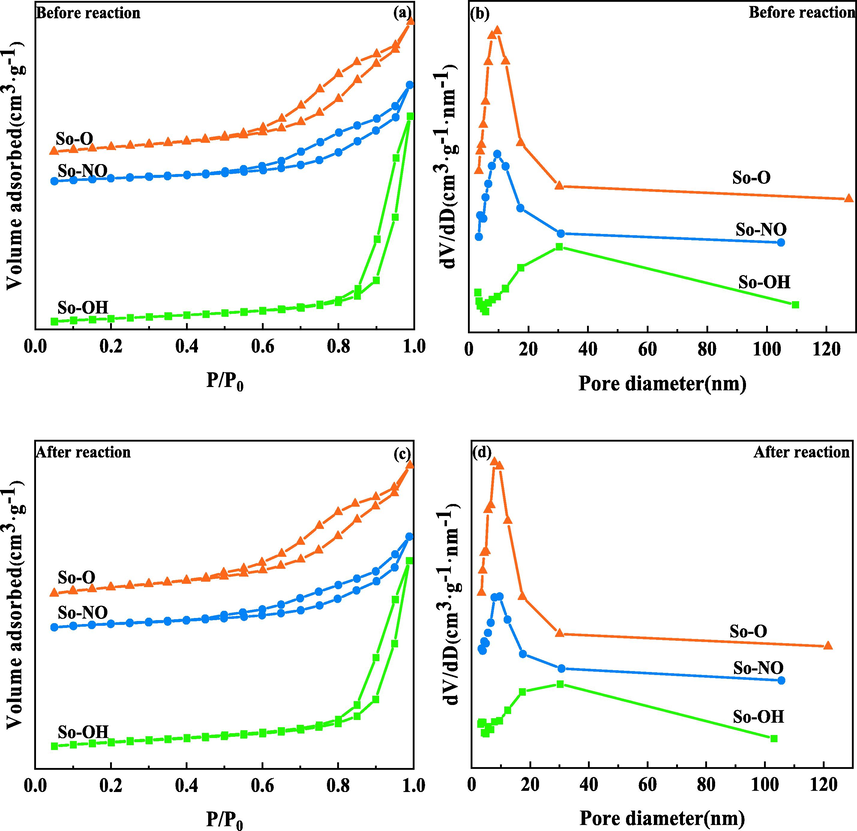
N2 absorption–desorption curves and pore size distribution of CuAl2O4 spinel before (a, b) and after (c, d) the reaction.
3.3 SEM characterization
Fig. 3 showed SEM images of different CuAl2O4 spinel catalysts before the reaction. It can be seen that the dispersion of the three catalysts was significantly different. The So—OH catalyst was homogeneously distributed with slightly agglomerated, and the particle size was mainly less than 3 μm. The agglomeration of the So—NO catalyst was severe with large particles (>10 μm). The So—O catalyst was homogeneous and uniform with small particles (≤3 μm).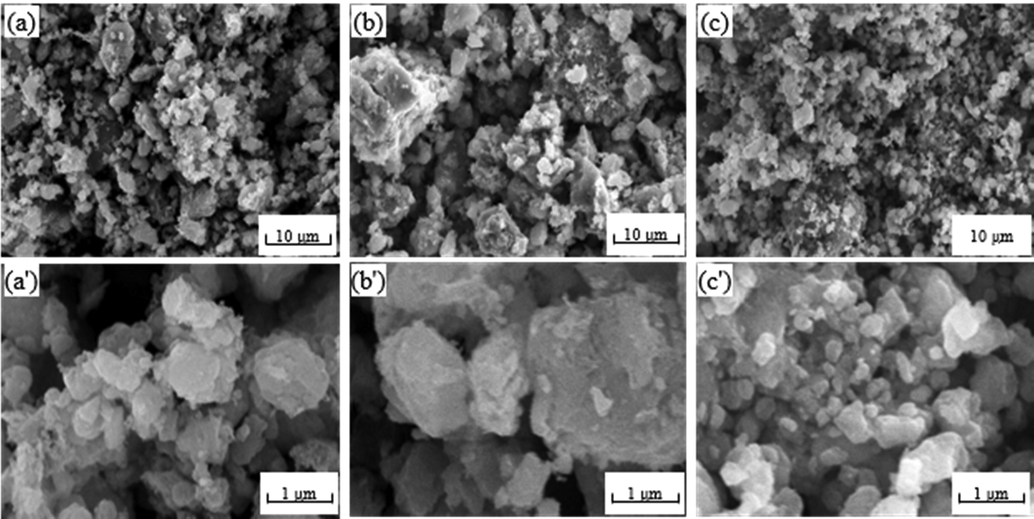
SEM images of different CuAl2O4 spinel before the reaction. (a, a') So-OH; (b, b') So-NO; (c, c') So-O.
Fig. 4 exhibited SEM images of different CuAl2O4 spinel catalysts after the reaction. The particle size of the So—OH and So—O catalysts increased after reaction due to the slight sintering of the Cu0 particles during the reaction process. However, the particle size of the So—NO catalyst decreased and the dispersion improved after reaction. It was speculated that this phenomenon may be related with the decomposition of seriously agglomerated CuAl2O4 spinel during the reaction, which could gradually release high dispersion Cu species (Maiti et al., 2016, Wan et al., 2016, Li et al., 2018, Liu et al., 2022a, 2022b).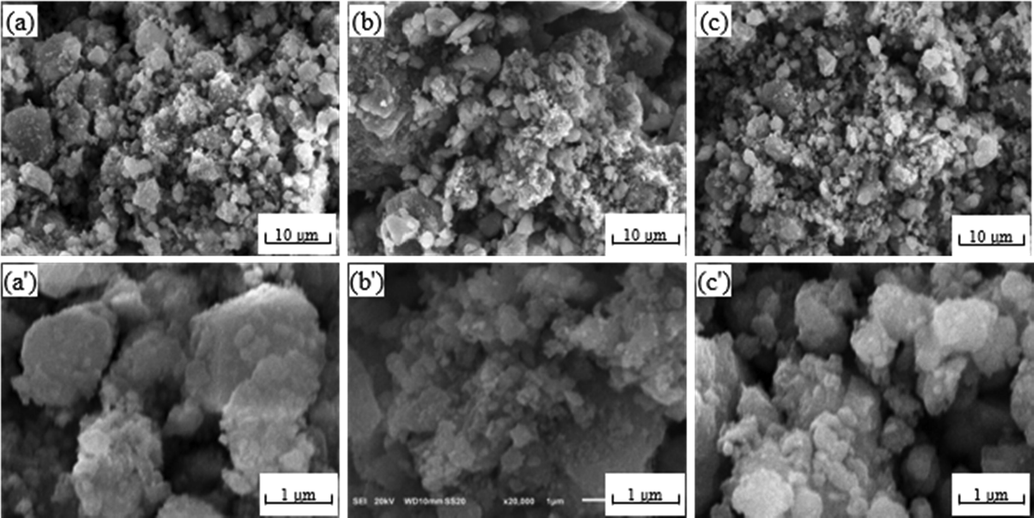
SEM images of different CuAl2O4 spinel after the reaction (a, a') So-OH; (b, b') So-NO; (c, c') So-O.
3.4 TEM characterization
TEM images of different CuAl2O4 spinel catalysts before the reaction were shown in Fig. 5. All catalysts were stacked in irregular flakes. The diameter of So-OH [Fig. 5(a)] and So-NO [Fig. 5(b)] catalysts were mainly distributed in the range of 20 ∼ 30 nm. Moreover, their accumulation was relatively thin, and individual flakes can be observed. However, the diameter of the So-O catalyst was small and primarily within 10 ∼ 15 nm. Compared with So-OH and So-NO catalysts, its accumulation was thick, and no single flakes appeared [Fig. 5(c)], which was consistent with the SEM result that the So-O catalyst was uniformly dispersed with small particles.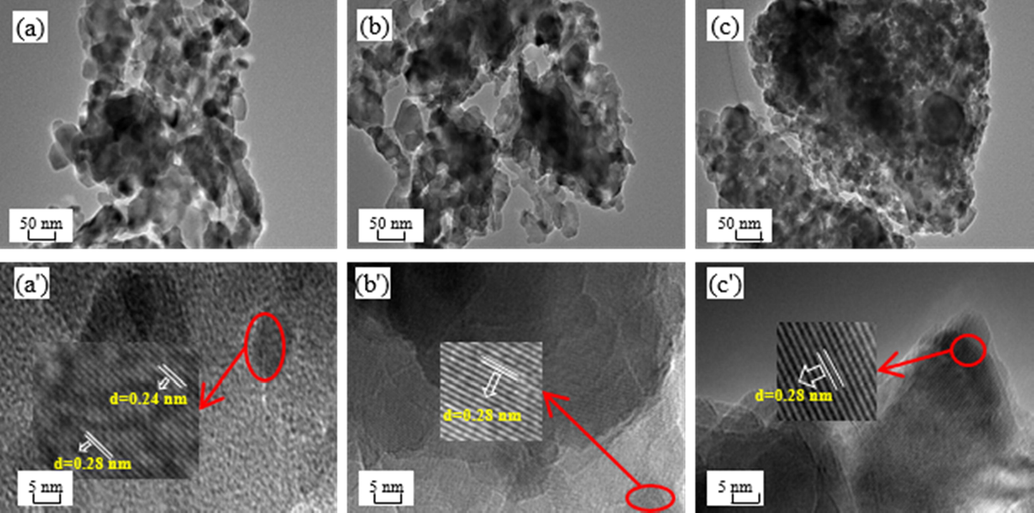
TEM and HRTEM images of different CuAl2O4 spinel before the reaction. (a, a') So-OH; (b, b') So-NO; (c, c') So-O.
In the HRTEM, the So-OH catalyst [Fig. 5(a')] exhibited 0.24 nm and 0.28 nm lattice spaces, which was ascribed to CuAl2O4(3 1 1) and CuAl2O4(2 2 0) facet, respectively (Ding et al., 2009). However, the So-NO [Fig. 5(b')] and So—O [Fig. 5(c')] catalysts only presented a lattice space of 0.28 nm, indicating that the copper source affected the facets exposure of CuAl2O4 spinel.
3.5 H2-TPR characterization
Fig. 6(a) displayed the H2-TPR profiles of different CuAl2O4 spinel catalysts before the reaction. Three reduction peaks were observed and named as α1 (170–220 °C), α2 (225–280 °C), and β (290–470 °C), respectively. According to the above XRD characterization, α1 was ascribed to the reduction of highly dispersed free CuO species, α2 was assigned to the reduction of free CuO species with large crystallite size, and β was attributed to the reduction of CuAl2O4 (Luo et al., 2005, Kwak et al., 2012, Hou et al., 2021). The molar percentage of CuO in all copper species [CuO/(CuO + CuAl2O4)] calculated according to the reduction peak area ratio in the H2-TPR spectra were 15.0 %, 30.5 % and 24.9 % for the So-OH, So-NO and So-O catalysts, respectively (See Table 2). The amount of CuO in the So-OH catalyst was the least among the three catalysts. No CuO diffraction peaks were observed in XRD characterization, indicating that CuO was highly dispersed or amorphous. Meanwhile, it can be founded that the molar percentage of CuAl2O4 in all copper species [CuAl2O4/ (CuO + CuAl2O4)] was 85 %, 69.5 %, and 75.1 % in the So-OH, So-NO, and So-O catalysts, respectively, indicating that the copper sources showed obvious influence on the purity of the CuAl2O4 spinel.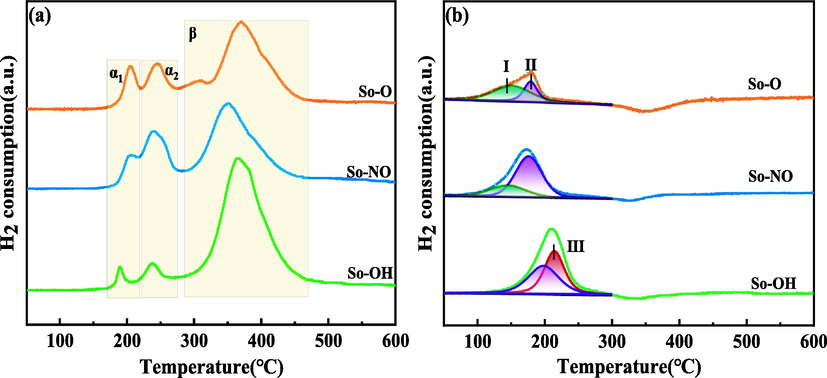
H2-TPR profiles of different CuAl2O4 spinel before (a) and after (b) the reaction.
Catalyst
Before reaction(bulk)a
Before reaction(surface)b
After reactionc
CuO(%)
CuAl2O4(%)
CuO(%)
CuAl2O4(%)
Cu+(%)
Cu0(%)
So-OH
15.0
85.0
23.4
76.6
93.1
6.9
So-NO
30.5
69.5
31.0
69.0
91.4
8.6
So-O
24.9
75.1
58.9
41.1
83.5
16.5
The H2-TPR spectra of different CuAl2O4 spinel catalysts after the reaction were shown in Fig. 6(b). Compared with the catalysts before the reaction, the reduction peak of CuAl2O4 spinel around 360 ℃ disappeared, and the peak area of about 200 ℃ obviously increased, indicating that the CuAl2O4 spinel was decomposed and reduced during the reaction. Agzamova et al. reported that CuAl2O4 spinel could decompose to produce CuO and Al2O3 at 6 MPa, 800 ℃ (Agzamova et al., 2020). In this work, based on XRD results, the CuAl2O4 spinel decomposed at 4 MPa, 280 ℃ because the reaction atmosphere (CO + H2) could accelerate the decomposition. In order to distinguish from free CuO, the CuO produced by the CuAl2O4 spinel decomposition was named as the released CuO. Our previous work reported that CuO would be completely reduced to Cu after heat-treated in liquid paraffin at 280 ℃ for 1 h (Fan et al., 2013). Based on this result, it was reasonable to assume that free CuO in the CuAl2O4 spinel catalysts was reduced to Cu during 144 h reaction at 280 ℃ in liquid paraffin.
After peak fitting of Fig. 6(b), the reduction peaks around 200 ℃ can be classified into three peaks. Peak Ⅰ belonged to the reduction of the released CuO. Compared with the reduction of free CuO before the reaction, it could be seen that, after the reaction, the reduction temperature of the released CuO was shifted to a lower temperature, implying there existed a difference between the structure of free CuO and released CuO. It was reported that the CuAl2O4 spinel gradually released CuO during the reaction, and the released CuO had high dispersion and could avoid agglomeration (Maiti et al., 2016, Wan et al., 2016, Li et al., 2018, Liu et al., 2022a, 2022b). Further studies will be needed to obtain the precise difference between the structure of free CuO and the released CuO. Moreover, it can be seen from Fig. 6(b) that peak Ⅰ did not appear for the So-OH catalyst, indicating the released CuO could be further reduced to low valence copper species. According to the XRD patterns [Fig. 1(b)] and Cu LMM spectra [Fig. 7(c)], there existed Cu2O and Cu for So-OH catalyst after the reaction. Hence, peak Ⅱ was assigned to the reduction of highly dispersed Cu2O, and peak III corresponded to the reduction of Cu2O with a good crystallite shape. It can be founded that the reduction peaks of the released CuO and highly dispersed Cu2O appeared for the So-NO and So-O catalysts. As for the So-OH catalysts, the reduction peak of the released CuO disappeared, and the reduction peaks of the highly dispersed Cu2O and crystalline Cu2O appeared, illustrating that CuO released by the CuAl2O4 spinel decomposition was rapidly reduced. Hence, it can be concluded that the CuAl2O4 spinel prepared by different copper sources possessed various decomposition and reduction abilities during the reaction. Specifically, the So-OH catalyst with the highest purity of CuAl2O4 promoted the released CuO in the bulk phase completely reduced to low valence copper species, and Cu2O with good crystallization appeared. While catalysts with lower purity of CuAl2O4 resulted in part of the released CuO not being timely reduced and no crystalline Cu2O formed.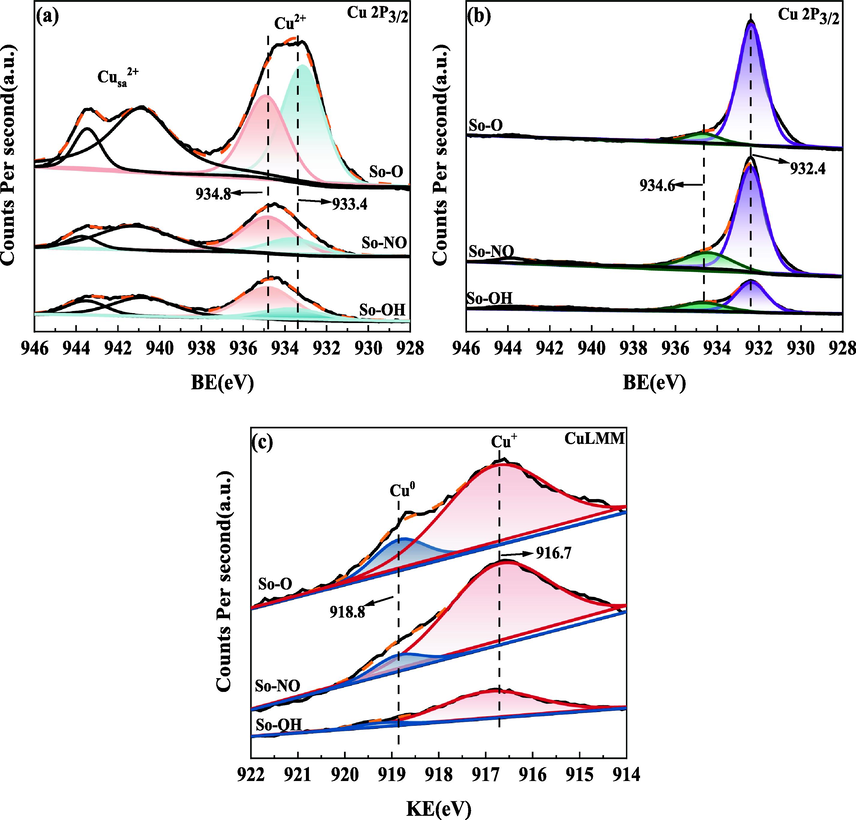
XPS spectra of different CuAl2O4 spinel before (a) and after (b, c) the reaction.
3.6 XPS characterization
To further explore the changes in the chemical valence state and existing forms of the elements over the catalyst surface before and after the reaction, the catalysts were characterized by XPS. As shown in Fig. 7(a), all catalysts before the reaction displayed shake-up satellite peaks between 941 ∼ 943 eV, indicating the existence of Cu2+ on the catalyst surface. After peak fitting, the binding energies of Cu 2p3/2 located at 934.8 eV and 933.4 eV were attributed to CuAl2O4 and CuO, respectively (Severino et al., 1998). The molar percentage of CuAl2O4 on the catalyst surface calculated by the area ratio of Cu 2p3/2 followed the order: So-OH > So-NO > So-O (See Table 2). Moreover, compared with the molar percentage of CuAl2O4 in catalyst bulk, that of CuAl2O4 on the catalyst surface over the So-OH and So-O catalysts decreased by 8.4 % and 34.0 %, respectively. But the molar percentage of the So-NO catalyst was almost the same. It can be concluded that different copper sources brought a profound influence on CuAl2O4 enrichment degree.
The XPS spectra of different CuAl2O4 spinel after the reaction were shown in Fig. 7(b). After peak fitting, the Cu 2p3/2 exhibited two peaks with the binding energy at 932.4 eV and 934.6 eV. Owing to the absence of shake-up satellite of Cu2+, the peak located at 932.4 eV was attributed to Cu+ or Cu0, which was further corroborated by the X-ray excited Auger electron spectroscopy spectra of Cu LMM [see Fig. 7(c)]. The peaks at 916.7 eV and 918.8 eV were assigned to Cu+ and Cu0, respectively (Dai et al., 2001, Faungnawakij et al., 2009, Sun et al., 2020). As shown in Table 2, there were differences in the Cu+/Cu0 ratio on the catalyst surface, following the order: So-OH > So-NO > So-O, which was similar to the molar percentage of CuAl2O4 on the catalyst surface. Thus, it can be concluded that a higher CuAl2O4 enrichment degree favored the formation of more Cu+ on the catalyst surface. It was worth noting that the CuO reduction peak was observed in the H2-TPR profiles of the So-NO and So-O catalysts after the reaction, but the binding energy peak of CuO did not appear in Cu 2p3/2, illustrating that CuO on the catalyst surface was easy to be reduced to Cu+ or Cu0, while CuO in the catalyst bulk was more difficult to be reduced, in other words, the Cu2+ mainly existed in the bulk phase. The peak located at 934.6 eV corresponded to CuAl2O4, indicating part of CuAl2O4 spinel was not completely decomposed during the reaction. Moreover, the enrichment of Cu+ over the catalyst surface after the reaction caused the binding energy of the CuAl2O4 spinel was slightly shifted towards the low binding energy (Faungnawakij et al., 2009).
3.7 Activity evaluation result
Fig. 8 presented the activity evaluation results of different CuAl2O4 spinel catalysts for CO hydrogenation. The carbon balance of all catalysts was higher than 85 %. It can be seen from Fig. 8(a) that the copper source obviously affected the catalytic activity. The CO conversion of the catalysts followed the sequence of So-OH < So-NO < So-O. Combined with XPS characterization, it was found that the higher CuAl2O4 enrichment degree on the catalyst surface, the lower CO conversion. Moreover, more Cu0 in the catalysts resulted in higher CO conversion. The reason may be that the presence of a higher amount of free CuO on the catalyst surface can be played a role as an initiator to promote more CuAl2O4 to be decomposed and reduced to Cu0. The Cu0 was considered as the active site for CO activation and H2 dissociation and adsorption (Liu et al., 2017a).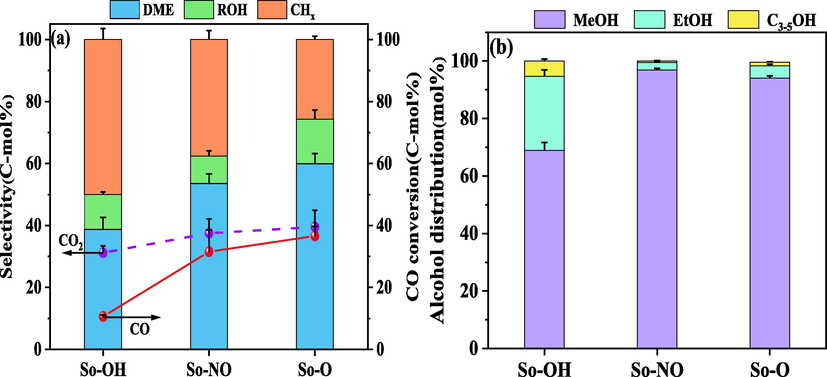
CO conversion, product selectivity (a) and alcohol distribution (b) of different CuAl2O4 spinels.
Without considering CO2, the total selectivity of dimethyl ether and alkane for the three catalysts was over 85 %. The highest selectivity of dimethyl ether reached 59.9 %, while the highest total alcohols selectivity was only 14.4 %. The selectivity of dimethyl ether followed the order: So-OH < So-NO < So-O, which was consistent with the order of the Cu0 content of the catalyst surface after the reaction. According to the above characterization, the CuAl2O4 spinel can be decomposed and reduced to Cu0 and γ-Al2O3 during the reaction. Cu0 was the active site for methanol synthesis, and γ-Al2O3 was the traditional methanol dehydration catalyst. The intense interaction between Cu0 and γ-Al2O3 favored the production of dimethyl ether. Moreover, with the selectivity of dimethyl ether increasing, the alkanes selectivity showed a downward trend. It was reported that the -CHxO intermediate was formed during the CO hydrogenation, and then -CHxO can be directly hydrogenated to methanol, which can be further dehydrated to dimethyl ether. Besides, -CHxO can also be polarized and deoxygenated by Cu+ to form -CHx and then hydrogenated to alkanes (Gong et al., 2012, Wang et al., 2015, Li et al., 2017). Therefore, the competitive relationship between dimethyl ether and alkanes existed.
As shown in Fig. 8(b), the alcohol distribution of the three catalysts was significantly different. C2+OH fraction reached 31.1 % for the So-OH catalyst, but only 3.1 % for the So-NO catalyst. According to the literature reports, the synergistic effect of Cu0 and Cu+ promoted the synthesis of C2+OH (Zuo et al., 2014, Liu et al., 2017b, Guo et al., 2022, Jia et al., 2022). The more Cu+ were favorable to polarize C—O in -CHxO, making it break and increase the content of -CHx. The CO* generated by Cu0 activation combined -CHx to generate -CHxCO, and then hydrogenated to C2+OH. In this work, the Cu+/Cu0 ratio on the catalyst surface was 13.49, 10.63 and 5.06 for So-OH, So-NO, and So-O, respectively, which was not the same order for the C2+OH fraction. Thus, it can be shown that the Cu+/Cu0 ratio was not the only factor in improving the C2+OH fraction. Combined with the texture characterization of the catalysts, the So-OH catalyst showed a different pore structure compared with the other two. Meanwhile, its pore volume and pore size were maximum. It was reported that the larger pore size was conducive to the diffusion of syngas, and the much more pore volume helped to reduce the space restriction and facilitate the growth of the carbon chain (Liu et al., 2012, Gao et al., 2017). It can be concluded that the catalysts with higher Cu+/Cu0 ratio and larger pore volume and pore size favored the formation of higher alcohols.
4 Conclusions
In the paper, the structure of CuAl2O4 spinel was regulated by different copper sources using the solid-phase method. Characterization results showed that the CuAl2O4 spinel was gradually decomposed to CuO and Al2O3 during the reaction for CO hydrogenation. It was discovered that the CuAl2O4 spinel structure relied highly on the copper sources. The difference in surface enrichment degree resulted in dissimilar decomposition and reduction ability of the CuAl2O4 spinel, which will further tune the ratio of Cu+/Cu0 and thus change the alcohol distribution of CO hydrogenation. The CuAl2O4 spinel, selecting copper hydroxide as the copper source, possessed a large pore size and much more pore volume. Moreover, the highest enrichment degree of CuAl2O4 spinel promoted the reducibility of the released CuO, which favored the formation of more Cu+ on the catalyst surface. Activity evaluation results suggested the higher ratio of Cu+/Cu0 with larger pore size and pore volume was conducive to enhancing the chain growth and promoting C2+OH selectivity. Meanwhile, the synergistic effect between Cu0 and γ-Al2O3 improved the selectivity of dimethyl ether. In contrast, the catalysts using copper nitrate and copper oxide as copper sources had a relatively smaller pore size and pore volume, and much more Cu0 on the catalyst surface, facilitating the formation of methanol and dimethyl ether. The research of this paper will provide a new strategy to regulate the ratio of Cu+/Cu0 and texture property by changing the structure of the CuAl2O4 spinel, thus achieving different product distribution for CO hydrogenation.
Acknowledgments
The authors are very thankful for the support from the National Natural Science Foundation of China (22078214) and Key R&D Program of Shanxi Province (201803D121043).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Structural stability of CuAl2O4under pressure. J. Phys. Condens. Matter. 2020;33:035403
- [CrossRef] [Google Scholar]
- Facile and green synthesis of silver quantum dots immobilized onto a polymeric CTS–PEO blend for the photocatalytic degradation of p-Nitrophenol. ACS Omega. 2021;6:30432-30441.
- [CrossRef] [Google Scholar]
- Enhanced catalytic activity for CO oxidation by highly active Pd nanoparticles supported on reduced graphene oxide/copper metal organic framework. J. Taiwan Inst. Chem. E.. 2021;128:194-208.
- [CrossRef] [Google Scholar]
- Low temperature CO oxidation over highly active gold nanoparticles supported on reduced graphene oxide@Mg-BTC nanocomposite. Catal. Lett. 2022
- [CrossRef] [Google Scholar]
- Evaluation of CuAl2O4 as an Oxygen carrier in chemical-looping combustion. Ind. Eng. Chem. Res.. 2012;51:13924-13934.
- [CrossRef] [Google Scholar]
- Cu–Al spinel as a highly active and stable catalyst for the reverse water gas shift reaction. ACS Catal.. 2019;9:6243-6251.
- [CrossRef] [Google Scholar]
- XPS studies of Cu/ZnO/Al2O3 ultra-fine catalysts derived by a novel gel oxalate co-precipitation for methanol synthesis by CO2+H2. Appl. Surf. Sci.. 2001;177:172-179.
- [CrossRef] [Google Scholar]
- Heat treatment of CuZnAl catalyst under high pressure to synthesize higher alcohols. Energ Source Part A.. 2019;47:532-539.
- [CrossRef] [Google Scholar]
- In-situ synthesis of photocatalytic CuAl2O4-Cu hybrid nanorod arrays. Chem. Commun. (Camb). 2009;6:3588-3590.
- [CrossRef] [Google Scholar]
- Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol.. 2022;18:1963-1976.
- [CrossRef] [Google Scholar]
- Chemical change of copper species in liquid paraffin. Chinese J. Appl. Chem.. 2013;30:67-72.
- [CrossRef] [Google Scholar]
- Crystal structure and surface species of CuFe2O4 spinel catalysts in steam reforming of dimethyl ether. Appl. Catal. B-Environ.. 2009;92:341-350.
- [CrossRef] [Google Scholar]
- Steam reforming of dimethyl ether over coupled catalysts of CuO-ZnO-Al2O3-ZrO2 and solid-acid catalyst. Chinese J. Chem. Eng.. 2009;17:64-71.
- [CrossRef] [Google Scholar]
- CuZnAl catalysts prepared by precipitation-hydrothermal method for higher alcohols synthesis from syngas. Energy Source Part A.. 2017;39:1625-1631.
- [CrossRef] [Google Scholar]
- Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc.. 2012;134:13922-13925.
- [CrossRef] [Google Scholar]
- Modulation of CuO surface properties for selective electrocatalytic reduction of CO2 to HCOOH. J. Inorg. Mater.. 2022;37:29-37.
- [CrossRef] [Google Scholar]
- Cu1−xMgxAl3 spinel solid solution as a sustained release catalyst: one-pot green synthesis and catalytic performance in methanol steam reforming. Fuel. 2021;284:119041
- [CrossRef] [Google Scholar]
- Effect of Si/Al ratio on the performance of Cu-Zn-Si-Al catalyst prepared by complete liquid-phase technology for dimethyl ether synthesis from syngas. Chem. J. Chinese U.. 2011;32:118-123.
- [CrossRef] [Google Scholar]
- Insight into the structural sensitivity of CuZnAl catalysts for CO hydrogenation to alcohols. Fuel. 2022;323:124265
- [CrossRef] [Google Scholar]
- Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol–gel method for the hydrogenolysis of glycerol. Catal. Commun.. 2012;24:90-95.
- [CrossRef] [Google Scholar]
- Hydrogen production from methanol decomposition using Cu-Al spinel catalysts. J. Clean. Prod.. 2018;183:415-423.
- [CrossRef] [Google Scholar]
- Facile preparation of Cu-Al oxide catalysts and their application in the direct synthesis of ethanol from syngas. ChemistrySelect. 2017;2:10365-10370.
- [CrossRef] [Google Scholar]
- CO hydrogenation to higher alcohols over CuZnAl catalysts without promoters: effect of pH value in catalyst preparation. Fuel Process. Technol.. 2017;167:575-581.
- [CrossRef] [Google Scholar]
- Higher alcohols synthesis from syngas over P-promoted non-noble metal Cu-based catalyst. Fuel. 2017;208:423-429.
- [CrossRef] [Google Scholar]
- Synergy between active sites of ternary CuZnAlOOH catalysts in CO hydrogenation to ethanol and higher alcohols. ACS Sustain. Chem. Eng.. 2020;8:6634-6646.
- [CrossRef] [Google Scholar]
- Structure and performance of Cu-Fe bimodal support for higher alcohol syntheses. Acta Phys-Chim Sin.. 2012;28:1964-1970.
- [CrossRef] [Google Scholar]
- Effect of ZnO morphology on the performance of CuZnAl slurry catalysts for the ethanol synthesis from syngas. Fuel Process. Technol.. 2022;238
- [CrossRef] [Google Scholar]
- Synthesis of Cu-Al spinels and its non-isothermal formation kinetics analysis. Fuel Chem. Technol.. 2020;48(3):338-348.
- [CrossRef] [Google Scholar]
- Sustained release catalysis: dynamic copper releasing from stoichiometric spinel CuAl2O4 during methanol steam reforming. Appl. Catal. B-Environ.. 2022;122043
- [CrossRef] [Google Scholar]
- In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A-Chem.. 2005;239:243-248.
- [CrossRef] [Google Scholar]
- Combustion synthesized copper-ion substituted FeAl2O4 (Cu0.1Fe0.9Al2O4): a superior catalyst for methanol steam reforming compared to its impregnated analogue. J. Power Sources. 2016;304:319-331.
- [CrossRef] [Google Scholar]
- Sulfamic acid supported on mesoporous MCM-41 as a novel, efficient and reusable heterogenous solid acid catalyst for synthesis of xanthene, dihydropyrimidinone and coumarin derivatives. Colloid Surf. A.. 2021;628:127261
- [CrossRef] [Google Scholar]
- Palladium supported on mixed-metal-organic framework (Co-Mn-MOF-74) for efficient catalytic oxidation of CO. RSC Adv.. 2021;11:4318-4326.
- [CrossRef] [Google Scholar]
- Nature of copper active sites in the carbon monoxide oxidation on CuAl2O4 and CuCr2O4 spinel type catalysts. J. Catal.. 1998;177:82-95.
- [CrossRef] [Google Scholar]
- Combustion products of calcium carbide reused by Cu-based catalysts for acetylene carbonylation. ACS Omega. 2020;5:27692-27701.
- [CrossRef] [Google Scholar]
- Hydrogen production from steam reforming of methanol over CuO/ZnO/Al2O3 catalysts: Catalytic performance and kinetic modeling. Chinese J Chem Eng.. 2016;24:1186-1194.
- [CrossRef] [Google Scholar]
- Insight into the balancing effect of active Cu species for hydrogenation of carbon–oxygen bonds. ACS Catal.. 2015;5:6200-6208.
- [CrossRef] [Google Scholar]
- Spinel AFe2O4 catalysts: Preparation and catalytic combustion of toluene. J. Inorg. Mater.. 2017;32:1068-1074.
- [CrossRef] [Google Scholar]
- Cu-Al spinel catalyst prepared by solid phase method for methanol steam reforming. J. Fuel Chem. Technol.. 2013;41:998-1002.
- [CrossRef] [Google Scholar]
- Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming. Angew. Chem. Int. Ed. Engl.. 2014;53:11886-11889.
- [CrossRef] [Google Scholar]
- Effect of CuAl2O4 spinel structure on CO hydrogenation in slurry reactor. Chem. J. Chinese U.. 2021;42:1846-1854.
- [CrossRef] [Google Scholar]
- Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy. 2013;38:9541-9552.
- [CrossRef] [Google Scholar]
- Experimental and theoretical studies of ethanol synthesis from syngas over CuZnAl catalysts without other promoters. J. Phys. Chem. C. 2014;118:12890-12898.
- [CrossRef] [Google Scholar]







