Translate this page into:
Anthemis cotula L. from Jordan: Essential oil composition, LC-ESI-MS/MS profiling of phenolic acids - flavonoids and in vitro antioxidant activity
⁎Corresponding author. mahmoud.qudah@yu.edu.jo (Mahmoud A. Al-Qudah) MAAlQudah@imamu.edu.sa (Mahmoud A. Al-Qudah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Essential oils of the leaves and flowers of Anthemis cotula L. (family Asteraceae) grown in Jordan were extracted by hydro-distillation and then analyzed by GC–MS. Sesquiterpenes hydrocarbons (SH) were the dominant components in the oils extracted from leaves and flowers of A. cotula. γ-Muurolene and aromadendrene, were the major compounds that were obtained from the flowers oil, while γ-muurolene and trans-cadinene ether were detected as major ingredients in the leaves extract. LC-MS analysis was carried out to identify the significant compounds from each extract. Additionally, butanol (B), aqueous methanol (M) and water (W) extracts prepared from the flowers and the leaves of A. cotula were analysed by LC-MS/MS. Apigenin and chlorogenic acid were the main constituents detected in the flowers’ alcoholic extracts and leaves’ aqueous extract. Moreover, the essential oils and all prepared extracts were assayed for their total antioxidant activity using the DPPH, ABTS, and ferrous ion chelating effect (FIC) assay methods. All investigated oils and extracts showed interesting activity as compared to the positive controls employed (α-tocopherol and ascorbic acid).
Keywords
Anthemis cotula L.
Chemical composition
Antioxidant activity
Essential oil
LC-MS/MS
1 Introduction
The genus Anthemis belonging to the Asteraceae (Compositae) family. is nowadays defined as the second largest genus of the Anthemideae tribe, comprising approximately 210 species widely distributed throughout Europe and the Mediterranean basin, spanning to the south of the Arabian Peninsula and some tropical regions (Fernandes 1976; Bremer 1994; Maggio et al. 2014). It represents a heterogeneous combination of annual and perennial herbs and dwarf shrubs of attractive and beautiful heads (Maggio et al. 2014). In Jordan, sixteen Anthemis species were reported to grow wild all over the country (Al-Eisawi 1982; Oran and Al-Eisawi, 1998). Folk herbal medicine uses Anthemis species mainly for the treatment of gastrointestinal disorders, hemorrhoids, dysmenorrhea, and stomach ache. Also, members of this genus have been found to possess antibacterial, antispasmodic, anti-inflammatory, hepatoprotective, anticholinesterase, antibiofilm and antioxidant activities (Fernandes 1976; Bremer 1994; Saroglou et al. 2006; Ghafoor 2010; Riccobono et al. 2017; Chemsa et al. 2018). Phytochemical investigation of several Anthemis plants revealed the presence of sesquiterpene lactones, polyacetylenes, flavonoids, and essential oils (Bohlmann et al. 1963; Bohlmann et al. 1965a; Bohlmann et al. 1965b; Bohlmann and Kleine 1966; Bulatuvic et al. 1997; Williams et al. 2001; Van et al. 2003; Pavlovíc et al. 2006; Staneva et al. 2008; Saroglou et al. 2010; Gonenc et al. 2012; Tuba et al. 2014; Dŏgan et al. 2015; Chemsa et al. 2018)).
A. cotula L. is an annual glandular plant, characterized by its harsh taste and intensive acrid smell with 12 to 24 in. in height (Zohary 1966; Oran and Al-Eisawi, 1998). A. cotula is well-known in folk herbal medicine for the treatment of psoriasis, fever, gastrointestinal problems, dysentery, and gouty arthritis (Quarenghi et al. 2000).
Previous investigations on the aerial parts of A. cotula revealed the presence of 7-methoxy-6-acetyl-2,2-dimethylchromene, quercetagetin, quercetagetin-7-glucoside, quercetin, quercetin-7-glucoside, patuletin, patuletin-7-glucoside, kaempferol, kaempferol-7-glucoside, kaempferol-3-rutinoside, apigenin and hispidulin in its extract (Quarenghi et al. 2000). From the aerial parts of A. cotula, six linear sesquiterpene lactones were isolated (Van et al. 2003). In addition, investigation of the extracts obtained from the roots of A. cotula afforded four acetylenes, three prenylated acetophenones, and polyacetylenes (Bohlmann et al. 1965a; Bohlmann et al. 1965b; Van et al. 2003; Vucković et al. 2006). A previous work by Rezaei and Jaymand showed variable qualitative and quantitative composition of the essential oils obtained from the flowers and the leaves (Rezaei and Jaymand 2007). The major components of the flower oil were n-nonadecane, cedrane, and (E, E)-α-farnesene, while the leaves oil contained 1-eicosane, benzyl salicylate, and aromadendrene (Rezaei and Jaymand 2007). It is worth mentioning that A. cotula from Jordan has neither been investigated for its essential oil, phenolic acids and flavonoids content nor evaluated for its antioxidant activity.
In the current study, essential oils extracted by hydrodistillation (HD) from the flowers and leaves were investigated for their chemical composition by GC/MS. Additionally, these two organs were also subjected separately to extraction with methanol and water. The obtained extracts were then analysed for their phenolic and flavonoid compounds by LC-MS/MS. Moreover, the hydrodistilled essential oils, butanol (B), aqueous methanol (M) and water (W) extracts were screened for their in vitro antioxidant activities using the DPPH, ABTS and FIC assay methods.
2 Results and discussion
2.1 Essential oil composition
Hydrodistillation (HD) of from the A. cotula flowers and and leaves afforded afforded yellow-colored oils (yields: 0.05 % and 0.08 % (w/w), respectively). The obtained HD-essential oils were then analyzed using GC/MS and GC/FID techniques (Fig. 1). The main components, their Kovats retention indices (KIs) and relative concentration values are summarized in Table 1. The compounds are arranged in order of their elution from the DP-5 column (Table 1).
GC chromatogram of essential oils from the flowers (A) and leaves (B) of A. cotula L. (1: α-terpineol; 2: Z-caryophyllene; 3: β- cedrene; 4: neryl acetate; 5: aromadendrene; 6: γ-muurolene; 7: Germacrene D; 8: δ- Selinene; 9: trans-cadinene ether; 10: ledol; 11: β-oplopenone; 12: 1epi-cubenol; 13: β- eudesmol; 14: n-heptadecanol.
No.
1KIexp
Name of compounds
Relative percentage amounts (%)
2Identification
Leaves
Flowers
1
806
4E-Octene
0.13
0.89
a, b
2
813
2Z-Octene
0.14
0.68
a, b
3
818
2E-Octene
0.9
–
a, b
4
830
Perenyl acetate
0.05
0.98
a, b
5
835
isoValeric acid
0.18
–
a, b
6
849
3E-Hexenol
0.12
–
a, b
7
856
2EHexenal
0.34
–
a, b
8
930
α- Pinene
0.56
–
a, b, c
9
970
Verbenene
0.31
–
a, b, c
10
975
Sabinene
0.18
–
a, b, c
11
987
β-Pinene
0.57
–
a, b, c
12
990
cis-meta-Menta-2,8-diene
0.1
–
a, b
13
1015
α- Terpinene
0.09
–
a, b, c
14
1023
p-Cymene
0.08
–
a, b, c
15
1028
1-p-Menthene
0.48
–
a, b
16
1029
o-Cymene
0.4
–
a, b
17
1037
3E-Octene-2-one
0.32
–
a, b
18
1050
E-β- Ocimene
0.49
–
a, b, c
19
1055
γ- Terpinene
0.63
–
a, b, c
20
1079
cis- Vertocitral C
1.38
1.89
a, b
21
1083
Terpinolene
0.09
–
a, b
22
1097
Linalool
0.44
–
a, b, c
23
1107
α-Thujone
1.71
2.18
a, b, c
24
1108
2E,4E-Octadienol
0.16
–
a, b
25
1112
trans-Thujone
0.68
1.34
a, b
26
1131
α-Campholenol
0.17
–
a, b
27
1188
α-Terpineol
3.76
6.8
a, b
28
1203
γ- Terpineol
0.82
1.35
a, b
29
1215
trans-Pulegol
1.05
0.67
a, b
30
1232
Nerol
0.21
–
a, b, c
31
1238
E-Ocimenone
0.18
–
a, b
32
1244
Hexyl-2E-butanoate
0.39
–
a, b
33
1247
2E-Hexenyl isovalerate
0.12
–
a, b
34
1257
Thymoquinone
0.25
–
a, b, c
35
1260
Benzyl propanoate
0.08
0.1
a, b
36
1270
n-Decanol
0.14
–
a, b, c
37
1315
cis-Dihydro-α-terpinylacetate
0.11
–
a, b
38
1350
Citronellyl acetate
0.39
–
a, b
39
1359
neoiso-Dihydrocarveol acetate
0.75
–
a, b
40
1368
Cyclosativene
0.75
–
a, b
41
1379
α-Copaene
0.21
–
a, b
42
1408
Z-Caryophyllene
3.18
1.65
a, b, c
43
1413
β- Cedrene
3.23
6.81
a, b, c
44
1415
β-Duprezianene
0.08
–
a, b
45
1429
Methylundecanoate
–
0.77
a, b
46
1431
Neryl acetate
2.41
3.74
a, b
47
1446
Aromadendrene
3.93
7.18
a, b
48
1448
epi-Cedrene
1.12
–
a, b
49
1478
γ-Muurolene
19.03
34.2
a, b, c
50
1486
Germacrene D
6.02
1.66
a, b
51
1494
δ- Selinene
2.59
5.94
a, b
52
1496
γ- Amorphene
1.4
2.26
a, b
53
1504
α-Muurolene
0.53
1.65
a, b
54
1508
E,E-α-Farnesene
0.68
–
a, b
55
1517
Z-α-Bisabolene
1.1
–
a, b
56
1523
δ- Cadinene
0.97
–
a, b
57
1547
Selina-3,7(11)-diene
1.38
–
a, b
58
1553
cis-Cadinene ether
1.6
1.77
a, b
59
1565
trans-Cadinene ether
6.49
3.93
a, b
60
1572
α-Cedrene epoxide
0.87
1.17
a, b
61
1581
n-Hexylbenzoate
1.59
–
a, b
62
1587
Caryophellene oxide
0.87
–
a, b, c
63
1595
Globulol
1.21
–
a, b
64
1604
Ledol
3.41
2.56
a, b, c
65
1613
β-Oplopenone
3.08
1.44
a, b
66
1619
epi-Cedrol
–
0.7
a, b
67
1626
1-epi-Cubenol
4.57
2.07
a, b
68
1627
Davanol D2
–
1.13
a, b
69
1636
γ- Eudesmol
0.81
–
a, b
70
1651
β- Eudesmol
3.64
0.76
a, b
71
1669
E-Bisabol-11-ol
0.29
–
a, b
72
1673
Spathulenol
0.25
–
a, b
73
1687
Davanone
0.52
–
a, b
74
1698
Acorenone B
0.27
–
a, b
75
1703
n-Heptadecane
0.56
–
a, b, c
76
1773
n-Heptadecanol
2.41
–
a, b
77
1869
n-Hexadecanol
–
0.78
a, b
Total
A total of 73 compounds were identified in the leaves hydrodistilled essential oils that amounted to 100 % of the total oil content. On the other hand, 30 compounds (accounting for 99.05 % of the total content) were identified in the flowers’ hydrodistilled-essential oil. The components were classified based on their chemical structures into 5 classes (Fig. 2): monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, and others (aliphatic alkane, alkene, alcohols, acids, and esters). The main class of compounds detected in the leaves and flowers oils was sesquiterpenes hydrocarbons (leaves HD-oil: 54.29 %; flowers HD-oil: 65.39 %). Oxygenated monoterpenes accounted for 14.31 %, 17.97 %) of the total content of HD-oils of the leaves and flowers oils respectively.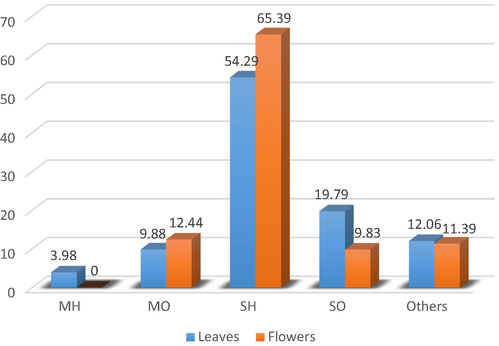
Variation of essential oils composition between leaves and flowers oil from A. cotula L. Monoterpenes hydrocarbons (MH), Oxygenated monoterpenes (OM), Sesquiterpenes hydrocarbons (SH), Oxygenated sesquiterpenes (OS).
The major constituents in leaves oil were γ-muurolene (19.03 %), trans-cadinene ether (6.49 %), germacrene D (6.02 %), 1-epi-cubenol (4.57 %), and aromadendrene (3.93 %). For the flowers oil, γ-muurolene (34.20 %), aromadendrene (7.18 %) β- cedrene (6.81 %), α-terpineol (6.80 %), and δ-selinene (5.94 %).
Results of the current investigation indicated noticeable qualitative and quantitative differences between the essential oils obtained from leaves and flowers. Many components were characteristic in one organ. n-Heptadecanol (2.41 %), selina-3,7(11)-diene (1.38 %), epi-cedrene (1.12 %), α-pinene (0.56 %) and linalool (0.44 %), were detected in the leaves, while davanol D2 (1.13 %), n-hexadecanol (0.78 %), methyl undecanoate (0.77 %) and epi-cedrol (0.70 %) were detected in the flowers oil. Of all 77 compounds detected in the oils of both organs, only 26 compounds were common.
Previous investigations related to essential oil composition of Anthemis species revealed the richness of oxygenated monoterpenes, such as α- and β-thujone, yomogi alcohol, borneol, terpinen-4-ol, and α-terpineol, and by terpene esters, especially cis-chrisanthenyl acetate and other chrysanthenyl esters (Pavlovíc et al. 2006; Saroglou et al. 2006; Gonenc et al. 2012; Bardaweel et al. 2014; Dŏgan et al. 2015; Tawaha et al., 2015). The findings of the current study were quite different. The essential oils obtained from the flowers and leaves of A. cotula from Gilan province included each of n-nonadecane and cedrane (flowers oil), and 1-eicosane and benzyl salicylate (leaves oil) (Rezaei and Jaymand 2007). The chemical variability between the oils reported in these studies could be due to the environmental factors (Barra 2009).
2.2 LC-MS/MS profiling of phenolic acids and flavonoids alcoholic and water extracts
The leaves and flowers of A. cotula from Jordan were subjected separately to the extraction procedure described in the experimental section to prepare the butanol (B), aqueous methanol (M) and water (W) fractions. Then these fractions were subjected to analysis using LC-ESI-MS/MS technique to determine their chemical profiles. Twenty constituents numbered 1–20 were detected and tentatively identified belonging to both phenolic acid and flavonoid. The molecular formulas of all identified compounds and their relative concentrations are shown in Table 2. By comparing the extract's contents with standard references, the phenolic components were identified. The results showed that the B extracts of leaves and flowers of A. cotula contained appreciable amounts of phenolic acids (5.25%) and flavonoids (26.32%) (Table 2). The M extracts of leaves contained slightly lower contents of phenolic acids (3.93 %) but much greater amounts of flavonoids (67.82 %). Flowers M extract contained the highest content of flavonoids (77.53 %) when compared to all other investigated extracts. On the other hand, the W extract of the leaves contained the highest content of chlorogenic acid (77.25 %), as compared to all other extracts. The leaves M extract contained cinnamic acid, vitexin, luteolin, and apigenin as major components. While B extract was characterized by the presence of apigenin, quercetin, and luteolin.
No.
Rt (min)
Compound
Molecular Formula
M.wt
Relative peak area percentage (%)
Leaves
Flowers
B*
M*
W*
B*
M*
W*
1
1.9
Gallic acid
C7H6O5
170.12
–
–
–
2.31
–
–
2
3.2
Syringic acid
C9H10O5
198.17
–
–
–
1.37
–
–
3
3.5
Chlorogenic acid
C16H18O9
354.31
0.57
–
77.25
22.55
1.43
5.38
4
3.9
Aesculetin
C9H6O4
178.14
1.32
–
2.77
3.39
–
–
5
4.0
4-Hydroxy coumarin
C9H6O3
162.14
–
–
–
11.43
–
–
6
4.0
Catechin
C15H14O6
290.27
0.74
–
–
0.39
–
–
7
4.5
Ellagic acid
C14H6O8
302.19
–
–
–
–
0.67
6.83
8
4.6
Vitexin
C21H20O10
432.38
7.42
20.39
5.88
2.16
20.85
7.79
9
4.6
Isoorientin
C21H20O11
448.38
0.88
3.95
2.92
0.73
4.53
–
10
4.7
Gallic acid ethyl
C9H19O5
198.17
–
–
–
0.58
–
–
11
4.7
Cinnamic acid
C9H8O2
148.16
1.61
26.53
–
–
16.77
48.69
12
4.9
7-Hydroxy coumarin
C9H6O3
162.14
0.87
1.71
–
11.42
3.56
–
13
5.0
Naringin
C27H32O14
580.54
0.90
–
4.45
0.88
3.00
8.00
14
5.2
Hyperoside
C21H20O12
464.40
4.68
3.93
–
0.09
–
2.46
15
5.2
Hesperidin
C28H34O15
610.57
1.19
2.82
3.05
2.14
3.20
–
16
5.3
Rutin
C27H30O16
610.52
0.77
1.59
–
2.10
0.63
–
17
6.5
Quercetin
C15H10O7
302.24
27.30
2.52
3.67
2.82
1.69
–
18
6.8
Luteolin
C15H10O6
286.24
14.87
15.29
–
16.95
8.46
–
19
7.6
Apigenin
C15H10O5
270.24
35.44
12.99
–
8.97
32.54
28.83
20
7.6
Kaempferol
C15H10O6
286.24
1.41
8.27
–
0.70
2.63
–
The B extract of the flowers was characterized by the presence of chlorogenic acid, apigenin, Luteolin, and 7-hydroxy coumarin, The M extract of flowers revealed the presence of apigenin, vitexin, cinnamic acid, and luteolin. In addition, in the W extract of flowers cinnamic acid, apigenin, vitexin, and ellagic acid were detected.
Due to the high content of phenolic acids and flavonoids in the extracts of A. cotula, the plant could be considered as a promising source for natural antioxidant agents. In the light of the current results, the oils and extracts were assayed for their antioxidant activities using the DPPH, ABTS and FIC assay methods.
2.3 Antioxidant activity
Several methods are used to evaluate the antioxidant and the free radical scavenging activity of plant extracts (Al-Jaber et al. 2014; Al‐Qudah et al. 2014; Al-Qudah 2016; Al-Jaber et al. 2018; Al‐Qudah et al. 2018a; Al‐Qudah et al. 2018b; Al-Humaidia et al. 2019; Abu-Orabi et al. 2020). In the present study, the antioxidant and radical scavenging capacity of the hydrodistilled essential oils and all prepared extracts including the M, B, and W extracts of the leaves and flowers of A. cotula were evaluated. Three different methods were used including the DPPH, ABTS radical scavenging, and the ferrous ion chelating activity (FIC) assay methods. The measurements and results are given in Fig. 3. The IC50 values were compared with those of α-tocopherol and ascorbic acid as positive controls (Table 3). Higher radical scavenging activities are associated with lower IC50 values.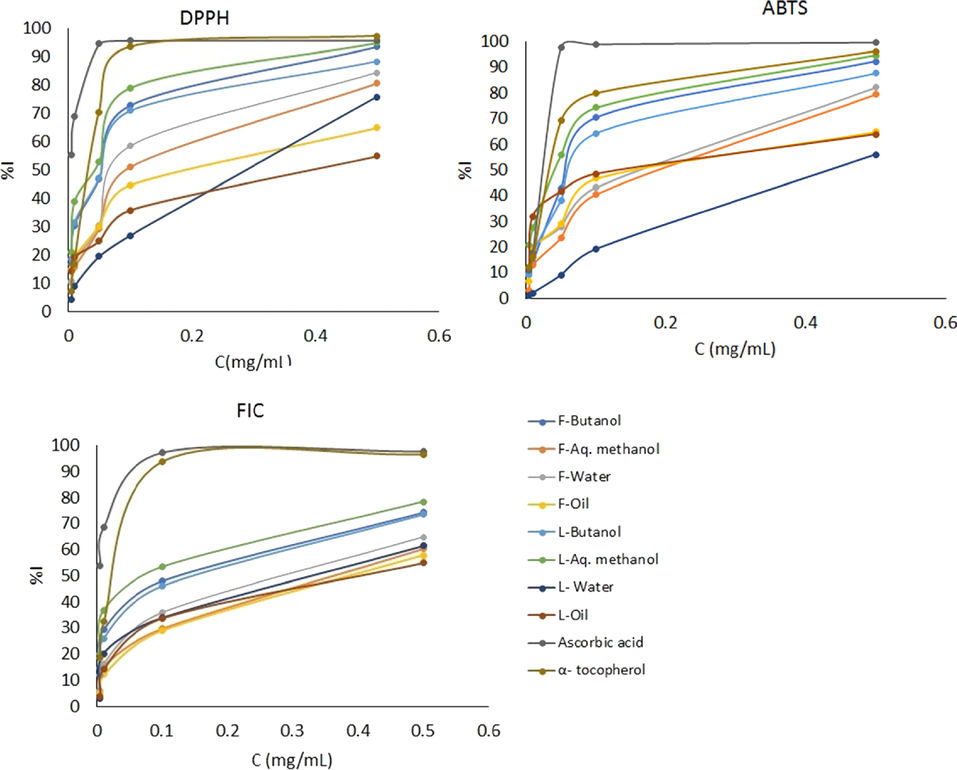
Antioxidant activities of essential oils and crude extracts of the leaves and flowers from A. cotula and standards by using DPPH, ABTS and FIC methods.
Parts of plant
Crude
IC50 (mg/L)
DPPH
ABTS
FIC
Leaves
Aq. Methanol
(23.00 ± 0.20) * 10-2
(23.00 ± 0.60) * 10-2
(29.00 ± 0.50) * 10-2
Butanol
(24.00 ± 0.10) * 10-2
(25.00 ± 0.30) * 10-2
(31.00 ± 0.40) * 10-2
Water
(62.80 5.40) * 10-2
(75.20 ± 6.30) * 10-2
(41.20 ± 0.30) * 10-2
Oil
(54.80 ± 3.46) * 10-2
(14.60 ± 1.18) * 10-2
(28.40 ± 1.80) * 10-2
Flowers
Aq. Methanol
(9.40 ± 0.30) * 10-2
(13.34 ± 1.18) * 10-2
(18.40 ± 3.18) * 10-2
Butanol
(3.68 ± 0.8) * 10-2
(5.17 ± 0.25) * 10-2
(7.60 ± 0.50) * 10-2
Water
(26.00 ± 0.2) * 10-2
(28.00 ± 0.2) * 10-2
(20.90 ± 0.50) * 10-2
Oil
(17.00 ± 2.80) * 10-2
(21.08 ± 1.60) * 10-2
(36.70 ± 5.90) * 10-2
Ascorbic acid
(1.80 ± 0.06) *10-3
(1.90 ± 0.06) *10-3
(1.90 ± 0.02) *10-3
α-tocopherol
(2.30 ± 0.04) *10-3
(1.80 ± 0.01) *10-3
(2.90 ± 0.02) *10-3
The DPPH radical scavenging activities of the essential oil and crude extracts of leaves and flowers of A. cotula quantified in terms of inhibition percentage (%) are presented graphically in Fig. 3. The results show that the DPPH radical scavenging activities are concentration dependent and had the following order: M > B > essential oil > W. The DPPH radical scavenging activity of the flowers' different extracts had the following order: B > W > M > essential oil (Table 3).
The ABTS+. radical scavenging of the leaves and flowers extracts, as well as the standards increased with increasing concentrations (Fig. 3). The order of ABTS+. radical scavenging activity of the different extracts obtained from the leaves had the following order: M > B > essential oil > W. For the flowers extracts the order was B > W > M > essential oil (Table 3).
The essential oil and crude extracts from leaves and flowers of A. cotula were evaluated for their ferrous ion chelating activity, which was found to increase with increasing concentrations, as shown in Fig. 3. The values of IC50 of FIC of the essential oils and extract fractions of A. cotula L. on Fe2+ and ferrozine complex formation are shown in Table 3. The results showed that the M extracts obtained from the leaves had the best chelating activity for ferrous ions (IC50 = 29.00 ± 0.50 × 10-2 mg/L) as compared with the other crude extracts. Yet, the flowers B extracts had the highest chelating activity for ferrous ions (IC50 = 7.60 ± 0.50 × 10-2 mg/L).
The antioxidant efficiency of different extracts obtained from the leaves and flowers of A. cotula has been attributed mainly to their high phenolic and flavonoids contents, being especially rich in chlorogenic acid, cinnamic acid, vitexin, apigenin, quercetin, and luteolin (Romanova et al. 2001; Babaei et al. 2020). For the essential oils, the observed activity could be related to their high sesquitepene hydrocarbon contents that are recognized as strong antioxidants (Victoria et al. 2012).
3 Materials and methods
3.1 Instrumentation
Gas chromatography-mass spectrometry (GC–MS) analysis was performed using Agilent 6890 series II – 5973 mass spectrometers interfaced with HP chemstation. The UV-spectra were recorded on wave light II UV–visible spectrophotometer. A Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF System equipped with Bruker Dalotonik Elute UHPLC system (Bremen, Germany) was used for screening flavonoids and phenolics compounds.
3.2 Materials and chemicals
1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-P,P’-disulfonic (Ferrozin), Iron(II) chloride, Potassium persulfate, anhydrous sodium carbonate, Aluminum chloride, Sodium hydroxide, Methanol, n-Butyl Alcohol, Petroleum ether and Hexane were used as purchased without further purification.
3.3 Plant material
Plant material was collected from Irbid (AL Koura region (N 32.458559; E 35.676982), Jordan in 2019. Botanical authentication of the plant species was confirmed by the botanist Prof. Jamil N. Lahham (Yarmouk University, Department of Biological Sciences). The aerial parts were dried at room temperature (in the shade for about a month). A reference specimen (AC/C/2019–1) was stored in Prof. Mahmoud A. Al-Qudah Laboratory, Yarmouk University, Irbid, Jordan.
3.4 Extraction and fractionation
The fresh aerial parts of the plant material were dried at room temperature in a shady place for a month. The air-dried powdered plant samples were successively extracted in Soxhlet extractor with petroleum ether to remove fatty acids and followed by methanol. The plant material was dried before the following extraction process with the next solvent system. The extracts were concentrated by a rotary vacuum evaporator and then dried. The alcohol residue was partitioned between CHCl3 and H2O (1:1). After the separation of CHCl3 and H2O phases, the dried CHCl3 fraction was partitioned between 10 % aqueous methanol and hexane (H) yielding the aqueous methanol (M) and hexane (H) fractions. The polar organic compounds were extracted from water by n-butanol affording Water (W) and butanol (B) fractions. The extracts obtained were used directly for the estimation of the assessment of antioxidant potential through various chemical assays.
3.5 Essential oil extraction
Fresh leaves or flowers (200 g) of A. cotula were finely chopped and subjected to the classical hydrodistillation method using a Clevenger-type apparatus for 4 h. Subsequently, oils were dried over anhydrous sodium sulfate and immediately stored in GC-grade n-hexane at 4 °C by gas chromatography/mass spectrometry (GC/MS).
3.6 GC–FID analysis
The oils were analyzed by an Agilent (Palo Alto, USA) 6890 N gas chromatograph fitted with a 5 % phenyl–95 % methyl silicone (HP5, 30 m × 0.25 mm × 0.25 μm) fused silica capillary column. The oven temperature was programmed from 60 °C to 240 °C at 3 °C/minute and hydrogen was used as a carrier gas (1.4 mL/minute), and 1.0 μL of a 1 % solution of the oils in hexane was injected in split mode (1:30). The injector was kept at 250 °C and the flame ionization detector (FID) at 280 °C. Concentrations (% contents) of oil ingredients for A. cotula L. species were determined using their relative area percentages obtained from GC chromatogram, assuming a unity response by all components.
3.7 GC–MS analysis
Chemical analysis of the essential oils was carried out using gas chromatography-mass spectrometry (GC–MS). The chromatographic conditions were as follows: column oven program, 60 °C (1 min, isothermal) to 246 °C (3 min, isothermal) at 3 °C /min, the injector and detector temperatures were 250 °C and 300 °C, respectively. Helium was the carrier gas (flow rate of 0.90 mL/min) and the ionization voltage was maintained at 70 eV. An HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm) was used. Retention indices (RIs) were calculated by the injection of a series of n-alkanes (C8-C20) in the same column under the same conditions specified above for gas chromatography analysis. Identification of oil components was based on computer search using the library of mass spectral data and comparing the calculated retention indices (RIs) with those of the available authentic standards and literature data. Standard solutions of α-pinene, β-pinene, α-terpineol, β-cedrene, and γ-muurolene were also injected for confirmation.
3.8 LC-MS/MS profiling of phenolic acids and flavonoids
A Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF System equipped with Bruker Dalotonik Elute UHPLC system (Bremen, Germany) was used for screening flavonoids and phenolics compounds of interest in both positive (M + H) and negative (M − H) electrospray ionization modes. The Full Sensitivity Resolution (FSR) was 50000, the mass accuracy was < 1 ppm, and the TOF repetition rate was up to 20 kHz. Chromatographic separation was conducted using a 120, C18 reversed phase column, 100 × 2.1 mm, 1.8 µm (120 A°) from Bruker Daltonik (Bremen, Germany) at 30 °C, autosampler temperature 8.0 °C with total run time 20.0 min using gradient grade. The elution gradient consisted of mobile phase A (water / methanol (90:10 %) with 5 mM ammonium formate and 0.1 % formic acid)) and solvent B (methanol with 5 mM ammonium formate and 0.1 % formic acid). The gradient program with the following proportions of solvent B was applied (%B, min): 40–90 % B (0.00–6.00 min), isocratic 90 % (6.00–10.00 min), isocratic 40 % (10.01–15.00 min). The solvent flow rate was 0.5 mL/min and the injection volume was 10 µL. MS/MS analysis was performed in negative ion mode with an ion spray voltage of − 4,500 V. Nitrogen gas at a pressure of 60 psi was used as the nebulizing and drying gas. The mass spectra were obtained over the m/z range of 100–1,000 amu. Each crude extract was dissolved in 2.0 mL DMSO and then completed to 50 mL using acetonitrile. After that, each sample was centrifuge at 4000 rpm for 2.0 min. 1.0 mL was taken and transfer to the autosampler in order to inject 3.0 ul.
3.9 Determination of antioxidant activity
The antioxidant activity of the essential oils was determined by DPPH, ABTS radical scavenging, and Ferrous Ion Chelating (FIC) assay methods using the procedure described in the literature (Al-Jaber et al. 2014; Al‐Qudah et al. 2014; Al-Qudah 2016; Al-Jaber et al. 2018; Al‐Qudah et al. 2018a; Al‐Qudah et al. 2018b; Al-Humaidia et al. 2019; Abu-Orabi et al. 2020; Al-Qudah et al. 2020a). The percentage of scavenging activity was calculated using the equation: where Ac is the absorbance of the control and As is the absorbance in the presence of either extracts or control substance.
Non-linear regression analysis of GraphPad Prism 6 (GraphPad Software, San Diego, California, USA) was applied for the determination of IC50 in all of the antioxidant assays from the sigmoidal curve which was obtained by plotting the percentages of scavenging relative to the control versus logarithmic concentration of test essential oils. Each concentration was tested three times in 3 independent experiments.
4 Conclusion
In this study, the chemical composition of the essential oils obtained from the leaves and flowers of A. cotula L. grown in Jordan were analysed by GC/MS has been reported here for the first time. Additionally, fractions of different polarities were also screened for the presence of selected phenolic acids and flavonoids using LC-ESI-MS/MS. Sesquiterpenes hydrocarbons (SH) were the dominant components in the oils extracted from both organs. All investigated extracts were generally rich in phenolic acids and flavonoids with notable qualitative and quantitative differences. The high phenolic acids and flavonoids content contributed to the high antioxidant activities observed for all investigated extracts.
Acknowledgments
We would like to thank the Deanship of Scientific Research and Graduate Studies at Yarmouk University for funding this research project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem.. 2020;13(7):6256-6266.
- [Google Scholar]
- Antioxidant activity and chemical composition of essential oils of selected cleome species growing in Saudi Arabia. Jordan J. Chem.. 2019;14(1):29-37.
- [Google Scholar]
- Volatile oil composition and antiplatelet activity of Jordanian Achillea biebersteinii collected at different growth stages. J. Essent. Oil-Bear Plants. 2014;17(4):584-598.
- [Google Scholar]
- Essential oil composition and anticholinesterase activity evaluation of Achillea fragrantissima growing wild in Jordan. J. Herbs Spices Med. Plants. 2018;24(3):272-281.
- [Google Scholar]
- Antioxidant activity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Active Prod. Nat.. 2016;6(2):101-111.
- [Google Scholar]
- Antioxidant activity and chemical composition of essential oils from Jordanian Ononis natrix L. and Ononis Sicula Guss. J. Biol. Active Prod. Nat.. 2014;4(1):52-61.
- [Google Scholar]
- Al-Qudah MA, Allahham FE, Obeidat SM, Al-Jaber HI, Lahham JN, Abu Orabi ST. 2020a. in vitro antioxidant activities, total phenolics and total flavonoids of the different extracts of Capparis spinosa L. and Capparis decidua Edgew (Forssk.) from Jordan. International Journal of Pharmaceutical Research. 12 (3): 1226-1236.
- Volatile components analysis, total phenolic, flavonoid contents, and antioxidant activity of Phlomis species collected from Jordan. J. Essent. Oil-Bear Plants. 2018;21(3):583-599.
- [Google Scholar]
- Intercomparative investigation of the total phenols, total flavonoids, in vitro and in vivo antioxidant activities of Capparis cartilaginea (Decne.) maire and weiller and Capparis ovata Desf. from Jordan. Pharmacogn. Mag.. 2018;14(55):154-160.
- [Google Scholar]
- Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr.. 2020;8(6):2569-2580.
- [Google Scholar]
- Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement. Altern. Med.. 2014;14(1):1-8.
- [Google Scholar]
- Factors affecting chemical variability of essential oils: a review of recent developments. Nat. Prod. Commun.. 2009;4(8):1147-1154.
- [Google Scholar]
- Polyacetylene compounds. XLVIII. the polyynes of the genus Anthemis. Chem. Ber.. 1963;96(6):1485-1494.
- [Google Scholar]
- Polyacetylene compounds. LXXXVII. structure and biogenesis of a thioether isolated from Anthemis species. Chem. Ber.. 1965;98(9):3087-3091.
- [Google Scholar]
- Polyacetylenic compounds. LXXVIII. New constituents of the genus Anthemis. Chem. Ber.. 1965;98(5):1616-1622.
- [Google Scholar]
- Polyacetylene compounds. CVI. Some new acetylenic compounds from the genus Anthemis. Chem. Ber.. 1966;99(7):2096-2103.
- [Google Scholar]
- Bremer K. 1994. Asteraceae, Cladistics & Classification. Timber Press. Portland. Oregon. 435-478.
- Highly oxygenated guainolides from Anthemis carpatica. J. Nat. Prod.. 1997;60(12):1222-1228.
- [Google Scholar]
- Chemsa, A. E., Zellagui, A., Öztürk, M., Erol, E., Ceylan, O., Duru, M. E., & Lahouel, M. (2018). Chemical composition, antioxidant, anticholinesterase, antimicrobial and antibiofilm activities of essential oil and methanolic extract of Anthemis stiparum subsp. sabulicola (Pomel) Oberpr. Microbial Pathogenesis, 119, 233-240.
- Dŏgan G, Demirpolat A, Băgcı E. 2015. Composition of the volatile oils of Anthemis coelopoda var. coelopoda from Turkey. Hacettepe J Biol Chem. 43 (4): 259–265.
- Fernandes R. 1976. Genus Anthemis L., In Flora Europaea. Vol. 4. Tutin TG. Heywood VH. Burges NA (Eds). Cambridge University Press. Cambridge. UK. 145-149.
- The genus Anthemis L. (Compositae-anthemideae), Arabian Peninsula: a taxonomic study. Pak. J. Bot.. 2010;42:79-98.
- [Google Scholar]
- Chemical composition of the essential oils of Anthemis coelopoda var. bourgaei. and Anthemis aciphylla var. aciphylla. Chem. Nat. Compd.. 2012;48:332-334.
- [Google Scholar]
- Pavlovíc M, Kovaˇcevíc N, Tzakou O, Couladis M. 2006. Essential oil composition of Anthemis triumfetti (L.) DC. Flavour Fragr J. 21 (2): 297–299.
- Chemical composition of the essential oils of three endemic species of Anthemis Sect. Hiorthia (DC.) R.Fern. growing wild in Sicily and chemotaxonomic volatile markers of the genus Anthemis L.: an update. Chem Biodivers. 2014;11:652-672.
- [Google Scholar]
- Antimicrobial activity of flowers from Anthemis cotula. Fitoterapia. 2000;71(6):710-712.
- [Google Scholar]
- Chemicalc of essential oils from leaves and flowers of Anthemis cotula L. from Gilan Province. J. Med. Plants. 2007;6(22):99-105.
- [Google Scholar]
- Study of antioxidant effect of apigenin, luteolin, and quercetin by DNA protective method. Neoplasma. 2001;48(2):104-107.
- [Google Scholar]
- Riccobono, L., Maggio, A., Bruno, M., Spadaro, V., & Raimondo, F. M. 2017. Chemical composition and antimicrobial activity of the essential oils of some species of Anthemis sect. Anthemis (Asteraceae) from Sicily. Nat. Prod. Res. 31(23), 2759-2767.
- Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A. 2006;1104(1–2):313-322.
- [Google Scholar]
- Saroglou V, Karioti, A Rancik A, Dimas K, Koukoulitsa C, Zervou M. 2010. Sesquiterpene lactones from Anthemis melanolepis and their antibacterial and cytotoxic activities: Prediction of their pharmacokinetic profile. J Nat Prod. 73 (2): 242-246.
- Sesquiterpene lactones as chemotaxonomic markers in genus Anthemis. Phytochemistry. 2008;69(3):607-618.
- [Google Scholar]
- Chemical composition and general cytotoxicity evaluation of essential oil from the flowers of Anthemis palestina Reut. ex Boiss., growing in Jordan. J. Essent. Oil-Bear Plants. 2015;18(5):1070-1077.
- [Google Scholar]
- Fatty acid composition and preclinical researches on Anthemis wiedemanniana Fisch. & Mey: discovery of a new anti-inflammatory agent. Pharmacogn. Mag.. 2014;10(37):53-60.
- [Google Scholar]
- Biosynthesis of anthecotuloide, an irregular sesquiterpene lactone from Anthemis cotula L. (Asteraceae) via a non-farnesyl diphosphate route. Org. Biomol. Chem.. 2003;1(9):1503-1508.
- [Google Scholar]
- Essential oil of the leaves of Eugenia uniflora L.: antioxidant and antimicrobial properties. Food Chem. Toxicol.. 2012;50(8):2668-2674.
- [Google Scholar]
- Phytochemical investigation of Anthemis cotula. J. Serb. Chem. Soc.. 2006;71(2):127-133.
- [Google Scholar]
- The role of lipophilic and polar flavonoids in the classification of temperate members of the Anthemideae. Biochem. Systemat. Ecol.. 2001;29(9):929-945.
- [Google Scholar]
- Zohary M, Feinbrun-Dothan N. 1966. Flora Palaestina.







