Translate this page into:
Synthesis of 1-((4-methoxyphenyl)-3-alkynes)-1H-pyrrole-2,5-diones and functionalization to triazoles
⁎Corresponding author. Tel.: +55 11 3091 3654; fax: +55 11 3815 441. hstefani@usp.br (Hélio A. Stefani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of alkynyl maleimides were prepared via one-step cross-coupling reaction using bromomaleimide and acetylenes under the Sonogashira conditions, affording 1-((4-methoxyphenyl)-3-alkynes)-1H-pyrrole-2,5-diones in good to high yields. These products were subsequently converted in the corresponding 1,2,3-triazole using conventional click chemistry approach. The alkynyl maleimide compound (8g) crystallized in the triclinic space group P1 with unit cell parameters a = 5.3692(6), b = 9.2513(10), c = 10.3070(11) Å, α = 85.349(4), β = 86.892(4), γ = 86.892(4)°, V = 507.31(10) Å3, and Z = 1. In the crystal the molecules are stacked parallel to the c axis and held together through a C–H⋯π and a C–H⋯O interaction.
Keywords
Maleimide
Halomaleimide
Cross-coupling
Sonogashira
1,2,3-Triazole
X-ray structure
1 Introduction
Maleimides and their related compounds have invaluable properties, especially biologically, with high cytotoxicity and inhibitory activity toward protein kinase C and topoisomerase I (Lakatosh et al., 2003). Maleimides and their derivatives are potential building blocks in the frontiers of organic chemistry, such as medicinal chemistry, polymer chemistry, and material science.
Cyclic oxopyrrolidine compounds are building blocks and are versatile intermediates for the total synthesis of a wide variety of natural and unnatural compounds with interesting biological activities (Deore and Argade, 2014). Maleimides have countless pharmaceutical properties e.g. antibacterial, anticancer, antimicrobial, antiviral, and antigenic activities (Sanchez et al., 2006). Rebeccamycin, which has an oxopyrrolidine structure, is known topoisomerase I inhibitor, and exhibits antitumor activity against L1210 leukemia, B16 melanoma implanted in mice, and P388 leukemia and also inhibits the growth of human lung adenocarcinoma cells (A549) (Prudhomme, 2003; Bush et al., 1987; Nettleton et al., 1985) (Fig. 1). Among the maleimides, 3,4-disubstituted maleimides have also found applications in material science as components of red light-emitting diodes (LEDs) because of their intense color (Kaletas et al., 2005a,b,c). These compounds have also been used in the development of photocatalysts immobilized on surfaces (Kaletas et al., 2005a,b,c; Souffrin et al., 2012).
Maleimides with pharmaceutical properties.
Our group is also working on the development of diverse structures of oxopyrrolidines (Stefani et al., 2014a,b; Ali et al., 2015a,b) and maleimide compounds (Stefani et al., 2014a,b; Ali et al., 2015a,b; Caracelli et al., 2015). To the best of our knowledge, few practical routes are known for the synthesis of alkynyl maleimides (Pews-Davtyan et al., 2008; Brennführer et al., 2009; Bouissane et al., 2009).
Sonogashira coupling is one of the most important carbon–carbon bond forming cross-coupling reaction involving alkynes (Izgu and Hoye, 2012; Awuah and Capretta, 2011; Roshchin, 2010). Although, the Sonogashira coupling of alkynes with halomaleimides has not been described previously, only a few practical routes for palladium-catalyzed cross-coupling reactions have been reported (Deore and Argade, 2012; Banwell et al., 2010; Stewart et al., 2007; Dubernet et al., 2005). In this context, we herein report our results as the synthesis of 1-((4-methoxyphenyl)-3-triazololyl)-1H-pyrrole-2,5-diones and their functionalization.
2 Material and methods
All reactions were carried out under a nitrogen atmosphere; all compounds were characterized by 1H NMR, 13C NMR and electrospray ionization-mass spectrometry (ESI-MS). NMR spectra were recorded on a 300 MHz instrument. All 1H NMR experiments are reported in δ units, parts per million (ppm), and were measured relative to the signals for tetramethylsilane (TMS) (0.00 ppm). All 13C NMR spectra are reported in ppm relative to deuteron-chloroform (77.23 ppm), unless otherwise stated, and all were obtained with 1H decoupling. For HRMS, previously lyophilized samples were dissolved in methanol and deposited into the 96 well plate of the SIL-20A autosampler for ESI-MS analysis in an IT-TOF mass spectrometer system (Shimadzu). The compounds were detected in positive mode. Typically, 0.1 μL sample aliquots were injected and infused into the instrument in 50% acetonitrile, containing 0.5% formic acid under a constant flow rate of 0.2 mL min−1. Instrument control, data acquisition and processing were performed by the LCMS Solution suite (Shimadzu). ESI conditions are as follows: source temperature 200 °C, cone voltage 4.5 kV, detector voltage 1.57 kV, nebulizing gas flow 1.5 L min−1. Solvents and reagents were of analytical grade or the highest grade commercially available and were used without further purification.
2.1 Crystal structure determination
A suitable crystal for X-ray crystal structure analysis of 2-(4-methoxyphenyl)-4-[2-(4-pentylphenyl)- ethynyl]cyclopent-4-ene-1,3-dione (8g) was obtained by slow evaporation from ethyl acetate at room temperature. Data were collected on a Bruker Kappa APEXII CCD diffractometer using Mo Kα radiation (λ = 0.71073 Å). The structure was solved by direct methods using SIR2014 (Burla et al., 2015), and refined by full matrix least-squares on F2 using SHELXL2014/7 (Sheldrick, 2015). All non-hydrogen atoms were refined anisotropically and H-atoms were placed in calculated positions (C—H = 0.93 to 0.97 Å) and were included in the refinement in the riding model approximation, with Uiso(H) = 1.2–1.5Ueq(C). The programs WinGX (Farrugia, 2012), PLATON (Spek, 2009) and ORTEP-3 for Windows (Farrugia, 2012) were also used in the study. The crystal and structure refinement data of 8g are summarized in Table 3.
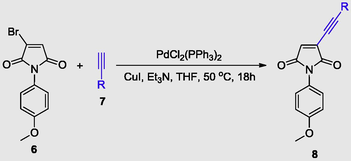
2.2 General procedure for Sonogashira coupling
1-(4-methoxyphenyl)-3-(p-tolylethynyl)-1H-pyrrole-2,5-dione (8j) – To a solution of 3-bromo-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (6) (280.96 mg, 1.0 mmol, 1.0 eq) in THF (5 mL) at 0 °C under nitrogen atmosphere were added PdCl2(PPh3)2 (35 mg, 0.049 mmol, 5 mol%), CuI (9.5 mg, 0.05 mmol, 5 mol%) and 1-ethynyl-4-methylbenzene (7j) (174.1 mg, 1.5 mmol, 1.5 eq) and stirred for 5 min. Then, Et3N (1.5 eq) was added dropwise, ice bath was removed, the temperature was raised to 50 °C and the reaction was monitored by TLC. After completion, the reaction was cooled to room temperature, then quenched with ethylacetate (30 mL), and the organic phase was washed with saturated NH4Cl (10 mL) and then dried over MgSO4. Evaporation under reduced pressure followed by column chromatography on silica gel (20% ethyl acetate in hexanes) afforded the product.
3-(4-hydroxybut-1-yn-1-yl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8a), Gummy solid; Yield (206 mg, 76%); 1H NMR (300 MHz, CDCl3) δ ppm 2.39–2.41 (m, 2H), 3.54–3.61 (m, 5 H), 6.77 (br. s., 1 H), 6.91 (d, J = 6.78 Hz, 2 H), 7.15 (d, J = 6.00 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ ppm 25.55, 55.49, 67.94, 96.21, 108.08, 114.45 (2C), 123.92, 127.58, 129.35, 132.22 (2C), 159.11, 169.83 (2C); (+)-HRESI-MS calcd for C15H14NO4 (M+H+): 272.0923; found: 272.0925.
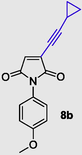
3-(cyclopropylethynyl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8b), Gummy solid; Yield (221 mg, 83%); 1H NMR (300 MHz, CDCl3) δ ppm 0.92–0.98 (m, 2 H), 1.19 (d, J = 7.16 Hz, 1 H), 1.59–1.64 (m, 2 H), 3.76 (s, 3 H), 6.76 (s, 1 H), 6.91–6.94 (m, 2 H), 7.16–7.19 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ ppm 8.80 (2C), 10.11, 54.39, 96.53, 112.77, 113.38 (2C), 122.74, 126.48 (2C), 130.39, 133.04, 158.06, 168.72 (2C); (+)-HRESI-MS calcd for C16H14NO3 (M+H+): 268.0974; found: 268.0980.
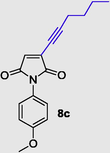
3-(hex-1-yn-1-yl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8c), Gummy solid; Yield (183 mg, 65%); 1H NMR (300 MHz, CDCl3) δ ppm 0.91 (d, J = 6.78 Hz, 3 H), 1.23–1.40 (m, 2 H), 1.71 (dd, J = 15.45, 6.97 Hz, 2 H), 3.45 (t, J = 6.00, 3.00 Hz, 2 H), 3.82 (br. s., 3 H), 6.96–7.45 (m, 5 H); 13C NMR (75 MHz, CDCl3) δ ppm 12.92, 18.41, 20.77, 31.23, 55.56, 96.11, 109.72, 119.66 (2C), 125.99 (2C), 131.69 (2C), 135.62, 157.07, 171.20 (2C); (+)-HRESI-MS calcd for C17H18NO3 (M+H+): 284.1287; found: 284.1295.
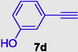
3-((3-hydroxyphenyl)ethynyl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8d), Gummy solid; Yield (271 mg, 85%); 1H NMR (300 MHz, CDCl3) δ ppm 3.79 (br. s., 3 H), 6.81–7.46 (m, 9H); 13C NMR (75 MHz, CDCl3) δ ppm 55.50, 95.26, 109.16, 113.96, 113.96, 114.57 (2C), 119.37, 121.46, 123.68, 124.23, 127.61 (2C), 129.71, 136.10, 155.90, 159.93, 168.83; (+)-HRESI-MS calcd for C19H14NO4 (M+H+): 320.0923; found: 320.0930.
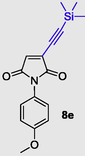
1-(4-methoxyphenyl)-3-((trimethylsilyl)ethynyl)-1H-pyrrole-2,5-dione (8e), Gummy solid; Yield (179 mg, 60%); 1H NMR (300 MHz, CDCl3) δ ppm 0.01 (s, 9 H), 3.59 (s, 3 H), 6.59 (s, 1H), 6.71–6.81 (d, J = 9.04 Hz, 1 H), 7.00 (d, J = 9.04 Hz, 9 H); 13C NMR (75 MHz, CDCl3) δ ppm 0.05, 0.56, 55.92, 96.24, 111.12, 114.92 (2C), 128.01 (2C), 129.67, 132.71, 134.57, 159.58, 170.27 (2C); (+)-HRESI-MS calcd for C16H18NSiO3 (M+H+): 300.1056; found: 300.1060.
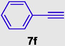
1-(4-methoxyphenyl)-3-(phenylethynyl)-1H-pyrrole-2,5-dione (8f), Gummy solid; Yield (242 mg, 80%); 1H NMR (300 MHz, CDCl3) δ ppm 3.79 (br. s., 3 H), 6.77–7.49 (m, 10 H); 13C NMR (75 MHz, CDCl3) δ ppm 55.50, 95.43, 111.59, 114.64 (2C), 121.27, 123.60, 127.56 (2C), 127.87 (3C), 129.47 (2C), 131.37 (2C), 136.31, 159.89, 171.49 (2C); (+)-HRESI-MS calcd for C19H14NO3 (M+H+): 304.0974; found: 304.0980.
1-(4-methoxyphenyl)-3-((4-pentylphenyl)ethynyl)-1H-pyrrole-2,5-dione (8g), Colorless crystal; mp (101–102 °C); Yield (261 mg, 70%); 1H NMR (300 MHz, CDCl3) δ ppm 0.89 (t, J = 6.00, 12.00 Hz, 3 H), 1.30–1.34 (m, 4 H), 1.60 (d, J = 7.54 Hz, 2 H), 2.61 (d, J = 7.90 Hz, 2 H), 3.82 (s, 3 H), 6.77 (s, 1 H), 6.96–6.99 (m, 2 H), 7.22–7.27 (m, 4 H), 7.50–7.53 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ ppm 14.20, 22.45, 31.46, 33.64, 37.52, 55.55, 97.21, 112.44, 114.46 (2C), 120.53, 124.88, 127.52 (2C), 130.01 (2C), 131.41, 134.20 (2C), 159.23, 171.12 (2C);); (+)-HRESI-MS calcd for C24H24NO3 (M+H+): 374.1756; found: 374.1765.
3-((6-methoxynaphthalen-2-yl)ethynyl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8h), Gummy solid; Yield (317 mg, 83%); 1H NMR (300 MHz, CDCl3) δ ppm 3.78 (br. s., 3 H), 3.82 (br. s., 3 H), 6.74–8.12 (m, 11 H); 13C NMR (75 MHz, CDCl3) δ ppm 55.41, 55.52, 97.46, 105.29, 114.44 (2C), 117.15 (2C), 127.15, 127.81 (2C), 128.91 (2C), 128.94, 130.02, 132.18 (2C), 132.99, 134.58, 142.20, 159.05, 159.67, 167.42 (2C); (+)-HRESI-MS calcd for C24H18NO4 (M+H+): 384.1236; found: 384.1240.
3-([1,1′-biphenyl]-4-ylethynyl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (8i), Gummy solid; Yield (299 mg, 79%); 1H NMR (300 MHz, CDCl3) δ ppm 3.79 (s, 3 H), 6.81 (d, J = 8.67 Hz, 1H), 6.96 (d, J = 8.85 Hz, 2 H), 7.30–7.66 (m, 11 H), 8.03 (d, J = 8.29 Hz, 1 H); 13C NMR (75 MHz, CDCl3) δ ppm 55.53, 96.40, 114.44 (2C), 124.12, 126.29, 127.06 (8C), 128.91 (3C), 128.97, 132.18 (2C), 140.08, 142.20, 159.67, 167.40 (2C); (+)-HRESI-MS calcd for C25H18NO3 (M+H+): 380.1287; found: 380.1297.
1-(4-methoxyphenyl)-3-(p-tolylethynyl)-1H-pyrrole-2,5-dione (8j), Yellow solid; mp (107-108 °C); Yield (291 mg, 92%); 1H NMR (300 MHz, CDCl3) δ ppm 2.32 (br. s., 3 H), 3.78 (s, 3 H), 6.70 (s, 1 H), 7.04–7.16 (m, 4 H), 7.25–7.49 (m, 4 H); 13C NMR (75 MHz, CDCl3) δ ppm 23.54, 55.46, 96.03, 106.06, 114.46 (3C), 123.85, 127.58 (2C), 129.21 (3C), 132.28 (2C), 133.58, 139.74, 159.19, 171.18 (2C); (+)-HRESI-MS calcd for C20H16NO3 (M+H+): 318.1130; found: 318.1142.
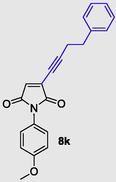
1-(4-methoxyphenyl)-3-(4-phenylbut-1-yn-1-yl)-1H-pyrrole-2,5-dione (8k), Gummy solid; Yield (252 mg, 76%); 1H NMR (300 MHz, CDCl3) δ ppm 2.40–3.04 (m, 4 H), 3.76 (s, 3 H), 6.77–7.40 (m, 10 H); 13C NMR (75 MHz, CDCl3) δ ppm 18.37, 34.53, 55.54, 114.52, 127.61, 127.71, 128.39, 128.56, 128.68, 128.97, 132.28, 139.78, 141.81, 159.63, 174.67; (+)-HRESI-MS calcd for C20H16NO3 (M+H+): 332.1287; found: 332.1295.
2.3 General procedure for triazole formation
3-(1-benzyl-1H-1,2,3-triazol-4-yl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (10a) – To a solution of 1-(4-methoxyphenyl)-3-((trimethylsilyl)ethynyl)-1H-pyrrole-2,5-dione (8e) (299.09 mg, 1.0 mmol, 1.0 eq) in THF (5 mL) at 25 °C under a nitrogen atmosphere were added benzyl azide (160 mg, 1.2 mmol, 1.2 eq) and CuI (28 mg, 0.15 mmol, 15 mol%). PMDETA (208 mg, 1.2 mmol, 1.2 eq) was followed by TBAF (1.2 eq) and the reaction was sonicated for 1 h. TLC analysis revealed no starting material. Next, the reaction was quenched with CH2Cl2 (20 mL) and the organic phase was washed with a saturated solution of NH4Cl (10 mL), then dried over MgSO4. Evaporation under reduced pressure followed by column chromatography provided the desired product.
3-(1-benzyl-1H-1,2,3-triazol-4-yl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (10a), Gummy solid; Yield (234 mg, 65%); 1H NMR (300 MHz, CDCl3) δ ppm 3.62–3.80 (m, 5 H), 6.75–7.38 (m, 10 H) 8.33 (s, 1 H); 13C NMR (75 MHz, CDCl3) δ ppm 55.53, 57.75, 96.57, 114.44, 119.1, 127.06 (3C), 127.50, 128.91, 130.02, 132.18 (2C), 132.99, 134.57, 159.67, 167.40 (2C); (+)-HRESI-MS calcd for C20H17N4O3 (M+H+): 361.1300; found: 361.1312.
3-(1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)-1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (10b), Gummy solid; Yield (209 mg, 55%); 1H NMR (300 MHz, CDCl3) δ ppm 3.66 (s, 3 H), 6.72–7.59 (m, 9 H), 8.33 (s, 1 H); 13C NMR (75 MHz, CDCl3) δ ppm 55.51, 100.67, 114.37 (2C), 124.65, 126.49, 127.84 (2C), 127.84, 129.13 (2C), 129.45 (2C), 131.1, 131.56, 134.14, 159.59, 169.41(2C); (+)-HRESI-MS calcd for C19H14ClN4O3 (M+H+): 381.0754; found: 381.0759.
1-(4-methoxyphenyl)-3-(1-(3-methoxyphenyl)-1H-1,2,3-triazol-4-yl)-1H-pyrrole-2,5-dione (10c), Gummy solid; Yield (188 mg, 50%); 1H NMR (300 MHz, CDCl3) δ ppm 3.63 (m, 3 H), 3.78 (s, 3 H), 6.77–7.76 (m, 9 H), 8.07 (s, 1 H); 13C NMR (75 MHz, CDCl3) δ ppm 54.80, 55.58, 97.19, 106.86, 114.22 (2C), 118.67, 125.83 (2C), 126.41 (3C), 131.63, 136.47, 141.50, 154.67, 157.76 (2C), 168.9(2C); (+)-HRESI-MS calcd for C20H17N4O4 (M+H+): 377.1250; found: 377.1254.
1-(4-methoxyphenyl)-3-(1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)-1H-pyrrole-2,5-dione (10d), Gummy solid; Yield (203 mg, 52%); 1H NMR (300 MHz, CDCl3) δ ppm 6.63–6.72 (m, 2 H), 6.80–6.85 (m, 2 H), 7.04–7.08 (m, 2 H), 7.32–7.39 (m, 2 H), 8.08–8.11 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ ppm 53.39, 100.38, 116.86 (2C), 118.83 (2C), 126.11(3C), 128.91 (2C), 132.21, 135.85 (2C), 144.83 (2C), 158.52, 169.20 (2C); (+)-HRESI-MS calcd for C19H14N5O5 (M+H+): 392.0995; found: 392.1002.
3 Results and discussion
Typical Sonogashira reaction conditions involve the use of PdCl2(PPh3)2 or Pd(PPh3)4 as the catalyst, CuI as the cocatalyst, and an excess amount of base used as the solvent. Often, an excess amount of the terminal alkyne is needed to obtain the final products in good yields with halogenated substrates.
We prepared maleimide acid 3 from maleic anhydride 1 by the treatment with amine 2 in the presence of water (Dulla et al., 2014). Thus the obtained 3 was reacted with acetic anhydride and sodium acetate (Kaur et al., 2015) to get the cyclic maleimide 4. This cyclic maleimide was treated with bromine to obtain the dibromo intermediate 5, which further on treatment with triethylamine afforded the desired halomaleimide 6 (Rulev et al., 2013) Scheme 1.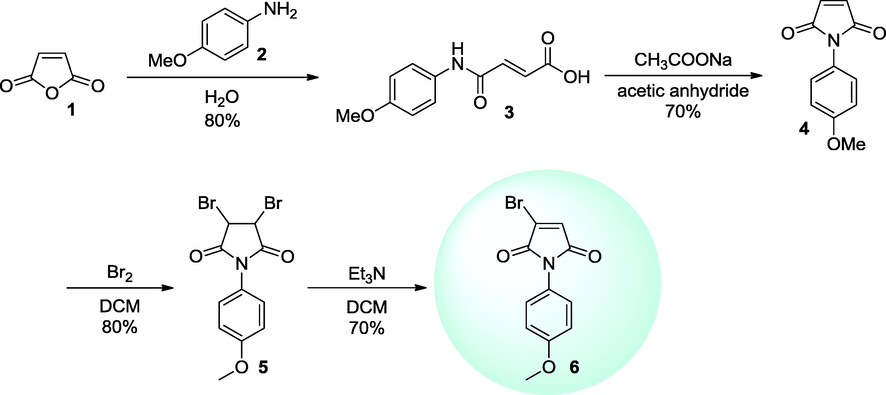
Synthesis of halomaleimide 6.
Initially, we surveyed transition metal-catalyzed cross-coupling reactions of a vinylic leaving group by a carbon nucleophile, the substitution nucleophilic vinylic (SNV) reaction, by developing a simple methodology using bases and solvents. The most representative results obtained using transition metal-catalyzed cross-coupling reactions are presented in entries 1–9 (Table 1).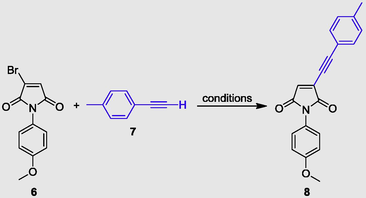
#
Catalyst (mol%)
Bases (eq)
Conditions (°C, h)
Solvent
Yield (%)a
1
Pd(OAc)2 (10)
Cs2CO3 (2)
rt, 24
MeOH
nr
2
Pd(OAc)2 (10)
K2CO3 (2)
rt, 24
MeOH
nr
3
Pd(OAc)2 (10)
K2CO3 (2)
rt, 24b
THF:H2O
nr
4
Pd(OAc)2 (10)
Cs2CO3 (2)
rt, 24b
DMF
nr
5
Pd(OAc)2 (10)/CuI (5)
Cs2CO3 (2)
rt, 4b
DMF
nr
6
PdCl2(dppf) (10)
K2CO3 (2)
rt, 5b
THF:H2O
nr
7
PdCl2(PPh3)2 (5)/CuI (5)
Et3N (1.5)
50, 18
THF
92
8
PdCl2(PPh3)2 (5)
Et3N (1.5)
50, 18
THF
40
9
CuI (5)
Et3N (1.5)
50, 18
THF
30
When 6 was reacted with 1-ethynyl 4-methylbenzene 7 in the presence of Pd(OAc)2 (10%), using Cs2CO3 (entry 1, Table 1) in methanol and K2CO3 (2 eq) in methanol and THF:H2O, at room temperature for 24 h (entries 2–3, Table 1), the reaction did not work.
Similar conditions were also applied for refluxing the reaction mixture, but no progress was made (entries 3–4, Table 1); using CuI as a co-catalyst did not affect the reaction (entry 5, Table 1). PdCl2(dppf) also did not give the acetylenic compound (entry 6, Table 1).
Using the catalyst combination of PdCl2(PPh3)2/CuI in THF in the presence of Et3N at room temperature the reaction also failed to give the desired coupling product,6c,11a but when temperature was elevated to 50 °C, the product was obtained in 92% yield (entry 7, Table 1). The reaction yield has proven to be dependent upon the presence of both catalysts, palladium and copper, whereas when used separately, only copper or palladium, the desired product was obtained in only 40% and 30% of isolated yields (entries 8 and 9, Table 1).
Having found the best conditions, to further investigate their catalytic role, PdCl2(PPh3)2 and CuI were used alone, giving poor yields (40% and 30%, respectively, entries 8–9, Table 1), which indicates that the mixture of catalysts (entry 7, Table 1) at 50 °C and Et3N has prime importance in the coupling of acetylene with halomaleimide in our synthetic strategy.
As shown in Table 2, several aliphatic and aromatic alkynes (7) were found to react smoothly with halomaleimide 6 in the presence of PdCl2(PPh3)2/CuI in THF, and the desired products 8a−k were obtained in yields (entries 1–11) ranging from 60% to 92%.
It was found that the reaction carried out with alkyl alkynes worked well to produce 76% and 65% yields (Table 2, entries 1 and 3), and showed that 3-butyne-1-ol gives moderate yields (Table 2, entry 1). Cyclopropene resulted in a good yield of 83% (Table 2, entry 2). Similarly, trimethylsilyl alkyne yielded 60% (Table 2, entry 5).
Generally, coupling reactions with alkyl acetylenes yielded the expected products in moderate yields (Table 2, entries 2 and 3). For aryl alkynes, the product yield depended on the substitution pattern of the phenyl ring. Arylacetylenes having electron-rich moieties gave better yields (85% and 83%; Table 2, entries 4 and 8) than other alkynes (70–80%, respectively; Table 2, entries 6, 7 and 9). A crystal of 8g was obtained by slow evaporation from ethyl acetate at room temperature.
3.1 X Crystal structure analysis of 8g
The molecular structure of 8g is shown in Fig. 2 together with the atom-labeling scheme. The crystal and structure refinement data are collated in Table 3. The cyclopent-4-ene-1,3-dione ring makes a torsion angle with the phenyl ring attached to it of 64.46(11)° being almost coplanar with the other one [dihedral angle = 6.08(14)°]. In the crystal the three-dimensional architecture is stabilized by intermolecular C–H⋯O (H9⋯O3i = 2.82 Å, C9–H9⋯O3 = 111°; symmetry operation i = x, y 1 + z) and C–H⋯π [H18⋯Cg(C1–C6)i = 2.95 Å, C18–H18⋯Cg(c1–C6)i; symmetry operation i = x, y 1 + z) interactions, as seen in Fig. 3.![The molecular structure of 2-(4-methoxyphenyl)-4-[2-(4-pentylphenyl)ethynyl]cyclopent-4-ene-1,3-dione.](/content/184/2017/10/4/img/10.1016_j.arabjc.2016.06.003-fig5.png)
The molecular structure of 2-(4-methoxyphenyl)-4-[2-(4-pentylphenyl)ethynyl]cyclopent-4-ene-1,3-dione.
Color/dimensions (mm)
Colorless/0.20 × 0.25 × 0.3
Chemical formula
C24H23NO3
Formula weight
373.43
Crystal system
Triclinic
Space group
P1
Unit cell dimensions
a (Å)
5.3692(6)
b (Å)
9.2513(10)
c (Å)
10.3070(11)
α (°)
85.349(4)
β (°)
84.420(4)
γ (°)
86.892(4)
Volume (Å3)
507.31(10)
Z
1
Density (calculated) (g/cm3)
1.222
Absorption coefficient (mm−1)
0.080
F (0 0 0)
184
θ range for data collection (°)
1.99–25.24
Reflections measured
13205
Independent/observed reflections
4186/3355 (Rint = 0.024)
Data/restraints/parameters
4186/3/255
Goodness of fit on F2
1.076
Final R indices [I > 2_(I)]
R1 = 0.052, wR2 = 0.151
R indices (all data)
R1 = 0.066, wR2 = 0.164
Δρ max, min, (e.Å−3)
0.37/−0.20

Crystal packing in 8g showing the C–H⋯O and C–H⋯π interactions.
As a concern regarding the synthetic utility of general synthesis of 1-(4-methoxyphenyl)-3-((trimethylsilyl)ethynyl)-1H-pyrrole-2,5-dione (8e), different azides were reacted in order to obtain 1,2,3-triazoles. The conducted reactions provided the desired products in good yield, similar to the methodology developed earlier by our group (Scheme 2).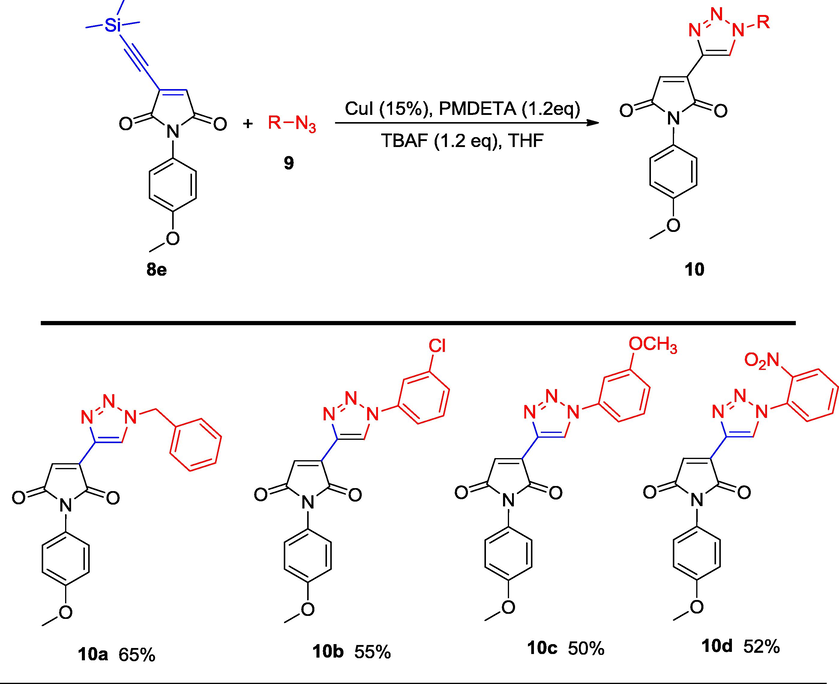
Synthesis of 1,2,3-triazole rings from alkynyl maleimide (8e) (Isolated yield.).
4 Conclusion
In summary, we have described a simple method where bromomaleimide undergoes a cross-coupling reaction under the Sonogashira conditions with acetylenes in the presence of PdCl2(PPh3)2/CuI catalysis. The alkynyl maleimides were obtained in good to very good yield range. The alkynylated products were then subsequently converted into the corresponding 1,2,3-triazoles via a click chemistry approach in moderate yields. This simple approach may find interest in the development of small molecules incorporating triazoles. The crystal structure of 8g showed that the five membered ring makes a torsion angle with the phenyl ring attached to it of 64.46(11)° being almost coplanar with the other one. The molecules are arranged in the crystal in layers parallel to z through C–H⋯O and C–H⋯π interactions.
Acknowledgments
The authors are grateful for the financial support provided by FAPESP – São Paulo Research Foundation (Grant 2012/00424-2 and fellowships to BA – 2012/17954-4 and SNSV – 2013/17960-7) and CNPq – the National Council for Scientific and Technological Development (308.320/2010-7 to HAS, 305626/2013–2 to JZ-S). We thank Professor Regina H.A. Santos from IQSC-USP for the X-ray data collection.
References
- Development of methods for the synthesis of libraries of substituted maleimides and α, β-unsaturated-γ-butyrolactams. J. Org. Chem.. 2011;76:3122-3130.
- [CrossRef] [Google Scholar]
- Synthesis and applications of 4-substituted 1-(4-iodophenyl)pyrrolidine-2,5-diones. Tetrahedron Lett.. 2015;56:4234-4241.
- [CrossRef] [Google Scholar]
- Lewis-acid catalyzed N-acyliminium ion cyclodimerization: synthesis of symmetrical 1,4-dioxanes. Tetrahedron Lett.. 2015;56:1153-1158.
- [CrossRef] [Google Scholar]
- A Pd[0]-catalyzed Ullmann cross-coupling/reductive cyclization approach to C-3 mono-alkylated oxindoles and related compounds. Tetrahedron. 2010;66:9252-9262.
- [CrossRef] [Google Scholar]
- Synthesis of 3,4-disubstituted maleimides by selective cross-coupling reactions using indium organometallics. Org. Lett.. 2009;11:1285-1288.
- [CrossRef] [Google Scholar]
- Catalytic and stoichiometric synthesis of novel 3-aminocarbonyl-, 3-alkoxycarbonyl-, and 3-amino-4-indolylmaleimides. Eur. J. Org. Chem.. 2009;1:38-42.
- [CrossRef] [Google Scholar]
- Crystal structure determination and refinement via SIR2014. J. Appl. Cryst.. 2015;48:306-309.
- [CrossRef] [Google Scholar]
- Production and biological activity of rebeccamycin, a novel antitumor agent. J. Antibiot.. 1987;40:668-678.
- [Google Scholar]
- Crystal structure of 1-benzyl-2-hydroxy-5-oxopyrrolidin-3-yl acetate. Acta Crystallogr., Sect. E. 2015;71:o582-o583.
- [CrossRef] [Google Scholar]
- Short review metal-catalyzed cross-coupling reactions of halomaleic anhydrides and halomaleimides: synthesis of structurally interesting and biologically important natural and unnatural products. Synthesis. 2014;46:281-289.
- [CrossRef] [Google Scholar]
- Sonogashira coupling reactions of bromomaleimides: route to alkyne/cis-alkene/alkyl maleimides: synthesis of Luffarin X and Cacospongionolide C. J. Org. Chem.. 2012;77:739-746.
- [CrossRef] [Google Scholar]
- Synthesis of substituted bis(heteroaryl)maleimides. Tetrahedron. 2005;61:4585-4593.
- [CrossRef] [Google Scholar]
- Catalyst/surfactant free chemoselective acylation of amines in water. Curr. Green Chem.. 2014;1:73-79.
- [CrossRef] [Google Scholar]
- O-(trialkylstannyl)anilines and their utility in Migita–Kosugi–Stille cross-coupling: direct introduction of the 2-aminophenyl substituent. Tetrahedron Lett.. 2012;53:4938-4941.
- [CrossRef] [Google Scholar]
- WinGX and ORTEP for windows: an update. J. Appl. Cryst.. 2012;45:849-854.
- [CrossRef] [Google Scholar]
- Asymmetric indolylmaleimide derivatives and their complexation with Zinc(II)−Cyclen. J. Phys. Chem. A. 2005;109:9443-9455.
- [CrossRef] [Google Scholar]
- Sensitization of nanocrystalline TiO2 films with carboxy-functionalized bis(indolyl)maleimide. Eur. J. Org. Chem. 2005:3443-3449.
- [CrossRef] [Google Scholar]
- Unexpected photophysical properties of symmetric indolylmaleimide derivatives. J. Phys. Chem. A. 2005;109:6440-6449.
- [CrossRef] [Google Scholar]
- One-pot regioselective synthesis of novel 1-N-methyl-spiro[2,3′]oxindole-spiro[3,3″]-1″-N-arylpyrrolidine-2″,5″-dione-4-arylpyrrolidines through multicomponent 1,3-dipolar cycloaddition reaction of azomethine ylide. J. Het. Chem.. 2015;52:827-833.
- [CrossRef] [Google Scholar]
- Synthesis of 6H-pyrrolo[3′,4′:2,3][1,4]diazepino[6,7,1-hi]indole-8,10(7H,9H)-diones using 3-bromo-4-(indol-1-yl)maleimide scaffold. Org. Biomol. Chem.. 2003;1:826-833.
- [CrossRef] [Google Scholar]
- Isolation and structure of rebeccamycin – a new antitumor antibiotic from nocardia aerocoligenes. Tetrahedron Lett.. 1985;26:4011-4014.
- [CrossRef] [Google Scholar]
- Efficient palladium-catalyzed synthesis of 3-aryl-4-indolylmaleimides. Org. Biomol. Chem.. 2008;6:992-997.
- [CrossRef] [Google Scholar]
- Rebeccamycin analogues as anti-cancer agents. Eur. J. Med. Chem.. 2003;38:123-140.
- [CrossRef] [Google Scholar]
- Arylmaleic anhydrides via Heck arylation of fumaric acid. Tetrahedron Lett.. 2010;51:3633-3635.
- [CrossRef] [Google Scholar]
- Reaction of α-bromo enones with 1,2-diamines. Cascade assembly of 3-(trifluoromethyl)piperazin-2-ones via rearrangement. Org. Lett.. 2013;15:2726-2729.
- [CrossRef] [Google Scholar]
- Indolocarbazole natural products: occurrence, biosynthesis, and biological activity. Nat. Prod. Rep.. 2006;23:1007-1045.
- [CrossRef] [Google Scholar]
- Efficient and general protocol for Sonogashira cross-coupling reactions of maleimides. Eur. J. Org. Chem. 2012:2499-2502.
- [CrossRef] [Google Scholar]
- Structure validation in chemical crystallography. Acta Cryst.. 2009;D65:148-155.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted addition of alcohols to N-acyliminium ions mediated by In(OTf)3 and synthesis of 1,2,3-triazoles. Tetrahedron Lett.. 2014;55:3400-3405.
- [CrossRef] [Google Scholar]
- Synthesis of functionalized N-triazolyl maleimides. Tetrahedron Lett.. 2014;55:4355-4358.
- [CrossRef] [Google Scholar]
- A concise synthesis of maleic anhydride and maleimide natural products found in Antrodia camphorata. Tetrahedron Lett.. 2007;48:2241-2244.
- [CrossRef] [Google Scholar]