Translate this page into:
Effects of inert gas CO2/N2 injection on coal low-temperature oxidation characteristic: Experiments and simulations
⁎Corresponding author. Dingdc123@163.com (Cong Ding)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
To deeply understand the mechanism of inert gases in inhibiting coal spontaneous combustion, the effects of dry air, CO2, and N2 on coal spontaneous combustion were analyzed experimentally. To this end, bituminous coal prepared from Dongrong No. 2 Coal Mine was considered the research object. Based on the adsorption configuration of the oxygen-containing coal, the displacement behavior of O2 by CO2 /N2 was studied using the grand canonical Monte-Carlo (GCMC) and molecular dynamics (MD) methods. The obtained results show that the injection of CO2 and N2 reduces the ability of spontaneous combustion of coal. It is found that among the studied gases, CO2 has a stronger inhibition effect on coal spontaneous combustion, which increases the temperature of CO occurrence by 5℃, decreases the concentration of CO by 29.91%, and inhibits low-temperature oxidation of coal. From the microscopic point of view, CO2 /N2 gases can effectively displace O2 by diffusion and occupying adsorption sites. It is found that after the injection of CO2, the concentration of O2 molecules increases significantly in the vacuum layer. Compared with N2, injection of CO2 increases the diffusion activation of O2 by 5.89%. This indicates that the injection of an inert gas significantly reduces the oxygen absorption capacity of coal, thereby decreasing the coal-oxygen combination reaction and preventing the spontaneous combustion of coal. The performed analyses demonstrate that CO2 outperforms N2 in restraining the spontaneous combustion of coal.
Keywords
Low-temperature oxidation of coal
Inert gas injection
Inhibition effect on coal spontaneous
Gas injection desorption
1 Introduction
Coal mine fire is one of the main disasters that threaten the safety of coal extraction (Shi et al., 2022; Perera et al., 2011; Adamus et al., 2011). This hard-to-control disaster seriously threatens the safety of underground personnel, equipment, and engineering facilities, easily spoils coal resources, leads to environmental problems, affects coal production, and causes economic losses. Moreover, the coal fire may cause disorders in the ventilation system, reverse the normal airflow, and even ignite the explosion of gas and coal dust (Nakamura, 1995; Xia et al., 2019; Lin et al., 2016). In this regard, N2 and CO2 inert gas fire prevention and extinguishing technology have been proposed to effectively inhibit spontaneous combustion of the coal seam in goaf.
In terms of fire prevention methods using an inert gas, numerous experimental investigations have been carried out (Meng et al., 2018; Wang et al., 2010; Shao et al., 2014; Zhou et al., 2022). Wang (Liu, 2016) analyzed the performance of CO2 and N2 gases in inhibiting spontaneous combustion of coal and the variations of spontaneous combustion parameters such as CO production rate and oxygen consumption rate in coal samples. Shao (Zhang et al., 2009) studied the effect of CO2 and N2 gases in inhibiting coal spontaneous combustion. The obtained results for apparent activation energy showed that CO2 outperforms N2 in inhibiting coal spontaneous combustion. Zhou (You, 2016) studied the mechanism and inhibitory effect of N2 and CO2 gases on the CSC and found that the inhibition rate of CO2 is higher than that of N2.
Generally, most investigations in this area are focused on analyzing the influence of inert gas on coal spontaneous combustion through experiments. It should be indicated that coal is composed of inorganic and organic substances, and has complex internal surface structure characteristics. Accordingly, it is necessary to study the gas injection displacement mechanism at the molecular scale (Cui, 2005; Gao et al., 2020; Xiang, 2014; Wang et al., 2017). In this regard, molecular simulation has been widely used to study the gas adsorption by coal, providing theoretical support for goaf fire prevention technologies (Li et al., 2019; Liu et al., 2016; Zhang et al., 2014; Chen, 2016). Wu (Wu et al., 2019) used the grand canonical ensemble Monte-Carlo method to analyze the ability and competitive difference of coal to adsorb CO2, O2, and N2 gases according to the actual situation of fire prevention and storage of the power plant flue gas injected into goaf. Lou et al. (Lou and Jia, 2020) established a macromolecule-scale model to simulate the surface adsorption of different gases, including O2, CO2, and N2 by the coal surface. Then the effect of the gas competition difference of mixed gas molecules on the surface adsorption was analyzed. Tan et al. (Tan et al., 2022) applied GCMC and DFT methods and systematically studied the physical adsorption characteristics of O2 in low-rank coal.
The performed literature survey reveals that most investigations are focused on the inhibition of coal spontaneous combustion by inert gas and reducing the coal-oxygen reaction rate. It is worth noting that the injection of inert gases such as CO2 and N2 affects the adsorption of oxygen in the coal-oxygen reaction. However, this mechanism has not yet been elucidated. In the present study, it is intended to perform thermal analysis experiments to study the influence of dry air, CO2, and N2 on the spontaneous combustion of bituminous coal. Taking wiser bituminous coal as an example, the interaction between CO2 and N2 gases injected into the coal is studied using molecular simulation, and the adsorption mechanism and behavior of coal for inert gas are explored to provide theoretical support for goaf fire prevention technologies.
2 Methods
2.1 Experimental method
2.1.1 Preparation and treatment of coal samples
The bituminous coal prepared from Dongrong No. 2 coal mine in Shuangyashan, Heilongjiang Province, China is used as the research object. Table 1 shows the main properties of coal samples. The particle size of the coal sample is 100 ∼ 250 mesh. The coal samples were divided into 3 parts. Then samples were placed in CO2, N2, and dry air incubators to absorb gas at 30 ℃ for 24 h. After the adsorption process, the coal samples were placed in the sample tank to perform the thermal analysis tests in a vacuum.
Sample Total
Moisture (%)
Ash (%)
Volatile (%)
Sulfur (%)
DR
3.15
19.585
25.68
0.68
2.1.2 Adiabatic oxidation and index gas test
According to the prediction model of adiabatic oxidation experiments, an oxidation experimental device was developed to collect the CO gas produced during coal oxidation. Fig. 1 schematically shows the main parts of the test setup.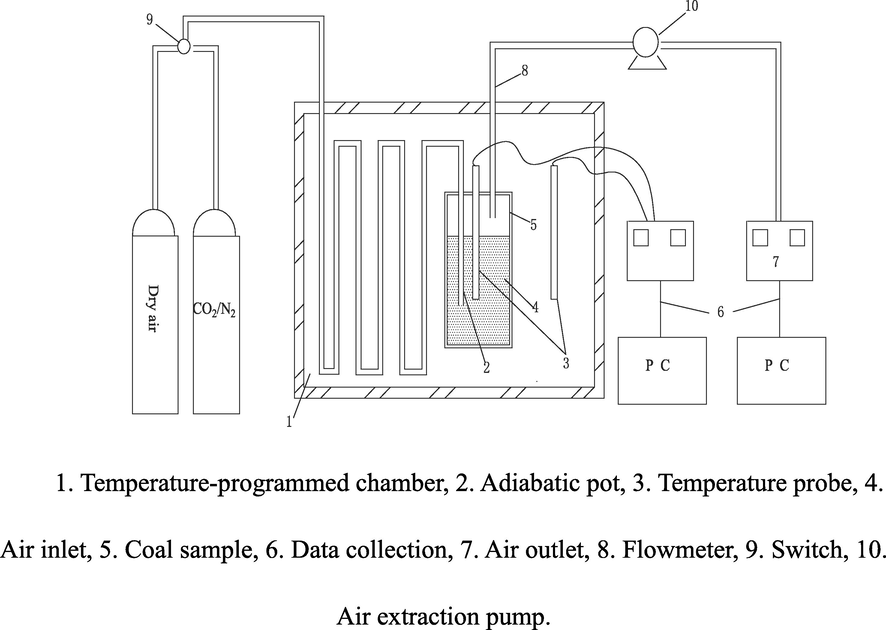
Schematic diagram of the self-heating experimental device.
During the experiment, the treated coal sample is placed in the sample tank, and a thermocouple is installed at the tank center to monitor the temperature during the low-temperature oxidation of coal. Under the action of the air pump, the gas concentration of the test coal sample in the low-temperature oxidation process is detected in real-time using a chromatograph. Then gas samples were extracted every 5 ℃ to perform further analyses. The temperature of the adiabatic oxidation bottle and the adiabatic furnace is detected using a temperature detector, and the temperature of the two parts was kept constant (error less than 0.2 °C) using a controller.
2.2 Simulation method
2.2.1 Construction of the coal structure model
Coal rock is a complex, strained, heterogeneous, cross-linked macromolecular solid, which has an amorphous macromolecular structure (Xia, 2020). Based on physical and chemical analyses, different molecular models have been proposed for bituminous coal. In this regard, the Wiser model (Wiser et al., 1967) is a widely adopted model for bituminous coal. In the present study, this model is used to study the coal samples. Table 2 shows the structural parameters of the Wiser model.
Molecular formula
Molecular weight
Element content
C
H
O
N
S
C192H165O20N5S8
3115
73.96
10.27
5.30
2.25
8.22
2.2.2 Model optimization
In the Wiser bituminous coal macromolecular model, CO2, O2, and N2 are optimized to obtain the initial configuration with the lowest energy. Considering the energy change in the system, the new configuration is formed using the Metropolis operation rule. In the three-dimensional direction restricted by periodic boundary conditions, the unit cell is a cube with an edge length of 21.9 Å.
The COMPASSⅡ force field was used to geometrically optimize the three-dimensional structure, and a global energy minimization configuration was obtained by Annealing. The setting of MD parameters is presented in Table 3. The final model is shown in Fig. 2. Then the cell was expanded and a 50 Å vacuum layer was added. The model construction process is shown in Fig. 1. The coal density is set to 1.21 g/cm3 (the actual bituminous coal density is generally in the range of 1.2–1.4 g/cm3 (Meng, 2020).
Dynamics
Forcefield
COMPASSⅡ (Zhang et al., 2020)
Charges
Use current
Electrostatic
Ewald (Karasawa and Goddard, 1992)
Production
106
Van der Waals
Atom based (Zhang et al., 2016)
Step size/fs
1
Canonical ensemble
NVT (Parrinello, 1981)
Steps Total time/ps
1000
Temperature
Nose (Nosé, 1991)
Quality
Fine
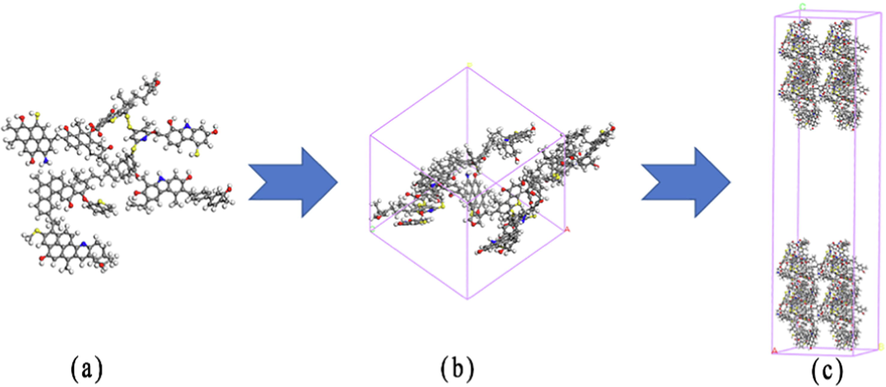
Schematic construction of the coal structure model: (a) single-molecule model, (b) bituminous coal surface model, and (c) supercell model.
2.3 Determination of initial configuration of the displacement
To determine the initial configuration of the displacement, it is necessary to calculate the adsorption characteristic parameters of the cell model. In the Sorption module, the Adsorption Isotherm was used as the task item while the range of the fugacity and temperature were set to 10–1000 kPa and 293–333 K, respectively. The number of steps from simulation loading to equilibrium state is 1 × 108, and the total number of steps is 2 × 108. The metropolis and the charge balance (QEq) methods were used to calculate the configuration and charge, respectively. The applied methods to calculate the force field, van der Waals force, hydrogen bond force, and Coulomb forces were consistent with those in the structural optimization of the above model. Meanwhile, the sorption module was applied to calculate the adsorption amount through fugacity and converts fugacity and pressure based on the P-R equation of state (Long et al., 2021). Through simulation at different temperatures, the adsorption characteristic parameters of the lowest energy adsorption configuration were obtained as the initial configuration for the oxygen adsorption by coal (see Table 4).
Temperature(K)
System energy(kcal/mol)
Number of adsorbed molecules (per cell)
Average adsorption heat(kcal/mol)
293
−181.89
147
1.80
303
−146.01
127
1.77
313
−144.16
122
1.73
323
−129.06
114
1.71
333
−115.69
104
1.78
Fig. 3(a) shows the adsorption capacity of single component O2 at different temperatures and fugacity. It is observed that when the fugacity increases from 10 kPa to 1000 kPa, the adsorption capacity of O2 increases rapidly. Meanwhile, it is found that as the temperature increases, the corresponding adsorption of O2 decreases gradually. Fig. 3(b) shows the distribution between the adsorption heat and the adsorption capacity at different temperatures. These obtained results are consistent with the presented curves (Wu et al., 2019). In Fig. 3(b), the adsorption heat has an exponential correlation with the adsorption capacity. Moreover, the average adsorption heat has an inverse correlation with the adsorption capacity and the temperature.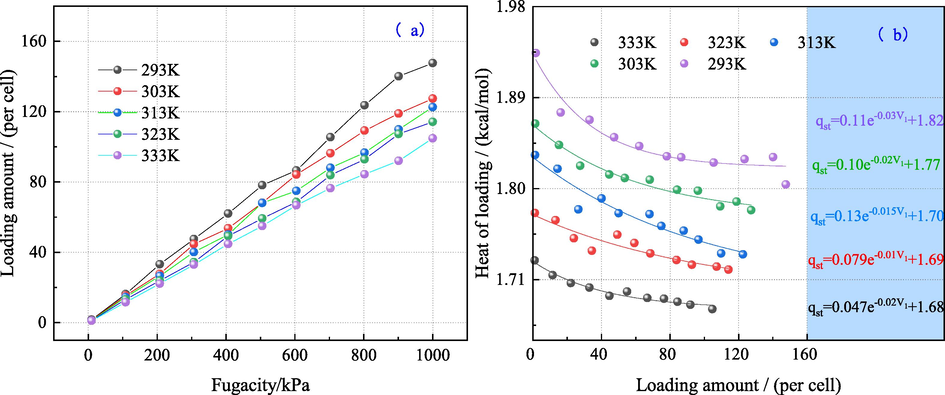
Distribution of the adsorption capacity of bituminous coal and the floating heat against pressure at different temperatures.
2.4 Injection of inert gas into the initial configuration of displacement
Generally, CO2 and N2 gases are injected to prevent the spontaneous combustion of coal. The initial configuration of displacement is based on the adsorption configuration determined in Section 2.2.3. The Sorption module with a temperature in the range of 293–333 K was adopted, and the initial configuration of the displacement was used in simulating the adsorption of two inert gases. Table 5 shows the characteristics of the injected gases. Focite module was used in the simulation calculation of O2 displacement. In this module, dynamics properties are set and the temperature was set to 293–333 K. The temperature control method, ensemble, and force field were consistent with the Dynamics parameters.
Gas type
System energy(kcal/mol)
Number of adsorbed molecules (per cell)
Average adsorption heat(kcal/mol)
CO2
−694.35
255
5.26
N2
−113.59
86
1.23
3 Experimental results and analysis
3.1 Effect of CO2/N2 on the heat release from the coal self-reaction
The main objective of the self-heating method is to eliminate the energy transfer between the coal and the external environment so the heat generated by the natural oxidation of coal can be retained in its own body. In this case, the self-oxidation process improves and spontaneous combustion of coal can be characterized. In the present study, the time from 40 ℃ self-heating to 110 ℃ is selected as the shortest ignition time.
Fig. 4 reveals that the minimum ignition time of the coal sample injected with dry air is 26.5 h. After CO2/N2 injection, the minimum ignition time of coal samples was extended by 27.5 h and 20.5 h, respectively. This indicates that compared with N2, CO2 has a stronger ability to inhibit coal oxidation heat release.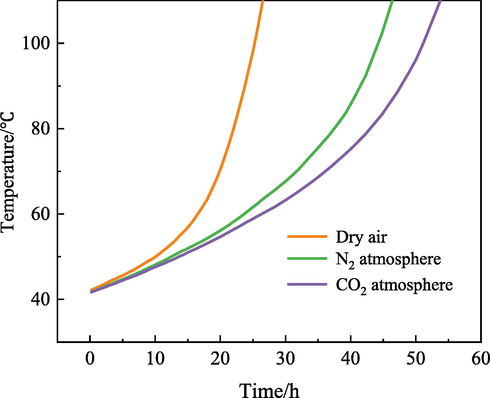
Autothermal oxidation curve of coal sample.
3.2 Effect of CO2/N2 on the CO generation during low-temperature oxidation of coal
The content of CO gas produced at different temperatures during the autothermal experiment was detected using a chromatograph, and the effect of CO2/N2 on low-temperature oxidation of coal was analyzed. Fig. 5 shows the volume fraction of gas products in the range of 40 ∼ 110 ℃.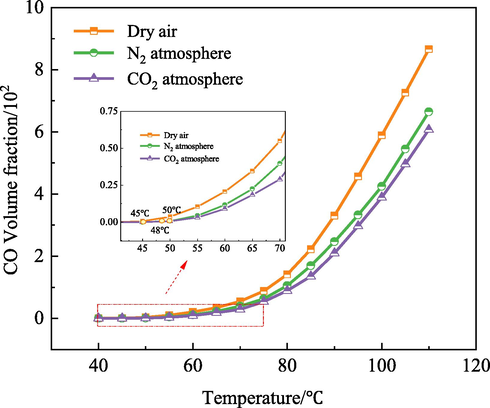
Variations of the CO volume fraction during coal low-temperature oxidation.
Fig. 5 shows that the coal sample is accompanied by the generation of CO and other gases during low-temperature oxidation. It is found that as the temperature of the test coal sample increases, the corresponding volume fraction of CO increases too.
When the temperature is lower than 70 ℃, the increase of the CO volume fraction is relatively gentle. However, when the temperature reaches 70 ℃, the slope of the curve begins to increase and the CO production increases rapidly. In the low-temperature stage, coal oxidation is slow, and a small amount of CO and other gases is produced in the reaction. As the temperature further increases, the chemical structure of coal destroys under the action of oxidation and pyrolysis, thereby producing a large number of carbon-containing active groups and producing a large amount of CO gas. When the coal sample is subjected to the CO2 and N2 atmosphere, the volume fraction of the CO gas generated by the low-temperature oxidation is lower than that of samples exposed to the dry air. In the initial oxidation stage, the initial temperature of CO in coal samples subjected to dry air, CO2, and N2 atmospheres is 45 ℃, 48 ℃, and 50 ℃, respectively. It indicates that CO2 has a strong inhibitory effect on coal spontaneous combustion, which increases the temperature of CO by 5 ℃. When the temperature of the test coal sample is 110 ℃, the volume fraction of CO gas in the samples subjected to CO2, and N2 atmospheres reduces to 6.07 × 102 and 6.65 × 102, respectively, which is 29.91 % and 23.21 % lower than that of the sample in the dry air. This shows that the inert gas reduces the low-temperature oxidation capacity of coal and effectively inhibits the spontaneous combustion of coal. Compared with N2 injection, CO2 injection has a higher inhibition effect on the spontaneous combustion of coal.
4 Analyzing the influence of the inert gas on the O2 displacement
Analyzing the characteristic parameters in the foregoing sections showed that CO2 has a promising inhibition ability on coal spontaneous combustion. In this section, it is intended to apply the GCMC and MD methods to analyze the inhibition of coal-oxygen reaction by an inert gas.
During the coal mining process, the coal body may be destroyed, which increases oxygen adsorption. The injection of inert gas reduces the O2 concentration in the environment, and displaces the adsorbed oxygen in the coal, thereby reducing the coal-oxygen reaction. In this regard, a model is proposed to analyze.
4.1 Energy and average relative concentration
Fig. 6 shows the analytical configuration of O2 in N2 and CO2 coal models when the temperature varies in the range of 293–333 K. It is observed that compared with the N2 injected configuration, there are many free O2 molecules in the CO2 injected configuration, indicating that CO2 injection has a better effect on promoting the replacement of O2.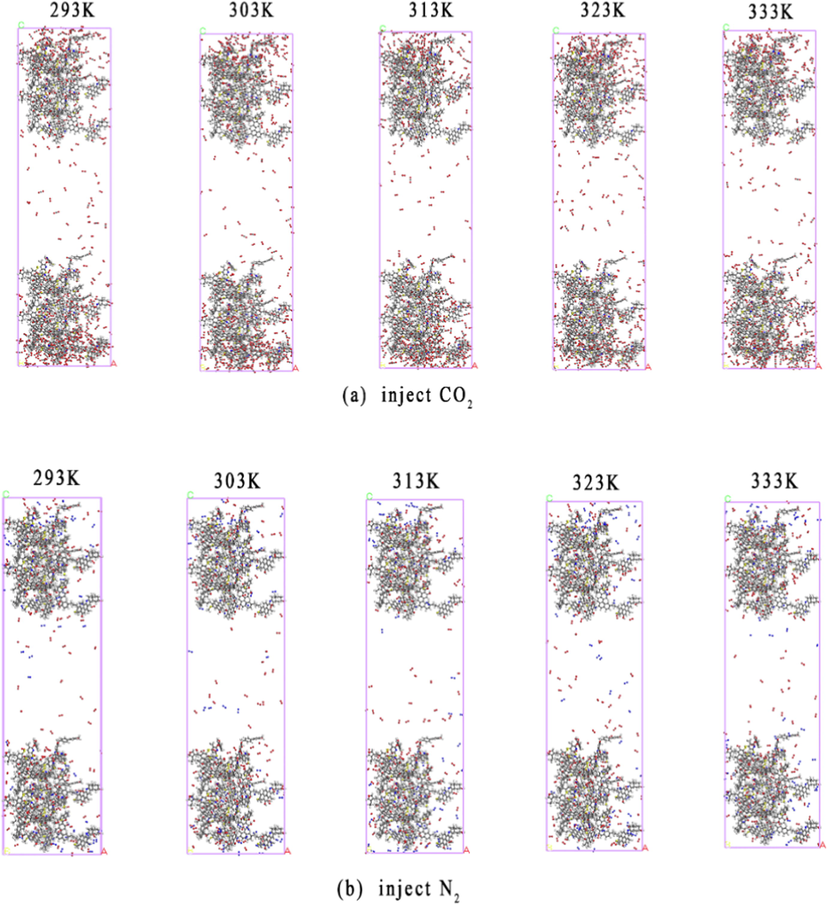
Configuration of oxygen displacement in coal.
Fig. 7 shows the energy level of different systems before and after displacement. Fig. 7 indicates that as the temperature increases, the total energy of the system before displacement increases rapidly. Meanwhile, the total energy of the system after displacement increases slowly. When the temperature reaches 293 K, the total energy of the O2-CO2 and N2-O2 system after displacement increases by 34.59 % and 47.58 %, respectively. Accordingly, the total energy growth rate of the O2-N2 system is higher than that of the O2-CO2 system at the same temperature. It is worth noting that this displacement is an endothermic reaction. The energy released in the adsorption process is less than that released in the displacement process, and the energy of the CO2 system is always higher than that of the N2 system, which indicates that the O2-CO2 system has a stronger intermolecular bonding (Zheng et al., 2020) (see Fig. 8).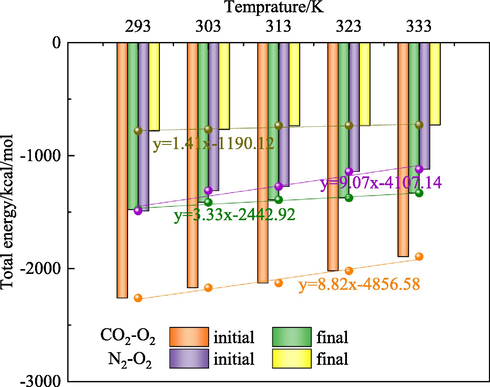
Energy level of O2-CO2 and O2-N2 systems.
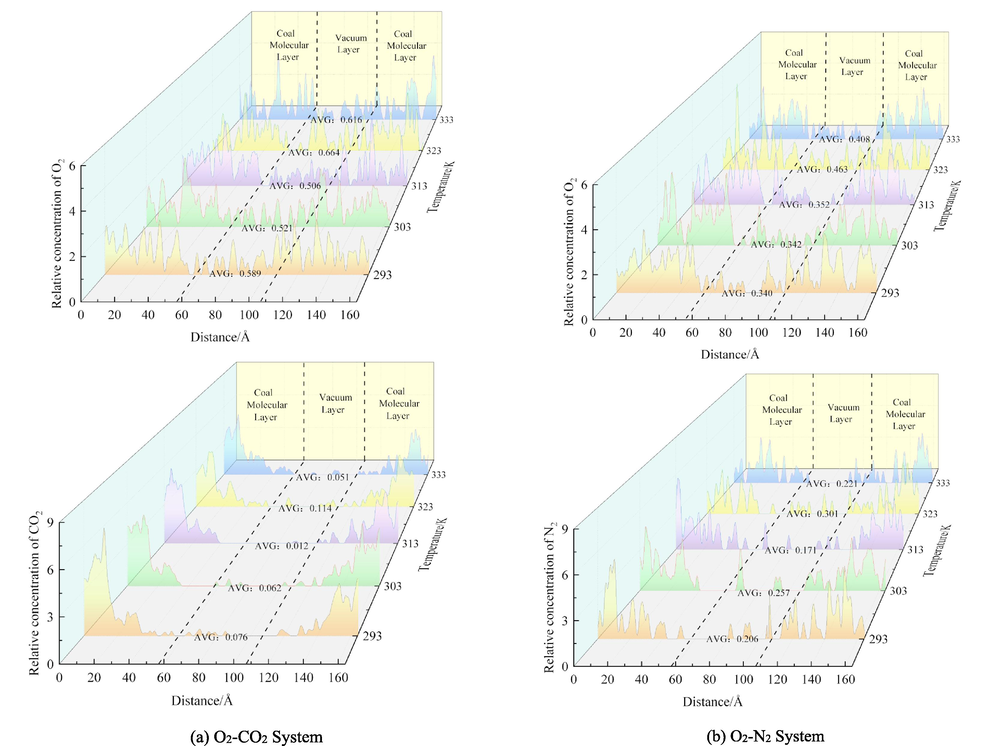
Relative concentration distribution of O2, CO2, and N2 in different systems.
In the present study, the effect of CO2 and N2 on the displacement of O2 in coal is studied from the microscopic point of view, and the average relative concentration of the two systems after kinetic optimization is analyzed using the Forcite module. Fig. 9 shows the average relative concentration of the three gases in coal molecules after injecting CO2 and N2 gases. 0–57 Å and 107–164 Å are the molecular layers of coal, and 57–107 Å is the vacuum layer. It is observed that all three gases diffuse into the vacuum layer. It is found that O2 molecules increase the most, followed by N2, and CO2 molecules have the lowest increment. In the range of 293–333 K, the average relative concentration of O2 increased by 4.58 % and 2.00 % after CO2 and N2 injection, respectively. In the O2-CO2 system, the concentration of O2 was relatively high in the vacuum layer. At the same temperature, the concentration of O2 displaced by CO2 injection was 42.28 % (293 K), 34.36 % (303 K), 30.43 % (313 K), 30.27 % (323 K), 33.77 % (333 K) higher than that by N2 injection. This indicates that CO2 promotes coal to have a better effect on the O2 displacement. These results are consistent with experimental data (Lou and Jia, 2020; Yin et al., 2020).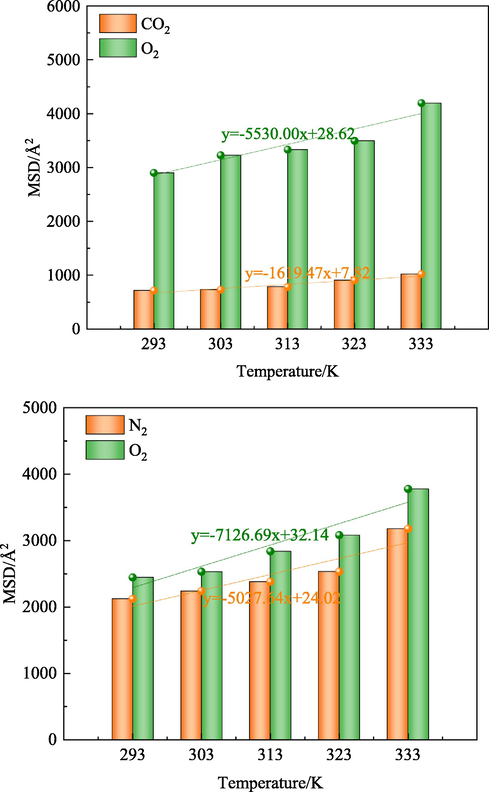
MSD Curves of Gas at Different Temperatures.
From the microscopic point of view, CO2 and N2 displace O2 by occupying the adsorption positions. Based on the competitive adsorption theory (Zheng et al., 2020), coal can absorb different gases simultaneously. However, the adsorption amount depends on the concentration of each component and the adsorption capacity of coal to each component. At a constant volume fraction of O2, the higher the adsorption capacity of coal to other components, the weaker its adsorption capacity to O2, and the lower the amount of absorbed oxygen. It should be indicated that the adsorption capacity of coal to CO2 is greater than that of N2. Therefore, at a constant O2 volume fraction, the oxygen absorption capacity of coal in the O2-N2 system is higher than that in the O2-CO2 system. Since coal has a different capacity to absorb oxygen, the coal-oxygen combination theory indicates that the oxygen absorption will be different. This phenomenon adversely affects the capability of the inert gas to inhibit the spontaneous combustion of coal. Therefore, the initial formation temperature of CO indicator gas in the O2-CO2 system is higher than that of N2. Moreover, the amount of CO production is relatively low and the oxidation time of coal is long. It is concluded that CO2 outperforms N2 in restraining the spontaneous combustion of coal.
4.2 Diffusion of O2, CO2, and N2
Mean square displacement (MSD) is an effective indicator to analyze the deviation of a particle's position over time from a reference position. MSD is the most commonly used measure of spatial extent in random motions and can be considered a measure of the part of the system that random walkers “explore”. In this section, the correlation between MSD and time (t) was obtained by simulating the particle motion trajectory, and the diffusion coefficient of particles in coal was calculated through the Einstein equation (Mosher et al., 2013). To this end, the Forcite module was used to carry out the dynamics calculation of the system, and the Analysis task item of the Focite module was used to carry out calculation after optimization.
where and are the instantaneous and initial position vectors of the ith particle, respectively. Moreover, N is the total number of particles in the system.
Fig. 9 shows that as the temperature increases, the MSD of O2, CO2, and N2 in the O2-N2 and O2-CO2 systems increases indicating that the diffusion degree of gas molecules in the system has a direct correlation with temperature. At a constant temperature, the MSD of O2 in the O2-CO2 system is larger than that in the O2-N2 system, indicating that CO2 outperforms N2 in the O2 displacement.
The diffusion coefficient of gas can be obtained from the following expressions (Zhao, 2017):
where D is the diffusion coefficient of the gas, N is the number of particles in the system, and KMSD is the slope of the MSD curve.
Fig. 10 reveals that the root means square displacement of the gas in the O2-N2 and O2-CO2 systems continuously increases over time. Meanwhile, as the temperature increases, the corresponding root means square displacement of gas in the system increases. After injecting N2/CO2 into coal, O2 is adsorbed on coal molecules under the influence of the pressure gradient. Moreover, N2/CO2 gases dilute the O2 concentration in the vacuum layer. Therefore, the O2 concentration in the molecular layer on the coal surface is higher than that in the vacuum layer, and the O2 adsorbed in the molecular layer gradually diffuses into the vacuum layer. On the contrary, the concentration of N2 and CO2 gases in the vacuum layer increases and exceeds that in the coal molecular layer. Under this circumstance, N2 and CO2 molecules will diffuse into the coal molecular layer and will be absorbed by the coal molecules. Subsequently, N2 and CO2 molecules displace O2 molecules through the diffusion mechanism. At a certain temperature, the overall order of root mean square displacement of CO2, N2, and O2 is O2 > N2 > CO2. This is because the molecular structure of coal and the strong adsorption capacity of CO2 molecules hinders the diffusion of CO2. This is consistent with the published results in this area.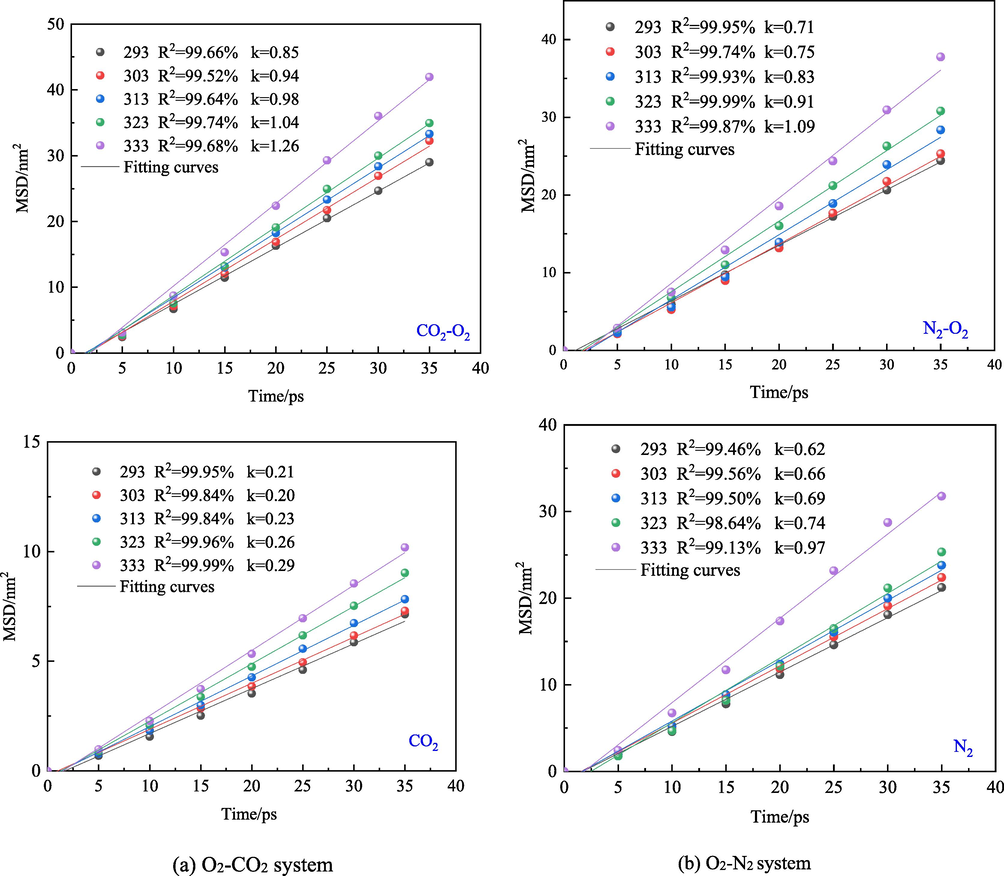
The fitting curves of diffusion coefficients of CO2, O2, and N2 in the coal model at different temperatures.
Fig. 11 reveals that the diffusion coefficient of O2 increases linearly with the N2/CO2 injection temperature. The average diffusion coefficient of O2 in the O2-CO2 and O2-N2 systems are 1.69 × 10-9m2/s and 1.43 × 10-9 m2/s, respectively. Moreover, the average diffusion coefficient of N2 and CO2 are 1.23 × 10-9m2/s and 0.40 × 10-9m2/s, respectively. Among the studied gases, the largest diffusion coefficient belongs to O2, followed by N2, and CO2 has the lowest diffusion coefficient. Since the diffusion coefficient of N2 is larger than that of CO2, the concentration of N2 diffused into the vacuum layer is higher than that of CO2. Compared with the O2-N2 system, the O2 diffusion coefficient is larger in the O2-CO2 system, indicating that from the aspect of displacement effect, CO2 outperforms N2.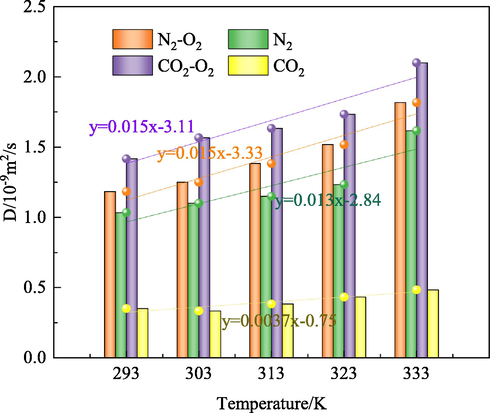
Diffusion coefficient of gas at different temperatures.
4.3 Diffusion activation energies of O2, CO2, and N2
The diffusion of different gases in the coal structure can be simplified as an activation process in accordance with Arrhenius law (Yang et al., 2007), where the diffusion activation energy can be calculated as follows:
Where D0 is the pre-diffusion factor, Ea is the apparent activation energy of diffusion, and R is the gas constant.
Fig. 12 shows that among the studied gases in the two systems, the largest diffusion activation energy belongs to O2 followed by N2, and CO2 has the lowest diffusion activation energy. It is observed that the increase in temperature promotes gas diffusion, increases the self-diffusion coefficient of the gas, and decreases the diffusion activation energy. As the temperature rises, the molecular thermal motion intensifies, thereby increasing the diffusion of gas molecules in the vacuum layer and increasing the self-diffusion coefficient. The diffusion activation energy Ea of O2 in the N2-CO2 and N2-CO2 systems are 7.89 kJ/mol and 8.45 kJ/mol, respectively. It is found that the diffusion activation energy of O2 in the O2-N2 system is 5.89 % higher than that in the O2-CO2 system, indicating that CO2 has a higher inhibitory effect than N2.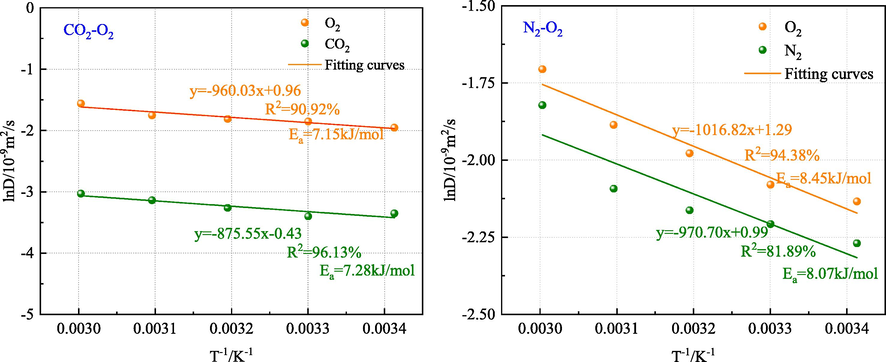
Diffusion activation energy of gas.
5 Conclusion
In the present study, the effects of dry air, CO2, and N2 on coal spontaneous combustion were analyzed experimentally. Moreover, the effects of O2 displacement after CO2 and N2 injection at different temperatures were studied using GCMC and MD methods. Based on the obtained results, the main conclusions can be summarized as follows:
Compared with the injection of dry air, the concentration of CO generated by low-temperature oxidation in the coal samples injected with CO2/N2 decreased by 29.91 % and 23.21 %, respectively. The spontaneous combustion time of coal was prolonged by 27.5 h and 20.5 h, respectively. It is concluded that N2/CO2 inert gases have a good inhibitory effect on coal spontaneous combustion. Meanwhile, the inhibitory effect of CO2 is much higher than that of N2.
CO2 and N2 can effectively displace O2 by occupying adsorption sites. The energy released in the adsorption process is less than that released in the displacement process, and the energy of the CO2 system is higher than that of the N2 system, indicating stronger intermolecular bonds in the O2-CO2 system. The overall concentration order of CO2, N2, and O2 in the vacuum layer is O2 > N2 > CO2. The CO2 injected model exhibited significantly free O2 molecules, suggesting a promising effect of CO2 displacing O2. It is found that after injecting CO2/N2, the oxidation of coal slows down or even stops. This phenomenon originates from the lack of oxygen due to inhibiting the absorption of O2 molecules by coal, thus effectively preventing coal's spontaneous combustion.
The performed analyses show that N2 and CO2 can effectively displace O2 by diffusion. This process is especially more pronounced at high temperatures. The diffusion coefficients and activation energies of O2 are greater than those of N2 and CO2. After injecting CO2 and N2, the diffusion activation energies of O2 reach 7.98 and 8.45 kJ/mol, respectively. Compared with N2, the diffusion activation of O2 after injecting CO2 increases by 5.89 %. It is concluded that CO2 outperforms N2 in displacing O2.
Future research will combine specific coal and rock parameters to refine the simulation research results. The influence of inert gas on low temperature oxidation characteristics of coal with different metamorphic degrees was studied.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (No.51774170). The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 51774170].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- An investigation of the factors associated with interpretation of mine atmosphere for spontaneous combustion in coal mines. Fuel Process. Technol.. 2011;92(3):663-670.
- [Google Scholar]
- Density functional calculation of the adsorption of different amine/ammonium cations on the surface of kaolinite (001) J. China Coal Soc.. 2016;41(12):3115-3121.
- [Google Scholar]
- Adsorption of single component gas CH4, N2 and CO2 on various rank coals. Nat. Gas Ind.. 2005;25(1):61-65.
- [Google Scholar]
- Molecular simulation of gas adsorption characteristics and diffusion in micropore of lignite. Fuel. 2020;269:117443
- [Google Scholar]
- Force fields, structures, and properties of polyvinylidene fluoride crystal. Macromolecules. 1992;25:7268-7281.
- [Google Scholar]
- Molecular simulation of adsorption of gas in coal slit model under the action of liquid nitrogen. Fuel. 2019;255:115775
- [Google Scholar]
- Structure Characterization and Model Construction of Indonesian Brown Coal. Energy Fuels. 2016;30:3809-3814.
- [Google Scholar]
- Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci.. 2016;389:894-905.
- [Google Scholar]
- Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci.. 2016;389:894-905.
- [Google Scholar]
- Molecular simulation of the competitive adsorption characteristics of CH4, CO2, N2, and multicomponent gases in coal. Powder Technol.. 2021;385:348-356.
- [Google Scholar]
- Competitive adsorption differences during coal spontaneous combustion process in noble gas atmosphere. China Safety Science Journal. 2020;30(4):60-67.
- [Google Scholar]
- Insight on adsorption mechanism of coal molecules at different ranks. Fuel. 2020;267(1):117234
- [Google Scholar]
- Molecular Model Construction and Study on Gas Adsorption of Zhaozhuang Coal. Energy Fuel. 2018;32(9):9727-9737.
- [Google Scholar]
- Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol.. 2013;109:36-44.
- [Google Scholar]
- Constant temperature molecular dynamics methods. Prog Theor Phys Suppl. 1991;103(103):1-46.
- [Google Scholar]
- Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys.. 1981;52(12):7182-7190.
- [Google Scholar]
- Sub- and super-critical carbon dioxide flow behavior in naturally fractured black coal: An experimental study. Fuel. 2011;90(11):3390-3397.
- [Google Scholar]
- Comparative experimental study on the effect of carbon dioxide and nitrogen on spontaneous combustion performance of coal. J. China Coal Soc.. 2014;39(11):2244-2249.
- [Google Scholar]
- Numerical simulation of coal dust self–ignition and combustion under inclination conditions. Energy. 2022;239:122227
- [Google Scholar]
- Molecular simulation for physisorption characteristics of O2 in low-rank coal. Energy. 2022;242:122538
- [Google Scholar]
- The influence of methane and CO2 adsorption on the functional groups of coals: Insights from a Fourier transform infrared investigation. J. Nat. Gas Sci. Eng.. 2017;45:358-367.
- [Google Scholar]
- Comparative experimental study on CO2 and N2 inhibition of coal oxidation and spontaneous combustion. Safety in Coal Mines. 2010;2:14-17.
- [Google Scholar]
- Kinetic study of pyrolysis of high volatile bituminous coal. Ind. Eng. Chem. Process Des. Dev.. 1967;6(1):133-138.
- [Google Scholar]
- Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel. 2019;239:87-96.
- [Google Scholar]
- Role of molecular simulation in understanding the mechanism of low-rank coal flotation: A review. Fuel. 2020;262:116535
- [Google Scholar]
- Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel. 2019;239:145-152.
- [Google Scholar]
- Molecular simulation of the adsorption of CH4/CO2/H2O in the molecular structure of coal. Sci. Sin. Terrae. 2014;44(7):1418-1428.
- [Google Scholar]
- Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J. Membr. Sci.. 2007;291:1-9.
- [Google Scholar]
- A new constructed macromolecule-pore structure of anthracite and its related gas adsorption: a molecular simulation study. Int. J. Coal Geol.. 2020;220:103415
- [Google Scholar]
- Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study. Chin. J. Chem. Eng.. 2016;24(9):1275-1282.
- [Google Scholar]
- Combined Monte Carlo and molecular dynamics simulation of methane adsorption on dry and moist coal. Fuel. 2014;122:186-197.
- [Google Scholar]
- Molecular dynamics study of CO2 sorption and transport properties in coal. Fuel. 2016;177:53-62.
- [Google Scholar]
- Adsorption-desorption experiments of CH4 and CO2 with different consistency. J. China Coal Soc.. 2009;34(4):551-555.
- [Google Scholar]
- Molecular simulation of the adsorption characteristics of methane in Zhaozhuang 3# coal. Chinese Science and Technology Paper. 2020;15(1):94-99.
- [Google Scholar]
- Modeling and experiments for transient diffusion coefficients in the desorption of methane through coal powders. Int. J. Heat Mass Transf.. 2017;110:845-854.
- [Google Scholar]
- Effect of multi-component gases competitive adsorption on coal spontaneous combustion characteristics under goaf conditions. Fuel Process. Technol.. 2020;208:106510
- [Google Scholar]
- Variation characteristics of active groups and macroscopic gas products during low-temperature oxidation of coal under the action of inert gases N2 and CO2. Fuel. 2022;307:121893
- [Google Scholar]







