Translate this page into:
Investigation of optical and electrical properties of copper oxide - polyvinyl alcohol nanocomposites for solar cell applications
⁎Corresponding author. mkharma@bau.edu.jo (Mahmoud Abu-Kharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Copper oxide nanoparticles (CuO-NPs) were synthesized at room temperature via a green method that is easy, efficient, eco-friendly, and low-cost. The aqueous extract of Bougainvillea leaves was used as a reducing and capping agent. Pure polyvinyl alcohol (PVA) and CuO-PVA nano-composite thin films of , , , and were prepared by the solution casting method. The optical and electrical properties of the prepared CuO-NPs, pure PVA, and CuO-PVA thin films were analyzed using a UV–visible spectrophotometer and Keithley electrometer.
The band-gap energy (Eg) value for the synthesized CuO-NPs was determined to be 2.74 eV. This band separation lies in a range that is suitable for a variety of optical and optoelectronic applications such as solar cells for energy conversion. Further, increasing the dispersion of CuO-NPs in a PVA matrix extended the absorption spectrum of PVA distinctly into the visible region and decreased the optical band gap exponentially from 4.42 eV for pure PVA to 3.34 eV for CuO-PVA. In addition, an increase in the DC electrical conductivity with CuO-NPs dispersion was observed. The decrease in the band gap energy was usually accompanied by an increase in the DC electrical conductivity making the CuO-PVA nanocomposite more applicable in thin-film solar cell applications.
Keywords
CuO-PVA Nanocomposite
Optical Absorption
Optical Band Gap
Electrical Conductivity

1 Introduction
Copper oxide nanoparticles (CuO-NPs) are semiconducting materials with direct band gaps and high absorption properties. They have been applied in many areas including magnetic storage media (Kumar et al., 2000), photodetectors (Wang et al., 2011), field emissions (Zhu et al., 2004), solar cells (Santos-Cruz et al., 2005), nanofluid (Zhou et al., 2010). Additional applications involve biosensors (Rahman et al.,2010), gas sensors (Choi and Jang, 2010), super-capacitors (Zhang et al.,2011), photocatalysis (Liu et al., 2012), antibacterial activity (Al-Fa’ouri et al., 2021), and antiviral properties (Zhou et al., 2019). CuO-NPs can cause the virus's genome to degrade and its integrity to be compromised. It was reported that CuO-NPs can attack the COVID-19 virus's membrane and damage the virus's DNA structure (Fakhroueian et al., 2020). CuO-NPs can be impregnated into a N95 mask to create an antiviral respiratory safe facial mask, which in turn enhanced the masks antiviral function by five orders of magnitude (Zhou et al., 2020).
The most common methods for fabricating nanomaterials can be broadly classified into two groups: top-down methods (physical methods) and bottom-up methods (chemical and bio-assisted). However, these methods have various limitations that can be decreased by the green synthesis method which is becoming popular because of its simplicity and low cost.
Furthermore, polyvinyl alcohol (PVA) is a synthetic polymer that has recently gained significant attention due to its particular physical and chemical properties. It is both a nontoxic and environmentally friendly water-soluble polymer. It is also oil, grease, and solvent resistant, has high dielectric strength, is ductile but rigid, elastic, and acts as an excellent oxygen and odor barrier. In addition, its effective film-forming nature has been employed in developing inorganic–organic nanocomposites with better performance properties than typically filled polymer composites, which are made by loading inorganic nanoparticles as additives in a polymer matrix (Abd El-Kader and Orabi, 2002). Furthermore, PVA is also utilized in the production of surface coatings, artificial sponges, cosmetics, pharmaceutical aids, and ophthalmic lubricants as well as other products.
Controlling the band gap is essential when developing new organic solar cell materials. Researchers tried to develop and synthesize new materials with the greatest possible overlap between their absorption and solar emission spectra (Bredas, 2014). The addition of nano-inorganic particles such as CuO-NPs will regulate these two properties of organic materials. In addition, the balance between light absorption and charge carrier transfer is a major challenge for metal oxide semiconductors in solar energy harvesting. Both are based on band structure and are combined to assess the final solar conversion efficiency. Therefore, studying the optical and electrical properties of CuO-PVA nanocomposites is very important to decrease the band gap and enhance the harvesting of solar energy (Divya et al., 2014; Tessema et al., 2018).
PVA has a relatively wide band gap that limits visible-light absorption and reduces light harvesting. Consequently, this makes it inappropriate to be applied as an active layer in organic solar cells. The band gap and optical absorption of a semiconducting material are significant factors for its use as an active layer in solar cells. Combining the properties of organic PVA and inorganic CuO-NPs would assist in developing new nanomaterials with enhanced physical and chemical properties that can resolve the limitations of their counterparts (Sperling, 2005). It has been reported that inorganic–organic nanocomposites enhanced the band gap and the optical absorption and therefore found potential applications in gas sensors, super capacitors, solar cells, and flexible electronic devices (Srikanth et al., 2016; Al-Harbi et al., 2023).
In the present study, a facile, non-toxic, and eco-friendly method was used for the preparation of CuO-NPs using Bougainvillea leaves aqueous extract. In addition, CuO-PVA nanocomposite thin films were prepared using the solution casting approach to investigate the effects of adding CuO-NPs at various concentrations to PVA on solar energy harvesting. Hence, studying the optical and electrical properties of the prepared CuO-NPs, pure PVA, and CuO-PVA thin films is the main objective of the present work.
In the literature, AC Conductivity and dielectric constant were studied without investigating any applications for this work (Manjunath al., 2016). This is the first work done, according to our knowledge, to investigate the optical and electrical properties (DC-Conductivity) of copper oxide - polyvinyl alcohol nanocomposites for solar cell applications using the green route for the synthesis of copper oxide nanoparticles.
2 Experimental details
The experimental procedure to fabricate CuO-NPs from the Bougainvillea leaves and to prepare CuO-PVA nanocomposite films are presented in our previous study in which the green synthesized nanoparticles were subjected to characterization by XRD, FT-IR, and TEM, well crystalline, spherical shape and crystalline size in the range of 8–20 nm with an average size equal to 15 nm are presented (Al-Fa’ouri et al., 2021). The optical properties of the prepared CuO-NPs, pure PVA, and CuO-PVA thin films were studied and analyzed using a UV–visible spectrophotometer (Schimadzu 1601). The UV–vis absorption spectrum recorded for these samples is in the wavelength range of 200–800 nm. For the electrical properties, the two-probe technique with the aid of the Keithley electrometer (Model 6430 Sub-Femtoamp) was used to measure the temperature-dependent DC electrical conductivity for the pure PVA and the four CuO-PVA thin films over the temperature range of . The thickness of each film has been measured using Swanepoel's envelope method and the results for pure PVA, 15 wt% CuO-PVA, 30 wt% CuO-PVA, 40 wt% CuO-PVA, 51 wt% CuO-PVA were 1730.84 nm, 1787.3 nm, 1996 nm, 2545.3 nm, and 2824.2 nm, respectively.
3 Results and discussion
The investigation of the optical absorption spectra in semiconducting materials provides essential information about the band structure and the associated energy gap. The optical band gap can be estimated from the absorption coefficient α of the films under investigation over a wide range of wavelengths (Tauc, 1970). The value of
specifies how far light of a specific wavelength can penetrate a material before being absorbed depending on the known Beer-Lambert's law
, where
is the incident intensity and
the transmitted one. It can be calculated from absorbance data using the relation
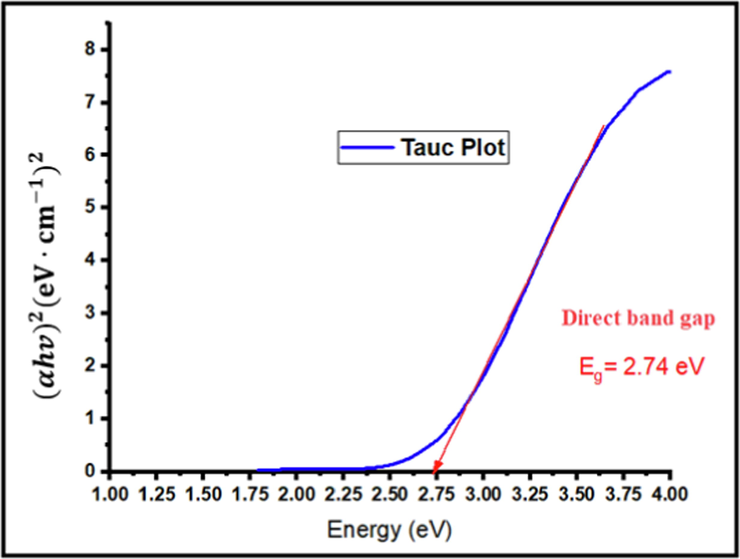
Tauc plot for green synthesized CuO-NPs.
In addition, UV–vis absorption spectra of pure PVA and four prepared CuO-PVA (
,
,
, and
) nanocomposite films were recorded using a Schimadzu 1601 spectrophotometer in the range of 200–800 nm. These spectra are shown in Fig. 2. One can obtain from these figures that pure PVA films exhibit no apparent absorption in the visible light region (wavelength over 400 nm). However, films of PVA doped with different concentrations of CuO-NPs extend the absorption spectrum distinctly into the visible region with increasing concentration, as it appears from the absorption spectrum of
CuO-PVA. This result reveals that doping CuO-NPs into a PVA matrix enhances the harvesting of the visible part of solar radiation promising thereby a possible potential impact on solar-cell applications. The absorption spectra in Fig. 2 display that all films have relatively poor absorbance in the visible light region, and the absorbance rapidly increases with decreasing wavelength due to the direct transition of electrons.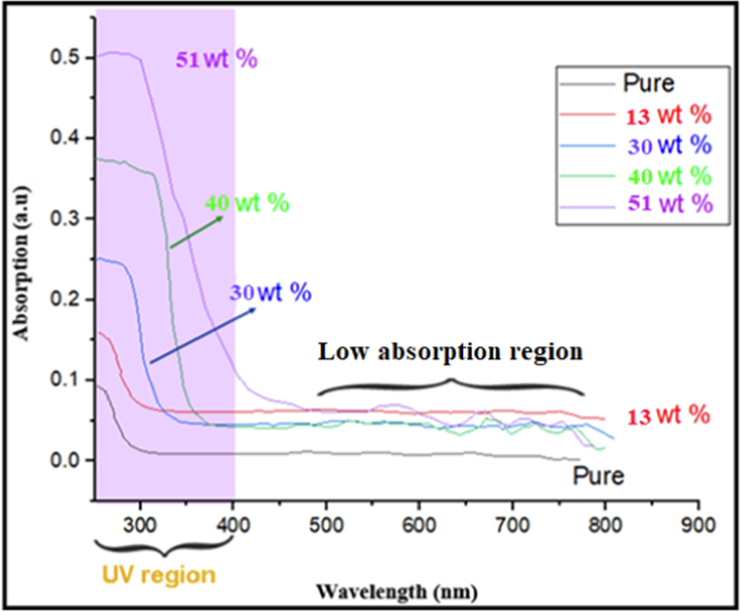
UV–vis absorption spectra of pure PVA and four CuO-PVA (
,
,
, and
) thin films.
Figs. 3-7 demonstrate the calculated band gaps of pure PVA and the four prepared CuO-PVA (
,
,
, and
) nanocomposite films. CuO dispersion in the PVA matrix decreases the optical band gap from 4.42 eV for pure PVA to 3.34 eV for 51 wt% CuO- PVA. In other words, the optical band gap of the films decreases with the increasing concentration of the CuO nanofiller which is in good agreement with the literature ((Divya, et al.,2014; Radhakrishnan et al., 2014)). Apart from the predicted CuO-NPs aggregation, this could be due to the complex formation of the salt in the polymer matrix (hydroxyl groups of PVA). Besides, charge transfer transitions may cause a decrease in the optical band gap and an increase in the absorption spectrum (Divya et al., 2014a; Selvi, 2019). The shift in the absorption peaks in the UV region was employed to investigate the change in the optical energy band gap for pure PVA and CuO-PVA nanocomposite thin films. A favorable band separation of CuO-PVA nanocomposite thin films of about 3.34 eV makes it suitable for solar cell applications. As a result, the optical band gap of CuO-PVA nanocomposite thin films can be tuned by changing the concentration of the CuO nanofiller in the PVA matrix, as well as changing the crystal size of CuO-NPs. This can be beneficial in the design of solar cell photoactive layers.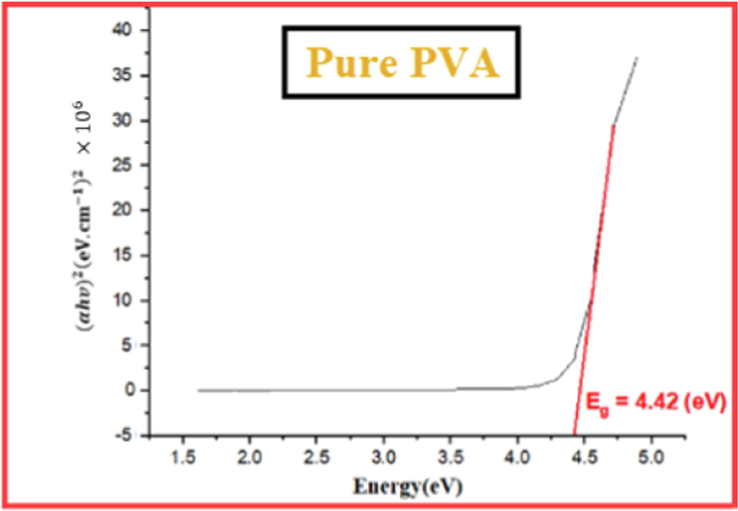
Tauc plot for pure PVA film.
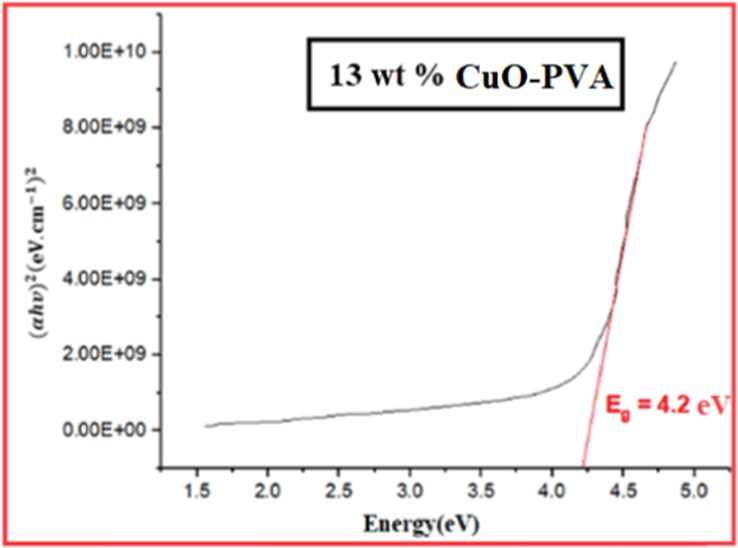
Tauc plot for 13 wt% CuO-PVA film.
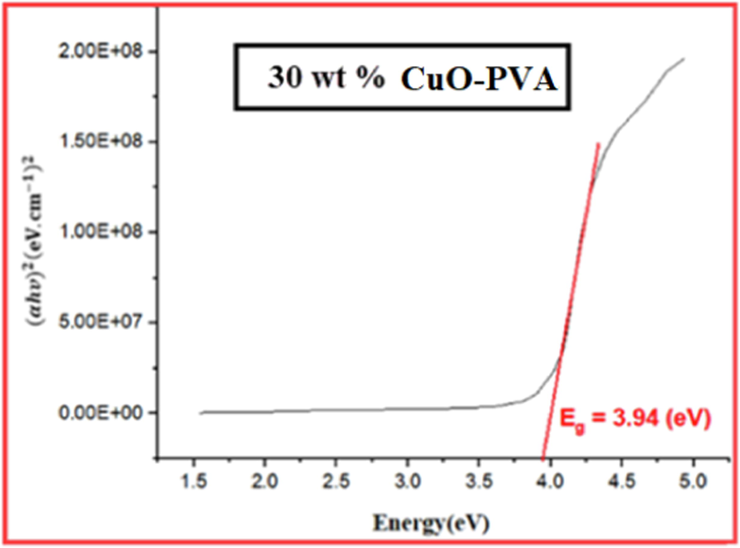
Tauc plot for 30 wt% CuO-PVA film.
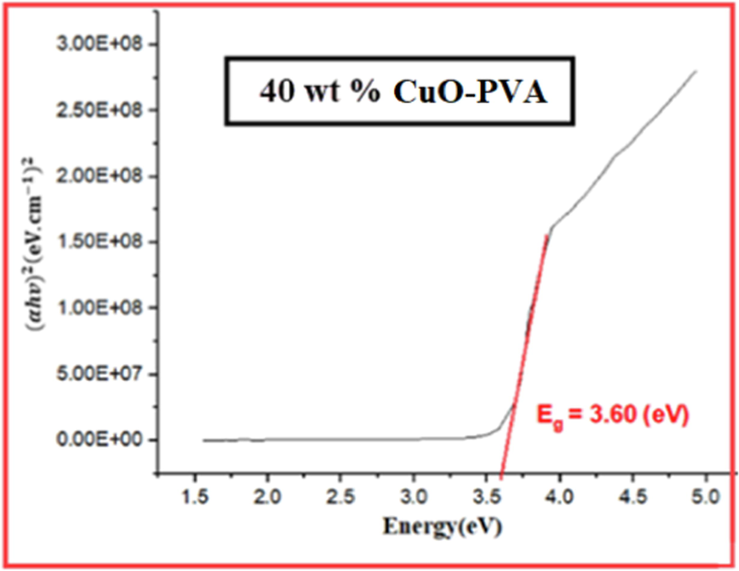
Tauc plot for 40 wt% CuO-PVA film.
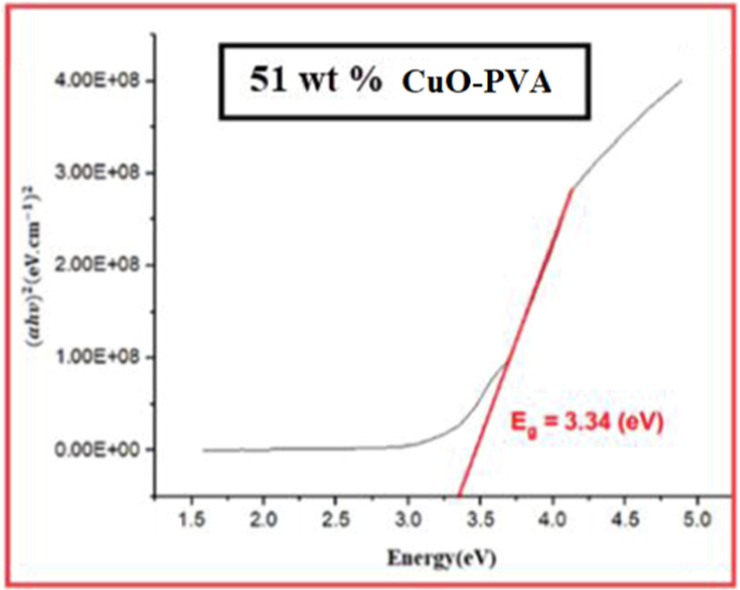
Tauc plot for 51 wt% CuO-PVA film.
To explore the transport of charge carriers in the prepared samples, the DC electrical conductivity was measured at different temperatures T in the case of the planner. The typical current–voltage I-V characteristics of the studied samples were recorded at different T, ranging from 35 °C to 95 °C, and in the voltage range (0–200 V). The I-V characteristics of the studied samples exhibit non-linear behavior, particularly at large electric fields. The DC electrical conductivity
can be calculated using the simple relation
where
is the direct current through the thin film,
is the voltage drop across the film,
is the length of the film (
),
is the cross-sectional area
,
being the width of the film (
) and
its thickness. Here, the values of I/V are obtained from Fig. 8. Note that
versus T for pure PVA exhibits nonlinear behavior. Similarly, in the four CuO-PVA nanocomposites, I/V curves vary with T as shown in Fig. 8. It is clear from this figure that
increases with T and with the concentration of the CuO nanofiller. The formation of charge-transfer complexes (CTCs) in the PVA polymer chain leads to the formation of new conducting routes inside the amorphous region of PVA, thus enhancing the conductivity (Mott & Davis. 1974). This implies that dispersing CuO nanofiller acts as a conductive filler in the PVA host matrix which considerably increases electronic conduction.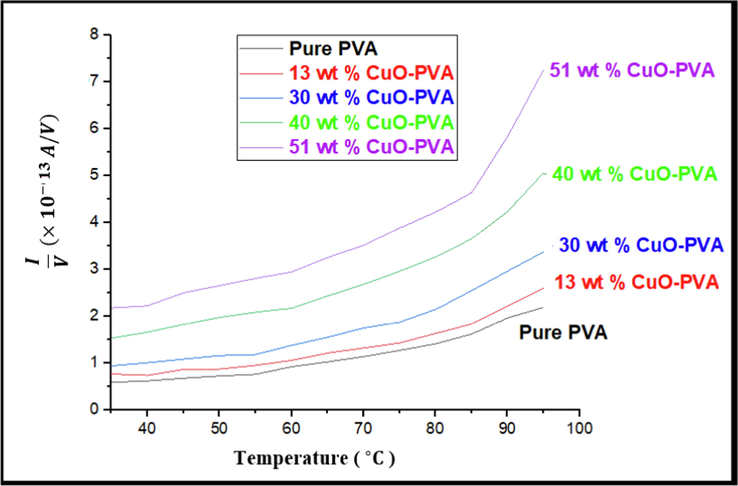
Current / voltage I/V variation with temperature T for pure PVA and the four CuO-PVA nanocomposites films.
It is known that for semiconductors the conductivity follows the well-known Arrhenius equation (Mott and Davis, 1979)
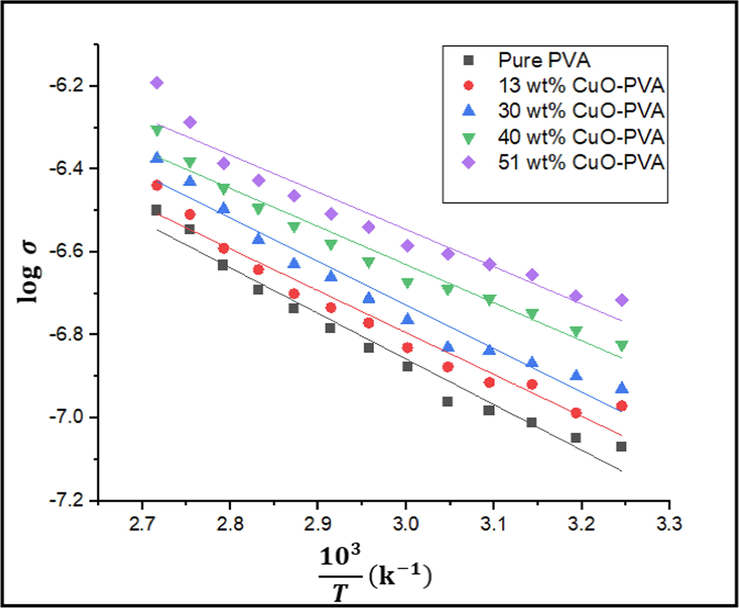
log σ vs
of the pure PVA and the four CuO-PVA nanocomposites.
DC conductivity electrical measurement showed that; as the temperature increases the DC conductivity increases. Also, DC conductivity increases gradually as the concentration of the CuO nanofiller increases. This implies that dispersing CuO nanofiller acts as conductive fillers in the PVA host matrix, and considerably increases electronic conduction. As a result, the produced CuO-PVA nanocomposites will be more useful in electronic and solar cell devices.
It would be interesting to study the effect of different sizes of CuO nanofiller on tuning and engineering the optical energy band gap of CuO-PVA nanocomposite. Incorporating a conductive component into nanocomposite and creating a hybrid composite material will improve the electrical conductivity of CuO-PVA nanocomposites, so it would be beneficial to study the effect of adding Cu or Au nanofiller on the absorption spectrum and band gap of CuO-PVA nanocomposites. It is recommended to study different coating techniques in future research to lower the manufacturing costs of photovoltaic systems.
4 Conclusion
Green synthesized CuO-NPs, pure PVA, and CuO-PVA nanocomposite thin films were prepared and characterized through spectrophotometry. The DC electrical conductivity at different temperatures T has been measured and analyzed using a Keithley electrometer. The key results and conclusions are as follows:
-
The peak of the UV–vis absorption spectrum of synthesized CuO-NPs indicates that they have maximum absorption in the ultraviolet range and the estimated value of the optical band gap was determined to be Eg = 2.74 eV. This value of Eg makes CuO-NPs suitable for solar cell applications.
-
The UV–vis absorption spectrum of PVA films showed that it extends distinctly into the visible region with increasing concentrations of CuO-NPs. This enhances the harvesting of the visible part of solar radiation that could promise a potential impact on solar cell applications.
-
The values of the optical band gap for (0, 15, 35, 56, and 51) wt % CuO- PVA composite films were obtained to be (4.42, 4.20, 3.94, 3.60, and 3.34) eV, respectively. This means that the optical band gap of CuO-PVA nanocomposite films enhanced its visible light absorption.
-
The DC electrical conductivity increases with the temperature and also gradually with the concentration of the CuO nanofiller. In addition, the variation of the estimated values of the activation energy gives the same trend as that of the optical band gap values.
-
Results of this work showed that the prepared samples can be used to develop a good candidate material for photoactive layers in solar cell applications, with more safety since NPs could be prepared using green synthesis.
Acknowledgments
The authors would like to thank the Department of Materials Science at the Royal Scientific Society and the German Jordanian University for their collaborations on the advanced characterization of the nanocomposite.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spectroscopic behavior of poly (vinyl alcohol) films with different molecular weights. Polym. Test.. 2002;21(5):591-595.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using bougainvillea leaves aqueous extract and antibacterial activity evaluation. Chem. Int.. 2021;7(3):155-162.
- [Google Scholar]
- Tuning optical, dielectric, and electrical properties of Polyethylene oxide/Carboxymethyl cellulose doped with mixed metal oxide nanoparticles for flexible electronic devices. J. Mol. Struct.. 2023;1272:134244
- [Google Scholar]
- Tailoring the structural, optical, dielectric, and electrical properties of PEO/PVA blend using graphene nanoplates for energy storage devices. J. Mater. Sci. Mater. Electron.. 2022;33:22196-22207.
- [Google Scholar]
- One-Dimensional oxide nanostructures as gas-sensing materials: review and issues. Sensors. 2010;10:4083-4099.
- [Google Scholar]
- Preparation and optical properties study of CuO thin film as applied solar cell on LAPAN-IPB Satellite. Procedia Environ. Sci.. 2016;33:661-667.
- [Google Scholar]
- Investigation on CuO dispersed PVA polymer films. J. Eng. Res. Applications. 2014;4(5):1-7.
- [Google Scholar]
- The mirror strategy of nanoparticles against the coronavirus. Preprints 2020:2020070443.
- [CrossRef] [Google Scholar]
- Electrical conductivity, density of states and optical band gap in Se90Te6Sn4 glassy semiconductor. Phys. B Condens. Matter. 2013;410:201-205.
- [Google Scholar]
- Sonochemical synthesis and characterization of nanometer-size transition metal oxides from metal acetates. Chem. Mater.. 2000;12(8):2301-2305.
- [Google Scholar]
- Correlation of some opto-electrical properties of Se- Te-Sn glassy semiconductors with the average single bond energy and the average electronegativity. J. Alloys Compounds. 2016;660:503-508.
- [Google Scholar]
- Tailoring CuO nanostructures for enhanced photocatalytic property. J. Colloid Interface Sci.. 2012;384(1):1-9.
- [Google Scholar]
- Synthesis and characterization of CuO nanoparticles and CuO doped PVA nanocomposites. Adv. Mater. Phys. Chem.. 2016;6(10):263.
- [Google Scholar]
- Electronic Processes in Non-Crystalline Materials. Oxford: Clarendon Press; 1979.
- Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME) Sustainability. 2021;13(2):796.
- [Google Scholar]
- Structural and optical absorption analysis of CuO nanoparticles. Indian J. Adv. Chem. Sci. 2014;2(2):158-161.
- [Google Scholar]
- A Comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors. 2010;10:4855-4886.
- [Google Scholar]
- Dependence of electrical and optical properties of sol-gel prepared undoped cadmium oxide thin films on annealing temperature. Thin Solid Films. 2005;493(1–2):83-87.
- [Google Scholar]
- Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials. 2020;10(2):312.
- [Google Scholar]
- Optical, thermal, mechanical properties, and non-isothermal degradation kinetic studies on PVA/CuO nanocomposites. Polym. Compos.. 2019;40(9):3737-3748.
- [Google Scholar]
- Introduction to physical polymer science (Fourth Edition). John Wiley and Sons; 2005.
- Characterization and DC conductivity of novel ZnO doped polyvinyl alcohol (PVA) nano-composite films. J. Adv. Phys.. 2016;5(2):105-109.
- [Google Scholar]
- The Optical Properties of Solids. Amsterdam: North-Holland; 1970.
- High-Power and high-energy-density flexible pseudocapacitor electrodes made from porous CuO Nanobelts and single-walled carbon nanotubes. ACS Nano. 2011;5(3):2013-2019.
- [Google Scholar]
- Progress and perspective of antiviral protective material. Adv. Fiber Mater.. 2020;2:123-139.
- [Google Scholar]
- Synthesis and characterization of size-controlled Nano-Cu2O Deposited on alpha-zirconium phosphate with excellent antibacterial property. Mater. Sci. Eng. C. 2019;101:499-504.
- [Google Scholar]
- Large-scale synthesis and field emission properties of vertically oriented CuO nanowire films. Nanotechnology. 2004;16(1):88.
- [Google Scholar]






