Translate this page into:
Effect of sodium bicarbonate and sodium chloride on protein conformation and gel properties of pork myofibrillar protein
⁎Corresponding author. kzlnj1988@163.com (Zhuang-Li Kang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
To explore the potential of using sodium bicarbonate to take the place of sodium chloride, the changes in endogenous fluorescence, ultraviolet absorption spectrum, water holding capacity, gel properties and water distribution of pork myofibrillar protein were investigated. Because of the increase in pH and the exposure of more tryptophan and tyrosine residues with increasing sodium bicarbonate, the cooking yield and gel strength of pork myofibrillar protein significantly increased (P < 0.05), and centrifugal loss significantly decreased (P < 0.05). Meanwhile, the colour of the cooked pork myofibrillar protein was darker with increasing sodium bicarbonate, the bound water and immobile water were bound more tightly to the protein matrix, and the mobility of the water was lower, except for in the sample with 0.4 % sodium bicarbonate. Moreover, free water significantly increased (P < 0.05) when 0.4 % sodium bicarbonate was added, indicating that the mobility of the water was enhanced. Overall, the use of sodium bicarbonate could induce the protein conformation change, and improve the bond water content and texture properties of reduced-salt pork myofibrillar protein.
Keywords
Sodium bicarbonate
Myofibrillar protein
Reduced-salt
Texture properties
Mobility

1 Introduction
Myofibrillar protein accounts for 50–55 % of the total muscle protein content. As a vital functional protein, it dissolves in high-ionic-strength solutions; thus, sodium chloride determines the texture properties, shelf life, and sensory quality of meat products (Wu, Xiong & Chen et al., 2009; Shen, Huang, Zhao & Sun, 2019; Li, Kang, Sukmanov & Ma, 2021). However, excess intake of sodium chloride increases the incidence of hypertension and other diseases (Citterio, Carpini, Lupoli, Brioni & Lanzani, 2020; Adolf et al., 2021). Previous studies reported that direct reducing sodium chloride causes the quality of meat products to become worse (Shen, Huang, Zhao & Sun, 2019; Li, Kang, Sukmanov & Ma, 2021; Kang et al., 2021a). Thereby, establishing novel approach to reduce sodium chloride in meat products is a vital area.
Sodium bicarbonate is a frequently-used food additive, and it is inexpensive and convenient. Because sodium bicarbonate is a strong buffering substance, capable of adjusting pH and ionic strength, it has been used in chicken (Saleem, Hasnain & Ahmad, 2015; Kaewthong & Wattanachant, 2018; Zhu et al., 2018; Lu et al., 2021), pork (Kang et al., 2021a; Zou et al., 2022), beef (Hsieh, Cornforth, Pearson, & Hooper, 1980; Mohan, Jaico, Kerr & Singh, 2016), and seafood (Chantarasuwan, Benjakul, Visessanguan, 2011; Kaewjumpol, Thawornchinsombut, Ahromrit & Rojanakorn, 2013; Gabriel, Erasmus & Namwoond, 2020) to improve processing properties and sensory quality. Our previous work found that using sodium bicarbonate instead of sodium chloride significantly increased the pH and solubility of target materials, exposing more active sulfhydryl and hydrophobic groups, and dissociating pork myofibrillar protein (Li, Kang, Sukmanov & Ma, 2021). This led to an increase in salt soluble protein concentration and cooking yield, improve texture properties of pork batters, lower water mobility, and increase immobilized water with increasing sodium bicarbonate from 0 to 0.28 % (Kang et al., 2021a). However, as far as we know, there is few study regarding the changes in gel properties of cooked myofibrillar protein when sodium chloride was partially replaced by sodium bicarbonate. Thus, the objective of the study was to investigate the changes in the protein conformation, colour, cooking yield, texture, and water distribution of pork myofibrillar protein with different sodium chloride (0.8–2.0 %) and sodium bicarbonate (0–0.4 %), to obtain a new method of improving reduced-salt myofibrillar protein processing properties.
2 Materials and methods
2.1 Materials
In each repetition, 8 sets (500 g/set) of chilled pork [Duroc × (Landrace × Yorkshire)] leg meat (Semitendinosus, biceps femoris, mesoglutaeus; at 24 h–48 h postmortem, core temperature 2 ± 2 °C, pH 5.65 ± 0.01) were obtained from 8 pigs (180 ± 3 d old, 100 ± 5 kg) in a local slaughter house (Xinxiang Gaojin Food Co., ltd., Xinxiang, China). In this study, 24 sets of pork meat were purchased at different times. Removed the tendons and fat, the meat was ground (JR-120, Shandong, China) and mixed uniformly. Following, approximately 200 g of ground meat was vacuum packaged in a nylon/PE bag, and stored at 2 ± 2 °C within 1 d. All the chemical reagents were analytical grade.
2.2 Extraction of myofibrillar protein and preparation of cooked gel
Referring to the way of Kang et al. (2021b), pork myofibrillar protein was obtained, and solutions were utilized within 48 h. Then, approximately 60 mg/mL pork myofibrillar protein solutions with different sodium chloride and sodium bicarbonate were prepared using the phosphate buffer solution (50 mmol/L K2HPO4/KH2PO4, pH = 6.0). Therein, T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate. Following, each sample was mixed uniformly using a T25 digital homogenizer (IKA ltd., Germany) at 2000 rpm for 10 s. After leaving 2 ± 2 °C for 30 min, approximately 5 g solution was put into a beaker, and heated to the core temperature was 72 °C. Finally, the cooked samples were cooled using running water, and stored overnight at 2 ± 2 °C.
2.3 Endogenous fluorescence
The myofibrillar protein was diluted to a protein concentration of 0.4 mg/mL with 50 mmol/L phosphate buffer (pH 6.0), and 3.5 mL of supernatant was aspirated and placed in a quartz cuvette with an optical range of 1 cm, and detected using an F-180 FluoroMax-3 fluorescence spectrophotometer (Tianjin Gangdong Science and Technology Development Co., ltd., Tianjin, China). The excitation was performed at 283 nm at room temperature, and the emission spectra were recorded at 300–400 nm for subsequent analysis. The excitation and emission slit widths were both set to 10 nm, and the data collection rate was 500 nm/min. Solvent emission spectra were obtained under the same conditions to eliminate interference.
2.4 Ultraviolet absorption spectrum
Referring to the method of Zhang et al. (2017) and minor modifications. The myofibrillar protein was diluted to a protein concentration of 0.4 mg/mL with 50 mmol/L phosphate buffer (pH 6.0), with phosphate buffer as blank, and detected using a Ut-1810 Ultraviolet Spectrophotometer (Beijing General Analysis Instrument Co., ltd., Beijing, China). The parameters were set as follows: the scanning speed was 240 nm/min, the interval was 1 nm, and the scanning wavelength was 190–400 nm. The structural changes of myofibrillar protein can be characterized vividly by ultraviolet second-order conductance spectroscopy.
2.5 pH
Approximately 5 g of each cooked treatment was homogenized with 20 mL of distilled water (4 °C) by a homogenizer at 15000 rpm for 15 s in an ice bath (T25 digital, IKA ltd., Germany), and then it was measured by a digital pH meter (Hanna, Italy).
2.6 Colour
The colour (L*, a* and b*) of cooked sample slices was determined using a CR-400 colorimeter (Minolta Camera Co., Japan) with a pulse xenon lamp (the aperture is a diameter of 11 mm), a 10◦ observer angle, and illuminant D65 calibrated against a white colorimeter was used as a standard. According to the formula was calculated for the whiteness:
2.7 Cooking yield and centrifugal loss
The lost water from the cooked treatment was wiped away, and weighed. After that, the formula was calculated for cooking yield as follows:
Cooking yield (%) = The cooked protein solution weight / The raw protein solution weight × 100 %.
After being stored overnight at 2 ± 2 °C, the cooked treatment was centrifugated (Model 225, USA) at 4000 × g (30 min, 4 °C), removed the water and weighed. Then, the formula was calculated for centrifugal loss as follows:
Centrifugal loss (%) = (Weight of cooked treatment before centrifugation - Weight of cooked treatment after centrifugation) / Weight of cooked treatment before centrifugation × 100 %.
2.8 Gel strength
The samples were left at 20 °C for about 2 h. The gel strength was determined using a TA-XT.plus texture analyzer (Stable Micro System ltd., Surry, England) through a P/0.5 probe. The parameters were set as follows: pre-test speed: 1 mm/s, mid-test speed 2 mm/s, post-test speed: 10 mm/s, test distance 10 mm, trigger force: 10 g. Record the peak value of the test peak, which is the breaking force (g) of the cooked treatment.
2.9 Low-field nuclear magnetic resonance
Approximately 2 g of cooked myofibrillar protein solution was placed in Ziplock bags (PE), and left at 32 °C thermostats for about 30 min. According to the method of Kang, Zhu, Li, Ma, and Song (2017), NMR relaxation (T2) measurements were measured using a Pulsed NMR analyzer (NMI20-040 V-1, Niumag Electric Corporation, China).
2.10 Statistical analysis
The experiment was based on the preparation of 3 independent batches of the base mixture (n = 3), using different samples (sodium chloride and sodium bicarbonate). All results were shown in mean ± standard error (SE). The data were analyzed using the one-way ANOVA program (SPSS v.18.0) and the general linear model (GLM) procedure, considering the treatments (different sodium chloride and sodium bicarbonate levels) as a fixed effect and the replicates as a random effect. Significant differences between means were identified by the LSD procedure. The difference between means was considered significant at P < 0.05.
3 Results and discussion
3.1 Endogenous fluorescence characteristics
Protein endogenous tryptophan fluorescence intensity and maximum absorption wavelength (λmax) are very sensitive to the polarity of its surrounding micro-environment and can be used to monitor the changes in protein conformation (Cao, True, Chen, & Xiong, 2016). As shown in Fig. 1, the maximum fluorescence emission wavelength of tryptophan was near 336 nm. With the increase of sodium bicarbonate and the decrease of sodium chloride, the fluorescence intensity of myofibrillar protein decreased significantly, and the emission spectrum showed an obvious redshift phenomenon. The result indicated that the increase in sodium bicarbonate content caused the conformation change of myofibrillar protein, and the exposure of endogenous tryptophan residues to a hydrophilic environment (Cao & Xiong, 2015). However, compared with the T3, the fluorescence intensity of T4 was enhanced and there was a slight blue shift (338.35 ∼ 337.37 nm). The reason is possible due to the exposure of the hydrophobic groups of myofibrillar protein caused by the increase of sodium bicarbonate addition, but the exposure may not be all tryptophan, so the λmax became shorter (Jia et al., 2019).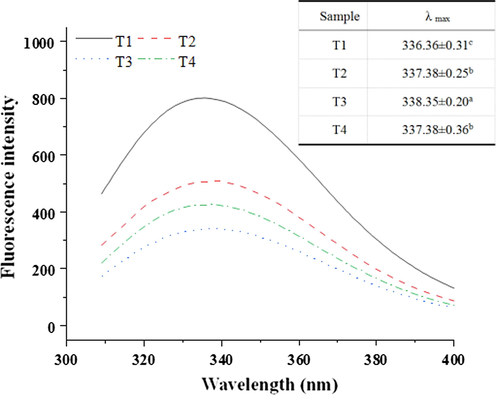
The endogenous fluorescence characteristics of myofibrillar protein solute with different sodium chloride and sodium bicarbonate. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate.
3.2 Ultraviolet absorption spectrum
The second derivative of the ultraviolet spectrum has two peaks and troughs compared with the relatively uncharacteristic ultraviolet spectrum, mainly caused by the electronic transition of peptide bonds. It reflects the conformation of the main chain of the peptide chain, can better reflect the changes of amino acid residues in the microenvironment, and is often used for the analysis of secondary structure changes in proteins (Mach & Middaugh, 1994; Hou et al., 2019). For tyrosine and tryptophan, the positions of the peaks and troughs are blue shifted (shorter wavelengths) when the solvent polarity changes from non-polar (when the residue is buried in the protein) to polar (when exposed to the solvent). At the same time, the amplitude also changes, so the relative amount of amino acids and the average polarity of proteins in the microenvironment can be reflected by calculating the ratio between the two peaks and valleys (R = a/b) (Ragone, Colonna, Balestrieri, Servillo, & Irace, 1984). As shown in Fig. 2, two peaks appeared at about 288 nm and 296 nm. Compared with the sample of T1, with the increase of sodium bicarbonate, the peak position at 288 nm shifted to a shorter wavelength (blue shift), the peak value of the two absorption peaks became lower and lower, and the “R” value increased significantly (P < 0.05) from 0.548 (T1) to 0.857 (T4). It indicates the increased polarity of the environment around tyrosine and tryptophan residues (Hou et al., 2019). It may be that the increase of sodium bicarbonate caused the conformation change of some proteins, and the tyrosine and tryptophan residues were exposed to more hydrophobic environment due to the unfolding of proteins, which was consistent with the change of hydrophobic forces (Li, Zhang, Lu, & Kang, 2021).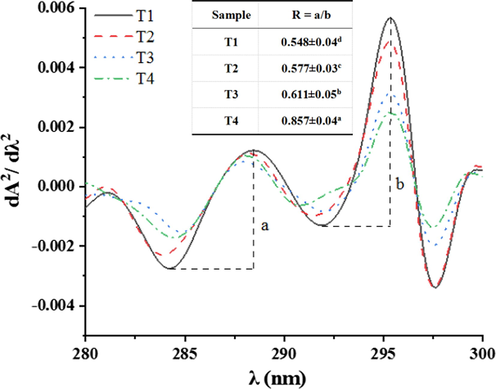
The ultraviolet second derivative spectra of myofibrillar protein solute with different sodium chloride and sodium bicarbonate. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate.
3.3 pH
The changes in pH of the cooked myofibrillar protein solute with different sodium chloride and sodium bicarbonate are shown in Table 1. The pH was increased from 5.96 ± 0.01 (T1) to 7.17 ± 0.01 (T4) with the increase of sodium bicarbonate while the decrease of sodium chloride. The reason is possible that sodium bicarbonate has a good buffer capacity, and the pH of 1 % aqueous solution is approximately 8.31, the other, sodium chloride has no effect on the pH of cooked myofibrillar protein solute. Thus, the pH significantly increased (P < 0.05) with increasing sodium bicarbonate. Our previous studies reported that the pH of raw myofibrillar protein solute increased by approximately 1.20 units when sodium bicarbonate was added from 0 % to 0.40 % (Li, Zhang, Lu, & Kang, 2021), and the pH of the raw pork batter increased by approximately 0.60 units when sodium bicarbonate was added from 0 % to 0.42 % (Kang et al., 2021). In addition, sodium bicarbonate could be used in broiler breast meat (Kaewthong &Wattanachant, 2018), chicken meat batters (Li, Zhang, Lu, & Kang, 2021), pork meat batters (Li, Zou, Kang, Ma, 2022), actomyosin (Saleem, Hasnain, & Ahmad, 2015), and ground beef (Mohan, Jaico, Kerr, & Singh, 2016) to increase the pH. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate. Each value represents the mean value ± SE, n = 3. a-d different letters indicate significant differences between groups (P < 0.05).
Sample
pH
L*
a*
b*
Whiteness
T1
5.96 ± 0.01d
88.82 ± 0.22a
−1.40 ± 0.02a
3.58 ± 0.10a
86.44 ± 0.28a
T2
6.67 ± 0.02c
86.45 ± 0.26b
−1.82 ± 0.01b
1.74 ± 0.14b
84.12 ± 0.24b
T3
7.92 ± 0.02b
84.07 ± 0.18c
−1.99 ± 0.03c
0.73 ± 0.08c
82.25 ± 0.18c
T4
8.17 ± 0.01a
80.81 ± 0.20d
−2.20 ± 0.02d
−0.16 ± 0.04d
80.12 ± 0.23d
3.4 Colour
From Table 1 we can observe that the L*, a*, b*, and whiteness values of cooked myofibrillar protein solution decreased significantly (P < 0.05) with increasing sodium bicarbonate. A similar study showed that the L* values in the salted cod decreased significantly after the addition of sodium bicarbonate (Asli & Mørkøre, 2012). In a separate study, the use of bicarbonate to marinate broiler breast meat produced a lower L* value, and significantly darker meat was observed (Sen, Naveena, Muthukumar, Babji, & Murthy, 2005; Petracci et al., 2012). However, other studies have reported that the L* values of cooked ground beef or pork batters were not changed with the increasing sodium bicarbonate while decreasing sodium chloride. Meanwhile, the a* values were decreased, and b* values were increased (Mohan et al., 2016; Kang et al., 2021a). It is possible that the myoglobin of pork and beef was higher than the myofibrillar protein and chicken breast meat, partially explaining these results.
3.5 Cooking yield and centrifugal loss
According to Fig. 3, the cooking yield and centrifugal loss of the raw and cooked myofibrillar protein solution were significantly affected with increasing sodium bicarbonate while decreasing sodium chloride. The cooking yield of myofibrillar protein significantly increased (P < 0.05) with increasing sodium bicarbonate: the T4 group had the highest cooking yield. Our previous research showed that the pH of myofibrillar protein improved by about 1.20 units with increasing sodium bicarbonate, from 0 % to 0.4 %, and the turbidity and particle size of myofibrillar protein decreased significantly at the same time (Li et al., 2021). In addition, Chantarasuwan, Benjakul and Visessanguan (2011) reported that the solubility of natural actomyosin increased with the increase in concentration of sodium bicarbonate, and the actomyosin complex was dissociated, while the denaturation of myosin head was produced. Thus, more myofibrillar protein were used to form the gel structure, so the water-holding capacity was improved. Our previous work (Kang et al., 2021a) reported a similar result, the salt soluble protein concentration and cooking yield of pork batter significantly increased with increasing sodium bicarbonate. Furthermore, Lu et al. (2021) found that the protein solubility and gel properties of chicken breast meat batters with sodium bicarbonate were higher compared with samples incorporating sodium tripolyphosphate.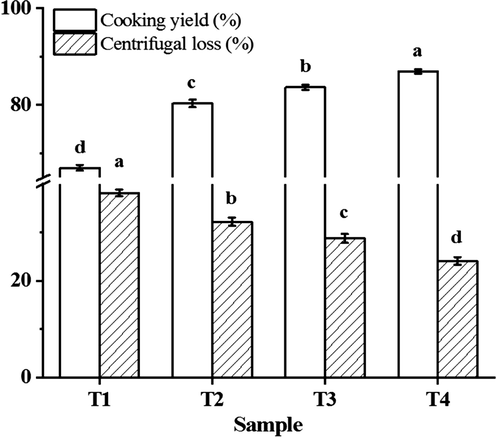
The cooking yield and centrifugal loss of myofibrillar protein solute with different sodium chloride and sodium bicarbonate. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate. Each value represents the mean value ± SE, n = 3. a-d different letters indicate significant differences between groups (P < 0.05).
Corresponding to these results, the centrifugal loss of cooked myofibrillar protein solution decreased significantly (P < 0.05) with increasing sodium bicarbonate: the T4 group had the fewest centrifugal loss. Centrifugal loss is closely related to the gel structure formed by protein denaturation by noncovalent cross-linking and covalent interactions. Chantarasuwan, Benjakul and Visessanguan (2011) discovered that the surface hydrophobicity and sulfhydryl groups of natural actomyosin increased, and the more negative charge was formed with increasing sodium bicarbonate. Li et al. (2021) also showed that the sulfhydryl group and surface hydrophobicity of myofibrillar protein solution increased significantly as increasing sodium bicarbonate. The results of endogenous fluorescence (Fig. 1) and ultraviolet absorption spectrum (Fig. 2) were in agreement with the result. In addition, the myofibrillar protein is more negatively charged after the pH value is kept away from the isoelectric point, causing the electrostatic repulsion between the protein molecules were increased, then bound more water molecules in the gel system (Bertram, Kristensen & Andersen, 2004).
3.6 Gel strength
Gel strength could influence the texture characteristics of the gel (Xiong, 2017). The gel strength of cooked myofibrillar protein solution was affected with increasing sodium bicarbonate while decreasing sodium chloride (Fig. 4). The gel strength of cooked myofibrillar protein solution increased significantly (P < 0.05) with the increase of sodium bicarbonate: the T4 group had the strongest gel strength. Our previous research reported that the pH, surface hydrophobicity and sulfhydryl groups increased with increasing sodium bicarbonate (Li et al., 2021). In other work, Zhu et al. (2018) found that the β-sheet, β-turn, and random coil structures from chicken meat proteins with sodium bicarbonate alone increased significantly, and the content of α-helices decreased significantly compared with that of sodium chloride-treated meat; the I850/I830 ratio and surface hydrophobicity was increased. In addition, the electrostatic repulsion and swelling of myofibrillar protein increased as the pH increased; gel formed under neutral pH conditions is stronger than gel formed under acidic conditions (Feng & Hultin, 2001).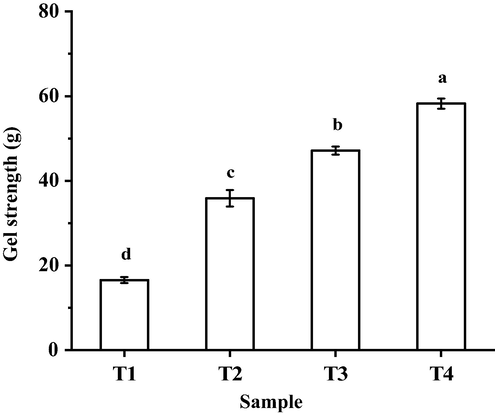
The gel strength on cooked myofibrillar protein solute with different sodium chloride and sodium bicarbonate.. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate. Each value represents the mean value ± SE, n = 3. a-d different letters indicate significant differences between groups (P < 0.05).
3.7 Low-field nuclear magnetic resonance
Low-field nuclear magnetic resonance can be utilized to determine the physicochemical state of water fluidity and distribution in protein gel systems (Bertram, Karlsson & Rasmussen, 2004; Li, Kang, Sukmanov & Ma, 2021). Table 2 and Fig. 5 show the influence of sodium bicarbonate concentration on the initial relaxation time and peak ratio of the cooked myofibrillar protein solution. According to previous studies, T2b, T21, and T22 represent tie water, immobile water, and free water, respectively (Kang, Li, Ma & Chen, 2016; Wang, Lin, Cheng, & Tan, 2020). T2b represents the water closely linked to the protein and macro-molecular constituents; T21 represents the water bound to a protein-intensive myofibrillar network and had greater fluidity; T22 represents the water located in extra-myofibrillar space (Grossi et al, 2016; Guo et al, 2019). The initial relaxation times of T2b and T21 decreased significantly (P < 0.05) after adding the sodium bicarbonate, and they were not changed as increasing sodium bicarbonate. Meanwhile, the initial relaxation time of T22 was not a significant difference (P > 0.05). This result implies that water molecules of cooked myofibrillar protein solution with sodium bicarbonate were bound more tightly to the protein matrix than those of T1 (Pearce, Rosenvold, Andersen & Hopkins, 2011). In addition, when sodium bicarbonate was added, the absolution values of the zeta potential, solubility, surface hydrophobicity, sulfhydryl groups of pork myofibrillar protein were increased significantly, forming a better cooked gel during heating (Li, Kang, Sukmanov & Ma, 2021; Tang & Ma, 2009). However, the gel structure was destroyed during processing due to CO2, which reduced the degree of binding between water and the protein matrix (Kang et al., 2021a; Lu et al., 2021). T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate. Each value represents the mean value ± SE, n = 3. a-d different letters indicate significant differences between groups (P < 0.05).
Sample
Initial relaxation time(ms)
Peak ratio (%)
T2b
T21
T22
P2b
P21
P22
T1
2.47 ± 0.36a
42.87 ± 3.11a
438.67 ± 23.22a
1.35 ± 0.18a
86.76 ± 0.66c
11.71 ± 0.55b
T2
1.73 ± 0.26b
36.61 ± 2.24b
416.40 ± 25.05a
1.46 ± 0.26a
92.51 ± 0.45b
5.88 ± 0.47c
T3
1.52 ± 0.17b
33.13 ± 2.43b
360.56 ± 24.62a
1.33 ± 0.30a
94.53 ± 0.29a
4.05 ± 0.50d
T4
1.44 ± 0.20b
30.88 ± 1.79c
429.64 ± 23.90a
1.41 ± 0.17a
84.32 ± 0.47d
13.76 ± 0.79a
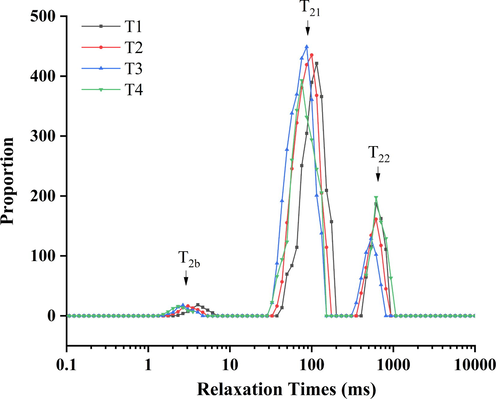
The changes in LF NMR relaxation times of the cooked myofibrillar protein solute with different sodium chloride and sodium bicarbonate. T1 = 2 % sodium chloride; T2 = 1.6 % sodium chloride, 0.13 % sodium bicarbonate; T3 = 1.2 % sodium chloride, 0.27 % sodium bicarbonate; T4 = 0.8 % sodium chloride, 0.4 % sodium bicarbonate.
The peak ratio of P2b was not significantly different with increasing sodium bicarbonate; the P21 was increased significantly, and the P22 significantly decreased (P < 0.05), except in T4. The reason is possible that high levels of sodium bicarbonate produce more CO2, and a few big cavities were formed and held water, leading to the increase in free water and accompanying decrease in immobile water (Kang et al., 2021a; Kang, Shang, Li, & Ma, 2022). This result was in agreement with the water-holding capacity of pork myofibrillar protein solution (Fig. 3), implying that between water and protein matrix were closely bonded, decreasing mobility, and that the cooking yield and centrifugal loss were increased during heat-processing (Ma et al., 2015). Therefore, the cooking yield and centrifugal loss of pork myofibrillar protein could be improved by adding sodium bicarbonate.
4 Conclusion
The endogenous fluorescence, ultraviolet absorption spectrum, water-holding capacity, and gel properties of pork myofibrillar protein solution were significantly affected with the increasing sodium bicarbonate while decreasing sodium chloride. The fluorescence intensity, L*, a*, b*, and white values, as well as centrifugal loss, decreased significantly with the increasing sodium bicarbonate, while the “r” value, cooking yield and gel strength significantly increased. The initial relaxation times of T2b and T21 were faster after sodium bicarbonate was added, and the peak ratio of P21 increased significantly when 0.13 % and 0.27 % sodium bicarbonate were added. However, P21 decreased significantly when 0.40 % sodium bicarbonate was added. In conclusion, sodium bicarbonate could cause more tyrosine and tryptophan residues exposed, and improve the water-holding capacity and gel strength of pork myofibrillar protein solution.
Acknowledgments
This study was supported by National Natural Science Foundation of China (NSFC, Grant No. 32272365), Natural Science Foundation of Henan Province (no. 212300410344), and Joint Innovative, Scientific and Technological Research Program for Yangzhou City and Yangzhou University (YZ2020267).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Altered taste perception for sodium chloride in patients with primary aldosteronism: a prospective cohort study. Hypertension. 2021;77(4):1332-1340.
- [Google Scholar]
- Brines added sodium bicarbonate improve liquid retention and sensory attributes of lightly salted Atlantic cod. LWT - Food Sci. Tech.. 2012;46:196-202.
- [Google Scholar]
- Functionality of myofibrillar proteins as affected by pH, ionic strength and heat treatment - a low-field NMR study. Meat Sci.. 2004;68(2):249-256.
- [Google Scholar]
- Dual role (anti- and pro-oxidant) of gallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. J. Agr. Food Chem.. 2016;64:3054-3061.
- [Google Scholar]
- Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem.. 2015;180:235-243.
- [Google Scholar]
- The effects of sodium bicarbonate on conformational changes of natural actomyosin from Pacific white shrimp (Litopenaeus vannamei) Food chem.. 2011;129(4):1636-1643.
- [Google Scholar]
- Citterio, L., Carpini, S. D., Lupoli, S., Brioni, E., Lanzani, C. 2020. Klotho gene in human salt-sensitive hypertension. Clinical J. Amer. Soc. Nephrology 15(3). CJN.08620719.
- Effect of pH on the rheological and structural properties of gels of water-washed chicken-breast muscle at physiological ionic strength. J. Agr. Food Chem.. 2001;49(8):3927-3935.
- [Google Scholar]
- Effects of different fish sizes, temperatures and concentration levels of sodium bicarbonate on anaesthesia in Mozambique tilapia (Oreochromis mossambicus) Aquaculture. 2020;529:735716
- [Google Scholar]
- The effect of high pressure on the functional properties of pork myofibrillar proteins. Food Chem.. 2016;196:1005-1015.
- [Google Scholar]
- Effect of low-frequency magnetic field on the gel properties of pork myofibrillar proteins. Food Chem.. 2019;274(15):775-781.
- [Google Scholar]
- Activation and conformational changes of chitinase induced by ultrasound. Food Chem.. 2019;285:355-362.
- [Google Scholar]
- Ultrastructural changes in pre-rigor and post-rigor beef muscle caused by conventional and microwave cookery. Meat Sci.. 1980;4:299-311.
- [Google Scholar]
- The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food chem.. 2019;301:125206
- [Google Scholar]
- Effects of Bicarbonate, Xanthan Gum, and Preparation Methods on Biochemical, Physicochemical, and Gel Properties of Nile Tilapia (Oreochomis niloticus Linn) Mince. J. Aquatic Food Product Tech.. 2013;22(3):241-257.
- [Google Scholar]
- Optimizing the electrical conductivity of marinade solution for water-holding capacity of broiler breast meat. Poultry Sci.. 2018;97(2):701-708.
- [Google Scholar]
- Effect of pre-emulsified sesame oil on physical-chemical and rheological properties of pork batters. Food Sci. Tech. Int.. 2017;37(4):620-626.
- [Google Scholar]
- Effects of sodium bicarbonate on the gel properties, water distribution and mobility of low-salt pork batters. LWT - Food Sci. Tech.. 2021;139:110567
- [Google Scholar]
- Effect of ultrasound-assisted sodium bicarbonate treatment on gel characteristics and water migration of reduced-salt pork batters. Ultrasonics Sonochem.. 2022;89:106150
- [Google Scholar]
- The effects of sodium chloride on proteins aggregation, conformation and gel properties of pork myofibrillar protein Running Head: Relationship aggregation, conformation and gel properties. J. Food Sci. Tech.. 2021;58(6):2258-2264.
- [Google Scholar]
- Effect of sodium bicarbonate and sodium chloride on aggregation and conformation of pork myofibrillar protein. Food Chem.. 2021;350(8):129233
- [Google Scholar]
- Effect of sodium bicarbonate on techno-functional and rheological properties of pale, soft, and exudative (PSE) meat batters. Meat Sci.. 2022;194:108990
- [Google Scholar]
- Effect of sodium bicarbonate on gel properties and protein conformation of phosphorus-free chicken meat batters. Arab. J. Chem.. 2021;14(2):102969
- [Google Scholar]
- Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT - Food Sci. Tech.. 2015;61(2):377-384.
- [Google Scholar]
- Simultaneous monitoring of the environment of tryptophan, tyrosine, and phenylalanine residues in proteins by near-ultraviolet second-derivative spectroscopy. Analytical Biochem.. 1994;222(2):323-331.
- [Google Scholar]
- Functional properties of bicarbonates on physicochemical attributes of ground beef. LWT - Food Sci. Tech.. 2016;70:333-341.
- [Google Scholar]
- Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes - A review. Meat Sci.. 2011;89(2):111-124.
- [Google Scholar]
- The use of sodium bicarbonate for marination of broiler breast meat. Poultry sci.. 2012;91(2):526-534.
- [Google Scholar]
- Determination of tyrosine exposure in proteins by second-derivative spectroscopy. Biochem.. 1984;23(8):1871-1875.
- [Google Scholar]
- Changes in some biochemical indices of stability of broiler chicken actomyosin at different levels of sodium bicarbonate in presence and absence of sodium chloride. Int. J. Food Properties. 2015;18(6):1373-1384.
- [Google Scholar]
- Effect of chilling, polyphosphate and bicarbonate on quality characteristics of broiler breast meat. British Poultry Sci.. 2005;46:451-456.
- [Google Scholar]
- Interactions of selected ketone flavours with porcine myofibrillar proteins: The role of molecular structure of flavour compounds. Food Chem.. 2019;298:125060
- [Google Scholar]
- Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT - Food Sci. Tech.. 2009;42(2):606-611.
- [Google Scholar]
- Water dynamics changes and protein denaturation in surf clam evaluated by two-dimensional LF-NMR T1–T2 relaxation technique during heating process. Food Chem.. 2020;320:126622
- [Google Scholar]
- Rheological and microstructural properties of porcine myofibrillar protein–lipid emulsion composite gels. J. Food Sci.. 2009;74(4):207-217.
- [Google Scholar]
- Structure-function relationships of muscle proteins. In: Food Proteins and Their Applications. CRC Press; 2017. p. :341-392.
- [Google Scholar]
- Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci.. 2017;133:10-18.
- [Google Scholar]
- Effect of sodium chloride or sodium bicarbonate in the chicken batters: A physico-chemical and Raman spectroscopy study. Food Hydrocolloids. 2018;83:222-228.
- [Google Scholar]
- Effect of sodium bicarbonate on solubility, conformation and emulsion properties of pale, soft and exudative meat myofibrillar proteins. LWT - Food Sci. Tech.. 2022;157:113097
- [Google Scholar]






