Translate this page into:
Furfuryl alcohol functionalized graphene for sorption of radionuclides
⁎Corresponding authors. xiekun_79@163.com (Kun Xie), chemlx11@163.com (Xia Liu),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, graphene was functionalized with furfuryl alcohol (FA) through the Diels–Alder reaction. The as-prepared FA functionalized graphene (FAG) was characterized by XRD, Raman, TEM, AFM, FT-IR and TG. The sorption performance of the obtained FAG toward radionuclides was evaluated. The maximum sorption capacities from the Langmuir model were calculated to be 1.178, 1.947, 1.431 and 1.608 mmol g−1 for U(VI), Eu(III), Sr(II), and Co(II), respectively, at T = 303 K and pH = 3.0, indicating the hierarchical binding affinity of the FAG surface toward the radionuclides in the order Eu(III) > Co(II) > Sr(II) > U(VI). Based on the experimental results presented in this study, it is clear that FAG can be potentially used as a promising candidate for the sorption of radionuclides.
Keywords
Furfuryl alcohol
Graphene
Sorption
Radionuclides
1 Introduction
Since its discovery in 2004, graphene has attracted extensive research interests due to its extremely large surface-to-volume ratio and outstanding electronic (Zhu et al., 2010; Chen et al., 2012), mechanical, thermal and chemical properties (Jahan et al., 2010). These extraordinary and superior properties broadened the applications of graphene in versatile fields, such as touch screens, capacitors, spintronic devices, fuel cells, batteries, sensors, transparent conductive films, high-frequency circuits, and toxic material removal (Park et al., 2009). Currently, several methods were developed to produce the well-defined graphene sheets, including epitaxial growth, liquid phase exfoliation, and chemical reduction of exfoliated graphite oxide (Georgakilas et al., 2012). The high cost and low yield of epitaxial growth and the damage of structure and property of chemical reduction of exfoliated graphite oxide made these two methods inappropriate for bulk applications both academically and industrially (Han et al., 2011). The liquid phase exfoliation route seems to be promising for large scale production by using intercalant technique from graphite in solvent (Li et al., 2010).
With the peaceful utilization of nuclear energy and the rapid development of nuclear industry, the safe treatment and disposal of high-level wastes in nuclear waste management has always been an environmental concern to the public. In the last decades, several methods, such as precipitation, coprecipitation, oxidation, ion-exchange, reverse osmosis, membrane electrolysis, and sorption, were employed to remove Co(II) ions from large volumes of aqueous solutions. Although these methods afforded moderate to efficient removal of heavy metal ions, sorption technology is one of the most effective choices for the removal of heavy metal ions from aqueous solutions due to its simplicity of design, convenience, low cost, high sorption efficiency, and wide adaptability (Liu et al., 2011a,b). The removal of radionuclides from aqueous systems has been extensively studied using versatile types of adsorbents such as clay minerals, metal oxides, biomaterials and carbon nanomaterials. Dahn et al. (2002) demonstrated that the uptake of Ni(II) on montmorillonite resulted in the neoformation of a phyllosilicate. Tan studied the adsorption of Eu(III) onto TiO2. Romanchuk et al. (2013) also demonstrated that the GO presented high adsorption capacity for radionuclides such as Am(III) and U(VI). However, these materials suffer from either low sorption capacities or efficiencies. Graphene, a kind of one or several atomic layered graphite, possesses special two-dimensional structure and excellent mechanical, thermal, and electrical properties.
Despite the fact that graphene holds great promise for various applications, there are still two major shortcomings to its practical applications. The zero-band gap of graphene is a pitfall since graphene based field effect transistors would be working under a very small on/off ratio. Meanwhile, the physical handling of graphene sheets is challenging due to its poor solubility in most solvents. Graphene sheets tend to form irreversible agglomerates or restack to form graphite via π–π stacking and van der Waals interactions (Kuila et al., 2011). Chemical functionalization of graphene is often used to address the above challenges (Hummers et al., 1958). When organic molecules are covalently attached on the graphene surface, its extended aromatic character is perturbed and a band gap is thus developed. Moreover, the functionalization of pristine graphene sheets with organic functional groups can enhance the dispersity of graphene in common organic solvents (Wu et al., 2010). The dispersion of graphene sheets in organic solvents is a crucial move toward the formation of nanocomposite materials with graphene. In addition, organic functional groups can offer new properties that could be combined with the properties of graphene.
Among various methods used to functionalize graphene, the Diels–Alder reaction has received special attention due to the facile reactivity of graphene as a diene or dienophile (Goods et al., 2014). The Diels–Alder transformation is a powerful reaction in organic chemistry that allows the saturation of conjugated sp2-hybridized carbon atoms, and it has been extensively used in fullerene and carbon nanotube functionalization. As a result of the scope of Diels–Alder chemistry and the dual nature of the reactivity of graphene, dienophiles and dienes with a wide range of modifiable chemical functionalities can be employed, providing a platform for post grafting modification of graphene. The covalent functionalization of graphene via Diels–Alder reactions is a simple and efficient approach for reversibly engineering the properties of graphene for a wide range of applications (Li et al., 2009).
Based on the intriguing physical and chemical properties of graphene and its derivatives, there has been a steadily growing interest in the use of these materials for water treatment and purification, reflected by an enormous increase in the number of research articles in the recent literature (Dreyer et al., 2010). In this work, graphene was functionalized with furfuryl alcohol (FA) through the Diels–Alder reaction and used to remove radionuclides from aqueous solutions. The FA functionalization can not only enhance the dispersity of graphene in aqueous solutions but also introduce the oxygen-containing hydroxyl groups, which offer active sites for sorption of metal ions. Graphene was obtained via solvothermal expansion of graphite followed by sonication assisted exfoliation. Afterward, Diels–Alder reaction between graphene and FA was carried out without addition of any catalysts. XRD, Raman, TEM, AFM, FT-IR and TG were used to investigate the exfoliation process and morphologies of graphene. The sorption performance of the obtained FA functionalized graphene (FAG) toward radionuclides was then evaluated.
2 Materials and instruments
All chemical materials were of analytical grade and used without further purification unless otherwise stated. Microcrystalline graphite was purchased from Kermel Chemical Reagent Co., Ltd., Tianjin, China. The others were obtained from Sinopharm Chemical Reagent (Beijing, China). Raman spectra measurements were carried out with a Raman microspectroscopic setup (RamLab-010) (LabRam, Horiba-Jobin-Yvon, Bensheim, Germany) spectrometer. The AFM sample was prepared by dropping the sample on the freshly cleaved mica and dried naturally at room temperature for at least 24 h, and then measured with the instrument of NT MDT (Ntegra Prima) using a tapping mode. TEM images were recorded on a JEM-3010 microscopy. Fourier transform infrared (FT-IR) spectra were recorded on a WQF-410 spectrophotometer by using KBr pellets. Thermal properties of the samples were measured using a HCT-1 (HENVEN, Beijing, China) comprehensive thermal analyzer in flowing (50 mL/min) nitrogen atmosphere.
3 Experimental
3.1 Preparation of graphene dispersion
The preparation starts by mixing natural graphite flakes (5.0 g) and N-methyl-2-pyrrolidone (NMP, 100 mL), and transferred into an autoclave. The autoclave was purged with nitrogen and pressurized to 2 MPa. The reactor was heated to 250 °C and maintained for 8 h before cooling to room temperature. The crude filter cake was obtained after suction filtration, which was then placed into muffle furnace and heated at 400 °C for 4 h. After heating, the obtained graphene was dispersed into organic solvents (NMP, DMSO, DMF, and methanol) and PVP was added as a dispersant. After sonicating for 2 h, the graphene dispersion was obtained. The as-prepared graphene dispersion was settled for a week and then vacuum-filtered through a 0.22 μm PVDF membrane and washed with acetone. Weight difference was recorded. The concentration of graphene dispersion was calculated as follows: C = (Mafter − Mbefore)/V.
3.2 Functionalization of graphene
Functionalization of graphene was performed as follows (Hossein Roghani-Mamaqani et al., 2014): furfuryl alcohol (2.5 mL) was added into graphene/NMP dispersion (100 mL) under nitrogen protection, which was then heated to 130 °C and maintained for 72 h. After reaction, the solution was cooled to room temperature, vacuum-filtered through a 0.22 μm PVDF membrane and washed with tetrahydrofuran (THF) and acetone. The graphene-FA was kept overnight in the vacuum drying chamber. The possible mechanism of exfoliation of graphite and functionalization processes of graphene is shown in Fig. 1.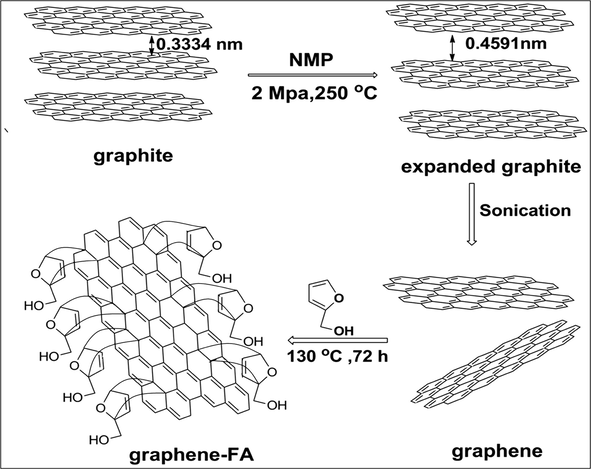
The possible mechanism of exfoliation of graphite and its functionalization processes.
3.3 Batch adsorption experiments
The batch experiments of radionuclides adsorption on FAG were carried out in polyethylene test tubes, where the stock suspensions of FAG (0.2 g/L), radionuclides (0.2 mmol/L) and NaClO4 (0.01 mol/L) solutions were added in the polyethylene test tubes to achieve the desired concentrations of different components. NaClO4 was chosen as a background electrolyte because of the noncompeting behavior of ClO4− ions with metal ions and numerous sorbent surfaces. Negligible amount of high concentrated HClO4 or NaOH solution was added into the suspension solutions to adjust pH to the desired values. Then the tubes were placed in an oscillator and kept shaking for a desired time. The concentration of radionuclides was analyzed by the Dichlorophosphonazo III Spectrophotometer (V-1600 Mapada Shanghai) method at the specific wavelength.
4 Results and discussion
4.1 Characterization
To the best of our knowledge, NMP could not intercalate into graphite by itself under regular conditions. However, at high temperature and under high pressure the interlayer spacing of graphite can be expanded through the intercalation of NMP. The XRD patterns of graphite and expanded graphite are shown in Fig. 2A and B. In these spectra, the interlayer distance of expanded graphite increased from 0.3334 to 0.4591 nm, indicating that NMP molecules can soak through the graphite layer to expand the graphite layers. To investigate the structural information of the obtained graphene, Raman spectroscopy is utilized (Fig. 2C). The disorder mode (D-band) at 1340 cm−1 is attributed to the breathing vibration of the sp2 carbon ring and exhibits Raman-active when structure defects or lattice vacancy in graphitic materials is formed (Zhang et al., 2010a,b). The intensity ratio of D-band to G-band (1570 cm−1) (ID/IG) could be used to observe the structural defects at surfaces of graphene. Compared with graphite, the ID/IG ratio of graphene showed no significant increase, demonstrating that no considerable defect is introduced to the graphene surface during the intercalation and exfoliation process (Zhang et al., 2009; Huang et al., 2014). Meanwhile, the 2D peak of graphene is red-shifted, indicating that the interaction between new-formed interlayers is weakened to some extent. The graphene dispersion can be obtained by sonication of expanded graphite in some organic solvents such as NMP, DMF, DMSO, or CH3OH at a short time. The concentration of dispersed graphene solution is approximately 0.38–0.75 mg mL−1 (Fig. 2D), which is higher than that of untreated graphite. The stability of these solutions was confirmed by settling for a few weeks without observing any aggregations.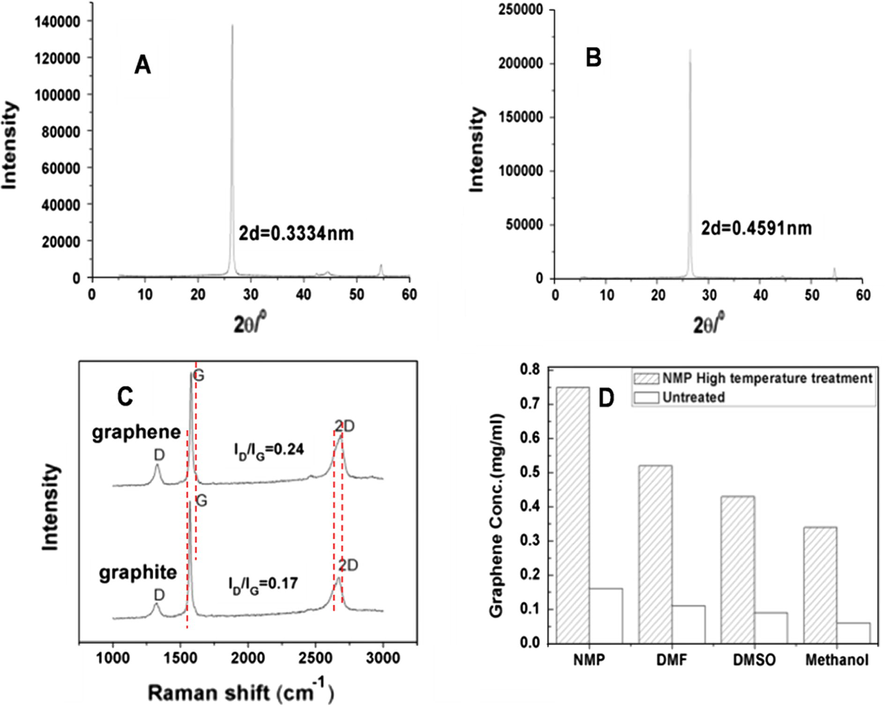
XRD patterns of untreated graphite (A) and the obtained expanded graphite (B), Raman spectra of untreated graphite and the obtained graphene (C), and concentrations of exfoliated graphene sheets dispersed in organic solvents (D).
Both atomic force microscopy (AFM) and transmission electron microscopy (TEM) are used to characterize the morphologies of graphene. The representative AFM images along with height profiles for monolayer graphene sheets and graphene functionalized with furfuryl alcohol are shown in Fig. 3A and B, respectively. No obvious height difference can be observed between the AFM images of graphene and FAG, indicating that the functionalization process has not brought strong destruction to the graphene sheet. TEM (Fig 3C) is utilized for detailed characterization by transferring obtained graphene onto the TEM grid. The edges of graphene are shown by using HRTEM and the results demonstrate that the graphene is made up of about two layers (Fig. 3D) and no obvious agglomeration or other defects are detected on the base-plane. Results also show that the thickness of graphene is about 2 nm, comprising of a few layers (Zhao et al., 2011a,b).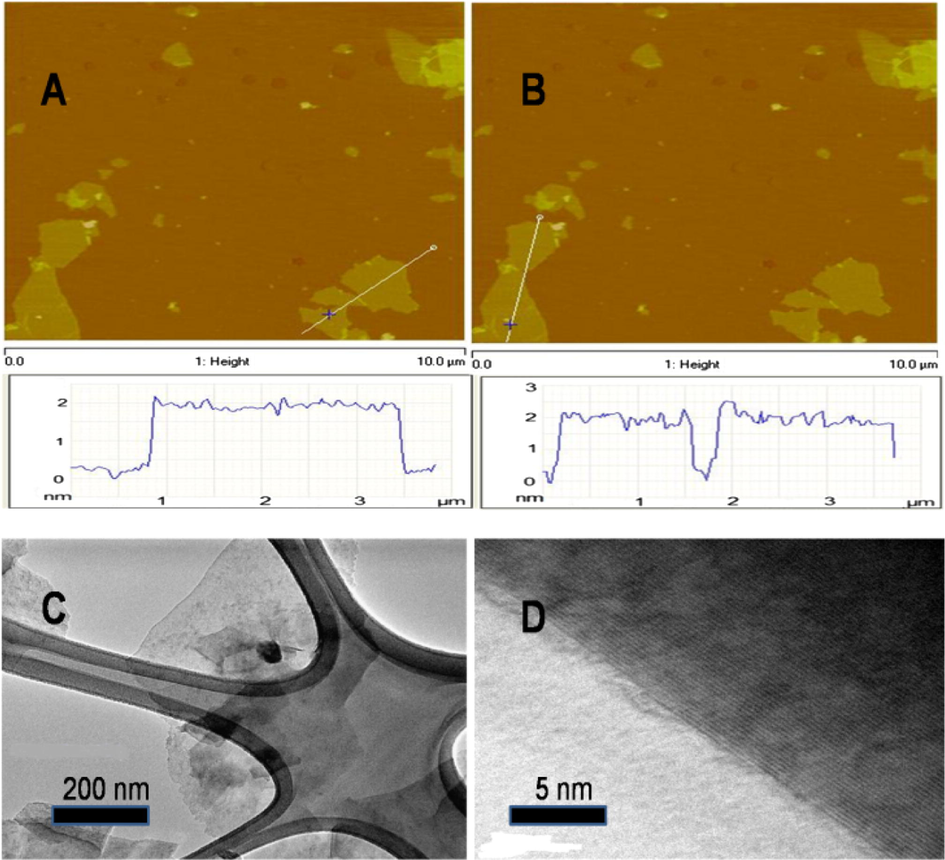
AFM images of the obtained graphene (A) and untreated graphite (B). TEM and HRTEM images of the obtained graphene (shown in C and D, respectively).
The FT-IR spectra of FAG (Fig 4A) exhibit characteristic absorption peaks at 3440, 1642, 1575 and 1154 cm−1, among which the strong peak at 3440 cm−1 originates from stretching vibration of free or associated hydroxyl groups, while peaks at 1642, 1575 and 1154 cm−1 correspond to stretching vibrations of C⚌C, C⚌O and C—O functional groups, respectively (Veerapandian et al., 2012), indicating the successful functionalization of graphene with furfuryl alcohol. In the Raman spectrum (Fig. 4B), two main peaks at 1350 and 1580 cm−1 are representatives of D and G bands, respectively (Zhao et al., 2011a,b). It is noted that the value of ID/IG for FAG, is 0.72, which is higher than that of graphene (0.24), suggesting that grafting of furfuryl alcohol via Diels–Alder reaction has been accomplished. In this case, some of the sp2 hybridized carbon atoms are successfully transformed to sp3 hybridized atoms. In the TG analysis (Fig. 4C), FAG has a sharp weight loss in the range of 200–500 °C. However, the exfoliated graphene demonstrates gradual weight loss with the increase of temperature, which was mainly derived from oxidation in high temperature. The degradation behavior of the former implies the presence of organic portion in graphene and the grafted furfuryl alcohol ratio can be calculated to be approximately 14% by weight (Zhao et al., 2013). The photographs of graphene and FAG dispersed in water are shown in Fig. 4D. FAG shows a better hydrophilic ability than unmodified graphene.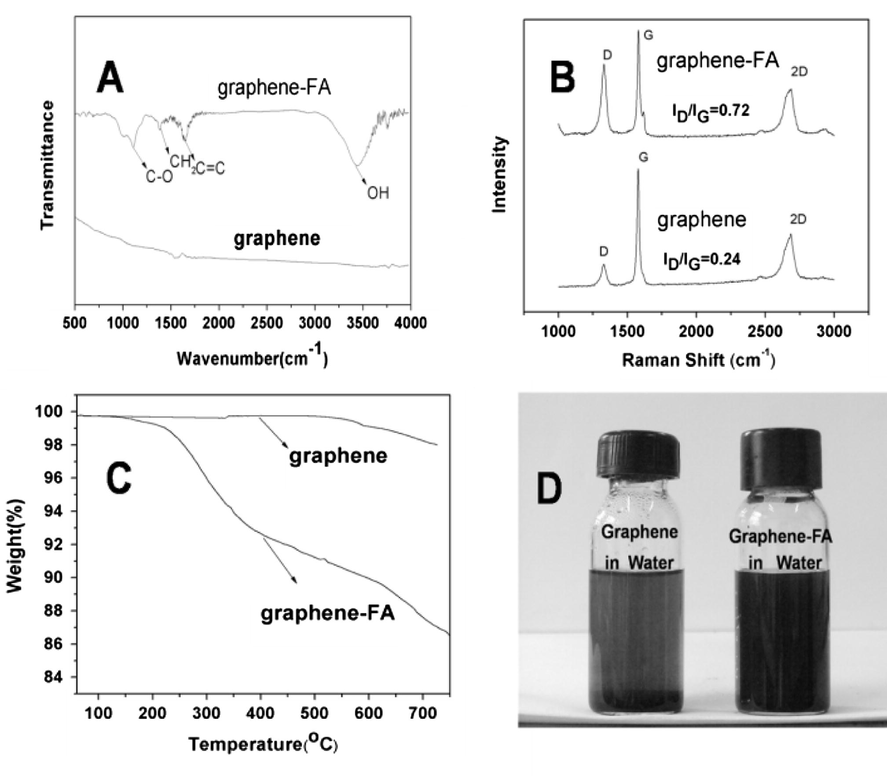
FTIR spectra of graphene and graphene-FA(A), Raman spectra of graphene and graphene-FA(B), TG curves of graphene and FAG (C), and the photographs of graphene and FAG dispersed in water (D).
4.2 Time-dependent sorption
Fig. 5 shows the kinetic data of radionuclide sorption on FAG at pH 3.0 and 298 K. The amount of adsorbed radionuclides (mmol g−1) increases sharply within the first hour and thereafter it proceeds at a slow rate and finally reaches equilibrium after 2 h. The initial rapid sorption may be attributed to the existence of plenty binding sites on FAG surfaces. The increase in concentration gradient tends to increase radionuclides sorption rate at the initial stages. As contact time increases, the concentration gradient is gradually reduced due to the accumulation of radionuclides on FAG surfaces, leading to the decrease of sorption rate at the later stages (Liu et al., 2011a,b). Overall, the sorption reaches equilibrium after 2 h. The fast sorption kinetics implies that FAG may have good potentialities for continuous sewage disposal systems. Based on the above-mentioned kinetic data, the shaking time in the following experiments was fixed to 12 h to ensure complete equilibrium (Ho et al., 1998).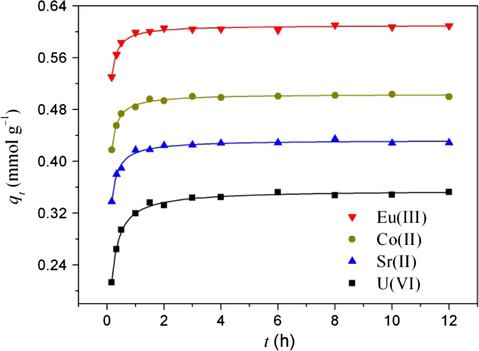
Sorption of radionuclides on FAG as a function of contact time. pH = 3.0, T = 303 K, I = 0.01 mol L−1 NaClO4, Cinitial = 0.2 mmol L−1, m/V = 0.2 g L−1.
The sorption kinetics was simulated by pseudo-first-order and pseudo-second-order models (Liu et al., 2014; Ho, 2006):
Sample
Eu(III)
Co(II)
Sr(II)
U(VI)
Adsorption kinetics
Pseudo-first order
k1 (h−1)
5.43
3.56
2.67
4.43
qe (mmol g−1)
0.1625
0.103
0.091
0.091
R2
0.76
0.80
0.83
0.81
Pseudo-second order
k (gmmol−1 h−1)
63.468
67.444
61.600
26.866
qe (mmol g−1)
0.609
0.503
0.431
0.354
R2
0.999
0.999
0.998
0.999
4.3 Effects of pH on sorption
It has been clearly established that the solution pH has a great impact on the sorption process. As shown in Fig. 6, the sorption percentage of U(VI) increases gradually as pH increases from 2.0 to 7.0. Afterward, the sorption percentage sharply decreases when pH is higher than 8.0. Increasing the solution pH can decrease the electrostatic repulsion between UO22+ and the positively charged surface of FAG, and consequently, promote the sorption amounts (Liu et al., 2015). Nevertheless, when pH values exceed 8.0, the sorption amount begins to fall off with increasing pH values. This may be attributed to the hydrolysis of U(VI) leading to non-complexible species such as UO2(OH)3− and the electrostatic repulsion of these anions with the negatively charged surfaces of FAG at high pH (Chen et al., 2012). Sorption percentage of Co(II) increases slowly in pH range 2.0–8.0 and the high level of sorption remains at pH > 8.5. The same phenomenon can also be observed for Eu(III) sorption. For Eu(III) and Co(II), precipitation can be witnessed at high pH values, and thus, the sorption amount maintains a high level. Sorption of Sr(II) keeps a stable increase in the whole pH range (Langmuir, 1918). The main species of Sr(II) are positively charged cations Sr2+ and no precipitation forms in the whole pH range. Therefore, the sorption of Sr(II) increases gradually due to the electrostatic attraction between the positive charge of cations and the increasing negative charge of sorbents.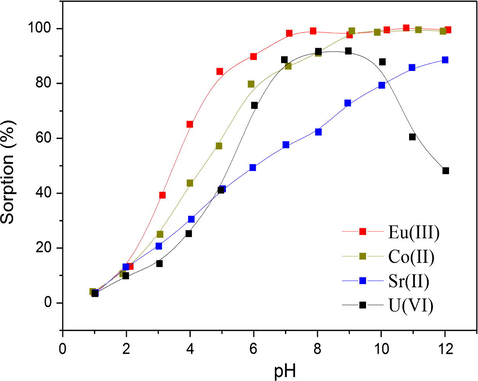
Effect of pH on radionuclide sorption on FAG. T = 303 K, I = 0.01 mol L−1 NaClO4, Cinitial = 0.2 mmol L−1, m/V = 0.2 g L−1.
4.4 Sorption isotherms
The sorption isotherms of U(VI), Eu(III), Sr(II), and Co(II) on FAG at T = 303 K and pH = 3.0 are shown in Fig. 7. Langmuir and Freundlich models were used to fit radionuclide sorption isotherms (Zhang et al., 2013):
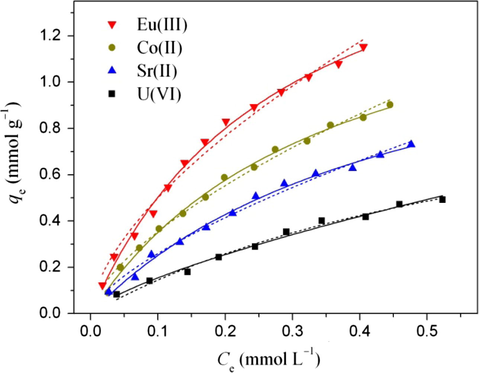
Sorption isotherms of radionuclide sorption on FAG. T = 303 K, I = 0.01 mol L−1 NaClO4, m/V = 0.2 g L−1. The scattered points represent experiment data, the solid lines represent the Langmuir model and the dashed lines represent the Freundlich model.
Sorbates
Langmuir
Freundlich
qmax (mmol g−1)
b (Lmmol−1)
R2
kF (mmol1−n Ln g−1)
n
R2
Eu(III)
1.947
3.486
0.995
2.066
1.623
0.986
Co(II)
1.608
2.792
0.996
1.556
1.667
0.990
Sr(II)
1.431
2.125
0.997
1.220
1.493
0.980
U(VI)
1.178
1.403
0.995
0.812
1.389
0.988
The sorption ability of FAG for different radionuclides should be affected by a series of factors, including ionic size and ionic charge (Huang et al., 2014).
A higher valence ion is more easily adsorbed on FAG; therefore, Eu(III) has the highest affinity to FAG surface. The radius of Co(II) is smaller than that of Sr(II), and both of which are smaller than that of linear UO22+. Smaller ions adsorbed occupy smaller surface area of sorbents, thus leading to higher sorption amounts. Therefore, the relative affinity of the FAG surface for the radionuclides can be ranked in the order Eu(III) > Co(II) > Sr(II) > U(VI).
5 Conclusions
The analysis results of SEM, FT-IR, and XPS indicated the successful addition of FA onto GO through the Diels–Alder reaction. The as-prepared FAG was applied to adsorb radionuclides from acidic wastewater. All the adsorption kinetics followed the pseudo-second-order model rather than pseudo-first-order model. The thermodynamic parameters calculated from the temperature dependent isotherms indicated an endothermic and spontaneous adsorption process. The relative binding affinity of the FAG surface toward the radionuclides can be ranked in the order Eu(III) > Co(II) > Sr(II) > U(VI).
Acknowledgment
We are grateful for the project supported by the Natural Science Foundation of Chongqing Science & Technology Commission (No. cstc2013jcyjA1225), China.
References
- Poly(acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co(II) removal from aqueous solution. Chem. Eng. J.. 2012;210:475-481.
- [Google Scholar]
- Neoformation of Ni phyllosilicate upon Ni uptake on montmorillonite: a kinetics study by powder and polarized extended X-ray absorption fine structure spectroscopy. Geochim. Cosmochim. Acta. 2002;66:2335-2347.
- [Google Scholar]
- Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev.. 2012;112:6156-6214.
- [Google Scholar]
- Phosphate functionalized graphene with tunable mechanical properties. Adv. Mater.. 2014;26:718-723.
- [Google Scholar]
- Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym.. 2011;83:653-658.
- [Google Scholar]
- Review of second-order models for adsorption systems. J. Hazard. Mater.. 2006;136:681-689.
- [Google Scholar]
- Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot.. 1998;76:183-191.
- [Google Scholar]
- Carbon nanomaterial based composites in wastewater purification. Nano Life. 2014;4:1441006.
- [Google Scholar]
- Synthesis and anticoagulant activity of polyureas containing sulfated carbohydrates. Biomacromolecules. 2014;15:4455-4466.
- [Google Scholar]
- Structure-directing role of graphene in the synthesis of metal-organic framework nanowire. J. Am. Chem. Soc.. 2010;132:14487-14495.
- [Google Scholar]
- Preparation of functionalized graphene/linear low density polyethylene composites by a solution mixing method. Carbon. 2011;49:1033-1037.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [Google Scholar]
- Effect of pH, ionic strength, foreign ions and temperature on the adsorption of Cu(II) from aqueous solution to GMZ bentonite. Colloid Surf. A. 2009;349:195-201.
- [Google Scholar]
- Organo-and water-dispersible graphene oxide − polymer nanosheets for organic electronic memory and gold nanocomposites. J. Phys. Chem. C. 2010;114:12742-12748.
- [Google Scholar]
- Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal. J. Phys. Chem. C. 2011;115:25234-25240.
- [Google Scholar]
- Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J. Phys. Chem. C. 2011;115:25234-25240.
- [Google Scholar]
- Facile synthesis of hierarchical Fe4(P2O7)3 for removal of U (VI) J. Mol. Liq.. 2014;200:311-318.
- [Google Scholar]
- High performance of phosphate-functionalized graphene oxide for the selective adsorption of U (VI) from acidic solution. J. Nucl. Mater.. 2015;466:56-64.
- [Google Scholar]
- Graphene oxide sheets chemically cross-linked by polyallylamine. J. Phys. Chem. C. 2009;113:15801-15804.
- [Google Scholar]
- Furfuryl alcohol functionalized graphene nanosheets for synthesis of high carbon yield novolak composites. J. Appl. Polm. Sci.. 2014;131:40273.
- [Google Scholar]
- Graphene oxide for effective radionuclide removal. Phys. Chem. Chem. Phys.. 2013;15:2321-2327.
- [Google Scholar]
- Synthesis, characterization and electrochemical properties of functionalized graphene oxide. Carbon. 2012;50:4228-4238.
- [Google Scholar]
- Graphene-based modified electrode for the direct electron transfer of cytochrome c and biosensing. Electrochem. Commun.. 2010;12:175-177.
- [Google Scholar]
- Size-controlled synthesis of graphene oxide sheets on a large scale using chemical exfoliation. Carbon. 2009;47:3365-3368.
- [Google Scholar]
- Controlled synthesis of few-layered graphene sheets on a large scale using chemical exfoliation. Carbon. 2010;48:2367-2371.
- [Google Scholar]
- Polyaniline nanorods dotted on graphene oxide nanosheets as a novel super adsorbent for Cr (VI) Dalton Trans.. 2013;42:7854-7858.
- [Google Scholar]
- Removal of Pb (II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans.. 2011;40:10945-10952.
- [Google Scholar]
- Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol.. 2011;45:10454-10462.
- [Google Scholar]
- Efficient enrichment of uranium(VI) on amidoximated magnetite/graphene oxide composites. RSC Adv.. 2013;3:18952-18959.
- [Google Scholar]
- Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater.. 2010;22:3906-3924.
- [Google Scholar]







