Translate this page into:
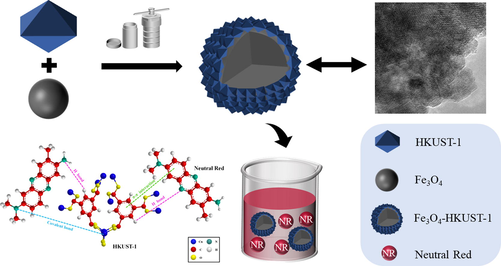
Synthesis of magnetic Fe3O4-HKUST-1 nanocomposites for azo dye adsorption
⁎Corresponding author at: School of Chemistry and Environment, Yunnan Minzu University, Kunming 650500, PR China. liu-chenhui@hotmail.com (Chenhui Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The magnetic Fe3O4-HKUST-1 nanocomposites were synthesized using a simple and green hydrothermal technique, utilizing Fe3O4 and the chemically stable metal–organic framework HKUST-1. The Fe3O4-HKUST-1 nanocomposites were synthesized with a varied proportion of Fe3O4 and subjected to advanced characterization, which included XRD, IR, BET, TG, SEM, TEM, EDS, and VSM. The magnetic nanocomposite 0.4 Fe3O4-HKUST-1 (FH4) had a rough surface with spherical morphology and a good proportion of micro and mesopores. The magnetic nanocomposite had a mean pore size of 2.58 nm, with pore volume of 0.35 cm3/g, having excellent magnetic saturation performance (26.39 emu/g). The adsorption isotherm and adsorption kinetics of magnetic nanocomposites on azo dye neutral red (NR) wastewater were assessed in detail. In contrast to the Freundlich model, the Langmuir model better described the adsorption isotherms, while the pseudo-second-order kinetic model better captured the adsorption kinetics. The FH4 magnetic nanocomposite was the most effective adsorbent with the highest adsorption capacity for NR separation.
Keywords
Metal organic framework
HKUST-1
Fe3O4
Nanocomposite
Adsorption
1 Introduction
The rapid industrialization of the 20th Century, although it improved the standard of living, was the root cause for the deterioration of environment due to indiscriminate discharge of pollutants. Industrial manufacturing generates all three forms of solid, liquid and gaseous discharges to the environment (Cheng 2021, Pona et al., 2021, Huang et al., 2022). Although they are designed to meet environmental standards, the overall load on the environment is still considerable, which augurs the development of efficient waste management technologies. It is reported that about 400 billion cubic meters of sewage flow into rivers worldwide every year, contributing to 40% river pollution globally (Gallego-Schmid and Tarpani 2019, du Preez and van Huyssteen 2020, Yin et al., 2021, Lin et al., 2022). In China, nearly 70% of rivers are polluted while the urban water resources are about 90% polluted (Liu and Yang 2012, Xu et al., 2014, Huang et al., 2022, Liu et al., 2022). A significant portion of water pollution can be attributed to the indiscriminate discharge of untreated industrial and domestic wastewater into sewage, which has severely damaged the flora and fauna of the water bodies, endangering the life around them (Han et al., 2016, Xu et al., 2019, Shi et al., 2020). The United Nations (UN) has directed the countries to halve the untreated wastewater, through adoption of 3R principles of waste management, to meet the Sustainable Development Goals (Ortigara et al., 2018, Xu et al., 2019).
The discharged wastewaters are known to contain various widespread hazardous contaminant like metal ions, drugs, nitrogen and phosphorus and organic dyes (Song et al., 2022, Tang et al., 2022). Among the industrial wastewater contaminants, organic dyes in particular are the most discharged pollutant, as they are very widely used in small-scale industries (Zhan et al., 2018, Mashkoor and Nasar 2021). Organic dyes are widely utilized in printing (Schneider and Sostar-Turk 2003), textile (Wiraningtyas et al., 2020), paint (Mahmoodi et al., 2017), chemical (Yigit and Turhan 2021), and pharmaceutical (Hirachan 2019) industries, which are organic compounds consisting of chromophores and auxiliary chromophores (Tara et al., 2020). The structure of organic dyes is usually complex and non-biodegradable, which can disrupt the ecological balance and exacerbate the pollution problem (Sobhanardakani and Zandipak 2015, Huong et al., 2020, El-Ghobashy et al., 2022). The presence of these colored organic dyes not only increased the chromaticity of polluted water bodies but also restricted sunlight penetration, affecting the ecosystem of water bodies (Allen et al., 2004, Donia et al., 2009). Organic dyes are carcinogenic and teratogenic, that are established to accumulate as well in the food chain (McMullan et al., 2001, Fonovich 2013, Olas et al., 2021).
Chemical oxidation (Krull and Hempel 2001), biological treatment (Rehorek and Plum 2006), membrane separation (Ciardelli et al., 2001), electrochemical (Jovic et al., 2014) and catalyst (Mondal et al., 2020) are various technologies that are in practice to treat organic dyes from wastewater. However, these technologies inevitably suffer from high resource consumption, high capital and operating costs, and a low chromaticity removal rate, which limit their applications. Moreover, organic dyes are resistant to photodegradation, chemical and biological treatment, rendering conventional techniques ineffective (Chung 2016, Sobhanardakani et al., 2016). In addition to the above treatment methods, adsorption has proven to be the most effective and are popularly used due to its apparent operational simplicity, low energy consumption, low process cost, high efficiency and recovery (Melendez-Ortiz et al., 2019, Alguacil and Lopez 2021, Soltani et al., 2021). Adsorbents are porous materials having suitable surface properties to attract specific molecules of target. Commercially, a large number of adsorbents are manufactured, which makes it an attractive option (Sobhanardakani et al., 2013). Some of the popular adsorbents are silica gel (Zhang et al., 2021), zeolites (Robles-Mora et al., 2021), activated carbons (Mei et al., 2019) etc.
Metal-organic frameworks (MOFs) are a form of porous hybrid functional material consisting of metal ions or metal clusters and organic ligands through coordination bonds and weak interaction forces (Buso et al., 2011, Wei et al., 2017). There are thousands of different types of MOF that are being explored for their suitability for different applications. MOF are easy to synthesis with varied pore structure, surface area and topology tailored to meet specific applications (Chae et al., 2004, Hirscher et al., 2010, Zhang et al., 2022). In recent years, MOF and MOF-derived materials have been increasingly explored to assess their effectiveness for treatment of pollutants in wastewater (Zhang et al., 2018, Ru et al., 2021). Lian et al. (Lian and Yan 2016) have synthesized series of lanthanide MOF-76 via solvothermal method and reported its suitability for removing cationic dyes such as methylene blue (MB). Ghosh et al. (Ghosh and Das 2020) have synthesized MOF based on Sn using hydrothermal technique, having good water stability and efficient in removing toxic anionic dyes. Yang et al. (Yang and Bai 2019) have synthesized bimetallic Ni-Zn MOF microspheres using solvothermal technique and reported its effectiveness for adsorption of Congo Red (CR) molecules.
MOF materials have also been reported to remove other pollutants, such as heavy metal ions (Wang et al., 2022), thiophenes (Chen et al., 2019), phenols (Lin and Hsieh 2015), and gases (Jia et al., 2021). Although MOF materials have many practical applications, its application is limited by the ability to quickly remove from the wastewater system, that demands development of improved solid–liquid separation techniques. Magnetic separation methods are well known to be handy, economical and effective for wastewater treatment (Shen et al., 2004, Moghaddam et al., 2015, Zandi Pak and Sobhan Ardakani 2016). The MOF with induced magnetic properties is easy to separate after treatment, which could save resources, protect the environment, and reduce the cost of wastewater treatment (Zandipak and Sobhanardakani 2016). Hence, an attempt has been made to combine magnetic particles with MOF to induce magnetic properties.
The azo dye neutral red (NR) is a dark green crystalline powder that are easily soluble in water. NR is widely used for staining cells and as a pH indicator in laboratories. It releases dangerous substances like nitrogen oxides upon decomposition (Iram et al., 2010). The molecular properties of NR are shown in Table 1. HKUST-1 (MOF-199) is a popular MOF known for its good thermal and chemical stability (Chui et al., 1999). Magnetic Fe3O4 with low toxicity and high thermal aqueous stability was selected to prepare magnetic MOF composites. In this study, a simple and green two-step hydrothermal method was used to synthesize magnetic Fe3O4-HKUST-1 nanocomposites to improve the adsorption and removal ability of HKUST-1 for NR. The prepared materials were subjected to characterization using infrared spectroscopy (IR), thermogravimetric analysis (TG), scanning electron microscopy (SEM), transmission electron microscopy (TEM), x-ray diffractometer (XRD), vibrating sample magnetometer (VSM), N2 adsorption and desorption (BET) and energy spectrum analysis (EDS). The effects of different proportion of magnetic Fe3O4 on the morphology, crystal phase, thermal stability and specific surface area of the materials were assessed. The factors affecting the adsorption capacity of synthesized adsorbents were investigated under various conditions, including contact time, initial NR concentration, adsorbent dosage, pH, and temperature. A comprehensive evaluation of adsorption kinetics, adsorption isotherms, and adsorption mechanisms was also carried out. The rate of adsorption of NR is modeled using appropriate isotherm model and kinetic model. To adsorption mechanism of NR on the Fe3O4-HKUST-1 composite has not been reported in open literature and hence efforts are made to understand the adsorption mechanism. The regeneration performance and reusability of the synthesized materials were also investigated.
Generic
nameChemical
formulaChemical
structureMolecular
weight
(g/mol)Boiling
Point
(℃)Melting
Point
(℃)Density
(g/cm3)Character
Solubility
Neutral
RedC15H17ClN4
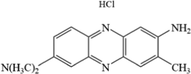
288.75
290
497.3
1.159
Dark green crystalline powder
Soluble in water and alcohol
2 Experimental
2.1 Reagents and chemicals
Ferric chloride (FeCl3·6H2O), ethylene glycol (EG), sodium acetate anhydrous (CH3COONa), N,N-Dimethylformamide (DMF), trimesic acid (H3BTC), anhydrous ethanol, polyethylene glycol (PEG), copper nitrate trihydrate (Cu (NO3)2·3H2O) and neutral red (NR) were procured by Aladdin reagent company, China. Without any additional purification, all of the reagents that were bought were of analytical grade. The water for the experiment is ultrapure water prepared in the laboratory.
2.2 Adsorbent synthesis
2.2.1 Synthesis of HKUST-1
Cu (NO3)2·3H2O (6.21 mmol) and H3BTC (3.57 mmol) were placed in 90 mL of mixed solution (anhydrous ethanol: DMF: ultrapure water, V: V: V, 1:1:1) in an ultrasonic bath and processed until the solute was entirely dissolved. The solution was poured into a hydrothermal reactor and maintained at 80 °C for 24 h. The mixture was filtered and dried at 160 °C overnight. This synthesis method was based on that derived from the work of Chowdhury et al. (Chowdhury et al., 2009).
2.2.2 Synthesis of magnetic Fe3O4
The magnetic Fe3O4 was synthesized via the hydrothermal method (Sun et al., 2011). FeCl3·6H2O (7.76 mmol), CH3COONa (0.073 mol) and PEG (0.041 mmol) were dispersed in 100 mL of EG and magnetically stirred until the solute was entirely dissolved. The mixture was poured into a hydrothermal reactor and maintained at 200 °C for 20 h. The samples were rinsed with ultrapure water and ethanol, and were separated applying a magnetic field. The product was a black powder, which was dried overnight at 60 °C.(See Fig. 1).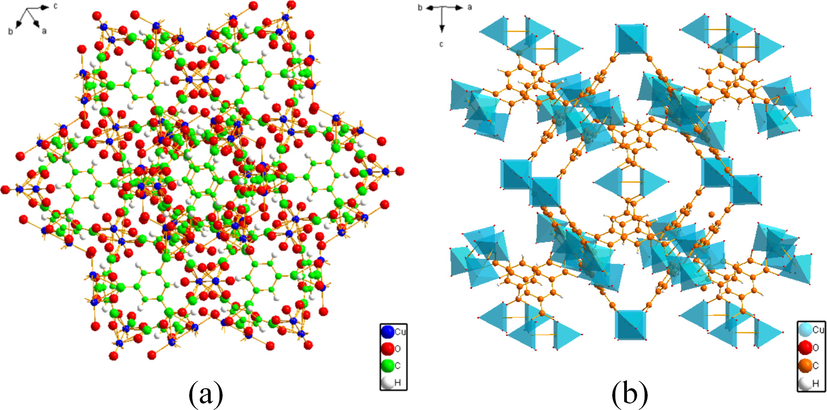
Crystal structure of HKUST-1.
2.2.3 Synthesis of magnetic × Fe3O4-HKUST-1 nanocomposites
The preparation method of magnetic xFe3O4-HKUST-1 nanocomposites is based on the previous research results (Ahmadipouya et al., 2021), and some changes have been made. X g of magnetic Fe3O4 (x = 0.1, 0.2, 0.3, 0.4, 0.5, 0.6), Cu (NO3)2·3H2O (6.21 mmol) and H3BTC (3.57 mmol) were dispersed in 90 mL of mixed solution (anhydrous ethanol: DMF: ultrapure water, on 1:1:1 vol basis) in an ultrasonic bath until the solute was entirely dissolved. The mixture was poured into a hydrothermal reactor and maintained at 80 °C for 24 h. The mixture was rinsed with ultrapure water and ethanol, and separation was affected by applying a magnetic field. The final product brown-black powder was dried at 60 °C overnight (Fig. 2). The samples were abbreviated as FH1, FH2, FH3, FH4, FH5, FH6, for future reference in the manuscript.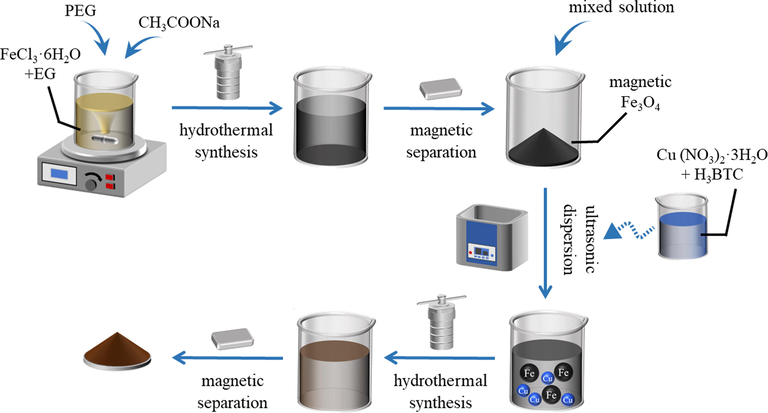
Preparation process of magnetic Fe3O4-HKUST-1 nanocomposites.
2.3 Characterization
The adsorbents were characterized by X-ray diffractometer (XRD), using Cu Kα as a radiation source. The diffraction pattern of the products was obtained by scanning in the 2θ angle range of 5 ∼ 80°. The functional groups of the adsorbents were obtained by Fourier transform infrared spectroscopy (FT-IR). The adsorbents were processed with potassium bromide pellet method to measure the infrared values in the range of 500 to 4000 cm−1. The adsorption desorption diagram, pore volume and pore size trend diagram of the adsorbents were analyzed by N2 adsorption–desorption (BET) at a liquid nitrogen temperature of 77 K. A certain position of the sample was characterized by Energy dispersive spectrum (EDS), and the elements contained in the adsorbents were obtained. The surface morphology and size images of the particles were obtained by Scanning electron microscopy (SEM) and Transmission electron microscopy (TEM). The samples were characterized using thermogravimetric analysis (TG) at temperatures between 25 and 800 °C. The spectra were obtained to analyze the thermal stability and composition of the adsorbents. The magnetic response value of the adsorbents was characterized by Vibrating sample magnetism intensity (VSM) to obtain the hysteresis loop of the sample.
2.4 Adsorption experiment
Adsorption experiments were conducted to assess the adsorption characteristics of the magnetic nanocomposites towards NR. The standard curve of concentration with respect to absorbance was generated using a UV spectrophotometer at a maximum absorption wavelength of 535 nm, and it was found to follow the Lambert-Beer law. The adsorption experiments were performed covering the initial concentration of NR, mass of adsorbents, and contact time at a constant temperature of 298 K. The NR solution concentration ranged from 10 to 100 mg/L, the adsorbent dosage was varied in the range of 10 to 50 mg, and the contact time ranged from 10 to 160 min. The adsorption equilibrium was established well within 120 min. The clear solution on the upper layer was collected at intervals of time to calculate the concentration from the standard curve.
To evaluate the effectiveness of the adsorption course, the adsorption rate of samples was calculated using Eq. (1) as the following:
The amount of adsorbed dye was defined as follows Eqs. (2) and (3):
The pH was adjusted to a range of 4 to 10 with 0.1 M NaOH and HCI solution at room temperature. The effect of pH on the adsorption properties of magnetic Fe3O4-HKUST-1 nanocomposites was examined. The temperature of the adsorption experiments was set at 25 to 50 °C. To investigate the effect of different temperatures on the adsorption properties of magnetic Fe3O4-HKUST-1 nanocomposites.
2.5 Desorption experiment
To evaluate the recycling performance of the prepared magnetic Fe3O4-HKUST-1 nanocomposites, desorption experiments were carried out. The adsorbent was treated with 0.1 M HCl and ethanol as eluent. 10 mL of eluent was added to the container, and the mixed solution underwent ultrasonic treatment at 298 K for 3 h. After the desorption process was completed, the magnetic Fe3O4-HKUST-1 nanocomposites were separated from the solution with an external magnet. It was also rinsed with ultrapure water to neutralize the acidity and dried overnight at 60 °C. The recovered adsorbent is prepared for the next cycle, and the cycling step is repeated five times.
3 Result and discussion
3.1 Characterization analysis
The synthesized adsorbent XRD spectrum is shown in Fig. 3 (a). The XRD patterns of the prepared samples were consistent with those of HKUST-1 reported in previous literature (Chui et al., 1999, Pan et al., 2022). The presence of all the diffraction peaks of HKUST-1 is seen, without appearance of spurious peaks. The characteristic peaks of adsorbent correspond to diffraction angles of 6.7°, 9.5°, 11.6°, 13.4°, 17.5°, and 19.0°, with sharp and high intensity peaks, indicating successful preparation of HKUST-1 having high crystallinity.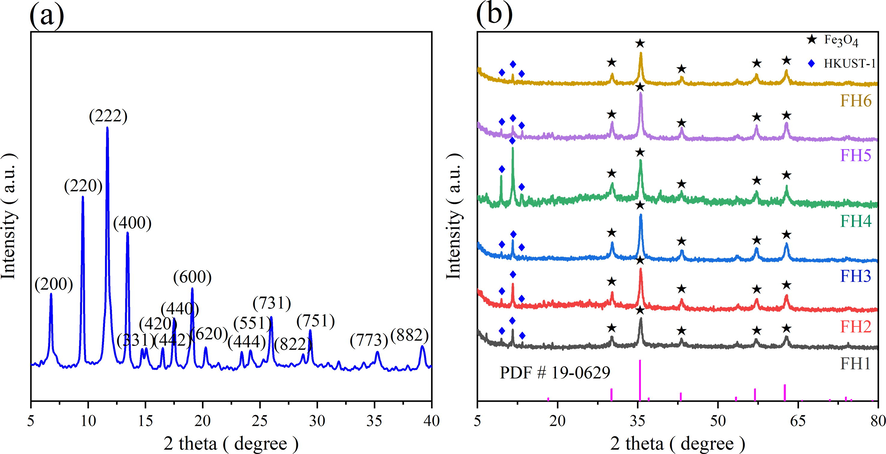
XRD patterns of HKUST-1 (a), magnetic xFe3O4-HKUST-1 nanocomposites (b).
In order to determine the formation of the magnetic xFe3O4-HKUST-1 nanocomposite. The XRD examination results are depicted in Fig. 3 (b). The distinct peak appearing at a diffraction angle of 11.6° corresponds to the (2 2 2) crystal plane of HKUST-1. The intensity of other diffraction peaks of HKUST-1 was reduced. The distinctive diffraction peaks of Fe3O4 appeared at diffraction angles of 30.2°, 35.6°, 43.3°, 57.2°, and 62.8° corresponding to the (2 2 0), (3 1 1), (4 0 0), (5 1 1), and (4 4 0) crystallographic planes of Fe3O4. This matched the standard card (PDF # 19-0629). It indicates that Fe3O4 has been successfully incorporated and that the composite was successfully prepared.
The FT-IR spectrum of the HKUST-1 and the composites of the xFe3O4-HKUST-1 were shown in Fig. 4. The main peaks were due to organic ligand H3BTC. The stretching vibration of the hydroxyl could be the cause of the broader absorption peak appearing around 3445 cm−1. The stretching vibration of the aromatic ring in the organic ligand BTC was what caused the absorption peak around 1300–1700 cm−1. The stretching vibration of O = C–C could be responsible for the absorption peak at 1634 cm−1. The stretching vibration of the skeleton of the benzene ring could be the cause of the absorption peak at 1445 cm−1. The stretching vibration of C = C could be the cause of the absorption peak at 1372 cm−1, which at around 1109 cm−1 could be the cause of the C-O-C stretching vibration. The absorption peak near 729 cm−1 could be ascribed to the stretching vibration of Cu–O.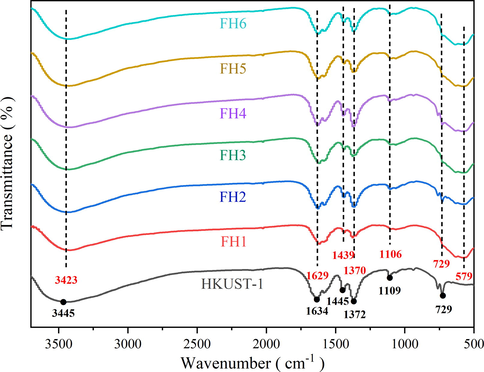
FT-IR spectra of HKUST-1 and magnetic xFe3O4-HKUST-1 nanocomposites.
The IR spectra of the previously examined HKUST-1 matched the functional groups of the magnetic xFe3O4-HKUST-1 nanocomposites. The appearance of these peaks confirms that the introduction of magnetic Fe3O4 did not affect the formation of HKUST-1. Besides, the existence of characteristic adsorption band around 579 cm−1 could be ascribed to the stretching vibration of Fe-O.
The N2 adsorption–desorption isotherm of MOF-199 is depicted in Fig. 5(a). It clearly indicates that the isotherm belongs to type I adsorption isotherm as per IUPAC classification (Sing 1985). This exhibited a typical microporous structure, as evidenced by the rapid increase in N2 adsorption in the low-pressure zone and the relatively stable adsorption at higher relative pressures. Additionally, the pore size distribution revealed presence of only micropores in the range of 0.4 to 1.5 nm, with the maximum at 1 nm. The specific surface area was 977 m2/g, the total pore volume was roughly 0.387 cm3/g, and the mean pore size was roughly 1.58 nm.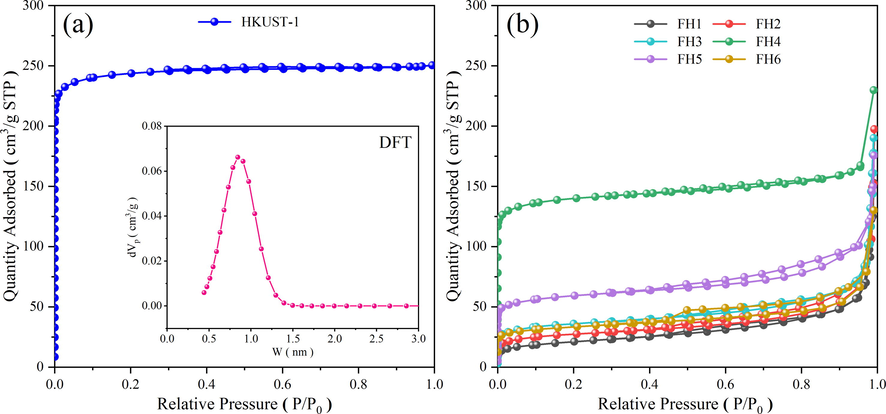
N2 adsorption–desorption isotherms curves of HKUST-1 (a), magnetic xFe3O4-HKUST-1 nanocomposites (b).
The N2 adsorption–desorption isotherm of the effect of Fe3O4 addition on the pore structure of HKUST-1 was shown in Fig. 5 (b). The xFe3O4-HKUST-1 adsorption isotherm pattern was significantly different from HKUST-1. According to IUPAC, the adsorption isotherm clearly shifted form type I to type IV. The sharp rise in the adsorption capacity at high relative pressure and the presence of the hysteresis loop in the 0.5–0.9 relative pressure region, clearly indicate the pore size enlargement that belong to material with good proportion of mesopores. These types of adsorption isotherms are characteristic of material with a mixture of micro and mesopores, often referred to as “bottle-neck” type pores. In Table 2, the parameters for the total pore volume, mean pore size, and specific surface area were listed.
Sample
BET/
(m2/g)Mean pore diameter/
(nm)Total pore volume/
(cm3/g)
HKUST-1
977.92
1.5831
0.3870
FH1
74.65
12.3880
0.2312
FH2
98.14
9.9316
0.2437
FH3
127.54
8.9740
0.2861
FH4
548.86
2.5803
0.3541
FH5
220.93
4.6648
0.2576
FH6
122.63
6.5122
0.1996
Table 2 showed that the pore size decreased and the surface area increased with increase in the Fe3O4 content in the nanocomposites until the optimal 0.4 (FH4). At Fe3O4 contents higher than 0.4, the pore diameter increased significantly with a drastic reduction in the surface area. Compared with HKUST-1, the pore volume and BET of magnetic nanocomposites were found to be significantly lower, which could be due to the presence of magnetic Fe3O4 nuclei. Cao et al. (Cao et al., 2014) have reported that adding excessive metal ions to MOF materials may reduce the specific surface area.
The thermogravimetric curve of MOF-199 was shown in Fig. 6 (a). The MOF-199 exhibited three distinct weight loss phases, the weight loss of the MOF-199 was mainly the volatilization of free molecules of solvent ethanol and water molecules within 25–130 °C, and the weight loss was mainly the volatilization of solvent molecules inside the pore channels within 130–260 °C. With a further rise in temperature to about 300 °C, the material began to decompose, and the fastest thermal weight loss occurred at 352 °C. The frame structure of HKUST-1 collapsed completely at about 367 °C. During this phase skeletal structure collapsed completely and was oxidized to form the end product metal oxide Cu2O, leading to a rapid decrease in mass, from which the thermal stability of the material was known to be around 300 °C.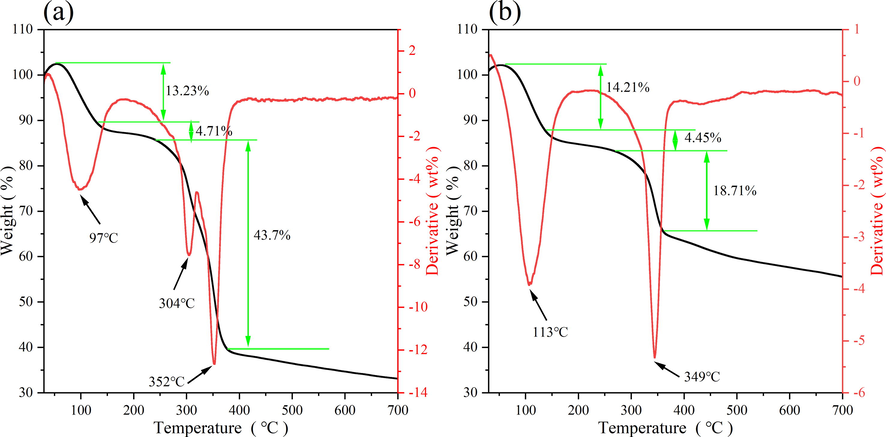
TG and DTG curves of HKUST-1 (a), FH4 (b).
The thermogravimetric curve of the magnetic nanocomposites FH4 was shown in Fig. 6 (b). It exhibited a similar trend to HKUST-1. The nanocomposites FH4 started to decompose at about 330 °C, and the temperature at which the thermal weight loss was fastest was at about 350 °C. The collapse of the framework structure was complete at around 380 °C, with the formation of product metal oxide residue, leading to a dramatic decrease in quality. The thermal stability of the magnetic nanocomposites FH4 increased around 30 °C relative to that of pure HKUST-1.
Fig. 7 (a), (b), (c), (d) and (e) presented show the SEM images of HKUST-1 at different magnifications. The synthesized metal–organic framework material HKUST-1 showed sharp edges and regular ortho-octahedral structure with complete morphology, which corroborated well with the high crystallinity characterized by XRD spectra. Fig. 8 (a), (b), (c), (d) and (e) showed FH4 nanocomposite at different magnifications. The surface was relatively rough, close to spherical, with an average diameter of 220 nm having many projections. This could be due to the growth of many HKUST-1 on the outer surface of magnetic Fe3O4, which corroborated well with the findings of XRD and IR spectra.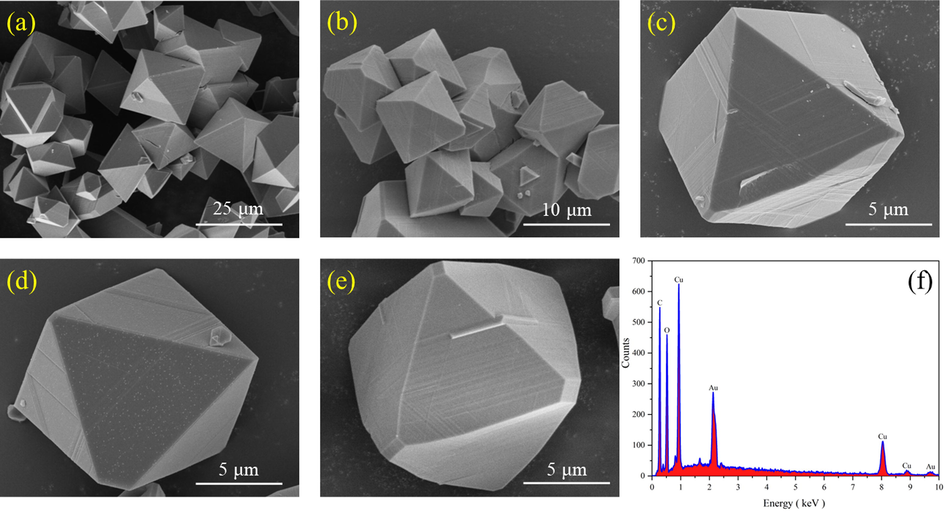
SEM images of HKUST-1 (a, b, c, d, e), EDS analysis of HKUST-1 (f).
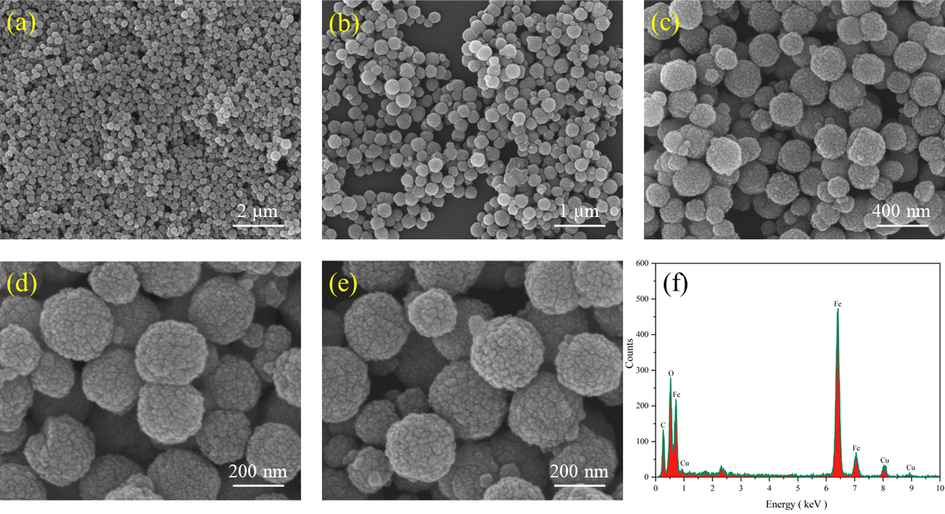
SEM images of FH4 (a, b, c, d, e), EDS analysis of FH4 (f).
The energy spectrum analysis of the synthesized materials shows existence of C, O, and Cu elements in the HKUST-1. In the FH4 nanocomposite, it demonstrates the existence of C, O, Fe, and Cu elements. The proportion of Fe increased significantly as compared to Cu. The presence of Au elements is due to the spraying of gold treatment before testing.
The TEM morphologies of HKUST-1 and magnetic 0.4 Fe3O4-HKUST-1 nanocomposites were shown in Figs. 9 and 10, respectively. The image data showed that the prepared HKUST-1 has an octahedral shape with sharp edges. The surface was smooth, and the edges were clear. This is consistent with previous reports (Kubo et al., 2022, Wu et al., 2022). Magnetic Fe3O4 did not significantly change the morphology of HKUST-1, but the particle size of HKUST-1 decreased (Min et al., 2017). According to the TEM image (Fig. 10), the surface of the FH4 nanocomposite was rough with the surface covered with irregular bumps. This confirmed that HKUST-1 successfully adhered to the outer surface of Fe3O4, which is consistent with the morphology reflected in the SEM image of the FH4 nanocomposite and further confirms the uniform size of 220 nm.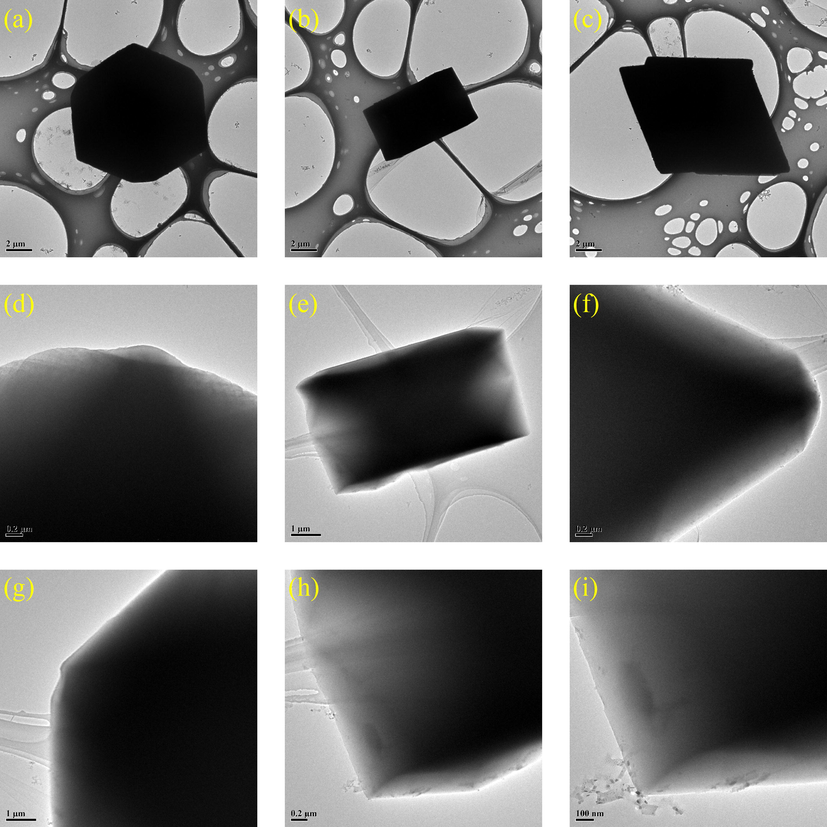
TEM images of HKUST-1.
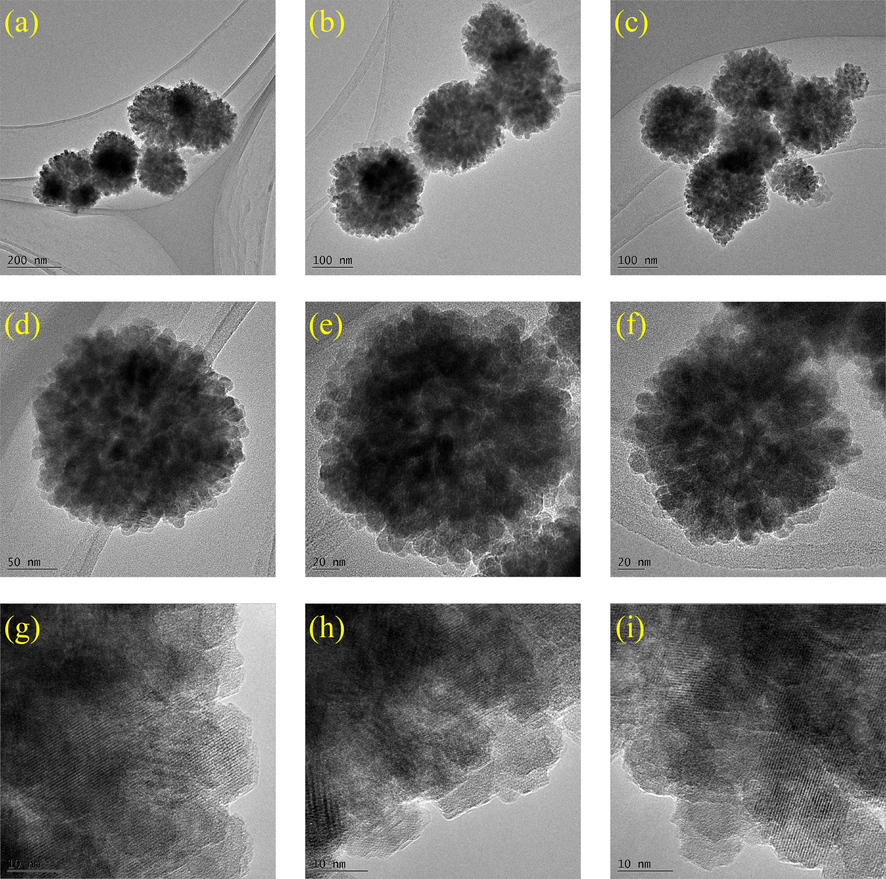
TEM images of magnetic 0.4 Fe3O4-HKUST-1 nanocomposites (FH4).
The hysteresis loop (MH curve) of FH4 was obtained by vibrating the sample magnetometer to measure the magnetism of the material. The M stands for the magnetic saturation level of the material, which reflected the magnetic strength of the material. From Fig. 11, the magnetic saturation level of FH4 was given as 26.39 emu/g, indicating very good magnetic properties. The remanence magnetism and the coercivity of the composite was particularly small and tend to be close to zero, indicating the composite to be superparamagnetic. This was an important targeted property of the composite so as to be suitable for adsorption processes. Superparamagnetic particles themselves are nonmagnetic, however on exposure to external magnetic field, they act as magnetic particles (Aghayi-Anaraki and Safarifard 2020). Thus, the aggregation of magnetic particles can be prevented which allow to redisperse rapidly after removal of magnetic field. This property facilitates separation of magnetic particles exposed to an external magnetic field. The magnetic nanoparticle (FH4) can be successfully utilized as it can facilitate easy removal of the adsorbate through the application of a magnetic field. The elimination of the expensive centrifugation and filtration processes contributed to the improvement of the economy of the wastewater treatment system.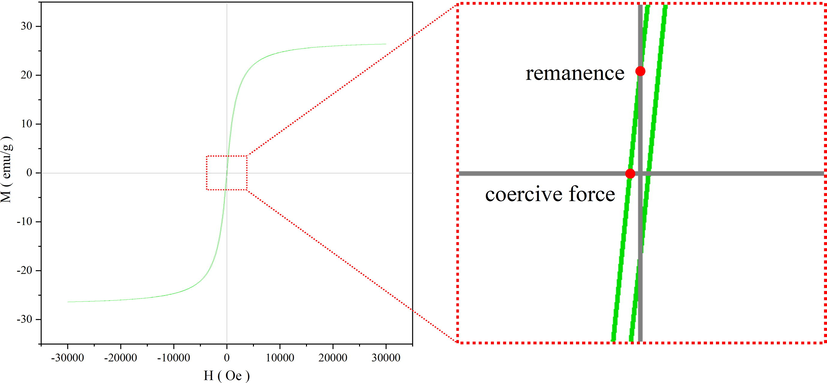
Hysteresis loop of magnetic 0.4 Fe3O4-HKUST-1 nanocomposites (FH4).
3.2 Adsorption performance study
3.2.1 Impact of contact time
The impact of contact time on the adsorption of NR on the different nanocomposite were presented in Fig. 12 (a). The initial dye concentration was maintained at 60 mg/L, and the adsorbent dosage was 50 mg. The rate of adsorption was high at low duration while it was found to decrease with increase in the duration. The high adsorption rate at an earlier part of adsorption could be due to the high mass transfer concentration driving force and availability of large active adsorption sites in the adsorbent. The adsorbent and the adsorbate were found to equilibrate at an adsorption duration of around 120 min, the adsorption capacity did not increase further. The addition of magnetic Fe3O4 improved the adsorption performance and adsorption rate with the maximum relating to 0.4 g of magnetic Fe3O4. The adsorption capacity was the highest for FH4, which correlated well with the high surface area. The HKUST-1 had the lowest adsorption capacity, while the nanocomposites exhibited a trend in coherence with the surface area.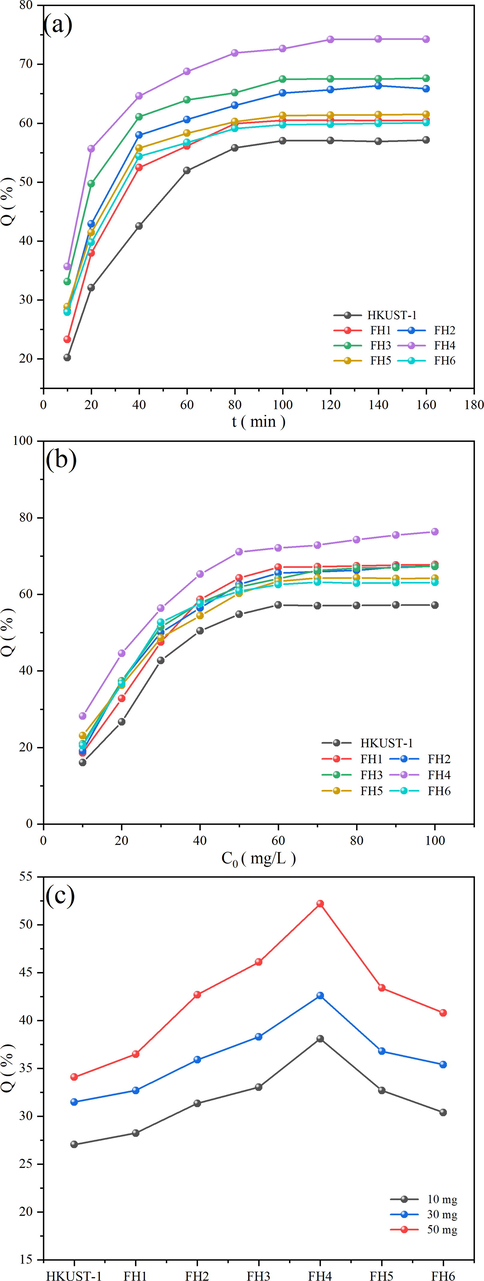
Effect of contact times on adsorption capacity of HKUST-1 and magnetic xFe3O4 HKUST-1 nanocomposites (a), effect of initial dye concentration (b), effect of the amount of adsorbent (c).
3.2.2 Impact of initial dye concentration
The influence of initial concentration of NR on the adsorption process was obtained from Fig. 12 (b), while the adsorption contact time and the adsorbent amount kept constant at 45 min and 50 mg. An increase in the concentration of NR increased the adsorption capacity of the adsorbent until a critical concentration was reached. The quantity of active adsorption sites limited the process of adsorption, due to the fixed mass of adsorbent dosage. As the concentration increased, the rate of adsorption would be faster due to the higher concentration driving force, but it would reach its maximum due to the limitation of the active sites to further accommodate the adsorbent. The highest adsorption capacity was for FH4, in concurrence with the earlier observation owing to the same cause as discussed earlier.
3.2.3 Impact of the amount of adsorbent
Fig. 12 (c) compared the adsorption capacities of the different nanocomposites with respect to the amount of adsorbent, while the contact time and the initial dye concentration were kept at 30 min and 60 mg/L. The adsorption capacity increased with increase in the Fe3O4 content of the nanocomposite up to FH4, with a decrease for FH5 and FH6. This trend was synonymous with the other results, and it related to the decrease in surface area beyond FH4. A clear increase in the NR uptake was exhibited with rise the adsorbent dosage for all the nanocomposites. With an increase in adsorbent dosage, the quantity of active adsorption sites increased, and the adsorbent could accommodate more adsorbate molecules.
3.2.4 Impact of pH
PH is an important factor affecting the adsorption of NR by magnetic Fe3O4-HKUST-1 nanocomposites. Therefore, it is essential to determine the ideal pH for adsorption. The pH at which the surface charge of the adsorbent is zero is called pHpzc. When pH is less than pHpzc, the material surface is positively charged, and when pH is greater than pHpzc, the material surface is negatively charged. As shown in Fig. 13 (a), the pHpzc of FH4 was 4.67. When the pH was lower than 4.67, the surface of FH4 has a positive charge and produces electrostatic repulsion with neutral red cationic dyes. It was not conducive to the adsorption of NR, resulting in a low adsorption rate. When the pH was higher than 4.67, the surface negative charge of FH4 increased, resulting in electrostatic attraction with neutral red cationic dyes. The FH4 can better adsorb neutral red cationic dyes, thus increasing the adsorption rate. While the pH reached about 6, the adsorption rate reached its maximum. With the increase in pH, there was no significant effect on the adsorption of NR. The pH of the untreated neutral red solution was approximately 6 in the experiment. Therefore, there was no need to change the pH of the neutral red dye solution.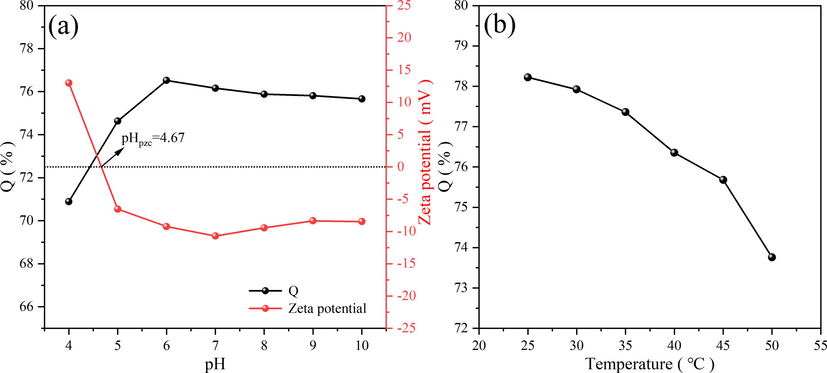
Effect of pH on adsorption capacity of FH4 and zeta potential of FH4 (a), effect of temperature (b).
3.2.5 Impact of temperature
The results are presented in Fig. 13 (b). Temperature is also a key factor affecting the adsorption performance of magnetic Fe3O4-HKUST-1 nanocomposites. The experimental data showed that the adsorption rate of NR by FH4 decreased with the gradual increase in temperature. It indicated that the adsorption of NR by FH4 was an exothermic process. Due to its exothermic properties, the increase in temperature accelerated the flow of dye ions and reduced the interaction of the adsorbent with the dye (Sobhanardakani et al., 2017). The increase in temperature was not conducive to the adsorption mass transfer. Therefore, the high temperature was not required for the adsorption of NR by magnetic Fe3O4-HKUST-1 nanocomposites. The lower temperature facilitated adsorption and increased the adsorption rate.
3.3 Adsorption isotherms
On behalf of clarifying the adsorption mechanism of dye NR simulated wastewater on MOF-199 and magnetic xFe3O4-HKUST-1 nanocomposites, magnetic composite materials FH4 and HKUST-1 are selected to test its fit with the Langmuir and Freundlich models. The Langmuir adsorption isotherm is given below in Eq. (4):
By using Eq. (4), Langmuir linear fit graph is obtained by plotting ce/qe against ce. The Freundlich adsorption isotherm has been given by Eq. (5):
With Eq. (5), Freundlich linear fit graph is plotted ln qe against ln ce. Equation (6) is acquired from Eq. (4) by deforming the equation, and Eq. (7) is obtained from Eq. (5). The nonlinear fit plots of the Langmuir and Freundlich models were generated by software fitting and are presented on a graph as qe versus ce.
The fitting results by the Langmuir and Freundlich models were presented in Fig. 14. The obtained parameters and the calculated data were listed in Table 3 and Table 4.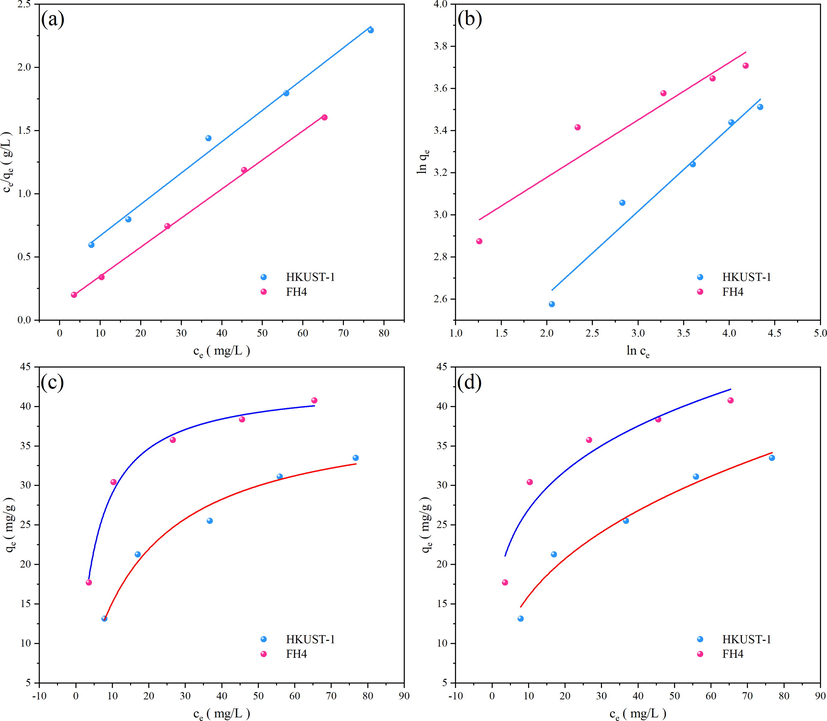
(a) Fit of Langmuir isotherm model in linearized form, (b) Fit of Freundlich isotherm model in linearized form, (c) Fit of Langmuir isotherm model to equilibrium data, (d) Fit of Freundlich isotherm model to equilibrium data.
Adsorbent
Langmuir model
Freundlich model
qL/
(mg/g)KL/
(L/mg)R2
KF
n
R2
HKUST-1
40.35
0.0339
0.9924
6.21
3.67
0.9144
FH4
53.49
0.1936
0.9988
13.92
2.52
0.9680
Adsorbent
Langmuir model
Freundlich model
qL/
(mg/g)KL/
(L/mg)R2
KF
n
R2
HKUST-1
39.57
0.0624
0.9786
6.82
2.69
0.9209
FH4
53.04
0.2079
0.9925
15.61
4.20
0.9752
Fig. 14 showed that both the linearized form as well as the fit to the raw equilibrium data show that the Freundlich model is less appropriate as compared to the Langmuir model. The Freundlich adsorption model has a lower correlation coefficient (R2) than the Langmuir adsorption model. The fit to Langmuir model demonstrates that the active sites are evenly distributed and that adsorption is single-deck on the surface of the samples with numerous adsorption sites. This implies that the adsorption of NR by the adsorbents is monolayer adsorption and that it mostly takes place at the surface-active sites. As a result, it can be concluded that the Langmuir model is more appropriate for simulating the NR adsorption process on HKUST-1 and magnetic Fe3O4-HKUST-1 nanocomposites.
Table 5 showed the comparison of the results with the adsorption capacities of the adsorbents reported in the literature for NR. It can be seen that the synthesized adsorbent has a medium to high adsorption capacity for NR dyes in this work. For example, Li et al. (Li et al., 2022) prepared core–shell Fe3O4-PDA and functionalized with silver nanoparticles. Its maximum adsorption capacity for the cationic dye neutral red was 38.7 mg/g, which improved the adsorption performance of Fe3O4-PDA by more than 15%. Besides, Ma et al. (Ma et al., 2021) synthesized a novel magnetic gel bead adsorbent (SA/GO@Fe3O4/CS) using graphene oxide (GO)-modified Fe3O4 as magnetic nanoparticles doped with sodium alginate (SA) and chitosan (CS) gel. The maximum adsorption capacity of the material for neutral red is 44.65 mg/g. This resulted in an increase in the adsorption of NR by the initial SA/Fe3O4. Although magnetic Fe3O4-HKUST-1 nanocomposites in this study is slightly inferior, its recyclability makes it have considerable application prospects. Hence, the simple green synthetic magnetic Fe3O4-HKUST-1 nanocomposites in this study have the potential to remove NR adsorbents from the aquatic environment.
Adsorbent
Adsorption capacity (mg/g)
Reference
rice husk
25.69
(Zou et al., 2009)
boron nitride
26.2
(Zheng et al., 2019)
Ni0.5Zn0.5Fe2O4/SiO2
39.95
(Liu et al., 2016)
HNT–Fe3O4
13.62
(Xie et al., 2011)
PDA-Fe3O4-Ag
38.7
(Li et al., 2022)
chitosan and activated carbon
composite films30
(de Freitas et al., 2021)
SA/GO@Fe3O4/CS
44.65
(Ma et al., 2021)
sepiolite
36.32
(Coruh et al., 2011)
halloysite nanotubes
54.85
(Luo et al., 2010)
Pyrolytic Char
62.3
(Zou et al., 2012)
0.4 Fe3O4-HKUST-1 (FH4)
47.7
This work
3.4 Adsorption kinetics
To understand the adsorption behavior of MOF-199 and magnetic xFe3O4-HKUST-1 nanocomposites, the magnetic composites FH4 and HKUST-1 were selected to determine the kinetics of adsorption of both on dye NR simulated wastewater. The adsorption kinetics reflects the whole adsorption reaction process.
To analyze the adsorption data, the pseudo-first-order kinetic model (PFO) and the pseudo-second-order kinetic model (PSO) were utilized. In order to illustrate the most appropriate model for the experimental data and to identify the most ideal mechanism for the adsorption process. The PFO kinetic model equation was calculated by using Eq. (8):
By using Eq. (8), kinetics linear fit graph is plotted ln(qe-qt) against t. The PSO kinetic model equation are calculated as follows equation (9).
With Eq. (5), kinetics linear fit graph is plotted t/qt against t. By deforming the equation, Eq. (10) is obtained from Eq. (8), and Eq. (11) is obtained from Eq. (9). A graph is plotted qt against t, the nonlinear fit charts of the kinetics were obtained through software fitting.
where the amount of dye adsorbed at adsorption time t was indicated by qt (mg/g), the amount of dye adsorbed by the samples at adsorption equilibrium was indicated by qe (mg/g). The adsorption time was expressed in t (min), the PFO and the PSO kinetic constant was represented by K1 (1/min) and K2 (g/min·mg), respectively.
The fitting results by the two kinetic model were obtained in Fig. 15. The acquired and calculated results for kinetic model were listed in Table 6 and Table 7.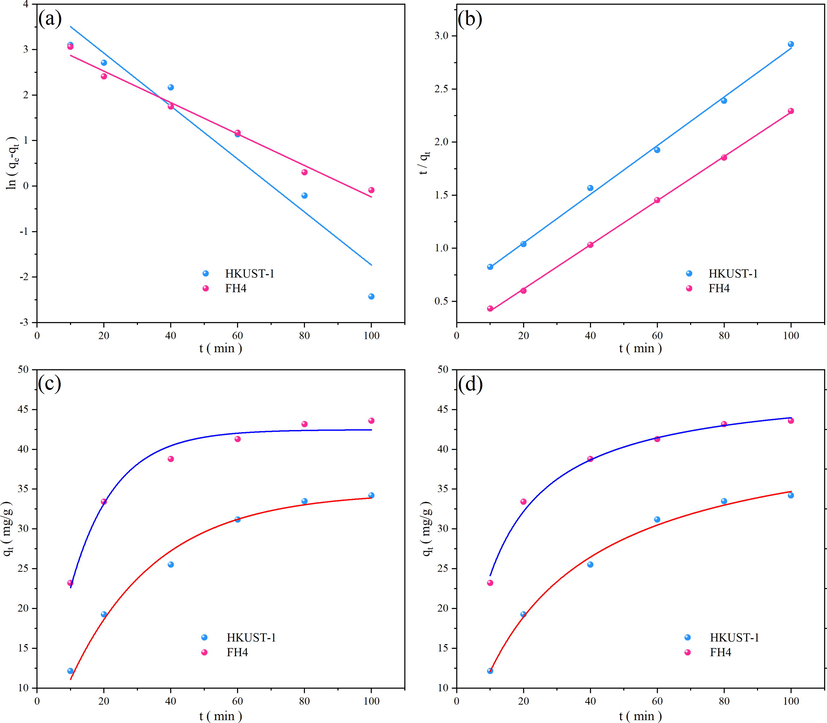
Kinetic plots, PFO linearized form (a), PSO linearized form (b), PFO raw data (c), PSO raw data (d).
Adsorbent
qe, exp/
(mg/g)Pseudo-first-order
kinetic modelPseudo-second-order
kinetic model
K1/
(1/min)qe, cal/
(mg/g)R2
K2/
(g/min·mg)qe, cal/
(mg/g)R2
HKUST-1
34.3
0.0582
59.62
0.9411
0.0229
45.45
0.9974
FH4
47.7
0.0346
25.02
0.9861
0.0021
48.04
0.9975
Adsorbent
qe,exp/
(mg/g)Pseudo-first-order
kinetic modelPseudo-second-order
kinetic model
K1/
(1/min)qe,cal/
(mg/g)R2
K2/
(g/min·mg)qe,cal/
(mg/g)R2
HKUST-1
34.3
0.0387
34.61
0.9878
0.0009
43.79
0.9950
FH4
47.7
0.0761
42.47
0.9814
0.0020
48.39
0.9917
The correlation coefficient (R2) value closest to 1 obtained by fitting was indicated as the most suitable kinetic adsorption. The results of both linear and nonlinear fits demonstrated that the adsorption of NR by both HKUST-1 and FH4 adhered to the PSO kinetic model. It showed a better fit to the kinetic data, with R2>0.99. The fit was significantly larger than the PFO kinetic model, and the experimental value differed greatly from the theoretical adsorption value derived by the linear fitting of the PFO kinetic model. In contrast, the experimental value was closer to the theoretical adsorption value of the PSO kinetic model. According to the results, the PSO kinetic model adequately described the entire adsorption process for HKUST-1 and magnetic xFe3O4-HKUST-1 nanocomposites.
The chemisorption mechanism was in charge of regulating the adsorption rate, which formed the basis of the PSO kinetic model, which also indicated that the adsorption of NR by HKUST-1 and magnetic Fe3O4-HKUST-1 nanocomposites was influenced by chemisorption. The aromatic ring on the ligand in HKUST-1 undergoes π-π interactions with the aromatic ring on the organic dye NR, hydrogen bonding between HKUST-1 and organic dye NR, and pore filling (Fig. 16). The adsorption process of azo dye neutral red on HKUST-1 is mainly based on intermolecular forces, electrostatic interaction, and ion exchange in solution as the main mechanisms.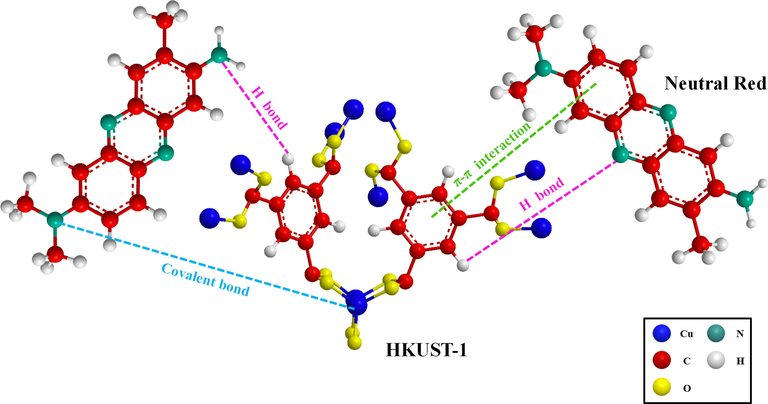
Adsorption mechanism of NR on magnetic Fe3O4-HKUST-1 nanocomposites.
3.5 Desorption and reusability
Regeneration and reuse of adsorbents are important indicators in practical applications, and excellent reuse performance is beneficial to energy savings and the long-term development of adsorbents. As can be seen from Fig. 17, the effect of the first and second cycles was close. The first removal rate was 95.8%, while the second and third removal rates decreased by 1.4% and 4.7%, respectively. The removal rate decreased slightly as the number of regeneration cycles increased but remained at a high level. After five cycles of experiments, the removal rate of NR on FH4 remained above 83%. The obtained data confirmed that FH4 was an effective adsorbent for the adsorption of NR dyes in wastewater and has good regeneration behavior.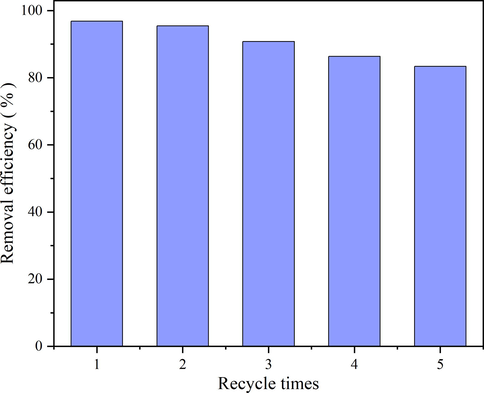
Reusability analysis of FH4.
After five cycles, the adsorbent was separated from the solution with an external magnet and dried, XRD analyses were performed. The XRD analysis results of FH4 after cyclic adsorption are shown in Fig. 18, where the characteristic peaks of FH4 and HKUST-1 can still be observed, but with lower intensity and hairy spectral peaks. After the adsorption process, the crystal structure of the composite still existed. It showed that FH4 has good stability. The presence of attached dye molecules in FH4 may be the cause of the reduction in XRD spectrum intensity (Martak et al., 2021).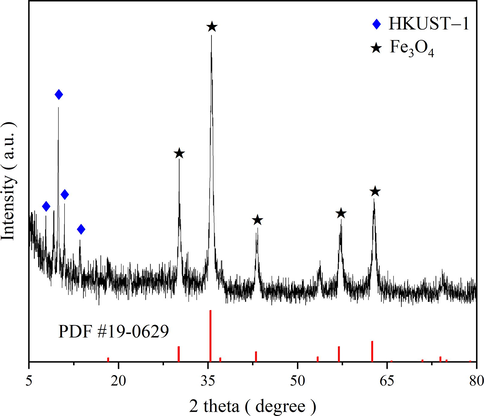
XRD patterns of FH4 after five cycles.
4 Conclusions
The magnetic Fe3O4-HKUST-1 nanocomposites were successfully synthesized starting from MOF material HKUST-1 and Fe3O4. The addition of magnetic Fe3O4 changed the mean pore size, specific surface area and total pore volume of HKUST-1, and the thermal stability of the material was enhanced. By varying the proportion of magnetic Fe3O4, a magnetic composite with addition of 0.4 g (FH4) was obtained. The FH4 morphology has a rough surface spherical in shape having good proportion of both micro mesopores. The total pore volume was 0.35 cm3/g, and the mean pore size of FH4 was 2.58 nm. The specific surface area was 548 m2/g, and the thermal decomposition temperature of 330 °C.
FH4 has good superparamagnetic properties of 26.39 emu/g. In the presence of a magnetic field from outside, the magnetic property of the nanocomposite facilitated easy separation from the solution. The adsorption capacity of FH4 for NR was the greatest as compared to other nanocomposites, with the actual adsorption capacity being 47.7 mg/g. The optimal conditions for adsorption of NR were a temperature of 25 °C and a pH of 6. The research is mainly in the laboratory. Further exploration was needed for practical applications. The adsorption isotherms of the HKUST-1 and magnetic Fe3O4-HKUST-1 nanocomposites were fitted with the Langmuir isothermal model, validating the homogeneous and monolayer adsorption properties. The adsorption kinetics was found to follow the PSO model. The process of adsorption was predominantly chemisorption due to intermolecular forces, electrostatic interaction and ion exchange. The FH4 has good regeneration performance and is an effective adsorbent for the adsorption of neutral red cationic dyes in wastewater.
CRediT authorship contribution statement
Zhiyu Ma: Writing – review & editing, Data curation. Chenhui Liu: Supervision, Project administration, Resources. C. Srinivasakannan: Conceptualization, Validation, Resources. Lingbo Li: Software, Validation. Yongli Wang: Formal analysis, Investigation, Conceptualization.
Acknowledgments
The authors acknowledge the financial supports from the National Natural Science Foundation of China (No. 51964046), and Yunnan Province Ten Thousand Youth Program Top Talents (No. YNWR-QNBJ-2019-066).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fe3O4@MOF Magnetic Nanocomposites: Synthesis and Applications. European Journal of Inorganic Chemistry.. 2020;2020:1916-1937.
- [CrossRef] [Google Scholar]
- Magnetic Fe3O4@UiO-66 nanocomposite for rapid adsorption of organic dyes from aqueous solution. Journal of Molecular Liquids.. 2021;322
- [CrossRef] [Google Scholar]
- Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science.. 2004;280:322-333.
- [CrossRef] [Google Scholar]
- Fast Synthesis of MOF-5 Microcrystals Using Sol-Gel SiO2 Nanoparticles. Chemistry of Materials.. 2011;23:929-934.
- [CrossRef] [Google Scholar]
- Alkali metal cation doping of metal-organic framework for enhancing carbon dioxide adsorption capacity. Journal of Energy Chemistry.. 2014;23:468-474.
- [CrossRef] [Google Scholar]
- A route to high surface area, porosity and inclusion of large molecules in crystals. Nature.. 2004;427:523-527.
- [CrossRef] [Google Scholar]
- Enhanced adsorption of thiophene with the GO-modified bimetallic organic framework Ni-MOF-199. Colloids and Surfaces a-Physicochemical and Engineering Aspects.. 2019;578
- [CrossRef] [Google Scholar]
- The impact of industrial pollution on urban groundwater resources. Desalination and Water Treatment.. 2021;241:269-275.
- [CrossRef] [Google Scholar]
- Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes. Microporous and Mesoporous Materials.. 2009;117:406-413.
- [CrossRef] [Google Scholar]
- A chemically functionalizable nanoporous material Cu-3(TMA)(2)(H2O)(3) (n) Science.. 1999;283:1148-1150.
- [CrossRef] [Google Scholar]
- Azo dyes and human health: A review. Journal of Environmental Science and Health Part C-Environmental Carcinogenesis & Ecotoxicology Reviews.. 2016;34:233-261.
- [CrossRef] [Google Scholar]
- Membrane separation for wastewater reuse in the textile industry. Resources Conservation and Recycling.. 2001;31:189-197.
- [CrossRef] [Google Scholar]
- Adsorption of neutral red dye from an aqueous solution onto natural sepiolite using full factorial design. Clays and Clay Minerals.. 2011;59:617-625.
- [CrossRef] [Google Scholar]
- Adsorption of neutral red dye by chitosan and activated carbon composite films. Heliyon.. 2021;7
- [CrossRef] [Google Scholar]
- Effect of structural properties of acid dyes on their adsorption behaviour from aqueous solutions by amine modified silica. Journal of Hazardous Materials.. 2009;161:1544-1550.
- [CrossRef] [Google Scholar]
- Threats to soil and water resources in South Africa. Environmental Research.. 2020;183
- [CrossRef] [Google Scholar]
- Eco-Friendly NiO/Polydopamine Nanocomposite for Efficient Removal of Dyes from Wastewater. Nanomaterials.. 2022;12
- [CrossRef] [Google Scholar]
- Sudan dyes: are they dangerous for human health? Drug and Chemical Toxicology.. 2013;36:343-352.
- [CrossRef] [Google Scholar]
- Life cycle assessment of wastewater treatment in developing countries: A review. Water Research.. 2019;153:63-79.
- [CrossRef] [Google Scholar]
- Green synthesis of Sn(II)-BDC MOF: Preferential and efficient adsorption of anionic dyes. Microporous and Mesoporous Materials.. 2020;297
- [CrossRef] [Google Scholar]
- Deep challenges for China's war on water pollution. Environmental Pollution.. 2016;218:1222-1233.
- [CrossRef] [Google Scholar]
- Use of toluidine blue dye in detection of anogenital injuries in consensual sexual intercourse. Journal of Forensic and Legal Medicine.. 2019;64:14-19.
- [CrossRef] [Google Scholar]
- Metal-organic frameworks for hydrogen storage. Microporous and Mesoporous Materials.. 2010;129:335-339.
- [CrossRef] [Google Scholar]
- Does the Government's Environmental Attention Affect Ambient Pollution? Empirical Research on Chinese Cities. Sustainability.. 2022;14
- [CrossRef] [Google Scholar]
- Water pollution incidents and their influencing factors in China during the past 20 years. Environmental Monitoring and Assessment.. 2022;194
- [CrossRef] [Google Scholar]
- Removal of cationic dye waste by nanofiber membrane immobilized with waste proteins. International Journal of Biological Macromolecules.. 2020;164:3873-3884.
- [CrossRef] [Google Scholar]
- Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. Journal of Hazardous Materials.. 2010;181:1039-1050.
- [CrossRef] [Google Scholar]
- Hierarchically Porous Boron Nitride/HKUST-1 Hybrid Materials: Synthesis, CO2 Adsorption Capacity, and CO2/N-2 and CO2/CH4 Selectivity. Industrial & Engineering Chemistry Research.. 2021;60:2463-2471.
- [CrossRef] [Google Scholar]
- Electrochemical Treatment of Reactive Blue 52 Using Zirconium, Palladium and Graphite Electrode. Clean-Soil Air Water.. 2014;42:804-808.
- [CrossRef] [Google Scholar]
- Treatment of dyehouse liquors in a biological sequencing batch reactor with recursive chemical oxidation. Water Science and Technology.. 2001;44:85-92.
- [CrossRef] [Google Scholar]
- Spray synthesis of Pd nanoparticle incorporated HKUST-1, and its catalytic activity for 4-nitrophenol reduction. Advanced Powder Technology.. 2022;33:103701
- [CrossRef] [Google Scholar]
- Separation/degradation behavior and mechanism for cationic/anionic dyes by Ag-functionalized Fe3O4-PDA core-shell adsorbents. Frontiers of Environmental Science & Engineering.. 2022;16
- [CrossRef] [Google Scholar]
- A lanthanide metal-organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. Rsc Advances.. 2016;6:11570-11576.
- [CrossRef] [Google Scholar]
- Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. Journal of the Taiwan Institute of Chemical Engineers.. 2015;50:223-228.
- [CrossRef] [Google Scholar]
- Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Frontiers in Environmental Science.. 2022;10
- [CrossRef] [Google Scholar]
- Adsorption Optimization and Mechanism of Neutral Red Onto Magnetic Ni0.5Zn0.5Fe2O4/SiO2 Nanocomposites. Journal of Nanoscience and Nanotechnology.. 2016;16:8252-8262.
- [CrossRef] [Google Scholar]
- Study on the present situation and prevention measures of water pollution in a drainage basin in Eastern Guangdong. Desalination and Water Treatment.. 2022;249:174-182.
- [CrossRef] [Google Scholar]
- Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Research.. 2010;44:1489-1497.
- [CrossRef] [Google Scholar]
- Magnetic graphene oxide-containing chitosan-sodium alginate hydrogel beads for highly efficient and sustainable removal of cationic dyes. International Journal of Biological Macromolecules.. 2021;193:2221-2231.
- [CrossRef] [Google Scholar]
- A hybrid dye-clay nano-pigment: Synthesis, characterization and application in organic coatings. Dyes and Pigments.. 2017;147:234-240.
- [CrossRef] [Google Scholar]
- Direct synthesis of Al-HKUST-1 and its application as adsorbent for removal of congo red in water. Nano-Structures & Nano-Objects.. 2021;27
- [CrossRef] [Google Scholar]
- Environmental Application of Agro-waste Derived Materials for the Treatment of Dye-polluted Water: A Review. Current Analytical Chemistry.. 2021;17:904-916.
- [CrossRef] [Google Scholar]
- Microbial decolourisation and degradation of textile dyes. Applied Microbiology and Biotechnology.. 2001;56:81-87.
- [CrossRef] [Google Scholar]
- N-doped activated carbon from used dyeing wastewater adsorbent as a metal-free catalyst for acetylene hydrochlorination. Chemical Engineering Journal.. 2019;371:118-129.
- [CrossRef] [Google Scholar]
- Adsorption performance of mesoporous silicas towards a cationic dye. Influence of mesostructure on adsorption capacity. International Journal of Applied Ceramic Technology.. 2019;16:1533-1543.
- [CrossRef] [Google Scholar]
- Fe3O4@ ZIF-8: a magnetic nanocomposite for highly efficient UO22+ adsorption and selective UO22+/Ln(3+) separation. Chemical Communications.. 2017;53:4199-4202.
- [CrossRef] [Google Scholar]
- Gold immobilized onto poly(ionic liquid) functionalized magnetic nanoparticles: a robust magnetically recoverable catalyst for the synthesis of propargylamine in water. Rsc Advances.. 2015;5:34502-34510.
- [CrossRef] [Google Scholar]
- Water soluble gold-polyaniline nanocomposite: A substrate for surface enhanced Raman scattering and catalyst for dye degradation. Arabian Journal of Chemistry.. 2020;13:4009-4018.
- [CrossRef] [Google Scholar]
- The Effects of Natural and Synthetic Blue Dyes on Human Health: A Review of Current Knowledge and Therapeutic Perspectives. Advances in Nutrition.. 2021;12:2301-2311.
- [CrossRef] [Google Scholar]
- A Review of the SDG 6 Synthesis Report 2018 from an Education, Training, and Research Perspective. Water.. 2018;10
- [CrossRef] [Google Scholar]
- HKUST-1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance. Environmental Research.. 2022;205
- [CrossRef] [Google Scholar]
- Environmental health situation in Nigeria: current status and future needs. Heliyon.. 2021;7
- [CrossRef] [Google Scholar]
- Online LC-MS-MS process monitoring for optimization of biological treatment of wastewater containing azo dye concentrates. Analytical and Bioanalytical Chemistry.. 2006;384:1123-1128.
- [CrossRef] [Google Scholar]
- Polycyclic Aromatic Hydrocarbon Sorption by Functionalized Humic Acids Immobilized in Micro- and Nano-Zeolites. Sustainability.. 2021;13
- [CrossRef] [Google Scholar]
- UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicology and Environmental Safety.. 2021;208
- [CrossRef] [Google Scholar]
- Good quality printing with reactive dyes using guar gum and biodegradable additives. Dyes and Pigments.. 2003;57:7-14.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of 3-aminopropyltriethoxysilane-modified superparamagnetic magnetite nanoparticles. Chemistry Letters.. 2004;33:1468-1469.
- [CrossRef] [Google Scholar]
- The Impacts of Water Pollution Emissions on Public Health in 30 Provinces of China. Healthcare.. 2020;8
- [CrossRef] [Google Scholar]
- Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984) Pure and applied chemistry.. 1985;57:603-619.
- [CrossRef] [Google Scholar]
- Assessing of Removal Efficiency of Indigo Carmine from Wastewater Using MWCNTs. Iranian Journal of Science and Technology Transaction a-Science.. 2017;41:1047-1053.
- [CrossRef] [Google Scholar]
- Removal of anionic dyes (direct blue 106 and acid green 25) from aqueous solutions using Oxidized Multi-Walled Carbon Nanotubes. Iranian Journal of Health Sciences.. 2015;3:48-57.
- [CrossRef] [Google Scholar]
- Removal of Janus Green dye from aqueous solutions using oxidized multi-walled carbon nanotubes. Toxicological and Environmental Chemistry.. 2013;95:909-918.
- [CrossRef] [Google Scholar]
- Removal of cationic dyes from aqueous solutions using NiFe2O4 nanoparticles. Journal of Water Supply Research and Technology-Aqua.. 2016;65:64-74.
- [CrossRef] [Google Scholar]
- A water-stable functionalized NiCo-LDH/MOF nanocomposite: green synthesis, characterization, and its environmental application for heavy metals adsorption. Arabian Journal of Chemistry.. 2021;14
- [CrossRef] [Google Scholar]
- Zirconia nano-powders with controllable polymorphs synthesized by a wet chemical method and their phosphate adsorption characteristics & mechanism. Ceramics International.. 2022;48:6591-6599.
- [CrossRef] [Google Scholar]
- Sun, D. H., Zhang, J. L. and Sun, D. X. 2011. Solvothermal Synthesis and Magnetic Properties of Fe3O4 Microspheres. International Conference on Biotechnology, Chemical and Materials Engineering (CBCME 2011), Kunming, PEOPLES R CHINA.
- Twenty years of China's water pollution control: Experiences and challenges. Chemosphere.. 2022;295
- [CrossRef] [Google Scholar]
- Nano-Engineered Adsorbent for the Removal of Dyes from Water: A Review. Current Analytical Chemistry.. 2020;16:14-40.
- [CrossRef] [Google Scholar]
- Highly efficient and selective removal of Pb2+ by ultrafast synthesis of HKUST-1: Kinetic, isotherms and mechanism analysis. Colloids and Surfaces a-Physicochemical and Engineering Aspects.. 2022;633
- [CrossRef] [Google Scholar]
- Synthesis and characterization of metal–organic frameworks fabricated by microwave-assisted ball milling for adsorptive removal of Congo red from aqueous solutions. RSC advances.. 2017;7:46520-46528.
- [CrossRef] [Google Scholar]
- Application of Natural Dyes and Sodium Alginate From Sargassum Sp. Sea weed In Coloring Bima Woven Fabric. Oriental Journal of Chemistry.. 2020;36:964-967.
- [CrossRef] [Google Scholar]
- Tunning heterostructures interface of Cu2O@HKUST-1 for enhanced photocatalytic degradation of tetracycline hydrochloride. Separation and Purification Technology.. 2022;303
- [CrossRef] [Google Scholar]
- Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chemical Engineering Journal.. 2011;168:959-963.
- [CrossRef] [Google Scholar]
- Effects of pollution on macroinvertebrates and water quality bio-assessment. Hydrobiologia.. 2014;729:247-259.
- [CrossRef] [Google Scholar]
- The lag effect of water pollution on the mortality rate for esophageal cancer in a rapidly industrialized region in China. Environmental Science and Pollution Research.. 2019;26:32852-32858.
- [CrossRef] [Google Scholar]
- Urban river pollution control in developing countries. Nature Sustainability.. 2019;2:158-160.
- [CrossRef] [Google Scholar]
- Flower-like hierarchical Ni-Zn MOF microspheres: Efficient adsorbents for dye removal. Colloids and Surfaces a-Physicochemical and Engineering Aspects.. 2019;582
- [CrossRef] [Google Scholar]
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells. Heterocyclic Communications.. 2021;27:155-163.
- [CrossRef] [Google Scholar]
- Urban river pollution in the densely populated city of Dhaka, Bangladesh: Big picture and rehabilitation experience from other developing countries. Journal of Cleaner Production.. 2021;321
- [CrossRef] [Google Scholar]
- Evaluation of kinetic and equilibrium parameters of NiFe2O4 nanoparticles on adsorption of reactive orange dye from water. Iranian Journal of Toxicology.. 2016;10:51-58.
- [CrossRef] [Google Scholar]
- Synthesis of NiFe2O4 nanoparticles for removal of anionic dyes from aqueous solution. Desalination and Water Treatment.. 2016;57:11348-11360.
- [CrossRef] [Google Scholar]
- Ligands-Coordinated Zr-Based MOF for Wastewater Treatment. Nanomaterials.. 2018;8
- [CrossRef] [Google Scholar]
- Studies on the removal of phosphate in water through adsorption using a novel Zn-MOF and its derived materials. Arabian Journal of Chemistry.. 2022;15
- [CrossRef] [Google Scholar]
- Adsorption-Desorption Behaviors of Methanol and Ethyl Acetate on Silica Gel: Modeling and Experimental Tests. Industrial & Engineering Chemistry Research.. 2021;60:1829-1838.
- [CrossRef] [Google Scholar]
- Effect of the Synergetic Interplay between the Electrostatic Interactions, Size of the Dye Molecules, and Adsorption Sites of MIL-101(Cr) on the Adsorption of Organic Dyes from Aqueous Solutions. Crystal Growth & Design.. 2018;18:7533-7540.
- [CrossRef] [Google Scholar]
- Functional group driven adsorption of neutral red by boron nitride nanoparticles: experimental and theoretical studies. Materials Research Express.. 2019;6
- [CrossRef] [Google Scholar]
- Equilibrium, kinetic and mechanism study for the adsorption of neutral red onto rice husk. Desalination and Water Treatment.. 2009;12:210-218.
- [CrossRef] [Google Scholar]
- Competitive Adsorption of Neutral Red and Cu2+ onto Pyrolytic Char: Isotherm and Kinetic Study. Journal of Chemical and Engineering Data.. 2012;57:2792-2801.
- [CrossRef] [Google Scholar]







