Translate this page into:
African and Holy Basil - a review of ethnobotany, phytochemistry, and toxicity of their essential oil: Current trends and prospects for antimicrobial/anti-parasitic pharmacology
⁎Corresponding author at: Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nsukka, Enugu State 410001, Nigeria. timothy.ezeorba@unn.edu.ng (Timothy Prince Chidike Ezeorba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The increased global health burden and mortality rate due to synthetic drug side effects and microbial resistance requires immediate attention for safer and better agents. This quest has fueled the search for phytotherapeutic alternatives, such as essential oils (EOs). Ocimum (Basil) essential oil has pleiotropic health-promoting potential in the treatment of a variety of diseases. This review focused on the ethnobotany, phytochemicals, antimicrobial properties, and toxicity of African and Holy Basil essential oils. African Basil EOs have been used to treat malaria, typhoid, yellow fever candidiasis, influenza, tooth gargle, sore eyes, and ear infections, among other things. Similarly, Holy Basil is used locally as a remedy for diseases such as colds, coughs, malaria, asthma, genitourinary infections, stomach acidity, diabetes, and influenza. This potency could be attributed to the abundance of phytochemicals in the plants, such as eugenol, linalool, and 1, 8-cineole. Experimental evidence has shown that the phytonutrients found primarily in their EOs have antimicrobial activity against many bacteria, fungi, viruses, and protozoans. This study discusses the multi-targeted approach of these compounds in eliminating microorganisms by distorting their cellular architecture, which leads to membrane permeability disruption, denaturation of key proteins for survival, damage to the microbial DNA and replication machinery, and ultimately cell lysis and organism death. Their antimicrobial pharmacology invariably positions them as a new, effective, and safer Phyto antimicrobial agent to reduce morbidity and mortality due to microbial resistance.
Keywords
African Basil (Ocimum gratissimum)
Holy Basil (Ocimum sanctum)
Ocimum tenuiflorum
Mosquitocidal/anti-malarial
Ethnopharmacology
Essential oil extraction

- Eos
-
Essential oils
- Nrf2
-
Nuclear factor erythroid-2-related factor 2
- NF-қB
-
nuclear factor-kappa B
- PRISMA
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- OGEO
-
Ocimum gratissimum Essential Oil
- OSEO
-
Ocimum Sanctum Essential Oil
- DPPH
-
2,2-diphenylpicrylhydrazyl
- MIC
-
Minimum Inhibitory Concentration
- BVH-1
-
Bovine Herpes Virus-type-1
- FMDV
-
Foot and Mouth disease virus
- NDV
-
Newcastle Disease Virus
Abbreviations
1 Introduction
Substances derived from aromatic plants, particularly essential oils (EOs), have been used since antiquity and are currently extensively utilized by the cosmetics, food, and pharmaceutical industries (Chimnoi et al., 2018a; Melo et al., 2019a; Melo et al., 2019; Tran et al., 2018). African Basil (Ocimum gratissimum) and holy Basil (Ocimum sanctum), both members of the Lamiaceae family's Ocimum genus, are rich in EOs (Avetisyan et al., 2017). Ocimum gratissimum, also known as African Basil, clove Basil, and wild Basil, is native to Asia, India, and Africa (Mohr et al., 2017). African Basil is grown in home gardens in South East Asia and Africa and used in cooking local foods/dishes, soups, pasta, jellies, salads, and vinegar (Nweze and Eze, 2009). However, it has recently been commercially cultivated in many parts of the world. Ocimum sanctum is native to Asia and Central and Western Africa (Saharkhiz et al., 2015). It is grown worldwide mainly for religious and therapeutic reasons (Choudhary, 2020). Although both African and Holy Basil is members of the same genus, emerging data indicate significant differences in their morphology and ethnobotany.
African Basil leaves are ovate or ovate-lanceolate in shape, cuneate and decurrent at the base, with a coarsely crenate, serrate margin and glandular trichomes (Gupta et al., 2021; Prabhu et al., 2009a). At the same time, the stem and flowers are dark brown and creamy white, respectively (Rawat et al., 2016). Holy Basil differs from African Basil by having internally glabrous calyces and spreading pedicels, as well as slightly toothed, hairy, sub-ovate, and sub-serrate leaves (Kumar et al., 2021, 2022; Malav et al., 2015; Rawat et al., 2016). The stem and flower colours are purple, green, and purple white, respectively (Rawat et al., 2016). African Basil has been utilized to treat digestive disorders, stomach disorders, malaria, typhoid, and yellow fever (Lal et al., 2021; Mukaila et al., 2021). Based on other reports, African Basil can be used to treat ear infections, tooth decay, sore eyes, fever, convulsions, coughs, measles, and tuberculosis (Bankole et al., 2018; Faustin et al., 2021). Holy Basil is traditionally regarded as a sacred plant and is used in religious rituals (Rupani and Chavez, 2018). Among the ethnobotany applications of Holy Basil are cough, cold, asthma, and kidney stone treatment; tooth, skin, and eye protection; and the prevention of lung disease, heart disease, and cancer (Choudhary, 2020). The health benefits of these two Ocimum species could invariably be anchored on their abundant plant-derived phytochemicals.
Although the phytochemical profile of African and Holy Basil varies depending on geographical origin, cultivar type, drying method, and distillation method, eugenol, 1,8-cineole (Melo et al., 2019b; Mohr et al., 2017), linalool, cis-ocimene (Chimnoi et al., 2018b), thymol, p-cymere (Kpoviessi et al., 2014), and β -caryophyllene are the major constituents found in their EOs (Ashish et al., 2022; Kumar et al., 2010; Srivastava et al., 2021). Eugenol, a phenylpropanoid synthesized via the shikimic acid pathway and the most active component of the essential oil of the Basil genus, has antioxidant, cardioprotective, and antimicrobial properties against a variety of microbes (Srivastava et al., 2021; Wang et al., 2018). In addition, it regulates unsaturated lipid oxidation and free radical scavenging, extending the shelf life of food products (Kumar et al., 2010). Eugenol's antimicrobial action is mediated mainly through inhibition of energy generation and the conversion of cytochrome P-450 into the cytotoxic quinine methide (Saharkhiz et al., 2015). 1,8-cineole is a monoterpenoid with anticancer, antimicrobial, antioxidant, and anti-inflammatory properties (Ashish et al., 2022; Cai et al., 2020; Lal et al., 2022). It induces apoptosis through MAPK-mediated and caspase-dependent pathways, targets L-asparaginase in spoilage organisms and many pathogens, and reduces oxidative stress and inflammatory aberrations through regulation of the nuclear factor erythroid-2-related factor 2 (Nrf2) and nuclear factor-kappa B (NF-қB) signaling pathways, respectively (Cai et al., 2020). Linalool, a monoterpenoid produced by the mevalonic acid pathway, has antimicrobial and antioxidant properties against various microorganisms (Avetisyan et al., 2017).
Using essential oils as antimicrobial agents may be a permanent solution to the recent global health challenge and cost burden caused by antibiotic and multidrug resistance in clinical practice. Approximately 700,000 deaths are recorded annually due to infections caused by drug-resistant microorganisms. The value is expected to increase to 10 million annually by 2050 if effective proactive measures are not taken (Somtochukwu et al., 2021). In addition, antibiotic resistance tends to increase hospitalization time and patient costs, increasing morbidity and mortality rates (Melo et al., 2019b). The antimicrobial potentials of African and Holy Basil using different analytical models show that their EOs had maximal inhibitory activities against several bacteria, fungi, viruses, protozoa, and mosquito larvae. Moreover, recent studies have shown that the nano-derived formulation or nano-conjugation of their essential oils not only improves their biological activities but as well are advantageous to improving their stability, controlling/sustaining their release over time, and achieving targeted delivery (Badea et al., 2019; Predoi et al., 2018; Raita et al., 2020).
Although several empirical research on African and Holy Basil have been undertaken, there is still a demand for detailed comparative studies of ethnobotany, phytochemistry, antimicrobial pharmacology, and toxicity of their essential oils. This study reviews and presents a comparative overview of the ethnobotanical applications, morphological characteristics of African and Holy Basil, and the phytochemical variation of their essential oils. Moreover, emphasis was placed on examining the antimicrobial pharmacology of their EOs with a special focus on their antibacterial, antifungal, antiviral, anti-protozoan, and mosquitocidal activities comprehensively. Finally, we reviewed studies on their possible toxicities to establish possible acceptable dietary intake and prospects for application in clinical practice and industries.
2 Search strategy
This study retrieved relevant literature by adapting the set down principles of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol. The primary search was performed on Scopus, Science Direct, and PubMed using focused keywords such as Ocimum gratissimum, Ocimum sanctum/tenuiflorum, anti-microbial, anti-fungal, anti-bacterial, anti-parasitic, anti-helminthic, mosquitocidal, essential oil, and others. Whereas the Google Scholar database was used as a secondary search source. The Boolean connectors AND or OR were applied to achieve a focused search for useful literature for this review. The inclusion criteria include the following; first, the article must be focused on essential oils fraction of either African or Holy Basil plants. Secondly, selected articles must be reported in the English language. And finally, the article should report anti-microbial or anti-parasitic bioactivities. Preferences were given to recently published papers (after 2010), and non-focused studies were excluded. A total of 402 works of literature were obtained using the focused keywords and combined with the Boolean connectors. The duplicates were removed before screening the full text and abstract for eligibility. Finally, a total of 113 works published between 2010 and 2022 were used for the study after excluding works that were too old, not written in English, peer-reviewed, and informative regarding the topic (Fig. 1).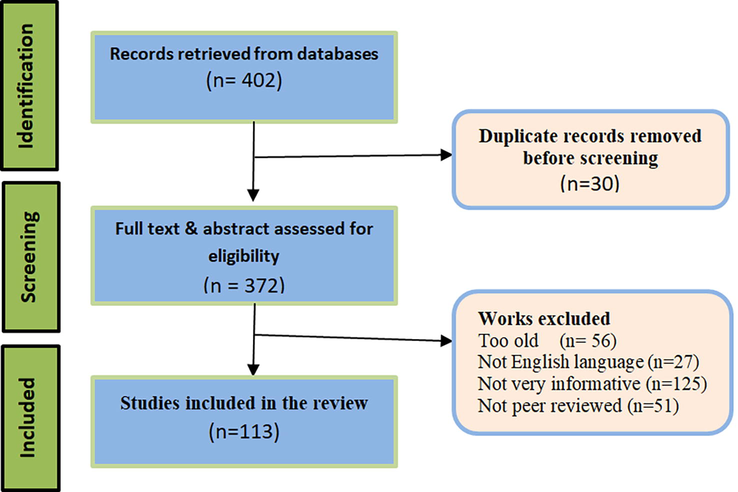
Flowchart showing the steps of published data selection for inclusion in this study.
3 Ethnobotanical and morphological variations between African and holy Basils
Ethnobotany studies the direct relationship between plants and man in his culture (Rahman et al., 2019). It refers to the various local uses of plants alongside their medicinal purposes. Plant morphology, on the other hand, refers to the study of plants' physical and external features. It is one of the significant steps in establishing the true identity of plants (Akinyemi and Ayodele, 2020), and it is essential to the taxonomy for phylogenetic studies and understanding plant growth (Heslop-Harrison, 2017). Morphology is also crucial for standardizing crude drugs (Agarwal et al., 2013; Lal et al., 2022). Morphological features include size, arrangement, texture, venation, surface characters, markings, and hardness of plant parts (Agarwal et al., 2013). The morphology of African and Holy Basil helps to distinguish the closely related species from each other so as not to misappropriate their usage.
Ocimum gratissimum and Ocimum sanctum belong to the same botanical family (Lamiaceae; formerly Labiatae), as highlighted in the Taxonomical classification in Table 1. However, despite the similarity in taxonomical classification, variation exists in the ethnobotany and morphology of holy and African Basils. The ethnobotanical uses of both Basils are addressed in the subsequent section.
Botanical classification
Ocimum gratissimum
Ocimum sanctum
Kingdom
Plantae
Plantae
Subkingdom
Tracheobionta
Tracheobionta
Super division
Spermatophyta
Spermatophyta
Division
Magnoliophyta
Magnoliophyta
Class
Magnoliopsida
Magnoliopsida
Subclass
Asteridae
Asteridae
Order
Lamiales
Lamiales
Family
Lamiaceae
Lamiaceae
Genus
Ocimum
Ocimum
Species
Gratissimum
O. Sanctum
References
(Imosemi, 2020)
(Kumar et al., 2022)
3.1 Ethnobotanical variation between African and holy Basils
African Basil is used as medicine, food, ritual, chemical, and ornamental plant (Kpètèhoto et al., 2017). It is used in the North East of Brazil for culinary and medicinal purposes. The decoction of its roots is used in tropical forest Brazil as a sedative for children. Due to their essential oil content, flowers and leaves are used in preparing teas and infusions. In combination with Xylopia aethiopica, African Basil is used to preparing potions and teas for women during puerperium (Prabhu et al., 2009b). Half a glass of African Basil leaf juice with salt, taken once daily for five days, treats digestive disorders in the Ibarapa area of Oyo State, Nigeria (Oyelakin et al., 2020). Its leaves also treat stomach problems in Enugu State, Nigeria. It is used to cure the pile by taking its extracted leaves and root of Zanthoxylum xanthoxyloides twice daily for one week. Together with Psidium guajava, African Basil leaves are used for curbing diabetes (Aiyeloja and Bello, 2006). Tincture of O. gratissimum leaves in alcohol treats measles and tuberculosis in Ogun State, Nigeria (Bankole et al., 2018). In Ile-Ife, Osun State, Nigeria, its leaf juice is used to treat fresh wounds, and maceration of the leaf in water is used to cure malaria, typhoid, and yellow fever (Mukaila et al., 2021). The leaf is also applied in treating typhoid, malaria, dysentery, diarrhea, abdominal pain, and nausea in Uromi, Delta State, Nigeria (Igberaese and Ogbole, 2018; Lal et al., 2023). In Kenya and other sub-Saharan African communities, rubbing the leaves between the palms and sniffing is used to cure blocked nostrils. They also use the plant for abdominal pains, ear infections, tooth gargling, sore eyes, fever, convulsions, coughs, barrenness, regulation of menstruation, and to cure prolapse of the rectum. In Benin, African Basil is a flavoring, aids digestion, and combats intestinal worms (Faustin et al., 2021). Decoction and pounding of the leaf stem are also used in Southern Benin to treat candidiasis (Fanou et al., 2020). African Basil is used in Cameroon for treating hemorrhoids, witchcraft problems, incurable wounds, and breast pain (Japhette et al., 2019) and as an insect repeller (Youmsi et al., 2017). The whole African Basil plant is used in India to treat sunstroke, influenza, and headache. In Côte d'Ivoire, the leaves are recommended against sore throat, sinusitis, influenza, colds, coryza, and dizziness. The leafy stem treats hypertension, diabetes, and varicella (Yvette et al., 2014).
Holy Basil and its essential oil are cultivated for religious and medicinal purposes (Choudhary, 2020). It is mainly used as healing remedies, cosmetics, preservatives, and herbal tea preparation. It is considered a sacred plant and may be taken in water as a daily tonic or as part of a religious ritual (Rupani and Chavez, 2018). O. sanctum is used to relieve fear, stress, pain, insect bites, and stings; treatment of cough, cold, asthma, and kidney stones; protection of the teeth, skin, and eyes; prevention of lung disorder, heart disease, and cancer (Choudhary, 2020). The whole plant of Ocimum tenuiflorum is used by the people of Thoppampatti, Tamilnadu, India, for the treatment of cardiopathy, leucoderma, asthma, genitourinary diseases, and ophthalmia (Sivasankari et al., 2014). Specifically, its leaves are used in Mauritius to treat stomach acidity, insomnia, abdominal pain, dark spots, high blood pressure, stress, diabetes, influenza, and flatulence (Mahomoodally, 2014). The paste of its leaves is used to treat scorpion stings and chest pains by the Paliyar tribe in India (Ignacimuthu et al., 2008). The presence of eugenol in its leaves contributes to its medicinal properties as a painkiller and reduction of blood glucose levels in type-2 diabetics (Malav et al., 2015). The juice of the fresh leaves (10 ml) is also taken twice a day to relieve patients suffering from kidney stones. Its leaves are used together with the stem of Tinospora cordifolia to treat fever in Chhattisgarh, India (Pandey, 2021). Its leaves and roots are prepared as juice and used by the Kani tribe in India to treat colds, coughs, and fevers (Ayyanar & Ignacimuthu, 2011; Xavier et al., 2014). Decoction of the leaves and roots supplemented with honey is used twice daily before meals to treat malaria in Jonai, India (Wangpan et al., 2016). Chewing the leaf or its juice is also used for malaria prevention in Odisha, India (Nagendrappa et al., 2013). It is also used in North India as a mosquito repellant (Kantheti and Padma, 2017). In Malaysia, the decoction of the leaves and seeds is also used to cure malaria (Al-adhroey et al., 2010).
3.2 Morphological variation between African and holy Basils
Although Africa and Holy Basil have the same taxonomical family, several distinct morphological variations exist for identification and survival advantages. Some of these morphological variations between the two plants are summarized in Table 2. African Basil is a shrub, more strongly scented than other Ocimum species, growing up to 102.6 cm in height (Rawat et al., 2016). Its leaves are ovate or ovate-lanceolate, measure up to 10 × 5 cm, and sub-acuminate to acuminate at the apex, with dots on both sides. They are also cuneate and decurrent at the base, having a coarsely crenate, serrate margin (Prabhu et al., 2009b). There are glandular trichomes on the leaves. The ordinary trichomes are few. Long trichomes - up to 6-celled - are mainly present on the margin, while the 2-celled trichomes (short ones) are found majorly on the lamina. Stomata are present on the lower surface but rare or absent on the upper surface of the leaves (Prabhu et al., 2009b). The petioles are up to 6 cm long, and the racemes are up to 18 cm long. Lamina is ovate-lanceolate,12.4 cm long, and possesses a serrated margin. The peduncles are densely pubescent. The calyx is campanulate, up to 5 mm long, and greenish-white to greenish-yellow in color. When wet, the nutlets are mucilaginous. Seeds are globose and brown, with an average 1000-seed weight of 0.9 g. The stem and flower colors are dark brown and creamy white, respectively. The plant height and canopy are 102.6 cm and 787.53 cm2, respectively (Fig. 2) (Rawat et al., 2016).
Morphological character
African Basil
Holy Basil
Plant height (cm)
102.6
51.46
Lamina length (cm)
12.4
4.3
Lamina shape
Ovate-lanceolate
Sub-ovate
Lamina margin
Serrate
Sub-serrate
Flower color
Creamy white
Purple white
Stem color
Dark brown
Purple green
1000-seed weight average (g)
0.9
0.3
Plant canopy average (cm2)
787.53
692.24

A snapshot of African and Holy Basil plants showing some morphological features.
Holy Basil (O. tenuiflorum/sanctum) is an erect, many-branched, about 60 cm in height, aromatic and sweet-scented sub-shrub cultivated in India at a large scale (Baseer and Jain, 2016; Choudhary, 2020). It is readily differentiated from the other Ocimum species by its internally glabrous calyces and spreading pedicels (Malav et al., 2015). The leaves are simple, purple or green, ovate, acute or obtuse, slightly toothed, and possess sub-serrate, entire, or dentate margins. They are hairy and sub-quadrangular, with a dark purple to black color on the outside and a cream color on the inside (Malav et al., 2015; Rawat et al., 2016; Kumar et al., 2022). Both sides of the leaves are pubescent, dotted with minute glands, and have hairy slender petioles. Lamina is sub-ovate, 4.3 cm in length, with a sub-serrate margin (Fig. 2). The inflorescence has purplish white, zygomorphic, and hermaphrodite flowers arranged in elongate racemes in close whorls. Pedicels of the flower are longer than the calyx, and the calyx is ovoid or campanulate, 3–4 mm bilipped (Kumar et al., 2022). The seeds are globose to subglobose, brownish-reddish-yellow, with a shining seed coat that becomes mucilaginous when wet. Seeds are globose and brown, with an average 1000-seed weight of 0.3 g. The stem and flower colors are purple, green, purple, and white. The plant canopy is 692.24 cm2 (Rawat et al., 2016).
4 Essential oil from African and Holy Basil - extraction and phytochemical composition
Essential oil is one of African and Holy Basil's most valuable components and metabolites, with vast biological activities and industrial usefulness (Kunihiro et al., 2022). Generally, the EO from Africa and Holy Basil are extracted by either hydrodistillation or steam distillation. However, some studies have reported energy-assisted systems, such as microwave-assisted and ultrasound-assisted distillation extraction (Boumahdi et al., 2021; Ghazanfari et al., 2020; Sneha et al., 2022). The nature and composition of EO from different Basil species are strongly affected by the distillation techniques, distillation time, cultivars type, method of drying, ecotypes, and chemotypes (Shiwakoti et al., 2017), in addition to climate, ecological zone, vegetative stage, time of harvesting, genetic results, water, and nutrients availability (Maurya et al., 2022; Melo et al., 2019b; Verma et al., 2011). A substantial number of studies conducted on holy and African Basil essential oils revealed variations in oil from different distillation techniques. Tables 3 and 4 show the major constituents identified from OGEO and OSEO, respectively. The significant compounds out of the 37 constituents identified in SD of OGEO were eugenol (55.6%), cis-ocimene (13.9%), γ-muurolene (11.6%), (Z,E)-α-farnesene (5.6%), α-trans-bergamotene (4.1%),and caryophyllene (2.7%) (Chimnoi et al., 2018b). On the other hand, Melo (Melo et al., 2019b) obtained 19 compounds from HD of OG leaves belonging to several chemotypes, namely phenylpropanoid chemotype (74.83%), oxygenated monoterpenes chemotype (16.09%), hydrocarbon sesquiterpenes chemotype (6.96%), and minor amounts of hydrocarbon monoterpenes chemotype (0.92%) and oxygenated sesquiterpenes chemotype (0.62%) with 74.83 % eugenol, 1,8-Cineole (15.16%), β-Selinene (2.82 %) and (E)-Caryophyllene (2.20 %) being the highest. In the same vein, it was also reported that out of the 30 volatile compounds extracted with HD EG by Mohr (Mohr et al., 2017), oxygenated monoterpenes (72.30 %), sesquiterpene hydrocarbons (10.16 %), phenylpropanoids (7.42 %), oxygenated sesquiterpenes (5.18 %) and monoterpene hydrocarbons (4.82 %) were recorded (Fig. 3). Similarly, a study on the chemical composition of OG grown in Togo extracted with HD recorded monoterpene hydrocarbons (56.21 %), oxygenated monoterpenes (37.85 % and sesquiterpene hydrocarbons (3.80 % with thymol (31.79 %), p-cymene (15.57 %) and γ-terpinene (12.34 %), myrcene (6.94 %) and α-thujene (6.11 %) as the major components (Koba et al., 2009). *HD - Hydrodistillation, SD – Steam distillation, GC–MS – Gas Chromatography Mass Spectrometry. HD - Hydrodistillation, SD – Steam distillation, GC–MS – Gas Chromatography Mass Spectrometry.
S/N
Compounds
Phytochemical
class
Abundance
(%)
Extraction methods
Analytical methods
Molecular
formulae
MW (g/mol)
References
Eugenol
Phenylpropanoid
7.42–74.83
SD and HD
GC–MS
C10H12O2
164.2
(Chimnoi et al., 2018b; Melo et al., 2019b; Mohr et al., 2017)
Linalool
Monoterpenoid.
0.47–32.95
SD and HD
GC–MS
C10H18O
154.25
(Chimnoi et al., 2018b; Kobenan et al., 2021b; Melo et al., 2019a; Mohr et al., 2017)
1,8-Cineole
Monoterpenoid
0.1–21.91
HD and SD
GC–MS
C10H18O
154.25
(Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019a; Mohr et al., 2017)
Thymol
Monoterpenoid phenol
18.7
SD
GC–MS
C10H14O
150.22
(Kobenan et al., 2021b)
cis-Ocimene
Monoterpenes
0.43–13.89
SD and HD
GC–MS
C10H16
136.23
(Chimnoi et al., 2018b; Koba et al., 2009; Kobenan et al., 2021b)
γ-Muurolene
Sesquiterpenoids.
11.55
SD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b)
(Z,E)-α-farnesene
sesquiterpene isoprenoid
5.58
SD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b)
α- Cadinol
sesquiterpenoids
0.06–5.18
SD and HD
GC–MS
C15H26O
222.37
(Chimnoi et al., 2018b; Mohr et al., 2017)
β-Selinene
sesquiterpenoids
2.82–5.0
HD
GC–MS
C15H24
204.35
(Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019a; Mohr et al., 2017)
Germacrene D
sesquiterpenoids
0.1–4.76
SD and HD
GC–MS
C15H24
204.35
(Kobenan et al., 2021b; Mohr et al., 2017)
α-Bergamotene-trans
sesquiterpenoids.
0.2–4.12
SD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b; Kobenan et al., 2021b)
α-Terpineol
monoterpenoids
0.31–3.36
HD
GC–MS
C10H18O
154.25
(Koba et al., 2009; Melo et al., 2019a)
β-Caryophyllene
sesquiterpenoids
1.68–2.67
HD and SD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b; Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019a; Mohr et al., 2017)
α-Pinene
monoterpenoid
0.08–2.4
SD and HD
GC–MS
C10H16
136.23
(Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019a; Mohr et al., 2017)
trans-Ocimene
Monoterpenoid
0.10–1.75
HD and SD
GC–MS
C10H16
136.23
(Chimnoi et al., 2018b; Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019a)
α-Copaene
Sesquiterpenes
0.85–1.22
SD and HD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b; Koba et al., 2009; Kobenan et al., 2021b)
β-Pinene
monoterpenoid
0.43–1.25
SD and HD
GC–MS
C10H16
136.23
(Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019b; Mohr et al., 2017)
Terpinen-4-ol
Monoterpenoid
0.16–1.13
HD
GC–MS
C10H18O
154.25
(Koba et al., 2009; Melo et al., 2019b; Mohr et al., 2017)
Sabinene
monoterpenoids
0.56–1.2
HD and SD
GC–MS
C10H16
136.23
(Koba et al., 2009; Kobenan et al., 2021b; Melo et al., 2019b; Mohr et al., 2017)
α-cis-Bergamotene
monoterpenoids.
0.03–1.07
SD and HD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b; Mohr et al., 2017)
γ-Cadinene
sesquiterpenoids
0.04–1.05
SD
GC–MS
C15H24
204.35
(Chimnoi et al., 2018b; Mohr et al., 2017)
Caryophyllene oxide
sesquiterpenoid oxide
0.05–1.0
SD and HD
GC–MS
C15H24O
220.35
(Chimnoi et al., 2018b; Kobenan et al., 2021b; Melo et al., 2019a)
S/N
Compounds
Ocimum sanctum
Phytochemcal
Class
Abundance
(%)
Extraction method
Analytical methods
Molecular
Formulae
MW (g/mol)
References
Methyl eugenol
phenylpropanoid
92.4
SD
GC–MS
C11H14O2
178.23
(Joshi, 2013)
Eugenol
phenylpropanoid
2.4–61.30
HD and SD
GC–MS
C10H12O2
164.2
(Joshi, 2013; Kumar et al., 2010; Saharkhiz et al., 2015)
1,8-Cineole
Monoterpenoid
0.01–19.41
HD and SD
GC–MS
C10H18O
154.25
(Joshi, 2013; Saharkhiz et al., 2015)
β-Caryophyllene
sesquiterpenoids
11.89
HD
GC–MS
C15H24
204.35
(Kumar et al., 2010)
Germacrene D
sesquiterpenoids
9.14
HD
GC–MS
C15H24
204.35
(Kumar et al., 2010)
β-Elemene
sesquiterpenoids
0.4–7.7
HD and SD
GC–MS
C15H24
204.35
(Joshi, 2013; Kumar et al., 2010; Saharkhiz et al., 2015)
α-Cubebene
sesquiterpenoids.
2.54
HD
GC–MS
C15H24
204.35
(Kumar et al., 2010)
Carvacrol
phenolic monoterpenes
2.04
HD
GC–MS
C10H14O
150.22
(Kumar et al., 2010)
β-Pinene
terpenoid
0.06–1.53
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010; Saharkhiz et al., 2015)
β-Selinene
sesquiterpene
1.34
HD
GC–MS
C15H24
204.35
(Kumar et al., 2010)
α-Humulene
sesquiterpene
0.22–1.29
HD
GC–MS
C15H24
204.35
(Kumar et al., 2010; Saharkhiz et al., 2015)
α-Pinene
monoterpene
0.41–0.61
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010; Saharkhiz et al., 2015)
p-Cymene
monoterpene
0.83
HD
GC–MS
C10H14
134.22
(Kumar et al., 2010)
Sabinene
monoterpenoids
0.54
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010)
γ-Terpinene
monoterpenoids
0.56
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010)
Limonene
monoterpenoids
0.71
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010)
Thymol
monoterpenoid
0.82
HD
GC–MS
C10H14O
150.22
(Kumar et al., 2010)
α-Terpinolene
monoterpenoids
0.74
HD
GC–MS
C10H16
136.23
(Kumar et al., 2010)
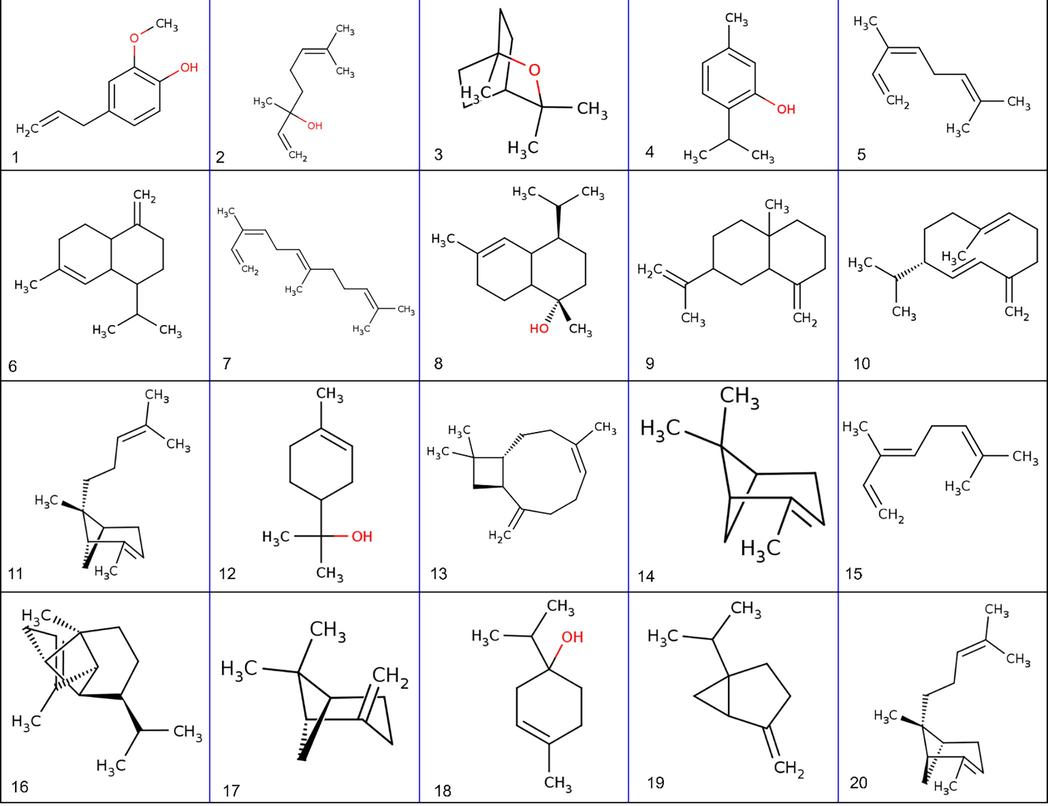
2D structure of Major phytochemical constituents of African Basil Essential Oil. 1) Eugenol 2) Linalool 3) 1,8-Cineole 4) Thymol 5) cis-Ocimene 6) γ-Muurolene 7) (Z,E)-α-farnesene 8) α-Cadinol 9) β-Selinene 10) Germacrene D 11) α-Bergamotene-trans 12) α-Terpineol 13) β-Caryophyllene 14) α-Pinene 15) trans-Ocimene 16) α-Copaene 17) β-Pinene 18) Terpinen-4-ol 19) Sabinene 20) α-cis-Bergamotene.
The compound group in EO of holy Basil were predominantly oxygenated monoterpenes (75% in HD; 67% in SD), sesquiterpene hydrocarbon (17% in HD; 27% in SD), and a trace amount of monoterpene hydrocarbons. Specifically, methyl eugenol (68% in HD; 59% in SD), caryophyllene (8% in HD; 13% in SD), and eugenol (7% in HD; 9% in SD) were the major identified compounds in holy Basil EO (Shiwakoti et al., 2017). The high content of EO in SD compared to HD is due to the direct contact of the plant with heat, which easily ruptures the oil gland and higher heat transfer in an index which leads to fast elution of volatiles (Fig. 4) (Shiwakoti et al., 2017).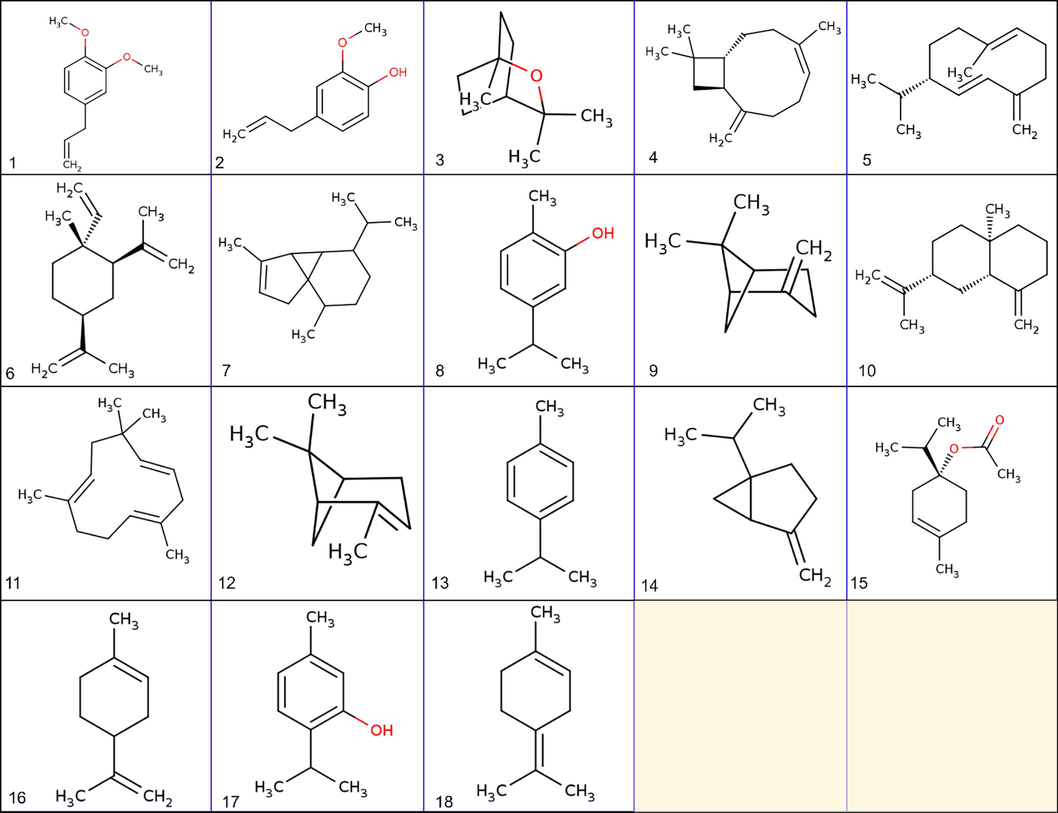
2D structure of Major phytochemical constituents of Holy Basil Essential Oil 1) Methyl eugenol 2) Eugenol 3) 1,8-Cineole 4) β-Caryophyllene 5) Germacrene D 6) β-Elemene 7) α-Cubebene 8) Carvacrol 9) β-Pinene 10) β-Selinene 11) α-Humulene 12) α-Pinene 13) p-Cymene 14) Sabinene 15) DL-γ-Terpinene 16) Limonene 17) Thymol 18) α-Terpinolene.
Results of several studies have also proved that vegetative stages can influence the constitution of EOs (Melo et al., 2019b). Specifically,(Saharkhiz et al., 2015) realized variations in the prevalence of constituents at the different stages of growth and development from Iran OSEO. From their study, eugenol (37.15 %), 1.8-cineole (26.45 %), and β-Bisabolen (20.99 %) were the most abundant compounds in floral budding (emergence of flower buds), full flowering, and vegetative stages respectively.
Taken together, a study carried out by (Joshi, 2013) to characterize EO from OG and OS showed that the composition of 31 constituents identified in OGEO were 5 phenyl derivatives (76.0%), ten monoterpene hydrocarbons (15.5%), nine sesquiterpene hydrocarbons (6.1%), 6 oxygenated monoterpenes (1.6%), and 1 oxygenated sesquiterpene (0.1%) with eugenol (75.1 %) as the most abundant. However, in OSEO, the compositions of the 25 constituents were 3 phenyl derivatives (94.9%), 9 sesquiterpene hydrocarbons (2.6%), 5 oxygenated sesquiterpenes (0.9%), 5 monoterpene hydrocarbons (0.3%), and 3 oxygenated monoterpenes (0.2%) with was methyl eugenol (92.4%) as the most abundant (Joshi, 2013). Thus, phenyl derivatives are the most abundant class in OGEO and OSEO, but the composition of other chemotypes varies in the two species.
Besides, irrespective of the distillation techniques, several studies have identified eugenol and thymol-rich compounds as the most abundant constituents of EO from OG and OS (Chimnoi et al., 2018b). For instance, 55.55 and 74.83 % of eugenol were obtained in OGEO by (Chimnoi et al., 2018b; Melo et al., 2019b), respectively, while 37.15 and 61.30 % of eugenol were identified in OSEO by (Kumar et al., 2010; Saharkhiz et al., 2015), respectively. Eugenol, with 54.0, 34.6, and 73.1, was also reported as the most abundant constituent in OGEO obtained with SD, HD, and SFE, respectively (Prabhu et al., 2009a). However, the second and third compounds may vary. Examples are 1,8-Cineole (Melo et al., 2019b; Mohr et al., 2017), cis-ocimene (Chimnoi et al., 2018b), thymol, p-cymere (Kpoviessi et al., 2014) and β-caryophyllene (Kumar et al., 2010). Thus, the variation in EO calls for the chemical characterization of EO to maximize its use. Eugenol has demonstrated antimicrobial and antioxidant potency and thus can control the oxidation of unsaturated lipids and free radical scavenging, thereby enhancing the shelf life of food products (Kumar et al., 2010).
5 Antimicrobial pharmacology of African and Holy Basils essential oil
Pathogenic microorganisms (bacteria, fungi, and viruses) usually cause several diseases and debilitating health states. Efforts have been made for decades to achieve a lasting weapon against these microbes in the form of antibiotics. However, these pathogenic microbes resist these synthetic antibiotics (Sodhi et al., 2021). Antibiotic and multidrug resistance have been the major cause of death due to microbial infections, as treatments usually fail to annihilate infecting pathogens. Scientists have taken the bull by the horn to investigate alternative routes or treatment regimens that would be long-lasting and effective. Natural products have gained tremendous application as antimicrobial agents and have interested the wider scientific population (Okeke et al., 2021). Plant EO is one of many other forms of plant extract that are vastly known for potencies against pathogenic microbes. One interesting fact about plant extract as an antimicrobial is the multi-targeting mode of action of the plant extract due to the numerous phytochemical constituent of the extracts (Ezeorba et al., 2022). Among several mechanisms of action by plants, EO is disrupting the microbial cell membranes, fostering the increase in ROS for oxidative damage to the microbes, damage of the microbial DNA and its replication machinery, denaturing key proteins for survival, and many more (Fig. 5) (Srivastava et al., 2022; Vaishampayan and Grohmann, 2021). Recent studies showing the antimicrobial activities of African and Holy Basil EO are concisely reviewed in this section (Table 4).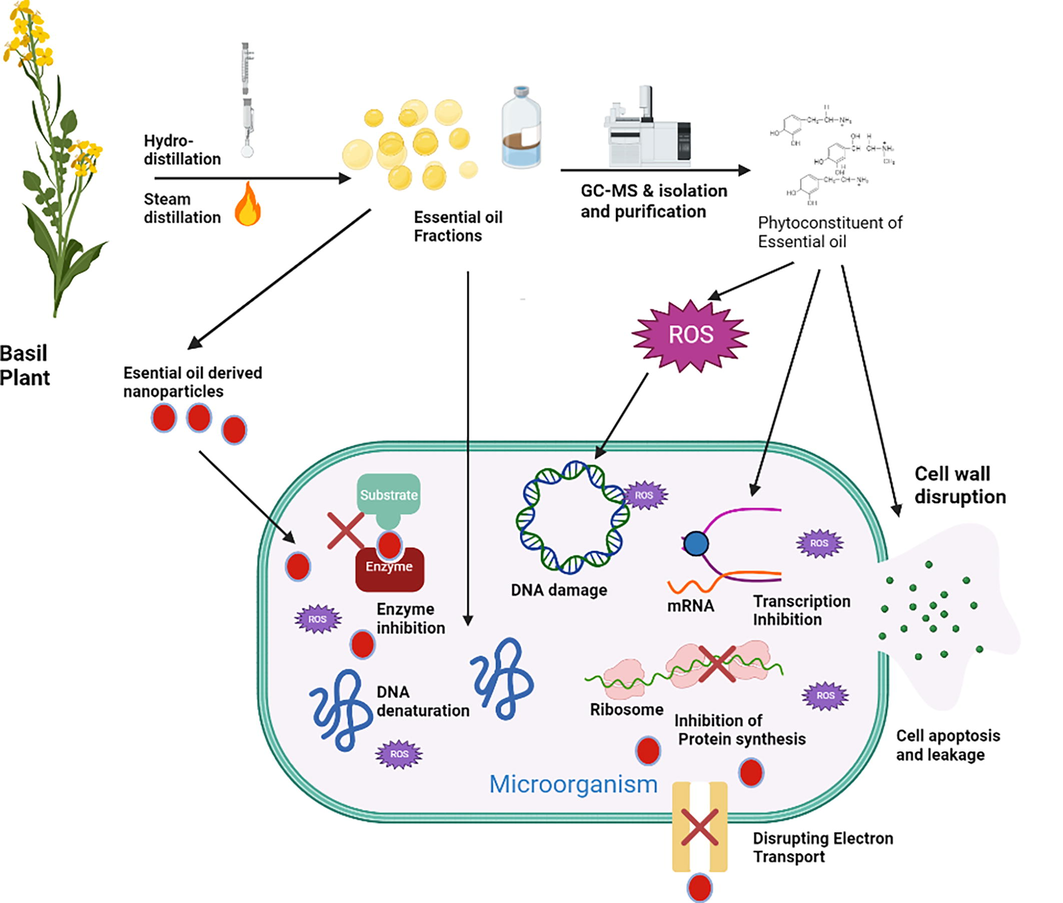
Possible mechanisms of antimicrobial activities of essential oil from African and Holy Basil.
5.1 Antibacterial studies
In a study by Sneha et al. (2022), the EO extracted from African Basil leaves by hydrodistillation and characterized by GC–MS showed antibacterial potencies against S. aureus, S. enteritidis, E. coli, and P. aeruginosa. The minimum inhibitory concentration (MIC) ranges from 2 to 4 μg/mL, and the zone of inhibition (ZI) was between 5 and 10. Moreover, the EO containing majorly p-Cymene (15.1%), camphor (10.1%), Thymol (32.8%), Eugenol (12.3%), and Methyl Chavico (9.3%) also showed larvicidal activities against Aedes aegypti and Culex tritaeniorhynchus causing the mortality of the third instar larva with an LC50 of fewer than 50 μg/mL for both mosquitoes (Sneha et al., 2022). Finally, the antioxidant capacity of the EO was estimated using several assays such as the DPPH radical scavenging, Hydrogen peroxide scavenging, ABTS cation radical scavenging, FRAP, Nitric oxide assay, and Lipoxygenase inhibition assay. The IC50 for the antioxidant scavenging activities was reasonable and was presented in Table 5 (Sneha et al., 2022). HD - Hydrodistillation, SD – Steam distillation, GC–MS – Gas Chromatography Mass Spectrometry, EO – Essential oils, DPPH − 2,2-diphenylpicrylhydrazyl, NO – Nitric oxide, ABTS − 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid, FRAP - Ferric ion reducing antioxidant power.
Class of Basil
Biological activities
Purified or characterized compound
Extraction method
Microbes
Assay/Treatment /dosage
Assays and bioactivities
Ref.
African Basil
Antibacterial
Larvicidal
Antioxidant Capacity/Radical scavenging activitiesEO from leaves having
GC/MS characterized compounds (p-Cymene (15.1%), Camphor (10.1%), Thymol (32.8%), Eugenol (12.3%), Methyl Chavico
(9.3%)
HD
S. aureus, S. enteritidis, E. coli and P. aeruginosa
Aedes aegypti, Culex tritaeniorhynchus
10 μL of EO on a 6 mm diameter filter paper to 100 μL of microbial isolates (107 CFU/mL)
Mortality of the Third instar larva
DPPH, HP, ABTS, FRAP, NO, Lipoxygenase inhibition assayMIC range of 2–4 μg/mL and a zone of inhibition of 5–10 mmLarvicidal
(LC50) – Less than 50 μg/mL for both mosquito larva
IC50 Values (µg/mL) – DPPH (44.2); H2O2 Scavanging (32.1); ABTS (20.3); others (>50)(Sneha et al., 2022)
Antibacterial and
Antioxidant capacityProcured EO with specific gravity of 0.96456- containing majorly Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol (16.22%); Phenol, 2-methoxy-3-(2-propenyl)- (14.71%); γ-Muurolene (6.57%); 9-Methoxycalamenene (6.51%).
Procured
E. coli, Enterobacter cloacae and Staphylococcus aureus
Disc diffusion methods
DPPH assayMIC of S. aureus (12.5), E. coli (6.25) and Enterobacter cloacae (25%)DPPH Scavanging (EC50)
− 9.43 ng/ml(Salvi et al., 2022)
Antibacteria
EO from leaves and inflorescences containing majorlyeugenol (43.3%), 1,8-cineole (28.2%) and β-selinene
(5.5%)HD
Aeromonas hydrophila
Microdillution methods
MIC and MBC – 1250–5000 μg/mL
(Monteiro et al., 2020)
Antibacteria and anti-biofilm
EO from leaves and inflorescences containing majorly
Eugenol (74.83%); 1,8-cineole (15.16%)HD
Staphylococcus aureus and Escherichia coli
Disk diffusion,
Microdillution, growth under sub MIC-exposure,Zone of inhibition diameter –
a. E. coli – 12–13 mm
b. S. aureus – 15 – 20 mm
MIC and MBC – 1000 – 2000 µg/mL(Melo et al., 2019a)
African Basil
Antibacteria (gastroenteritis pathogens)
EO from the leaves containing majorly eugenol (55.6%); cis-ocimene (13.9%), γ-muurolene (11.6%), (Z,E)-α-farnesene (5.6%), α-trans-bergamotene (4.1%), and β-caryophyllene (2.7%).
SD
Staphylococcus aureus, Escherichia coli, Salmonella Typhimurium, and Shigella flexneri
Disk diffusion and membrane disruption
MICs of 1–2 mg ml−1
Killings at 5 S at 4 times the MICs(Chimnoi et al., 2018b)
Antibacterial against oral bacteria pathogen
EO from the leaves containing majorly β-Cubebene (10.9%); Eugenol (56.4 %); trans-β-Ocimene (7.6%)
SD
Periodontopathic Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans and cariogenic Streptococcus mutans and Lactobacillus acidophilus
Broth dilution methods
Less than 10 CFU as % of control at conc of 1.0, 0.1 and 0.01% EO in text solution for all the tested strain
(Ocheng et al., 2015)
Antibacterial
Uncharacterized EO from the leaves
HD
Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, MDR- Acinetobacter baumannii
Agar diffusion methods
MBC90 of 2 mg/ml against MDR-A. baumanniiDiameter for zone of inhibition
(6.0–17.3 mm)(Intorasoot et al., 2017)
Antibacterial
EO from the Aerial parts containing majorly estragol (43.0–44.7%) and linalool (24.6–29.8%) in O. Basilicum oils; carvacrol (12.0–30.8%) and p-cymene (19.5–26.2%) in O. canum oils; thymol (28.3–37.7%) and γ-terpinene (12.5–19.3%)
HD
L. monocytogenes and S. Typhimurium
Disc diffusion and broth microdilution assays
MICs and MBCs 0.34–2.5 μL/ mL
(Mith et al., 2016)
Antibacterial
Uncharacterized EO from the leaves
HD
Multiresistant strains of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa
Microdillution technique
MIC against i) E. coli ATCC10536 – 128 μg/mL, S. aureus ATCC25923 – 512 μg/mL,
(Do Nascimento Silva et al., 2016)
Holy Basil
Antibacterial
Larvicidal
Antioxidant Capacity/Radical scavenging activitiesEO from leaves having majorly camphor (26.2%), Methyl Chavicol (18%), Eugenol (29.0%), Thymol (7.2%)
HD
S. aureus, S. enteritidis, E. coli and P. aeruginosa
Aedes aegypti, Culex tritaeniorhynchus
Disc Diffusion method;
Mortality of the Third instar larva;
DPPH, HP, ABTS, FRAP, NO, Lipoxygenase inhibition assay
10 μL of EO on a 6 mm diameter filter paper to 100 μL of microbial isolates (107 CFU/mL)MIC range of 4– 8 μg/mL and a zone of inhibition of 6–10 mmLarvicidal
(LC50) – 50–60 μg/mL for both mosquito larva
H2O2 Scavenging (39.4); ABTS (26.3); others (>50)(Sneha et al., 2022)
Antibacterial and
Antioxidant capacityProcured EO with a specific gravity of 0.92
Containing majorly Phenol, 2-methoxy-3-(2-propenyl)- (8.52%), γ-Muurolene (7.43%); α-Citral (14.75%); β-Elemene (11.81%); Caryophyllene (8.91%); α-Amorphene (7.78%);Procured
E. coli, Enterobacter cloacae, and methicillin-resistant Staphylococcus aureus
Disc diffusion methods
DPPH assay
MIC (v/v) of S. aureus (12.5), E. coli (12.5) and Enterobacter cloacae (25%)
DPPH Scavenging (EC50) − 9.48 ng/ml(Salvi et al., 2022)
Antibacterial
Uncharacterized EO from the leaves
HD
Anti-mycobacterium against H37Rv and isolates of M. tuberculosis
BD BACTEC MGIT method
The MIC was 2.931 μg for H37Rv and 0.5 μL (1.4655 μg) to 6 μL (5.862 μg) for the different isolates.
(Jayapal et al., 2021)
Antibacterial and anti-biofilms
EO from the leaves containing majorly Estragole, eugenol, methyleugenol, benzofuran, citral
HD
–
In silico (Molecular docking of compounds to csgA gene
Benzofuran was the most potent with a docking energy of −9.27Kcal/Mol
(Saishree Anchana et al., 2021)
Holy Basil
Antibacterial
EO from the leaves with GC/MS analysis Most abundant - m-eugenol (69.1 %)
SD
S. aureus, S. epidermidis and E. faecalis, P. aeruginosa and E. coli
Disc diffusion and broth micro-dilution methods
Zone of inhibition diameter>15 mm for all organism.
MIC ranging from 0.03 % to 0.12 %, and MBC of 0.12 % to 0.25 %(Bugayong et al., 2019)
Antibacterial
(Dental protection and mouthwash)Uncharacterized EO from the leaves
HD
Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum
Agar well diffusion methods
Zone of inhibition diameter (48–55 mm)
(Kalra et al., 2019)
Antibacterial
EO from the flower spikes, leaves with Major bioactive compounds such as camphor, eucalyptol and eugenol
HD
Staphylococcus aureus (including MRSA) and Escherichia coli
Broth micro-dilution method
MIC of 2.25% of E) against the tested strains
(Yamani et al., 2016)
Antimicrobial
EO from the leaves
HD
Methicillin sensitive and resistance S. aureus, E. coli, K. pneumonia, P. aeruginosa
S. pyogenes, S. agalactiae, Cutibacterium acne, Bacteroides fragilisagar punch well method
Zone of inhibition diameter of 42 mm
(Subramanian et al., 2021)
African Basil
Antifungal
EO from the leaves containing majorly 1,8-Cineol (23.8%); Eugenol (51.84%); β-Selenine (8.88%); β-Cariofileno (5.79%)
HD
Candida species
modified broth microdilution method
Checkerboard methods
Crystal violet membrane permeability assayMIC of 1–8 g/ml, after 24 h resulted to a reduction of 70.1–80.8% microbial growth
(OLIVEIRA et al., 2016)
Antifungal
EO from the leaves, containing majorly 0.18% essential oil yield containing linalool majorly (32.9%) and 1,8-cineole (21.9%)
HD
Fusarium sp
broth microdilution method
MIC of the essential oil 31.25 to 125 µg/mL
(Mohr et al., 2017)
Antifungal
EO from the aerial parts majorly with thymol (28.1%)
Para-cymene (21.2%)
And terpinene (16.5%)HD
A. flavus, A. niger, A. tamarii, A. terreus, Fusarium poae, F. verticillioides, P. citrinum, P. griseofulvum, Aspergillus aculeatus, A. ustus, Penicillium brevicompactum and Scopulariopsis brevicaulis
Agar medium assay with Minimum Fungicidal concentration and Mycelia growth inhibition
800–1000 g/Ml of EOIt was reported that the mucelial growth was inhibited 91.1–100% and MFC of 50–100%
(Sessou et al., 2013)
Antifungal
EO from the leaves with nanoformulation by non-ionic surfactant and water by ultrasonication
(Oil: Surfactant − 1:1 ration (v/v)HD
Penicillium digitatum
agar medium assay with Minimum Fungicidal concentration
Growth inhibition of 85% pure oil and 96% nano emulation after 15 days
(Mahajan et al., 2021)
Holy Basil
Antifungal
EO from the leaves packaged in Poly Lactic Acid, having its major constituent as eugenol
HD
Aspergillus ochraceus; Aspergillus westerdijkiaeFumigation test
Inhibit the fungal growth and production of Ochratoxin A
(Brandão et al., 2022; Kumar et al., 2020)
Antifungal
O.4% yield of EO from the aerial parts with Eugenol (53.3%), Estragole (19.11%), and Camphor (10.01%) as the major component incorporated inside Chitosan Nanomatrix
HD
Aspergillus flavus
poisoned food technique
MIC of 0.75 μL/ml was obtained
(Brandão et al., 2022)
Antifungal
Uncharacterized EO from the aerial parts
HD
Penicillium chrysogenum, Aspergillus tubingensis, A. minutus)
MIC estimation following CLSI M38-A2 protocol
A MIC of 0.25 – 5.04 μg/Ml
(Angelini et al., 2019)
In another recent study, procured EO of Basil with specific gravity 0.96456, containing majorly Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahedron-naphthalene-2-ol (16.22%); Phenol, 2-methoxy-3-(2-propenyl)- (14.71%); γ-Muurolene (6.57%); 9-Methoxycalamenene (6.51%), was shown to possess antibacterial activities against E. coli, Enterobacter cloacae and Staphylococcus aureus (Salvi et al., 2022). Using the Disc diffusion methods, the MIC for S. aureus was 12.5 μg/mL, while E. coli was 6.25 μg/mL and Enterobacter cloacae, 25 μg/Ml.The EO also showed antioxidant activities through the DPPH radical scavenging assay, with an EC50 of 9.43 ng/ml (Salvi et al., 2022). Essential oil of African Basil extracted from steam distillation was also reported with antibacterial activities against gastroenteritis pathogens (Staphylococcus aureus, Escherichia coli, Salmonella Typhimurium, and Shigella flexneri) as well as against oral/Periodontopathic/cariogenic bacteria pathogens (Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Streptococcus mutans, and Lactobacillus acidophilus) (Chimnoi et al., 2018a; Ocheng et al., 2015).
Similarly, Holy Basil's EO has also been shown to portray excellent antibacterial activities. By adopting the BD BACTEC MGIT assay method, Jayapal et al. (2021) investigated the anti-mycobacteria activities of the EO fraction. Their results showed that the EO has impressive potencies against isolates of M. tuberculosis and H37Rv with MIC ranging from 2.931 μg − 5.862 μg (Jayapal et al., 2021). In another study, the antibacterial activities of Holy Basil were investigated using the Disc diffusion and broth micro-dilution method against S. aureus, S. epidermidis and E. faecalis, P. aeruginosa, and E. coli. The diameter of the zone of inhibition was greater than 15 mm for all organisms and a MIC ranging from 0.03 % to 0.12 % and MBC of 0.12 % to 0.25 %. The Essential oil had an abundance of m-eugenol (69.1 %), according to the GC–MS analysis of the leaves EO (Bugayong et al., 2019). Similarly, as reported in Subramanian, Saumya 2021, the EO from the leaves of Holy Basil should have impressive inhibition against Methicillin-sensitive and resistant S. aureus, as well as other organisms such as E. coli, K. pneumonia, and P. aeruginosa. S. pyogenes, S. agalactiae, Cutibacterium acne, and Bacteroides fragilis. For all the organisms, the Zone of Inhibition diameter estimated from the agar punch well method was grater than 42 mm (Subramanian et al., 2021).
5.2 Antifungal studies
The Essential Oil of African Basil has been mentioned in several studies to be potent against some pathogenic fungi such as Candida, Fusarium, Penicillium, Aspergillus strains, and others. Different fungi species such as A. flavus, A. niger, A. tamarii, A. terreus, Fusarium poae, F. verticillioides, P. citrinum, P. griseofulvum, Aspergillus aculeatus, A. ustus, Penicillium brevicompactum, and Scopulariopsis brevicaulis are susceptible to the EO from the aerial part of African Basil, having thymol (28.1%), Para-cymene (21.2%), and terpinene (16.5%) as the major components (Sessou et al., 2013). From the study, the mycelial growth inhibition for all the fungi strains fell between 91.1 and 100%, while the minimal fungicidal concentration of 50–100% was also reported (Sessou et al., 2013). In a different study, several strains of Fusarium species were inhibited by the EO from the African Basil leaves containing majorly linalool (32.9%) and 1,8-cineole (21.9%). Using the broth microdilution technique, the MIC for the different strains was between 31.25 and 125 µg/mL (Mohr et al., 2017). In a more recent study, the agar medium assay evaluated the minimum fungicidal concentration of African Basil EO nanoformulation against Penicillium digitatum. The EO nanoformulation was fabricated from the Basil EO and non-ionic surfactant in a 1:1 ratio by ultrasonication (Mahajan et al., 2021). In the study, the nanoformulation showed more potency than the pure EO of African Basil. After 15 days of incubation of the EO with the fungi, a growth inhibition of 85% was recorded for the pure oil, while a 96% inhibition was reported for the nanoformulation (Mahajan et al., 2021). Similarly, Several other studies showing the antimicrobial activities of African Basil EO are summarized in Table 5.
Moreover, a few studies have reported the antifungal activities of Holy Basil EO. The EO of Holy Basil, having eugenol as its major constituent, was packaged in Poly Lactic Acid and used in a fumigation study against Aspergillus ochraceus and Aspergillus westerdijkiae. It was reported that the EO formulation significantly inhibited the fungal growth as well as its ochratoxin A production (Brandão et al., 2022; Kumar et al., 2020). Similarly, Aspergillus flavus, one common cause of food poisoning, showed a MIC of 0.75 μL/ml when targeted against EO from the aerial parts Holy Basil incorporated inside Chitosan Nanomatrix, with Eugenol (53.3%), Estragole (19.11%), and Camphor (10.01%) as the major component (Brandão et al., 2022). In summary, the constituents found in the EO of African and Holy Basil confer the antimicrobial activities to the EO, and this can be harnessed as a friend and less toxic alternative to combat disease-causing pathogens.
5.3 Antiviral activities
The use of natural products as antiviral therapy is steadily increasing. Although numerous studies have shown the antiviral potencies of several plants' bioactive chemicals and essential oils, there are limited or no studies revealing the antiviral properties of African and Holy Basil (Ezema et al., 2022). Researchers can consider covering this knowledge void by exploring both plants' antiviral potencies. There are possibilities that findings from such future research endeavors may provide a path toward lasting solutions to several endemic and pandemic viral disturbances. On the other hand, there have been several exciting findings from the few studies which have evaluated the antiviral potencies of solvent extracts of the plant leaves. Ghoke et al. (2018) reported the antiviral activities of holy Basil leaves against the H9N2 virus using an embryonated chicken egg model. The hydro-methanol (1:1) crude extract of holy Basil leaves and its terpenoid-enriched fraction showed the best antiviral properties in limiting the viral replication after 48–72 h post-inoculation. Moreover, a significant decrease in viral genome copy was reported after treatment with the leave extract (Ghoke et al., 2018; Rai et al., 2023). In more recent studies, the cytopathic inhibition and haemagglutination inhibition assay was used to show the antiviral potencies of extracts of Holy Basil against different animal diseases such as Bovine Herpes Virus-type-1 (BVH-1), Foot and Mouth disease virus (FMDV) and Newcastle Disease Virus (NDV). A concentration lower than the non-toxic doses of O. sanctum leaves caused 85.3% and 98.4% protection of BHV-1 and NDV, whereas there were no significant observable effects on the FMD virus (Goel and Bhatia, 2022). Just as the above-discussed illustrative studies, Similar research in the future can perform with the essential oils of Holy and African Basils, and their potencies can be compared.
5.4 Anti-parasitic activities – A focus on mosquitocidal properties of African and Holy Basil essential oil
Mosquitoes are members of a problematic group of insect vectors of human disease causative agents. They belong to the Culicidae family comprising about 3600 flies species. Some diseases and parasites dispersed by mosquitoes are Zika, West Nile, Chikungunya, dengue, and plasmodium (malaria) (Agboli et al., 2021). These disease-causing organisms, transmitted by mosquito vectors, cause serious debilitating health conditions, systemic and metabolic damage, and even death (Chala and Hamde, 2021; Okagu et al., 2022). Essential oil from African and Holy Basil has been shown in several studies to possess mosquitocidal activities, either as a repellent or with insecticidal, larvicidal, or ovicidal activities. This section summarizes key findings from some of these studies to expose the applicability of this oil to reduce the prevalence of some of these diseases, such as malaria, dengue, and zika virus infections (Table 6).
Class of Basil
Biological activities
Purified or characterized compound
Method of extraction
Experimental model
Treatment/dosage and time
Assays and bioactivities
References
African Basil
Mosquitocidal and Larvicidal activities
Uncharacterized EO from leaves
HD
Anopheles subpictus, Aedes aegypti, and Culex quinquefasciatus
Mosquito knockdown after 1–2h exposure
Inhibition of larva mortality94–97% of Mosquitocidal activities
LC50 = 40.08 ± 1.60 ppm(Sharma et al., 2021)
mosquito-repellent and mosquitocidal activities
EO from leaves
HD
Anopheles gambiae
Human bait for evaluation of repellency 30% EO in olive oil
95.7–97.2% repellency
(Oparaocha et al., 2010)
MosquitocidalEO fraction form aerial parts containing majorly eugenol (67.38%) and Z-β-ocimene (14.95 %)
HD
Aedes aegypti
Oviposition deterrence
0.5 – 3.5 mg/mlOviposition deterrence between 8 and 27 d
(Harikarnpakdee and Chuchote, 2018)
mosquito-repellent
EO fraction from the leaves containing majorly (Z)-Ocimene (29.73%), eugenol* (21.76), and Germacrene D (9.65)
HD
Anopheles gambiae
Repellency RD50 = 2.77 × 10-5 mg cm−2
(Ywaya et al., 2020)
Holy Basil
Larvicidal activities
Uncharacterized EO from the leaves
SD
Aedes aegypti
larva mortality
0.075 – 0.175 ppm81.0–97% larva mortality
(Chaiphongpachara et al., 2020)
mosquito-repellent
Uncharacterized EO from the leaves
HD
Aedes aegypti
20% EO in ethanol
100% repellency for 6 h
(Lalthazuali and Mathew, 2017)
Ovicidal
Uncharacterized EO from the leaves
HD
Aedes aegypti
100–1000 ppm exposure for 24–72 h
LC50 − 8.40 ppm
(Sarma et al., 2020)
In a recent study, Sharma et al. (2021) reported the mosquitocidal and larvicidal activities of an uncharacterized EO obtained via hydro distillation from the leaves of African Basil against Anopheles subpictus, Aedes aegypti, and Culex quinquefasciatus mosquito species. It was observed that about 94–97% of mosquitoes were knockdown after exposure for 1–2 h and the concentration at 50% lethality (LC50) was 40.08 ± 1.60 ppm. The EO also caused inhibition of mosquito larva motility (Sharma et al., 2021). In another study, African Basil essential oils acted as an effective repellent against Anopheles gambiae mosquito at an RD50 of 2.77 × 10-5 mg cm−2 (Ywaya et al., 2020). GC–MS characterized the essential oil fractions obtained from the leaves of African Basil. They were found to contain majorly (Z)-Ocimene (29.73%), Eugenol (21.76%), and Germacrene D (9.65%) (Ywaya et al., 2020). On the other hand, Harikarnpakdee and Chuchote (2018) reported the oviposition deterrence activities of African Basil essential oil. In their study, Essential oil from the aerial part of the plants containing majorly eugenol (67.38%) and Z-β-ocimene (14.95 %) promoted oviposition deterrence in Aedes aegypti mosquito, for about 8 – 27 d, at a concentration of 0.5 – 3.5 mg/ml (Harikarnpakdee and Chuchote, 2018).
In the same vein, in recent pieces of literature, holy Basil essential oil has shown larvicidal activities, mosquito-repellent, and ovicidal activities against Aedes aegyptii. In a study by Chaiphongpachara et al. (2020), uncharacterized essential oils from Basil, extracted through steam distillation, promoted the mortality (81 – 97%) of Aedes aegyptii larvae within a concentration range of 0.075 – 0.175 ppm. Another study that used hydrodistillation to obtain the EO from Holy Basil leaves showed significant ovicidal effects on Aedes aegypti eggs when exposed to the oil within a 100–1000 ppm concentration 24 to 72 h (Sarma et al., 2020). Finally, an earlier study obtained 100% repellency of Aedes aegyptii adult mosquito, which only had a concentration of 20% EO of Holy Basil in ethanol (Lalthazuali and Mathew, 2017). From these studies, it is very clear that the essential oils of both African and Holy Basils are very potent against mosquito vectors and may have similar applications against other insect pests of humans and agro-produce. Future studies may focus more on improving the potency, which out any harm or toxicity to humans or non-targeted species.
5.5 Other anti-parasitic activities of African and Holy Basil
Basil EO may be potent against other parasites infesting aquaculture and livestock animals. A few studies have recently been performed to investigate the efficacies of essential oils from African Basil as well as its components against several varying classes of parasites (Table 7). However, only sparsely available old studies have investigated similar anti-parasitic activities with Holy Basil Essential Oil (Asha et al., 2001).
Class of Basil
Biological activities
Purified or characterized compound
Method of extraction
Parasite
Experimental model
Treatment/dosage and time
Assays and bioactivities
References
African Basil
Anti-parasite (antihelminthic effects)
Eugenol (43.3%), 1,8-cineole (28.2%) and beta-selinene (5.5%)
Hydro-distillation
Monogenean (Aquaculture)
Monogenean parasite count on tambaqui (Colossoma macropomum) fish gills
0, 5, 10, and 15 mg L− 1 EO in alcohol
Antihelmintic activities at a minimum conc of 15 mg L− 1
(de Lima Boijink et al., 2016)
Anti-parasitic (antihelminthic activities)
Eugenol (91.47%) and Z-β-ocimene (5.93%)
Hydro-distillation
monogenean parasite Gyrodactylus sp
(Aquaculture)monogenean count silver catfish (Rhamdia quelen) maxillary barbells
5 and 10 mg L−1 of EO dissolved by a ratio of 1:10 in 96% ethanol.
Exposure time is 1 h before parasite count under a stereomicroscopeParasite mortality is approximately 35% and 50% in 5 and 10 mg L−1 EO
(Bandeira et al., 2017)
Anti-parasite (acaricidal activity)
Thymol (33.4 – 39.5%); γ-Terpinene (26.2 – 35.1%);
p-Cymene (6.9 – 17.0%)Hydro-distillation
Rhipicephalus microplus larvae (Cattle Ticks)
(Livestock farming)Larval immersion test, followed by dead and live larva count
0.66 to 5.0 mg/mL EO for 10 min
The LC50 was 0.84 mg/mL to 2.57 mg/mL
(Silva Lima et al., 2018)
Anti-parasitic (antihelminthic activities)
Eugenol (42.3%); 1,8-cineole (20.4) and β-selinene (12.9%)
Hydro-distillation
monogenean parasite (Cichlidogyrus tilapiae)
Aquaculture
Monogenean motality in Nile tilapia
0 – 100 mg/l for every 6 h
80% of parasite mortality at LC50 (96 h) of 40.70 mg.L-1 at 2 h
(Meneses et al., 2018)
Anti-parasitic (pulicidal and acaricide activity)
Eugenol (89.68%) and Apofarnesol (2.68%)
Hydro-distillation and Eugenol purification
Fleas (Ctenocephalides felis felis) and Ticks (Rhipicephalus sanguineus)
(Livestock farming)Mortality of immature and adult stage Flea and Tick
Conc of 25–1500 µg/ml for immature stage (Egg) and 25 to 10000 µg/ml for mature stage (Larvae, pupae, and adult)
Mature stage (Larvae, pupae and adult) -- 50–10,000 µg/ml EO and 100–1000 µg/ml EugenolImmature (egg)
− 25–1500 µg/ml EO and 2.3–1200 µg/ml Eugenol(Oliveira et al., 2022)
Basil EO has been effective in aquaculture as an antihelminthic agent in combating different monogenean parasites affecting various fishes. A study by Meneses et al. (2018) reported an impressive 80% mortality of the monogenean parasite (Cichlidogyrus tilapiae) isolated from Nile tilapia after a 2 h incubation with 40.70 mg/l African Basil EO. The major components of the EO were Eugenol (42.3%), 1,8-cineole (20.4), and β-selinene (12.9%) detected by GC–MS analysis after the hydrodistillation extraction (Meneses et al., 2018). Furthermore, in a similar study, the EO of Basil was shown to be efficacious against the monogenean parasite Gyrodactylus sp, isolated from silver catfish (Rhamdia quelen) maxillary barbells. About 50% mortality of the parasite was reported after a 1 h exposure of 10 mg/l EO, whereas the EO content had a significant quantity of eugenol (91.47%) (Bandeira et al., 2017). Other interesting studies on the anti-parasitic activities of Basil EO are summarized in Table 7.
Like aquaculture, many parasites affect livestock animals causing detrimental effects on their health, reproduction, and viability. Essential Oils from Basil are effective phototherapy against some of these livestock parasites, such as fleas, ticks, and other insect parasites, at different stages of their development (egg, larvae, pupa, or adult). A recent study by Oliveira et al. (2022) has shown that African Basil EO and its Eugenol-enriched fractions were very effective in causing mortality of the egg, larvae, pupa, and adult of Fleas (Ctenocephalides felis felis) and Ticks (Rhipicephalus sanguineus). About 50–10,000 µg/ml EO and 100–1000 µg/ml eugenol were effective in causing significant mortality (>40%) in the mature stage of the parasite (Larvae, pupae, and adult). A 25–1500 µg/ml EO concentration and 2.3–1200 µg/ml eugenol effectively managed the immature stage (ovicidal) of both the Flea and Tick parasites (Oliveira et al., 2022). These results have shown the potencies of African Basil EO act as effective pulicidal and acaricidal agents in causing mortality in all the developmental stages of the parasites. In an older study, Silva Lima et al. (2018a) showed the larvicidal activities of African Basil EO against Rhipicephalus microplus larvae (Cattle Ticks). The larva immersion test was performed, followed by a stereomicroscopic count of dead and live larvae after a 10 min incubation with EO (0.66 to 5.0 mg/mL). The study reported a variation in larvicidal activities based on seasons (rainy and dry), with an LC50 ranging between 0.84 mg/mL to 2.57 mg/ml (Silva Lima et al., 2018). Future studies should consider more research into the crop and animal protection derived from Basil essential oil.
6 Toxicity of African and holy Basils (essential oils)
Toxicity studies determine the short-, medium- and long-term effects of administering specific substances on an organism. Despite the beneficial effects of the essential oils of African and holy Basils, they may have toxic effects. Perhaps, the beneficial effects of the toxicity of the essential oils of African and holy Basils are manifested in its control of pests, as demonstrated by previous research (Cruz et al., 2016; Bhavya et al., 2018; Benelli et al., 2019; Thodsare et al., 2019; Bhavya et al., 2020; Essoung et al., 2020; Kobenan et al., 2021a; Žabka et al., 2021).
The median lethal dose (LD50) of African Basil essential oil in rats was 1750 mg/kg body weight (Fandohan et al., 2008). For the 14-day sub-acute toxicity, rats administered 1500 mg/kg b.w. of the oil died within four days of the experiment, with morphological alterations in the stomach. However, no liver changes were observed even at higher doses (Fandohan et al., 2008). A study by Degla et al. (2021) also showed that 500 mg/kg b.w. of essential oil of African Basil caused a 10% reduction in rats' weight and had a toxic effect on hematological and biochemical parameters after 14 days of administration.
Estimating oral and intraperitoneal acute toxicities of the essential oil of African Basil in mice and rats revealed intraperitoneal LD50 values of 0.27 and 0.43 g/kg, respectively. Similarly, in mice and rats, intraperitoneal LD100 values were 0.59 and 0.74 g/kg, respectively. In mice, oral LD50 and LD100 values were 1.41 and 2.50 g/kg, respectively, and 2.29 and 4.07 g/kg, respectively, in rats (Orafidiya et al., 2004). Enlargement in organ weights was observed in the sub-chronic toxicity study. No deaths were recorded in the group administered orally compared to the intraperitoneally-administered groups within the 30-day test period (Orafidiya et al., 2004).
As revealed by Parandin & Haeri (2010), the oil extracts of African Basil produced negative impacts on male reproductive function and fertility as manifested by the reduction in sperm motility and viability, daily sperm production (DSP), epididymal sperm reserve (ESR), gonadosomatic index (GSI), testosterone concentration and fertility, after 60 days administration at 300 mg/kg b.w.
Fourteen-day acute toxicity study of the essential oil of holy Basil in male Swiss albino rats revealed that the LD50 was greater than 1100 mg/kg b.w (Rangasamy et al., 2012). In mice, the LD50 of holy Basil essential oil was found to be 4571.43 µL/kg (Kumar et al., 2010). Following the Organization for Economic Cooperation and Development (OECD) guidelines 423 in female Wistar rats, the toxic effect of the essential oils of holy Basil was revealed at 2,000 mg/kg bw. However, no mortality was observed (Jayapal et al., 2022). This was in contrast with the acute oral and dermal acute toxicity studies carried out in Sprague Dawley rats by Chil et al. (2017), as no toxic manifestations were observed at 2, 000 mg/kg b.w, following the OECD 423 and 402 guidelines. Only weight gain in male and female Sprague Dawley rats was observed after the dermal toxicity test. The oil was also non-toxic to cardiomyocytes in vitro at 1200 µg/ml (Chil et al., 2017).
7 Conclusion and prospect
According to the findings of this study, EOs from Africa and Holy Basil have a wide range of morphological differences and ethnobotanical applications in treating human diseases. Morphologically, basil leaves are ovate-lanceolate in shape, cuneate, and decurrent at the base, with a coarsely crenate, serrated margin, and glandular trichomes. Whereas Holy Basil differs in having internally glabrous calyces and spreading pedicels, as well as slightly toothed, hairy, sub-ovate, and sub-serrate leaves. Regarding the stem and flowers, African basil have a brown ark stem and creamy white flowers, while Holy Basils have a purple-green stem and purple-white flowers. This study critically evaluated essential oil phytochemical composition variations and their respective antimicrobial pharmacology. Both essential oils contain diverse phytochemicals with a multi-targeted approach against several microbial strains, making them excellent candidates for developing novel antimicrobial agents. In principle, they exert antimicrobial actions mainly by distorting their cellular architecture, which leads to membrane permeability disruption, denaturation of critical proteins for survival, damage to the microbial DNA/replication machinery, and ultimately cell lysis and organism death. The reports of this study open a window for the food industry to carry out an extensive experiment on the large-scale use of these EOs as food preservatives. Also, more in vivo studies, standardization of its dose for application, and the cost-benefit ratio must be determined before it can be formulated as an antimicrobial agent. Finally, we recommend that future studies delve more into nano-formulation or nano-derived conjugation of these essential oils products for effective delivery and improved antimicrobial activities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pharmacognostical Characterization of Ocimum Spp. J. Pharmacogn. Phytochem.. 2013;2:219-224.
- [Google Scholar]
- Mosquito-associated viruses and their related mosquitoes in West Africa. Viruses. 2021;13
- [CrossRef] [Google Scholar]
- Ethnobotanical potentials of common herbs in Nigeria : a case study of Enugu state. Educ. Res. Rev.. 2006;1:16-22.
- [Google Scholar]
- Seedling morphology and molecular characterisation of some Ocimum L. species (Lamiaceae) in Southwest states of Nigeria. Feddes Repert.. 2020;131:213-224.
- [CrossRef] [Google Scholar]
- Ethnobotanical study on some Malaysian anti-malarial plants : a community based survey. J. Ethnopharmacol.. 2010;132:362-364.
- [CrossRef] [Google Scholar]
- Screening of the antifungal activity of essential oils against human and plant pathogenic filamentous fungi. Flora Mediterr.. 2019;29:5-12.
- [CrossRef] [Google Scholar]
- Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia. 2001;72:669-670.
- [CrossRef] [Google Scholar]
- Intercropping and mixed herb distillation for high-quality oil yield using lemon-scented basil (Ocimum africanum Lour.) cv. CIM-Jyoti and lemongrass (Cymbopogon flexuous (Nees ex Steud.) cv. Krishna. Acta Ecol. Sin.. 2022;42:269-273.
- [CrossRef] [Google Scholar]
- Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med.. 2017;17:60-67.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol.. 2011;134:851-864.
- [CrossRef] [Google Scholar]
- Peppermint essential oil-doped hydroxyapatite nanoparticles with antimicrobial properties. Mol.. 2019;2019(24):2169.
- [CrossRef] [Google Scholar]
- Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind. Crops Prod.. 2017;97:484-491.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of medicinal plants sold in markets of Ijebu-ode and Ijebu-igbo, Ogun state, Nigeria. Niger. J. Sci. Res.. 2018;17:245-256.
- [Google Scholar]
- Review of botany, phytochemistry, pharmacology, contemporary applications and toxicology of ocimum sanctum. Int. J. Pharm. Life Sci.. 2016;7:4918-4929.
- [Google Scholar]
- Insecticidal activity of the essential oil and polar extracts from Ocimum gratissimum grown in Ivory Coast: Efficacy on insect pests and vectors and impact on non-target species. Ind. Crops Prod.. 2019;132:377-385.
- [CrossRef] [Google Scholar]
- Ocimum tenui fl orum oil, a potential insecticide against rice weevil with anti- acetylcholinesterase activity. Ind. Crop. Prod.. 2018;126:434-439.
- [CrossRef] [Google Scholar]
- Efficacy of Ocimum tenuiflorum essential oil as grain protectant against coleopteran beetle, infesting stored pulses. J. Food Sci. Technol.. 2020;58:1611-1616.
- [CrossRef] [Google Scholar]
- Microwave-assisted hydrodistillation of the essential oil from Algerian Pimpinella anisum seeds. Flavour Fragr. J.. 2021;36:34-46.
- [CrossRef] [Google Scholar]
- Antifungal and physicochemical properties of Ocimum essential oil loaded in poly(lactic acid) nanofibers. Lett. Appl. Microbiol.. 2022;74:765-776.
- [CrossRef] [Google Scholar]
- Antibacterial Activity and Chemical Composition of Essential Oils from Leaves of Some Aromatic Plants of Philippines. J. Essent. Oil Bear. Plants. 2019;22:932-946.
- [CrossRef] [Google Scholar]
- 1, 8-Cineole : a review of source, biological activities, and application. J. Asian Nat. Prod. Res.. 2020;23:938-954.
- [CrossRef] [Google Scholar]
- Screening seven commercial essential herb oils for larvicidal activity against the mosquito Aedes aegypti (Linnaeus), a vector of the dengue virus ARTICLE INFO. J. Appl. Pharm. Sci.. 2020;10:43-050.
- [CrossRef] [Google Scholar]
- Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Heal.. 2021;9:1466.
- [CrossRef] [Google Scholar]
- Chemical Composition and Toxicity of Ocimum sanctum L. Var. Cubensis Essential Oil Up-Growing in the Eastern of Cuba. Int. J. Pharmacogn. Phytochem. Res.. 2017;9:1021-1028.
- [CrossRef] [Google Scholar]
- Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. Microb. Pathog.. 2018;118:290-300.
- [CrossRef] [Google Scholar]
- Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. Microb. Pathog. 2018
- [CrossRef] [Google Scholar]
- Ecotoxicology Sublethal Effects of Essential Oils From Eucalyptus staigeriana (Myrtales : Myrtaceae), Ocimum gratissimum (Lamiales : Laminaceae), and Foeniculum vulgare (Apiales : Apiaceae) on the Biology of Spodoptera frugiperda (Lepidoptera : Noc. J. Econ. Entomol.. 2016;109:660-666.
- [CrossRef] [Google Scholar]
- Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum) Aquaculture. 2016;457:24-28.
- [CrossRef] [Google Scholar]
- Comparative study of acute oral toxicity of essential oils of Ocimum gratissimum, Hyptis suaveolens and Psidium guajava in wistar rats. Int. J. Pharmacogn. Life Sci.. 2021;2:1-5.
- [Google Scholar]
- Repellence and fumigant toxicity of essential oils of Ocimum gratissimum and Ocimum kilimandscharicum on Tuta absoluta (Lepidoptera : Gelechiidae) Environ. Sci. Pollut. Res.. 2020;27:37963-37976.
- [Google Scholar]
- Therapeutic benefits of Salvia species: A focus on cancer and viral infection. Heliyon. 2022;8:e08763.
- [Google Scholar]
- Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res. - Mod. Chinese Med.. 2022;3:100075
- [CrossRef] [Google Scholar]
- Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol.. 2008;46:2493-2497.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey on plants used in the treatment of candidiasis in traditional markets of southern Benin. BMC Complement. Med. Ther.. 2020;20:1-18.
- [Google Scholar]
- Ethnobotany of Aromatic Plants used by Phytotherapists in the District of Glazoue, Ouesse and Save in Benin. Annu. Res. Rev. Biol.. 2021;36:74-83.
- [CrossRef] [Google Scholar]
- Microwave-assisted hydrodistillation extraction of essential oil from coriander seeds and evaluation of their composition, antioxidant and antimicrobial activity. Heliyon. 2020;6:e04893.
- [Google Scholar]
- Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complement. Altern. Med.. 2018;18:1-10.
- [CrossRef] [Google Scholar]
- Ocimum sanctum: in vitro antiviral potential against animal viruses. Indian J. Tradit. Knowl.. 2022;21:120-125.
- [CrossRef] [Google Scholar]
- DNA Fingerprinting and Genetic Relationships Similarities Among the Accessions/Species of Ocimum Using SCoT and ISSR Markers System. Mol. Biotechnol.. 2021;63:446-457.
- [CrossRef] [Google Scholar]
- Oviposition Deterrent Efficacy and Characteristics of a Botanical Natural Product, Ocimum gratissimum (L.) Oil-Alginate Beads, against Aedes aegypti (L.) Sci. World J. 2018
- [CrossRef] [Google Scholar]
- Ethnobotanical Survey of Plants used in the Treatment of Typhoid and its Complication(s) in Esan North East Local Government Area, Uromi, Edo State. Nig J Pharm Res. 2018;14:175-188.
- [Google Scholar]
- Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia. 2008;79:562-568.
- [CrossRef] [Google Scholar]
- A Review of the medicinal values, pharmacological actions, morphological effects and toxicity of ocimum gratissimum linn a review of Ocimum gratissimum LINN. Eur. J. Pharm. Med. Res.. 2020;7:29-40.
- [Google Scholar]
- Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacter baumannii. J. Intercult. Ethnopharmacol.. 2017;6:218-222.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of anti- hemorrhoidal plants in the Bamboutos division, West region of Cameroon. J. Pharmacogn. Phytochem.. 2019;8:2287-2292.
- [Google Scholar]
- In-vitro anti-Mycobacterium tuberculosis effect of essential oil of Ocimum sanctum L. (Tulsi/Basil) leaves. Indian J. Tuberc.. 2021;68:470-473.
- [CrossRef] [Google Scholar]
- Acute oral toxicity of Essential oil extracted from the leaves of Ocimum sanctum L. (Thulasi) in female Wistar albino rats. Res. J. Pharm. Technol.. 2022;15:1827.
- [Google Scholar]
- Chemical composition, In vitro Antimicrobial and antioxidant Activities of Essential Oils of Ocimum gratissimum, O santum and major Constituents. Indian J. Pharm. Sci.. 2013;75:457-462.
- [CrossRef] [Google Scholar]
- Antibacterial efficacy of essential oil of two different varieties of ocimum (tulsi) on oral microbiota-an invitro study. Indian J. Public Heal. Res. Dev.. 2019;10:188-193.
- [CrossRef] [Google Scholar]
- Ethnobotanical tribal practices for mosquito repellency followed by people of north India. J. Pharmacogn. Phytochem.. 2017;6:942-944.
- [Google Scholar]
- Antifungal Activity of the Essential Oils from Ocimum gratissimum L. Grown in Togo. J. Sci. Res.. 2009;1:164-171.
- [CrossRef] [Google Scholar]
- Chemical composition and spectrum of insecticidal activity of the essential oils of Ocimum gratissimum L. and Cymbopogon citratus Stapf on the main insects of the cotton entomofauna in Côte d’Ivoire. Chem. Biodivers.. 2021;18:e2100497.
- [Google Scholar]
- Chemical composition and spectrum of insecticidal activity of the essential oils of Ocimumgratissimum L. and Cymbopogoncitratus Stapf on the main insects of the cotton entomofauna in Côte d’Ivoire. Chem. Biodivers.. 2021;18:e2100497.
- [Google Scholar]
- Étude ethnobotanique, phytochimique et écotoxicologique de Ocimum gratissimum Linn (Lamiaceae) à Cotonou. J. Appl. Biosci.. 2017;109:10609-10617.
- [Google Scholar]
- In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. J. Ethnopharmacol.. 2014;155:1417-1423.
- [Google Scholar]
- The pre-eminence of agro-parameters and chemical constituents in the influence of harvest interval by traits × environment interaction over the years in lemon-scented basil (Ocimum africanum Lour.) Ind. Crops Prod.. 2021;172:113989
- [CrossRef] [Google Scholar]
- A Systemic Review of Ocimum sanctum (Tulsi): Morphological Characteristics, Phytoconstituents and Therapeutic Applications. Int. J. Res. Appl. Sci. Biotechology. 2022;9:121-126.
- [Google Scholar]
- Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem. Toxicol.. 2010;48:539-543.
- [CrossRef] [Google Scholar]
- Assessing the antifungal and aflatoxin B1 inhibitory efficacy of nanoencapsulated antifungal formulation based on combination of Ocimum spp. essential oils. Int. J. Food Microbiol.. 2020;330:108766
- [CrossRef] [Google Scholar]
- Characteristic of aroma components and antioxidant activity of essential oil from Ocimum tenuiflorum leaves. Flavour Fragr. J.. 2022;37:210-218.
- [CrossRef] [Google Scholar]
- Delineating photosynthesis, essential oil yield performance, and their component traits under genotype × traits × environments interactions in Ocimum genotypes. South African J. Bot.. 2021;141:54-65.
- [CrossRef] [Google Scholar]
- Evaluation of yield and agronomic components by triallel cross and selection of high essential oil yielding hybrids in Basil. Ind. Crops Prod.. 2022;177:114486
- [CrossRef] [Google Scholar]
- The nature and extent of heterosis, combining ability under the influence of character associations, and path analysis in Basil (Ocimum basilicum L.) Ind. Crops Prod.. 2023;195:116421
- [CrossRef] [Google Scholar]
- Mosquito repellent activity of volatile oils from selected aromatic plants. Parasitol. Res.. 2017;116:821-825.
- [CrossRef] [Google Scholar]
- Nanoemulsion formulation of ocimum gratissimum essential oil and its antifungal activity against penicillium digitatum. J. Nanosci. Nanotechnol.. 2021;21:3556-3565.
- [CrossRef] [Google Scholar]
- A quantitative ethnobotanical study of common herbal remedies used againST 13 human ailments catergories in mauritius. Afr. J. Tradit Complement Altern Med.. 2014;11:1-32.
- [Google Scholar]
- Morphological variability in holy basil (Ocimum tenuiflorum L.) from India Morphological variability in holy basil (Ocimum tenuiflorum L.) from India. Genet Resour. Crop Evol.. 2015;62:1245-1256.
- [CrossRef] [Google Scholar]
- Investigation of monoterpenoids rich essential oils of two Ocimum basilicum L. varieties at different agro-climatic conditions in India. Acta Ecol. Sin.. 2022;42:1-10.
- [CrossRef] [Google Scholar]
- Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Mol.. 2019;24:3864.
- [CrossRef] [Google Scholar]
- E ff ectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Mole. 2019;24:3864-3880.
- [CrossRef] [Google Scholar]
- Efficacy of Ocimum gratissimum essential oil against the monogenean Cichlidogyrus tilapiae gill parasite of Nile tilapia. Arq. Bras. Med. Veterinária e Zootec.. 2018;70:497-504.
- [CrossRef] [Google Scholar]
- Chemical Composition and Antimicrobial Activity of Essential Oils of Ocimum basilicum, Ocimum canum and Ocimum gratissimum in Function of Harvesting Time. J. Essent. Oil Bear. Plants. 2016;19:1413-1425.
- [CrossRef] [Google Scholar]
- Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genet. Mol. Res.. 2017;16
- [CrossRef] [Google Scholar]
- Antimicrobial activity of essential oils from Lippia sidoides, Ocimum gratissimum and Zingiber officinale against Aeromonas spp. J. Essent. Oil Res.. 2020;33:152-161.
- [CrossRef] [Google Scholar]
- Which Plants for What Ailments : A Quantitative Analysis of Medicinal Ethnobotany of Ile-Ife, Osun State. Southwestern Nigeria. Evidence-Based Complement. Altern. Med.. 2021;2021:5711547.
- [Google Scholar]
- Ethnobotanical survey of malaria prophylactic remedies in Odisha. India. J. Ethnopharmacol.. 2013;146:768-772.
- [CrossRef] [Google Scholar]
- Justification for the use of Ocimum gratissimum L in herbal medicine and its interaction with disc antibiotics. BMC Complementary Alternative Med.. 2009;6:1-6.
- [CrossRef] [Google Scholar]
- Essential Oils from Ugandan Aromatic Medicinal Plants: Chemical Composition and Growth Inhibitory Effects on Oral Pathogens. Evidence-based Complement. Altern. Med.. 2015;2015
- [CrossRef] [Google Scholar]
- Molecular mechanisms of hematological and biochemical alterations in malaria: A review. Mol. Biochem. Parasitol.. 2022;247:111446
- [CrossRef] [Google Scholar]
- Okeke, E.S., Nweze, E.J., Chibuogwu, C.C., Anaduaka, E.G., Chukwudozie, K.I., Ezeorba, T.P.C., 2021. Aquatic Phlorotannins and Human Health: Bioavailability, Toxicity, and Future Prospects. Nat. Prod. Commun. 16, 1934578X2110561. https://doi.org/10.1177/1934578X211056144.
- Atividade antifúngica e possível mecanismo de ação do óleo essencial de folhas de Ocimum gratissimum (Linn.) sobre espécies de Candida. Rev. Bras. Plantas Med.. 2016;18:511-523.
- [CrossRef] [Google Scholar]
- Ocimum gratissimum essential oil and eugenol against Ctenocephalides felis felis and Rhipicephalus sanguineus: In vitro activity and residual efficacy of a eugenol-based spray formulation. Vet. Parasitol.. 2022;309:109771
- [CrossRef] [Google Scholar]
- Preliminary study on mosquito repellent and mosquitocidal activities of Ocimum gratissimum (L.) grown in eastern Nigeria. J. Vector Borne Dis.. 2010;47:45-50.
- [Google Scholar]
- Studies on the acute and sub-chronic toxicity of the essential oil of Ocimum gratissimum L. leaf. Phytomedicine. 2004;11:71-76.
- [Google Scholar]
- Ethnobotanical survey and phytochemical analysis of selected medicinal plants used in treating digestive disorder. J. Med. Plants Stud.. 2020;8:38-42.
- [Google Scholar]
- An Ethnobotanical Study of Medicinal Plants in Atal Nagar (New Raipur) of An Ethnobotanical Study of Medicinal Plants in Atal Nagar (New Raipur) of Chhattisgarh. India. Int. Res. J. Plant Sci.. 2021;12:1-18.
- [CrossRef] [Google Scholar]
- Effect of the Oil Extract of Ocimum gratissimum Leaves on the Reproductive Function and Fertility of Adult Male Rats Effect of the Oil Extract of Ocimum gratissimum Leaves on the Reproductive Function and Fertility of Adult Male Rats. J. Appl. Biol. Sci.. 2010;4:45-48.
- [CrossRef] [Google Scholar]
- Ocimum gratissimum : A Review of its Chemical, Pharmacological and Ethnomedicinal Properties. Open Complement. Med. J.. 2009;1:1-15.
- [Google Scholar]
- Ocimum gratissimum : A Review of its Chemical, Pharmacological and Ocimum gratissimum : A Review of its Chemical, Pharmacological and Ethnomedicinal Properties. Open Complement. Med. J.. 2009;1:1-15.
- [CrossRef] [Google Scholar]
- Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomater.. 2018;2018(8):291.
- [CrossRef] [Google Scholar]
- Historical perspectives of ethnobotany. Clin. Dermatol.. 2019;37:382-388.
- [CrossRef] [Google Scholar]
- Comprehensive Metabolomic Fingerprinting Combined with Chemometrics Identifies Species- and Variety-Specific Variation of Medicinal Herbs: An Ocimum Study. Metabolites. 2023;13:122.
- [CrossRef] [Google Scholar]
- Multifunctional Hydroxyapatite Coated with Arthemisia absinthium Composites. Mol.. 2020;2020(25):413.
- [CrossRef] [Google Scholar]
- Essential Oil Composition, Antimicrobial Potential, Phytochemical Profile and Toxicity of Essential Oils and Crude Extracts of Sweet Basil Prior and After Flowering. Int. J. Biotechnol. Wellness Ind.. 2012;1:85-89.
- [CrossRef] [Google Scholar]
- A comparative study of morphological and anatomical structures of four ocimum species in Uttarakhand, India. J. Drug Deliv. Ther.. 2016;6:1-6.
- [CrossRef] [Google Scholar]
- Medicinal Plants with Traditional Use: Ethnobotany in the Indian Subcontinent. Clin Dermatol. 2018;36:306-309.
- [CrossRef] [Google Scholar]
- Chemical Compositions and Antimicrobial Activities of Ocimum sanctum L. Essential Oils at Different Harvest Stages. Jundishapur J Microbiol.. 2015;8:1-7.
- [CrossRef] [Google Scholar]
- Detection of csgA gene in carbapenem-resistant Acinetobacter baumannii strains and targeting with Ocimum sanctum biocompounds. Iran. J. Basic Med. Sci.. 2021;24:690.
- [CrossRef] [Google Scholar]
- Antimicrobial Potential of Essential Oils from Aromatic Plant Ocimum sp.; A Comparative Biochemical Profiling and In-Silico Analysis. Agron.. 2022;12:627.
- [CrossRef] [Google Scholar]
- Twenty Essential Oils as Ovicidal Agent Against Aedes aegypti (Diptera: Culicidae) Natl. Acad. Sci. Lett.. 2020;43:497-500.
- [CrossRef] [Google Scholar]
- Comparative study of antifungal activities of six selected essential oils against fungal isolates from cheese wagashi in Benin. Pakistan J. Biol. Sci.. 2013;16:1751-1757.
- [CrossRef] [Google Scholar]
- Mosquito repellent and larvicidal perspectives of weeds Lantana camara L. and Ocimum gratissimum L. found in central India. Biocatal. Agric. Biotechnol.. 2021;34:102040
- [CrossRef] [Google Scholar]
- Yield, Composition and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil as Influenced by Distillation Methods. Chem. Divers.. 2017;14
- [CrossRef] [Google Scholar]
- Chemical Profile of Essential oil of Ocimum gratissimum L. and Evaluation of Antibacterial and Drug Resistance-modifying Activity by Gaseous Contact Method. Pharmacogn. J.. 2016;8:4-9.
- [CrossRef] [Google Scholar]
- Seasonal analysis and acaricidal activity of the thymol-type essential oil of Ocimum gratissimum and its major constituents against Rhipicephalus microplus (Acari: Ixodidae) Parasitol. Res.. 2018;117:59-65.
- [CrossRef] [Google Scholar]
- An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu. India. J. Ethnopharmacol.. 2014;153:408-423.
- [CrossRef] [Google Scholar]
- Antimicrobial and Larvicidal Activities of Different Ocimum Essential Oils Extracted by Ultrasound-Assisted Hydrodistillation. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Perspectives on the antibiotic contamination, resistance, metabolomics, and systemic remediation. SN Appl. Sci.. 2021;3:1-13.
- [CrossRef] [Google Scholar]
- Antibacterial activity of Cyperus esculentus (Tiger nut) and Cucumis sativus (Cucumber) against multiresistant bacteria isolates. J Basic Pharmacol Toxicol. 2021;5:14-19.
- [Google Scholar]
- Chemical diversity of essential oil among basil genotypes (Ocimum viride Willd.) across the years. Ind. Crops Prod.. 2021;173:114153
- [CrossRef] [Google Scholar]
- Chemical composition of phenylpropanoid rich chemotypes of Ocimum basilicum L. and their antimicrobial activities. Ind. Crops Prod.. 2022;183:114978
- [CrossRef] [Google Scholar]
- Antimicrobial activity of some Essential oils and Extracts from Natural sources on Skin and Soft tissue infection causing microbes: An In-vitro Study. Res. J. Pharm. Technol.. 2021;14:3603-3609.
- [CrossRef] [Google Scholar]
- Residual toxicity of essential oils from Murraya koenigii and Ocimum tenuiflorum against Spodoptera litura. J. Entomol. Zool. Stud.. 2019;7:905-908.
- [Google Scholar]
- Optimization of Microwave-Assisted Extraction of Essential Oil from Vietnamese Basil (Ocimum basilicum L.) Using Response Surface Methodology. Process. 2018;6:206-217.
- [CrossRef] [Google Scholar]
- Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorg.. 2021;10:61.
- [CrossRef] [Google Scholar]
- Chemical composition and antibacterial activity of essential oil from two Ocimum spp grown in sub-tropical India during spring-summer cropping season. Asian J. Trad. Med.. 2011;6:211-217.
- [Google Scholar]
- Wang, Y., Kong, L., Liu, J., Ma, H., 2018. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains 1–9. https://doi.org/10.1186/s13756-018-0303-7.
- Anti-Malarial Plants of Jonai, India : an Ethnobotanical Approach. Not Sci Biol. 2016;8:27-32.
- [CrossRef] [Google Scholar]
- Ethnobotanical study of Kani tribes in Thoduhills of Kerala, South India. J. Ethnopharmacol.. 2014;152:78-90.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front. Microbiol.. 2016;7
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of medicinal plants used as insects repellents in six malaria endemic localities of Cameroon. J. Ethnobiol. Ethnomed.. 2017;13:1-14.
- [CrossRef] [Google Scholar]
- Phamacognostic study of Ocimum gratissimum Linn.: Pharmafood plant. J. Pharmacogn. Phytochem.. 2014;2:74-79.
- [Google Scholar]
- Repellency and Composition of Essential Oils of Selected Ethnobotanical Plants Used in Western Kenya against Bites of Anopheles gambiae Sensu Stricto. J. Essent. Oil-Bearing Plants. 2020;55–64
- [CrossRef] [Google Scholar]
- Antifungal and Insecticidal Potential of the Essential Oil from Ocimum sanctum L. against Dangerous Fungal and Insect Species and Its Safety for Non-Target Useful Soil Species Eisenia fetida (Savigny, 1826) Plants. 2021;10:2180.
- [Google Scholar]






