Translate this page into:
Silver-platinum bimetallic nanoparticles as heterogeneous persulfate activator for the oxidation of malachite green
⁎Corresponding author. zoya.zaheer@gmail.com (Zoya Zaheer) zzkhan@kau.edu.sa (Zoya Zaheer)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fabrication of noble metal nanoparticles by using green chemical method with plant extract as reducing agent to the in situ oxidation toxic water contaminants have been the subject of various investigators due to their enhanced catalytic efficiencies. This paper describes the extraction of caffeic acid from Artemisia herba-alba aqueous extract, and preparation of silver (Ag), platinum (Pt), and silver-platinum (Ag-Pt) nanoparticles with caffeic acid as a reducing and capping agent. The as-prepared Ag-Pt NPs was used as a heterogeneous activator of persulphate (K2S2O8) for the in situ chemical oxidation of malachite green (MG). The Ag-Pt NPs shows no surface Plasmon resonance absorption band. The malachite green dye was completely decolorized in presence of Ag-Pt/K2S2O8 system. SO4-• and HO• radicals generated by the cleavage of S2O82- peroxide activation with Ag-Pt were responsible for the MG decolorization as well as mineralization. The Ag-Pt NPs acted as a sacrificial electron donor. The Ag-Pt NPs were characterized by using conventional techniques such as, UV–visible, FTIR, SEM, TEM, EDX, XPS, and XRD spectroscopic methods.
Keywords
Ag-Pt
Bimetallic
K2S2O8
Malachite green
Chemical oxidation

1 Introduction
Artemisia herba-alba was known for medicinal and therapeutic properties due to the presence of many bioactive compounds such as flavonoids, sesquiterpenes, and polyphenolic acids. Herbal tea from artemisia herba-alba has been used as analgesic, antibacterial, antispasmodic, antioxidant, hemostatic, and nematicidal agents (Al-Shamaony et al., 1994; Boriky et al., 1996; Laid et al., 2008). Caffeic acid is one of the most water soluble common phenolic acid of Artemisia herba-alba, and used in coffee, tea, olive oil and wine (Iglesias et al., 2009; Gulcin, 2006). The aqueous extract of Artemisia herba-alba increased gastro-intestinal transit time. Generally, the aqueous extract of various parts of natural plants (Iravani, 2011), fruits (Ghodake et al., 2010), green tea (Zaheer et al., 2019), betanin (Kosa and Zaheer, 2019), flowers (Shankar et al., 2003), and biomass (Gardea-Torresdy et al., 2003) were used to the fabrication of nanomaterials. Bakshi and his coworkers reported the synthesis of nanomaterials by using different natural and synthetic stabilizers for different applications (Bakshi, 2014; Bakshi, 2018; Gurtu et al., 2020). The natural biomolecules present in an aqueous extract acted as a capping agents and avoid the necessity of externally added stabilizer for the protection the nanoparticles aggregation (Riedesel et al., 2021).
Advanced oxidation technologies (hydroxyl and sulfate free radical based) are ecofriendly and based on the generation of reactive radical species (HO• and SO4-•). Persulfate is a solid strong oxidant (E0 = 2.01 V) than hydrogen peroxide (E0 = 1.76 V). It is highly stable at ambient temperature, easy to transport and relatively low cost. Generally, transition metals and metal nanoparticles were used as peroxide bond activator to the generation of radical species for the wastewater treatment (Anipsitakis and Dionysiou, 2004; Xu and Li, 2010; Deng et al., 2008; Oh et al., 2009; Al-Shehri et al., 2021; Park et al., 2018). For example, Anipsitakis and Dionysiou were used different metal ions such as Ag+, Co2+, Cu2+, Fe2+, Mn2+, Ni2+, Ce3+, Fe3+, V3+, and Ru3+ to the generation of reactive inorganic radicals by the activation of three oxidants, namely, hydrogen peroxide, potassium peroxymonosulfate, and persulfate (Anipsitakis and Dionysiou, 2004).
Literature contained abundant reports and reviews regarding the applications and importance of using biorenewable resources in biofules from natural biomass (Singh et al., 2022), organic waste reagents in synthetic chemistry (Venkateswarlu et al., 2021), greenhouse gas emissions in BIMSTEC region (Sharma et al., 2021), magnetic nanoparticles for wastewater treatment (Vallinayagam et al., 2021), and biomass derived catalyst for the oxidation of α-hydroxy ketones (Naidu et al., 2022). The reduced graphene oxide/ZnFe2O3 (Rani et al., 2017), graphene oxide(RGO)/Fe3O4 (Vinothkannan et al. 2015) nanocomposites were prepared and used as an efficient catalyst for the degradation of methylene blue under different experimental conditions. The bio-assisted, β-cyclodextrin-functionalized, and piper betle biomass capped silver NPs were prepared and used as sensor for the colorimetric and selective detection of copper(II), melamine, and nitrite, respectively (Kirubaharan et al., 2012; Xavier et al., 2014; Ramachandaran et al. 2016). The carbon nanodots were prepared using lemon and utilized for the selective and sensitive detection of Fe(III) ions (Xavier et al., 2019). Bio-assisted synthesis of platinum (Mohamadi et al., 2020), silver (Asadi et al., 2018), and CdO NPs (Kaveh et al., 2021) were reported by using the natural plant extract. The platinum and CdO NPs were used as sensor for the detection of hydrogen peroxide and quantification of cefixime, respectively.
Xu and Li reported the degradation of orange G azo dye using HO• reactive oxygen species generated by K2S2O8/ferrous ions (Xu and Li, 2010). These investigators also discussed the role of inorganic salts (Cl-, NO3–, HCO3–, and H2PO4-) on the rates of dye degradation. Co3O4-N doped carbon nanotubes were used as persulfate activation catalyst to the in situ chemical decomposition of azo dyes (Jiang et al., 2020). Various mono-, and bimetallic nanomaterials such as Co3O4, Co(OH)2, CuO, Fe3O4, ZnO, CuFe2O4, Mn3O4, CoFe2O4, MnFe2O4, and carbon nanotubes have been used to the generation of SO4-•, and HO• radicals for the removal of wastewater contaminants (Yao et al., 2013; Al-Anazi et al., 2018; Lee et al., 2015; Zhu et al., 2013; Zhu et al., 2011). Ultrasound radiations combined with heterogeneous titanium oxide and homogenous photocatalysis method was used for the oxidation of MG in water (Berberidou et al., 2007). Hameed and Lee reported the degradation of malachite green by using Fenton’s reagent (Fe2++ H2O2) for the generation of HO• reactive species (Hameed and Lee, 2009). Wu et al. utilized the iron NPs as a catalyst for the heterogeneous oxidation of MG with H2O2 (Wu et al., 2015). The Fe3O4/graphene aerogels and CuFe2O4@biochar composite were used as S2O82- activators for the degradation of MG (Lu et al., 2020; Huang et al., 2021). The use of Ag-Pt bimetallic as a heterogeneous S2O82- activator has not been reported in the literature for the in situ chemical oxidation of toxic MG at room temperature.
Caffeic acid (3,4-dihydroxycannamic acid) isolated from the aqueous extract of Artemisia herba-alba at room temperature, and used for the fabrication of Ag, Pt, and Ag-Pt NPs for the first time. We determined the catalytic efficiency of Ag-Pt in the heterogeneous activation of K2S2O8 for the degradation of MG (triphenyl methane basic dye, widely used in food and dyeing industries as coloring agent, and difficult to remove from wastewater). The main objectives of this study were to provide (i) simple cost effective green method for the synthesis of Ag-Pt NPs using natural medicinal plant and (ii) insights into the kinetics of dye degradation by SO4-• based advanced oxidation processes under environmentally relevant conditions. Bimetallic Ag-Pt acted as an excellent heterogeneous S2O82- activator for the in situ chemical oxidation of MG at 25 °C within 50 min of reaction time.
2 Experimental section
2.1 Materials
The aerial parts of Artemisia herba-alba were collected from plants growing wild in Yanbo region of Saudi Arabia, dried in dark at room temperature, ground to fine powder by using an electric mill, and conversed in a desiccator. Metal salt precursor (silver nitrate and potassium tetrachloroplatinate (K2PtCl4, molar mass = 415.09 g/mol ≥ 99.9%)), potassium persulfate, malachite green, sodium hydroxide, and other chemicals were used as received. Deionized water was used as solvent to the preparation of all reagents solution. Silver nitrate solution was stored in a brown glass container to prevent the photochemical reactions. Methanol, tertiary butyl alcohol, and potassium iodide were employed to scavenge the formation of reactive radical species.
2.2 Extraction of caffeic acid
For the extraction of caffeic acid, the 10.0 g of dry powder of Artemisia herba-alba plant was taken in a stoppered round bottom reaction vessel flask. The 250 mL of deionized water into the vessel, and the solution was heated for 60 min at 70 °C with constant stirring. The resulting reaction mixture was kept for 24 h at 25 °C for complete extraction of caffeic acid, filtered through a Whatmam filter paper, and pale yellow color solution stored in an amber glass container until analyzed. The photo images of Artemisia herba-alba, dry plant, and resulting pale yellow extract are given in Scheme 1. UV–visible spectra of pale yellow color was measured in the range of 200 to 800 nm on the UV–visible spectrophotometer, and compared with an authentic sample of caffeic acid purchased from the Sigma-Aldrich.
Photo images (green and dry) Artemisia herba-alba plant and its water extract.
2.3 Synthesis of Ag, Pt, and Ag-Pt NPs
Green chemical reduction method was utilized for the synthesis of water soluble silver and platinum sols at room temperature. Artemisia herba-alba plant water extract was employed as reducing and capping agent (Zaheer et al., 2019). In order to prepare water soluble silver sols (Ag NPs), the required amount of silver nitrate and an aqueous extract of plant were placed into the vial, and required volume of deionized water was added for dilution. The pale yellow to dark brown color appeared as the time increases. The resulting brown color stored in a container. We did not observe any type of turbidity and precipitate during the storage time. For PtNPs, the aqueous solution of potassium tetrachloroplatinate (5.0 mL of 0.01 M) was added into the solution of aqueous extract. The reaction mixture was stirred for 1 h at room temperature, and solid PtNPs were obtained after the centrifugation. Seedless co-reduction method was used for the synthesis of Ag-Pt NPs (Zaheer et al., 2019). The solutions of metal salts precursors (silver nitrate and potassium tetrachloroplatinate) were added into the aqueous extract, and heated for 1 h at 40 °C. The solid Ag/Pt was collected by using centrifugation.
2.4 Morphology determination
Surface morphology and elemental composition of Ag, Pt, and Ag-Pt NPs were determined by recording the SEM images and EDX of as-prepared NPs. The size and the size distribution were determined from the transmission electron microscope. Rigaku X-ray diffractometer equipped with a peltier-cooled solid state detector was used to determine the x-ray diffraction patterns (XRD). UV– visible spectra of an extract, Ag, Pt, and Ag/Pt NPs were measured on a Shimadzu UV–vis multi Spec-1501 spectrophotometer. The FT-IR spectra were recorded with Varian FTIR-640 spectrometer to establish the capping action of caffeic acid in-situ fabrication of Ag/Pt NPs. Shimadzu TOC-VCPN equipped with IR spectrometer for carbon dioxide quantification was used to the measurements of total organic carbon of decomposed MG.
2.5 Degradation of MG dye
The dye degradation experiments were performed in 40 mL reactor. The K2S2O8 solution (16.0 mL of 0.01 mol/L) was added to the reactor, then the dye solution (5.0 mL of 0.01 mol/L), then the Ag-Pt (activator, 16 mg/L), and deionized water (19.0 mL) for dilution. The pH of the solution was adjusted with standard hydrochloric acid and sodium hydroxide. At a given time intervals, the sample aliquots were taken from the reactor and sodium azide (1.0 mL of 0.01) was added to quench the oxidation of dye (Lau et al., 2007). A calibration plot based on the Beer-Lambert’s law (absorbance at 620 nm versus [MG]) was prepared at 620 nm by recording the absorbance to standard malachite green solution concentration. For the range of concentrations, a linear relationship between absorbance and concentration was obtained. The concentration of unreacted MG was calculated from the absorbance at 620 nm (wavelength maxima of MG). All measurements were performed at a room temperature (25 °C) unless otherwise stated. Several sets of batch experiments (such as effects of dye concentration, pH, persulfate concentration, temperature and dosage of Ag/Pt NPs) were carried out to establish the role of each parameter in the dye degradation. Eq.(1) was used to determine the degradation percentage of malachite green.
The pseudo-first order rate equation was used for the calculation of degradation rate constant (kapp) (Eq. (2) (Pouretedal and Keshavarz, 2010).
For the stability determination of the activator, Ag-Pt was separated from the reaction mixture by using centrifugation after each degradation experiment, washed with deionized water, ethanol several times, and then dried in oven at 60 °C for another kinetic runs under the similar experimental conditions.
3 Results and discussion
3.1 UV–visible spectra of aqueous extract
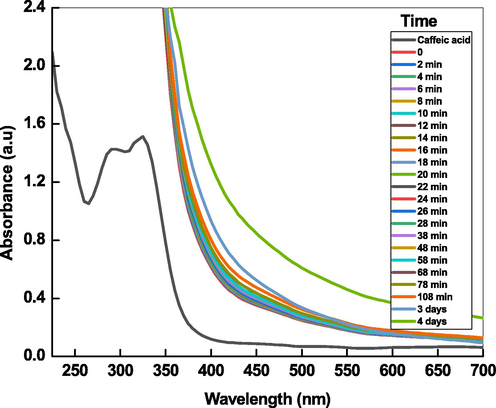
Fig. 1A shows the UV–visible spectra of the pale yellow color (extracted from the Artemisia herba-alba), which exhibits two absorption bands at 289 and 326 nm. These peaks attributed to the π → π* transitions of –COOH in the caffeic acid and an aromatic benzene moiety (Swislocka, 2013). The calibration plot was constructed between the absorbance at 326 nm and [caffeic acid] by using Beer-Lambert law. The molar extinction coefficient was determined from the calibration plot (Fig. 1B) and found to be 15,782 L/mol/cm. FT-IR spectroscopy proved to be a useful tool to get structural information of organic compounds. The FT-IR spectra of isolated and authentic caffeic acid were recorded for the comparison (Fig. 1C). The broad and intense peaks at 3440 cm−1 and 3135 cm−1 were ascribed to the –OH and carbon–carbon single bond stretching vibrations. The strong band at 1763 cm−1 assigned to the stretching vibrations of carbonyl group of caffeic acid. For aromatic and carbon–carbon double bonds stretching vibrations, the peaks were observed at 1384 cm−1, 1269 cm−1 and 1080 cm−1 (Swislocka, 2013).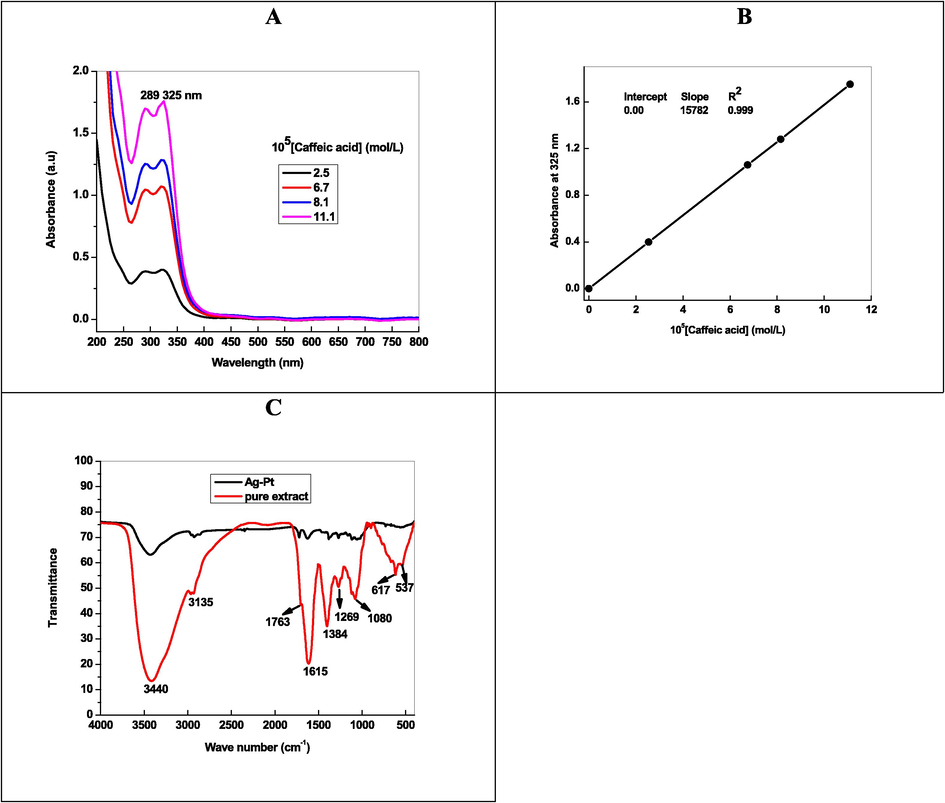
UV–visible spectra of (A), Beer-Lambert plot (B), and FT-IR spectra of caffeic acid extracted from Artemisia herba-alba.
3.2 Preparation of metal NPs
UV–visible spectra of Ag and Pt NPs were recorded at different time intervals to establish the stability of NPs. Fig. 2A shows that the spectra of AgNPs has intense broad absorption band from 400 to 600 nm, which indicates the formation of AgNPs with mixed morphology (spherical, triangular, and truncated triangular. Inspection of Fig. 2A clearly indicates that the intensity of caffeic acid peaks decrease with time and new absorption peak was appeared. Table 1 shows the effects of different parameters ([metal ions], and [caffeic acid]) on the appearance, position of SPR band and stability of Ag NPs. In order to see the effect of storage time, optical images of AgNPs recorded as a function of time (Scheme 2). These visual observations can be ascribed due to the formation of colored silver sols during the redox reaction between silver ions and caffeic acid.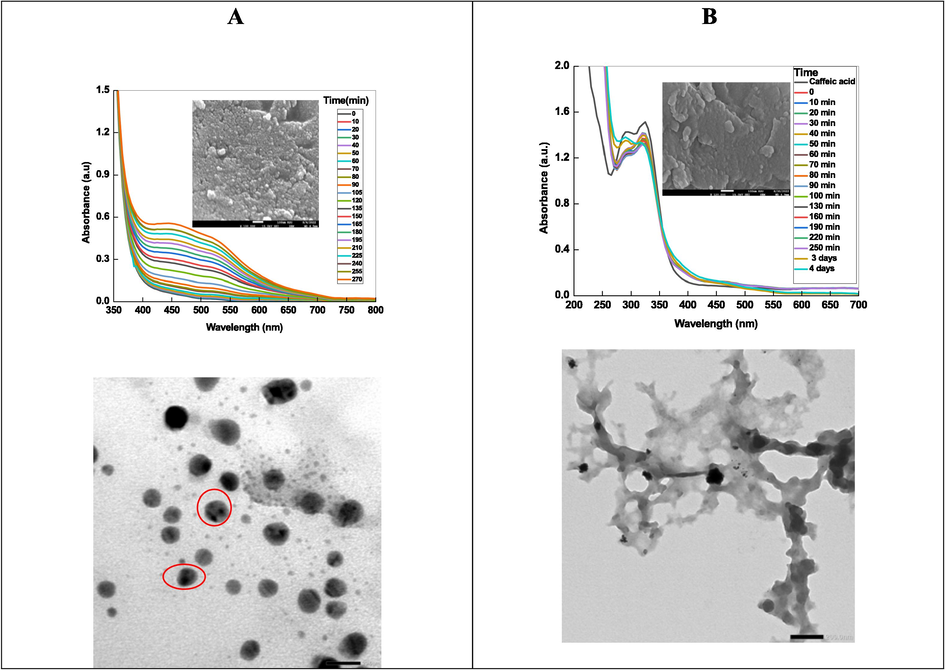
Time resolved spectra Ag NPs (A) and Pt NPs (B) along with TEM images. In set- SEM images for both NPs.
[Caffeic acid] (mM)
[Metal ions] (mM)
Optical properties of NPs
0.15
Ag+ 1.0
Pale yellow, stable, SPR band = 400–600 nm, Ag0
0.15
Ag+ 2.0
Yellow, stable, SPR band = 400–600 nm, Ag0
0.25
Ag+ 2.0
Brown, stable, SPR band = 400–600 nm, Ag0
0.40
Ag+ 2.0
Brown red, stable, SPR band = 400–600 nm, Ag0
0.15
PtCl42- 1.0
Pale yellow, stable, no SPR band, Pt0
0.15
PtCl42- 2.0
Pale gray, stable, no SPR band, Pt0
0.15
PtCl42- 3.0
Gray, stable, no SPR band, Pt0
0.25
PtCl42- 2.0
Gray, stable, no SPR band, Pt0
0.40
PtCl42- 2.0
Gray, stable, no SPR band, Pt0
0.15
Ag++ PtCl42- 1.0
Pale yellow, stable, no SPR band, Ag-Pt
0.15
Ag++ PtCl42- 2.0
Yellowish gray, stable, no SPR band, Ag-Pt
0.15
Ag++ PtCl42- 3.0
Dirty gray, stable, no SPR band, Ag-Pt
0.25
Ag++ PtCl42- 2.0
Dirty gray, stable, no SPR band, Ag-Pt
0.40
Ag++ PtCl42- 2.0
Dirty gray, stable, no SPR band, Ag-Pt
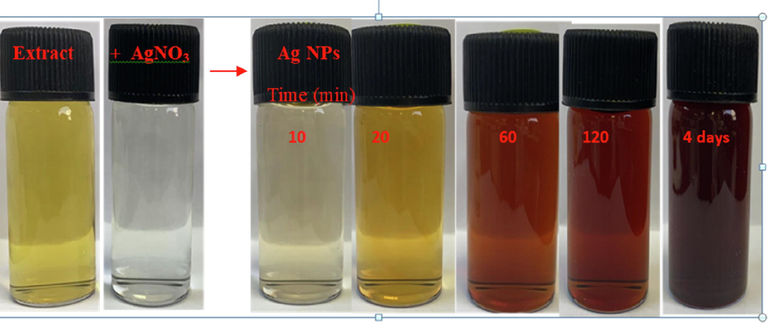
Visual observation to the formation of AgNPs by silver ions-caffeic acid redox reaction at room temperature.
The resulting silver sols became perfect transparent and no precipitate was observed during the observations, indicates that the AGNPs was capped with caffeic acid, which has strong tendency to formed complex with metal ions (Scheme 3) (Cornard et al., 2006).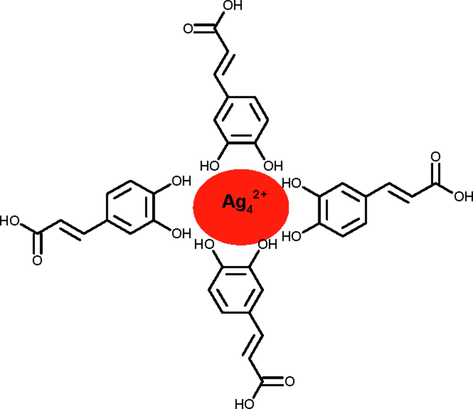
Capping action of caffeic acid with AgNPs.
Fig. 2B shows the time-resolve UV–visible spectra of Pt NPs. The absorption intensity of caffeic acid at 326 nm decreases after the addition of an aqueous solution of [PtCl4]2-, which suggests formation of Pt0 by the reduction of [PtCl4]2- with caffeic acid (Eq. (3). UV–visible spectra of metallic Pt0 was featureless in the entire visible region (Table 1). Our results are in accordance to the suggestion of Creighton and Eadon regarding the UV–visible spectra of Pt NPs (Creighton and Eadon, 1991).
Optical images of caffeic acid, [PtCl4]2-, and Pt NPs are given in Scheme 4.
Optical images of Pt NPs after 120 min at 30 °C.
Ag-Pt bimetallic NPs were fabricated using seedless co-reduction method under various concentration of caffeic acid and metal ions precursor (Table 1). In a typical experiment, an equimolar solutions of silver nitrate and [PtCl4]2- (2.0 mM) were added into the conical flask containing the 0.11 mM caffeic acid at 22 °C. The progress of the redox reaction was monitored by recording the UV–visible spectra of Ag/Pt NPs with time. Surprisingly, no SPR band was appeared in the vicinity of 400 to 600 nm, which is the characteristic of mono-metallic AgNPs, indicating the formation of Ag-Pt NPs. Optical images of reaction mixture containing silver nitrate, [PtCl4]2-, and caffeic acid were recorded. The colorless solution became pale yellow to gray-orange, which indicates the formation of Ag-Pt NPs (Scheme 5).
Optical images of Ag-Pt NPs formation as a function of time.
In a seedless co-reduction of Ag+ and [PtCl4]2- by caffeic acid, there is a competition between the metal ions to gains electrons from the reducing agent (Goia and Matijevic, 1998); Cheng et al., 2012). The Ag+ ions were first reduced by caffeic acid to generate Ag NPs, because Ag+ ions had a higher reducing potential (reduction potential (E0) = 0.799 V for Ag+/Ag0) than [PtCl4]2- ions (reduction potential (E0) = 0.755 for [PtCl4]2-/Pt0). Consequently, the [PtCl4]2- ions would be reduced on the surface of the Ag NPs under potential deposition (Liu et al., 2014). Inspection of Fig. 3 clearly suggests that the color and position of SPR band of the resulting Ag-Pt NPs were dominated by the outer surface of the Pt NPs. Thus we may state confidently that the Ag-Pt NPs were formed during the seedless co-reduction of silver and [PtCl4]2- ions by caffeic acid (Eqs. (4) and (5) (Liu et al, 2014).
Time resolved spectra of Ag-Pt NPs.
[PtCl4]2- ions was deposited on to the positive surface of AgNPs via electrostatic and van der Waals interactions (Eq. (4)). In the next step, [PtCl4]2- ions were reduced under potential deposition, and Ag-Pt NPs were formed (Eq. (5).
3.3 Morphology of NPs
Surface morphology and elemental composition of Ag, Pt, and Ag-Pt NPs were determined by recording the SEM images and EDX of as-prepared NPs. Fig. 2A and 2B (in set) shows the SEM images with UV–visible spectra of Ag, and Pt NPs, respectively. The particles are spherical in shape and aggregated to each other. Ag/Pt NPs are also spherical in shape and formed a layered sheet like structure due to the deposition of one metal onto the other (Fig. 4, indicated by red circle). Such type of morphology was not observed in case of mono-metallic Ag and Pt NPs. TEM image of AgNPs indicates the formation spherical, triangular, irregular, and poly-dispersed NPs (Fig. 2A TEM image), while the PtNPs are interconnected to each other and formed beautiful chain of metallic Pt (Fig. 2B, TEM image). For Ag-Pt NPs, small granules adsorbed onto the surface of each other, and leads to the formation of large size NPs. Inspection of Fig. 4 TEM image suggests that the grains of metals formed a flower like morphology of metallic Ag0 and Pt0 at 20 nm scale. High resolution TEM image clearly indicates the presence of both metals in Ag-Pt NPs (Fig. 4). EDX spectra and percentage of elements present in all samples are given in Fig. 5, which shows the presence of Ag, Pt, and Ag-Pt along with the carbon and oxygen. The XRD patterns of AgNPs, PtNPs and Ag-PtNPs are given in Fig. 6A, which clearly suggests the formation of pure Ag, Pt, and Ag-Pt NPs. For AgNPs, the diffraction peaks observed at 2θ = 42.2 (1 1 1), 54.6 (2 0 0), 63.5 (2 2 0), and 77.40 (3 1 1) planes of Ag0 (Fig. 6A). For Pt NPs, three peaks located as 2θ = 39.80, 46.70, and 67.60, which are the characteristic of the (1 1 1), (2 0 0), and (2 2 0) diffraction planes of the crystalline Pt0 NPs, respectively, (JCPDS card no. 04–0802). For Ag-Pt NPs, all diffraction peaks are merged together, which indicates the formation of Ag-Pt NPs (Fig. 6A). The average crystallite size of Ag-Pt NPs was determined with Debye-Scherrer equation (D = C λ / β cosθ, where D = size in Å, C = shape factor constant equal to 0.94, λ = 1.5418 Å, β = full-width at half-maximum in radians, and θ = Bragg’s diffraction angle for the peak in degrees). The size of Ag-Pt was found to be 20 nm.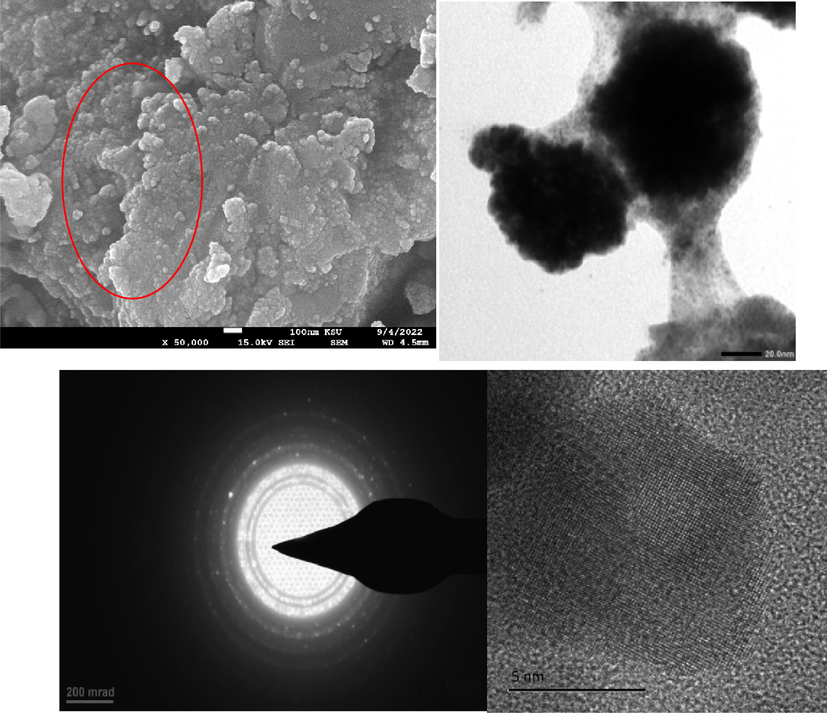
SEM and TEM (high resolution, and corresponding lattices) images of Ag-Pt NPs.
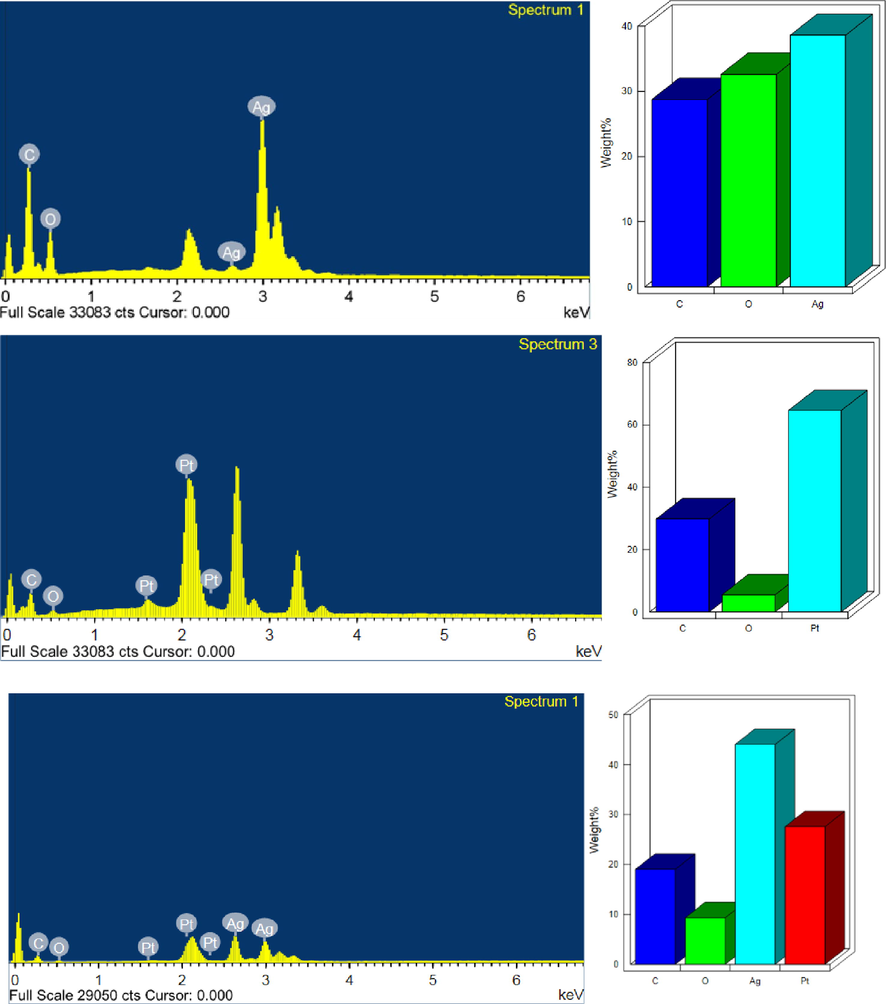
EDX spectra of Ag (A), Pt (B), and Ag-Pt NPs (C).
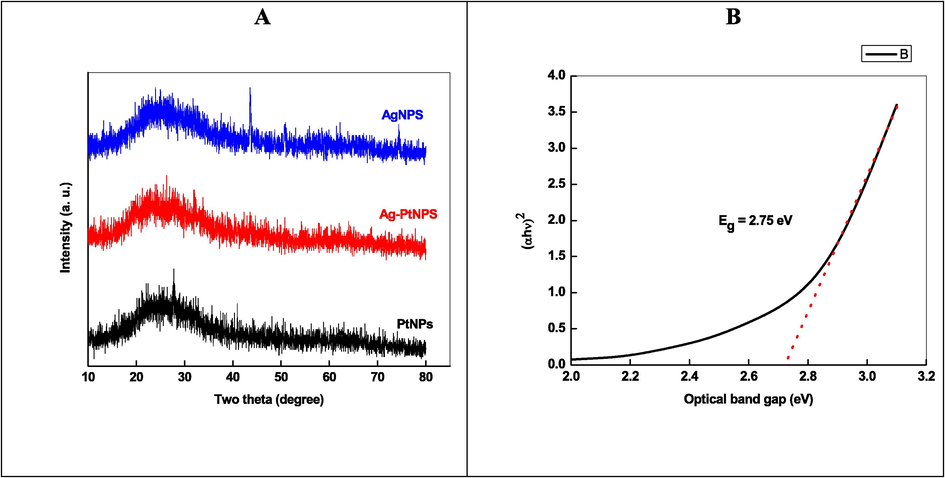
XRD (A), and optical band gap plot (B) of Ag-Pt NPs.
From the UV–visible spectrum of Ag-Pt NPs, the optical band gap (Eg) was calculated with (Eq. (6)).
In order to determine the oxidation states of Ag and Pt in the Ag-Pt NPs, XPS analyses were carried out. The XPS spectra for survey, Ag 4d, Pt 4f, O1s, and C 1 s are shown in Fig. 7A. The overall spectrum consists the main characteristic peaks appeared at the binding energy of Pt 4f at 75.98 eV, O 1 s at 533.68 eV, Ag 3d at 369.17 eV, and C 1 s at 286.15 eV. As displayed, the Ag 3d spectrum can be deconvoluted into two doublet at 368.23 and 374.18 eV (spin–orbit separation = 6.0 eV) corresponds to 3d5/2 and Ag 3d3/2, respectively, for metallic Ag state (Fig. 7B). The high resolution spectrum was recorded in the Pt 4f (4f5/2 and 4f7/2) region. The doublet peaks at 80.1 eV and 87.67 eV with spin–orbit separation of 3.67 ev, which are ascribed to the binding energies of metallic Pt 4f7/2 and Pt 4f5/2, respectively (Fig. 7C). The high resolution XPS peak of C 1 s, and O 1 s, respectively, at 286.23 eV, and 533.68 eV were observed (Fig. 7D and 7E).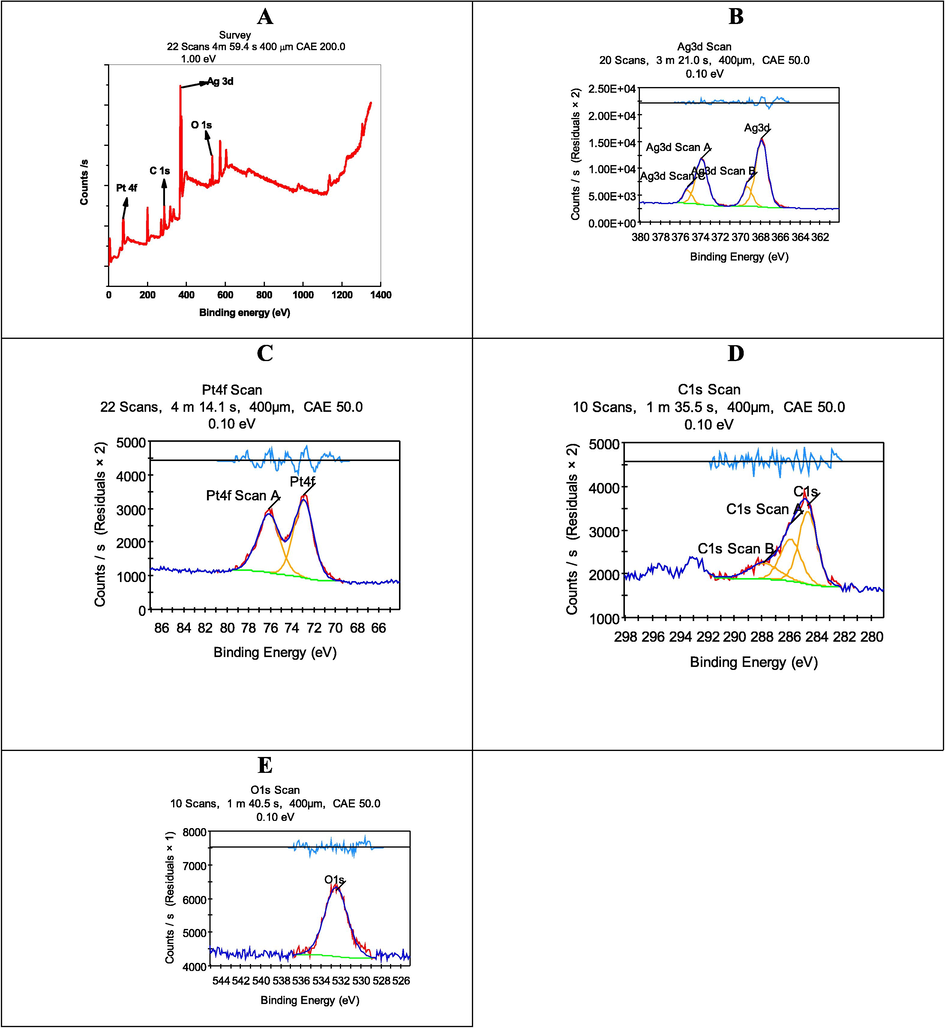
XPS of Ag-Pt NPs.
3.4 Degradation of MG
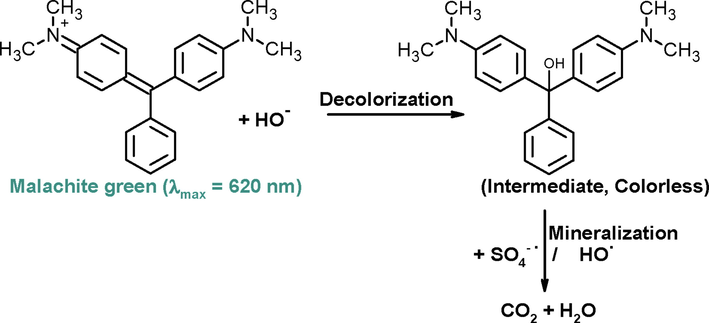
UV–visible spectra of MG exhibits three absorption band at 314, 424 and 620 nm in water (Kitching et al., 2014). Out of these, the absorbance was maximum at 620 nm. Therefore, degradation of green color of dye was monitored with Ag-Pt as activator for K2S2O8 as a model dye by advanced oxidation processes (Scheme 6).
Structure of MG.
In order to see insight into the role of Ag-Pt and K2S2O8 on the degradation of MG, a series of experiments was performed with various conditions for example: (1) MG + Ag-Pt NPs, (2) MG + K2S2O8 and (3) MG + Ag-Pt + K2S2O8 (Table 2). It was observed that the degradation of dye was not occurred with Ag-Pt NPs and/or K2S2O8. The optical images demonstrate that no significant MG oxidation takes place with Ag-Pt and K2S2O8. As shown in Fig. 8, when small amount of K2S2O8 (4.0 mM) was added to the reaction mixture of MG and Ag-Pt, the absorbance at 620 nm decreases with reaction time, and green color reaction solution became colorless within 50 min. The spectral changes indicate that the peroxide bond of K2S2O8 activated by Ag-Pt NPs, and reactive radical species generated, which decolorized and mineralized the MG. These observations are in good agreement to the results of other investigators (Gokulakrishnan et al, 2012; Lu et al. 2020) regarding the heterogeneous oxidation of MG with activated persulfate by different methods. The primary intermediate(s) and degradation by products of dye depends on the nature of the reactive radical species as well as method of the oxidation (chemical and biochemical) (Saquib and Muneer, 2003; Azmi and Sani, 1998). Benzophenone derivatives, benzaldehyde, benzene, nitrobenzene, p-aminobenzoic acid, aniline, and others were identified as the intermediate(s) degradation products of MG under different experimental conditions (Berberidou et al. 2007; Gokulakrishnan et al, 2012; Lu et al. 2020; Saquib and Muneer, 2003).
Ag-Pt (mg)
[MG] (mM)
[K2S2O8] (mM)
pH
Temperature (0C)
kapp (min−1)
R2
6.0
1.25
4.0
3.0
25
0.016
0.996
10.0
1.25
4.0
3.0
25
0.024
0.996
12.0
1.25
4.0
3.0
25
0.038
0.992
16.0
1.25
4.0
3.0
25
0.055
0.994
16.0
0.07
4.0
3.0
25
0.092
0.994
16.0
1.0
4.0
3.0
25
0.081
0.991
16.0
2.0
4.0
3.0
25
0.036
0.993
16.0
2.5
4.0
3.0
25
0.012
0.995
16.0
1.25
4.0
4.0
25
0.078
0.995
16.0
1.25
4.0
7.0
25
0.095
0.997
16.0
1.25
4.0
8.0
25
0.062
0.998
16.0
1.25
4.0
10.0
25
0.032
0.992
16.0
1.25
1.0
3.0
25
0.022
0.996
16.0
1.25
6.0
3.0
25
0.082
0.998
16.0
1.25
7.0
3.0
25
0.043
0.992
16.0
1.25
4.0
3.0
35
0.082
0.994
16.0
1.25
4.0
3.0
45
0.123
0.994
16.0
1.25
4.0
3.0
55
0.162
0.996
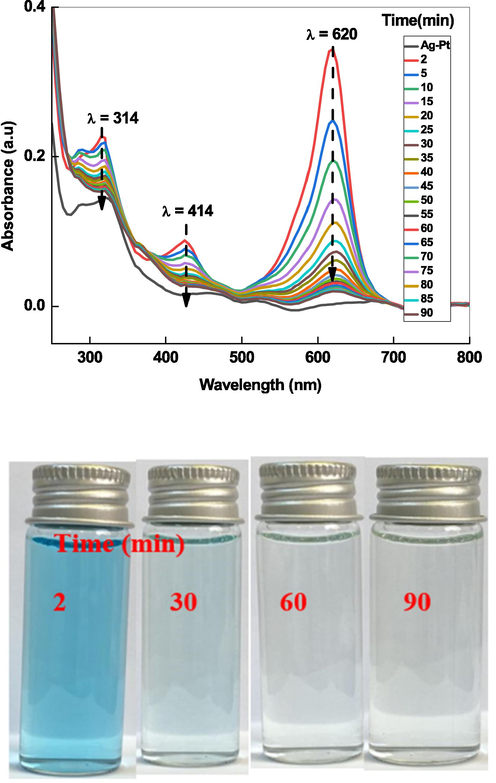
UV–visible spectra of MG oxidized by K2S2O8/Ag-Pt system at room temperature.
The effects of Ag-Pt concentration (=8.0 mg to 16.0 mg) on MG decolorization were investigated at fixed [MG] = 1.25 mM, [S2O82-] = 4.0 mM, and temperature = 30 °C, and the results obtained are presented in Fig. 9A. The Mg degradation % was significantly increased over the concentration of Ag-Pt dosage. The degradation efficiency was also increases with the amount of the activator (Table 2). The 94.2% dye was decomposed at 16 mg of Ag-Pt after 50 min of reaction time. The effects of MG concentration were investigated at different initial concentrations in the range of 0.75 to 2.5 mM at fixed amount of activator (16 mg/L), pH (7.0) and temperature (25 °C). As shown in Fig. 9B, the rate of dye degradation decreases with increasing initial concentration of MG, which was also reported in other metal NPs activated persulfate oxidation of toxic water contaminants (Zhang et al., 2020; Liang et al., 2013; Hu et al., 2019). The degradation of MG was also investigated over the pH range of 4 to 10 with fixed concentration of dye (1.25 mM), amount of Ag-Pt (16 mg), K2S2O8 (4.0 mM) at 30 °C. The following sequence in the degradation of MG in the S2O82-/Ag-Pt combined system at different initial pH was observed: pH 7 > pH 4 > pH 8 > pH 13 > pH 10 (Fig. 9C). The neutral aqueous medium was the optimal initial pH for the dye degradation in the S2O82-/Ag-Pt. At low and high pH, the decrease in the oxidation capacity of S2O82- has been attributed to the involvement of SO4-• in other reactions (Liang et al., 2013; Al-Shehri et al., 2021).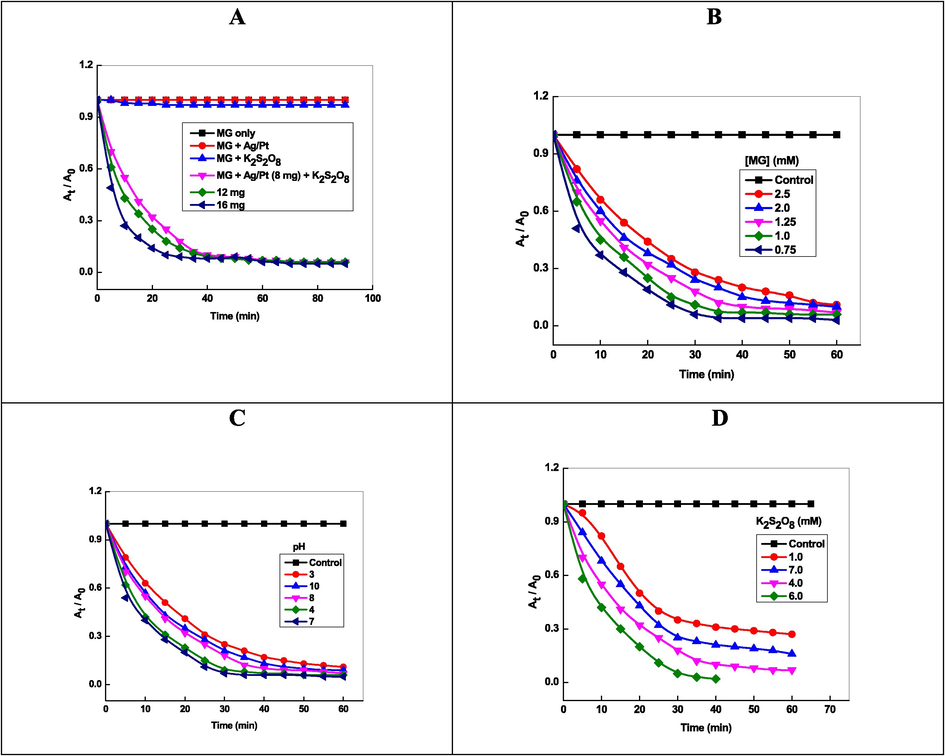
Effects of Ag-Pt dosage (A), dye concentration (B), initial pH (C), and temperature (D) for activating K2S2O8 to decolorize MG.
The complete degradation of MG was observed at lower S2O82- concentration (≥1.0 mM). The rate of degradation increased with increasing S2O82- from 1.0 to 6.0 mM. On further increasing the S2O82- concentration from 7.0 mM, decreased in the MG decolorization (Fig. 9D). The degradation rate increases and decreases with the [S2O82-], which indicates the participation of S2O82- in SO4-• generator (S2O82- + Heat/ metal NPs → 2SO4-•) and SO4-• scavenger (2SO4-• →S2O82-). For [S2O82-] = 4.0 mM, the kapp and % degradation were found to be 0.055 min−1 and 94.2 %, respectively. The efficiency of % degradation depends on the persulfate activator (metal ions, metal NPs, temperature, UV light), structure of dye, and pH (Behnajady et al., 2008; Colonna et al., 1999; Deng et al., 2013).
The K2S2O8 was activated by heat and generates reactive radical species. In order to study to effect of temperature on the cleavage of peroxide bond of K2S2O8 and degradation of malachite green, the effect of temperature was carried out at 25, 35, 45 and 55 °C. The rate of degradation increased from 0.055 min−1 to 0.162 min-1as a consequence of increasing the temperature from 25 to 55 °C (Table 2). This is because of higher temperature increased the rate of decomposition of peroxide linkage of K2S2O8, thus increasing the rate of generation of reactive radical species such as SO4-• and HO•, which in turn, enhanced the degradation of malachite green. The Arrhenius equation (lnkapp = lnA – Ea/ RT, where kapp = rate constant, Ea = activation energy, R = gas constant, and T = temperature in Kelvin) was used for the calculation of activation energy. From the slope of the lnkapp versus 1/ T, the activation energy is found to be 29.7 k J mol−1 with R2 = 0.996. The lower value of activation energy suggests that the two effects, namely, temperature as well as Ag-Pt NPs operates simultaneously and activates the cleavage of peroxide bond (Ahn et al., 2019). Table 3 shows that the degradation efficiency was found to be 0.0% 55.2%, 94.2%, respectively, for Pt, Ag, and Ag-Pt NPs in 30, 50, and 50 min in presence of 4.0 mM S2O82-. The best S2O82- activator for MG oxidation was found to be Ag-Pt NPs [Ag-Pt ˃ Ag0 ˃ Pt0], which might be due to the synergistic effect of both metals. The bimetallic system was more promising than monometallic for the heterogeneous activation of S2O82- (Al-Shamsi, et al., 2013). The second metal serves as an additional electron donor, increase the surface area of monometallic NPs by depositing a non-uniform layer on the surface of the NPs, and form a galvanic corrosion system on the surface of NPs, which enhances the catalytic ability of the NPs (Zhang, et al., 1998).
Catalysts
Conditions
Results
Ref.
Fe73.5Si13.5B9Cu1Nb3
[MG] = 22 ppm, catalyst = 0.4 g/L, [S2O82-] = 1.0 mM, UV–visible irradiation
Degradation = 100%,Time = 30 min, Rate constant = 0.0849 min−1
Liang, et al., 2017
Fe3O4/graphene
[MG] = 20 mg/L, [S2O82-] = 1.0 mM, catalyst = 0.2 g/L, T = 25 °C, pH = 3.0,Sun light
Degradation = 91.7%,Time = 12 min, Rate constant = 0.213 min−1
Lu et al., 2020
Fe3O4/graphene
[MG] = 20 mg/L, [S2O82-] = 1.0 mM, catalyst = 0.2 g/L, T = 25 °C, pH = 3.0, dark
Degradation = 82.18Time = 12 min, Rate constant = 0.1474 min−1
Lu et al., 2020
CuFe2O4@biochar
[MG] = 100 mg/L, [S2O82-] = 0.25 mM, catalyst = 0.2 g/L, T = 25 °C, pH = 7.0
Degradation = 98.9%,Time = 90 min, Rate constant = 0.040 min−1
Huang et al., 2021
Ni(II) perchlorate complex
[MG] = 10 mg/L, [S2O82-] = 1 g/L, complex = 200 μM, pH = 7.0
Degradation = 97.9%,Time = 60 min, Rate constant = 0.93 min−1
Gokulakrishnan et al., 2012
CuO
[MG] = 10 μM/L, [S2O82-] = 250 μM/L, catalyst = 200 mg/L, pH = 7.0
Degradation = 79.07%, Time = 60 min
Qi et al., 2017
Ag-Pt
[MG] = 1.25 mM, [S2O82-] = 4.0 mM, catalyst = 16 mg/L, T = 25 °C, pH = 7.0
Degradation = 94.2%,Time = 50 min, Rate constant = 0.095 min−1, Ea = 29.7 kJ mol−1
Present work
Ag0
[MG] = 1.25 mM, [S2O82-] = 4.0 mM, catalyst = 16 mg/L, T = 25 °C, pH = 7.0
Degradation = 55.2%,Time = 50 min
Present work
Pt0
[MG] = 1.25 mM, [S2O82-] = 4.0 mM, catalyst = 16 mg/L, T = 25 °C, pH = 7.0
Degradation = 0.0%,Time = 30 min
Present work
The degradation efficiency percentage was evaluated from Eq.(1) and found to be increased with reaction time at fixed concentrations of other parameters. The 94.2% MG was degraded in 50 min (Fig. 10A), and green color of dye disappeared completely after 90 min (Fig. 8 optical images. Fig. 8 shows that the some organic intermediate(s) are present in the residual solution for longer reaction time. This indicates that a lower mineralization efficiency is attained as a result of the existence of small toxic oxidation products of the used dye. It has been established that the decolorization and mineralization (formation of CO2 and water) were the two different steps of the complete dye decomposition (Aleboyeh et al., 2008; Lu et al., 2020; Huang et al., 2021). Fig. 10B shows the decay of absorbance at 320 nm (related to the absorbance of poly aromatic rings), and 620 nm (related to the destruction of conjugated system of dye) for the mineralization and decolorization of dye, respectively (Behnajady et al., 2008). At 620 nm, the absorbance decreases from 0.34 to 0.036 (94.2% degradation) after 50 min of reaction time and remained unchanged approximately after it, which might be related to the destruction of conjugated system of MG green as well as N-demethylation processes. On the other hand, absorbance decreases from 0.22 to 0.016 (60 % degradation) at 320 nm after the same reaction time, which can be ascribed due to the destruction of benzene moieties of dye. The mineralization efficiency was monitored by recording the change in the total organic content of MG. The mineralization efficiency was evaluated with the following relation (Eq. (7).
![Plots of degradation (A), decay of absorbance (B), TOC percent removal (B), and TOC/TOC0 (D) for the degradation of MG under K2S2O8/Ag-Pt system. Reaction conditions: [MG] = 1.25 mM, and [Ag-Pt] = 16 mg.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104863-fig16.png)
Plots of degradation (A), decay of absorbance (B), TOC percent removal (B), and TOC/TOC0 (D) for the degradation of MG under K2S2O8/Ag-Pt system. Reaction conditions: [MG] = 1.25 mM, and [Ag-Pt] = 16 mg.
In situ chemical oxidation of MG by S2O82-/Ag-Pt system.
In Scheme 7 represents the decolorization of MG into colorless MG intermediate due to the addition reaction between HO– and MG. For the decolorization of dye, the first step is the addition of HO– on to the MG, which diminished the chromophoric structure of MG (Huang, et al., 2021). The 12 and 20 degradation intermediates of MG with Fe3O4/GAs + S2O82- + light (Lu et al. 2020) and CuFe2O4@biochar/S2O82- (Huang et al., 2021) systems were detected by using LC-MS and UPLC-MS, respectively. These investigators suggested that the MG was completely degraded via two possible routes, and eventually mineralized into CO2 and H2O. On the basis of UPLC-MS data, Huang et al. also proposed the similar mechanism for the degradation of MG, and suggests that the addition of HO– with MG is the first step (Huang, et al., 2021). The intermediate was slowly mineralized into the CO2 and H2O by the generated radical species (Gokulakrishnan et al., 2012; Xu and Le, 2010; Li et al., 2015).
3.5 Ag-Pt as activator of K2S2O8
Fig. 7 optical images show that the green color of malachite green became colorless after the treatment with S2O82-/Ag-Pt system. The color and suspension of Ag-Pt NPs was not visualized after the degradation of dye, indicating the metallic silver and platinum were oxidized into the corresponding metal ions (Ag+ and Pt+). The generated HO• species, formed after the activation of S2O82- by Ag-Pt, may initiates a series of radical chain reactions. The possible degradation mechanism of MG with S2O82-/Ag-Pt might occur as Eqs. (8) to (11) listed below (Anipsitakis and Dionysiou, 2004).
The Ag+ ions onto the surface of Ag0 acted a heterogeneous S2O82- activator and generates SO4-• through the cleavage of peroxide bond of S2O82-. First, Ag+ on the surface of Ag-Pt will activate S2O82- to generate SO4-• and transfer the Ag+ ions to higher valence state (Eq. (8). AgNPs also formed cage complex with S2O82- (Eq.9). The HO• radicals were generated in solution after the reaction of SO4-• with water (Eq. (10). Both radicals (HO• and SO4-•) possesses strong oxidizing activity and oxidized target organic water contaminants to CO2 and H2O (Eq. (11). The presence of Pt NPs accelerated the generation of SO4-• radicals due to their strong galvanic metal displacement cell reaction with zero valent silver NPs, in which Pt NPs and Ag NPs acts as catalyst (cathode), and an electron donor (anode), respectively. As a result, the Pt0 helps to increase the ability of AgNPs. Ahn et al. suggested that the surface metal such as Pt and Au were the most appealing activator to generate SO4-• radicals in the M/Al2O3 composite (Ahn et al., 2019). The reason behind the role of metal type is unknown, while the efficient activation by Pt in Ag-Pt might be related to the intrinsic surface catalytic property of these metals (Eqs. (12) to (14).
The Pt0 and Ag0 were oxidized to produce corresponding ions by hydrogen peroxide, and air + ascorbic acid, respectively, in an aqueous solution through oxidation etching process (Okamoto, et al., 2012; Wang, et al., 2013). The oxidative dissolution of NPs could be due to the direct redox reaction with the reactive oxygen radical species and/or natural organic materials in aquatic system (Wang, et al., 2016). S2O82- activation by Ag-Pt NPs involves the oxidation of both metals (Ag0 and Pt0) and subsequent their aqueous dissolution (Aljadaani, et al., 2021). Thus the generation of SO4-• by the decomposition of S2O82- with transition metals and/or metal NPs is not truly a catalytic process. Ag-Pt NPs serves as a sacrificial electron donor. On the basis of observed results and pervious existing literature, Scheme 8 mechanism was proposed for the activation and degradation of S2O82- and MG in Ag-Pt/S2O82- system (Liang, et al., 2017; Lu, et al., 2020; Huang, et al., 2021).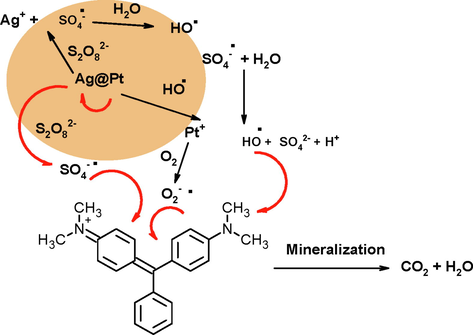
Mechanism of persulfate activation and degradation of MG.
3.6 Scavenger test and reusability
It has been established that the SO4-• and HO• were the two main reactive radical species produced by S2O82- decomposition (Gu et al., 2018; Lu et al., 2020). Quenching tests were performed with ethanol, tertiary butanol (TBA), and potassium iodide to quench both SO4-• and HO•, only HO•, and both HO• and SO4-•, respectively. Fig. 11A displaced the effect of different quenchers on the oxidation of MG. The degradation efficiency was dropped 6.2% after adding 10 mM ethanol, indicating that ethanol quench the both HO• and SO4-• radicals. The MG removal efficiency was dropped only 3.4% with 10 mM TBA, suggested that a small quantity of HO• species were generated. This resulted from the reaction of generated SO4-• with water in an aqueous solution. As depicted in Fig. 11A, only ca. 32.4% MG was removed from the reaction solution in presence of 10 mM potassium iodide. These observations can be rationalized due to the presence of numerous SO4-• on the surface of Ag-Pt, and the heterogeneous Fenton-like reaction was mainly occurred on the catalyst surface (Wan and Wang, 2017).![Effect of radical scavengers (A) and reusability of Ag-Pt over six cycles (B) on the oxidation of malachite green. Reaction conditions: [MG] = 1.25 mM,[Ag-Pt] = 16 mg/L, [K2S2O8] = 4.0 mM, pH = 7.0, Temperature = 25 °C.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104863-fig19.png)
Effect of radical scavengers (A) and reusability of Ag-Pt over six cycles (B) on the oxidation of malachite green. Reaction conditions: [MG] = 1.25 mM,[Ag-Pt] = 16 mg/L, [K2S2O8] = 4.0 mM, pH = 7.0, Temperature = 25 °C.
The surface stability and catalytic reusability are the two most important capabilities of the using Ag-Pt NPs as persulfate activator for MG oxidation in the Ag-Pt/S2O82- system. All recycling experiments in this work operated under the same condition: 1.25 mM MG concentration, 4.0 mM S2O82- concentration, 3.0 pH, 16.0 mg Ag-Pt, and 25 °C temperature. Fig. 11B showed that the MG oxidation efficiency was ca. 94.2% after 50 min in the first recycle process, and was over 92.3%, 90.4%, 88.0%, 86.4%, and 85.3% in the next five kinetic runs after 50 min. The above results revealed that the decrease of Ag-Pt activation efficiency may be due to the oxidation of Ag-Pt. The activation of S2O82- by transition metal and/or NPs to generate reactive SO4-• species is not a true catalytic process (Ahn et al., 2019; Zaheer et al., 2023).
In order to compare the persulfate activation efficiency of Ag-Pt with other catalysts, the degradation parameters (time, degradation efficiency, rate constant, and activation energy) of MG are summarized in Table 3. The degradation % and oxidation rates of MG depends on the concentration of MG, pH, amount of catalyst, temperature, and source of light irradiation (Liang, et al., 2017; Lu et al., 2020; Huang, et al., Gokulakrishnan et al., 2012, and Qi, et al. 2017). Table 3 clearly shows that the nature of catalyst, and concentration of S2O82- have significant impact on the degradation efficiency of MG within a suitable time reaction time. The metal NPs/S2O82- system is a better approach to generate reactive radicals for the complete degradation of MG and other water contaminants.
4 Conclusion
Caffeic acid was extracted from an aqueous solution of Artemisia herba-alba, and used for the fabrication of stable sols of Ag, Pt, and Ag-Pt NPs at room temperature. The SPR band nature and optical band gap of Ag-Pt NPs were determined by UV–visible spectroscopy. We demonstrated the use of Ag-Pt as heterogeneous S2O82- activator efficiently, when the initial pH ranged from 3.0 to 10.0. Complete MG decolorization (94.2%) was observed in 50 min reaction time. The MG decomposition percentage increases with amount of Ag-Pt dosage, and reaction temperature. Under optimal conditions ([S2O82-]0 / [dye]0 = 4.0 mM, and Ag-Pt dosage = 16 mg), nearly 90% TOC removal of dye can be achieved in 4 h. This study contributes to developing a bimetallic activator for SO4-• based advanced oxidation processes in wastewater treatment.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at the King Abdulaziz University, Jeddah, under grant no. (G-50-247-1441). The authors, therefore, acknowledges with thanks to DSR for technical and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahn, Y.-Y., Bae, H., Kim, H.-Il., Kim, S.-H., Kim, J.-H., Leef, S.-G., Lee, J., 2019. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B: Environ. 241, 561-569
- Cobalt ferrite nanoparticles with controlled composition-peroxymonosulfate mediated degradation of 2-phenylbenzimidazole-5-sulfonic acid. Appl. Catal. B. 2018;221:266-279.
- [Google Scholar]
- Electrical energy determination for an azo dye decolorization and mineralization by UV/H2O2 advanced oxidation process. Chem. Eng. J.. 2008;137:518-524.
- [Google Scholar]
- Aljadaani, A. H. A., AL-Thabaiti, S. A., Khan, Z., 2021. SDS capped Cu nanorods: photosynthesis, stability, and their catalytic activity for trypan blue oxidative degradation, J. Mater. Res. Technolo., 15, 6841-6854
- Hypoglycemic effect of Artemisia herba alba II: effect of a valuable extract on some blood parameters in diabetic animals. J. Ethnopharmacol.. 1994;43:167-171.
- [Google Scholar]
- Iron based bimetallic nanoparticles to activate peroxygens. Chem. Eng. J.. 2013;232:555-563.
- [Google Scholar]
- Photo-oxidative Decolorization of brilliant blue with AgNPs as an activator in the presence of K2S2O8 and NaBH4. ACS Omega. 2021;6:27510-27526.
- [Google Scholar]
- Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol.. 2004;38:3705-3712.
- [Google Scholar]
- Green synthesis and characterization of silver nanoparticles (Ag NPs) using white water Lily flower extraction species Nymphaea Alba of Nymphaeaceae family. Iran. J. Organ. Chem.. 2018;10:2403-2408.
- [Google Scholar]
- Biodegradation of triphenylmethane dyes. Enzyme Microbial. Technol.. 1998;22:185-191.
- [Google Scholar]
- Colloidal micelles of block copolymers as nanoreactors, templates for gold nanoparticles, and vehicles for biomedical applications. Adv. Colloid Interface Sci.. 2014;213:1-20.
- [Google Scholar]
- Engineered nanomaterials growth control by monomers and micelles: From surfactants to surface active polymers. Adv. Colloid Interface Sci.. 2018;256:101-110.
- [Google Scholar]
- Effect of operational parameters on degradation of malachite green by ultrasonic irradiation. Ultrason. Sonochem.. 2008;15:1009-1014.
- [Google Scholar]
- Berberidou, C., Poulios, I., Xekoukoulotakis, N. P., Mantzavinos, D., 2007. Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions. Appl. Catal. B: Environ. 74, 63-72.
- Seedless, silver-induced synthesis of star-shaped gold/silver bimetallic nanoparticles as high efficiency photothermal therapy reagent. J. Mater. Chem.. 2012;22:2244-2253.
- [Google Scholar]
- Oxidative degradation of dyes by ultraviolet radiation in the presence of hydrogen peroxide. Dyes Pigments. 1999;41:211-220.
- [Google Scholar]
- UV-visible and synchronous fluorescence spectroscopic investigations of the complexation of Al(III) with caffeic acid, in aqueous low acidic medium. Polyhedron. 2006;25:2215-2222.
- [Google Scholar]
- Ultraviolet-visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans.. 1991;87:3881-3891.
- [Google Scholar]
- FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl. Catal. B. 2008;84:468-473.
- [Google Scholar]
- Thermally activated persulfate (TAP) oxidation of antiepileptic drug carbamazepine in water. Chem. Eng. J.. 2013;228:765-771.
- [Google Scholar]
- Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357-1361.
- [Google Scholar]
- Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf. B. 2010;75:584-589.
- [Google Scholar]
- Degradation of malachite green by potassium persulphate, its enhancement by 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane nickel(II) perchlorate complex, and removal of antibacterial activity. J. Hazard. Mater.. 2012;213–214:19-27.
- [Google Scholar]
- Degradation of phenanthrene in sulfate radical based oxidative environment by nZVI-PDA functionalizedrGO catalyst. Chem. Eng. J.. 2018;354:541-552.
- [Google Scholar]
- Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213-220.
- [Google Scholar]
- Functionalized iron oxide-metal hybrid nanoparticles for protein extraction from complex fluids. Ind. Eng. Chem. Res.. 2020;59:1045-1055.
- [Google Scholar]
- Degradation of malachite green in aqueous solution by Fenton process. J. Hazard. Mater.. 2009;164:468-472.
- [Google Scholar]
- Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ.. 2019;647:352-361.
- [Google Scholar]
- Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: efficiency, stability and mechanism. J. Environ. Chem. Eng.. 2021;9:105800
- [Google Scholar]
- Caffeic acid as antioxidant in fish muscle: mechanism of synergism with endogenous ascorbic acid and alpha-tocopherol. J. Agric. Food Chem.. 2009;57:675-681.
- [Google Scholar]
- Green synthesis of metal nanoparticles using plants. Green Chem.. 2011;13:2638-2650.
- [Google Scholar]
- Strong synergistic effect of Co3O4 encapsulated in nitrogen-doped carbon nanotubes on the nonradical-dominated persulfate activation. Carbon. 2020;158:172-183.
- [Google Scholar]
- Phytochemical synthesis of CdO nanoparticles: fabrication of electrochemical sensor for quantification of cefixime. J. Mater. Sci.: Mater. Electron.. 2021;32:8932-8943.
- [Google Scholar]
- Biomediated silver nanoparticles for the highly selective copper(II) ion sensor applications. Ind. Eng. Chem. Res.. 2012;51:7441-7446.
- [Google Scholar]
- The interaction of gold and silver nanoparticles with a range of anionic and cationic dyes. Phys. Chem. Chem. Phys.. 2014;16:6050-6059.
- [Google Scholar]
- Betanin assisted synthesis of betanin@silver nanoparticles and their enhanced adsorption and biological activities. Food Chem.. 2019;298:125014
- [Google Scholar]
- Laid, M.. Hegazy, M-E. F., Ahmed, A. A., 2008. Sesquiterpene lactones from Algerian Artemisia herbaalba. Phytochem. lett. 1, 85-88
- The aqueous degradation of butylated hydroxyanisoleby UV/S2O82−: study of reaction mechanisms via dimerization and mineralization. Environ. Sci. Technol.. 2007;41:613-619.
- [Google Scholar]
- Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism. Chem. Eng. J.. 2015;266:28-33.
- [Google Scholar]
- Photodegradation of malachite green under simulated and natural irradiation: kinetics, products, and pathways. J. Hazard. Mater.. 2015;285:127-136.
- [Google Scholar]
- Liang, C., H.-W., Su, 2009. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 48, 5558-5562
- Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV-Vis light. Mater. Design. 2017;119:244-253.
- [Google Scholar]
- Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J.. 2013;218:384-391.
- [Google Scholar]
- Bimetallic Fe nanoparticles: recent advances in synthesis and application in catalytic elimination of environmental pollutants. Chem. Eng. J.. 2014;236:448-463.
- [Google Scholar]
- Fe3O4/graphene aerogels: a stable and efficient persulfate activator for the rapid degradation of malachite green. Chemosphere. 2020;251:126402
- [Google Scholar]
- Bio-directed synthesis of platinum nanoparticles by Nymphaea alba extract: fabrication of a novel non-enzymatic hydrogen peroxide sensor. J. Mater. Sci.: Mater. Electronics. 2020;31:18721-18731.
- [Google Scholar]
- WEPA: a reusable waste biomass-derived catalyst for external oxidant/metal-free quinoxaline synthesis via tandem condensation–cyclization–oxidation of α-hydroxy ketones. Green Chem.. 2022;2022(24):6215-6223.
- [Google Scholar]
- Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J. Hazard Mater.. 2009;168:346-351.
- [Google Scholar]
- Oxidative deterioration of platinum nanoparticle and its prevention by palladium. Experiment. Dermatol.. 2012;21(Suppl. 1):5-7.
- [Google Scholar]
- Heterogeneous activation of persulfate by reduced graphene oxide–elemental silver/magnetite nanohybrids for the oxidative degradation of pharmaceuticals and endocrine disrupting compounds in water. Appl. Catal. B.. 2018;225:91-99.
- [Google Scholar]
- Synthesis and characterization of Zn1-XCuXS and Zn1 - XNiXS nanoparticles and their applications as photocatalyst in Congo red degradation. J. Alloys Compoun.. 2010;501:130-135.
- [Google Scholar]
- Degradation of malachite green by persulfates activated with CuO. Chinese J. Environ. Eng.. 2017;11:2084-2090.
- [Google Scholar]
- A facile green synthesis of silver nanoparticles using Piper betle biomass and its catalytic activity toward sensitive and selective nitrite detection. J. Ind. Eng. Chem.. 2016;35:29-35.
- [Google Scholar]
- Reduced graphene oxide/ZnFe2O4 nanocomposite as an efficient catalyst for the photocatalytic degradation of methylene blue dye. Res. Chem. Intermed.. 2017;43:2669-2690.
- [Google Scholar]
- Distinguishing nanoparticle-nanoparticle interactions between gold and silver nanoparticles controlled by gemini surfactants: Stability of nanocolloids. J. Phys. Chem. C. 2021;125:5399-5411.
- [Google Scholar]
- TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes Pigments. 2003;56:37-49.
- [Google Scholar]
- Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater. Chem.. 2003;13:1822-1826.
- [Google Scholar]
- Exploring the nexus between agriculture and greenhouse gas emissions in BIMSTEC region: the role of renewable energy and human capital as moderators. J. Environ. Manag.. 2021;297:113316
- [Google Scholar]
- Biofuels from inulin-rich feedstocks: a comprehensive review. Bioresour. Technol.. 2022;346:126606
- [Google Scholar]
- Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++G** level) studies on alkali metal salts of caffeic acid. Spectrochim. Acta Part A. 2013;100:21-30.
- [Google Scholar]
- Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J. Environ. Chem. Eng.. 2021;9:106553
- [Google Scholar]
- Ashes from organic waste as reagents in synthetic chemistry: a review Environ. Chem. Lett.. 2021;19:3887-3950.
- [Google Scholar]
- One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim. Acta A. 2015;136:256-264.
- [Google Scholar]
- Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater.. 2017;324:653-664.
- [Google Scholar]
- Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano. 2016;3:240-255.
- [Google Scholar]
- The pH-dependent interaction of silver nanoparticles and hydrogen peroxide: a new platform for visual detection of iodide with ultra-sensitivity. Talanta. 2013;107:146-153.
- [Google Scholar]
- Heterogeneous Fenton-like oxidation of malachite green by iron-based nanoparticles synthesized by tea extract as a catalyst. Sep. Puri. Technol.. 2015;154:161-167.
- [Google Scholar]
- Xavier, S. S. J, Kumar, T. R., Ranjani, M., Yoo, D. J., Archana, V., Charles, L., Annaraj, J., G. Kumar, G. G.. 2019. Environmentally benign carbon nanodots prepared from lemon for the sensitive and selective fluorescence detection of Fe(III) and tannic acid. J. Fluoresc. 29, 631-643
- Colorimetric detection of melamine using β-cyclodextrin-functionalized silver nanoparticles. Anal. Methods. 2014;6:8165-8172.
- [Google Scholar]
- Degradation of azo dye orange G in aqueous solutions by persulfate with ferrous ion. Sep. Puri. Technolo.. 2010;72:105-111.
- [Google Scholar]
- Facile synthesis of Mn3O4–reduced grapheme oxide hybrids for catalytic decomposition of aqueous organics. Ind. Eng. Chem. Res.. 2013;52:3637-3645.
- [Google Scholar]
- Adsorption of methyl red on biogenic Ag@Fe nanocomposities adsorbent: Isotherms, kinetics and mechanistic approach. J. Mol. Liq.. 2019;283:287-298.
- [Google Scholar]
- Silver-ruthenium bimetallic nanoparticles as sacrificial heterogeneous persulfate activator in situ chemical oxidation of dye. J. Mol. Liq.. 2023;377:121549
- [Google Scholar]
- Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today. 1998;40:387-395.
- [Google Scholar]
- Carbon nanofibers supported Co/Ag bimetallic nanoparticles for heterogeneous activation of peroxymonosulfate and efficient oxidation of amoxicillin. J. Hazard. Mater.. 2020;400:123290
- [Google Scholar]
- Core–shell Fe-Fe2O3 nanostructures as effective persulfate activator for degradation of methyl orange. Sep. Purif. Technol.. 2013;108:159-165.
- [Google Scholar]
- Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J. Phys. Chem. C. 2011;115:18923-18934.
- [Google Scholar]






