Translate this page into:
Nigericin attenuates lipopolysaccharides induced sepsis and alleviates acute lung injury in in vivo model
⁎Corresponding author. yangyong00372@sina.com (Yong Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nigericin is a potent antibiotic derived from Streptomyces hygroscopicus which acts as a polyether ionophore. The anticancer property of nigericin was extensively studied, and it has been proven to inhibit cancer cell proliferation, progression, and invasion in prostate, colorectal, nasopharyngeal, and lung cancer. It also stimulates bacterial clearance in macrophages. The protective role of nigericin in sepsis, which is the foremost risk factor for acute lung injury and acute respiratory distress syndrome. Since bacteremia is frequently associated with lung injury, in this study we evaluated the potency of nigericin against an lipopolysaccharide (LPS)-induced sepsis mice model. BALB/c mice were challenged with intratracheal administration of LPS and simultaneously treated with 10 mg/kg and 20 mg/kg nigericin for 3 consecutive days. The mice were euthanized, and the lung tissue was excised to determine the lung wet/dry ratio. Bronchoalveolar lavage fluid was collected and assessed for protein content, LDH, and immune cell count. The antioxidant activity of nigericin was evaluated by measuring the MPO, MDA, SOD, and GSH activities in the lung tissues. The proinflammatory cytokines TNF-α and IL-6 were quantified to assess the anti-inflammatory potency against LPS-induced inflammation. iNOS, COX-2 and PGE-2 levels in lung tissue of nigericin-treated LPS-challenged mice were estimated to assess the immunomodulatory activity of nigericin. Finally, the antiapoptotic potency and cytoprotective effect of nigericin were analyzed by estimating apoptotic protein levels in lung tissue and assessing lung tissue histopathology with hematoxylin & eosin staining. Nigericin treatment significantly reduced immune cell infiltration, synthesis of proinflammatory cytokines, immunomodulatory proteins, and proapoptotic proteins in LPS-challenged mice. It also increased antioxidants and antiapoptotic protein Bcl2, thereby ameliorating lung tissue from LPS-induced inflammation. Our results indicate nigericin suppressed the LPS induced sepsis in an in vivo model and has the therapeutic potency to cure sepsis induced acute lung injury.
Keywords
Sepsis
Acute lung injury
Lipopolysaccharides
Inflammation
Antibiotic
Nigericin
1 Introduction
Sepsis is a life-threatening disease that is often underestimated as an inflammatory disorder. Sepsis, the multifaceted syndrome, occurs when the primary host defense mechanism against an infection is uncontrollable and causes host tissue damage (Jarczak et al., 2021). The dysregulated host defense mechanism also leads to organ failure in the host. 26% of fatalities were reported in sepsis patients residing in high-income countries (Fleischmann et al., 2016). Globally, about 50 million people were affected by sepsis, and 11 million sepsis related deaths were reported. Geriatric and immunocompromised patients are prone to sepsis attacks (Gaieski et al., 2013; Vincent et al., 2019; Rudd et al., 2020). Due to the increased incidence of sepsis-related mortalities by the year 2017, World Health Organization (WHO) had identified sepsis as a global health risk and passed resolutions on the diagnosis and treatment of sepsis (Reinhart et al., 2017).
More than 2.5 million deaths were reported globally due to sepsis-induced lower respiratory tract infections (Angus and van der Poll, 2013). In western countries about 6–7% of patients with sepsis are identified with acute respiratory distress syndrome (ARDS). In severe sepsis conditions, the acute lung injury and acute respiratory distress syndrome progression is rapid, and the mortality rate is high (Gajic et al., 2011; Mikkelsen et al., 2013). Pneumonial infection seems to be the primary cause of severe sepsis leading to pulmonary dysfunction (Menéndez et al., 2012). Lipopolysaccharides (LPS) are prominent structural components present on the outer cell membrane of gram-negative bacteria and are responsible for their antigenicity. The host phagocytes and immune cells recognize pathogen-associated molecular patterns present in the structural components of bacteria with pattern recognition receptors, which trigger the host defense system (Hargreaves and Medzhitov, 2005). In this study, we have induced sepsis in mice with LPS since it is a well-accepted animal model to initiate sepsis-induced acute lung injury. Several earlier studies already supported the choice of LPS-induced sepsis in experimental animal models to study sepsis-induced acute lung injury (Thomas et al., 2014; Park et al., 2021).
Innate immune response of host against an infection activates macrophages, antigen presenting cells and triggers the cytokine secretion. The increase in proinflammatory cytokines causes inflammation during sepsis (Cavaillon and Adib-Conquy, 2005; Chaudhry et al., 2013). Prostaglandins are inflammatory mediators that are derived from arachidonic acid by cyclooxygenase activity. The synthesis of prostaglandins is reported to be vital for signaling in the inflammation process (Lin et al., 2017). Inducible nitric oxide synthase (iNOS) is another prime inflammatory mediator that has been targeted by research to suppress inflammation. Inhibition of iNOS has proven to attenuate inflammatory signaling in animal models (Takatani et al., 2018). Severe sepsis causes lung endothelial and epithelial cell disruption, causing increased membrane permeability and leading to excess neutrophil infiltration (Johnson and Matthay, 2010), which is the hallmark event for the initiation of acute lung injury.
At present, treating an acute lung injury induced by sepsis is a severe task for health care professionals. No precise drug was proven to be potent against treating acute lung injury, and most of the drugs have side effects (Jarczak et al., 2021). Steroids are prescribed for acute lung injury patients, but delayed administration of steroids in ARDS patients worsens the disease. The major reason for drug failure is the pro-inflammatory signaling that occurs due to secondary sepsis attacks that cause systemic inflammatory syndrome (Steinberg et al., 2006). Nigericin is an antibiotic polyether ionophore derived from Streptomyces hygroscopicus. It is extensively used as non-hormonal growth regulator in veterinary medicine, and it is also used as a coccidiostatic agent in poultry breeding (Callaway et al., 2003; Kevin Ii et al., 2009). Nigericin has been proven to be a potent anticancer drug that inhibits cancer cell proliferation, invasion, and progression in prostate (Mashima et al., 2010), colorectal (Liu et al., 2018), nasopharyngeal (Deng et al., 2013), and lung cancer in vitro and in vivo models (Andersson et al., 2005; Gao et al., 2021). Hence, in the present study, we evaluated the nigericin potency to attenuate LPS-induced sepsis and acute lung injury in an in vivo model.
2 Materials and methods
2.1 Chemicals
Nigericin and lipopolsaccharides were procured from Sigma Aldrich, USA. The ELISA kits were procured from Abcam, USA. All the other chemicals used in the current study are of analytical grade.
2.2 Animals
Four-week-old male BALB/c mice were housed at 22 ± 2 °C, 55 ± 5% relative humidity, and 12 h light–dark cycle. The animals were acclimatized in the animal house for 7 days before the initiation of the experiment. Sterile conditions were maintained in the animal house throughout the experiment period. The animals were fed ad libitum with a standard laboratory pellet diet.
2.3 Experimental design
The acclimatized mice were divided randomly into four groups, each consisting of six mice. Group I are control group mice, which are treated with sterile saline. Group II are lung inflammation-induced mice challenged intratracheally with 5 mg/kg of LPS for 3 days. Groups III and IV are LPS-challenged mice treated with 10 and 20 mg/kg of nigericin, respectively, for 3 days, followed by the LPS administration. The animals were euthanized 24 h after the last treatment, and the lung tissue was isolated for further analysis.
2.4 Measurement of lung Wet/dry ratio
Pulmonary edema induced by LPS treatment was assessed by measuring lung tissue weight. Immediately after excision of the lungs, the lung tissue weight was measured, and the lungs were subjected to drying by placing them in an oven set at 80 °C for 72 h. The dried lung tissue was then weighed again to determine the lung wet/dry weight ratio.
2.5 Analysis of broncholalevolar lavage fluid (BALF)
The mice were euthanized and placed on their backs on the surgical table. An incision was made in the sterilized neck region near the trachea, and the trachea was exposed gently. A 5 µm catheter was carefully pierced into the trachea, and 1 ml of balanced salt/EDTA solution was injected via the catheter. The solution was then gently aspirated, and the process was repeated three times. The collected BALF was stored @ −20 °C for further analysis (Van Hoecke et al., 2017). The protein concentration of BALF was quantified using the Braford reagent.
2.6 Quantification of LDH activity
The LDH activity in BALF was measured by an LDH colorimetric assay kit procured from Sigma Aldrich, USA. The reduction of NAD to NADH by LDH was quantified by measuring the final absorbance at 450 nm, and LDH levels were determined with the standard curve plot.
2.7 BALF total and differential cell counts
The collected BALF was pooled and subjected to centrifugation at 2500 rpm for 15 min at 4 °C. The cell pellet was collected and suspended in 500 µl sterile saline solution for the quantification of total and differential cell counts. The total cell count was quantified using a hemocytometer, and a differential count was performed with Diff-Quik stain (ThermoFisher Scientific, USA). The cell pellets were smeared on a clean glass slide, fixed with 100% methanol, and stained with Diff-Quik stain. The stained slides were subjected to a differential cell count using a light microscope.
2.8 Quantification of Myeloperoxidase (MPO) activity
Lung tissue homogenate was prepared by homogenizing 50 mg of lung tissue with 1 ml cell lysing buffer using tissue homogenizer. The tissue homogenate was centrifuged at 10,000 rpm for 15 min @ 4 °C and the supernatant was collected for further analysis. Myeloperoxidase levels in the lung tissue homogenate was quantified using the colorimetric assay kit procured form Elabscience, USA. Myeloperoxidase in sample reduces hydrogen peroxide which reacts with o-dianisidine and the final absorbance was measured at 460 nm.
2.9 Quantification of lipid peroxidation
The level of lipid peroxidation was measured by quantifying the levels of malondialdehyde (MDA) in the tissue homogenate (Devasagayam and Tarachand, 1987). Malondialdehyde, the end product formed due to lipid peroxidation, forms an adduct by reacting with thiobarbituric acid. The MDA-TBA 2 adduct was measured at 532 nm, and the MDA levels in samples were calculated using a standard curve plot.
2.10 Quantification of antioxidants
The superoxide dismutase (SOD) activity in the lung tissue homogenate was measured using the method of Marklund & Marklund, (Marklund and Marklund, 1974; Marklund and Marklund, 1974). The rate of pyrogallol oxidation due to SOD in the tissue sample was measured at 470 nm. Glutathione levels were measured using the DTNB method as per the protocol of the Rahman et al. (Rahman et al., 2006; Rahman et al., 2006). The conversion of sulfhydryl reagent 5;5 ′-dithio-bis(2-nitrobenzoic acid) to 5′-thio-2-nitrobenzoic acid (TNB) by glutathione was measured at 412 nm. The levels of SOD and GSH were calculated using the standard curve plot.
2.11 Enzyme-linked immunosorbent assay (ELISA)
The levels of proinflammatory cytokines TNF-α, IL-6, iNOS, COX-2, PGE-2 and apoptotic signaling proteins Bax, Bcl2 and Caspase-3 were quantified using commercially available ELISA kits procured from Abcam, USA. 100 µl of tissue homogenate was added to coated ELISA plates and incubated for 1 h at 37 °C. After incubation period 100 µl of enzyme labeled antibody was added to the plates and incubated for 1 h 37 °C. Substrate solution was added and incubated for 30 min and finally the reaction was stopped with the addition of stop solution. The final absorbance of the solution was measured at 450 nm.
2.12 Histopathological analysis
The excised lung tissue was fixed for 24 h with 10% neutral buffered formalin, and then the fixed tissue was subjected to dehydration with graded alcohol concentrations. The dehydrated lung tissue was embedded with paraffin wax in a wax bath. The paraffinized tissue blocks were sectioned into 4-µm thickness using a rotary microtome. The tissue sections were deparaffinized by placing them in a hot water bath. The deparaffinized tissue sections were coated on albumin-coated glass sides, and the sections were stained with haematoxylin and eosin stain. The stained tissues were viewed and photographed under a light microscope (Olympus).
3 Statistical analysis
The data obtained in each experiment were analyzed using SPSS 18.0 statistical software. The intergroup difference was analyzed with one-way ANOVA, and the intragroup difference was analyzed with Tukey’s post hoc test. The statistically analyzed data were illustrated as the mean ± standard deviation. Statistical significance was considered to be p < 0.05.
3.1 Nigericin prevented pulmonary edema in LPS challenged mice
Fig. 1A depicts the results of the lung wet/dry ratio in nigericin-treated and untreated LPS-challenged mice. LPS treatment significantly caused pulmonary edema in the mouse model, which was evidenced by a significant increase in the lung wet/dry ratio compared to the control rats. Nigericin treatment mice showed a significant decrease in lung wet/dry ratio compared to LPS-only challenged mice. Compared to 10 mg/kg nigericin-treated mice, the 20 mg/kg nigericin-treated mice showed a decreased lung wet/dry ratio.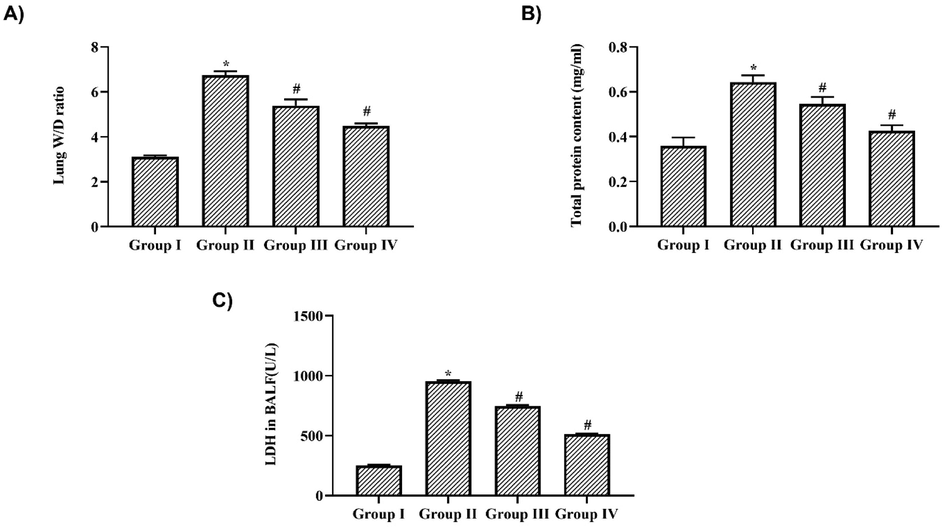
Nigericin prevented pulmonary edema & suppressed BALF LDH activity in LPS challenged mice. A) Lung Wet/Dry ratio, B) Total protein content, C) Lactate dehydrogenase activity in BALF of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.2 Nigericin suppressed LDH activity in BALF of LPS challenged mice
LPS-challenged mice's BALF showed a significant increase in protein concentration and LDH activity, indicating the occurrence of interstitial pulmonary injury. The protein levels were increased to 0.52 ± 0.02 mg/ml in LPS alone-challenged mice, whereas nigericin treatment significantly decreased the protein levels to 0.38 ± 0.01 mg/ml and 0.32 ± 0.02 mg/ml in 10 mg/kg and 20 mg/kg nigericin-treated mice, respectively (Fig. 1B). Nigericin treatment also decreased the levels of LDH in the BALF of LPS-challenged mice. 6.2 ± 0.08U/L of LDH levels were detected in 10 mg/kg nigericin-treated mice, whereas they were further decreased to 4.8 ± 0.6 U/L in 20 mg/kg nigericin-treated mice. LPS-only challenged mice showed a significant increase of 7.9 ± 0.5 U/L LDH in BALF compared to the control, which was observed to be 2.2 ± 0.3 U/L (Fig. 1C).
3.3 Nigericin inhibited immune cells infiltration in BALF of LPS challenged mice
LPS treatment significantly increased the count of immune cells in BALF, indicating the induction of inflammation. The total cell count in the BALF of LPS-only challenged mice was counted at 22 ± 0.05 × 10−5 cells/ml, which was significantly higher than the control mice, which were to have 7 ± 0.02 × 10−5 cells/ml. Nigericin treatment significantly decreased the total cell count in BALF of LPS-challenged mice to 13 ± 0.04 × 10−5 cells/ml and 8 ± 0.02 × 10−5 cells/ml in 10 and 20 mg/kg nigericin-treated mice, respectively (Fig. 2A). Nigericin treatment also decreased the neutrophil count (6 ± 0.02 × 10−5 cells/ml and 5 ± 0.03 × 10−5 cells/ml) and eosinophil count (0.14 ± 0.001 × 10−5 cells/ml and 0.09 ± 0.002 × 10−5 cells/ml) in 10 and 20 mg/kg nigericin-treated mice, respectively whereas LPS alone-challenged mice showed 12 ± 0.03 × 10−5 cells/ml and 0.18 ± 0.002 × 10−5 cells/ml of neutrophils and eosinophils respectively (Fig. 2B and C). LPS treatment significantly increased the count of both lymphocytes (8 ± 0.05 × 10−5 cells/ml) and macrophages (0.32 ± 0.003 × 10−5 cells/ml) compared to control lymphocytes (2 ± 0.01 × 10−5 cells/ml) and macrophages (0.18 ± 0.001 × 10−5 cells/ml). Nigericin treatment significantly decreased the lymphocytes (5 ± 0.02 × 10−5 cells/ml & 3 ± 0.01 × 10−5 cells/ml) and macrophage count (0.22 ± 0.001 × 10−5 cells/ml and 0.19 ± 0.001 × 10−5 cells/ml) in 10 mg/kg and 20 mg/kg nigericin-treated mice (Fig. 2D &E).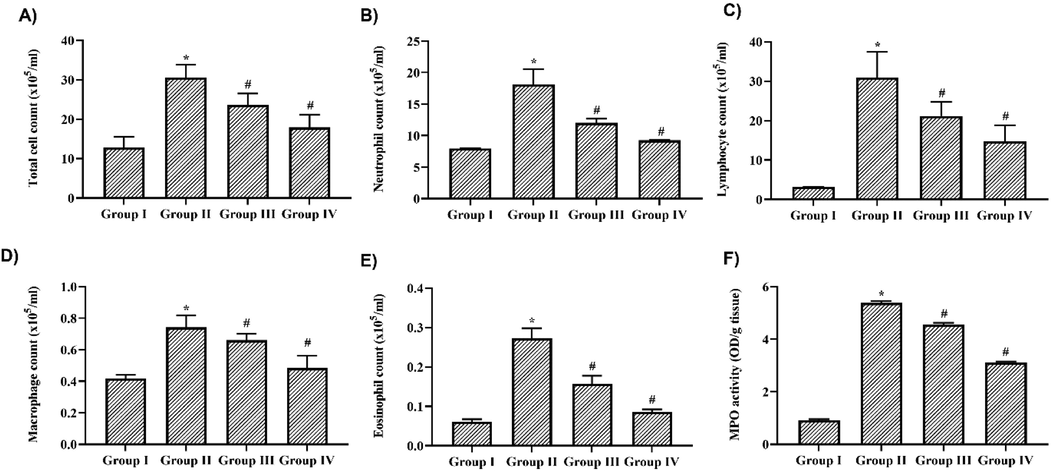
Nigericin inhibited immune cells infiltration & MPO in BALF of LPS challenged mice. A) Total cell count B) Neutrophil count C) Eosinophil count D) Lymphocyte count F) Macrophage count in BALF of nigericin treated and LPS alone challenged mice. F) MPO activity in lung tissue of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.4 Nigericin obstructed Myeloperoxidase activity in LPS challenged mice
Myeloperoxidase synthesized by neutrophils induces the synthesis of free radicals, which eventually cause cellular damage. Fig. 2F depicts the levels of MPO in nigericin-treated and untreated LPS-challenged mouse lung tissue. LPS alone challenged mice showed an increased level of 4.2 ± 0.05 OD/g tissue compared to the control 0.8 ± 0.008 OD/g tissue of MPO. Nigericin treatment significantly decreased the levels of MPO in a dose-dependent manner. 10 mg/kg nigericin treatment showed 2.9 ± 0.007 OD/g tissue and 1.8 ± 0.002 OD/g tissue in 20 mg/kg nigericin-treated mice.
3.5 Nigericin suppressed oxidative stress induced damage in LPS challenged mice
Fig. 3A illustrates the levels of MDA, an end product of lipid peroxidation, in nigericin-treated and untreated LPS alone-challenged mice. LPS treatment significantly increased the levels of MDA to 42 ± 0.6 mmol/g tissue compared to the control 5 ± 0.02 mmol/g tissue). Nigericin treatment significantly decreased the MDA levels to 6 ± 0.02 mmol/g tissue in the 10 mg/kg treatment and 5.4 ± 0.01 mmol/g tissue in 20 mg/kg treatment. Nigericin treatment significantly increased the levels of antioxidants SOD (17 ± 0.5 mmol/g tissue and 19 ± 0.4 mmol/g tissue) and GSH (8 ± 0.2 mmol/g tissue and 11 ± 0.4 mmol/g tissue) in both 10 & 20 mg/kg treated mice. Significant decrease in the levels of SOD (11 ± 0.3 mmol/g tissue) and GSH (4 ± 0.2 mmol/g tissue) were observed in LPS-only challenged mice compared to the control mice's SOD (27 ± 0.8 mmol/g tissue) and GSH (15 ± 0.4 mmol/g tissue) levels.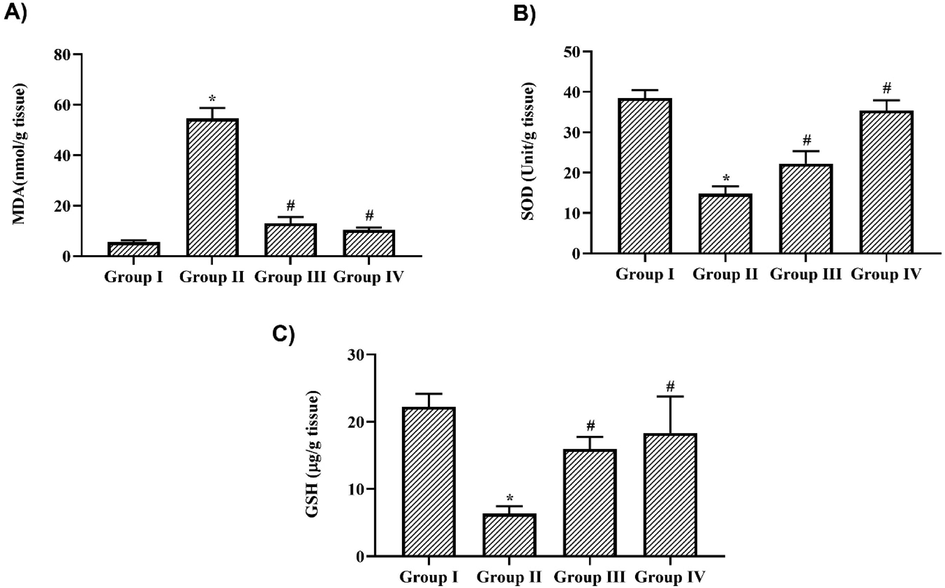
Nigericin suppressed oxidative stress induced damage in LPS challenged mice. A) Malondialdehyde B) Superoxide dismutase C) Glutathione levels in lung tissue of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.6 Nigericin prevented proinflammatory cytokines synthesis in LPS challenged mice
Nigericin acted as a potent anti-inflammatory antibiotic that significantly decreased the levels of the proinflammatory cytokines TNF-α and IL-6. LPS treatment increased the levels of TNF-α to 6.3 ± 0.1 pg/ml and IL-6 to 4.7 ± 0.08 pg/ml compared to the control mice which exhibited TNF-α levels of 1.9 ± 0.07 pg/ml and IL-6 levels of 1.1 ± 0.003 pg/ml. 10 mg/kg Nigericin treatment significantly decreased the levels of TNF-α to 3.8 ± 0.03 pg/ml and IL-6 to 1.5 ± 0.02 pg/ml and it was further to 6.3 ± 0.1 pg/ml and 4.7 ± 0.08 pg/ml respectively, in 20 mg/kg nigericin-treated mice (Fig. 4).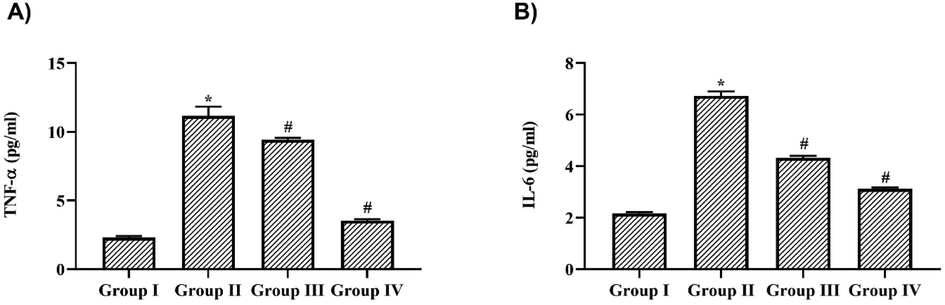
Nigericin prevented proinflammatory cytokines synthesis in LPS challenged mice. A) Tumor Necrosis Factor – α, B) Interleukin – 6 levels in lung tissue of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.7 Nigericin inhibited iNOS, COX-2 & PGE-2 synthesis in LPS challenged mice
Fig. 5 illustrates the levels of immunomodulatory iNOS, COX-2 & PGE-2 in nigericin treated and untreated LPS challenged mice. LPS treatment had significantly increased the levels of iNOS (7.2 ± 0.2 pg/ml), COX-2 (135 ± 8 pg/ml) and PGE-2 (6520 ± 40 pg/ml) compared to the control mice which exhibited iNOS levels as 0.3 ± 0.001 pg/ml, COX-2 levels as 50 ± 4 pg/ml and PGE-2 level as 300 ± 15 pg/ml. 10 mg/kg Nigericin treatment significantly decreased the the levels of iNOS to 4.4 ± 0.01 pg/ml, COX-2 to 84 ± 6 pg/ml and PGE-2 to 3450 ± 27 pg/ml and it was further to 3.8 ± 0.2 pg/ml, 73 ± 5 pg/ml and 2390 ± 36 pg/ml respectively in 20 mg/kg nigericin treated mice.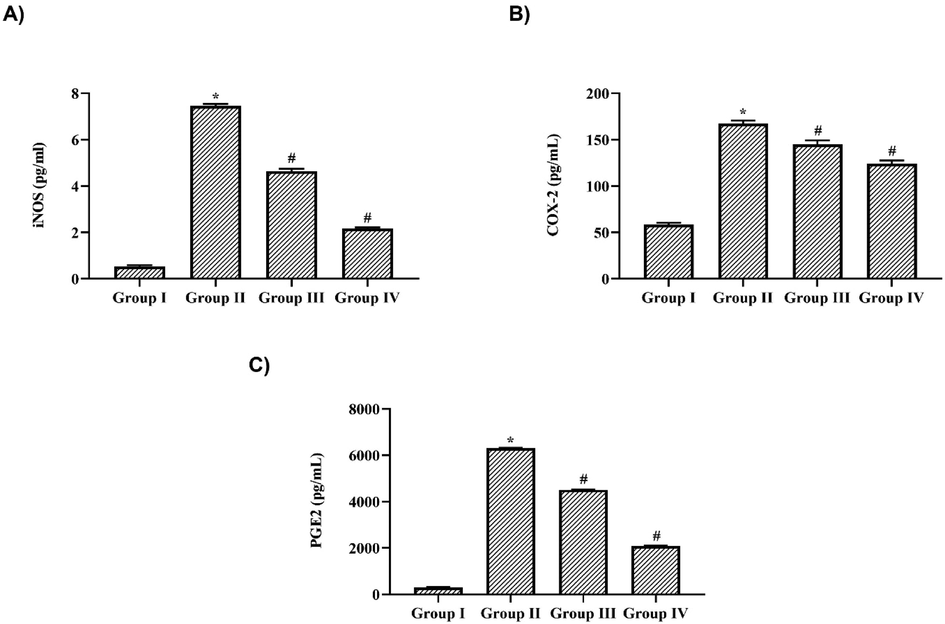
Nigericin inhibited iNOS, COX-2 & PGE-2 synthesis in LPS challenged mice. A) inducible nitric oxide synthase B) Cyclooxygenase-2 C) ProstaglandinE-2 in lung tissue of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.8 Nigericin attenuated apoptotic proteins in LPS challenged mice
The levels of pro-apoptotic protein BAX, Caspase-3 and anti-apoptotic protein Bcl2 levels in nigericin treated and untreated LPS challenged mice (Fig. 6). Nigericin treatment significantly attenuated the levels of pro-apoptotic proteins BAX and Caspase-3 in a dose dependent manner. 10 mg/kg Nigericin treatment significantly decreased the levels of BAX to 3.8 ± 0.02 pg/ml and Caspase-3 to 1.8 ± 0.03 pg/ml and it was further to 1.9 ± 0.08 pg/ml and 1.4 ± 0.02 pg/ml respectively in 20 mg/kg nigericin treated mice. LPS treatment significantly increased the levels of BAX and caspase −3 to 5.8 ± 0.3 pg/ml and 5.4 ± 0.2 pg/ml respectively. Antiapoptotic protein Bcl2 was significantly decreased in LPS alone challenged mice (1.8 ± 0.02 pg/ml) whereas it is increased in both 10 (3.7 ± 0.04 pg/ml) & 20 mg/kg (4.1 ± 0.01 pg/ml) nigericin treated mice.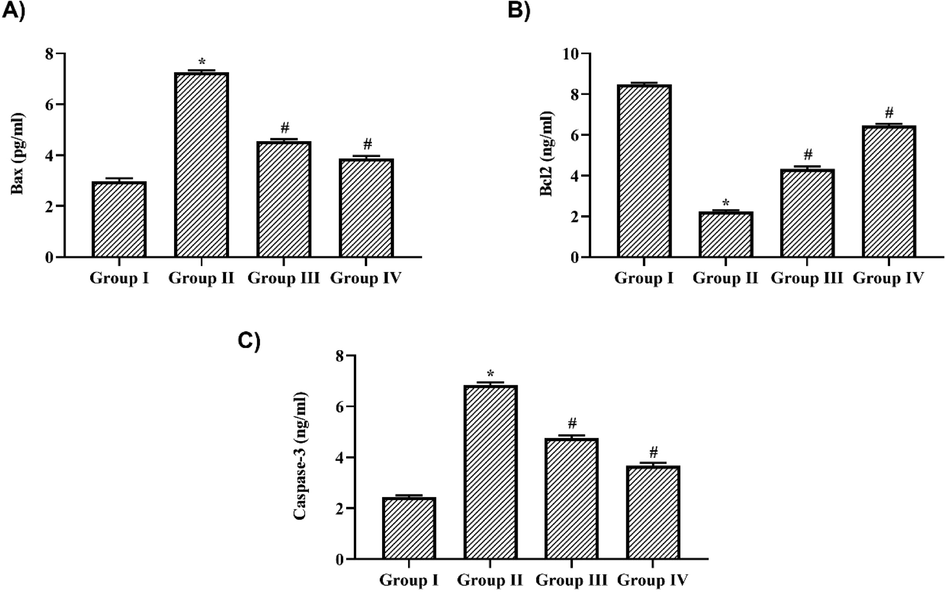
Nigericin attenuated apoptotic proteins in LPS challenged mice. A) BAX, B) Bcl2, C) Caspase-3 levels in lung tissue of nigericin treated and LPS alone challenged mice. Data were analyzed with One Way ANOVA and post hoc Tukey’s test. The results were depicted as mean ± standard deviation.
3.9 Nigericin protected lung tissue histomorphology of LPS challenged mice
Fig. 7 depicts representative images of H&E-stained lung tissue sections of nigericin-treated and untreated LPS-challenged mice. Control mice exhibited normal lung histoarchitecture with undisrupted alveolar walls, whereas pulmonary vessels with neutrophil infiltration and thick alveolar septa were observed in the LPS-only challenged mice. Both doses of nigericin treatment ameliorated LPS-induced lesions in lung tissue. Compared to LPS alone challenged mice, the nigericin-treated mice had reduced pulmonary edema with decreased neutrophil infiltration and alveolar septa thickness.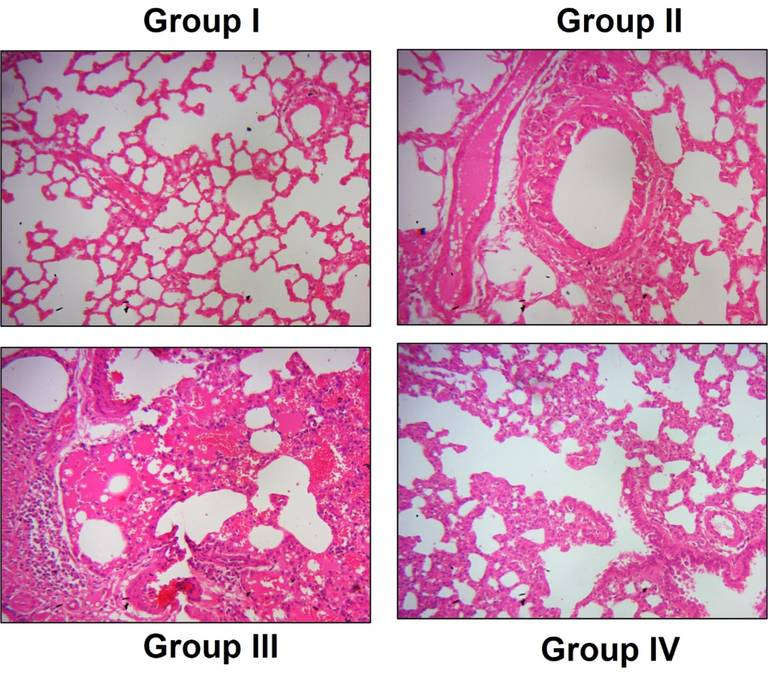
Nigericin protected lung tissue histomorphology of LPS challenged mice. A) Control, B) LPS alone challenged C) LPS + 10 mg/kg nigericin treated, D) LPS + 20 mg/kg nigericin treated. Intact lung histoarchitecture without the evidence of fibrosis, edema was observed in Control group. Thickened alveolar septa with increased neutrophils infiltration in LPS alone challenged mice. Nigericin treated mice shown reduced fibrosis and neutrophil infiltration with decrease in alveolar thickness. Magnification: 40×.
4 Discussion
Sepsis is one of the foremost diseases that affect millions of people globally every year. Even though the mortality rates due to sepsis were slightly declining, an average of 25% of patients still suffer from sepsis, and the hospitalized sepsis patient’s mortality rate had increased to 60% (Vincent et al., 2019). Uncontrolled host defense responses to infectious pathogens are the primary cause of sepsis and its related diseases. Severe sepsis leads to acute lung injury and acute respiratory distress syndrome. To date, no specific therapeutics are available to treat sepsis; only broad-spectrum antibiotics are prescribed (Levy et al., 2015). Therefore, in this study, we assessed the potency of nigericin, an antibiotic, against LPS-induced sepsis and acute lung injury.
Severe sepsis leads to acute lung injury, which is characterized by pulmonary edema, respiratory distress, and hypoxemia (Trepte et al., 2016; Saguil and Fargo, 2020). Untreated acute lung injury progresses to a clinical condition called acute respiratory distress, which causes increased morbidity and mortality (Schmickl et al., 2015). LPS-induced animal models are widely used to assess the drug's efficacy against acute lung injury (Chen et al., 2010). LPS is a prime component present on the outer cell wall membrane of gram-negative bacteria. LPS treatment in animals triggers an immune and inflammatory response that mimics the acute lung injury condition in humans (Hu et al., 2017; Niu et al., 2017). Hence, in our study, we also used an LPS-induced sepsis model to assess the efficacy of nigericin to treat acute lung injury.
The recognition of PAMPs or DAMPs by the host defense cells is the prime signal for the defense mechanism. Monocytes of antigen-presenting cells possess TLR, which is activated by PAMPs, thereby initiating the synthesis of inflammatory cytokines and adaptive immunity (Rubio et al., 2019). Neutrophil infiltration is the first line of defense after the recognition of PAMPs (Drifte et al., 2013; Bermejo-Martin et al., 2016). During sepsis, an increased number of immature neutrophils and delayed neutrophil apoptosis are reported (van der Poll et al., 2017). The increased production and release of neutrophils triggers platelet agonists, chemokines, and cytokines, which result in hypercoagulation and host endothelial tissue damage (Cox et al., 2020; Ortmann and Kolaczkowska, 2018). In our study nigericin treatment significantly decreased the neutrophils count in BALF which was triggered by the LPS. This proves the potency of nigericin to inhibit neutrophil-induced endothelial damage in alveolar cells. Nigericin also decreased the count of eosinophils, macrophages, and lymphocytes in BALF; this was the reason for reduced pulmonary edema in nigericin-treated LPS challenged mice. Acute eosinophilic inflammation due to eotaxin ruptures cells and increases the level of LDH, which acts as a biomarker of cytotoxicity (Gerlofs-Nijland et al., 2005; Lee et al., 2016). Nigericin treatment significantly reduced the LDH levels in LPS challenged mice, which indicates its cytoprotective effect.
Activated infiltration of neutrophils releases myeloperoxidase, which triggers the synthesis of free radicals and hypochlorous acid, causing cellular damage (Haegens et al., 2008). The imbalance in antioxidant status leads to increased synthesis of proinflammatory cytokines and neutrophil infiltration, which leads to mitochondrial impairment in cells (Andrades et al., 2011). In our study, LPS-challenged mice significantly increased the levels of MPO and LPO. The antioxidant levels of SOD and glutathione were also drastically decreased in LPS-challenged mice, confirming the oxidative stress in lung tissues. Whereas nigericin significantly increased the levels of antioxidants glutathione, SOD and prevented lung tissue from MPO induced lipid peroxidation.
The host's innate immune system is the prime warrior for most pathogenic infections. The innate immune system triggers cytokine and chemokine release immediately after an encounter with pathogens. In sepsis patients, this innate immune system is deregulated, and excessive proinflammatory cytokines are secreted, which damage the host tissue (Iwasaki and Medzhitov, 2004; Takeuchi and Akira, 2010; van der Poll et al., 2017). Immunomodulatory and anti-inflammatory therapies seem to be effective in treating sepsis patients (Vincent et al., 2002; Annane et al., 2015). Hence, in this study, we analyzed the anti-inflammatory potency of nigericin against LPS-induced proinflammatory cytokine synthesis. Nigericin treatment significantly decreased the levels of TNF-α and IL-6 in LPS-challenged mice, signifying its anti-inflammatory property.
Sepsis patients exhibit increased levels of nitric oxide and prostaglandins, along with proinflammatory cytokines (Russell, 2006). The increase in nitric oxide and prostaglandin E2 leads to sepsis-induced systemic inflammation. Synthesis of prostaglandin E2 requires cyclooxygenase 2, which is reported to be triggered by LPS (Murakami and Ohigashi, 2007; Chang et al., 2012). Hence, a drug that inhibits these immunomodulators (iNOS, COX-2, and PGE-2) may effectively attenuate the sepsis-induced inflammation. Nigericin significantly decreased the levels of iNOS, COX-2, PGE-2, and proinflammatory cytokines.
Nigericin significantly decreased the levels of the pro-apoptotic proteins BAX and Caspase-2 and increased the levels of the anti-apoptotic Bcl2 protein in LPS-challenged mice. This indicates nigericin significantly scavenged the free radicals, myeloperoxidase, and lactate dehyderogenase activity induced by LPS and thereby prevented alveolar cells from apoptosis. Our macroscopic examination of lung tissue in nigericin-treated LPS challenged mice confirms that the nigericin treatment significantly prevented the LPS-induced endothelial and epithelial alveolar cell barrier disruption. Nigericin inhibited neutrophil infiltration and prevented LPS-induced pulmonary edema and acute lung injury in a mouse model.
5 Conclusion
Nigericin, an antibiotic polyether ionophore often used as a non-hormonal growth regulator in medicine, possesses anti-inflammatory properties. In this study, we assessed the potency of nigericin to inhibit LPS induced sepsis and alleviate acute lung injury in an in vivo model. Nigericin treatment significantly prevented neutrophil and eosinophilic infiltration, the hallmark event in sepsis, and decreased LDH and MPO activity in LPS challenged mice. It also effectively suppressed oxidative stress and inhibited pro-inflammatory cytokines and inflammatory mediators. Nigericin prevented apoptosis in alveolar cells and lung tissue damage in LPS-challenged mice, as evidenced by our macroscopic analysis of lung tissue sections. Over all, our results confirm that nigericin is a potent antibiotic that inhibits sepsis-induced acute lung injury in an LPS-challenged mouse model.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP2023R383), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pharmacological modulation of lung cancer cells for potassium ion depletion. Anticancer. Res.. 2005;25:2609-2616.
- [Google Scholar]
- Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J. Surg. Res.. 2011;167(2):e307-e313.
- [Google Scholar]
- Defining immunological dysfunction in sepsis: A requisite tool for precision medicine. J. Infect.. 2016;72(5):525-536.
- [Google Scholar]
- Ionophores: their use as ruminant growth promotants and impact on food safety. Curr. Issues Intest. Microbiol.. 2003;4(2):43-51.
- [Google Scholar]
- Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem.. 2012;134(2):972-979.
- [Google Scholar]
- The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med.. 2010;4(6):773-783.
- [Google Scholar]
- Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol.. 2020;20(1):15.
- [Google Scholar]
- Nigericin selectively targets cancer stem cells in nasopharyngeal carcinoma. Int. J. Biochem. Cell Biol.. 2013;45:1997-2006.
- [Google Scholar]
- Decreased lipid peroxidation in the rat kidney during gestation. Biochem. Biophys. Res. Commun.. 1987;145(1):134-138.
- [Google Scholar]
- Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med.. 2013;41(3):820-832.
- [Google Scholar]
- International forum of acute care trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med.. 2016 Feb 1;193(3):259-272.
- [Google Scholar]
- Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med.. 2013;41(5):1167-1174.
- [Google Scholar]
- U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am. J. Respir. Crit. Care Med.. 2011;183(4):462-470.
- [Google Scholar]
- Evidence of nigericin as a potential therapeutic candidate for cancers: A review. Biomed. Pharmacother.. 2021;137:111262
- [Google Scholar]
- Effects of particulate matter on the pulmonary and vascular system: time course in spontaneously hypertensive rats. Part. Fibre Toxicol.. 2005;2(1):2.
- [Google Scholar]
- Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J.. 2008;31(2):252-260.
- [Google Scholar]
- Liver X Receptor Agonist TO901317 Attenuates Paraquat-Induced Acute Lung Injury through Inhibition of NF-κB and JNK/p38 MAPK Signal Pathways. Biomed Res. Int.. 2017;2017:4652695.
- [Google Scholar]
- Toll-like receptor control of the adaptive immune responses. Nat. Immunol.. 2004;5:987-995.
- [Google Scholar]
- Sepsis-Pathophysiology and Therapeutic Concepts. Front Med (Lausanne).. 2021;14(8):628302
- [Google Scholar]
- Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv.. 2010;23(4):243-252.
- [Google Scholar]
- Polyether ionophores: broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin. Drug Discov.. 2009;4(2):109-146.
- [Google Scholar]
- Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part. Fibre Toxicol.. 2016;13(1):30.
- [Google Scholar]
- Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit. Care Med.. 2015;43(1):3-12.
- [Google Scholar]
- Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci.. 2017;18(12):2637.
- [Google Scholar]
- Nigericin exerts anticancer effects on human colorectal cancer cells by inhibiting Wnt/β-catenin signaling pathway. Mol. Cancer Ther.. 2018;17(5):952-965.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-474.
- [Google Scholar]
- Pharmacological targeting of constitutively active truncated androgen receptor by nigericin and suppression of hormone-refractory prostate cancer cell growth. Mol. Pharmacol.. 2010;78:846-854.
- [Google Scholar]
- Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur. Respir. J.. 2012;39(1):156-162.
- [Google Scholar]
- The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40(5):375-381.
- [Google Scholar]
- Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int. J. Cancer. 2007;121(11):2357-2363.
- [Google Scholar]
- Cavidine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via NF-κB Signaling Pathway in vivo and in vitro. Inflammation. 2017;40(4):1111-1122.
- [Google Scholar]
- Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res.. 2018;371(3):473-488.
- [Google Scholar]
- Comparison of response to LPS-induced sepsis in three DBA/2 stocks derived from different sources. Lab Anim Res.. 2021;37(1):2.
- [Google Scholar]
- Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc.. 2006;1(6):3159-3165.
- [Google Scholar]
- Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N. Engl. J. Med.. 2017;377(5):414-417.
- [Google Scholar]
- Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect. Dis.. 2019;19(12):e422-e436.
- [Google Scholar]
- Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211.
- [Google Scholar]
- Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician.. 2020;101(12):730-738.
- [Google Scholar]
- Comparison of hospital mortality and long-term survival in patients with acute lung injury/ARDS vs cardiogenic pulmonary edema. Chest. 2015;147(3):618-625.
- [Google Scholar]
- Lung, and blood institute acute respiratory distress syndrome (ARDS) Clinical TRIALS network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med.. 2006;354(16):1671-1684.
- [Google Scholar]
- Inducible nitric oxide synthase during the late phase of sepsis is associated with hypothermia and immune cell migration. Lab. Invest.. 2018;98(5):629-639.
- [Google Scholar]
- Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides. 2014;61:56-60.
- [Google Scholar]
- Electrical impedance tomography (EIT) for quantification of pulmonary edema in acute lung injury. Crit. Care. 2016;22(20):18.
- [CrossRef] [Google Scholar]
- The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol.. 2017;17(7):407-420.
- [Google Scholar]
- Bronchoalveolar lavage of murine lungs to analyze inflammatory cell infiltration. J. Vis. Exp.. 2017;123:55398.
- [Google Scholar]
- Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis.. 2002;34:1084-1093.
- [Google Scholar]
- Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit. Care. 2019;23(1):196.
- [Google Scholar]







