Translate this page into:
Novel supercapacitor electrodes based semiconductor nanoheterostructure of CdS/rGO/CeO2 as efficient candidates
⁎Corresponding authors. a.bumajdad@ku.edu.kw (Ali Bumajdad), f.alsagheer@ku.edu.kw (Fakhreia Al Sagheer)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, we have synthesized metal oxide/metal sulphide based nanoheterostuctures mediated with graphene nanosheets. The synthesized nanoheterostructures were characterized via different techniques such as XRD, XPS, and TEM. The electrochemical characteristics of the investigated nanoheterostucture (CdS/rGO/CeO2) were investigated through electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and galvanostatic charge-discharge. The specific capacitance of the single rGO, binary CdS/CeO2 and ternary CdS/rGO/CeO2 heterostructures were measured. The CdS/rGO/CeO2 nanoheterostucture showed the most excellent cycling stability with high specific capacitance of 407 F g−1 achieved at a charge-discharge rate of 1 A/g. The investigated supercapacitor retained about 96% of the initial energy density after charge-discharge at a 10 A/g for 5000 cycles. The ternary CdS/rGO/CeO2 nanoheterostucture revealed the best specific capacitance as the graphene nanosheets increased interfacial electron transfer. The results revealed that the investigated novel nanoheterostucture is among the best reported ones in the literature.
Keywords
Nanoheterostucture
Semiconductors
Galvanostatic charge-discharge
Specific capacitance
1 Introduction
It is of great importance to develop a new non-polluting (environmentally friendly) renewable energy source and energy storage systems to overcome the fossil fuels related problems and the energy demand. One of the most important energy storage systems recently used are the supercapacitors which known also as ultracapacitors or electrochemical capacitors that can provide large amounts of energy in short time to be used in different important applications (Miller and Simon, 2008). The main advantages of using supercapacitors are their fast charging-discharging process, high power density, long cycle life, and the ability to use in wide range of temperatures. Carbonaceous based supercapacitors which have high electrical conductivity, surface area and porosity showed high power density and energy (Frackowiak, 2007). Carbonaceous materials including carbon nanotubes (CNT), fullerenes and graphene (rGO) have remarkable and unique chemical, physical, and electronic characteristics that make them attractive for next generation miscellaneous field of research such as supercapacitors, electronics, batteries, fuel cells, electrochemical sensors, biosensors, and medicinal applications (Li et al., 2012). Once discovered in 2004, Graphene has attracted a great attention owing to its exceptional thermal, mechanical and optoelectronic characteristics. Graphene exhibits excellent charge carrier mobility and a large surface area, which enables it to be a targeted candidate to be used as an electron mediator to accomplish efficient charge separation in electron transfer processes (Wang et al., 2013). Graphene-based supercapacitors offer good flexibility and thermal stability (Li et al., 2013). To enhance the specific capacitance and the electrochemical properties of the prepared graphene based supercapacitors, different strategies was investigated by researchers including studying ternary composites to be uses as supercapacitors (Sankar and Selvan, 2015; Han et al., 2014). The fabrication of a heterostructure is an effectual approach to enhance the electrical activity because of the blend of merits of each component (Han et al., 2014). In the heterostructure; graphene could acts as an excellent electron mediator and a gap linker to improve the charge separation efficiency (Wang et al., 2010).
Also, metal oxides have been widely used for application in supercapacitors due to their different oxidation states for pseudocapacitance generation (Huang et al., 2015). From these metal oxides, MnO2, RuO2 and IrO2 have been widely studied as redox supercapacitor candidates depending on their high pseudocapacitance resulted from their fast redox reactions (Xue et al., 2007). The most important one is RuO2 but its toxicity, high cost and abundance limit its use in different applications (Chen et al., 2004). Cerium oxide, Ceria, (CeO2), as one of the rare earth metal oxides, with a wide band gap (Eg = 3.15 eV), has attracted extreme significance. Ceria has the fluorite structure, which is stable from room temperature till melting point. It plays an essential role in environmental and energy correlated applications, such as solar cells, catalysis, capacitors and fluorescence (Bumajdad et al., 2009). CeO2 is considered as good alternative to be used due to its abundance, environmental safe, low cost and excellent electrochemical redox properties (Bumajdad et al., 2006; Kalubarme et al., 2013). Moreover, the cerium oxidation state can change quickly between III and IV which facilitate its application as superconductors (Su et al., 2013). The main problem of using metal oxides is their poor electrical conductivity which makes their surface part only contribute in the total capacitance while the other interior parts are difficult to contribute in the charge storage process which decreases the total performance of the supercapacitors. Also using carbonaceous ternary nanocomposites was one of the effective strategies to solve this problem. On contrast, CdS semiconductor nanoparticles have excellent electrical, physical and optical properties, which make it a good candidate for wide range of applications such as solar cells, electrochemical biosensor, light detectors, photocatalysis and supercapacitors (Zhang and Zhu, 2010; Cao et al., 2010; Yang et al., 2010). Zhang et al., studied CdS nanoparticles with 3D rGO which exhibited a high specific capacitance of 300 F/g at a scan rate 5 mV/s, which confirms the possibility of 3D rGO/CdS architecture to be used in energy storage applications (Zhang et al., 2016).
In this study, a nanoheterostucture based on metal oxide/metal sulphide semiconductors mediated with rGO nanosheets have been synthesized. To best of our knowledge, this nanoheterostructure has not been ever studied. Moreover, the pseudocapacitive performance of single rGO, binary CdS/CeO2 and ternary CdS/rGO/CeO2 heterostructures in 2 M KOH has been investigated and reported. The ternary CdS/rGO/CeO2 nanoheterostucture revealed the best specific capacitance as the graphene nanosheets increased interfacial electron transfer. The results revealed that the investigated novel nanoheterostucture is among the best reported materials in the literature.
2 Experimental
2.1 Materials
All chemicals (cerium(III)nitrate hexahydrate, sodium hydroxide, sodium nitrate, graphite powder,sulphuric acid, potassium permanganate,hydrazine hydrate, hydrogen peroxide, sodium sulphide, polyvinylalcohol, polyvinyl pyrrolidone,cadmium nitrate) and solvents (ethanol and acetone) were of analytical grade (Sigma-Aldrich) and were used as received without any further purification.
2.2 Synthesis of nanoparticles
2.2.1 Preparation of graphene oxide
Graphene oxide was prepared according to the modified Hummer's method (Hummers and Offeman, 1958; Liang et al., 2011) in which 2.0 g of graphite powder was added into a 250 ml beaker, followed by the addition of 1.0 g sodium nitrate NaNO3 and 46 ml H2SO4 into it sequentially under stirring in an ice-bath at 0 °C. Then KMnO4 (6.0 g) was added slowly into the beaker under stirring and control the temperature below 20 °C. The ice-bath was removed after 5.0 min and heated at 35 °C for 30 min, then 92 ml D.I H2O was added slowly into the system and the temperature increased up to 98 °C and stirring was continued for another 30 min. Then 280 ml hot water with 60 °C and 30% Hydrogen peroxide, H2O2, (30 ml) aqueous solution were added to reduce the residual KMnO4 till no bubble was appeared. Finally, the system was centrifuged at 7200 rpm for 10 min, and the obtained powder was washed by warm water until the initially black colored mixture changes into a reddish-brown color. For preparation of graphene, 0.1 g of the prepared GO was sonicated in 20 ml deionized water until obtaining a homogeneous yellow dispersion. 100 ml of hydrazine hydrate was added as reducing agent into the solution and transferred inside a conventional microwave. The microwave oven was operated at a full power (1000 W) in 30 s cycles (on for 10 s, off for 20 s) for a total reaction time of 60 s. The yellow color of GO was changed to a black color indicating complete reduction to graphene.
2.2.2 Synthesis of CdS quantum dots nanoparticles
The CdS QDs were prepared by adding 0.2 M cadmium nitrate to a 5% aqueous solution of PVA/PVP (50:50, %wt) under continuous stirring. After that, an equal volume of 0.2 M sodium sulphide was added dropwise to the solution with continuous stirring. Then, the solution was transferred to a 100 ml Teflon-lined stainless steel autoclave inside an oven at 110 °C for 5 h. Finally, the product was washed three times with distilled water then centrifuged at a speed of 8000 rpm. The obtained yellow precipitate of CdS QDs was dried overnight in a vacuum oven at 40 °C.
2.2.3 Synthesis of CeO2 nanoparticles
CeO2 nanoparticles were prepared by a modified precipitation method. In a typical procedure, a 0.03 M of Ce(NO3)3·6H2O was dissolved in distilled water. Then, this solution was transferred portion wise to a beaker containing 100 ml of NaOH solution with a constant pH value of 13. A precipitate was formed, washed with distilled water, then centrifuged at a speed of 8000 rpm for 10 min. The washed precipitate was dried overnight in a vacuum oven at 40 °C.
2.2.4 Synthesis of rGO/CdS heterostructured nanoparticles
To a 5% aqueous solution of PVA/PVP (50:50,%wt), 2% rGO suspension (v/v) (6 mg) was added and the solution allowed stirring. rGO/CdS NPs were prepared by adding certain volume of 0.2 M cadmium nitrate to the prepared solution under continuous stirring. After that, certain volume of 0.2 M sodium sulphide was added dropwise to the solution under continuous stirring. Then, the solution mixture was transferred to a 100 ml Teflon-lined stainless steel autoclave inside an oven at 110 °C for 5 h. Finally, the product was washed three times with distilled water then centrifuged at a speed of 8000 rpm. The obtained precipitate of rGO/CdS NPs was dried overnight in a vacuum oven at 40 °C.
2.2.5 Synthesis of hybrid heterostructures of CdS/CeO2 and CdS/rGO/CeO2
Firstly, 0.03 M of Ce(NO3)3·6H2O was dissolved in a dispersion of CdS and CdS/rGO suspensions (0.45 g for each) with stoichiometric amounts in order to obtain CdS/CeO2 and CdS/rGO/CeO2 heterostructures with 1:2 and 1:0.01:2 respectively. Then, this solution was transferred to a beaker containing 100 ml of NaOH solution. The pH value of the solutions during experiment should maintained constant to around 13. A precipitate was formed, washed with distilled water, then centrifuged at a speed of 8000 rpm for 10 min. The washed precipitate was dried overnight in a vacuum oven at 40 °C.
2.3 Characterization
The X-ray powder diffraction (XRD) analysis was conducted using D8 Advance diffractometer with a copper target and nickel filter with CuKα radiation (λ = 0.154056 nm). The shape of the nanoparticles was investigated using transmission electron microscopy (TEM) using a JEOL JEM 1230 operating at 120 kV. X-ray photoelectron spectroscopy (XPS) measurements were conducted using Thermo ESCA Lab 250xi equipped with Mg Kα radiation (1253 eV).
2.4 Electrochemical measurements
Potentiostat/Galvanostat model Gamry 3000 was used for electrochemical measurements such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge-discharge. Glassy carbon electrode coated with the investigated CdS/rGO/CeO2 nanoheterostructure was used as working electrode. Also, saturated calomel electrode and platinum foil were used as reference and counter electrodes, respectively. 2 M KOH solution was used as electrolyte. Electrochemical impedance spectroscopy measurements were conducted at Eapp = 0.0 V with ac voltage of 5 mV in the frequency range from 0.01 Hz to 100 kHz. Echem analyst software was used for data analysis. For preparing the working electrode, mixture of 2 mg of CdS/rGO/CeO2 powder in 700 µL isopropyl alcohol with 6 µL Nafion was vigorously sonicated for 2 h. Then the working electrode was prepared by drop casting of 10 µL of the mixture onto glassy carbon electrode.
3 Results and discussion
3.1 Charaterization of nanoparticles
X-ray photoelectron spectroscopy (XPS) spectra of the prepared CdS/rGO/CeO2 nanoheterostructure Fig. 1) indicates the presence of Cd, S, O, Ce, and C signals. The binding energy of peaks corresponding to Cd 3d5/2 and Cd 3d3/2 peaks for CdS are observed at 405.5 eV and 412.2 eV in Fig. 1A (Zhao et al., 2010). The two obvious peaks observed at 161.7 eV and 162.9 eV in Fig. 1B correspond to the characteristic S 2p3/2 and S 2p1/2 peaks of S2− for CdS NPs (Jing and Guo, 2007). The C1s spectrum of rGO sheets shown in Fig. 1C reveals a sharp peak at 284.5 eV that corresponds to C—C bonds of carbon atoms in the honeycomb lattice. Peaks at 285.6, and 287.7 eV could be assigned to different C—O bonding configurations due to the harsh oxidation which destroyed the sp2 atomic structure of graphite (Some et al., 2012).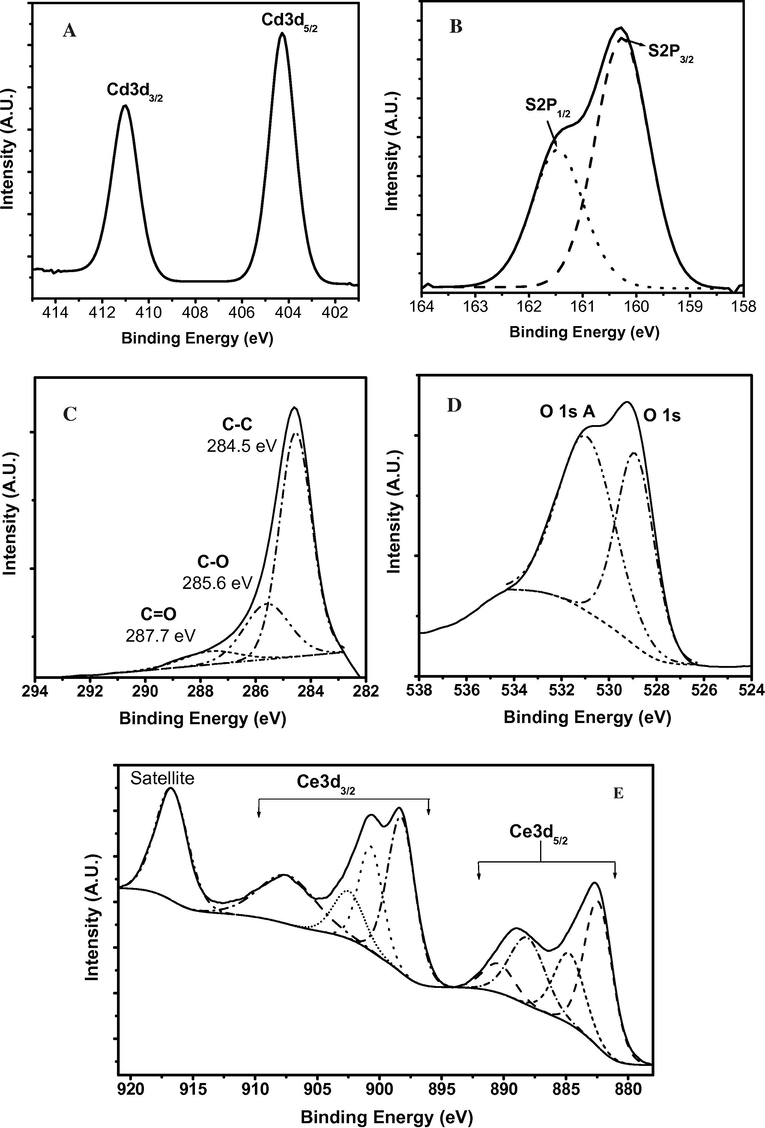
XPS spectra for Cd 3d, S 2p, C 1s, O 1s and Ce 3d for CdS/rGO/CeO2 nanoheterostructure.
The O 1s spectrum in Fig. 1D showed three peaks at 529.1 eV, 531.9 eV and 533.7 eV. The shoulder appeared at 531.9 eV was assigned to the adsorbed oxygen and weakly bonded oxygen species. The peak at 533.7 eV is belonged to oxygen from hydroxide or adsorbed water on the catalyst surface. Both peaks confirmed the existence of surface oxygen vacancies in CeO2. However, the weakest peak, located at 529.1 eV, was attributed to lattice oxygen in the anchored CeO2 nanocrystals (Wang et al., 2011; Wang et al., 2013; Shan et al., 2012). Generally, surface adsorbed oxygen is more reactive than lattice oxygen in oxidation reactions because of its higher mobility (Shan et al., 2012). Regarding the Ce 3d spectrum Fig. 1E), the six peaks observed and denoted as (882.5 eV), (889.3 eV), (898.5 eV), (901.0 eV), (907.5 eV) and (916.8 eV), can be assigned to Ce 3d of CeO2, which are identical with the reported values (Primo et al., 2011; Yang et al., 2014).
Fig. 2 presents the typical XRD patterns of the as-prepared rGO, CeO2, CdS, CdS/CeO2 and CdS/rGO/CeO2 nanoheterostructures. Diffraction peaks of the bare CeO2 at 28.8°, 33.3°, 47.6° and 56.4° can be indexed as the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of the face-centered cubic structure of CeO2 (JCPDS No. 34-0394), respectively (You et al., 2016). And the peaks of the bare CdS at 26.5°, 44.0° and 52° can be characterized as the (1 1 1), (2 2 0) and (3 1 1) planes of the cubic structure of CdS (JCPDS No.42-1411), respectively (You et al., 2016). The crystallite sizes of the synthesized nanoheterostructures were calculated using the Debye-Scherrer equation Eq. (1):
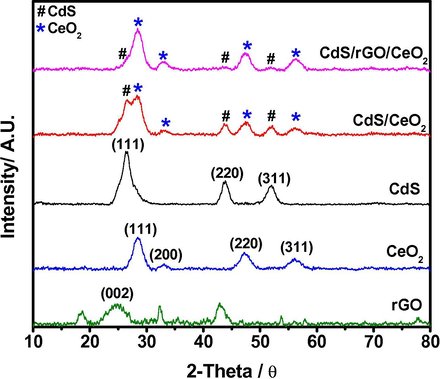
XRD patterns of the as-prepared rGO, CeO2, CdS, CdS/CeO2 and CdS/rGO/CeO2 nanoheterostructure.
To have better insight on the morphology, TEM analysis was carried out. From TEM images, non-aggregated and well dispersed spherical nanoparticles were obtained for CdS and CeO2 nanoparticles. From Fig. 3B, it can be found that the CeO2 NPs with an average diameter around 3.9 nm. As shown in Fig. 3C, the highly ordered CdS QDs displayed an average diameter of about 3.4 nm. Fig. 3D shows a typical TEM image of CdS/CeO2 heterostructure which showed homogeneous distribution of the heterostructure with an average particle size of 4.8 nm which is most likely resulted from the adjacent contact of CdS and CeO2 nanoparticles. The CdS/CeO2 heterostructures are seen along with smooth rGO sheets as shown in Fig. 3E and F.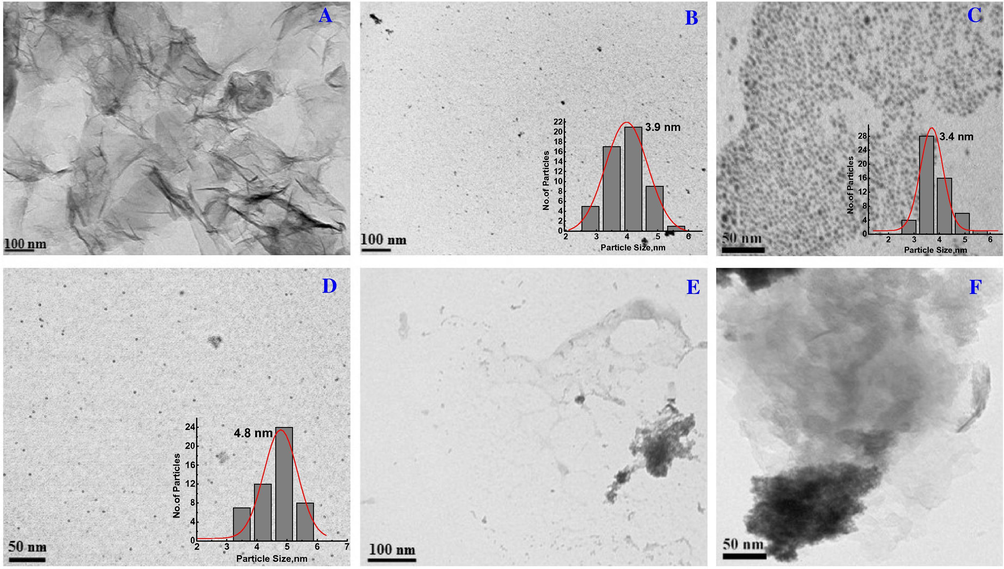
TEM images of the as-prepared (A) rGO, (B) CeO2, (C) CdS, (D) CdS/CeO2 and (E) and (F) CdS/rGO/CeO2 nanoheterostructure.
3.2 Electrochemical performance
The electrochemical behavior of rGO, CdS/CeO2 and CdS/rGO/CeO2 nanoheterostructures was studied by cyclic voltammetry (CV) measurements in 2 M KOH aqueous electrolyte in a potential window of 0–1 V at a scanning rate of 10 mV s−1 as shown in Fig. 4A. The cyclic voltammetric curve of rGO features perfect rectangular and symmetric shaped curve, indicative of the perfect electrical double-layer capacitance behavior (Chen et al., 2012). In case of CdS/rGO/CeO2 the CV curve shows a remarkable increase in the current density and the electrochemical area compared with CdS/CeO2. Introducing the reduced graphene oxide to CdS/CeO2 pronouncedly increased the electron transfer and also the peak current between the electrode and the investigated compound (Lei et al., 2014). Obtaining one pair of redox peaks in cyclic voltammetric curve confirms that the capacitance behavior of CdS/rGO/CeO2 is typical pseudocapacitance. By increasing the scan rate, CV curves for CdS/rGO/CeO2 nanoheterostructure maintained its shape emphasizing the good capacitive behavior and reversibility and hence the electrochemical stability Fig. 4B). The specific capacitance (C, in F g−1) of the electrode was calculated using cyclic voltammetry according to the following equation:
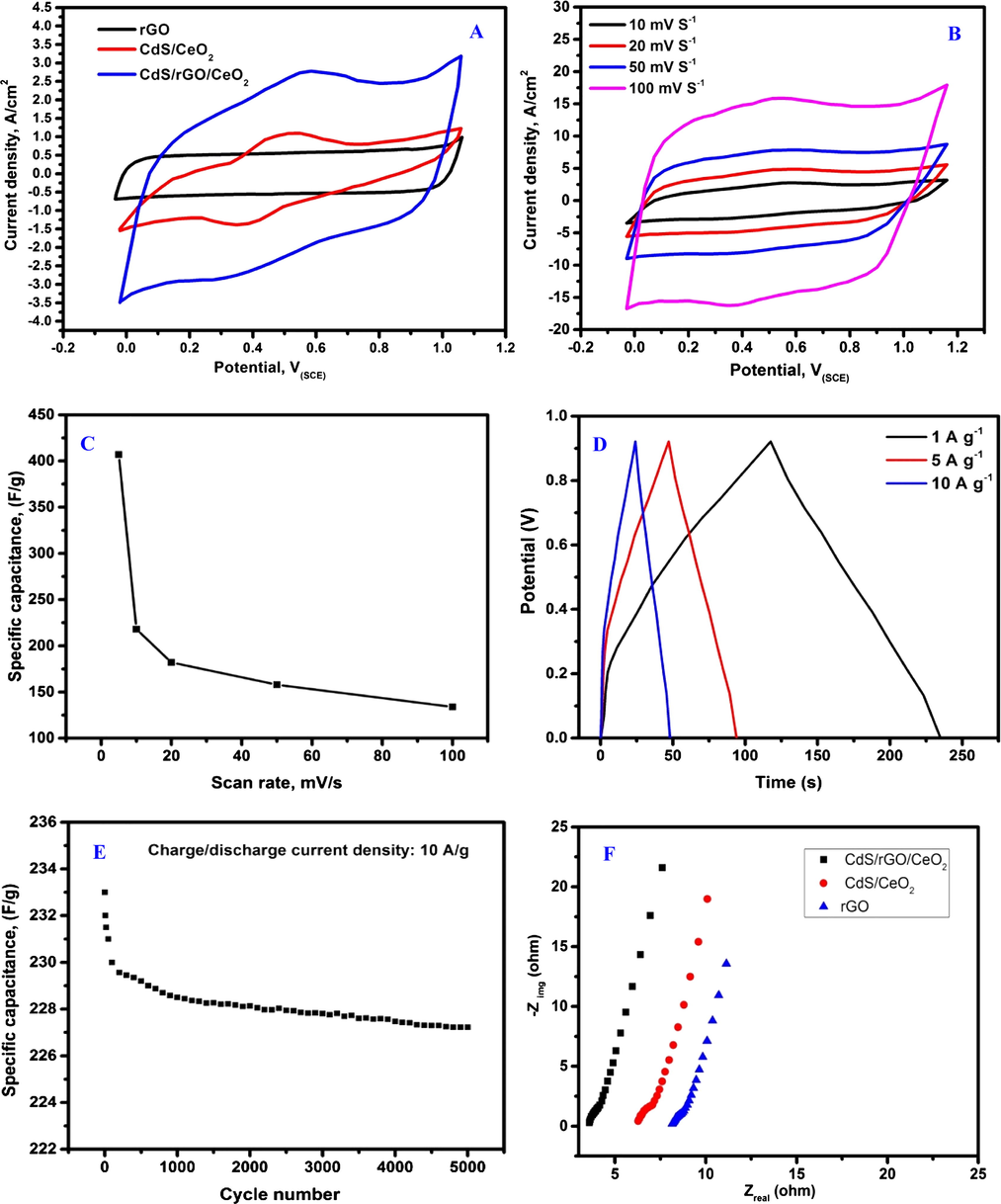
Electrochemical performance of the CdS/rGO/CeO2 nanoheterostructure measured in 2 M KOH solution at room temperature. (A) CV curves of the rGO, CdS/CeO2 and CdS/rGO/CeO2 electrodes measured at a scan rate of 10 mV s−1, (B) CV curves of CdS/rGO/CeO2 at different scan rates, (C) plots of the scan rate versus the specific capacitance of the CdS/rGO/CeO2 electrode, (D) charge/discharge curves at different current densities, (E) cycling performance of the CdS/rGO/CeO2 electrode at a current density of 10 A g−1 and (F) Nyquist plots of the rGO, CdS/CeO2 and CdS/rGO/CeO2 electrodes.
Fig. 4C showed the relation between the specific capacitance of the CdS/rGO/CeO2 nanoheterostructure and the scan rate. From this figure it is clear that increasing the scan rate (5–100 mV s−1 resulted in decreasing the calculated specific capacitance. Different factors affect the specific capacitance value such as the diffusion of ion in the electrolyte, the adsorption of ions on the electrode surface and the ability to charge transfer in the studied electrode material (Deng et al., 2017).
In our case, decreasing the capacitance with increasing the scan rate explained with the insufficient electrolyte ions limited by the diffusion and time constraints and in this case the charge storage will performed using the ions of outer active surface only. While at low scan rate, the charge storage increases due to the diffusion of the electrolyte ions into the electrode surface using all the active site for charge storage and hence the specific capacitance increases. From the results obtained, the highest specific capacitance achieved for CdS/rGO/CeO2 electrode at scan rate 5 mV S−1 was found to be 407 F g−1.
Improving the electrochemical performance of CdS/rGO/CeO2 nanoheterostructure is related to its unique structure due to the improved conductivity of CdS and CeO2 and the good utilization of graphene which facilitate the electron transport through its conductive network (Deng et al., 2017). Also the good dispersion of the nanoparticles of CdS and CeO2 in rGO pronouncedly enhances the diffusion of electrolyte ions without significant loss or recombination. As shown in Fig. 4, the value of specific capacitance was constant at 134 F g−1 after increasing the scan rate up to 100 mV/s. The specific capacitance obtained using the investigated nanoheterostructure was found to be higher than the reported values for different graphene based nanoheterostructure at the same conditions (Saravanan et al., 2015; Chen and Xue, 2015; Dezfuli et al., 2015). The electrochemical capacitance of CdS/rGO/CeO2 electrode was also calculated using galvanostatic charge-discharge measurements at current densities of 1, 5, 10 A/g as depicted in Fig. 4D. The charge-discharge curves showed the same behavior indicating its electrochemical reversibility. Due to the pseudocapacitance behavior of the CdS/rGO/CeO2 nanoheterostructure, nonlinear discharge curve was obtained resulting from the redox reaction at electrode-electrolyte interface (Peng et al., 2011).
The specific capacitances calculated using the equation reported elsewhere (Nagamuthu et al., 2013) are 407, 280 and 233 F/g at the current density of 1, 5, 10 A/g, respectively. The results confirms that the capacitance still high even after increasing the applied current. The enhanced specific capacitance of CdS/rGO/CeO2 could be attributed to the better conductivity of CdS/rGO/CeO2 due to the presence of rGO in the heterostructure. Studying the cyclic stability is an important measurement for supercapacitor materials. Fig. 4E depicted the effect of the continuous galvanostatic charge-discharge for the CdS/rGO/CeO2 architecture on the specific capacitance for 5000 cycle at current density of 10 A/g. From this figure it is obvious that the CdS/rGO/CeO2 retains about 96% of its initial values after 5000 cycle. CdS/rGO/CeO2 electrode showed large specific capacitance, high stability and reversibility which might be helps in development of a promising supercapacitor.
The electrochemical impedance spectroscopy (EIS) technique was used to understand the reason for the significant electrochemical behavior of the CdS/rGO/CeO2 nanoheterostructure compared with CdS/CeO2, in the frequency range from 0.01 Hz to 100 kHz. Fig. 4F, shows Nyquest plots of rGO alone and the investigated nanoheterostructure in 2 M KOH solution. From this figure it is clear that all curves in the low frequency region contain a straight line resulted from Warburg impedance while in the high frequency region a semicircle was obtained resulted from the resistance and capacitance.
In comparison, CdS/rGO/CeO2 heterostructure showed EIS plot with vertical slope higher than the plot obtained in case of CdS/CeO2 heterostructure or rGO at low frequency, illustrating the highest capacitive behavior of CdS/rGO/CeO2 heterostructure and the lower diffusion resistance of electrolyte ions in the electrode compared with CdS/CeO2 or rGO, which agreed well with the cyclic voltammetric data. Also from EIS plot it is clear that the CdS/rGO/CeO2 showed shorter semicircles radius at the high frequency region compared with CdS/CeO2 or rGO, which means decreasing the charge transfer resistance on the electrode surface and hence increasing interfacial electron transfer which suggest great conductivity for ion transport resulted from the presence of graphene which has excellent conductivity (Ye et al., 2015).
4 Conclusion
In summary, we have successfully fabricated a CdS/rGO/CeO2 nanoheterostructure via a hydrothermal method. The investigated nanoheterostructure showed a well-distributed particle size and exhibited excellent electrochemical performance, such as excellent cycling stability, and good reversibility and stability which is a critical factors in practical applications for supercapacitors. The investigated nanoheterostructure showed high specific capacitance of 407 F/g with retention of about 96% of the initial value even after 5000 charge-discharge cycle. The results confirmed that the prepared electrode material may be used in a wide range of energy storage applications.
Acknowledgements
We would like to gratefully acknowledge the support of Kuwait University, research sector, Project No. SC 10/16 and SAF Facilities No. (GS 03/01 and GS 01/05). Also, the support provided by the nanoscopy science centre at Kuwait University is highly acknowledged.
References
- Characterization of nano-cerias synthesized in microemulsions by N2 sorptiometry and electron microscopy. J. Colloid Interface Sci.. 2006;302(2):501-508.
- [Google Scholar]
- Cerium oxide nanoparticles prepared in self-assembled systems. Adv. Colloid Interface Sci.. 2009;147–148(Supplement C):56-66.
- [Google Scholar]
- Adv. Mater.. 2010;22:103.
- J. Power Sources. 2004;125:292.
- In-situ electrochemical route to aerogel electrode materials of grapheme and hexagonal CeO2. J. Colloid Interface Sci.. 2015;446:77-83.
- [Google Scholar]
- High-performance supercapacitors based on a graphene–activated carbon composite prepared by chemical activation. RSC Adv.. 2012;2:7747-7753.
- [Google Scholar]
- Phys. E: Low-Dimension. Syst. Nanostruct.. 2017;86:284-291.
- A high performance supercapacitor based on a ceria/graphene nanocomposite synthesized by a facile sonochemical method. RSC Adv.. 2015;5:46050-46058.
- [Google Scholar]
- Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys.. 2007;9:1774-1785.
- [Google Scholar]
- MnO2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Sci. Rep.. 2014;4:4824-4830.
- [Google Scholar]
- Synthesis of Co3O4/SnO2@MnO2 core-shell nanostructures for high-performance supercapacitors. J. Mater. Chem. A. 2015;3:12852-12857.
- [Google Scholar]
- J. Am. Chem. Soc.. 1958;80(6):1339.
- Efficient hydrogen production by a composite CdS/mesoporous zirconium titanium phosphate photocatalyst under visible light. J. Phys. Chem. C. 2007;111:13437-13441.
- [Google Scholar]
- One step hydrothermal synthesis of a carbon nanotube/cerium oxide nanocomposite and its electrochemical properties. Nanotechnology. 2013;24:365401-365408.
- [Google Scholar]
- One-step in situ synthesis of CeO2 nanoparticles grown on reduced graphene oxide as an excellent fluorescent and photocatalyst material under sunlight irradiation. PCCP. 2016;18(16):11157-11167.
- [Google Scholar]
- Hydrothermal synthesis of graphene–CdS composites with improved photoelectric characteristics. J Mater Sci: Mater Electron. 2014;25:3057-3061.
- [Google Scholar]
- A nanocomposite of graphene/MnO2 nanoplatelets for high-capacity lithium storage. J. Appl. Electrochem.. 2012;42:1065-1070.
- [Google Scholar]
- Reduced graphene oxide-linked stacked polymer forests for high energy-density supercapacitor. Nano Energy. 2013;2:628-635.
- [Google Scholar]
- J. Mater. Chem.. 2011;21:17654.
- Synthesis of Mn3O4/amorphous carbon nanoparticles as electrode material for high performance supercapacitor applications. Energy Fuel. 2013;27:3508-3515.
- [Google Scholar]
- Synthesis of electrochemically-reduced graphene oxide film with controllable size and thickness and its use in super-capacitor. Carbon. 2011;49:3488.
- [Google Scholar]
- Efficient visible-light photocatalytic water splitting by minuteamounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method. J. Am. Chem. Soc.. 2011;43:123-125.
- [Google Scholar]
- The ternary MnFe2O4/graphene/polyaniline hybrid composite as negative electrode for supercapacitors. J. Power Sources. 2015;275:399-407.
- [Google Scholar]
- Facile synthesis of graphene-CeO2 nanocomposites with enhanced electrochemical properties for supercapacitors. Dalton Trans.. 2015;44:9901-9908.
- [Google Scholar]
- Catal. Today. 2012;184:160-165.
- Dual functions of highly potent graphene derivative poly-L-lysine composites to inhibit bacteria and support human cells. ACS Nano. 2012;6:7151-7161.
- [Google Scholar]
- In situ TEM observation of the electrochemical process of individual CeO2/Graphene anode for lithium ion battery. J. Phys. Chem. C. 2013;117:4292-4298.
- [Google Scholar]
- Appl. Catal. B: Environ.. 2013;130–131:277-284.
- Appl. Catal., B. 2010;100:179.
- CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton Trans.. 2011;40:6388-6391.
- [Google Scholar]
- ChemPhysChem. 2011;12:2763-2770.
- Nanoscale. 2013;5:11291.
- J. Power Sources. 2007;164:953.
- J. Phys. Chem. C. 2010;114:11414.
- Novel Ag3PO4/CeO2 composite with high efficiency and stability for photocatalytic applications. J. Mater. Chem. A. 2014;2:1750-1756.
- [Google Scholar]
- 3D reduced graphene oxide coated V2O5 nanoribbon scaffolds for high-capacity supercapacitor electrodes part. Part. Syst. Charact.. 2015;32:817-821.
- [Google Scholar]
- CdS nanoparticles/CeO2 nanorods composite with high-efficiency visible-light-driven photocatalytic activity. Appl. Surf. Sci.. 2016;363:154-160.
- [Google Scholar]
- Morphological control of RGO/CdS hydrogels for energy storage. Cryst. Eng. Comm.. 2016;18:1090-1095.
- [Google Scholar]
- J. Phys. Chem. C. 2010;114:5822.
- Sunlight photocatalytic activity of CdS modified TiO2 loaded on activated carbon fibers. Appl. Surf. Sci.. 2010;256:3493-3498.
- [Google Scholar]







