Translate this page into:
Protective effects of Pimpinella diversifolia DC. root essential oil against lipopolysaccharide/D-galactosamine-induced acute liver injury in mice through inhibiting inflammation and reducing oxidative stress
⁎Corresponding authors. 20081026@zcmu.edu.cn (Chunchun Zhang), 20141025@zcmu.edu.cn (Meiya Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute liver injury (ALI) is a life-threatening syndrome with few therapeutic drugs are available. Excessive inflammatory response and imbalance of the antioxidant system are the hallmark of ALI. Therefore, anti-inflammatory and anti-oxidant treatment would be beneficial for ALI. Pimpinella diversifolia DC. is a perennial aromatic herb of the Umbelliferae family. The whole herb has been used as a medicine for thousands of years by the She ethnic minority of China, and is claimed to have beneficial health effects on liver disease. Its roots are rich in essential oil, but there are few reports on its pharmacological study. Therefore, the aim of the current work was to reveal the hepatoprotective effects of P. diversifolia root essential oil (PDREO). PDREO was prepared by a simultaneous distillation–extraction method and characterized by gas chromatography–mass spectroscopy (GC–MS). The potential application of PDREO in detoxification of liver injury was evaluated using a lipopolysaccharide (LPS)- and D-galactosamine (D-GalN)-induced acute liver injury mouse model. The results showed that the major constituents of PDREO were isodaucene (29.97%), 4-methoxy-2-(3-methyloxiranyl)-phenyl isobutyrate (19.53%), geijerene isomer (12.33%), and 2-methyl-4-methoxy-2-(3-methyl-2-oxiranyl) phenyl butyrate (6.88%). Isodaucene was identified in the essential oil of the Pimpinella species for the first time. Pre-treatment with PDREO (50 mg/kg) and (150 mg/kg) can effectively reduce the serum levels of alanine aminotransferase and aspartate aminotransferase, and dramatically alleviate liver edema, hemorrhage, hepatocyte apoptosis, and necrosis, which may be attributed to the significant anti-inflammatory activity (inhibiting the secretion of inflammatory cytokines and chemokines and reducing the infiltration of monocyte-derived macrophages and neutrophils) and the regulatory effects on oxidative stress (upregulating the expression of antioxidant enzymes such as GPX4, SOD1, and CAT) of PDREO. In addition, PDREO (150 mg/kg) can completely block the death of mice induced by LPS/D-GalN, while the survival rate of mice treated with the clinical first-line drug N-acetylcysteine (300 mg/kg) was only 10%. In light of the results obtained, PDREO is a promising natural detoxification agent, with potential applications in the food and pharmaceutical industries.
Keywords
Acute liver injury
Antioxidant
Apoptosis
Essential oil
Inflammation
Pimpinella diversifolia DC.
1 Introduction
Acute liver injury (ALI) is a life-threatening syndrome with an acute onset that is associated with immune dysfunction and a high mortality rate (nearly 80%). Clinically, although a variety of causes can lead to ALI, such as viruses, hepatic toxins (including acetaminophen and herbal medicine), alcohol, and idiosyncrasy, the following clinical symptoms will eventually appear: massive hepatocyte apoptosis, necrosis, liver dysfunction, and multiple organ failure (Sowa et al., 2016). However, few therapeutic strategies and drugs other than liver transplantation are available for ALI patients; thus, there is a need for new and effective therapeutic drugs (Kumar et al., 2021).
Clinical studies have shown that intestinal bacterial translocation from the gut into the circulation and the liver is one of the major pathological features of ALI patients. The invading bacteria and their products (e.g., lipopolysaccharide [LPS]) activate the immune system, resulting in the release of large amounts of inflammatory factors, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β (Bajaj et al., 2022). Excessive TNF-α can induce hepatocyte apoptosis, and the above mentioned hepatic insults can further promote the sensitivity of hepatocytes to TNF-α-induced apoptosis by reducing glutathione levels and inhibiting protein expression and RNA transcription, resulting in hepatocyte apoptosis, necrosis, and finally ALI (Janssen et al., 2003; Kim et al., 2005; Matsumaru et al., 2003). In clinical and animal experiments, hepatocyte injury often manifests as elevated levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), which has become a recommended indicator for liver injury evaluation by the US Food and Drug Administration (Shi et al., 2010). Therefore, a mouse model of ALI induced by D-galactosamine (D-GalN) as a hepatocyte sensitizer and LPS as a macrophage activator has been established and widely used to study the pathogenesis of ALI and develop new drugs for ALI therapy (Kusakabe et al., 2021; Nowak et al., 2000). And Serum ALT, AST, and ALP levels are usually measured to evaluate liver injury (Shi et al., 2010) and the hepatic protective effect of drugs (Ferah et al., 2022).
The liver tissue of LPS/D-GalN-induced ALI mice often exhibits high monocyte and neutrophil infiltration, an excessive inflammatory response, imbalance of the antioxidant system, and massive hepatocyte apoptosis (Kusakabe et al., 2021), which is basically consistent with the pathological changes of ALI (Bajaj et al., 2022). Therefore, it is not surprising that anti-inflammatory (Xia et al., 2021), antioxidant (Bemeur et al., 2010), and anti-apoptotic (Jia et al., 2021) agents are used in the treatment of ALI. Essential oils are volatile components produced by various plants that are rich in terpenoids and phenols, with a variety of unique aromatic smells. Many studies have shown that essential oils display a wide variety of biological activities, including anti-inflammatory, antioxidant, anti-bacterial, anti-viral, and anti-tumor effects (Ludwiczuk and Asakawa, 2020; Wilkin et al., 2020). Therefore, essential oils are widely used in the perfume, cosmetics, food, and pharmaceutical industries. In addition, many essential oils have shown potential hepatoprotective effects, which are mainly attributed to their anti-inflammatory (Zhao et al., 2021) and antioxidant activities (Koubaa et al., 2021). Thus, plant essential oils are valuable resources for the screening of anti-ALI drugs.
Pimpinella diversifolia DC. is a perennial herb belonging to the Umbelliferae family. It is mainly distributed in China, Japan, and Southeast Asian countries (Chinese Academy of Sciences Editorial Committee of Flora of China, 2005). Similar to those of Pimpinella anisum L., one of the most extensively and in-depth researched species (Mahboubi and Mahboubi, 2021), the seeds of P. diversifolia are rich in essential oil, making P. diversifolia an aromatic crop with high economic value (Zhejiang Food and Drug Administration, 2016). In China, especially among the She ethnic minority, the whole herb of P. diversifolia is used as a medicine, which has the effects of regulating qi flow to relieve pain, dispelling wind and ventilating the lungs, reinforcing the spleen to promote digestion, promoting blood circulation and menstruation, eliminating dampness, and removing toxic substances. It is often used to treat conditions including cough and asthma, lung abscess, pain, indigestion, dysentery, enteritis, irregular menstruation, and dysmenorrhea, and it is used externally to treat conditions including traumatic injuries, snake and insect bites, and eczema (Zhejiang Food and Drug Administration, 2016). Pimpinella diversifolia is rich in flavonoids and essential oil, which may be responsible for its multiple applications in ethnic medicine. Its whole herbal extracts show anti-inflammatory, analgesic, hemostatic, and procoagulant activities (Chen et al., 2015; Wang and Wang, 2017); the total flavonoids show strong antioxidant activity (Zhao et al., 2019); and the 50% alcohol extract of its seeds displays remarkable anti-fungal activity (Tabanca et al., 2007). The roots, stems, leaves, and seeds of P. diversifolia are rich in essential oils. The essential oils of the aboveground part contain a variety of terpenoids, such as 1H-benzocycloheptene, sesquiphellandrene, and β-chamigrene (Xu et al., 2012). The root part contains a high content of phenylpropanoid derivatives (Bottini et al., 1985), especially eight phenylpropanoid compounds, accounting for 78% of the total essential oil (Melkani et al., 1990).
As is well known, Pimpinella species are rich in essential oils, which show remarkable anti-inflammatory, antioxidant, anti-bacterial, and anti-fungal effects (Shojaii and Abdollahi, 2012; Tabanca et al., 2007). However, to the best of our knowledge, there have been few reports on the pharmacological study of the essential oil of P. diversifolia. In the folk tradition, P. diversifolia is often drunk as a tea, which is believed to have detoxification effects and to prevent liver-related disease. Thus, the aim of the current work was to reveal the effects of P. diversifolia root essential oil (PDREO) in an LPS/D-GalN-induced ALI mouse model.
2 Material and methods
2.1 Reagents
Lipopolysaccharide (LPS) and C7-C40 saturated alkanes standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-acetylcysteine (NAC), Dichloromethane and D-galactosamine (D-GalN) were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). M−PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA). Bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Rockford, USA). Superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), malondialdehyde (MDA), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) commercial kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). BD mouse inflammation cytometric bead array (CBA) kit (BD Biosciences, San Diego, USA). Antibodies against F4/80 (ab111101), CD11b (ab133357) and Ly6G (ab238132) and HRP-conjugated secondary antibody (ab205719) were purchased from Abcam (Cambridge, MA, USA). Anti-caspase 3 antibody (14220 T) and anti-β-actin antibody (4970S) were obtained from CST (MA, USA). DAB solution was derived from cwbiotech (Beijing, China). TUNEL apoptosis detection kit was purchased from Beyotime biotechnology (Shanghai, China).
2.2 Plant material and preparation of PDREO
The root parts of P. diversifolia were harvested at Shouning County, Ningde City, Fujian Province, China, in September 2021. Samples were dried at 60 °C (Fig. 1A), powdered, and sieved through a 40-mesh screen (Fig. 1B). A voucher specimen (PDR20210909) was stored in the laboratory of the College of Life Science, Zhejiang Chinese Medical University.
(A) The root part of P. diversifolia. Its powder (B). (C) Its essential oil is extracted by the SDE method.
PDREO was prepared by a simultaneous distillation–extraction (SDE) method. Briefly, 500 g powder was submitted to SDE in a classical Likens–Nickerson apparatus for 5 h with 5 L water, dichloromethane (100 mL) was used as the extractant. The dichloromethane phase was collected, dried with anhydrous sodium sulfate, and the organic phase was removed under reduced pressure at 25 °C. Finally, 1.41 g of brownish-yellow essential oil was obtained (Fig. 1C).
2.3 Chemical composition
The chemical profile of PDREO was characterized by gas chromatography (GC) as we previously reported (Wang et al., 2022). PDREO was separated using an Agilent Technologies 7890B system (Agilent Technologies, MA, USA) equipped with an HP-5 capillary column (30 m × 0.25 mm × 0.25 μm) coupled to an Agilent 7250 quadrupole time-of-flight mass spectrometry. With helium as carrier gas, the flow rate was 1.0 mL/min. Then, 1 uL sample was injected at a split ratio of 10:1, keep the temperature of injector at 250 °C. The initial column temperature was 50 °C, maintained for 3 min, then increased to 280 °C at a rate of 5 °C/min, and held for 5 min.
The PDREO components were determined by comparing their Kovats retention index (RI) values (calculated using the RI value of a C7–C40 n-alkane series [O2si, USA] obtained on the HP-5MS column) with the values in the National Institute of Standards and Technology (NIST) database and those reported in the literature. The relative content of each ingredient in PDREO was determined according to the peak area of GC–MS without correction.
2.4 Animals
C57BL/6 mice (male, 18–22 g) were obtained from the Experimental Animal Center of Zhejiang Academy of Medical Sciences (Hangzhou, China). All animals were kept without specific pathogen conditions, with controlled temperature and humidity, 12/12-h light/dark cycle and free access to water and food. The experiment was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Ethical Committee of Zhejiang Chinese Medical University (IACUC-20191202–04).
2.5 Establishment of the LPS/D-GalN-induced ALI model
Forty male C57BL/6 mice were randomly divided into five groups: the control group (vehicle), the model group, the N-acetylcysteine (NAC) (300 mg/kg) group, the PDREO (50 mg/kg) group, and the PDREO (150 mg/kg) group. In the NAC and PDREO groups, mice were injected intraperitoneally with NAC or PDREO (both dissolved with PBS containing 1% Tween 80) once a day for three consecutive days, while mice in the control and model groups were injected with vehicle (PBS containing 1% Tween 80). One hour after the last administration, LPS (40 µg/kg, Sigma) and D-GalN (500 mg/kg, Shanghai Macklin Biochemical Co., Ltd.) were injected intraperitoneally to induce ALI. Mice were sacrificed after 6 h. Blood serum was obtained by centrifuging the blood at 1000 g for 15 min after coagulation. Liver tissue was harvested, and liver sample was fixed in a 10% formaldehyde /PBS solution; the remaining parts were snap-frozen in liquid nitrogen, and stored at − 80 °C.
For the animal survival assay, 50 male C57BL/6 mice were randomly divided into five groups, and treated as described above. After LPS (40 µg/kg) and D-GalN (500 mg/kg) were injected, mice were weighed every 24 h and continuously observed for 120 h, and death was recorded.
2.6 Serum biochemistry
Serum AST and ALT activities were determined by the colourimetric method of Reitman and Frankel using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the instructions. According to the method principle described by (POWELL and SMITH, 1954), the ALP level in serum was measured using commercial kit purchased from Nanjing Jiancheng Bioengineering Institute. The levels of serum inflammatory factors, such as TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1), were measured using a BD mouse inflammation cytometric bead array (CBA) kit (BD Biosciences, San Diego, USA) with a slight modification. Briefly, three capture bead populations were mixed together in equal volumes, and 25 μL of each sample was added, following mixed with 25 μL of PE detection reagent. The mixture was incubated for 2 h at room temperature in the dark. The beads were washed with wash buffer, and detected on a Cytoflex S flow cytometer (Beckman, USA). Data was analyzed using FCAP Array software (BD Biosciences).
2.7 Liver index and biochemistry
The liver was weighed and the liver index (liver weight/body weight, mg/kg) was calculated to measure the edema of the liver tissue. According to the method reported by (Maimaitimin et al., 2018), SOD and CAT activities, GSH and MDA contents in the liver were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China)r.
2.8 Histological analysis
Fixed liver tissues were embedded in paraffin, cut into 4-µm-thick sections, and stained with hematoxylin and eosin (HE). The severity of liver damage was graded on a four-point scale according to (Lv et al., 2019), where 1-no damage, 2-mild damage, 3-moderate damage, and 4-severe damage.
2.9 Immunochemistry staining
For immunohistochemical studies, sections were subjected to antigen repair in citrate buffer (pH 6.0, 10 mM) and treated in 3% H2O2 for 15 min. Then blocked with 2% bovine serum albumin (Amresco, Solon, OH) for 1 h at 25 °C, followed by incubation with primary antibodies against F4/80 (ab111101, Abcam, 1:100), CD11b (ab133357, Abcam, 1:1000), or Ly6G (ab238132, Abcam, 1:1000) overnight at 4 °C. After washing, sections were incubated with HRP-conjugated secondary antibody (ab205719, Abcam, 1:2000) for 1 h at 25 °C. Positive staining was then observed using DAB solution (Cwbiotech, Beijing, China) with hematoxylin counterstaining.
2.10 TUNEL assay
Hepatocyte apoptosis was evaluated by TUNEL assay. A one-step TUNEL apoptosis detection kit (Beyotime biotechnology, China) was used according to the manufacturer's instructions. Positive staining cells were quantified using Image-Pro Plus software (media controlnetics, Rockville, MD) based on three different random fields of each sample.
2.11 Quantitative real-time PCR (qRT-PCR)
Liver tissue (90–100 mg) was homogenized in 1 mL TRIzol reagent (Invitrogen, USA) using a MagNA Lyser (Roche, Germany) at 6000 rpm for 20 s. Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions and quantified by spectrophotometry. cDNA was obtained using the High-Capacity cDNA Reverse Transcription Kit (Cwbiotech, Beijing, China). qRT-PCR was performed on an LC480 Real-Time PCR Detection System (Roche, Germany) using SYBR Green PCR Master Mix (Cwbiotech, Beijing, China). Primers were produced by Sangon Biotech (Shanghai, China) and are listed in Table 1. The β-actin gene (B661302, Sangon Biotech, Shanghai) was used as an endogenous reference. Gene expression levels were calculated using the
method.
Gene Name
Forward primer (5′-3′)
Revers primer (5′-3′)
TNF-α
CGAGTGACAAGCCTGTAGCCC
GGGCAGCCTTGTCCCTTGA
IL-6
AGTTGCCTTCTTGGGACTGA
TTCTGCAAGTGCATCATCGT
MCP-1
AGGTGTCCCAAAGAAGCTGT
GACCTTAGGGCAGATGCAGTT
IL-1β
CAAATCTCGCAGCAGCACATC
TCATCTCGGAGCCTGTAGTGC
iNOS
CTTGGAGCGAGTTGTGGATTGTC
TAGGTGAGGGCTTGGCTGAGTGA
COX2
AGAAGGAAATGGCTGCAGAA
GCTCGGCTTCCAGTATTGAG
NLRP3
TCTGCACCCGGACTGTAAAC
CATTGTTGCCCAGGTTCAGC
SOD1
GAGCATTCCATCATTGGCCG
ACTGCGCAATCCCAATCACT
SOD2
GTGTGGGAGCACGCTTACTA
CTATAAACCAGCCCGGAGCC
CAT
CACTGACGAGATGGCACACT
TGTGGAGAATCGAACGGCAA
GPX4
CCTTCCCCTGCAACCAGTTT
GTGGGCATCGTCCCCATTTA
HO-1
CAGAAGAGGCTAAGACCGCC
TCTGACGAAGTGACGCCATC
2.12 Western blotting analysis
Liver samples were weighted and total proteins were prepared according to the instructions of the M−PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA). Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, USA). The levels of capspase 3 and cleaved-caspase 3 proteins were measured by immunoblotting with specific antibodies on a simple western system (ProteinSimple, San Jose, CA, USA) according to our previous report (Jiang et al., 2019).
2.13 Statistical analysis
All data are expressed as mean ± SD. Statistical differences among multiple groups were determined by one-way ANOVA followed by Tukey's multiple comparisons test using GraphPad Prism version 8.0 software (San Diego, CA, USA). P < 0.05 were considered to be statistically significant.
3 Results
3.1 Chemical composition of PDREO
PDREO is a transparent brownish yellow liquid that emits a unique fragrance. It accounts for about 0.28% of the dry weight of the root, which is higher than that of most other Pimpinella species (Tabanca et al., 2005; Tabanca et al., 2004). A total of 27 components (Fig. 2 and Table 2) were identified from PDREO, accounting for 95.74% of the total volatile components, including terpene components accounting for 45.87%, aromatic components accounting for 32.57%, and aliphatic components accounting for 17.30%. The compounds isodaucene, 4-methoxy-2-(3-methyloxiranyl)-phenyl isobutyrate, and 2-methyl-4-methoxy-2-(3-methyl-2-oxiranyl) phenyl butyrate are the main components of PDREO, with contents of 29.97%, 19.53%, and 6.88%, respectively. In addition, the content of the aliphatic compound geijerene and its isomer is also high, accounting for 15.31% of the total essential oil content. Pseudoisoeugenol type phenylpropanoid derivatives are the characteristic components in the essential oils of Pimpinella species. They are mainly distributed in the roots, and the content of these components in the essential oil of some species is more than 80% (Tabanca et al., 2005). The current work also confirmed the presence of pseudoisoeugenol type phenylpropanoid compounds in the root of P. diversifolia, but the number and content of these compounds are significantly lower than those reported by (Bottini et al., 1985). Most of the other components such as geijerene, β-bisabolene, copaene, and β-caryophyllene have been identified and reported in the essential oils of various Pimpinella species (Bottini et al., 1985; Tabanca et al., 2005; Tabanca et al., 2004). However, it is surprising that the compound isocarotene accounts for 29.97% of PDREO, but has not been reported in the essential oils of other Pimpinella species and the essential oil of P. diversifolia leaves from Zhejiang Province (Xu et al., 2012), which may be attributed to the variety of species, different geographical origins, different harvest times, and other factors. aRetention index experimentally calculated based on the C7-C40 n-alkanes standard. bRetention indices were estimated using the NIST GC-RI database. Retention index taken from NIST.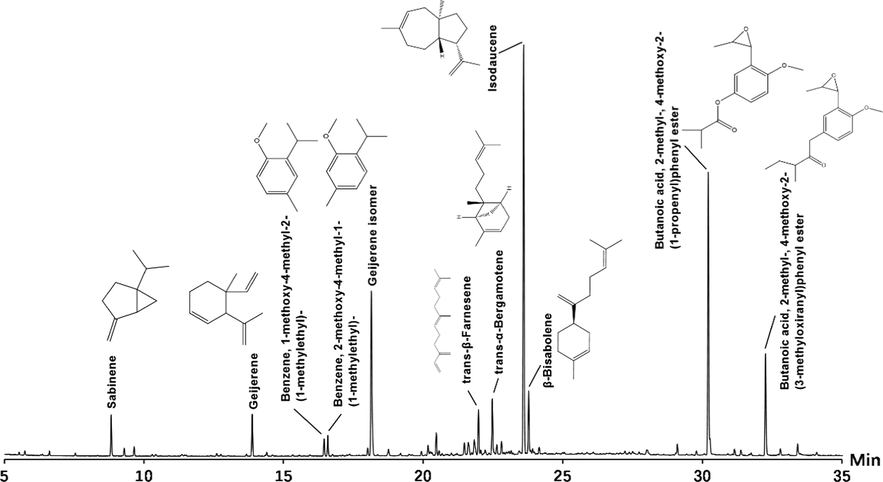
Total ion current chromatogram of PDREO by GC-Q/TOF-MS.
RT(min)
Component
Molecular formula
Area (%)
RIa
RI (NIST)b
8.82
Sabinene
C10H16
2.22
976
974 ± 2
9.30
2-Amylfuran
C9H14O
0.37
992
993 ± 2
9.65
Octanal
C8H16O
0.59
1003
1003 ± 2
13.88
Geijerene
C12H18
2.55
1142
1143 ± 4
16.45
Benzene, 1-methoxy-4-methyl-2-(1-methylethyl)-
C11H16O
0.84
1231
1230 ± 15
16.58
Benzene, 2-methoxy-4-methyl-1-(1-methylethyl)-
C11H16O
1.07
1236
1235 ± 2
18.01
2,3,4,5-Tetramethyltricyclo[3.2.1.02,7]oct-3-ene
C12H18
0.43
1287
/
18.15
Geijerene isomer
C12H18
12.33
1291
/
18.19
Benzaldehyde, 2-hydroxy-5-methoxy-
C8H8O3
1.21
1293
1319
18.76
2-Methoxy-4-vinylphenol
C9H10O2
0.31
1314
1317 ± 5
20.18
6-Methoxy-3-methylbenzofuran
C10H10O2
0.58
1368
/
20.47
Copaene
C15H24
1.36
1379
1376 ± 2
21.48
Biccyclo[5.3.0]decane,2-methylene-5-(1-methylvinyl)-8-methyl
C15H24
0.85
1418
/
22.48
trans-β-Farnesene
C15H24
3.6
1458
1457 ± 2
21.61
Caryophyllene
C15H24
0.88
1424
1419 ± 3
21.83
3,9-Dimethyltricyclo[4.2.1.1(2,5)]dec-3-en-9-ol
C12H18O
1.03
1432
/
21.98
trans-α-Bergamotene
C15H24
2.34
1438
1435 ± 3
22.64
(E)-2-methoxy-4-(prop-1-enyl)phenol
C10H12O2
0.62
1465
1454 ± 6
22.81
Naphthalene, 1,2,4a,5,6,7,8,8a-octahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, (4aS,7R,8aS)-
C15H22
0.79
1472
1477
23.60
Isodaucene
C15H24
29.97
1503
1503
23.78
β-Bisabolene
C15H24
3.48
1511
1509 ± 3
28.01
Acorenone 1
C15H24O
0.38
1695
1668 ± 36
29.10
Phenol, 2-methoxy-4-(1-propenyl)-, acetate
C12H14O3
0.64
1746
/
30.21
4-Methoxy-2-(3-methyloxiranyl)-phenyl isobutyrate
C14H18O4
19.53
1799
/
30.28
Propan-2-one, 1-(4-isopropoxy-3-methoxyphenyl)-
C13H18O3
0.53
1802
/
31.16
Butanoic acid, 2-methyl-, 4-methoxy-2-(1-propenyl)phenyl ester
C15H20O3
0.36
1845
1834 ± 12
32.26
Butanoic acid, 2-methyl-, 4-methoxy-2-(3-methyloxiranyl)phenyl ester
C15H20O4
6.88
1900
1892 ± 7
Totally
95.74
3.2 PDREO can effectively reduce the levels of serum biomarkers of liver injury
Serum biochemical indicators, including ALT, AST, and ALP, are important markers to evaluate liver injury (Shi et al., 2010). As depicted in Fig. 3, the serum ALT, AST, and ALP levels in the model group were significantly higher than those in the control group. Treatment with NAC effectively lowered the serum levels of ALT, AST, and ALP. Surprisingly, PDREO (50 mg/kg) significantly reduced the serum levels of ALT, AST, and ALP; those of ALT and ALP were even significantly lower than those of the NAC group. In the PDREO (150 mg/kg) group, the serum levels of ALT and ALP were almost equal to those in the control group. Studies have shown that ALT is mainly located in the liver (Sherman, 1991), while AST is more widely distributed in the liver, heart, bone, kidney, and brain (Cai et al., 2019). Therefore, ALT is a far more specific than AST in diagnosing liver injury (Xu et al., 2015), which indicates that PDREO exhibits potential hepatoprotective activity.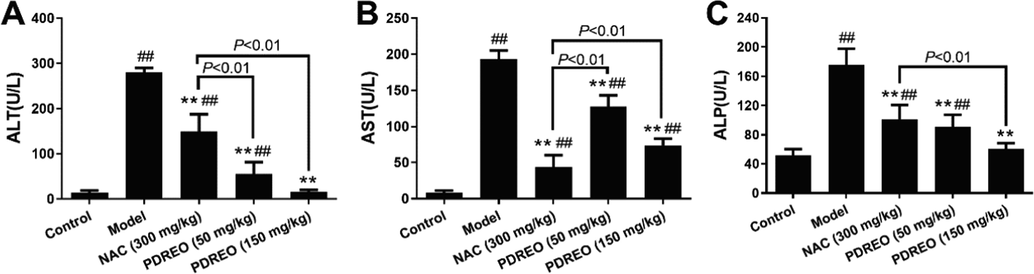
Effects of PDREO on serum biomarkers of liver injury. (A) ALT. (B) AST. (C) ALP. Data are expressed as mean ± SD (n = 8). ##P < 0.01 vs. control group; **P < 0.01 vs. model group.
3.3 PDREO can effectively reduce the liver index and ameliorate liver tissue damage
The livers of mice in the control group were pink, with complete structure and no pathological changes (Fig. 4A). In the model group, the liver was dark red and black. Compared with the model group, the liver of the NAC treatment group was significantly lighter but was still dark red. In the PDREO (50 mg/kg) and (150 mg/kg) groups, liver morphology and color were basically similar to those of the control group. HE staining of liver tissue sections more intuitively reflects the pathological changes. As depicted in Fig. 4B, the hepatocytes in the model group were much smaller than in the control group, the space between hepatocytes was enlarged, and massive red blood cells were exuded, which may be one of the reasons for the deepening of liver color. After NAC treatment, the liver tissue lesions were alleviated, but a large number of red blood cells were still visible. The morphology of hepatocytes in the PDREO (50 mg/kg) treatment group was basically normal; only a few red blood cells were occasionally seen. In the PDREO (150 mg/kg) group, no obvious hepatic injury was observed. The HE staining results were basically consistent with the overall color changes in different treatment groups. The liver damage score (Fig. 4C) in the NAC treatment group was improved compared with the model group, but the injury was still significant compared with the control group. However, no statistically significant difference between the PDREO (50 mg/kg) and (150 mg/kg) treatment groups and the control group were observed. The severity of liver tissue lesions and tissue edema is also reflected in the liver index. As shown in Fig. 4D, the liver index was 53.713 ± 3.642 mg/g BW in the model group, which was significantly higher than that of the control group (36.251 ± 1.286 mg/g BW), which was consistent with the above liver color and HE staining results. The liver index was effectively reduced in all treatment groups, and it was significantly lower in the PDREO (150 mg/kg) group than in the NAC treatment group. These data further confirmed the hepatoprotective activity of PDREO.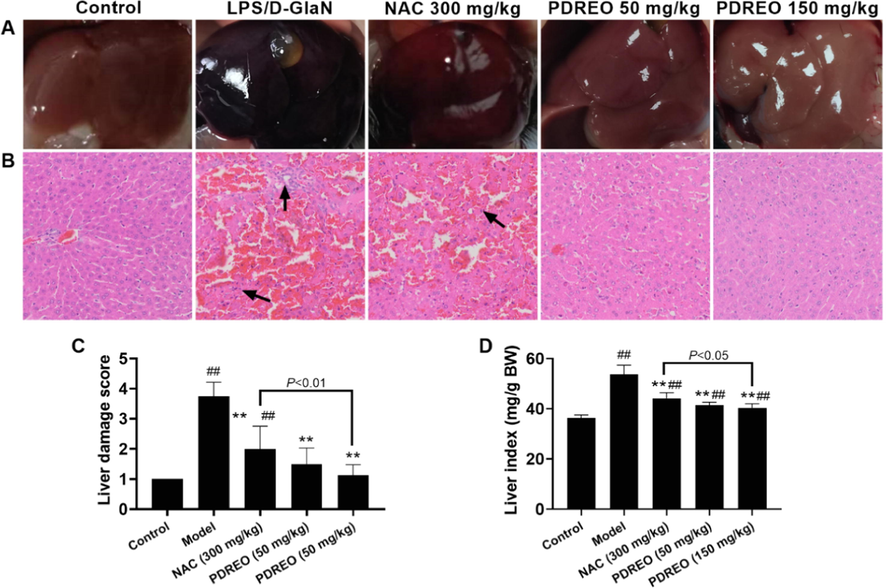
Effects of PDREO on (A) liver morphology, (B) liver histopathology, (C) the liver damage score, and (D) the liver index. Data are expressed as mean ± SD (n = 8). ##P < 0.01 vs. control group; **P < 0.01 vs. model group.
3.4 PDREO can effectively reduce the serum levels of inflammatory cytokines and inhibit the expression of inflammatory genes in liver
Inflammation is a hallmark of ALI (Triantafyllou et al., 2018); therefore, the serum levels of inflammatory cytokines were determined. As shown in Fig. 5A, TNF-α, IL-6, and MCP-1 levels were significantly higher in the model group compared with the control group. NAC treatment effectively reduced the serum levels of all inflammatory cytokines, but these levels were still significantly higher than in the control group. In the PDREO (50 mg/kg) and (150 mg/kg) groups, the serum levels of inflammatory cytokines were lower than in the NAC group; in the PDREO (150 mg/kg) group, serum levels of inflammatory cytokines were almost reduced to control levels. These inflammatory cytokines are often derived from the damaged liver; therefore, the mRNA levels of some important inflammation-related genes in liver tissues were analyzed. Consistent with the serum levels of inflammatory cytokines, the expression levels of the genes encoding TNF-α, IL-6, IL-1β, NLRP3, iNOS, and COX2 were significantly increased in the liver tissue of the model group, and all drugs effectively antagonized this effect (Fig. 5B). PDREO can reduce the expression of inflammatory genes at the mRNA and protein levels and hence exert anti-inflammatory activity.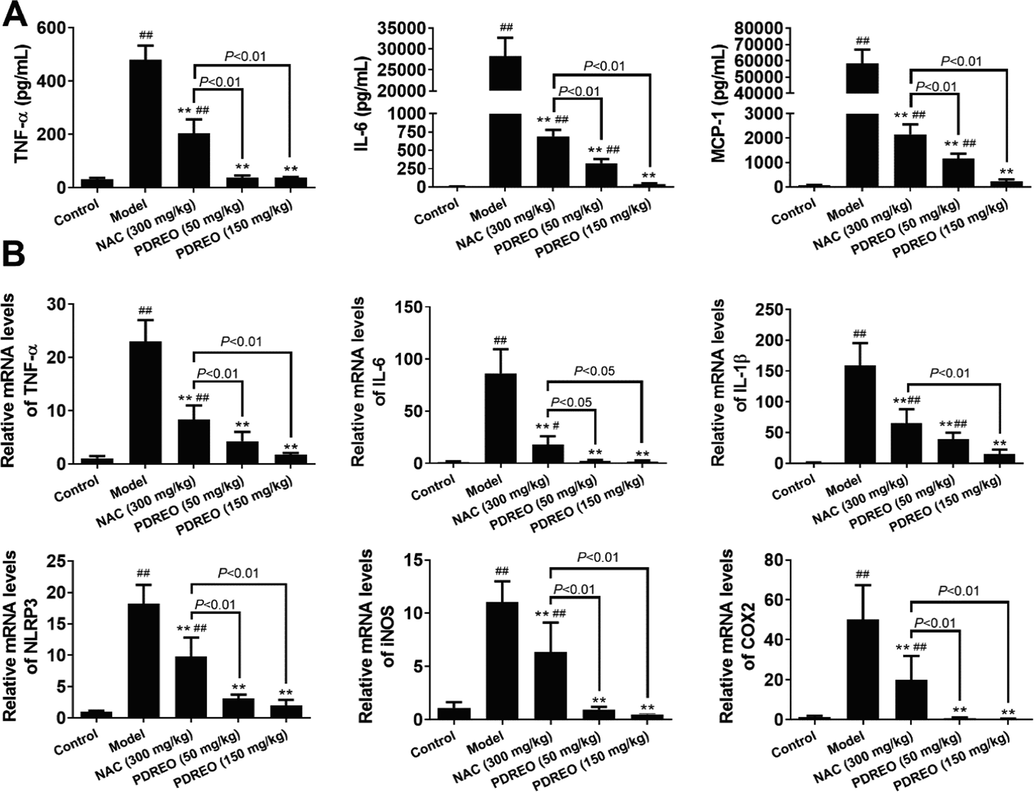
Anti-inflammatory effects of PDREO. (A) Serum levels of inflammatory cytokines. (B) Expression levels of inflammatory genes in liver. Data are expressed as mean ± SD (n = 8). ##P < 0.01 vs. control group; **P < 0.01 vs. model group.
3.5 PDREO can regulate antioxidant enzymes and relieve hepatic oxidative stress
Oxidative stress caused by excessive production of reactive oxygen species (ROS) plays a central role in the pathology of ALI (Li et al., 2018). To determine the effects of PDREO on LPS/D-GalN-induced oxidative stress in the liver, changes in the antioxidant system were analyzed. As shown in Fig. 6A, LPS/D-GalN exposure resulted in dramatically increased content of MDA (2.628 ± 0.464 mmol/mg protein) in liver homogenate compared with the control group (0.903 ± 0.146 mmol/mg protein). Pre-treatment with NAC effectively reduced the levels of MDA (1.511 ± 0.326 mmol/mg protein) compared with the model group. Surprisingly, both PDREO (50 mg/kg) (0.943 ± 0.239 mmol/mg protein) and (150 mg/kg) (0.820 ± 0.122 mmol/mg protein) almost completely inhibited the increase in MDA levels. Additionally, the toxicity of LPS/D-GalN led to a significant decrease in the content of GSH and the activities of SOD and CAT, resulting in imbalance of the liver antioxidant system. Compared with the model group, NAC and PDREO pre-treatment effectively restored GSH levels and SOD and CAT activities in liver tissue. The qRT-PCR results showed that NAC and PDREO upregulate the gene expression of Gpx4, Sod1, and Cat (Fig. 6B).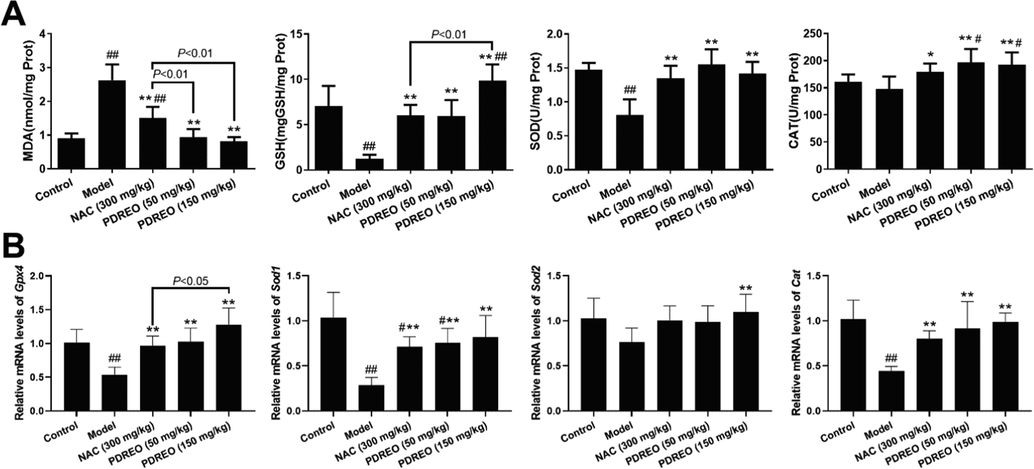
Inhibition of PDREO on oxidative stress induced by LPS/D-GalN. (A) MDA and GSH contents and SOD and CAT activities in liver tissue. (B) Normalized mRNA levels of Gpx4, Sod1, Sod2, and Cat in liver tissue. Data are expressed as mean ± SD (n = 8). #P < 0.05, ##P < 0.01 vs. control group; *P < 0.05, **P < 0.01 vs. model group.
3.6 PDREO can alter populations of liver-resident and infiltrating macrophages
Inflammatory cytokines are usually secreted by activated macrophages, but studies have shown that the number of liver-resident F4/80 + macrophages (Kupffer cells) was highly decreased in LPS/D-GalN-induced ALI mice. On the contrary, numerous CD16b + macrophages were infiltrated and induced inflammatory responses (Tacke, 2017; Zigmond et al., 2014). As shown in Fig. 7A, in the present study, the number of F4/80 + cells were dramatically decreased in the model group, but this decrease was significantly inhibited in the NAC and PDREO groups. In contrast, CD11b + cells were massively recruited into the liver of the model group, and this was significantly inhibited by NAC and almost completely blocked by PDREO (50 mg/kg) and (150 mg/kg) (Fig. 7B).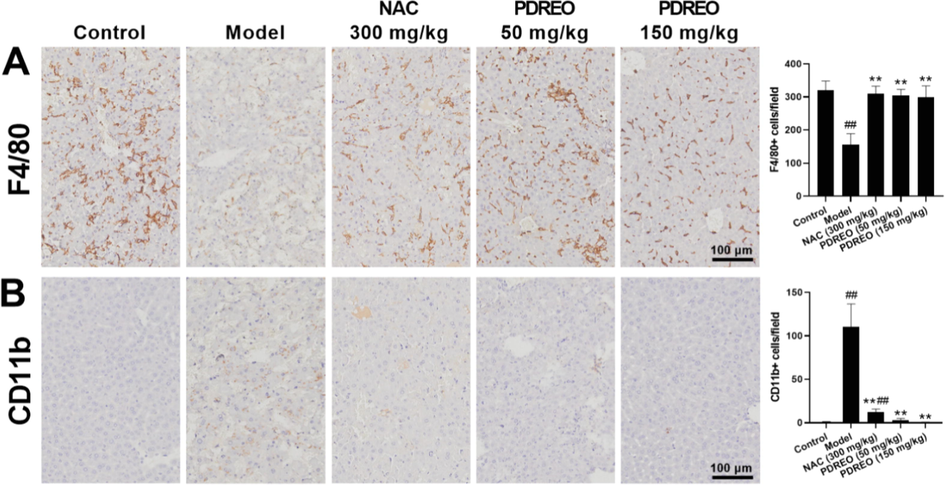
Effects of PDREO on the number of resident and infiltrating macrophages in the liver. Immunohistochemical staining for (A) F4/80 + and (B) CD11b + cells. Cells were quantified using IPP9.0. Data are expressed as mean ± SD (n = 8). ##P < 0.01 vs. control group; **P < 0.01 vs. model group.
3.7 PDREO can effectively reduce neutrophil infiltration
Activated macrophages can secrete a variety of chemokines, which can recruit neutrophils into damaged liver tissues, thus aggravating inflammatory reaction and liver injury (Ilyas et al., 2016). Consistent with a previous study (Kusakabe et al., 2021), compared with 0.88 ± 0.99 cells/field in the control group, the number of Ly6G + neutrophils significantly increased to 78.00 ± 12.46 cells/field after challenge with LPS/D-GalN (Fig. 8A, B). After pre-treatment with NAC (300 mg/kg) or PDREO (50 mg/kg) and (150 mg/kg) for 3 days, the number of Ly6G + cells decreased to 37.25 ± 10.69, 19.75 ± 10.51, and 2.13 ± 1.25 cells/field, respectively. Tissue myeloperoxidase (MPO) is a biomarker of neutrophil aggregation in tissues (Rennekampff et al., 1995). Therefore, MPO levels in liver tissues were determined. As depicted in Fig. 8C, the MPO level in the model group was significantly increased, and NAC and PDREO pre-treatment could effectively reduce the MPO level, which was basically consistent with the cell count results.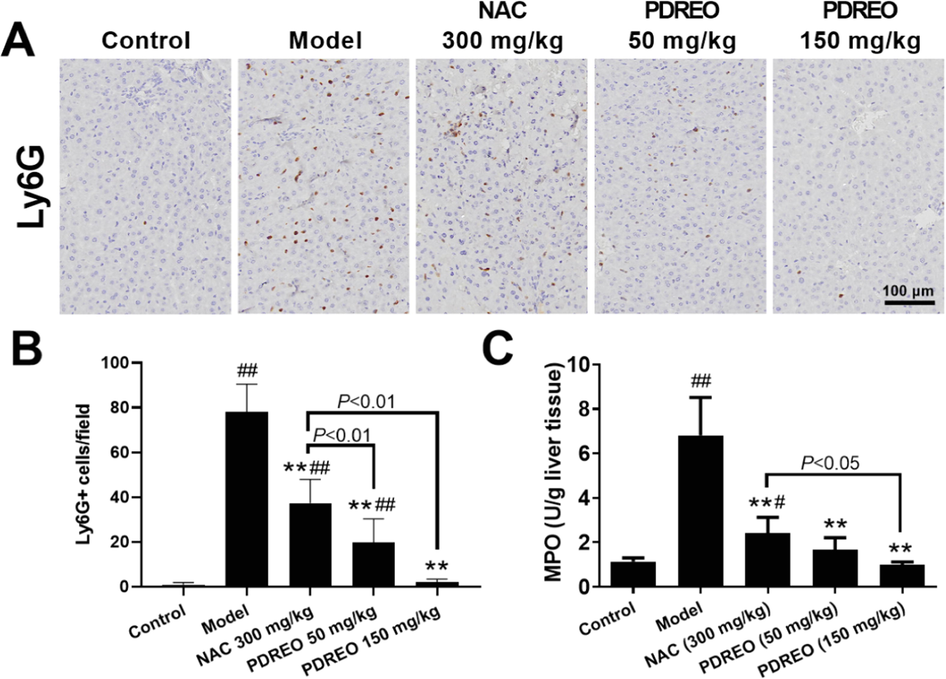
Inhibitory effects of PDREO on neutrophil infiltration. (A) Immunohistochemistry of Ly6G + neutrophils. (B) Quantification of the results in (A). (C) MPO activity in liver tissues. Data are expressed as mean ± SD (n = 8). #P < 0.05, ##P < 0.01 vs. control group; **P < 0.01 vs. model group.
3.8 PDREO can effectively prevent liver cell apoptosis
LPS/D-GalN treatment can lead to massive hepatocyte apoptosis, resulting in ALI (Kusakabe et al., 2021). TUNEL staining showed that the apoptosis rate of hepatocytes in the model group reached (35.979 ± 2.928)% (Fig. 9A, B), and the apoptosis rate decreased to (5.446 ± 4.225)% after NAC pre-treatment. PDREO treatment could highly efficiently prevent hepatocyte apoptosis; the apoptosis rate in the PDREO (150 mg/kg) group was (0.019 ± 0.036)%, which was even lower than that of the control group (0.114 ± 0.0.114)%. Caspase 3 is essential for apoptosis, and the appearance of cleaved caspase 3 is a hallmark of apoptosis (Porter and Janicke, 1999). Western blot analysis revealed that LPS/D-GalN injection significantly promoted caspase 3 activation, which was verified by the presence of the cleaved caspase 3 bands in Fig. 9C. Consistent with the TUNEL results, weak cleaved caspase 3 bands were still observed in the NAC group, while no cleaved caspase 3 bands were observed in the PDREO (50 mg/kg) and (150 mg/mL) and control groups. The above data show that PDREO has strong anti-apoptotic activity.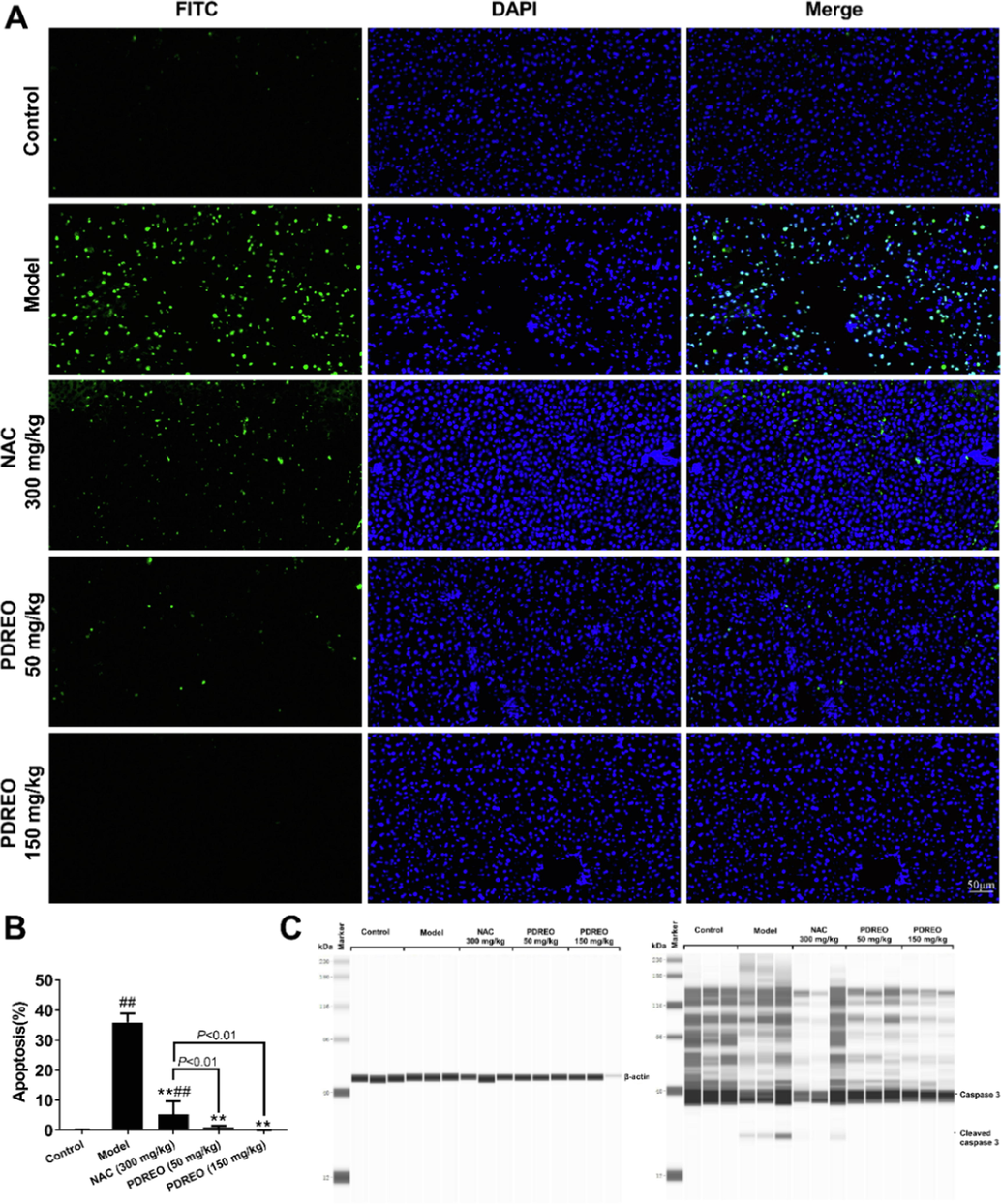
Inhibitory effects of PDREO on LPS/D-GalN-induced apoptosis in acute liver injury. (A) TUNEL staining of apoptotic cells (FITC and DAPI double positive cells). (B) Quantification of the results in (A). Data are expressed as mean ± SD (n = 8). #P < 0.05, ##P < 0.01 vs. control group; **P < 0.01 vs. model group. (C) Western blot analysis of caspase 3 and cleaved caspase 3 (n = 3).
3.9 PDREO significantly reduced LPS/D-GalN-induced mortality in mice
In the model group, mice began to die 8 h after LPS/D-GalN injection, and all mice were dead about 10 h later (Fig. 10A). The mortality rates in the NAC and PDREO (50 mg/kg) groups were 20% and 10%, respectively, at about 14 h, and no more mice died in the PDREO (50 mg/kg) group after that. However, 72 h after modeling, mice in the NAC group began to die one after another, and the survival rate was only 10% at 120 h. In addition, the appearance and activity of mice in the NAC group changed significantly, which was manifested by dull hair, reduced activity, reduced food intake, and significantly reduced body weight, especially at 72 h (Fig. 10B). The PDREO (50 mg/kg) and (150 mg/kg) groups were generally similar to the control group, except that the state was slightly worse and the body weight decreased within 24 h after modeling (Fig. 10B). Surprisingly, all mice in the PDREO (150 mg/kg) group survived. This result further confirms that PDREO has a significant protective effect on LPS/D-GalN-induced liver injury in mice, indicating great potential for use in the pharmaceutical industry.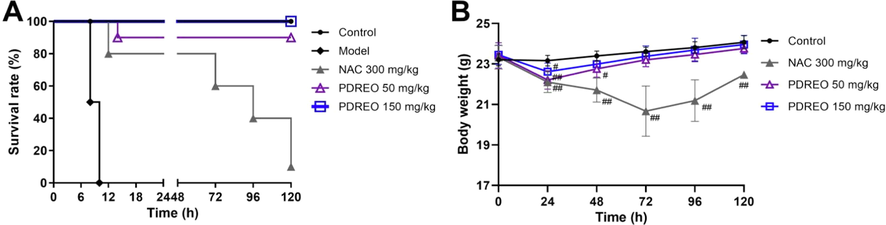
Effects of PDREO on (A) the survival rate and (B) the body weight of mice induced by LPS/D-GalN. Data are expressed as mean ± SD (n = 1–10). #P < 0.05, ##P < 0.01 vs. control group of the corresponding time point.
4 Discussion
Pimpinella diversifolia is an aromatic plant which has been used in traditional Chinese medicine for thousands of years. All parts of the plant are rich in essential oil, especially the root part, which contains more essential oil than the roots of other Pimpinella species. In the present study, a total of 27 compounds were identified in PDREO (Fig. 2 and Table 1), accounting for 95.74% of the total essential oil content. The major constituents of PDREO were isodaucene (29.97%), 4-methoxy-2-(3-methyloxiranyl)-phenyl isobutyrate (19.53%), geijerene isomer (12.33%), and 2-methyl-4-methoxy-2-(3-methyl-2-oxiranyl) phenyl butyrate (6.88%). It is worth noting that isodaucene, with a content as high as 29.97%, was identified in the essential oil of Pimpinella species for the first time. Phenylpropanoid compounds are the second major class in PDREO, and they are also the phytochemical markers of the genus Pimpinella that separate this genus from all the other Umbelliferae genera investigated so far (Tabanca et al., 2005). In general, the chemical constituents of the essential oil of P. diversifolia have been extensively studied (Bottini et al., 1985; Melkani et al., 1990; Xu et al., 2012), but pharmacological research is lagging behind.
LPS/D-GalN-induced ALI in mice is a well-established model for studying the mechanisms of clinical liver dysfunction and screening potential therapeutic drugs (Nowak et al., 2000). Therefore, an LPS/D-GalN-induced ALI mouse model was used in the present study to investigate the hepatoprotective effects of PDREO. The mortality assay indicated that injection of LPS and D-GalN at doses of 40 µg/kg and 500 mg/kg, respectively, resulted in the death of all mice in the model group within 10 h, and 90% of mice in the NAC (300 mg/kg) group died within 120 h. In the PDREO (50 mg/kg) group only 10% of the mice died, and no death was observed in the PDREO (150 mg/kg) group within 120 h (Fig. 10A). As is well known, NAC is an excellent antioxidant and also a first-line drug for clinical treatment of ALI (Khanam and Kottilil, 2021). Although it can prolong the death time of mice induced by LPS/D-GalN, it cannot effectively reduce the mortality of mice (Fig. 10A). On the contrary, PDREO (50 mg/kg) and (150 mg/kg) pre-treatment effectively alleviated the death of mice (Fig. 10B). These results indicate that PDREO shows great potential for ALI treatment.
In addition to mortality, liver injury is another important index to evaluate the hepatoprotective activity of drugs. LPS/D-GalN exposure dramatically increased the serum levels of ALT, AST, and ALP in the model group. NAC and PDREO pre-treatment significantly reduced the serum levels of all biochemical indicators (Fig. 3). In parallel, massive necrosis of parenchymal hepatocytes accompanied by obvious hemorrhage and inflammation was observed in the liver tissue sections of the model group. NAC and PDREO pre-treatment significantly alleviated LPS/D-GalN-induced liver injury (Fig. 4). In particular, PDREO is superior to NAC in reducing ALT levels (a specific index of liver injury) and attenuating hepatic necrosis and hemorrhage.
LPS is often used to simulate microbial infection. It can activate macrophages, which subsequently release a large number of inflammatory cytokines such as TNF-α, IL-6, and MCP-1, which is consistent with the explosive inflammatory response in clinical ALI (Bajaj et al., 2022). As expected, LPS/D-GalN injection significantly increased not only the serum levels of TNF-α, IL-6, and MCP-1 (Fig. 5A), but also the mRNA levels of TNF-α, IL-6, IL-β, NLRP3, iNOS, and COX2 (Fig. 5B). NAC has anti-inflammatory activity, but its ability to inhibit inflammatory factors is still controversial. Some studies demonstrated that NAC can significantly inhibit LPS-induced release of TNF-α (Li et al., 2002; Peristeris et al., 1992), but others showed that its inhibitory activity is limited (Wang et al., 2007), and the results of the present study are consistent with the latter. On the contrary, PDREO (150 mg/kg) could significantly reduce the expression of all inflammatory cytokines, both at the mRNA and protein levels, while PDREO (50 mg/kg) had a weaker effect, but nevertheless showed a stronger inhibitory effect on most inflammatory cytokines than NAC (Fig. 5).
Inflammatory factors related to LPS/D-GalN-induced liver injury mainly originate from Kupffer cells and infiltrated monocyte-derived macrophages (Zimmermann et al., 2012). In general, LPS binds to TLR4 receptors on the surface of Kupffer cells, and activated Kupffer cells secrete a large variety of inflammatory cytokines and chemokines, inducing monocyte-derived macrophages (such as CD16b + cells) and neutrophils (Ly6G + cells) to infiltrate into liver tissue, aggravating the inflammatory response and leading to hepatic cell damage and apoptosis (Zimmermann et al., 2012). However, studies indicated that hepatic insults with agents such as LPS/D-GalN and acetaminophen not only activate Kupffer cells, but also significantly reduce the number of Kupffer cells and increase the number of infiltrating macrophages, which is one of the pathological features of ALI (Kusakabe et al., 2021; Zigmond et al., 2014). Therefore, the populations of liver-resident (F4/80+, mainly Kupffer cells) and infiltrating (CD11b + ) macrophages were analyzed. As expected, LPS/D-GalN exposure remarkably reduced F4/80 + cell numbers and recruited CD11b + cells in the model group. In contrast, F4/80 + cells were significantly retained and the infiltration of CD11b + cells was dramatically attenuated by pre-treatment with NAC and PDREO (Fig. 7). These data imply that PDREO not only has significant anti-inflammatory activity, but also has a great protective effect on Kupffer cells.
Following migration and activation, the infiltrated neutrophils produce abundant oxygen free radicals via oxidative bursts, which plays a pivotal role in inflammatory responses and hepatocyte injury in ALI (Ilyas et al., 2016). Free radicals are responsible for cellular lipid peroxidation, protein oxidation, and mitochondrial disfunction, leading to apoptosis and further tissue damage (Kukreja and Hess, 1992). After LPS/D-GalN challenge, massive neutrophils and large amounts of MDA (an end product of lipid peroxidation) were detected in the model group, which were dramatically attenuated by pre-treatment with NAC and PDREO (Fig. 8). In addition to excessive production of free radicals, imbalance of the antioxidant system (such as GSH depletion, inhibition of antioxidant enzyme expression, etc.) is another important reason for ALI caused by various liver insults (Kim et al., 2017; Li et al., 2018). Concordant with a previous study (Li et al., 2018), a significant reduction in SOD activity and GSH content was observed in the model group, but all these disturbances in the antioxidant system were restored by pre-treatment with PDREO. Our results indicate that PDREO pre-treatment can protect mice from LPS/D-GalN-induced ALI by alleviating liver oxidative stress.
Apoptosis is a hallmark of liver injury (Kusakabe et al., 2021). Apoptosis is usually mediated by two major pathways, the extrinsic death receptor-dependent pathway and the intrinsic mitochondrial-dependent pathway. Caspase 3 is a key downstream apoptosis effector shared by these two apoptotic induction pathways (Cao et al., 2016). An increasing body of evidence indicates that the cytokine TNF-α plays a pivotal role in LPS-induced liver injury. Neutralization of TNF-α or knockout of the TNF-α receptor (TFNR1) can significantly attenuate LPS/D-GalN-induced hepatocyte apoptosis (Hishinuma et al., 1990; Leist et al., 1995). The current study showed that PDREO significantly decreased TNF-α serum levels (Fig. 5A), which could partially contribute to the alleviation of liver injury. Western blot analysis and TUNEL staining confirmed that LPS/D-GalN treatment led to massive caspase 3 activation and hepatocyte apoptosis, which was effectively blocked by pre-treatment with PDREO (Fig. 9). In addition to TNF-α, the excessive ROS released by activated macrophages and infiltrating neutrophils are another important factor that causes apoptosis and necrosis of liver cells, leading to liver injury (Wang et al., 2008). However, PDREO can significantly upregulate the expression of antioxidant genes Gpx4, Sod1, and Cat and dramatically reduce the levels of the lipid peroxidation product MDA (Fig. 6), effectively alleviating the oxidative stress induced by LPS/D-GalN, which may also contribute to reducing apoptosis of hepatocytes. Collectively, these data lend support to the notion that PDREO is capable of protecting against LPS/D-GalN-induced liver injury via modulation of TNF-α and ROS levels (Fig. 11).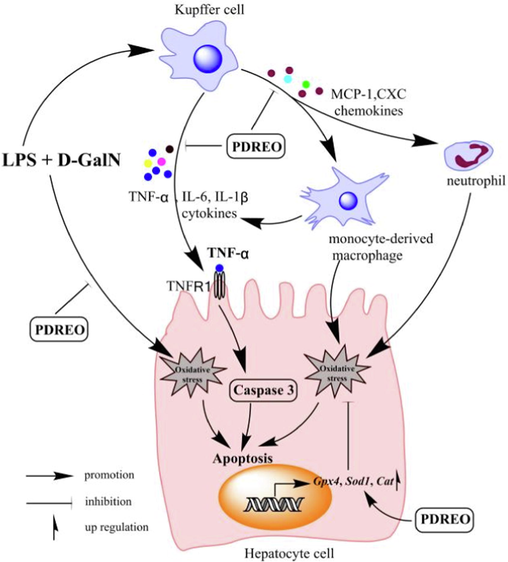
Schematic diagram of the protective mechanism of PDREO on LPS/D-GalN-induced ALI.
In the past few decades, the survival rate of patients with acute liver failure has increased from approximately 20% to over 60%. And the significant improvement in survival rate is mainly due to the combined effects of improved medical practices and the use of emergency liver transplantation in selected patients (Arshad et al., 2020). There have been many literature reports that some plant extracts (Yu et al., 2022; Jeong et al., 2021) and purified compounds (Tang et al., 2023; Farghali et al., 2009; Liu et al., 2022) have shown potential application value in the protection of acute liver injury, and silymarin has been applied in clinical liver disease treatment (Gillessen and Schmidt, 2020). In addition, some plant essential oils (Aati et al., 2022; Chen et al., 2021) have also shown good protective effects on experimental liver injury. However, due to differences in animal types, liver injury inducers, and induction doses used by various studies, it is difficult to directly compare the activity of drugs. At least, in the present study, PDREO (150 mg/kg) was significantly superior to clinical first-line drug NAC (300 mg/kg) in inhibiting LPS/D-GalN induced liver injury and lethal protection in C57BL/6 mice, demonstrating good development and application prospects. But its exact functional components still need further in-depth and systematic disclosure.
5 Conclusions
In summary, the current study confirmed that P. diversifolia roots are rich in essential oils and have a high content of compound isodaucene (29.97%), which has not been reported in Pimpinella species. PDREO (150 mg/kg) showed a strong protective effect on LPS/D-GalN induced liver injury and death in C57BL/6 mice, and its effect was significantly better than the positive control NAC (300 mg/kg). The protective effects of PDREO could be attributed to its inhibitory effects on the inflammatory response and activation of the antioxidant system. These findings suggest that PDREO might be used as a natural detoxification agent and that PDREO has great potential for application in the food and pharmaceutical industries.
6 Funding
The present research was carried out with the financial support of the Natural Science Foundation of Zhejiang Province [grant number LY23H280004] and a grant from the Zhejiang Provincial Key Laboratory Project [grant number 2012E10002].
7 Ethics statement
The animal study protocol was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Ethical Committee of Zhejiang Chinese Medical University (IACUC-20191202–04).
8 CRediT authorship contribution statement
Chenglong Hua: Methodology, Writing – review & editing, Investigation, Validation. Fusheng Jiang: Methodology, Writing – original draft, Funding acquisition. Linyan Wang: Methodology, Writing – original draft. Suyu Peng: Methodology, Investigation. Hang Gao: Software, Validation. Wanting Mo: Methodology. Sijia Yan: Methodology, Validation. Xiaobing Dou: Resources. Shiqing Li: Writing – review & editing, Software, Data curation. Chunchun Zhang: Conceptualization, Supervision, Project administration. Meiya Li: Conceptualization, Funding acquisition, Project administration.
9 Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
10 Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
- Chemical composition of Tagetes Patula flowers essential oil and hepato-therapeutic effect against carbon tetrachloride-induced toxicity (in-vivo) Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Acute-on-chronic liver failure clinical guidelines. Am. J. Gastroenterol.. 2022;117:225-252.
- [CrossRef] [Google Scholar]
- N-Acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab. Brain Dis.. 2010;25:241-249.
- [CrossRef] [Google Scholar]
- Oxiranylphenyl esters from Pimpinella Diversifolia. Phytochemistry. 1985;25:207-211.
- [CrossRef] [Google Scholar]
- Clinical significance of elevated liver transaminases in Hiv-infected patients. Aids. 2019;33:1267-1282.
- [CrossRef] [Google Scholar]
- Essential oil from the raw and vinegar-processed Rhizoma Curcumae Ameliorate Ccl(4)-induced liver fibrosis: integrating network pharmacology and molecular mechanism evaluation. Food Funct.. 2021;12:4199-4220.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and analgesic effects of Primpinellae Diversifoliae Dc. and anti-inflammatory activities in Lps-Stimulatedraw 264.7 cells in vitro. Chin. Arch. Tradit. Chin. Med.. 2015;33:2595-2597.
- [CrossRef] [Google Scholar]
- Chinese Academy of Sciences Editorial Committee of Flora of China, 2005. Flora of China, Science Press, Beijing
- Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-Galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide-Biol. Ch.. 2009;21:216-225.
- [CrossRef] [Google Scholar]
- Syringic acid protects against thioacetamide-induced hepatic encephalopathy: behavioral, biochemical, and molecular evidence. Neurosci. Lett.. 2022;769:136385
- [CrossRef] [Google Scholar]
- Silymarin as supportive treatment in liver diseases: a narrative review. Adv. Ther.. 2020;37:1279-1301.
- [CrossRef] [Google Scholar]
- Involvement of tumor necrosis factor-alpha in development of hepatic injury in galactosamine-sensitized mice. Hepatology. 1990;12:1187-1191.
- [CrossRef] [Google Scholar]
- Macrophage autophagy limits acute toxic liver injury in mice through down regulation of Interleukin-1Beta. J. Hepatol.. 2016;64:118-127.
- [CrossRef] [Google Scholar]
- Hepatitis B virus enhances tumor necrosis factor-related apoptosis-inducing ligand (Trail) cytotoxicity by increasing trail-R1/death receptor 4 expression. J. Hepatol.. 2003;39:414-420.
- [CrossRef] [Google Scholar]
- Lysimachiae Herba inhibits inflammatory reactions and improves Lipopolysaccharide/D-Galactosamine-induced hepatic injury. Antioxidants-Basel. 2021;10
- [CrossRef] [Google Scholar]
- Danshensu derivative Adtm Ameliorates Ccl4Induced acute liver injury in mice through inhibiting oxidative stress and apoptosis. Pathol. Res. Pract.. 2021;228:153656
- [CrossRef] [Google Scholar]
- Coelonin, an anti-inflammation active component of Bletilla Striata and its potential mechanism. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Acute-on-chronic liver failure: pathophysiological mechanisms and management. Front. Med. (Lausanne). 2021;8:752875
- [CrossRef] [Google Scholar]
- Kim, W.H., Hong, F., Jaruga, B.et al., 2005. Hepatitis B Virus X Protein Sensitizes Primary Mouse Hepatocytes to Ethanol- And Tnf-Alpha-Induced Apoptosis by a Caspase-3-Dependent Mechanism. Cell Mol. Immunol. 2, 40-48. https://doi.org/http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16212910&query_hl=1.
- Selenoprotein Msrb1 deficiency exacerbates acetaminophen-induced hepatotoxicity via increased oxidative damage. Arch. Biochem. Biophys.. 2017;634:69-75.
- [CrossRef] [Google Scholar]
- Anti-oxidant and hepatoprotective effects of salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. Int.. 2021;28:11001-11015.
- [CrossRef] [Google Scholar]
- The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc. Res.. 1992;26:641-655.
- [CrossRef] [Google Scholar]
- Liver transplantation in acute liver failure: dilemmas and challenges. World J. Transplant.. 2021;11:187-202.
- [CrossRef] [Google Scholar]
- Complement-5 inhibition deters progression of fulminant hepatitis to acute liver failure in murine models. Cell Mol. Gastroenter.. 2021;11:1351-1367.
- [CrossRef] [Google Scholar]
- Leist, M., Gantner, F., Jilg, S.et al., 1995. Activation of the 55 Kda Tnf Receptor is Necessary and Sufficient for Tnf-Induced Liver Failure, Hepatocyte Apoptosis, and Nitrite Release. J. Immunol. 154, 1307-1316. https://doi.org/http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7822799&query_hl=1.
- Supplementation of N-Acetylcysteine normalizes lipopolysaccharide-induced nuclear factor kappab activation and proinflammatory cytokine production during early rehabilitation of protein malnourished mice. J. Nutr.. 2002;132:3286-3292.
- [CrossRef] [Google Scholar]
- Diallyl sulfide protects against Lipopolysaccharide/D-Galactosamine-induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Food Chem. Toxicol.. 2018;120:500-509.
- [CrossRef] [Google Scholar]
- Ginsenoside Rb1 reduces D-Galn/Lps-induced acute liver injury by regulating Tlr4/Nf-Kappab signaling and Nlrp3 inflammasome. J. Clin. Transl. Hepato.. 2022;10:474-485.
- [CrossRef] [Google Scholar]
- Terpenoids and aromatic compounds from bryophytes and their central nervous system activity. Curr. Org. Chem.. 2020;24:113-128.
- [CrossRef] [Google Scholar]
- Nrf2 signaling and autophagy are complementary in protecting Lipopolysaccharide/D-Galactosamine-induced acute liver injury by Licochalcone A. Cell Death Dis.. 2019;10:313.
- [CrossRef] [Google Scholar]
- Pimpinella Anisum and female disorders: a review. Phytomed. Plus. 2021;1:100063
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Alhagi Sparsifolia against alcoholic liver injury in mice. Braz. J. Pharm. Sci.. 2018;54:e17732.
- [Google Scholar]
- Mechanisms for sensitization to Tnf-induced apoptosis by acute Glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425-1434.
- [CrossRef] [Google Scholar]
- Composition of the Root Essential Oil From Pimpinella Diversifolia. International Congress of Essential Oils; 1990.
- Lps-induced liver injury in D-Galactosamine-sensitized mice requires secreted Tnf-Alpha and the Tnf-P55 receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2000;278:R1202-R1209.
- [CrossRef] [Google Scholar]
- N-Acetylcysteine and Glutathione as inhibitors of tumor necrosis factor production. Cell. Immunol.. 1992;140:390-399.
- [CrossRef] [Google Scholar]
- Emerging roles of Caspase-3 in apoptosis. Cell Death Differ.. 1999;6:99-104.
- [CrossRef] [Google Scholar]
- The determination of serum acid and alkaline phosphatase activity with 4-Aminoantipyrine (a.a.P.) J. Clin. Pathol.. 1954;7:245-248.
- [CrossRef] [Google Scholar]
- Effects of early and delayed wound excision on pulmonary Leukosequestration and neutrophil respiratory burst activity in burned mice. Surgery. 1995;118:884-892.
- [CrossRef] [Google Scholar]
- Alanine Aminotransferase in clinical practice. a review. Arch. Intern. Med.. 1991;151:260-265.
- [CrossRef] [Google Scholar]
- Biomarkers for drug-induced liver injury. Expert Rev. Gastroent.. 2010;4:225-234.
- [CrossRef] [Google Scholar]
- Review of pharmacological properties and chemical constituents of Pimpinella Anisum. Isrn Pharm.. 2012;2012:510795
- [CrossRef] [Google Scholar]
- Acute liver failure-it's just a matter of cell death. Digest. Dis.. 2016;34:423-428.
- [CrossRef] [Google Scholar]
- Estrogenic activity of isolated compounds and essential oils of Pimpinella species from Turkey, evaluated using a recombinant yeast screen. Planta Med.. 2004;70:728-735.
- [CrossRef] [Google Scholar]
- Gas chromatographic-mass spectrometric analysis of essential oils from Pimpinella Aurea, Pimpinella Corymbosa, Pimpinella Peregrina and Pimpinella Puberula gathered from Eastern and Southern Turkey. J. Chromatogr. A. 2005;1097:192-198.
- [CrossRef] [Google Scholar]
- Effect of essential oils and isolated compounds from Pimpinella Species On Nf-Kappab: a target for antiinflammatory therapy. Phytother. Res.. 2007;21:741-745.
- [CrossRef] [Google Scholar]
- Targeting hepatic macrophages to treat liver diseases. J. Hepatol.. 2017;66:1300-1312.
- [CrossRef] [Google Scholar]
- The novel hepatoprotective mechanisms of Silibinin-Phospholipid complex against D-Galn/Lps-induced acute liver injury. Int. Immunopharmacol.. 2023;116:109808
- [CrossRef] [Google Scholar]
- The role of monocytes and macrophages in acute and acute-on-chronic liver failure. Front. Immunol.. 2018;9:2948.
- [CrossRef] [Google Scholar]
- Naltrexone protects against Lipopolysaccharide/D-Galactosamine-induced hepatitis in mice. J. Pharmacol. Sci.. 2008;108:239-247.
- [CrossRef] [Google Scholar]
- Chemical characterization and antioxidant, anti-inflammatory, and anti-septic activities of the essential oil from the aerial parts of Atractylodes Macrocephala Koidz. Arab J. Chem.. 2022;15:104215
- [CrossRef] [Google Scholar]
- Experimental study on the anti-inflammatory, hemostatic and coagulation effects of extracts from Pimpinella Diversifolia. Yunnan. J. Tradit. Chin. Med. Mater. Med.. 2017;38:75-77.
- [CrossRef] [Google Scholar]
- N-Acetylcysteine attenuates Lipopolysaccharide-induced apoptotic liver damage in D-Galactosamine-sensitized mice. Acta Pharmacol. Sin.. 2007;28:1803-1809.
- [CrossRef] [Google Scholar]
- The undiscovered potential of essential oils for treating SARS-Cov-2 (Covid-19) Curr. Pharm. Des.. 2020;26:5261-5277.
- [CrossRef] [Google Scholar]
- Withaferin a alleviates fulminant hepatitis by targeting macrophage and Nlrp3. Cell Death Dis.. 2021;12:174.
- [CrossRef] [Google Scholar]
- Limiting the testing of Ast: a diagnostically nonspecific enzyme. Am. J. Clin. Pathol.. 2015;144:423-426.
- [CrossRef] [Google Scholar]
- Study on the chemical components of essentiale oil from Zhejiang Pimpinella Diversifolia. China Pharm.. 2012;21:3-4.
- [CrossRef] [Google Scholar]
- Hepatoprotective effects of different mulberry leaf extracts against acute liver injury in rats by alleviating oxidative stress and inflammatory response. Food Funct.. 2022;13:8593-8604.
- [CrossRef] [Google Scholar]
- Cinnamon and eucalyptus oils suppress the inflammation induced by lipopolysaccharide in vivo. Molecules. 2021;26:7410.
- [CrossRef] [Google Scholar]
- Antioxidant activity of total flavonoids from Pimpinella Diversifolia Dc. Tianjin Agric. Sci.. 2019;25:8-13.
- [CrossRef] [Google Scholar]
- Zhejiang Food and Drug Administration, 2016. Zhejiang Traditional Chinese Medicine Processing Specification. 2015 ed., China Pharmaceutical Science and Technology Press
- Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol.. 2014;193:344-353.
- [CrossRef] [Google Scholar]
- Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol.. 2012;3:56.
- [CrossRef] [Google Scholar]







