Translate this page into:
Gas chromatography mass spectrometry couple with quadrupole time-of-flight (GC-QTOF MS) as a powerful tool for profiling of oxygenated sesquiterpenes in agarwood oil
⁎Corresponding authors at: Bio Aromatic Research Centre, Universiti Malaysia Pahang, 26300 Kuantan, Pahang Darul Makmur (A.K.M. Moyeenul Huq). moyeenul@ump.edu.my (A.K.M. Moyeenul Huq), saifulnizam@ump.edu.my (Saiful Nizam Tajuddin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Agarwood (Aquilaria malaccensis) is very well known as the most expensive wood in the world due to its wide applications in perfumery, cosmetic traditional medicine, and religious ceremonies. The study aimed to give an in-depth characterisation focusing on marker compounds in A. malaccensis from different places in Malaysia. The establishment of an oxygenated sesquiterpenes chemical profile of the fungus-infected agarwood oil was achieved by gas chromatography mass spectrometry (GC–MS) coupled with quadrupole time (QTOF) technique. Aroma compounds were identified as sesquiterpenes and oxygenated sesquiterpenes where agarospirol was found in samples of all locations (3.12%, 3.54%, 3.36% and 2.26% from Melaka, Pahang, Kelantan A and Kelantan B respectively) and also N-hexadecanoic acid as one of the major compounds. Both compounds were further isolated by Prep-GC and confirmed by NMR. This study provides a reference for agarwood oil analysis from different origins in Malaysia.
Keywords
Aquilaria malaccensis
Agarwood oil
phytochemical
Sesquiterpene
Gas chromatography-mass spectrometry
Gas chromatography quadrupole time-of-flight
1 Introduction
Aquilaria malaccensis is one of 15 tree species in the genus Aquilaria (family Thymelaeceae). Fungus-infected A. Malaccensis agarwood oil is highly sought after by the perfumery industry for its “warm, unique, balmasic notes with sandalwood-ambergris tonalities” (Naef, 2011). There is no common grading standard or method for agarwood oil at present. Several studies carried out on oil characterization and recognized a relationship between the agarwood oil of different origins and volatile and semi-volatile compounds namely, oxygenated sesquiterpenes (Ahmaed, 2017; Ahmaed and Kulkarni, 2017; Tajuddin et al., 2013).
The major chemical constituents of Malaysian agarwood oils were previously reported to be 4-phenyl-2butanone (1), α-bulnesene (2), α-guaiene (3), agarospirol (15), and γ-eudesmol (18) (Haslina et al., 2018; Ismail et al., 2016; Tajuddin et al., 2013) as shown in Fig. 1. Generally, the major components of the oils are sesquiterpenes and chromone derivatives (Yoneda et al., 1984). Other workers have reported sesquiterpenes, sesquiterpenes furanoids, tetradecanoic acid and pentadecanoic acid to be present in all species (Ishihara et al., 1991, 1993b; Ngan et al., 2020; Ueda et al., 2006; Yoneda et al., 1984). Agarospirol (15), jinkohol-eremol, jinkohol and kusunol are compounds contributing to its characteristic aroma (Ishihara et al., 1993b). Such compounds have specific aroma descriptors, e.g. agarospirol (15), which exhibit a spicy, peppery and woody aroma. While some compounds do not contribute directly to the aroma, they are reactive and can break down to give pleasant-smelling volatiles. Agarwood oil is generally obtained in 0.2–0.3% yield (dry weight basis) by steam or hydrodistillation (Tajuddin et al., 2013). Eleven compounds had been reported present in agarwood oils from peninsular Malaysia via GC-FID analysis previously (Nor Azah et al., 2008) and seventeen major compounds were identified in different agarwood oil via two-dimensional gas chromatography (Wong et al., 2015).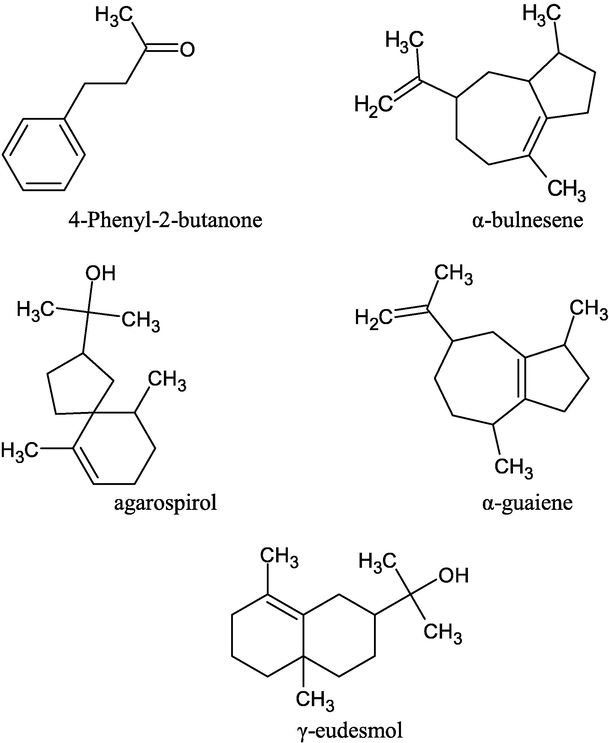
Some chemical constituents of Malaysian agarwood oil.
Despite of the great separation power of the conventional one-dimensional modern chromatographic technique, the complex nature of the sample arising from the compact oxygenated sesquiterpenes fraction requires extended GC runs. Furthermore, deep analyses of the chromatograms frequently indicate that some peaks are the result of two or more co-eluting compounds. As a consequence of co-elution, reliable MS identification is not possible. Although one-dimensional chromatographic processes often provide rewarding analytical results, the complexity of many naturally occurring matrices exceeds the capacity of any single separation system. As a result, much research has been dedicated to hyphenated techniques to strengthen resolving power (Eyres et al., 2008; Ong and Marriott, 2002; Park et al., 2012; Ryan and Marriott, 2003; Tajuddin et al., 2013).
Comprehensive two-dimensional gas chromatography (GC × GC) employing two orthogonal mechanisms to separate the constituents of the sample within a single analysis has also been used to analyse agarwood oil. The TOF-MS brings other advantages such as full mass spectra acquisition at trace level sensitivity and mass spectral continuity, which allows for deconvolution of spectra of co-eluting peaks (López-Cobo et al., 2017; Wang et al., 2014). This study aims to develop a methodology based on a novel GC-QTOF MS technique to obtain a deep qualitative and quantitative characterization of the oxygenated sesquiterpenes in the agarwood oil obtained from difference sources of origin. Considering the complexity of the data obtained, this manuscript is focused only on the oxygenated sesquiterpenes as marker compound present in the samples for grading purpose by proper identification and quality analysis of commercial agarwood oil.
2 Material and method
2.1 Samples preparation
In this study, A. malaccensis (agarwood) oil from various places in Malaysia was analysed. The climate for Malaysia was categorised as equatorial with an average rainfall of 250 cm (98 in) a year, and an average temperature of 27 °C. The samples were collected from different origins in peninsular Malaysia; Melaka city (2.1896° N, 102.2501° E)., Rompin district, Pahang (2.7945° N, 103.4862° E) and two samples were of Kelantan, Gua Musang district and Kota Bharu (4.8843° N, 101.9682° E and 6.1248° N, 102.2544° E respectively) origin. Agarwood was subjected to hydrodistillation with 5L distilled water for 24 h, using Clavenger-type apparatus. The essential oils were isolated by extraction with hexane, distillate collected and dried over anhydrous sodium sulphate. The oils collected were stored in amber vials at 4 °C until analysis.
2.2 GC-Mass spectrometry (GC–MS)
Analysis was performed using Agilent 7890A Network System gas chromatography coupled to a mass spectrometer (Agilent 5975C) with a detector in full scan mode under electron impact ionization (EI, 70 eV) and fitted with a capillary column (DB-1 30 m × 0.25 mm, i.d.; 0.25 μm film thickness). The oven temperature was programmed at 50 °C for 0 min, then ramped at 10 °C/min to 160 °C and lastly, at 3 °C/min to 220 °C. Injector inlet and detector temperatures were set at 320 °C. Each sample was diluted in dichloromethane and then injected in 1 µL volume in the split mode (ratio 50:1) using helium as carrier gas (1 mL/min).
2.3 Comprehensive GC-QTOF
An Agilent’s 7200, the world’s first Q-TOF designed specifically for GC–MS were used. The non-polar column, DB-1 (30 m × 0.25 mm, i.d 0.25 µm film thickness) was purchased from J&W Scientific (Folsom, California). While handling the Q-TOF, software from Mass Hunter acquisition B.06, Mass Hunter Qualitative B.06 and NIST Mass Spectra Library 2011 were used. Helium was used as carrier gas with electronic impact (EI) ionization sources. The transfer line of the TOF was kept at 280 °C, ion source temperature at 230 °C and the mass range was around 45–500 amu. The resolution used was ∼ 12,000 (FWHM), while the acquisition rate was 5 spectra per second and the acquisition time was 200 ms per spectrum. For the injection, the inlet temperature was 250 °C, using split mode with a split ratio of 150:1. The GC oven temperature was programmed with the initial temperature at 50 °C (0 min), then ramp to 160 °C (0 min) at 10 °C/min, and the final temperature was 220 °C (0 min) at 3 °C/min.
2.4 Preparative gas chromatography (Prep-GC)
Isolation of the compound was carried out on preparative gas chromatography Agilent 7890B model. The following conditions were applied: non– polar DB-5 ms column (30 m × 0.25 mm, i.d 0.53 µm film thickness), oven programmed from 80 °C to 280 °C, ramping at 5 °C/min; temperatures of injector, switching device and transfer line were set at 250 °C. High purity nitrogen (N2) was used as carrier gas at a constant flow rate. The inlet and FID temperatures were 250 °C, respectively.
3 Results and discussion
3.1 Analysis of the chemical composition
Two methods were adopted in this study to detect and confirm the marker compounds in A. Malaccensis oil using GC–MS and GC-QTOF-MS. Table 1 shows the peak identification for the components. About 21 compounds have been identified whereas most of the compounds detected were either sesquiterpenes or oxygenated sesquiterpenes. This finding is also in good agreement with a previous report which found oxygenated sesquiterpenes as the major component in agarwood oil (Atikah et al., 2015; Haslina et al., 2018). Agarwood oil from Melaka contained a greater number of compounds as compared to that of Pahang and Kelantan. The sample from Melaka appeared darker with higher viscosity compared to others. Based on the present study, selinene (14), agarospirol (15), and n-hexadecanoic acid (21) were observed to be the main components present in all agarwood oil samples as matched with the NIST library. Selinene (14) contributes to the herbal smell, while agarospirol (15) gives out the smell of spicy, peppery and woody according to literature. Thus, agarospirol can be considered to be chosen as one of the marker compounds of agarwood oil. Therefore, agarospirol in A. malaccensis oil was targeted for isolation in this study due to its characteristic woody odor that may contribute to the strong smell of agarwood oil (Naef, 2011). This result also aligned with another study that identified the presence of agarospirol in agarwood oil (Nät et al., 1993; Okugawa et al., 1996; Pripdeevech et al., 2011). This compound was reported to give positive effects on the central nervous system and may decrease methamphetamine and apomorphine-induced spontaneous motility (Okugawa et al., 1996). A decade ago, previous studies have reported the isolation of this compound using column chromatography but none of these used preparative gas chromatography for isolation of agarospirol from A. malaccensis (agarwood) oil. The presence of agarospirol compound was proven based on further experimental analysis using GC-QTOF-MS and also spectroscopy analysis. The QTOF collects data using accurate mass in EI spectra and provides fragmentation data to identify the compound while GC–MS identified the compounds by unit mass allowing library search by NIST database. QTOF helps to confirm the compounds with mass accuracy below 5 ppm. Mass fragmentation of the main compounds was deduced based on the mass spectrum obtained.
No.
Compound
Chemical Formula
Area (%)
Odor Description
Ref.
Melaka
Pahang
Kelantan A
Kelantan B
1
4-phenyl-2-butanone
C10H12O
1.62
2.84
1.65
–
floral, jasmine, herbal, fruity, balsam
(Buttery and Ling, 1994; Kessler and Baldwin, 2007)
2
α-bulnesene
C15H24
4.79
2.42
1.61
–
woody, warm,
(Ishihara et al., 1993a, 1993b), (Salum et al., 2009)
3
α-Guaiene
C15H24
4.33
–
0.70
–
sweet, woody, balsam, peppery
(Jirovetz et al., 2002; McGrath and Karahadian, 1994)
4
α-humulene
C15H24
0.51
–
–
–
woody
(Jirovetz et al., 2002)
5
β-humulene
C15H24
–
–
–
0.28
woody
(Jirovetz et al., 2002)
6
α-gurjunene
C15H24
0.62
2.16
1.53
–
wood, balsamic
(Jirovetz et al., 2002)
7
β-maaliene
C15H24
4.45
8.31
–
–
–
–
8
Longiflene
C15H24
9.74
–
–
–
–
–
9
Alloaromadendrene
C15H24
–
–
–
1.17
woody
(Chung et al., 1993)
10
Aromandendrene
C15H24
–
–
–
0.20
woody
(Chung et al., 1993)
11
γ-cadinene
C15H24
–
–
–
9.45
dry-woody, weak medicinal
(Jirovetz et al., 2002)
12
Eremophilene
C15H24
–
–
–
2.07
–
–
13
Farnesene
C15H24
–
2.61
–
–
citrus, green
(Choi et al., 2001)
14
Selinene
C15H24
1.27
1.46
1.08
7.59
herb
(Adedeji et al., 1991)
15
Agarospirol
C15H26O
3.12
3.54
3.36
2.30
spicy, peppery, woody
(Ishihara et al., 1993a, 1993b)
16
γ-gurjunene
C15H24
2.51
9.26
2.26
–
–
–
17
Bulnesol
C15H26O
3.23
–
–
–
weak woody, weak spicy
(Jirovetz et al., 2002)
18
γ-eudesmol
C15H26O
1.15
13.39
17.23
–
waxy, sweet
(Jirovetz et al., 2002)
19
α-elemol
C15H26O
–
10.03
–
–
green, wood
(Choi et al., 2001)
20
Myristic acid
C14H28O2
3.11
–
–
–
–
–
21
n-Hexadecanoic acid
C16H32O2
10.87
8.59
7.05
18.42
–
–
3.2 Agarospirol
Sample with high percentage of agarospirol was selected for isolation purpose. This is important to maintain the concentration and target for high volume collection. The retention time of agarospirol was established on prep-GC for heart cutting selection and multiple injections. In this study, agarospirol was successfully isolated as colorless oil using Prep-GC, whereas the cooling trap condition was set at −20 °C. Preparative gas chromatography is a green, simple, convenient, and highly efficient method for sample collection trap (Nojima et al., 2008; Ochiai & Sasamoto, 2011; Sciarrone et al., 2015). Many researchers have proven the capability of the preparative method in isolation, including essential oil (Ball et al., 2012; Rühle et al., 2009; Sutton et al., 2005). Carotol was successively isolated from carrot seed oil using one-dimensional Prep-GC and prep-MDGC (multi-dimensional) (Sciarrone et al., 2015). In this study, the heart cutting method with multiple times injections was applied. The most crucial consideration for this isolation method is selecting the cutting time, sample concentration, and times of injection (Kim et al., 2013; Kim & Marriott, 2012). The flow of chromatogram from starting until the end product was shown in Fig. 2. Shown in Fig. 3 is the EI-MS spectrum for agarospirol. The EI-MS spectrum exhibited an [M] + m/z 222 and fragments ions appeared at m/z 204, 189, 162, 147, 120 and 105 as shown. Losing of isopropanol radical [CH3CH2CH2OH]+ and hydride transfer H+ from parent ion (I) resulted in the formation of the base peak of ion [C12H18]+ (II), represented as m/z peak 162, with the maximum intensity (100%). Next, m/z 204 [C15H24]+ was formed from the loss of water molecule and fragment ion of m/z 189 (IV) [C14H21]+ was derived from the loss of methyl group. After the loss of propene radical (IV), the ion with m/z 147, [C11H15]+ was formed (V), which underwent the Retro-Diels Alder reaction, resulting in the peak of m/z 120 (VI). The ion at m/z 59 (VII) was produced from the loss of radical group (•C12H19) to form [C3H7O]+. Full mass fragmentation of agarospirol is shown in Fig. 4 (Bercht et al., 1976; Rabe & Dickschat, 2016). Based on the mass fragmentation pattern, the compound can be assigned as agarospirol. The accurate mass of the agarospirol was measured using GC-QTOF MS with the mass deviation in the range −4.87 to 0.48 as shown in Table 2.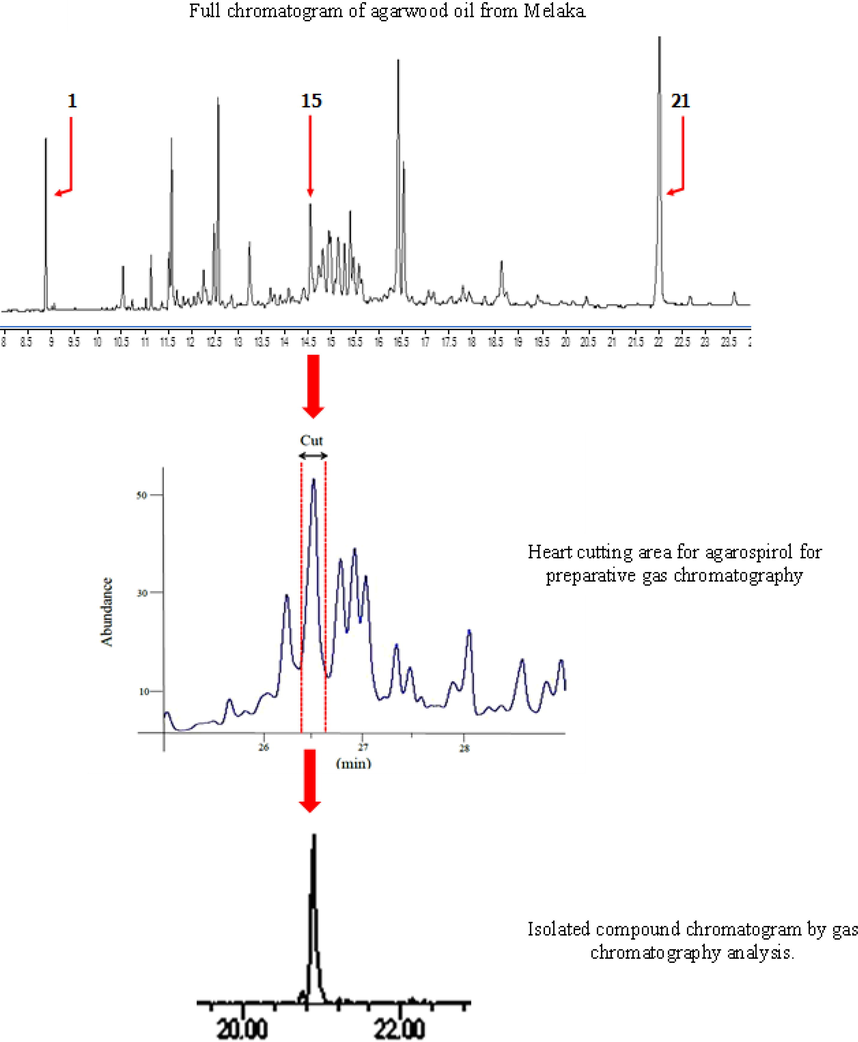
Chromatogram flow of agarospirol isolation.
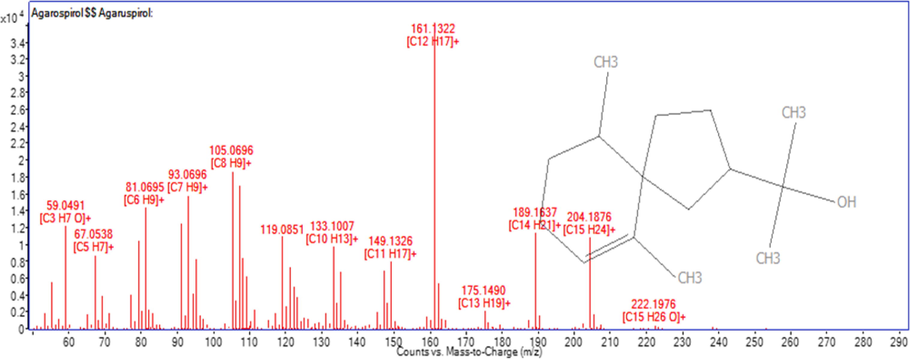
EI-MS spectrum for agarospirol.
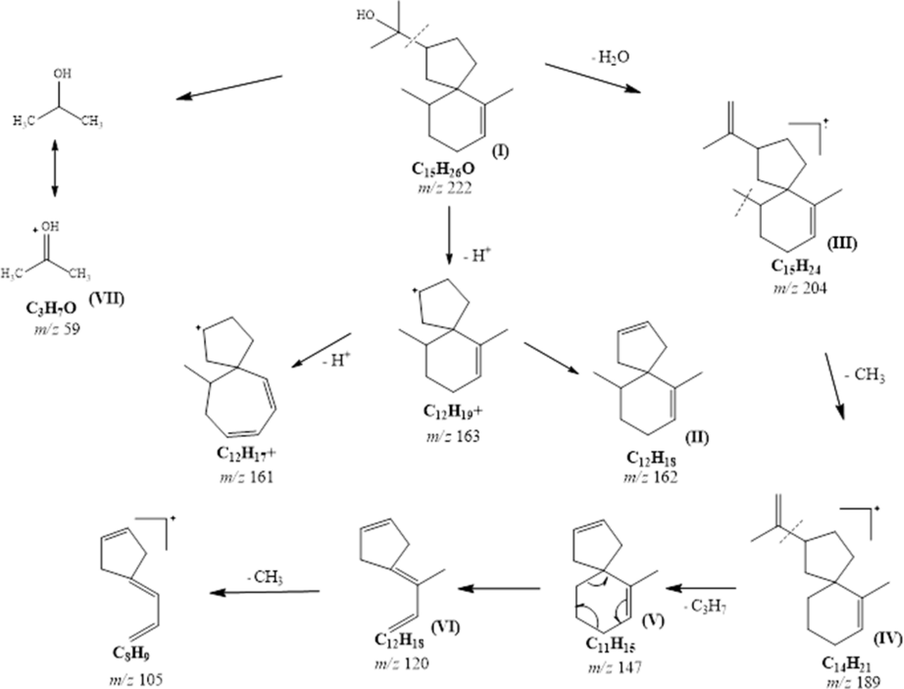
Full mass fragmentation of agarospirol.
Experiment mass (Da)
Calculated mass (Da)
Mass error (ppm)
Formula
59.0594
59.0491
−4.38
C3H7O
105.0700
105.0699
−1.17
C8H9
107.0856
107.0855
−0.68
C8H11
119.0857
119.0855
−1.45
C9H11
147.1173
147.1168
−3.22
C11H15
161.1324
161.1325
0.48
C12H17
189.1648
189.1638
−5.41
C14H21
204.1882
204.1873
−4.64
C15H24
222.1989
222.1978
−4.87
C15H26O
3.3 n-Hexadecanoic acid
Under the cooling trap condition setup at −20 °C by Prep-GC, this compound was obtained as a colorless oil. Further isolation method of this compound refers to the agarospirol method, except that the heart cutting method was applied at different times. Fig. 5 show the mass spectrum of n-Hexadecanoic acid from GCQTOF-MS, which exhibited a molecular ion peak at an m/z value of 256 corresponding to the molecular formula C16H32O2. The fragmentation of n-hexadecanoic acid is shown in Fig. 6. The base peak showed a fragment at an m/z value of 60 (C2H4O2). The fragment confirmed the presence of carboxylic acid group, while the other fragment is proven as the removal of ethyl group from the long-chain hydrocarbon. Therefore, from the spectroscopic analysis, the fragmentation pattern of MS, and the comparison of the data with the literature of the same compound isolated (Krishnan et al., 2016), this compound is assigned as n-hexadecanoic acid.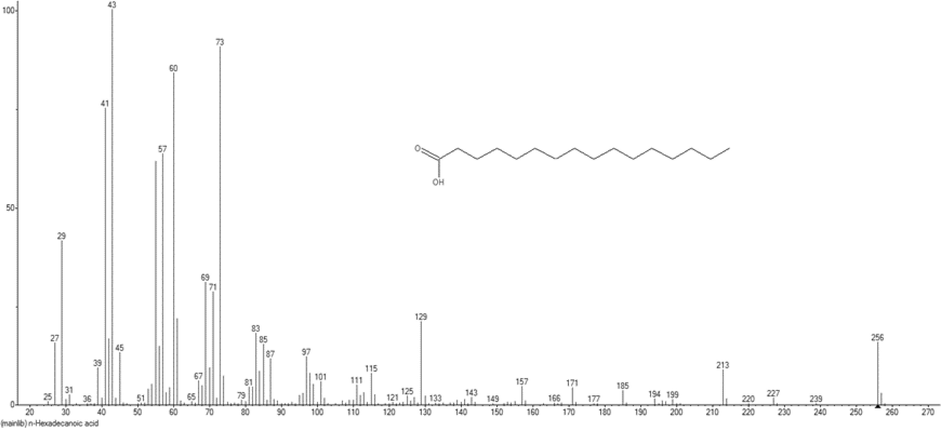
Mass Spectrum of n-Hexadecanoic acid from GCQTOF-MS.
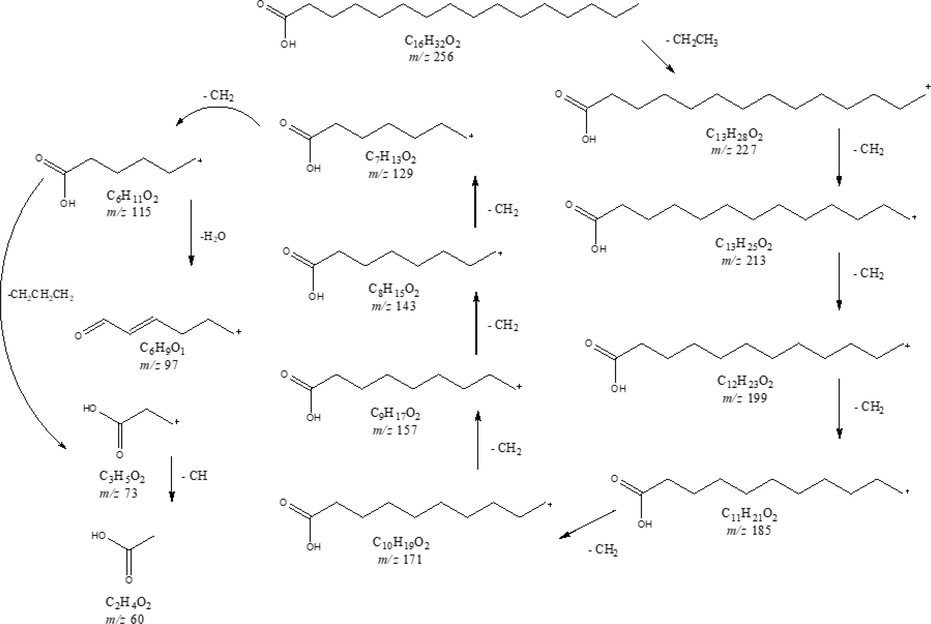
Mass fragmentation of n-Hexadecanoic acid.
4 Conclusion
In this study, the combined use of gas chromatography spectrometry (GC–MS) and quadrupole time of flight (QTOF) was demonstrated in the successful identification of the major compounds in agarwood oil collected from different origins. GC-QTOF-MS is a highly sensitive, accurate mass detector giving high resolution mass compared to a standalone GC–MS. A number of sesquiterpens were identified and quantified successfully from different samples of agarwood oil. Three compounds agarospirol, n-hexadecanoic acid and selinene were identified in all the samples. The identification of compounds was further validated by isolating Agarospirol and n-hexadecanoic acid where agarospirol is responsible for the characteristic woody smell of Oud.
Acknowledgement
This research was supported by grant received from Ministry of Science (02-01-16-SF0092), Technology and Innovation (MOSTI), Malaysia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Free and glycosidically bound aroma compounds in hog plum (Spondias mombins L.) J. agric. food chem.. 1991;39:1494-1497.
- [Google Scholar]
- Sesquiterpenes and chromones of agarwood: a review. Malay. J. Chem.. 2017;19:33-58.
- [Google Scholar]
- Investigation of agarwood compounds in Aquilaria malaccensis & Aquilaria rostrata chipwood by using solid phase microextraction. Biomed. J. Sci. Technic. Res.. 2017;1:1609-1616.
- [Google Scholar]
- A two-dimensional, heart-cutting preparative gas chromatograph facilitates highly resolved single-compound isolations with utility towards compound-specific natural abundance radiocarbon (14C) analyses. J. Chromatograp. A.. 2012;1220:122-131.
- [Google Scholar]
- Cannabispirone and cannabispirenone, two naturally occurring spiro-compounds. Tetrahedron.. 1976;32:2939-2943.
- [Google Scholar]
- Importance of 2-Aminoacetophenone to the Flavor of Masa Corn Flour Products. J. Agric. Food Chem.. 1994;42:1-2.
- [Google Scholar]
- Characterization of the odor-active volatiles in citrus Hyuganatsu (Citrus tamurana Hort. ex Tanaka) J. Agric. Food Chem.. 2001;49:2404-2408.
- [Google Scholar]
- Volatile compounds isolated from edible Korean Chamchwi (Aster scaber Thunb) J. Agric. Food Chem.. 1993;41:1693-1697.
- [Google Scholar]
- Method for small-molecule discovery based on microscale-preparative multidimensional gas chromatography isolation with nuclear magnetic resonance spectroscopy. Anal. Chem.. 2008;80:6293-6299.
- [Google Scholar]
- Analysis of different quality agarwood oil (Aquilaria Malaccensis) and sensory study. J. Telecommun. Electron. Comput. Eng.. 2018;10:57-61.
- [Google Scholar]
- Components of the volatile concentrate of Agarwood. J. Essent. Oil Res.. 1993;5:283-289.
- [Google Scholar]
- Ismail, N., Rahiman, M. H. F., Taib, M. N., Ibrahim, M., Zareen, S., Tajuddin, S. N., 2016. A review on agarwood and its quality determination. In: Proceedings - 2015 6th IEEE Control and System Graduate Research Colloquium. ICSGRC. 2015, 103–108.
- Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J. Chromatograph. A.. 2002;976:265-275.
- [Google Scholar]
- Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J.. 2007;49:840-854.
- [Google Scholar]
- Kim, L., Marriott, P. J., 2012. Preparative Gas Chromatography. In: Gas Chromatography. Elsevier Inc.
- Quantitative preparative gas chromatography of caffeine with nuclear magnetic resonance spectroscopy. J. Separat. Sci.. 2013;36:1774-1780.
- [Google Scholar]
- Isolation and characterization of n-hexadecanoic acid from Canthium parviflorum leaves. J. Chem. Pharmaceut. Res.. 2016;8:614-617.
- [Google Scholar]
- Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int.. 2017;100:423-434.
- [Google Scholar]
- Evaluation of headspace volatiles and sensory characteristics of ripe pawpaws (Asimina triloba) from selected cultivars. Food Chem.. 1994;51:255-262.
- [Google Scholar]
- The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: a review. Flavour Fragr. J.. 2011;26:73-87.
- [Google Scholar]
- New compounds identified in agarwood (Aquilaria agallocha Roxb.) Flavour Fragr. J.. 1993;8:307-313.
- [Google Scholar]
- Chemical components of agarwood (Aquilaria crassna) essential oils grown in various regions of Asia. Asian J. Chem.. 2020;32:36-40.
- [Google Scholar]
- A simple, convenient, and efficient preparative GC system that uses a short megabore capillary column as a trap. J. Chem. Ecol.. 2008;34:418-428.
- [Google Scholar]
- Comparison of chemical profiles of selected gaharu oils from peninsular Malaysia. Malaysian J. Anal. Sci.. 2008;12(2):338-340.
- [Google Scholar]
- Selectable one-dimensional or two-dimensional gas chromatography-olfactometry/mass spectrometry with preparative fraction collection for analysis of ultra-trace amounts of odor compounds. J. Chromatograph. A.. 2011;1218:3180-3185.
- [Google Scholar]
- Effect of Jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Phytomed. : Int. J. Phytother. Phytopharmacol.. 1996;3:147-153.
- [Google Scholar]
- A review of basic concepts in comprehensive two-dimensional gas chromatography. J. Chromatograph. Sci.. 2002;40:276-291.
- [Google Scholar]
- Simple preparative gas chromatographic method for isolation of menthol and menthone from peppermint oil, with quantitative GC-MS and 1H NMR assay. J. Separat. Sci.. 2012;35:416-423.
- [Google Scholar]
- Identification of odor-active components of agarwood essential oils from thailand by solid phase Microextraction-GC/MS and GC-O. J. Essent. Oil Res.. 2011;23:46-53.
- [Google Scholar]
- The EIMS fragmentation mechanisms of the sesquiterpenes corvol ethers A and B, epi-cubebol and isodauc-8-en-11-ol. Beilstein J. Organ. Chem.. 2016;12:1380-1394.
- [Google Scholar]
- Multiple component isolation in preparative multidimensional gas chromatography with characterisation by mass spectrometry and nuclear magnetic resonance spectroscopy. J. Chromatograph. A.. 2009;1216:5740-5747.
- [Google Scholar]
- Comprehensive two-dimensional gas chromatography. Analytic. Bioanalytic. Chem.. 2003;376:295-297.
- [Google Scholar]
- Determination of volatiles produced during radiation processing in Laurus cinnamomum. Radiat. Phys. Chem.. 2009;78:635-637.
- [Google Scholar]
- Advanced preparative techniques for the collection of pure components from essential oils. Naut. Volat. Essent. Oils.. 2015;2:1-15.
- [Google Scholar]
- Isolation of individual hydrocarbons from the unresolved complex hydrocarbon mixture of a biodegraded crude oil using preparative capillary gas chromatography. Organ. Geochem.. 2005;36:963-970.
- [Google Scholar]
- Characterization of the chemical constituents of agarwood oils from Malaysia by comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. Mendeleev Communicat.. 2013;23:51-52.
- [Google Scholar]
- New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorgan. Med. Chem.. 2006;14:3571-3574.
- [Google Scholar]
- Identification of unknown organosulfur compounds with GC/QTOF-MS in the water-soluble portion from mildly oxidized Jincheng No. 15 anthracite. Fuel.. 2014;135:188-190.
- [Google Scholar]
- Evaluation of comprehensive two-dimensional gas chromatography with accurate mass time-of-flight mass spectrometry for the metabolic profiling of plant-fungus interaction in Aquilaria malaccensis. In J. Chromatograph. A.. 2015;1387:104-115.
- [Google Scholar]
- Sesquiterpenoids in two different kinds of agarwood. Phytochem.. 1984;23:2068-2069.
- [Google Scholar]







