Translate this page into:
Bioactive secondary metabolites produced by fungi of the genus Diaporthe (Phomopsis): Structures, biological activities, and biosynthesis
⁎Corresponding author at: 492 Jinxi Rd., Hecheng District, Huaihua 418000, China. ph0745@126.com (Hong Pu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Diaporthe and its anamorph Phomopsis, a genus of endophytic, saprotrophic, and plant pathogenic fungi, are found in many different ecosystems worldwide. Diaporthe (Phomopsis) fungi generate natural products such as pyrones, polyketides, alkaloids, and terpenoids. Most of these natural products show antibacterial, anti-inflammatory, and/or cytotoxic activity. In this review, we describe the 331 bioactive secondary metabolites isolated from 75 known species and various unidentified species of Diaporthe and Phomopsis from 2016 to 2021. These products comprise 143 bioactive compounds from Diaporthe and 188 from Phomopsis, including quinones, alkaloids, terpenoids, pyrones, polyketides, diphenyl ketones, diphenyl ethers, steroids, and fatty acids. The major activities of these compounds are as cytotoxic, antibacterial, and anti-inflammatory chemicals. All 21 fungi in the genus Diaporthe (Phomopsis) with available whole genome sequencing data contain several gene clusters for secondary metabolite biosynthesis. Such gene clusters and biosynthetic mechanisms have been identified for rugulosin A, terpestacin, and sch-642305. Diaporthe (Phomopsis) fungi produce abundant novel active natural products with great potential for drug development. In addition, these fungi provide important resources for research on the biosynthesis of secondary metabolites.
Keywords
Fungi
Diaporthe
Phomopsis
Natural products
Biological activities
Biosynthesis
1 Introduction
Diaporthe belongs to the family Diaporthaceae, which includes nearly 800 fungal species. Its asexual state is called Phomopsis (Dissanayake et al., 2020). Diaporthe is a common plant pathogenic fungus that produces natural products, including polyketides, alkaloids, peptides, terpenoids, and nucleosides (Tanney et al., 2016). These natural products possess antibacterial, anti-inflammatory, and cytotoxic properties. For example, the dimer anthraquinone compound epi-cytoskyrin A (Agusta et al., 2015a) has significant antibacterial activity; the terpenoids eupenifeldin and pycnidione have significant cytotoxic activity (Chen et al., 2020a, 2020b, 2020c, 2020d; Hsiao et al., 2012); terpestacin has angiogenic activity (Jung et al., 2010); sch-642305 has significant cytotoxic activity (Chu et al., 2003a); and libertellenone M has significant anti-inflammatory activity (Fan et al., 2020). To date, the genome sequences of 21 fungi from the Diaporthe (Phomopsis) genus have been published in the National Center for Biotechnological Information (NCBI) database. Among these fungi are Diaporthe sp. HANT25 (Tulsook et al., 2020), which produces mycoepoxydiene, and Phomopsis sp. CMU-LMA (Trenti et al., 2020), which produces the cytotoxic compound sch-642305. According to the genomic data, fungi of the genus Diaporthe (Phomopsis) represent important sources of abundant bioactive secondary metabolites.
Chepkirui et al. (Chepkirui and Stadler, 2017) reviewed our understanding of the structures and biological activities of natural products produced by fungi from the genus Diaporthe (Phomopsis) prior to 2015. Xu et al. (Xu et al., 2021a, 2021b) reviewed the progress made on the secondary metabolites of Diaporthe and Phomopsis fungi from 2010 to 2019 and their biological activities, and Nagarajan et al. (Nagarajan et al., 2020) reviewed research progress on the secondary metabolites of Diaporthe from 2015 to 2020 and their biological activities. However, these reviews do not discuss the biosynthesis of these important active compounds.
In the current review, we discuss the structures and biological activities of secondary metabolites isolated from Diaporthe (Phomopsis) fungi between 2016 and 2021. We also describe the biosynthetic pathways of important bioactive molecules produced by these fungi. This review will provide a reference for genome mining of novel active natural products derived from Diaporthe (Phomopsis) fungi as well as in-depth research on the biosynthesis and pharmacological mechanisms of known important active molecules.
2 Bioactive secondary metabolites from Diaporthe and Phomopsis
Diaporthe and Phomopsis fungi are valuable sources of bioactive chemicals for drug development, with several medical applications. In this review, we summarize the structures, biological activities, and biosynthesis of new natural products from the genus Diaporthe (Phomopsis). Between 2016 and 2021, 331 compounds isolated from the genus Diaporthe and its anamorph Phomopsis have been studied. The 143 compounds obtained from Diaporthe consisted of 50 pyrones (35%), 28 polyketides (19.6%), 27 alkaloids (18.9%), 21 fatty acids (14.7%), 6 terpenoids (4.2%), 4 diphenyl ketones (2.8%), 3 quinones (2.1%), 2 diphenyl ethers (1.4%), and 2 steroids (1.4%) (Fig. 1A). The primary sources of these chemicals include pyrones, polyketides, alkaloids, and fatty acids. Phomopsis produced 188 natural compounds, comprising 52 terpenoids (27.7%), 42 polyketides (22.3%), 41 alkaloids (21.8%), 29 pyrones (15.4%), 5 diphenyl ethers (2.7%), 4 diphenyl ketones (2.1%), 4 quinones (2.1%), 4 steroids (2.1%), and 7 others (3.7%) (Fig. 1B). The major sources of these chemicals are terpenoids, polyketides, alkaloids, and pyrones.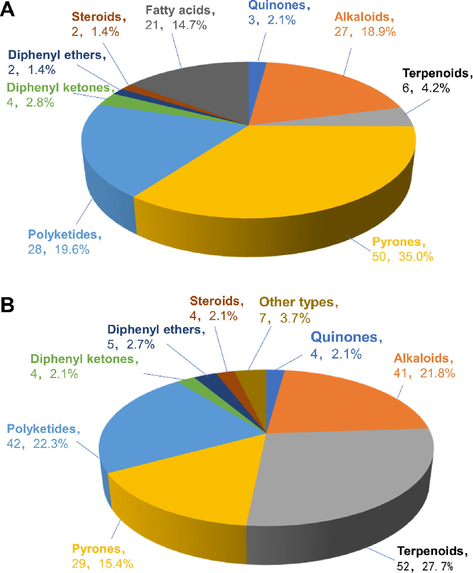
Classes of bioactive secondary metabolites isolated from Diaporthe and Phomopsis from 2016 to 2021. (A) Distribution of 143 bioactive secondary metabolites isolated from Diaporthe from 2016 to 2021; (B) Distribution of 188 bioactive secondary metabolites isolated from Phomopsis from 2016 to 2021.
The bioactivities of the compounds identified from Diaporthe and Phomopsis are shown in Fig. 2. These bioactivities primarily include cytotoxic, anti-inflammatory, antibacterial, antiviral, antioxidant, neuroprotective, anti-α-glucosidase, anti-β-site amyloid precursor protein cleaving enzyme 1 (anti-BACE1), anti-acetylcholinesterase (anti-AchE), anti-pulmonary fibrosis, and anti-hyperlipidemic activities. As shown in Table 1, secondary metabolites of Diaporthe and Phomopsis primarily exhibit anti-inflammatory, antibacterial, and cytotoxic activities. Notably, a growing number of compounds with anti-inflammatory, antibacterial, and cytotoxic properties were recently investigated for their effects on significant human disorders.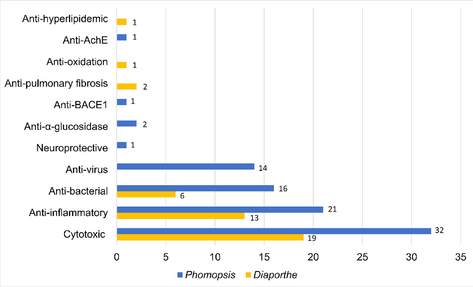
Distribution of the major bioactivities of compounds isolated from Diaporthe and Phomopsis from 2016 to 2021.
Compound
Strain
Habitat
Activity
Refs.
epi-Cytoskyrin A (1)
Diaporthe sp. ARL-09
Anoectochilus roxburghii
Cytotoxic
(Tian et al., 2018)
Cytoskyrin C (2)
Diaporthe sp. ARL-09
Anoectochilus roxburghii
Cytotoxic
(Tian et al., 2018)
Biatriosporin N (3)
Diaporthe vochysiae
Red-clawed crab (Chiromantes haematocheir)
Anti-inflammatory
(Liu et al., 2019a)
Epoxyquinophomopsins A–B (4–5)
Phomopsis sp.
Morus cathayana
Cytotoxic
(Hermawati et al., 2021)
Compounds 6–7
Phomopsis sp.
Nicotiana tabacum L.
Antibacterial
(Wu et al., 2021)
Diaporisoindoles A–E (8–12)
Diaporthe sp. SYSU-HQ3
Excoecaria agallocha
Antibacterial (8); Anti-inflammatory (9–10)
(Cui et al., 2017b, 2018)
Diaporphasines A–D (13–16)
Diaporthe phaseolorum SKS019
Acanthus ilicifolius
Cytotoxic
(Cui et al., 2017c)
Meyeroguillines C–D (17–18)
Diaporthe phaseolorum SKS019
Acanthus ilicifolius
Cytotoxic
(Cui et al., 2017c)
Compounds 19–20
Diaporthe sp. GDG-118
Sophora tonkinensis
Antibacterial (20)
(Huang et al., 2019)
Diaporthichalasins A–C (21 ∼ 23)
Diaporthe sp. GZU-1021
Chiromantes haematocheir
Cytotoxic (22)
(Liu et al., 2019b)
Diaporthichalasin D–H (24–28)
Diaporthe sp. SC-J0138
Cyclosorus parasiticus
Cytotoxic
(Yang et al., 2020a, 2020b)
Deacetyl-19-epi-cytochalasin P1 (29–30)
Diaporthe sp. RJ-47
Dracaena cochinchinensis
Antibacterial (29)
(Yan et al, 2021)
Vochysiamides A–B (31–32)
Diaporthe vochysiae
Vochysia divergens
Antibacterial (32)
(Noriler et al., 2019)
(±)-Diaporthin C (33–34)
Diaporthe phragmitis
Actinidia chinensis
–
(Yu et al., 2021a, 2021b)
Cytochalasins J1–J3 (35–37)
Phomopsis sp. CMB-M0042F
Marine sediment
Cytotoxic
(Shang et al., 2017)
Cytochalasins H1–H2 (38– 39)
Phomopsis sp. CMB-M0042F
Marine sediment
Cytotoxic
(Shang et al., 2017)
Phomopsichalasins D–E (40–41)
Phomopsis spp. xy21 and xy22
Xylocarpus granatum
Cytotoxic
(Luo et al., 2016)
Phomopsichalasins F–G (42–43)
Phomopsis spp. xy21 and xy22
Xylocarpus granatum
Cytotoxic (43)
(Luo et al., 2016)
Phomopchalasins A ∼ C (44–46)
Phomopsis sp. shj2
Isodon eriocalyx var. laxiflora
Cytotoxic (44–45); Anti-inflammatory (46)
(Yan et al., 2016)
Phomocytochalasin (47)
Phomopsis theicola BCRC 09F0213
Litsea hypophaea Hayata
–
(Hsiao et al., 2016)
Phomopsisins A (48–50)
Phomopsis sp. sh917
Isodon eriocalyx var. laxiflora
Anti-inflammatory (50)
(Tang et al., 2020)
(±)-Farinomalein F (51 ∼ 52)
Phomopsis sp. SYSU-QYP-23
Marine Kandelia candel
Anti-inflammatory
(Chen et al., 2020a, 2020b, 2020c, 2020d)
Farinomalein H (53)
Phomopsis sp. SYSU-QYP-23
Marine Kandelia candel
Anti-inflammatory
(Chen et al., 2020a, 2020b, 2020c, 2020d)
(±)-Farinomalein G (54 ∼ 55)
Phomopsis sp. SYSU-QYP-23
Marine Kandelia candel
Anti-inflammatory
(Chen et al., 2020a, 2020b, 2020c, 2020d)
Phomoamide (56)
Phomopsis sp. SYSU-QYP-23
Marine Kandelia candel
Anti-inflammatory
(Chen et al., 2020a, 2020b, 2020c, 2020d)
Phochrodines A–B (57–58)
Phomopsis sp. 33#
Rhizophora stylosa
–
(Chen et al., 2018)
Phochrodines C–D (59 –60)
Phomopsis sp. 33#
Rhizophora stylosa
Anti-inflammatory
(Chen et al., 2018)
Phomopsol A (61)
Phomopsis sp. xy21
Thai Xylocarpus granatum
Neuroprotective
(Li et al., 2019)
(±)-Tersone A–E (62 –71)
Phomopsis tersa FS441
Sediment
Antibacterial (70 –71)
(Chen et al., 2019)
Tersone F–G (72–73)
Phomopsis tersa FS441
Sediment
–
(Chen et al., 2019)
Prenylcyclotryprostatin A (74)
Phomopsis asparagi CICC2706
China Center of Industrial Culture Collection
–
(Zhou et al., 2021)
7-hydroxy-cis-L(-)-3,6-dibenzyl-2,5-Dioxopiperazine (75)
Phomopsis asparagi CICC2706
China Center of Industrial Culture Collection
–
(Zhou et al., 2021a)
Lithocarins B–D (76–78)
Diaporthe lithocarpus A740
Morinda officinalis
Cytotoxic
(Liu et al., 2019)
Diaporpenoids A–C (79–81)
Diaporthe sp. QYM12
Kandelia candel
Anti-inflammatory (79)
(Yan et al, 2021)
Phomeroids A–B (82–83)
Phomopsis tersa FS441
Sediment
Cytotoxic
(Chen et al., 2020a, 2020b, 2020c, 2020d)
Eupenifeldin (84)
Phomopsis tersa FS441
Sediment
Cytotoxic
(Chen et al., 2020a, 2020b, 2020c, 2020d)
Pedinophyllols K–L (85 ∼ 86)
Phomopsis sp. S12
Illigera rhodantha
–
(Xu et al., 2019a)
Libertellenone T (87)
Phomopsis sp. S12
Illigera rhodantha
Anti-inflammatory
(Xu et al., 2019b)
Photeroids A–B (88–89)
Phomopsis tersa FS441
Sediment sample
Cytotoxic
(Chen et al., 2020b)
Phomopoxides A–F (90 ∼ 96)
Phomopsis sp. YE3250
Paeonia delavayi
Anti-α-glucosidase (90 ∼ 91)
(Huang et al., 2018)
Phomophyllins A–K (97–107)
Phomopsis sp. TJ507A
Phyllanthus glaucus
Anti-β-site amyloid precursor protein cleaving enzyme 1 (97)
(Xie et al., 2018)
Phomophyllins L–N (108–110)
Phomopsis sp. TJ507A
Phyllanthus glaucus
–
(Xie et al., 2018)
Eremofortin G (1 1 1)
Phomopsis sp. SYSU-QYP-23
Kandelia candel
Anti-inflammatory
(Chen et al., 2021a, 2021b)
Eremofortins I–K (112–114)
Phomopsis sp. SYSU-QYP-23
Kandelia candel
Anti-inflammatory
(Chen et al., 2021a, 2021b)
Altiloxins C–E (115–117)
Phomopsis sp. SYSU-QYP-23
Kandelia candel
Anti-inflammatory
(Chen et al., 2021a, 2021b)
Phomomane (1 1 8)
Phomopsis sp. SYSU-QYP-23
Kandelia candel
Anti-inflammatory
(Chen et al., 2021a, 2021b)
Carneic acids C–O (119–131)
Phomopsis sp. SNB-LAP1-7–32
Diospyros carbonaria
Antiviral
(Peyrat et al., 2020)
Hydroxylithocarin A (1 3 2)
Phomopsis asparagi CICC 2706
China Center of Industrial Culture Collection
–
(Shi et al., 2020)
Lithocarin A (1 3 3)
Phomopsis lithocarpus FS508
Deep-sea sediment
Cytotoxic
(Xu et al., 2018a, 2018b)
Compounds 134–136
Diaporthe sp. F2934
Siparuna gesnerioides
–
(Sousa et al., 2016)
Diaporchromanones A–D (137–140)
Diaporthe phaseolorum SKS019
Acanthus ilicifolius
Anti-inflammatory
(Cui et al., 2017a)
(±)-Phomopsichin A (141–142)
Diaporthe phaseolorum SKS019
Acanthus ilicifolius
Anti-inflammatory
(Cui et al., 2017a)
(±)-Diaporchromone A (1 4 3)
Diaporthe phaseolorum SKS019
Acanthus ilicifolius
Anti-inflammatory
(Cui et al., 2017a)
Phaseolorins A–H (144–151)
Diaporthe phaseolorum FS431
Marine sediment
–
(Guo et al., 2019)
Phomotide A (1 5 2)
Phomopsis sp. CFS42
Cephalotaxus fortunei Hook
–
(Ma et al., 2020)
Pestalotiopsone H (1 5 3)
Diaporthe sp. SCSIO 41011
Mangrove
–
(Luo et al., 2018b)
Methyl-Convolvulopyrone (1 5 4)
Diaporthe sp. SCSIO 41011
Mangrove
–
(Luo et al., 2018b)
Diaporpyranes A–C (155 ∼ 157)
Diaporthe sp. QYM12
China Center of Industrial Culture Collection
Anti-inflammatory (1 5 5)
(Yan et al, 2021)
Compounds (158–162)
Diaporthe foeniculina BZM-15
Leptospermum brachyandrum
–
(Yu et al., 2021a, 2021b)
Ellagic acid B (1 6 3)
Diaporthe sp. CB10100
Sinomenium acutum (Thunb.)
–
(Pu et al., 2021)
Diaporpyrones A–D (164–167)
Diaporthe sp. CB10100
Sinomenium acutum (Thunb.)
–
(Pu et al., 2021)
Compounds 168–169
Diaporthe phaseolorum FS459
Deep-sea sediment
–
(Hu et al., 2021)
Compounds 170–172
Diaporthe phaseolorum FS459
Deep-sea sediment
–
(Hu et al., 2021)
Foeniculins A–K (173–183)
Diaporthe foeniculina SCBG-15
Leptospermum brachyandrum
–
(Lu et al., 2021)
Phomaspyrones A–E (184–188)
Phomopsis asparagi SWUKJ5.2020
Kadsura angustifolia
Cytotoxic (1 8 6)
(Song et al., 2017)
Phomopsichins A–D (189–192)
Phomopsis sp. 33#
Rhizophora stylosa
–
(Huang et al., 2016)
(10S)-10-O-b-D-40-Methoxymanno-pyranosyldiaporthin (1 9 3)
Phomopsis sp. sh917
Isodon eriocalyx var. laxiflora
–
(Tang et al., 2017)
Clearanol H (1 9 4)
Phomopsis sp. sh917
Isodon eriocalyx var. laxiflora
–
(Tang et al., 2017)
Phomochromenones A –K (195–203)
Phomopsis sp. HNY29-2B
Acanthus ilicifolius Linn
–
(Ding et al., 2017)
Phomoisocoumarins C–D (204 –205)
Phomopsis prunorum
Hypericum ascyron
Antibacterial (2 0 5)
(Qu et al., 2020)
Phomotide A (2 0 6)
Phomopsis sp. CFS42
Cephalotaxus fortunei Hook
–
(Ma et al., 2020)
Phomopsinins B–C (207–208)
Phomopsis sp. CAM212
Garcinia xanthochymus
–
(Jouda et al., 2020)
Phomochromenones D –G (209–212)
Phomopsis asparagi DHS-48
Rhizophora mangle
–
(Wei et al., 2021)
Acetoxydothiorelone B (2 1 3)
Diaporthe pseudomangiferaea
Tylophora ouata
Anti-pulmonary fibrosis
(Liu et al., 2018)
(15S)-acetoxydothio relone A (2 1 4)
Diaporthe pseudomangiferaea
Tylophora ouata
–
(Liu et al., 2018)
Dothiorelones K–N (215 ∼ 218)
Diaporthe pseudomangiferaea
Tylophora ouata
Anti-pulmonary fibrosis (2 1 6)
(Liu et al., 2018)
5-Hydroxy-7-methoxy-4,6-dimethyl-2-Phenyliso in doline-1,3-dione (2 1 9)
Diaporthe pseudomangiferaea
Tylophora ouata
–
(Liu et al., 2018)
(13R)-diaporphthalide A (2 2 0)
Diaporthe pseudomangiferaea
Tylophora ouata
–
(Liu et al., 2018)
(9S, 17R, 19S, 6Z, 10E, 14E)-diaporlactone A (2 2 1)
Diaporthe pseudomangiferaea
Tylophora ouata
–
(Liu et al., 2018)
Isochromophilones A–F (222 ∼ 227)
Diaporthe sp. SCSIO 41011
Mangrove plant
Cytotoxic (2 2 5)
(Luo et al., 2018a)
Isochromophilone G (2 2 8)
Diaporthe perseae sp.
Pongamia pinnata (L.)
Antibacterial/Antioxidation
(Niaz et al., 2020)
11-Hydrochermesinone B (2 2 9)
Diaporthe phaseolorum FS459
deep-sea sediment
–
(Hu et al., 2021)
Diaportones A–C (230–232)
Diaporthe foeniculina BZM-15
Leptospermum brachyandrum
–
(Kang et al., 2021)
(15R)-Acetoxydothiorelone A (2 3 3)
Diaporthe sp. SCSIO 41011
Mangrove
–
(Luo et al., 2018b)
(±)-Microsphaerophthalides H∼I (234–235)
Diaporthe sp. SCSIO 41011
Mangrove
–
(Luo et al., 2018b)
Diaporindenes A–D (236–239)
Diaporthe sp. SYSU-HQ3
Mangrove
Anti-inflammatory
(Cui et al., 2018)
Isoprenylisobenzofuran A (2 4 0)
Diaporthe sp. SYSU-HQ3
Mangrove
–
(Cui et al., 2018)
Phomopsiketones A–C (241–243)
Phomopsis sp. sh917
Isodon eriocalyx var. laxiflora
Cytotoxic (241 ∼ 242)
(Tang et al., 2017)
Compounds 244–245
Phomopsis sp. PSU-H188
Hevea brasiliensis
–
(Kongprapan et al., 2017)
Phomopones A–C (246 –248)
Phomopsis sp. D15a2a
Alternanthera bettzickiana
–
(Yu et al., 2019)
Koninginins T–U (249 –250)
Phomopsis stipata
Styrax camporum Pohl
Antibacterial/anti- acetylcholinesterase (2 4 9); Antibacterial (2 5 0)
(Biasetto et al., 2020)
Compounds 251–253
Phomopsis sp.
–
Antiviral (2 5 1)
(Du et al., 2017)
Compounds 254–258
Phomopsis fukushii
Nicotiana tabacum
Antibacterial
(Yang et al., 2017), (Li et al., 2021)
Phomopsol B (2 5 9)
Phomopsis sp. xy21
Xylocarpus granatum
–
(Li et al., 2019)
Compound 260
Phomopsis sp. xy21
Xylocarpus granatum
–
(Li et al., 2019)
(±)-Phomopsisin A (261 ∼ 262)
Phomopsis asparagi CICC 2706
China Center of Industrial Culture Collection
–
(Zhou et al., 2021)
Lithocaldehydes A–B (263 ∼ 264)
Phomopsis lithocarpus FS508
Marine sediment
Antibacterial
(Liu et al., 2020)
Phomopsones A–C (265 ∼ 267)
Phomopsis sp. CGMCC No.5416
Achyranthes bidentata
Antiviral/Cytotoxic (266 ∼ 267)
(Yang et al., 2020a, 2020b)
Tersaphilones B–D (268 ∼ 271)
Phomopsis tersa FS441
Sediment
Cytotoxic (2 7 0)
(Chen et al., 2021a, 2021b)
Diaporindenes E–I (272–276)
Phomopsis lithocarpus FS508
Deep-sea sediment sample
–
(Liu et al., 2021a, 2021b)
Tenellones J–M (277–280)
Phomopsis lithocarpus FS508
Deep-sea sediment sample
Cytotoxic (2 7 8)
(Liu et al., 2021a, 2021b)
Lithocarpinols A–B (281 ∼ 282)
Phomopsis lithocarpus FS508
Cytotoxic (2 8 1)
(Xu et al., 2019a, 2019b)
Tenellones C–D (283–284)
Diaporthe sp. SYSU-HQ3
Excoecaria agallocha
Anti-inflammatory (2 8 3)
(Cui et al., 2018)
Tenllone I (2 8 5)
Diaporthe lithocarpus A740
Morinda officinalis
–
(Liu et al., 2019)
Tenellones D–H (286–290)
Phomopsis lithocarpus FS508
Deep-sea sediment sample
Cytotoxic (2 9 0)
(Xu et al., 2018a, 2018b)
Diaporthols A–B (291 –292)
Diaporthe sp. ECN-137
Phellodendron amurense
Cytotoxic
(Nakashima et al., 2018)
Phomopsinin A (2 9 3)
Phomopsis sp. CAM212
Garcinia xanthochymus
–
(Jouda et al., 2020)
Compound 294
Phomopsis sp. CAM212
Garcinia xanthochymus
Anti-inflammatory
(Jouda et al., 2020)
Compounds 295–297
Phomopsis fukushii
Paris polyphylla var. yunnanensis
Antibacterial
(Gao et al., 2019)
Diapolic acids A–B (304–305)
Diaporthe terebinthifolii
G. glabra
–
(Yedukondalu et al., 2017)
Eucalyptacid A (3 0 6)
Diaporthe eucalyptorum
Melia azedarach
Antibacterial
(Gao et al., 2020a)
Eucalactam B (3 0 7)
Diaporthe eucalyptorum
Melia azedarach
–
(Gao et al., 2020b)
Diaporthsins A–K (308 –318)
Diaporthe sp. JC-J7
Dendrobium nobile Lindl
Antihyperlipidemic (3 1 2)
(Hu et al., 2018a, 2018b)
Diaporthesters A–D (319–322)
Diaporthe sp. T24
Ligularia fischeri
Cytotoxic (3 1 9)
(He et al., 2021)
Diaportheolides A–B (323 –324)
Diaporthe sp. SXZ-19
Camptotheca acuminata
–
(Liu et al., 2021a, 2021b)
Lithocarpins A–G (325 –331)
Phomopsis lithocarpus FS508
Marine sediment
Cytotoxic (327–329)
(Xu et al., 2018a, 2018b), (Xu et al., 2021a, 2021b)
2.1 Quinones
Quinones and anthraquinones are widely found in nature, primarily in microorganisms and plants. Their pharmacological activities include antitumor, anti-inflammatory, anti-HIV (human immunodeficiency virus), antioxidant, antiviral, antibacterial, and other related activities. The drugs on the market with a quinone structure with antitumor effects include mitomycin and adriamycin.
A novel anthraquinone dimer derivative with a cage skeleton known as epi-cytoskyrin A (1) and its derivative, cytoskyrin C (2) were isolated from Diaporthe sp. ARL-09, an endophytic fungus of the Jewel orchid (Anoectochilus roxburghii) (Tian et al., 2018) (Fig. 3). Biatriosporin N (3), a new quinone derivative isolated from the endophytic fungus Diaporthe vochysiae, inhibited nitric oxide (NO) production by RAW 264.7 cells exposed to lipopolysaccharide (LPS) with an IC50 (the concentration at which NO production is repressed by 50%) value of 11.5 μM (positive control drug indomethacin, IC50 = 29.7 μM) (Liu et al., 2019a). Two new benzoquinone compounds, epoxyquinophomopsin A and B (4 and 5), were isolated from the fermentation products of the deciduous tree Morus cathayana infected by the endophytic fungus Phomopsis sp. Compound 4 significantly inhibited Bruton's tyrosine kinase (BTK) activity (Hermawati et al., 2021). New anthraquinone derivatives 6 and 7 were identified in the fermentation products of rice (Oryza sativa) infected by Phomopsis sp. This endophytic fungus is found in tobacco (Nicotiana tabacum) (Wu et al., 2021). These two compounds showed antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). (See Figs. 4-7)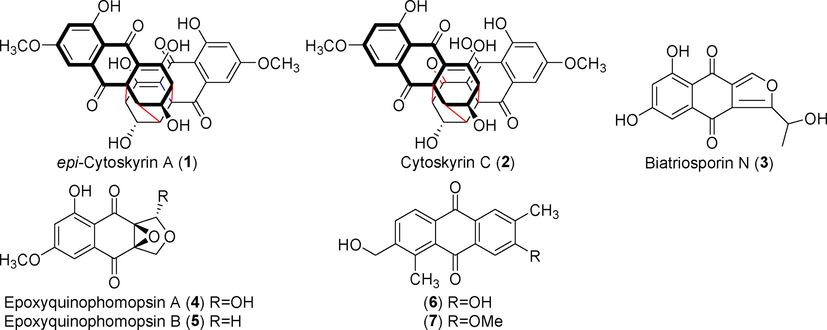
Anthraquinones isolated from Diaporthe and Phomopsis (1–7).
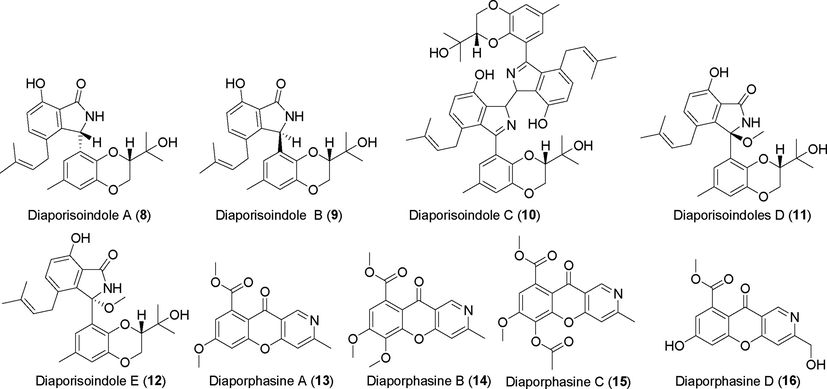
Alkaloids isolated from fungi of the genus Diaporthe (8–16).
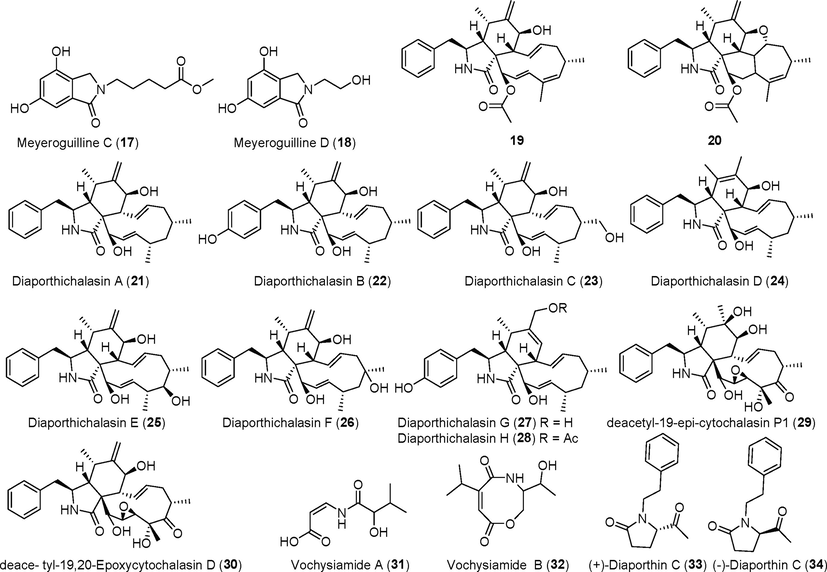
Alkaloids isolated from fungi of the genus Diaporthe (17–34).
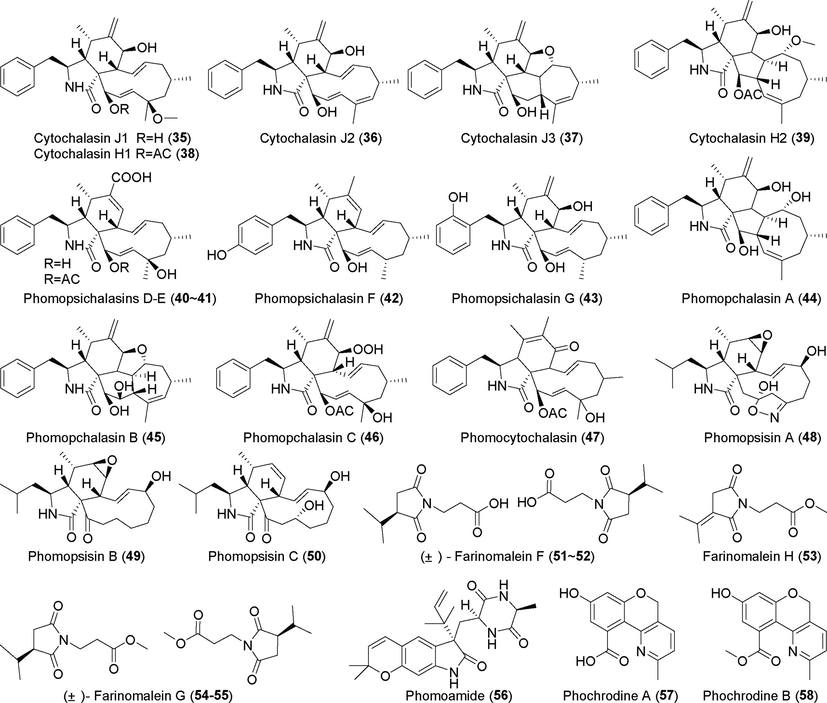
Alkaloids isolated from fungi of the genus Phomopsis (35–58).
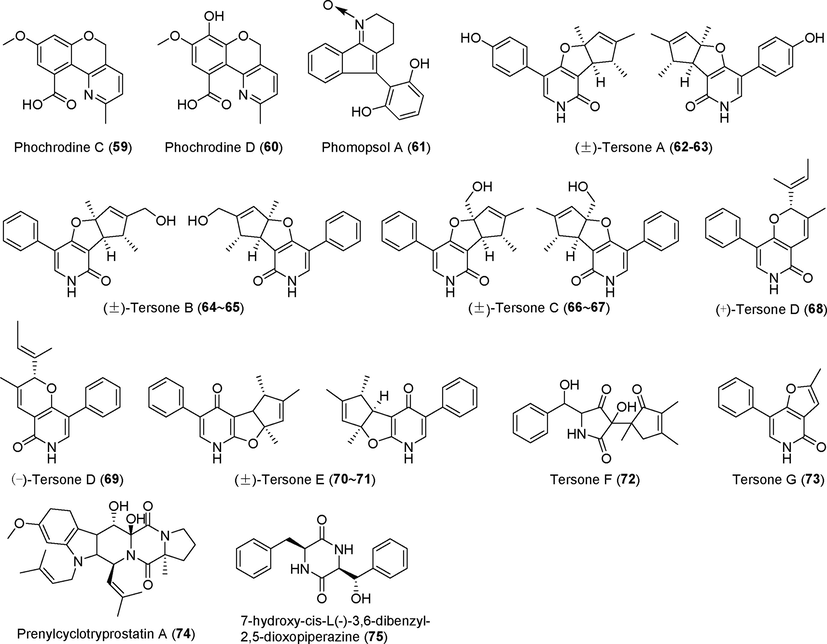
Alkaloids isolated from fungi of the genus Phomopsis (59–75).
2.2 Alkaloids
Alkaloids are a class of nitrogen-containing organic compounds that widely exist in plants, microorganisms, and animals (Mishra et al., 2017). A total of 68 alkaloids was isolated from fungi of the genus Diaporthe (Phomopsis) (Fig. 1), accounting for 20.5% of the total compounds recently identified. Their major activities include antibacterial, anti-inflammatory, and cytotoxic properties.
2.2.1 Alkaloids isolated from Diaporthe
Diaporisoindoles A–E (8–12) were discovered among the metabolites of the endophytic fungus Diaporthe sp. SYSU-HQ3, which originated from mangroves (Cui et al., 2017b, 2018). Compounds 8 and 9 are novel isoprene isoindole alkaloids, and compound 10 is a unique dimer of an isoprene isoindole alkaloid. Compound 8 showed significant inhibitory activity against protein tyrosine phosphatase B of Mycobacterium tuberculosis, with IC50 values of 4.2 μM, whereas compounds 9 and 10 showed inhibitory activity against NO production, with IC50 values of 22.7 μM and 18.2 μM, respectively. Diaporphasines A–D (13–16) and two new isoindolinones, meyeroguillines C–D (17, 18), were discovered in the metabolites of Diaporthe phaseolorum SKS019 (Cui et al., 2017c), which was originally derived from holy mangrove (Acanthus ilicifolius). These compounds were tested on five tumor cell lines and showed no significant inhibitory effects. Two new cytorelaxin derivatives (19, 20) were identified in the metabolites of the endophytic fungus Diaporthe sp. GDG-118 from the traditional Chinese medicine herb Sophora tonkinensis (Huang et al., 2019). Compound 20 showed significant activity against Bacillus anthracis and Escherichia coli, with a MIC (minimum inhibitory concentration, allowing complete inhibition of bacterial growth) value of 12.5 μg/mL. Three novel cytosolasin alkaloid derivatives, diaporthichalasins A–C (21–23), were identified in the metabolites of the endophytic fungus Diaporthe sp. GZU-1021 (Liu et al., 2019a). These compounds inhibited NO production in LPS-induced RAW 264.7 cells.
Five novel cytoflasin derivatives, diaporthichalasins D–H (24–28), were identified in the fermentation metabolites of the endophytic fungus Diaporthe sp. SC-J0138 (Yang et al., 2020a, 2020b), which was derived from parasitic maiden fern (Cyclosorus parasiticus). Compounds 24–28 demonstrated varying degrees of cytotoxicity against A549, HeLa, HepG2, and MCF-7 tumor cells. Surprisingly, compounds 24 and 28 showed strong cytotoxic activity against HepG2 cells, with IC50 values of 8.8 ± 1.7 μM and 9.9 ± 1.6 μM, respectively.
Two new cytochalasin alkaloids, deacetyl-19-epi-cytochalasin P1 (29) and deacetyl-19, 20-epoxycytochalasin D (30), were identified in the fermentation metabolites of the endophytic fungus Diaporthe sp. RJ-47 (Yan et al, 2021); compound 29 showed antibacterial activity. Two new alkaloid derivatives, vochysiamides A and B (31, 32), were identified in the metabolites of the newly identified endophytic fungus Diaporthe vochysiae (Noriler et al., 2019). Compound 32 showed certain activity against Klebsiella pneumoniae. A new pair of pyrrolidone derivatives (±), diaporthin C (33, 34), were identified in the fermentation products of the endophytic fungus Diaporthe phragmitis from kiwi (Actinidia chinensis) (Yu et al., 2021a, 2021b); these compounds did not show any significant antibacterial activity.
2.2.2 Alkaloids isolated from Phomopsis
Five cytochalasins, J1–J3 (35–37) and H1–H2 (38, 39), were identified in the metabolites of Phomopsis sp. CMB-M0042F (Shang et al., 2017), which originated from marine sediments. Compounds 35 and 36 have rare 5/6/6/7/5 four-ring fused skeletons, and compounds 37 and 39 have rare 5/6/5/8 ring systems. Compounds 35–39 exhibited significant cytotoxic activity against the human cell lines SW620, NCI-H460, HepG2, and HEK, with IC50 values of 0.1 µM to 12.9 μM. Four new alkaloid cytochalasin derivatives, phomopsichalasins D–G (40–43), were identified in the metabolites of the endophytic fungi Phomopsis sp. xy21 and xy22 originating from mangrove plants (Luo et al., 2016). These compounds showed significant cytotoxic activity against five tumor cell lines, HCT-8, HCT-8/T, A549, MDA-MB-231, and A2780, with IC50 values of 7.5 μM, 8.6 μM, 6.4 μM, 3.4 μM, and 7.1 μM, respectively. Three new alkaloid derivatives, phomopchalasins B–D (44–46), were identified in the metabolites of the endophytic fungus Phomopsis sp. shj2 derived from the medicinal plant Isodon eriocalyx var. laxiflora (Yan et al., 2016); compound 44 has a unique 5/6/5/8 tetracyclic thickened skeleton, whereas compound 45 has a novel 5/6/6/7/5 pentacyclic thickened skeleton. Compound 45 showed moderate cytotoxic activity against HL-60, SMMC-7721, and A-549 cells, with IC50 values of 14.9 µM, 22.7 µM, and 21.1 μM, respectively. Compound 46 showed inhibitory activity against NO production by LPS-induced RAW 264.7 cells, with an IC50 value of 11.2 μM.
Phomocytochalasin (47), a novel alkaloid, was discovered in the metabolites of Phomopsis theicola BCRC 09F0213 (Hsiao et al., 2016). Three novel leucine-derived cytochalasin alkaloids, phomopsisins A–C (48–50), were identified in the fermentation metabolites of the endophytic fungus Phomopsis sp. sh917 derived from Isodon eriocalyx var. laxiflora (Tang et al., 2020); compound 48 is a cytochalasin compound with a rare 2H–iso-xazole group, and compound 50 was shown to inhibit NO production. Maleamide derivatives (±)-farinomalein F (51–52), (±)-farinomalein G (53–54), farinomalein H (55), and one new linearly fused prenylated indole alkaloid phomoamide (56) were found in the metabolites of the endophytic fungus Phomopsis sp. SYSU-QYP-23, which originated from the mangrove plant Kandelia candel. (±)-farinomalein G (53–54), farinomalein H (55), and phomoamide (56) showed no significant anti-inflammatory activity (Y. Chen et al., 2020a, 2020b, 2020c, 2020d).
Four new tryptopyridine derivatives, phochrodines A–D (57 –60), were identified in the metabolites of Phomopsis sp.33#, an endophytic fungus found in mangrove forests (Chen et al., 2018). There was no evidence of significant anti-inflammatory, antioxidant, or cytotoxic activities. Phomopsol A (61), a highly oxidized alkaloid polyketide, was found in the metabolites of Phomopsis sp. xy21 (Li et al., 2019), but there are no reports of its activity. Seven new pyridinone derivatives, (±)-tersones A–E (62–71) and tersones F-G (72–73), were discovered in the metabolites of the deep-sea fungus Phomopsis tersa FS441 (Chen et al., 2019). Compounds 62–65 have a rare 6/6/5/5 ring system. Neither antibacterial nor cytotoxic activities were detected for these compounds. Prenylcyclotryprostatin A (74) and 7-hydroxy-cis-L(-)-3,6-dibenzyl-2,5-dioxopiperazine (75), two new diketopiperazine alkaloids isolated from the fermentation metabolites of Phomopsis asparagi CICC2706, showed no significant inhibitory activity against glucosidase (Zhou et al., 2021).
2.3 Terpenoids
Terpenoids, the most abundant natural organic compounds based on isoprene, are found in plants, fungi, and other organisms. Monoterpenes, sesquiterpenes, diterpenes, and triterpenes are the most common types of terpenoids. Fifty-eight terpenoids were isolated from fungi of the genus Diaporthe (Phomopsis) between 2016 and 2021, accounting for 17.5% of all metabolites (Figs. 8–11). Terpenoids have significant cytotoxic activity, α-glycosidase inhibitory activity, and an anti-inflammatory effects.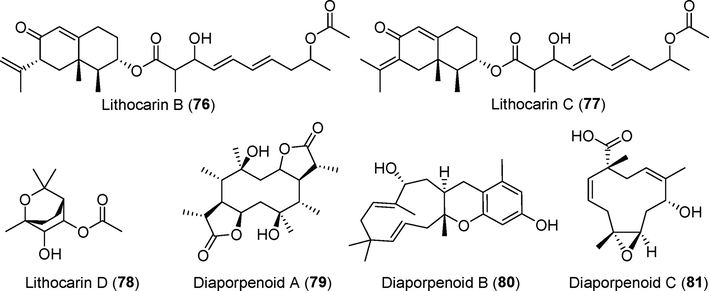
Terpenoids isolated from fungi of the genus Diaporthe (76–81).
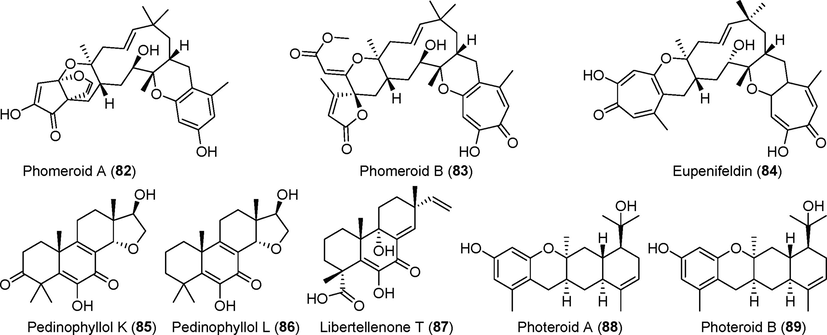
Terpenoid derivatives isolated from fungi of the genus Phomopsis (82–89).
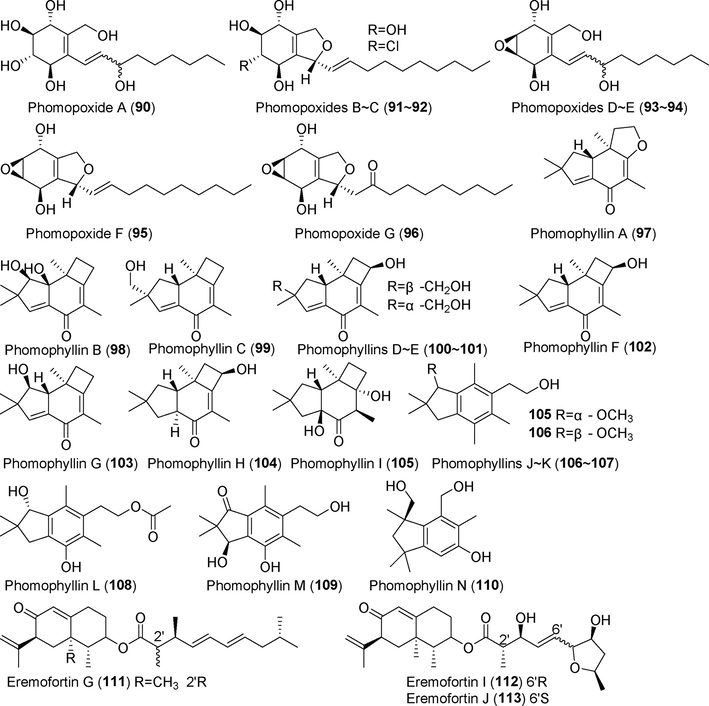
Terpenoid derivatives isolated from fungi of the genus Phomopsis (90–113).
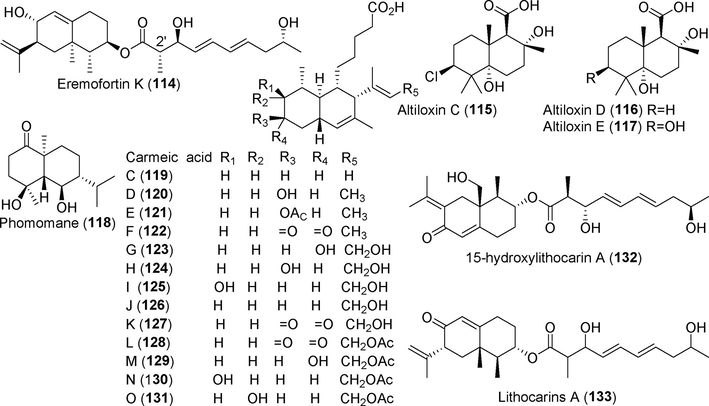
Terpenoid derivatives isolated from fungi of the genus Phomopsis (114–133).
2.3.1 Terpenoids isolated from fungi of the genus Diaporthe
Two new eremophilane derivatives, lithocarins B–C (76, 77), and the new monoterpenoid lithocarin D (78) were isolated from the endophytic fungus Diaporthe lithocarpus A740 (Liu et al., 2019), which originated from the medicinal plant Indian mulberry (Morinda officinalis). Compounds 76–78 showed weak inhibitory activity against HepG-2, MCF-7, SF-268, and A549 tumor cells. The new diterpenoid diaporpenoid A (79) and two new sesquiterpenoids B–C (80, 81) were discovered in the fermentation products of Diaporthe sp. QYM12 (Yan et al, 2021). Compound 79 inhibited NO production from LPS-induced RAW264.7 cells, with an IC50 value of 21.5 μM.
2.3.2 Terpenoids from Phomopsis fungi
Two terpenoids with new types of skeletons, phomeroid A and B (82–83), were isolated from the deep-sea-derived fungus Phomosis tersa FS441, along with one known terpenoid, eupenifeldin (84) (Chen et al., 2020a, 2020b, 2020c, 2020d). Compound 82 is a sesquiterpenoid derivative with a 6/6/11/6/5/5 ring; compound 83 is a sesquiterpenoid derivative with a unique 7/6/11/6–5 screw ring. Compound 83 showed significant inhibitory activity against HepG-2, MCF-7, SF-268, and A549 tumor cells, with IC50 values of 0.5 µM, 0.3 µM, 1.0 µM, and 1.1 μM, respectively. A study of three new diterpenoids, pedinophyllol K (85), pedinophyllol L (86), and libertellenone T (87), and two known compounds from metabolites of the endophytic fungus Phomopsis sp. S12 (Xu et al., 2019a, 2019b) showed that compound 87 had anti-inflammatory activity against RAW 264.7 cells incubated with LPS. Two photeroids, A–B (88, 89), with a 6/6/6/6 tetracyclic highly dense skeleton, were isolated from the deep-sea fungus Phomopsis tersa FS441 (Chen et al., 2020b). These compounds had inhibitory activity against SF-268, MCF-7, HepG-2, and A549 tumor cells, with IC50 values between 20 µM and 26 μM.
Seven new polyoxygenated cyclohexenoids, phomopoxides A–G (90–96), were isolated from the fermentation broth extract of the endophytic fungus Phomopsis sp. YE3250 from the medicinal plant Delavay's tree peony (Paeonia delavayi Franch) (Huang et al., 2018). Phomopoxides A–C (90–92) inhibited glucosidase activity in a manner similar to that of acarbose. Thirteen new sesquiterpenoids, phomophyllins A–N (97–110), were discovered in the metabolites of the endophytic fungus Phomopsis sp. TJ507 from Phyllanthus glaucus (Xie et al., 2018). Compound 97 showed an excellent inhibitory effect on β-site amyloid precursor protein lyase 1 (BACE1) and no hepatotoxicity. Eight new sesquiterpenoids, eremofortin G (1 1 1), eremofortins I–K (112–114), altiloxins C–E (115–117), and phomomane (1 1 8), were obtained from the fermentation metabolites of Phomopsis sp. SYSU-QYP-23, which was derived from the mangrove species Kandelia candel (Chen et al., 2021a, 2021b). These compounds showed a potent inhibitory effect on NO production by LPS-induced RAW 264.7 cells, with an IC50 value of 8.6–14.5 μM. Thirteen new sesquiterpenoid acid derivatives, carneic acids C—O (119–131), were isolated from the fermentation metabolites of Phomopsis sp. SNB-LAP1-7–32 (Peyrat et al., 2020), an endophytic fungus derived from Diospyros carbonaria. Five of these compounds significantly inhibited dengue polymerase activity, with IC50 values ranging from 10 µM to 20 μM with no cytotoxicity. The novel sesquiterpenoid 15-hydroxylithocarin A (132) was identified in the fermentation metabolites of Phomopsis asparagi CICC 2706 (Shi et al., 2020); this compound did not inhibit glucosidase activity. Lithocarin A (133) is a new sesquiterpenoid isolated from metabolites of Phomopsis lithocarpus FS508, a fungus isolated from deep-sea sediment (Xu et al., 2018a, 2018b). This compound showed weak inhibitory activity against HepG-2, MCF-7, SF-268, and A549 cells.
2.4 Pyrones
Pyrone, an active group present in some drugs, is divided into two types: α-pyrone and γ-pyrone. Between 2016 and 2021 (Figs. 12–14), 79 compounds were discovered in this genus of fungi, accounting for 23.9% of the total identified compounds.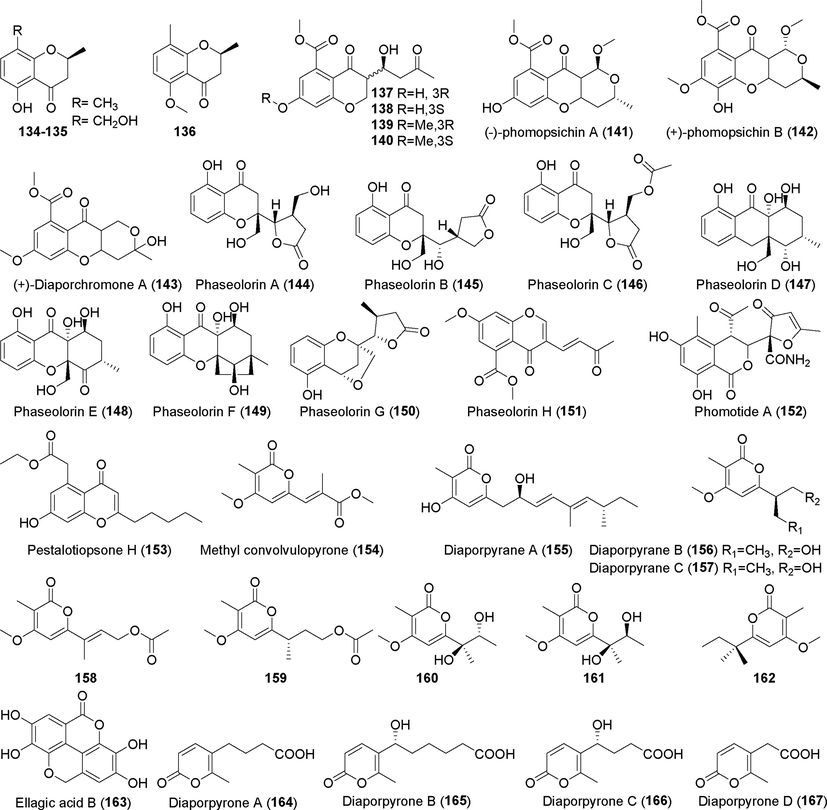
Pyrone derivatives isolated from fungi of the genus Diaporthe (134–167).
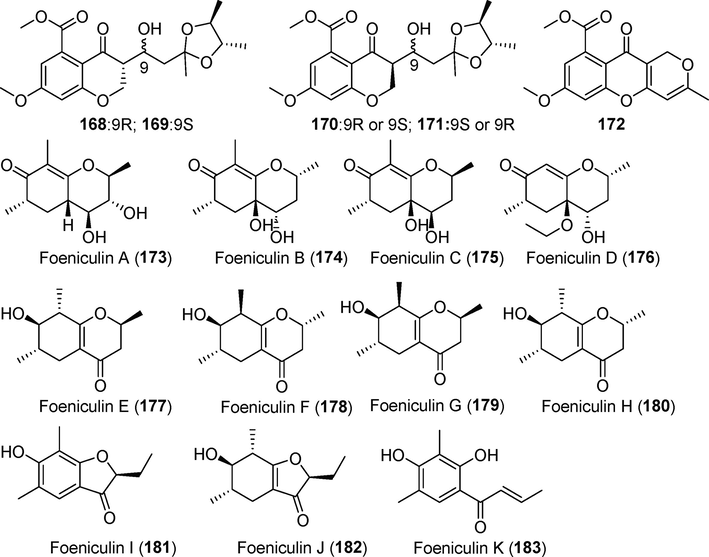
Pyrone derivatives isolated from fungi of the genus Diaporthe (168–183).
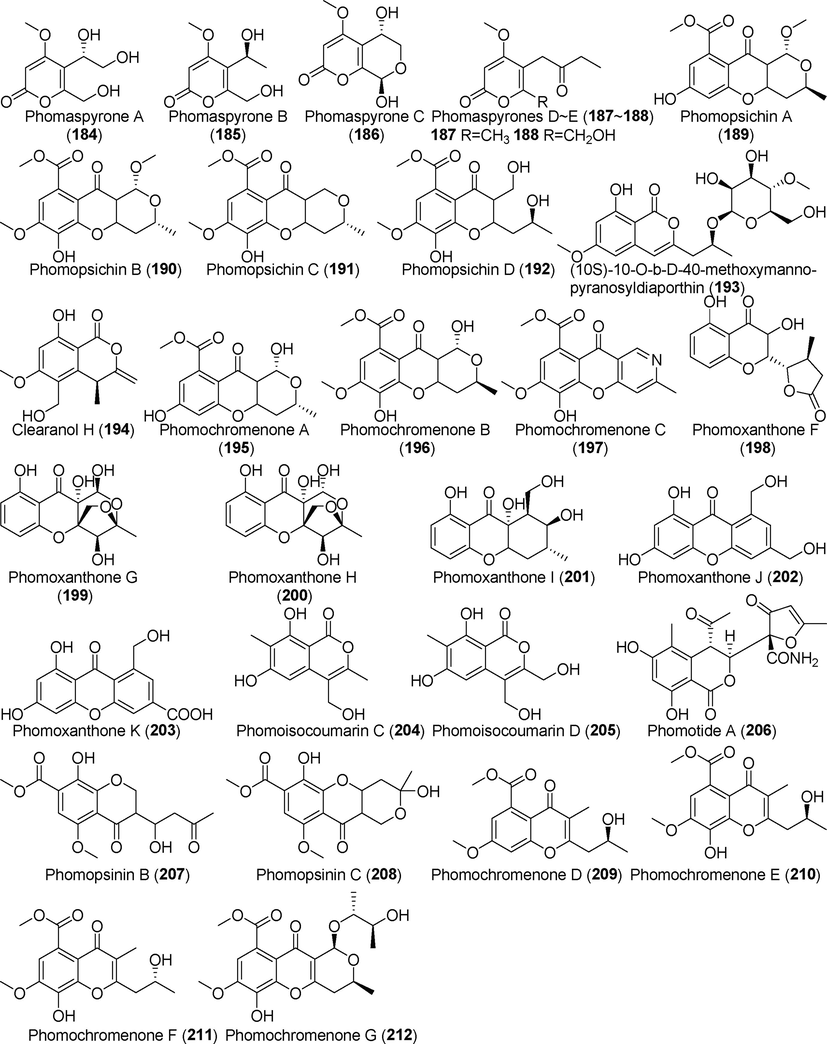
Pyrone derivatives isolated from fungi of the genus Phomopsis (184–212).
2.4.1 Pyrones isolated from fungi of the genus Diaporthe
Three pyrone derivatives (134–136) were identified from metabolites of the endophytic fungus Diaporthe sp. F2934 (Sousa et al., 2016). No obvious antibacterial activity was found. Seven novel chromogenone derivatives, diaporchromanones A–D (137–140), (–)-phomopsichin A (1 4 1), (+)-phomopsichin B (1 4 2), and (±)-diaporchromone A (1 4 3), were found in the metabolites of the endophytic fungus Diaporthe phaseolorum SKS019 (Cui et al., 2017a). These compounds moderately inhibited NF-kappaB (NF-κB) activity in RANKL (receptor activator of NF-κB ligand)-induced RAW 264.7 cells. Five new chromogenone derivatives, phaseolorins A–F (144–149), were identified in the metabolites of the deep-sea fungus Diaporthe phaseolorum FS431 (Guo et al., 2019), but they displayed weak cytotoxic activity. Two pyrone derivatives, phaseolorin G and H (150–151), were isolated from the metabolites of the deep-sea fungus Diaporthe phaseolorum FS431 (Niu et al., 2019). These compounds showed weak inhibitory activity against MCF-7, HepG-2, and A549 tumor cells. Phomolide A (1 5 2), a compound discovered in the metabolites of Phomopsis sp. CFS42 (Ma et al., 2020), showed no biological activity. Pestalotiopsone H (1 5 3) and methyl convolvulopyrone (1 5 4), two previously unreported chrogenic ketone derivatives, were identified in the metabolites of Diaporthe sp. SCSIO 41011 (Luo et al., 2018b). No obvious inhibitory effect against the influenza A virus was detected.
Three previously unknown diaporpyran derivatives (155–157) were identified in the fermentation metabolites of Diaporthe sp. QYM12 (Yan et al, 2021). Compound 155 showed anti-inflammatory activity, with an IC50 value of 12.5 µM. Four derivatives of 2-pyrone (158–162), including a pair of enantiomers (160 and 161), were isolated from rice fermentation products of the endophytic fungus Diaporthe foeniculina BZM-15, which was derived from the weeping tea tree (Leptospermum brachyandrum) (Yu et al., 2021a, 2021b). These compounds showed no significant cytotoxic activity against SF-268, MCF-7, or HepG-2 tumor cells. Five new pyrone derivatives are produced by the endophytic fungus Diaporthe sp. CB10100, which was derived from Sinomenium acutum (Thunb.) (Pu et al., 2021). Among these, the novel dibenzo α-pyrone derivative ellagic acid B (1 6 3) and four α-pyrones, diaporpyrones A–D (164–167) showed no obvious inhibitory activity against cyclooxygenase (COX-2) or inducible nitric oxide synthase (iNOS). Three new tryptophan derivatives (168, 169, and 172) and a pair of new heteroisomer mixtures (170 and 171) were obtained from rice fermentation products of the deep-sea-derived fungus Diaporthe phaseolorum FS459 (Hu et al., 2021). Compounds 168–171 have rare 2ʹ,3ʹ-dimethyl-dioxopentyl structures. No significant activity was observed in vitro. Eight novel cyclohexanone derivatives, foeniculins A–H (173–183), were identified from rice fermentation products of the endophytic fungus Diaporthe foeniculina SCBG-15, which was derived from Leptospermum brachyandrum (Lu et al., 2021). Three novel phenolic acid derivatives, foeniculins I–K (181–183), showed moderate cytotoxic activity against SF-268, MCF-7, and HepG-2 tumor cells, with IC50 values of 27.73 µM, 42.54 µM, and 25.12 μM, respectively.
2.4.2 Pyrones isolated from fungi of the genus Phomopsis
Five new pyranoid ketone derivatives, phomaspyrones A–E (184–188), were found in the metabolic products of the endophytic fungus Phomopsis asparagi SWUKJ5.2020 (Song et al., 2017), which was derived from the evergreen shrub Kadsura angustifolia. Compound 186 showed significant cytotoxic activity against various cancer cell lines, which include A549, Raji, HepG2, MCF-7, HL-60 and K562, with IC50 values of 1.2, 2.0, 1.6, 2.2, 1.0, and 1.2 μg/mL. Four previously unreported pyrone derivatives, phomopsichins A–D (189–192), were identified in the metabolites of the endophytic fungus Phomopsis sp. 33# from mangrove forests (Huang et al., 2016). These four compounds failed to inhibit α-glucosidase and showed no antibacterial or cytotoxic properties. Two new pyranone derivatives, (10S)-10-O-B-D-40-methoxymanno-pyranosyldiaporthin (1 9 3) and clearanol H (1 9 4), were identified in the metabolites of the endophytic fungus Phomopsis sp. sh917, which was derived from Isodon eriocalyx var. laxiflora. These compounds showed no significant antiangiogenic activity (Tang et al., 2017). Three pyrone derivatives, phomochromenones A–C (195–197), were isolated from metabolites of the endophytic fungus Phomopsis sp. HNY29-2b, which was derived from the mangrove plant Acanthus ilicifolius Linn (Ding et al., 2017). Six new pyrone derivatives, phomoxanthones F–K (198–203), were identified in the metabolites of the endophytic fungus Phomopsis sp. xy21 (Hu et al., 2018a, 2018b). Among these, compounds 199 and 200 are highly oxidized xanthones. No cytotoxic activity was detected against tumor cell lines.
Two previously undescribed pyrone derivatives, phomoisocoumarins C and D (204–205), are metabolites of the endophytic fungus Phomopsis prunorum (Qu et al., 2020); phomoisocoumarin D (2 0 5) showed antibacterial activity. Phomotide A (2 0 6) is a previously unreported pyranone derivative from the metabolites of the endophytic fungus Phomopsis sp. CFS42 of Chinese plum yew (Cephalotaxus fortunei Hook) (Ma et al., 2020); this compound showed no significant antimicrobial activity. Phomopsinin B and C (207, 208) are novel pyrone natural products derived from fermentation metabolites of Phomopsis sp. CAM212. These compounds showed no anti-inflammatory properties.
Two novel pyrone natural products, phomopsinin B and C (207, 208), were identified in the fermentation metabolites of the endophytic fungus Phomopsis sp. CAM212 (Jouda et al., 2020), which originated from the medicinal plant gamboge (Garcinia xanthochymus). These compounds did not appear to have any anti-inflammatory properties. Four new chromenones, phomochromenones D–G (209–212), were obtained from rice fermentation products of Phomopsis asparagi DHS-48, which was derived from the Chinese mangrove plant Rhizophora mangle (Wei et al., 2021). These compounds showed no obvious immunosuppressive activity.
2.5 Polyketides
2.5.1 Polyketides isolated from fungi of the genus Diaporthe
Nine new polyketide compounds, acetoxydothiorelone B (2 1 3), (15S)-acetoxydothiorelone A (2 1 4), dothiorelone K–N (215–218), 5-hydroxy-7-methoxy-4,6-dimethyl-2-phenyliso in doline-1,3-dione (2 1 9), (13R)-diaporphthalide A (2 2 0), and (9S, 17R, 19S, 6Z, 10E, 14E)-diaporlactone A (2 2 1) were identified in the metabolites of the endophytic fungus Diaporthe pseudomangiferaea from the medicinal plant Indian Sarsaparilla (Tylophora ouata) (Liu et al., 2018) (Fig. 15). These compounds showed no significant cytotoxicity against tumor cell lines. Six new highly oxidized isochromophilones, A–F (222–227), were identified in the metabolites of the endophytic fungus Diaporthe sp. SCSIO 41011 (Luo et al., 2018a), which was derived from mangrove. Compound 225 inhibited 786-O tumor cell growth, with an IC50 value of 8.9 μM.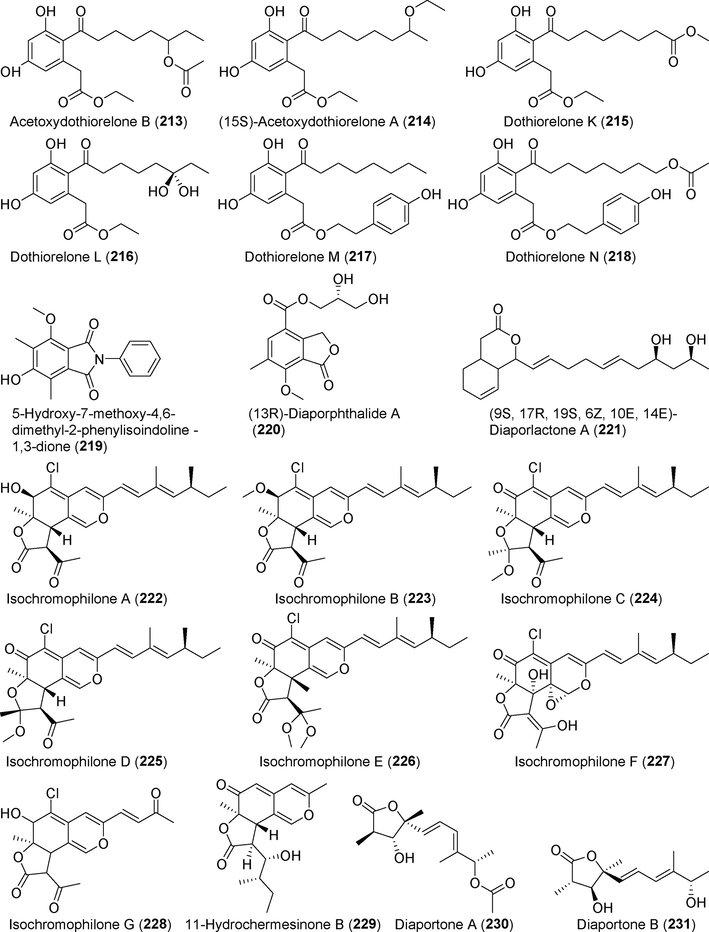
Polyketides isolated from fungi of the genus Diaporthe (213–231).
Isochromophilone G (2 2 8) is an azaphilone derivative isolated from the endophytic fungus Diaporthe perseae of the mangrove plant Pongamia pinnata (Niaz et al., 2020). This compound exhibited antibacterial and antioxidant properties. A new azaphilone (2 2 9) was identified in rice fermentation products of the deep-sea fungus Diaporthe phaseolorum FS459 (Hu et al., 2021). No significant cytotoxicity, antibacterial activity, or inhibitory effect on NO production was detected for this compound. Diaportone A (2 3 0) is a novel γ-butyrolactone derivative isolated from rice fermentation products of Diaporthe foeniculina BZM-15, an endophytic fungus derived from Leptospermum brachyandrum (Kang et al., 2021).
The novel cyclopentenone derivative diaportone B (2 3 1) showed no significant antibacterial or cytotoxic properties. Four novel polyketides, dothiorelone O (2 3 2), (15R)-acetoxydothiorelone A (2 3 3), microsphaerophthalide H (2 3 4), and microsphaerophthalide I (2 3 5) were identified in metabolites of the mangrove fungus Diaporthe sp. SCSIO 41011 (Fig. 16) (Luo et al., 2018b). No significant inhibitory effects were detected against influenza A virus. Diaporindenes A–D (236–239), four new polyketide derivatives with 2,3-dihydro-1H–indene chemical structures, were isolated from the metabolites of Diaporthe sp. SYSU-HQ3 (Cui et al., 2018). All four compounds significantly inhibited NO production, with IC50 values ranging from 4.2 µM to 9.0 μM.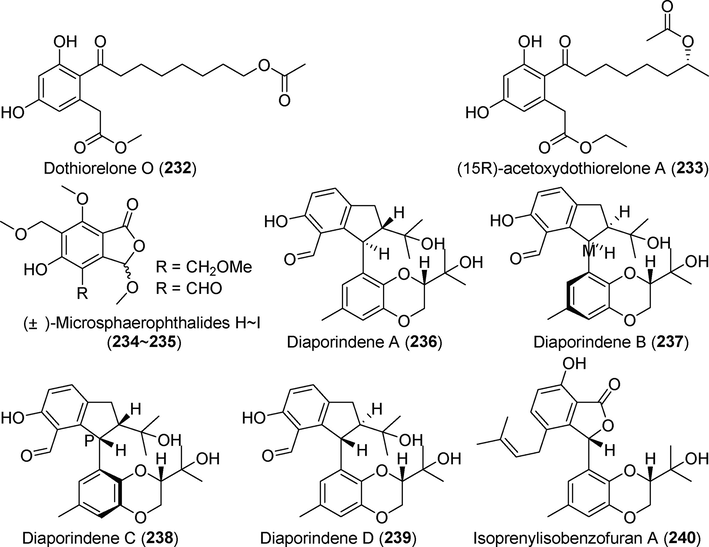
Polyketides isolated from fungi of the genus Diaporthe (232–240).
2.5.2 Polyketides isolated from Phomopsis
Three new polyketides, phomopsiketones A–C (241–243), were isolated from the metabolites of the endophytic fungus Phomopsis sp. sh917 from Isodon eriocalyx var. laxiflora (Fig. 17) (Tang et al., 2017). Compounds 241 and 242 showed certain antiangiogenic activities. Two previously unreported polyketides, 244 and 245, were found in the metabolites of the endophytic fungus Phomopsis sp. PSU-H188 from rubber tree (Hevea brasiliensis) (Kongprapan et al., 2017). These compounds showed no significant antibacterial activity or tumor cytotoxicity. Three new polyketides, phomopones A–C (246–248), were identified in the metabolites of the endophytic fungus Phomopsis sp. D15a2a (Yu et al., 2019), which originated from calico-plant (Alternanthera bettzickiana). The polyketide derivatives koninginin T and U (249, 250) were isolated from the endophytic fungus Phomopsis stipata from the shrub Styrax camporum Pohl (Biasetto et al., 2020). Compounds 249 and 250 showed moderate antifungal activity, and compound 249 also inhibited acetylcholinesterase activity. (See Fig. 18)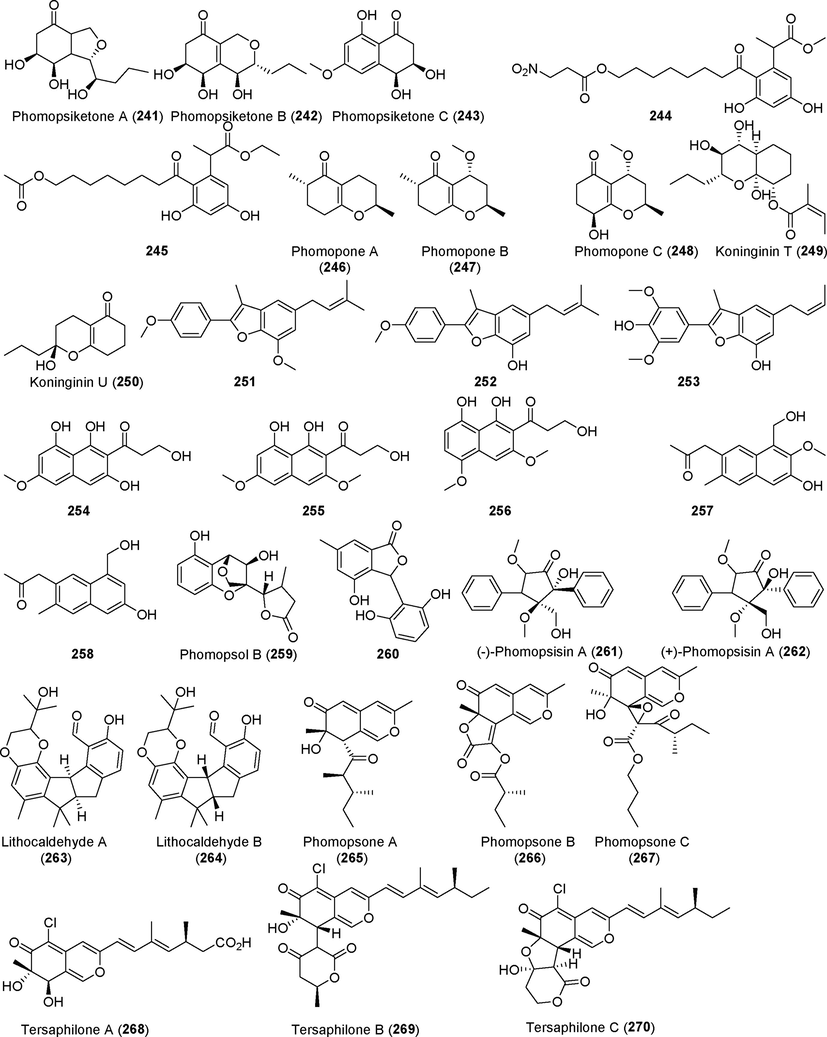
Polyketides isolated from fungi of the genus Phomopsis (241–270).
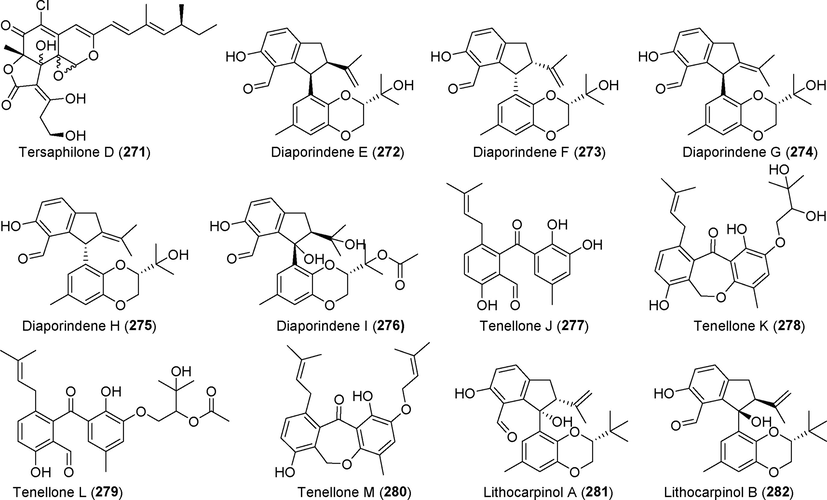
Polyketides isolated from fungi of the genus Phomopsis (271–282).
Three new arylbenzofuran derivatives, 251–253, were isolated from the metabolites of the endophytic fungus Phomopsis sp. (Du et al., 2017). Compound 251 displayed anti-tobacco mosaic virus (TMV) activity, with an inhibition rate of 35.2%. The polyketide derivatives 254–256 were found in the metabolites of the endophytic fungus Phomopsis fukushii (Yang et al., 2017), which originated from Zhonglu, Yunnan Province. Compound 254 displayed excellent antimicrobial activity against MRSA. Two new naphthalene derivatives (257–258) were isolated from the fermentation products of the endophytic fungus Phomopsis fukushii from the roots of tobacco (Nicotiana tabacum) (Li et al., 2021). These compounds showed certain inhibitory effects on Staphylococcus aureus. Two polyketides, phomopsol B (2 5 9) and 260, were found in the metabolites from Phomopsis sp. xy21 (Li et al., 2019), but no activity was reported.
Two new polyketides, (±)-phomopsisin A (261, 262), were isolated from the fermentation metabolites of Phomopsis asparagi CICC 2706 (Zhou et al., 2021). These compounds did not exhibit significant inhibitory activity against glycosidase. Two new polyketoids with a 6/6/5/5/6 highly fused ring skeleton, lithocaldehydes A and B (263, 264), were detected in the fermentation metabolites of the deep-sea fungus Phomopsis asparagi FS508 (Liu et al., 2020). These compounds showed antifungal activity. Three new azaphilone-like compounds, phomopsones (265–267), were isolated from the fermentation metabolites of the endophytic fungus Phomopsis sp. CGMCC No.5416 from ox knee (Achyranthes bidentata) (Yang et al., 2020a, 2020b). Compounds 266 and 267 displayed significant anti-HIV activity, with IC50 values of 7.6 µM and 0.5 μM, respectively. In addition, compounds 266 and 267 showed moderate cytotoxic effects against A549, MDA-MB-231, and PANC-1 cells, with IC50 values ranging from 3.2 µM to 303 μM. Compound 267 induced early apoptosis in PANC-1 cancer cells, with an apoptosis rate of 28.54%.
The novel chlorine-containing azaphilone derivatives tersaphilones A–D (268–271) were found in the fermentation metabolites of the deep-sea fungus Phomopsis tersa FS441 (Chen et al., 2021a, 2021b). Among these compounds, compound 269 has a unique 6/6/6 carbon skeleton and a cleaved tetrahydrofuran ring, and compounds 270 and 271 are diastereomers with epoxide ring groups, which are rarely found in azaphilones. Compound 270 showed significant inhibitory activity against tumor cell lines MCF-7, SF-268, and A549, with IC50 values ranging from 5.4 µM to 8.3 μM. Five new 2,3-dihydro-1H–ninhydrin derivatives, diaporindenes E–I (272–276), and four new benzophenone derivatives, tenellones J–M (277–280), were isolated from the rice fermentation products of the deep-sea fungus Phomopsis lithocarpus FS508 (Liu et al, 2021a, 2021b). Compound 278 showed cytotoxicity against SF-268 cells, with an IC50 value of 11.36 μM. Two new polyketide derivatives, lithocarpinol A and B (281, 282), were isolated from metabolites of the deep-sea fungus Phomopsis lithocarpus FS508 (Xu et al., 2019a, 2019b). Compound 281 had good inhibitory effects on HepG-2 and A549 tumor cell lines, with IC50 values of 9.4 µM and 10.9 μM, respectively.
2.6 Phenyl ketones
Two novel benzophenone derivatives, tenellones C–D (283, 284), were found in the metabolites of Diaporthe sp. SYSU-HQ3 (Cui et al., 2018). Tenellone C (2 8 3) inhibited NO production (Fig. 19). The new benzophenone derivative tenellone I (2 8 5) was isolated from the metabolites of the endophytic fungus Diaporthe lithocarpus A740 from Morinda officinalis (Liu et al., 2019); this compound showed no significant cytotoxic activity against tumor cells. Five new benzone derivatives, tenellones D–H (286–290), were found in the metabolites of the deep-sea fungus Phomopsis lithocarpus FS508 (Xu et al., 2018a, 2018b). Tenellone H (2 9 0) exhibited cytotoxic activity against cell lines HepG-2 and A549, with IC50 values of 16.0 µM and 17.6 µM, respectively.(See Fig. 20)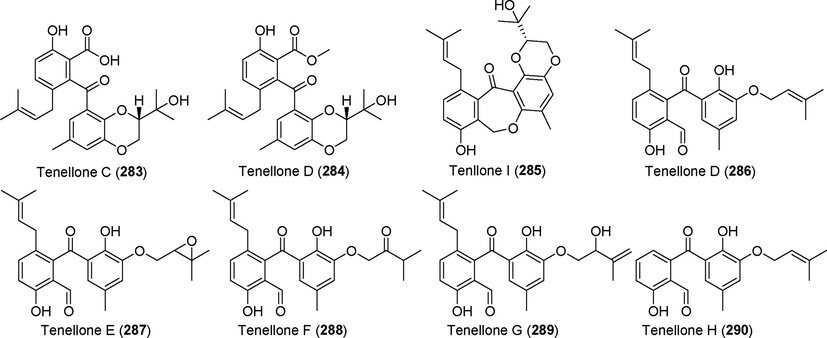
Diphenyl ketone derivatives isolated from fungi of the genus Diaporthe (Phomopsis) (283–290).
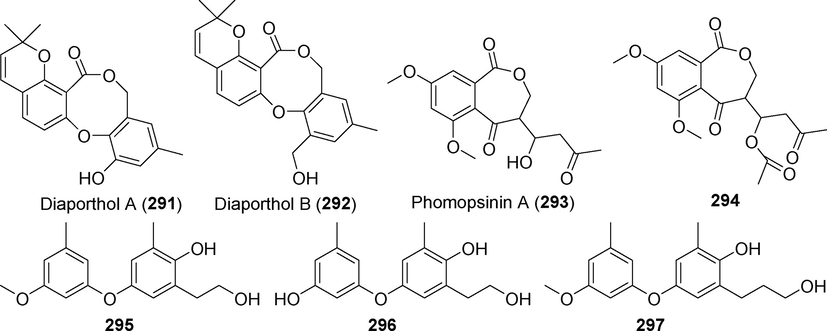
Diphenyl ethers isolated from fungi of the genus Diaporthe (Phomopsis) (291–297).
2.7 Diphenyl ethers
The new diphenyl ether compounds Diaporthols A and B (291–292) are produced by the endophytic fungus Diaporthe sp. ECN-137 of Amur cork tree (Phellodendron amurense) (Nakashima et al., 2018) (Fig. 19). Compounds 291 and 292 exhibited an anti-migration effect against TGF-1 (transforming growth factor beta-1)-induced MDA-MB-231 breast cancer cells at 20 μM. Phomopsinin A (2 9 3), a new diphenyl ether natural product, was discovered in the metabolites of Phomopsis sp. CAM212, an endophytic fungus of the medicinal plant Garcinia xanthochymus (Jouda et al., 2020). These chemicals did not show anti-inflammatory properties. However, compound 294 considerably suppressed NO generation once its chemical structure was acetylated, with an IC50 value of 14.80 µM. The association between the structure and activity of this compound indicates that the anti-inflammatory effects of 294 are significantly influenced by acetyl groups. Compound 294 inhibited the expression of iNOS, COX-2, and IL-6 but had no effect on the expression of IL-1. The novel diphenyl ether derivatives 295–297 were isolated from the metabolites of the endophytic fungus Phomopsis fukushii, which was derived from Paris polyphylla var. yunnanensis (Gao et al., 2019). Both 295 and 297 showed potent anti-MRSA activity.
2.8 Nonsteroidal compounds
Two novel steroid derivatives, diaporthins A and B (298 and 299), were detected in Diaporthe phragmitis, an endophytic fungus isolated from Actinidia chinensis (Yu et al., 2021a, 2021b) (Fig. 21). These two compounds failed to exhibit any appreciable antibacterial activity. A novel steroid derivative (3 0 0), as well as five known steroid derivatives, were produced from the rice fermentation products of Phomopsis sp. MGF222, which was discovered in mangrove plants (Zhu et al., 2021). These compounds showed no substantial antibacterial activity.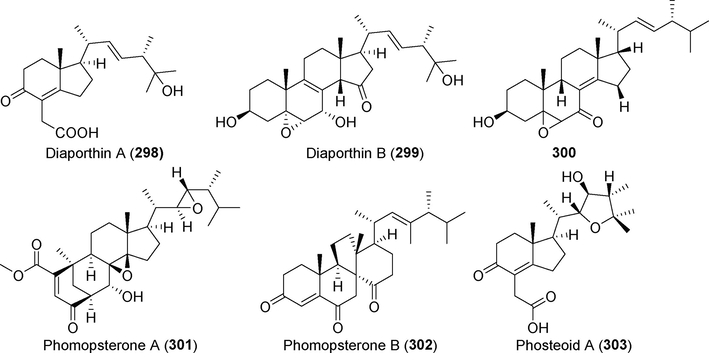
Steroids isolated from fungi of the genus Diaporthe (Phomopsis) (298–303).
Homopsterones A and B (301, 302) were found in the metabolites of the endophytic fungus Phomopsis sp. TJ507A from the medicinal plant Phyllanthus glaucus (Hu et al., 2017). Compound 301 is a distinct ergosteroid with a skeleton made of rearranged bicyclic nonane components. iNOS protein abundance and NO production were both suppressed by compounds 302, with IC50 values of 4.65 µM and 1.49 μM, respectively. The metabolites of Phomopsis tersa FS441 (Chen et al., 2020a), which were tested for cytotoxic and anti-inflammatory activities, contained a highly oxidized steroid derivative named phosteoid A (3 0 3) with a distinctive 6/5/5 tricyclic structure. This compound clearly demonstrated inhibitory activity at a concentration of 100 μM.
2.9 Fatty acids
The new fatty acid derivatives diapolic acids A and B (304, 305) were isolated from metabolites of the endophytic fungus Diaporthe terebinthifolii (Yedukondalu et al., 2017) (Fig. 22). These two compounds showed no significant cytotoxic activity. Eucalactam B (3 0 7) and eucalyptacid A (3 0 6), two novel compounds isolated from the metabolites of the fungus Diaporthe eucalyptorum (Gao et al., 2020a), showed specific antifungal activity.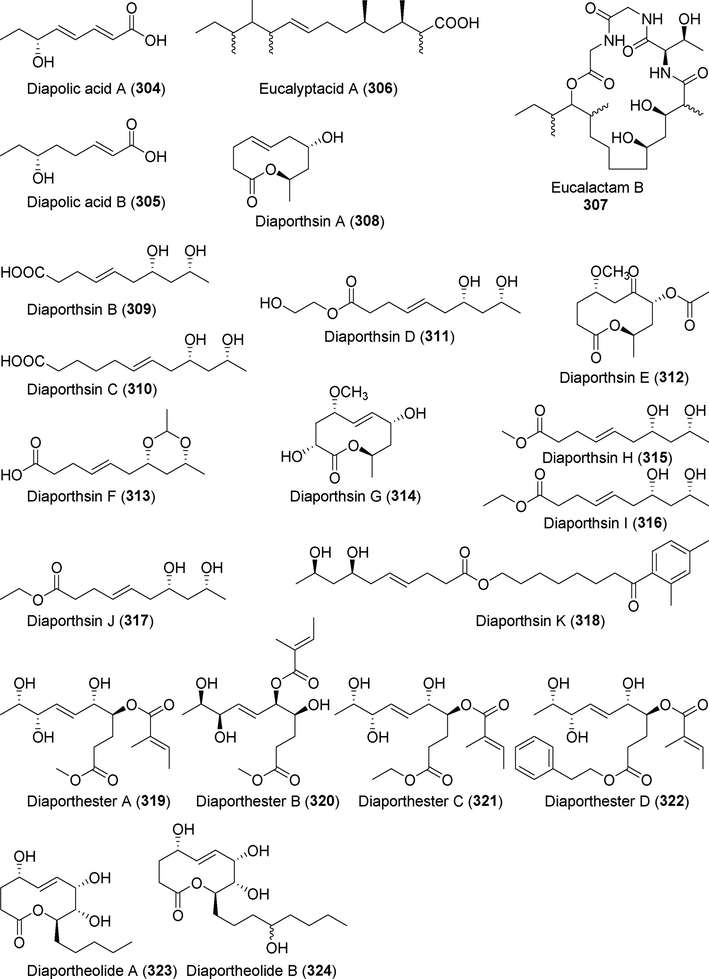
Fatty acids isolated from fungi of the genus Diaporthe (304–324).
Diaporthsins A–K (308–318) were identified as alternate derivatives of fatty acids from metabolites of the endophytic fungus Diaporthe sp. JC-J7 from Dendrobium nobile (Hu et al., 2018a, 2018b). Compound 312 had antilipemic (lipid limiting) effects comparable to lovastatin. Diaporthesters A–D (319–322) are four novel fatty acid derivatives isolated from the fermentation products of the endophytic fungus Diaporthe sp. T24. This fungus was obtained from the stems of the medicinal plant Ligularia fischeri (He et al., 2021). Compound 319 showed cytotoxicity against HCT-8 and MCF-7 cells, with IC50 values of 0.06 μM and 0.65 μM, respectively. Diaportheolides A and B (323, 324) were isolated from the fermentation metabolites of the endophytic fungus Diaporthe sp. SXZ-19 from Happy tree (Camptotheca acuminata) (Liu et al., 2021a, 2021b). These compounds showed no antibacterial activity.
2.10 Other types of compounds
Four new macrolide derivatives, lithocarpins A–D (325–328), were extracted from the metabolites of the deep-sea fungus Phomopsis lithocarpus FS508 (Xu et al., 2018a, 2018b) (Fig. 23). Compounds 327 and 328 showed antitumor effects against the HepG-2, MCF-7, and SF-268 cell lines, with IC50 values ranging from 17.0 µM to 21.6 μM. Three novel macrolide skeleton derivatives, lithocarpins E–G (329–331), were identified from the secondary metabolites of the deep-sea fungus Phomopsis lithocarpus FS508 (Xu et al., 2021a, 2021b). Compound 329 considerably inhibited HepG-2 cell growth, with an IC50 of 6.30 μM. The activation of p-Erk, Bax, and Caspase-3 gene expression by this chemical induced apoptosis in HepG2 cells.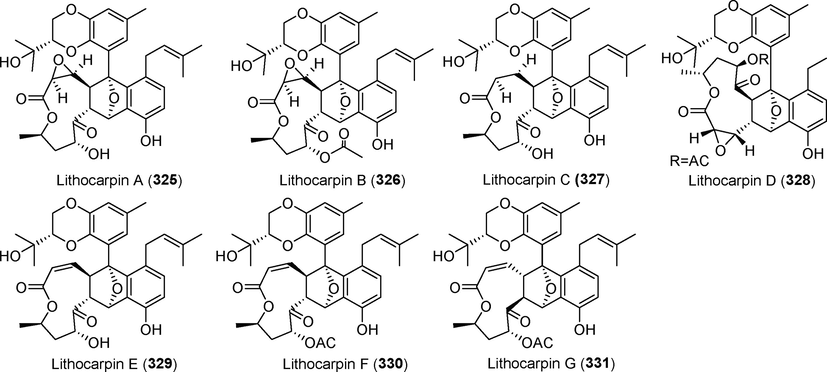
Diphenyl ethers isolated from fungi of the genus Phomopsis (325–331).
3 Biosynthesis of important compounds
Based on all genomes deposited in the NCBI database as of December 2021, the full genome sequences of 21 Diaporthe fungi had been published. The genome size of members of this genus typically ranges from 50 Mb to 65 Mb (Table 2). This information paves the way for investigating secondary metabolites and their biosynthesis in these species. NA, not available.
No.
Strain
Genome size (Mb)
Year
GenBank acc. number
Reference
1
Diaporthe longicolla TWH P74
64.71
2014
GCA_000800745.1
(Li et al., 2015)
2
Diaporthe longicolla MSPL 10–6
129.0
2014
GCA_000498855.2
(Li et al., 2015)
3
Diaporthe ampelina DA912
47.3
2015
GCA_001006365.1
(Morales-Cruz et al., 2015)
4
Diaporthe ampelina I2T2
59.9
2015
GCA_001006365.1
(Savitha et al., 2016)
5
Diaporthe aspalathi MS-SSC91
55.03
2015
GCA_001447215.1
(Li et al., 2016)
6
Diaporthe ampelin S3MP
59.50
2016
GCA_001630405.1
(Savitha et al., 2016)
7
Diaporthe helianthin 7/96
63.67
2018
GCA_001702395.2
NA
8
Diaporthe sp. HANT25
55.34
2020
GCA_013435955.1
(Tulsook et al., 2020)
9
Diaporthe sp. NJD1
58.33
2020
GCA_013842865.1
NA
10
Diaporthe capsica GY-Z16
57.56
2020
GCA_013364905.1
(Fang et al., 2020)
11
Diaporthe citri NFHF-8–4
63.69
2020
GCA_014595645.1
(Gai et al., 2021)
12
Diaporthe citr ZJUD2
60.38
2020
GCA_014872965.1
(Gai et al., 2021)
13
Diaporthe citr ZJUD14
53.97
2020
GCA_014872985.1
(Gai et al., 2021)
14
Diaporthe citr Q7
63.64
2020
GCA_014873005.1
(Gai et al., 2021)
15
Diaporthe citriasiana ZJUD30
53.41
2020
GCA_014872975.1
(Gai et al., 2021)
16
Diaporthe citriasiana ZJUD30
50.67
2020
GCA_016432825.1
(Gai et al., 2021)
17
Diaporthe citrichinensis ZJUD34
54.5
2020
GCA_014872995.1
(Gai et al., 2021)
18
Diaporthe destruens CRI305-2
56.11
2021
GCA_016859255.1
NA
19
Diaporthe batatas CRI 302–4
54.2
2021
GCA_019321695.1
NA
20
Diaporthe sp. DP-2020a
57.82
2021
GCA_018137895.1
NA
21
Diaporthe vexans PV4
59.77
2021
GCA_021188095.1
NA
3.1 epi-cytoskyrin a and its analogs
epi-cytoskyrin A (1) is a dimeric anthraquinone derivative with a unique cage-like skeleton that was first isolated by Agusta et al. (Agusta, 2006; Agusta et al., 2015b) from the metabolites of the endophytic fungus Diaporthe phaseolorum SW-93–13 in tea plants (Fig. 24). This compound exhibited considerable anti-MRSA (MIC = 0.25 g/mL) and anti-E. coli activity (MIC = 1.00 g/mL). Brady et al. (Brady et al., 2000) isolated cytoskyrins A and B (332 and 333) from the endophytic fungus Cytospora sp. CR200 in 2000. Tian et al. (Tian et al., 2018) isolated epi-cytoskyrin A (1) and cytoskyrin C (3 3 4) from the metabolites of the endophytic fungus Diaporthe sp. in 2018. Rugulosins A–C (335–337) were identified from Penicillium radicum FKI-3765–2 metabolites in 2010 (Yamazaki et al., 2010).(See Figs. 25 and 26)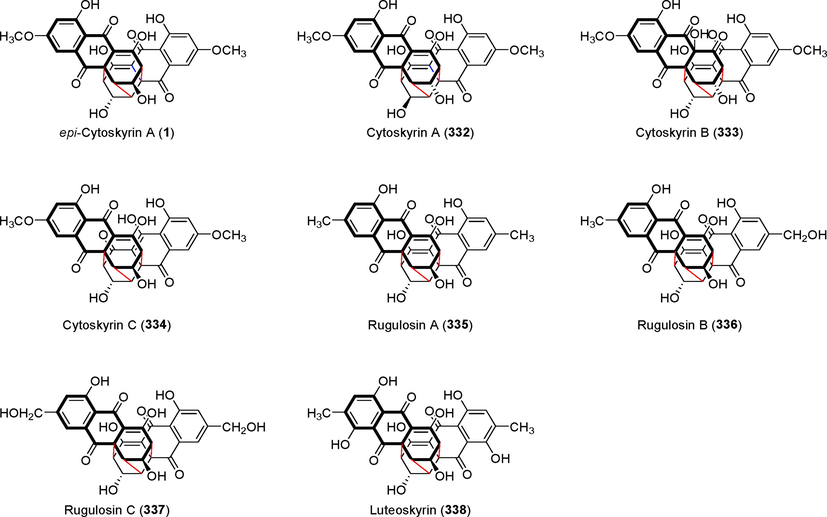
Derivatives of the cytoskyrins series containing unique cage dimer anthraquinone skeletons (1, 332–338).
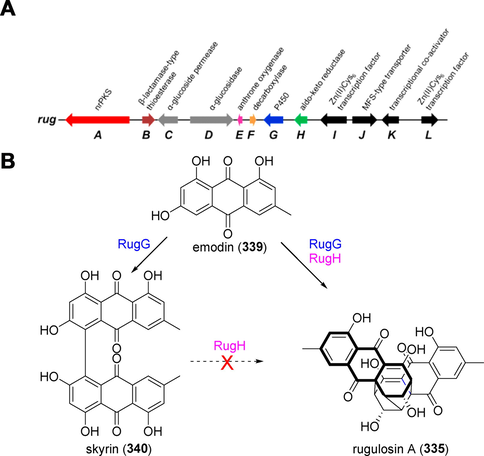
rug gene cluster governing the intertwined biosynthesis of rugulosin A (3 3 5) (Han et al., 2021).
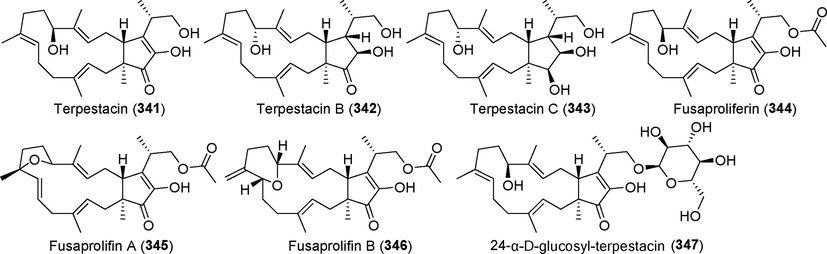
Terpestacin and its related derivatives (341–347).
Rugulosin A (3 3 5) is an analog of epi-cytokyrin A (1) with anti-MRSA activity, with MIC values as low as 0.125 g/mL. The gene cluster that controls the simultaneous biosynthesis of rugulosin A (3 3 5) in Talaromyces sp. YE3016 was first described by Han et al. in 2021 (Han et al., 2021). The discovery of the gene functions, biosynthetic precursors, and enzymes involved in the creation of their molecular architecture was made possible using a combination of genome sequencing, gene inactivation, heterologous expression, and biotransformation assays. Skyrin is formed via the 5,5′-dimerization of emodin radicals, which is catalyzed by RugG. Dimeric polyphenols can be created (chemo)enzymatically by cytochrome P450 monooxygenases. The fungal aldo–keto reductase RugH hijacks the closest skyrin precursor (CSP) immediately following emodin radical coupling and catalyze CSP ketone reduction to inactivate its tautomerization into skyrin, thus allowing a spontaneous intramolecular Michael addition to cyclize the ketone-reduced form of CSP into rugulosin A (3 3 5). Penicillium islandicum Sopp. produces luteoskyrin, a hepatotoxic anthraquinone. Long-term feeding of mice (Mus musculus) with P. islandicum Sopp-infected rice and isolated luteoskyrin elicited pathological changes in the liver, including adenoma and hepatoma (Ueno and Ishikawa, 1969). These findings could facilitate the manufacturing of epi-cytokyrin A (1) and its analogs.
3.2 Terpestacin
Terpetacin (3 4 1), a disesquiterpenoid that exhibits HIV-inhibitory and anti-angiogenic activity by binding to the 13.4-kDa subunit of mitochondrial complex III (ubiquinol-cytochrome c reductase binding protein, UQCRB), was discovered in 1993 from metabolites of the fungus Arthrinium sp. SF1744 (Iimura et al., 1993; Narita et al., 2018; Oka et al., 1993). The metabolites of the fungi Fusarium culmorum (Schlegel et al., 2001), Arthrinium sp. (Ye et al., 2019), Mariannaea humicola IG100 (Schlegel et al., 2001), and Aplosporella javeedii (Gao et al., 2020b) were subsequently obtained. In 2020, our research team also discovered this compound in the metabolites of Diaporthe sp. CB10100 (publication in preparation). Related derivatives reported thus far include terpetacins B and C (342, 343) (Gao et al., 2020b), fusaproliferin (3 4 4) (Santini et al., 1996), fusaproliferin A and B (345, 346) (Liu et al., 2013), and 24-α-d-glucosyl-terpestacin (3 4 7) (Liu et al., 2013). The structure of these compounds includes a unique binary ring architecture of 5/15 rings, which may be valuable for future development in addition to their remarkable biological activity.
In 2018, Narita et al. (Narita et al., 2018) verified the biosynthetic gene cluster of terpestacin (3 4 1) in Aspergillus oryzae by heterologous gene expression (tpcA–D) (Fig. 27). Two cytochrome P450s (tpcBC) catalyze the biosynthesis of three hydroxyl groups at the A-ring in a stereoscopic and spatially selective manner in the presence of bifunctional terpenoid synthase (tpcA). Subsequently, a flavin-dependent oxidase (TpcD) catalyzes the oxidation of the vicinal diol molecule to generate α-diketone, which is then enolized to yield terpstacin (3 4 1).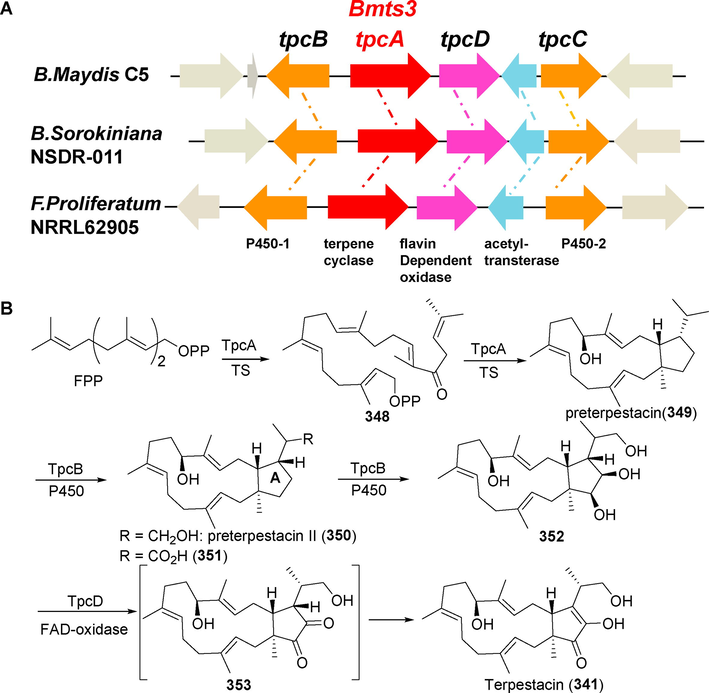
(A) Biosynthetic gene clusters of terpestacin in fungi such as Bipolaris maydis, Bipolaris sorokiniana, and Fusarium proliferatum; (B) A possible biosynthetic pathway for terpestacin (Narita et al., 2018).
3.3 Sch-642305
Sch-642305 (3 5 4) is a distinctive bicyclic 10-membered macrolide produced by Phomopsis sp. CMU-LMA and other fungi (Trenti et al., 2020). Chu et al. (Chu et al., 2003b) isolated sch-642305 (3 5 4) from the metabolites of Penicillium verrucosum for the first time in 2003. In 2005, Jayasuriya et al. (Jayasuriya et al., 2005) discovered that this compound exhibited anti-HIV activity, with an IC50 value of 1 µM. Dermenci et al. (Dermenci et al., 2011) discovered in 2018 that sch-642305 (3 5 4) inhibits glioma cell proliferation, which has significant development and application potential. Trenti et al. (Trenti et al., 2020) used heterologous expression, gene knockdown, and precursor feeding to identify the biosynthetic gene cluster responsible for sch-642305 (3 5 4) production in Phomopsis sp. CMU-LMA in 2020. An analysis using targeted gene disruption and pathway reconstruction revealed the crucial chemical steps and a succession of redox activities that occur in this pathway (Fig. 28).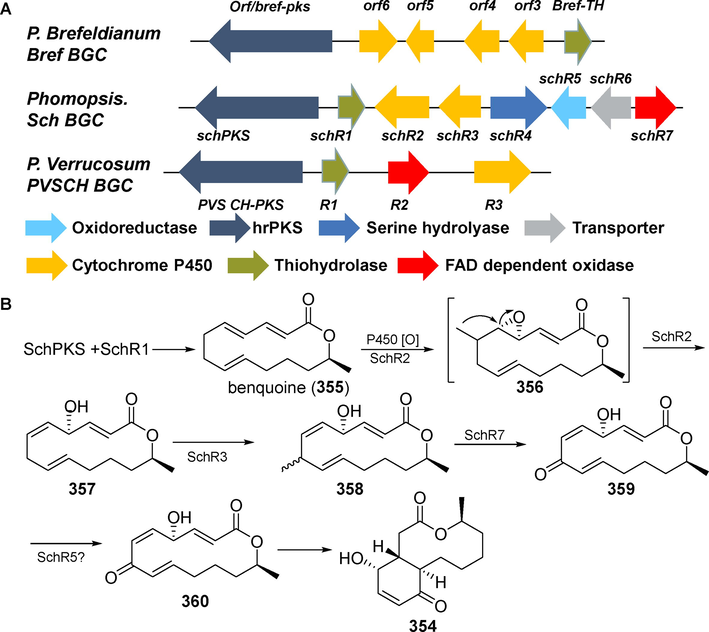
Gene cluster and biosynthetic pathway of sch-642305 (3 5 4) (Trenti et al., 2020).
4 Conclusions and future prospects
In this review, we summarized the structures, biological activities, and biosynthesis of new natural products from the genus Diaporthe and its anamorph Phomopsis. The 331 compounds isolated from Diaporthe and Phomopsis between 2016 and 2021, comprising 143 compounds from Diaporthe and 188 from Phomopsis, have diverse chemical structures and bioactivities. The major categories of these compounds include alkaloids, terpenoids, pyrones, and polyketides. These chemicals have a wide range of biological effects, including anti-inflammatory, antibacterial, and anticancer properties. These findings pave the way for future pharmaceutical research.
In addition, we described the biosynthesis of three compounds generated by Diaporthe (Phomopsis) and listed the 21 strains of Diaporthe (Phomopsis) fungi whose full genome sequences are available in the NCBI database. Many novel substances may be created by activating the expression of suppressed gene clusters using advanced molecular techniques including heterologous expression, gene deletion, and other approaches. We also described the biosynthesis of rugulosin A (3 3 5), terpestacin (3 4 1), sch-642305 (3 5 4), and other chemicals with anticancer effects. By studying the biosynthesis of these chemicals, fascinating biosynthetic enzymes have been revealed, expanding the pool of biological elements for synthetic biology. Furthermore, in light of the growing interest in the biosynthetic routes of natural products originating from fungi, identifying certain unique catalytic enzymes from Diaporthe (Phomopsis) is of particular interest. The literature on secondary metabolites of Diaporthe (Phomopsis) fungi greatly expanded between 2016 and 2021. These investigations have substantially aided in the identification of novel active natural products derived from these fungi, as well as the development and use of existing active natural products.
Therefore, Diaporthe (Phomopsis) fungi represent important resources for the discovery of novel natural active substances and research on natural compound biosynthesis. To date, research on Diaporthe (Phomopsis) fungi has mainly focused on the isolation and identification of novel natural products and preliminary biological activity screening, whereas only a few studies have focused on the key biosynthetic genes and enzymes involved in their production. In addition, the bioactivities of most of these compounds have only been studied in vitro. To fully develop and exploit Diaporthe (Phomopsis) fungi, further investigations are needed on the biosynthesis and pharmacological mechanisms of highly active compounds from this genus.
CRediT authorship contribution statement
Lin Jiang: Conceptualization, Writing – original draft. Qingxian Ma: Writing – original draft. Aijie Li: Writing – review & editing. Runze Sun: Writing – review & editing. Genyun Tang: Writing – review & editing. Xueshuang Huang: . Hong Pu: Conceptualization, Writing – original draft.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (82104055) (to L. J.) and (81703821) (to G. T.); the Hunan Provincial Natural Science Foundation of China (2022JJ50294) (to H. P.); the Hunan Provincial Department of Education Science Research Project (22B1036) (to H. P.); the China Postdoctoral Science Foundation (2020M671163) (to L. J.); the Hunan University of Medicine High-Level Talent Introduction Startup Funds 202203 (to H. P.); and National Innovation and Entrepreneurship Training for University of PRC (202212214002). (to Q. X. M.).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bisanthraquinone metabolites produced by the endophytic fungus Diaporthe sp. Chem. Pharm. Bull.. 2006;54:579-582.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mode of action of (+)-2,2’-epi-cytoskyrin A. Microbiology Indonesia. 2015;9:35-43.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mode of action of (+)-2,2’-epi-cytoskyrin A. Microbiology Indonesia. 2015;9:35-43.
- [CrossRef] [Google Scholar]
- The new koninginins T-U from Phomopsis stipata, an endophytic fungus isolated from Styrax camporum Pohl. Phytochem. Lett.. 2020;36:106-110.
- [CrossRef] [Google Scholar]
- Cytoskyrins A and B, new BIA active bisanthraquinones isolated from an endophytic fungus. Org. Lett.. 2000;2:4047-4049.
- [CrossRef] [Google Scholar]
- Phochrodines A-D, first naturally occurring new chromenopyridines from mangrove entophytic fungus Phomopsis sp. 33#. Fitoterapia. 2018;124:103-107.
- [CrossRef] [Google Scholar]
- Tersone A-G, new pyridone alkaloids from the deep-sea fungus phomopsis tersa. Mar. Drugs. 2019;17:1-14.
- [CrossRef] [Google Scholar]
- Phosteoid A, a highly oxygenated norsteroid from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron Lett.. 2020;61:151555
- [CrossRef] [Google Scholar]
- Phomeroids A and B: two novel cytotoxic meroterpenoids from the deep-sea-derived fungus Phomopsis tersa FS441. Org. Chem. Front.. 2020;7:557-562.
- [CrossRef] [Google Scholar]
- Photeroids A and B, unique phenol-sesquiterpene meroterpenoids from the deep-sea-derived fungus: Phomopsis tersa. Org. Biomol. Chem.. 2020;18:642-645.
- [CrossRef] [Google Scholar]
- Tersaphilones A-E, cytotoxic chlorinated azaphilones from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron. 2021;78:131806
- [CrossRef] [Google Scholar]
- Anti-inflammatory activities of alkaloids from the mangrove endophytic fungus Phomopsis sp. SYSUQYP-23. Bioorg. Chem.. 2020;97:103712
- [CrossRef] [Google Scholar]
- Bioactive sesquiterpene derivatives from mangrove endophytic fungus Phomopsis sp. SYSU-QYP-23: Structures and nitric oxide inhibitory activities. Bioorg. Chem.. 2021;107:104530
- [CrossRef] [Google Scholar]
- The genus Diaporthe: a rich source of diverse and bioactive metabolites. Mycol. Prog.. 2017;16:477-494.
- [CrossRef] [Google Scholar]
- Isolation and structure elucidation of Sch 642305, a novel bacterial DNA primase inhibitor produced by Penicillium verrucosum. J. Nat. Prod.. 2003;66:143-145.
- [CrossRef] [Google Scholar]
- Chroman-4-one and pyrano[4,3-b]chromenone derivatives from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. RSC Adv.. 2017;7:20128-20134.
- [CrossRef] [Google Scholar]
- Diaporisoindoles A-C: Three isoprenylisoindole alkaloid derivatives from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. Org. Lett.. 2017;19:5621-5624.
- [CrossRef] [Google Scholar]
- Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorg. Med. Chem. Lett.. 2017;27:803-807.
- [CrossRef] [Google Scholar]
- Diaporindenes A-D: four unusual 2,3-dihydro-1 H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem.. 2018;83:11804-11813.
- [CrossRef] [Google Scholar]
- Quasi-biomimetic ring contraction promoted by a cysteine-based nucleophile: Total synthesis of Sch-642305, some analogs and their putative anti-HIV activities. Chem. Sci.. 2011;2:1568-1572.
- [CrossRef] [Google Scholar]
- Three new chromone derivatives produced by Phomopsis sp. HNY29-2B from Acanthus ilicifolius Linn. Chin. J. Chem .. 2017;35:1889-1893.
- [CrossRef] [Google Scholar]
- Unravelling diaporthe species associated with woody hosts from karst formations (Guizhou) in china. J. Fungi. 2020;6:1-29.
- [CrossRef] [Google Scholar]
- Three new 3-methyl-2-arylbenzofurans from the fermentation products of an endophytic fungus Phomopsis sp. and their anti-TMV activity. Phytochem. Lett.. 2017;21:287-290.
- [CrossRef] [Google Scholar]
- Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF- κ B and NLRP3 inflammasome activation. Int. Immunopharmacol.. 2020;80:106144
- [CrossRef] [Google Scholar]
- Whole genome sequence of Diaporthe capsici, a new pathogen of walnut blight. Genomics. 2020;112:3751-3761.
- [CrossRef] [Google Scholar]
- The genome sequence of the citrus melanose pathogen Diaporthe citri and two citrus-related Diaporthe species. Phytopathology. 2021;111:779-783.
- [CrossRef] [Google Scholar]
- Isolation and characterization of antifungal metabolites from the melia azedarach-associated fungus Diaporthe eucalyptorum. J. Agric. Food Chem.. 2020;68:2418-2425.
- [CrossRef] [Google Scholar]
- Sesterterpenes and macrolide derivatives from the endophytic fungus Aplosporella javeedii. Fitoterapia. 2020;146:104652
- [CrossRef] [Google Scholar]
- Three new diphenyl ether derivatives from the fermentation products of an endophytic fungus Phomopsis fukushii. J. Asian Nat. Prod. Res.. 2019;21:316-322.
- [CrossRef] [Google Scholar]
- Chromone-derived polyketides from the deep-sea fungus Diaporthe phaseolorum FS431. Mar. Drugs. 2019;17:1-12.
- [CrossRef] [Google Scholar]
- Intertwined biosynthesis of skyrin and rugulosin A underlies the formation of cage-structured bisanthraquinones. J. Am. Chem. Soc.. 2021;143:14218-14226.
- [CrossRef] [Google Scholar]
- Four new fatty acid derivatives from Diaporthe sp. T24, an endophytic fungus isolated from Ligularia fischer. J. Asian Nat. Prod. Res. 2021:1-14.
- [CrossRef] [Google Scholar]
- Epoxyquinophomopsins A and B from endophytic fungus Phomopsis sp. and their activity against tyrosine kinase. J. Nat. Med.. 2021;75:217-222.
- [CrossRef] [Google Scholar]
- Secondary metabolites and bioactivity of the endophytic fungus Phomopsis theicola from Taiwanese endemic plant. Rec. Nat. Prod.. 2016;10:189-194.
- [Google Scholar]
- Pycnidione, a fungus-derived agent, induces cell cycle arrest and apoptosis in A549 human lung cancer cells. Chem. Biol. Interact.. 2012;197:23-30.
- [CrossRef] [Google Scholar]
- Polyketides from the deep-sea-derived fungus Diaporthe phaseolorum FS459. Chin. J. Org. Chem.. 2021;41:1591-1598.
- [CrossRef] [Google Scholar]
- Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21. Fitoterapia. 2018;131:265-271.
- [CrossRef] [Google Scholar]
- Phomopsterones A and B, two functionalized ergostane-type steroids from the endophytic fungus Phomopsis sp. TJ507A. Org. Lett.. 2017;19:258-261.
- [CrossRef] [Google Scholar]
- Potential antihyperlipidemic polyketones from endophytic: Diaporthe sp. JC-J7 in Dendrobium nobile. RSC Adv.. 2018;8:41810-41817.
- [CrossRef] [Google Scholar]
- Polyoxygenated cyclohexenoids with promising α-glycosidase inhibitory activity produced by Phomopsis sp. YE3250, an endophytic fungus derived from Paeonia delavayi. J. Agric. Food Chem.. 2018;66:1140-1146.
- [CrossRef] [Google Scholar]
- Phomopsichin A-D; Four new chromone derivatives frommangrove endophytic fungus Phomopsis sp. 33#. Mar. Drugs. 2016;14:1-11.
- [CrossRef] [Google Scholar]
- Cytochalasins from endophytic Diaporthe sp. GDG-118. Nat. Prod. Res.. 2019;35(20):3396-3403.
- [CrossRef] [Google Scholar]
- Terpestacin, a novel syncytium formation inhibitor, isolated from Arthrinium species. Tetrahedron Lett.. 1993;34:493-496.
- [CrossRef] [Google Scholar]
- Identification of diverse microbial metabolites as potent inhibitors of HIV-1 Tat transactivation. Chem. Biodivers.. 2005;2:112-122.
- [CrossRef] [Google Scholar]
- Natural polyketides isolated from the endophytic fungus Phomopsis sp. CAM212 with a semisynthetic derivative downregulating the ERK/IκBα signaling pathways. Planta Med.. 2020;86:1032-1042.
- [CrossRef] [Google Scholar]
- Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J. Biol. Chem.. 2010;285:11584-11595.
- [CrossRef] [Google Scholar]
- Diaportones A-C: three new metabolites from endophytic fungus Diaporthe foeniculina BZM-15. Front. Chem.. 2021;9:755351
- [CrossRef] [Google Scholar]
- Cytosporone derivatives from the endophytic fungus Phomopsis sp. PSU-H188. Phytochem. Lett.. 2017;22:219-223.
- [CrossRef] [Google Scholar]
- Phomopsols A and B from the mangrove endophytic fungus Phomopsis sp. xy21: structures, neuroprotective effects, and biogenetic relationships. Org. Lett.. 2019;21:7919-7922.
- [CrossRef] [Google Scholar]
- Antibacterial naphthalene derivatives from the fermentation products of the endophytic fungus Phomopsis fukushii. Chem. Nat. Compd.. 2021;57:293-296.
- [CrossRef] [Google Scholar]
- Draft genome sequence of phomopsis longicolla type strain TWH P74, a fungus causing Phomopsis seed decay in soybean. Genome Announc.. 2015;3:2-3.
- [CrossRef] [Google Scholar]
- Draft genome sequence of Diaporthe aspalathi isolate MS-SSC91, a fungus causing stem canker in soybean. Genomics Data. 2016;7:262-263.
- [CrossRef] [Google Scholar]
- Four new metabolites from the endophytic fungus Diaporthe lithocarpus A740. Fitoterapia. 2019;137:104260
- [CrossRef] [Google Scholar]
- Two new nonenolides from Diaporthe sp. SXZ-19, an endophytic fungus of Camptotheca Acuminata. Chem. Biodivers.. 2021;18:e2001055.
- [Google Scholar]
- Sesterterpenes and 2H-pyran-2-ones (=α-pyrones) from the mangrove-derived endophytic fungus Fusarium proliferatum MA-84. Helv. Chim. Acta. 2013;96:437-444.
- [CrossRef] [Google Scholar]
- Lithocaldehydes A and B, polyketones from the deep sea-derived fungus: Phomopsis lithocarpus FS508. Org. Biomol. Chem.. 2020;18:7326-7329.
- [CrossRef] [Google Scholar]
- Cytotoxic diaporindene and tenellone derivatives from the fungus Phomopsis lithocarpus. Chin. J. Nat. Med.. 2021;19:874-880.
- [CrossRef] [Google Scholar]
- Cytochalasins and polyketides from the fungus Diaporthe sp. GZU-1021 and their anti-inflammatory activity. Fitoterapia. 2019;137:104187
- [CrossRef] [Google Scholar]
- Dothiorelone derivatives from an endophyte Diaporthe pseudomangiferaea inhibit the activation of human lung fibroblasts MRC-5 cells. Fitoterapia. 2018;127:7-14.
- [CrossRef] [Google Scholar]
- Cyclohexanone and phenolic acid derivatives from endophytic fungus Diaporthe foeniculina. Front. Chem.. 2021;9:738307
- [CrossRef] [Google Scholar]
- Isochromophilones A-F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod.. 2018;81:934-941.
- [CrossRef] [Google Scholar]
- Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front. Chem.. 2018;6:282.
- [CrossRef] [Google Scholar]
- Cytochalasins from mangrove endophytic fungi Phomopsis spp. xy21 and xy22. Phytochem. Lett.. 2016;17:162-166.
- [CrossRef] [Google Scholar]
- Phomotide A, a novel polyketide, from the endophytic fungus Phomopsis sp. CFS42. Tetrahedron Lett.. 2020;61:151468
- [CrossRef] [Google Scholar]
- Drug development against tuberculosis: impact of alkaloids. Eur. J. Med. Chem.. 2017;137:504-544.
- [CrossRef] [Google Scholar]
- Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genomics. 2015;16:1-22.
- [CrossRef] [Google Scholar]
- Potential of endophytic Diaporthe sp. as a new source of bioactive compounds. J. Microbiol. Biotechnol.. 2020;31:1-8.
- [CrossRef] [Google Scholar]
- Diaporthols A and B: bioactive diphenyl ether derivatives from an endophytic fungus Diaporthe sp. Tetrahedron Lett.. 2018;59:1212-1215.
- [CrossRef] [Google Scholar]
- Total biosynthesis of antiangiogenic agent (-)-terpestacin by artificial reconstitution of the biosynthetic machinery in Aspergillus oryzae. J. Org. Chem.. 2018;83:7042-7048.
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant chlorinated azaphilones from mangrove Diaporthe perseae sp. isolated from the stem of chinese mangrove Pongamia pinnata. J. Asian Nat. Prod. Res. 2020:1-8.
- [CrossRef] [Google Scholar]
- Cytotoxic polyketides from a deep-sea sediment derived fungus Diaporthe phaseolorum FS431. Molecules. 2019;24:1-9.
- [CrossRef] [Google Scholar]
- Vochysiamides A and B: two new bioactive carboxamides produced by the new species Diaporthe vochysiae. Fitoterapia. 2019;138:104273
- [CrossRef] [Google Scholar]
- Terpestacin, a new syncytium formation inhibitor from Arthrinium sp. J. Antibiot.. 1993;46:367-373.
- [CrossRef] [Google Scholar]
- Carneic Acids from an endophytic Phomopsis sp. as dengue virus polymerase inhibitors. J. Nat. Prod.. 2020;83:2330-2336.
- [CrossRef] [Google Scholar]
- Bioactive α-pyrone derivatives from the endophytic fungus Diaporthe sp. CB10100 as inducible nitric oxide synthase inhibitors. Frontiers. Chemistry. 2021;9:679592
- [CrossRef] [Google Scholar]
- Antibacterial bisabolane sesquiterpenoids and isocoumarin derivatives from the endophytic fungus Phomopsis prunorum. Phytochem. Lett.. 2020;37:1-4.
- [CrossRef] [Google Scholar]
- Structure and absolute stereochemistry of fusaproliferin, a toxic metabolite from Fusarium proliferatum. J. Nat. Prod.. 1996;59:109-112.
- [CrossRef] [Google Scholar]
- Complete genome sequence of the endophytic fungus Diaporthe (Phomopsis) ampelina. Genome Announc.. 2016;4:1-2.
- [CrossRef] [Google Scholar]
- (-)-Terpestacin and L-tenuazonic acid, inducers of pigment and aerial mycelium formation by Fusarium culmorum JP 15. J. Basic Microbiol.. 2001;41:179-183.
- [CrossRef] [Google Scholar]
- Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M0042F): acid-mediated intramolecular cycloadditions enhance chemical diversity. J. Org. Chem.. 2017;82:9704-9709.
- [CrossRef] [Google Scholar]
- Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food Chem.. 2020;68:15440-15448.
- [CrossRef] [Google Scholar]
- Bioactive 2-pyrone metabolites from an endophytic Phomopsis asparagi SWUKJ5.2020 of Kadsura angustifolia. Phytochem. Lett.. 2017;22:235-240.
- [CrossRef] [Google Scholar]
- Chemical constituents and their antibacterial activity from the tropical endophytic fungus Diaporthe sp. F2934. J. Appl. Microbiol.. 2016;120:1501-1508.
- [CrossRef] [Google Scholar]
- Polyketides from the endophytic fungus Phomopsis sp. sh917 by using the one strain/many compounds strategy. Tetrahedron. 2017;73:3577-3584.
- [CrossRef] [Google Scholar]
- Phomopsisins A-C: three new cytochalasans from the plant endophytic fungus Phomopsis sp. sh917. Tetrahedron. 2020;76:131475
- [CrossRef] [Google Scholar]
- Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol.. 2016;120:1448-1457.
- [CrossRef] [Google Scholar]
- Cytoskyrin C, an unusual asymmetric bisanthraquinone with cage-like skeleton from the endophytic fungus Diaporthe sp. Fitoterapia. 2018;128:253-257.
- [CrossRef] [Google Scholar]
- Investigating the biosynthesis of Sch-642305 in the fungus: Phomopsis sp. CMU-LMA. RSC Advances. 2020;10:27369-27376.
- [CrossRef] [Google Scholar]
- Draft genome sequence of Diaporthe sp. strain HANT25, an endophytic fungus producing mycoepoxydiene. Microbiology Resource Announcements. 2020;9:3-4.
- [CrossRef] [Google Scholar]
- Production of luteoskyrin, a hepatotoxic pigment, by Penicillium islandicum Sopp. Appl. Microbiol.. 1969;18:406-409.
- [CrossRef] [Google Scholar]
- Four new chromones from the endophytic fungus Phomopsis asparagi DHS-48 isolated from the chinese mangrove plant Rhizophora mangle. Mar. Drugs. 2021;19:348.
- [CrossRef] [Google Scholar]
- Two new antibacterial anthraquinones from cultures of an endophytic fungus Phomopsis sp. Chem. Nat. Compd.. 2021;57:823-827.
- [CrossRef] [Google Scholar]
- Protoilludane, illudalane, and botryane sesquiterpenoids from the endophytic fungus Phomopsis sp. TJ507A. J. Nat. Prod.. 2018;81:1311-1320.
- [CrossRef] [Google Scholar]
- Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Mar. Drugs. 2018;16:1-12.
- [CrossRef] [Google Scholar]
- Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms. 2021;9:1-49.
- [CrossRef] [Google Scholar]
- Lithocarpins A-D: four tenellone-macrolide conjugated [4 + 2] hetero-adducts from the deep-sea derived fungus: Phomopsis lithocarpus FS508. Org. Chem. Front.. 2018;5:1792-1797.
- [CrossRef] [Google Scholar]
- Lithocarpinols A and B, a pair of diastereomeric antineoplastic tenellone derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Chin. Chem. Lett.. 2019;30:439-442.
- [CrossRef] [Google Scholar]
- Lithocarpins E—G, Potent anti-tumor tenellone-macrolides from the deep-sea fungus Phomopsis lithocarpus FS508. Chin. J. Chem .. 2021;39:1104-1112.
- [CrossRef] [Google Scholar]
- Anti-inflammatory diterpenoids from an endophytic fungus Phomopsis sp. S12. Tetrahedron Lett.. 2019;60:151045
- [CrossRef] [Google Scholar]
- New rugulosins, anti-MRSA antibiotics, produced by Penicillium radicum FKI-3765-2. Org. Lett.. 2010;12:1572-1575.
- [CrossRef] [Google Scholar]
- Metabolites with anti-inflammatory activity from the mangrove endophytic fungus Diaporthe sp. QYM12. Mar. Drugs. 2021;19:2-11.
- [CrossRef] [Google Scholar]
- Phomopchalasins A and B, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus Phomopsis sp. shj2. Org. Lett.. 2016;18:1108-1111.
- [CrossRef] [Google Scholar]
- Three new napthalene derivatives from the endophytic fungus Phomopsis fukushii. Phytochem. Lett.. 2017;22:266-269.
- [CrossRef] [Google Scholar]
- Cytochalasans from endophytic fungus Diaporthe sp. SC-J0138. Fitoterapia. 2020;145:104611
- [CrossRef] [Google Scholar]
- New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No.5416. Fitoterapia. 2020;145:104573
- [CrossRef] [Google Scholar]
- Two new sesterterpenes from marine-derived fungus Arthrinium sp. Chem. Nat. Compd.. 2019;55:281-284.
- [CrossRef] [Google Scholar]
- Diapolic acid A-B from an endophytic fungus, Diaporthe terebinthifolii depicting antimicrobial and cytotoxic activity. J. Antibiot.. 2017;70:212-215.
- [CrossRef] [Google Scholar]
- Polyketides and nitrogenous metabolites from the endophytic fungus Phomopsis sp. D15a2a. Tetrahedron Lett.. 2019;60:151325
- [CrossRef] [Google Scholar]
- 2-Pyrones from endophytic fungus Diaporthe foeniculina BZM-15. Nat. Prod. Res.. 2021;1–9
- [CrossRef] [Google Scholar]
- Secondary metabolites from cultures of the kiwi-associated fungus Diaporthe phragmitis and their antibacterial activity assessment. Phytochem. Lett.. 2021;46:143-148.
- [CrossRef] [Google Scholar]
- Five new secondary metabolites from the fungus Phomopsis asparagi. Fitoterapia. 2021;150:104840
- [CrossRef] [Google Scholar]
- One new α, β-unsaturated 7-ketone sterol from the mangrove-derived fungus Phomopsis sp. MGF222. Nat. Prod. Res.. 2021;35:3970-3976.
- [Google Scholar]







