Translate this page into:
Extraction, characterization and functionality assessment of Aloe vera, chitosan and silk sericin
⁎Corresponding author. mihmondal@gmail.com (Md. Ibrahim H. Mondal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
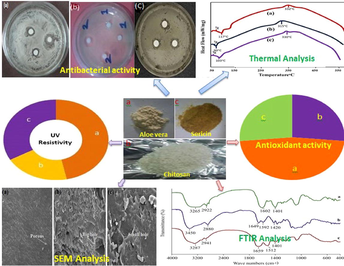
Aloe vera, chitosan, and silk sericin have been investigated for their physicochemical properties as well as functional characteristics such as antibacterial, antioxidant, and UV resistance. Aloe vera was extracted by methanol as a solvent. Silk sericin was prepared from a boiled water solution of silk cocoons through ethanol precipitation, while chitosan was synthesized by deacetylation of chitin. FT-IR, TGA, DSC, EDX, UV–vis, and XRD were used to characterize the extracted Aloe vera, chitosan, and sericin. Antibacterial activity, antioxidant activity and UV protection factors were also investigated. The TGA graph revealed that the significant weight loss at 400 °C was 39%, 51%, and 52%. for Aloe vera, chitosan, and sericin, respectively. For E. coli, Aloe vera, chitosan, and silk sericin, the zones of inhibition were 2.5 mm, 3 mm, and 1 mm, respectively, whereas for S. aureus, the zones of inhibition were 4 mm, 5 mm, and 1.5 mm. The free radical scavenging activity of 10 mg/ml Aloe vera, chitosan and sericin was 93%, 53% and 52%, respectively. All three UV radiation zones were absorbed by an Aloe vera solution. Chitosan and silk sericin solution exclusively absorbed UV-C rays. These almost priceless natural resources can contribute to health-related beneficial issues.
Keywords
Aloe vera
Chitosan
Silk sericin
Antibacterial activity
Anti-oxidant property and ultraviolet protection factor
1 Introduction
Bacteria availability in our surroundings is categorized into two types, one is pathogenic, and another is non-pathogenic. Between them, pathogenic bacteria are very harmful, especially in the hospital, as it causes dangerous infection in surgical patient despite the enormous advancement in the medical and pharmaceutical sectors. The surgical gown, masks, theatre drapes, bed sheets, and pillowcases are the most common sources of cross-infection (Ristić et al. 2011, Shahidi et al. 2007). The United States expends above $120 billion per year on the treatment of infectious diseases.
Conversely, the US government spends $5 billion/year exclusively to treat resistant pathogens (Boucher 2010). Recently large pharmaceutical companies did not show enough interest in inventing new antibiotics. It is time-consuming, expensive and risky, contrary to, searching for smart solutions to this problem, both inexpensive and effective. Besides the above problems, the food industry faces a great threat of a microbial attack, which can lead to quality deterioration and reduce the food products' shelf life (Ding et al. 2013, Amit 2017). As well as both pathogenic and non-pathogenic bacteria also grow in textile clothing. It creates strain, bad odor, discoloration, and deterioration of textile clothing. The antimicrobial agent is a potential factor, to control, eradicate or repress the development of microorganisms and their negative effects of odor, staining and deterioration.
Humans and other living beings retain their body function through a chemical reaction known as metabolism. The breakdown of food and its conversion into energy is specifically referred to as metabolism. In this chemical reaction, some molecules lose an electron; it becomes to be free radical. Free radical molecules possess an uneven number of electrons in their backbone which can initiate chain reactions in our bodies. These reactions are called oxidation. Free radicals exhibited electrically charged molecules due to their unstable form in atomic structure. As a result, free radical contained molecules in the living body cells attack the other molecules (like DNA, protein, lipid, and fatty tissue) that can cause significant damage to the living tissue and cell (Sharifi-Rad et. al., 2020; Boulebd et al., 2022). Consequently, a large number of diseases like diabetes, high blood pressure, cancer, and heart disease are caused by the chain reaction of free radicals (Milatovic et al., 2021). On the other hand, UV rays create free radicals in the body’s skin that are supposed to contribute to the development of various diseases, such as inflammatory disorders, skin cancer, allergies, and premature skin ageing (Sangamesha et al., 2022).
Oxidation also breaks down the polymer chain of textile dyes. As a result, dyed textile materials are going to fade very quickly. Oxidation also creates a problem in food processing companies. The degradation of processed food has taken place rapidly through the oxidation process. Recently natural antioxidant agents have gained considerable attention in the food industry since natural antioxidant agents are safe, non-toxic and environment-friendly (Gutiérrez-del-Río et al., 2021; Dutta and Sit, 2022).
Additionally, natural antioxidant agents are also used in the paint, plastic and textile industries. In addition, antioxidant agents are also used as food preservatives to enhance the shelf life of packaging food products (Spruit and Almenar 2021). The presence of the antioxidant agent reduces textile dye polymer degradation. As a result, it will be helpful to retain the colorfastness of dyed textile materials (Crews and Clark 1990). Finally, the antioxidant agent can be used in garment finishing (Mondal et al. 2021). ln that case, it will be very helpful to the wearer as it is expected that antioxidant-finished clothing minimizes or stabilize the free radicals of the outer side of the body. The principal function of antioxidant agents is to provide electrons to free radicals for neutralization. The role of antioxidants is shown in Fig. 1A.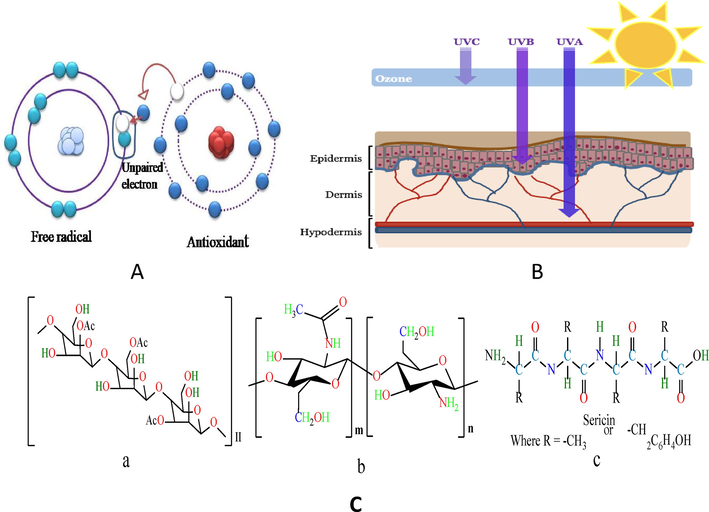
(A) Role of the antioxidant agent. (Source: https://www.stapleton-spence.com/nutrition/ antioxidants-and-free-radicals/), (B) Penetration intensity of UV rays into the layers of the skin. (Source: https://www.mondoscience.com/blog/2017/4/22/sunlight-and-your-skin) and (C) Chemical structure of (a) Aacemannan of Aloe vera, (b) Chitosan and (c) Silk sericin.
Ultraviolet light is electromagnetic radiation with a wavelength between visible light and X-rays which ranges from 40 nm to 400 nm and has energies from 3 eV to 124 eV. Ultraviolet light is further categorized into UV-A (320 to 400 nm), UV-B (290 to 320 nm), and UV-C (200 to 290 nm). UV-B rays exhibited shorter wavelengths compared to UV-A rays but are more dangerous than UV-A rays. Unlike the penetration intensity/ depth of UV-A rays are more than UV-B rays on the human skin. The penetration intensity of UV-A and UV-B in the human body is shown in Fig. 1B. Generally, the ozone layer absorbed the UV-C range light. UV rays come to earth easily at present, owing to the depletion of the ozone layer. The UV-A rays are associated with skin wrinkles and human ageing. And UV-B is mainly responsible for causing skin burning and dangerous skin cancer (Sarkar 2004, Griffon et al. 2011). UV-A rays are present all over the year and can penetrate through clouds and glass. The intensity of UV-B rays varies according to the season, location and time.
The Aloe vera gel almost contains 99% water and 1% solid material. Aloe vera gel consists of a range of compounds, namely saccharides (mannose, glucomannan, acemannan.), vitamins (B1, B2), phenolics (anthraquinones, flavonoids), enzymes (amylase, carboxypeptidase), low molecular weight substances (cholesterol, salicylic acid) and so on. The acemannan is the key functional component of Aloe vera which exhibits a long-chain polymer consisting of randomly acetylated linear D-mannopyranosyl units (Sharma et al. 2022), as shown in Fig. 1Ca. It has antibacterial, antifungal, antioxidant, anti-inflammation, anti-cancer, anti-diabetic, anti-allergic, immuno-stimulation, and antitumor properties (Khavari et al., 2021; Ray et al., 2013b; Rodriguez et al., 2010). Several solvents are available to extract Aloe vera like water, ethanol, methanol, chloroform, petroleum ether etc (Añibarro-Ortega et al. 2021). Among the solvents, methanol extraction showed the best antibacterial activity (Das and Srivastav, 2015).
Chitosan is regarded to be the second most abundant biopolymer after cellulose. Chitosan is derived from chitin as a result of N-deacetylation and has a linear chain of β-(1, 4) linked 2-acetamino-2-deoxy-β-D-glucopyranose, and β-(1, 4) linked 2-amino-2-deoxy-β-Dglucopyranose. Its chemical structure (dos Santos et al. 2009) is shown in Fig. 1Cb. Chitin is present in shrimp shells waste which is available in Bangladesh. Originally, the crude shrimp's head and skin materials possessed low economic value and were treated as bio-waste or sold to animal feed manufacturers. As a result, large amounts of shrimp bio-waste are found in the shrimp processing zone, approximately 45–55% of the weight of raw shrimp. However, this bio-waste can be converted to value-added products such as chitosan (Islam and Bhuiyan 2016). Chitosan has obtained a great interest in various fields like food, chemistry, biotechnology, veterinary, cosmetic, medicine, dentistry, agriculture, environmental protection, textiles, paper making, and packaging due to its exclusive characteristics such as non-toxicity, biodegradability, biocompatibility, wound healing properties and hemostatic activity (Yen et al. 2009, De Holanda and Netto 2006, Seo et al. 2007, Morin–Crini 2019).
Silk sericin is a water-soluble globular protein that is another natural biopolymer, derived from the silkworm Bombyx mori. The chemical structure of silk sericin is shown in Fig. 1Cc. There are two types of protein found in Bombyx mori silk, namely silk sericin and silk fibroin. Silk sericin and silk fibroin contribute near about (20–30%) and (70–80%) in respect of the total weight of the silk cocoon (Ki et al. 2007, Poza et al. 2002, Wu et al. 2007, Kim 2007). In the silk industry, a large amount of silk sericin is wasted during the degumming process. When this effluent is discharged into rivers, lakes, ponds, and other bodies of water, it raises the BOD and COD levels. About 400,000 tons of dry cocoons are generated all over the world per year. No less than 50,000 tons (12.5%) of sericin are discarded into water. As a result, water gets contaminated in the presence of silk sericin (Vaithanomsat and Kitpreechavanich 2008). Many functional properties like moisture-absorbing, antitumor activities, antioxidant and antibacterial, properties are found in silk sericin. In textile industries, sericin is used to improve the functional properties of natural and artificial fiber. Silk sericin is also used in cosmetics industries, pharmaceuticals, and biomedical applications (El Shafay, 2022, Sasaki et al. 2000, Zhaorigetu et al. 2003, Gulrajani 2005).
Some researchers investigated the antibacterial property of Aloe vera (Agarry et al., 2005; Athiban et al., 2012; Danish et al., 2020; Das and Srivastav, 2015; Khavari et al., 2021; Lawrence et al., 2009; Ray et al., 2013b; Rodriguez et al., 2010) and chitosan (Islam et al. 2011, Gerasimenko et al. 2004, Kim et al. 2003, Chien and Chou 2006; Ke 2021, Park et al. 2004). Where few articles were found on the antimicrobial and antioxidant activity of sericin (Saha et al. 2019, Jassim and Al-saree 2010). But the comparative study of functionalities of Aloe vera, chitosan, and silk sericin was not found in the literature, especially the UV resistance activity. Among these raw materials, chitosan and silk sericin were derived from waste resources and another one was aloe vera, a very affordable and readily available substance. In addition, these three materials are also non-toxic, safe for biological use, and environmentally friendly. This study aims to investigate the structural aspects as well as functional qualities in terms of antibacterial, antioxidant and UV resistance of these three biomaterials. This comparative study will help to decide which biopolymer is most suited for a specific application, as these materials are widely used in textiles, pharmaceuticals, food, medical, and health care sectors. Furthermore, all the functional properties are not found in a single biomaterial at desired level but it is possible to develop required properties by blending two or more materials together. For example, one agent has good antibacterial and poor UV resistance characteristics while the other has vice versa i.e. poor antimicrobial and excellent UV resistance properties. When these two biomaterials blended together, they may be able to achieve their maximum potential. Thus this study will also help to formulate various blended products with desired functionalities.
2 Experimental
2.1 Materials
Shrimp shell debris was collected from the Khulna shrimp processing area for chitosan manufacturing. Aloe vera leaves were collected from Rajshahi's local market. Silk cocoons of Bombyx mori were gathered from the Bangladesh Sericulture Research Training Institute (BSRTI) in Rajshahi. All essential chemicals, such as methanol, sodium hydroxide, hydrochloric acid, sodium hypochlorite, acetic acid, potassium bromide (KBr), nutrient agar, nutrient broth and 2.2-diphenyl-1-picryl-hydrazyl (DPPH) etc were obtained from BDH, Sigma-Aldrich, and Merck and used without further purification.
2.2 Extraction of Aloe vera
Aloe vera leaves were cleaned, with distilled water. Next, the Aloe vera gel was separated from the leaves and dried in an oven at 60˚C. The dried Aloe vera gel was ground by ceramic mortar and pestle. After that, the powder was soaked in methanol for seven days. During this duration, the bottles were enveloped with aluminium foil and put in a dark place. Following the one week, the solutions were filtered with the use of Whatman filter paper. Then a rotary evaporator was used to evaporate the methanol (Ghayempour et al. 2016).
2.3 Preparation of chitosan
At first, the shrimp shells were washed with tap water followed by hot distilled water and then dried at 105˚C for 2 h using an air-circulating oven dryer. Using a crushing machine, the dried shrimp shells were broken down into fine particles. Then deproteinization, demineralization and decolorization processes were done step by step using 1 M NaOH, 1 M HCl and 0.3% sodium hypochlorite bleaching agent to produce water-insoluble chitin. At last, the water-insoluble chitin was transferred into chitosan through deacetylation. Deacetylation was accomplished by heating with high concentrated sodium hydroxide solution (Alam et al. 2008).
2.4 Silk sericin extraction
According to Khalifa et al., extraction of silk sericin can be executed by a method subject to some modification (Khalifaa et al. 2012). Initially, 50 gm silk cocoons were cut into small pieces. The chopped segments of the cocoon were then immersed in deionized water before being autoclaved at 120 °C for 20 min at 0.1 MP pressure. After completing the autoclave, the silk fibroin was isolated from the solution by using a filter net. A motor-driven Bruckner breaker filtering method was used to collect sericin from the solution. Eventually, sericin is dried at 105 °C in an oven.
2.5 Estimation of solubility
Solubility is one of the most important parameters of the antibacterial agent as the add-on percentage on fabric depends on the solubility of raw material. Solubility of raw material is measured by either visual inspection or filtration or instrumental analysis. The solubility of Aloe vera and sericin was evaluated by visual inspection. In a 1 percent acetic acid solution, chitosan powder was dissolved. The following equation was used to determine the solubility of the chitosan solution (Rastogi et al. 2001).
W1 is the initial chitosan weight and W2 is the residue chitosan weight after filtration.
2.6 Determination of degree of deacetylation
A potentiometric titration procedure was used to assess the degree of deacetylation (DD) of chitosan. After dissolving 0.2 g of chitosan in 10 ml of 0.30 M HCl, it was diluted to 50 ml with ultrapure water and titrated with 0.10 M NaOH. The discrepancy between two inflection points is used to calculate the absorbed volume of NaOH solution, which refers to the number of amine groups in chitosan (Tolaimate et al. 2000). The degree of deacetylation was determined using the formulae below.
Where, m is the weight of the sample, V1 and V2 are the volumes of sodium hydroxide solution corresponding to the deflection points, 2.03 is the coefficient resulting from the molecular weight of the chitin monomer unit, 0.0042 is the coefficient resulting from the difference between molecular weights of chitin and chitosan monomer units.
2.7 Fourier transform infrared spectrophotometer (FTIR)
The dried sample was mixed with potassium bromide (KBr) using a mortar and pestle to make powder in the mass ratio of 1:100 (1 mg specimen and 100 mg KBr). A sample disc was prepared from the powder (Bagheri-Khoulenjani et al. 2009). The spectra of the sample were recorded using an FTIR spectrophotometer (Spectrum-100, Perkin Elmer, USA) at a resolution of 4 cm−1 in the scanning range of 4000–400 cm−1.
2.8 UV absorption measurement
Each Aloe vera extract and sericin (5 mg/ml) was dissolved in distilled water and made into five times dilute solution. Besides, chitosan was dissolved in a 1% acetic acid solution. The UV absorption spectra of the sample solution were recorded from 200 to 400 nm using UV–vis Spectrophotometer of the Shimadzu 1650 model (Japan).
2.9 X-Ray diffraction (XRD) analysis
The crystallite diameter, crystalline percentage and plane spacing of Aloe vera, chitosan and silk were evaluated from X-ray diffraction pattern and its XRD patterns were determined using dry powder samples on PAN Analytical X Pert PRO X-ray diffractometer (New Zealand) employing Cu-Kα radiation with wavelength 1.5406 Å as the X-ray source. The sample holder was placed on the X-ray goniometer and 2 scanning was done at the rate of 4°/min over a range of 5⁰–60°. The operating voltage and current were used at 40 kV and 30 mA, respectively at room temperature.
The ‘d’ spacing was evaluated according to Bragg’s law:
Where 0.15406 nm is the X-ray wavelength and ‘d’ is the spacing between two planes.
The crystal diameter of the specimen was determined by the following Scherrer formula (Wang et al. 2013).
Where K (=0.9) is the Scherrer constant, is the Braggs diffraction angle, and β is the full width of the peak in the X-ray pattern line at half peak height in radians.
The crystalline percentage (Cr %) of Aloe vera, chitosan and silk sericin samples was calculated from the XRD pattern, using the following equation (Sagheer et al., 2009)
Where, Icr is the maximum intensity of the crystalline region of the material, and Iam is the amorphous diffraction at 16° of the XRD pattern.
2.10 Thermal analysis
A simultaneous TGA, DSC thermal analyzer (STA 449 F3 Jupiter, Germany) carried out a thermal analysis of Aloe vera, chitosan, and sericin samples in powder form. For thermal analysis, 10 °C/min of heating rate and a temperature range from 30 to 600 °C have been employed under a constant flow of nitrogen atmosphere.
2.11 Scanning electron microscopy (SEM)
A scanning electron microscope was used to study the surface morphology of the Aloe vera, chitosan, and sericin powder. SEM (Model- Phenom G2 pro, Netherlands) was performed to investigate the surface morphology at 3000 magnification.
2.12 Energy-dispersive X-ray spectroscopy
The energy-dispersive X-ray (EDX) represents the different elements composing the materials of the sample. The contents of carbon (C), nitrogen (N), and oxygen (O) of the treated cotton were measured by ED (JEOL-6300F, Germany).
2.13 Antibacterial activity test by qualitative method
The disc diffusion process is involved in testing a zone of inhibition of a sample solution (AATCC Test Method: TM 147–2004, 2010). 250 ml of nutrient agar medium and paper discs were arranged and autoclaved. Subsequently, the nutrient agar was poured into a sterile petri dish and placed in the laminar airflow. 20 µl of S. aureus and E. coli bacterial solution was spread over the nutrient agar of the petri dish. Paper discs of 1 mm diameter were immersed in a 10 g/L sample solution and placed in the petri dish. The petri dish was incubated at 37 °C for 24 h to form a zone of inhibition.
2.14 Determination of bacterial growth by optical density
Bacterial growth was determined according to Ai et al. (2019) method. The bacterial growth was determined by optical density (OD) using a UV spectrophotometer (T60 UV–Visible spectrophotometer, UK) at 600 nm. At first, the bacterial solution was diluted to an absorbance of 0.02 solution and then added to each Aloe vera, chitosan, and sericin solution. The solution was cultured at 37 °C for 2, 4, 6, 8 and 10 h, and then absorbance of the sample corresponding sample was taken. For each experiment, the assay was carried out in triplicate. The transmitted light decreases as the bacterial cell population increases, while the absorbance rises. The bacterial reduction percent was measured using the equation below.
Where, A is the absorbance of the control sample solution and B is the absorbance of mixed with the sample solution.
2.15 Antioxidant activity measurement
The 2.2-diphenyl-1-picryl-hydrazyl (DPPH) reagent was used to test antioxidant activity. DPPH is a stable free radical which a characteristic absorption peak is shown at 517 nm. A system defined by Wu et al. (2007) was used to investigate the free radical scavenging effects of Aloe vera, chitosan, and sericin with slight modification. The sample solution was formulated in five separate amounts of 2, 4, 6, 8, and 10 mg/ml. In methanol, 0.004% DPPH solution was prepared, and 3 ml of it was combined with 1 ml of sample solution and vortexed. The mixture solution was kept in the dark for 25 min at room temperature (25⁰C) to complete the reaction. However, the reaction mixtures were centrifuged at 6000 rpm for 2 min. The absorbance of the decolorized solution and control sample was then measured at 517 nm against a blank sample using a UV–visible spectrophotometer. The following equation was used to calculate the free radical scavenging operation.
Where control absorbance means methanol solution of DPPH and sample absorbance means decolorized solution. In case of measurement of sample absorbance, the blank sample is prepared by 3 ml pure methanol, without DPPH, mixed with 1 ml Aloe vera or chitosan or sericin sample solution for each respective concentration.
2.16 Measurement of UV protection factor (UPF)
A film of the sample was prepared to measure the UPF. 5 (five) and 10 (ten) mg/ml concentration of sample solution was spread on a 10 mm length and 1 mm width glass slide to prepare a film. The prepared film was dried at room temperature. The transmittance value of the films was taken with the help of a UV spectrophotometer. The UPF of the film was calculated according to the AATCC test method (AATCC Test Method: TM 183–2004) by the following equation:
3 Results and discussion
Aloe vera, chitosan, and silk sericin are biopolymers with enormous applications in medical, pharmaceutical, healthcare, food, textiles, etc. Different structural and functional features of Aloe vera, chitosan, and sericin were explored and discussed their functional and structural properties.
3.1 Solubility
Generally, Aloe vera extract is partially soluble in deionized water. When Aloe vera extraction is done in presence of methanol, then methanolic Aloe vera extract is easily soluble in deionized water. The appearance of Aloe vera solution is like as brown-yellowish.
Chitosan solubility is determined by the degree of deacetylation. Peter (1995) discovered that chitin with a deacetylation level of more than 50% can be defined as chitosan, which is soluble in 1% acetic acid. A higher degree of deacetylation of chitosan ensures higher solubility as the reactive site expands when the degree of deacetylation increases. The dominating reactive groups of chitosan are considered as - NH2 and –OH. The solubility % was measured according to Eqn. no. 1. The solubility of chitosan was 88% where the initial chitosan weight was 0.25 g and after filtration, the chitosan weight was 0.03 g. Since chitosan has a higher proportion of protonated free amino groups compared to chitin, which attracts ionic compounds and causes it to dissolve easily in acetic acid. Apart from that, chitosan with insufficient protonated amino groups due to a lack of deacetylation degree (DDA) was poorly soluble in an acidic medium. In cold water, sericin is insoluble, but in hot water, it dissolves. Gulrajani (1988) found a similar result. It is assumed that long protein molecules of sericin are broken down into smaller fractions that can be dispersed or dissolved in hot water.
3.2 Degree of deacetylation
On the basis of potentiometric titration of chitosan solution, the graph with the variation of pH versus the added volume of the base has two inflection points is obtained in Fig. 2. The first and second inflection points are found after adding 7 ml (V1) and 18 ml (V2) NaOH solution respectively. The first inflection point corresponds to the neutralization of HCl, and the second inflection point to the neutralization of the ammonium ions from chitosan. The difference between the volumes V2 and V1 corresponds to the acid consumed by the amine groups. And finally, the degree of deacetylation was found 90% according to Eqn. no. 2.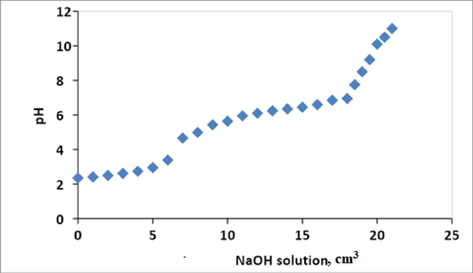
pH versus volume of NaOH solution.
3.3 FTIR of Aloe vera, chitosan and sericin
The FTIR spectra of Aloe vera are shown in Fig. 3(Aa). The peaks of 2922 cm−1 and 1401 cm−1 correspond to - CH2 and COO − groups, respectively. The prominent broad peak around 3265 cm−1 was assigned to phenolic –OH stretching, associated with the presence of Aloe species phenolics e.g., flavonoids, and anthraquinones. Another moderate-intensity peak at 1602 cm−1 was assigned the C = O stretching vibration indicating the presence of carbonyl compounds. The broad FTIR spectra of chitosan are at around 3450 cm−1 indicating O–H and N–H stretching vibration in Fig. 3(Ab). The moderate peak at 2880 cm−1 and 1420 cm−1 represent the stretching and bending vibrations of C–H (CH3 and CH2) respectively. The most important peaks of chitosan are observed at 1649 cm−1, and 1592 cm−1 representing the C = O stretching vibration (amide l) of acetyl groups and N–H bending vibration (amide II) of amino groups, respectively. The peak at around 3500–3000 cm−1 (3287 cm−1) is indicated by the N–H stretching vibration of sericin shown in Fig. 3(Ac). The peak at 1659 cm−1 revealed the stretching vibration of C = O. The characteristics peak at 1512 cm−1 confirms the presence of N–H bending functional groups in sericin. The peak at 1401 cm−1 represents the CH3 bending and CH2 deformation. Thus the above-identified FTIR peaks indicate the characteristics structures of Aloe vera, chitosan and sericin(See Fig. 4).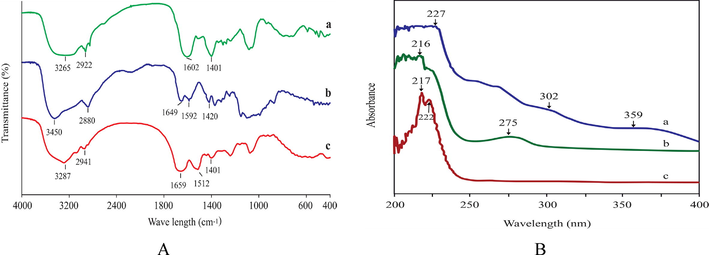
(A) FTIR spectra of (a) Aloe vera, (b) Chitosan and (c) Silk sericin and (B) UV spectra of (a) Aloe vera, (b) Silk sericin and (c) Chitosan solution.
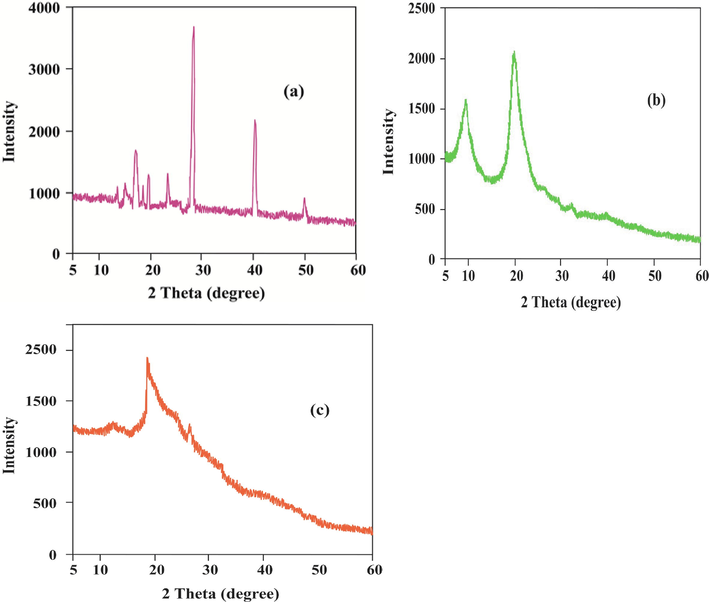
XRD Pattern of (a) Aloe vera, (b) Chitosan and (c) Silk sericin.
The presence of OH, COOH, and N–H, amide functional groups indicates that these biopolymers are hydrophilic. Chitosan and sericin revealed the N–H bending functional groups. It is assumed that the amino group is responsible for antibacterial, antioxidant, and UV-resistant properties.
3.4 UV absorption of specimens
Aloe vera extract solution showed UV absorption peaks at 227, 302, and 359 nm, as shown in Fig. 3(Ba). Ray et al., 2013b found UV spectra of Aloe vera absorption peak at 270 nm (UV-C region), 290 nm (UV-B region), and 350 nm (UV-A region). Ozsoy et al. (2008) identified the phenolic group of Aloe vera at the absorption band between 320 and 380 nm. The UV-B range radiation (315–280 nm) at prolonged exposure can cause various detrimental impacts on human skin, and immune systems and sometimes may cause skin cancer. Overexposure to UV radiation also amplifies the chance of malignant melanoma by DNA damaging activity. UV radiation is a prominent factor in 92 % of melanoma diseases (Davies et al. 2002).
The absorption peak of silk sericin solution shows at 216 and 275 nm of wavelength and is shown in Fig. 3(Bb). Silk sericin is mainly made of protein, and its amount is almost 90% of its composition, which exhibits 18 kinds of amino acids. Sericin contains approximately 32% serine and other amino acids like aspartic acid, threonine, glycine etc., which are attributed to sericin's physicochemical and functional properties. About 70% of hydrophilic amino acids have existed in 18 kinds of amino acids responsible for the good solubility and water absorbability of sericin (Zhu et al., 2022). On the contrary, only 6.6% of aromatic amino acids absorb harmful UV irradiation among the 18 kinds of amino acids (Wu et al. 2007). Aromatic amino acids like tryptophan, tyrosine, and phenylalanine absorb UV radiation in the range of 260–290 nm (Gupta et al. 2014).
UV absorption of chitosan solution is observed at 217 and 222 nm wavelengths in Fig. 3(Bc). It is assumed that absorption around this region is due to the presence of amide linkages in chitosan. Chitosan exhibits two chromophoric groups N-acetylglucosamine and glucosamine, which absorb UV range light (Tyagi et al. 1996). Generally, solar UV radiation is classified as UV-A (320 to 400 nm), UV-B (290 to 320 nm), and UV-C (200 to 290 nm). As stated above, chitosan absorbs UV-C region radiation. Usually, the ozone layer absorbs UV-C region rays. Increased environmental pollution, especially CO2 emission, has caused a depletion of the ozone layer. As a result, UV-C region rays come to earth easily, and are absorbed by chitosan. Aloe vera solution absorbed three regions of UV radiation. On the other hand, silk sericin and chitosan solution absorbed the UV-C region radiation only. Therefore, it can be said that Aloe vera is very much effective in resisting all regions of UV radiation.
3.5 XRD analysis
The ‘d’ spacing and crystal diameters of Aloe vera, chitosan, and sericin determined from XRD analysis are listed in Table 1. The ‘d’ spacing of Aloe vera, chitosan, and sericin was 0.43, 0.31, and 0.47 nm, respectively. The peak intensity of Aloe vera, chitosan, and silk sericin was 3382, 2142, and 1936, respectively. The highest sharp peak intensity of the Aloe vera sample indicates the highest crystallinity (Table 1). According to Scherrer (Eqn. no. 4) formula, the full length at half maximum (FWHM) value is inversely proportional to the crystal diameter. Full length at half maximum value decreases crystal diameter of specimen increases. The crystal diameter of Aloe vera is the highest compared to other specimens because the full length at half the maximum value of Aloe vera is the minimum (Table 1). The crystal diameter of chitosan and silk sericin was 0.31 and0.47 nm. The crystal diameter of chitosan and silk sericin is much lower than that of Aloe vera. These results indicate that chitosan and sericin are more amorphous. The maximum intensity of chitosan was 2142counts at 2θ = 20.07°and the amorphous intensity was 751 counts at 2θ = 16°. The 60.53% of crystallinity index of chitosan was found according to the formulae number 5 (five). Similar way crystallinity index of silk sericin and aloe vera were calculated. The crystallinity index of aloe vera and silk sericin was 83.44 and 39.66%. # Full width of the peak at half maximum (FWHM).
Observation
2 theta value (°)
Peak intensity at crystalline region
Amorphous diffraction at 16°
FWHM (
in (°)
Crysta llinity (%)
Crystal Diameter (D) in nm
d spacing in nm
Aloe vera
28.33
3382
560
0.162
83.44
50.36
0.43
Chitosan
20.07
2142
751
2.861
60.53
3.38
0.31
Silk sericin
18.86
1936
1168
3.703
39.66
2.17
0.47
3.6 Thermal analysis
From Fig. 5(A-C) it can be observed that the mass loss of Aloe vera, chitosan, and sericin is 11%, 7.5%, and 6% respectively at 100 °C due to loss of moisture, low molecular weight solvent, and gas. The weight loss at 200 °C was 17%, 9%, and 14% for Aloe vera, chitosan, and sericin, respectively. The 28%, 38%, and 30% weight losses occurred at 300° C due to the decomposition of the specimen. At 400 °C, there was a significant weight loss of 39%, 51%, and 52%. 17%, 19%, and 11% weight loss between 400° C and 500° C. Eventually, 45%, 15%, and 34% ash were found for Aloe vera, chitosan, and sericin specimens, respectively. In the case of sericin, a similar amount of ash was determined by Chollakup et al. (2015). The decomposition temperature of all the samples which were determined by DTG thermograph. The decomposition temperature of Aloe vera, chitosan, and sericin was 430, 295, and 300° C, respectively. As a result, Aloe vera has the highest thermal stability, whereas sericin comes in second. In the case of silk sericin, Tsukada et al. (Tsukada 1998) observed maximum decomposition temperatures were 320 °C.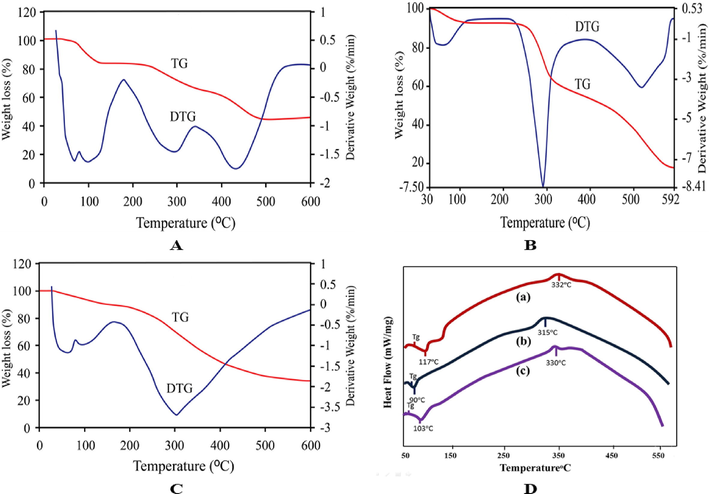
TG and DTG curves of (A) Aloe vera, (B) Chitosan and (C) Silk sericin, and (D) DSC curves of (a) Aloe vera, (b) Chitosan and (c) Silk sericin.
Differential scanning calorimetry has been used to measure the glass transition temperature (Tg) and thermal stability of extracted biopolymers samples. Normally these biopolymer samples contain moisture and bound water, which is not completely removed during drying. This bound water affects many properties of polymers, such as rheological, transport properties, and glass transition temperature. In presence of different amounts of moisture, the material gives an endothermic peak at different positions. Dry and wet samples of the same material will give different Tg as water is present in different forms in the macromolecules of biopolymer (Schubnell and Schawe 2011). Normally, the glass transition temperature is identified at the initial change in the slope of the heat capacity of the DSC curve.
The DSC thermograph of Aloe vera in Fig. 5Da showed that one endothermic peak at 117 °C is due to removing the absorbed water, and one exothermic peak at 332 °C is associated with the degradation of the material. In Fig. 5Da, the Tg value of Aloe vera was found nearly at 80 °C. Chakraborty et al. found a sharp endothermic peak obtained at 120 °C, and an exothermic peak was at 337 °C, which is associated with the breakage of the saccharide ring and the bond rupture of linked groups of Aloe vera extract (Chakraborty et al. 2019, Nindo et al. 2010). Nindo et al. (2010) found that the Tg of Aloe vera was nearly 65 °C temperature.
Chitosan showed an endothermic peak at 90 °C and a glass transition temperature at 81 °C. The other characteristic exothermic peak was revealed at 315 °C in Fig. 5Db. The endothermic peak is also called dehydration temperature, assigned to the loss of water associated with the hydrophilic groups of chitosan (Cheung et al. 2002; Kittur et al. 2002). Chitosan is a polysaccharide-based biopolymer and has a good affinity to water. It is because chitosan can be easily hydrated (Cardenas and Miranda 2004). The exothermic peak identified the degradation of acetyl and deacetylated units, and glycoside bond cleavage of chitosan (Sreenivasan 1996, Deng et al. 2007). Dey et al. (2016) found an endothermic peak almost at 80 °C and an exothermic peak at 304 °C from the DSC thermograph of chitosan.
The silk sericin showed one endothermic peak at 103 °C, one exothermic peak at 330 °C, and a Tg value at 70 °C in Fig. 5Dc. Dutta et al. (2012) found two endothermic peaks of silk sericin at 65.5° C and the 363° C temperature. Two endothermic peaks of silk sericin in the range of 210––220° C and 310–320° C, correspond to the thermally induced molecular motion of an amorphous region and thermal decomposition of silk sericin, respectively observed by Tao et al. (2016) and Oh et al. 2011).
3.7 SEM analysis
The morphology of chitosan powder showed a uniform, smooth and non-aggregated arrangement of its particles, as shown in Fig. 6a. Additionally, the surface morphology of chitosan possesses a tiny, porous structure. The Aloe vara sample exhibits an uneven surface morphology and roughness with high compactness, as shown in Fig. 6b. On the other hand, silk sericin exhibits a more sponge-like appearance (Fig. 6c). More cracked and big hole structure is visible in Aloe vera extract powder compared to silk sericin powder(See Fig. 7).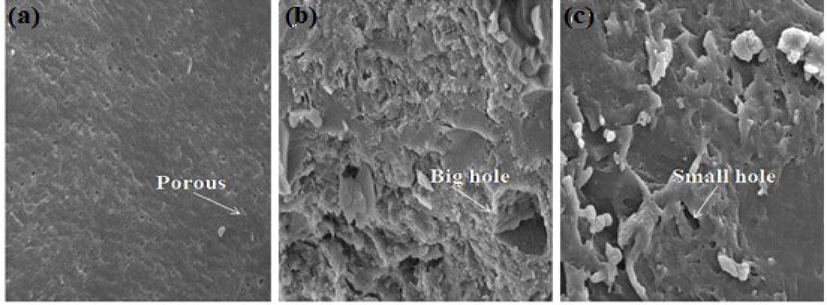
SEM image of (a) Chitosan, (b) Aloe vera and (c) Silk sericin at 3000 magnification.
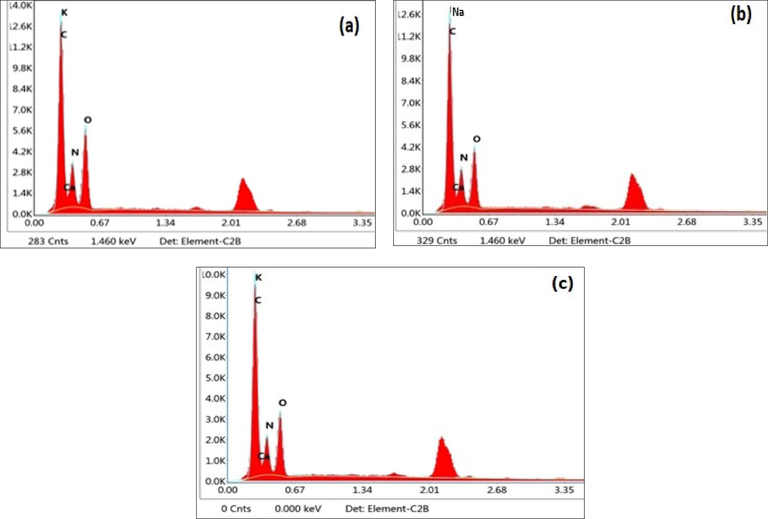
Energy-dispersive X-ray spectroscopy (EDX) of (a) Aloe vera (b) Chitosan (c) Sericin.
3.8 Energy-dispersive X-ray spectroscopy (EDX)
Atomic % is the % as a function of the number of atoms, whereas Wt % is the % as a function of weight. Wt. % gives the concentration of the element in terms of the mass fraction of that element in the sample. The percentage of elements of Aloe vera, chitosan and sericin determined by Energy Dispersive X-ray spectroscopy (EDX) is listed in Table 2. The energy-dispersive X-ray spectroscopy (EDX) spectra of the Aloe vera, chitosan, and sericin. The percentage of the elements is presented in Table 2. The main elements of the three natural biopolymer samples are C, O, and N. The Aloe vera powder exhibits a high amount of carbon (42.58%). The amount of N elements was 25.70, 28.19, and 25.06 for Aloe vera, chitosan, and sericin powder, respectively. In the case of the N element, Aloe vera and sericin powder presented similar weights and atomic percentages.
Elements
Wt.% of Aloe vera
Atomic % of Aloe vera
Wt.% of Chitosan
Atomic % of chitosan
Wt.% of sericin
Atomic % of sericin
C K
42.58
48.90
40.72
46.24
39.09
45.22
N K
25.70
25.31
28.19
27.44
25.06
24.85
O K
28.69
24.74
30.50
25.98
33.52
29.11
Na K
0.00
0.00
0.54
0.32
0.00
00
K K
0.86
0.30
0.00
0.00
0.99
0.35
Ca K
2.17
0.75
0.05
0.017
1.34
0.46
Total
100
100
100
100
100
100
On the other hand, chitosan contains a maximum N element, and sericin contains the highest wt.% regarding the O element. Minor elements like Na, K and Ca were observed in Aloe vera, chitosan and sericin. Chitosan only exhibited 0.54% Na elements among the samples. Aloe vera and sericin powder exhibited K (0.86%), K (0.99%), and Ca (2.17%), Ca (1.34%), respectively. The amount of the mentioned elements represents their structural and functional characteristics.
3.9 Antimicrobial activity evaluated by qualitative method
The zone of inhibition (ZOI) of Aloe vera, chitosan, and silk sericin was evaluated for 10 g/L concentration with E. coli gram-negative and S. aureus gram-positive bacteria. E. coli is shaped like a bacillus and lives in the intestines of humans. It can spread during the handling and eating of raw food. The symptoms of E. coli are severe diarrhea, especially in children and kidney damage. S. aureus is one type of harmful bacteria that may be responsible for 19% of total surgical infections in the hospital. This organism can cause skin infections, such as pimples, impetigo, boils, cellulitis folliculitis, carbuncles, scalded skin syndrome, life-threatening diseases such as pneumonia, osteomyelitis, endocarditis, toxic shock syndrome (TSS), and meningitis (Barrett et al. 1993, Boyce 1990, Hartstein et al. 1995, Panlilio et al. 1992).
Aloe vera, chitosan, and silk sericin showed the zone of inhibition in Fig. 8 against E. coli gram-negative and S. aureus gram-positive bacteria. The zone of inhibition of Aloe vera, chitosan, and silk sericin was 2.5, 3, and 1 mm respectively for E. coli and that of Aloe vera, chitosan, and silk sericin was 4, 5, and 1.5 mm for S. aureus (Fig. 8). The zone of inhibition of chitosan was greater than that of Aloe vera and silk sericin solution. On the other hand, the antimicrobial activity of Aloe vera was better than that of silk sericin solution. The zone of inhibition of Aloe vera, chitosan, and silk sericin was greater for S. aureus than for E. coli. The reasons are, that gram-negative bacteria are harder than Gram-positive bacteria because of the extra outer membrane of gram-negative bacteria. In addition, gram-negative bacteria contain 10% peptidoglycan in their cell wall and high lipid content in the outer membrane. On the other hand, Gram-positive bacteria contain 90% peptidoglycan in their cell wall and have low lipid content (Krasner 2010, Kumari 2012).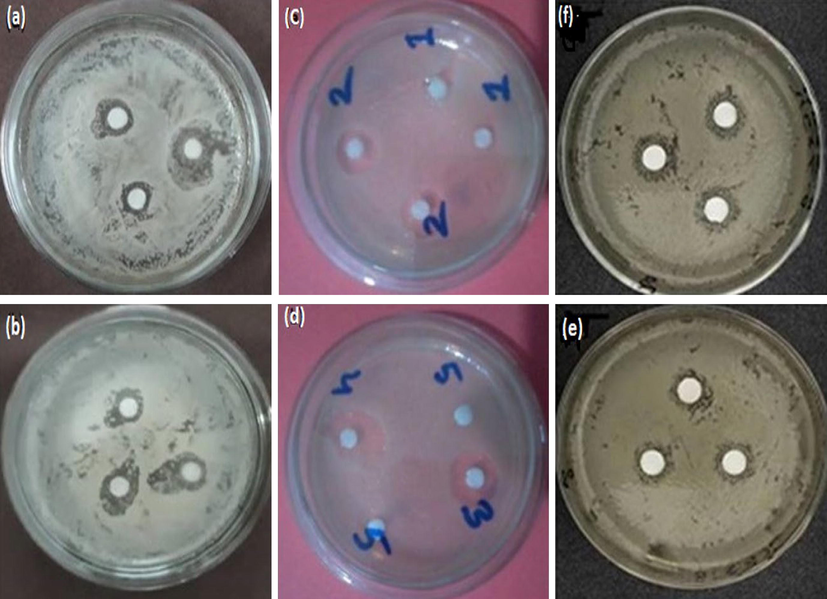
Zone of inhibition of 10 g/L Aloe vera solution against (a) E. coli and (b) S. aureus; 10 g/L chitosan solution against (c) E. coli and (d) S. aureus; and 10 g/L sericin solution against (e) E. coli and (f) S. aureus.
Aloe vera holds different ingredients like polysaccharide acemannan, anthraquinones, and tannins which have antibacterial properties. It is assumed that these elements can inactivate enzymes, denature proteins and disrupt the membrane to limit the growth of microbes, by disabling cell functioning or reproduction (de Rodrı́guez 2005).
Many hypotheses have been explained on the mechanism of the antibacterial activity of chitosan. Three reactive functional groups are exhibited in chitosan (amino/acetamide group at the C2 position of each deacetylated unit and both primary and secondary hydroxyl groups at the C6 and C3 positions).
The interaction between the positively charged amino group (–NH 3+) of chitosan and the negatively charged carboxylate group (–COO-) found on bacterial cell surfaces gave antimicrobial characteristics to chitosan. As a result, cell surface alteration and altered cell permeability are happened by the above electrochemical binding leading to disruption of the cell membrane of bacteria followed by leakage of intracellular substances, such as electrolytes, proteins, amino acids, glucose, UV absorbing materials, and lactate dehydrogenase. In this way, chitosan shows its bacteriostatic and bactericidal effects (Lim and Hudson 2004, Helander et al., 2001).
Senakoon et al. (2009) studied the mode of antimicrobial action of Eri silk sericin through scanning electron microscopy against S. aureus and E. coli bacteria. They observed that silk sericin inhibits cell division and cell growth in bacteria media, which is expected to cause the early declining phase, together with the induction of membrane dysfunction. Moreover, the observation of scanning electron microscopy also illustrated that silk sericin causes cell deformation, cells with reduced sizes, and cell shrinkage of bacteria. In this way metabolism of bacteria is brought to a close.
3.10 Antimicrobial activity evaluated by optical density
The absorbance of the sample is directly proportional to the concentration of the bacterial cells in the sample. The absorbance values indicate the optical density (OD) of the bacterial cells. The optical density of bacterial control solution and control solution plus Aloe vera, chitosan, and silk sericin solution was determined by UV-spectrophotometer.
The results showed no significant difference in the bacterial growth between the Aloe vera and chitosan (Fig. 9). On the other hand, bacterial growth in the presence of sericin was higher compared to Aloe vera and chitosan (Fig. 9). At the end of 10 h’s incubation, the peak or highest growth for S. aureus (control) and the absorbance was 1.675. In contrast, the lowest growth was found in chitosan containing S. aureus medium, and its absorbance was 0.745, and the absorbance of chitosan containing E. coli medium was 0.872. The bacteriostatic growth curves indicate that chitosan has the highest antimicrobial effects on both E. coli and S. aureus. Lower optical density means higher antimicrobial activity.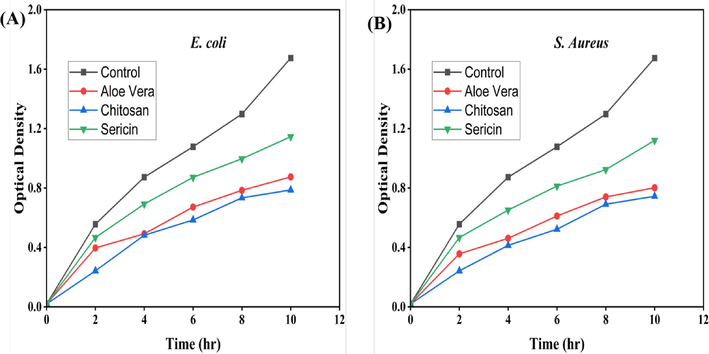
The growth curves of bacterial solution in the presence of 1% Aloe vera, 1% chitosan and 1% sericin solution: (A) E. coli and (B) S. aureus.
3.11 Antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), a stable free radical, is used for antioxidant assessment. The DPPH radical contains an odd electron, responsible for the maximum absorbance at 517 nm and shows a visible deep purple color. It reacts with suitable reducing agents, to form the hydrogen bond. When free radicals accept an electron donated by an antioxidant compound, the DPPH is decolorized, and the absorbance is reduced (Sultana et al. 2009, Cuendet et al. 1997, Burits and Bucar 2000, Amarowicz et al. 2004.
The action mechanism of antioxidant agents against DPPH free radicals is shown in Fig. 10.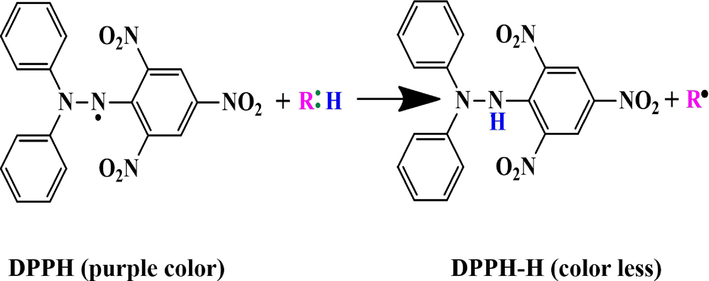
The action mechanism of the antioxidant agent on 2, 2 di-phenyl 1-picryl hydrazyl (DPPH) free radicals (R: H represents antioxidant) (Liang and Kitts 2014).
The free radical scavenging activity of Aloe vera, chitosan, and sericin solutions is shown in Table 3. The result shows that the percentage of radical scavenging activity increased with the increasing concentration of Aloe vera, chitosan, and sericin solution. The UV absorption decreased when Aloe vera, chitosan, and sericin concentration increased, indicating the increase of free radical scavenging activity. On the other hand, absorption decreased gradually with increasing concentration of specimen solution as the specimen donate protons to this free radical. Aloe vera extract solution showed the highest scavenging percentage compared to other solutions. Ray et al., (2013b) observed that the phenolics present in Aloe could scavenge DPPH free radicals, and phenolics are the major contributor to the antioxidant activity in Aloe gel. Phenol compounds can trap free radicals directly or scavenge them through a series of reactions with antioxidant enzymes (Reynolds and Dweck 1999). The abundance of the integral phenolic OH in Aloe vera extract contributes to its antioxidant activity (Ray et al., 2013a).
Concentration of specimen
RSA % of Aloe vera
RSA % of Chitosan
RSA % of Sericin
2 mg/ml
65
40
35
4 mg/ml
76
44
39
6 mg/ml
85
47
43
8 mg/ml
90
49
47
10 mg/ml
93
53
52
Table 3 also revealed that the free radical scavenging activity of chitosan and sericin is almost the same. The free radical scavenging activity of 8 mg/ml sericin was 47%. This study isolated silk sericin from Bombyx Mori silk cocoons by autoclaving for only 20 min at 120 °C. Fan et al. (2009) extracted silk sericin from bombyx Mori silk cocoon by a heating process at 95℃ for 120 min. They found that the DPPH scavenging rate of 8 mg/ml of silk sericin solution was nearly 75% higher than the present study's result. It is assumed that extraction time is the main responsible factor for DPPH scavenging activity.
10 mg/ml of chitosan solution showed 53% free radical scavenging activity (Kanatt et al. 2008). Chitosan has poor antioxidant activity due to the very low scavenging percentage obtained by the DPPH assay. It is assumed that the residual free –NH2 group of chitosan is responsible for antioxidant activity. The DPPH radicals can react with the residual free –NH2 group of chitosan to form stable molecules. The –NH2 groups can form ammonium groups (NH3+) by capturing a hydronium ion from the solution (Yen et al., 2008).
The antioxidant activity of the substance can be expressed as its ability to scavenge the DPPH radicals. Therefore, it is not surprising to conclude that Aloe vera, chitosan, and sericin solutions possibly contained electron donors, which reacted with free radicals to convert them to more stable products and terminate the radical chain reaction. These results indicated that Aloe vera, chitosan, and sericin solutions were natural antioxidants with potent anti-oxidative activity. Several oxidative stress-related diseases have been happened by reactive oxygen species. It is hoped that these natural antioxidants will be helpful to prevent oxidative stress like cardiovascular diseases, rheumatoid arthritis, ulcerative colitis, and neurological degenerative diseases. These natural antioxidant agents can be used in food supplements, cosmetics, biomedical applications, pharmaceuticals, and medical industries.
3.12 UV protection factor
From Table 4, it can see that the UV protection factor of Aloe vera extract film was the best compared to other materials. On the other hand, the UV protection factor of silk sericin producing the film is second. UV protection factor increased with the increase of concentration for all samples. The UPF rating of 10 mg/ml concentrated Aloe vera, chitosan, and silk sericin film were 10.4, 4.46, and 7.4, respectively.
Observation
UV Protection Factor (UPF) rating
5 mg/ml Aloe vera
8.23
10 mg/ml Aloevera
10.4
5 mg/ml Chitosan
3.64
10 mg/ml Chitosan
4.46
5 mg/ml sericin
6.3
10 mg/ml sericin
7.4
The UPF rating of Aloe vera was twofold higher than chitosan. UPF rating order was Aloe vera Chitosan had the lowest UPF values among the three samples. Equation number (7) revealed that the UPF rating is inversely proportional to the transmittance value of the specimens. So it can say that the transmittance value of the chitosan-producing film was the highest. One of the desired characteristics properties of packaging materials is protecting the foods from harmful UV rays as UV rays are responsible for lipid oxidation. UV light in the range of 200 to 280 nm is the common initiator of oxidation in the food system (Garavander al., 2017). These oxidations are mainly responsible for creating undesirable flavors and appearances in food. Hence chitosan, Aloe vera, and sericin films absorb UV range light, so these films will be used as UV light barriers. As a result, these films will be helpful to reduce the oxidation rate and increase the shelf life of the food product. Among the films, Aloe vera-based film absorbs the most UV rays. Consumers appreciate transparent packaging because it allows them to see the product's contents and assess its visual quality. Chitosan film had the finest transparency of all the films. As a result, UV absorbers Aloe vera, chitosan, and sericin could be used as food preservatives.
4 Conclusion
Antioxidant, UV resistant and antibacterial characteristics have been demonstrated in Aloe vera, Chitosan, and silk sericin. Among these, chitosan showed the highest antibacterial activity against both gram-positive and gram-negative bacteria. The highest free radical scavenging activity was recorded for aloe vera whereas sericin exhibited the maximum UV resistance property. Due to having these sparking properties, these biomaterials can be used to develop various precious products like UV protective sunscreen creams and clothes, antimicrobial packaging film for food preservation and medical textiles such as surgical sutures, hand gloves, gauges, bandages, aprons, bedsheets, etc. Antioxidant properties have also been recorded in these biomaterials. These valuable agents might be used as a food preservatives and in the cosmetic and pharmaceutical sectors. It is hoped that these wastage and cheap biomaterials can be used to develop present-time congruent products for the healthcare sector and would be helpful for the economy. At the same time, these waste materials can also minimize environmental pollution.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AATCC Test Method: TM 147-2004, 2010. Determination of Zone of Inhibition by Qualitative Method. AATCC Technical Manual, 251-252.
- AATCC Test Method: TM 183-2004, 2010. Transmittance or blocking of erythemally weighted ultraviolet radiation through fabric. AATCC Technical Manual, Research Triangle Park, NC, USA, 85, 318-321.
- Comparative antimicrobial activities of Aloe vera gel and leaf. Afr. J. Biotechnol.. 2005;4(12):1413-1414.
- [Google Scholar]
- Rational design and fabrication of ZnO NPs functionalized sericin/PVA antimicrobial sponge. Int. J. Mol. Sci.. 2019;20(19):4796.
- [CrossRef] [Google Scholar]
- Study on the physico mechanical properties of photo-cured chitosan films with oligomerand acrylate monomer. J. Environ. Polym. Degrad.. 2008;16(3):213-219.
- [CrossRef] [Google Scholar]
- Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem.. 2004;84:551-562.
- [CrossRef] [Google Scholar]
- A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Security. 2017;6:51.
- [CrossRef] [Google Scholar]
- Extraction of Aloesin from Aloe vera rind using alternative green solvents: process optimization and biological activity assessment. Biology. 2021;10(10):951.
- [CrossRef] [Google Scholar]
- Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J. Conserv. Dentent.. 2012;15(3):246-248.
- [CrossRef] [Google Scholar]
- An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym.. 2009;78(4):773-778.
- [CrossRef] [Google Scholar]
- Methicillin resistant Staphylococcus aureus in three adjacent Health Districts of south-east England 1986–1991. J. Hosp. Infect.. 1993;24(4):313-325.
- [CrossRef] [Google Scholar]
- Challenges in anti-infective development in the era of bad bugs, no drugs: A regulatory perspective using the example of bloodstream infection as an indication. Clin. Infect. Dis.. 2010;50:S4-S9.
- [CrossRef] [Google Scholar]
- Assessment of the free radical scavenging potential of cannabidiol under physiological conditions: Theoretical and experimental investigations. J. Mol. Liq.. 2022;346:118277
- [CrossRef] [Google Scholar]
- Increasing prevalence of methicillin resistant Staphylococcus aureus in the United States. Infect. Control Hosp. Epidemiol.. 1990;11(12):639-642.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Nigella sativa essential oil. Phytother. Res.. 2000;14:323-328.
- [CrossRef] [Google Scholar]
- FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc.. 2004;49(4):291-295.
- [CrossRef] [Google Scholar]
- Development of antimicrobial and liquid/blood repellent surgical gown: Synthesis and characterization of chitosan-Aloe vera bionanocomposite. Int. J. Curr. Biotechnol.. 2019;7(1):1-12.
- [Google Scholar]
- Miscibility and morphology of chiral semicrystalline poly-(R)-(3-hydroxybutyrate)/chitosan and poly-(R)-(3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan blends studied with DSC, 1H T1 and T1ρ CRAMPS. J. Appl. Polym. Sci.. 2002;86(5):1253-1258.
- [CrossRef] [Google Scholar]
- Antifungal activity of chitosan and its application to control post-harvest quality and fungal rotting of Tankan citrus fruit (Citrus tankan Hayata) J. Sci. Food Agric.. 2006;86(12):1964-1969.
- [CrossRef] [Google Scholar]
- Characterization of sericin biomaterial from silk cocoon waste. Mater. Sci. Appl.. 2015;1(2):45-50.
- [Google Scholar]
- Evaluating UV absorbers and antioxidants for topical treatment of upholstery fabrics. Text. Res. J.. 1990;60(3):172-179.
- [CrossRef] [Google Scholar]
- Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv. Chim. Acta. 1997;80:1144-1152.
- [CrossRef] [Google Scholar]
- Antifungal and antibacterial activity of aloe vera plant extract. Biol. Clin. Sci. Res. J.. 2020;2020:4.
- [CrossRef] [Google Scholar]
- Phytochemical extraction and characterization of the leaves of Aloe vera barbadensis for its anti-bacterial and anti-oxidant activity. Int. J. Sci. Res., (IJSR). 2015;4(6):658-661.
- [Google Scholar]
- Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J. Food Sci.. 2006;71(5):298-303.
- [CrossRef] [Google Scholar]
- de Rodrı́guez, D.J., Hernández-Castillo, D., Rodrı́guez-Garcı́a, R., Angulo-Sánchez, J.L., 2005. Antifungal activity in vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Industrial Crops and Products, 21(1), 81–87. Doi: 10.1016/j.indcrop.2004.01.002.
- Investigation on the properties of methoxy poly (ethylene glycol)/chitosan graft co-polymers. J. Biomater. Sci.: Polym. Ed.. 2007;18(12):1575-1589.
- [Google Scholar]
- Preparation, characterization and performance evaluation of chitosan as an adsorbent for remazol red. Int. J. Latest Res. Eng. Technol., (IJLRET). 2016;2(2):52-62.
- [Google Scholar]
- Microbial contamination in sprouts: how effective is seed disinfection treatment. J. Food Sci.. 2013;78(4):R495-R501.
- [CrossRef] [Google Scholar]
- Determination of deacetylation degree of chitosan: a comparion between conductometric titration and CHN analysis. Corbohydr. Res.. 2009;344(18):2591-2595.
- [CrossRef] [Google Scholar]
- Purification and characterization of glue like sericin protein from a wild silkworm Antheraea assamensis Helfer. Global J. Biosci. Biotechnol.. 2012;1(2):229-233.
- [Google Scholar]
- Application of natural extracts as active ingredient in biopolymer based packaging systems. J. Food Sci. Technil.. 2022;2022:1-9.
- [CrossRef] [Google Scholar]
- Antioxidant, antidiabetic, anti-inflammatory and anticancer potential of some seaweed extracts. Food Sci. Technol.. 2022;42:e20521.
- [Google Scholar]
- Antioxidant activities of silk sericin from silkworm Bombyx mori. J. Food Biochem.. 2009;33(1):74-88.
- [CrossRef] [Google Scholar]
- Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol.. 2017;104:687-707.
- [CrossRef] [Google Scholar]
- Antibacterial effects of water-soluble low-molecular weight chitosans on different microorganisms. Appl. Biochem. Microbiol.. 2004;40(3):253-257.
- [CrossRef] [Google Scholar]
- Simultaneous encapsulation and stabilization of Aloe vera extract on cotton fabric for wound dressing application. RSC Adv.. 2016;113:111895-111902.
- [CrossRef] [Google Scholar]
- The role of natural dyes in the UV protection of fabrics made of vegetable fibres. Dyes Pigm.. 2011;91(3):279-285.
- [CrossRef] [Google Scholar]
- Gulrajani M.L., ed. Silk Dyeing, Printing, and Finishing. New Delhi, India: Hauz Khas; 1988.
- Gulrajani, M.L., 2005. Sericin: A bio-molecule of value. Souvenir of 20th Congress of the International Sericultural Commission, Bangalore, India, 15-18 December, 2005, pp. 21-29.
- Extraction and characterization of silk sericin. Indian J. Fibre Text. Res.. 2014;39(4):364-372.
- [Google Scholar]
- Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 2021;10(8):1264.
- [CrossRef] [Google Scholar]
- Control of methicillin-resistant Staphylococcus aureus in a hospital and an intensive care unit. Infect. Control Hosp. Epidemiol.. 1995;16:405-411.
- [CrossRef] [Google Scholar]
- Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol.. 2001;71(2–3):235-244.
- [CrossRef] [Google Scholar]
- Impact scenarios of shrimp farming in coastal region of Bangladesh: an approach of an ecological model for sustainable management. Aquac. Int.. 2016;24:1163-1190.
- [CrossRef] [Google Scholar]
- Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J. Adv. Scient. Res.. 2011;2(4):63-66.
- [Google Scholar]
- Study of the antimicrobial activity of silk sericin from silk worm bombyx mori. Iraqi J. Community Med.. 2010;23(2):130-133.
- [Google Scholar]
- Chitosan and mint mixture: A new preservative for meat and meat products. Food Chem.. 2008;107(2):845-852.
- [CrossRef] [Google Scholar]
- Antimicrobial actions and applications of chitosan. Polymers. 2021;13:904.
- [CrossRef] [Google Scholar]
- Application of sericin to modify textile supports. J. Text. Inst.. 2012;103(4):370-377.
- [CrossRef] [Google Scholar]
- Microalgae: therapeutic potentials and applications. Mol. Biol. Rep.. 2021;48:4757-4765.
- [CrossRef] [Google Scholar]
- The effect of residual silk sericin on the structure and mechanical property of regenerated silk filament. Int. J. Biol. Macromol.. 2007;41(3):346-353.
- [CrossRef] [Google Scholar]
- Gas permeation through water-swollen sericin/ PVA membranes. Ontario, Canada: University of Waterloo; 2007. Master Thesis
- Antimicrobial activity of native chitosan, degraded chitosan, and o-carboxymethylated chitosan. J. Food Prot.. 2003;66(8):1495-1498.
- [CrossRef] [Google Scholar]
- Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym.. 2002;49(2):185-193.
- [CrossRef] [Google Scholar]
- The microbial challenge: Science disease and public health ((2nd Edition)). Jones and Bartlett Publishers; 2010.
- Text book of microbiology. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd; 2012.
- Isolation, purification and evaluation of antibacterial agents from Aloe vera. Brazilian Microbiol.. 2009;40(4):906-915.
- [CrossRef] [Google Scholar]
- Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules. 2014;19(11):19180-19208.
- [CrossRef] [Google Scholar]
- Application of a fibre reactive chitosan derivatives to cotton fabric as an antimicrobial textile finish. Carbohydr. Polym.. 2004;56(2):227-234.
- [CrossRef] [Google Scholar]
- Milatovic, D., Zaja-Milatovic, S., Gupta, R.C., 2021. Ch 29 - Oxidative Stress and Excitotoxicity: Antioxidants from Nutraceuticals. In: Gupta, R., Lall, R., Srivastava, A. (Eds.), Nutraceuticals - Efficacy, Safety and Toxicity (2nd Edn.), Elsevier, pp. 401-403. Doi: 10.1016/b978-0-12-802147-7.00029-2.
- Functional applications of Aloe vera on textiles: A review. J. Polym. Environ.. 2021;29:993-1009.
- [CrossRef] [Google Scholar]
- Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett.. 2019;17:1667-1692.
- [CrossRef] [Google Scholar]
- Thermal properties of Aloe vera powder and rheology of reconstituted gels. Am. Soc. Agric. Biol. Eng.. 2010;53(4):1193-1200.
- [Google Scholar]
- Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol.. 2011;48(1):32-37.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem.. 2008;110(3):571-583.
- [CrossRef] [Google Scholar]
- Methicillin-resistant Staphylococcus aureus in U.S. hospitals 1975–1991. Infect. Control Hosp. Epidemiol.. 1992;13(10):582-586.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J. Microbiol. Biotechnol.. 2004;14(2):317-323.
- [Google Scholar]
- Applications and environmental aspects of chitin and chitosan. J. Macromol. Sci., Part A: Pure Appl. Chem.. 1995;A32(4):629-640.
- [CrossRef] [Google Scholar]
- Fractographic analysis of silkworm and spider silk. Eng. Fract. Mech.. 2002;69(9):1035-1048.
- [CrossRef] [Google Scholar]
- Photofading of reactive dyes on silk and cotton: effect of dye—fibre interactions. Color. Technol.. 2001;117(4):193-198.
- [CrossRef] [Google Scholar]
- Isolation and characterization of potent bioactive fraction with antioxidant and UV absorbing activity from Aloe barbadensis Miller gel. J. Plant Biochem. Biotechnol.. 2013;22(4):483-487.
- [CrossRef] [Google Scholar]
- Reynolds, T., Dweck, A.C., 1999. Aloe vera leaf gel, a review update. Journal of Ethnopharmacology, 68(1-3), 3-37. DOI: 10.1016/s0378-8741(99)00085-9.
- Strnad, S., 2011. Antimicrobial efficiency of functionalized cellulose fibers as potential medical textiles. In: Mendez-Vilas, A. (Ed.), Science against microbial pathogens: Communicating current research and technological advances (Vol. 1.), FORMATEX, Spain, pp. 36-51.
- Evaluation of anti-oxidative activity and UV absorption potential of the extracts of Aloe vera L. gel from different growth periods of plants. Ind. Crop. Prod.. 2013;3(49):712-719.
- [CrossRef] [Google Scholar]
- Aloe vera as a functional ingredient in foods. Crit. Rev. Food Sci. Nutr.. 2010;50(4):305-326.
- [CrossRef] [Google Scholar]
- Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym.. 2009;77(2):410-419.
- [Google Scholar]
- Extraction, structural and functional properties of silk sericin biopolymer from bombyx mori silk cocoon waste. J. Text. Sci. Eng.. 2019;9(1):1-5.
- [CrossRef] [Google Scholar]
- Sangamesha, M.A., Ranganatha V.L., Rashmi V., Ramu, R., Mallikarjunaswamy, C., 2022. Synthesis and characterization of 4-Hydroxy benzophenone (Ph2CO)/ Polyvinyl alcohol (PVA) composites for ultraviolet (UV)-Shielding applications. Materials Today: Proceedings, 2022 62, Part 8, 5250-5257, Doi: 10.1016/j.matpr.2022.03.217.
- An evaluation of UV protection imparted by cotton fabrics dyed with natural colorants. BMC Dermatol.. 2004;4:15.
- [CrossRef] [Google Scholar]
- Silk protein, sericin, suppresses colon carcinogenesis induced by 1, 2-dimethylhydrazine in mice. Oncol. Rep.. 2000;7(5):1049-1052.
- [CrossRef] [Google Scholar]
- Quantitative determination of the specific heat and the glass transition of moist samples by temperature modulated differential scanning calorimetry. Int. J. Pharm.. 2001;217(1–2):173-181.
- [CrossRef] [Google Scholar]
- Senakoon, W., Nuchadomrong, S., Sirimungkararat, S., Senawong, T., Kitikoon, P., 2009. Antibacterial action of eri (samiaricini) sericin against Escherichia coli and staphylococcus aureus. Asian Journal of Food and Agro-Industry, Special Issue, S222-S228.
- Simultaneous depolymerisation and decolorization of chitosan by ozone treatment. J. Food Sci.. 2007;72(9):C522-C526.
- [CrossRef] [Google Scholar]
- Investigation of antibacterial activity on cotton fabrics with cold plasma in the presence of a magnetic field. Plasma Processes Polym.. 2007;4:S1098-S1103.
- [CrossRef] [Google Scholar]
- Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol.. 2020;11:694.
- [CrossRef] [Google Scholar]
- Sustained activity of stimuli-responsive curcumin and acemannan based hydrogel patches in wound healing. ACS Appl. Bio Mater.. 2022;5(2):598-609.
- [CrossRef] [Google Scholar]
- First market study in e-commerce food packaging: Resources, performance, and trends. Food Packag. Shelf Life. 2021;29:100698
- [CrossRef] [Google Scholar]
- Thermal stability studies of some chitosan metal ion complexes using differential scanning calorimetry. Polym. Degrad. Stab.. 1996;52(1):85-87.
- [CrossRef] [Google Scholar]
- Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167-2180.
- [CrossRef] [Google Scholar]
- Preparation and characterization of silver nanoparticles composited on polyelectrolyte film coated sericin gel for enhanced antibacterial application. Sci. Adv. Mater.. 2016;8(8):1547-1552.
- [CrossRef] [Google Scholar]
- On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer. 2000;41:2463-2469.
- [CrossRef] [Google Scholar]
- Thermal decomposition behavior of sericin cocoon. J. Appl. Polym. Sci.. 1978;22(2):543-554.
- [Google Scholar]
- Chitosan as an affinity macroligand for precipitation of N-acetyl glucosamine binding proteins/enzymes. Isolat. Purif.. 1996;2:217-226.
- [Google Scholar]
- Sericin separation from silk degumming wastewater. Sep. Purif. Technol.. 2008;59(2):129-133.
- [CrossRef] [Google Scholar]
- Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba) Carbohydr. Polym.. 2013;92(1):90-97.
- [CrossRef] [Google Scholar]
- Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem.. 2007;103(4):1255-1262.
- [CrossRef] [Google Scholar]
- Antioxidant properties of chitosan from crab shells. Carbohydr. Polym.. 2008;74(4):840-844.
- [CrossRef] [Google Scholar]
- Physicochemical characterization of chitin and chitosan from crab shells. Corbohydr. Res.. 2009;75:15-21.
- [CrossRef] [Google Scholar]
- Zhaorigetu, S., Yanaka, N., Sasaki, M, Watanabe, H., Kato, N., 2003. Silk protein, sericin, suppresses DMBA-TPA-induced mouse skin tumorigenesis by reducing oxidative stress, inflammatory responses and endogenous tumor promoter TNF-α. Oncology Reports, 10(3), 537-543 (2003).
- Recent advances in environmentally friendly and green degumming processes of silk for textile and non-textile applications. Polymers. 2022;14(4):659.
- [CrossRef] [Google Scholar]







