Translate this page into:
Bilobetin induces apoptosis in human hepatocellular carcinoma cells via ROS level elevation and inhibition of CYP2J2
⁎Corresponding authors at: Department of Pathology, Jeonbuk National University Medical School, Jeonju 54896, Republic of Korea (Kyu Yun Jang), BK21 FOUR Community-Based Intelligent Novel Drug Discovery Education Unit, College of Pharmacy and Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu 41566, Republic of Korea (Kwang-Hyeon Liu) and Department of Bio and Chemical Engineering, Hongik University, Sejong 30016, Republic of Korea (See-Hyoung Park). kyjang@jbnu.ac.kr (Kyu Yun Jang), dstlkh@knu.ac.kr (Kwang-Hyeon Liu), shpark74@hongik.ac.kr (See-Hyoung Park)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bilobetin is a biflavonoid isolated from the leaves of Ginkgo biloba. Bilobetin displays several biological effects, however, the activities of bilobetin against cancer have been solely demonstrated. Thus, the aim of this study is investigating the apoptotic effects of and anticancer mechanism of bilobetin in Huh7 and HepG2 cells, both of human hepatocellular carcinoma (HCC) cell lines. MTT, cell counting, and colony formation assay showed that anti-proliferation effect of bilobetin. Cell cycle analysis revealed that bilobetin induces increase of population of the sub G1 phase. Annexin V/PI staining and TUNEL assay showed that bilobetin treatment promotes apoptotic cell death and DNA fragmentation. Bilobetin also induces ROS elevation and DNA damage in HCC cells. Western blot analysis elucidated that bilobetin displays apoptotic signaling pathway in HCC cells via upregulating cleaved PARP, cleaved caspase3 and Bax and downregulating CYP2J2. Bilobetin inhibited CYP2J2-catalyzed terfenadine and ebastine hydroxylase activities with IC50 values of 0.81 and 2.21 μM, respectively. Transfection of CYP2J2 siRNA increased the anticancer effect of bilobetin in HCC cells through the inhibition of CYP2J2 expression. Taken together, this study suggests that bilobetin induces apoptosis against HCC cells via ROS level elevation and inhibition of CYP2J2.
Keywords
Bilobetin
CYP2J2
Hepatocellular carcinoma
Apoptosis
Anticancer
Reactive oxygen species
1 Introduction
Hepatocellular carcinoma (HCC) is known to be the main subtype cancer among liver cancer in some areas of Asia and Africa (Lee et al., 2021). The prevalence of HCC is expanding extremely in the western areas including Europe and America (Llovet et al., 2021). The earliest treatment option for HCC is surgical resection and transplant (Cheng and Lv 2013). Moreover, various treatments such as radiofrequency and chemotherapy ablation for HCC have been develop (Lee et al., 2020). Nevertheless, these treatments have some drawbacks, such as detrimental effects of radiation therapy for normal liver tissue (Lee et al., 2018). Therefore, it is necessary for research and doctor to improve alternative therapeutic method to treat HCC (Huang et al., 2020).
One of the alternative methods is treatment of natural products such as bioflavonoids and phytochemicals to liver cancer patient (Kim et al., 2022). For example, EGb761 belonging to ginkgo biloba extract significantly can inhibit proliferation in HCC HepG2 and Hep3B cells (Chao and Chu 2004). In addition, it is reported that ginkgolic acid in ginkgo biloba leaf extract suppress proliferation and migration of HCC HepG2 and Huh7 cells by inducing G0/G1 cell cycle arrest (Chena et al., 2021). Bilobetin is a kind of active component of Ginkgo biloba (Li et al., 2019). It is reported that it could reduce blood lipids and improve the effects of hypoglycemic agent, insulin (Hirata et al., 2015). In previous studies, bilobetin ameliorated resistance of insulin, increased the hepatic uptake and oxidation of lipids, reduced very-low-density lipoprotein triglyceride secretion and blood triglyceride levels. It also increased the activity of enzymes concerned in β-oxidation and attenuated the accumulation of triglycerides and their metabolites in tissues (Zhang and Wang 2020). However, in cancer biology, effect of bilobetin have not been investigated against HCC cells.
Cytochrome P450 2 J2 (CYP2J2) is the one of the cytochromes P450 enzyme subfamily (Berlin et al., 2011, Zou and Mo 2021). It is secreted from vascular endothelium, and it is an important enzyme regulating metabolism of endogenous polysaturated fatty acids (Askari et al., 2013, Xu et al., 2013). Recent studies, it is reported that CYP2J2 is a new crucial enzyme in cyclophosphamide bioactivation and a potential biomarker for hematological malignancies (El-Serafi et al., 2015). CYP2J2 is known as a crucial biomarker in cancer biology (Zou and Mo 2021). In previous study, acetylshikonin promoted anti-cancer effects in HepG2 cells via downregulation of CYP2J2 (Park et al., 2017). It is reported that broussochalcone A induced apoptosis in HCC cells via activation of FOXO3 and inhibition of CYP2J2 (Park et al., 2018). In addition, CYP2J2 has a protective effect in MDA-MB-468 breast cancer cells against cell death mediated by reactive oxygen species (ROS) (Allison et al., 2017). Consequently, CYP2J2 is spotlighted as a novel biomarker to develop anticancer drugs.
In this study, we demonstrated that bilobetin as new CYP2J2 enzyme inhibitor. It inhibits cell proliferation and induces apoptotic effects in human HCC cell lines. To support suppression of cell proliferation, we performed MTT, cell counting, and colony formation assay. To demonstrate the apoptotic effect, annexin V/ PI staining, cell cycle analysis, and TUNEL assay were performed. To verify the expression of apoptosis-related markers such as PARP, caspase3, and Bax, western blot analysis was carried out. CYP2J2 siRNA transfection experiments were performed to prove the crucial role of bilobetin as a CYP2J2 inhibitor. Taken together, the aim of this study is to investigate the anticancer effect of bilobetin against human HCC Huh7 and HepG2 cells via inhibition of CYP2J2.
2 Materials and methods
2.1 Chemical reagents and antibodies
Bilobetin was purchased from the MedChemExpress and dissolved in DMSO (Sigma-Aldrich, St. Louis). A concentration of 40 mM stock solution of bilobetin was stored at −80℃. Mouse anti-ß-actin (1:5000 dilution), mouse anti-CYP2J2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-Bax (1:1000 dilution), rabbit anti-cleaved caspase-3 (1:1000 dilution), rabbit anti-caspase-3 (1:1000 dilution), rabbit anti-cleaved PARP (1:1000 dilution), and rabbit anti-PARP (1:1000 dilution) were purchased from Cell Signaling Technology (Cell Signaling, Danvers, MA, USA). Terfenadine, ebastine, terfenadine alcohol, and hydroxyebastine were purchased from Toronto Research Chemicals (North York, Canada). Danazol was obtained from Sigma-Aldrich. Pooled human liver microsomes were obtained from XenoTech (Lenexa, KS, USA). Solvents were of mass spectrometer grade.
2.2 Cell culture
HCC Huh7 and HepG2 cells were purchased from the Korean cell line bank (Seoul, Korea). The cells were maintained under standard conditions (5% CO2, 37℃, and 95% humidity). Huh7 and HepG2 cells were cultured in RPMI-1640 media (GIBCO, Grand Island, New York, USA) containing 10% heat-inactivated fetal bovine serum (GW biotech, Korea) and 1% penicillin/streptomycin (GIBCO).
2.3 MTT assay
Huh7 and HepG2 cells were seeded 96-well plates at a density of 5 × 103 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for 24 h. Subsequently, the cells were treated with various concentrations of bilobetin (0, 2.5, 5, 10, 20, and 40 μM). After 24 h, 48 h, and 72 h of treatment, 20 μL of MTT dye (5 mg/mL) was added to each well and the cells were incubated for 1 h 30 min at 37℃. The supernatants were removed, and the cells were treated with DMSO to forming formazan. The plates were incubated in shaker at room temperature (25℃) for 30 min. After that, the absorbance was measured at 570 nm using a microreader.
2.4 Cell counting assay
Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 5, 10, and 20 μM) for 24, 48, and 72 h. Next, the cells were stained using trypan blue solution. Cell numbers were counted using a hemocytometer.
2.5 Colony formation assay
Huh7 and HepG2 cells (1000 cells in each well) were seeded in 6-well plates and incubated at 37℃ in a humidified incubator containing 5% CO2 in air until cells were attached and stable. The cells were treated to various concentration of bilobetin (0, 5, 10, and 20 μM) for 24 h, the media was replaced with fresh media and Huh7 and HepG2 cells were incubated for 14 days under standard conditions. Then the cells were washed with PBS (Phosphate Buffer Saline), twice for 5 min each and fixed with 4% formaldehyde for 30 min at 4℃. Following fixation, the cells were again washed twice with PBS for 3 min and stained with 1% crystal violet (Sigma-Aldrich) solution for 30 min. The number of colonies were counted.
2.6 Cell cycle analysis
Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 5, 10, and 20 μM) for 24 and 48 h. The cells were harvested with trypsinization and fixed with 70% EtOH overnight. Subsequently, the cells were centrifuged and stained with 50 μg/mL of propidium iodide (PI) and 200 μg/mL of RNase in PBS at 37℃ for 30 min. Then, the cells were analyzed using flow cytometry (Beckman Coulter, Brea, CA, USA).
2.7 Annexin V/PI staining assay
Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 5, 10, and 20 μM) for 24 and 48 h. The cells were harvested with trypsinization and stained with 5 μL of annexin V and 5 μL of PI in 1 × binding buffer, and incubated at room temperature for 10 min. Subsequently, the cells were analyzed using flow cytometry (Beckman Coulter).
2.8 TUNEL assay
We detected DNA fragmentation in apoptotic cells using DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI, USA). Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 10, and 20 μM) for 24 and 48 h. The cells were fixed with 4% formaldehyde solution for 25 min at 4℃ and permeabilized with Triton X-100 (0.2%). The cells were treated with 50 μL TdT enzyme buffer (Equilibration buffer 45 μL, Nucleotide mix 5 μL, and TdT enzyme 1 μL). The nuclei of cells were stained by Hoechst 33,342 stain solution (Sigma Aldrich). DNA fragmentations were detected by fluorescence microscope (Nikon Eclipse TE 2000-U, Tokyo, Japan).
2.9 DCFDA assay
Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 5, 10, and 20 μM) for 24 and 48 h. Additionally, cells were treated with or without 5 mM N-acetyl cysteine (NAC) as the control and 20 μM of bilobetin. The cells were harvested by trypsinization, stained with 10 μL of 2′, 7′-dichlorofluorescin diacetate (DCF-DA) solution in 4 mL of PBS for 30 min at room temperature (25℃C), and analyzed by flow cytometry (Beckman Coulter).
2.10 Comet assay
Huh7 and HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight to attach. After incubation, the cells were treated with different concentrations of bilobetin (0, 10, and 20 μM) for 24 h. The assay was performed using the Comet assay kit (Abcam ab238544), according to the manufacturer’s instructions with minor modifications. The DNA-damaged cells were mixed with comet agarose in a 1/10 ratio (v/v). Thereafter, the slides were immediately blotted onto glass slides covered with a comet agarose base layer. After incubation with ice-cold lysis buffer, the slides were subjected to electrophoresis in a TAE electrophoresis solution. Subsequently, the slides were stained with Vista Green DNA dye. Damaged DNA was detected using a fluorescence microscope (Nikon Eclipse TE 2000-U).
2.11 Western blot assay
Huh7 and HepG2 were seeded in in 6-well plates at a density of 3 × 105 cells and incubated at 37℃ in a humidified incubator containing 5% CO2 in air for overnight. After incubation, the cells were treated with various concentrations of bilobetin (0, 5, 10 and 20 μM) for 24 h. All cells were gathered with cold PBS, and lysates were harvested using RIPA (Radioimmunoprecipitation assay) buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Sigma Aldrich). After centrifugation, the supernatant was transferred to EP tube. The protein concentrations were measured by Bradford assay. The protein samples were separated by SDS-PAGE (Sodium dodecyl sulfate polyacrylamide gel electrophoresis) at 120 V for 2 h. The proteins were transferred to methanol activated PVDF membrane at 25 V (4℃) for overnight. After that, membranes were blocked with 3% bovine serum albumin (BSA) for 30 min at room temperature and incubated with the specific primary antibodies against at 4℃ overnight. The membranes were washed with TBS-T three times every 10 min and incubated with a secondary antibody. Chemiluminescence was detected using a Chemi-doc detection system (Bio-Rad, Hercules, CA, USA).
2.12 siRNA transfection
CYP2J2-siRNA or control-siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The cells were transfected with CYP2J2 or control siRNA using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol.
2.13 CYP2J2 activity assays
All incubations were performed in triplicate, and data are presented as average values. The inhibitory potential of bilobetin against CYP2J2-mediated terfenadine and ebastine hydroxylation activities were determined using pooled human liver microsomes (HLMs) in the absence and presence of bilobetin. Danazol, a known CYP2J2 inhibitor was used as a positive control. In brief, the incubation mixtures (final volume, 100 μL) containing pooled HLMs (0.25 mg/mL), CYP2J2 substrate (0.2 μM terfenadine or 5 μM ebastine), and bilobetin (0, 0.2, 0.5, 2, 5, and 20 μM) were preincubated for 5 min at 37℃. The reaction was initiated by the addition of a NADPH-generating system (1.3 mM NADP+, 3.3 mM glucose-6-phosphate, 3.3 mM MgCl2, and 500 units/mL glucose-6-phosphate dehydrogenase) after preincubation, and the reactions were maintained for 20 min in a thermoshaker. The reactions were terminated by the addition of 100 μL of ice-cold acetonitrile containing 10 nM trimipramine (internal standard, IS) into the mixtures. After mixing and centrifuging at 13,000 g for 5 min at 4℃, aliquots of the supernatants were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
2.14 Statistical analysis
The results are expressed as mean ± SEM (the standard error of the mean). To compare the statistical meaning between the groups, student’s t-test was used for statistical analysis, and p-value < 0.05 was considered statistically significant. Experiments were repeated three times. And the representative data were shown.
3 Results
3.1 Bilobetin inhibits the cell proliferation in Huh7 and HepG2 cells.
To demonstrate the antiproliferative effects of bilobetin, we performed the MTT, cell counting, and colony formation assay. Huh7 and HepG2 cells treated with the indicated dose of bilobetin. As shown in Fig. 1, the cell viability decreased after bilobetin treatment. The cell viability of Huh7 cells treated with 0, 2.5, 5, 10, 20, and 40 μM of bilobetin was 100, 96.2, 91.3, 85.5, 58.5, and 51.2% for 24 h, and 100, 97,4, 86.2, 84.9, 42.5, and 25.7% for 48 h, and 100, 94.7, 85.8, 77, 42.3, and 25.9% for 72 h. The cell viability of HepG2 cells treated with 0, 2.5, 5, 10, 20, and 40 μM of bilobetin was 100, 98.5, 88.9, 84, 79.6, and 78.5% for 24 h, and 100, 96,6, 85.5, 82, 70.2, and 55.1% for 48 h, and 100, 89.6, 80.2, 69.4, 48,2, and 37.2% for 72 h. According to MTT results, the 50% inhibitory concentration of bilobetin was about 18.28 μM (IC50 = 18.28 μM) at 48 h in Huh7 cells and 19 μM (IC50 = 19 μM) at 72 h in HepG2 cells. The dose and time-dependent cell counting assay results showed that the anti-proliferation ability of bilobetin (0, 5, 10, and 20 μM). The cell number in Huh7 and HepG2 cells treated with bilobetin (20 μM) was significantly lower than that of the control at 72 h incubation time. Colony formation assay also performed using the same treatment condition of bilobetin against Huh7 and HepG2 cells. Clonogenicity in Huh7 and HepG2 cells treated with bilobetin (20 μM) was also much less than that of the control. Taken together, these results suggested that bilobetin induced inhibition of cell proliferation against Huh7 and HepG2 cells.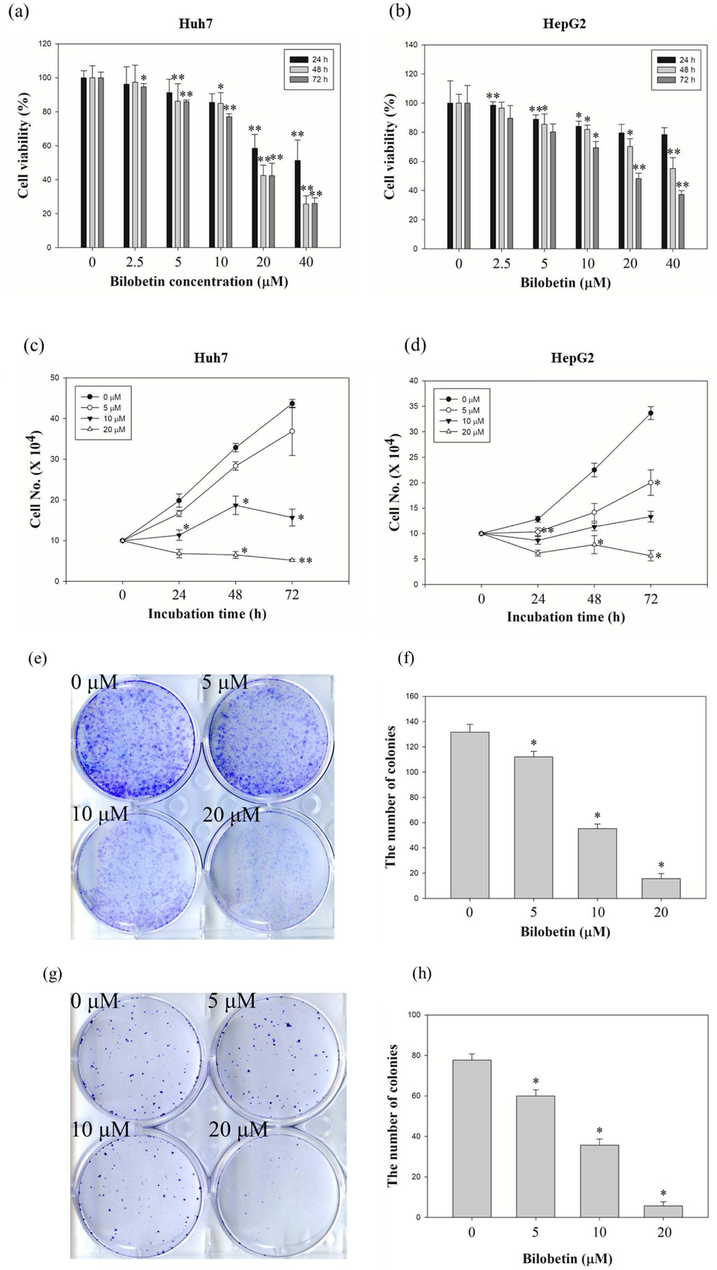
Antiproliferative effects of bilobetin against HCC cell lines. Dose- and time-dependent effects of bilobetin (0, 2.5, 5, 10, 20, and 40 μM) in (a) Huh7 and (b) HepG2 cells after bilobetin treatment for 24 h, 48 h, and 72 h. The cell viability was examined using the MTT assay. Cell counting assay of (c) Huh7 and (d) HepG2 cells after treatment of bilobetin (0, 5, 10, and 20 μM) for 24 h, 48 h, and 72 h. Colony formation assay of (e) Huh7 and (g) HepG2 cells after treatment of bilobetin (0, 5, 10, and 20 μM) following 2 weeks. (f, h) The graphical bar displayed the standard error. Significant differences between bilobetin treatment groups and DMSO control groups are showed as * (* means p < 0.05 and ** means p < 0.01).
3.2 Bilobetin induces apoptosis in Huh7 and HepG2 cells.
To evaluate the bilobetin-induced apoptosis, cell cycle analysis and annexin V/PI assay were performed using flow cytometry. Huh7 and HepG2 cells were treated with various concentration of bilobetin (0, 5, 10, and 20 μM) for 24 and 48 h and the cells were analyzed. We could find the increased sub G1 population of both cells after treating cells with bilobetin with dose- and time-dependent. As shown in Fig. 2, the sub G1 population of Huh7 cells was 0.8, 0.9, 1.3, and 8.4% at 24 h and 1.8, 1.2, 8.0, and 17.8% at 48 h, respectively. The sub G1 population of HepG2 cells was 1.8, 2.0, 3.0 and 4.1% at 24 h and 1.7, 2.0, 3.3, and 12.5% at 48 h, respectively. Annexin V/PI assay results showed that apoptotic cell death rates were increased after treatment of bilobetin in dose- and time-dependent manner (Fig. 3). The total apoptotic cell rate of Huh7 cells treated with 0, 5, 10, and 20 μM of bilobetin was 2.2, 2.2, 1.9, and 10.6% for 24 h and 1.7, 1.5, 7.5, and 22.5% for 48 h, respectively. The total apoptotic cell rate of HepG2 cells treated with 0, 5, 10, and 20 μM of bilobetin was 1.1, 1.2, 1.6, and 3.0% for 24 h and 1.5, 1.1, 3.2, and 14.2% for 48 h, respectively. To investigate DNA fragmentation in Huh7 and HepG2 cells after treatment of bilobetin, TUNEL assay was performed. As shown in Fig. 4, Huh7 and HepG2 cells were treated with different concentrations of bilobetin (0, 10, and 20 μM) for 24 h and 48 h. TUNEL-positive cells were increased in dose- and time-dependent manner. After treatment with 0, 10, and 20 μM of bilobetin, the percentage of TUNEL-positive cells of Huh7 was 0, 8, and 17.3% for 24 h and 0, 9, and 20.7% for 48 h. The percentages of TUNEL-positive cells of HepG2 after treatment with 0, 10, and 20 μM of bilobetin was 0, 1, and 4.3% for 24 h and 0, 6, and 15.7% for 48 h. These overall results suggested that bilobetin induced apoptosis of Huh7 and HepG2 cells.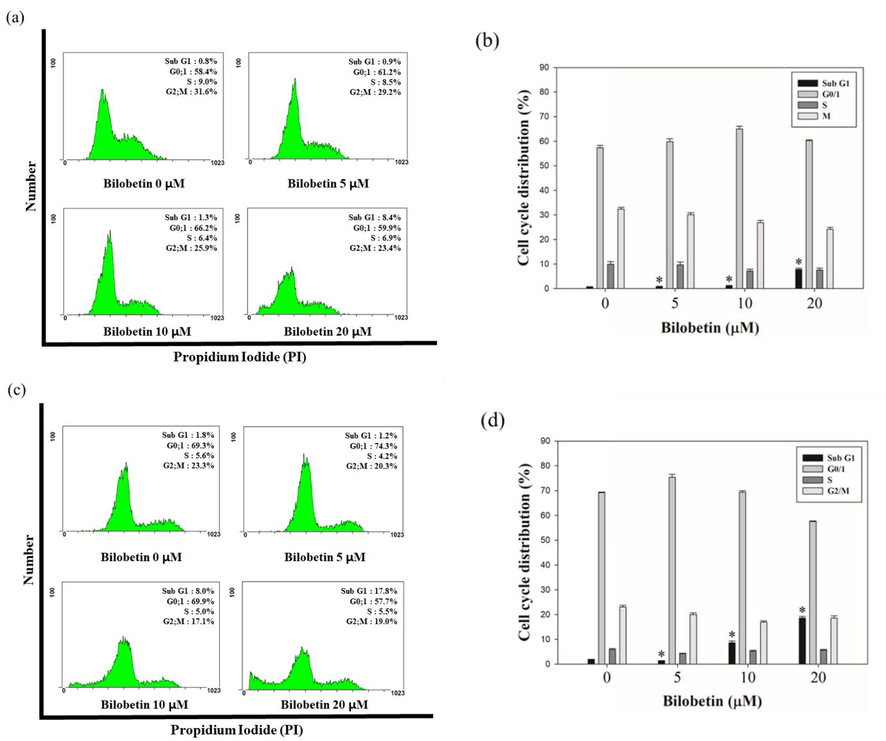
Cell cycle analysis of bilobetin against HCC cell lines. Increase of Sub G1 population in Huh7 and HepG2 cells. Huh7 and HepG2 cells were treated with 0, 5, 10, and 20 μM of bilobetin for 24 h and 48 h and stained with propidium iodide and specific RNase A. Subsequently, the cells were analyzed with flow cytometry. The cytogram results show that the cell cycle distribution and percentages of cells in each proportion of cell cycle indicated for (a, c) Huh7 and (e, g) HepG2 cells were treated with bilobetin for 24 h and 48 h. Distribution of (b, d) Huh7 and (f, h) HepG2 cells of each proportion of the cell cycle was represented after bilobetin treatment. Significant differences between bilobetin treatment groups and DMSO control groups are showed as * (* means p < 0.05).
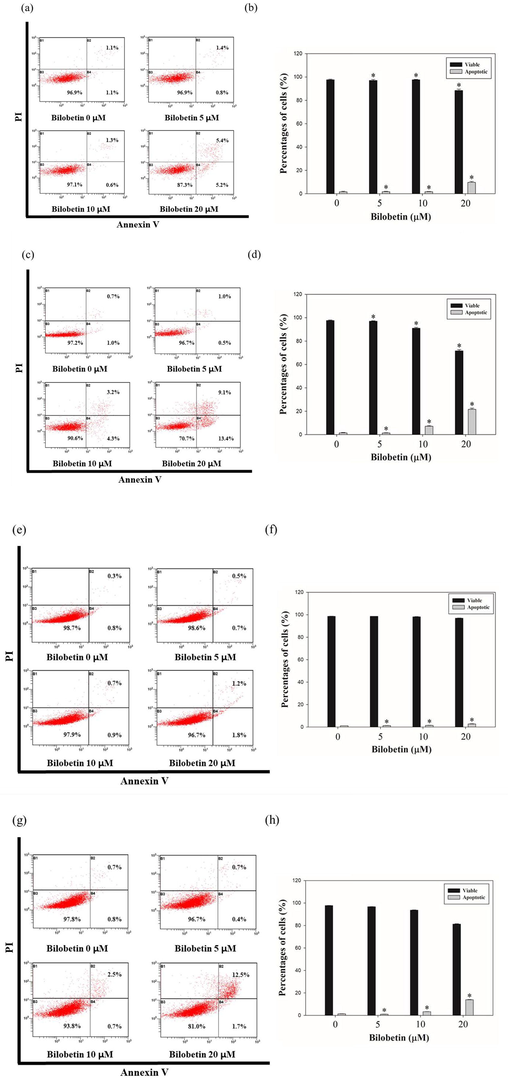
Annexin V/PI staining results following 24 h and 48 h of treatment of bilobetin (0, 5, 10, and 20 μM) in Huh7 and HepG2 cells. The percentages of apoptotic cells against (a, c) Huh7 and (e, g) HepG2 cells are showed in cytogram. Percentages of viable and apoptotic (b, d) Huh7 cells and (f, h) HepG2 cells were indicated in bar graph. The error bar indicated the standard error. Significant differences between bilobetin treatment groups and DMSO control groups are showed as * (* means p < 0.05).
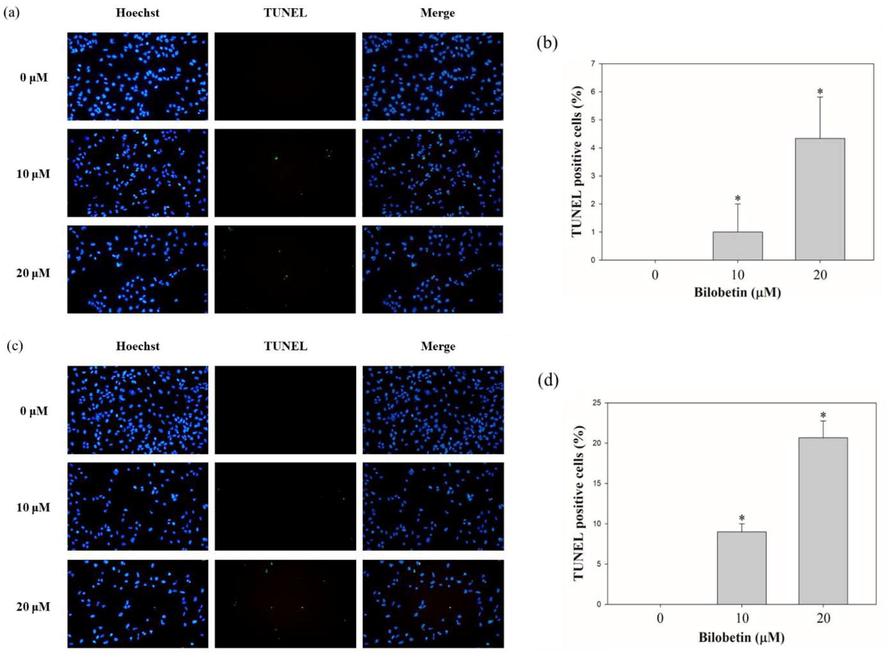
TUNEL assay results of Huh7 and HepG2 cells treated with bilobetin (0, 5, 10, and 20 μM) for 24 h and 48 h. DNA fragmentation was shown by fluorescence microscopy. (a, c, e, and g) Blue fluorescence represents the nuclei stained using Hoechst 33342, and green fluorescence indicates the TUNEL-positive cells. (b, d, f, and h) The bar graph showed that the percentages of TUNEL-positive cells after BCA treatment. The error bar represented the standard error. Significant differences between BCA treat and DMSO control groups are indicated as ∗ (∗means p < 0.05).
3.3 Bilobetin promotes apoptotic signaling pathways in Huh7 and HepG2 cells.
To elucidate the apoptotic effect of bilobetin in Huh7 and HepG2 cells, western blot analysis was performed. We revealed the expression levels of proteins related to cancer cell apoptosis after treatment of bilobetin. As shown in Fig. 5, caspase3 cleavage and PARP cleavage expression levels were increased after treatment of 0, 5, 10, and 20 μM of bilobetin. However, that of pro-forms expression levels were decreased after bilobetin treatment. The expression levels of proapoptotic protein such as Bax were increased. Furthermore, we demonstrated that the expression levels of CYP2J2 were decreased after treatment of bilobetin. Therefore, these results elucidate bilobetin stimulated apoptotic signaling pathways in Huh7 and HepG2 cells.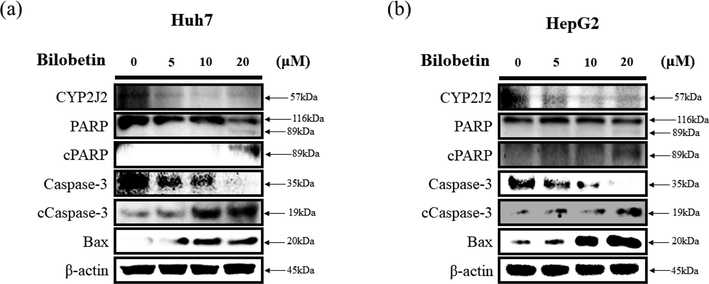
Western blot assay of (a) Huh7 and (b) cells after bilobetin treatment (0, 5, 10, and 20 μM) for 24 h. The expression levels of specific proteins associated with apoptosis were analyzed by western blot assay. ß-actin was used as a loading control protein.
3.4 Bilobetin induces accumulation of ROS and DNA damage in Huh7 and HepG2 cells.
We performed the DCFDA assay to measure the intracellular ROS levels in Huh7 and HepG2 cells. As shown in Fig. 6, bilobetin treatment induced a significant increase of intracellular ROS generation in both cell lines. DCF-DA analysis revealed that the mean fluorescence intensity (MFI) values of Huh7 cells treated with 0, 5, 10, and 20 μM of bilobetin, NAC, and cotreated with NAC and 20 μM of bilobetin were 21.4, 27.4, 47.4,68.4, 16.5, and 46.2. The ROS level was increased by 27.5, 120.1, 215.2% in Huh7 cells compared to control group. However, treatment of NAC and cotreated with NAC and bilobetin decreased by 24.5 and 101% compared to control group and 20 μM of bilobetin treated group. The MFI values of HepG2 cells treated with 0, 5, 10, and 20 μM of bilobetin, NAC, and cotreated with NAC and 20 μM of bilobetin were 21.1, 30.1, 64.6, 86.3, 21.8, and 56.8. The ROS level was increased by 44.9, 213.8, 298.9% in HepG2 cells compared to control group. Likewise, cotreatment with NAC and bilobetin decreased by 93.4% compared to 20 μM of bilobetin treated group. Furthermore, we investigated the cell viability of cells cotreated with bilobetin and NAC using the MTT assay. Based on the results, the cell viabilities owing to the control, bilobetin, NAC, and NAC + bilobetin treatments were 100, 60.1, 120.2, and 87.8% for Huh7 cells and 100, 89.9, 111, and 97.4% for HepG2 cells. These results suggested that BCA treatment induced the accumulation of ROS in HCC cells, which might be associated with DNA damage in cells, thereby activating the apoptosis signaling pathways. Hence, we performed the comet assay to determine whether bilobetin induced DNA damage against Huh7 and HepG2 cells. As shown in Fig. 7, the length of comet tail of 0, 10, and 20 μM of bilobetin was 6.4, 24, and 52.6 in Huh7 cells and 2.3, 7, and 39.8 in HepG2 cells, Therefore, the length of the comet tail was found to increase as the bilobetin concentration increased. Taken together, these overall results suggested that bilobetin increased ROS generation and induced DNA damage in Huh7 and HepG2 cells.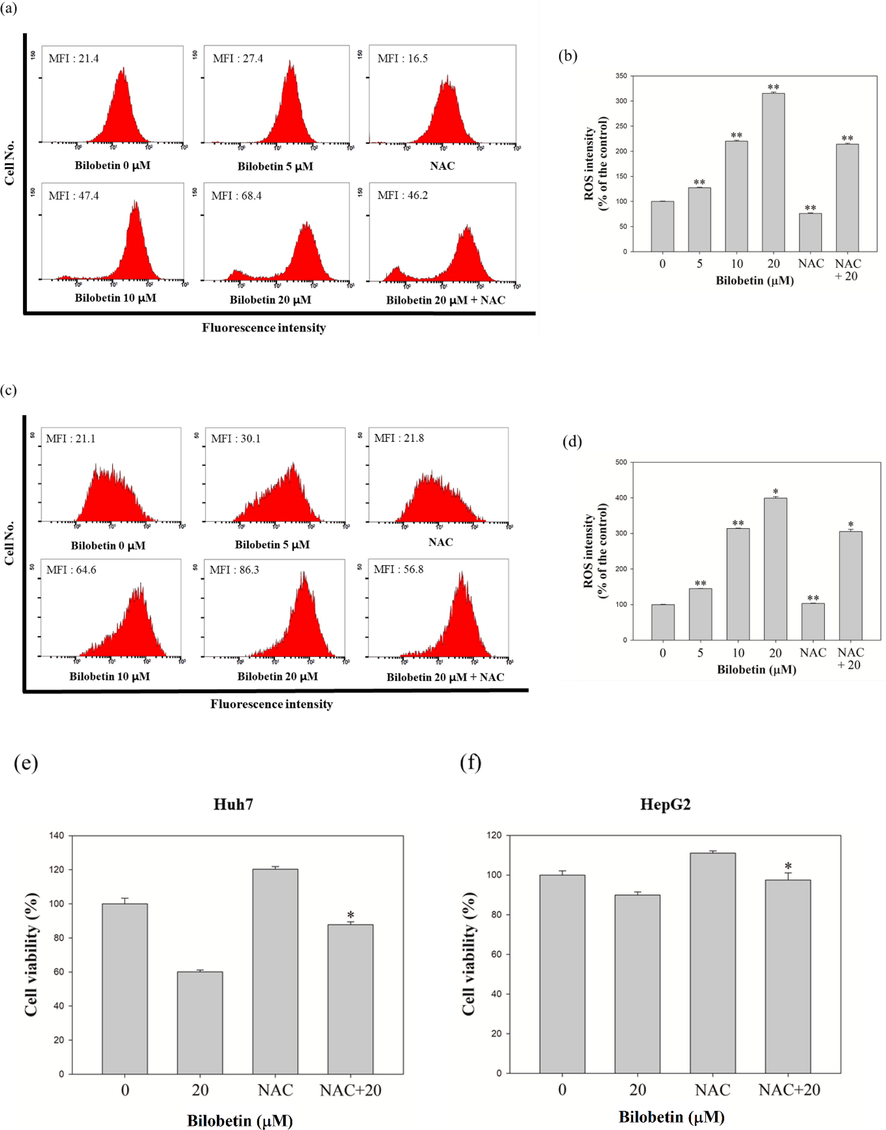
Measurement of intracellular ROS intensity in (a) Huh7 and (c) HepG2 cells. The bar graph represented that the percentage of control of MFI according to treatment of bilobetin and NAC in (b) Huh7 and (d) HepG2 cells. (e) The cell viability of Huh7 and HepG2 cells in response to control, bilobetin, NAC, and cotreatment of bilobetin and NAC. Significant differences between bilobetin treatment groups and DMSO control groups are showed as * (* means p < 0.05 and ** means p < 0.01).
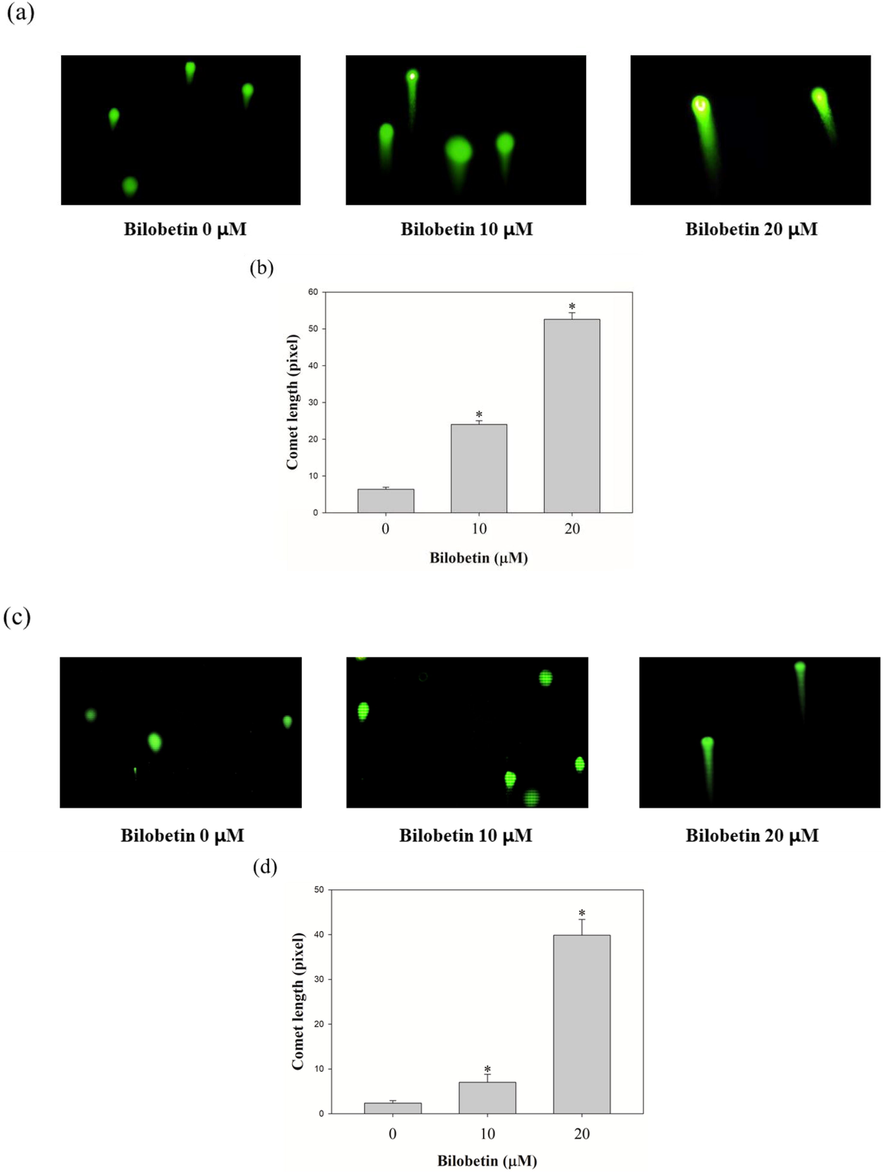
Comet assay was performed to identify bilobetin induces DNA damage in (a) Huh7 and (c) HepG2 cells. The cells were treated with bilobetin (0, 10, and 20 μM) of 24 h. Bilobetin-induced DNA damage was revealed using a comet assay. The length of comet tail dose-dependently enhanced after treatment of bilobetin (0, 10, and 20 μM) in (b) Huh7 and (d) HepG2.
3.5 Bilobetin inhibits CYP2J2 activity
To elucidate the mechanistic target for the anticancer activity of bilobetin, we evaluated the inhibitory potential of bilobetin against the CYP2J2 enzyme using HLMs. Bilobetin strongly inhibited CYP2J2-mediated terfenadine hydroxylase activity with an IC50 value of 0.81 μM (Fig. 8 (a)). Danazol, a well-known strong CYP2J2 inhibitor, also inhibited CYP2J2-mediated terfenadine hydroxylase activity with an IC50 value of 0.49 μM (Fig. 8 (b)), which was similar to the previous data (IC50 = 0.18 ∼ 2.14 μM) (Lee et al., 2015, Lee et al., 2018). To determine whether the inhibition by bilobetin was substrate-specific, we examined the inhibitory effect of bilobetin on CYP2J2-mediated ebastine hydroxylation and found that bilobetin also inhibited it with an IC50 value of 2.21 M (Fig. 8 (c)).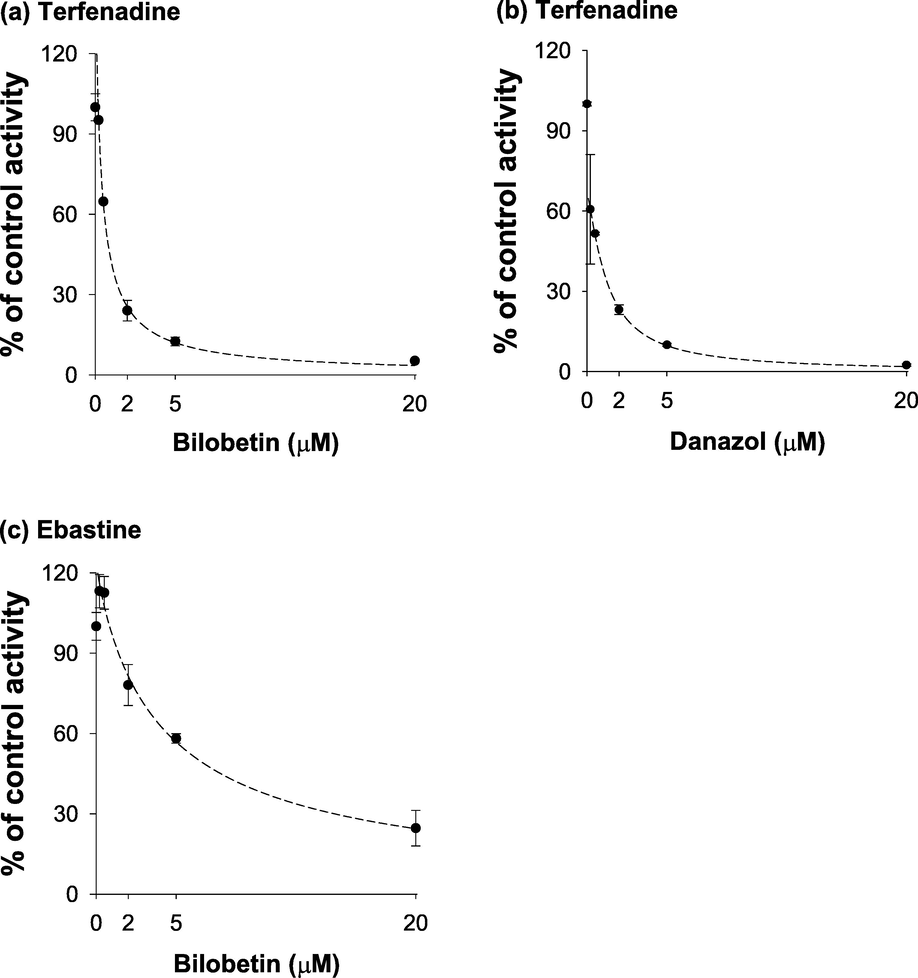
Inhibitory effects on CYP2J2-catalyzed (a) terfenadine (by bilobetin), (b) terfenadine (by danazol), (c) ebastine (by bilobetin) hydroxylation in pooled human liver microsomes. Pooled human liver microsomes (0.25 mg/ml) were incubated with terfenadine (0.2 μM) or ebastine (5.0 μM) in the presence or absence of various concentrations of bilobetin (0 – 20 μM) at 37 °C for 20 min. The activity was calculated as the percentage of control sample activity and plotted versus the bilobetin concentration. Each data point is the average ± S.D. of triplicate experiments.
3.6 CYP2J2 siRNA transfection enhances the apoptotic effects of bilobetin in Huh7 and HepG2 cells
We performed cell counting assay to evaluate cell viability after transfection of CYP2J2 siRNA and treatment of bilobetin against Huh7 and HepG2 cells. As shown in Fig. 9, bilobetin treatment after transfection with CYP2J2 siRNA induced the combined cytotoxicity and antiproliferative effect in Huh7 and HepG2 cells. Furthermore, we evaluated western blot assay to evaluate additive anticancer mechanism of bilobetin after transfection with CYP2J2 siRNA. We analyzed the expression levels of protein related to cancer cell apoptosis. As shown in Fig. 10, bilobetin treatment after CYP2J2 siRNA transfection decreased synergistically the expression levels of CYP2J2, and pro-forms of PARP and caspase3. However, treatment of bilobetin after transfection with CYP2J2 siRNA increased synergistically the expression levels of cleaved PARP, cleaved caspase3 and Bax. Therefore, these results elucidated that downregulation of CYP2J2 modulated Huh7 and HepG2 cells to bilobetin treatment and CYP2J2 is associated with cancer cell apoptosis in HCC cells treated with bilobetin.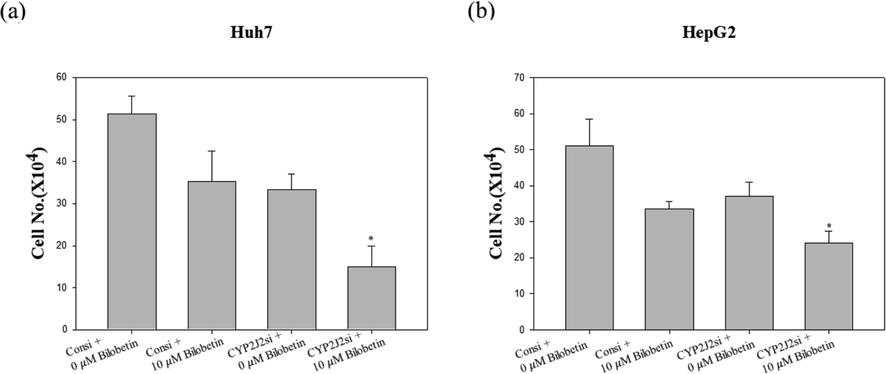
Cytotoxic and antiproliferative effect of the combination of CYP2J2 siRNA transfection and bilobetin treatment in Huh7 and HepG2 cells. (a) Huh7 and (b) HepG2 cells were transfected with the control or siRNA against CYP2J2 followed by treatment with bilobetin for 24 h for cell counting assay. The graphical bar displayed the standard error. Significant differences between bilobetin treatment groups and DMSO control groups are showed as * (* means p < 0.05).
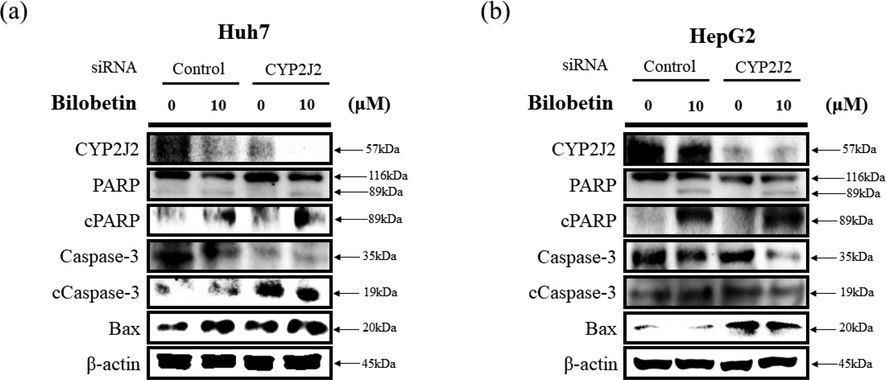
Western blot analysis was performed to determine apoptotic signaling pathway in (a) Huh7 and (b) HepG2 cells. The cells were transfected with control or CYP2J2-siRNA for 24 h. Subsequently, the transfected cells were treated with bilobetin (0 and 10 μM) for 24 h. The expression levels of specific proteins associated with apoptosis were analyzed by western blot assay. ß-actin was used as a loading control protein.
4 Discussion
Cancer is one of the most common diseases that lead to human death in the United States as well as globally (Fitzmaurice et al., 2015). HCC is one of a kind malignant carcinoma among cancer. The prevalence of HCC is expanding quickly in western countries, particularly in some developing countries, where HCC is a major source of the death among males (Lee et al., 2019). Recently, phytochemicals are well-known as anticancer drugs to treat HCC including polyphenol, terpenoids, and thiols (Zhang et al., 2015). However, there are still various side effects of the cancer therapies against the advanced metastasized cancer (Aslam et al., 2014). Thus, developing an efficient and alternative therapies are necessary for cancer patients to reduce the detrimental effects and improve current cancer therapies.
Bilobetin is a kind of the natural bioflavonoids isolated from the frond of Gingko biloba (Krauze-Baranowska and Wiwart 2003). In previous studies, it is reported that bilobetin induces kidney injury by upregulating cGMP-mediated AQP-2 trafficking and G2/M cell cycle arrest in podocyte cells (Wang et al., 2019). Bilobetin treatment ameliorates insulin resistance, increases phosphorylation and activity of PPARα via elevating PKA activity in rats fed a high-fat diet (Kou et al., 2012). It is reported that bilobetin acts as a novel small molecule inhibitor targeting influenza virus polymerase acidic endonuclease (Zhang and Wang 2020). In cancer biology, it is reported that bilobetin inhibited cell proliferation and G2/M cell cycle arrest in HeLa cells. In addition, bilobetin induces upregulation of proapoptotic protein Bax, downregulation of antiapoptotic protein, Bcl2 and accumulation of critical executioner in the downstream signaling pathway of apoptosis, cleaved caspase 3 in HeLa cells (Li et al., 2019). In this study, we focus on investigating the anti-cancer effects of bilobetin against HCC Huh7 and HepG2 cells.
In previous study, it is reported that bilobetin induces apoptotic effects such as suppressing the cell viability, G2/M cell cycle arrest, and working anticancer mechanisms in cervix adenocarcinoma HeLa cells (Li et al., 2019). Our results also suggested that bilobetin induced apoptotic effects via ROS level elevation and inhibition of CYP2J2. Our MTT, cell counting, and colony formation assay results displayed the inhibition of cell proliferation in HCC cells (Fig. 1). We revealed that Huh7 cells are slightly more sensitive to bilobetin compared to HepG2 cells by results of MTT assay. We demonstrated that increase of sub G1 population of both cell lines according to treatment of bilobetin (Fig. 2). As bilobetin concentration increased, accumulation of annexin V and PI positive cells, which is apoptotic cell rate is straight evidence of cancer cell apoptosis (Fig. 3). TUNEL positive cells were also detected after treatment of bilobetin (Fig. 4). Furthermore, we elucidated anticancer mechanism using western blot assay. Our results showed that the expression levels of cleaved PARP, cleaved caspase3, and Bax protein were increased (Fig. 5). Therefore, bilobetin is highly novel anticancer drug and CYP2J2 inhibitor to treat HCC, because it has never been investigated against Huh7 and HepG2 cells.
Reactive oxygen species (ROS) is an important factor in cell survival and proliferation, particularly cancers because the ROS intensity affects cell functions such as differentiation, cell cycle and DNA repair (Weiss and Ito 2014, Perillo et al., 2020). It is reported that Auricularia auricular increased an apoptosis in HCC cells via regulation of the peroxiredoxin1 related to ROS expression (Kang et al., 2020). Intracellular ROS induced DNA damage, which means it affects interpretation and transmission of genetic information (Rowe et al., 2008, Srinivas et al., 2019). In our study, we also detected expression level of ROS after treatment of bilobetin using by DCF-DA assay (Fig. 6). Bilobetin induced ROS level elevation in HCC cells while leading to apoptotic cell death. Additionally, intracellular ROS caused DNA damage in HCC cells. The results of comet assay represented that DNA damage occurred because length of comet tails was increased after treatment of bilobetin (Fig. 7). Therefore, these results might be crucial evidence that bilobetin induced increase of intracellular ROS level and DNA damage in Huh7 and HepG2 cells.
CYP2J2 is a member of the monooxygenase cytochrome P450 family and the only member of the human CYP2J subfamily (Solanki et al., 2018). It plays many functions such as modulation of oxidative stress, inflammation, apoptosis, and immune responses (Narjoz et al., 2014, Murray 2016). It has reported that CYP2J2 is also greatly expressed in several cancer cell lines, and it could advance human cancer metastasis and cancer cell growth (Jiang et al., 2007). Thus, CYP2J2 may be a crucial biomarker to develop the effective therapeutic methods for carcinoma. Indeed, it is reported that terfenadone is potent inhibitor of CYP2J2 present in the human liver and intestinal microsomes (Lee et al., 2018). In addition, it is reported that inhibition of CYP2J2 by tanshinone IIA induces apoptosis in HCC cells (Jeon et al., 2015). Therefore, the aim of our study is elucidating the apoptotic effect and anticancer mechanism of bilobetin via inhibition of CYP2J2 against HCC Huh7 and HepG2 cells. We observed that bilobetin inhibited CYP2J2-catalyzed terfenadine and ebastine hydroxylase activity with IC50 values of 0.81 and 2.21 μM, respectively. The inhibitory potential of bilobetin was less potent than that of danazol (IC50 = 0.18 μM for ebastine) (Lee et al., 2015), while it was similar or more potent than those of decursin (IC50 = 6.95 μM for astemizole) (Lee et al., 2014), 6,8-diprenylorobol (IC50 = 2.61 μM for ebastine) (Lee et al., 2020), LKY-047 (IC50 = 1.7 μM for astemizole) (Phuc et al., 2017), and terfenadone (IC50 = 2.11 μM for terfenadine) (Lee et al., 2018).
Additionally, polyphenol which are extracted from plant or attracting attention as medicinal plants, have an antioxidant effect and are issued currently for anticancer drugs. It is reported that Broussochalcone A induce apoptosis in renal cancer cells via ROS level elevation and activation of FOXO3 and it also induces anticancer effect in HCC cells via inhibition of CYP2J2 (Park et al., 2018, Lee et al., 2021). Acetylshikonin promotes anticancer activity in HCC cells via CYP2J2 inhibition. Furthermore, 6,8-diprenylorobol induces apoptosis in HCC cells via activation of FOXO3 and inhibition of CYP2J2 (Lee et al., 2020). Thus, these various polyphenol induces apoptotic effects in cancer cells, particulary HCC cells.
Apoptosis is a type of cell death, and there are various types of cell death in addition to apoptosis, necrosis, ferroptosis, and autophagy-dependent cell death (Galluzzi et al., 2018). In Fig. 1, cell viability was investigated via MTT assay. The MTT assay is a cell survival test through the mitochondrial dehydrogenase reaction (Buranaamnuay 2021). Therefore, the results referred to cell viability itself and belonged to the upstream concept of apoptosis (Darzynkiewicz et al., 1994). Moreover, the dose was adapted up to 40 μM and the time was set up to 72 h to determine the cell viability change by bilobetin through the MTT assay. However, for the apoptosis assay, the experiment was designed by figuring out the scale for which the death interval was determined through the MTT assay. Since both early and late apoptosis are induced at the dose of 10 μM and 20 μM of bilobetin, the dose range was used as shown in Fig. 6. In addition, the same dose range was applied to cell cycle or DNA fragmentation analysis as well as apoptosis assay.
Meanwhile, we investigated that bilobetin extracted from the leaves of Gingko biloba induced apoptotic effect in Huh7 and HepG2 cells via ROS level elevation and inhibition of CYP2J2. Until now, the anticancer effect of bilobetin has not been studied much. In this study, we analyzed in depth the anticancer effect of bilobetin, especially the apoptotic effects in Huh7 and HepG2 cells. In addition, we also demonstrated that bilobetin is selective CYP2J2 inhibitor. CYP2J2 is expressed predominantly in the heart and, to a lesser extent, in other tissues such as liver, pancreas, lung, and central nervous system (Karkhanis et al., 2017). Its high overexpression contributes to promoting the growth and proliferation of cancer cells (Gui et al., 2020). Therefore, we tried to investigate the synergic anticancer effect by the treatment of bilobetin and transfection of CYP2J2 siRNA and elucidated that it was effective to promote apoptotic effects against Huh7 and HepG2 cells. Therefore, it is believed that this study suggests a widespread understanding of apoptotic mechanisms of bilobetin in HCC and supplies an alternative candidate in improving anticancer therapeutic method.
5 Conclusion
Taken together, bilobetin displayed anticancer activity against HCC Huh7 and HepG2 cells. Bilobetin inhibited cancer cell proliferation in HCC cells. We revealed that bilobetin-induced apoptosis in HCC cells in dose- and time-dependent manner. Furthermore, we evaluated apoptotic pathways and anticancer mechanism by performing western blot assay. Also, we investigated the inhibitory activity of bilobetin against CYP2J2, which could be related to the apoptotic effects of bilobetin in Huh7 and HepG2 cells. For additional study, we are currently designing to demonstrate the detailed anticancer mechanisms of bilobetin such as anti-migration and anti-invasion and carry out xenograft mouse experiments.
Acknowledgments
This research was supported by the Basic Science Research Program (NRF-2014R1A6A3A04054307, NRF-2022R1A2C1008642, and NRF-2017R1A5A2015061) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIP).
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Authors' Contributions
HKL, SBB, JSL, HSC, MJN, JBL, KMP, YHY, KYJ, KHL, and SHP conceived the presented idea, carried out the experiments, and wrote the manuscript. HKL, SBB, JSL, HSC, MJN, JBL, KMP, YHY, KYJ, KHL, and SHP contributed to analysis and interpretation of the results. HKL, SBB, JSL, HSC, MJN, JBL, KMP, YHY, KYJ, KHL, and SHP provided critical feedback, discussed the results, and contributed to writing the first draft and final manuscript. Han Ki Lee, Subin Bae, and Jongsung Lee contributed equally to this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Activation of ALDH1A1 in MDA-MB-468 breast cancer cells that over-express CYP2J2 protects against paclitaxel-dependent cell death mediated by reactive oxygen species. Biochem. Pharmacol.. 2017;143:79-89.
- [CrossRef] [Google Scholar]
- Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat.. 2013;107:56-63.
- [CrossRef] [Google Scholar]
- Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J. Cancer Ther.. 2014;05(08):6.
- [CrossRef] [Google Scholar]
- PharmGKB summary: cytochrome P450, family 2, subfamily J, polypeptide 2: CYP2J2. Pharmacogenet Genomics.. 2011;21:308-311.
- [CrossRef] [Google Scholar]
- The MTT assay application to measure the viability of spermatozoa: A variety of the assay protocols. Open veterinary journal.. 2021;11:251-269.
- [Google Scholar]
- Effects of Ginkgo biloba extract on cell proliferation and cytotoxicity in human hepatocellular carcinoma cells. World J Gastroenterol.. 2004;10:37-41.
- [CrossRef] [Google Scholar]
- Ginkgolic acid inhibits proliferation and migration of human hepatocellular carcinoma cells by inducing G0/G1 cell cycle arrest. ScienceAsia. 2021;47:11-18.
- [Google Scholar]
- New progress of non-surgical treatments for hepatocellular carcinoma. Med Oncol.. 2013;30:381.
- [CrossRef] [Google Scholar]
- Darzynkiewicz, Z., X. Li and J. Gong, 1994. Chapter 2 Assays of Cell Viability: Discrimination of Cells Dying by Apoptosis. Methods in Cell Biology. Z. Darzynkiewicz, J. Paul Robinson and H. A. Crissman, Academic Press. 41: 15-38.
- Cytochrome P450 2J2, a new key enzyme in cyclophosphamide bioactivation and a potential biomarker for hematological malignancies. Pharmacogenomics J. 2015;15:405-413.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ.. 2018;25:486-541.
- [CrossRef] [Google Scholar]
- CYP2J2 promotes the development of hepatocellular carcinoma by increasing the EETs production to improve HIF-1α stability. Am J Transl Res.. 2020;12:7923-7937.
- [Google Scholar]
- <i>Ginkgo biloba</i> Extract Improves Insulin Signaling and Attenuates Inflammation in Retroperitoneal Adipose Tissue Depot of Obese Rats. Mediators Inflamm.. 2015;2015:419106
- [CrossRef] [Google Scholar]
- Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther.. 2020;5:146.
- [CrossRef] [Google Scholar]
- Inhibition of cytochrome P450 2J2 by tanshinone IIA induces apoptotic cell death in hepatocellular carcinoma HepG2 cells. Eur. J. Pharmacol.. 2015;764:480-488.
- [CrossRef] [Google Scholar]
- Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res.. 2007;67:6665-6674.
- [CrossRef] [Google Scholar]
- Auricularia auricula increases an apoptosis in human hepatocellular carcinoma cells via a regulation of the peroxiredoxin1. J Food Biochem.. 2020;e13373
- [CrossRef] [Google Scholar]
- Inhibition and inactivation of human CYP2J2: Implications in cardiac pathophysiology and opportunities in cancer therapy. Biochem. Pharmacol.. 2017;135:12-21.
- [CrossRef] [Google Scholar]
- Natural Products for Liver Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Journal. 2022;14
- [CrossRef] [Google Scholar]
- Bilobetin ameliorates insulin resistance by PKA-mediated phosphorylation of PPARα in rats fed a high-fat diet. Br. J. Pharmacol.. 2012;165:2692-2706.
- [CrossRef] [Google Scholar]
- Antifungal Activity of Biflavones from Taxus baccata and Ginkgo biloba. Zeitschrift für Naturforschung C.. 2003;58:65-69.
- [CrossRef] [Google Scholar]
- Broussochalcone A Induces Apoptosis in Human Renal Cancer Cells via ROS Level Elevation and Activation of FOXO3 Signaling Pathway. Oxid. Med. Cell. Longev.. 2021;2021:2800706.
- [CrossRef] [Google Scholar]
- Indole-3-carbinol induces apoptosis in human hepatocellular carcinoma Huh-7 cells. Food Chem. Toxicol.. 2018;118:119-130.
- [CrossRef] [Google Scholar]
- Terfenadone is a strong inhibitor of CYP2J2 present in the human liver and intestinal microsomes. Drug Metab Pharmacokinet.. 2018;33:159-163.
- [CrossRef] [Google Scholar]
- Anticarcinogenic effect of indole-3-carbinol (I3C) on human hepatocellular carcinoma SNU449 cells. Hum Exp Toxicol.. 2019;38:136-147.
- [CrossRef] [Google Scholar]
- 6,8-Diprenylorobol Induces Apoptosis in Human Hepatocellular Carcinoma Cells via Activation of FOXO3 and Inhibition of CYP2J2. Oxid. Med. Cell. Longev.. 2020;2020:8887251.
- [CrossRef] [Google Scholar]
- Knockout of Hepatocyte Growth Factor by CRISPR/Cas9 System Induces Apoptosis in Hepatocellular Carcinoma Cells. Journal of Personalized Medicine.. 2021;11
- [CrossRef] [Google Scholar]
- Potential of decursin to inhibit the human cytochrome P450 2J2 isoform. Food Chem Toxicol.. 2014;70:94-99.
- [CrossRef] [Google Scholar]
- Danazol Inhibits Cytochrome P450 2J2 Activity in a Substrate-independent Manner. Drug Metab Dispos.. 2015;43:1250-1253.
- [CrossRef] [Google Scholar]
- Anticancer Effects of Five Biflavonoids from Ginkgo Biloba L. Male Flowers In Vitro. Molecules.. 2019;24
- [CrossRef] [Google Scholar]
- Hepatocellular carcinoma. Nature Reviews Disease Primers.. 2021;7:6.
- [CrossRef]
- CYP2J2 - regulation, function and polymorphism. Drug Metab Rev.. 2016;48:351-368.
- [CrossRef] [Google Scholar]
- Important role of CYP2J2 in protein kinase inhibitor degradation: a possible role in intratumor drug disposition and resistance. PLoS One. 2014;9:e95532-e.
- [CrossRef] [Google Scholar]
- Identification of acetylshikonin as the novel CYP2J2 inhibitor with anti-cancer activity in HepG2 cells. Phytomedicine. 2017;24:134-140.
- [CrossRef] [Google Scholar]
- The inhibitory potential of Broussochalcone A for the human cytochrome P450 2J2 isoform and its anti-cancer effects via FOXO3 activation. Phytomedicine. 2018;42:199-206.
- [CrossRef] [Google Scholar]
- ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med.. 2020;52:192-203.
- [CrossRef] [Google Scholar]
- Phuc, N. M., Z. Wu, Y. O, et al., 2017. LKY-047: First Selective Inhibitor of Cytochrome P450 2J2. Drug Metab Dispos. 45, 765-769. https://doi.org/10.1124/dmd.117.075036.
- DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med.. 2008;45:1167-1177.
- [CrossRef] [Google Scholar]
- Cytochrome P450 2J2: Potential Role in Drug Metabolism and Cardiotoxicity. Drug Metab. Dispos.. 2018;46:1053.
- [CrossRef] [Google Scholar]
- Bilobetin induces kidney injury by influencing cGMP-mediated AQP-2 trafficking and podocyte cell cycle arrest. Phytomedicine. 2019;64:153073
- [CrossRef] [Google Scholar]
- DNA damage response, redox status and hematopoiesis. Blood Cells Mol Dis.. 2014;52:12-18.
- [CrossRef] [Google Scholar]
- Cytochrome P450 2J2: distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab Rev.. 2013;45:311-352.
- [CrossRef] [Google Scholar]
- Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules (Basel, Switzerland).. 2015;20:21138-21156.
- [CrossRef] [Google Scholar]
- Bilobetin, a novel small molecule inhibitor targeting influenza virus polymerase acidic (PA) endonuclease was screened from plant extracts. Nat Prod Res.. 2020;1–4
- [CrossRef] [Google Scholar]
- CYP2J2 Is a Diagnostic and Prognostic Biomarker Associated with Immune Infiltration in Kidney Renal Clear Cell Carcinoma. Biomed Res Int.. 2021;2021:3771866.
- [CrossRef] [Google Scholar]







