Translate this page into:
Isolation of antimicrobial peptides from seed harvester ant, Trichomyrmex scabriceps (Mayr) (Hymenoptera: Formicidae) and their antimicrobial assay
⁎Corresponding authors. shiva.kt@icar.gov.in (Kadanakuppe Thammayya Shivakumara), helansary@ksu.edu.sa (Hosam O. Elansary),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Antimicrobial peptides (AMPs) are used to treat many infectious diseases. Nowadays, AMPs enjoy special attention due to their novel mode of action and kill various pathogens such as fungi, bacteria and viruses. Among insects, the ant genus can provide a potential antimicrobial peptide against various microorganisms. In the present study, AMPs compounds were isolated and purified from the ant species Trichomyrmex scabriceps using reversed-phase high performance liquid chromatography (RP-HPLC). The extract from abdomen showed the highest protein content (4.60 µg/µl) compared to head and thorax. The HPLC profile of the abdomen showed 14 different peaks, of which the peaks eluting at the 6th, 8th, 17th and 23rd minutes had a higher intensity. The number of peaks was comparatively lower in the extracts from the head and thorax. The antimicrobial activity of the head, thorax and abdomen extracts of ants was evaluated against Gram-positive Staphylococcus aureus (MTCC 3160) and Gram-negative Escherichia coli (MTCC 2692) bacterial strains. Among the extracts tested, the abdomen alone exhibited a distinct zone of inhibition against E. coli (176.98 ± 3.36 mm2). However, the extracts of the head and thorax showed no zone of inhibition. In addition, none of the extracts showed a zone of inhibition against S. aureus. To our knowledge, this is the first report observed AMPs in T. scabriceps ant and their antimicrobial property against these bacterial strains.
Keywords
Trichomyrmex scabriceps
Formicidae
Antimicrobial peptides
RP-HPLC
Escherichia coli
Staphylococcus aureus
1 Introduction
The peptide science is steadily growing and this tremendous progression shows the significance of peptides and proteins as therapeutics (Chandudu et al., 2013). Several peptide drugs have been approved by Food and Drug Administration (FDA) and have reached the market, which demonstrates as effective drugs. The breadth of opportunity offered by peptide therapeutics demonstrates the present and future potential of the peptide chemistry field (Chandudu et al., 2013). In nature, AMPs are found in all classes of life (Reddy et al., 2004). The AMPs viz., cecropins, drosocin, attacins, diptericins, defensins, ponericins, drosomycin and metchnikowin are most explored in insect species (Mylonakis et al., 2016; Zang and Gallo, 2016). Anti-microbial peptides support the insects in the elimination of viral infections. AMPs help to regulate brain-controlled activities like sleep and non-associative learning. By influencing neuronal health, communication, and activity, they can affect the functioning of the insect nervous system. Expansion of the AMP repertoire and loss of their specificity is connected with the aging process and lifespan of insects. Furthermore, AMPs contribute to gut homeostasis by regulating the quantity of endosymbionts and decreasing the number of foreign microbiota. In turn, the presence of AMPs in insect venom limits infection spread among social insects, where prey may be a source of pathogens. (Staczek et al., 2023). Ants are the most prevalent venom-producing animals (Aili et al., 2014). Because ants live in high-density colonies, they are vulnerable to pathogen infection and transmission. A potential source of infection in predatory species can be food; therefore, in addition to cytolytic peptides, potent antimicrobial molecules must be present in the venom. Of major importance are linear cationic peptides with antibacterial activity against Gram-positive bacteria like, Bacillus cereus CIP 6624, B. megaterium ATCC 9885, B. stearothermophilus CIP 675, B. subtilis ATCC 6623, Enterococcus faecalis CIP 636, Lactococcus lactis ssp. Cremoris 116, Streptococcus pyogenes CIP 561, S. sanguinis CIP 55128, Listeria ivanovii LMA 94, L. monocytogenes ATCC 15313, Micrococcus luteus CIP 5345, S. aureus CIP 677, S. aureus LMA, S. epidermidis CIP 53134 and Gram-negative bacteria Escherichia coli CIP 548, E. coli, Enterobacter cloacae CIP 6085, Klebsiella pneumoniae CIP 8291, Proteus mirabilis LMA TP, Salmonella enterica CIP 813, Serratia marcescens LMA TP, Yersinia entericolitica CIP 6529, Alcaligenes faecalis CIP 6723, Flavobacterium meningosepticum CIP 6057, Pseudomonas aeruginosa CIP A22, P. putida LMA. Also show inhibition activity against Yeast like Saccharomyces cerevisiae LMA 720 and fungi like Aspergillus niger LMA 910, Botrytis cinerea LMA 950 (Aili et al., 2014). The Australian ant Myrmecia pilosula is responsible for 90% of life-threatening allergic reactions to ant stings. The venom of these ants contains two major protein allergens: pilosulin 1, which has cytolytic and hemolytic action and pilosulin 2 (pilosulin 3a), which only appears in the venom as a heterodimer (with pilosulin 3b) termed pilosulin 3a. It accounts for 80% of the peptides in venom and has antibacterial properties against (E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, B. subtilis, L. garvieae) and antifungal activity against (Candida albicans and Saccharomyces cerevisiae) (Yacoub et al.,2020). A few novel peptides named ponericins with antibacterial and insecticidal properties were purified and characterized from predatory ant Pachycondyla goeldii (Orivel et al., 2001). In the venom, the estimated peptide concentrations appear to be compatible with antibacterial activity in vivo. This suggests that in the ant colony, the peptides exhibit a defensive role against microbial pathogens arising from prey introduction and/or ingestion (Orivel et al., 2001). Recently identified a novel decapeptide U-MYRTX-MANr1 (NH2-IDPKVLESLV-CONH2) using a combination of Edman degradation and de novo peptide sequencing by using the crude venom of the predatory ant, Manica rubida and this observed severe fitness costs in the parthenogenetic pea aphid, Acyrthosiphon pisum (Heep et al., 2019). Three species of ants Viz., Solenopsis geminata, Solenopsis invicta, and Camponotus compressus were collected and test the antimicrobial activities of the whole body extract by using 10% of acetic acid solution against test microorganism viz. Escherichia coli, Staphylococcus mutans, Vibrio cholarae and Candida albicans and strong antimicrobial activity was marked in all the extracts in all the microorganisms (Chakraborthy et al., 2021). The glycine-rich and proline-rich peptides are active against Gram-negative strains of bacteria (Rozgonyi et al., 2009). Insect AMPs are very potent since their IC50 ranges in the submicromolar or low micromolar range. Presently, there are still no insect-derived AMPs on the market yet. However, insect AMPs can be exploited as an alternative to antibiotics (Jozefiak, 2017). The possibility of seed harvester ants having AMPs to protect the seeds stored in the granaries and antimicrobial properties. We hypnotised the interesting possibilities in Trichomyrmex scabriceps(Mayr) (Myrmicinae: Formicidae) and to date not been addressed. Based on the above facts in view, the present study was taken up to investigate the possible role of glandular secretions of the seed harvester ant, T. scabriceps for antimicrobial study in peninsular India.
2 Material and methods
2.1 Study site and collection of ants
The antimicrobial peptide compounds were analysed and tested for activity against Gram-positive and Gram-negative bacterial strains using the materials and techniques described. Antimicrobial peptides isolated and characterised from the seed harvester ant Trichomyrmex scabriceps (Mayr), as well as their bioassay, were reported. The current study was conducted at the University of Agricultural Sciences, Gandhi Krishi Vignana Kendra in Bengaluru, Karnataka, India (12° 57˝ N 72° 35˝ E, 930 m MSL), T. scabriceps workers were collected while foraging or by digging the nest in the open field. An aspirator was used to gather ants from the field. The ants were identified, and voucher specimens were stored at the University of Agriculture, Bengaluru, India. The ants were identified and voucher specimens were deposited at UAS, Bengaluru, India. The ants were immobilized at 4°c in a refrigerator at 10 min, rinsed with Millipore water and transferred to an aqueous solution containing 0.1% trifluroacetic acid (TFA) for further study (Fig. 1).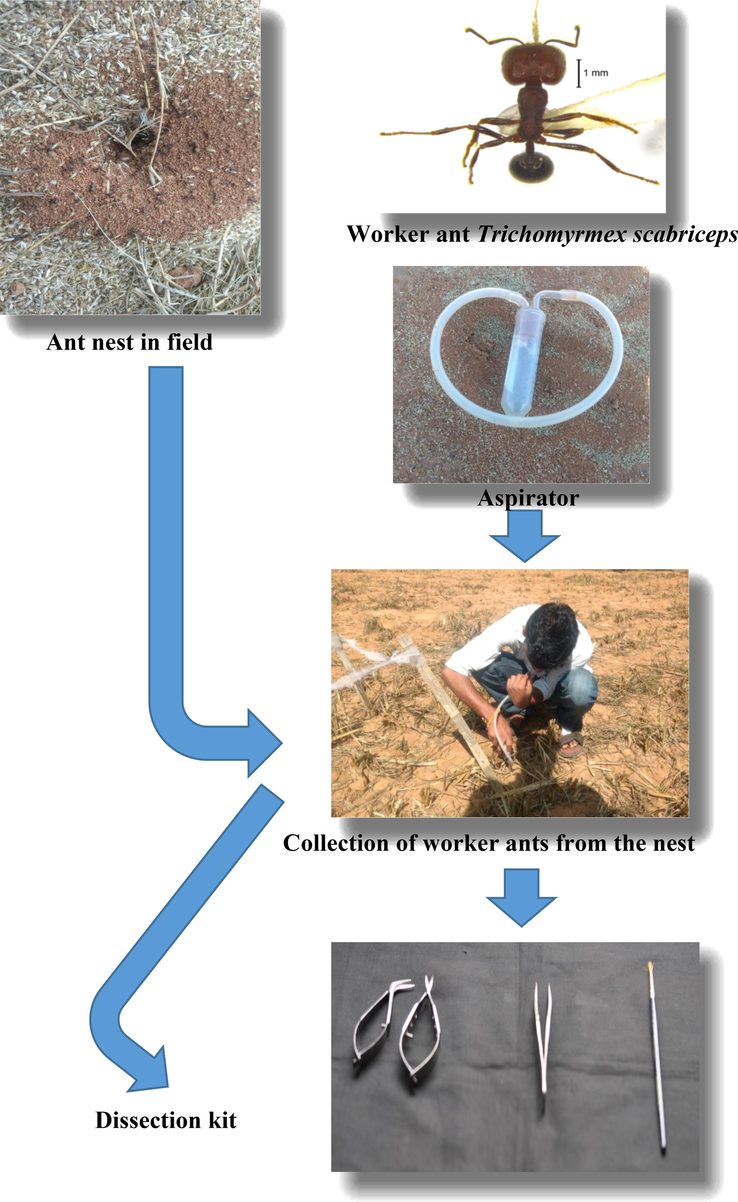
Collection of worker ants of Trichomyrmex scabriceps from the field.
2.2 Isolation of antimicrobial peptides compound from ants
The ants' body parts adhered with the soil and waste particle were removed properly and kept in ice flakes to inactive. Further, a known quantity of worker ants were taken and with the help of sterilized micro-scissors the whole insect body parts were dissected into three segments viz., the head, thorax and abdomen were separated and stored in 50 per cent acetonitrile solvent ((50 ml Acetonitrile solvent + 50 ml Distilled water) kept at −80 °C for further processing. Sonication is process of the act of applying sound energy to agitate particles in as ample. Ultrasonic frequencies (>20 kHz, Ultrasonic water bath Sidilu, India) were used for the uniform dissolution of the ant sample in an aqueous solution containing 50% of acetonitrile (ACN) solvent. Sonication was carried out for about 30 min to elute polar and nonpolar peptides from the cuticle of ants. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top (Susan et al., 2004). The ant samples were centrifuged (iMark™ Microplate Absorbance Reader-Bio-Rad) for 10 min at 10,000 × g rpm under 4 °C. After centrifugation, the supernatant was transferred to separate tubes and concentrated using a vacuum concentrator and the concentrated sample was freeze-dried/lyophilized and stored at −20 °C for further analysis.
2.3 Protein estimation
Protein estimation was performed by using the head, thorax and abdomen of worker ants by standard protocol Bradford assay (Bradford et al., 1976). Take 100 ml of Bradford reagent and the solution is mixed with 10 ml of 85 per cent ortho-phosphoric acid the volume will make up to 100 ml of distilled water, once its turns to brown colour finally it is ready for use. The reagent was then filtered through Whatman filter paper No. 1. Protein standards were prepared using Bovine Serum Albumin (BSA) at a concentration of 2 mg/ml using distilled water and stored at −20°C. Bio-Rad iMark™ Microplate Absorbance Reader and Bio-Rad's Costar 96–Well Flat–Bottom EIA Plate were used for monitoring the optical density (OD) of the samples which treated with reagent. The sample (5 µl) was taken in 96 well ELISA plate to which 290 µl of Bradford reagent was added, upon mixing the colour of the sample turns to blue from brown colour, resulting from the binding of the dye to the proteins, which in turn indicates the abundance of the proteins/peptides. The reading absorbance of the mixture was measured at 595 nm using a Shimadzu UV–Visible Spectrophotometer (UVmini-1240). Finally protein content was estimated by a using regression equation.
2.4 Isolation and purification of the active fractions
To find out peptide fractions, RP-HPLC was used on the samples. The column (C18), was used with acetonitrile (CH3CN, ACN) HPLC grade, Milli-Q water, Trifluoro acetic acid (TFA), Methanol and HPLC solvent delivery system with binary gradient capability and a UV detector. The injection volume was 20 μl, and the sample temperature and column temperature were 4˚C and 40˚C respectively. Diode array detector (DAD) containing 226 nm, 4 nm (1.00). Buffer A: 0.1% (v/v) TFA in water and Buffer B: Acetonitrile/ACN (CH3CN) − 80%:20% (Water: 0.1%TFA) served as the mobile phase solvents. The liquid chromatography technique is 0–5 % ACN for 5 min, 5–95 % ACN for 50 min, 95 % ACN for 5 min and 95–5% for 5 min. The crude extracts of the head, thorax and abdomen were prepared at a concentration of 1 μg of protein in 1 μl of 50% ACN: water for RP-HPLC analysis.
Using a C-18 reverse-phase analytical column (Phenomenex: 5 µm particle size; 250 × 4.6 mm column) installed on a Shimadzu HPLC, inject 20 µl of crude extract in to the column. The crude samples were fractioned using the linear binary gradient method at a flow rate of 1 ml/min. A UV-DAD detector was used to monitor the UV detection of the eluted peptide peaks at 280 nm during the 65 min run period for the linear gradient elution of solvent B using UV-DAD detector (SPD-M20A). For all the HPLC separations of extract components, the peptide column, gradient protocol, and UV detection were kept constant.
2.5 Identify the antimicrobial peptide constituents of the exocrine gland secretions
The abdomen extract was considered for further consideration for the separation and collection of various peptide fractions using RP-HPLC. The repeated fractionation of abdominal extracts was carried out based on the retention time and peak profile. A total of 13 RP-HPLC isolated peptide fractions were collected from RP-HPLC, they were lyophilized to concentrate the samples by reducing their volume. The peptide fractions were initially concentrated using a roto-evaporator, followed by lyophilisation/freeze-drying. Lyophilised fractions were subjected to protein estimation and (2 μg protein/1μl concentration) to determine the active fractions, which exhibit antimicrobial activity.
2.6 Antimicrobial assay
In the present study, the antimicrobial activity was evaluated against Gram-positive Staphylococcus aureus (MTCC 3160) and Gram-negative Escherichia coli (MTCC 2692) strains received from the Institute of Microbial Technology (IMTECH), Chandigarh, India. These cultures were maintained on nutrient agar in slants. The strains were frequently sub-cultured and used in the study.
2.6.1 Protocol of antimicrobial assay
A spot-on-lawn assay fallowing a standard procedure was used to measure antimicrobial activity. In 20 ml of nutrient broth medium, a pure culture of standard microorganisms was cultivated overnight before being serially diluted to a concentration of 1 × 106 colony-forming units (CFU)/ml. A pre-sterilised 90 mm Petri plates was filled with 50 μl of a broth culture of overnight-grown microorganisms, and then 15–20 ml of nutrient agar was added and left to set. 5 μl of the test samples were applied on the surface of semisolid agar after the nutritional agar media had solidified. Fallowing this, 2.5 μl of 2.5 μg of tetracycline (Himedia SD037) was used as appositive control for Gram-Positive Staphylococcus aureus (MTCC 3160) and Gram-negative Escherichia coli (MTCC 2692), additionally, the crude extract reconstitution with acetonitrile serves as negative reference. The head, thorax and abdomen lyophilized extracts were collected in triplicates at a protein concentration of 20 μg protein concentration in 10 μl (i.e.2 μg/1μl concentration of protein). Bacterial growth was monitored overnight in petri plates at 37˚C. Using a digital vernier caliper, the diameter of the zone of inhibition against microorganisms in petri plates was determined. The inhibition area was calculated using the following formula and expressed in mm2.
Where, r = radius of the inhibition zone in mm.
Π = 22/7 peaks observed during the run time and finally confirm the presence of peptides in extracted samples. (Andrews, 2002).
3 Results
3.1 Extraction of antimicrobial peptides from worker ants
In the current study, 11,000 T. scabriceps worker ants were dissected and extracts were processed. The protein concentration was measured using the Bradford assay. Further, the antimicrobial activity of these extracts was evaluated.
3.2 Protein content of crude extracts
The protein concentration of the extracts was determined using the protein standard curve Bovine Serum Albumin (BSA) (Fig. 2). The abdomen extract had the greatest total protein content (4.60 g/l), followed by the head (3.84 g/l) and thorax (2.44 g/l) (Fig. 3).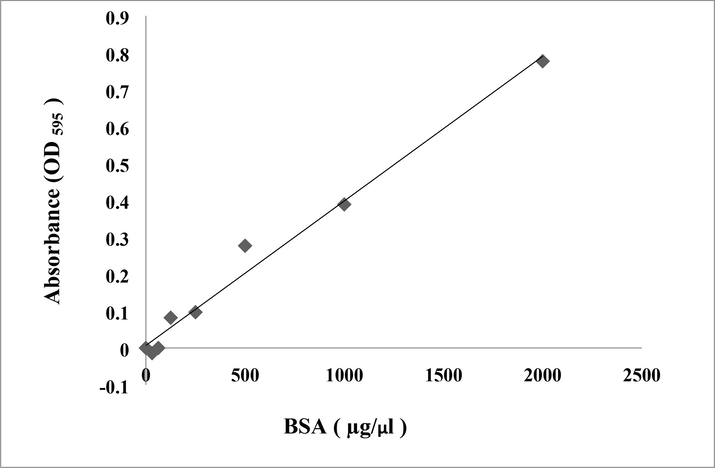
Standard curve of bovine serum albumin (BSA) at concentration of 2 mg/ml using Bradford method.
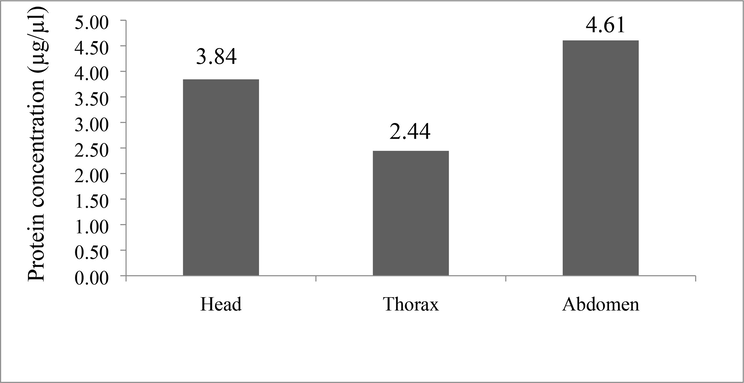
The protein concentration of ants body parts viz., head, thorax and abdomen estimated by Bio-Rad protein micro assay using Bovine Serum Albumin (BSA) at concentration of 2 mg/ml, OD595 using Bradford method.
3.3 Peptide profiling of crude extracts from ants by RP-HPLC
The crude extracts were separated using RP-HPLC with a C18 column mounted on Shimadzu HPLC and a flow rate of 0.8 ml/min, and the eluting peaks were observed at 280 nm. The HPLC column and wavelength are designed specifically for identifying peptide molecules. The peptide profiling was done twice, once with a batch of extracts produced from 4000 ants and once with 7000 ants. The HPLC profile of head, thorax, and abdomen extracts revealed the presence of several potential peptide peaks that eluted at various retention times. The RP-HPLC profiles of two batches of ant extracts (4000 and 7000 ants) were examined. (Fig. 4a & b). The RP-HPLC profile of 7000 and extracts belly revealed 14 peaks. However, the intensity of the peptide peaks and retention times varied, suggesting the presence of various types of peptides. The HPLC profile of the abdomen revealed 14 unique peaks, with the peaks eluted at the 6th, 8th, 17th, and 23rd minutes being the most intense (Fig. 5). The number of peaks in the extracts from the head and thorax were relatively lower (Fig. 6 a&b). After analysing the chromatogram peaks, the fractions were gathered by HPLC to determine the potential fraction of the abdomen extract exhibiting antimicrobial activity. A total of 14 samples were obtained, as shown in the chromatogram. Repeated RP-HPLC of abdomen extract was performed to gather a sufficient amount of each of the 14 extracts for bioassays to evaluate antimicrobial activity.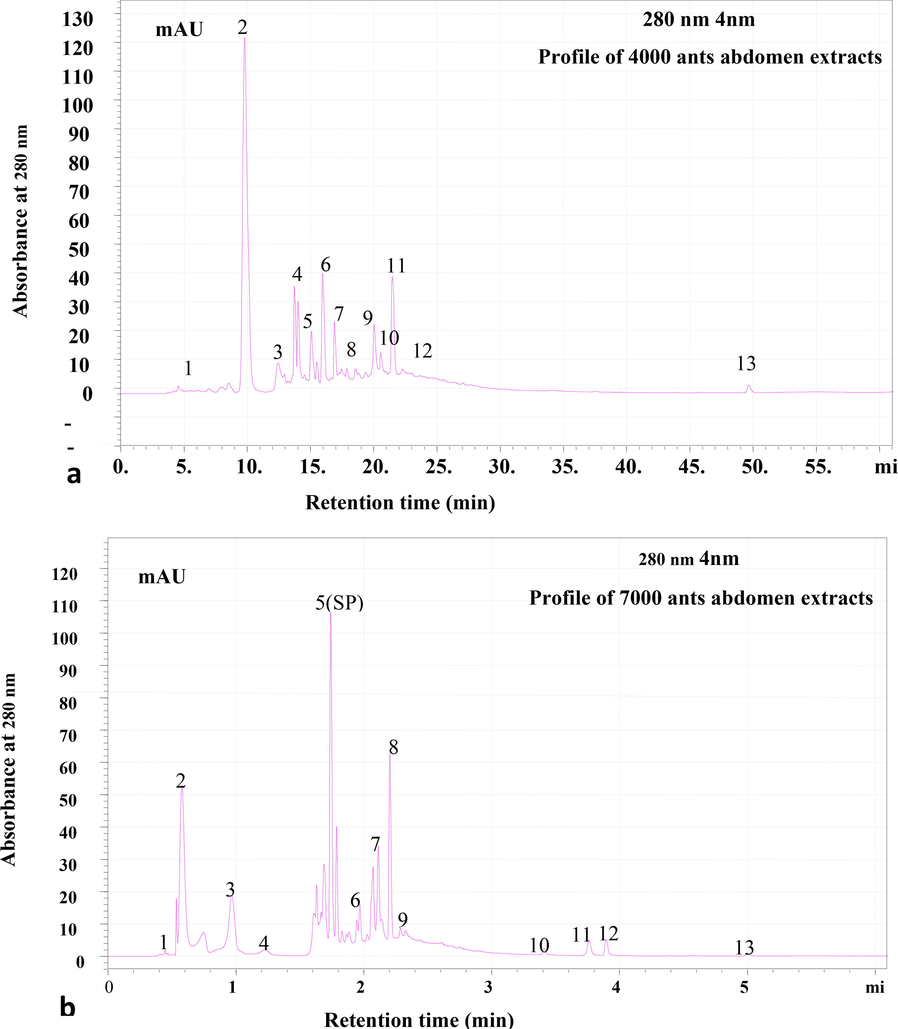
RP-HPLC profile of (a) 4000 and (b) 7000 ants abdomen extracts. Lyophilized extract of abdomen was subjected to Reverse- Phase HPLC with gradient (0–5 % ACN for 5 min, 5–95 % for 50 min, 95 % ACN for 5 min and 95–5 % for 5 min) of Acetonitrile (ACN) and water containing 0.1 % TFA over 61 min at flow rate of 0.8 ml /min. Eluted peaks were monitored at 280 nm.
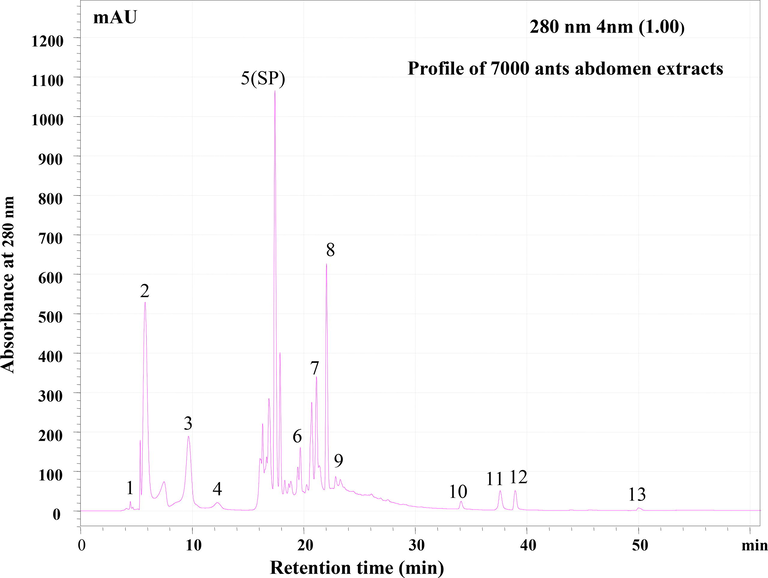
RP-HPLC profile of 7000 ants abdomen extracts. Lyophilized extract of abdomen was subjected to Reverse- Phase HPLC with gradient (0–5 % ACN for 5 min, 5–95 % for 50 min, 95 % ACN for 5 min and 95–5 % for 5 min) of Acetonitrile (ACN) and water containing 0.1 % TFA over 61 min at flow rate of 0.8 ml /min. Eluted peaks were monitored at 280 nm. Each peaks represented with number.
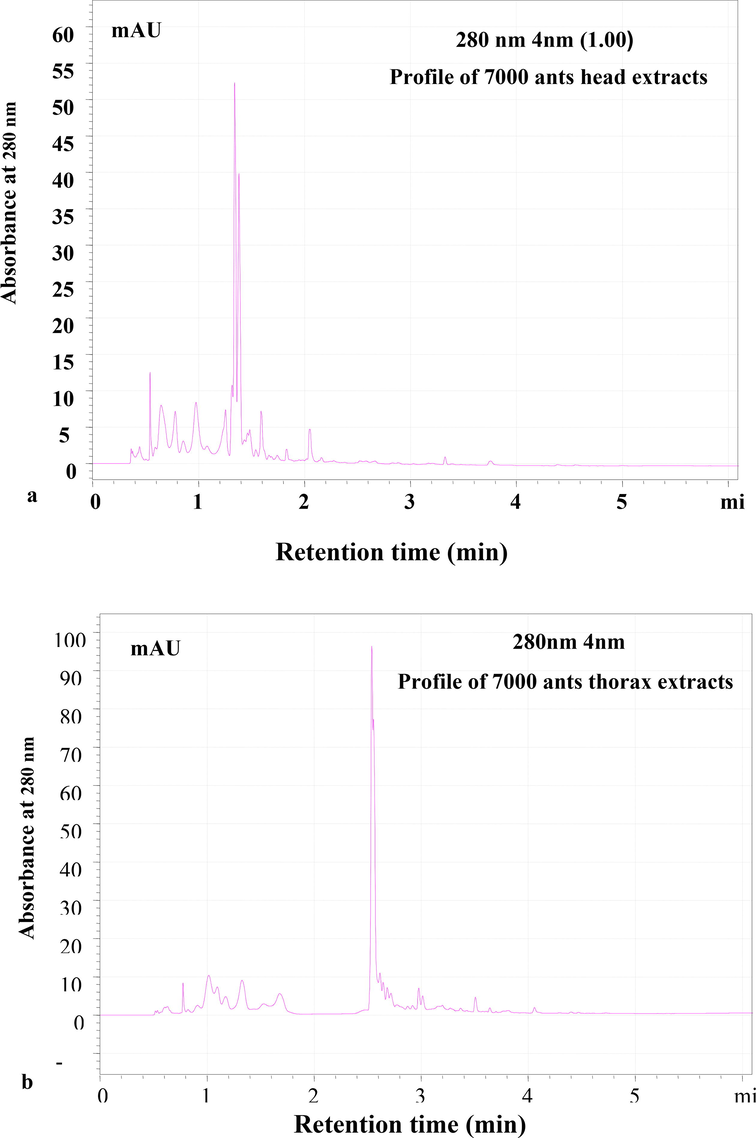
RP-HPLC profile of 7000 ants a. head extracts and b. thorax; Lyophilized extract was subjected to Reverse- Phase HPLC with gradient (0–5 % ACN for 5 min, 5–95 % for 50 min, 95 % ACN for 5 min and 95–5 % for 5 min) of Acetonitrile (ACN) and water containing 0.1 % TFA over 61 min at flow rate of 0.8 ml /min. Eluted peaks were monitored at 280 nm.
3.4 Antimicrobial assay
The crude extract was tested for antimicrobial action against S. aureus and E. coli. Among the extracts examined, extracts of the abdomen alone demonstrated a clear zone of inhibition against E. coli (176.98 3.36 mm2). However, the zone of inhibition was not visible in the extracts of the head and thorax (Fig. 7). However, none of the preparations demonstrated an inhibition zone against S. aureus (Fig. 8). Furthermore, neither the head nor the thorax extracts inhibited bacterial growth in vitro to any visually detectable amounts in the spot-on-lawn assay against either test organism, whereas the abdomen extract did not inhibit S. aureus. However, the antimicrobial activity of abdominal extracts against E. coli was relatively modest when compared to the positive control, tetracycline (1 g/l). Tetracycline inhibited E. coli with a zone of inhibition of 294.99 19.90 mm2 and S. aureus with a zone of inhibition of 232.73 6.24 mm2 (Fig. 9).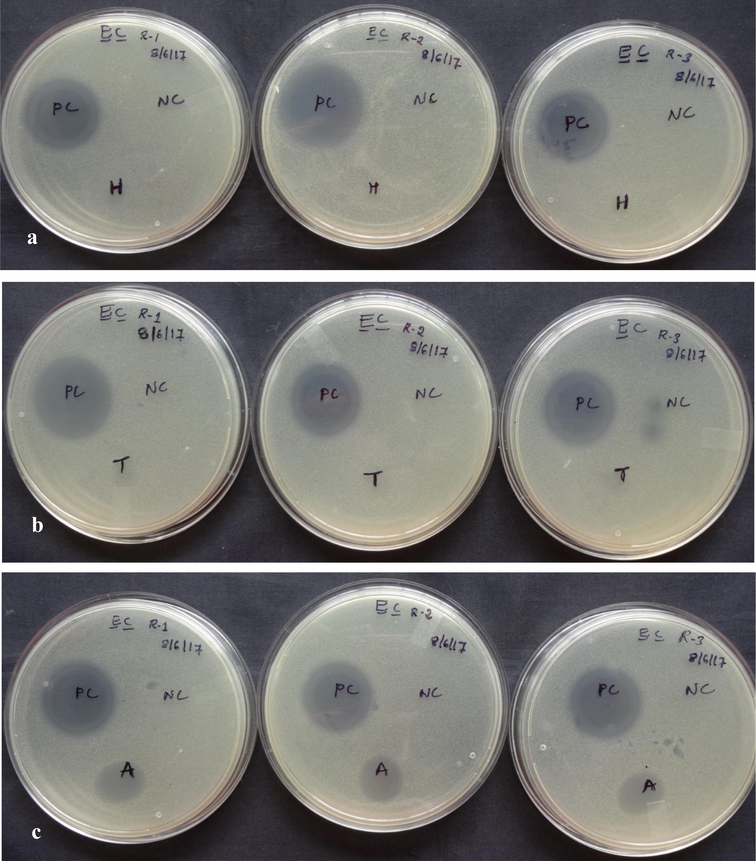
Antimicrobial activity of crude peptides a. head, b. thorax and c. abdomen of Trichomyrmex scabriceps using Escherichia coli (gram –ve bacteria) by spot on lawn bioassay method after 24 h of treatment (in vitro) (PC-Positive Control-Tetracycline 1 µg/1µl) (NC-Negative Control-Acetonitrile 50%), H-Head, T-Thorax and A-Abdomen.
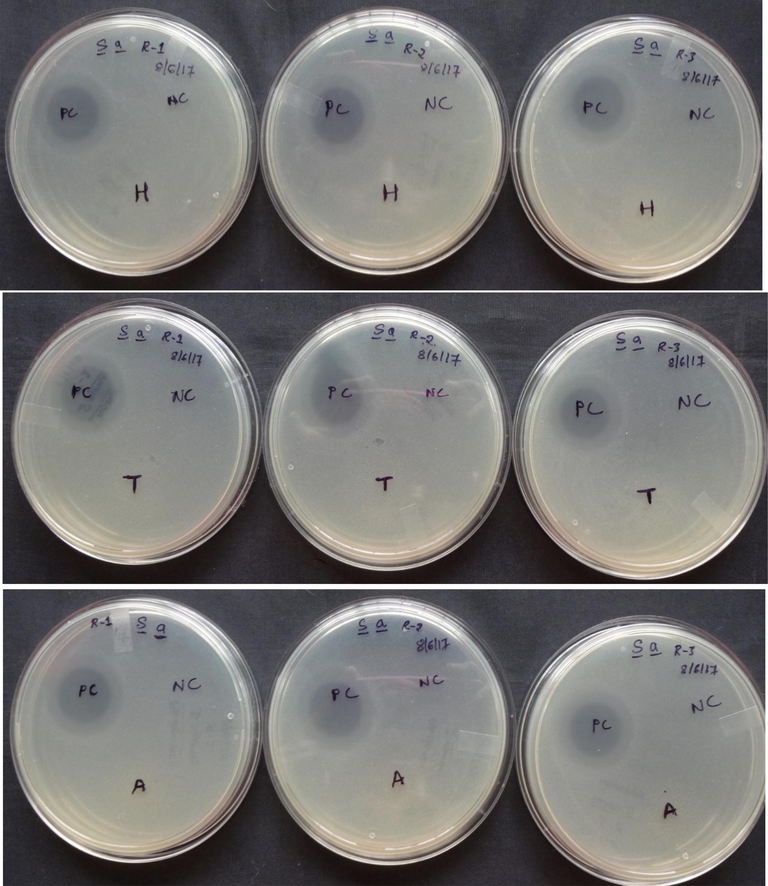
Antimicrobial activity of crude peptides a. head, b. thorax and c. abdomen of Trichomyrmex scabriceps using Staphylococcus aureus (gram + ve bacteria) by spot on lawn bioassay method after 24 h of treatment (in vitro) (PC-Positive Control-Tetracycline 1 µg/1µl) (NC-Negative Control-Acetonitrile 50%), H-Head, T-Thorax and A-Abdomen.
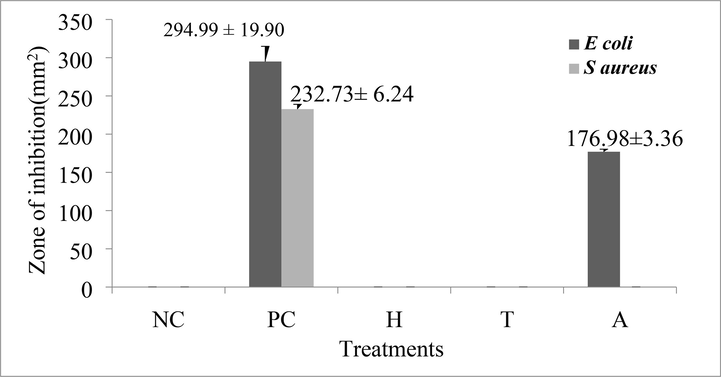
Antimicrobial activity (in vitro) of crude peptides extract of Trichomyrmex scabriceps in spot on lawn bioassay method using Escherichia coli (gram –ve bacteria) and Staphylococcus aureus (gram + ve bacteria) at 24 h of post treatment (PC-Positive Control-Tetracycline 1 µg/1µl) (NC-Negative Control-Acetonitrile 50%). H-Head, T-Thorax, A-Abdomen.
3.5 Protein estimation of peptide fractions
The fractions collected were pooled as independent peaks and lyophilized to decrease volume. Thus obtained dry samples were dissolved in 50% ACN in water and protein estimation was performed using the Bradford technique. The protein standard curve was created using BSA, and the protein concentration of different fractions is shown (Fig. 10). Protein concentrations were greatest in fractions 3, 9, and 6, which eluted at the 9th, 23rd, and 20th minutes (5.74, 5.45, and 5.14 ug/ul, respectively). However, fraction 8 had the lowest protein content of 1.90 g/l (Fig. 11).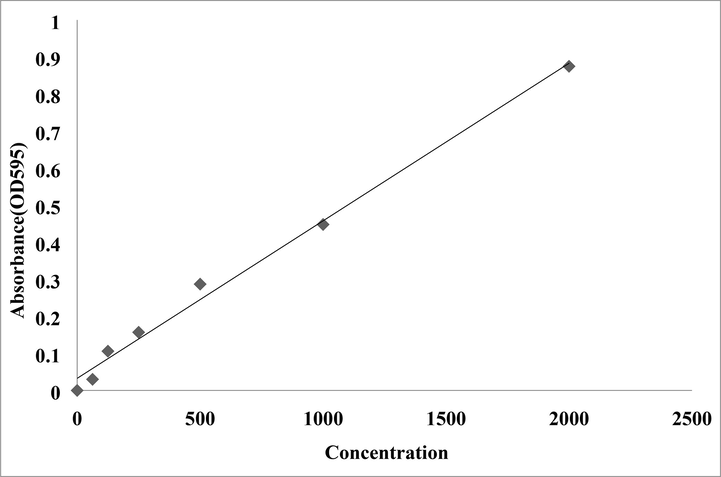
Standard curve of abdomen extract fractions obtained from RP-HPLC using Bovine Serum Albumin (BSA) at concentration of 2 mg/ml, OD595, by Bradford method.
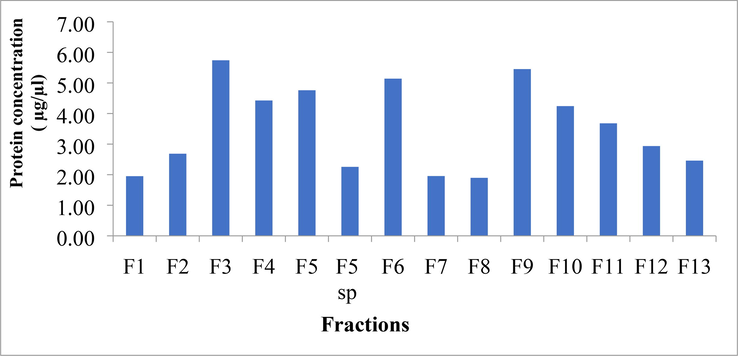
Protein concentration of individual fractions (F) of abdomen extracts obtained from RP-HPLC. (F5 SP-Fraction 5 Sharp peak).
3.6 Antimicrobial activity of individual fractions against Escherichia coli
All the fractions collected from extracts of the abdomen were reconstituted at 2 µg/µl and 10 µl of the reconstituted fraction was used to assess antimicrobial activity against E. coli. Among the 14 fractions tested, only two fractions, fraction-1 and fraction-2 exhibited antimicrobial activity. Among the two fractions, fraction-2 exhibited antimicrobial activity with inhibition of clear zone (475.39 ± 11.72 mm2) when compared to fraction 1 (22.06 ± 17.09 mm2) and the positive control tetracycline (191.26 ± 11.21 mm2) (Fig. 12).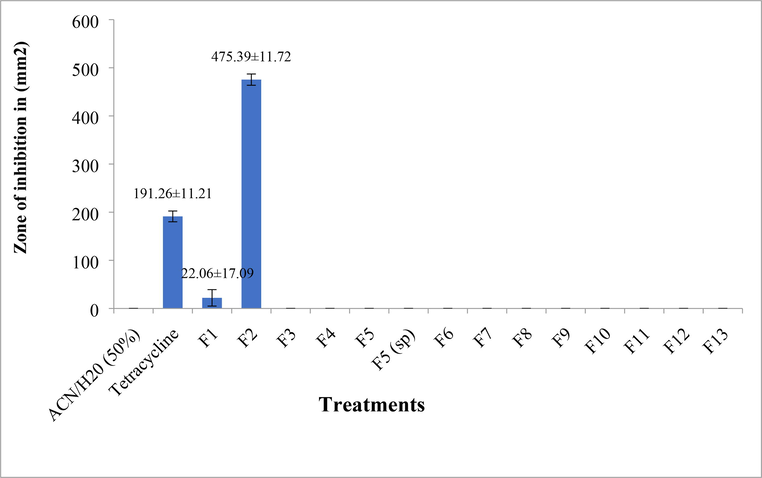
Antimicrobial activity (in vitro) of isolated peptide HPLC fractions of Trichomyrmex scabriceps in spot on lawn bioassay method, using Escherichia coli (gram –ve bacteria) at 24 h of post treatment (PC-Positive control-Tetracycline 1 µg/1µl) (NC-Negative control-Acetonitrile 50%).
4 Discussion
Insect defence mechanisms primarily consist of AMPs (Brey and Hultmark, 1998). Anti-microbial peptides have a wide range of activity methods that help insects clear microbial infections. (Ganz, 2003; Hoffmann, 2003). Ants (Hymenoptera: Formicidae) are a taxonomically diverse collection of hymenopterans, with over 16,032 species currently extant (Antweb, 2012). Ants' immune systems contain AMP-containing metapleural gland fluids (Schluns and Crozier, 2009). The capacity of ants to colonise and exploit every geographical location (Wilson, 1990). As hypothesised by the emerging field of ecological immunology, continuous exposure to microbes associated with their resources may have resulted in the selection of a strong immune system. (Rolff and Siva-Jothy, 2003; Schulenburg, 2009). Ants, particularly harvester ants, play an important role in the dynamics of plant communities by serving as seed dispersal agents, seed predators, or both. These ants forage for vegetation and plant seeds, which they then transport back to their colony. Myrmecochory, or seed dispersal mediated by the elaiosome, a lipid-rich seed appendage that primarily attracts non-granivorous ants and provides rewards for seed dispersal, and diszoochory, or seed dispersal performed by seed-harvesting ants that is not mediated by any particular seed structure, are the two main mechanisms by which ants disperse seeds. While the former has historically been regarded as a mutualism, the latter is commonly regarded as an antagonism. (Arnan et al., 2012). However, what is fascinating about the interplay of seed harvester ants is that the ants store the seeds in underground nests in granaries. The seeds are kept for at least a year, until the following crop a year later. It's intriguing to speculate on how the ants keep the seeds without contaminating them with microbes. Preliminary research with the harvester ant Trichomyrmex criniceps from India has revealed that ant whole-body preparations contain at least two antimicrobial peptides. (Bhagavathula et al., 2017). However, there is no indication that these AMPs are used by T. criniceps to protect the seeds. This research was undertaken in order to determine the source of AMPs in the head, thorax, and abdomen. If the ants are AMPs, then the extract of the head, where the salivary glands are found, should be a source of AMPs. The research was thus carried out to obtain a quantitative description in order to identify the source of AMPs in insect bodies, namely the head, thorax, and abdomen. Furthermore, real experiments were conducted to evaluate ants' ability to inhibit microbial growth. T. scabriceps worker ant crude extracts were evaluated for antimicrobial action. The extract from the belly inhibited E. coli but not S. aureus. Head and thorax extracts, on the other hand, did not suppress either pathogen. Thus, it is hypothesised that the antimicrobial activity of abdomen extract is due to secretions from different glands located in the abdominal area.
The ant abdomen extract was fractionated using RP-HPLC to visualise the peptide profile and isolate the peptide peaks. The extract from the abdomen alone had more peptide/protein peaks than the other two extracts, indicating the existence of different/unique peptide fractions in the abdomen extract. It's conceivable that the peptides found in the head and thorax extracts aren't truly antimicrobial. The second possibility is that the extracts recovered peptides at extremely low concentrations, possibly well below the Minimum inhibitory concentration (MIC), and thus the bioassays failed to identify antimicrobial activity. Because the abdomen extract alone demonstrated antimicrobial activity, an effort was made to isolate the fraction responsible for the activity. Based on the retention time for each eluting peak, the abdomen extract was submitted to repeated RP-HPLC, yielding a total of 14 peptide fractions. The antimicrobial activity of these components against E. coli was investigated. Only fractions 1 and 2 demonstrated antimicrobial activity, while the remaining fractions did not suppress microbial growth. Despite having lower protein concentrations than the other fractions, fractions 1 and 2 showed antimicrobial activity, which could be attributed to the presence of peptide(s) with significant antimicrobial activity. Previously, Bhagavathula et al. (2017) used a similar protocol to report two new AMPs from the related species T. criniceps (Crinicepsin-1 and Crinicepsin-2). Ponericins peptide was previously isolated from Pachycondyla goeldii and demonstrated antimicrobial action as well as neurotoxicity. (Orivel et al., 2001). Rifflet et al. (2012) extracted bicarinalin from the Myrmecine ant Tetramorium bicarinatum. Ant venom peptides have a wide spectrum of antimicrobial activity, and these peptides play an essential role in in vivo microbial activity. (Orivel et al., 2001; Heep et al., 2019; Chakraborty et al., 2021; Hoffmann, 2010). The isolation and characterization of AMPs from T. scabriceps could be useful in the development of powerful drugs for the treatment of microbial infections linked with a variety of diseases.
5 Conclusions
Insects have powerful antibacterial defence reactions and primarily depend on innate immunity to defend themselves against pathogens. T. scabriceps extracts from the head, thorax, and abdomen were tested for antimicrobial activity against Escherichia coli and Staphylococcus aureus. Surprisingly, the extract of the abdomen alone was antimicrobial against E. coli. None of the other extracts demonstrated antimicrobial activity, suggesting that secretions from various glands in the abdomen are significant antimicrobial agents. RP-HPLC was used to separate the extract from the abdomen with substantial antimicrobial activity into 14 fractions based on retention time. Only two of these 14 fractions, fractions-1 and fraction-2, performed well. These AMPs will be promising candidates for different biological applications in the future.
Author contribution statement
HA and KC Conceptualisation and designed the experiments. HA performed the experiments. KTS performed statistical analyses and original draft preparation KTS, RC, HOE, SRMS, AME and RAT provided editorial advice. All authors agreed on final approval for publication.
Acknowledgements
The authors are thankful to the Head, Department of Entomology, and University of Agricultural Science Bengaluru for providing the research facilities. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R743), King Saud University, Riyadh, Saudi Arabia.
Funding
Research supporting project (RSPD2023R743), King Saud University for publication of this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Andrews, J. M., 2002. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother 49(6), 1049-1049.
- Antweb. Retrieved from: http://www.antweb.org 2012 (12.08.12).
- Arnan, X., Horas, R. M., Rodrigo, A., Retana, J., Adler., 2012. Uncoupling the effects of seed predation and seed dispersal by granivorous Ants on plant population dynamics. In: Frederick R, editor. Plos one. pp. 1-11.
- Characterization of two novel antimicrobial peptides from the cuticular extracts of the ant Trichomyrmex criniceps (Mayr) (Hymenoptera: Formicidae) Arch. Insect. Biochem. Physiol.. 2017;94:21381.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analy. Chemi.. 1976;72:248-254.
- [Google Scholar]
- Molecular mechanisms of immune responses in insects. London: Chapman & Hall; 1998.
- Characterization of antimicrobial peptides from local forest dwelling ants: in-vitro screening for antimicrobial activity. Eur. J. Biol. Biotechnol.. 2021;2:20-25.
- [Google Scholar]
- The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol.. 2003;43:300-304.
- [Google Scholar]
- Identification and functional characterization of a novel insecticidal decapeptide from the myrmicine ant Manica rubida. Toxins. 2019;11:562.
- [Google Scholar]
- Engberg RM. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed Sci.. 2017;26:87-99.
- [Google Scholar]
- Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. Lond. B. 2016;371:20150290.
- [Google Scholar]
- Ponericins, new antibacterial and insecticidal peptides from the venom of the ant Pachycondyla goeldii. J. Bio. Chem.. 2001;276:17823-17829.
- [Google Scholar]
- Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents. 2004;24:536-547.
- [Google Scholar]
- Identification and characterization of a novel antimicrobial peptide from the venom of the ant Tetramorium bicarinatum. Peptides. 2012;38:363-370.
- [Google Scholar]
- The antibacterial effect of a proline-rich antibacterial peptide A3-APO. Curr. Med. Chem.. 2009;16:3996-4002.
- [Google Scholar]
- Molecular and chemical immune defenses in ants (Hymenoptera: Formicidae) Myrmecol. News. 2009;12:237-249.
- [Google Scholar]
- Introduction. Ecological immunology. Philos. Trans. R. Soc. Lond., B Biol. Sci. PHILOS. T. R. SOC. B. 2009;364:3-14.
- [Google Scholar]
- Staczek, S., Cytrynska, M., Zdybicka-Barabas., 2023. Unravelling the Role of Antimicrobial Peptides in Insects. Int. J. Mol. Sci.24, 5753.
- Susan, R., Mikkelsen., Corton, E., 2004. Bio analytical Chemistry, Ch. 13. Centrifugation Methods. John Wiley & Sons, pp. 247-267.
- Success and dominance in ecosystems: the case of the social insects. Nordbunte, Germany: Ecology Institute; 1990.







