Translate this page into:
Antioxidative effect of Aloe vera against malathion induced neurotoxic response in Wistar rats
⁎Corresponding authors. yunsuk.huh@inha.ac.kr (Yun Suk Huh), sharmabi@yahoo.com (Bechan Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Insight about the impact of malathion on human physiology is still a challenge in environmental health. The present work was focused on the neurotoxic effects of malathion followed by impact of Aloe vera, if any, which is not known. The malathion has significantly altered the levels of the body weight, brain weight and relative weight of the brain. The significant levels of alteration were also observed in the levels of antioxidant potential and oxidative stress biomarkers such as NO, PCO, MDA, GSH, SOD, CAT, GST, and GPx antioxidants. The increased levels of SOD, CAT, GPx and decreased levels of GST were observed in the malathion treated experimental rats. In addition of these, the contents of inflammatory markers such as IL-6, COX-2, TNF-α, and NF-κB were also found to be altered significantly. A significant alteration was also recorded in the activity of AChE. The histological examination of liver tissue sections revealed the severe injury to the central vein and hepatic cords due to malathion toxicity. However, the pre-administration of A. vera markedly ameliorated the neurotoxic effect of malathion. These results suggested that the metabolites present in A. vera may be utilized as a potential and sustainable supplement in the proper management of pesticide induced neurotoxicity in association with the relevant therapeutics.
Keywords
Malathion
Free radicals
Oxidative stress
Inflammatory response
Antioxidants
Phytochemicals
1 Introduction
There are certain compounds which are used for the control of pests that are referred to as pesticides. The organophosphate-based pesticides (OP-Ps) are the most commonly used group of pesticides (Zeng et al., 2021). The residual amounts of these OP-Ps have been found in the water, soil, vegetables, grains, and other foods products as one of the major contaminants (Li et al., 2021). The exposure of these chemicals can be toxic and leading to the several adverse impacts on the health of organisms. According to the reports, it has been estimated that, only 2–5% of the overall sprayed insecticides reach to the pest and the remainder spreads in the biotic and abiotic environment (Khuman et al., 2020).
The OP-Ps has been shown to impose its toxic effects primarily on the nervous system by disrupting the activity of acetylcholinesterase (AChE) (Phillips et al., 2021). The inhibition of AChE activity has been facilitated via covalent interaction between phosphate group of pesticide and the OH group of serine present in the catalytic site of AChE (Bartling et al., 2007). They disturb the balance of Na+/K+ ion on the nerve fibers (Gutierres et al., 2012), and thereby making them to transmit constantly (Bartling et al., 2007) which lead in the development of diseases. Due to the lipophilic nature of OP-Ps, their intestinal assimilation and distribution to tissues and organs are very high, which eventually lead to several pathological anomalies (Selmi et al., 2018). OP-Ps exposure have been reported to affect the biotransformation process of different xenobiotics (Lasram et al., 2009) and induce oxidative stress (OS) by producing the ROS (Yang et al., 2000).
Malathion is a member of OP-Ps, which is a usually applied to get rid of parasites, flies, pests to protect the stored grains and disease-causing arthropods, agricultural and veterinary practices (Abdelfattah and El-Bassiony, 2022). Major routes for the malathion exposure include consumption of contaminated water and food, inhalation during application, and dermal contact (Sapbamrer and Hongsibsong, 2019). Currently the management of malathion toxicity involves sanitization, antidotes (atropine and pralidoxime), and circulatory or/and respiratory support. However, the disadvantages of these agents include the requirement of their administration within restricted time period after the exposure of malathion and they mainly emphasis on easing the muscarinic and nicotinic effects of intoxication, without any substantial influence on the oxidative injury (Purser et al., 1992). The primary mechanism of OP-Ps is to induce toxicity through inhibiting the AChE, disturbing the functions of mitochondria and disrupting the hormonal secretion and activity (Mnif et al., 2011). In addition, several reports have highlighted the OS as a probable mechanism of malathion toxicity (Coban et al., 2015, Ince et al., 2017).
Traditionally, the herbs were identified as the any flora that is being utilized in the diet for the aroma devoid of any nutritious value (Giannenas et al., 2020). However, in recent times, herbs are known as the sources of a variety of active phytochemicals (Guldiken et al., 2018). Antioxidants present in plants are the constituent nutrients along with their established free radical-quenching abilities. Thus, in addition to α-tocopherol, carotenoids, and ascorbate, plant-based medications may also comprise flavonoids, polyphenols, and flavoproteins. The plant derived medicines have been an integral component of our conventional system for medication since ancient civilizations. Due to the least side effects and low cost, the herbal medicines have arose as the greatest source of antioxidative molecules which can be utilized as a sustainable additive with various therapeutic agents against many disorders (Gupta et al., 2015).
Most of the herbal principles including A. vera are the rich source of antioxidant molecules (Kumar et al., 2019). A. vera was shown to contain huge quantity of secondary metabolites with antioxidative property (López et al., 2017). This plant has been shown to exhibit numerous pharmacological activities for instance antimicrobial, immune booster, antioxidant, hypoglycemic, antitumor, hypolipidemic, antiseptic, and anti-inflammatory (Gupta et al., 2021). Thus, A. vera formulations can act as multi-faceted therapeutic agent against several disorders. However, extensive investigations are required to investigate the therapeutic potential of A. vera for developing a new drug molecule to deal with the health conditions. This finding may be fruitful in the development and better understanding of potential and harmless plant-based medication. A. vera-based principles might be further improved and authenticated for it’s enhanced actions by means of bioinformatic tools. Since it is easy to grow this plant, its products can prove highly profitable, safe, and more efficient against various diseases.
The extensive literature survey indicated that malathion has the potential to induce OS. However, the ameliorating potential of A. vera has not been explored in contrast to the neurotoxicity induced by malathion. Considering this, the current investigation was attempted to depict the differential impacts of malathion and A. vera in rat brain.
2 Materials and methods
2.1 Chemicals
Malathion was procured from Hindustan Insecticides Ltd. (a Government of India Enterprise) New Delhi-India in liquid form having 50% W/W purity. Acetylthiocholine iodide and hydrogen peroxide was of from TCI, Japan. Triton X-100, pyrogallol, ethylenediaminetetraacetic acid, sodium acetate, sodium carbonate, sodium bicarbonate, sodium hydrogen phosphate, sodium dihydrogen phosphate, and sodium hydroxide were from MERK. Follin’s reagent, reduced nicotinamide adenine dinucleotide, reduced glutathione, 1-chloro-2,4-dinitrobenzene, glycerol, sodium pyruvate, tris-base, sodium chloride, and bovine serum albumin were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. ELISA kit for COX-2, IL-6, TNF-α and NF-κB procured from KRISHGEN Biosystems, India. All other chemicals used were of analytical grade.
2.2 Animals
The male Wistar rats aging about 8 weeks, weighing 150 ± 5 g were procured from the Central Drug Research Institute (CDRI)-Lucknow, India. All the animals were acclimatized at ambient temperature and environmental conditions in polypropylene cages in the laboratory conditions. Each animal group contained six rats in polypropylene cage. The animals had allowed free access to water and food. The experimental procedures were designed and performed according to the guidelines of Institutional Animal Ethical Committee (IAEC), University of Allahabad, Prayagraj (CPCSEA Registration Number: 839/GO/Re/04/CPCSEA, 1/10/2004).
2.3 Collection of plant and storage of plant extract
The fresh young leaves of the A. vera (family Asphodelaceae) were collected from Roxburgh Botanical Garden, University of Allahabad-Prayagraj, India during the period of March 2022 and freed from foreign matter. The plant was identified and authenticated by our eminent Professor of Taxonomy, University of Allahabad, Prayagraj, U.P. India. The leaves were dried in shade at room temperature. These shade dried Aloe leaves were grinded into fine powder followed by the preparation of extract (1:10 w/v ratio of Aloe powder and water) using Soxhlet apparatus. The obtained aqueous extract was lyophilized and stored at −20 °C for the long-term use.
2.4 Experimental design
The animal treatment with subacute dose of malathion and amelioration by aqueous extract of A. vera leaf was attempted. The experimental animals (male Wistar rats) were divided into 4 groups. Group 1 was served as control (C), had free access to rat feed and water only; Group 2 was A. vera extract treated control (Av), received 420 mg.kg−1 body weight; Group 3 was malathion treated (M), received 10% LD50 (29 mg.kg−1 body weight); Group 4 received the pretreatment of A. vera extract (420 mg.kg−1 body weight) followed by malathion (10% LD50, i.e. 29 mg.kg−1 body weight) treated (Av + M).
The oral treatment was given for all groups up to 15 days at regular intervals of 24 h. During this period, the body weight was also monitored. At the end of treatment, all animals were euthanized and sacrificed as per the guidelines of IAEA-University of Allahabad, India. After sacrificing the animals, the brain tissue from the rats were dissected out, washed with phosphate buffer saline (PBS), and used for further biochemical estimations.
2.5 Preparation of homogenate in sucrose solution
The brain tissue homogenate (10% w/v) was prepared in ice-cold 0.25 M sucrose solution. The cytosolic fraction i.e., post-nuclear supernatant (PNS) of rat brain tissue was separated by centrifuging the homogenate at 9000g for 30 min at 4 °C.
2.6 Extraction of acetylcholinesterase (AChE) from rat brain tissue
The brain of rat was removed and washed with ice cold saline (0.9% NaCl) blotted to dry. For the estimation of AChE acetylcholinesterase activity, the tissues were homogenized (10% w/v) in 50 mM phosphate buffer, pH 7.4 containing 0.2% Triton X-100 (v/v), followed by the centrifugation at 10000g for 30 min at 4 °C.
2.7 Estimation of total antioxidant potential: Ferric reducing antioxidant potential (FRAP)
FRAP analysis indicates the reducing power of the cytoplasm. FRAP assay was estimated in the PNS of rat brain tissue (Benzie and Strain, 1996). The absorbance was measured at 593 nm. The reducing ability of the brain PNS was calculated with reference to the reaction signal given by Fe(II) solution of known concentration. The values were expressed as μmol.g−1 of tissue.
2.8 Determination of nitric oxide (NO) level
The level of nitrite in the PNS of rat brain homogenate, an indicator of the production of NO. The nitrite level was determined in the PNS of rat brain (Green et al., 1982). The absorbance was measured at 540 nm using UV–visible spectrophotometer. The sodium nitrite standard curve was used for calculating the concentration of nitrite in the supernatant and the result was expressed as μmol.g−1 protein.
2.9 Determination of protein carbonyl (PCO) level
The protein carbonyl content was evaluated in the PNS by DNPH method (Singh et al., 2002). The absorbance was measured at 370 nm. The result was expressed as μmol.mg−1 of protein.
2.10 Estimation of inflammatory markers (COX-2, NF-κB, TNF-α and IL-6)
The levels of inflamatory cytokines such as COX-2, NF-κB, TNF-α and IL-6, are considered as the marker of inflammation. The levels of COX-2, NF-κB, TNF-α and IL-6 in the PNS of rat brain were determined using GENLISATM ELISA kits procured from KRISHGEN Biosystems, India. The procedures were pursued as stated in kit protocol. The optical density of colored complex of the total reaction mixture was observed by using an ELISA microtiter plate reader at 450 nm.
2.11 Estimation of malondialdehyde (MDA) level
Lipid peroxidation (LPO) was measured in the PNS of the rat brain (Niehaus Jr. and Samuelsson, 1968) and the results were calculated by using 1.56 × 105 M−1 cm−1 as the extinction coefficient and the unit was expressed as nmol MDA mg−1 protein.
2.12 Estimation of reduced glutathione (GSH) level
The total thiol content in the PNS of rat brain was determined by the method of Ellman (1959) modified by Sedlak and Lindsay (1968) (Sedlak and Lindsay, 1968). The optical density of the colored complex present in the supernatant of reaction mixture was measured at 412 nm. The result was expressed as µg GSH mg−1 protein.
2.13 Activity assay of catalase (CAT)
The CAT activity was measured in the PNS of rat brain (Beers and Sizer, 1952) by measuring the decrease in the absorbance for H2O2 consumption at the interval of 30 sec for 3 min at 240 nm. The unit of enzyme activity was defined as µmoles of H2O2 decomposed min−1. The enzyme activity was calculated by using the molar extinction coefficient (43.6 M−1 cm−1) of H2O2.
2.14 Activity assay of superoxide dismutase (SOD)
The specific activity of SOD was measured in the PNS of rat brain (Marklund and Marklund, 1974). The optical density of pyrogallol auto-oxidation at 412 nm for 3 min at the interval of 30 sec was measured spectrophotometrically. The unit of enzyme activity was expressed as the 50% inhibition of auto-oxidation of pyrogallol min−1.
2.15 Activity assay of glutathione-S-transferase (GST)
The specific activity of GST was determined in the PNS of rat brain (Habig et al., 1974) in the PNS of rat brain. The change in optical density of the reaction mixture was recorded at the interval of 30 sec at 340 nm for 3 min. The activity of GST was calculated using the molar extinction coefficient (9.6 × 103 µmol−1.cm−1). The unit of results was expressed as µmol.ml-1min-1mg−1 protein or IU.mg−1 protein.
2.16 Activity assay of glutathione peroxidase (GPx)
The specific activity of GPx was assessed in the PNS of the rat brain (Paglia and Valentine, 1967) method. The rate of oxidized glutathione formation was measured spectrophotometrically at 340 nm using NADPH. The enzyme activity was expressed as IU.mg−1Hb.
2.17 Oxidative stress index (OSI)
The oxidative stress index (OSI) has been calculated as the ratio of prooxidant/antioxidant (P/A) by using the formula described elsewhere (Jaiswal et al., 2016).
Oxidative stress index (OSI) = Levels of pro-oxidant (MDA)/Levels of antioxidants (specific activities of SOD + CAT + GST + GPx).
2.18 Activity assay of AChE
The activity of AChE was determined in the PNS of rat brain, prepared in phosphate buffer containing Triton X-100 (Ellman et al., 1961). The AChE activity was calculated using extinction coefficient 13.6 × 103 M−1cm−1 and expressed as µmoles of acetylthiocholine (ATI) hydrolyzed ml−1 min−1 or units (U). The specific activity of this enzyme was expressed in IU.mg−1 of protein. The assay system without substrate/enzyme was considered as a substrate/enzyme blank, respectively.
2.19 Histopathological study of the liver tissue
Rat liver tissues from experimental animals were quickly dissected out, washed in PBS, and blotted, followed by the fixation in Bouin’s solution for 24 h. The washing of fixed liver tissue was performed under running water for 24 h followed by dehydration in graded ethyl alcohol (100%, 90%, 70%, 50% and 30%). The dehydrated tissue was embedded in wax and serially sectioned (10 μm) using rotary microtome (model number SP‐1120/SP‐1120A). The tissue sections were stretched on gelatin coated glass slides and stained with hematoxylin and eosin (H&E) using the standardized protocol of our laboratory. The glass slides containing H&E-stained liver tissue sections were mounted using dibutylphthalate polystyrene xylene (DPX) and monitored under a light microscope (model-YJ-2016). The tissue sections were examined for the extent of vacuolar degeneration, vascular congestion, hemorrhage, and necrosis. The aberrations were scored using the scale of absence of any aberration (−), mild (+), moderate (++) and severe (+++).
2.20 Determination of total protein content (TPC)
The TPC was measured in the PNS of rat brain (Lowry, 1951). Bovine serum albumin (BSA) was used as standard for calculating the TPC.
2.21 Statistical analysis
The spectrophotometric assays of enzymatic and nonenzymatic biomarkers were performed and analyzed on UV–visible double beam spectrophotometer (Thermo Scientific Spectroscan UV 2700).
The Graph Pad Prism 5.01 was employed for analyzing the data using one-way analysis of variance (ANOVA) and Bonferroni’s Multiple Comparison Test was used to compare different groups. The calculated data was presented as the values of mean ± standard deviation of three different experiments and were considered significant at P ≤ 0.05.
3 Results
3.1 Impact of A. vera against malathion on the body weight (BW) and relative brain weight (RBW) of rats
The impact of malathion and A. vera on the levels of body and organ weight (brain) was monitored as mentioned in section 2. At the end of the fifteen days treatment regimen, no death was found in any experimental animal group. However, the malathion treated group exhibited significant changes in the BW by +25.22% and RBW by −20.96% in comparison to the control. However, when the malathion treated rats were pretreated by extract from A. vera leaves, the BW (+3.87%) and the RBW (−1.61%) remained almost near control (Table 1). The pre-administration of A. vera extract to the malathion treated group showed an ameliorating effect on the BW and RBW. Abbreviations: IBW: initial body weight; FBW: final body weight; CBW: change in body weight; RBW: relative brain weight; ABW: absolute brain weight; P: parameters; g: grams; C: control; Av: A. vera treated control; M: malathion treated; Av + M: malathion with pre-treatment of A. vera. The data are presented as mean ± SD of six rats. CBW (%)/week = [FBW- IBW/IBW)/number of weeks × 100] and % RBW = (ABW/FBW) × 100 as described in materials and methods.
P (g)
C
Av
M
Av + M
IBW(g)
153.3 ± 2.16
153.5 ± 3.082ns
153.0 ± 2.608ns
154.7 ± 0.816ns
FBW (g)
153.7 ± 1.633
154.0 ± 2.608ns
172.3 ± 1.966***
157.70 ± 2.944ns
% CBW
+5.21
+0.65
+25.22
+3.87
ABW (g)
1.914 ± 0.070
1.951 ± 0.110ns
1.692 ± 0.124*
1.937 ± 0.154ns
% RBW
1.24
1.26 (+1.61%)
0.98 (-20.96%)
1.22 (-1.61%)
3.2 Impact of A. vera against malathion on the NO level
The effect of A. vera extract against malathion was assesed in rat brain as mentioned in Section 2. The oral administration of malathion significantly raised the levels of NO in the rat brain by 111.69%, in comparison to the control. Conversely, the intake of A. vera along with malathion have non-significantly changed the level of NO production and the values were found to be near control (Fig. 1A).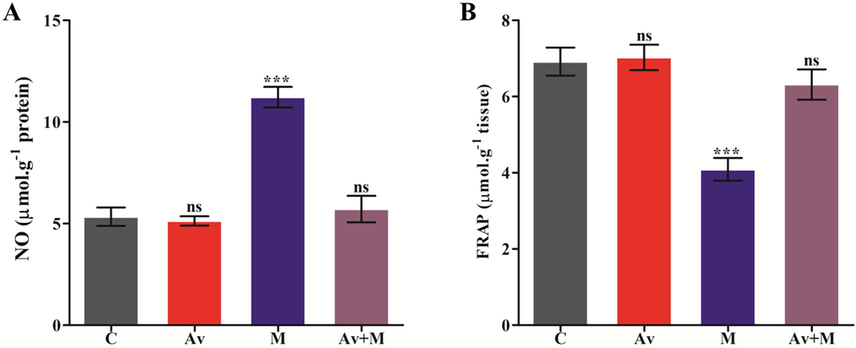
Effect of A. vera extract and malathion on the levels of NO (A) and FRAP (B) in rat brain. The treatment procedures of A. vera extract and malathion and the assay of NO and FRAP were same as indicated in Materials and Methods. The unit for the NO was expressed as μmol.g−1 of protein. The FRAP was stated as μmol.g−1 of tissue. The data represented as mean ± SD of three distinct experiments. The values were significant at P < 0.05 compared to control.
3.3 Impact of A. vera against malathion on the FRAP level
Ferric reducing antioxidant potential (FRAP) offers an index of antioxidant potential of cell plasma and is widely used as a marker of antioxidant reserve. The ability of cell plasma antioxidants has been used for the estimation of antioxidant potential to predict the level of cellular OS. In this study, a significant decline (40.81%) in antioxidant potential was observed in brain tissue of rats trated with malathion in contrast with control. Furthermore the supplementation of A. vera extract restored/ameliorated the alterd level of FRAP (8.68%) in response to the malathion near normal. The non-significant alteration (1.73%) was observed in the levels of FRAP in A. vera leaf extract treated rats. Our results show that A. vera leaf extract effectivevely restored the antioxidant capacity in brain tissue when it was preadminitered along with the malathion (Fig. 1B).
3.4 Impact of A. vera against malathion on MDA, and GSH content
In order to evaluate the effect of malathion (10% LD50), the rats were treated for fifteen days as described in section 2. The malathion treated rats were also subjected to receive the oral administration of A. vera to evaluate it’s ameliorating effect. The findings from present study showed extensive increase in MDA level (i.e., 102.88%) and decrease the level of GSH (i.e., 57.08%) in the brain (Table 2). The oral administration of A. vera did not exhibit any substantial alteration in the concentrations of these indices (i.e., 3.34% for MDA, and 1.56% for GSH). When the animals treated with malathion were pre-treated with A. vera, the levels of both indices were observed to remain near control. The procedure for the administration of malathion and A. vera extract as well as evaluation of levels of MDA, GSH, and PCO in rat brain was the same as mentioned in Materials and Methods. The MDA level was expressed as nmol.mg−1 protein, the GSH was expressed as µg.mg−1 protein. and the PCO was expressed as μmol.mg−1 protein in the rat brain tissues. (+) and (−) sign shows increase and decrease, respectively, in the levels of MDA, GSH, and PCO. The data represent mean ± SD of three independent experiments. The values significant (*) at P < 0.05 as compared to control group. Abbreviations: P: Parameters; C: control; Av: A. vera extract treated control; M: malathion treated; Av + M: malathion with pretreatment of A. vera extract.
P
C
Av
M
Av + M
MDA
10.76 ± 0.6684
11.12 ± 0.7865ns
(+3.34%)21.83 ± 2.074***
(+102.88%)11.85 ± 0.5784ns
(+10.13%)
GSH
2.104 ± 0.244
2.137 ± 0.2245ns
(+1.56%)0.902 ± 0.0908***
(−57.12%)1.99 ± 0.1955ns
(−5.41%)
PCO
15.34 ± 0.4515
14.93 ± 0.5502ns
(−2.67%)21.22 ± 0.5059
(+38.33%)***15.71 ± 0.6511ns
(+2.41%)
3.5 Impact of A. vera against malathion on PCO level
The impact of malathion on the PCO level in rat brain was assessed as described in Section 2. The malathion exposure had signficantly increased the content of PCO in the rat brain. The PCO content was found to be increased by 38.33% in the malathion exposed rat brain, in comparison to control. The animals pre-treated with leaf extract of A vera has reflected an improvement in the content of PCO in rat brain. The change in the content of PCO level was very small or non-significant (2.41%) in the rats co-treated with A. vera and malathion compared to malathion treated. There was non-significant changes observed in the group treated with leaf extract of A. vera (Table 2).
3.6 Impact of A. vera against malathion on specific activity levels of CAT, SOD, GST and GPx
In order to assess the effect of A. vera and malathion (10% LD50) on the specific activity levels of CAT, SOD, GST and GPx, the experimental rats were treated for fifteen days. Their activity assays were carried out by utilizing the PNS of rat brain. The results indicated that the exposure of malathion was able to cause a significant reduction in the activity levels of CAT by 39.13%, SOD by 35.26%, and GST by 59.94% (Fig. 2A, 2B and 2C) and increase in the activity level of GPx by 47.42% (Fig. 2D) when compared to control. The pre-treatment of A. vera has been found to offer complete amelioration to the rats from the toxic effect of malathion. When the malathion exposed animals were subjected to the pre-treatment of A. vera, the activity levels of all these enzymes were observed to be nearly equal to control. The A. vera itself showed non-significant change in the levels of all indices even after 15 days of treatment.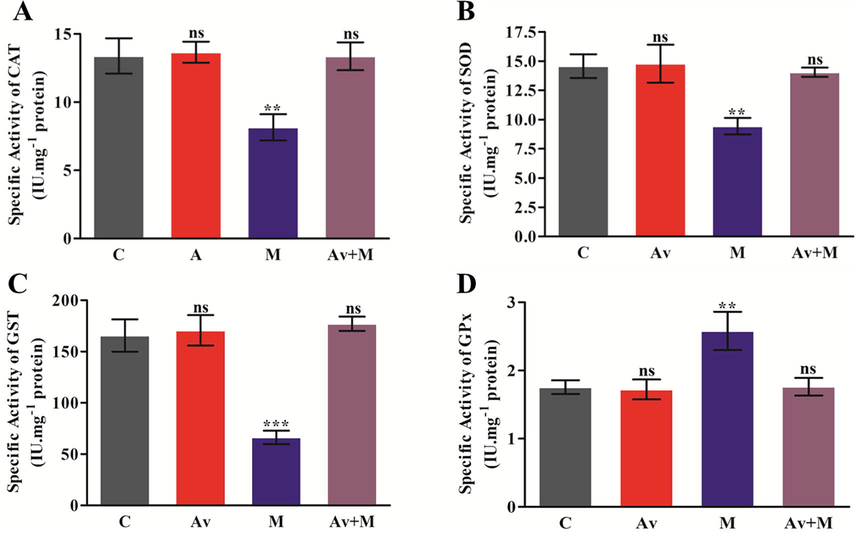
Effect of A. vera extract and malathion on the activity of CAT (A), SOD (B), GST (C) and GPx (D) in rat brain. The treatment procedures of A. vera extract and malathion and the assay of CAT, SOD, GST and GPx activity were same as described in Materials and Methods. The activity unit was stated as IU.mg−1 protein. The data represented as the mean ± SD of three distinct experiments. The data were significant at P < 0.05 as compared to the control.
3.7 Effect of A. vera against malathion on the level of OSI
The OSI status was calculated for the experimental rat brains, treated for fifteen days at sub-lethal dose of malathion. The computed values of OSI were shown as prooxidant/antioxidant (P/A). The P/A ratio was determined to be elevated in brain of malathion exposed rats (358.72%) as compared to that of control. The data of present investigation indicated that the pre-treatment of A. vera potentially ameliorated the altered OSI level and able to bring it back close to the level of control group (Table 3). The OSI was computed by the P/A ratio as shown in Materials and Methods section. The (+) sign indicates significant rise in the level of OSI. Abbreviations: P: Parameters; P/A: prooxidant/antioxidant; C: control; Av: A. vera extract treated control; M: malathion treated; Av + M: malathion with pre-treatment of A. vera extract.
P
C
Av
M
Av + M
OSI (P/A)
0.0550
0.0554
(+0.72%)0.2523
(+358.72%)0.0574
(+4.63%)
3.8 Impact of A. vera against malathion on the levels of IL-6, COX-2, TNF-α and NF-κB
The concentration of cytokines in cells has been considered as the potential inflamatory markers. The impact of malathion at sublethal doses was determined by monitoring the contents of IL-6, COX-2, TNF-α, and NF-κB in brain. The protecting effect of A. vera was also assessed in the rats, if any. The content of these cytokines in the malathion treated rat brain was found to be altered significantly. The results presented in Fig. 3A, 3B, 3C, and 3D respectively, indicated that the rats administred with malathion had increased the levels of IL-6, COX-2, TNF-α, and NF-κB by 203.03%, 165.42%, 111.43%, and 261.38%, respectively, in comparison to the control. However, the use of A. vera before malathion had potentially ameliorated the altered levels of IL-6, COX-2, TNF-α, and NF-κB when in contarst to malathion treated rats. The contents of these markers were found to be ameliorated by A. vera extract.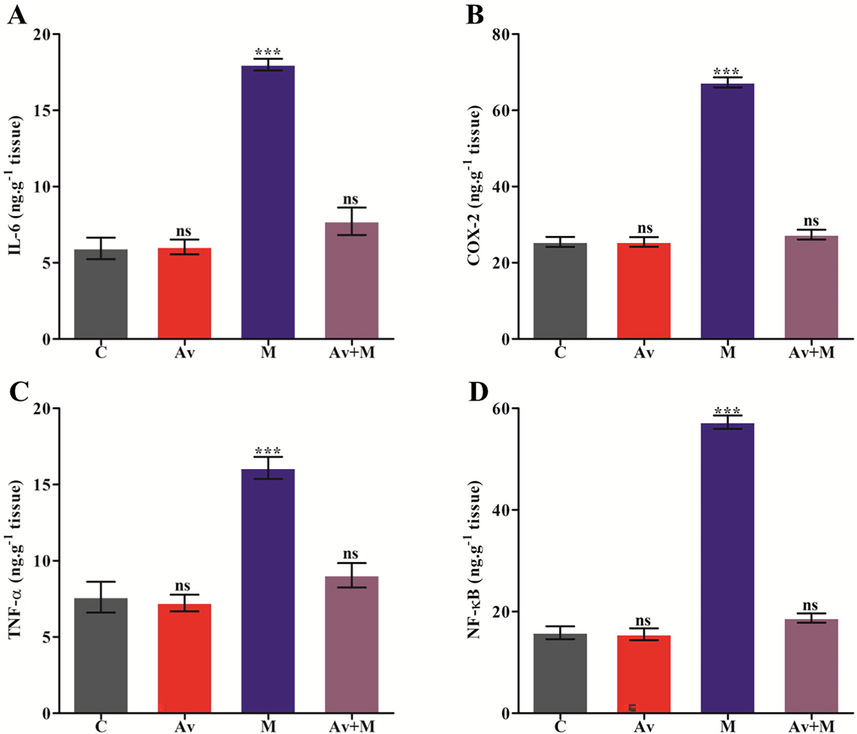
Effects of A. vera extract and malathion on the levels of cytokines i.e., IL-6 (A), COX-2 (B), TNF-α (C) and NF-κB (D) in rat brain. The treatment procedures of A. vera extract and malathion and the assay for inflammatory markers was as described in Materials and Methods. The unit of the concentration was stated as ng.g−1 tissue. The data represented as mean ± SD of three distinct experiments. The data were significant at P < 0.05 as compared to the control.
3.9 Impact of A. vera against malathion on the level of AChE activity
The effect of sublethal dose of malathion was determined by monitoring the level of specific activity of the AChE in brain. The protecting efficacy of A. vera leaves was also evaluated in the rats, if any. The data presented in Fig. 4A suggested that malathion was potentially able to inhibit the AChE activity by 38.96% in rat brain as compared to control. This inhibition was restored to nearby the control level by pre-treatment of A. vera. However, the A. vera extract solitary did not exhibit any impact on the level of AChE activity.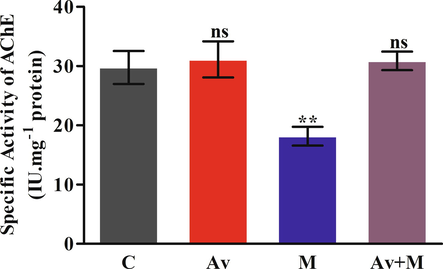
Effect of A. vera extract and malathion on the activity levels of AChE in rat brain. The treatment procedures of A. vera extract and malathion and the activity assay of AChE was the same as indicated in Materials and Methods. The activity units were stated as IU.mg−1 protein. The data represented as mean ±SD of three distinct experiments. The data were significant at P < 0.05 as compared to the control.
3.10 Impact of A. vera on the cellular architecture of rat liver against malathion
The effect of malathion and A. vera on the levels of changes of cellular architecture were monitored by hematoxylin and eosin staining. The treatment regimen of malathion and A. vera to rats and the staining procedures of liver tissue sections were same as mentioned in Section 2. At the end of administration, the rat liver was excised and processed to study the histological aberrations. The light microscopic observations of liver sections are presented in Fig. 5. Fig. 5A, and 5B indicated the normal organization of liver cells. The hexagonal lobules of hepatic tissue were centered towards the central vein containing a portal triad, having a portal vein, hepatic artery, and bile duct. Fig. 5C showed the presence of the disorganization of the central vein and hepatic cord. The figure from A. vera-treated rats displayed significant protection (Fig. 5D) against malathion-induced injury to cellular organization of liver. These changes in the rat liver tissue sections were scored and compiled in Table 4. Abbreviations: DH: degenerating hepatocytes; DCV: degeneration of central vein; NH: necrosis of hepatic cells; DHT: degeneration in hepatic triad; (−): absent; (+): present; P: Parameters; C: control; Av: A. vera treated; M: malathion treated; Av + M: malathion with pre-treatment of A. vera extract.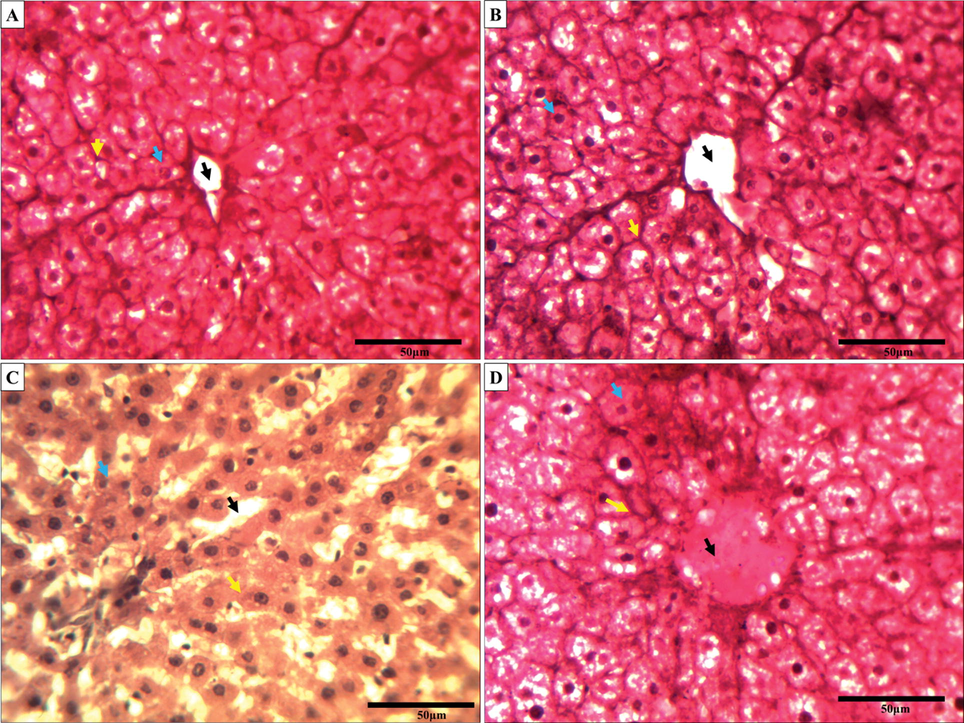
The changes in rat liver tissue section are due to the exposure of malathion and A. vera (H&E staining, ×40). A micrograph of control liver tissue section (A); A. vera treated (B); Cartap treated (C); and A. vera + malathion treated (D) groups. The treatment procedures were the same as indicated in Materials and Methods. The black arrow, yellow arrow and blue arrow indicated the disorganization of central vacuole, hepatic cords, and the normal cellular architecture, respectively, of the rat liver tissue under malathion exposure.
S. No.
P
C
Av
M
Av + M
1
DH
–
–
++
–
2
DCV
–
–
++++
+
3
NH
–
–
++
–
4
DHT
–
–
+++
–
4 Discussion
The extensive use of pesticides has led to the exposure of non-target animals, invertebrates to these chemical and the adulteration of water and food sources (Suresh Babu et al., 2006). The non-target animals such as mammals are prone to be adversely affected by dermal, oral and nasal inhalation of the pesticides (Lasram et al., 2009). The application of pesticide is a two-edged sword in agricultural practices. The application of pesticide can control the target pests and help to increase the crop yields, whereas on the other hand they contaminate water, soil, air, and the foodstuffs. The application of pesticides has been resulted in several toxicological outcomes on the health of various nontarget animals including humans (Kalyabina et al., 2021). The malathion exposure to living organisms has been reported to damage the several key activities of an organism such as the energy metabolism, redox and cholinergic systems. The variations in these functions can help in the development of several disorders related to the roles of corresponding markers in the brain. Therefore, restoration of these key markers is needed for the treatment of neurodegenerative disorders. In this context, the therapeutic application of plant based principals have shown the great potential (Xie et al., 2021). The organ weight and body weight has also been considered as the significant criteria for the assessment of toxicity induced by pesticide. The results from the present study indicated significant changes in the levels of RBW and BW in rats treated to malathion. This may be due to the generation of OS along with the occurrence of cholinergic deficiency. The findings from the present study was corroborated with the earlier findings (Gupta et al., 2020). However, the rats pre-treated with A. vera potentially ameliorated the changes in RBW and BW of the malathion exposed rats. The protective impact offered by A. vera may be attributable to the presence of several antioxidants in it.
Reactive radicals such as NO has been reported to induce death in a variety of cells including immune (Fehsel et al., 1995), inflammatory (Sarih et al., 1993) and neural (Gunasekar et al., 1995, Bolaños et al., 1997) cells. However, considering the limited self-renewal capacity of brain cells, the cell death may result in neurodegenerative disorders (Thompson, 1995). Furthermore, the reaction between NO and superoxide anion forms highly reactive and more cytotoxic peroxynitrite (Beckman et al., 1990, Xia et al., 1996). In the present study, the results indicated a significant rise in the level of NO in the malathion treated rat brain, which could be associated with tissue damage caused by malathion induced rise in the NO level. Our finding is corroborated with the similar reports, in which they have shown that the administration of malathion can induce NO mediated delayed apoptosis in brain tissue (Coban et al., 2015). However, the pre-administration of rats with A. vera was potentially able to protect the rat brain from malathion toxicity as the NO level came close to control.
In physiologic conditions, the level of FRAP plays a crucial role in quenching ROS. It has been monitored mainly for the assessment of antioxidant system status (Feng et al., 2022). A substantial decline in FRAP level may be due to the generation of excess ROS and prevailing OS under malathion exposure in rat brain. In current study, the cytoplasmic FRAP level was substantially declined in the malathion treated rats in contrast to control. The decreased capacity of antioxidant potential after malathion exposure may be due to the alteration in markers of the antioxidant protection system (Salam et al., 2023). The reduced FRAP level may lead to the rise in the levels of PCO and LPO, which account for the establishment of malathion induced OS in the rat brain. The results from current study suggest that malathion may rapidly trigger their oxidation processes by targeting the proteins and lipids. At the same time, excess production of ROS can directly affect the antioxidant enzymes and may cause an imbalance of cellular redox reactions. In this condition, the LPO products may also promote the dysfunction of antioxidant enzymes.
Exposure to environmental pollutants has been shown to trigger the generation of excess ROS. The excess cellular ROS result into the establishment of OS and decrease in the synthesis of cellular antioxidative agents (Vidyasagar et al., 2004). The OP-Ps can easily accept an electron and then transfer it to O2 to form superoxide ions and hence H2O2 through dismutation reaction. In addition, the OP-Ps compounds contain the thion (P⚌S) which get transformed into oxon by microsomal oxidases including cytochrome P450. These oxons are responsible for the OP-Ps mediated cytotoxicity. Brain tissues are most vulnerable to the oxidative damage because of it’s low mitotic rate, high content of unsaturated fatty acids which can be easily peroxidized and its need for high amount of O2 per unit weight (Floyd and Hensley, 2002). Moreover, despite having high rate of metabolism, the brain tissues have low content of celular antioxidants (Guignet et al., 2020). Carboxylesterases, which is the mainly accountable for the rapid breakdown of malathion, also maintain a lower activity in the brain (Hazarika et al., 2003). This coupled the great susceptibility of the brain tissue to malathion exposure, which partly, explains the maximum production MDA in the brain. The LPO produces various thiobarbituric acid reactive substances (TBARS) which are routinely monitored as the biomarkers of oxidative damage including MDA (An et al., 2018). The increased level of LPO under the pesticide exposure is an indicator of oxidative damage in a living system. The findings of the present study showed that the exposure of malathion has potentially raised the MDA level in brain tissue. Our data was found to be in agreement with earlier reports in which the malathion treated rats showed a significant increase in MDA level in brain (Akbel et al., 2018). However, the pre-treatment of rats with A. vera preparation was potentially able to offer protection of rat brain from malathion as the MDA level came close to normal.
The GSH is known as a potential antioxidant marker of cellular OS (Ross, 1988). In current investigation, a significant decrease in the content of GSH in brain indicated the induction of OS in the malathion treated rats. The malathion exposure leads to the decline in the content of GSH in rat brain, which was protected by A. vera. These results were strongly corroborated with the recent work presented by other workers (Akbel et al., 2018). Similar findings was reported in the rat treated with sublethal dose of cartap (Gupta et al., 2022). The pre-treatment of A. vera substantially restored the GSH content in the malathion treated rat brain. The co-treatment of malathion with A. vera overturned the toxic effects of malathion perhaps by guarding the content of GSH from reduction under malathion stress. LPO in the brain may result in membrane stiffness of nervous cells. Depletion of GSH content and production of ROS directly support to the damage of biomolecules, leads to the apoptosis, and necrosis (Brocardo et al., 2005). The reduction in GSH content under malathion induced OS may have resulted due to the reduction of lipid hydroperoxides to form lipid alcohols by GPx activity. This reaction utilizes GSH as a source of reducing analogue. Instead, GSH content might be reduced by the direct utilization of it in quenching ROS produced by malathion.
Disturbances in the normal redox status can trigger toxic impacts through that can damage the cell. The PCO has been thought of as a marker of protein oxidation. In the current study, the oxidative damage to proteins was evaluated by the amount of carbonyl groups present in malathion exposed to rats. The content of PCO was significantly raised in malathion treated brain of rat. This data received support from some previous published studies (Brocardo et al., 2005, Fortunato et al., 2006). The malathion induced oxidative damage on the levels of protein carbonyls occurring could be related with the level of malathion induced toxicity. The co-administration of A. vera and malathion was observed to inhibit the protein oxidation in rat brain.
The enzymes which are involved in the attenuation of the ROS/RNS producing molecules into non-toxic species are referred as enzymatic antioxidants such as CAT, SOD, GST, and GPx. SOD catalyze the dismutation of .O2– to H2O2 which go through degradation by CAT, producing H2O and O2 (Costa et al., 2021). Any alterations in the activity levels of these antioxidative enzymes may lead to severe pathophysiological outcomes in the key organs of mammals (Gupta et al., 2019). Our findings also suggested that the malathion has modulated the SOD and CAT activities in the brain. Our findings were strongly corroborated with Brocardo et al., (2005). The activities of antioxidant enzymes may either increase, reduce, or not altered in the malathion exposed brain (Brocardo et al., 2005). It may be suggested that involvement of ROS is responsible for the neuronal damage induced by malathion exposure. Present study showed the substantial decline in the activities of both enzymes that indicated the failure of antioxidant defense system. Our results are corroborated with the similar findings reported in the malathion treated rats (Akbel et al., 2018). However, the pre-treatment of rats with A. vera has offered substantial protection from malathion toxicity as the activities of CAT, and SOD be found close to control.
The enzymes of glutathione redox system (GST and GPx) are involved in keeping the cellular redox status normal as well as the xenobiotics detoxification in the living system. The decrease in GSH level in malathion treated rats indicated it’s utilization in the detoxification of malathion through activity of GST. Conjugation of GSH and pesticide could be a major pathway in the process of detoxification (Vijaya Padma et al., 2011) and the GPx actively engaged in the redox reaction of GSH. GST and GPx are also known to be involved with the rapid removal of the ROS formed due to pesticide exposure. In the present study, the reduction in GST and raise in the GPx activities in the malathion exposed rat brain suggested the failure of glutathione redox system. Similar findings have been reported when rats were treated with malathion (Ahmed et al., 2000, Selmi et al., 2018). However, the rats pre-treated with A. vera indicated significant level of attenuation of malathion toxicity as the activities of GST and GPx appeared near control.
The association of prooxidant and antioxidants has been considered as an index of OS. It can be best expressed by the ratio of P/A (Gupta et al., 2019). The values of P/A imply an index of net ROS scavenging capacity of cell. The raised value of P/A indicated a rise in the prooxidant level thereby imposing OS in the rat brain treated with malathion. The elevated P/A value implied the greater oxidative ability of the malathion. This trend shows that ROS production depends on the persistent nature of malathion in rat brain. Brain may be the highly vulnerable to malathion induced OS (López et al., 2017). The P/A was restored to nearby control upon the pre-treatment of A. vera in the rats treated with malathion. The antioxidant power of A. vera may be due to the presence of flavonoids and polysaccharide (López et al., 2017).
Cytokines are peptides or proteins that work as an immunomodulatory as well as the signaling molecules. Cytokines include chemokines, tumor necrosis factors, lymphokines, interferons and interleukins. They play a vital role in health through inflammatiory immune response, trauma, and cancer. It has been reported that the accumulation of ROS may activate stress-induced production of inflammatory cytokines, following the deterioration of stress response and appearance of histopathological signs (Birben et al., 2012). The data from our study have displayed that the content of IL-6, COX-2, TNF-α, and NF-kB was potentially altered by malathion exposure in rat brain. These factors might be activated due to the rise in the level of malathion induced OS. Furthermore, this study also showed the ameliorating potential of A. vera against malathion toxicity. The pre-treatment of A. vera potentially ameliorated the content of IL-6, COX-2, TNF-α, and NF-kB in rat brain. The malathion induced changes in the levels of different inflamatory markers have been demonstrated earlier (Gupta et al., 2020). The incresed contents of TNF-α and IL-6 may occur either by COX or lipoxygenase pathway. The glycoproteins reported in A. vera have been reported to inhibit the activity of COX-2 (Yagi et al., 2003). The phytosterols, anthraquinones and chromones found abundantly in the A. vera have been shown as the enhancers of anti-inflammatory reactions (PARK et al., 2009).
The OP-Ps are recognized as to potentially inhibit AChE activity (Čadež et al., 2021), resulting in the hyper-stimulation of cholinergic receptors (Tsai and Lein, 2021). It evokes a series of physical symptoms, and not excluding psychological and neurobehavioral effects, such as anxiety, depression, and cognitive impairments (Sinha et al., 2022). This group of pesticides have the ability to covalently block AChE (Kamal, 1997). The AChE catalyzed hydrolysis of acetylcholine to acetic acid and choline at synapse and is necessary for the normal conduction of nerve signals. The results from current study suggested that AChE was membrane-bound and may be solubilized by using Triton X-100. The similar reports have been reported that AChE is present as the membrane-bound form in brain of rats and erythrocyte’s membrane (Gupta et al., 2015, Gupta et al., 2016). In this study, the significant decline in the activity level of AChE has indicated the great inhibitory ability of the malathion in brain of rat. Similar remarks have been made by several other workers in the malathion treated rats (Hazarika et al., 2003, Abdollahi et al., 2004, Assini et al., 2005, Brocardo et al., 2005, Ramos et al., 2006, Selmi et al., 2018). These findings may accord with the idea that malathion is able to produce oxidative stress by producing excess ROS and inhibit the AChE activity. This response can be more clearly supported by the activity of the antioxidant enzymes, NO and MDA levels studied which positively correlated with the decrease in AChE activity (Figs. 1, 2, and 4). However, the pre-treatment of A. vera protected AChE activity from the malathion in rat’s brain.
In light of the above biochemical findings against the malathion in the rat brain, it was envisaged to study the status of rat liver tissue under the same treatment regimen. Severe changes were observed in the liver tissues due to malathion exposure. Our findings were in corroboration with the results from previous reports (Handy et al., 2002, İşeri et al., 2007). In their results they have shown that the exposure of rats OP-Ps caused acute degeneration in hepatocytes by activating the Kupffer cells. The toxic effects of malathion can be supported by several reports. The concentration of malathion was reported to be highly concentrated in the liver compared to the other tissues (Rumiza Abd et al., 2008). Malathion exposure has also been reported to affect the mineral status in rat liver which may be the cause of cellular toxicity (Lasram et al., 2014). The malathion induced histological alterations in liver has been reported to be protected by A. vera (Selmi et al., 2018). In the present study, the rat liver was protected from malathion toxicity by A. vera extract which could be due to the presence of antioxidants in it (Wintola and Afolayan, 2011).
5 Conclusion
Brain has been the keen target of assault by any OP-Ps like malathion. The outcomes from current study suggested that malathion was able to generate toxicity in rat brain at sub-lethal concentration. Wistar rats exposed to malathion showed significant variations in the BW and RBW. The levels of NO, PCO, and FRAP were found to be significantly altered. The concentration of cytokines (IL-6, COX-2, TNF-α, and NF-kB) were also found to be potentially altered in the malathion exposed rats. Malathion was observed to substantially alter the redox status by altering the levels of MDA, GSH, CAT, SOD, GST, and GPx in the brain of the exposed rats. The alterations in oxidative status suggested the prevalence of OS in rats. The OSI was computed to be highly elevated in the malathion treated rat brain. The substantial changes in the activity level of AChE were also noticed which indicated that the functions of cholinergic system may also be hampered due to the exposure of this pesticide. The histopathological examination of rat liver treated with malathion registered severe damage to hepatocytes. The malathion induced toxic effects on the level of biochemical and histological parameters were potentially ameliorated by the A. vera extract in rat brain and liver. These findings indicated that A. vera extract contains bioactive compounds which can be used as the potential molecule in the management of malathion intoxication. The antioxidative ability of A. vera might be utilized as a plant based medicinal supplement for ameliorating the malathion induced neurotoxicity without any side effect.
Social relevance of the study
Hazardous chemicals like pesticides are used tremendously to overcome the damage caused by pests to crops and also for the preservation of these crops yield for longer storage. The exposure to pesticide such as malathion can cause a serious risk to the various key organs of non-target organisms and causes numerous diseases such as cardiovascular diseases such as diabetes, hypertension, and hyperlipidemia. In the present work, we focused on malathion induced toxic effects mediated alterations in the rat brain and the ameliorating impact of A. vera to find out the plant-based treatment of such diseases. Though we have several medications available, they have some side effects also. The results suggested that A. vera can be used as the potential supplement for the appropriate management of malathion toxicity in association with other relevant therapeutics.
Author’s contributions
This article was envisaged and designed by BS and VKG. VKG, UCP, EK, SK, NJS, YSH and BS also structured and analyzed regarding data and wrote the manuscript.
Funding information
This work was supported by a grant from the Korea Environmental Industry & Technology Institute (KEITI), funded by the Ministry of Environment (MOE) of the Republic of Korea (RE202201777). This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2021R1A5A6002853 and 2022M3J7A1062940). The work also sponsored by the ICMR-New Delhi and UPCST-Lucknow India, India. The Department of Biochemistry, University of Allahabad is sponsored by UGC-SAP and DST-FIST. UCP gratefully thanks the Department of Biological Sciences and Bioengineering, Inha University, for their favor. NJS gratefully thanks the King Saud University, Riyadh, for their assistance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of malathion toxicity on the oxidative stress parameters of the black soldier fly Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae) Sci. Rep.. 2022;12:4583.
- [CrossRef] [Google Scholar]
- Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2004;137:29-34.
- [CrossRef] [Google Scholar]
- Influence of dietary ginger (Zingiber officinales Rosc) on oxidative stress induced by malathion in rats. Food Chem. Toxicol.. 2000;38:443-450.
- [CrossRef] [Google Scholar]
- The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats: the protective role of resveratrol. Toxicol. Res.. 2018;7:503-512.
- [CrossRef] [Google Scholar]
- Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. Psychiatry. 2018;8:258.
- [CrossRef] [Google Scholar]
- Behavioral effects and ChE measures after acute and repeated administration of malathion in rats. Environ. Toxicol. Pharmacol.. 2005;20:443-449.
- [CrossRef] [Google Scholar]
- Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007;233:166-172.
- [CrossRef] [Google Scholar]
- Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci.. 1990;87:1620-1624.
- [CrossRef] [Google Scholar]
- A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol chem.. 1952;195:133-140.
- [Google Scholar]
- The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [CrossRef] [Google Scholar]
- Oxidative stress and antioxidant defense. World Allergy Organ. J.. 2012;5:9-19.
- [CrossRef] [Google Scholar]
- Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J. Neurochem.. 1997;68:2227-2240.
- [CrossRef] [Google Scholar]
- Antioxidant defenses and lipid peroxidation in the cerebral cortex and hippocampus following acute exposure to malathion and/or zinc chloride. Toxicology. 2005;207:283-291.
- [CrossRef] [Google Scholar]
- Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci Rep. 2021;11:21486.
- [CrossRef] [Google Scholar]
- Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem. Toxicol.. 2015;38:391-399.
- [CrossRef] [Google Scholar]
- The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med.. 2021;162:615-635.
- [CrossRef] [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [Google Scholar]
- Nitric oxide induces apoptosis in mouse thymocytes. J. Immunol.. 1995;155:2858-2865.
- [CrossRef] [Google Scholar]
- Research on melatonin in fruits and vegetables and the mechanism of exogenous melatonin on postharvest preservation. Food Biosci.. 2022;50:102196
- [CrossRef] [Google Scholar]
- Oxidative stress in brain aging Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795-807.
- [Google Scholar]
- Malathion-induced oxidative stress in rat brain regions. Neurochem. Res.. 2006;31:671-678.
- [CrossRef] [Google Scholar]
- Chapter 1 - The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In: Florou-Paneri P., Christaki E., Giannenas I., eds. Feed Additives. Academic Press; 2020. p. :1-18.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem.. 1982;126:131-138.
- [CrossRef] [Google Scholar]
- Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol. Dis.. 2020;133:104431
- [CrossRef] [Google Scholar]
- Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol.. 2018;119:37-49.
- [CrossRef] [Google Scholar]
- NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implications for cell death. J. Neurochem.. 1995;65:2016-2021.
- [CrossRef] [Google Scholar]
- Gupta, V.K., Pathak, A.K., Siddiqi, N.J., et al., 2016. Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro.
- Acetylcholinesterase from human erythrocytes as a surrogate biomarker of lead induced neurotoxicity. Enzyme Res.. 2015;2015:370705
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Aloe vera against cartap- and malathion-induced toxicity in Wistar rats. J. Cell. Physiol.. 2019;234:18329-18343.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and antioxidative potential of Aloe vera on the cartap and malathion mediated toxicity in Wistar Rats. Int. J. Environ. Res. Public Health. 2020;17:5177.
- [Google Scholar]
- Recent advances in ethnopharmacological and toxicological properties of bioactive compounds from Aloe barbadensis (miller), Aloe vera. Curr. Bioact. Compd.. 2021;17:2-23.
- [Google Scholar]
- Ameliorative impact of Aloe vera on cartap mediated toxicity in the brain of Wistar rats. Indian J. Clin. Biochem.. 2022;37:51-59.
- [CrossRef] [Google Scholar]
- Protective effect of α-Tocopherol on memory deficits and Na+, K+-ATPase and acetylcholinesterase activities in rats with diet-induced hypercholesterolemia. Biomed. Aging Pathol.. 2012;2:73-80.
- [CrossRef] [Google Scholar]
- Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [CrossRef] [Google Scholar]
- Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology. 2002;172:13-34.
- [CrossRef] [Google Scholar]
- Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology. 2003;185:1-8.
- [CrossRef] [Google Scholar]
- Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed. Pharmacother.. 2017;96:263-268.
- [CrossRef] [Google Scholar]
- Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256-264.
- [CrossRef] [Google Scholar]
- Curcumin mediated attenuation of carbofuran induced oxidative stress in rat brain. Biochem. Res. Int.. 2016;2016:7637931.
- [CrossRef] [Google Scholar]
- Pesticides: formulants, distribution pathways and effects on human health – a review. Toxicol. Rep.. 2021;8:1179-1192.
- [CrossRef] [Google Scholar]
- Effect of malathion on kinetic parameters of acetylcholinesterase (EC 3.1.1.7) in vitro. Biochem. Mol. Biol. Int.. 1997;43:89-97.
- [CrossRef] [Google Scholar]
- Spatial distribution and compositional profiles of organochlorine pesticides in the surface soil from the agricultural, coastal and backwater transects along the south-west coast of India. Chemosphere. 2020;254:126699
- [CrossRef] [Google Scholar]
- Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine. 2019;60:152996
- [CrossRef] [Google Scholar]
- Metabolic disorders of acute exposure to malathion in adult Wistar rats. J. Hazard. Mater.. 2009;163:1052-1055.
- [CrossRef] [Google Scholar]
- Effects of N-acetyl-l-cysteine, in vivo, against pathological changes induced by malathion. Toxicol. Mech. Methods. 2014;24:294-306.
- [CrossRef] [Google Scholar]
- The present situation of pesticide residues in China and their removal and transformation during food processing. Food Chem.. 2021;354:129552
- [CrossRef] [Google Scholar]
- Antioxidant and cytotoxicological effects of Aloe vera food supplements. J. Food Qual.. 2017;2017:7636237.
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-474.
- [Google Scholar]
- Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health. 2011;8:2265-2303.
- [Google Scholar]
- Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem.. 1968;6:126-130.
- [CrossRef] [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70:158-169.
- [Google Scholar]
- Evaluation of Aloin and Aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotech. Bioch.. 2009;73:828-832.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase activity and thyroid hormone levels in Ecuadorian adolescents living in agricultural settings where organophosphate pesticides are used. Int. J. Hyg. Environ. Health. 2021;233:113691
- [CrossRef] [Google Scholar]
- Purser, D., Ballantyne, B., Marrs, T., 1992. Combustion toxicology of anticholinesterases. Butterworth-Heinemann, Stoneham, MA (USA).
- Influence of malathion on acetylcholinesterase activity in rats submitted to a forced swimming test. Neurotox. Res.. 2006;9:285-290.
- [CrossRef] [Google Scholar]
- Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol. Ther.. 1988;37:231-249.
- [CrossRef] [Google Scholar]
- Determination of malathion levels and the effect of malathion on the growth of Chrysomya megacephala (Fibricius) in malathion-exposed rat carcass. Trop. Biomed.. 2008;25:184-190.
- [Google Scholar]
- Protective effect of rutin against thiram-induced cytotoxicity and oxidative damage in human erythrocytes. Pestic. Biochem. Physiol.. 2023;189:105294
- [CrossRef] [Google Scholar]
- Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ. Sci. Pollut. Res.. 2019;26:18267-18290.
- [CrossRef] [Google Scholar]
- Nitric oxide synthase induces macrophage death by apoptosis. Biochem. Biophys. Res. Commun.. 1993;191:503-508.
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep.. 2018;5:189-195.
- [CrossRef] [Google Scholar]
- Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem.. 2002;50:81-86.
- [CrossRef] [Google Scholar]
- Investigation of acute organophosphate poisoning in humans based on sociodemographic and role of neurotransmitters with survival study in South India. Sci Rep. 2022;12:16513.
- [CrossRef] [Google Scholar]
- Effects of subchronic malathion exposure on the pharmacokinetic disposition of pefloxacin. Environ. Toxicol. Pharmacol.. 2006;22:167-171.
- [CrossRef] [Google Scholar]
- Apoptosis in the pathogenesis and treatment of disease. Science (New York, N.Y.). 1995;267:1456-1462.
- [CrossRef] [Google Scholar]
- Mechanisms of organophosphate neurotoxicity. Curr Opin Toxicol. 2021 Jun;26:49-60.
- [CrossRef] [Google Scholar]
- Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J. Pharmacol.. 2004;36:76-79.
- [Google Scholar]
- Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol.. 2011;49:991-998.
- [CrossRef] [Google Scholar]
- Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag.. 2011;7:325-333.
- [CrossRef] [Google Scholar]
- Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl. Acad. Sci.. 1996;93:6770-6774.
- [CrossRef] [Google Scholar]
- Neuroprotective effect for cerebral ischemia by natural products: A review. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from aloe vera gel. Planta Med.. 2003;69(3):269-271.
- [Google Scholar]
- Hepatic disposition of neurotoxins and pesticides. Pharmacol. Toxicol.. 2000;87:286-291.
- [CrossRef] [Google Scholar]
- Protective effects of dietary flavonoids against pesticide-induced toxicity: A review. Trends Food Sci. Technol.. 2021;109:271-279.
- [CrossRef] [Google Scholar]







