Translate this page into:
Bakuchiol reduces the severity of Mycoplasma pneumoniae-induced pneumonia in mice by attenuating inflammatory responses
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Pneumonia has become a leading cause of death due to its high morbidity rates worldwide. Pneumonia is caused by the bacterium Mycoplasma pneumoniae (MP), which is a prominent airway pathogen. MP infection is usually self-limiting, but an alarming increase in cases has recently developed into refractory, severe, and even fatal pneumonia.
Objective
The objective of the current work was to study the anti-inflammatory and antioxidant properties of bakuchiol in ameliorating MP-induced pneumonia in mice.
Methodology
BALB/c mice were challenged with MP (100 µl) via nasal drops for 2 days to induce pneumonia, and bakuchiol (25 mg/kg) was given concurrently to the mice for 3 days. Thereafter, measurements of lung weight, MPO, and NO concentrations were done. The corresponding kits were used to analyze the GSH, SOD, and MDA concentrations. The DNA contents of MP were assessed by PCR, and the total cells in BALF were counted. The corresponding kits were used to measure NF-κB levels and examine the inflammatory cytokine levels in the BALF samples. The lung histopathology was assessed to identify the histological changes.
Results
In pneumonia-induced mice, the lung weight was significantly reduced after treatment with 25 mg/kg of bakuchiol. The bakuchiol treatment exhibited an effective antioxidant and anti-inflammatory properties on the pneumonia mice. Bakuchiol also lowered the NF-κB level and attenuated histological alterations in the lungs of pneumonia-induced mice. Conclusion: In summary, our findings exhibited that bakuchiol significantly reduced inflammation induced by MP in a mouse model of pneumonia. As a result, it has potential as a future salutary agent for the pneumonia treatment.
Keywords
Airway pathogen
Inflammation
Bakuchiol
Myeloperoxidase
M. pneumoniae
1 Introduction
Pneumonia is an acute lung disease with severe symptoms. Pneumonia can be caused by a variety of factors, the most common of which is microbial infection. Choking on food, inhaling hydrocarbons, swallowing a foreign object, having too much stomach acid, or having an allergic reaction are some of the common causes of pneumonia. About 4 million children die each year from pneumonia, making it the major infectious cause of child mortality worldwide, particularly in underdeveloped nations (Miyashita, 2022). Mycoplasma pneumoniae (MP), a frequent pathogen of pneumonia in children and adolescents, is responsible for 10 to 40% of incidences. It is capable of causing not just pneumonia but also a wide range of extra-pulmonary and systemic consequences (Rueda et al., 2022). MP infection is usually self-limiting, but an alarming increase in cases has recently developed into refractory, severe, and even fatal pneumonia. Symptoms of MP infection include a recurrent fever, a dry cough, a headache, and a sore throat. A prolonged dry cough or a cough with significant phlegm is the most common respiratory symptom of MP infection (Megumi et al., 2020). Recently, there has been a progressive rise in the frequency of severe pneumonia, especially in younger patients. The pathophysiology of MP pneumonia is not fully understood. The initial attachment of MP occurs on the airway epithelial cell surface. Due to the lack of a cell wall, the MP membrane is able to make direct contact with its host and engage in membrane-to-membrane exchanges (Essandoh et al., 2016).
The pathogenesis of MP involves intracellular colonization, adherence to host cells, and rupture of cell membranes, all of which can injure alveoli and alter the structure of epithelial cells, impairing lung function (Tsai et al., 2021). The etiology of MP infection is generally associated with an imbalanced immune-inflammatory response following infection. In response to MP infection, airway epithelial cells release a array of inflammatory cytokines, including TNF-α, which recruit other immune cells and cause inflammation, remodeling of the airway, obstruction of airflow, and a decrease in lung activity (Lai et al., 2019). Dysregulation of cytokines such as TNF-α, IL-6, and IL-10 contributes to the pathogenesis of severe pneumonia by activating systemic inflammation (Gao et al., 2015). IL-6, TNF-α, and IL-10 are major inflammatory mediators that have been shown to be secreted in response to MP infection. These cytokines recruit inflammatory cells, linking the adaptive and intrinsic immune responses (Sun et al., 2020). It was known that the immune reaction has a dual effect in many different types of infections, acting both as an early-stage antibacterial agent and as a long-lasting agent that damages tissue (Yu et al., 2018).
Treatment with glucocorticoids and immunoglobulins has been shown to be effective in suppressing inflammatory responses and alleviating clinical symptoms in patients with refractory MP pneumonia, supporting the idea that immune inflammatory damage plays a pivotal function in the progression of MP pneumonia (Shan et al., 2017). The most frequently prescribed drug for MP pneumonia is azithromycin, a member of a class of drugs known as macrolides. Azithromycin is effective against a wide variety of bacteria and yeast infections; however, it might cause gastrointestinal distress in children when used for an extended period of time. Treatment for pneumonia is complicated and ineffective for a variety of reasons, including mixed infection and mycoplasma colonization (Izumikawa et al., 2014). Hence, the need for novel and effective medications with fewer side effects has emerged in recent times for the successful treatment of pneumonia.
Bakuchiol is a phenolic monoterpene compound obtained from the seeds of Psoralea corylifolia (Leguminosae). It has been shown that bakuchiol has a wide variety of biological properties, including antioxidant (Seo et al., 2013), anti-inflammatory (Lim et al., 2019), antidiabetic (Xin et al., 2019), anti-aging (Chaudhuri and Bojanowski, 2014), and anticancer (Li et al., 2017) properties. Bakuchiol treatment may also reduce oxidative stress, edema, and inflammation in sepsis-induced mice (Zhang et al., 2017). Additionally, bakuchiol may lessen myocardial ischemia–reperfusion damage (Feng et al., 2016). Apart from these effects, the ameliorative effects of bakuchiol against mycoplasmal pneumonia remain to be explored. Hence, the current study sought to discover the protective effects of bakuchiol against MP-induced pneumonia in mice.
2 Materials and methods
2.1 Chemicals
Bakuchiol, azithromycin, and other chemicals were purchased from Sigma-Aldrich, USA. The corresponding assay kits for biochemical markers were attained from Thermofisher, USA.
2.2 Animals
4–6 weeks aged BALB/c mice were used in the current work. The mice used in the investigation were housed in hygienic polypropylene cabins. Throughout the experiment, mice were kept in a organized milieu with a temperature of 23 ± 5 °C, air moisture of 50%-60%, and a light/dark series of 12 h. During the course of the study, the mice were allowed to access the pellet food and distilled water. Before beginning trials, the mice were acclimated to the laboratory environment for one week. All the animal experiments were approved by the institutional animal ethical committee.
2.3 Experimental groups
A total of 24 mice were divided evenly among four groups. Group I served as a non-treated control group. Mice in Group II were given 100 µl of MP in their nostrils twice daily for two days to induce pneumonia. Group III mice were given an MP exposure to induce pneumonia, and bakuchiol (25 mg/kg) was given to them via oral gavage route concurrently for three days. Mice in group IV were given an MP while also receiving three days of treatment with azithromycin (100 mg/kg). After all of the treatments were completed, the mice were anesthetized and sacrificed. The blood and lung tissues were then collected for biochemical analysis.
2.4 Quantification of myeloperoxidase (MPO) and nitric oxide (NO) levels
When the lungs were taken from the test mice, they were homogenized with buffer and centrifuged at 10,000 rpm for 10 min at 4°Celsius. The supernatant was then used to measure NO concentration and MPO activity using the kits in the guidelines specified by the manufacturers (Thermofisher, USA).
2.5 Determination of oxidative and antioxidant biomarkers
Using the corresponding assay kits in agreement with the manufacturer's recommendations, the malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) concentrations in the lung tissue homogenate of both control and treated mice were examined (Biocompare, USA).
2.6 Collection of BALF and total cell count
By injecting 30 ml aliquots of saline into the right middle lobe of the mice used in the experiment and control groups, the BALF was obtained. The cell pellets were then separated from the collected BALF by immediately centrifuging it at 6000 rpm for 5 min. The total cells in the samples was then enumerated using a hemocytometer and a optical microscope.
2.7 Detection of MP DNA amount
Homogenates of lung tissue were taken from the experimental mice and placed into the DNA extraction buffer for 10 min at 37 °C. Finally, DNA was extracted from the samples by centrifuging the solution at 13,000 rpm for 5 min at 4 °C. After that, the PCR method was used to analyze how much MP DNA was in each sample (Williamson et al., 1992).
2.8 Quantification of pro-inflammatory cytokines
The contents of inflammatory biomarkers were examined in the obtained BALF from the control and treated animals. Using the corresponding assay kits and the manufacturer's specified procedures, the status of IL-1, IL-6, IL-8, TNF-α, and TGF in animal BALF was measured (Thermofisher Scientific, USA).
2.9 Detection of NF-κB levels
According to the manufacturer's instructions, we used the kit to determine the amount of NF-κB in the lung tissue homogenate of both control and treated mice (Thermofisher, USA).
2.10 Statistical analysis
The outcomes were analyzed statistically using SPSS software, and the findings was reported as the mean ± SD of triplicates. One-way ANOVA and Tukey's post hoc assay were employed to assess the values and p < 0.05 was fixed as statistical significance.
3 Results
3.1 Bakuchiol decreases lung weight, MPO, and NO concentrations in the pneumonia-induced mice
The MPO activity, NO, and lung weight index of control and MP-induced pneumonia mice are shown in Fig. 1. Compared to controls, the MP-infected pneumonia mice revealed a significant increase in lung weight, NO levels, and MPO activity. After receiving 25 mg/kg of bakuchiol, pneumonia mice showed a significant decrease in lung weight, MPO, and NO concentrations. Similar decreases were also observed in the azithromycin-treated pneumonia mice.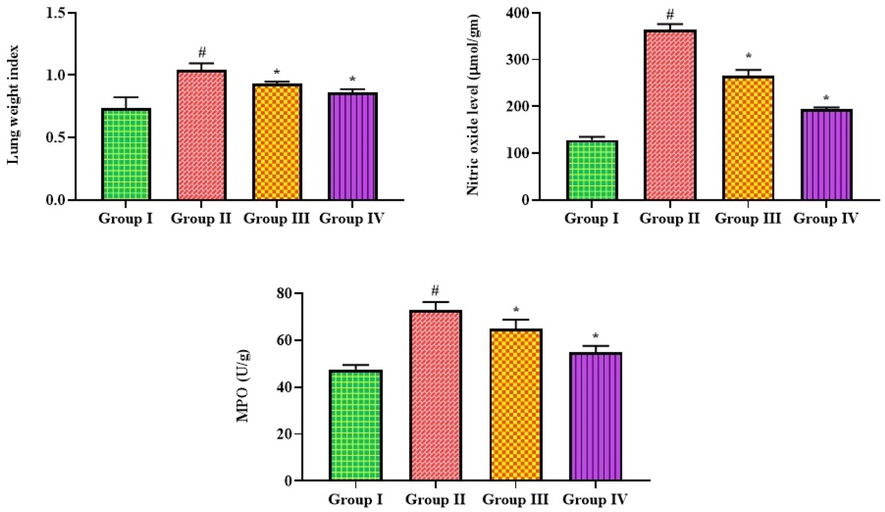
Effect of bakuchiol on the lung weight, NO concentration, and MPO activity in the pneumonia-induced mice. The results were shown as the mean ± SD of three independent tests. One-way ANOVA and Tukey's post hoc test were used for statistical analysis of the data. An asterisk (#) denotes a significant difference (p < 0.01) from the control group, while a (*) denotes a significant difference (p < 0.05) from pneumonia mice. Note. Group I. Control mice; Group II. Pneumonia-induced mice; Group III. Pneumonia + Bakuchiol (25 mg/kg)-treated mice; Group IV. Pneumonia + Standard drug azithromycin (100 mg/kg)-treated mice.
3.2 Bakuchiol increases antioxidants while decreasing the MDA level in the lung tissues of pneumonia-induced mice
The results of bakuchiol on MDA, GSH, and SOD levels in pneumonia mice are exhibited in Fig. 2. The pneumonia mice had a considerably increased MDA content and decreased GSH and SOD concentrations than the control. Bakuchiol at a dose of 25 mg/kg remarkably reduced the MDA and boosted the GSH and SOD concentrations in the mice with pneumonia. Azithromycin, a standard drug, also reduced MDA and boosted GSH and SOD in pneumonia-induced mice.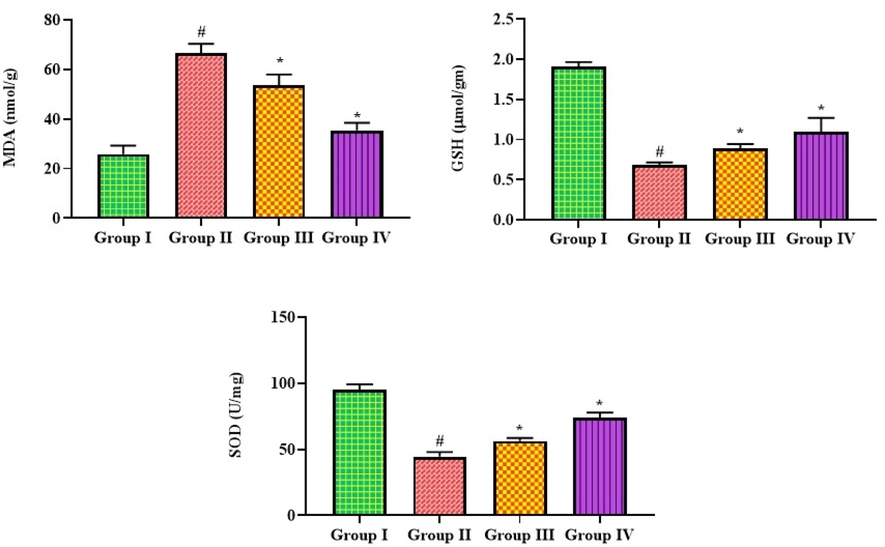
Effect of bakuchiol on the antioxidants and MDA level in the lung tissues of pneumonia-induced mice. The results were shown as the mean ± SD of three independent tests. One-way ANOVA and Tukey's post hoc test were used for statistical analysis of the data. An asterisk (#) denotes a significant difference (p < 0.01) from the control group, while a (*) denotes a significant difference (p < 0.05) from pneumonia mice. Note. Group I. Control mice; Group II. Pneumonia-induced mice; Group III. Pneumonia + Bakuchiol (25 mg/kg)-treated mice; Group IV. Pneumonia + Standard drug azithromycin (100 mg/kg)-treated mice.
3.3 Bakuchiol decreases the DNA amount and total cells in the lung tissues of pneumonia-induced mice
The total cell count and DNA content of the MP in the untreated and treated mice are shown in Fig. 3. Mice with pneumonia induced by MP had a striking elevation in the total cell numbers and the quantity of DNA in their lung tissues. The treatment with 25 mg/kg of bakuchiol remarkably depleted both the total cells and the amount of MP DNA in the lungs. Both the DNA amount and total cells in the lung tissues were reduced by the azithromycin treatment, which corroborated the effects of bakuchiol.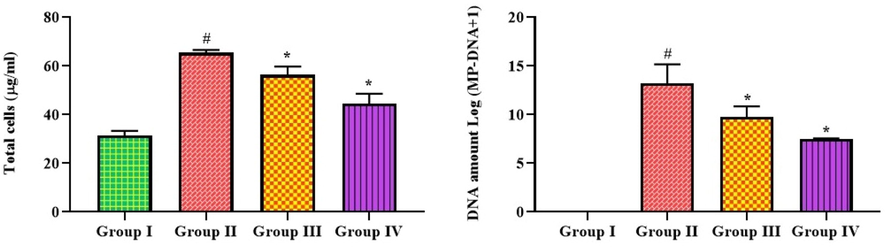
Effect of bakuchiol on the total cells and DNA amount in the lung tissues of pneumonia-induced mice. The results were shown as the mean ± SD of three independent tests. One-way ANOVA and Tukey's post hoc test were used for statistical analysis of the data. An asterisk (#) denotes a significant difference (p < 0.01) from the control group, while a (*) denotes a significant difference (p < 0.05) from pneumonia mice. Note. Group I. Control mice; Group II. Pneumonia-induced mice; Group III. Pneumonia + Bakuchiol (25 mg/kg)-treated mice; Group IV. Pneumonia + Standard drug azithromycin (100 mg/kg)-treated mice.
3.4 Bakuchiol decreases the levels of inflammatory cytokines in the BALF of pneumonia-induced mice
The effects of bakuchiol administration on the levels of inflammatory markers in the BALF of both control and treated mice are shown in Fig. 4. BALF levels of IL-6, IL-1, IL-8, TGF, and TNF-α were dramatically elevated in pneumonia mice compared to controls. Meanwhile, BALF from pneumonia mice administered with 25 mg/kg of bakuchiol showed a significant decrease in these cytokine levels. The activity of bakuchiol was supported by the results of azithromycin, which show that the status of these cytokines in the MP-infected pneumonia mice was also remarkably depleted after the azithromycin treatment (Fig. 4).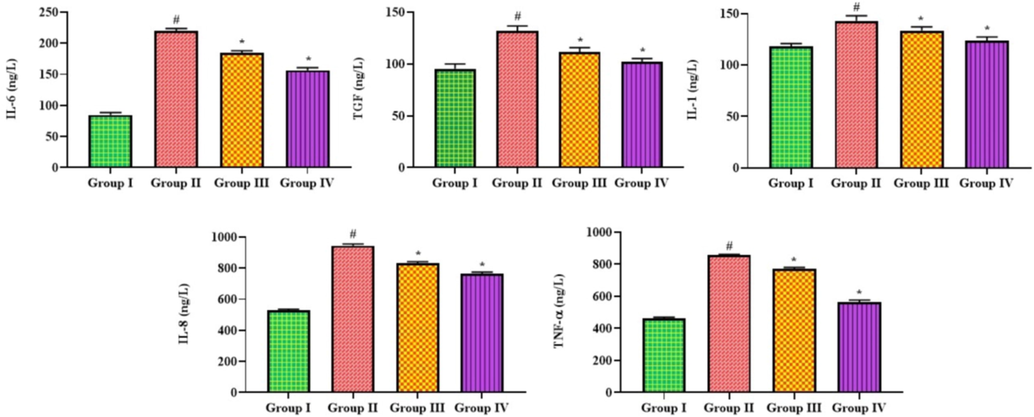
Effect of bakuchiol on the levels of inflammatory cytokines in the BALF of pneumonia-induced mice. The results were shown as the mean ± SD of three independent tests. One-way ANOVA and Tukey's post hoc test were used for statistical analysis of the data. An asterisk (#) denotes a significant difference (p < 0.01) from the control group, while a (*) denotes a significant difference (p < 0.05) from pneumonia mice. Note. Group I. Control mice; Group II. Pneumonia-induced mice; Group III. Pneumonia + Bakuchiol (25 mg/kg)-treated mice; Group IV. Pneumonia + Standard drug azithromycin (100 mg/kg)-treated mice.
3.5 Bakuchiol treatment decreases the NF-κB expression in the lung tissues of pneumonia-induced mice
Fig. 5 shows the relative amount of NF-κB in mice with and without MP-induced pneumonia. When compared to controls, the NF-κB expression in the lungs of the mice exposed to pneumonia showed a significant increase. While, the treatment of 25 mg/kg of bakuchiol significantly reduced NF-κB levels in mice with pneumonia. A similar reduction in NF-κB level was also seen in the pneumonia mice when treated with azithromycin, which supports the activity of bakuchiol.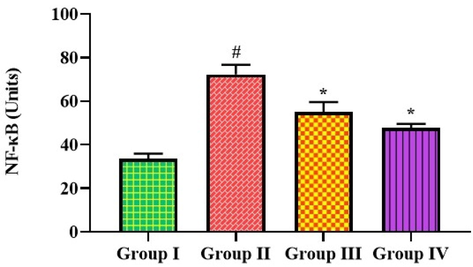
Effect of bakuchiol on the NF-κB expression in the lung tissues of pneumonia-induced mice The results were shown as the mean ± SD of three independent tests. One-way ANOVA and Tukey's post hoc test were used for statistical analysis of the data. An asterisk (#) denotes a significant difference (p < 0.01) from the control group, while a (*) denotes a significant difference (p < 0.05) from pneumonia mice. Note. Group I. Control mice; Group II. Pneumonia-induced mice; Group III. Pneumonia + Bakuchiol (25 mg/kg)-treated mice; Group IV. Pneumonia + Standard drug azithromycin (100 mg/kg)-treated mice.
3.6 Bakuchiol improves the lung histopathology
The differences between the lung histology of control and treated animals are shown in Fig. 6. The lungs of the control mice had typical alveolar patterns without any lesions or inflammatory symptoms. The enhanced inflammatory cell infiltrations, thickening of alveolar walls, and constricted bronchial tubes in the lungs were seen in mice that had developed pneumonia. The treatment with bakuchiol significantly reduced the indications of hyperemia, congested bronchial tubes, and inflammatory cell infiltration in the pneumonia mice (Fig. 6). Treatment with azithromycin also significantly attenuated the lung tissue histological changes in pneumonia-induced mice.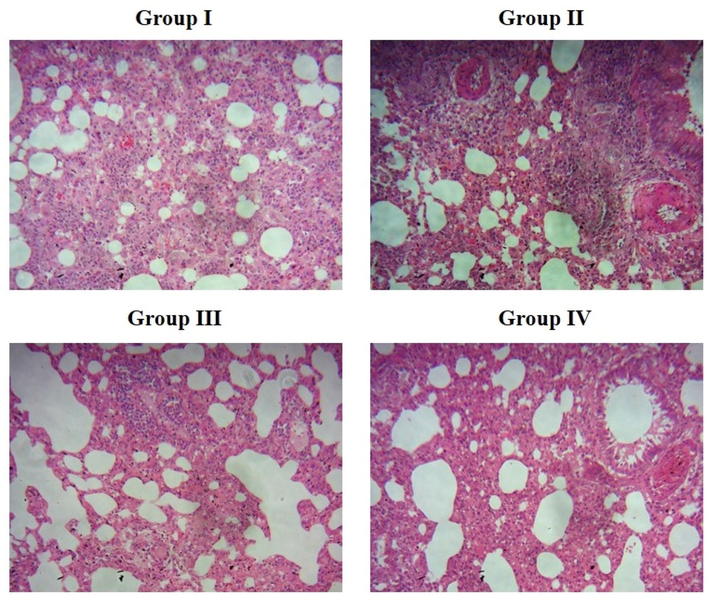
Effect of bakuchiol on the lung histopathology of pneumonia-induced mice. Group I. Alveolar structures in control mice are normal, and there are no symptoms of inflammation or lesions. Group II. Greater inflammatory cell infiltrations, thickening of alveolar walls, and constricted bronchial tubes were observed in the lung tissues of MP-infected pneumonia mice. Group III. Treatment with 25 mg/kg of bakuchiol significantly decreased the histological changes in the lung tissues of pneumonia mice. Group IV. Histological changes in the lungs were substantially suppressed by standard drug azithromycin treatment.
4 Discussion
MP is one of the common bacterial species that can cause life-threatening pneumonia. The clinical presentation of an infection caused by this bacterium is slow-progressing tracheobronchitis accompanied by a dry cough and agitation. This bacterium can cause acute pneumonia as well as less severe episodes of pharyngitis and tracheobronchitis (Becker et al., 2015). The clinical manifestations of MP infection range from asymptomatic infection to lethal pneumonia. Children and young adults are more vulnerable to MP, which can lead to acute bronchitis, community-acquired pneumonia (CAP), and acute asthma (Maselli et al., 2018). In the airway epithelium, MP can multiply, aggregate, and release inflammatory factors and chemotactic mediators, and even take part in the inflammatory process and immune response, all of which can lead to chronic airway inflammation and hyperresponsiveness (Narita, 2016). Inflammation is a crucial part of the innate immune system because it helps protect the body against external pathogens. Inflammation caused by the host's immune mechanisms is a major factor in the pathogenesis of MP infection and its clinical manifestations (Waites et al., 2008; Naik et al., 2021). Inflammation involves many different types of inflammatory cells, and the removal of MP from the lungs is mostly dependent on macrophages rather than neutrophils (Lai et al., 2010). The outcomes of the current work exhibited an increased total cell count in the lungs of the pneumonia mice, which proves the severe inflammatory cell influx and infiltrations into the lungs. Interestingly, the pneumonia mice treated with bakuchiol effectively depleted the total cells in the lung tissues, demonstrating its anti-inflammatory effects.
Inflammation of the respiratory system is often caused by cytokine production in the airway epithelial cells. Increased inflammatory responses to pathogen invasion can harm the lungs, leading to poor clinical results in pneumonia (Dolinay et al., 2012; Theerthagiri et al., 2019 Sep 27). Upon exposure to MP antigen or live MP, bronchial epithelial cells produce inflammatory cytokines, which subsequently chemoattract and activate neutrophils. The pathogenesis of pneumonia relies on an inflammatory response triggered by an imbalance between pro- and anti-inflammatory cytokines (Shi et al., 2017). The secretion of pro-inflammatory mediators and the formation of pulmonary fibrosis are increasingly linked to MP-induced pneumonia symptoms (Kurai et al., 2013). Increased secretion of TNF-α, IL-6, and other chemokines at the infection site, as well as leukocyte aggregation (mostly neutrophils), can cause lung injuries and pulmonary fibrosis caused by MP infection (Lin et al., 2018). IL-8 is an influential cytokine that is linked to the onset and persistence of inflammation. Human IL-8 is predominantly secreted by the epithelium lining the airways, and its expression is correlated with the pathogenesis of pneumonia (Wang et al., 2018). Serum IL-6 levels were found to be significantly higher in MP-infected patients, making it a useful predictor of therapy outcomes (Tian et al., 2020). The pneumonia mice showed a significant increase in IL-1, IL-6, IL-8, TNF-α, and TGF levels in the current study. Meanwhile, bakuchiol treatment significantly reduced the status of these cytokines in pneumonia mice. The activity of bakuchiol was corroborated by the results of the standard drug azithromycin, which supports the anti-inflammatory effects of bakuchiol. An earlier study done by Zhang et al. (Zhang et al., 2021 Aug) found that baicalin treatment effectively reduced the inflammatory cytokine levels in the pneumonia mice. These findings corroborated the current findings and supported the anti-inflammatory role of bakuchiol.
The mediators released by the accumulating leukocytes at the site of inflammation have a significant influence on the onset and regulation of inflammation. MPO is a common neutrophil hemoprotein believed to play a pivotal function in mediating host defense mechanisms (Nussbaum et al., 2013). Lung MPO accumulation has the potential to modulate the redox-sensitive signaling that regulates inflammatory mechanisms, such as the catabolism of NO. Furthermore, the high MPO concentration may result in oxidative stress and cell injury (KubalaL et al., 2013). The outcomes of the present work unveiled that the pneumonia mice showed a drastic increase in the concentrations of MPO and NO than the control. The bakuchiol treatment significantly reduced MPO and NO concentrations in pneumonia mice, which is similar to the results of azithromycin treatment.
The airways are vulnerable to oxidative attack, the severity of which is determined by the body's ability to counter or upregulate the defensive ROS scavenging mechanisms (Theerthagiri et al., 2021). Even though the lungs have anti-oxidants like GSH and SOD to defend against damage from inhaled ROS, their activities can be compromised (Comhair et al., 2000). GSH is a powerful intracellular antioxidant with a wide range of roles in regulating cellular redox homeostasis. The epithelium lining of the respiratory tract has been observed to have higher GSH concentrations than those seen in plasma. In addition, the lungs have specialized proteins, including peroxiredoxins, thiodoxins, glutaredoxins, haeme oxygenases, and reductases, which all work together to defend against oxidative damage (Domej et al., 2014). The outcomes of this work demonstrated the considerable reduction in SOD and GSH concentrations while increased levels of MDA in the pneumonia mice. Interestingly, the bakuchiol treatment remarkably improved the SOD and GSH concentrations and diminished the MDA concentration in the pneumonia mice. These outcomes support the antioxidant properties of bakuchiol. Fu et al. (Fu et al., 2022 Apr) have found that the treatment with geraniol showed an antioxidant property by considerably boosting the antioxidants in the pneumonia mice. These findings are in line with the present results of the bakuchiol treatment and support its antioxidant properties in the pneumonia mice.
The pro-inflammatory effects of pneumonia require NF-κB activation. The lipoprotein subunits of MP are capable of recognizing toll-like receptors and triggering NF-κB activation (Shimizu et al., 2008). In bronchial epithelial cells, MP has been shown to trigger the NF-κB pathway, which in turn increases inflammation (Chmura et al., 2008). The protective inflammatory genes are expressed in conjunction when NF-κB is activated (Andrades et al., 2011). Reducing the inflammatory response is hypothesized to have a protective effect on the lungs. It was reported that the stimulation of NF-κB in airway epithelial cells is especially critical for regulating inflammatory reactions, which can occur in a variety of lung cell types (Sadikot et al., 2006). It is becoming clear that the lung epithelium is an important site where NF-κB is stimulated due to the several pathogens in the airways. Multiple microbial and host-derived factors have been reported to stimulate NF-κB in epithelial cells (Poynter et al., 2003). Reports have revealed a tight connection between NF-κB signaling and lung disorders, including acute lung damage (Gu et al., 2020; Arora et al., 2019). In the current work, we observed that the pneumonia mice revealed increased NF-κB expression in the lungs. However, the bakuchiol administration considerably reduced the NF-κB expression in the lungs of the pneumonia mice, which supports its anti-inflammatory roles. The previous report found a decrease in NF-κB expression in the pneumonia mice after treatment with morusin (Chen et al., 2019). These findings supported the current findings of the bakuchiol treatment, which also reduced NF-κB expression in the pneumonia mice.
5 Conclusion
In summary, our findings exhibited that bakuchiol successfully reduced inflammation and oxidative stress induced by MP in a mouse model of pneumonia. Antioxidant levels were significantly increased after treatment with bakuchiol, whereas inflammatory biomarkers were significantly reduced, and histological alterations were improved in the pneumonia mice. This proposes that bakuchiol may be a talented salutary agent to treat the pneumonia. Nevertheless, more studies are required to fully understand the processes through which bakuchiol exerts its therapeutic effects against pneumonia.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R677), King Saud University, Riyadh, Saudi Arabia for financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J. Surg. Res.. 2011;167:307-313.
- [Google Scholar]
- Structure of CARDS toxin, a unique ADP-ribosylating and vacuolating cytotoxin from mycoplasma pneumonia. Proc. Natl. Acad. Sci. USA. 2015;112(16):5165-5170.
- [Google Scholar]
- Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmetic Sci.. 2014;36(3):221-230.
- [Google Scholar]
- Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling. Biosci. Rep.. 2019;39(6) (BSR20190190)
- [Google Scholar]
- Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. Am. J. Physiol. Lung Cellular Mol. Physiol.. 2008;295(1):L220-L230.
- [Google Scholar]
- Differential induction of extracellular glutathione peroxidise and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O2 or cigarette smoke. Am. J. Respir. Cell Mol. Biol.. 2000;23:350-434.
- [Google Scholar]
- Inflammasome-Regulated cytokines are critical mediators of acute lung. Am. J. Respir. Crit. Care Med.. 2012;185(11):1225-1234.
- [Google Scholar]
- Oxidative stress and free radicals in COPD-implications and relevance for treatment. Int. J. Chron. Obstruct. Pulmon. Dis.. 2014;9:1207-1224.
- [Google Scholar]
- MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46:122-131.
- [Google Scholar]
- Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: the role of silent information regulator 1. Apoptosis. 2016;21(5):532-545.
- [Google Scholar]
- Geraniol relieves mycoplasma pneumonia infection-induced lung injury in mice through the regulation of ERK/JNK and NF-κB signaling pathways. J. Biochem. Mol. Toxicol.. 2022;36(4):e22984.
- [Google Scholar]
- Mycoplasma pneumonia infection and asthma: a clinical study. Pakistan J. Med. Sci. Quart.. 2015;31:548-551.
- [Google Scholar]
- LncRNA MALAT1 affects mycoplasma pneumoniae pneumonia via NF-kB regulation. Front. Cell Dev. Biol.. 2020;8:563693
- [Google Scholar]
- Clinical features, risk factors andtreatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J. Infection Chemother.. 2014;20(3):181-185.
- [Google Scholar]
- The potentiation of myeloperoxidase activity by the glycosaminoglycan- dependent binding of myeloperoxidase to proteins of the extracellular matrix. Biochimica et Biophys Acta (BBA)—General Subjects. 2013;1830(10):4524-4536.
- [Google Scholar]
- Mycoplasma pneumoniae extract induces an IL-17-associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation. 2013;36:285-293.
- [Google Scholar]
- A Novel TNF-a-Targeting Aptamer for TNF-a-Mediated acute lung injury and acute liver failure. Theranostics. 2019;9(6):1741-1751.
- [Google Scholar]
- Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS One. 2010;5:e14417.
- [Google Scholar]
- Mechanistic study of bakuchiol-induced anti-breast cancer stem cell and in vivo anti-metastasis. Front. Pharmacol.. 2017;8:746.
- [Google Scholar]
- Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int. J. Mol. Sci.. 2019;20(14):E3574.
- [Google Scholar]
- The protective effect of naringenin on airway remodeling after Mycoplasma pneumoniae infection by inhibiting autophagy-mediated lung inflammation and fibrosis. Mediators Inflamm.. 2018;2018:8753894.
- [Google Scholar]
- The immunopathologic effects of Mycoplasma pneumoniae and community-acquired respiratory distress syndrome toxin. A primate model. Am. J. Respir. Cell Mol. Biol.. 2018;58:253-260.
- [Google Scholar]
- Transgenic chlamydomonas expressing human transient receptor potential Ankyrin 1 (TRPA1) channels to assess the effect of agonists and antagonists. Front. Pharmacol.. 2020;11:578955
- [Google Scholar]
- Atypical pneumonia: pathophysiology, diagnosis, and treatment. Respir. Investig.. 2022;60(1):56-67.
- [Google Scholar]
- Rapid and highly selective electrochemical sensor based on ZnS/Au-decorated f-multi-walled carbon nanotube nanocomposites produced via pulsed laser technique for detection of toxic nitro compounds. J Hazardous Mat.. 2021;418(15):126269
- [Google Scholar]
- Classification of extrapulmonary manifestations due to mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front. Microbiol.. 2016;7:23.
- [Google Scholar]
- Myeloperoxidase: a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid. Redox Signal.. 2013;18(6):692-713.
- [Google Scholar]
- A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway inflammation. J. Immunol.. 2003;170:6257-6265.
- [Google Scholar]
- Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatrics. 2022;22(1):169.
- [Google Scholar]
- Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J. Immunol.. 2006;176:4923-4930.
- [Google Scholar]
- Protective role of Psoralea corylifolia L. seed extract against hepatic mitochondrial dysfunction induced by oxidative stress or aging. Evid. Based Complement Alternative Medicine Studies 2013678028
- [Google Scholar]
- Effects of methylprednisolone or immunoglobulin when added to standard treatment with intravenous azithromycin for refractory Mycoplasma pneumoniae pneumonia in children. World J. Pediatric Surg.. 2017;14:321-327.
- [Google Scholar]
- Downregulation of caspase3 alleviates Mycoplasma pneumoniae induced apoptosis in alveolar epithelial cells. Mol. Med. Rep.. 2017;16:9601-9606.
- [Google Scholar]
- Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun.. 2008;76:270-277.
- [Google Scholar]
- Data mining and systematic pharmacology to reveal the mechanisms of traditional Chinese medicine in Mycoplasma pneumoniae pneumonia treatment. Biomed. Pharmacother.. 2020;125:109900
- [Google Scholar]
- A review on ZnO nanostructured materials: energy, environmental and biological applications. Nanotechnology. 2019;30(39):392001
- [Google Scholar]
- Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J Hazardous Mat.. 2021;412(15):125245
- [Google Scholar]
- Differences of TNF-α, IL-6 and Gal-3 in lobar pneumonia and bronchial pneumonia caused by mycoplasma pneumoniae. Technol. Health Care.. 2020;28:711-719.
- [Google Scholar]
- Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J. Microbiol. Immunol. Infect.. 2021;54:557-565.
- [Google Scholar]
- New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol.. 2008;3:635-648.
- [Google Scholar]
- Serum tumor necrosis factor- α and interferon- γ levels in pediatric Mycoplasma pneumoniae pneumonia: a systematic review and meta-analysis. Can Respiratory J. 20188354892
- [Google Scholar]
- Laboratory diagnosis of mycoplasma pneumoniae infection. 4. Antigen capture and PCR-Gene amplification for detection of the mycoplasma: problems of clinical correlation. Epidemiol. Infect.. 1992;109(3):519-537.
- [Google Scholar]
- Bakuchiol: a newly discovered warrior against organ damage. Pharmacol. Res.. 2019;141:208-213.
- [Google Scholar]
- Inhibition of microRNA-143-3p attenuates myocardial hypertrophy by inhibiting inflammatory response. Cell Biol. Int.. 2018;42:1584-1593.
- [Google Scholar]
- Bakuchiol protects against acute lung injury in septic mice. Inflammation. 2017;40(2):351-359.
- [Google Scholar]
- Baicalin relieves Mycoplasma pneumoniae infection-induced lung injury through regulating microRNA-221 to inhibit the TLR4/NF-κB signaling pathway. Mol. Med. Rep.. 2021;24(2):571.
- [Google Scholar]







