Translate this page into:
Evaluation of cytotoxic activity of Syringodium isoetifolium against human breast cancer cell line - an in silico and in vitro study
⁎Corresponding authors. amudhaa85@gmail.com (P. Amudha), mprabhaharan@hotmail.com (M. Prabhaharan), sasijanaki123@gmail.com (P. Sasikumar), ghassan.m.sulaiman@uotechnology.edu.iq (Ghassan M. Sulaiman),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Seagrass is a natural, renewable, and much unexplored marine resource, which are capable, and reliable sources in the field of pharmaceuticals and drug discovery. The seagrass, Syringodium isoetifolium is our target plant for the study, which was collected from the Ramanathapuram district, Tamil Nadu. The current study is focused to evaluate the cytotoxic, cell cycle arrest, and apoptotic induction activities of the hydroalcoholic extract of S. isoetifolium against the human breast cancer (MCF-7) cell line. The cytotoxic evaluation revealed that the extract inhibited MCF-7 proliferation with IC50 value of 230.32 μg/ml. Evaluation of MCF-7 cell cycles demonstrated that the extract arrested the cell cycle in the S phase and continued to the G2/M phase at half of the IC50 value. The extract induced apoptotic of MCF-7 cells about 30.61% which was nearly the same with aripiprazole as a positive control (52.35%). Nootkatone significantly binds to the target proteins – HSP 90 and HER2 kinase with the least binding energy was predicted as the most active anticancer compounds by a molecular docking study. In light of the findings, it can be said that the hydroalcoholic extract of S. isoetifolium has the potential to be a therapeutic agent for breast malignancies by acting as an anticancer component. It can be used as an anticancer agent on its own and/or as a scaffold for analog synthesis to develop novel anticancer agents with improved therapeutic efficacy.
Keywords
Syringodium isoetifolium
MCF-7
Apoptosis
Insilico
HSP 90 and HER2 Kinase receptor
Nootkatone and Zerumbone
1 Introduction
Cancer ranks as the second highest cause of death globally, with mortality and morbidity rates on the rise due to new cases. (Liu et al., 2016). Among women worldwide, breast cancer stands out as the most commonly occurring cancer. Current treatment options for breast cancer include radiotherapy and chemotherapy. However, these therapies often come with various side effects such as nausea, vomiting, hair loss, changes in sexual function, and cognitive dysfunction. Therefore, it is crucial to emphasize the importance of complementary medicine as a natural approach to cancer treatment. (Smolarz et al., 2022).
A novel drug derived from marine sources has recently been developed and holds great promise as a future pharmaceutical resource. (Rhyaf et al., 2023, Singh et al., 2018). Extensive research has validated the efficacy and safety of these drugs. The marine environment is renowned for its breathtaking coral reefs, dolphins, ornamental fishes, sharks, and other fascinating marine life, captivating the interest of people worldwide. Among the marine flora, seagrass stands out as a submerged flowering plant that originated on land and successfully adapted to an underwater existence. (Deepak et al., 2019).
In India, researchers have identified 13 distinct types of seagrass, among which Syringodiumisoetifolium, known as tube grass, exhibits larvicidal and scavenging activities. (Venkataraman et al., 2015). S. isoetifolium holds significant pharmacological potential, displaying antibacterial, antifungal, antibiotic, tumor-inhibiting, anti-hemolytic, and cytotoxic properties attributed to its phytoconstituents. (Gono et al., 2022). Notably, phyto-compounds present in S. isoetifolium exhibit diverse biological activities, such as antiviral, antibacterial, anti-inflammatory, anti-allergenic, anticancer, and antioxidant effects. (Pietta, P.G. 2000).
Traditionally, the concept of “one drug, one target, one disease” has prevailed, but researchers have recently recognized the potential of multi-target medications in treating certain disorders. Molecular docking serves as a computational tool used by researchers to develop multifunctional drugs. This methodology, known as Computer-Aided Drug Design, involves creating a complex between the ligand (e.g., tannin) and the target protein specific to a particular disease. (Scottiet al., 2017).
The purpose of this research was to evaluate the cytotoxicity activity of hydroalcoholic extract of S. isoetifolium on breast cancer cell MCF-7 by MTT assay and analyze the inducing apoptosis and inhibiting cell cycle of these by flow cytometry. By LC-MS analysis, the bioactive components present in the hydroalcoholic extract of S. isoetifolium can be identified. To support the anticancer activity of S. isoetifolium, in silico molecular docking was employed, wherein the phytochemical compounds of the plant were docked against the HSP 90 and HER2 kinase proteins.
2 Materials and methods
2.1 Collection and authentication of Syringodium isoetifolium
Syringodium isoetifolium has been collected from Devipattinam, Ramanadhapuram District, Tamilnadu, India, on June 2019. The identification of seagrass Syringodium isoetifolium was confirmed and validated by Dr. P. Jeyaraman, Ph.D., Director of the Plant Anatomy Research Centre, Retd., Professor, Presidency College (Autonomous), Chennai-600005 and also get authenticated by the Regional Scientist, Southern Regional Centre, Botanical Survey of India, Agriculture University Campus, Coimbatore, Tamilnadu-641 003, India.
2.2 Preparation and extraction of Syringodium isoetifolium
One kilogram of dried, powdered seagrass was extracted with 30:70 proportion of hydro ethanol for maceration periods (24 hrs). The extraction was carried out at room temperature with 150 rpm agitation. The extracts were filtered through the Whatman filter paper after the maceration period. The extracts were concentrated by using the Rotary Evaporator and the dry weight of the crude extracts was weighed and stored at 4 °C in a dark place for further analysis.
2.3 Maintenance of cell lines
The MCF-7 (Human breast adenocarcinoma cell lines) were purchased from National Centre for Cell Sciences (NCCS), University of Pune Campus, Pune, Maharastra-411 007, India. The cells were maintained in DMEM high glucose media supplemented with 10 % FBS along with the 1% antibiotic–antimycotic solution in the atmosphere of 5% CO2, 18–20% O2 at 37 °C temperature in the CO2 incubator and subcultured every 2 days.
2.4 MTT assay
MTT assay is a colorimetric assay used for the determination of cell proliferation and cytotoxicity, based on the reduction of the yellow-colored water-soluble tetrazolium dye MTT to formazan crystals. In a 96-well plate, 200 μl of cell suspension was added without test reagent and allowed for 24 hrs. Now the test reagents were added and incubated at 37 °C in 5% CO2 atmosphere, without light exposure incubate for 3 h, then the MTT reagent was removed with the addition of DMSO solution (100 μl). The reading was absorbed in 570 nm and IC50 values were calculated to check the cell viability percentage (Sulaiman et al., 2016, Al-jubori et al., 2021).
2.5 Cell cycle analysis
Culture the cells in 6 well plates at a density of 2 × 105 cells/ 2 mL and incubated in a CO2 incubator overnight at 37 °C for 24 hrs. Now the spent medium is aspirated and 2 mL of cultured medium is treated with the required concentration of cells with incubation of 24 hrs. By PBS wash remove the medium from all wells and 200 μl of trypsin-EDTA solution was added, with 3–4 min incubation at 37 °C. The cells directly into 12 × 75 mm polystyrene tubes which were centrifuged for five minutes at 300g at 25 °C and the supernatant was decanted carefully with PBS wash. Finally, the pellet cells were stained with propidium iodide, incubated for 15–20 min in the dark, and analyzed by flow cytometry (Mohammed et al., 2021, Sulaiman et al., 2015).
2.6 Cell cycle arrest and apoptosis
With the above standard methods, 5 μl of FITC Annexin V were added and incubated for 15 min at RT (25 °C) in the dark, now 5 μl of PI were added and 400 μl of 1X Binding Buffer to each tube and vortex gently. Now analyzed immediately with the addition of PI by flow cytometry method. Apoptosis can be evaluated by inducing proteins like Caspase 9, Caspase 3, and Bcl-2 expression. After washing pellets with PBS, 0.5 mL BD Cytofix/Cytoperm solution was added and allowed for 10 min. Now, wash it with 0.5% bovine serum albumin (BSA) in 1X phosphate-buffered saline (PBS) and 0.1% sodium azide. Add 20 μl of FITC Rabbit anti-active Caspase 3 antibody/PE Mouse Anti-Human Bcl2 antibody/PE Anti-Human Caspase 9 antibody and mix thoroughly, incubate for 30 min in the dark at room temperature (20 °C to 25 °C). Finally, wash with 1X PBS with 0.1% sodium azide, add 0.5 mL of PBS, mix thoroughly, and analyze by Flow Cytometry with the excitation and emission of 494 nm and 520 nm for FITC or FL-1 channel and excitation and emission of 488 nm and 578 nm for PE or FL-2 channel respectively.
2.7 Liquid chromatography-mass spectrometry analysis
All analytes were chromatographically screened using a ZORBAX Eclipse and a C18 column (2.1 × 100 mm, 5.0 μm molecule estimate; Agilent Technologies) at a flow rate of 0.5 mL/min and an infusion volume of 5 μl. To create the mobile phase, formic acid was combined with 10 mM ammonium formate, and 0.1% formic acid was added to methanol. (A: 80:20, B:10:90). The elution gradient started with 10% of B and progressed to 50% methanol after 0–7 min, 80% of B after 12–15 min, 100% of B after 15–18 min, 100% of B after 18–18.1 min, and 20% of B after 20–20 min. The oven was maintained at a temperature of 45 °C. The MS parameters were as follows: 325 °C for the drying gas; 11 L/min for the gas flow; 40 psi for the nebulizer; 350 °C for the sheath gas; 8 L/min for the sheath gas flow; 500 V for the delta EMV; and 4000 V for the capillary voltage. 3.2.5.1.
2.8 Molecular docking studies
In silico or molecular studies are to perform the interaction between the compounds identified from the HAE of S. isoetifolium. This study is to enhance the precision of biological tests, reliability and the compounds which interact are as follows: 7-Hydroxycoumarine, 4-Hydroxycoumarine, Phloretin, Zerumbone, Arecoline, and Nootkatone. The 3D structure of the PDB ID: 3RCD; 3TUH was downloaded from the protein data bank. Following Sribalan et al.(Sribalan et al., 2019) the discovery studio is used for molecular docking and visualization. Molecular docking was done with the help of Autodock 4.2. ChemDraw 13.0 and MMFF 94 were used to optimize the 3D structure of compounds. (Maximum number of interactions: 5000, minimum RMS gradient: 0.100). The enzyme was cleared of any unnecessary ligands and water, and the default docking settings were adjusted and used.
2.9 Autodock
For the interaction of the ligand with bio macromolecular target, an automated procedure is predicted which is termed an auto dock. This method is used to analyze the three-dimensional structure of the drug binding to the receptor. For docking studies, Genetic Algorithms are used for confirmational search. Along with modeling studies, auto dock tools are employed in the preparation, execution, and analysis of docking simulations (Rauf et al., 2015).
2.10 Ligand for docking preparation
The ligands chosen for this study are 7-Hydroxycoumarine, 4-Hydroxycoumarine, Phloretin, Zerumbone, Arecoline, and Nootkatone were identified by LC-MS analysis. These compounds were planned to dock with two breast cancer proteins such as HER2 Kinase Receptor and HSP90. By using the PubChem database, the physicochemical and structural characteristics of these compounds were recovered. PubChem is an open database, used to search for wide properties which may include hydrogen bond donor, hydrogen acceptor, name of the compound, structure, molecular weight, fragments, chemical formula, and X Log P. The chemical was converted to PDB format using an online editor that uses the Simplified Molecular Input Line Entry Specification (SMILES) format.
2.11 Protein preparation for docking
Large biological macromolecules like proteins and nucleic acids are stored in the RCSB PDB (Research Collaborator for Structural Bioinformatics, Protein Data Bank), which is a repository for their 3D structural information. After researching its metabolic pathway, the target proteins from the National Centre for Biotechnology Information NCBI protein database (https://www.rcsb.org/pdb) were determined to be the HER2 Kinase Receptor with the accession number (PDB ID: 3RCD) and HSP90 with the accession number (PDB ID: 3TUH).
3 Results and discussion
3.1 Cytotoxic activity in MCF-7 cells
Hydroalcoholic extract of S. isoetifolium shows a more cytotoxic effect on MCF-7 cells. The findings from statistical analysis of the cell cytotoxicity study show that the hydroalcoholic extract of S. isoetifolium demonstrated significant cytotoxic potency against MCF-7 cells with an IC50 (50% cell viability) concentration at 230.32 μg/ml in comparison to the standard drug, Aripiprazole. The percentage of cell viability was displayed in Table 1.
Concentration (μg/ml)
% of cell viability
0
100
25
95.96
50
91.13
100
74.47
200
53.95
400
14.84
Aripiprazole-35 mM
41.52
The cells treated with standard, control, and test compound show IC50 concentrations are high % of cells at S and G2/M stage arrest when compared with untreated cells. So, the cell cycle got arrested at S and G2/M stages. Hence, we evaluated the cell cycle study by Flow Cytometry to check the stages of cell cycle arrest and obtained the results by flow cytometry were tabulated in Table 2 and the percentage of cell cycle arrest was shown in Fig. 1.
Percentage of MCF-7 cells
Cell Cycle stage
Untreated
Aripiprazole
Plant Extract
Sub G0/G1
2.06
3.51
1.58
G0/G1
68.46
26.87
56.73
S
1.95
7.82
8.75
G2/M
26.33
52.55
30.61
Total Events Selected per each group −10000
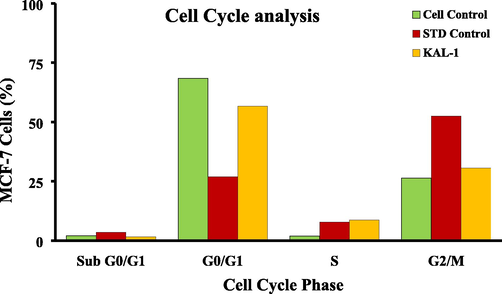
Overlay showing the percentage of cells that get arrested in the different stages of the MCF-7 cell cycle.
In the Sub G0/G1 phase (Apoptotic phase), 2.06%, 3.51%, and 1.58% of cells get arrested in untreated, standard, and hydroalcoholic extract of S. isoetifolium with IC50 concentration respectively. In G0/G1 phase (Growth Phase), 68.46%, 26.87%, and 56.73% of cells get arrested in untreated, standard, and hydroalcoholic extracts of S. isoetifolium with IC50 concentration respectively. In the S phase (synthetic phase), 1.95%, 7.82%, and 8.75% of cells get arrested in untreated, standard, and hydroalcoholic extracts of S. isoetifolium with IC50 concentration respectively. On the other hand, in the G2/M phase, 26.33%, 52.35%, and 30.61% of cells get arrested in the untreated, standard, and hydroalcoholic extract of S. isoetifolium with IC50 concentration respectively. The efficacy of the hydroalcoholic extract of S.isoetifolium on cell cycle arrest is examined and shown in Fig. 1. Hydroalcoholic extract of S. isoetifolium may also cause poly ribose polymerase cleavage caspase 3, and caspase 9 additionally. (Agarwal et al., 2006).
The loss of membrane integrity that follows the most recent stages of cell death brought on by either necrotic or apoptotic processes is preceded by FITC Annexin V staining. To enable the researcher to recognize early apoptotic cells, staining with FITC Annexin V is often employed in conjunction with a crucial dye like propidium iodide (PI) or 7-Amino-Actinomycin (7-AAD). (PI negative, FITC Annexin V positive). Without inhibiting the healthy cells hydroalcoholic extract of S. isoetifolium shows a more cytotoxic effect on cancer cells. It may also possess good anti-inflammatory and anti-malarial activity (Subramanian et al., 2015). In recent research, the authors prove the efficacy of gallic acid with the results obtained, it can induce cell cycle arrest at G2/M phase via Ch2K mediated phosphorylation in bladder carcinoma cell line (Ou et al., 2010). At an earlier stage, Annexin V/PI dye can identify apoptosis. The membrane integrity has been lost due to the stain. The study proves that Annexin V binds with the cells to inhibit the proliferation of cells and also detect apoptosis at different phases the expression of Annexin V with MCF 7 cells was shown in Fig. 2 and the meaning for quadrant was given in Table 3 (Kalpana et al., 2020). The percentage of cells that undergo apoptosis was shown in Fig. 2, which shows the live and apoptotic cell percentages.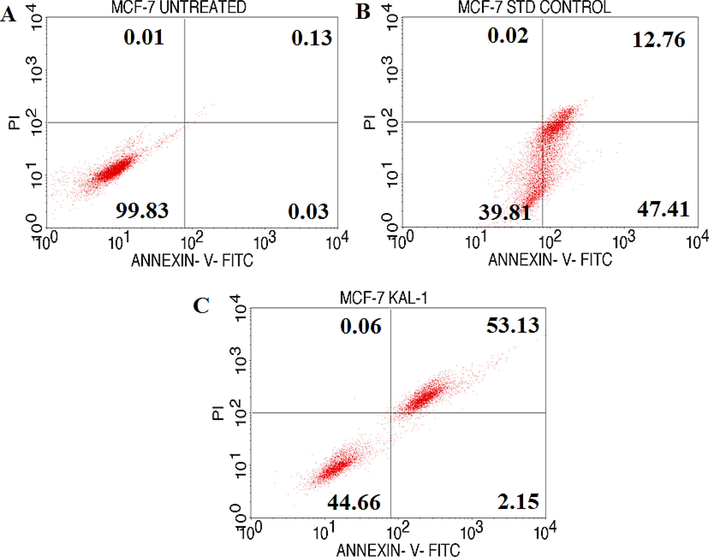
Quadrangular figure illustrating the Annexin V/PI expression in MCF-7 cells upon culture in the presence and absence of test chemical, GA coupled with std control. Cell Quest Pro and BD FACScalibur were used for the analysis. (Version: 6.0). Here, Annexin V- FITC - Primary Marker, PI- Propidium Iodide (Secondary fluorescence Marker) A-MCF-7 Untreated, B-MCF-7 std control, C-MCF-7 hydroalcoholic extract of S.isoetifolium. Lower left: % Viable Cells; Upper left: % of Necrotic Cells; Lower right: % of Early apoptotic cells; Upper right: % Late Apoptotic Cells.
S.No.
Compound Name
Molecular Formula
Molecular Weight
Retention Time
1
4-Dodecylbenzenesulfonic acid
C18H30O3S
326.19063
27.07
2
αα-trehalose
C24H38O4
390.2777
23.57
3
Dibutyl phthalate
C16 H0O4
278.15236
19.92
4
9-Oxo-ODE
C18 H30O3
294.2202
20.757
5
Dioctyl phthalate
C24 H38O4
390.2777
27.203
6
Arecoline
C8 H13 NO2
155.09508
1.088
7
Reserpine
C33 H40 N2O9
608.27422
14.973
8
Choline
C5H13NO
103.09999
0.954
9
2-Aminoanthraquinone
C14H9NO2
223.06403
17.403
10
Muramic acid
C9H17NO7
251.1014
1.108
11
Betaine
C5H11NO2
117.07925
1.062
12
Trigonelline
C7H7NO2
137.04808
1.065
13
3-Hydroxybenzoic acid
C7H6O3
138.03132
3.5
14
Caffeic acid
C9H8O4
180.04263
19.923
15
Octyl decyl phthalate
C26H42O4
418.30934
24.164
16
Myristyl sulfate
C14H30O4S
294.18574
27.2
17
Monobutyl phthalate
C12H14O4
222.08994
15.811
18
4-Hydroxycoumarin
C9H6O3
162.03205
19.924
19
D-Glucosamine
C6H13NO5
179.07982
1.079
20
L-Pyroglutamic acid
C5H7NO3
129.043
1.167
21
3-Hydroxyfluorene
C13H10O
182.07389
16.773
22
4-Dodecylbenzenesulfonic acid
C18H30O3S
326.19063
26.216
23
DL-Stachydrine
C7H13NO2
143.09513
1
24
Polygodial
C15H22O2
234.16277
19.183
25
2,2,6,6-Tetramethyl-4-piperidinol
C9H19NO
157.14722
16.081
26
4-Acetamidobutanoic acid
C6H11NO3
145.07431
1.072
27
Diisopentyl phthalate
C18H26O4
306.18407
21.109
28
1-Stearoylglycerol
C21H42O4
358.30929
23.651
29
Hexadecanamide
C16H33NO
255.25691
22.728
30
4-Hydroxycoumarin
C9H6O3
162.03205
23.584
31
Triphenyl phosphate
C18H15O4P
326.07173
19.16
32
OPEO
C16H26O2
250.19382
19.903
33
Diisodecyl phthalate
C28H46O4
446.34093
24.817
34
4-Oxoproline
C5H7NO3
129.04233
1.166
35
4-Pyridoxic acid
C8H9NO4
183.05359
24.467
36
Bis(4-ethylbenzylidene)sorbitol
C24H30O6
414.20485
18.325
37
Laurolactam
C12H23NO
197.17867
16.749
38
Glycerophospho-N-palmitoyl ethanolamine
C21H44NO7P
453.28676
22.562
39
4-Hydroxycoumarin
C9H6O3
162.03205
15.826
40
Cholest-4-en-3-one
C27H44O
384.3402
25.549
41
Citroflex A-4
C20H34O8
402.22655
20.911
42
Ethyl myristate
C16H32O2
256.23938
23.372
43
Stearamide
C18H37NO
283.28829
23.517
44
Oleamide
C18H35NO
281.27263
22.932
45
Betaine
C5H11NO2
117.07925
29.507
46
Leucine
C6H13NO2
131.095
1.161
47
L-Pyroglutamic acid
C5H7NO3
129.043
29.086
48
β-Estradiol
C18H24O2
272.1787
20.143
49
2,2,6,6-Tetramethyl-4-piperidinol
C9H19NO
157.14722
16.412
50
Phloretin
C15H14O5
274.08483
12.82
51
Dodecyltrimethylammonium
C15H33 N
227.26196
17.415
52
Docosanamide
C22H45NO
339.35127
18.114
53
Pyridoxal
C8H9NO3
167.05861
24.343
54
Nootkatone
C15H22O
218.1678
20.148
55
N,N'-Diphenylurea
C13H12N2O
212.09581
14.854
56
Dimethyl sebacate
C12H22O4
230.15251
14.892
57
Myristyl sulfate
C14H30O4S
294.18574
28.249
58
O-Desmethyltramadol
C15H23NO2
249.17352
20.619
59
Dibutyl phthalate
C16H22O4
278.15236
27.648
60
4-Methoxycinnamic acid
C10H10O3
178.06362
21.868
61
Astaxanthin
C40H52O4
596.38738
23.313
62
4-Hydroxycoumarin
C9H6O3
162.03205
21.112
63
Palmitoleic acid
C16H30O2
254.22539
21.992
64
19-Nortestosterone
C18H26O2
274.19426
19.639
65
Monobutyl phthalate
C12H14O4
222.08994
20.463
66
Zeatin-7-N-glucoside
C16H23N5O6
381.16451
1.018
67
Oleoyl ethanolamide
C20H39NO2
325.299
22.847
68
Sucrose
C12H22O11
342.11525
0.985
69
5α-Dihydrotestosterone
C19H30O2
290.22508
21.26
70
6-Hydroxynicotinic acid
C6H5NO3
139.02661
9.246
71
Meprednisone
C22H28O5
372.19509
16.336
72
(±)-Abscisic acid
C15H20O4
264.13713
13.707
73
Dihydrothymine
C5H8N2O2
128.05896
1.082
74
(+/-)-CP 47,497-C7-Hydroxy metabolite
C21H34O3
334.2517
23.931
75
D-(+)-Pyroglutamic Acid
C5H7NO3
129.043
28.773
Apoptotic investigation revealed that the hydroalcoholic extract of S. isoetifolium, effectively triggered programmed cell death, or apoptosis, in human breast cancer cells. This observation suggests that the hydroalcoholic extract may possess therapeutic potential against human breast cancer. To confirm the mechanism behind the anti-cancer effects of the test compound on human breast cancer cells, further preclinical research is needed. The study found that the hydroalcoholic extract of S. isoetifolium induces apoptosis in human breast cancer cells (MCF-7) through the activation of specific signaling pathways, namely caspase 3, caspase 9, and Bcl2 apoptotic signaling pathway. These pathways play a critical role in promoting programmed cell death, which is essential to prevent the development and progression of cancer cells. By measuring the amount of activated caspase(s), one can detect whether or not apoptosis is occurring. It is necessary to choose in advance the caspase(s) to be assayed. Among the various caspase(s), caspase 3 and 9 both initiate the cascade of apoptosis events. Caspase 9 is usually activated by cytotoxic agents that damage mitochondria, allowing cytochrome c leakage into the cytosol. Caspase 3 is a common downstream effector caspase associated with some forms of β-cell apoptosis (Yamada et al., 1999). It is worth noting that a previous study demonstrated that vinculin, another compound or factor under investigation, similarly induces apoptosis through the intrinsic caspase 9 pathway. By elucidating the underlying mechanisms by which these compounds induce apoptosis, we can gain a deeper understanding of their potential as anti-cancer agents in the context of human breast cancer (Lee et al., 2020).
A recent study proves that Bcl2 function in the antioxidant pathway and can inhibit lipid peroxidation. Bcl2 can be identified at the chromosomal translocation breakpoint and was mainly studied in lymphoma as well as in leukemia (Haldar et al., 1994). Fig. 3,4, and 5 show the activation of the apoptotic pathway via Bcl-2, caspase 3, and caspase 9 in the MCF-7 cells. On treating crude extract with MCF- 7 cells, Bcl2 may interfere with cytochrome c while the same cytochrome can be induced by the expression of Bax. Bcl2 which is an anti-apoptotic protein can induce the apoptotic expression in MCF- 7 breast cancer cells via an intrinsic apoptosis pathway (Rosse et al., 1998, Ekins et al., 2007).(See Figs. 4 and 5).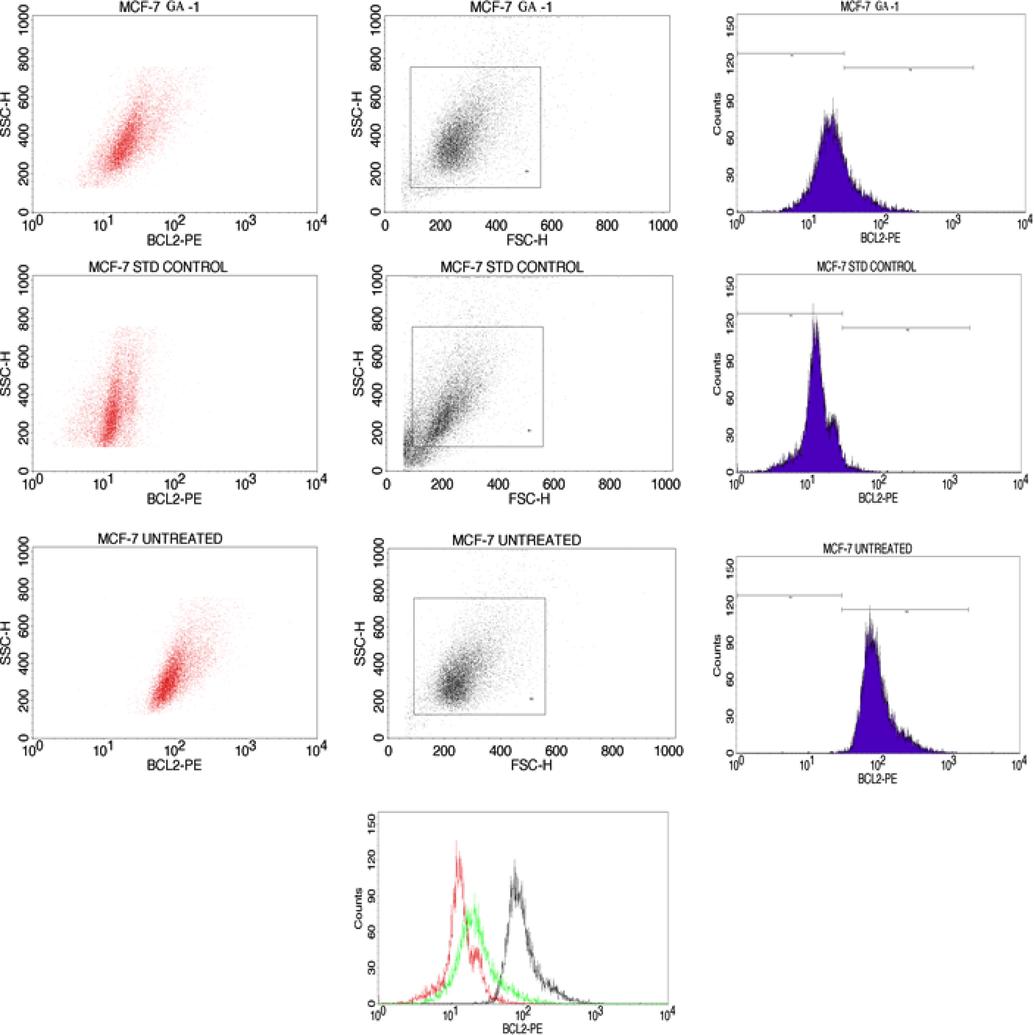
Hydroalcoholic extract of S.isoetifolium induces apoptosis via Bcl-2 activation in MCF-7 cells.
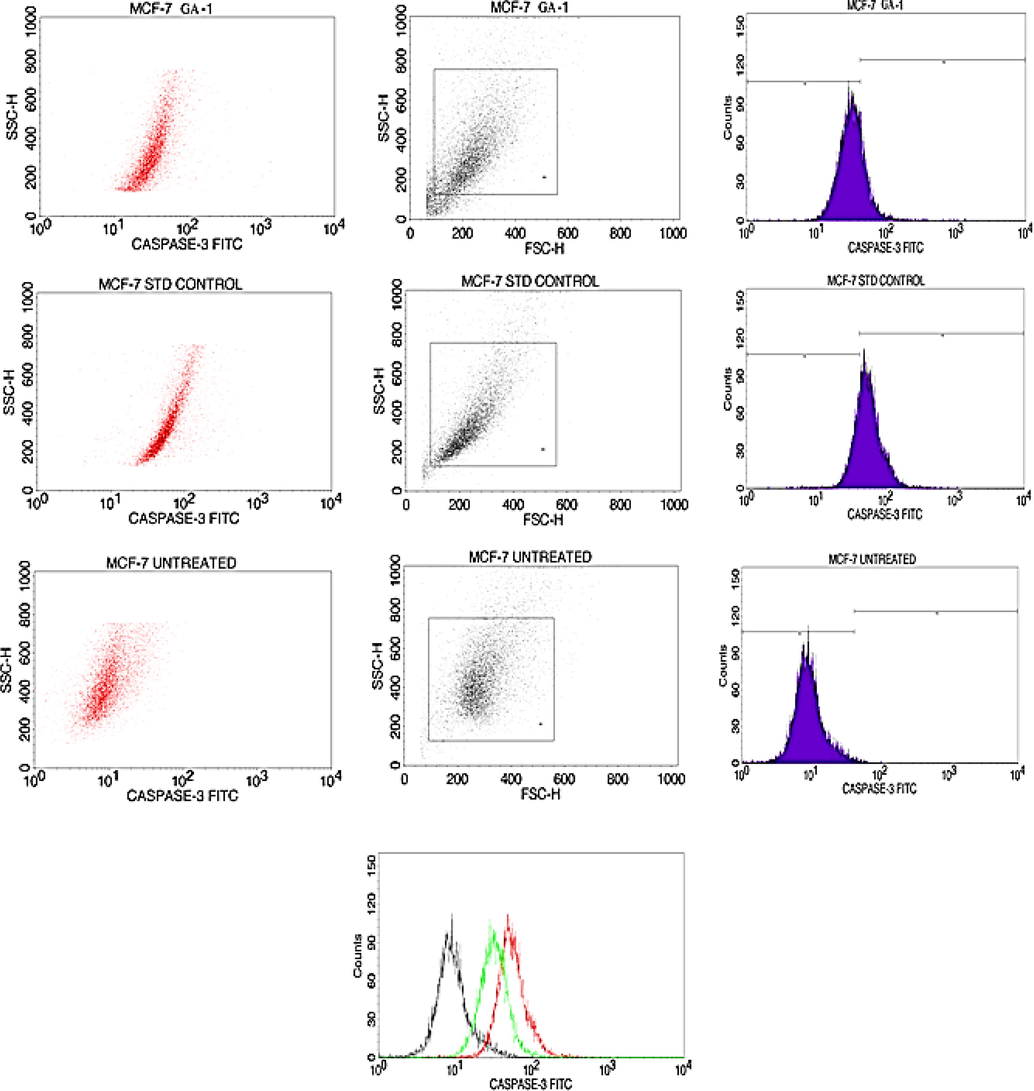
Hydroalcoholic extract of S.isoetifolium induces apoptosis via Caspase-3 activation in MCF-7 cells.
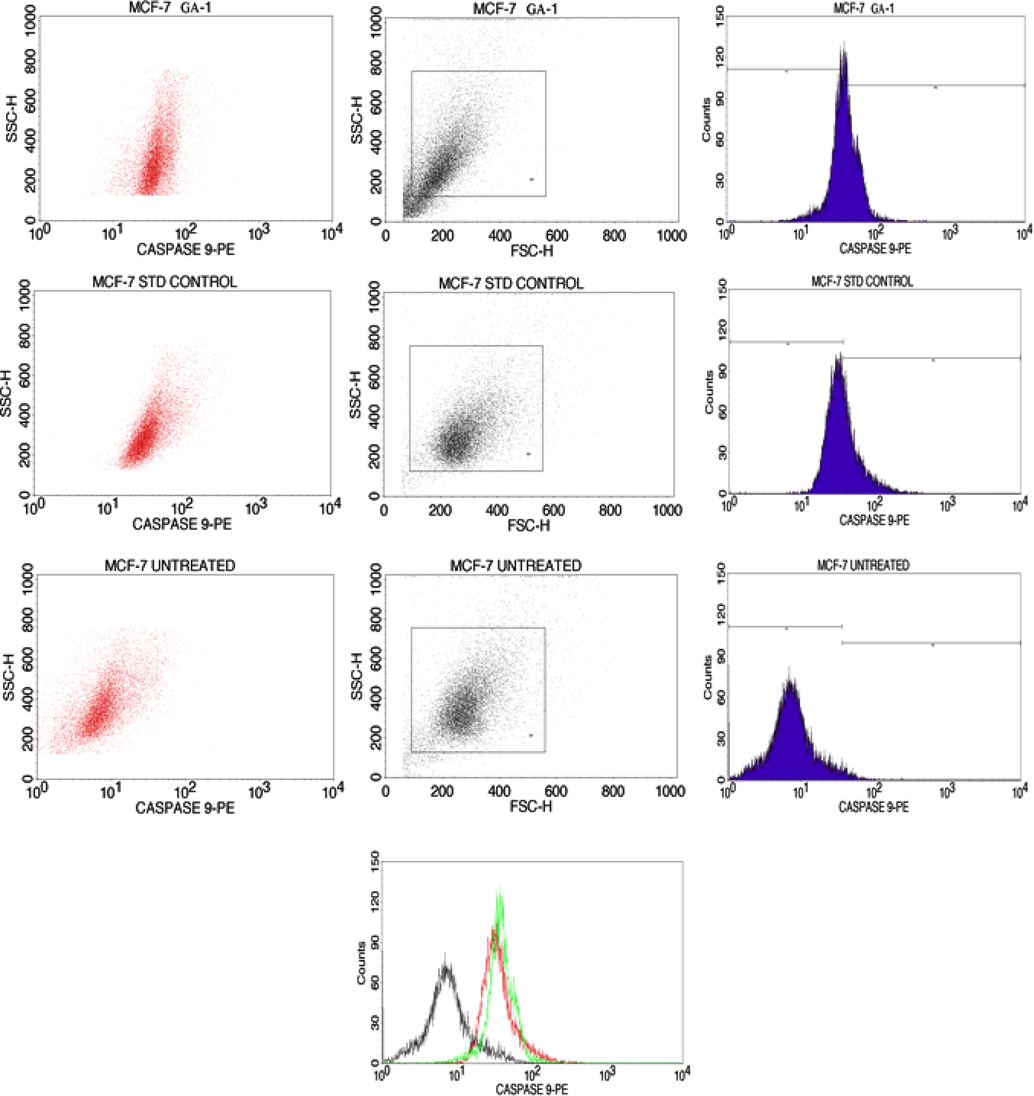
Hydroalcoholic extract of S. isoetifolium induces apoptosis via Caspase 9 activation in MCF-7 cells.
3.2 Liquid chromatography-mass spectroscopy analysis
About seventy-five chemical components were recognized in HAE of S. isoetifolium, along with their retention time, molecular weight, and molecular formula were listed in Table 3 and the chromatogram was represented in Fig. 6. The biological activity of selected compounds was presented in Table 4. The prevalent compounds were 4-Dodecylbenzenesulfonic acid showing a retention time of 27.07 min. The mass spectrum of Arecoline shows the peak at RT 1.088 with the ESI- MS spectrum at m/z 155 revealing the occurrence of this compound. Nootkatone showed the peak at a retention time of 20.148 and ESI-MS spectrum showed at m/z 19.924 indicating the presence of compound 4-Hydroxycoumarin. Similarly, the mass spectrum of 3-Hydroxybenzoic acid shows RT at 3.5. While the compound Reserpine shows the band at 14.973 and at 22.932 the compound present is Oleamide. Dibutyl phthalate showed the highest retention time peak at 27.648; the substance Choline showed the lowest retention time peak at 0.954. Compounds such as 9-oxo-ODE, Dioctyl phthalate, Myristyl sulfate, Diisodecyl phthalate, and Betaine show the highest value of RT at 20.757, 27.203, 27.2, 24.817, and 29.507, while lowest RT 1.018, 1.161, 1.166, 1.072 and 1 shows with the compounds Zeatin-7-N-glucoside, Leucine, 4-oxo proline, 4-Acetamidobutanoic acid, respectively.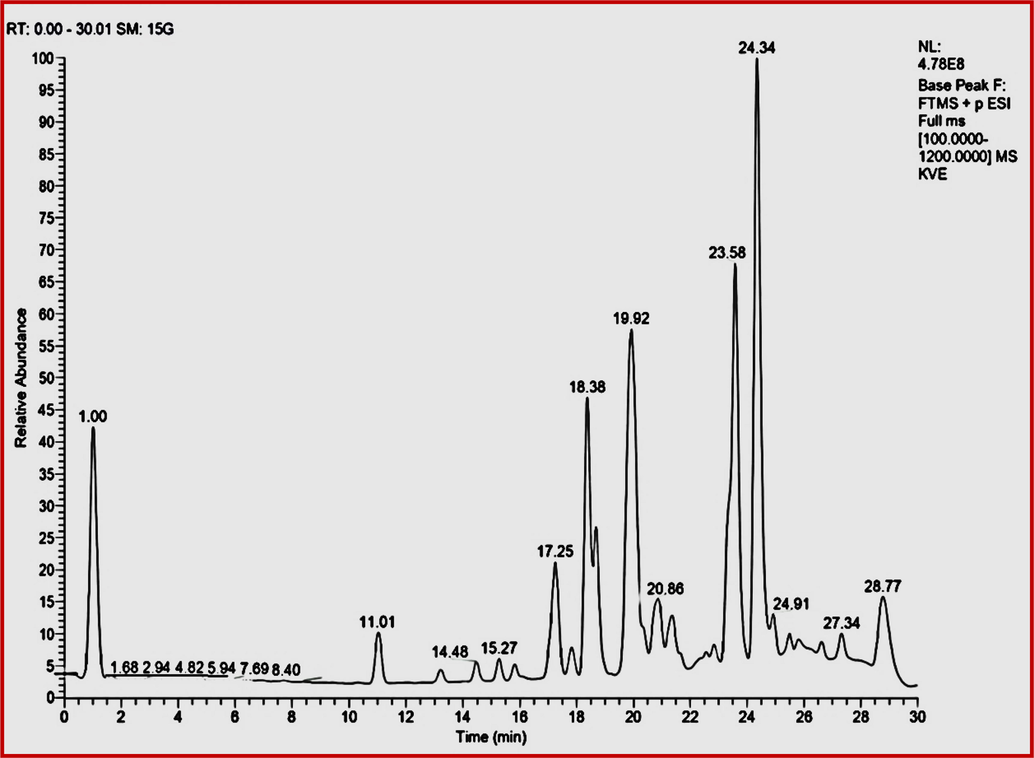
LC-MS Chromatogram of the hydroalcoholic extract of S. isoetifolium.
S. No.
R. T
Name of the Compounds
Pharmacological activities
1
23.57
αα-trehalose
Antitumor effects, suppression of bone loss, and migration of insulin resistance (Kapetanovic, 2008)
2
16.336
Meprednisone
Anti-inflammatory, neuroprotective agent, antiemetic, and androgenic agent (Shaker et al., 2021)
3
9.246
6-Hydroxynicotinic acid
Antitumor effects, anticancer activity
4
24.794
Cholecalciferol
suppress NF-kB activities, slowing down cancer growth
5
21.992
Palmitoleic acid
Antioxidant activity (Wada et al., 2014)
6
22.728
Hexadecanamide
Down-regulation of mast cell activation and inflammation
7
0.985
Sucrose
Antimicrobial and cytotoxic activity
8
23.313
Astaxanthin
Anti-oxidant, Anti-inflammatory, Anti-apoptotic activity
9
27.648
Dibutyl phthalate
Antifungal activity
10
19.923
Caffeic acid
Anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effect
11
1.079
D-Glucosamine
Mineralization of mature osteoblasts, reduction in expression of receptor activator- NF- kb
12
12.82
Phloretin
Anti-inflammatory, Anti-oxidative (Brodkiewicz et al., 2020)
3.3 Molecular docking studies
7-Hydroxy coumarin, 4-Hydroxy coumarin, Phloretin, Zerumbone, Nootkatone, and Arecoline are the ligands used for in silico study (Gao et al., 2021) from the LC-MS analysis against breast cancer target proteins HER2 Kinase and HSP90 respectively. Fig. 7(A and B) shows the three-dimensional structure of HER2 Kinase and HSP90 respectively. Monitoring characteristic features including docking score, binding energy, Van der Waals interactions, hydrophobic interactions, and unusual charge interactions can help to determine how well a ligand will bind to a receptor. The bigger the binding energy's negative value, the stronger the molecule's affinity for the receptor (Shamsee et al., 2019).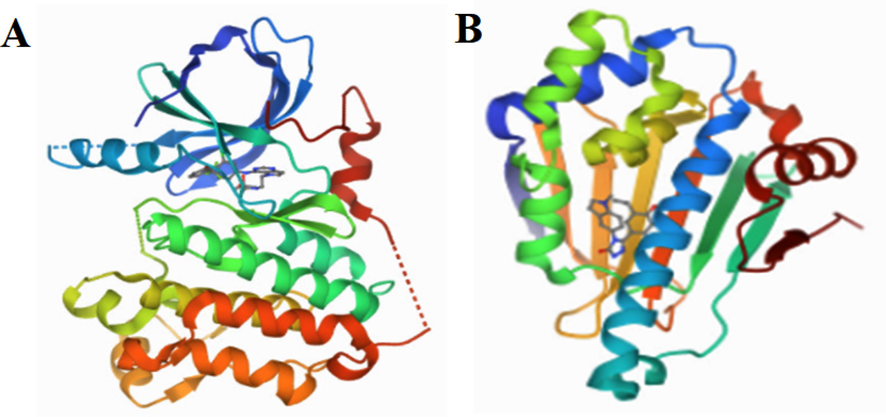
(A) 3D Structure of HER2 kinase (B) 3D Structure of HSP90.
The Protein Data Bank was used to obtain the receptor structures for molecular docking. In addition to the prioritized list of docked ligands and their binding poses, the docking positions were sorted based on their docking scores (Shaliza et al., 2007). Their binding energy was used to rank them. The outcomes for both breast cancer proteins are shown in Tables 5 and 6 and were determined by docking energy of 7-Hydroxycoumarine, 4-Hydroxycoumarine, Nootkatone, Arecoline, Zerumbone, and Phloretin Interactions with HER2 Kinase and HSP90.
Compound Name
Binding Energy (Kcal/mol)
Inhibition constant (μM)
Hydrogen bond Interactions
Distance
Binding residues (Around 5 Å)
Binding region of Ligand
4-Hydroxycoumarine
−5.79
56.64
Thr862(O)…H-OSer783
(O)…H-OThr798(N)-H…O1.8
3.03.0Leu796, Met774, Leu785, Thr798, Arg784, Asp863, Phe864, Ser783, Thr863
chromen-2-one4-hydroxy
7- Hydroxycoumarine
−5.32
125.07
Thr862(O)…HOSer783(O)
-H…OThr798(O)
H…OPhe864(O)…H-O3.3
2.7
2.82.1Leu796, Met774, Leu785, Thr798, Arg784, Ser783, Asp863, Ser783, Phe864, Lys753, Thr863
chromen-2-one7-hydroxy
Arecoline
−4.41
590.04
Thr862(O)H…NLys753(N)-H…O
3.03.1
Leu796, Leu785, Val797, Thr798, Arg784, Ser783, Asp863, Ser783, Thr862, Phe864, Lys753, Thr863
pyridinecarboxylate
Nootkatone
−7.72
2.2
–
–
Leu796, Met774, Leu785, Val797, Thr798, Arg784, Ser783, ASP863, Ser783, Thr862, Phe864, Lys753, The863
prop-1-en-2-ylhexahydronaphthalen-2-one
Phloretin
−6.1
33.58
Thr862(O)…H-OSer783(O)
-H…OLys753(N)
-H…OAsp863(O)…H-O2.6
2.0
3.03.4Leu785, Arg784, Ser783, ASP863, Ser783, Phe864, Met774, Lys753, Thr798, Leu796, Thr862
4-hydroxyphenyl2,4,6-trihydroxyphenyl
Zerumbone
−7.7
2.27
–
–
Leu796, Met774, Leu785, Val797, Thr798, Arg784, Ser783, Asp863, Ser783, Thr862, Phe864, Lys753, Thr863
tetramethylcycloundeca-2,6,10-trien-1-one
Compound Name
Binding Energy (Kcal/mol)
Inhibition constant (μM)
Hydrogen bond Interactions
Distance
Binding residues (Around 5 Å)
Binding region of Ligand
4-Hydroxycoumarine
−5.9
47.56
Thr109(O)-H…OGly135(O)…H-O
2.62.1
Asn106, Ile26, Thr109, Ile110, Ala111,Thr115, Lys112, Ser113, Phe134, Gly136, Asn51, Phe138, Tyr139, Leu107
chromen-2-one4-hydroxy
7- Hydroxycoumarine
−5.69
67.52
Asn51(O)-H…OPhe138(N)…H-O
3.02.8
Asn106, Ile26, Thr109, Ile110, Ala111, Thr115, Lys112, Ser113, Phe134, Gly136, Asn51, Phe138, Tyr139, Leu107
chromen-2-one7-hydroxy
Arecoline
−5.26
139.49
Phe138(N)…H-OAsn106(O)-H…N
3.43.0
Asn106, Ile26, Thr109, Ile110, Ala111, Thr115, Lys112, Ser113, Phe134, Gly136, Asn51, Phe138, Tyr139, Leu107
pyridinecarboxylate
Nootkatone
−7.5
3.19
–
–
Asn106, Ile26, Asn51, Asp54, Thr109, Ile110, Ala111, Thr115, Lys112, Ser113, Phe134, Gly136, Asn51, Phe138, Tyr139, Leu107
prop-1-en-2-ylhexahydronaphthalen-2-one
Phloretin
−6.18
29.43
Ser52(O)…H-OAsn51
(N)…H-OGly135(O)…H-O2.7
2.92.0Asn106, Ile26, Thr109, Ser113, Val136, Lys112, Ser113, Phe134, Gly135, Asn51, Ser52, Val186, Asp93, Phe138, Tyr139 Leu107
4-hydroxyphenyl2,4,6-trihydroxyphenyl
Zerumbone
−7.45
3.38
–
–
Asn106, Ile26, Thr109, Ser113, Val136, Lys112, Ser113, Phe134, Gly135, Asn51, Asp93, Phe138, Tyr139, Leu107, Met98
tetramethylcycloundeca-2,6,10-trien-1-one
3.4 Interaction of ligands with HER2 Kinase
The binding affinity of 4-hydroxy coumarin, 7-hydroxy coumarin, arecoline, nootkatone, phloretin, and zerumbone was identified through docking investigations, which supported the target protein HER2 Kinase receptor's restraint. The outcomes of the docked compound with HER2 kinase receptor were shown in Table 5 and Fig. 8. The docking score of 4-Hydroxycoumarine, 7-Hydroxycoumarine, Arecoline, Nookatone, Phloretin, Zerumbone was found to be −5.79, −5.32, −4.41, −7.72, −6.1, −7.7 Kcal/mol respectively. The order of binding energy was Nootkatone > Zerumbone > Phloretin > 4-Hydroxycoumarine > 7-Hydroxycoumarine > Arecoline. Among all the other compounds, Nootkatone was found to have more affinity. Nootkatone compounds possess both anticancer and antiplatelet effects which might be of therapeutic benefit for the prevention of platelet-associated cardiovascular diseases (Yoo et al., 2020). It is the most abundant component and possesses a wide range of beneficial effects mainly anti-proliferative and anti-inflammatory activities. It possesses anticancer activity especially in lung cancer via AMPK pathway and shows more activity against colorectal cancer (Zhu et al., 2020). Along with the ROS production nootkatone induce the cell cycle arrest at S-phase, it may also inhibit the retinoblastoma by inhibiting the Nf-kB signaling pathway and cell migration.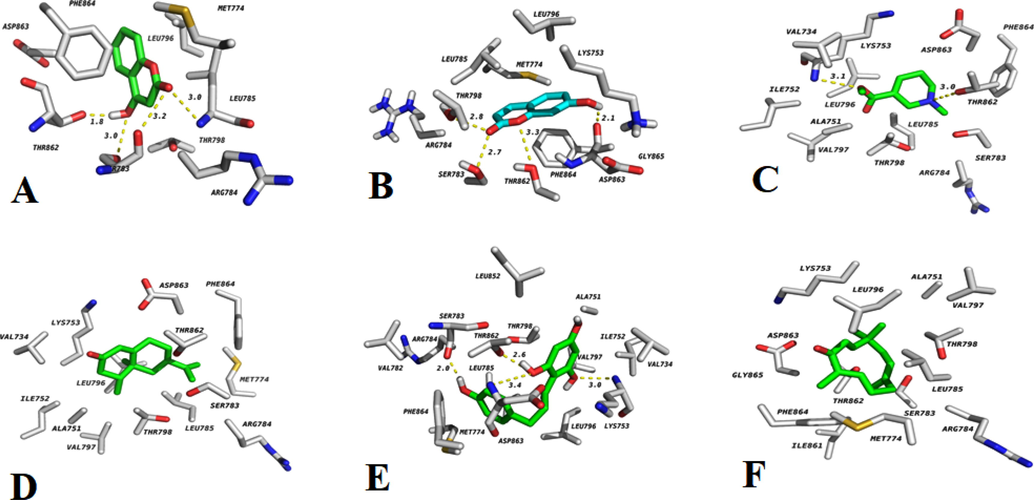
Interaction of 7-Hydroxycoumarine (A),4- Hydroxycoumarine (B), Nootkatone (C), Arecoline (D), Zerumbone (E), and Phloretin(F) with HER2 Kinase.
3.5 Interaction of ligands with HSP90
The docking score of Nootkatone, Zerumbone, 7-Hydroxycoumarine, 4-Hydroxycoumarine, phloretin, and arecoline against HSP90 protein was found to be −7.5, −7.45, −6.18, −5.9, −5.69, and 5.26 respectively. The order of binding energy was Nootkatone > Zerumbone > Phloretin > 4-Hydroxycoumarine > 7-Hydroxycoumarine > Arecoline. Heat Shock Proteins (HSP90) possess anti-parasitic and anticancer activity. The outcomes of the docked compound with HER2 kinase receptor were shown in Table 6 and Fig. 9 which shows the interaction of 4-Hydroxycoumarine, 7-hydroxycoumarine, arecoline, nookatone, phloretin, zerumbone. The inhibition of protein expression related to metastatic cancer and the induction of autophagy is attributed to the effects of nootkatone (Zho et al., 2020). Therefore, gaining a deeper understanding of the molecular mechanisms of nootkatone in anti-tumor activity could enhance our comprehension of metastatic cancer treatment and potentially improve therapeutic approaches. The docking studies confirmed the suppressive activity through suppression of target protein HER2 Kinase and HSP90. Among the various compounds, Nootkatone has more potential binding interactions than other compounds.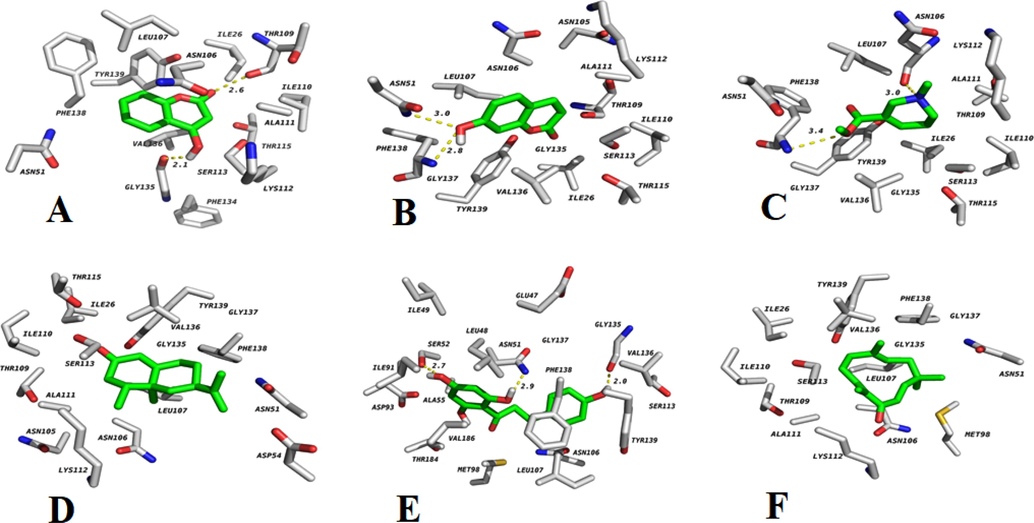
Interaction of 4-Hydroxycoumarine (A), 7-Hydroxycoumarine (B), Arecoline (C), Nookatone (D), Phloretin (E), and Zerumbone (F) with HSP90.
4 Conclusions
The hydroalcoholic extract of S. ifolium was found to be strong anticancer potential against human breast cancer cells. It exhibits prominent cell cycle phase arrest similar to the standard control, aripiprazole on MCF-7 cells. It may induce apoptosis via the activation of caspase 3, caspase 9, and Bcl-2 pathway. The in silico docking studies demonstrate the binding activity of the compound present in the HAE of S. isoetifolium to the breast cancer receptor proteins such as HER2 Kinase and HSP90, respectively. It supports the use of S. isoetifolium for the possible treatment of breast cancer. Further in vivo research, the success of this additional set of investigations will help to clarify how it is possible to mix the most potent extracts with the existing medication without running into problems with drug resistance and negative side effects. This study is the first scientific report that provides convincing anticancer and rich antioxidant sources as evidence for the relevance of S. isoetifolium thus providing scientific validity to its medicinal uses such as an anticancer agent.
CRediT authorship contribution statement
P. Kalaivani: Conceptualization, Methodology. P. Amudha: Conceptualization, Methodology. A. Chandramohan: . R. Vidya: Conceptualization, Methodology. M. Prabhaharan: Supervision, Conceptualization, Methodology. P. Sasikumar: Project administration, Supervision, Conceptualization, Methodology, Formal analysis. Salim Albukhaty: Writing – review & editing, Formal analysis, Investigation, Data curation. Ghassan M. Sulaiman: Project administration, Writing – review & editing, Formal analysis, Investigation, Data curation. Mosleh M. Abomughaid: Writing – review & editing, Investigation, Data curation, Validation. Mohammed Abu-Alghayth: Visualization, Validation, Investigation, Data curation.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther.. 2006;5:3294-3302.
- [Google Scholar]
- Layer-by-layer nanoparticles of tamoxifen and resveratrol for dual drug delivery system and potential triple-negative breast cancer treatment. Pharmaceutics. 2021;13:1098.
- [Google Scholar]
- In vivo evaluation of pharmacological properties of Argentine stingless bee geopropolis. Beni-Suef Univ. J. Basic Appl. Sci.. 2020;9:32.
- [Google Scholar]
- Phytochemical profiling of marine red alga, Halymenia palmata and its bio-control effects against dengue vector, Aedes aegypti. S. Afr. J. Bot.. 2019;121:257-266.
- [Google Scholar]
- In silico pharmacology for drug discovery: Applications to targets and beyond. Br. J. Pharmacol.. 2007;152:21-37.
- [Google Scholar]
- Synthesis and crystal structure of the one-dimensional chain triphenyltin compound bridged by 5-chloro-6-hydroxynicotinic acid. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing. 2021. p. :012010.
- [Google Scholar]
- A comprehensive update on the bioactive compounds from seagrasses. Mar. Drugs. 2022;20:406.
- [Google Scholar]
- Down-regulation of Bcl-2 by P53 in breast cancer cells. Cancer Res.. 1994;54:2095-2097.
- [Google Scholar]
- Deciphering the effect of hydro-alcoholic extract of cymodocea serrulata on the cell cycle arrest and apoptosis. Int. J. Res. Pharm. Sci.. 2020;11:3261-3271.
- [Google Scholar]
- Computer-aided drug discovery and development (CADDD): In silico-chemico-biological approach. Chem. Biol. Interact.. 2008;171:165-176.
- [Google Scholar]
- Aviculin isolated from lespedeza cuneata induce apoptosis in breast cancer cells through mitochondria-mediated caspase activation pathway. Molecules. 2020;25:1708.
- [Google Scholar]
- The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci.. 2016;17:1681.
- [CrossRef] [Google Scholar]
- Quercetin against MCF7 and CAL51 breast cancer cell lines: Apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine. 2021;16:1937-1961.
- [Google Scholar]
- Gallic acid induces G2/M phase cell cycle arrest via regulating 14-3-3β release from Cdc25C and Chk2 activation in human bladder transitional carcinoma cells. Mol. Nutr. Food Res.. 2010;54:1781-1790.
- [Google Scholar]
- Ligand docking and binding site analysis with pymol and autodock/vina. Int. J. Basic Appl. Sci.. 2015;4:168-177.
- [Google Scholar]
- In vitro and in vivo functional viability, and biocompatibility evaluation of bovine serum albumin-ingrained microemulsion: A model based on sesame oil as the payload for developing an efficient drug delivery platform. Pharmaceuticals. 2023;16:582.
- [Google Scholar]
- Bcl-2 prolongs cell survival after bax-induced release of cytochrome c. Nature. 1998;391:496-499.
- [Google Scholar]
- In silico methods and tools for drug discovery. Comput. Biol. Med.. 2021;137:104851
- [Google Scholar]
- Molecular modeling and docking studies of protein kinase C in prostate cancer for rational drug-design. Res. J. Biotechnol.. 2007;2(4):7-19.
- [Google Scholar]
- Cytotoxic and cell cycle arrest induction of pentacyclic triterpenoides separated from Lantana camara leaves against MCF-7 cell line in vitro. Mol. Biol. Rep.. 2019;46:381-390.
- [Google Scholar]
- Marine pharmacology: Potential, challenges, and future in India. J. Med Sci.. 2018;38:49.
- [Google Scholar]
- Breast cancer—Epidemiology, classification, pathogenesis and treatment (Review of literature) Cancers. 2022;14:2569.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and in silico studies of tetrazole heterocycle hybrids. J. Mol. Struct.. 2019;1175:577-586.
- [Google Scholar]
- Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv.. 2015;5:35608-35621.
- [Google Scholar]
- In vitro study of molecular structure and cytotoxicity effect of luteolin in the human colon carcinoma cells. Eur. Food Res. Technol.. 2015;241:83-90.
- [Google Scholar]
- Molecular structure and anti-proliferative effect of galangin in HCT-116 cells: In vitro study. Food Sci. Biotechnol.. 2016;25:247-252.
- [Google Scholar]
- Structure and biological properties of lentztrehalose: a novel trehalose analog. J. Antibiot. (Tokyo). 2014;67(4):319-322.
- [CrossRef] [Google Scholar]
- Essential role of caspase-3 in apoptosis of mouse beta-cells transfected with human Fas. Diabetes. 1999;48(3):478-483.
- [Google Scholar]
- Anti-proliferative activity of A. Oxyphylla and its bioactive constituent nootkatone in colorectal cancer cells. BMC Cancer. 2020;20:1-12.
- [Google Scholar]
- Inhibition of anticancer growth in retinoblastoma cells by naturally occurring sesquiterpene nootkatone is mediated via autophagy, endogenous ROS production, cell cycle arrest and inhibition of NF-κB signalling pathway. J. buon. 2020;25(1):427-431.
- [Google Scholar]







