Translate this page into:
Review of biomass derived-activated carbon for production of clean fuels by adsorptive desulfurization: Insights into processes, modifications, properties, and performances
⁎Corresponding author. gsadewale@kfupm.edu.sa (Saheed A. Ganiyu) gsadewale@gmail.com (Saheed A. Ganiyu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Activated carbon (AC) from renewable and low-cost agricultural byproducts is economical and prevents likely environmental degradation. Insight into surface chemistry modification of activated carbons is presented. The application of AC for adsorptive desulfurization offers a solution to one of the pressing global issues leading to environmental pollution. Regeneration of spent adsorbents conserves natural resources and reduces secondary pollution.
Abstract
Sulfur remains the universally acknowledged most populous element in crude oil after carbon and hydrogen. The deleterious impact of sulfur emission from transportation fuels on both the environment and the psychological well-being of humans is becoming worrisome due to the geometric increase in energy demands. Sulfur in liquid fuel oils combusts in the automobile engine to give oxides of sulfur (SOx) resulting in acidic rain, poisons catalytic converters used to reduce oxides of carbon (COx) and cut short the engine’s life due to corrosion. Additionally, heavier thiophenes are carcinogenic and mutagenic. Owing to these detrimental effects of sulfur contents present in the fuel, the concerned regulatory agencies set the minimum allowable sulfur concentration in transportation fuels to 10 ppmw and 15 ppmw for gasoline and diesel respectively. ADS process has been gaining an attractive interest towards achieving ultra-low sulfur removal from fuels due to its simplicity, fastness, environmental friendliness, reliability, and the use of cost-effective materials. This outstanding performance is due to its excellent adsorption affinity for recalcitrant sulfur compounds under moderate conditions. The ADS adsorption capacity is a function of the material used and AC produced from lignocellulosic biomass has proved to be a unique candidate for ultra-deep desulfurization applications. This review aims to expound researchers' views at a greater length on the recent development of ADS as a complementary process to traditional HDS or a freestanding technique using AC. This meticulous work would also serve as a guide to beginners who have picked an interest in material design and its applicability for pollutant control.
Keywords
Activated carbon
Carbonization
Activation
Surface modification
Adsorptive desulfurization
Regenerated activated carbon
- TB
-
Thiophene
- BT
-
Benzothiophene
- DBT
-
Dibenzothiophene
- MBT
-
Methylbenzothiophene
- MDBT
-
Methyldibenzothiophene
- DMDBT
-
Dimethyldibenzothiophene
- THDBT
-
Tetrahydrodibenzothiophene
- HPA
-
Heteropolyacid
- MDF
-
Model diesel fuel
- HDS
-
Hydrodesulfurization
- BDS
-
Biodesulfurization
- ADS
-
Adsorptive desulfurization
- ILS
-
Ionic liquid desulfurization
- ODS
-
Oxidative desulfurization
- HF
-
Hierarchical factor
- LHSV
-
Liquid hourly space velocity
Abbreviations
1 Introduction
Crude oil is known to be a complex mixture that majorly contains hydrocarbons, with an infinitesimal number of compounds that contain metals, sulfur, nitrogen, and oxygen. It contains substantially larger amounts of sulfur after carbon (C = 79.5 to 87.1%) and hydrogen (H = 11.5 to 14.8%) and the average sulfur content in crude oil varies in the range of 0.1 to 3.5% (Demirbas et al., 2015). It is generally classified as sour crude and sweet crude based on the amount of sulfur content present, usually expressed in part per million by weight or weight percent (wt. %). The former contains free sulfur in an amount greater than 1.0 wt.% while the latter contains little or no sulfur in an amount<0.5 wt.% (Demirbas et al., 2015). Generally, organic and inorganic are the two forms in which sulfur compounds are present in crude oil. The formal category includes carbonyl sulfide, elemental sulfur, dissolved pyrites, and hydrogen sulfide (H2S). The latter includes compounds therein sulfur is present as a heteroatom fused to a hydrocarbon molecule namely; sulfides, thiols, and thiophenic compounds, and undoubtedly, they are the major source of sulfur compounds present in crude oil (Svinterikos et al., 2019) and (Chandra Srivastava, 2012). Fig. 1 shows some of the most common classes of organosulfur compounds encountered in crude oil.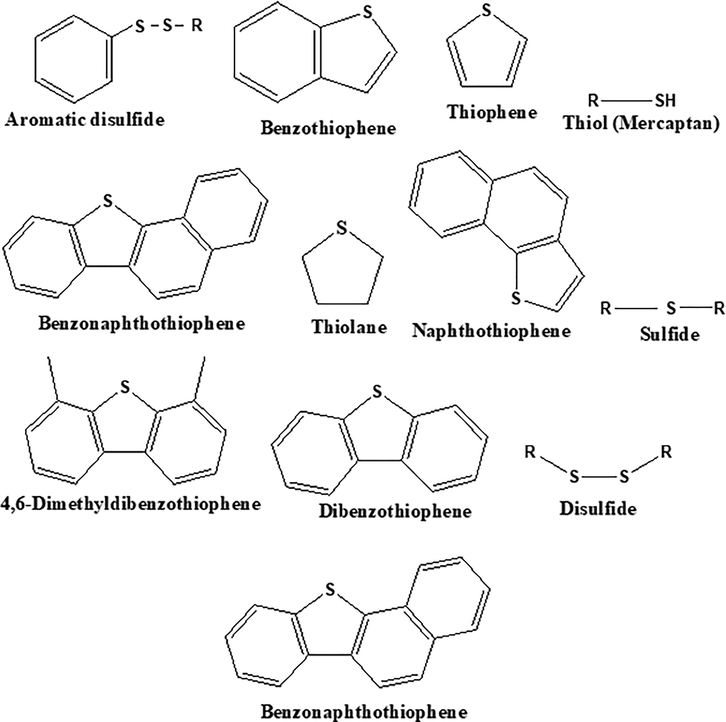
Crude oil major organic sulfur-containing compounds
It is an irrefutable fact that the presence of sulfur-containing compounds in fossil fuels poses a severe threat and endangers life and properties. For instance, sulfur (iv) oxides (SO2) are generated during the combustion of fuel oils resulting in acid rain by reacting with the air moisture and can also lead to photochemical smog. Acid rain is inimical to marine life owing to its tendency to augment the ecology of lakes and streams by reducing the pH with low buffer capacity having known that fish can’t live in water with pH < 4.5. Also, acid rain removes the protective coating of leaves, accelerate the erosion of historical building, and dissolves and wash away the soil nutrients (Soleimani et al., 2007). Similarly, hydrogen sulfide (H2S), mercaptans, and lower aliphatic sulfides cause numerous corrosion problems in pumping, pipeline, and refinery equipment (owing to the oxyacids formation from combustion products), as well as poisoning of the catalytic converters (that reduce particulates and NOx emission) used in automotive engines (Ahmad, 2015). Owing to these unpleasant impacts of sulfur on the health, economy, and the environment, the concern authorities in many countries gave a strict regulation to drastically lower the sulfur amount present in commercial fuels, which becomes stricter with time, precisely in the USA and EU. Thus, ultra-deep desulfurization becomes a crucial step in petroleum industries. Consequently, the highest sulfur content was set to 350 ppm and 150 ppm for diesel and gasoline respectively during the 90 s in the EU. Since 2009, the sulfur level limit in both fuels is 10 ppm. In the year 2003, Germany as one of the European Union adopted a 10 ppm sulfur limit for diesel first among other comity of nations and this was achieved through taxation of transportation fuel with more than 10 ppm residual sulfur content. The low sulfur content of 10 ppm was introduced from the year 2008 by other EU countries and Japan. Similarly, the dire need to ensure and maintain an ideal environment gravitated the United States Environmental Protection Agency (USEPA) to place the highest sulfur limit for diesel and gasoline at 15 ppm and 10 ppm respectively (Saleh and Danmaliki, 2016a). Also, the International Maritime Organization has determined to reduce sulfur levels for marine fuels from the current maximum of 3.5 wt.% to 0.5 wt.% starting in 2020. Lately, European Emission Standard (Euro VI) aims at 0 ppm sulfur in industrial and transportation fuels to further foster an ideal environment (Chandra Srivastava, 2012). With a few countries conceding to this new policy of sulfur-free fuel oils, it has become imperative for refineries to invest in research that will meet up with the new regulation standard of an infinitesimal amount of sulfur content in fuels. Researchers around the world have developed various technologies adapted to sulfur removal from fuel.
2 Desulfurization technologies
The most prominent desulfurization technologies are shown in Fig. 2 and these include conventional hydrodesulfurization (HDS), biodesulfurization (BDS), oxidative desulfurization (ODS), adsorptive desulfurization (ADS) and ionic liquid desulfurization (ILS).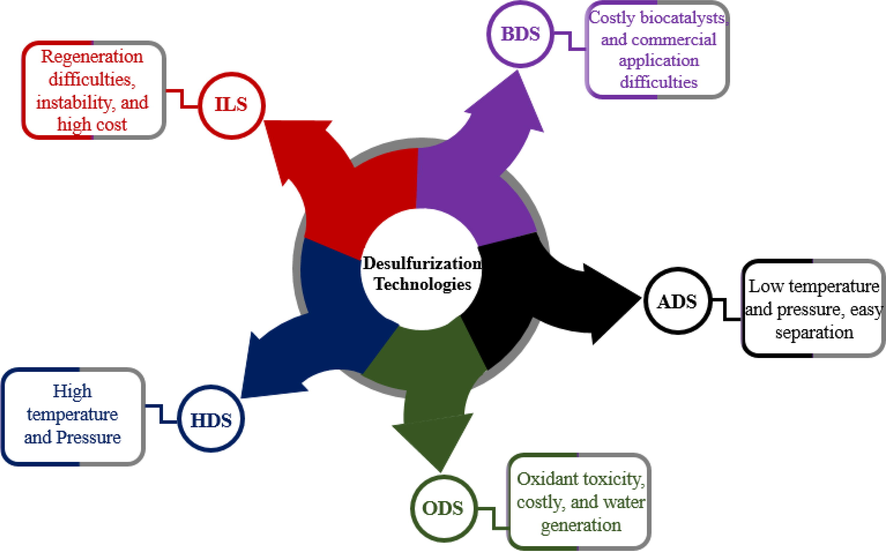
An overview of desulfurization technologies.
2.1 Hydrodesulfurization (HDS)
The conventional HDS technology, developed in the 1930 s, is largely utilized in the refineries to remove compounds of sulfur available in crude oil distillates into sulfur-free species and it works on a catalytic reaction with hydrogen customarily on Co-Mo or Ni-Mo catalysts (Song, 2007). The keen interest in HDS from inception can be attributed to the accessibility of hydrogen from catalytic reformers. Its process usually entails catalytic hydrotreatment with sulfur compounds at an elevated temperature and pressure to produce H2S and sulfur-deficient organic compounds. The resulting H2S is then ultimately transformed into elemental sulfur through an adjusted Claus process (Chandra Srivastava, 2012). HDS technology employed in refineries is carried out in a reactor operated at an elevated temperature and pressure in the range of 300–450 °C and 3.0–5.0 MPa respectively, typically with Co-Mo/Al2O3 or Ni-Mo/Al2O3 catalysts (Shafi and Hutchings, 2000). Octane rating is lost under this severe condition, much hydrogen is consumed, and olefins are hydrogenated. There is a tendency that H2S to react with olefins inside the HDS reactor to yield linear mercaptans or possibly branched thiols comprising 5–12 carbon atoms. The efficiency of the HDS process is affected owing to the yield of mercaptans which led to sulfur content being retained in the product.
A parallel relationship exists between the type of sulfur compounds and the effectiveness of the HDS process. A catalyst composed of sulfided CoO– MoO3/ɣ-Al2O3 was used for the HDS process on various types of organosulfur compounds and the resultant outcome indicated the order of reactivity as thus; thiophene (TP) > benzothiophene (BT) > benzonaphthothiophene > tetrahydrobenzothiophene > dibenzothiophene (DBT). Houalla et al proposed a universally accepted mechanism for the HDS process which is shown in Fig. 3.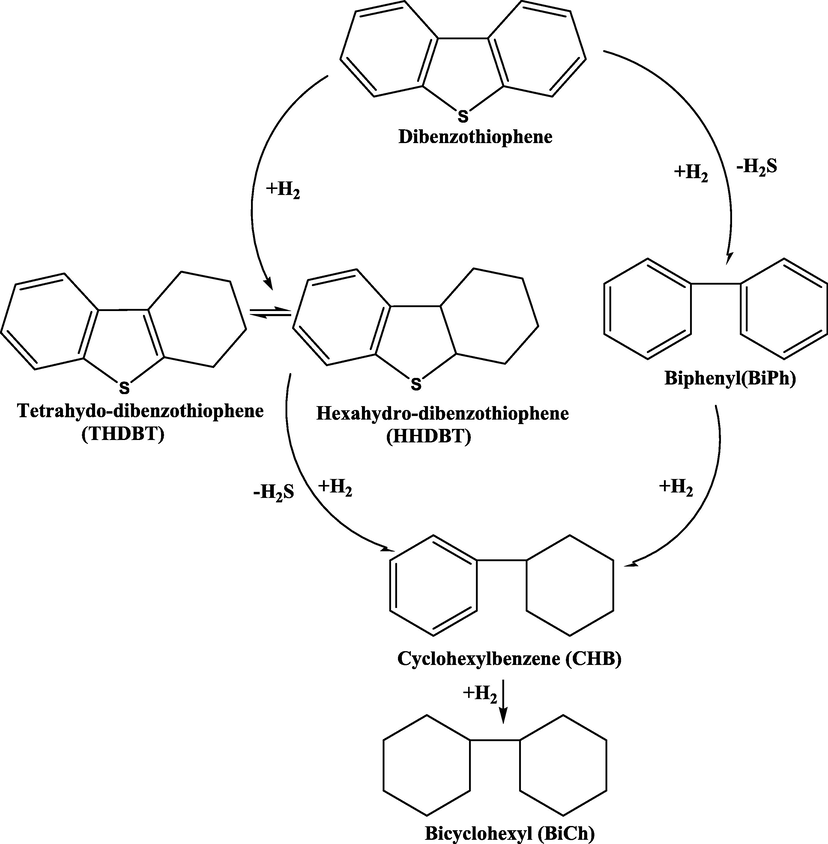
HDS reaction pathway for dibenzothiophene with CoMo/Al2O3 catalyst at a temperature of 300 °C and pressure of 102 atm (Gates, 1978).
Meanwhile, many investigators have reported the HDS process of DBT (Ali et al., 2020; Hu et al., 2020; Muhammad et al., 2019; Saleh, 2020; Wang et al., 2019). However, HDS is an outstanding process towards the ousting of sulfur compounds like mercaptans, sulfides, and disulfides (low boiling points compounds) but undesirable for organic compounds containing sulfur such as TP, BT, DBT, and their alkylated derivatives in particular, 4,6-DMDBT owing to steric hindrance (Liu et al., 2001). Also, cyclic, condensed multicyclic, and aromatic compounds are known to be refectory to the HDS process (Aida, 1998). HDS limitations also include severe operation conditions such as working at a higher pressure and elevated temperature of 3–7 MPa and 300–400 0C respectively, thereby resulting in excessive hydrogen consumption. Recent applications of HDS focus greatly on sterically hindered BT and DBT intending to get rid of them to a much lower extent in fuel (Otsuki et al., 2000a).
2.2 Oxidative desulfurization (ODS)
ODS is an essential alternative to HDS and has gained tremendous attention. The ODS process proceeds via two-steps that involve the oxidation of sulfur-containing compounds to sulfone and sulfoxides by oxidants like tBuOOH, H2SO4, H2O2, NO2, and O3 which are then evicted from crude oil by extraction owing to their higher polarity (Liu et al., 2001; Otsuki et al., 2000b; Yu et al., 2006). ODS technology is gaining increasing attention due to its excellent performance in reducing sulfur content in fuel to an infinitesimal amount at very low conditions temperatures and pressure (Ali et al., 2006). Fig. 4 illustrates the oxidation reaction of DBT to its corresponding sulfone.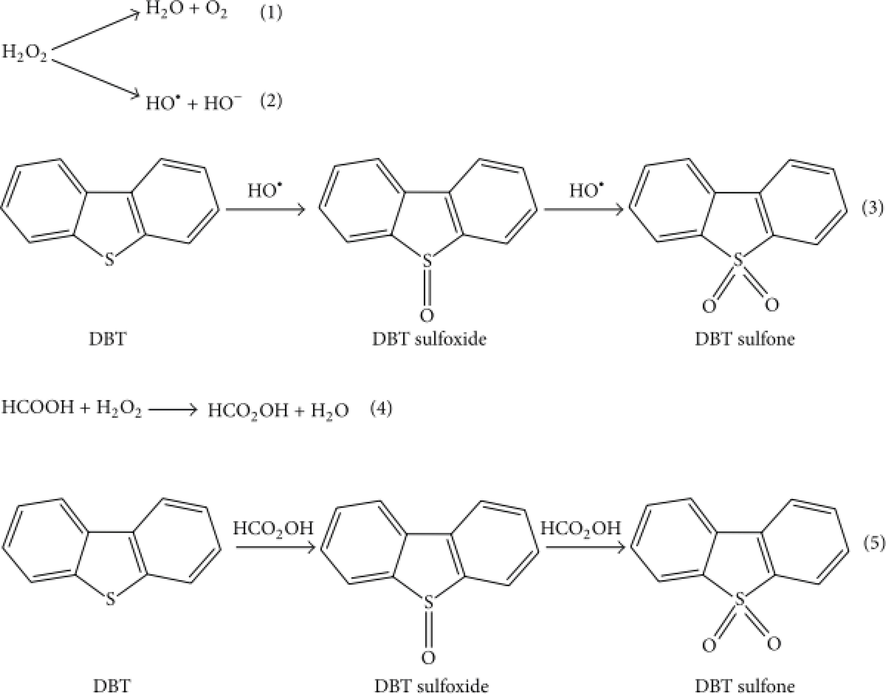
Oxidative desulfurization of DBT reaction pathway (Mamaghani et al., 2013).
ODS of DBT and its derivatives to their sulfone equivalent tends to have a significant increase in their molecular weight and polarity thereby enhancing their removal by adsorption (Haw et al., 2010), and extraction (Kittrell and Eldridge, 1990). Thus, these separation methods are excellent in removing sulfur concentrations from the organic phase. Many investigators have reported the desulfurization process by ODS using various oxidants. Mamaghani et al. used formic acid (CH2O2) and hydrogen peroxide (H2O2) as catalysts and oxidants respectively to examine the desulfurization of model fuel (BT, DBT, and 4,6-DMDBT) by ODS process which resulted in 100% conversion under the operating conditions of molar ratio 2:1 of H2O2 to sulfur, the temperature of 65 °C, and 22:1 of CH2O2 to sulfur and 56 min reaction time. The model fuels used in this study were removed by liquid–liquid extraction using acetonitrile in their oxidized form to corresponding sulfones (Mamaghani et al., 2013). Ti-MWW catalyst was studied on the removal efficiency of BT and DBT dissolve in isooctane for ODS using H2O2 as oxidants. Both DBT and BT are converted to their corresponding sulfoxide and sulfone which are extracted thereafter by acetonitrile. A conversion of 100% and 95% were achieved at 70 °C for BT and DBT respectively. The solvent effect was also investigated using acetonitrile, methanol, and water, of which DBT conversion was found to decrease in the order acetonitrile > methanol > water (Shifu et al., 2006). ODS has gained increasing attention from researchers across the globe as an alternative and complementary process to conventional HDS. However, the major limitations attributed to the ODS process include but are not limited to; the requirement of hours of stirring at high pressure and temperature selectivity, oxidant toxicity, cost, and water generation as a byproduct, of which excessive water in the fuel is harmful to an engine (Saleh and Danmaliki, 2016a).
2.3 Biodesulfurization (BDS)
BDS is a process adapted to the removal of sulfur contents present in fossil fuels under the influence of an enzyme-catalyzed reaction (Hou et al., 2005). Sulfur is an important constituent required for the biological activities and growth of microorganisms. Sulfur occurs generally in the structure of proteins and amino acids (such as disulfur bonds, methionine, and cysteine) and some enzyme cofactors (like biotin, Coenzyme A, and thymine) (Kertesz, 1999; Stoner et al., 1990). It should be mentioned that some microorganisms possess a high tendency to consume organosulfur compounds such as DBT thereby reducing sulfur contents present in fuels. BDS process results in low energy consumption emissions, and smaller residue production thereby making it environmentally friendly and can complement HDS to strengthen its efficiency. It is a known fact that bacteria use accumulated sulfur in a natural environment for their growth and maintenance. Sulfur is available in the organic sulfur form either as sulfate esters and sulfonate in both cultivated and arid soils and not as free inorganic sulfate and there are ample bacteria in nature capable of utilizing the carbon skeleton or sulfur through the transformation of sulfur-containing compounds (Timmis, 2010).
BDS technology is aimed at desulfurizing a variety of sulfur compounds available in petroleum fractions through the development of microbial systems. Though, no specific model compound is commonly used to mimic virtually several fractions of crude oil. It has been reported that 70% (w/w) and 40% (w/w) of DBT and its derivatives account for the total sulfur constituents of West Texas and some Middle East crude oils. In a nutshell, DBT as a simulated compound becomes the main choice on the ground that; (i) its alkyl-substituted derivatives tend to be the most challenging organosulfur compound to undergo ultra-deep desulfurization even with deep HDS process, certain isomers still manage to survive (ii) its derivatives constitute the main percentage of thiophenic sulfur in petroleum and its fractions (Sciences et al., 1998). Various bacteria species can metabolize sulfur-containing organic compounds following three main pathways: oxidative C–C bond cleavage, reductive C-S bond cleavage, and oxidative C-S bond cleavage. Typically, the two main pathways reported for BDS of alkyl-substituted sulfur compounds are; first, the Kodama pathway where at first, one of the carbon skeletons of DBT is attacked, and second, the 4S pathway in which the DBT carbon skeleton remains intact and the attack is launched towards the sulfur center (Chandra Srivastava, 2012). Many bacteria tend to degrade DBT aerobically as evidenced in the lowering of the sulfur amount present in diesel from (535–75) ppm in 24 h by sulfur-metabolizing bacteria.
DBT is widely employed in the isolation of microbes with desulfurization features (Agency, 2000). A number of DBT desulfurization microorganisms have been isolated, most especially mesophilic and a small number of thermophilic microbes. These include Pseudomonas delafieldii R-8 (Luo et al., 2003), Rhodococcus erythropolis H-2 (Hirata, 1996), Microbacterium sp. ZD-M2 (Li et al., 2005), Rhodococcus sp. IGTS8. (Gauagher et al., 1993), Bacillus subtilis WU-S28 (Kirimura et al., 2001), Gordonia sp. CYKS1(Chang et al., 1998), and Mycrobacterium sp. G3 (Nekodzuka et al., 1997). The proposed pathway named the “4S pathway” shows how DBT was desulfurized by bacteria (Gauagher et al., 1993; Ii, 2010). It was shown that DBT got converted to DBT sulfone after its initial oxidization to DBT sulfoxide, which is later followed by its transformation into 2’-hydroxylphenyl benzene sulfinate (HBPSi) by monooxygenase thereby resulting in cleavage of thiophene ring. Ultimately, the hydrolase enzyme reduced 2’-hydroxylphenyl benzene sulfinate by monooxygenase to 2-HBP leading to immediate release of sulfite and sulfate. DBT is selectively removed while its hydrocarbon skeleton is maintained in this pathway, thus, preventing a reduction in the fuel's thermal value (Bhatia and Sharma, 2010). BDS presents advantages as a complementary process to HDS technologies; however, it has not seen much needed to greenlight for industrial applications owing to several drawbacks in both the downstream and upstream processes. The major drawbacks of this process of BDS are the difficulties of commercial application in the industries owing to the exorbitant price of biocatalysts, reactor design and slow metabolism of sulfur containing compounds compared with chemical reactions. Furthermore, biological species have to be kept alive to function excellently in the refineries under varieties of given conditions which in turn extremely difficult to achieve due to the desulfurization rate that depend strongly on the parameters like temperature, pH and dissolved oxygen concentration (Chandra Srivastava, 2012).
2.4 Ionic liquids desulfurization (ILs)
Ionic liquids (ILs) desulfurization, an alternative to the conventional HDS process, is another fast-growing desulfurization technology first reported in 2001 (Bösmann et al., 2001a). The advantages attributed to this method that makes it an excellent extractant for sulfur compounds include inflammability, environmental friendliness, excellent chemical/thermal stability, recyclability, and solubility for organic and inorganic and non-volatility (Kêdra-Królik et al., 2011; Yu et al., 2011; Zhao et al., 2005). ILs are organic salts that comprise organic cations and organic/inorganic anions with a low melting point usually below 100 °C (Zhao et al., 2005). Several anions and cations have been employed in this desulfurization technology notably among them are PF6- EtSO4-, AlCL4-, BF4-, thiocyanate, ethanoate, Imidazolium, N-alky-3-methylimidazolium bis (trifluoromethylsulfonyl)imide. ILs system of 1-butyl3-methylimidazolium tetrafluoroborate (BMImBF4) and 1-butyl-3-methylimidazolium tetrachloroaluminate (BMImAlCl4) have been studied for the removal of sulfur contents present in transportation fuel (Bösmann et al., 2001b; Shuguang Zhang, Qinglin Zhang, 2004). AlCl3-based ionic liquids exhibited remarkable desulfurization but its major limitations include moisture sensitivity, air instability, and a tendency to undergo side reactions such as olefin polymerization in gasoline induced by the strong Lewis acidity of Al2Cl7. Thus, there is a need to investigate other forms of ionic liquid that are free from the said limitations. However, the major problem of AlCl3-free ionic liquids such as BMImBF4 used in desulfurization is low desulfurization performance. In light of this, Chen et al investigated the removal of sulfur compounds from gasoline by extraction with novel as-synthesized ionic liquids, which were prepared by mixing purified anhydrous CuCl with 1-butyl-3-methylimidazolium chloride. It was observed that Cu(I) anionic species (CuCl2 -, Cu2Cl3 - and Cu3Cl4-) that existed in the ionic liquid are stable in air, and moisture-insensitive. An excellent desulfurization capacity of gasoline was noticed with the use of CuCl- based ionic liquid as an extraction adsorbent. The exceptional desulfurization capacity was attributed to the π-complexation interaction of Cu(I) with TP. Furthermore, they concluded that there was no occurrence of olefinic polymerization reaction in the gasoline owing to the use of CuCl-based ionic liquid (Huang et al., 2004). Chen et al compared their research outcome using BMImCu2Cl3 ionic liquids with the result obtained with BMImAlCl4 and BMImBF4. The percentage of sulfur removal in model oil is 23.4 %, 16.0 %, and 11.0 % respectively. The major bottlenecks adapted to this process are corrosion problems, regeneration difficulty, high cost, instability, and undesirable fuel quality (CHU et al., 2008; Kêdra-Królik et al., 2011). Other approaches adapted to the removal of sulfur contents from fuel include adsorptive desulfurization.
2.5 Adsorptive desulfurization (ADS)
The ADS process has gained interest as a means of achieving ultra-low sulfur levels in fuels due to its simplicity, speed, environmental friendliness, reliability, and the use of cost-effective materials. This excellent performance is due to its excellent adsorption affinity for recalcitrant sulfur compounds with the usage of different materials under moderate conditions (energy-saving process) (Silva et al., 2014). Adsorption is a mass-transfer phenomenon in which molecules in a free state get attached to a surface by intermolecular attraction (Anthony L. Hines, 1985). The solids employed to facilitate the surface adhesion of dissolved substances are referred to as adsorbents while adsorbate is ascribed to adsorbed molecules. Most industrial adsorbents are generally classified into (i) carbon-based compounds with a typical example of carbon and graphite which are hydrophobic or non-polar. (ii) oxygen-containing compounds with a typical example of zeolites and silica gel which are hydrophilic and polar and (iii) polymer-based compounds which on the ground of functional groups in the polymer matrix, it could be polar or non-polar. Some of the major salient characteristics of an adsorbent are enumerated below:
-
It should be thermally stable and possess a high mechanical resistance.
-
It should have a relatively small pore diameter.
-
It should have a strong affinity for the adsorbate.
-
It should be readily available, cheap, and have a high surface area.
-
It should be regenerated easily.
The adsorption efficiency of any adsorbent material is a function of the size of the active pore in the interior area relative to the exterior area. It is worth mentioning that adsorbents must have a definite pore structure that enhances the rapid transport of the gaseous vapors. According to the International Union of Pure and Applied Chemistry (IUPAC), the pore sizes of porous materials are classified into three. Namely; microporous materials with a diameter<2 nm, mesoporous materials with a diameter between 2 nm and 50 nm, and macroporous with a diameter greater than 50 nm (Kirkby, 1988). ADS of organosulfur compounds is an excellent and reliable method especially when porous materials are employed. Its advantages over other processes are due to (i) excessive accessibility of adsorbents (ii) renewability of spent materials (iii) workability at moderate temperature and pressure, (iv) low-energy consumption (v) thermal stability, and (vi) promising textural characteristics like high surface area, desirable micropore volume, and well-developed pore size (Wang et al., 2009). Varieties of sorbent materials used in ADS studies have been reported. Among others, sorbents such as zeolites, amorphous silica-alumina, metal–organic framework (MOF), AC, silica gel, and metal oxides were studied for desulfurization of fluid catalytic cracking feedstock, model oils, coker naphtha and distillates, model oil and distillates (Adeyi and Aberuaga, 2012; Ahmed and Jhung, 2016; Saleh and Danmaliki, 2016b; Salem, 1994; Shi et al., 2010).
3 Activated carbon
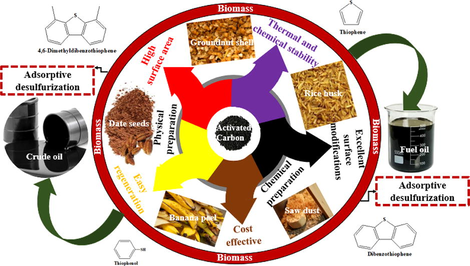
AC (also called activated coal or charcoal and sometimes solid sponge) is an amorphous form of carbon treated specially to produce a large surface area and a well-developed internal pore structure (Alhamed, 2006). It is a microcrystalline, non-graphite, amorphous, tasteless, form of carbon. It is a diverse adsorbent containing up to 90% carbon with unique textural properties which include a high degree of porosity and high surface area. On the ground of its physical features, it can be classified as powdered activated carbon (PAC), granulated activated carbon (GAC), pellet activated carbon, activated carbon cloths, fibrous activated carbon, extracted activated carbon (EAC), and others (Yahya et al., 2015). AC may be produced from industrial byproducts, agricultural waste, and livestock (Khadhri et al., 2019). AC production globally is estimated to be 100,000 tonnes (Seh-bardan, 2014). The prevalent source of AC on a large scale is bitumen and anthracite, coconut, charcoal, lignite, wood, and peat shells. Also, a number of precursors such as coal, and petroleum residua are used in AC production, all of which possess thermosetting features which upon thermal treatment in an inert atmosphere produce chars (carbonaceous residue) (Abotsi and Scaroni, 1989). Meanwhile, the usage of non-renewable, high-cost precursors namely; peat, petroleum residue, lignite, bituminous coal, and wood make commercial activated carbon expensive (Yahya et al., 2015). Thus, reasonably cheap environmentally friendly, and desirable adsorbents have been investigated using lignocellulosic biomass as precursors for AC production.
Lignocellulosic biomass is the most populous raw material on earth and can simply be considered as agricultural by-products and waste which has found its application in several pollution control strategies. It is essential to mention that the production of AC from lignocellulosic biomass intrinsically reduced global warming’s effect as compared to the AC derived from exorbitant precursors from fossil sources such as coal. Cellulose, hemicellulose, and lignin are the three different components under which lignocellulosic biomass are classified with cellulose and lignin being the major components responsible for the adsorption process (Suhas et al., 2007). The most prevalent renewable carbon precursor in the universe with a global production of 40–20 million tons annually is lignin-based biomass (Suhas et al., 2007). The agricultural by-products based precursors that have been investigated include but not limited to coconut shell and wood (Laine et al., 1989; Tsai et al., 2006), reedy grass leaves (Xu et al., 2014), corn stover (Fan et al., 2004; Zhang et al., 2004), oat hulls (Fan et al., 2004), palm shell (Lim et al., 2010), corncob (El-Hendawy et al., 2001; Njoku and Hameed, 2011; Tsai et al., 2001, 1998), Olive-tree (Ould-Idriss et al., 2011), apricot stones (Petrova et al., 2010), sour cherry stones (Angin, 2014), miscanthus (Minkova et al., 2001, 2000) birch wood (Minkova et al., 2000), rice hulls, sugarcane bagasse and pecan shells (Ahmedna et al., 2000), cotton stalk (Deng et al., 2010), date stems (Hadoun et al., 2013), date stone (Haimour and Emeish, 2006; Sekirifa et al., 2013) rice straw grape seeds (Al Bahri et al., 2012), rice husks (Malik, 2003; Yalçin and Sevinç, 2000), tomatoes processing solid waste (Sayğili and Güzel, 2016), cashew nuts (Spagnoli et al., 2017), olive residue (Haykiri-Acma et al., 2006), walnut shells (Aygün et al., 2003; Miyah et al., 2018), Almond shell (Ait Ahsaine et al., 2018; Aygün et al., 2003; Marcilla et al., 2000; Savova et al., 2001), wheat and corn straw (Lanzetta and Di Blasi, 1998), waste newspaper (Okada et al., 2003) and Bamboo waste (Zhang et al., 2014). The carbon composition of these materials isis is in the range of 40 to 90 wt% with a density range of 0.4 to 1.45 gm−3 (Jia and Gan, 2011; Khadhri et al., 2019). Generally, 800±1500 m2/g is the range of a typical AC surface area with mesopore volumes alternating between 0.1 and 0.2 cm3/g, exceptional cases could have a higher value up to 0.5 cm3/g and mesoporosity in the range of 10±200 m2/g.
Today, tremendous efforts have been directed towards exploiting waste as precursors in the production of AC which in turn has been used for several industrial applications which include wastewater treatment, removal of toxic substances, sugar refining, separation/purification of liquids and gases, as catalyst and catalyst support, deodorization of vegetable oil and fats, and other food industries (Alhamed, 2006; Fuente et al., 2001; Kazeem et al., 2018; Moon and Shim, 2006). Its efficiency in environmental remediation has been found infinitely better than other methods owing to the high quality of effluent achieved, insensitivity to toxic substances, ease of operation, and simplicity of design (Chen et al., 2002). It should be mentioned that the major functional groups accountable for the adsorption of contaminants in carbon structures include phenol, carboxyl, quinone, and lactone. AC is achieved by the partial gasification of the carbonaceous material in media like steam, oxygen, and CO2. Carbon gasification results in the creation of new pores, enlargement of existing pores, increase in surface heterogeneity, and the development of surface area and pore volume. AC are usually microporous materials thus limiting their application to reactions involving small molecules such as methanol production from CO2 and hydrogen (Abotsi and Scaroni, 1989). It is worth mentioning that AC performance is a function of desirable operating conditions during production or synthesis, which proceed via two distinct processes. First, char is produced from its precursor during the pyrolysis/carbonization process where the available moisture and volatile content of the biomass are eliminated. The second step involves the activation of the resultant material (char) produced. AC preparation can be achieved through two distinct methods with regard to the nature of the activation process, namely, physical and chemical activation (Ioannidou and Zabaniotou, 2007).
3.1 Preparation of activated carbon
3.1.1 Carbonization/pyrolysis
This is a stage in the preparation of AC which helps in the removal of non-carbon species to facilitate and enrich carbon content in the carbonaceous materials. The porosity of the resultant char could be drastically increased during the carbonization process by appropriate selection of carbonization parameters before proceeding to the final stage of activation where maximum porosity of the carbonaceous materials can be attained. The significance of carbonization parameters follows the order of pyrolysis residence time < nitrogen flow rate < heating rate < temperature < pyrolysis temperature (Ioannidou and Zabaniotou, 2007; Lua et al., 2006). Generally speaking, there is an inverse relationship between the pyrolysis temperature and carbon yield. The higher the carbonization temperature the lower the yield of the char and the resulting ACs. Also, it has been established that there is a parallel relationship between the fixed carbon content, ash, and pyrolysis temperature. Suffice it to say that higher pyrolysis temperature begets higher fixed carbon content and resulting ash but invariably, there is a reduction in the amount of volatile matter (Ioannidou and Zabaniotou, 2007; Lua et al., 2006; Pütün et al., 2005). Typically, elevated temperatures give rise to better quality charcoals but result in low char yield which could be attributed to either the initial decomposition of biomass at elevated temperature or the secondary decomposition of char residue (Ioannidou and Zabaniotou, 2007). Similarly, the char yield also decreases owing to the increased temperature of the primary degradation and/or the increase in the residence times of the primary vapors inside the rupture particles. To produce desirable ACs, carbon content is very essential; an indication of suitable char yield. Meanwhile, production of high char yield and low volatilization are functions of low heating rate usually in the range of 10–15 °C/min. This low heating rate advance not only the polymeric components stabilization but also augment dehydration (Ioannidou and Zabaniotou, 2007; Suhas et al., 2007). Table 1 below enumerates the proximate and elemental analysis of several AC derived from agricultural products and waste.
Agricultural wastes
Proximate analysis (% w/w)
Elemental analysis (% w/w)
Moisture
Ash
Volatile matter
C
H
N
O
S
Grape seeds
7.00
1.60
67.4
56.5
7.20
2.00
–
0.30
Tomato processing solid waste
9.00
26.0
11.0
79.4
1.76
1.70
17.1
0.10
Apricot stones
–
0.200
80.6
51.5
6.30
0.200
41.9
0.10
Sour cherry stones
2.67
0.170
78.5
48.72
6.41
1.85
43.02
–
Date stems
3.00
5.00
67.2
–
–
–
–
–
Corn cobs
7.10
5.34
–
46.3
5.60
0.570
42.2
0.00
Rice straw
25.0
13.4
69.3
48.1
4.63
0.700
36.6
0.080
Miscanthus pellets
12.10
2.40
–
48.9
4.60
0.400
46.7
–
Olive waste
–
7.70
2.10
49.5
6.30
0.500
43.7
–
Corn stalks
0
6.40
–
45.5
6.15
0.78
41.1
0.13
Barley straw
15.0
4.90
–
46.8
5.53
0.410
41.9
0.060
Sugar beet leaves
75.0
4.80
–
44.5
5.90
1.84
42.8
0.13
Sunflower straw
40.0
3.00
–
52.9
6.58
1.38
35.9
0.15
Cassava pill
11.4
0.300
59.4
59.3
9.78
2.06
28.7
0.11
Sugarcane bagasse
6.20
0.90
–
47.3
6.20
0.300
46.2
–
Grape stalk
15.7
10.2
51.1
46.1
5.74
0.37
36.6
0.00
Apricot tree prunings
40.0
0.200
80.4
51.4
6.29
0.800
41.2
0.10
Orange tree prunings
40.0
2.80
–
47.0
6.00
1.00
43.2
0.030
Olive tree prunings
7.10
4.75
–
49.9
6.00
0.700
43.4
–
Vineyward tree prunings
40.0
3.80
–
47.6
5.60
1.80
41.1
0.080
Peach tree prunings
40.0
1.00
79.1
53.0
5.90
0.320
39.1
0.050
Oats straw
15.0
4.90
–
46.0
5.91
1.13
43.5
0.015
Palm shell
7.96
1.10
72.5
50.0
6.90
1.90
41.0
0.00
Coconut shell
8.21
0.100
73.0.1
48.6
6.51
0.140
44.6
0.08
Bamboo
2.44
6.51
69.6
45.5
4.61
0.220
–
–
Wallnut shell
11.0
1.30
71.8
45.1
6.00
0.300
48.6
0.00
Olive stone
10.4
1.40
74.4
44.8
6.00
0.100
49.1
0.010
Bagasse
–
6.20
83.3
41.6
5.55
0.0300
52.7
–
Woody birch
6.60
0.200
81.2
48.4
5.60
0.200
45.8
–
Palm stem
6.06
4.02
72.5
50.0
6.90
0.82
47.7
–
Durian shell
11.3
4.84
–
39.3
5.90
1.00
53.7
0.0600
Straw pellets
7.00
6.20
47.0
6.10
0.500
46.4
–
3.2 Activation
3.2.1 Preparation of AC via chemical activation
Chemical activation (also known as wet oxidation) is used often for raw materials (otherwise called biomass resources) containing fruit pits, wood, sawdust, or cellulose. The precursor is impregnated with activating material (dehydrating agents) such as zinc chloride (ZnCl2 a Lewis acid), potassium hydroxide (KOH, a strong base), phosphoric acid (H3PO4, a weak acid) sodium hydroxide (NaOH, a strong base), and potassium carbonate (K2CO3, a Lewis base) and others, followed by carbonization under an inert atmosphere at a temperature range of 400–900 °C at which cellulose is degraded. A leaching process that involves repeated washing to recover the active substances of the resulting mixture is then employ and in the second step to remove the activator and AC is obtained (Seh-bardan, 2014). Chemical activation agents enhance pyrolytic decomposition with subsequent changes in the thermal degradation of precursors and augment the AC content by hindering bitumen formation thus facilitating porous structure development of carbon materials. The deep penetration of these activating agents into the carbon structure influences small pores' development hence, increasing the surface area (Gratuito et al., 2008). Chemical activation has many advantages over physical activation and this includes low activation temperature, higher yield, development of good porous structure, and simplicity (involvement of a single-step activation) (Yang et al., 2010). However, the main bottleneck of this method is embedded in the repeated washing of the resulting material to get rid of the spent activator which is time-consuming and labor-intensive yet toxic effluents are produced during the washing stage that is environmentally malign and requires secondary treatment (Wang et al., 2016; Yorgun et al., 2016). Meanwhile, AC synthesis has outstanding characteristics that to some extent depend on the nature of the precursor but to a large extent influenced by the operating conditions such as carbonization time, temperature, and the impregnation ratio (IR) (activator and precursor ratio).
3.2.2 Preparation of AC via physical activation
Typically, physical activation otherwise known as a dry method is a two-stage process that includes carbonization otherwise known as pyrolysis followed by activation. The formal takes place in a neutral atmospheric condition while the latter occurs in atmospheric oxidizing gases like steam, CO2, and air mixtures or nitrogen at an elevated temperature range of 800–1100 °C (Bouchelta et al., 2008). This method of AC production offers the advantage of excellent physical power and a well-developed porous structure yet it is regarded as a green approach owing to its chemical-free process (Pallarés et al., 2018). However, physical activation method limitations include low adsorption capacity, long activation time, and high energy consumption (Yahya et al., 2015).
3.3 Activated carbon surface chemistry
AC is an excellent adsorption material for a variety of compounds (Tan et al., 2008; Yang et al., 2010) and one of the commonly employed adsorbents in wastewater treatment (Kazeem et al., 2018; Malik, 2004; Wang et al., 2008). Several researchers have modified AC and tested its applicability as an efficient adsorbent for waste removal from water. For example, Sonal et al. (Sonal et al., 2020) modified the surface chemistry of a commercial AC and used it for the removal of anionic dye. Zirconium salt was used as an impregnating material with the view to make the carbon surface has a cationic counterpart that will attract the aimed anionic pollutant. Fig. 5 elucidates the proposed mechanism of the developed materials wherein the modification process proceeds via the wet oxidation method. First, to increase the AC surface interaction towards the metal counterpart, potassium permanganate solution (KMnO4) was used as an oxidizing agent to oxidize the AC surface. The carbonyl group present on the AC surface gets oxidized into a carboxylic group in the presence of an oxidizing agent KMnO4 which further readily reacts with the dichlorooxozirconium (ZrOCl2). This ZrOCl2 dissociates on hydrolysis to form a dimmer, [Zr4(OH)2(H2O)16] 8+ along with oxychloride forms and HCl. The dimmer reacts with the AC to form a complex while zirconium oxides (ZrO2) was formed from further oxidation of the oxychloride forms. Ultimately, an amphoteric substance sodium bicarbonate solution was employed and was used to treat the resultant solid formed which thus readily reacts with the remaining acidic group to prevent further complex formation. This modification process of AC resulted in novel materials (ZrAC) with high porosity, oxidative functionality and thermal stability which were validated by chemical and spectral analysis. A number of characterization techniques were used to authenticate the presence of metal oxide formation on the surface of AC, and this enhanced adsorption affinity for the targeted anionic dye. A number of AC modifications that would augment its porosity as well as surface area have been developed for efficient desulfurization (Seredych et al., 2009). There is a multitude of reported articles that show how AC surfaces were oxidized with the incorporation of metals to positively enhanced adsorption efficiency of DBTs (Ania and Bandosz, 2006; Danmaliki and Saleh, 2017; Ganiyu et al., 2016; Moosavi et al., 2012; Olajire et al., 2017; Seredych et al., 2009; Seredych and Bandosz, 2010; Xiao et al., 2010; Yu et al., 2007). It should be mentioned that surface chemistry modification and special treatments of AC which include both the physical and chemical modification of carbon surfaces play pivotal roles in adsorption capacity breakthrough as well as in the enhancement of carbon selectivity towards sulfur-containing compounds and the said treatments include oxidation, steaming, sulfidation, acidification and also metal loading and metal-oxides incorporation (Ganiyu et al., 2016).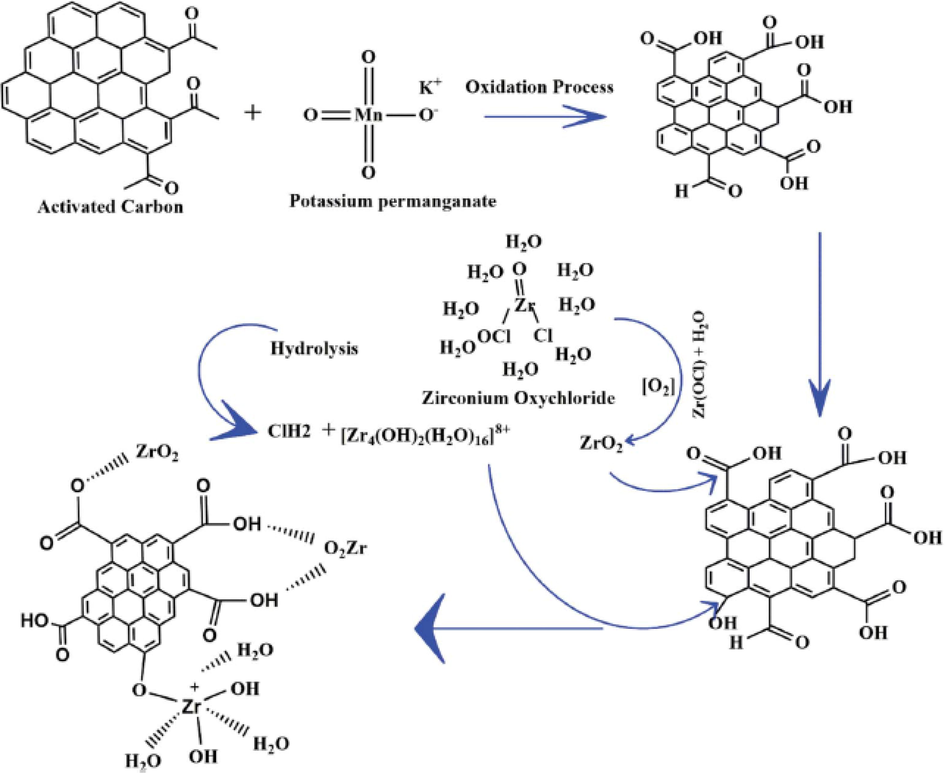
Schematic representation of a probable synthesis approach for zirconium-modified activated carbon (ZrAC) (Sonal et al., 2020).
The efficiency of AC for the removal of hazardous acidic gases from flue gas treatment was found to be minimal owing to low selectivity, low adsorption rate, and adsorption capacity of AC on the aimed gases. Low partial pressure and high flue gas temperature downstream are the major factors responsible for these limitations (Abdulrasheed et al., 2018; Ahmed et al., 2017; Wang et al., 2010). These limitations of flue gas treatment attained by physisorption influenced surface chemistry modification of AC by the creation of active sites and functional groups that will enhance a brilliant interaction between the targeted gases and the AC thus resulting in a better adsorption capacity and excellent selectivity. Surface modification of AC enables gas adsorption by two forces which include: (i) chemical reaction between the free and/or stationary chemical groups and the gases to be extracted (ii) occurrence of mass transfer phenomenon of the gases to the pores and the adsorbent surfaces. AC surface modification with respect to functional groups is classified into two based on their acidity or basicity.
3.3.1 Surface acidity
AC surface acidity heightens its affinity for gases having alkaline characteristics, such that a chemical reaction occurs on the adsorbent surface between acidic functional groups and ammonia (an alkaline gas). The surface acidity of AC is materialized by the oxygenated radicals intrinsically bound to the surface on the application of heat or via oxidation of the precursor by liquid phase or gas impregnation. Physical activation methods which can also be referred to as gas-phase oxidation constitute the usage of gaseous oxidizing agents such as CO2, pure oxygen, air, or steam to produce mainly carbonyl and hydroxyl functional groups. Conversely, oxidizing agents like H2SO4, H2PO4, H2O2, and HNO3 are used to produce higher amounts of carboxylic and phenolic groups on adsorbent surfaces in liquid-phase oxidation. Liquid phase oxidation is not only more efficient in the creation of surface functional groups than its gas phase counterpart but also requires the use of lower oxidation temperature which makes it more economical (Abdulrasheed et al., 2018). Oxygen, a heteroatom which upon introduction onto the surface of carbon during modification adheres to a graphite-like layer of carbon thereby resulting in radical group formation which includes carbonyl, carboxyl, hydroxyl, pyronic, lactonic, and ethoxy functional groups. Fig. 6 shows the most common oxygen-containing groups present on carbon-oxidized surfaces (Lo et al., 2013). AC carbon exposure to the oxidizing environment and air influences oxygenated groups available on several carbon surfaces to cause the chemisorption of oxygen into accessible pores and cracks on the AC surface. Carbon surface enhancement promotes gas separation due to the abundance of oxygen-containing groups which ordinarily are insignificant without carbon surface modification.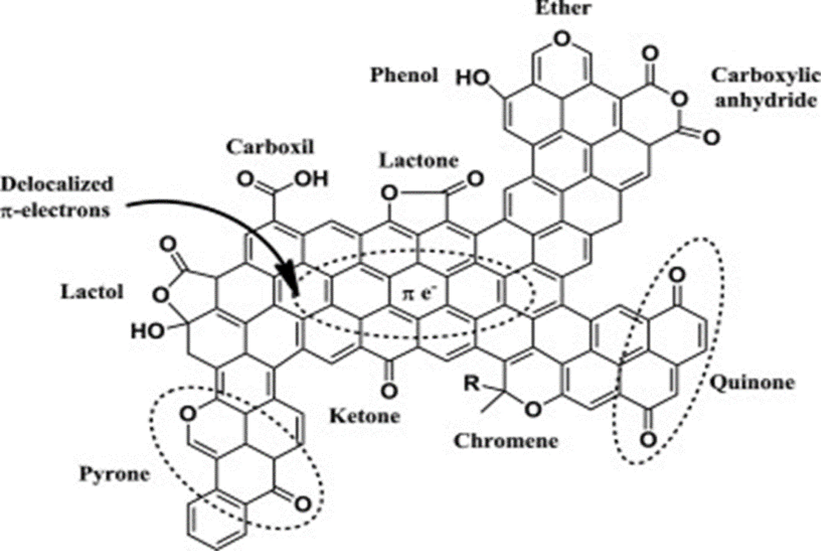
A scheme showing modes of carbon surface modification with oxygenated groups (Lo et al., 2013).
3.3.2 Surface basicity
The basicity of carbon surface is achieved through the introduction of certain oxygen-containing functional groups such as pyrone, chromene, and ketone radicals. Although the basic surface oxygen group is less significant than the most dominant surface oxygen acidic groups. The surface basicity of the adsorbent materials can thus be improved to augment its affinity and selectivity for the targeted gases through the incorporation of a basic nitrogen functional group (Abdulrasheed et al., 2018). Nitrogen functional groups are incorporated onto the carbon surface by members of the amine group and ammonia. It is also found that the nitrogen functional group introduction onto the carbon surface such that ammonia is impregnated which then react with the inherent surface oxygen on carbon to further yield more nitrogen surface group. Additionally, it has also been established that a parallel exists between surface oxygen groups and the number of nitrogen groups in that there has been a significant increase in the nitrogen groups amount when subjected to amination in the presence of oxygen groups. This assertion was corroborated by Shafeeyan et al. (Shafeeyan et al., 2011) in their work when they compared the quantity of nitrogen-containing groups available on the AC surface between an oxidized sample subsequently followed by impregnated with NH3 and the sample impregnated with NH3 without preceded by oxidation. The result obtained showed that the former significantly improved basic nitrogen groups thereby increasing the adsorption capacity for CO2 capture as compared with the latter. The adsorption capacity for the removal of SO2 gas from flue gas has been enhanced by the modification of AC with basic chemical agent having a high basicity or amphotericity such as amine groups as well as metallic oxides. In other words, to remove highly polarized SO2 gas by chemisorption, the surface chemistry of carbon surface (i.e. the basicity) needed to be enhanced (Arcibar-orozco et al., 2013). There has been a strong affinity and irreversible binding between acidic gases particularly SO2 and other nitrogen-containing functional groups on the surfaces of carbon. Surface area, pore volume, and size have been significantly influenced by amine compound incorporation into AC pores in the form of N2 functional groups thereby resulting in higher SO2 uptake (Tailor et al., 2014). A positive correlation was established between nitrogen content named the N-doping effect and SO2 sorption uptake by chemisorption (Sun et al., 2016).
4 Types of adsorptive desulfurization
ADS is the most prevalent HDS alternative often employed to attain ultra-deep desulfurization of fuel even below the regulated standard. Its mechanism of reaction is solely based on chemisorption or physisorption with which electrostatic attraction, chemical affinity, and Van der Waal's forces are answerable for adsorption. Various porous materials have been used for ADS with an impressive adsorption capacity. It is highly essential to mention that ADS has different forms (Fig. 7.) which include selective adsorption, π-complexation, polar adsorption, integrated adsorption, and reactive adsorption out of which selective adsorption and π-complexation are often used for ADS by aluminosilicate materials (zeolites). In selective adsorption, the organic sulfur compound interacts with metals via π-electrons. The π-complexation occurs between metal ions and zeolites. The chemistry behind this form of adsorption is that metal ions form σ-bonds with free s-orbitals and the d-orbitals will back-donate electron density to the antibonding π-orbitals in the sulfur-containing ring of thiophenes. It is a tenable fact that a stronger bond is formed as a result of this interaction which can be broken easily by alternating temperature or pressure and in doing so, adsorption capacity and selectivity to sulfur compounds are thereby enhanced.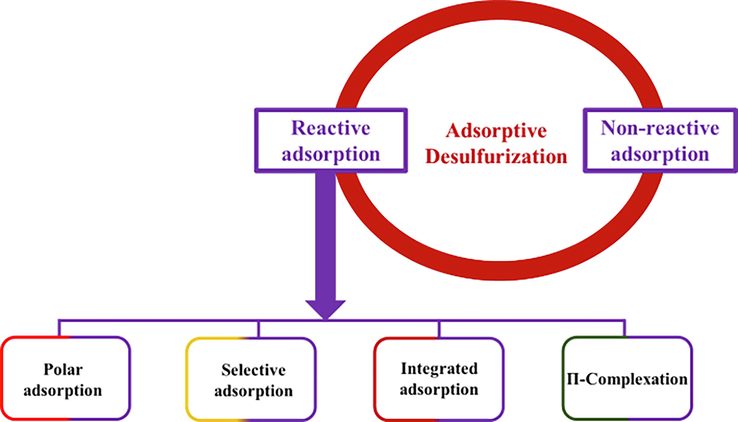
Types of adsorptive desulfurization .
Below is the scheme indicating various forms of adsorptive desulfurization.
It is obvious from Fig. 7 above that reactive adsorption is the most important form of ADS with which sulfur content in fuels is removed and is further categorized into four distinct forms as mentioned above. A detailed explanation of the types of ADS has been elucidated by Saleh et al (Saleh et al., 2016).
5 Activated carbon in desulfurization
AC is the most prominent adsorbent widely used in adsorption technologies due to its high surface area and enlarged porosity. It has been widely investigated for pollutant removal from both liquid and gas phases. Surface modification of AC instills some features which make it highly selective towards target compounds especially organosulfur and organo-nitrogen compounds. A horde of investigators studied ADS using a pure AC and a functionalized AC.
5.1 Pure activated carbon
Many investigators have employed AC synthesized from lignocellulosic biomass without modification of its surface chemistry and applied it for ADS. Danmaliki and Saleh investigated the influence of surface modification of AC derived from waste rubber tires for sulfur content removal in model fuel. The as-developed AC was treated with nitric acid or sodium hydroxide (activating agents) at ranges of temperature between 30 and 90 °C. Acid-treated AC at 90 °C showed the highest adsorption breakthrough capacity in the order of DBT > BT > TP which is also the order followed by all the ACs examined. It was reported that treated ACs displayed adsorption efficiency of at least 80% for DBT and this breakthrough was attributed to molecule size which is apt for the well-developed textural pores of the treated ACs as well as acidic functional groups on the surfaces of these pores that enhanced polar interactions (Saleh and Danmaliki, 2016b).
Alhamed and Bamufleh reported the ADS of model oil which contains DBT in n-decane using granulated activated carbon (GAC) synthesized from date’s stone activated by ZnCl2. The adsorptive power of GAC is evidenced in the adsorption breakthrough of DBT in which in the first 3 h more than 86% is adsorbed and subsequently increased gradually to 92.6% at the contact time of 48 h and no sulfur content is removed thereafter thus, equilibrium is said to have established. The adsorption efficiency of GAC was reduced to approximately 65% when treated with commercial diesel duel. The reduction in adsorption capacities was attributed to the presence of other competitive compounds like nitrogen and aromatic compounds to transit into the available adsorption site of the GAC surface. No report was provided on the regeneration of the spent adsorbent. (Alhamed and Bamufleh, 2009).
Bu and co-workers (Bu et al., 2011) employed AC to investigate ADS. The authors used both the commercial and simulated diesel containing sulfur and aromatics sulfur compounds in fixed-bed and batch adsorption systems. The ACs (denoted as AC1 to AC7) employed are derived from different carbon sources namely, wood, apricot coconut, and pitch with their surface area ranging from 713-1403 m2/g. It was observed that the adsorption capacity breakthrough follows the order AC1 < AC2 < AC5 < AC3 < AC6 < AC4 < AC7. The highest adsorption capacities recorded for AC7 whose carbon source is wood were attributed to π-π dispersive interaction between the graphene layer of AC and aromatic rings. They added that the electron donor–acceptor mechanism contributed a significant role in the adsorption affinities of S-containing molecules. The investigators claimed that adsorption selectivity follows the order of phenanthrene > anthracene greater than 4,6-DMDBT > DBT > fluorine > naphthalene. They concluded that the critical diameter of the adsorbate should be, at least, smaller than the pore size of the adsorbent before efficient adsorption of large molecules could be achieved.
Kim and co-workers established that activated carbon was excellent and superior in the removal of organosulfur compounds from model fuel as compared to nickel-silica alumina and activated alumina. AC was also observed to be more selective towards both the nitrogen and sulfur compounds (especially towards sulfur compounds containing methyl groups like 4,6-methyldinenzothiophene). This better adsorption breakthrough was attributed to hydrogen bonding interaction involving surface functional groups. The adsorption capacity breakthroughs are 0.049, 0.068, and 0.223 mmol of sulfur per gram of adsorbent (mmol-S/g-A) which correspond to 1.57, 2.18, and 7.15 mg of sulfur per gram of adsorbent (mg-S/g-A) for the activated alumina, Ni/SiO2-Al2O3, and the AC respectively (Kim et al., 2006).
Song and Zhou investigated the insight of surface chemistry modification on the adsorptive efficiency of organosulfur compounds of 4,6-MDBT, 4-MDBT, DBT, and BT in 10% aromatics as simulated diesel fuel using carbon materials which include ACs and carbon black from several precursors namely; wood, coconut, petroleum, coal and aromatic hydrocarbons with surface areas ranging from 254 – 2201 m2/g. Of all the carbon materials investigated, activated carbon derived from coal (ACD) showed the best adsorption capacity of 7.0 mg-S/g. Also, adsorption selectivity was conducted on the carbon materials and it was observed the surface functional group and textural characteristics of carbon materials play an essential role in sulfur adsorption-selective capacities with the order of 4,6-DMDBT greater than 4-MDBT > DBT > naphthalene > BT irrespective of the carbon materials. To test the regenerative power of the carbon materials, the fresh and the spent AC adsorbents derived from coconut and wood corresponding to ACC and ACNU respectively were investigated by solvothermal approach. Fig. 8 shows the breakthrough curves of spent adsorbent ACNU and ACC respectively, wherein the dashed line shown in the ACNU graph connotes the initial concentration of the model diesel fuel (MDF) at 398 ppmw. It could be seen from the breakthrough curve of ACNU that the adsorption capacity can be recovered by the solvothermal regeneration approach owing to the breakthrough curves of the fresh and spent adsorbent almost overlapping. Meanwhile, the breakthrough curves of the fresh and spent ACC adsorbent do not coincide with the observation that the adsorption capacity of fresh and spent adsorbent after regeneration of MDF are 16.5 g-MDF/g-A and 13 g-MDF/g-A respectively, which correspond to almost 21% of the adsorption capacity lost after regeneration (Zhou et al., 2006).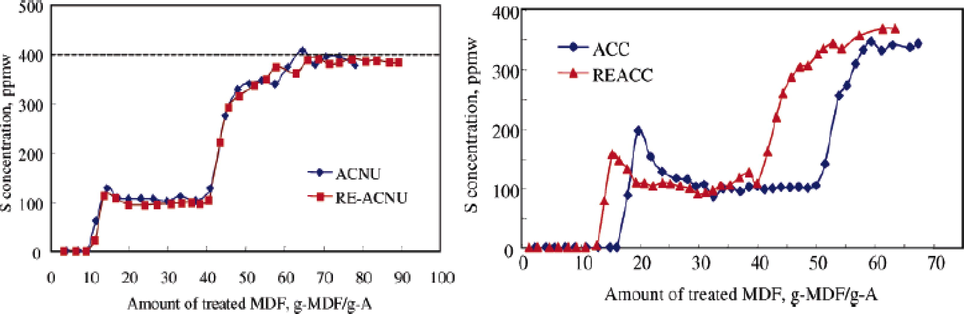
Total-sulfur-breakthrough curves for fresh and regenerated ACNU and ACC at 25 °C and 4.8 h−1 LHSV. MDF initial conc. = 398 ppmw (Zhou et al., 2006).
Nunthaprechachan et al. (Nunthaprechachan and Pengpanich, 2013) experimented with and studied the removal of DBT dissolved in n-octane using AC prepared from sewage sludge at ambient conditions. They investigated the influence of carbonization time (0.5–2 h), activating agent (KOH, ZnCl2, and HNO3), carbonization temperature (400–800 °C), activating agent: char weight ratio (0.5–6 w/w), on the adsorption affinity and physicochemical characteristics of the sorbents. They reported that a parallel correlation exists between the examined parameters and the surface chemistry of the synthesized activated carbons (S-ACs). The increase in the oxygen-containing functional group, especially the carbonyl group was observed to augment the adsorption capacity and that adsorbent prepared by KOH-activation displayed the best adsorption capacity for DBT removal with up to 14.12 mg/g at around 70.6 % which is around 1.28- and 1.22-fold greater when compared with the commercial activated carbon (C-AC). The adsorption data are well-fitted for the Langmuir isotherm.
Wen's research team studied the equilibrium, kinetics, and thermodynamics of AC adsorbent for the removal of nitrogen compounds and heterocyclic sulfur using light cycle oils, model diesel fuels, and shale oil. The nitrogen compounds (carbazole, indole, and quinoline) and the sulfur compounds (DBT and 4,6-DMDBT) in the diesel fraction were selected as model compounds. They observed that the adsorption of cyclic nitrogen compounds is favored by carbon and that total nitrogen compounds adsorbed by AC are higher than the sulfur compounds. The adsorption capacity breakthrough of nitrogen compounds follows the order quinoline > indole > carbazole. The isotherm followed Freundlich in the adsorption of DBT, indole, and quinoline on the AC surface but in contrast, Langmuir was the best fit in the adsorption of carbazole over the activated carbon surface (Wen et al., 2010).
ADS was studied by Seredych and co-workers with two commercial carbons and two synthetic, polymer-derived carbons, for the removal of 4,6-DMDBT and DBT from model diesel fuel having 20 ppm initial sulfur concentration. They observed that the polymer-derived carbons showed an excellent and higher adsorption capacity and selectivity than the commercial micro- and micro/mesoporous carbons. It was concluded that the acidic groups situated in larger pores played a crucial role in the adsorption affinities of DBT and 4,6-DMDBT through distinctive interactions (Seredych et al., 2009).
AC spheres derived from polystyrene (PACS) were produced via steam activation and were investigated for the adsorptive removal of DBT. The highest adsorption capacity obtained by PACS was 109.36 mg/g and was correlated with total pore volume, the volume of narrow micropores, and independent large surface area. The investigators added that there exists irreversible adsorption between PACS and DBT and that the sulfur may be incorporated into the carbon matrix or change to dimers or oligomers which cannot be removed easily from the carbon spheres (Wang et al., 2009).
A scientific study was conducted on an integrated adsorption process for ultra-clean sulfur removal from diesel fuel. AC fiber (ACF) was treated via an adsorption mechanism with traditionally hydrodesulfurized straight-run gas oil (HDS-SRGO) having<50 ppm sulfur. The sorbent ACF was employed primarily to remove the nitrogen and refractory sulfur species contained in straight-run gas oil SRGO to improve HDS reactivity over traditional CoMo catalysts. It was found that the integrated adsorption–reaction process employed in this technology made it possible for ACF to attain ultra-deep desulfurization of SRGO. ACF adsorbents used for desulfurization were regenerated with a traditional solvent and were confirmed efficient in bringing back their adsorption affinity (Sano et al., 2005).
ADS of 4,6-DMDBT and DBT from model diesel fuel was studied over a polymer-based carbon. A selectivity study was also investigated concerning DBT and 4,6-DMDBT relative to naphthalene. The result obtained was that selectivity and adsorption affinity for DBT and 4,6-DMDBT were hindered by the arrangement and content of heteroatoms but a modification of the adsorbent-surface which increased both oxygen and sulfur-containing groups enhanced adsorption capacity (Seredych and Bandosz, 2011).
Commercial AC prepared from a peanut-based precursor (AC-0) was employed for the removal of the sulfur-containing compound from gasoline and the other two composites were also derived from AC-0 through different silver loading labeled AC-1 AC-2 and AC-3. The reported adsorption capacity for AC-0, AC-1, and AC-3 follow the order 0.56 mg/g < 4.64 mg/g and 8.36 mg/g respectively. The activated carbon AC-3 has the lowest surface area of 830 m2/g with the highest adsorption capacity of 8.36 g/mg which was attributed to the possible formation of σ- and π-bonds between the impregnated silver particles and thiophenic ring of a sulfur compound. The normal σ-bonds could be formed by Ag+ cations through their empty s-orbitals while their d-orbitals could back-donate electron density to the antibonding (π*) orbitals of the TP rings. It was observed that a parallel relationship exists between the silver content of the AC and the adsorption capacity toward organosulfides. The higher the amount of silver loading the better the adsorption affinity towards sulfur compounds and AC-3 has the highest loading of silver content (Cao et al., 2008).
A study on ADS of sulfur-containing compound TP and DBT was reported using both commercial AC coconut-based precursor and a modified AC treated with 65% nitric acid (AC-H) in a batch system. A selectivity study was also conducted on the adsorbents with respect to benzene and 1-octene. The results obtained under the same operation conditions revealed that untreated AC carbon showed a higher adsorption capacity of 93.9 % for DBT than modified AC-H which gave 45.9 % adsorption. The better adsorption capacity breakthrough for virgin AC was linked to the molecular size of DBT which was relatively easy to be removed by the as-received AC via adsorption as well as the strong interactions between DBT and AC owing to electron-donating features of benzyl groups on the pentagonal rings. On the contrary, AC-Hs were observed to adsorb more TP which was linked not only to the abundance of surface oxygen instilled onto the carbon surfaces vial HNO3 treatment but also due to the increased hydrophilicity of modified AC–Hs which promotes strong interaction between the carbon materials and the thiophenic molecules. The regenerative power of the adsorbent materials was not provided but competitive adsorption was observed during the selectivity examination which reduced the adsorption capacity for DBT to 80% over the untreated AC (Yu et al., 2008).
Brazilian commercial AC derived from three different precursors pine wood, coconut shell, and babassu (Attalea speciosa) were investigated for the adsorptive removal of sulfur and nitrogen compounds from commercial diesel fuel AS500 samples. To understand the influence of surface chemistry modification on AC, the investigated AC samples were modified with three inorganic acids (HNO3, H2SO4, and HCl) and impregnated with palladium chloride. AC prepared from coconut shell (CAC) has the highest surface area of 784 m2/g and was observed to show the best adsorption capacity towards sulfur and nitrogen removal. The result obtained showed that surface acidity and textural characteristics are functions of adsorption capacity with the best adsorption efficiency of over 60 % recorded for AC modified with palladium chloride. The result obtained for sulfur removal follows the order pure CAC < CAC oxidized H2SO4 < CAC oxidized HCl < CAC oxidized HNO3 < Pd/CAC corresponding to 25.68 %, 26.47 %, 26.86 %, 31.71 %, and 64.70 % respectively. There is no report on the potential regeneration of the spent adsorbents (Lopes et al., 2016).
Shi et el. et al prepared a sucrose-based AC using hydrothermal carbonization and the resultant solid (char) was activated using potassium hydroxide KOH. The five different adsorbents prepared were denoted as char, C-0.5, C-1, C-2, and C-4 which correspond to the resulting char to KOH mass ratio of 1:0.5, 1:1, 1:2, or 1:4 respectively. The surface area of these four composites ranges between 3–2217 m2/g with the char and C-4 having the lowest and the highest surface area respectively. ADS of as-prepared adsorbents for the removal of thiophenic compounds revealed that sucrose-derived activated carbons are an excellent candidate for potential industrial applications. Adsorbents C-2 gave the maximum adsorption capacity of 41.5 mgS/g for 300 ppmwS model oil, excellent selectivity, and a fast adsorption rate (97% saturated within 5 min). This enhanced adsorption performance was attributed to several oxygenated groups present on the carbon surface, desirable mesopores, and abundant small micropores. Activated sucrose-derived carbon (C-2) was regenerated and found to show consistent sulfur removal after three runs. Fig. 9 shows the scanning electron microscope (SEM) images of the studied adsorbent as well as their percentage yield and elemental content. It should be mentioned that the percentage yield based on sucrose declined from 15.1% to 9.0 % when the char/potassium hydroxide ratio was increased from 0.5 to 4. This decrease was ascribed to the burn-off caused by more severe framework etching Shi et al., 2015.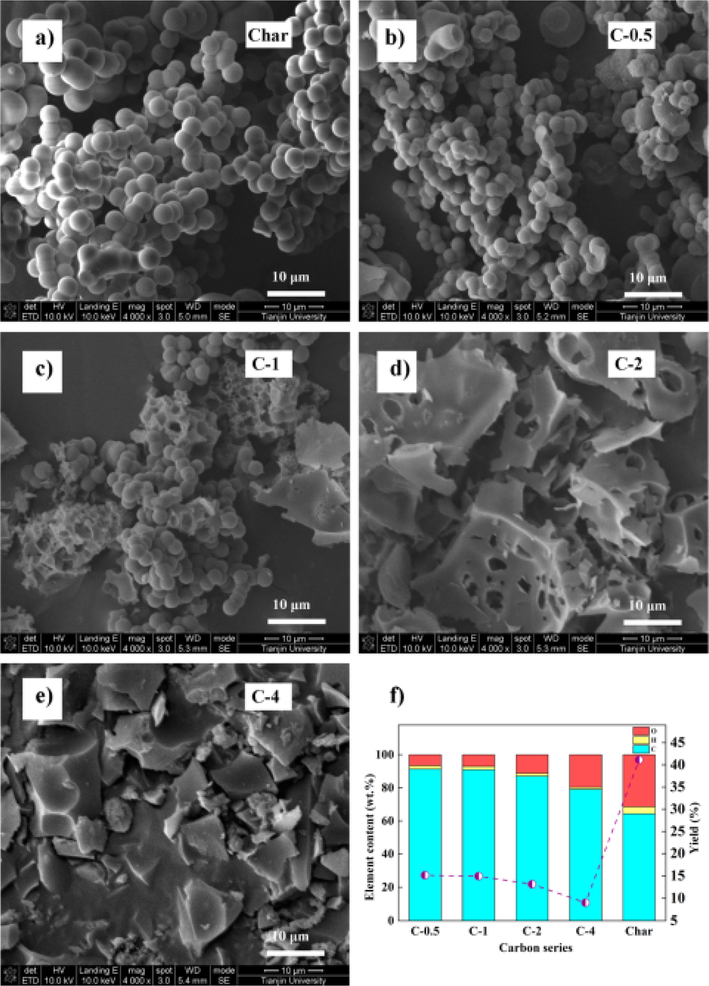
SEM images of as-synthesized adsorbents (a-e) and percentage yield and ultimate analysis of the carbon samples (f) (Shi et al., 2015).
5.2 Functionalized activated carbon
Salem and Hamid used AC, zeolite 13X, and zeolite 5A as solid adsorbents to investigate adsorptive desulfurization for the removal of sulfur from naphtha with which an initial sulfur level of 550 ppm was used. Activated carbon was reported as the least-performing adsorbent towards sulfur content removal but showed the highest capacity. Zeolite 13X was observed to be effective and superior towards sulfur removal at a very minimal concentration range yet at room temperature conditions. In light of the result obtained, a two-bed combination was proposed for an industrial application in which the first bed is loaded with AC which gives 65% sulfur removal at 80 °C and the second bed contains zeolite 13X that achieves complete removal of sulfur provided the sorbent to feed ratio is about 800 gL-1 at room temperature. Information on the regeneration of the adsorbent was not presented (Salem, 1994).
Danmaliki and Saleh studied adsorptive desulfurization using AC synthesized from waste rubber tires which were subsequently loaded with cerium and iron. In this study, the three composites prepared were named AC/Ce, AC/Fe, and AC/Ce/Fe and they were examined for the removal of organosulfur compounds of TP, BT, and DBT in a simulated fuel. They found out that the unmodified AC not only has the highest surface oxygen-containing group but also possesses the highest surface area and pore volume of 460.27 m2/g and 0.71 cm3/g respectively. But despite this promising feature of textural properties, it was observed to be the least performing adsorbent towards the examined sulfur-containing compounds with the adsorbent’s performance following the order of AC/Ce/Fe > AC/Ce > AC/Fe > AC. The best-performing sorbent AC/Ce/Fe showed adsorptive performance of DBT (75%), BT (30%), and TP (31%) which are attributed to the crystalline nature of iron and the acidic nature of cerium (Danmaliki and Saleh, 2017). The regeneration of the used adsorbent was conducted by a thermal method where the percentage adsorption of sulfur-containing compounds remains at a constant level after three cycles using the best-performing adsorbent AC/Ce/Fe. Fig. 10 elucidates the methods of preparation of AC/Ce/Fe composite.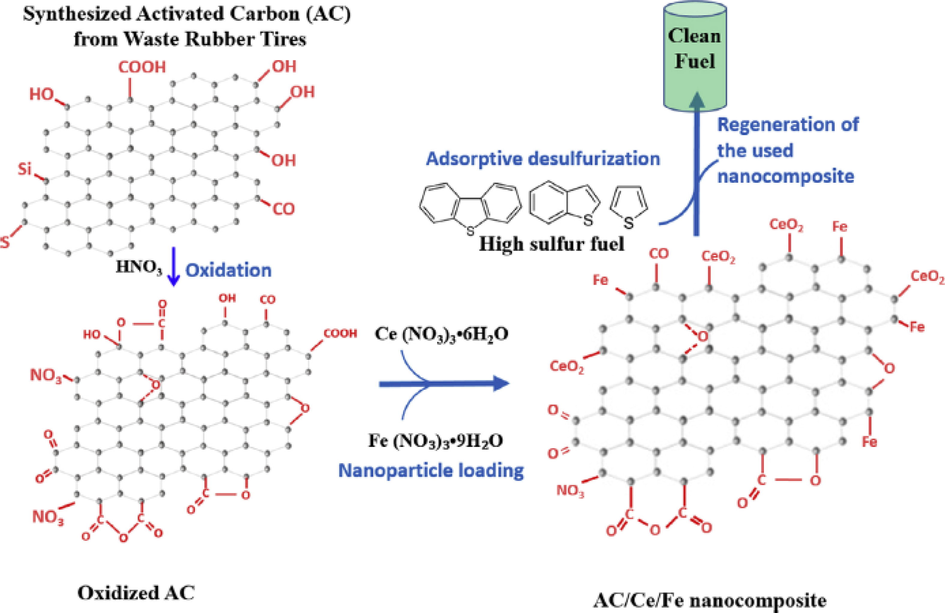
An illustration of the synthesis procedure of bimetallic cerium and iron AC composite (AC/Ce/Fe) (Danmaliki and Saleh, 2017).
Silver nanoparticles modified AC derived from Brewer’s spent grains was used for the application of ADS of model oil. The composites experimented with were AgNPscw/AC, AgNPskp/AC, and ACB where each represents AC modified with silver nanoparticles synthesized from cobwebs, AC modified with silver nanoparticles produced from Cola nitida plant, and AC from Brewer’s spent grains respectively. Adsorption capacity is of the order of AgNPscw/AC > AgNPskp/AC > ACB with a removal efficiency of 29.8, 25.7, and 13.9 mg DBT/gAds respectively at 298 K. The adsorption efficiency of the two best-performing composites was attributed to the loading of a weak Lewis acid Ag(1) ion that enhanced the additional adsorption site (Olajire et al., 2017). The scheme showing the synthesis procedure of AgNPscw/AC and AgNPskp/AC is elucidated in Fig. 11.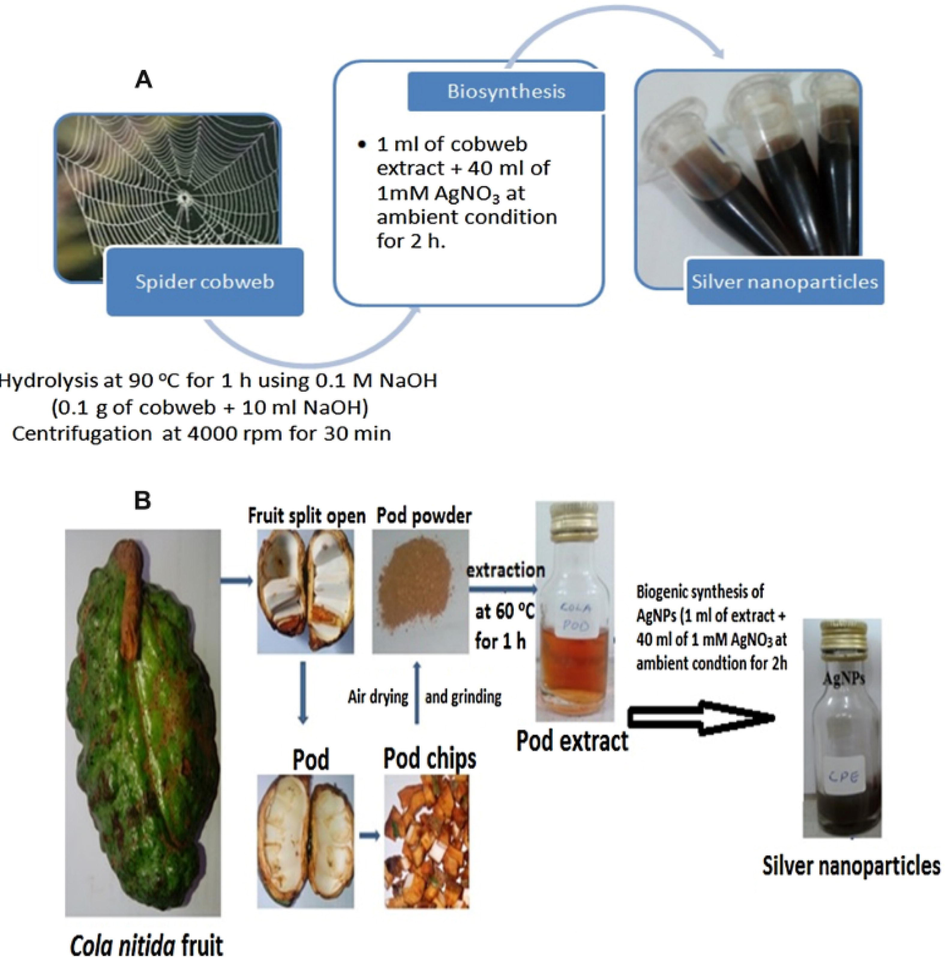
Schematic representation for the preparation of silver nanoparticles from cobweb (A) and Cola nitida fruit (B) (Olajire et al., 2017).
In a similar adsorptive desulfurization study by selective adsorption of thiophenic molecules, the carbon-based adsorbent was employed for the removal of organosulfur in model jet fuel. The sorbents investigated are pure AC and activated carbon impregnated with metal halides to give palladium-activated carbon Pd/AC, PdCl2/AC, and CuCl/AC. The pure commercial AC used has the highest surface area of 998 m2/g. It was reported that PdCl2 displayed an excellent selective sulfur adsorption affinity with the highest capacity for desulfurization in agreement with the molecular orbital results and was superior to that of CuCl and Pd0. The investigators discovered that in removing organosulfur compounds like MDBT and BT, AC remains the best support for π-complexation sorbents. PdCl2/AC has the highest capacity for the removal of organosulfur compounds in jet fuel and this was ascribed to cooperative adsorption explained by a geometric effect due to synergy between the supported π-complexation sorbents and the carbon substrate (Wang and Yang, 2007).
Ganiyu et al. reported the use of AC derived from lignocellulosic biomass of groundnut shell (GN-AC) for selective adsorption desulfurization. The AC was doped with melamine as nitrogen precursor which was subsequently functionalized with nickel nanoparticles precursor to enhance surface chemistry. This functionalization of AC gave rise to a reticulated morphology which contributed significantly to the adsorption capacity breakthrough for DBT. The three composites experimented with were denoted as AC, CN, and Ni-CN representing bare activated carbon, nitrogen-doped carbon, and nickel nanoparticles nitrogen-doped carbon respectively. Their sulfur removal efficiencies are 29.63, 40.49, and 44.79 mg-S/g-adsorbent respectively in 500 ppmw-S. The highest performance recorded for Ni-CN was attributed to the improved surface chemistry, rewarding textural features, and the synergy effect of nickel nanoparticles and nitrogen heteroatom. The best-performing adsorbent Ni-CN showed excellent selectivity with respect to aromatic naphthalene and regeneration potential for industrial viability. The scanning electron micrograph of the studied adsorbent is shown in Fig. 12 and the representative particle size and dispersion analysis by transmission electron microscope (TEM) for the examined adsorbents are displayed in Fig. 13. It could be seen in the TEM image of the best performing adsorbent Ni-CN that some of the vacant voids in CN have been filled by nickel nanoparticles which were observed to agree with the surface area and pore volume result of Ni-CN adsorbent. Also, Fig. 13 (d) shows the excellent dispersion of NiO nanoparticles on the CN (Ganiyu et al., 2020).
SEM images of adsorbent C, CN, and Ni-CN (Ganiyu et al., 2020).
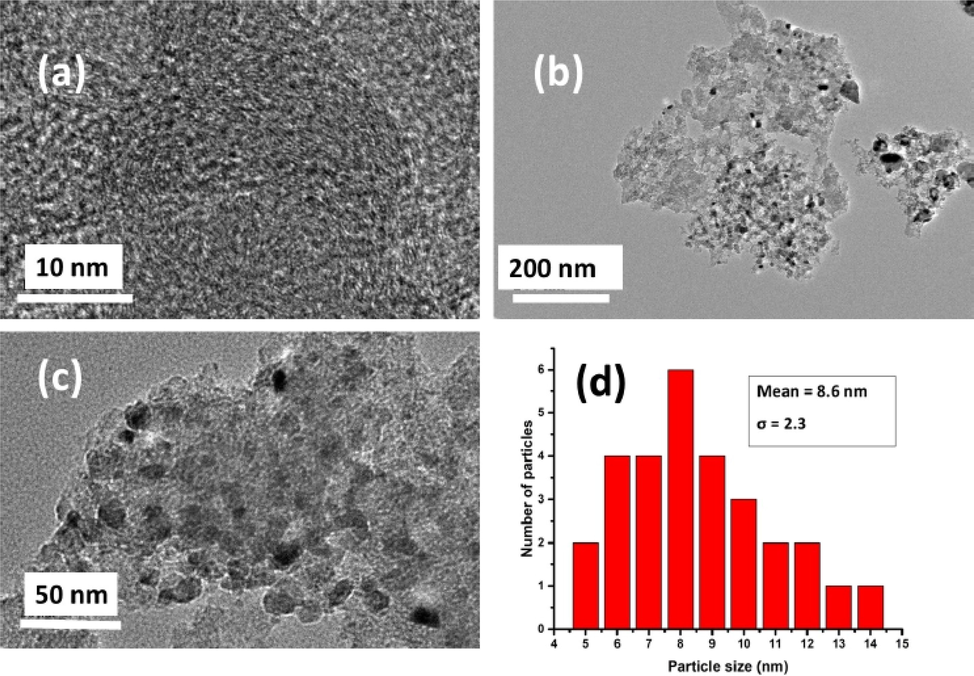
TEM images of (a) CN; (b) Ni-CN at 200 nm; (c) Ni-CN at 50 nm and (d) particle size distribution (Ganiyu et al., 2020).
Saleh 2017. (Saleh, 2018) published an article on ADS for the removal of sulfur-containing compounds namely; 4,6-DMDBT, 4-methyl dibenzothiophene (MDBT), 5-methyl-1-benzothiophene (MBT), DBT, BT, and TP using activated carbon incorporated with cobalt and copper nanoparticles in a batch and fixed-bed systems. Three composite Co/AC, Cu/AC, and CoCu/AC were prepared, and CoCu/AC was found to be the best-performing sorbent as it showed simultaneous removal of organosulfur compounds in the order of DBT > MDBT greater than 4,6-DMDBT > MBT > BT > T. It was concluded that direct sulfur-metal interactions and π-complexation mechanism could rationalize adsorption capacity trend and that the highest removal achieved for DBT could be as a result of a high level of interactions that existed between CoCu/AC and the delocalized π-electrons in the DBTs. The best-performing adsorbent was also investigated in a fixed-bed system Fig. 14 in which the reactor (laboratory scale) used comprised of a valve, a feed tank, a collecting tank, a low-flow liquid rotary piston pump, and a custom-made column backed with the material. The investigated organosulfur compounds were made to pass through the reactor in an up-flow direction and the effluent was periodically sampled. Subsequently, the eluted sulfur concentrations were evaluated by gas chromatography sulfur chemiluminescence detector (GC-SCD).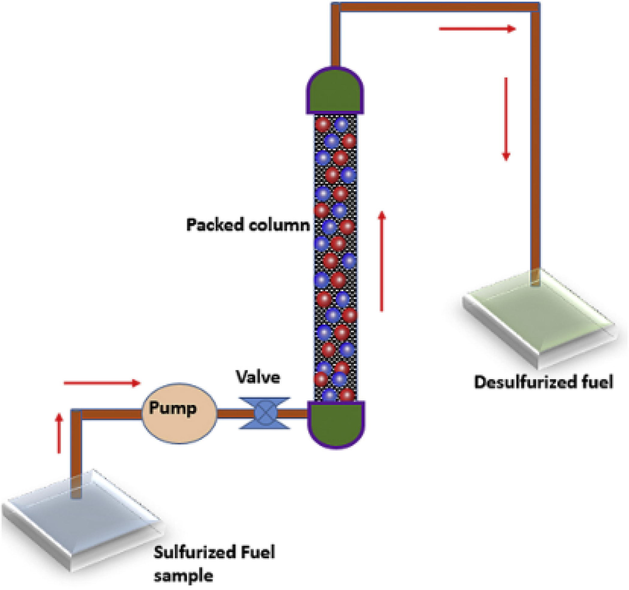
Diagrammatic experimental setup flowcharts of a fixed-bed system for adsorptive desulfurization (Saleh, 2018).
Carbon-doped porous boron nitride (C-BN) was investigated for the removal of sulfur compounds by Xiong and co-workers (Xiong et al., 2015). Metal-free porous C-BN showed higher adsorption efficiency for DBT than pure BN materials owing to the stronger Lewis acid-base interactions between carbon-doped porous BN and DBT as well as the significant exposure of more atoms along the edges of the pores. They reported that adsorption capacity of 35.2 mgSg−1 adsorbent for 500 ppm sulfur model oil for C-BN. It was also found that the porous material C-BN-0.005 was also selective towards DBT removal in the presence of 10 wt% (naphthalene and para-xylene). A comparison study of C-BN-0.005 with activated carbon, Cu(I)–Y zeolite, and Al2O3 was also investigated for DBT removal. It was found that the adsorption capacity of C-BN- 0.005 was the highest followed by AC (Fig. 15).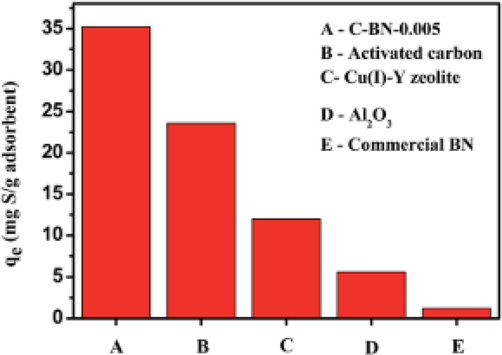
Comparison of the adsorption capacities with respect to C-BN-0.005 at working conditions: initial concentration of 500 ppmw-S, adsorbent weight = 0.05 g, V (oil) = 20 mL, 298 K, atmospheric pressure, contact time = 180 min (Xiong et al., 2015).
Nazal et al. (Nazal et al., 2015) conducted scientific research on ADS of DBT using several adsorbents namely; synthesized graphene oxide (GO), multiwall carbon nanotubes (CNT), and commercial coconut-activated carbon (AC). To enhance the chemical properties of adsorbents surfaces, 5% and 10.9% aluminum (Al) in the form of aluminum oxide (Al2O3) were loaded on each adsorbent and are designated as AC, ACAL10, ACAL5, CNT, CNTAL10, CNTAL5, GO, GOAL10, GOAL5. The introduction of Lewis acid (Al2O3), on sorbents, was said to have increased adsorption breakthrough capacities twofold due to the creation of an additional adsorption site. ACAL5 gave the highest adsorption efficiency of 85 mg/g for DBT. ACAL5 and CNTAL5 were used to investigate the selectivity study of DBT relative to TP as well as DBT relative to naphthalene. A relatively high selectivity factor of 54 mg/g was recorded for naphthalene using loaded AC and they concluded that modification of surface chemistry of the adsorbent AC, CNT, and GO with the Lewis acid (Al2O3), enhanced adsorption capacity towards DBT and improved selectivity towards competitive aromatics.
Aina and Bandosz reported sulfur content removal over activated carbon produced from various precursors (wood, coal, and waste) using adsorptive desulfurization technology. The importance of the structure and surface heterogeneity of AC was examined. Ammonium persulfate was oxidized to modify and augment carbon surface heterogeneity. The results obtained showed a parallel correlation between the AC and the surface chemistry for the adsorption of DBT from the liquid phase. The conclusion drawn was that surface chemistry modification which introduced surface functional groups promoted AC performance-specific interactions between the DBT molecule basic structure, sulfur-sulfur interactions, and the acidic centers of the carbon (Ania and Bandosz, 2005).
Xiong et al. (Xiong et al., 2010) prepared and characterized zirconium dioxide-modified activated carbon (ZrO2/AC) as a novel adsorbent and applied it for the adsorptive removal of refractory sulfur compound DBT from simulated diesel fuel. The Pure AC was treated thermally in distilled water and is denoted as PAC. This PAC was impregnated with varying amounts of ZrOCl2·8H2O solutions and the best-performing adsorbent denoted 1Zr/AC was treated with 0.01 M of the said solution. The adsorption capacity of ZrO2/AC was found to outperform bare AC by a magnitude of 17%.
A desulfurization experiment was conducted using synthesized AC loaded with cerium to remove the recalcitrant sulfur compound from model fuel (dissolution of DBT in petroleum ether). The three composites investigated are the AC, AC/Ce, and ACO corresponding to commercial activated carbon (coconut shell precursor) with impregnation, cerium-loaded activated carbon, and activated carbon without impregnation respectively. The three adsorbents showed adsorption capacity for sulfur-containing compounds in the order of 8.15 mg-S/g-A, 7.62 mg-S/g- 7.02 mg-S/g-A for Ce/AC(0.15), ACO, and AC respectively. This best-performing adsorbent was attributed to the new adsorption site introduced by the loading of cerium into the pure carbon. It was also noticed that, at higher cerium loading, the adsorption capacity breakthrough for Ce/AC decrease infinitesimally to 7.86 mg-S/g-A which was ascribed to pore volume reduction or perhaps pore blockage (Xiong et al., 2012).
Adsorption of DBT and 4,6-DMDBT from model diesel fuel was studied using polymer-derived AC loaded with nickel, silver, and cobalt species labeled as C-Ni, C-Co, and C-Ag respectively. The selectivity studied of the two organosulfur compounds was investigated with respect to aromatic naphthalene. The obtained result in percentage for adsorption affinity of DBT and 4,6-DMDBT are of the order 4.70 < 4.76 < 5.99 for C-Ag, C-Ni, and C-Co respectively. The three adsorbents investigated also remove organosulfur compounds selectively from model fuels with C-Co again displaying the highest adsorption capacity. The metal loading contributes significantly to the increased number of active sites that enhanced sulfur compounds' adsorption. The highest adsorption capacity of C-Co was ascribed to the availability of cobalt species in the mesopores of carbon which are filled first by the adsorbates and increase the uptake of sulfur compounds with higher surface coverage (Seredych and Bandosz, 2009).
Bandosz reported the usage of polystyrene (PS) as the precursor for AC synthesis for DBT removal in model oil. The - polystyrene-based AC was loaded with copper Cu, cobalt Co, silver Ag, and sodium Na metal. The metal content was properly controlled by selective washing. The result obtained from the metal-loaded PS based-adsorbents was compared with that of unmodified commercial ACs. PS-Cu and PS-Co displayed the highest adsorption capacity and the percentage of DBT adsorbed is 67 % and 55 % respectively. PS-Cu has the second lowest surface area of 566 m2/g after PS-Ag of 461 m2/g but yet showed the highest DBT uptake which was attributed to the tendency of copper to act as oxygen activator and enhance C-S bond cleavage in the thiophenic compound. Although explicit details to understand the mechanism of DBT adsorption by PS-Cu proved elusive and no information was reported on the recycling ability of the spent adsorbent. The selectivity of the adsorbents was studied for naphthalene and the outstanding performance of PS-Cu was again attributed to the presence of copper species and the availability of sulfur compound (sulfonic moiety) in the structure of carbon-based materials that facilitate sulfur-sulfur specific interaction thus resulting in the impressive DBT uptake (Ania and Bandosz, 2006).
The research was conducted to study the effect of bare AC and AC loaded by impregnation method with different metal ions on the activation energy for DBT desorption. The metals employed for this investigation are transition metals silver, zinc, copper, iron, and silver. Desorption activation energy, Ed, of DBT on the AC surfaces was estimated accordingly concerning temperature-programmed desorption (TPD) experiments that were conducted and subsequently used to measure the TPD curves of DBT on these modified ACs. The order of activation energy for DBT desorption follows: Fe(III)/AC < Cu(II)/AC < Ni(II)/AC < Zn(II)/AC < Ag(I)/AC. The desorption capacity of the best-performing adsorbent as compared to non-modified commercial AC was attributed to the loading of Ag+ on carbon surfaces which influenced chemical interaction between Ag(I)/AC surface and DBT, knowing well enough that Ag+ and DBT were soft acids and soft base respectively. The least performing adsorbent Fe(III)/AC was ascribed to the loading of Fe3+ which mitigate an excellent interaction between the carbon surfaces and DBT because Fe3+ was hard acid. The DBT desorption was enhanced to a certain extent when the borderline acid ion Zn2+, Ni2+, or Cu2 were loaded on the AC surfaces (Yu et al., 2007).
Yu et al. (Yu et al., 2010) investigated the effect of transition metal nickel, copper, or cobalt nitrate or chloride modification on activated carbon (AC 310) by impregnation method and examined its applicability for DBT removal in model oil. The result obtained revealed that calcination plays a pivotal role in the control of the metal species loaded on the carbon surface. Higher DBT uptake was recorded for the chlorides as compared to nitrates in all the three studied metal-loaded adsorbents, wherein CoCl2-modified activated carbon displayed the best adsorption capacity of 36.25 mgS/g which was attributed to adsorption by π-complexation. Among the calcined metal-modified ACs investigated, Ni(NO3)2 has the highest adsorption capacity breakthrough which was ascribed to the excessive availability of oxygen contacting functional groups on carbon surfaces resulting from nitrate ions decomposition after calcination under the influence of nitrogen gas.
Ganiyu et al. in their work investigated the influence of surface chemistry modifications on commercial AC by using varying amounts of aluminum (Al) concentration in the range of 0.5–10 wt.% and applied it for adsorption desulfurization. The simulated model oil employed comprises DBT dissolved in isooctane. It was observed that Al-loaded AC showed a better adsorption affinity towards DBT than the unmodified AC which led to their conclusion that textural properties and surface acidity have a parallel correlation with the adsorption capacity. Virgin carbon has a BET surface area of 1108 m2/g which is infinitely lesser than the surface area of 0.5 wt.% Al-AC having 1147 m2/g. At higher loading of aluminum beyond 0.5 wt.%, the surface area reduced significantly and was ascribed to the probable pore blockage of the activated carbon material. 2.5 wt.% Al-AC showed the best adsorption capacity breakthrough (14.19 mg/g at 200 ppm-S) which was attributed to its striking balance of micropore and mesopore as evidenced in the HF values in Fig. 16. The former harbor the acid sites, while the latter allows large sulfur molecules to transit easily. It should be mentioned that hierarchical factor HF can be represented mathematically from the result obtained from N2 physisorption analysis of the material sample as HF = (Vmicro / V total) * (Smeso/SBET). The 2.5 wt% Al-incorporated AC was regenerated using a solvothermal approach which showed no significant loss in the adsorption capacity after three cycles. It was observed from the post-activity analysis that regeneration of the used adsorbent restored its textural surface chemistry properties (Ganiyu et al., 2016).
(a) N2 adsorption–desorption (b) HF-factor derived from the physicochemical parameters of the adsorbent investigated (Ganiyu et al., 2016).
Saleh and co-workers studied the adsorptive desulfurization of sulfur-containing compounds using AC synthesized from waste rubber tires. Three composites were prepared through pure AC modifications by solid-state impregnation of cobalt and molybdenum nanoparticles. The composites are AC, AC/Co, AC/Mo, and AC/CoMo. The loading of bimetallic cobalt and molybdenum on AC enhanced not only the textural features such that it has the highest surface area but also the surface acidity of AC resulting in better adsorption capacity in comparison with other investigated adsorbents. AC/CoMo displayed the highest adsorption capacity of 90 % at an adsorbent dosage of 0.25 g which was attributed to the promising textural characteristics attained through bimetallic impregnation. The removal efficiency of bimetallic AC/CoMo follows the order of DMDBT > MDBT > MBT > DBT > thiophene as shown in Fig. 17.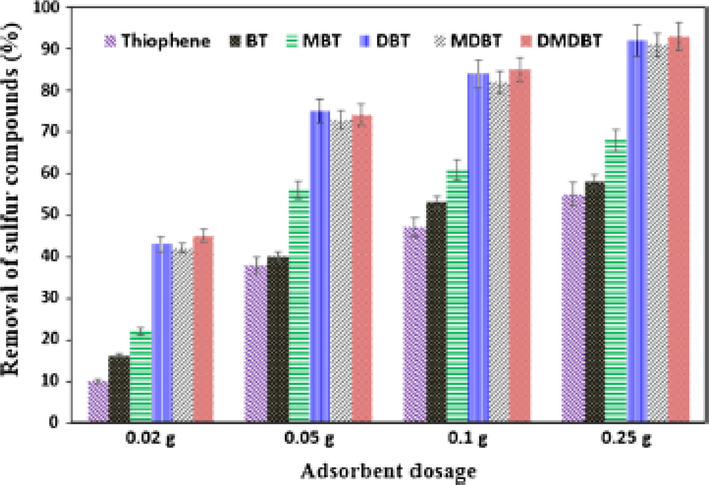
The effect of 5 wt% AC/CoMo as a function of the percentage of sulfur adsorbed (Saleh et al., 2018).
6 Regeneration of spent adsorbent
It is a tenable fact that the disposal of spent activated carbon (SAC) used for desulfurization applications seems to pose a detrimental impact on the environment owing to the amount of sulfur being released consistently into the environment. Regeneration of used adsorbent will not only conserve natural resources but also reduce secondary pollution (Guo and Du, 2012). In addition, the necessity of procuring a virgin activated carbon or perhaps the laborious processes surrounding the synthesis of a fresh AC (especially for industrial applications) due to the surface area and adsorption sites of SAC blockage is not economically viable and labor intensive. Thus, it is highly essential to study the regeneration potential and the reusability of a spent adsorbent. In a simple form, regeneration is a process of removing adsorbate adsorbed onto the SAC surface, restoring the adsorption site and surface area via desorption and/or decomposition (Nasruddin et al., 2018). In the year 2012, the disposed SAC in Malaysia is 1405.59 tones. To control the SAC environmental adverse effect, the concerned authorities in Malaysia set out a fine of RM 2, 790 per tonne for disposal cost for SAC at the designated waste location (Nasruddin et al., 2018). Researchers across the globe have investigated several regeneration techniques of SAC which include chemical regeneration (Li et al., 2015; Nahm et al., 2012), thermal regeneration (Danmaliki and Saleh, 2017; Duan et al., 2014; Ganiyu et al., 2020, 2016; Nahm et al., 2012), microbiological regeneration (Ha et al., 2000; Salvador et al., 2015), electrochemical regeneration (Narbaitz and McEwen, 2012; Wang and Balasubramanian, 2009), microwave-assisted regeneration (Li et al., 2008; Xia et al., 2014; Yuen and Hameed, 2009), vacuum stripping regeneration (Salvador et al., 2015) etcetera. It should be mentioned that microbiological regeneration involves two major important concepts biodegradation and bioregeneration. The formal involves the degradation of compounds that form the alimentary substrate of microorganisms while the latter involves a restoration of the adsorption capacity of activated carbons via the biodegradation of organic molecules trapped on the carbon surface (Chudyk and Snoeylnk, 1984; Salvador et al., 2015). However, the microbiological regeneration approach remains uncommon for industrial applications due to its long time requirement (Nasruddin et al., 2018). Vacuum regeneration is not feasible for industrial applications and has not gained interesting attention from investigators. Thermal and chemical regeneration are the most popular and essential regeneration types for carbonaceous materials. Traditional thermal regeneration is gaining increasing interest for ACs regeneration, and it is the most practicable method in the industry. It involves the use of a reasonable amount of energy to decompose the retained adsorbate on the saturated activated carbon(Salvador et al., 2015). For illustration, Nazal et al. (Nazal et al., 2015) thermally regenerated aluminum-loaded (ACAL5) and aluminum loaded carbon nanotube (CNTAL5) and investigated them for the DBT removal to establish their reusability efficiency and the results obtained after the five successive adsorption–desorption cycles (Fig. 18.) showed that the experimental errors in both cases are almost constant.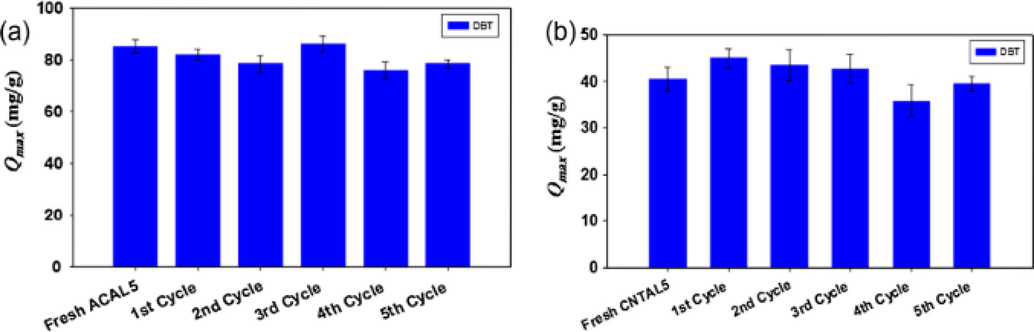
Regeneration potential of (a) ACAL5 adsorbent and (b) CNTAL5 adsorbent (Nazal et al., 2015).
Xiong et al. (Xiong et al., 2010) hydrothermally regenerated zirconium dioxide-modified activated carbon denoted as 1Zr/AC after it has been used to remove DBT from model fuel and was found to display a higher adsorptive performance than unmodified PAC after recycling three times (Fig. 19.) which was ascribed to the possibility of no significant loss of ZrO2 during the solvent-washing process. The recycling potential of 1Zr/AC could also be a result of the insolubility of ZrO2 in the solvent, simulated oil, and the bond formation (Zr-O-C) between the carbon matrix and ZrO2 during hydrothermal treatment. They concluded that surface acidic site impregnated ZrO2 could be responsible for the enhanced adsorption capacity in the desulfurization process.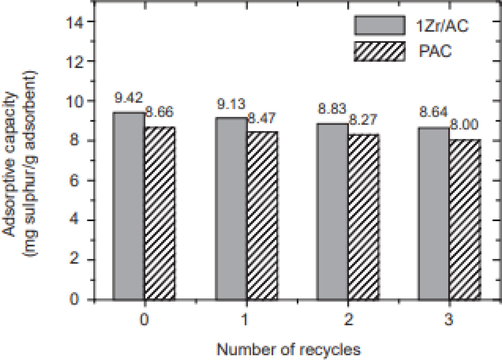
Regeneration ability of spent adsorbent PAC and 1Zr/AC at 300C, adsorbent dosage = 20 mg and oil volume of 0.5 (Xiong et al., 2010).
The major bottlenecks for thermal regeneration are that it is non-economical due to high energy requirements, time-consuming, and a larger amount of spent adsorbent required to attain economic attractiveness. In addition, during the thermal regeneration process, carbonaceous materials experience carbon loss, and deterioration in the adsorbent’s pore structures due to longer time and higher temperature thus limiting the reusability potential of the ACs (Ania et al., 2007). Chemical regeneration is a common alternative to conventional thermal regeneration owing to its attractive economic viability and it includes regeneration with wet air, HCl, NaOH, solvent, and liquid water still encompassing electrochemical regeneration, supercritical regeneration, oxidative regeneration, and pH changes. Xiong et al (Xiong et al., 2012) regenerated the spent adsorbent cerium-modified activated carbon (CeAC) employed for removing DBT from model fuel via a solvent-washing approach and reused in desulfurization three times as shown in Fig. 20. In comparison, the novel sorbent Ce/AC exhibited higher adsorption selectivity and capacity for DBT than that of a virgin carbon which was attributed primarily to the enhanced active site created through surface chemistry modification (Xiong et al., 2012). Fig 21 showed the versatility of chemical regeneration strategies.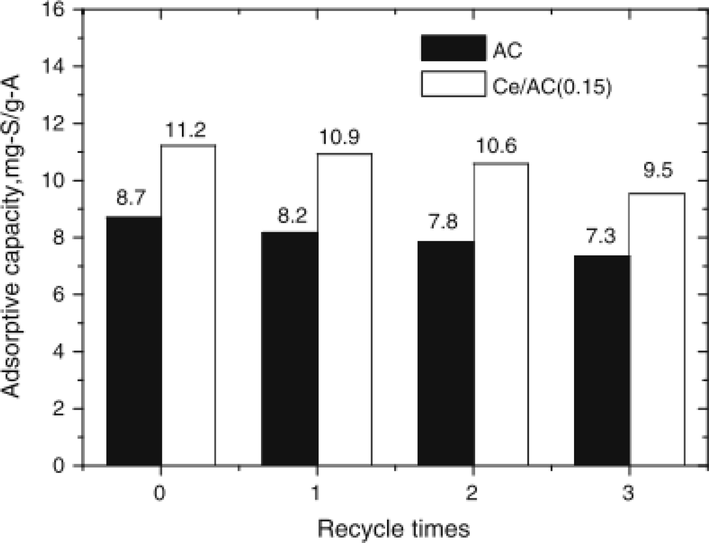
The regeneration potential of Ce/AC(0.15) and AC at 30 °C, adsorbent dosage = 0.5 g, and oil volume = 20 mL (Xiong et al., 2012).
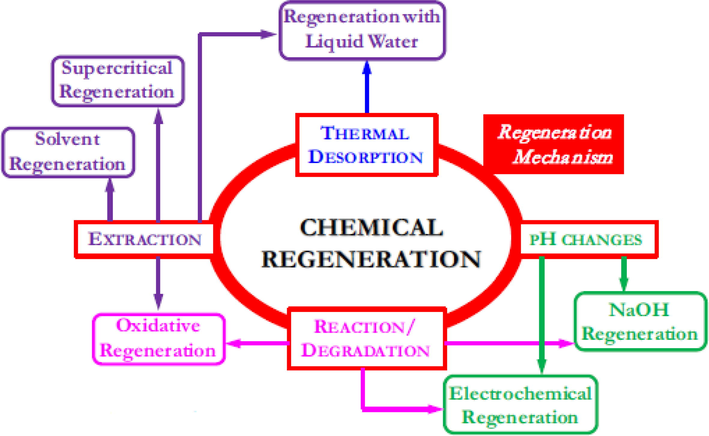
Schematic representation of carbonaceous materials regeneration using chemical methods (Salvador et al., 2015).
Chemical regeneration is accompanied by minimal carbon loss. The main drawbacks of chemical regeneration are embedded in its usage of large volumes of chemistry reagents and its eco-friendly way of disposing of the reagents which is very crucial. Microwave regeneration is an efficient, eco-friendly method owing to its clean form of energy. Its advantages over traditional approaches include fast heating, selective heating, small thermal inertial, easy automatic control, and all together lead to low treatment time, reduced pollution, and energy saving process (Thostenson and Chou, 1999; Xia et al., 2014). SAC has shown rewarding results with the use of microwave-assisted regeneration technology (Ania et al., 2004; Xin-hui et al., 2012).
7 Future directions and conclusion
It is an indisputable fact that crude oil is the world’s primary energy source, and the higher energy demand is a result of a persistent increase in the world’s population. Thus, energy consumption proliferation culminates in the release of pollutants into the environment of which a number of these dangerous substances pose a severe threat to mankind and an ideal environment. The multiplying effect of industrialization and urbanization have gravitated the interest of researchers across the globe to devise technologies adapted to the removal of these harmful pollutants. A notable pollutant with a serious environmental concern is sulfur content present in fossil fuels which is liberated into the environment during fuel combustion. The necessity to maintain an eco-friendly environment gives birth to various desulfurization technologies including conventional HDS, ODS, ILS, BDS, and ADS. Adsorptive desulfurization has gained increasing interest and has been established as an efficient option for the removal of sulfur-containing compounds present in transportation fuels. Also, it is a tenable fact that ADS is a complementary technology to the HDS and an alternative pretreatment in the traditional hydrotreatment process. In light of this, several sorbent materials have been investigated for the ADS process such that activated carbons have shown promising results. In this review, AC synthesized from a variety of lignocellulosic biomass has been discoursed and discussed. AC are usually microporous materials thus limiting their application to reactions involving small molecules such as methanol production from CO2 and hydrogen. Recent applications of activated carbon require a higher percentage of mesoporosity to facilitate the diffusion of larger molecules and ions that are too big to enter micropores into mesopores to attain effective adsorption efficiency. Thus, rewarding efforts have been directed towards the creation of mesoporosity into AC for expanded applications. Typically, locally available carbonaceous materials are good substitutes for the relatively expensive commercial activated carbons as rewarding results have been achieved with carbon-based agricultural residues. The enhancement of adsorption capacity for thiophenic compounds is a function of textural properties, surface oxygen, and surface acidity. The factors responsible for adsorption capacity in pure carbon are majorly attributed to the abundance of surface oxygen and desirable textural characteristics. The surface acidity of the carbon-based materials has been increased with the incorporation of metals to positively enhanced adsorption efficiency. Suffice it to say that an enhanced adsorption performance recorded for modified AC is mainly a function of increased surface acidity among others. The metal ions such as Zn2+, Cu2+, Ag2+, Ce2+, Fe2+, Na+ Zr4+, Pd2+, Co2+, Ni2+, Mo2+ and so on are loaded on activated carbon. It is also established from this work that the adsorption capacity of ACs can be enhanced via the synthesis method; by controlling conditions of activation and employing a suitable activating agent. Meanwhile, aromatic compounds such as pyridine and its derivatives, arenes, toluene, naphthalene, etc. usually coexist in middle distillate oil and have a significant tendency to interfere with and cause a reduction in the adsorption capacity breakthrough of sulfur-containing organic compounds. Considering this, the competitive effect of these aromatics in real fuels needs further investigation. ACs should be prepared and modified in such a way that it is very selective towards targeted organosulfur compounds as drastic reduction has been recorded for selectivity investigations. However, microwave-assisted regeneration technology should be thoroughly exploited due to its salient characteristics. It is therefore undoubtedly comprehensible that activated carbon-agricultural residues synthesis, optimization and applicability for ultra-deep desulfurization is feasible, promising, and worth further studying to prove its values for industrial applications.
Author contributions
Musa Azeez and Saheed Ganiyu both prepared and proofread the review article to the final stage.
Acknowledgment
The authors acknowledged the support from King Fahd University of Petroleum & Minerals (KFUPM) through interdisciplinary research support from the Center of Refining and Advanced Chemicals with project No. INRC2210.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdulrasheed, A.A., Jalil, A.A., Triwahyono, S., Zaini, M.A.A., Gambo, Y., Ibrahim, M., 2018. Surface modi fi cation of activated carbon for adsorption of SO 2 and NO X : A review of existing and emerging technologies 94, 1067–1085. https://doi.org/10.1016/j.rser.2018.07.011.
- A review of carbon-supported hydrodesulfurization catalysts. Fuel Process. Technol.. 1989;22:107-133.
- [CrossRef] [Google Scholar]
- Comparative analysis of adsorptive desulphurization of crude oil by manganese dioxide and zinc oxide. Res. J. Chem. Sci.. 2012;2:14-20.
- [Google Scholar]
- Agency, P., 2000. Biodesulfurization and the upgrading of petroleum distillates Daniel J Monticello 540–546.
- Ahmad, W., 2015. Sulfur in Petroleum 1–52. https://doi.org/10.4018/978-1-4666-9545-0.ch001.
- Adsorptive desulfurization and denitrogenation using metal-organic frameworks. J. Hazard. Mater.. 2016;301:259-276.
- [CrossRef] [Google Scholar]
- Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment : A review. Renew. Sustain. Energy Rev.. 2017;79:255-273.
- [CrossRef] [Google Scholar]
- Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bioresour. Technol.. 2000;71:113-123.
- [CrossRef] [Google Scholar]
- Aida, T., 1998. United States Patent 19.
- Ait Ahsaine, H., Zbair, M., Anfar, Z., Naciri, Y., El haouti, R., El Alem, N., Ezahri, M., 2018. Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Mater. Today Chem. 8, 121–132. https://doi.org/10.1016/j.mtchem.2018.03.004
- Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J.. 2012;203:348-356.
- [CrossRef] [Google Scholar]
- Activated carbon from dates’ stone by ZnCl2 activation. J. King Abdulaziz Univ. Sci.. 2006;17:75-98.
- [CrossRef] [Google Scholar]
- Sulfur removal from model diesel fuel using granular activated carbon from dates’ stones activated by ZnCl2. Fuel. 2009;88:87-94.
- [CrossRef] [Google Scholar]
- Carbon nanofiber-doped zeolite as support for molybdenum based catalysts for enhanced hydrodesulfurization of dibenzothiophene. J. Mol. Liq.. 2020;304
- [CrossRef] [Google Scholar]
- Deep desulphurization of gasoline and diesel fuels using non-hydrogen consuming techniques. Fuel. 2006;85:1354-1363.
- [CrossRef] [Google Scholar]
- Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel. 2014;115:804-811.
- [CrossRef] [Google Scholar]
- Importance of structural and chemical heterogeneity of activated carbon surfaces for adsorption of dibenzothiophene. Langmuir. 2005;21:7752-7759.
- [CrossRef] [Google Scholar]
- Metal-loaded polystyrene-based activated carbons as dibenzothiophene removal media via reactive adsorption. Carbon N. Y.. 2006;44:2404-2412.
- [CrossRef] [Google Scholar]
- Microwave-induced regeneration of activated carbons polluted with phenol. A comparison with conventional thermal regeneration. Carbon N. Y.. 2004;42:1383-1387.
- [CrossRef] [Google Scholar]
- Microwave-assisted regeneration of activated carbons loaded with pharmaceuticals. Water Res.. 2007;41:3299-3306.
- [CrossRef] [Google Scholar]
- Anthony L. Hines, R.N.M., 1985. Mass transfer fundamentals and applications. Prentice Hall.
- Reactive adsorption of SO 2 on activated carbons with deposited iron nanoparticles. J. Hazard. Mater.. 2013;246–247:300-309.
- [CrossRef] [Google Scholar]
- Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater.. 2003;66:189-195.
- [CrossRef] [Google Scholar]
- Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem. Eng. J.. 2010;50:104-109.
- [CrossRef] [Google Scholar]
- Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun.. 2001;23:2494-2495.
- [CrossRef] [Google Scholar]
- Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun.. 2001;23:2494-2495.
- [CrossRef] [Google Scholar]
- Bouchelta, C., Salah, M., Bertrand, O., Bellat, J., 2008. Preparation and characterization of activated carbon from date stones by physical activation with steam 82, 70–77. https://doi.org/10.1016/j.jaap.2007.12.009
- Bu, J., Loh, G., Gwie, C.G., Dewiyanti, S., Tasrif, M., Borgna, A., 2011. Desulfurization of diesel fuels by selective adsorption on activated carbons : Competitive adsorption of polycyclic aromatic sulfur heterocycles and polycyclic aromatic hydrocarbons 166, 207–217. https://doi.org/10.1016/j.cej.2010.10.063
- Adsorption desulphurization of gasoline by silver loaded onto modified activated carbons. Adsorpt. Sci. Technol.. 2008;26:225-231.
- [CrossRef] [Google Scholar]
- An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv.. 2012;2:759-783.
- [CrossRef] [Google Scholar]
- Chang, J.E.H., Chang, Y.K., Chang, H.O.N.A.M., 1998. Desulfurization of Dibenzothiophene and Diesel Oils by a Newly Isolated Gordona Strain , CYKS1 64, 2327–2331.
- Chen, X., Jeyaseelan, S., Graham, N., 2002. Physical and chemical properties study of the activated carbon made from sewage sludge 22, 755–760.
- Desulfurization of diesel fuel by extraction with [BF4]–based ionic liquids. Chinese J. Chem. Eng.. 2008;16:881-884.
- [CrossRef] [Google Scholar]
- Bioregeneration of activated carbon saturated with phenol. Environ. Sci. Technol.. 1984;18:1-5.
- [CrossRef] [Google Scholar]
- Effects of bimetallic Ce / Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem. Eng. J.. 2017;307:914-927.
- [CrossRef] [Google Scholar]
- API gravity, sulfur content, and desulfurization of crude oil. Pet. Sci. Technol.. 2015;33:93-101.
- [CrossRef] [Google Scholar]
- Optimization of preparation of activated carbon from cotton stalk by microwave assisted phosphoric acid-chemical activation. J. Hazard. Mater.. 2010;182:217-224.
- [CrossRef] [Google Scholar]
- Regeneration of spent catalyst from vinyl acetate synthesis as porous carbon: Process optimization using RSM. Chem. Eng. Res. Des.. 2014;92:1249-1256.
- [CrossRef] [Google Scholar]
- Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surfaces A Physicochem. Eng. Asp.. 2001;180:209-221.
- [CrossRef] [Google Scholar]
- Steam activation of chars produced from oat hulls and corn stover. Bioresour. Technol.. 2004;93:103-107.
- [CrossRef] [Google Scholar]
- Fuente, A.M., Pulgar, G., González, F., Pesquera, C., Blanco, C., 2001. Activated carbon supported Pt catalysts : effect of support texture and metal precursor on activity of acetone hydrogenation 208, 35–46.
- Influence of aluminium impregnation on activated carbon for enhanced desulfurization of DBT at ambient temperature : Role of surface acidity and textural properties. Chem. Eng. J.. 2016;303:489-500.
- [CrossRef] [Google Scholar]
- Ganiyu, S.A., Tanimu, A., Azeez, M.O., Alhooshani, K., 2020. Hierarchical Porous Nitrogen-Doped Carbon Modified with Nickel Nanoparticles for Selective Ultradeep Desulfurization 1–12.
- Gates, M.H.N.K.N.A.V.S.D.H.B.B.C., 1978. Hydrodesulfurization of dibenzothiophene catalyzed by sulfided CoO‐MoO3γ‐Al2O3: The reaction network. AIChE J. 24. https://doi.org/https://doi.org/10.1002/aic.690240611
- Gauagher, J.R., Olson, E.S., Stanley, D.C., 1993. Microbial desulfurization of dibenzothiophene : A sulfur-specific pathway 107, 31–35.
- Gratuito, M.K.B., Panyathanmaporn, T., Chumnanklang, R., 2008. Production of activated carbon from coconut shell : Optimization using response surface methodology 99, 4887–4895. https://doi.org/10.1016/j.biortech.2007.09.042
- The effects of thermal regeneration conditions and inorganic compounds on the characteristics of activated carbon used in power plant. Energy Procedia. 2012;17:444-449.
- [CrossRef] [Google Scholar]
- Bioregeneration by mixed microorganisms of granular activated carbon loaded with a mixture of phenols. Biotechnol. Lett.. 2000;22:1093-1096.
- [CrossRef] [Google Scholar]
- Characterization of mesoporous carbon prepared from date stems by H 3 PO 4 chemical activation. Appl. Surf. Sci.. 2013;280:1-7.
- [CrossRef] [Google Scholar]
- Haimour, N.M., Emeish, S., 2006. Utilization of date stones for production of activated carbon using phosphoric acid 26, 651–660. https://doi.org/10.1016/j.wasman.2005.08.004
- Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic diesel – acetonitrile system Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated. Fuel Process. Technol.. 2010;91:1105-1112.
- [CrossRef] [Google Scholar]
- Gasification of biomass chars in steam-nitrogen mixture. Energy Convers. Manag.. 2006;47:1004-1013.
- [CrossRef] [Google Scholar]
- Hirata, T., 1996. Desulfurization of dibenzothiophene derivatives by whole cells of Rhodococcus erythropolis H-2 142, 65–70.
- Biodesulfurization of dibenzothiophene by immobilized cells of Pseudomonas stutzeri UP-1. Fuel. 2005;84:1975-1979.
- [CrossRef] [Google Scholar]
- Hu, D., Li, Y., Mei, J., Wang, G., Xiao, C., Liu, C., Meng, Q., Li, H., 2020. High-dispersed Ni-Mo-S active phases within hierarchical pore materials by introducing the cationic protective shell during the impregnation process for hydrodesulfurization 263. https://doi.org/10.1016/j.fuel.2019.116701.
- Desulfurization of gasoline by extraction with new ionic liquids. Energy and Fuels. 2004;18:1862-1864.
- [CrossRef] [Google Scholar]
- Ii, J.J.K., 2010. Microbial biocatalyst developments to upgrade fossil fuels. https://doi.org/10.1016/j.copbio.2006.04.005
- Agricultural residues as precursors for activated carbon production-A review. Renew. Sustain. Energy Rev.. 2007;11:1966-2005.
- [CrossRef] [Google Scholar]
- Jia, X.Y.C.F., Gan, Y.X.C.J., 2011. Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment 1277–1285. https://doi.org/10.1007/s10646-011-0684-3.
- Kazeem, T.S., Lateef, S.A., Ganiyu, S.A., Qamaruddin, M., Tanimu, A., Sulaiman, K.O., Muhammad, S., Jillani, S., 2018. Aluminium-modi fi ed activated carbon as ef fi cient adsorbent for cleaning of cationic dye in wastewater 205, 303–312. https://doi.org/10.1016/j.jclepro.2018.09.114
- Extraction of thiophene or pyridine from n-heptane using ionic liquids. gasoline and diesel desulfurization. Ind. Eng. Chem. Res.. 2011;50:2296-2306.
- [CrossRef] [Google Scholar]
- Kertesz, M.A., 1999. Riding the sulfur cycle^metabolism of sulfonates and sulfate esters in Gram-negative bacteria 24.
- Khadhri, N., El Khames Saad, M., ben Mosbah, M., Moussaoui, Y., 2019. Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J. Environ. Chem. Eng. 7, 102775. https://doi.org/10.1016/j.jece.2018.11.020
- Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today. 2006;111:74-83.
- [CrossRef] [Google Scholar]
- Kirimura, K.O.H.T.A.R.O., Furuya, T., Nishii, Y., Ishii, Y., Kino, K., Usami, S., 2001. Biodesulfurization of Dibenzothiophene and Its Derivatives through the Selective Cleavage of Carbon-Sulfur Bonds by a Moderately Thermophilic Bacterium Bacillus subtilis WU-S2B 91, 262–266.
- Kittrell, J.R., Eldridge, J.W., 1990. Desulfurization of Fuel Oil by Oxidation and Extraction. Enhancement of Extraction Oil Yield 1–4. https://doi.org/10.1021/ie00099a002
- Laine, J., Calafat, A., labady, M., 1989. Preparation and characterization of activated carbons from coconut shell impregnated with phosphoric acid. Carbon N. Y. 27, 191–195. https://doi.org/10.1016/0008-6223(89)90123-1
- Pyrolysis kinetics of wheat and corn straw. J. Anal. Appl. Pyrolysis. 1998;44:181-192.
- [CrossRef] [Google Scholar]
- Li, W., Zhang, Y., Wang, M.D., Shi, Y., 2005. Biodesulfurization of dibenzothiophene and other organic sulfur compounds by a newly isolated Microbacterium strain ZD-M2 247, 45–50. https://doi.org/10.1016/j.femsle.2005.04.025
- Pilot-scale extraction of zinc from the spent catalyst of vinyl acetate synthesis by microwave irradiation. Hydrometallurgy. 2008;92:79-85.
- [CrossRef] [Google Scholar]
- Chemical regeneration of spent powdered activated carbon used in decolorization of sodium salicylate for the pharmaceutical industry. J. Clean. Prod.. 2015;86:424-431.
- [CrossRef] [Google Scholar]
- Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J. Anal. Appl. Pyrolysis. 2010;88:181-186.
- [CrossRef] [Google Scholar]
- Kinetics and mechanism of plasma oxidative desulfurization in liquid phase. Energy and Fuels. 2001;15:38-43.
- [CrossRef] [Google Scholar]
- Lo, J., Velo-gala, I., Sa, M., 2013. Surface modifications of activated carbon by gamma irradiation 7. https://doi.org/10.1016/j.carbon.2013.09.087.
- Lopes, A.R., Scheer, A. de P., Silva, G.V., Yamamoto, C.I., 2016. Pd-Impregnated activated carbon and treatment acid to remove sulfur and nitrogen from diesel. Rev. Mater. 21, 407–415. https://doi.org/10.1590/S1517-707620160002.0038
- Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J. Anal. Appl. Pyrolysis. 2006;76:96-102.
- [CrossRef] [Google Scholar]
- Luo, M.F., Xing, J.M., Gou, Z.X., Li, S., Liu, H.Z., Chen, J.Y., 2003. Desulfurization of dibenzothiophene by lyophilized cells of Pseudomonas delafieldii R-8 in the presence of dodecane 13, 1–6.
- Use of activated carbons prepared from sawdust and rice-husk for adsoprtion of acid dyes: A case study of acid yellow 36. Dye Pigment. 2003;56:239-249.
- [CrossRef] [Google Scholar]
- Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater.. 2004;113:81-88.
- [CrossRef] [Google Scholar]
- Mamaghani, A.H., Fatemi, S., Asgari, M., 2013. Investigation of Influential Parameters in Deep Oxidative Desulfurization of Dibenzothiophene with Hydrogen Peroxide and Formic Acid 2013.
- Influence of thermal treatment regime on the density and reactivity of activated carbons from almond shells. Carbon N. Y.. 2000;38:429-440.
- [CrossRef] [Google Scholar]
- Thermochemical treatment of biomass in a flow of steam or in a mixture of steam and carbon dioxide. Fuel Process. Technol.. 2000;62:45-52.
- [CrossRef] [Google Scholar]
- Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Process. Technol.. 2001;70:53-61.
- [CrossRef] [Google Scholar]
- Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surfaces and Interfaces. 2018;11:74-81.
- [CrossRef] [Google Scholar]
- Moon, S., Shim, J., 2006. A novel process for CO 2 / CH 4 gas separation on activated carbon fibers — electric swing adsorption 298, 523–528. https://doi.org/10.1016/j.jcis.2005.12.052
- Moosavi, E.S., Dastgheib, S.A., Karimzadeh, R., 2012. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. https://doi.org/10.3390/en5104233
- Boosting the hydrodesulfurization of dibenzothiophene e ffi ciency of Mn decorated (Co / Ni) -Mo / Al 2 O 3 catalysts at mild temperature and pressure by coupling with phosphonium based ionic liquids. Chem. Eng. J.. 2019;375:121957
- [CrossRef] [Google Scholar]
- Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J.. 2012;210:500-509.
- [CrossRef] [Google Scholar]
- Electrochemical regeneration of field spent GAC from two water treatment plants. Water Res.. 2012;46:4852-4860.
- [CrossRef] [Google Scholar]
- Regeneration of spent activated carbon from wastewater treatment plant application. J. Phys. Conf. Ser.. 2018;1116
- [CrossRef] [Google Scholar]
- The nature and kinetics of the adsorption of dibenzothiophene in model diesel fuel on carbonaceous materials loaded with aluminum oxide particles. Arab. J. Chem. 2015
- [CrossRef] [Google Scholar]
- Specific desulfurization of dibenzothiophene by Mycobacterium sp. strain G3. Biocatal. Biotransformation. 1997;15:17-27.
- [CrossRef] [Google Scholar]
- Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J.. 2011;173:391-399.
- [CrossRef] [Google Scholar]
- Adsorptive desulfurization of dibenzothiophene by sewage sludge-derived activated carbon. Chem. Eng. J.. 2013;228:263-271.
- [CrossRef] [Google Scholar]
- Okada, K., Yamamoto, N., Kameshima, Y., Yasumori, A., 2003. Adsorption properties of activated carbon from waste newspaper prepared by chemical and physical activation 262, 194–199. https://doi.org/10.1016/S0021-9797(03)00108-5
- Adsorptive desulphurization of model oil by Ag nanoparticles-modified activated carbon prepared from brewer’s spent grains. J. Environ. Chem. Eng.. 2017;5:147-159.
- [CrossRef] [Google Scholar]
- Otsuki, S., Nonaka, T., Takashima, N., Qian, W., Ishihara, A., Imai, T., Kabe, T., 2000a. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction 39, 1232–1239. https://doi.org/10.1021/ef000096i
- Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy and Fuels. 2000;14:1232-1239.
- [CrossRef] [Google Scholar]
- Preparation of activated carbons from olive-tree wood revisited. I. Chemical activation with H3PO4. Fuel Process. Technol.. 2011;92:261-265.
- [CrossRef] [Google Scholar]
- Biomass and Bioenergy Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass and Bioenergy. 2018;115:64-73.
- [CrossRef] [Google Scholar]
- Removal of aromatic hydrocarbons from water by activated carbon from apricot stones. Chem. Eng. J.. 2010;165:258-264.
- [CrossRef] [Google Scholar]
- Fixed-bed pyrolysis of cotton stalk for liquid and solid products. Fuel Process. Technol.. 2005;86:1207-1219.
- [CrossRef] [Google Scholar]
- Applied Catalysis B : Environmental Effect of carbon on the hydrodesulfurization activity of MoCo catalysts supported on zeolite / active carbon hybrid supports. Appl. Catal. B Environ.. 2020;263:117661
- [CrossRef] [Google Scholar]
- Influence of acidic and basic treatments of activated carbon derived from waste rubber tires on adsorptive desulfurization of thiophenes. J. Taiwan Inst. Chem. Eng.. 2016;60:460-468.
- [CrossRef] [Google Scholar]
- Ultra-deep adsorptive desulfurization of fuels on cobalt and molybdenum nanoparticles loaded on activated carbon derived from waste rubber. J. Colloid Interface Sci.. 2018;513:779-787.
- [CrossRef] [Google Scholar]
- Adsorptive desulfurization of dibenzothiophene from fuels by rubber tyres-derived carbons: Kinetics and isotherms evaluation. Process Saf. Environ. Prot.. 2016;102:9-19.
- [CrossRef] [Google Scholar]
- Saleh, T.A., Danmaliki, G.I., Shuaib, T.D., 2016. Nanocomposites and Hybrid Materials for Adsorptive Desulfurization. pp. 129–153. https://doi.org/10.4018/978-1-4666-9545-0.ch004.
- Saleh, A., 2018. Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon 172. https://doi.org/10.1016/j.jclepro.2017.11.208.
- Naphtha desulfurization by adsorption. Ind. Eng. Chem. Res.. 1994;33:336-340.
- [CrossRef] [Google Scholar]
- Regeneration of carbonaceous adsorbents. Part II: Chemical, microbiological and vacuum regeneration. Microporous Mesoporous Mater.. 2015;202:277-296.
- [CrossRef] [Google Scholar]
- Two-step adsorption process for deep desulfurization of diesel oil. Fuel. 2005;84:903-910.
- [CrossRef] [Google Scholar]
- Biomass conversion to carbon adsorbents and gas. Biomass and Bioenergy. 2001;21:133-142.
- [CrossRef] [Google Scholar]
- High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod.. 2016;113:995-1004.
- [CrossRef] [Google Scholar]
- Sciences, B., Eh, E., Oldfield, C., Wood, N.T., Gilbert, S.C., Murray, F.D., Faure, F.R., 1998. Desulphurisation of benzothiophene and dibenzothiophene by actinomycete organisms belonging to the genus Rhodococcus , and related taxa 119–132.
- Seh-bardan, A.W.S.F.S.B.J., 2014. Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals 967–976. https://doi.org/10.1007/s13762-013-0291-3.
- Preparation and characterization of an activated carbon from a date stones variety by physical activation with carbon dioxide. J. Anal. Appl. Pyrolysis. 2013;99:155-160.
- [CrossRef] [Google Scholar]
- Selective adsorption of dibenzothiophenes on activated carbons with Ag Co, and Ni species deposited on their surfaces. Energy and Fuels. 2009;23:3737-3744.
- [CrossRef] [Google Scholar]
- Adsorption of dibenzothiophenes on activated carbons with copper and iron deposited on their surfaces. Fuel Process. Technol.. 2010;91:693-701.
- [CrossRef] [Google Scholar]
- Investigation of the enhancing effects of sulfur and/or oxygen functional groups of nanoporous carbons on adsorption of dibenzothiophenes. Carbon N. Y.. 2011;49:1216-1224.
- [CrossRef] [Google Scholar]
- Textural and chemical factors affecting adsorption capacity of activated carbon in highly efficient desulfurization of diesel fuel. Carbon N. Y.. 2009;47:2491-2500.
- [CrossRef] [Google Scholar]
- Applied Surface Science Ammonia modification of activated carbon to enhance carbon dioxide adsorption : Effect of pre-oxidation. Appl. Surf. Sci.. 2011;257:3936-3942.
- [CrossRef] [Google Scholar]
- Shafi, R., Hutchings, G.J., 2000. Hydrodesulfurization of hindered dibenzothiophenes : an overview 59, 423–442.
- Characterization of sulfur compounds in oilsands bitumen by methylation followed by positive-ion electrospray ionization and fourier transform ion cyclotron resonance mass spectrometry. Energy and Fuels. 2010;24:3014-3019.
- [CrossRef] [Google Scholar]
- Shifu, C., Yueming, L.I.U., Jinbao, G.A.O., Lingling, W., Xiuli, L.I.U., Guohua, G.A.O., Peng, W.U., 2006. Catalytic Oxidation of Benzothiophene and Dibenzothiophene in Model Light Oil over Ti-MWW 27, 547–549.
- Activated Carbons Derived from Hydrothermally Carbonized Sucrose: Remarkable Adsorbents for Adsorptive Desulfurization. ACS Sustain. Chem. Eng.. 2015;3:2237-2246.
- [Google Scholar]
- Influence of the chemical composition of silica-alumina adsorbents in sulfur and nitrogen compounds removal from hydrotreated diesel. Ind. Eng. Chem. Res.. 2014;53:16000-16014.
- [CrossRef] [Google Scholar]
- Investigation of agricultural and animal wastes in Greece and their allocation to potential application for energy production. Renew. Sustain. Energy Rev.. 2007;11:1698-1719.
- [CrossRef] [Google Scholar]
- Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol. Adv.. 2007;25:570-596.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and sorption studies of a zirconium(IV) impregnated highly functionalized mesoporous activated carbons. RSC Adv.. 2020;10:13783-13798.
- [CrossRef] [Google Scholar]
- Song, C., 2007. Chunshan Song Uday T. Turaga Xiaoliang Ma 651–661. https://doi.org/10.1081/E-ECHP-120007732
- Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq.. 2017;229:465-471.
- [CrossRef] [Google Scholar]
- Stoner, D.L., Wey, J.E., Barrett, K.B., Jolley, J.G., Wright, R.B., Dugan, P.R., 1990. Modification of Water-Soluble Coal-Derived Products by Dibenzothiophene-Degrading Microorganisms 56, 2667–2676.
- Suhas, Carrott, P.J.M., Ribeiro Carrott, M.M.L., 2007. Lignin - from natural adsorbent to activated carbon: A review. Bioresour. Technol. 98, 2301–2312. https://doi.org/10.1016/j.biortech.2006.08.008
- Controllable nitrogen introduction into porous carbon with porosity retaining for investigating nitrogen doping effect on SO 2 adsorption. Chem. Eng. J.. 2016;290:116-124.
- [CrossRef] [Google Scholar]
- Selective removal of SO 2 over tertiary amine-containing materials. Chem. Eng. J.. 2014;240:462-468.
- [CrossRef] [Google Scholar]
- Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater.. 2008;154:337-346.
- [CrossRef] [Google Scholar]
- Microwave processing: fundamentals and applications. Compos. Part A Appl. Sci. Manuf.. 1999;30:1055-1071.
- [CrossRef] [Google Scholar]
- Timmis, K.N., 2010. Handbook of Hydrocarbon and Lipid Microbiology, Handbook of Hydrocarbon and Lipid Microbiology. Springer Berlin Heidelberg, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-77587-4.
- A low cost adsorbent from agricultural waste corn cob by zinc chloride activation. Bioresour. Technol.. 1998;64:211-217.
- [CrossRef] [Google Scholar]
- Cleaner production of carbon adsorbents by utilizing agricultural waste corn cob. Resour. Conserv. Recycl.. 2001;32:43-53.
- [CrossRef] [Google Scholar]
- Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis. 2006;76:230-237.
- [CrossRef] [Google Scholar]
- Electrochemical regeneration of granular activated carbon saturated with organic compounds. Chem. Eng. J.. 2009;155:763-768.
- [CrossRef] [Google Scholar]
- Chemical Engineering Research and Design Post-combustion CO 2 capture with chemical absorption : A state-of-the-art review. Chem. Eng. Res. Des.. 2010;89:1609-1624.
- [CrossRef] [Google Scholar]
- Preparation of polystyrene-based activated carbon spheres and their adsorption of dibenzothiophene. Xinxing Tan Cailiao/ New Carbon Mater.. 2009;24:55-60.
- [CrossRef] [Google Scholar]
- Understanding selectivity changes during hydrodesulfurization of dibenzothiophene on Mo 2 C / carbon catalysts. J. Catal.. 2019;369:427-439.
- [CrossRef] [Google Scholar]
- Desulfurization of liquid fuels by adsorption on carbon-based sorbents and ultrasound-assisted sorbent regeneration. Langmuir. 2007;23:3825-3831.
- [CrossRef] [Google Scholar]
- Preparation of sludge-based activated carbon and its application in dye wastewater treatment. J. Hazard. Mater.. 2008;153:22-27.
- [CrossRef] [Google Scholar]
- Facile, low-cost, and sustainable preparation of hierarchical porous carbons from ion exchange resin : An improved potassium activation strategy. FUEL. 2016;179:274-280.
- [CrossRef] [Google Scholar]
- A critical study on the adsorption of heterocyclic sulfur and nitrogen compounds by activated carbon : Equilibrium, kinetics and thermodynamics. Chem. Eng. J.. 2010;164:29-36.
- [CrossRef] [Google Scholar]
- Microwave assisted regeneration of spent activated carbon from petrochemical plant using response surface methodology. J. Porous Mater.. 2014;22:137-146.
- [CrossRef] [Google Scholar]
- Xiao, J., Bian, G., Zhang, W., Li, Z., 2010. Adsorption of Dibenzothiophene on Ag / Cu / Fe-Supported Activated Carbons Prepared by Ultrasonic-Assisted Impregnation 5818–5823. https://doi.org/10.1021/je1007795.
- Regeneration of microwave assisted spent activated carbon: Process optimization, adsorption isotherms and kinetics. Chem. Eng. Process. Process Intensif.. 2012;53:53-62.
- [CrossRef] [Google Scholar]
- Xiong, L., Yan, X., Mei, P., Engineering, E., 2010. Synthesis and Characterization of a ZrO 2 / AC Composite as a Novel Adsorbent for Dibenzothiophene 341–350.
- Xiong, L., Yan, F.C.X., Mei, P., 2012. The adsorption of dibenzothiophene using activated carbon loaded with cerium 713–719. https://doi.org/10.1007/s10934-011-9523-4.
- Xiong, J., Zhu, W., Li, H., Yang, L., Chao, Y., Wu, P., 2015. Carbon-doped porous boron nitride: metal-free adsorbents for sulfur removal from fuels 12738–12747. https://doi.org/10.1039/c5ta01346a.
- Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H 3 PO 4. Appl. Surf. Sci.. 2014;320:674-680.
- [CrossRef] [Google Scholar]
- Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev.. 2015;46:218-235.
- [CrossRef] [Google Scholar]
- Studies of the surface area and porosity of activated carbons prepared from rice husks. Carbon N. Y.. 2000;38:1943-1945.
- [CrossRef] [Google Scholar]
- Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol.. 2010;101:6163-6169.
- [CrossRef] [Google Scholar]
- Environmental Effects Activated carbon from paulownia wood : Yields of chemical activation stages. Energy Sources. Part A Recover. Util. Environ. Eff.. 2016;38:2035-2042.
- [CrossRef] [Google Scholar]
- Deep desulfurization of diesel fuels by catalytic oxidation. Gao Xiao Hua Xue Gong Cheng Xue Bao/J. Chem. Eng. Chinese Univ.. 2006;20:616-621.
- [CrossRef] [Google Scholar]
- Desorption activation energy of dibenzothiophene on the activated carbons modified by different metal salt solutions. Chem. Eng. J.. 2007;132:233-239.
- [CrossRef] [Google Scholar]
- Adsorption of dibenzothiophene on transition metals loaded activated carbon. Adv. Mater. Res.. 2010;132:141-148.
- [CrossRef] [Google Scholar]
- Deep desulfurization of fuel oils using low-viscosity 1-ethyl-3- methylimidazolium dicyanamide ionic liquid. Ind. Eng. Chem. Res.. 2011;50:2236-2244.
- [CrossRef] [Google Scholar]
- Adsorption removal of thiophene and dibenzothiophene from oils with activated carbon as adsorbent: Effect of surface chemistry. J. Porous Mater.. 2008;15:151-157.
- [CrossRef] [Google Scholar]
- Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv. Colloid Interface Sci.. 2009;149:19-27.
- [CrossRef] [Google Scholar]
- Shuguang Zhang, Qinglin Zhang, and Z.C.Z., 2004. Extractive Desulfurization and Denitrogenation of Fuels Using Ionic Liquids. Ind. Eng. Chem. Res. 231, 614–622. https://doi.org/10.1021/ie030561+
- Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J.. 2004;105:53-59.
- [CrossRef] [Google Scholar]
- Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl. Surf. Sci.. 2014;315:279-286.
- [CrossRef] [Google Scholar]
- Use of ionic liquids as “green” solvents for extractions. J. Chem. Technol. Biotechnol.. 2005;80:1089-1096.
- [CrossRef] [Google Scholar]
- Liquid-phase adsorption of multi-ring thiophenic sulfur compounds on carbon materials with different surface properties. J. Phys. Chem. B. 2006;110:4699-4707.
- [CrossRef] [Google Scholar]
- Svinterikos, E., Zuburtikudis, I., Al-Marzouqi, M., 2019. Carbon Nanomaterials for the Adsorptive Desulfurization of Fuels. J. Nanotechnol. 2019. https://doi.org/10.1155/2019/2809867







