Translate this page into:
Thiazole ring- the antimicrobial, anti-inflammatory, and anticancer active scaffold
⁎Corresponding author. aliramazani@znu.ac.ir (Ali Ramazani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The thiazole ring has the characteristic of resisting the inflammatory cells of various bacterial and fungal strains, as well as all types of cancer cells, and it can even be used to treat these pathogens in industrial drugs. Therefore, the thiazole ring is a suitable protective medicine for humans from these pathogenic agents.
Abstract
Background
Thiazole ring is one of the most important heterocycle scaffolds in organic and medicinal chemistry due to this scaffold being the backbone of numerous drugs and their significant activities against various diseases have made it one of the best candidates for industrial production. According to the literature, the number of drugs that have this ring on their structures is increasing. In fact, drugs containing thiazole scaffold have taken a significant share in the last decade, which reached its maximum number of 8 drugs in 2019. Objective: In This current review, we focus on essential advances of antimicrobial, anti-inflammatory, and anticancer activities of structures containing thiazole scaffold and various groups’ effects on this scaffold against the broad spectrum of bacterial strains, fungal strains, inflammatory cells, and cancer cells. Also, we analyzed and investigated thiazole hybrids with potential antimicrobial, anti-inflammatory, and anticancer activities. Results: More than 100 research articles were found, we selected about 100 research articles from 2014 to 2022 that had outstanding results.
Keywords
Antifungal
Antibacterial
Biological activity
Antitumor
Thiazoles
Drug discovery
1 Introduction
Organic and medicinal chemistry are important majors in the field of synthesis and development of structures with biological activity (Sani and Zanda, 2022; Wentrup, 2022). In recent years, researchers focusing on structures that have scaffolds containing heterocycles atoms, and rings. There are a lot of molecules with five, six, and so on membered heterocycle rings containing hetero atoms (Kaur, 2018a,b; Kaur and Kishore, 2014); Kaur et al., 2021). Meanwhile, the thiazole ring is one of the types of heterocyclic rings with different biological properties (Ali and Sayed, 2021).Scheme 1.
The thiazole ring has the characteristic of resisting the inflammatory cells of various bacterial and fungal strains, as well as all types of cancer cells, and it can even be used to treat these pathogens in industrial drugs. Therefore, the thiazole ring is a suitable protective medicine for humans from these pathogenic agents.
Research illustrates Thiazole rings have an excellent wide range of pharmaceutical applications such as antimalarial (Bueno et al., 2016), antifungal (Lino et al., 2018), anticancer (Jain et al., 2018), anti-inflammatory (Moldovan et al., 2011), antibacterial (Li et al., 2014), antioxidant (Grozav et al., 2017), and so on. According to the Njardarson group reports, five drugs with thiazole scaffold exist in the top two hundred brand name drugs by retail sales in 2015, namely: Viekira Pak, Sprycel, Augmentin, Kaletra, and Olysio/Sovriad. Five drugs exist in the top two hundred brand name drugs by retail sales in 2016, namely: Stribild, Sprycel, Genvoya, Myrbetriq, and Augmentin. In 2018, seven drugs exist in the top two hundred brand name drugs by retail sales, namely: Genvoya, Sprycel, Prezcobix, Myrbetriq, Tafinlar, Lixiana, and Augmentin. The number of drugs increased to eight in 2019, which are Genvoya, Sprycel, Prezista, Myrbetriq, Edoxaban, Tafinlar/Mekinist, Lixiana, and Augmentin. This trend decreased to 5 drugs in 2020, but again from 2021 this trend increased until it increased to 7 drugs in 2022. According to the information in the Fig. 1, it can be seen that after every decreasing trend, there is an increasing trend. Also, the highest number of drugs containing the thiazole ring was in 2019, and in 2015, 2016 and 2020, the number of drugs was fixed at 5 (Fig. 1) (J. Chem. Ed. 2010).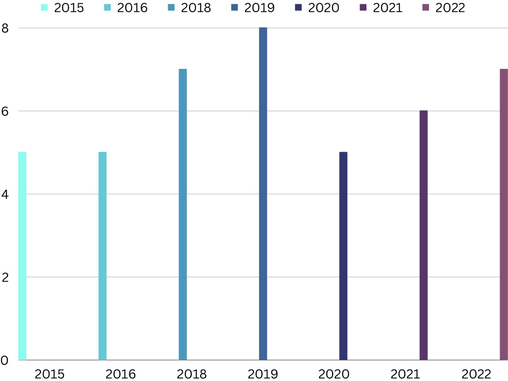
The number of drugs containing thiazole scaffold in between two hundred brand drugs by retail sales from 2015 to 2022.
The purpose of the present review is to collect and analysis of important studies that investigate of antimicrobial, anti-inflammatory, and anticancer activity of structures that have thiazole scaffold from 2014 to 2022 and show their advance for more effectiveness.
2 Thiazole derivatives with antimicrobial activity
For a long time, we didn't have very many new drugs to treat bacteria. This led to a lack of drugs that work against bacteria that are resistant to drugs. So now we need to synthesize new drugs that work in different ways and that can treat both sensitive and resistant strains of bacteria (Holmes et al., 2016).
In 2014, Liaras and co-workers synthesized two thiazole-based aminopyrimidine derivatives and eight N-phenylpyrazoline derivatives (Fig. 2). Ten derivatives were tested against 9 g-positive and negative bacteria and 9 fungal strains. The evaluations showed all structures have antibacterial activities. In addition, two structures (1a and 1b) among all structures had excellent activity but had no good yield (15% − 27%) (Table 1 and Fig. 2). Streptomycin and ampicillin were used as reference drugs. Most of the derivatives showed comparable or better activities than the reference drugs. Among all derivatives, structure 1a had the best activity (Table 1 and Fig. 2). Structure 1b showed the best inhibitory effect against gram-positive bacteria (Table 1 and Fig. 2). The best antibacterial activity against gram-positive and negative bacteria showed by structure 1a (Table 1 and Fig. 2). In addition, structure 2d displayed The lowest antibacterial activity, and had no activity against En. cloacae. Also, all derivatives had potent activity against S. aureus and L. monocytogenes compared to streptomycin (except 2 g) (Table 1 and Fig. 2). In the case of 2a-h, structures 2e, 2f and 2 h just increased the activity in comparison structure 2a, while structures 2d and 2 g showed the opposite effect (Table 1 and Fig. 2) (Liaras et al., 2014)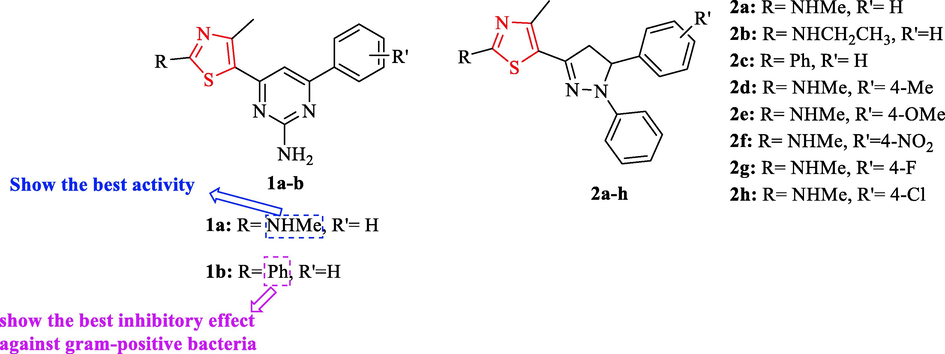
Chemical structures 1a-b and 2a-h (Liaras et al., 2014)
Cpd. no.
Gram-Psitive bacteria
Gram-Negative bacteria
S. a.
B. c.
M. f.
L. m.
Ps. aer.
S. typh.
E. coli
En. f.
En. cl.
MIC
8.41 ± 0.02
3.37 ± 0.02
3.37 ± 0.02
10.10 ± 0.10
3.37 ± 0.02
3.37 ± 0.02
3.37 ± 0.00
NT
6.74 ± 0.08
1a
MBC
10.10 ± 0.03
6.74 ± 0.10
8.41 ± 0.10
11.81 ± 0.10
6.74 ± 0.08
6.74 ± 0.02
6.74 ± 0.00
NT
8.41 ± 0.10
MIC
1.45 ± 0.00
5.80 ± 0.10
5.80 ± 0.10
1.45 ± 0.00
29.00 ± 0.30
29.00 ± 0.00
34.80 ± 0.10
29.00 ± 0.07
NT
1b
MBC
5.80 ± 0.07
40.60 ± 0.10
29.00 ± 0.00
36.25 ± 0.00
40.60 ± 0.00
34.80 ± 0.30
40.60 ± 0.20
34.80 ± 0.00
NT
Ampicillin
MIC
24.80 ± 0.07
24.80 ± 0.10
24.80 ± 0.02
37.20 ± 0.07
74.40 ± 0.10
24.80 ± 0.00
37.20 ± 0.07
24.80 ± 0.30
24.80 ± 0.20
MBC
37.20 ± 0.00
37.20 ± 0.07
37.20 ± 0.07
74.40 ± 0.10
124 ± 0.70
49.20 ± 0.07
49.20 ± 0.07
37.20 ± 0.07
37.20 ± 0.07
Streptomycin
MIC
17.20 ± 0.07
4.30 ± 0.10
8.60 ± 0.10
25.80 ± 0.10
17.20 ± 0.00
17.20 ± 0.07
17.20 ± 0.00
4.30 ± 0.10
4.30 ± 0.01
MBC
34.40 ± 0.10
8.60 ± 0.00
17.20 ± 0.00
51.60 ± 0.20
34.40 ± 0.00
34.40 ± 0.10
34.40 ± 0.10
8.60 ± 0.10
8.60 ± 0.10
S. a. – Staphylococcus aureus (ATCC 6538); B. c. – Bacillus cereus (clinical isolate); M. f. – Micrococcus flavus (ATCC 10240); L. m. – Listeria monocytogenes (NCTC 7973).Ps. aer. – Pseudomonas aeruginosa (ATCC 27853); S. typh. – Salmonella typhimurium (ATCC 13311); E. coli – Escherichia coli (ATCC 35210); En. f. – Enterococcus faecalis (human isolate); En. cl. – Enterobacter cloacae (human isolate).
Cpd. no.
Fungal yeast
A. o.
A. ver.
A. fl.
A. n.
A. fum.
T. v.
P. o.
P. f.
C. a.
MIC
3.37±0.10
NT
5.05±0.02
5.05±0.02
5.05±0.02
2.52±0.20
3.37±0.10
3.37±0.02
3.37±0.10
1a
MFC
6.74±0.10
NT
6.74±0.30
6.74±0.30
6.74±0.20
3.37±0.10
5.05±0.20
6.74±0.20
6.74±0.20
MIC
1.45±0.20
1b
MFC
5.80±0.30
Ketoconazole
MIC
15.70±0.20
21±0.00
NT
26.20±0.07
26.20±0.07
475±1.70
380±1.70
38±0.00
37.60±0.00
MFC
38±0.10
285±1.60
285±1.70
38±0.20
38±0.00
570±1.70
380±1.70
95±0.30
94±0.30
Bifonazole
MIC
48±0.00
48±0.20
48±0.20
48±0.20
48±0.00
64±0.00
48±0.00
64±0.20
32.20±0.07
MFC
80±1.60
64±0.30
64±0.00
64±0.20
64±0.30
80±0.03
64±0.03
80±1.00
48.30±0.10
A. o. – Aspergillus ochraceus (ATCC 12066); A. v. – Aspergillus versicolor (ATCC 11730); A. fl. – Aspergillus flavus (ATCC 9643); A. n. – Aspergillus niger (ATCC 6275); A. fum. – Aspergillus fumigatus (human isolate);T. v. – Trichoderma viride (IAM 5061); P. o. – Penicillium ochrochloron (ATCC 9112); P. f. – Penicillium funiculosum (ATCC 36839); C. a. – Candida albicans (human isolate).
On the other hand, the antifungal activity of derivatives was tested in comparison to ketoconazole and bifonazole as reference drugs. Structure 1a had the best activity. In the case of A. Ochraceus structures 1b had the best activity (Table 1 and Fig. 2) (Liaras et al., 2014)
A series of structures containing thiazole scaffold was reported by Rostom and co-workers and evaluated their in vitro antibacterial activity. Nineteen structures against gram (+) bacteria had good antibacterial activity. Among these derivatives, five structures (4, 7, 8b, 9, and 10b) had significant activities with broad-spectrum, and structure 10b (with 27% yield) had the best result compared to other ones (Fig. 3 and Table 2). Structures 4 and 10b showed equipotent activity to ampicillin as a reference drug against S. aureus ATCC 6538, while structures 8b, 9, and 11 exhibited half the potency of ampicillin (Fig. 3 and Table 2). In the case of B. subtilis, structure 10b showed equipotent activity to gentamicin sulfate and ampicillin, while structures 4, 8b, 9, and 11 had half the potency of ampicillin (Fig. 3 and Table 2). In addition, structures 4, 8b, and 10b showed good activity compared to ampicillin and gentamicin sulfate against E. Coli. Also, structures 3, 7, and 9 displayed moderate activity against the same organism compared to gentamicin sulfate and ampicillin (Fig. 3 and Table 2) (Rostom et al., 2014) S. a. - S. aureus ATCC 6538; B. s. - B. subtilis ATCC 6633; E. coli. - E. coli ATCC 25922; P. a. - P. aeruginosa ATCC 27853. NT = not tested. (-): totally inactive (MIC ≥ 200 μg/mL). C. a. - C. albicans ATCC 10231 (-): totally inactive (MIC ≥ 200 μg/mL).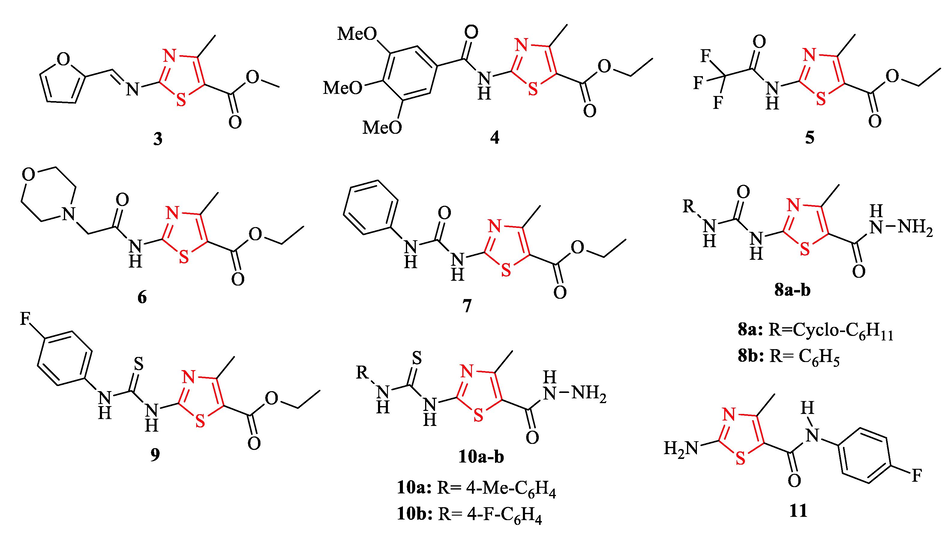
Chemical structures of 3–7, 8a-b, 9, 10a-b, and 11 (Rostom et al., 2014)
College
Gram positive bacteria
Gram negative bacteria
Fungus
S. a.
B. s.
E. coli
P. a.
C. a.
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
3
50
100
100
200
25
50
–
NT
4
6.25
6.25
25
50
12.5
12.5
50
100
5
100
100
6
100
100
7
25
25
50
50
25
25
50
100
25
25
8a
50
100
8b
12.5
12.5
25
25
12.5
12.5
50
100
25
25
9
12.5
12.5
25
25
25
25
100
100
25
50
10a
100
100
10b
6.25
6.25
12.5
12.5
12.5
12.5
50
50
12.5
12.5
11
12.5
25
25
50
50
100
–
NT
100
100
Ampicillin
Trihydrate
6.25
–
12.5
–
6.25
–
12.5
–
Gentamycin
3.12
–
12.5
–
3.12
–
12.5
–
Clotrimazole
6.25
–
Amphotericin-B
1.56
–
According to antifungal activity results, structures 5, 6, 7, 8a, 8b, 9, 10a, 10b, and 11 had significant growth inhibitory activity against C. Albicans compared to amphotericin B and clotrimazole as reference drugs (Fig. 3 and Table 2) (Rostom et al., 2014)
Prakash et al. synthesized twenty-seven structures containing thiazole scaffold. The antimicrobial activity of derivatives was tested against 4g-negative and positive bacteria and 2 fungal strains. (Table 3). Structures 16c and 17c displayed higher antibacterial activity compared to chloramphenicol as a reference drug, especially against Staphylococcus aureus (Fig. 4 and Table 3). In general, structures 12, 13b, 14a, 14b, 15, 16b, and 17b had moderate antibacterial activity, while structures 13a, 13c, 14c, 16a, and 17a showed good activity (Table 3 and Fig. 4) (Prakash et al., 2014) S. a. - Staphylococcus aureus; B. s. - Bacillus subtilis; P. a. -Pseudomonas aeruginosa; K. p. - Klebsiella pneumoniae, (-) No activity.
Cpd. no.
Zone of inhibition (mm)
Gram-positive bacteria
Gram-negative bacteria
S. a.
B. s.
P. a.
K. p.
Aspergillus niger
Penicillium chrysogenum
12
25 μg/well
15 ± 2
15 ± 2
8 ± 2
16 ± 2
50 μg/well
17 ± 1
16 ± 2
9 ± 2
17 ± 1
100 μg/well
19 ± 1
19 ± 2
12 ± 1
20 ± 2
13a
25 μg/well
22 ± 2
21 ± 2
12 ± 2
23 ± 1
50 μg/well
25 ± 1
22 ± 2
15 ± 2
25 ± 1
100 μg/well
26 ± 3
24 ± 3
17 ± 1
28 ± 2
13b
25 μg/well
11 ± 2
10 ± 1
–
11 ± 1
50 μg/well
13 ± 3
12 ± 2
6 ± 2
13 ± 1
100 μg/well
15 ± 3
14 ± 3
8 ± 2
16 ± 2
13c
25 μg/well
24 ± 1
22 ± 1
14 ± 1
26 ± 2
50 μg/well
26 ± 2
25 ± 1
16 ± 1
28 ± 2
100 μg/well
29 ± 1
26 ± 2
18 ± 1
31 ± 1
14a
25 μg/well
18 ± 3
18 ± 1
10 ± 1
19 ± 1
50 μg/well
20 ± 2
20 ± 2
12 ± 2
20 ± 1
100 μg/well
23 ± 2
22 ± 2
14 ± 1
24 ± 2
14b
25 μg/well
10 ± 3
9 ± 2
–
10 ± 2
50 μg/well
12 ± 2
10 ± 1
–
12 ± 2
100 μg/well
14 ± 2
13 ± 2
7 ± 1
14 ± 1
14c
25 μg/well
21 ± 1
19 ± 1
11 ± 2
20 ± 2
50 μg/well
23 ± 2
21 ± 1
13 ± 1
22 ± 2
100 μg/well
25 ± 2
23 ± 2
15 ± 2
26 ± 1
15
25 μg/well
17 ± 2
16 ± 2
9 ± 1
17 ± 1
50 μg/well
18 ± 3
18 ± 1
10 ± 1
19 ± 2
100 μg/well
21 ± 3
21 ± 1
13 ± 2
22 ± 1
16a
25 μg/well
29 ± 3
25 ± 2
17 ± 1
30 ± 3
50 μg/well
31 ± 2
26 ± 3
18 ± 1
32 ± 2
100 μg/well
32 ± 2
28 ± 2
21 ± 3
35 ± 2
16b
25 μg/well
14 ± 3
14 ± 1
7 ± 2
14 ± 2
50 μg/well
15 ± 2
15 ± 3
8 ± 1
15 ± 2
100 μg/well
18 ± 1
18 ± 1
11 ± 2
18 ± 1
16c
25 μg/well
34 ± 2
29 ± 2
20 ± 2
35 ± 2
50 μg/well
36 ± 2
32 ± 1
22 ± 2
37 ± 3
100 μg/well
38 ± 3
35 ± 2
25 ± 2
41 ± 1
17a
25 μg/well
27 ± 2
24 ± 2
15 ± 2
28 ± 1
32 ± 1
27 ± 1
50 μg/well
28 ± 1
26 ± 2
17 ± 2
29 ± 1
34 ± 2
29 ± 2
100 μg/well
30 ± 2
27 ± 1
20 ± 2
33 ± 2
37 ± 2
31 ± 1
17b
25 μg/well
12 ± 2
12 ± 2
6 ± 1
13 ± 1
50 μg/well
14 ± 2
13 ± 2
7 ± 1
14 ± 2
100 μg/well
17 ± 2
16 ± 2
10 ± 1
17 ± 1
17c
25 μg/well
32 ± 1
27 ± 1
18 ± 2
32 ± 2
33 ± 2
29 ± 2
50 μg/well
33 ± 3
29 ± 2
19 ± 2
34 ± 2
36 ± 1
30 ± 1
100 μg/well
35 ± 2
30 ± 3
23 ± 2
38 ± 1
39 ± 3
32 ± 2
Chloramphenicol
25 μg/well
30 ± 3
32 ± 3
25 ± 2
38 ± 1
50 μg/well
33 ± 1
34 ± 3
27 ± 3
40 ± 2
100 μg/well
35 ± 2
38 ± 1
30 ± 1
42 ± 3
Ketoconazole
25 μg/well
50 μg/well
100
μg/well
31 ± 3
33 ± 2
36 ± 235 ± 1
36 ± 2
38 ± 3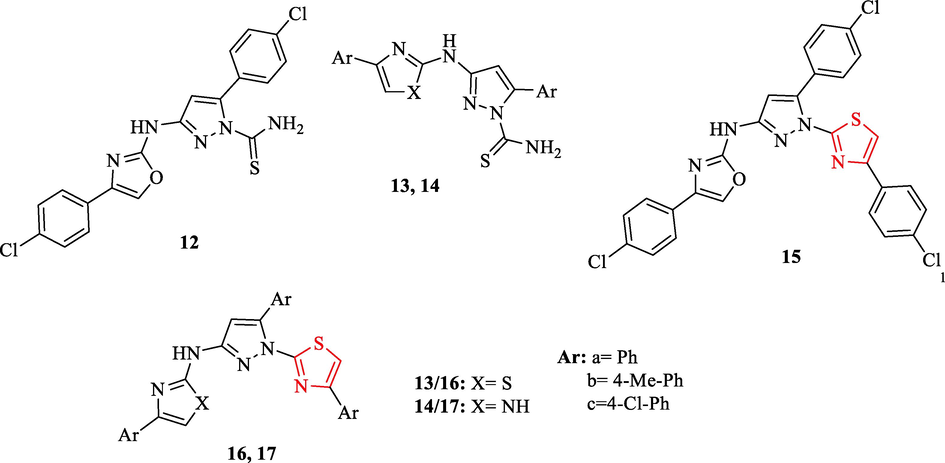
Chemical structures 12, 13a-c, 14a-c, 15, 16a-c, and 17a-c (Prakash et al., 2014)
Antifungal activity evaluations showed structures 17a and 17c had excellent activity, especially against A. niger greater than the reference drug (Table 3 and Fig. 4) (Prakash et al., 2014)
A novel series of derivatives containing thiazole were synthesized by Desai and co-workers. In this study, prepared twelve analogues and investigated their antimicrobial activities against 4 bacteria and 3 fungal strains. Structures 18a, 18b, and 18 h showed good antibacterial activity against S. pyogenes in comparison to chloramphenicol as a reference drug (Fig. 5 and Table 4). Whereas structures 18a and 18 g displayed good activity against S. Aureus (Fig. 5 and Table 4). Also, structures 18b and 18 h showed very good activity against S. aureus, E. coli, and P. Aeruginosa (Fig. 5 and Table 4). Whereas structures 18c and 18i had excellent activity against S. aureus and P. Aeruginosa (Fig. 5 and Table 4) (Desai et al., 2015)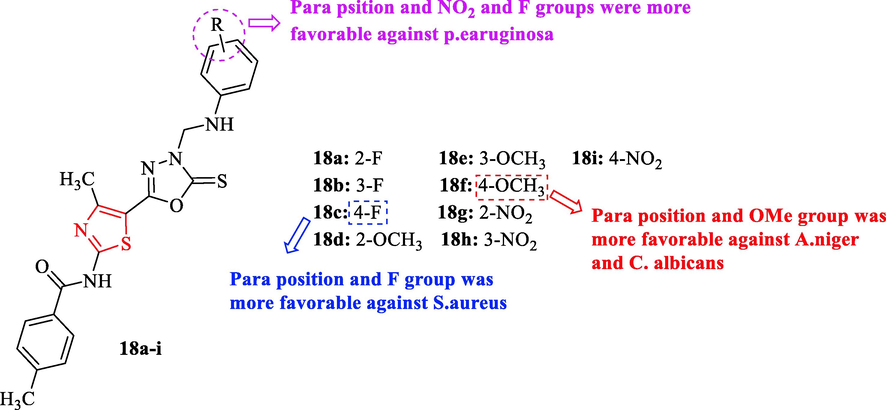
Chemical structures 18a-i (Desai et al., 2015)
Cpd. no.
MIC (μg/mL)
E. coli
MTCC 443S. aureus
MTCC 96P. aeruginosa MTCC 1688
S. pyogenes
MTCC 442A. niger
MTCC 282A. clavatus MTCC 1323
C. albicans
MTCC 227
18a
100
100
18b
50
50
50
100
18c
12.5
12.5
18d
100
18e
100
100
18f
25
25
18 g
100
18 h
50
50
50
18i
25
12.5
C
50
50
50
50
K
50
50
50
According to antifungal studies, structure 18f showed the most potent activity against C. albicans MTCC 227 and A. niger MTCC 282 in comparison to ketoconazole as a reference drug (Fig. 5 and Table 4). Among all derivatives, structures 18d and 18e had good activity against A. clavatus. Moreover, structure 18e exhibited good activity against A. niger (Fig. 5 and Table 4) (Desai et al., 2015)
Basha and co-workers synthesized a series of aroyl-4-heteroaryl pyrazoles and pyrroles containing thiazole scaffold and evaluated their antimicrobial activity against 4g-negative and positive bacteria and 2 fungal strains (Fig. 6 and Table 5). Structures 20c, 20d, 20f, 21d, and 21f were active against all bacteria strains, especially Bacillus subtilis in comparison to chloramphenicol as a reference drug (Fig. 6 and Table 5). Whereas structures 20d and 20f showed marginally higher activity against all tested concentrations (12.5, 25, 50, and 100 μg/well) toward the reference drug (Table 4). Structures 20a, 21a, and 21c displayed moderate activity, while 20b, 20e, 21b, and 21e exhibited the least activity. In addition, derivatives 20 and 21 (aromatized structures) showed higher activity than derivatives 19 (non-aromatized structures) (Fig. 6 and Table 5) (Basha et al., 2015)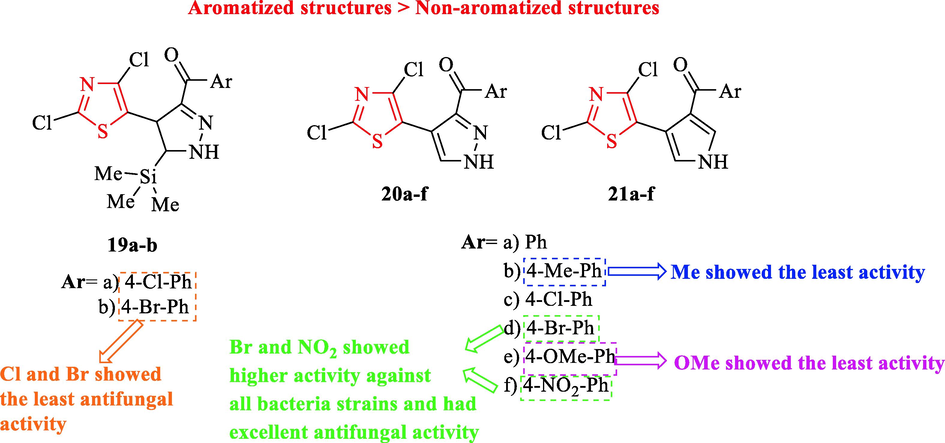
Chemical structures of 19a-b, 20a-f, and 21a-f (Basha et al., 2015)
Cpd. no.
concentrations
Zone of Inhibition (mm)
Gram-Psitive bacteria
Gram-Negative bacteria
Aspergillus niger
Staphylococcus aureus
Bacillus subtilis
Pseudomonas aeruginosa
Klebsiella pneumoniae
19a
100 μg/well
8 ± 2
19b
100 μg/well
9 ± 3
20b
12.5 μg/well
13 ± 3
12 ± 3
9 ± 3
14 ± 3
25 μg/well
15 ± 2
14 ± 2
11 ± 1
16 ± 2
50 μg/well
18 ± 3
16 ± 1
14 ± 1
18 ± 3
100 μg/well
22 ± 2
19 ± 1
15 ± 3
19 ± 1
10 ± 3
20c
12.5 μg/well
24 ± 1
28 ± 1
16 ± 2
24 ± 2
25 μg/well
26 ± 1
30 ± 2
18 ± 1
26 ± 3
50 μg/well
28 ± 2
32 ± 3
20 ± 2
28 ± 2
100 μg/well
30 ± 3
35 ± 2
23 ± 3
31 ± 1
34 ± 2
20d
12.5 μg/well
26 ± 1
31 ± 2
18 ± 2
27 ± 1
25 μg/well
27 ± 3
33 ± 4
19 ± 1
29 ± 3
50 μg/well
30 ± 1
36 ± 2
22 ± 3
32 ± 2
100 μg/well
32 ± 3
41 ± 1
24 ± 3
33 ± 2
36 ± 2
20e
12.5 μg/well
11 ± 2
11 ± 2
–
–
25 μg/well
13 ± 1
12 ± 2
–
–
50 μg/well
14 ± 3
15 ± 3
10 ± 3
–
100 μg/well
17 ± 3
16 ± 1
12 ± 2
10 ± 2
9 ± 1
20f
12.5 μg/well
27 ± 1
32 ± 2
17 ± 2
27 ± 1
25 μg/well
29 ± 3
35 ± 4
20 ± 1
30 ± 3
50 μg/well
31 ± 1
38 ± 2
22 ± 3
33 ± 2
100 μg/well
33 ± 3
42 ± 1
25 ± 3
34 ± 2
36 ± 2
21a
12.5 μg/well
15 ± 2
14 ± 3
11 ± 2
16 ± 2
25 μg/well
17 ± 1
16 ± 2
14 ± 3
17 ± 1
50 μg/well
19 ± 2
17 ± 2
15 ± 1
20 ± 3
100 μg/well
23 ± 1
21 ± 2
17 ± 3
22 ± 1
21b
12.5 μg/well
9 ± 2
10 ± 3
–
–
25 μg/well
11 ± 1
12 ± 2
–
–
50 μg/well
12 ± 2
14 ± 2
–
–
100 μg/well
14 ± 1
15 ± 1
10 ± 3
–
21c
12.5 μg/well
21 ± 2
20 ± 3
14 ± 2
20 ± 1
25 μg/well
23 ± 2
22 ± 2
15 ± 1
22 ± 2
50 μg/well
24 ± 1
25 ± 1
16 ± 2
23 ± 1
100 μg/well
27 ± 3
27 ± 1
19 ± 3
25 ± 2
21d
12.5 μg/well
23 ± 2
27 ± 2
15 ± 2
22 ± 2
25 μg/well
25 ± 1
28 ± 3
16 ± 2
23 ± 1
50 μg/well
26 ± 3
31 ± 1
18 ± 1
26 ± 3
100 μg/well
28 ± 4
34 ± 2
21 ± 5
29 ± 2
21e
12.5 μg/well
8 ± 2
9 ± 1
–
–
25 μg/well
10 ± 1
10 ± 3
–
–
50 μg/well
12 ± 1
12 ± 2
–
–
100 μg/well
13 ± 1
14 ± 3
9 ± 3
–
21f
12.5 μg/well
24 ± 2
29 ± 2
15 ± 2
24 ± 2
25 μg/well
27 ± 1
30 ± 3
17 ± 2
25 ± 1
50 μg/well
28 ± 3
32 ± 1
18 ± 1
27 ± 3
100 μg/well
29 ± 4
34 ± 2
21 ± 5
28 ± 2
chloramphenicol
12.5 μg/well
28 ± 1
30 ± 1
23 ± 1
36 ± 2
25 μg/well
30 ± 3
32 ± 3
25 ± 2
38 ± 1
50 μg/well
33 ± 1
34 ± 2
27 ± 3
40 ± 2
100 μg/well
35 ± 2
38 ± 1
30 ± 2
42 ± 3
Ketoconazole
100 μg/well
36 ± 1
On the other hand, Structures 20c, 20d, and 20f displayed excellent antifungal activity toward ketoconazole as a reference drug against Aspergillus niger at 100 μg (Fig. 6 and Table 5). In the case of Aspergillus niger at 100 μg, structures 19a, 19b, 21b, and 21e demonstrated the least activity (Fig. 6 and Table 5) (Basha et al., 2015)
El-Kady et al. reported some structures containing thiazole scaffold and evaluated their antimicrobial activity against 2g-negative and positive and 1 fungal strain (Fig. 7 and Table 6). Among all derivatives, structures 22, 23a, 24a, and 25a had a wide spectrum of antimicrobial activity against Staphylococcus aureus NCINB 50080 and E. coli ATCC 1177 compared to ciprofloxacin as a reference drug, and structures 26, 27, 23b, 24b, 25b, and 25c effective against E. coli ATCC 1177 (Fig. 7 and Table 6). In addition, 28 and 29 were effective against Staphylococcus aureus NCINB 50080 (Fig. 7 and Table 6) (El-Kady et al., 2016)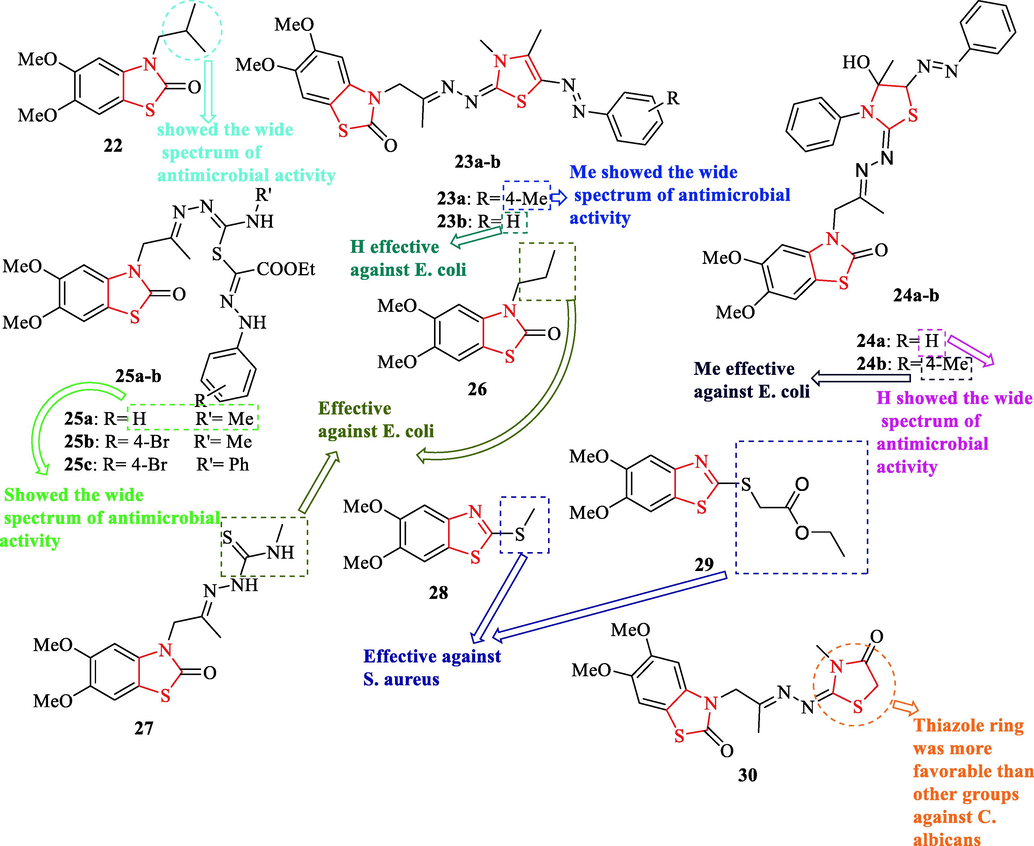
Chemical structures of 22, 23a-b, 24a-b, 25a-c, 26, 27, 28, 29, and 30 (El-Kady et al., 2016)
Cpd. no.
Inhibition zones (mm)
E. coli ATCC 1177
S. aureus NCINB 50,080
C. albicans ATCC10231
22
11
9
23a
10
10
23b
10
24a
10
9
24b
10
25a
10
8
25b
9
25c
10
26
10
27
8
28
10
29
10
30
19
Ciprofloxacin
20
21
AMB20
20
Antifungal activity evaluation showed structure 30 had remarkable activity toward AMB20 as a reference drug (Fig. 7 and Table 6) (El-Kady et al., 2016)
A series of ethyl 2-(2-(4-substituted)acetamido)-4-subtituted-thiazole-5-carboxylates (Fig. 8) were synthesized by Pawar and co-workers and their in vitro antimicrobial activities were tested against 3 bacteria (Table 7). In comparison, levofloxacin was used as a reference drug. The result of antibacterial activity revealed that structures 31a, 31b, 31c, 31d, and 32 had the most active and potent antimicrobial activity against B. subtilis, E. Coli, and S. aureus toward the reference drug (Fig. 8 and Table 7). Moreover, the same structures had potent antifungal activity against C. albicans, A. flavus, and A. niger as fungal strains which were chosen for the investigation of antifungal activity compared to miconazole and fluconazole as reference drugs (Fig. 8 and Table 7) (Pawar et al., 2016)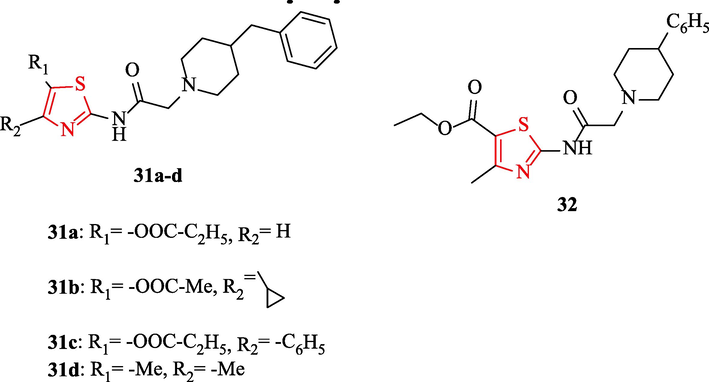
Chemical structures of 31a-d and 32 (Pawar et al., 2016)
Cpd. no.
MIC values (μg/mL)
B. subtilis
E. coli
S. aureus
C. Albicans
A. Flavus
A. Niger
31a
29
29
28
25
12.5
12.5
31b
29
29
28
25
12.5
12.5
31c
25
29
28
50
25
25
31d
28
29
30
25
12.5
12.5
32
28
29
30
25
12.5
25
Levofloxacin
29
29
28
Fluconazole
40
25
25
Miconazole
12.5
12.5
12.5
A novel series of 6-(5-Methyl-1H-1,2,3-triazol-4-yl)-5-[(2-(thiazol-2-yl)hydrazono)methyl]imidazo[2,1-b]thiazole derivatives (Fig. 9) were synthesized by Abdel-Wahab et al. All derivatives and vancomycine (As a reference drug) were tested against 6 bacteria and 2 fungal strains (Fig. 9). According to antibacterial studies, structure 33 exhibited significant antibacterial activity against all bacteria toward the reference drug. Whereas structures 34 and 35 demonstrated good activities against Staphelococcus aureus (Fig. 9). In addition, the same structures had good antifungal activities against Saccharomyces cervesia and Candida albicans NRRL Y-477 toward the reference drug (Fig. 9) (Abdel-Wahab et al., 2017)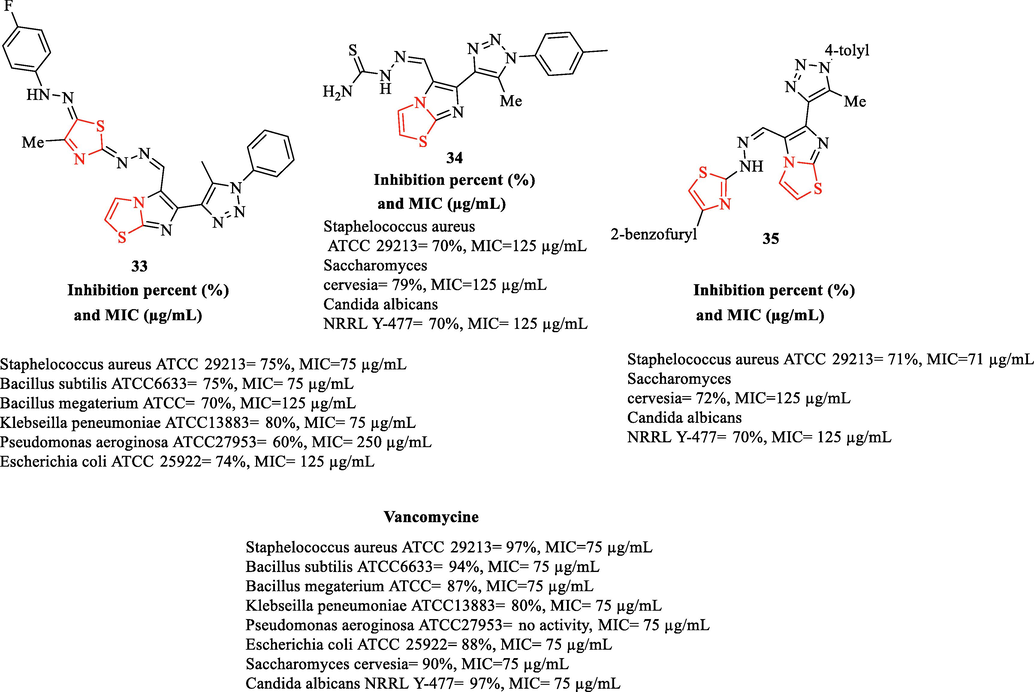
Chemical structures of 33–35 and inhibition and MIC values of the structures 33–35 (Abdel-Wahab et al., 2017)
Liao et al. synthesized a novel series of 2-substituted phenoxy-N-(4-substituted phenyl-5-(1H-1,2,4-triazol-1-yl)thiazol-2-yl)acetamide derivatives (Fig. 10). All derivatives were tested against 2 bacteria and three fungal strains (Table 8). Bismerthiazol and hymexazol were used as antibacterial and antifungal reference drugs, respectively. Antibacterial studies showed four structures (36a, 36b, 36c, and 36d) had potent inhibition effects against Xoo and exhibited potent inhibition effects against Xac toward the reference drug (Fig. 10 and Table 8). In addition, EC50 values of the same structures were good, which seem to be more potent than bismerthiazole. In addition, Structures 36b, 36c, and 36d (R1 = OMe) had better activity compared to structures 36 h, 36i, and 36j (R1 = 4-Cl) against xoo (Fig. 10 and Table 8). Whereas structures 36 h, 36i and 36j (R1 = 4-Cl) had better activity compared to structures 36e, 36f and 36 g (3,4-di-Cl) (Fig. 10 and Table 8) (Liao et al., 2017)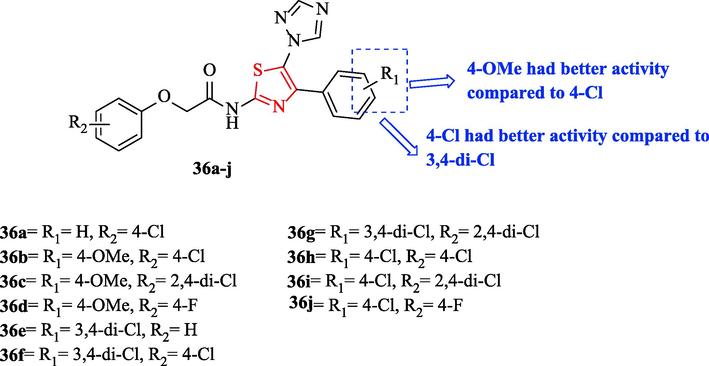
Chemical structures 36a-j (Liao et al., 2017)
Cdp. no.
Average values of inhibition rate (%)
Xanthomonas oryzae pv. oryzae
Xanthomonas axonopodis pv. citri
G. zeae
200 μg/mL
100 μg/mL
200 μg/mL
100 μg/mL
36a
100.0 ± 1.8
98.2 ± 2.2
80.2 ± 1.2
64.1 ± 1.8
36b
95.2 ± 1.5
78.1 ± 2.1
80.2 ± 2.1
57.4 ± 1.6
36c
100.0 ± 3.0
99.0 ± 1.2
55.2 ± 2.6
31.3 ± 3.1
36d
90.3 ± 2.2
77.6 ± 1.4
66.1 ± 0.8
46.8 ± 1.6
36e
75.0 ± 2.0
67.4 ± 4.1
36f
31.2 ± 1.8
26.6 ± 1.3
36 g
33.9 ± 1.7
21.3 ± 1.5
36 h
86.8 ± 1.7
61.3 ± 2.1
36i
37.0 ± 1.5
28.9 ± 1.1
36j
81.7 ± 1.8
60.8 ± 1.4
50.53 ± 1.72
Bismerthiazol
72.4 ± 3.1
54.2 ± 1.2
77.5 ± 1.4
50.0 ± 2.2
Hymexazol
52.23 ± 2.86
According to antifungal activity studies, structure 36j displayed high activity against G. zeae toward the reference drug (Fig. 10 and Table 8) (Liao et al., 2017)
Shankerrao et al. synthesized a novel series of imidazothiazoles of benzofuran. The antimicrobial activities of structures were tested against 4 bacteria and 2 fungal strains with respect to ampicillin and flucanozole as antibacterial and antifungal reference drugs, respectively (Fig. 11 and Table 9). Among all derivatives, structures 37e and 37d displayed the best result against E. coli and S. Aureus (Fig. 11 and Table 9). In addition, structures 38b and 37a had moderate activity against E. coli and Klbesilla pneumona, while the same structures had an excellent effect against S. aureus and P. aeruginosa (Fig. 11 and Table 9) (Shankerrao et al., 2017)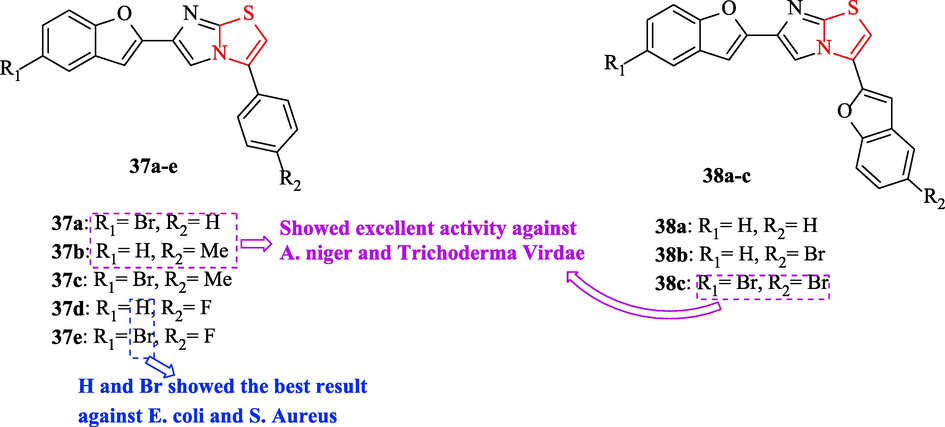
Chemical structures of 37a-e and 38a-c (Shankerrao et al., 2017)
Cpd. no.
Zone of inhibition (mm)
S. aureus
K. pneumona
P. aeruginosa
E. Coli
A. Niger
T. viradae
50 μL
100 μL
50 μL
100 μL
50 μL
100 μL
50 μL
100 μL
50 μL
100 μL
50 μL
100 μL
37a
20
27
16
22
18
25
15
18
19
22
15
19
37b
22
26
24
26
37c
14
20
23
25
37d
14
17
18
20
37e
18
22
16
20
38a
14
16
13
18
38b
23
28
13
18
18
24
12
16
38c
23
26
21
25
Ampicillin
24
30
28
32
20
28
28
31
Fulconozol
28
34
28
30
According to antifungal activity studies, three structures (37a, 37b, and 38c) had excellent activity against A. niger and Trichoderma Virdae compared to other structures and the reference drug (Fig. 11 and Table 9). Whereas two structures (37c and 38a) had moderate activity against A. niger and Trichoderma Virdae (Fig. 11 and Table 9) (Shankerrao et al., 2017)
A novel series of 2-(benzo[d]thiazol-2-yl)phenyl-4-nitrophenyl alkyl/aryl substituted phosphoramidates were reported by Reddy et al. The derivatives were tested against 3 bacteria and 2 fungal strains in comparison to ampicillin and antibiotic as antimicrobial and antifungal reference drugs, respectively (Fig. 12 and Table 10). Among the derivatives, structures 39c, 39d, and 39 h had the best inhibition activity against Escherichia coli, Streptococcus aureus ATCC-25923, and Bacillus subtilis ATCC-1789 toward the reference drug (Fig. 12 and Table 10). In addition, structure 39e demonstrated good activity against Bacillus subtilis (Fig. 12 and Table 10) (Reddy et al., 2018)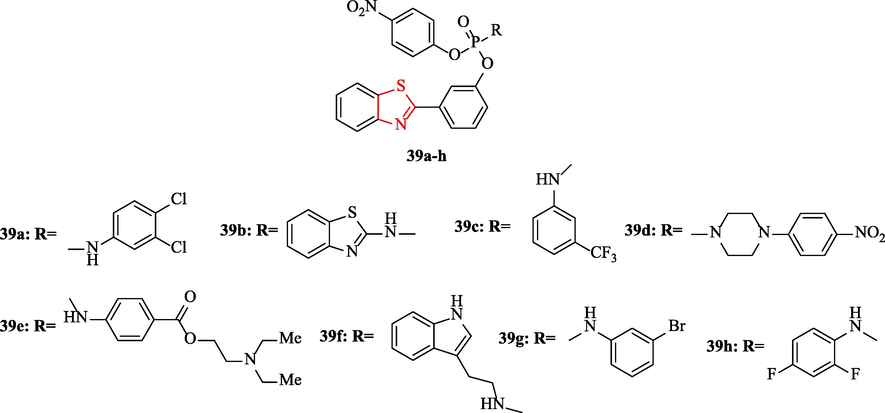
Chemical structures 39a-h (Reddy et al., 2018)
Cpd. no.
Zone of inhibition (mm)
Streptococcus aureus
(ATCC-25923)Bacillus subtilis
(ATCC-1789)Escherichia coli
(ATCC-9637)Aspergillus niger
(MTCC-1881)Candida albicans
(ATCC- 2091)
50 µg/mL
100 µg/mL
50 µg/mL
100 µg/mL
50 µg/mL
100 µg/mL
50 µg/mL
100 µg/mL
50 µg/mL
100 µg/mL
39a
10.7
20.2
9.4
18.7
39b
11.5
21.2
10.8
21.4
39c
14.5
21.7
12.4
22.1
11.8
24.2
12.9
19.8
11.5
20.7
39d
11.0
20.7
12.2
21.7
12.4
20.3
10.3
19.7
39e
10.5
19.6
39f
10.8
19.3
39 g
12.1
18.6
9.7
19.7
39 h
10.8
21.9
10.2
22.4
11.4
22.6
10.4
19.3
Ampicillin
15.9
22.0
17.5
24.0
16.9
23.0
Fulconozol
15.7
22.5
16.5
24.0
On the other hand, antifungal activity studies against 2 fungal strains at two concentrations, 50 and 100 μg/mL showed structures 39b, 39c, and 39 g had the best activity against C. albicans and A. niger at 100 µg/mL toward the reference drug (Fig. 12 and Table 10). In addition, structure 39a exhibited good activity against C. albicans and A. niger (Fig. 12 and Table 10). Whereas structures 39d and 39 h showed good activity against C. Albicans (Fig. 12 and Table 10). Moreover, structure 39f displayed good activity against A. niger (Fig. 12 and Table 10) (Reddy et al., 2018)
Ansari and Khan synthesized some of the structures containing thiazole scaffold. The derivatives were tested against 10g-negative and positive bacteria in comparison to ofloxacin as a reference drug. Structures 40a, 40c, and 40b had a higher activity with respect to other ones (Fig. 13 and Table 11). Structure 40c had the best activity against P. vulgaris, E. coli, and P. Aeruginosa (Fig. 13 and Table 11). Structure 40b had the highest activity against K. pneumonia and structure 40d displayed the highest activity against B. bronchiseptica. Moreover, structures 40a and 40b showed good activity against E. coli and P. Aeruginosa (Fig. 13 and Table 11) (Ansari and Khan, 2017)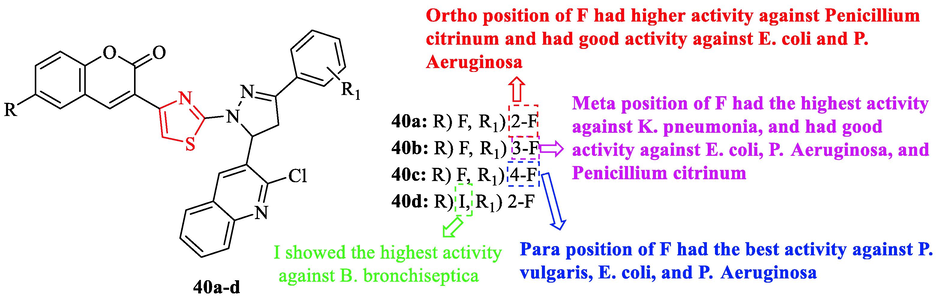
Chemical structures 40a-d (Ansari and Khan, 2017)
Cpd. no.
Zone of inhibition (mm) and MIC (μg/mL)
E. coli
P. aeruginosa
K. pneumonia
B. bronchiseptica
P. vulgaris
P. citrinum
40a
27.50 ± 0.33(50)
30.19 ± 0.32 (50)
30.94 ± 0.32 (50)
40b
26.67 ± 0.31 (50)
29.0 ± 0.41 (50)
31.30 ± 0.32 (50)
29.90 ± 0.31 (50)
40c
28.71 ± 0.30 (50)
30.28 ± 0.41 (50)
29.74 ± 0.28 (50)
40d
29.84 ± 0.31 (50)
Ofloxacin
31.61 ± 0.41 (12.5)
34.23 ± 0.14 (12.5)
31.55 ± 01.9 (12.5)
34.80 ± 0.24 (25)
32.0 ± 0.3 (12.5)
Ketoconazole
28.68 ± 0.31 (25)
All 25 derivatives were tested against 5 fungal strains with respect to Ketoconazole as a reference drug. According to the results obtained, structures 40a and 40b had higher activity against Penicillium citrinum toward the reference drug (Fig. 13 and Table 11) (Ansari and Khan, 2017).
In 2019, a series of novel benzothiazole linked to acetohydrazide, carboxamide, and sulfonamide scaffolds (Fig. 14 and Table 12) were reported by Fadda and co-workers. Four bacteria were used in the antimicrobial activity evaluation (Table 12). Chloramphenicol and cephalothin were chosen as reference drugs. Structures 41a and 41b had broad-spectrum antibacterial activity against all organisms (Fig. 14 and Table 12). The same structures showed equipotent activity to chloramphenicol in inhibiting the growth B. subtilis. Whereas their activities were fifty percent lower than chloramphenicol against Bacillus thuringiensis. In the case of 45, 46, 47a-d, and 48, all structures showed good to moderate activity against gram-positive bacteria (Fig. 14. and Table 12). Structures 43 and 44 showed weak growth inhibitory with respect to the reference drugs against B. subtilis and B. Thuringiensis (Fig. 14 and Table 12). Structures 41a and 41b displayed good to moderate growth inhibitory against B. subtilis and B. thuringiensis toward the reference drugs (Fig. 14 and Table 12) (Fadda et al., 2019)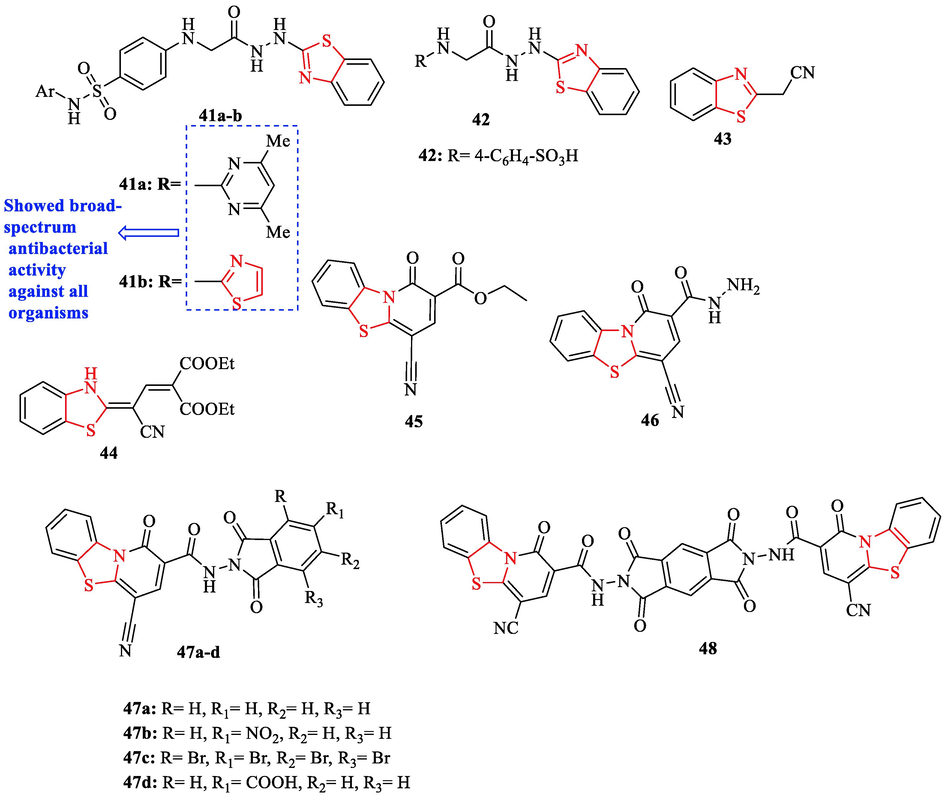
Chemical structures 41a-b, 42–46, 47a-d, and 48 (Fadda et al., 2019)
Cpd. no.
Minimal inhibitory concentration (MIC in μg/mL) and inhibition zone (mm)
Bacteria
Fungi
B. subtilis
B. thuringiensis
E. coli
P. aeruginosa
F. oxysporum
B. fabae
41a
3.125 (45)
6.25 (40)
12.5 (21)
50.0 (16)
12.5 (30)
12.5 (32)
41b
3.125 (44)
6.25 (45)
12.5 (26)
50.0 (18)
25.0 (30)
25.0 (33)
42
50.0 (20)
50.0 (21)
43
50.0 (24)
50.0 (26)
44
50.0 (20)
50.0 (25)
45
50.0 (25)
50.0 (22)
46
25.0 (24)
50.0 (26)
47a
12.5 (33)
50.0 (17)
47b
12.5 (33)
12.5 (34)
47c
12.5 (35)
50.0 (17)
47d
12.5 (36)
25.0 (27)
48
12.5 (35)
25.0 (26)
Chloramphenicol
3.125 (44)
3.125 (44)
6.25 (37)
6.25 (38)
Cephalothin
6.25 (36)
6.25 (37)
6.25 (38)
6.25 (37)
Cycloheximide
3.125 (43)
3.125 (42)
According to antifungal activity studies, all derivatives were tested against F. oxysporum and B. fabae. Cycloheximide served as a reference drug. structures 41b and 42 exhibited moderate activity against B. fabae and F. Oxysporum (Fig. 14 and Table 12). Moreover, structure 41a had the best inhibition compared to other structures (Fig. 14 and Table 12) (Fadda et al., 2019)
Deshineni et al. synthesized a new series of ethyl-2-(3-((2-(4-(4-aryl)thiazol-2-yl)hydrazono)methyl)-4-hydroxy/isobutoxyphenyl)-4-methylthiazole-5 carboxylates by one-pot multi-component approach (Fig. 15). In this investigation, 4 bacteria and 2 fungal strains were used. Levofloxacin and miconazole were used as antibacterial and antifungal reference drugs. The result showed structures 49a, 49c, 50b, and 50c had a wide spectrum of antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Azatobacter with respect to the reference drug (Fig. 15 and Table 13). Further, Structures 49a and 50a displayed good activity against S. aureus, B. subtilis, E. coli, and Azatobacter (Fig. 15 and Table 13) (Deshineni et al., 2020)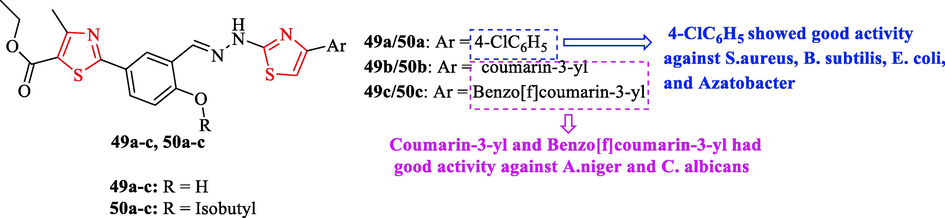
Chemical structures 49a-c and 50a-c (Deshineni et al., 2020)
Cpd. no.
MIC (μg/mL)
Staphylococcus
aureusBacillus
subtilisEscherichia
coliAzatobacter
Candida
albicansAspergillus
niger
49a
35
30
25
20
49b
15
20
20
25
12.5
12.5
49c
15
25
20
25
12.5
12.5
50a
25
25
20
15
50b
15
15
20
20
12.5
25
50c
20
20
20
25
12.5
12.5
Levofloxacin
15
20
16
18
Miconazole
12.5
12.5
Antifungal activity of all derivatives demonstrated structures 49b, 49c, 50b, and 50c had good activity against A.niger MTCC 282 and C. albicans MTCC 227 toward the reference drug (Fig. 15 and Table 13) (Deshineni et al., 2020)
Althagafi et al. synthesized a novel series of thiazole derivatives. As reference drugs, ampicillin and gentamicin were used. According to the results, structures 51, 53a, and 54 exhibited more potent activity against Staphylococcus aureus compared to ampicillin with preliminary antimicrobial activity values of 24.3 ± 0.63, 23.8 ± 0.72, and 24.1 ± 1.20 μg/ml, respectively (Fig. 16 and Table 14). Structures 55 and 56 displayed more potent activity against Salmonella typhimurium compared to gentamicin (Fig. 16 and Table 14) (Althagafi et al., 2019)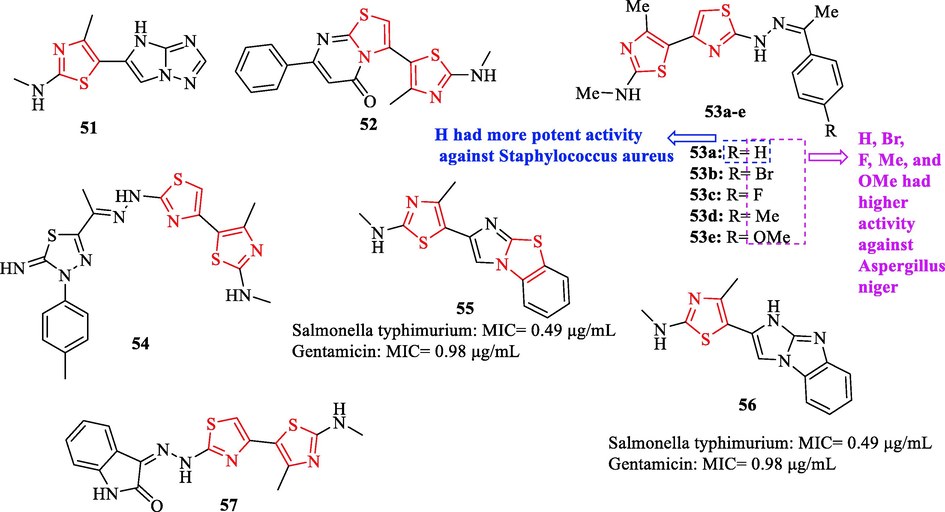
Chemical structures of 41–52, 53a-e, and 54–57 (Althagafi et al., 2019)
Cpd. no.
Preliminary antimicrobial activity
Aspergillus niger
51
25.4 ± 1.20
52
24.3 ± 1.20
53a
25.0 ± 0.72
53b
21.8 ± 0.43
53c
25.2 ± 2.10
53d
24.1 ± 0.85
53e
26.3 ± 0.63
54
25.2 ± 2.10
57
26.3 ± 0.63
Amphotericin B
23.3 ± 0.58
Among all derivatives tested against two fungal strains, structures 51, 52, 53a, 53b, 53c, 53d, 53e, 54, and 57 exhibited higher activity against Aspergillus niger compared to amphotericin B as an antifungal reference drug (Fig. 16 and Table 14) (Althagafi et al., 2019)
A new series of N‑(4‑(4‑bromophenyl)thiazol‑2‑yl)‑2‑chloroacetamides were synthesized by Sharma and co-workers. The derivatives were tested against 3 bacteria and 2 fungal strains. As antibacterial and antifungal reference drugs, norfloxacin and fluconazole were used, respectively. Among the derivatives, structures 58, 59, and 60 had the best antimicrobial activity (Fig. 17). The antimicrobial activity results showed structure 60 had remarkable activity against S. Aureus (Fig. 17). Structure 58 had high activity against E. Coli and structure 59 had potent activity against B. Subtilis and E.coli (Fig. 17) (Sharma et al., 2019a)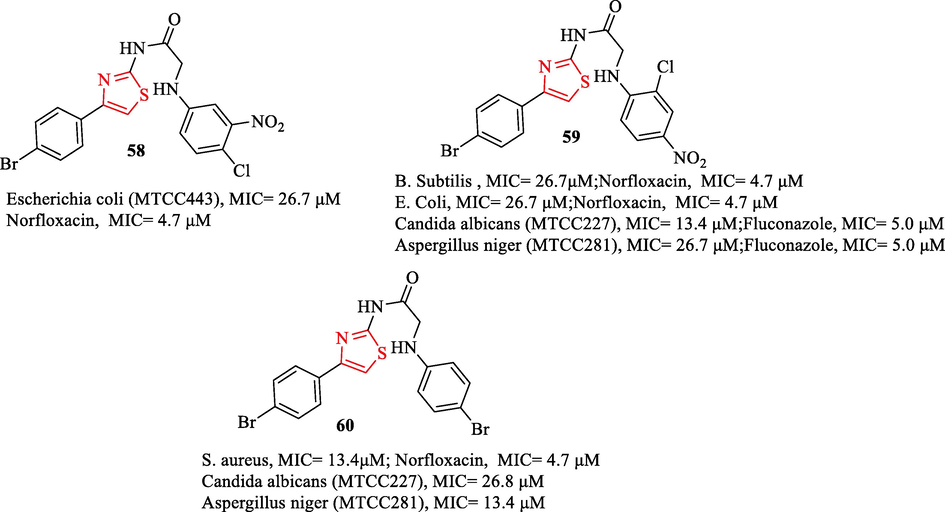
Antimicrobial activity of the structures 58–60 (Sharma et al., 2019a)
Antifungal activity results were found to structures 59 and 60 had high activity against 2 fungal strains (Fig. 17) (Sharma et al., 2019a)
A novel series of 4‑(4‑Bromophenyl)‑thiazol‑2-amines were synthesized by Sharma et al. All derivatives were tested against 3 bacteria and norfloxacin was used as a reference drug. Among all derivatives, structures 61, 62, 63, and 64 showed good antimicrobial activity toward the reference drug (Fig. 18). In vitro antimicrobial results showed structure 61 had good potential against E. Coli and S. Aureus (Fig. 18). Moreover, structure 63 had good potential against B. Subtilis (Fig. 18) (Sharma et al., 2019b)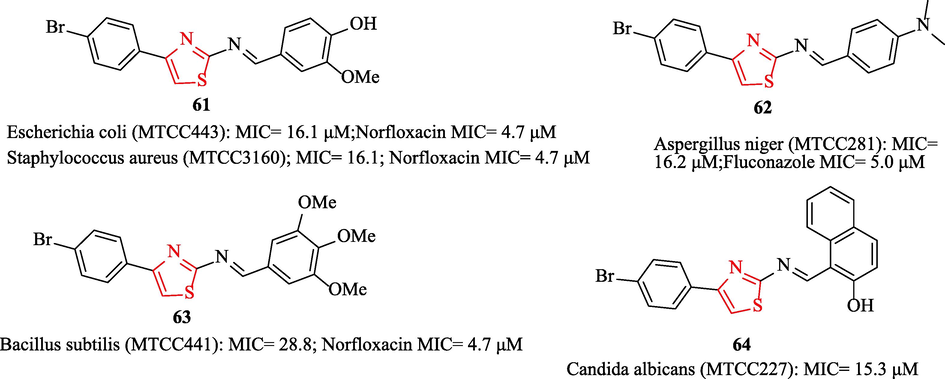
Antimicrobial activity of the structures 61–64 (Sharma et al., 2019b)
All derivatives were tested against 2 fungal strains in comparison to fluconazole as a reference drug. Antifungal activity results showed structure 64 had remarkable activity against C. Albicans and structure 62 had the highest potent against A. niger (Fig. 18) (Sharma et al., 2019b)
Sirakanyan et al. synthesized a novel series of derivatives containing thiazole scaffold which evaluated 11 derivatives against 6 bacteria with respect to ampicillin and streptomycin as reference drugs. Among all eleven derivatives synthesized, structures 65d, 65c, and 65f had lower activity against Pseudomonas aeruginosa toward streptomycin (Fig. 19 and Table 15). Structures 65f had lowes MIC against Bacillus cereus toward ampicillin (Fig. 19 and Table 15). Whereas structures 65b and 65c exhibited moderated activity against the same organism (Fig. 19 and Table 15). Structure 65f showed good activity against Listeria monocytogenes toward the reference drugs (Fig. 19 and Table 15) (Sirakanyan et al., 2021)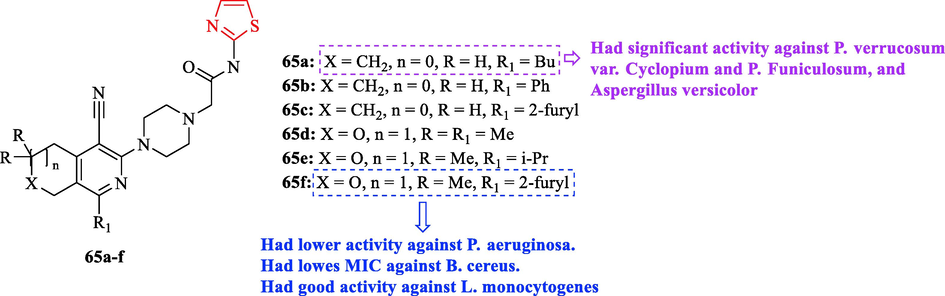
Chemical structures 65a-f (Sirakanyan et al., 2021)
Cpd. no.
Bacterial strains
Fungal strains
Pseudomonas
aeruginosaBacillus
cereusListeria
monocytogenesAspergillus
versicolorPenicillium
funiculosumPenicillium verrucosum
var. cyclopium
65a
MIC
0.17 ± 0.001
0.17 ± 0.001
0.17 ± 0.001
MFC
0.23 ± 0.001
0.23 ± 0.002
0.23 ± 0.002
65b
MIC
0.47 ± 0.002
MBC
0.94 ± 0.01
65c
MIC
0.23 ± 0.002
0.35 ± 0.003
0.23 ± 0.002
MBC
MFC
0.47 ± 0.003
0.47 ± 0.003
0.47 ± 0.003
65d
MIC
0.23 ± 0.002
MBC
0.47 ± 0.003
65e
MIC
0.23 ± 0.001
MFC
0.47 ± 0.004
65f
MIC
0.23 ± 0.001
0.06 ± 0.001
0.23 ± 0.002
MBC
0.47 ± 0.003
0.11 ± 0.001
0.47 ± 0.006
Streptomycin
MIC
0.05 ± 0.001
0.025 ± 0.0003
0.15 ± 0.001
MBC
0.1 ± 0.002
0.05 ± 0.001
0.3 ± 0.002
Ampicillin
MIC
0.2 ± 0.002
0.1 ± 0.002
0.15 ± 0.001
MBC
–
0.15 ± 0.002
0.3 ± 0.003
Bifon-
Azole
MIC
MFC
0.1 ± 0.001
0.2 ± 0.0020.2 ± 0.002
0.25 ± 0.0020.1 ± 0.001
0.2 ± 0.002
Ketoco-
nazole
MIC
MFC
0.2 ± 0.002
0.5 ± 0.0040.2 ± 0.001
0.5 ± 0.0020.2 ± 0.002
0.3 ± 0.01
All derivatives tested against six fungal strains toward ketoconazole and bifonazole as reference drugs. according to the results, structures 65a, 65c, and 65e had high activity against Aspergillus versicolor compared to ketoconazole (Fig. 19 and Table 15). Moreover, structure 65a had significant activity against P. verrucosum var. Cyclopium and P. Funiculosum toward ketoconazole (Fig. 19 and Table 15) (Sirakanyan et al., 2021)
In 2020, a series of novel thiazolyl-1,2,3-triazolyl-alcohol derivatives synthesized by Jagadale et al. In this study, were used 2 bacteria. Streptomycin was used as a reference drug. Among all derivatives, structures 66b, 66f and 76i showed good antibacterial activity against S. albus toward the reference drug (Fig. 20 and Table 16) (Jagadale et al., 2020)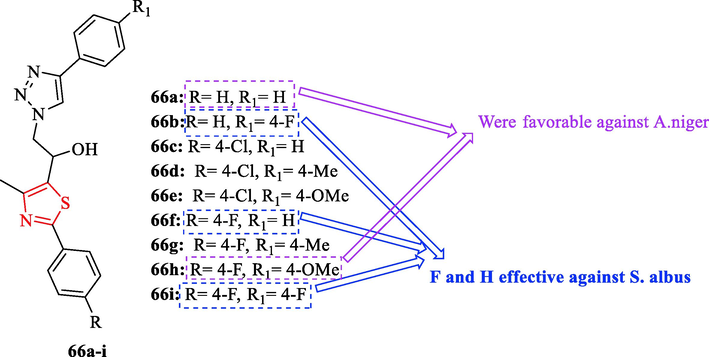
Chemical structures 66a-i (Jagadale et al., 2020)
Cpd. no.
MIC (μg/mL)
A.niger
S.albus
66a
31.25
66b
62.5
66c
62.5
66d
62.5
66e
62.5
66f
62.5
62.5
66 g
62.5
66 h
31.25
66i
62.5
62.5
Streptomycin
7.81
Fluconazole
7.81
Ravuconazole
31.25
Antifungal activity studies which were tested against 4 fungal strains showed structures 66a, 66c, 66d, 66e, 66f, 66 g, 66 h, and 66i had remarkable activity against A. niger compared to ravuconazole as a reference drug (Fig. 20 and Table 16) (Jagadale et al., 2020)
Patel and co-workers reported 4-thiazolidinone fused pyrimidines. Ten derivatives were tested against 4 bacteria with respect to ciprofloxacin as a reference drug. Among all derivatives, structures 67c and 67d (2 and 3-CH3) and 67a (3-NO2 had good activity against P.aeruginosa MTCC 741 and E.coli MTCC 442 toward the reference drug (Fig. 21 and Table 17). In addition, structures 67b (2-Cl) and 67d (3-CH3) showed good activity against S.aureus MTCC 96 and S.pyogenus MTCC 443 with respect to the reference drug (Fig. 21 and Table 17) (Patel et al., 2020)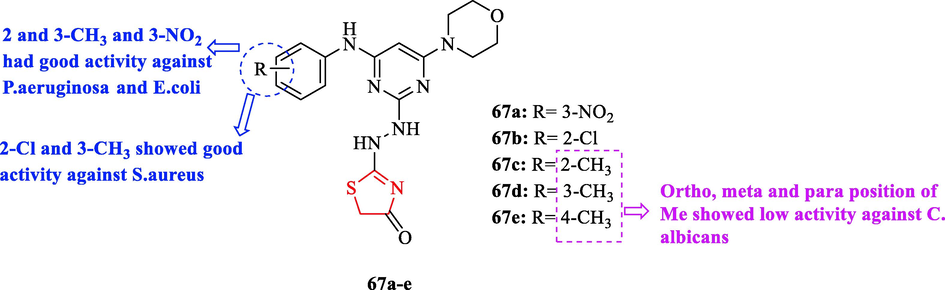
Chemical structures 67a-e (Patel et al., 2020)
Cpd. no.
MIC (μg/mL)
S.aureus
MTCC 96S.pyogenus
MTCC 443P.aeruginosa
MTCC 741E.coli
MTCC 442C.albicans
MTCC 227
67a
62.5
100
67b
100
250
67c
100
100
250
67d
100
100
100
125
250
67e
250
Ciprofloxacin
50
50
25
25
Nystatin
100
All derivatives were tested against 3 fungal strains. In between, structures 67c, 67d, and 67e had low activity to some extent against C. Albicans (Fig. 21 and Table 17) (Patel et al., 2020)
Shinde et al. evaluated the antibacterial activity of a series of 2-(4-(benzo[d]thiazol-5-ylsulfonyl) piperazine-1-yl)-N-substituted acetamides against 4 bacteria with respect to ciprofloxacin as a reference drug. Among all derivatives, structures 68a, 68c, 68e, 68 g, and 68 k showed moderate activity against E.coli and P. aeruginosa toward the reference drug (Fig. 22 and Table 18). In addition, structures 68b, 68 h, 68i, 68d, and 68j displayed superior activity against S. aureus and B.subtilis toward the reference drug (Fig. 22 and Table 18) (Shinde et al., 2020)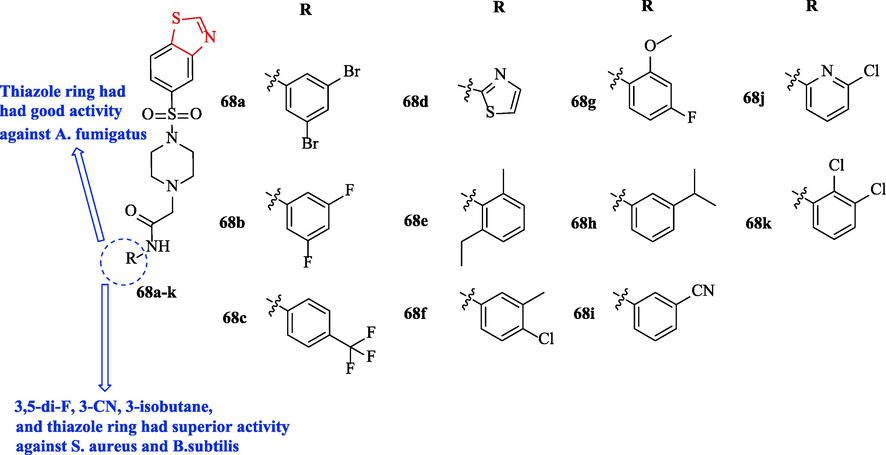
Chemical structures 68a-k (Shinde et al., 2020)
Cpd. no.
MIC (μg/mL)
S.aureus
B.Subtilis
E.coli
P.aeruginosa
C.albicans
A. fumigatus
68a
18
68b
21
17
17
68c
22
13
68d
26
13
21
68e
26
68f
19
68 g
22
15
68 h
18
18
68i
22
68j
17
10
22
68 k
25
Ciprofloxacin
19
20
36
34
Clotrimazole
25
25
All derivatives and clotrimazole (As reference drug) were tested against Aspergillus fumigatus and C. Albicans and results showed structures 68b, 68f, 68 g, and 68j had good activity against C. Albicans (Fig. 22 and Table 18). Moreover, structure 68d had good activity against A. fumigatus (Fig. 22 and Table 18) (Shinde et al., 2020)
A series of novel bis(azolyl)sulfonamidoacetamides were synthesized by Sankar P et al. Chloramphenicol as a reference drug and all derivatives were tested against 4 bacteria. Among all derivatives, structures 72a, 72c, and 74c showed low minimal inhibitory concentrations (MICs) against Bacillus subtilis, equal to the reference drug (Fig. 23 and Table 19). In general, derivatives 71 and 72 demonstrated greater activity compared to derivatives 69, 70, 73, and 74 (Fig. 23). In addition, derivatives 73 and 74 exhibited more activity compared to derivatives 69 and 70 (Fig. 23) (Ss et al., 2021)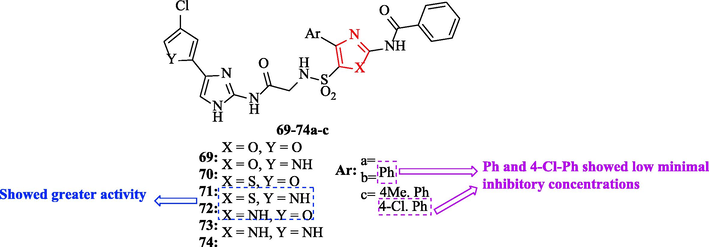
Chemical structures 69a-c-74a-c (Ss et al., 2021)
Cpd. no.
MIC (MBC/MFC) μg/well
Bacillus subtilis
Aspergillus niger
72a
6.25 (12.5)
72c
6.25 (12.5)
6.25 (12.5)
74c
6.25 (12.5)
6.25 (12.5)
Fluconazole
6.25
Ravuconazole
6.25
All derivatives were tested against Aspergillus niger and Penicillium chrysogenum except 69a, 69b, 70a, and 70b (Fig. 23). Structures 72c and 74c had low MICs against Aspergillus niger, but had equal MICs compared to ketoconazole as a reference drug (Fig. 23). In general, derivatives 73 and 74 exhibited greater activity compared to derivatives 69, 70, 71, and 72 (Fig. 23). Whereas derivatives 71 and 72 displayed higher activity compared to 69 and 70 (Fig. 23) (Ss et al., 2021)
Khidre and Radini. evaluated the antibacterial activity of a novel series of structures containing thiazole scaffold against 4 bacteria with respect to ampicillin as a reference drug. Among all derivatives, structure 75 showed good antimicrobial activity against B. subtilis, S. aureus, P. aeruginosa, and E. coli toward the reference drug (Fig. 24 and Table 20). Moreover, structure 76 showed great activity against P. aeruginosa and E.coli (Fig. 24 and Table 20) (Khidre and Radini, 2021)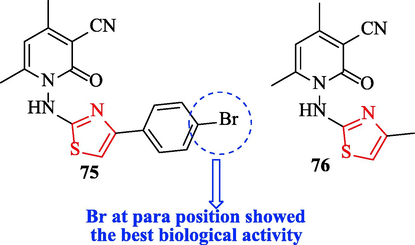
Chemical structures 75–76 (Khidre and Radini, 2021)
Cpd. no.
MIC (μg/mL)
E. coli
P. aeruginosa
S. aureus
B. subtilis
C. albicans
A. flavus
75
93.7 ± 0.95
62.5 ± 2.00
46.9 ± 0.84
62.5 ± 0.50
7.8 ± 0.17
5.8 ± 0.65
76
93.7 ± 0.95
93.7 ± 0.95
20
25
12.5
12.5
Ampicillin
125 ± 0.58
125 ± 3.51
187.5 ± 0.06
125 ± 1.73
Clotrimazole
5.8 ± 0.06
3.9 ± 0.06
Derivatives were tested against 2 fungal strains and as a reference drug, clotrimazole was used. According to the results, structure 75 exhibited the most activity against C. albicans and Aspergillus flavus toward the reference drug (Fig. 24 and Table 20). Generally, structure 75 had the best biological activity compared to other structures due to existing bromine at the para position of the phenyl ring (Fig. 24 and Table 20) (Khidre and Radini, 2021)
Shabaan et al. synthesized a novel series of 1,3,4-thiadiazines and 1,3-thiazoles derivatives. Some derivatives were tested against 2 bacterial and 2 fungal strains with respect to neomycin and cyclohexamide as antibacterial and antifungal reference drugs, respectively. Structures 77, 78b, 79a, 80, and 78a had lower MIC against S. aureus, while structures 81 and 82 showed higher MIC values (Fig. 25 and Table 21). In addition, structures 78a, 78b, and 77 demonstrated moderate activity against E. Coli, while structures 80 and 79a exhibited higher MIC values (Fig. 25 and Table 21). Structures 81 and 82 had higher MIC and MBC values against S. aureus and E. Coli (Fig. 25 and Table 21). Moreover, structures 78a showed the most potent activity against S. aureus and E. Coli toward the reference drug (Fig. 25 and Table 21). Structure 77 displayed significant activity against S. aureus, Whereas two structures (78b and 79b) exhibited moderate activity (Fig. 25 and Table 21). Also, structures 79a, 82, and 81 had low activities against S. aureus (Fig. 25 and Table 21). In contrast, Structures 83 and 84 displayed no activity against S. aureus (Fig. 25). Structures 81 had potent activity against E. coli, while structures 82, 77, 80, and 79b showed low activities toward the reference drug (Fig. 25 and Table 21) (Shabaan et al., 2021)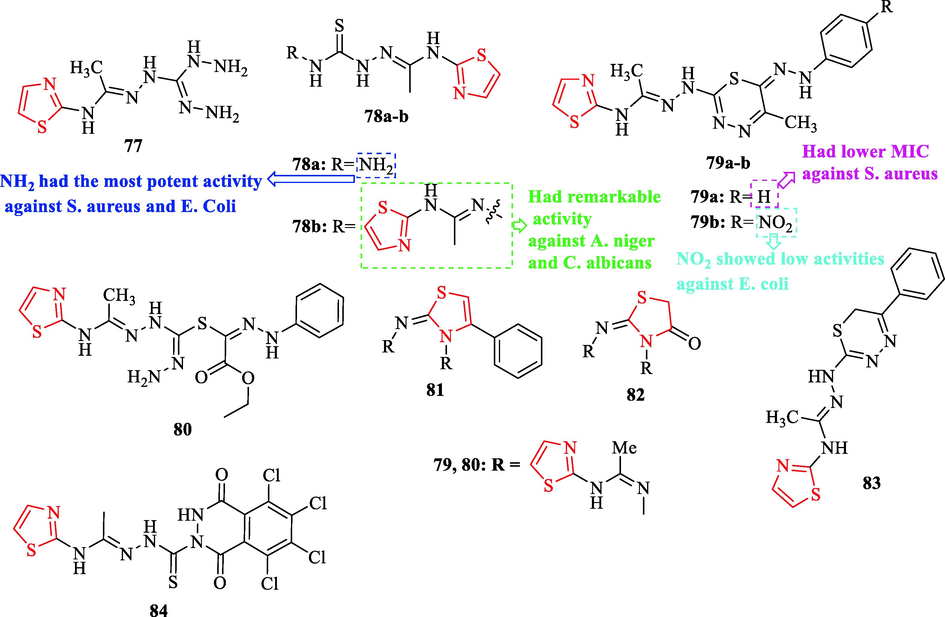
Chemical structures 77–84 (Shabaan et al., 2021)
Cpd. no.
Staphylococcus aureus Escherichia coli Growth inhibition zone (ϕmm)
MIC (μg/ml)
MBC (μg/ml)
MIC (μg/ml)
MBC (μg/ml)
S. aureus
E. coli
C. albicans
A. niger
77
39.06
39.06
156.25
312.50
32
23
31
30
78a
39.06
78.13
156.25
156.25
29
27
36
78b
78.13
31.5
156.25
625.00
29
27
32
36
79a
39.06
78.13
625.00
625.00
22
0
25
79b
19.06
39.06
25
21
24
35
80
9.77
19.06
625.00
1250.00
23
22
35
81
312.00
625
312.50
625.00
15
32
35
82
312.00
625
22
14
24
25
83
28
84
16
Neomycin
37
30
39
0
Cyclohe-xamide
0
0
0
39
On the other hand, five structures (78b, 81, 77, 80, and 79b) displayed remarkable activity against A. niger toward the reference drug (Fig. 25 and Table 21). Whereas structures 82, 83, and 79a displayed moderately active against A. niger (Fig. 25 and Table 21). Structures 78b and 77 had remarkable activities against C. albicans (Fig. 25 and Table 21). Whereas two structures (82 and 79b) displayed moderate activity (Shabaan et al., 2021)
A new series of 4-(6-substituted quinolin-4-yl)-Narylthiazol-2-amines were synthesized by Thakare et al. All derivatives were tested against 4 bacteria. As a reference drug, streptomycin was used. Structure 85f had moderated activity against E. Coli (Fig. 26 and Table 22). Furthermore, structures 85a, 85e, 85f, and 85 g displayed moderate activity against S. albus, while structures 85b and 85d had good activity (Fig. 26 and Table 22) (Thakare et al., 2021)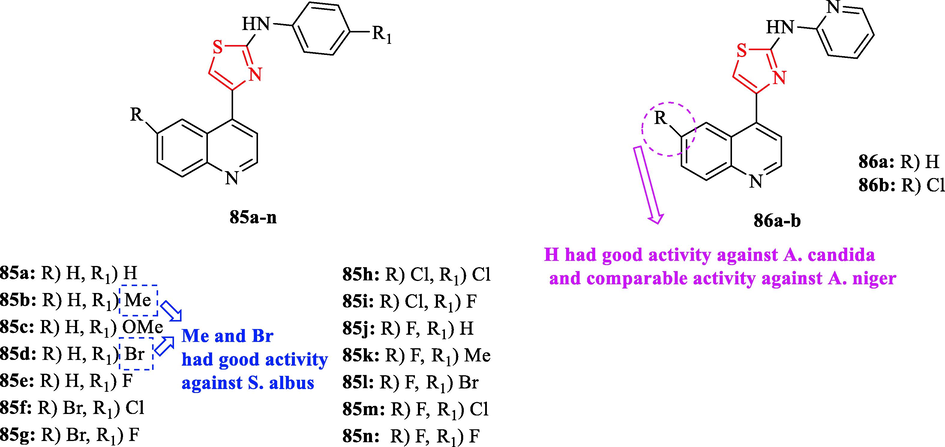
Chemical structures 85a-n and 86a-b (Thakare et al., 2021)
Cpd. no.
MIC (μg/mL)
E. coli
S. albus
A. Candida
A. niger
85a
125
85b
62.5
125
85c
62.5
62.5
85d
62.5
85e
125
85f
250
125
85 g
125
85 h
62.5
85i
125
85j
125
85 k
125
85 l
62.5
85 m
125
85n
125
86a
31.25
31.25
86b
250
Streptomycin
7.81
7.81
Fluconazole
7.81
7.81
Ravuconazole
7.81
31.25
The antifungal activities of derivatives were evaluated against 2 fungal strains with respect to ravuconazole and fluconazole as the reference drugs. According to the results, structures 85 h, 85i, 85j, 85 k, 85 l, 85 m, 85n, 86a and 86b were found to have moderate to good activity against A. niger toward ravuconazole (Fig. 26 and Table 22). Moreover, structure 86a showed good activity against A. candida and comparable activity against A. niger (Fig. 26 and Table 22). Structure 85b showed moderate activity against A. candida (Fig. 26 and Table 22). The structure 85c had good activity against A. candida and A. niger (Fig. 26 and Table 22) (Thakare et al., 2021)
In 2022, El-Hagrassey and co-workers synthesized a novel series of derivatives containing thiazole. Ampicillin as a reference drug and the novel derivatives were tested against 4 bacteria. According to antibacterial activity studies, structure 90 displayed the highest activity against both gram-negative and positive bacteria, Whereas structure 89 had a weak effect on E. coli and P. Aeruginosa (Fig. 27 and Table 23). Also, structures 87a, 87b, 87c, and 88 did not show an effect on E. coli and structures 87a, 87b, and 88 had no effect on S.aureus (Fig. 27). Structures 87b and 88 did not show effect on P. aeruginosa and B. Subtilis (Fig. 27) (El-Hagrassey et al., 2022)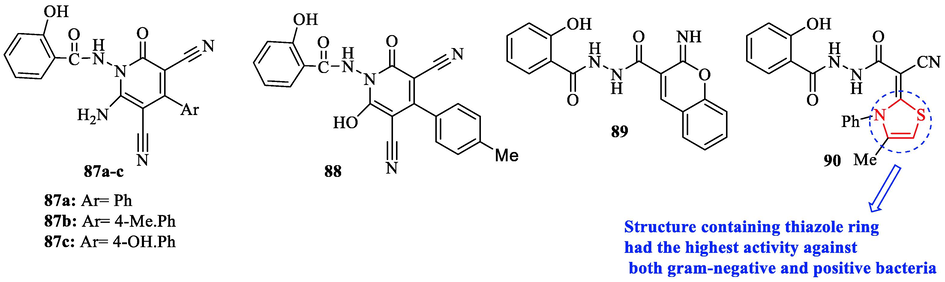
Chemical structure 87a-c and 88–90 (El-Hagrassey et al., 2022)
Cpd. no.
Inhibition zone, mm, (activity index %)
E. coli
P. aeruginosa
S. aureus
B. subtilis
C. albicans
89
6 (24.0%)
10 (43.5%)
14 (51.8%)
90
13 (52.0%)
16 (69.5%)
18 (75.0)
17 (73.9%)
18 (66.6%)
Ampicillin
25 (100.0%)
23 (100.0%)
24 (100.0%)
23 (100.0%)
Clotrimazole
27 (100%)
On the other hand, derivatives were tested against C. Albicans for investigation of antifungal activity. As a reference drug, clotrimazole was used. Structure 90 had the highest activity against C. albicans (Fig. 27 and Table 23). In addition, structures 87b and 88 had no effect on C. albicans (Fig. 27). Whereas structure 89 showed good activity against C. albicans (Fig. 27 and Table 23) (El-Hagrassey et al., 2022)
A novel series of 2,3-Dihydropyrido[2,3-d]pyrimidine-4-ones and Pyrrolo[2,1-b][1,3]benzothiazoles synthesized by Al-Mutairi et al. Cefotaxime as reference drug and all derivatives were tested against 6 bacteria (Fig. 28 and Table 13). The antibacterial evaluations of all derivatives exhibited 4 structures (91a, 91b, 92a, and 92d) that had higher activity toward the reference drug (Fig. 28 and Table 13). Structure 91a had an equipotent activity with respect to the reference drug against Chlamydia pneumonia and Bacillus subtilis, and structure 92a had equipotent activity against Chlamydia pneumonia and Bacillus subtilis, while had more activity against Staphylococcus aureus (Fig. 28 and Table 13). Moreover, structure 92d because of the presence of pyrrolobenzothiazole with p-fluorophenyl substituent exhibited significant activity against all bacteria compared to the reference drug (Fig. 28 and Table 13). In general, structures 92b, 92c, and 92e had good inhibition activity against all bacterial strains (Fig. 28 and Table 13) (Al-Mutairi et al., 2022)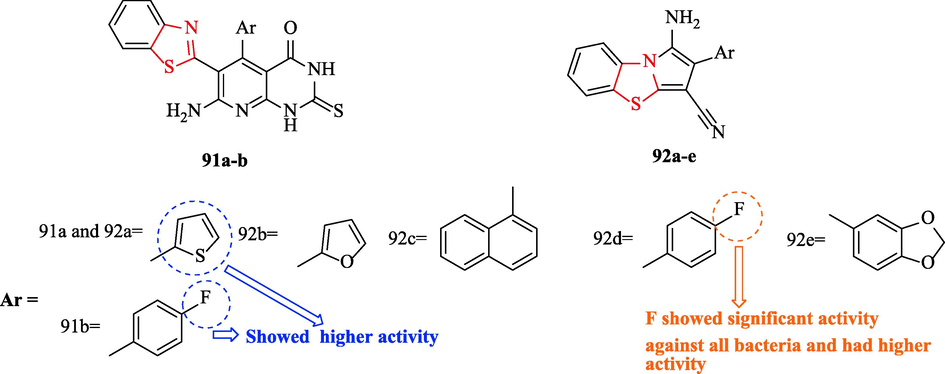
Chemical structures 91a-b and 92a-e (Al-Mutairi et al., 2022)
All derivatives were tested against 3 fungal strains. As a reference drug, fluconazole was used for the investigation of antifungal activity. According to studies, structures 91a, 91b, 92a, and 92d had high activity (Fig. 28 and Table 24). Structure 92d showed equipotent activity against Aspergillus flavus while had more high activity against Ganoderma lucidum and Candida albicans toward the reference drug (Fig. 28 and Table 24). Structure 92a showed significant activity against Ganoderma lucidum and Aspergillus flavus and had more potent activity against Candida albicans compared to the reference drug (Fig. 28 and Table 24). Also, structures 91a and 91b had good activity against Aspergillus Flavus, Candida Albicans, and Ganoderma Lucidum (Fig. 28 and Table 24) (Al-Mutairi et al., 2022)
Cpd. no.
MIC (μmol/L)
Staphylococcus Aureus
Streptococcus Pneumonia
Bacillus Subtilis
Chlamydia Pneumonia
Escherichia Coli
Salmonella Typhi
Aspergillus Flavus
Candida Albicans
Ganoderma Lucidum
91a
6
12
8
10
9
91b
8
10
10
92a
8
6
12
8
4
8
92b
14
10
10
14
10
12
92c
16
12
12
14
10
10
92d
10
4
6
8
5
8
6
5
4
92e
14
10
10
13
10
12
Cefotaxime
10
6
6
12
6
8
Fluconazole
6
8
6
Othman et al. synthesized and investigated the antimicrobial activity of a series of thiazole, thiophene, and thieno[2,3-d]pyrimidines against 4 bacteria and 2 fungal strains. As antibacterial and antifungal reference drugs, ampicillin and clotrimazole were used, respectively. Structure 93 against S. aureus and B. Subtilis had potent activity (Fig. 29 and Table 25). Structures 94, 95, and 96 showed weak sensitivity against P. aeruginosa and E. Coli (Fig. 29 and Table 25). In addition, structures 98 and 99 against P. aeruginosa exhibited moderate potency, while structures 97 and 93 had potent activity (Fig. 29 and Table 25). Moreover, structures 98 and 97 displayed less sensitivity against E. coli toward the reference drug (Fig. 29 and Table 25) (Othman et al., 2022)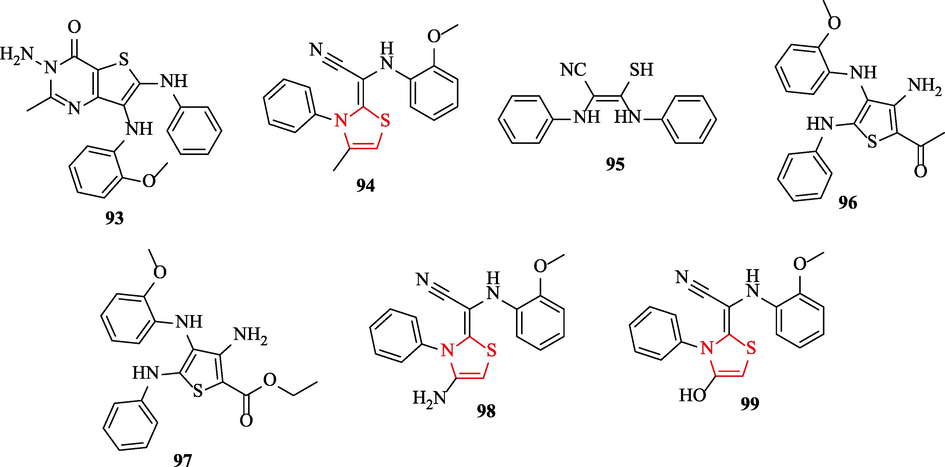
Chemical structures 93–99 (Othman et al., 2022)
Cpd. no.
Mean diameter of inhibition zones (Mean ± SEM (mm)) MIC (Mean ± SEM (μg/mL)
S. aureus
25,923B. Subtilis
6633P. aeruginosa
E. coli RCMB
010,052A. fumigatus
RCMP 02,568C. Albicans
ATCC10231
93
27.1 ± 0.11
(0.03 ± 0.18)25.1 ± 0.15
(0.05 ± 0.13)19.7 ± 0.14
(0.98 ± 0.55)
22.2 ± 0.17
(0.49 ± 0.32)21.4 ± 0.50
(0.98 ± 0.25)
94
14.3 ± 0.25
(125 ± 0.07)12.1 ± 0.25
(250 ± 0.62)
95
16.7 ± 0.13
(31.25 ± 0.46)15.2 ± 0.44
(62.5 ± 0.26)
22.1 ± 0.02
(0.49 ± 0.38)
96
12.7 ± 0.16
(250 ± 0.01)9.9 ± 0.25
(500 ± 0.04)21.6 ± 0.15
(0.49 ± 0.32)
97
8.9 ± 0.23
(0.50 ± 0.15)22.3 ± 0.17
(0.49 ± 0.28)
98
18.7 ± 0.45
(7.81 ± 0.41)21.8 ± 0.01
(0.49 ± 0.12)20.2 ± 0.10
(1.95 ± 0.52)
99
17.3 ± 0.43
(15.62 ± 0.31)
19.5 ± 0.12
(0.98 ± 0.05)
Ampicillin
27.7 ± 0.82
(0.03 ± 0.53)25.4 ± 0.18
(0.05 ± 0.22)20.3 ± 0.31
(1.95 ± 0.11)26.8 ± 0.15
(0.03 ± 0.48)
Clotrimazole
23.7 ± 0.10
(0.12 ± 0.28)22.8 ± 0.17
(0.49 ± 0.45)
Four structures (98, 99, 96, and 93) against A. fumigatus RCMB showed potent antifungal activity. Further, structures 95 and 93 showed remarkable antifungal activity against C. albicans toward the reference drug (Fig. 29 and Table 25) (Othman et al., 2022)
A novel series of structures containing thiazole scaffold were synthesized by Kartsev and co-workers and evaluated their antibacterial activity against 6 bacteria, using a microdilution method. As reference drugs, Streptomycin and ampicillin were used. Structure 100a displayed the best activity against E. coli with respect to the reference drugs (Fig. 30 and Table 26). Structure 101 exhibited good activity against B. cereus and S. typhimurium (Fig. 30 and Table 26). Also, structure 102 exhibited good activity against S. typhimurium (Fig. 30 and Table 26). In addition, structures 103b, 102, and 100a exhibited higher potential than ampicillin against E. coli, methicillin resistant Staphylococcus aureus, and P. aeruginosa (Fig. 30 and Table 26) (Kartsev et al., 2022)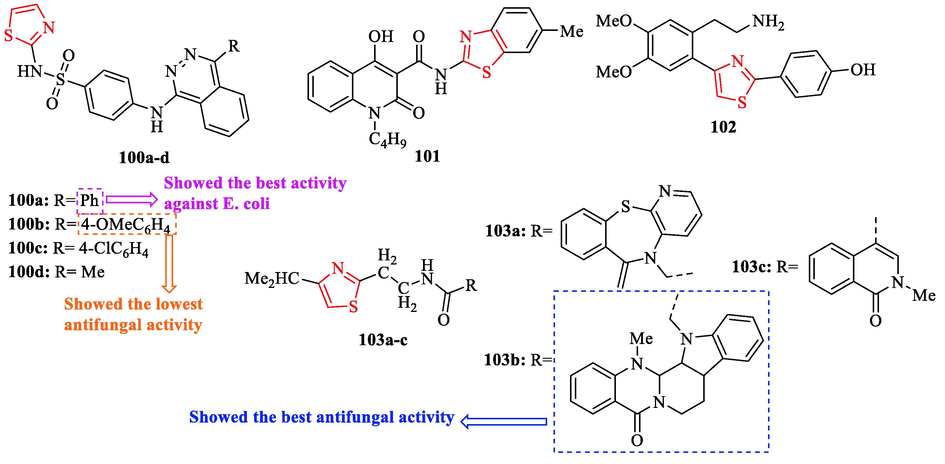
The structures of heteroaryl (aryl) thiazole derivatives 100a-d, 101–102, and 103a-c (Kartsev et al., 2022)
Cdp.
no.
MIC/MBC in mg/mL
B.c.
E.c.
S.T.
MRSA
P.a.
E. c.
A.n.
A.v.
P.f.
T.v.
P.v.c.
100a
MIC
0.17 ± 0.00
0.94 ± 0.00
0.23 ± 0.00
0.94 ± 0.00
MBC
0.23 ± 0.00
1.88 ± 0.00
0.47 ± 0.00
1.88 ± 0.00
100c
MIC
0.17 ± 0.00
MFC
0.23 ± 0.00
100d
MIC
0.47 ± 0.00
MFC
0.94 ± 0.00
101
MIC
0.17 ± 0.00
0.17 ± 0.19
0.17 ± 0.00
0.17 ± 0.00
0.17 ± 0.00
0.06 ± 0.00
0.17 ± 0.00
MBC
0.23 ± 0.00
0.23 ± 0.00
MFC
0.23 ± 0.00
0.23 ± 0.00
0.23 ± 0.00
0.11 ± 0.00
0.23 ± 0.00
102
MIC
0.23 ± 0.00
0.47 ± 0.00
0.23 ± 0.00
0.47 ± 0.00
0.11 ± 0.00
MBC
0.47 ± 0.00
0.94 ± 0.00
0.47 ± 0.00
0.94 ± 0.00
MFC
0.23 ± 0.00
103a
MIC
0.11 ± 0.00
MFC
0.23 ± 0.00
103b
MIC
0.23 ± 0.00
0.35 ± 0.08
0.23 ± 0.00
0.17 ± 0.00
0.35 ± 0.08
MFC
0.47 ± 0.00
0.47 ± 0.00
0.47 ± 0.00
0.23 ± 0.00
0.47 ± 0.00
103c
MIC
0.08 ± 0.00
MFC
0.11 ± 0.00
Streptomycin
MIC
0.02 ± 0.00
0.10 ± 0.00
0.10 ± 0.00
0.10 ± 0.00
0.05 ± 0.00
0.10 ± 0.00
MBC
0.05 ± 0.00
0.20 ± 0.00
0.20 ± 0.01
/
0.10 ± 0.00
0.20 ± 0.00
Ampicillin
MIC
0.10 ± 0.00
0.15 ± 0.00
0.10 ± 0.00
/
0.20 ± 0.00
0.20 ± 0.00
MBC
0.15 ± 0.00
0.20 ± 0.00
0.20 ± 0.00
/
/
/
Bifonazole
MIC
0.15 ± 0.00
0.10 ± 0.00
0.20 ± 0.00
0.15 ± 0.00
0.10 ± 0.00
MFC
0.20 ± 0.00
0.20 ± 0.00
0.25 ± 0.00
0.20 ± 0.00
0.20 ± 0.00
Ketoconazole
MIC
MFC
0.20 ± 0.00
0.5 ± 0.000.20 ± 0.00
0.5 ± 0.000.20 ± 0.00
0.5 ± 0.001.0 ± 0.01
1.5 ± 0.000.20 ± 0.00
0.03 ± 0.010
The antifungal activity of derivatives was evaluated against 6 fungal strains. As reference drugs, bifonazole and ketoconazole were used. Structure 103b displayed the best activity, while structure 100b showed the lowest activity (Fig. 30 and Table 26). Structures 103b and 101 against T. viride showed excellent activity nearly 29 times better compared to ketoconazole and four times better than bifonazole as well as against P. cyclpoium var. verucosum, P. funiculosum, A. versicolor, and A. niger (Fig. 30 and Table 26). Structures 103c and 102 against T. viride showed good activity, followed by structures 103a, 100c, and 100d (Fig. 30 and Table 26). All derivatives demonstrated better activity against T. viride than ketoconazole, while structures 100a and 100b had not a good activity. In general, most of the derivatives had more potent activity against all fungi toward ketoconazole, except for P. cyclpoium var. Verucosum which only structures 103c, 103b, and 101 were more active than ketoconazole (Fig. 30 and Table 26) (Kartsev et al., 2022)
A series of coumarin thiazoles were synthesized by Yang et al and their antimicrobial activities were evaluated against 9 bacteria. As antibacterial reference drugs, norfloxacin and ciprofloxacin were used. Structure 104d against S. aureus displayed equivalent antibacterial activity toward norfloxacin (Fig. 31 and Table 27). Structures 104a-b against E. coli ATCC 25922 showed the same MIC with respect to norfloxacin (Fig. 31 and Table 27). Structures 104b and 104c against most tested strains showed poor bioactivities (Fig. 31). In addition, structures 109b-d exhibited 2 to 8 times antibacterial potency against MRSA toward the reference drugs (Fig. 31 and Table 27). Structure 109a against E. coli ATCC 25,922 showed almost 2 times inhibition potency toward norfloxacin (Fig. 31 and Table 27). Structure 109c against A. baumanii showed high activity with respect to ciprofloxacin with the same MIC value and 8-fold more active than norfloxacin (Fig. 31 and Table 27). In the case of 107a-g, structure 107a against MRSA, Enterococcus faecalis, and Staphylococcus aureus showed the best antibacterial efficacy (Fig. 31 and Table 27). The same structure against MRSA was 8 times superior to ciprofloxacin and norfloxacin (Fig. 31 and Table 27). Structure 107b against MRSA, S. aureus, A. baumannii, and E. faecalis showed good activity which was 4 times higher than or equivalent to norfloxacin (Fig. 31 and Table 27). Structures 107c-g exhibited no bioactivity or remarkably declined inhibitory activity (Fig. 31). Further, structures 104 and 106a showed no sensitivity to the tested germs, while structure 108b showed 2-fold bacteriostatic activity toward ciprofloxacin and norfloxacin against MRSA (Fig. 31 and Table 27) (Yang et al., 2022) MRSA, methicillin-resistant Staphylococcus aureus (N315); E. f., Enterococcus faecalis; S. a., Staphylococcus aureus;A. b., Acinetobacter baumannii; Escherichia coli ATCC 25922; C. a., Candida albicans; C. t., Candida tropicalis; A. f., Aspergillus fumigatus; C. a. 90023, Candida albicans ATCC 90023, C. p. 22019, Candida parapsilosis ATCC 22019.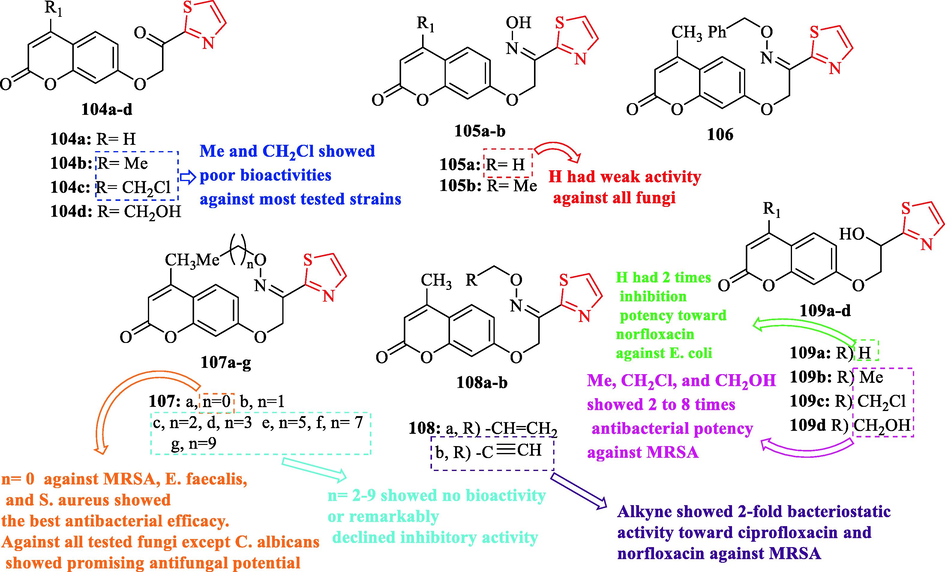
Chemical structures 104a-d, 105a-b, 106, 107a-g, 108a-b, and 109a-d (Yang et al., 2022)
Cpd. no.
MIC values (μg /mL)
MRSA
E. f.
S. a.
A. b.
E. c.
C. a.
C. t.
A. f.
C. a.
C. p
104a
8
104b
8
104d
8
105b
256
107a
1
2
16
64
4
8
64
4
107b
2
4
6
8
107d
32
107e
32
107f
32
107 g
64
108a
256
108b
128
109a
4
109b
1
109c
1
109d
4
Norfloxacin
8
4
8
8
8
Ciprofloxacin
8
2
2
1
2
Fluconazole
1
8
8
256
16
Antifungal activity was evaluated against 5 fungal strains. As an antifungal reference drug, fluconazole was used. Structure 107a against C. tropicalis, A. fumigatus, C. albicans ATCC 90023, and C. parapsilosis ATCC 22019 showed the best activity toward the reference drug, while structures 105a, 106, and 107b-c had weak activity against all fungi (Fig. 31). In addition, structures 105b, 107d-g, and 106a–b against C. albicans ATCC 90023 demonstrated moderate to good antifungal activity (Fig. 31 and Table 27). Structure 107a against all tested fungi except C. albicans showed promising antifungal potential and was selected as a potential drug candidate (Fig. 31 and Table 27) (Yang et al., 2022)
3 Thiazole derivatives with anti-inflammatory activity
The response to diverse stimuli by organisms is meaning Inflammation. There are a lot of diseases like asthma, psoriasis, and arthritis that deal with inflammation. Therefore, the need for anti-inflammatory drugs is needed more than ever. Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most important types of anti-inflammatory drugs., but their long-term use shows remarkable side effects such as nephrotoxicity and adverse cardiovascular events (Petrou et al., 2021)
Deb et al. evaluated in vivo anti-inflammatory activity of a new series of N-(benzo[d]thiazol-2-yl)-2-(substituted)acetamide derivatives and 2-substituted-N-(1,3-thiazole-2-yl)acetamide derivatives, using the carrageenan-induced rat paw edema model. According to the results, four structures (110a-d) were found to have significant activity with respect to other structures (about 84–93 % of the reference) (Fig. 32 and Table 28). Moreover, structures 110d and 110c with 4-methoxyaniline and 4-fluoroaniline substituent exhibited good activity toward structures 110b and 110a with imidazole and triazole scaffold (Fig. 32 and Table 28) (Deb et al., 2014)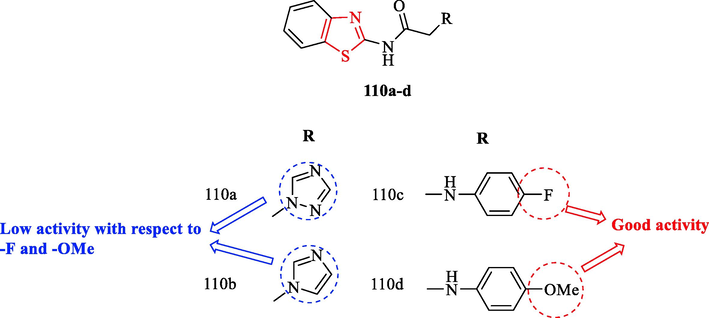
Chemical structures 110a-d (Deb et al., 2014)
Cpd. no.
2 h
4 h
110a
0.32 ± 0.019 (65.59)
0.29 ± 0.021 (67.77)
110b
0.29 ± 0.016 (68.81)
0.27 ± 0.070 (70.00)
110c
0.26 ± 0.019 (72.04)
0.23 ± 0.090 (74.44)
110d
0.27 ± 0.023 (70.97)
0.25 ± 0.013 (72.22)
Indomethacin
0.21 ± 0.023 (77.42)
0.19 ± 0.019 (78.89
A novel series of 1,2,3,4-tetrahydronaphthalen-6-yl-thiazoles and thiazolidinones were synthesized by Haiba and co-workers, but Twenty four structures were chosen for analgesic and anti-inflammatory activity. Anti-inflammatory activity study showed structures 114 and 118 had equipotent activity toward indomethacin as a reference drug with quicker action onset. In addition, six structures (111a, 111b, 113, 117, 119b, and 119c) showed dual analgesic and anti-inflammatory (Fig. 33). Among all derivatives, structure 113 (carrying coumarin-thiazole ring) in comparison with indomethacin exhibited remarkable dual analgesic and anti-inflammatory activity (Fig. 33 and Table 29). According to anti-inflammatory activity at 4 h, eleven structures (113, 118, 114, 111b, 119b, 111c, 112, 115, 116, 117, and 119a) showed the greatest anti-inflammatory potency (Fig. 33 and Table 29). Remarkable edema inhibition at the 1st h post-administration was observed by four structures (112, 114, 113, 118) (Fig. 33 and Table 29) (Haiba et al., 2014)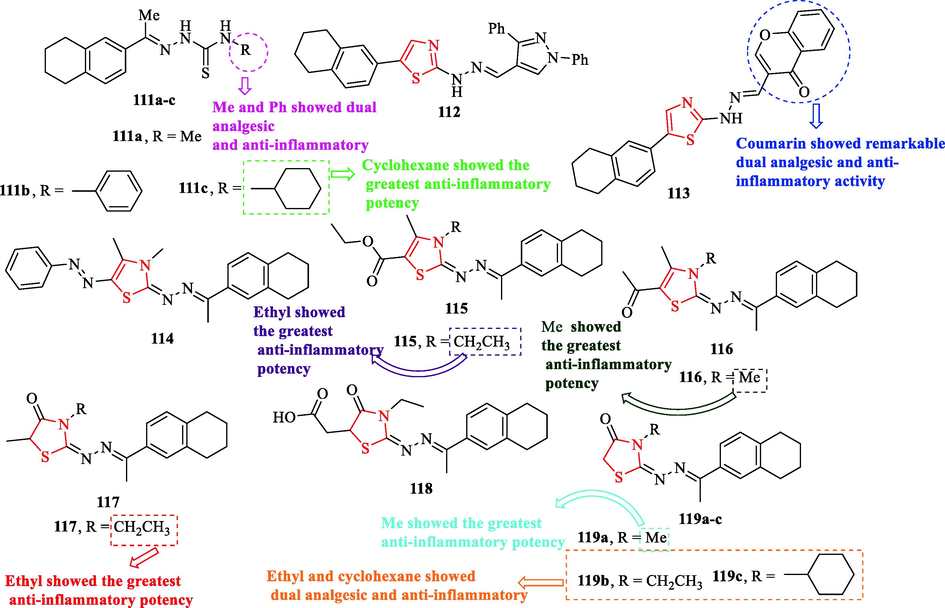
The structures of 1,2,3,4-tetrahydronaphthalen-6-yl-thiazole and thiazolidinone derivatives (111a-c, 112–118, and 119a-c) (Haiba et al., 2014)
Cpd. no.
1 h
4 h
111b
0.44 ± 0.03)
111c
0.41 ± 0.02
112
0.33 ± 0.01
0.41 ± 0.02
113
0.32 ± 0.00
0.36 ± 0.00
114
0.33 ± 0.01
0.39 ± 0.01
115
0.41 ± 0.01
116
0.41 ± 0.01
117
0.41 ± 0.01
118
0.33 ± 0.01
0.38 ± 0.01
119a
0.41 ± 0.01
119b
0.40 ± 0.02
Indomethacin
(0.03 mmol/kg)
0.37 ± 0.01
0.38 ± 0.00
In 2015, Khloya et al. evaluated the anti-inflammatory activity of a series of pyrazolylthiazole carboxylates and corresponding acid derivatives. Evaluation of thirty-two derivatives showed structure 120 h had the best activity and structures 121b, and 121i had remarkable anti-inflammatory activity after 3 h (Fig. 34 and Table 30). After three hours of carrageenan injection, eight structures (120a, 120c, 120f, and 121a-e) exhibited remarkable activity (Fig. 34 and Table 30). Nine structures (120d-e, 120 g–h, 121b, 121e–f, 121 h, and 121 g) were found to have significant activity compared to the reference drug after 4 h of carrageenan injection (Fig. 34 and Table 30). In general, the ester derivatives were better than the acids and the results after 4 h of carrageenan injection revealed that all derivatives do not get simply metabolized in the body (Fig. 34 and Table 30) (Khloya et al., 2015) a Values are expressed as mean ± SEM (number of animals = 6) and analyzed by ANOVA. b Values in parentheses (percentage anti-inflammatory activity, AI %).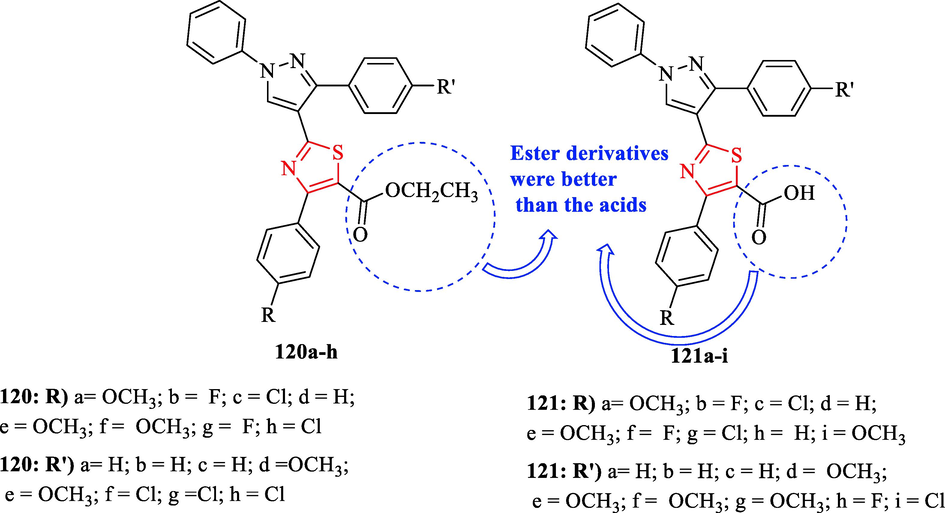
The structures of pyrazolylthiazole carboxylates (120a-h) and corresponding acid derivatives (121a–i) (Khloya et al., 2015)
Cpd. no.
Volume of edemaa (mL) and % AIb
3 h
4 h
Swelling
Inhibition (%)
Swelling
Inhibition (%)
120a
0.24 ± 0.10
86.12
120b
0.31 ± 0.06
82.08
0.42 ± 0.13
88.29
120c
0.24 ± 0.12
87.23
120d
0.24 ± 0.08
90.95
120e
0.17 ± 0.04
90.95
120f
0.25 ± 0.08
85.54
120 g
0.19 ± 0.04
89.89
120 h
0.12 ± 0.04
93.06
0.18 ± 0.07
90.42
121a
0.24 ± 0.05
86.12
121b
0.18 ± 0.04
89.59
0.17 ± 0.03
90.95
121c
0.20 ± 0.06
88.43
121d
0.21 ± 0.09
87.86
121e
0.20 ± 0.06
88.43
0.19 ± 0.06
89.89
121f
0.18 ± 0.04
90.42
121 g
0.20 ± 0.07
89.36
121 h
0.18 ± 0.04
90.42
121i
0.16 ± 0.05
90.75
Indomethacin
0.15 ± 0.03
91.32
0.16 ± 0.05
91.48
A novel series of structures thiazole bearing pyrazole were synthesized via a one-pot reaction method by D. Kamble and co-workers. According to in vivo anti-inflammatory activity results, structures 122d and 122e had stronger protection against inflammation by Carrageenan at 180 min, while at 90 min onwards structure 122e showed outstanding protection against inflammation (Fig. 35 and Table 31). In addition, structure 122e at 30, 90, 120, and 150 min exhibited unremarkable differences with respect to diclofenac as a reference drug (Fig. 35 and Table 31). Four structures (122a, 122b, 122c, and 122f) showed considerable differences compared to the control, while its anti-inflammatory activity at 30, 60, and 90 min had non-significant differences compared to the reference drug (Fig. 35 and Table 31). Moreover, structures 122c, 122e, and 120f had strong COX-II inhibitory activity (Fig. 35) (Kamble et al., 2016)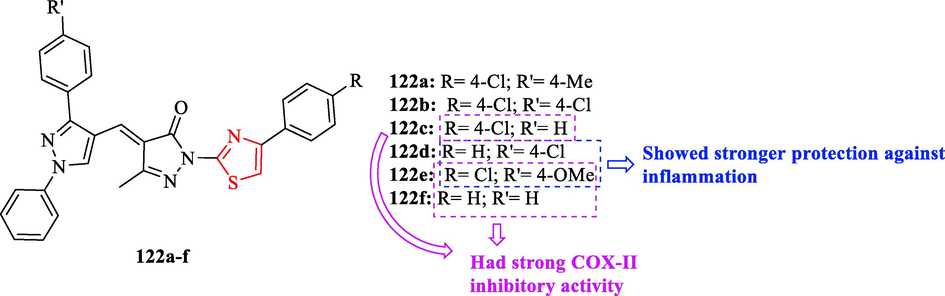
Chemical structures 122a-f (Kamble et al., 2016)
Cpd. no.
30 Min
60 Min
90 Min
180 Min
122a
0.4 ± 0.02
0.41 ± 0.016
0.39 ± 0.01
122b
0.39 ± 0.021
0.37 ± 0.013
0.39 ± 0.017
122c
0.41 ± 0.025
0.39 ± 0.036
0.38 ± 0.031
122d
0.35 ± 0.011
122e
0.34 ± 0.011
0.32 ± 0.006
122f
0.4 ± 0.02
0.41 ± 0.016
0.39 ± 0.01
Diclofenac
0.36 ± 0.005
0.4 ± 0.004
0.36 ± 0.007
0.29 ± 0.004
Kumar et al. reported N-[2-(1H-indol-3-yl)ethyl]-2amino/aminoaryl/alkyl/aryl/heteroarylthiazole-4-carboxamides. The anti-inflammatory activity of all derivatives were evaluated. Among all derivatives, structures 123b (p-tolyl), 123d (2-thienyl), 124a (NH2), and 124c (p-fluoro aminophenyl) displayed significant anti-inflammatory activity at 1 h compared to indomethacin as reference drug (Fig. 36 and Table 32). After 4 h, three structures (123b, 124a, and 124b) stayed active (Fig. 36 and Table 32). Also, five structures (123a, 123c, 123e, 124b, and 124d) showed good activity after 1 h (Fig. 36 and Table 32) (Kumar et al., 2016)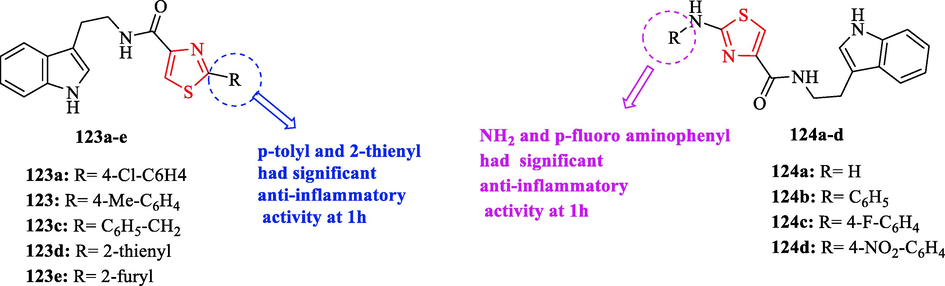
Chemical structures 123a-e and 124a-d (Kumar et al., 2016)
Cpd. no.
1 h
4 h
123a
0.23 ± 0.14 (68)
123b
0.13 ± 0.06 (81)
0.38 ± 0.15 (70)
123c
0.17 ± 0.12 (76)
123d
0.13 ± 0.06 (82)
123e
0.18 ± 0.10 (75)
124a
0.10 ± 0.02 (86)
0.23 ± 0.09 (82)
124b
0.16 ± 0.04 (77)
0.27 ± 0.01 (78)
124c
0.12 ± 0.01 (83)
124d
0.24 ± 0.02 (66)
Indomethacin
0.04 ± 0.02 (94)
0.17 ± 0.03 (86)
In 2017, a new series of structures containing thiazoles were synthesized by Toma et al. The mentioned derivatives were investigated for their anti-inflammatory activity. Among all derivatives, structures 126b, 126c, and 126d displayed good activity after 1 and 2 h compared to diclofenac as a reference (Fig. 37 and Table 33). Structures 125a, 125b, and 126a showed moderate activity after 1, 2, and 3 h (Fig. 37 and Table 33). In general, it seems that the 4-pyridyl substituent and the carboxylate ester group are effective in anti-inflammatory activity (Fig. 37 and Table 33) (Toma et al., 2017)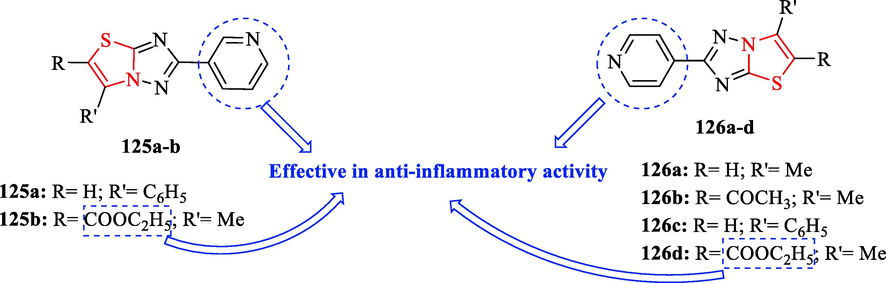
Chemical structures 125a-b and 126a-d (Toma et al., 2017)
Cpd. no.
1 h
2 h
3 h
125a
0.60 ± 0.46 (36.71)
1.26 ± 0.61 (30.35)
1.35 ± 0.64 (34.04)
125b
0.75 ± 0.29 (21.68)
1.13 ± 0.38 (36.99)
1.50 ± 0.43 (26.69)
126a
0.67 ± 0.27 (29.89)
1.16 ± 0.70 (35.80)
1.46 ± 0.71 (28.24)
126b
0.40 ± 0.18 (58.39)
0.89 ± 0.28 (50.28)
126c
0.38 ± 0.20 (60.84)
0.87 ± 0.40 (51.75)
126d
0.48 ± 0.32 (50.00)
0.65 ± 0.31 (63.56)
Diclofenac
0.74 ± 0.21 (2255)
0.98 ± 0.33 (45.48)
1.06 ± 0.36 (47.84)
Abdelazeem and co-workers evaluated the in vivo anti-inflammatory activity of a series of diphenylthiazole derivatives. According to the stated results, structure 127 had the best activity that was comparable with indomethacin as a reference drug after 1, 3, and 5 h (Fig. 38 and Table 34). Also, structure 128 showed excellent activity compared to the reference drug after 1 h (Fig. 38 and Table 34) (Abdelazeem et al., 2017)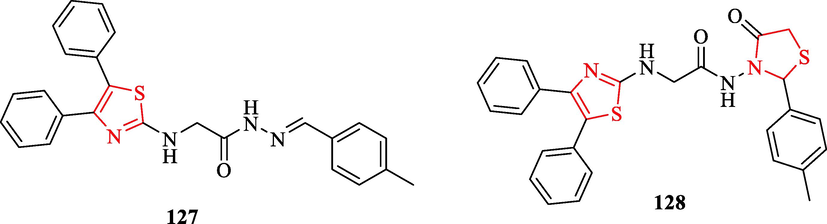
Chemical structures 127–128 (Abdelazeem et al., 2017)
Cpd. no.
1 h
3 h
5 h
127
0.196 ± 0.012 (71)
0.258 ± 0.013 (84)
0.630 ± 0.015 (80)
128
0.117 ± 0.014 (83)
Indomethacin
0.113 ± 0.004 (83)
0.221 ± 0.005 (86)
0.545 ± 0.003 (81)
Wang et al. evaluated the anti-inflammatory activity of a new series of new benzo[d]thiazole-hydrazones. Anti-inflammatory activity results demonstrated ten structures (129a, 129b, 129c, 129d, 129e, 129f, 129 g, 129 h, 129i, and 129j) had greater activity (Fig. 39 and Table 35). Moreover, structures 129f and 129 h exhibited the highest docking scores (Fig. 39 and Table 35) (Wang et al., 2017)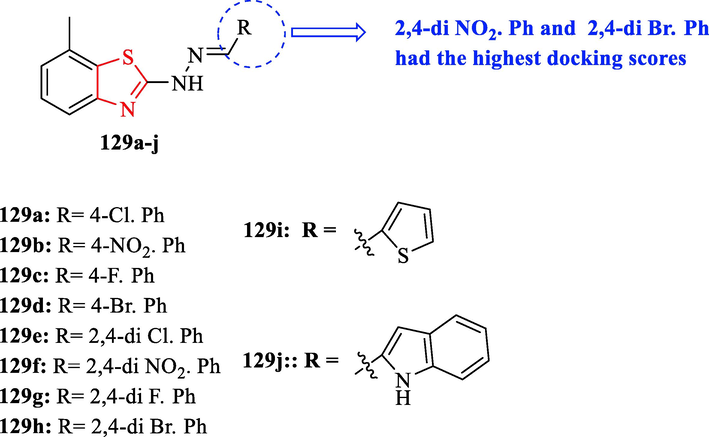
Chemical structure 129a-j (Wang et al., 2017)
Cpd. no.
IC50 μg mL−1
129a
28.74 ± 1.25
129b
24.07 ± 1.07
129c
32.15 ± 0.36
129d
20.07 ± 0.47
129e
18.17 ± 0.74
129f
12.74 ± 0.43
129 g
16.14 ± 0.45
129 h
10.12 ± 0.34
129i
28.74 ± 0.17
129j
30.18 ± 0.74
Indomethacin
40.04 ± 0.45
Mohareb et al. evaluated the anti-inflammatory activity of new structures of fused thiazole derivatives derived from 2-(2-oxo-2H-chromen-3-yl)thiazol-4(5H)-one (Fig. 40 and Table 36). The evaluation of anti-inflammatory activity showed structures 133, 134b, 136, 137a and 137b compared to other derivatives at 3 graded doses had high activity (Fig. 40 and Table 36). Structures 130a-c, 131a-c, 132, 133, 134a, 135, 137a, and 137b demonstrated the high antiulcer activity (Fig. 40 and Table 36) (Mohareb et al., 2017)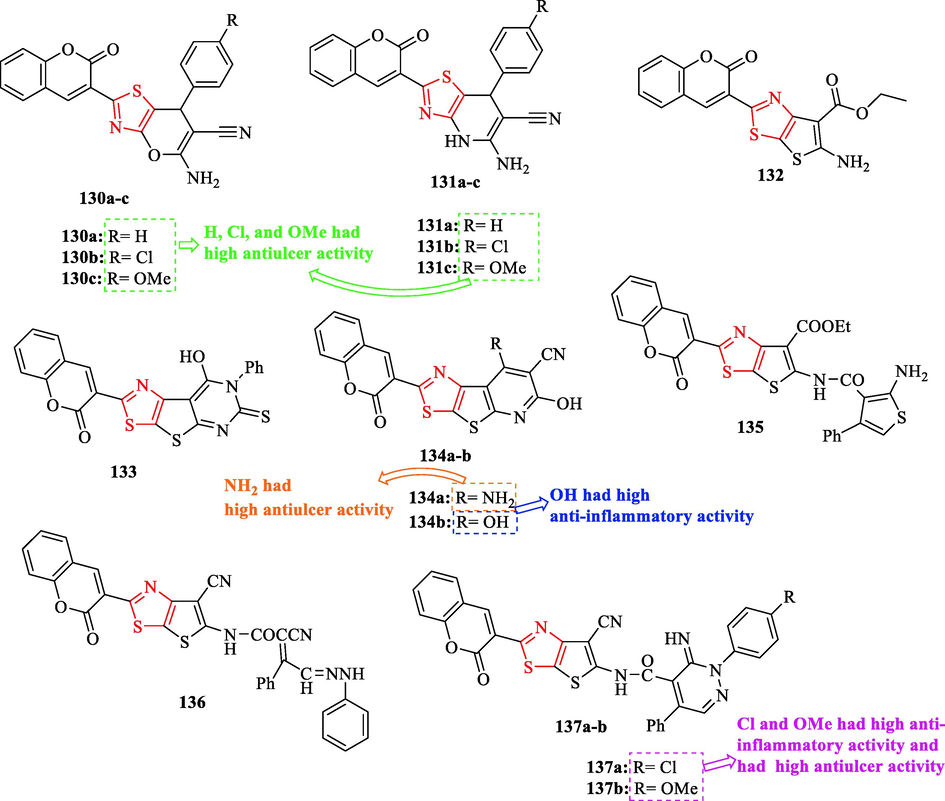
Chemical structures 130a-c, 131a-c, 132–133, 134a-b, 135–136, and 137a-b (Mohareb et al., 2017)
Cpd. no.
10 mg/Kg
20 mg/Kg
40 mg/Kg
133
0.09 ± 0.02 (96)
0.16 ± 0.08 (93)
0.19 ± 0.06 (92)
134b
0.39 ± 0.02 (83)
0.09 ± 0.01 (96)
0.39 ± 0.13 (83)
136
0.13 ± 0.07(94)
0.24 ± 0.04 (89)
0.07 ± 0.03 (97)
137a
0.18 ± 0.03 (92)
0.18 ± 0.09 (92)
0.09 ± 0.01 (96)
137b
0.29 ± 0.11 (87)
0.37 ± 0.07(84)
0.49 ± 0.11 (79)
Diclofenac
0.32 ± 0.09 (86)
0.31 ± 0.07 (88)
0.09 ± 0.29 (96)
Abu-Hashem et al. synthesized thiazolopyrimidines, 1,3,5-oxadiazepines, 1,3,5-triazines, and benzodifuranyl derivatives. Among all derivatives containing thiazole scaffold, ten structures (138a–f, 139a–b, and 140a–b) exhibited the highest anti-inflammatory activity compared to diclofenac as a reference drug after 3 h (Fig. 41 and Table 37) (Abu-Hashem et al., 2020)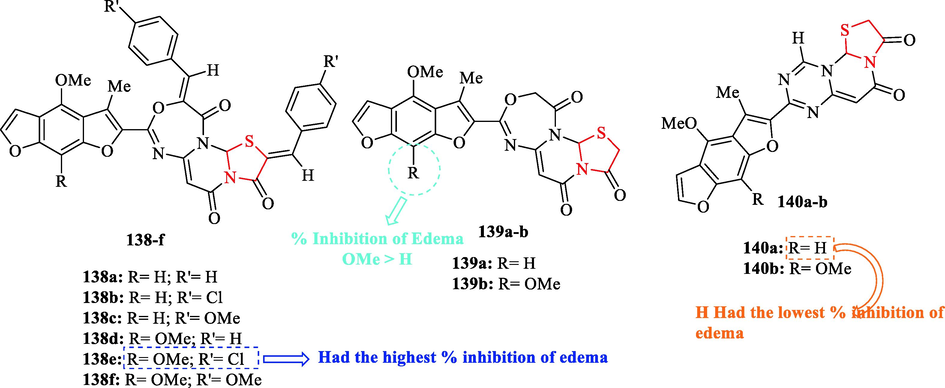
Chemical structures 138a-f, 139a-b, and 140a-b (Abu-Hashem et al., 2020)
Cpd. no.
% Change in Paw Height
(Mean ± SEM)
3 h
Anti-Inflammatory
Activity
(% Inhibition of Edema)
3 h
138a
0.24 ± 0.01
59
138b
0.20 ± 0.02
63
138c
0.22 ± 0.02
61
138d
0.23 ± 0.02
60
138e
0.19 ± 0.02
64
138f
0.21 ± 0.01
62
139a
0.26 ± 0.01
57
139b
0.25 ± 0.02
58
140a
0.28 ± 0.01
55
140b
0.27 ± 0.02
56
Diclofenac
0.20 ± 0.01
63
Kamat and co-workers evaluated the anti-inflammatory activity of a series of pyridine- and thiazole-based hydrazides, using denaturation of the bovine serum albumin method. Among all derivatives, three structures (141f, 141 g, and 141 h) showed good activity toward other structures (Fig. 42 and Table 38). The highest inhibition among all derivatives was achieved for structure 141 h at 5 different concentrations (Fig. 42 and Table 38). In addition, structures 141a, 141b, 141c, 141d, and 141e had moderate inhibition (Fig. 42 and Table 38) (Kamat et al., 2020)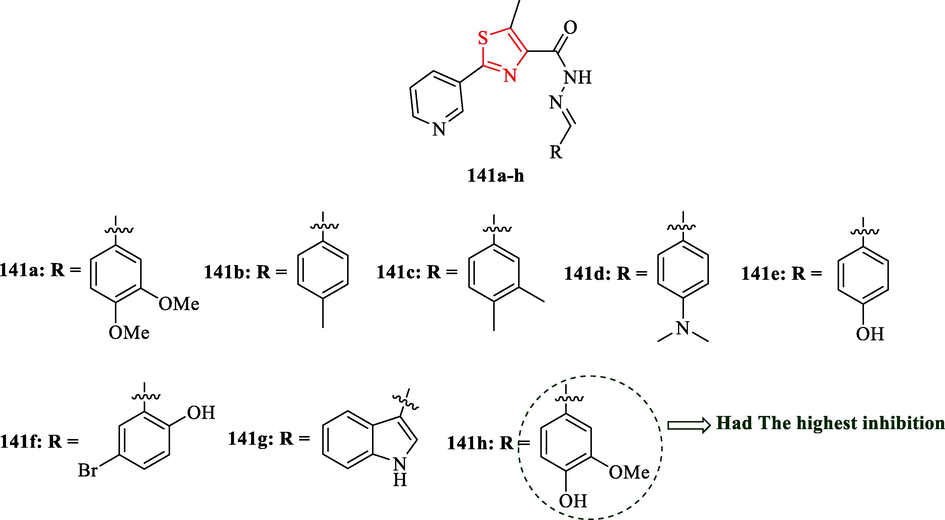
Chemical structures 141a-h (Kamat et al., 2020)
Cpd. no.
20(μg/mL)
40(μg/mL)
60(μg/mL)
80(μg/mL)
100(μg/mL)
IC50(μg/mL)
141a
22.66 ± 1.05
30.51 ± 2.22
42.86 ± 2.43
52.54 ± 0.90
69.65 ± 1.21
76.12
141b
24.46 ± 1.19
35.06 ± 0.56
48.94 ± 1.53
61.43 ± 1.24
73.82 ± 1.45
61.29
141c
17.65 ± 1.34
27.27 ± 1.52
35.26 ± 1.42
49.58 ± 0.98
62.06 ± 1.57
80.68
141d
19.18 ± 0.71
27.92 ± 1.59
37.33 ± 1.43
49.89 ± 1.45
65.78 ± 1.64
80.18
141e
25.49 ± 0.85
31.18 ± 1.28
49.24 ± 1.60
58.12 ± 0.56
71.07 ± 0.34
62.74
141f
30.66 ± 1.04
40.01 ± 1.33
53.98 ± 1.78
68.39 ± 1.00
80.34 ± 0.95
55.58
141 g
31.42 ± 0.95
41.00 ± 1.24
56.72 ± 2.00
71.47 ± 0.90
83.46 ± 2.21
52.89
141 h
32.63 ± 1.07
41.00 ± 1.24
59.51 ± 2.46
78.24 ± 1.37
87.97 ± 1.37
46.29
Diclofenac sodium
42.98 ± 1.28
57.08 ± 1.6
75.54 ± 1.26
83.56 ± 0.96
94.60 ± 0.90
35.03
Maghraby et al. reported and evaluated the anti-inflammatory activity of a series of structures containing thiazole scaffold. According to in vivo anti-inflammatory activity results, three structures (142, 143, and 144) showed significant activity (Fig. 43 and Table 39). Further, structure 144 showed the highest inhibition toward other structures compared to the reference drug after 4 h (Fig. 43 and Table 39) (Maghraby et al., 2020)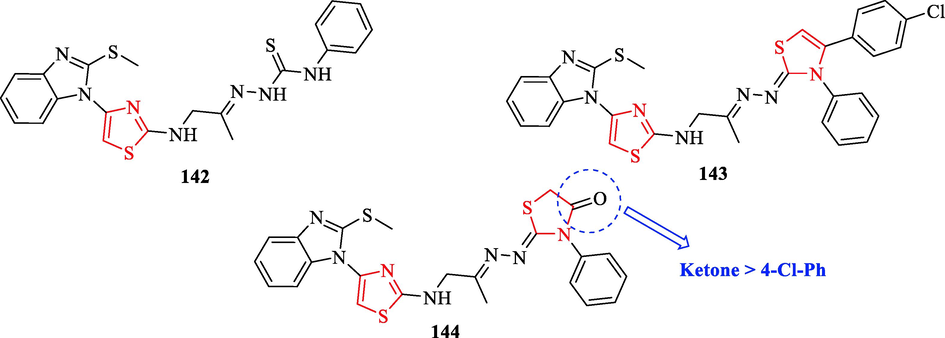
Chemical structures of 142–144 (Maghraby et al., 2020)
Cpd. no.
Thickness of right paw (cm) (Mean ± S.E.)
Zero time
0.5 h
1 h
2 h
3 h
4 h
142
7.33 ± 0.17
5.67 ± 0.44
5.00 ± 0.01
4.67 ± 0.17
4.83 ± 0.17
5.50 ± 0.29
143
7.17 ± 0.17
6.33 ± 0.17
5.33 ± 0.17
5.00 ± 0.29
4.67 ± 0.17
5.67 ± 0.17
144
7.00 ± 0.01
5.67 ± 0.17
4.67 ± 0.17
4.17 ± 0.17
3.83 ± 0.17
4.50 ± 0.29
Indomethacin
7.17 ± 0.17
5.83 ± 0.17
5.00 ± 0.29
4.67 ± 0.44
4.50 ± 0.29
4.17 ± 0.17
Han et al. evaluated the anti-inflammatory activity of a series of new 2-aminobenzo[d]thiazole derivatives, using the carrageenan-induced mouse paw edema method. The results of the study showed structures 145, 146, and 147 had the best activity compared to other structures and the same structures showed as potent sEH inhibitors, but structure 147in rat liver microsomes was unstable (Fig. 44 and Table 40) (Han et al., 2021)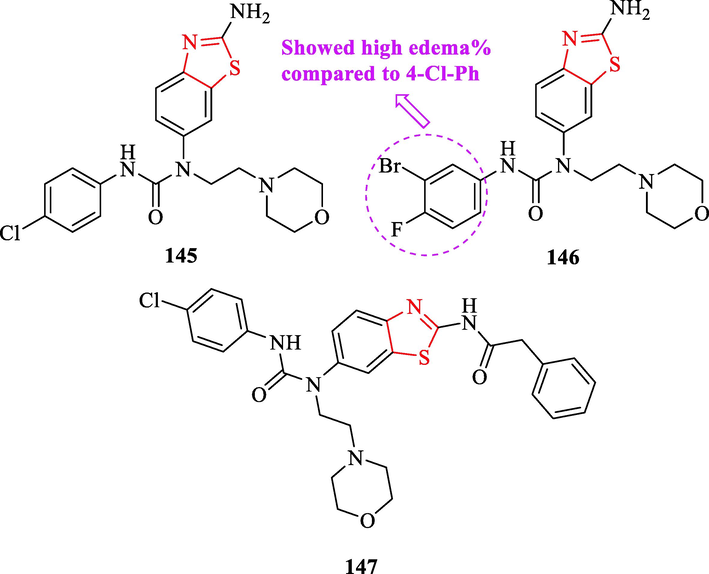
Chemical structures of 143–145 (Han et al., 2021)
Cpd. no.
In vivo anti-inflammatory
1 h
2 h
3 h
4 h
Paw thickness
(mm)Edema%
Paw thickness
(mm)Edema%
Paw thickness
(mm)Edema%
Paw thickness
(mm)Edema%
145
3.11 ± 0.17
20.99
3.06 ± 0.09
19.06
3.13 ± 0.21
21.64
3.17 ± 0.12
23.39
146
3.15 ± 0.04
30.86
3.16 ± 0.16
31.49
3.06 ± 0.07
27.39
3.06 ± 0.10
27.12
147
3.33 ± 0.16
21.56
3.22 ± 0.21
17.58
3.19 ± 0.19
16.48
3.30 ± 0.11
20.32
t-AUCB
3.30 ± 0.15
25.01
3.23 ± 0.15
22.61
3.29 ± 0.22
24.98
3.28 ± 0.15
24.71
Ankali et al. evaluated the anti-inflammatory activity of a novel series of 1,3-Thiazoles. According to the results, four structures (148a, 148b, 148c, and 148d) exhibited more potent anti-inflammatory activity with Cl, NO2, and Br substituting on the phenyl ring compared to diclofenac as reference drugs (Fig. 45 and Table 41). Moreover, structures 148e and 148f with CH3 and OH substitutes showed moderate activity compared to structures 148a, 148b, and 148d after 5 h (Fig. 45 and Table 41). It seems that structures with electron-withdrawing groups such as Cl and NO2 on the phenyl ring are greater anti-inflammatory activity toward structures with electron-donating groups (Ankali et al., 2021)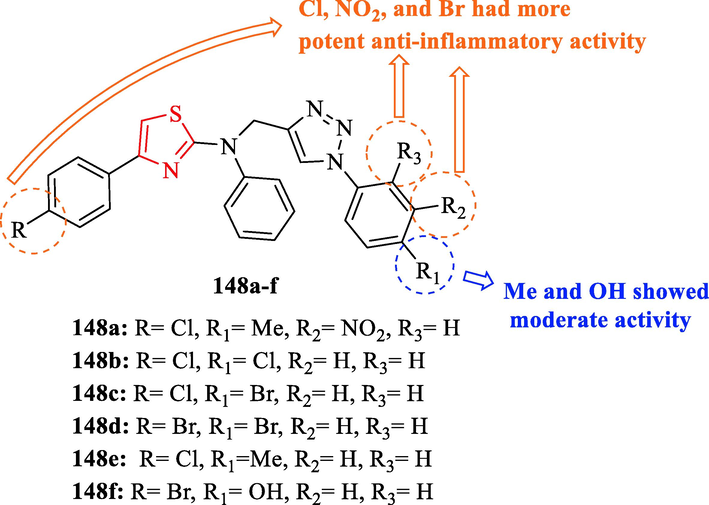
The structures of 1,3-thiazole derivatives(148a-f) (Ankali et al., 2021)
Cpd. no.
Paw edema volume in ml (% of edema inhibition)
0.5 h
1 h
3 h
5 h
148a
0.2433 ± 0.1041 (67.26%)
0.2100 ± 0.1305 (68.1%)
0.2167 ± 0.06333 (62.63%)
0.3767 ± 0.008819 (57.07%)
148b
0.1567 ± 0.03844 (78.91%)
0.3033 ± 0.1572 (54.04%)
0.1533 ± 0.09770 (73.56%)
0.3400 ± 0.0200 (61.21%)
148c
0.3533 ± 0.09597 (52.46%)
0.5200 ± 0.1484 (21.21%)
0.6633 ± 0.1241 (-14.36%)
0.5100 ± 0.08737 (41.82%)
148d
0.1000 ± 0.06245 (86.54%)
0.1633 ± 0.05897 (75.25%)
0.2300 ± 0.07211 (60.03%)
0.3633 ± 0.008819 (58.55%)
148e
0.2433 ± 0.09207 (67.26%)
0.1000 ± 0.06245 (84.84%)
0.1167 ± 0.01856 (96.8%)
0.4933 ± 0.2478 (43.72%)
148f
0.067 ± 0.02963 (91.03%)
0.1233 ± 0.03756 (81.31%)
0.1800 ± 0.05033 (91.32%)
0.6367 ± 0.1419 (27.37%)
Diclofenac
0.100 ± 0.06009 (86.54%)
0.123 ± 0.1418 (81.31%)
0.4967 ± 0.07860 (79.20%)
0.4533 ± 0.04702 (48.29%)
In 2021, a series of thiazole/oxazole substituted benzothiazole derivatives were synthesized by Kumar and Singh. All derivatives were tested against carrageenin at 3 different doses for their anti-inflammatory activity. According to the results, structure 149 with ethyl group at the 3-position of benzothiazole displayed greater activity compared to phenyl butazon as a reference drug (Fig. 46 and Table 42). In addition, derivatives 150a-h showed moderate anti-inflammatory activity (Fig. 46 and Table 42). Among the derivatives, structure 150e with chlorine at the 4-position of the phenyl azo group exhibited remarkable anti-inflammatory activity (Fig. 46 and Table 42) (Kumar and Singh, 2021)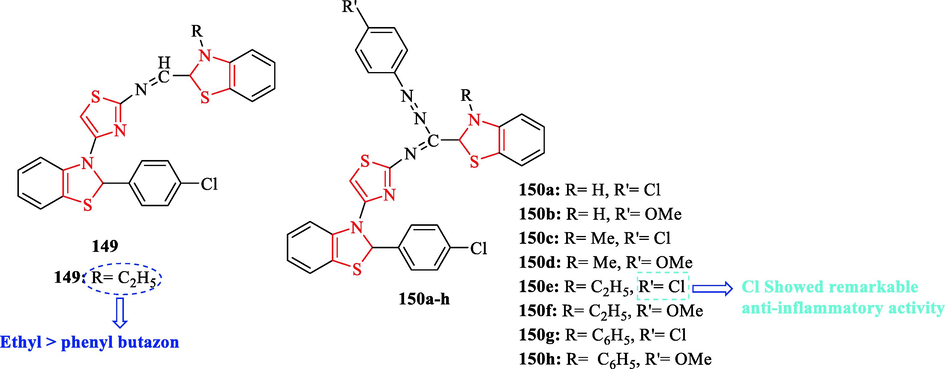
Chemical structures 149 and 150a-h (Kumar and Singh, 2021)
Cpd. no.
Dose (mg/kg
p.o.)
Anti-inflammation Activity
% of inhibition
25
38.5
149
50
55.4
100
69.6
25
150e
50
34.7
100
Phenyl butazone
25
50
10022.2
35.8
66.5
In 2022, Sukanya et al investigated the inhibition of novel series of thiazole derivatives against two enzymes. The investigation of anti-inflammatory activity exhibited structures 151a and 151b had significant activity compared to other structures (Fig. 47 and Table 43). The same structures had moderated activity against MMP-9 compared to positive control and had remarkable activity against MMP-2 compared to positive control (Fig. 47 and Table 43). It seems that the presence of NO2 substitute at both phenyls is effective in their anti-inflammatory activity (Sukanya et al., 2022)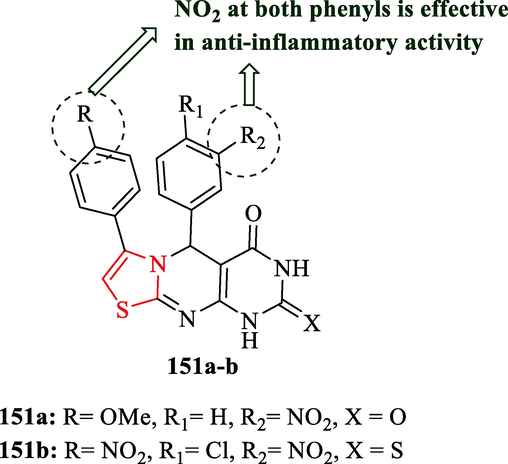
Chemical structures 151a-b (Sukanya et al., 2022)
Cpd. no.
Anti-inflammatory
activity against MMP-2
(% Inhibition)
Anti-inflammatory
activity against MMP-9
(% Inhibition)
151a
90 ± 0.41
45 ± 0.38
151b
85 ± 0.34
35 ± 0.54
Tetra- cycline
hydrochloride
99 ± 0.51
95 ± 0.35
Saliyeva and co-workers synthesized a series of derivatives containing thiazole scaffold and evaluated in vivo anti-inflammatory activity in white rats carrageenan-induced edema paw model. Diclofenac sodium was used as a reference drug. All derivatives showed moderate anti-inflammatory activity (Fig. 48 and Table 44). Structures 152a and 152b displayed the best activity compared to the reference drug (Fig. 48 and Table 44). Moreover, structures 152c, 152d, 152e, 152f, 152 g and 152 h exhibited moderate to good activity (Fig. 48 and Table 44) (Saliyeva et al., 2021)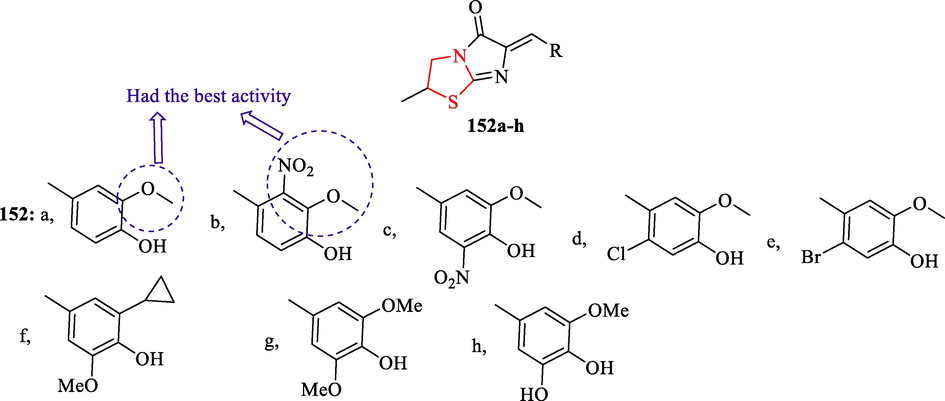
Chemical structures 152a-h (Saliyeva et al., 2021)
Cpd. no.
Rat hind limb volume
increase, 4 h, %
Inflammation
inhibition, %
152a
76.5 ± 6.3
40.3
152b
78.5 ± 6.0
38.8
152c
86.5 ± 7.1
33.4
152d
90.5 ± 8.9
33.9
152e
86.7 ± 6.4
31.6
152f
83.8 ± 5.9
33.9
152 g
85.5 ± 6.2
33.3
152 h
89.4 ± 7.0
30.3
Diclofenac sodium
64.8 ± 4.5
48.2
Yamsani and Sundararajan. investigated the anti-inflammatory activity of thirteen series of novel derivatives containing thiazole scaffold, using a carrageenan-induced paw edema method. Almost all derivatives demonstrated remarkable activity (Fig. 49). As a reference drug, diclofenac was used. Derivatives substituted at the para-position of phenyl ring attached at C-1 of pyrazole, namely 155e, 155f, and 155 g had excellent activity toward the reference drug (Fig. 49 and Table 45). Structure 155a with unsubstituted phenyl at C-1 of pyrazole had a nearly equipotent anti-inflammatory activity toward the reference drug (Fig. 49 and Table 45). In addition, Structures 155b, 155c, and 155d displayed moderated activity (Fig. 49 and Table 45). Also, Structures 153, 154a, 154b, 155 h, 155i, and 155j showed low activity (Fig. 49 and Table 45). In general, Structure 155f showed the most potent activity, and structure 153 displayed low activity (Fig. 49 and Table 45) (Yamsani and Sundararajan, 2022)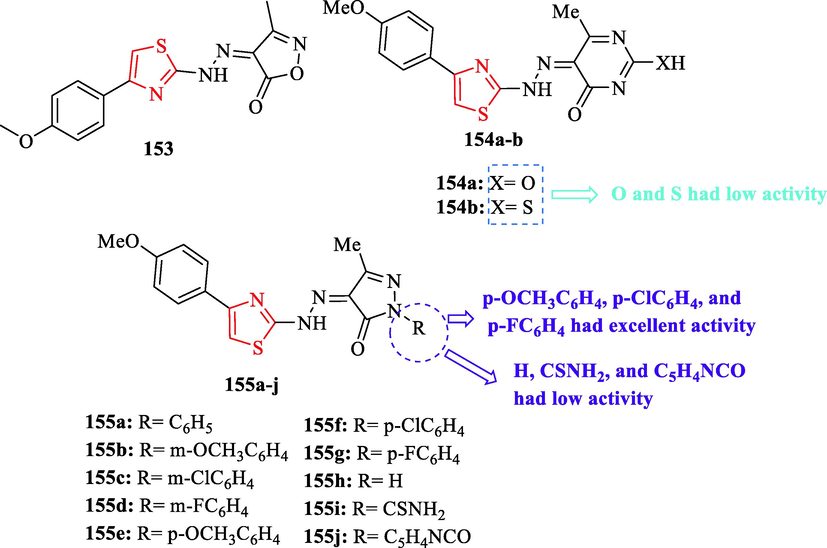
Chemical structures 153, 154a-b, and 155a-j (Yamsani and Sundararajan, 2022)
Cpd. no.
Dose
(mg/kg)
% Protection
30 min
1 h
2 h
3 h
153
10
9 ± 0.41
14 ± 1.85
16 ± 0.59
10 ± 0.20
20
11 ± 0.99
15 ± 0.42
18 ± 1.14
12 ± 0.6710
155a
10
36 ± 0.87
39 ± 0.95
49 ± 1.64
34 ± 0.40
20
44 ± 0.58
57 ± 0.14
73 ± 1.23
40 ± 0.76
155b
10
25 ± 0.70
28 ± 1.52
33 ± 0.99
25 ± 0.15
20
30 ± 2.16
39 ± 0.90
50 ± 0.67
28 ± 0.40
155c
10
29 ± 0.34
30 ± 0.79
35 ± 1.25
26 ± 0.98
20
33 ± 1.12
43 ± 0.30
55 ± 0.56
31 ± 1.31
155d
10
27 ± 0.78
30 ± 1.63
34 ± 0.41
2 5 ± 1.14
20
31 ± 0.95
40 ± 0.81
54 ± 0.43
31 ± 0.87
155e
10
38 ± 1.70
43 ± 0.45
50 ± 0.37
36 ± 0.59
20
46 ± 0.22
58 ± 0.16
75 ± 1.49
43 ± 0.42
155f
10
40 ± 0.71
46 ± 0.54
53 ± 0.16
38 ± 1.98
20
49 ± 0.49
60 ± 1.73
76 ± 0.35
45 ± 0.52
155 g
10
38 ± 1.15
44 ± 0.60
52 ± 0.89
38 ± 1.16
20
48 ± 0.37
59 ± 1.94
75 ± 0.72
45 ± 0.45
Tetra- cycline
hydrochloride
10
2037 ± 1.58
45 ± 0.9540 ± 0.95
57 ± 1.6049 ± 0.87
74 ± 0.5235 ± 1.84
42 ± 0.46
Abdel‐Aziz et al. evaluated COX-1/COX-2 inhibitory as well as anti-inflammatory activity of a series of pyrimidine/thiazole hybrids, using the carrageen‐stimulated paw edema bioanalysis. Structures 156c, 156d, 156 h, 156i, and 156j exhibited potent COX‐2 inhibitors (Fig. 50 and Table 46). Also, the same structures had significant COX‐2 inhibition over COX‐1 inhibition. Structures 156 h, 156i, and 156j compared to celecoxib as a control showed the most strong COX‐2‐inhibitory activity (Fig. 50 and Table 46). Structures 156b (R = NH2) and 156a (R = Ph) showed two times less potent activity against the COX‐2 isoform compared to structures 156j and 156i (Fig. 50 and Table 46). Moreover, structures 156e, 156f, and 156 g remarkably dwindled COX‐2‐inhibitory activity and selectivity (Fig. 50 and Table 46) (Abdel-Aziz et al., 2022)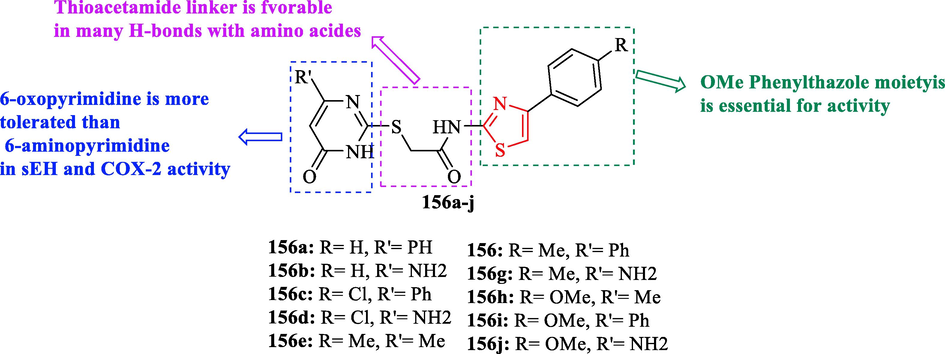
Chemical structures 156a–j (Abdel-Aziz et al., 2022)
Cpd. no.
COX‐1,
IC50 (μM)
COX‐2,
IC50 (μM)
156a
2.03
156b
2.33
156c
14.33
1.89
156d
14.53
1.74
156e
3.50
156f
2.93
156 g
13.88
3.09
156 h
14.98
1.43
156i
15.89
1.02
156j
15.31
1.13
Celecoxib
7.32
0.88
4 Thiazole derivatives with anticancer activity
Cancer is an illness that is caused by cells that grow and multiply too quickly. There are many different ways to try to treat cancer, but the most common is chemotherapy. Cancer is still a serious problem, because most cancer drugs do not have low toxicity to take, and cancer cells are often very resistant to them as well (Ayati et al., 2019)
In 2014, the anticancer activity of a series of spiro (cyclohexane1,20-thiazolopyridine) derivatives and spiro (cyclohexane-1,20-thiazolidine) derivatives were evaluated against 2 cell lines by Flefel et al. Among all derivatives, structure 157 against both cancer cell lines displayed the most potent activity with respect to LSD as a reference drug (Fig. 51). In addition, structure 158 showed the lowest activity against HepG-2 (Fig. 51) (Flefel et al., 2014)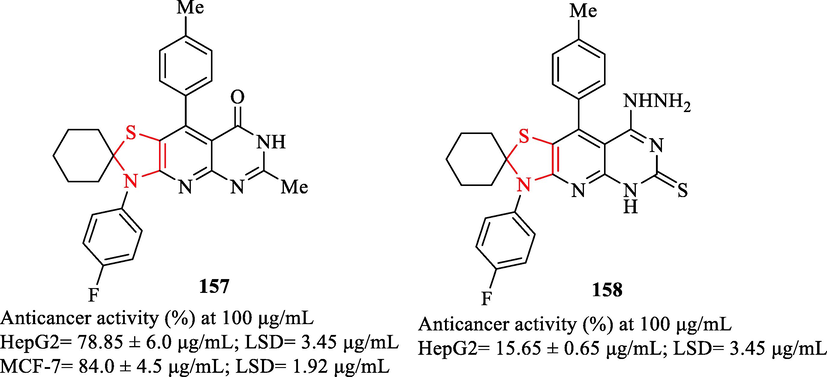
Anticancer activity of the structures 157 and 158 (Flefel et al., 2014)
Altıntop et al. evaluated in vitro cytotoxic effects of a series of nitro-substituted thiazolyl hydrazone derivatives on 2 cell lines, using the MTT method. Cisplatin was used as a reference drug. As a result, structure 159 showed the best activity against NIH/3T3 and MCF-7 compared to other structures, followed by structure 160 (Fig. 52)(Altıntop et al., 2014)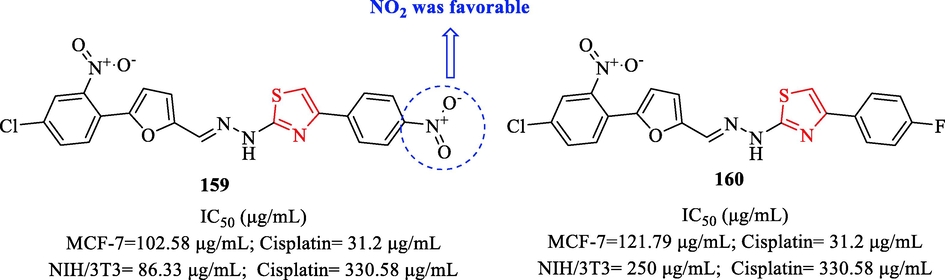
Anticancer activity of the structures 159 and 160 (Altıntop et al., 2014)
Nofal and co-workers evaluated the cytotoxic activity of a series of benzimidazole–thiazole derivatives against HepG2 and PC12. According to the results, structures 165 and 170 had remarkable activity against two cell lines. When derivatives were tested against HepG2 cells four structures (162, 166, 167, and 169) did not show any cytotoxic effects at two concentrations (10 and 30 mM) (Fig. 53). In PC12 cells at 0.3 mM, three structures (164, 166, and 167) did not display cytotoxicity. In HepC-2 cells, six structures (162, 161, 163, 164, 165, and 168) diminished the viability of the mentioned cells between 20 and 30% at 30 mM (Fig. 53). In addition, structure 168 against the PC12 cell showed the most activity, and structures 165 and 170 against the HepG2 cell line had the most activity (Fig. 53). Moreover, structures 165 and 170 exhibited antitumor activity against HepG2 and PC12 (Fig. 53) (Nofal et al., 2014)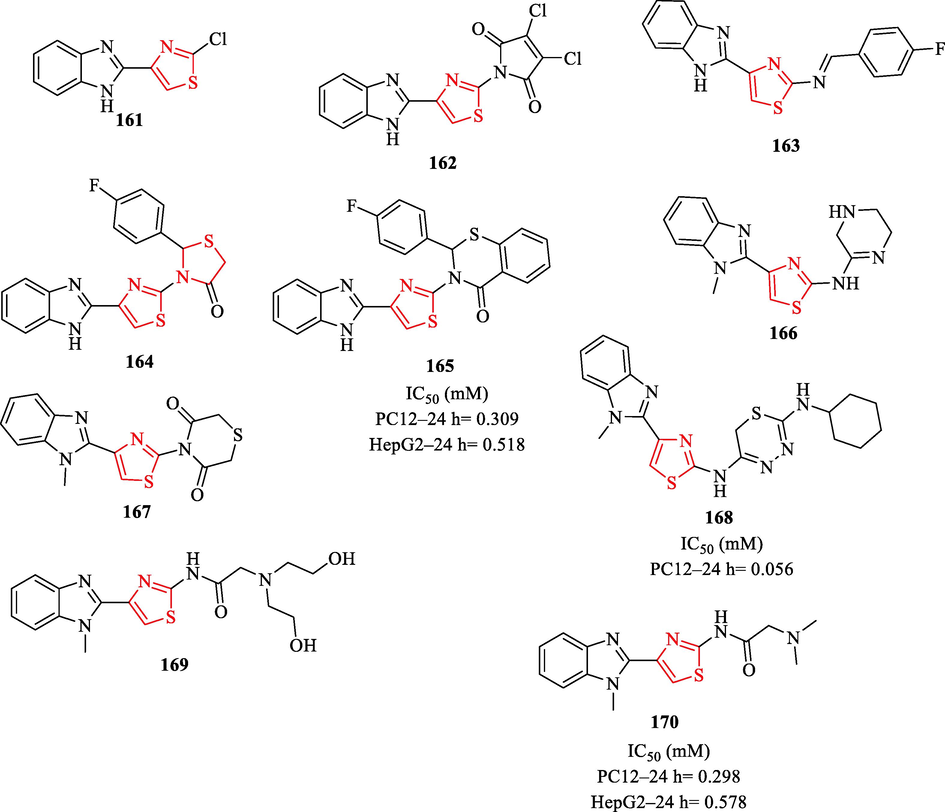
Chemical structures 161–170 and anticancer activity of the structures 165, 168, and 170 (Nofal et al., 2014)
Rostom and co-workers evaluated the in vitro antitumor activity of ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives against sixty subpanel tumor cell lines. According to the non-small cell lung cancer subpanel, structures 171, 173, 174, 175, 177, and 178 showed a changeable rate of sensitivity toward the Hop-92 cell line, especially to structures 175 and 178 (Fig. 54). Structures 171, 174, 175, 176, and 178 were tested against the NCI-H522 cell line within the same subpanel which structures 175, 176, and 178 displayed the highest growth inhibitory (Fig. 54). In the case of the melanoma subpanel of the SK-MEL-2 cell line, structures 172 and 178 exhibited substantial growth inhibitory (Fig. 54). Structures 172 and 174 against the IGROV1 cell line of ovarian cancer showed high growth inhibitory (Fig. 54). Moreover, two structures (171 and 172) against the colon cancer subpanel of the HCC-2998 cell line displayed significant growth inhibitory activity (Fig. 54). Most of the CNS, breast, and prostate cell lines were resistant to the selected derivatives. All structures were effective on inhibit the growth of the renal UO-31 cell line, but their percentages of growth inhibitory were low (Fig. 54). In general, structure 174 against most of the subpanel tumor cell lines had a broad spectrum of anticancer activity, especially against the non-small cell lung cancer Hop-92, melanoma SK-MEL-2, and ovarian cancer IGROV1 cell lines, and structure 172 exhibited substantial growth inhibitory potentials against melanoma SK-MEL-2, colon cancer HCC-2998 and ovarian cancer IGROV1 (Fig. 54) (Rostom et al., 2014)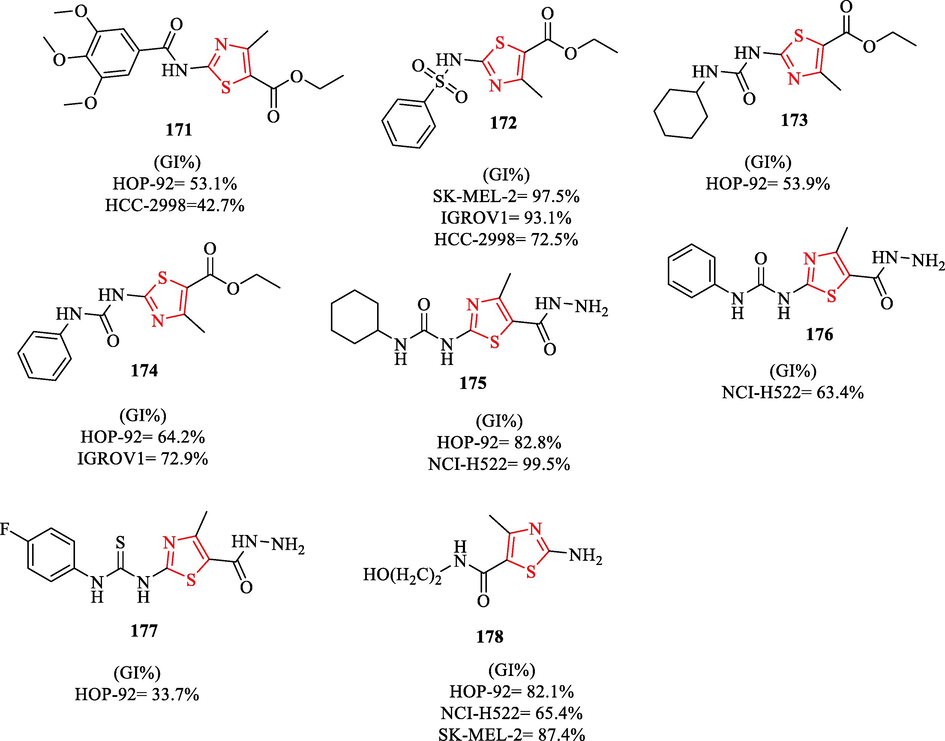
Chemical structures 171–178 and anticancer activity of the structures 171–178 (GI%= In vitro growth inhibitory percentage) (Rostom et al., 2014)
Ali et al. evaluated the anticancer activity of eleven structures out of imidazo[2,1-b]thiazole derivatives against the sixty cell lines. According to the results, structures 181b and 182b against all cell line panels showed the lowest mean percentage growth. Structures 179a and 180–182(a) against CNS SNB-75 and Renal UO-31 cancer cell lines had high growth percentages (Fig. 55). Structure 181a against Non-Small Cell Lung HOP-92 had significant potency (Fig. 55). Furthermore, structures 179b and 180–182(b) against Renal A498, Leukemia MOLT-4, and SR cancer cell lines showed substantial potency (Fig. 55). Structurec 182a with rapamycin (NSC S226080) had great correlation levels (Fig. 55). Moreover, structures 179a, 180a, 181a, 181b, 181c, and 182b with rapamycin demonstrated remarkable correlations (Fig. 55). Structure 181c with merbarone had a significant correlation (Fig. 55) (Ali et al., 2014)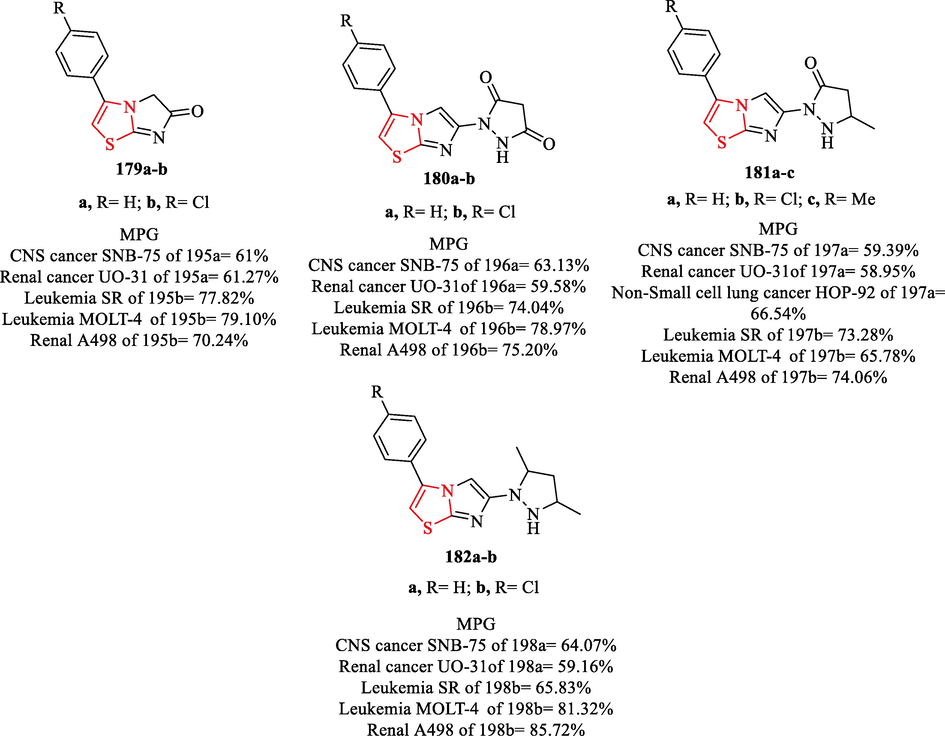
Chemical structures 179a-b, 180a-b, 181a-c, and 182a-b and their anticancer activity (MPG = Mean percentage growth) (Ali et al., 2014)
Koppireddi et al. evaluated the anti-proliferative activity of twelve novel derivatives containing thiazole scaffold against 4 human cancer cell lines with respect to doxorubicin as a reference drug. Structures 183b, 183c, 183d, 183e, and 183f had moderate antiproliferative activity against A549 and showed substantial activity against HeLa (Fig. 56 and Table 47). Structure 183d against all cell lines showed the best activity at a concentration range of 6.5 ± 0.56–17.4 ± 1.34 μM. Also, it caused considerable cytotoxicity in HeLa cells. Thereby, structure 183d caused caspase-8 and caspase-3 activation which guided to cell death of apoptotic (Fig. 56 and Table 47). According to FACS results, Structure 183d arrests cells in G0/G1 phase (Fig. 56 and Table 47). Generally, structures 183a and 183d against HeLa, A549, and THP1 displayed remarkable antiproliferative activity (Fig. 56 and Table 47) (Koppireddi et al., 2014)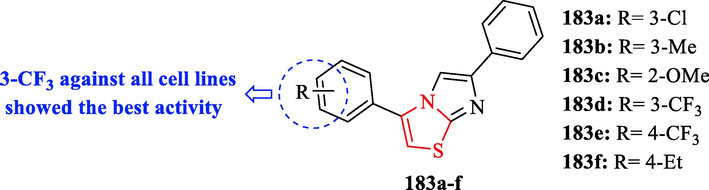
Chemical structures 183a-f (Koppireddi et al., 2014)
Cpd. no.
MIC50 values (μM)
HeLa1
A5492
MDAMB-2313
THP14
183a
14.0 ± 3.06
15.0 ± 3.1
21.7 ± 1.2
183b
13.7 ± 1.02
40.2 ± 4.03
183c
9.5 ± 1.09
39.0 ± 2.9
183d
6.5 ± 0.56
8.9 ± 0.46
10.9 ± 0.44
17.4 ± 1.34
183e
8.5 ± 2.43
21.5 ± 0.2
183f
11.3 ± 1.53
44.8 ± 1.21
Doxorubicin
6.0 ± 0.7
3.4 ± 0.9
2.0 ± 1.0
3.5 ± 0.8
Gali and and co-workers evaluated the anticancer activity of a series of coumarinylimidazo[2,1-b]thiazoles against 4 cancer cell lines with respect to doxorubicin as a reference drug. According to the in vitro cytotoxic activity results, all derivatives against all the tested cell lines were active. Structure 184c against HeLa, MCF-7, and HepG2 displayed broad spectrum activity (Fig. 57). Structure 187 showed remarkable activity against HeLa and MCF-7 (Fig. 57). In addition, structures 184a, 184b, and 185 exhibited significant activity against HeLa (Fig. 57). Whereas structure 186 showed prominent activity against NCI-H460 (Gali et al., 2014)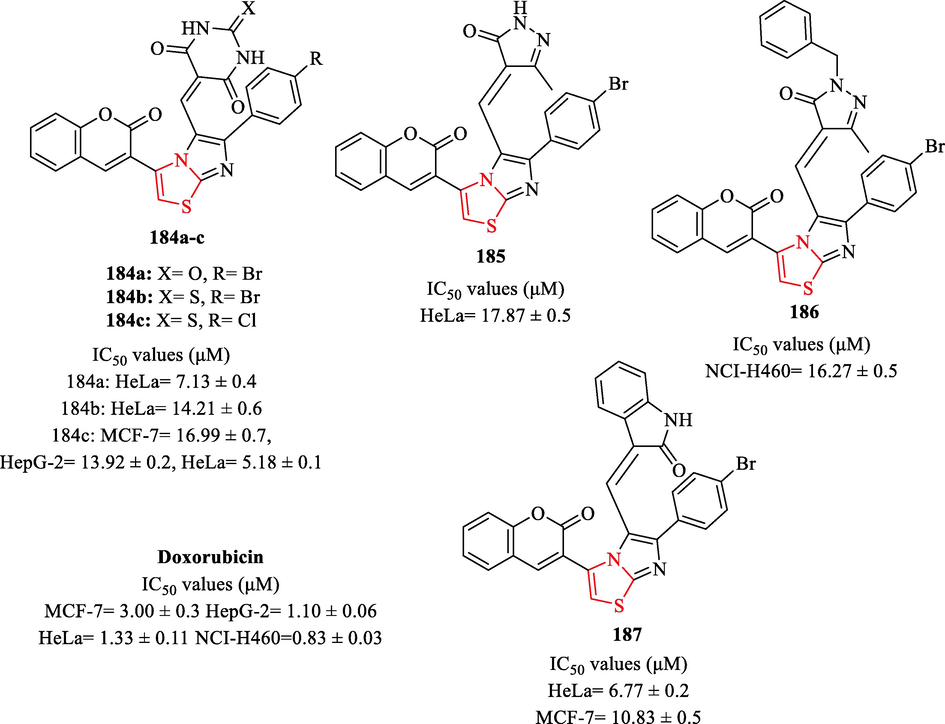
Chemical structures 184a-c, and 185–187 and their IC50 values (Gali et al., 2014)
In 2015, evaluated the anticancer activity of a series of derivatives containing thiazole scaffold against 2 cancer cell lines with respect to doxorubicin as a reference drug by Gomha and co-workers. According to the results, structures 189 and 190 against HEPG2-1 and HCT-116 showed significant activity (Fig. 58). Structures 188, 192, 193, and 194 against HCT-116 exhibited moderate activity (Fig. 58). In addition, structures 191 and 193 displayed moderate activity against HEPG2-1 (Gomha et al., 2015)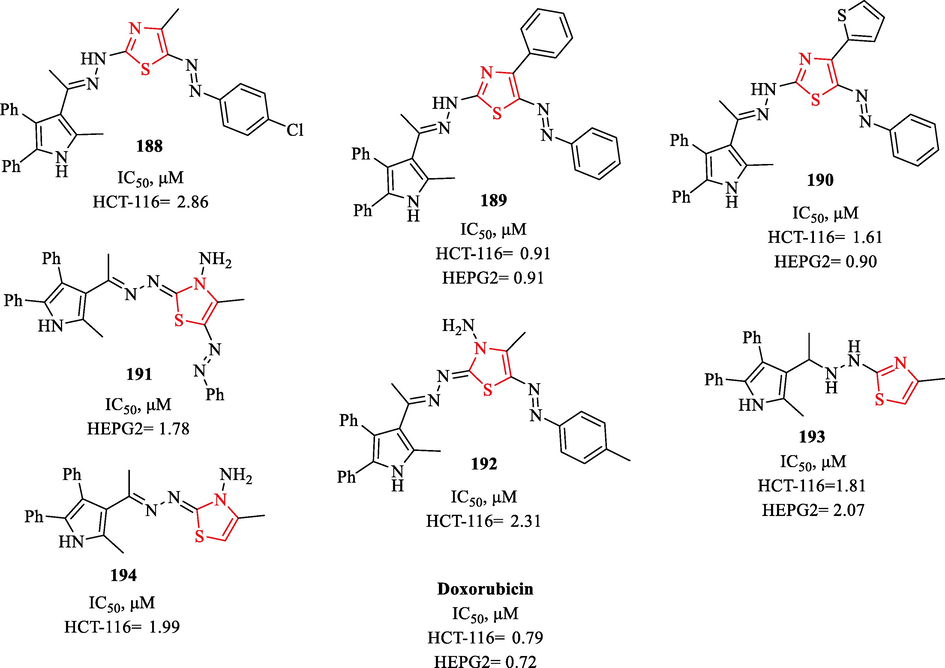
Chemical structures 188–194 and their IC50 values (Gomha et al., 2015)
Prabhu et al. reported and evaluated in vitro anticancer activity of a novel 8 derivatives containing thiazole scaffold against human cervical cancer cell line (HeLa) with respect to cisplatin as a reference drug. Structure 195b displayed better activity (Fig. 59 and Table 48). In general, structure 195b showed higher anticancer activity than structures 195e, 195 g, and 195 h (Fig. 59 and Table 48). Moreover, Structures 195d, 195c, 195f, and 195a exhibited less anticancer activity (Fig. 59 and Table 48) (Prabhu et al., 2015)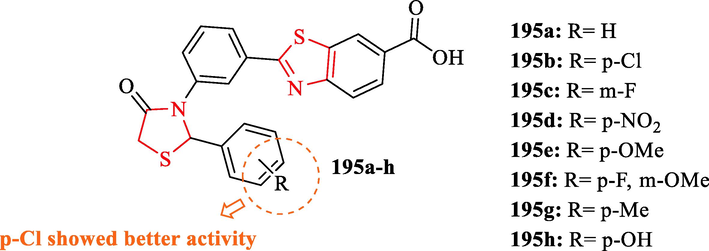
The structures of thiazolidinone substituted benzothiazole-6-carboxylic derivatives (195a-h) (Prabhu et al., 2015)
Cpd. no.
Compound concentration (μmol/L)
IC50
% Growth inhibition
5
12.5
25
40
195b
50.7
66.8
80.8
96.8
9.768
195e
45.7
70.8
77.9
88.45
14.566
195 g
40.8
66.7
70.7
82.68
16.456
195 h
44.9
68.8
76.9
81.7
17.768
Cisplatin
64.2
75.3
86.9
99.7
28.427
Tantak et al. investigated the anticancer activity of novel 16 derivatives containing thiazole scaffold against 6 cancer cell lines with respect to doxorubicin as a reference drug. Structure 196a was selective and potent against HEK293T and HeLa cells in the presence of FBS (Fig. 60 and Table 49). Structure 196b had an insignificant effect on HeLa cells but inhibited selectively HEK293T cells compared to structure 196a (Fig. 60 and Table 49). Structure 196c against HeLa had the most potent activity (Fig. 60 and Table 49) (Tantak et al., 2015)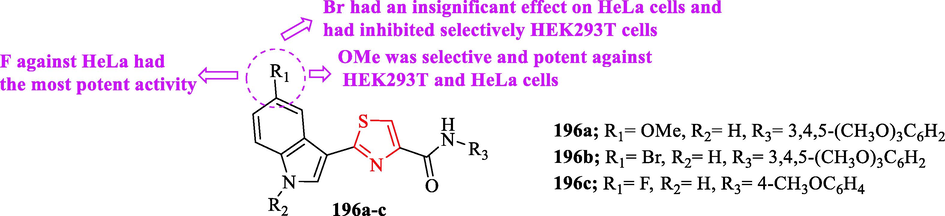
Chemical structures 196a-c (Tantak et al., 2015)
Cpd. no.
In vitro cytotoxicity (without FBS)
IC50 (μM)
In vitro cytotoxicity (with FBS)
IC50 (μM)
HEK 293 T
HeLa
HEK 293 T
HeLa
196a
8.60 ± 0.91
29.64 ± 1.44
8.74 ± 1.26
9.98 ± 0.01
196b
>100
55.79 ± 4.73
>100
93.03 ± 3.96
196c
12.10 ± 0.61
3.41 ± 0.07
33.48 ± 0.98
Doxorubicin
0.84 ± 0.05
0.45 ± 0.06
0.75 ± 0.03
0.43 ± 0.10
Metwally et al. evaluated the anticancer activity of 5 derivatives containing thiazole scaffold against 2 cancer cell lines. All the derivatives displayed remarkable anticancer activity. Structure 198a against the breast cancer cell line showed the most potent activity, while structure 197 against the liver cancer cell line had the most potent activity (Fig. 61 and Table 50). Also, structure 197 had the best reactivity toward HEPG-2 and MCF-7 (Fig. 61 and Table 50). Structure 198b against HEPG-2 and MCF-7 displayed less potent anticancer activity (Fig. 61 and Table 50) (Metwally et al., 2015)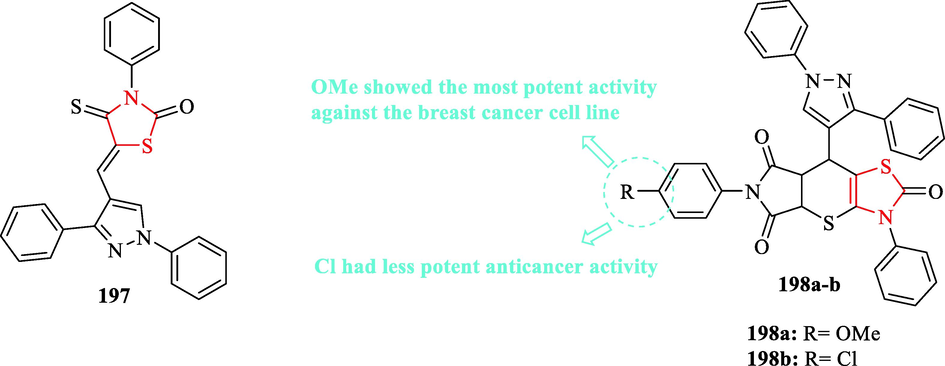
Chemical structures 197 and 198a-b (Metwally et al., 2015)
Cpd. no.
IC50 in μg/mL
MCF-7
HEPG-2
197
19.3
10.6
198a
12.3
21.4
198b
35.9
21.3
In 2016, evaluated anticancer activity of 10 derivatives containing thiazole scaffold against 5 human cancer cell lines which were synthesized by Vaddula et al via the one-pot method. Some derivatives displayed moderate activity. Structure 199d against BT-474 and MDA-MB-157 showed significant activity (Fig. 62 and Table 51). Structure 199a against BT-474 exhibited remarkable activity (Fig. 62 and Table 51). Structure 199b against MCF-7 displayed the best activity and structure 199c against BT-474 had the best activity (Fig. 62 and Table 51) (Vaddula et al., 2016)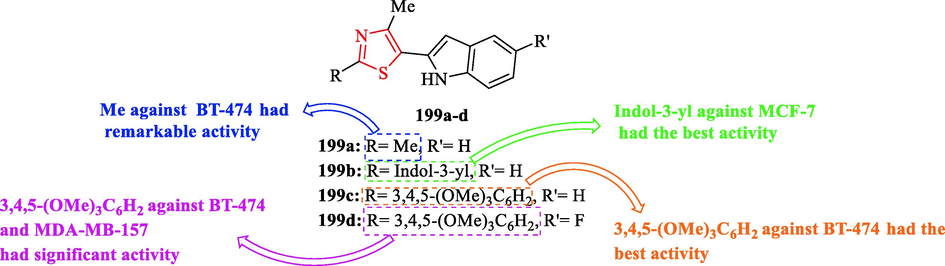
Chemical structures 199a-d (Vaddula et al., 2016)
Cpd. no.
IC50 in μM
BT-474
MCF-7
MDA-MB-157
199a
30
199b
10
199c
20
199d
30
30
Doxorubicin
0.3–0.4
Karaman and Ulusoy Güzeldemirci. evaluated the anticancer activity of a series of derivatives containing thiazole scaffold against sixty different cell panels with respect to cisplatin as a reference drug. Among all derivatives, structures 200a and 200b were chosen for the investigation of their anticancer activity (Fig. 63 and Table 52). Preliminary results showed structure 200b (R-2,5-dimethoxyphenyl) against prostate cancer cell line DU-145 and two leukemia cell lines K-562 and SR had the lowest growth percentages (Fig. 63 and Table 52). Further, structure 200a exhibited a better antiproliferative activity profile toward structure 200b. Structure 200a against some of the cancer cell lines displayed substantial inhibitory activity, especially against the prostate cancer cell line DU-145 and leukemia cell lines HL-60 (TB) and SR (Fig. 63 and Table 52). Structure 200a against all cancer cell lines except OVCAR-5, NCIH23, and SF-539 exhibited high potent activity (Fig. 63 and Table 52) (Karaman and Ulusoy Güzeldemirci, 2016)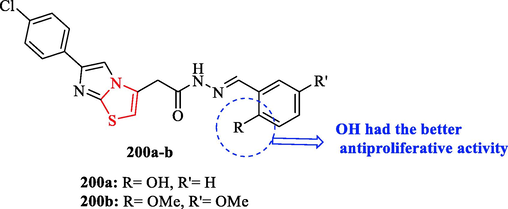
Chemical structures 200a-b (Karaman and Ulusoy Güzeldemirci, 2016)
Panel/cell line
Comparison of in vitro tumor cell GI
200a
Cisplatin
Sorafenib
Log10GI50
Log10TGI
Log10LC50
Log10GI50
Log10TGI
Log10LC50
Log10GI50
Log10TGI
Log10LC50
Leukemia
HL-60(TB)
−5.78
−5.07
>−4.00
−5.18
−4.047
−4
−5.793
−4.595
−4
K-562
−5.47
>−4.00
>−4.00
−4.577
−4.062
−4
−5.547
−4
−4
SR
−6.22
>−4.00
>−4.00
−5.113
−4.266
−4
−5.515
−4.885
−4
Prostate cancer
DU-145
−5.57
>−4.00
>−4.00
−5.165
−4.324
−4
−5.476
−4.899
−4.353
Cpd. no.
Growth %
DU-145
K-562
SR
HL-60 (TB)
200a
−8.65
−11.51
−3.50
200b
55.11
51.07
32.07
Anticancer activity of ten derivatives containing thiazole scaffold was evaluated against 4 cancer cell lines with respect to mitoxantrone as a reference drug by Turan-Zitouni et al. In general, all derivatives against C6 rat glioma cells displayed substantial cytotoxic and anticancer activity. In the case of the A549 cell line, structure 201d showed the most potent cytotoxic agent compared to the reference drug, which was followed by 201c, 201a, and 201b, while structure 201f had the lowest activity (Fig. 64 and Table 53). In the case of the C6 cell line, structure 201d showed the most effective anticancer agent compared to the reference drug, which was followed by structures 201b, 201c, and 201a, while structure 201e had the lowest activity (Fig. 64 and Table 53). In the case of the 5RP7 cell line, structure 201a showed the most potent cytotoxic activity compared to the reference drug, which was followed by 201 g and 201c, while structure 201d had the lowest anticancer activity (Fig. 64 and Table 53). Among all derivatives, structure 201d showed significant antiproliferative effects on C6 and A549 cell lines. Also, structure 201d against C6 and A549 cell lines displayed anticancer activity (Fig. 64 and Table 53) (Turan-Zitouni et al., 2016)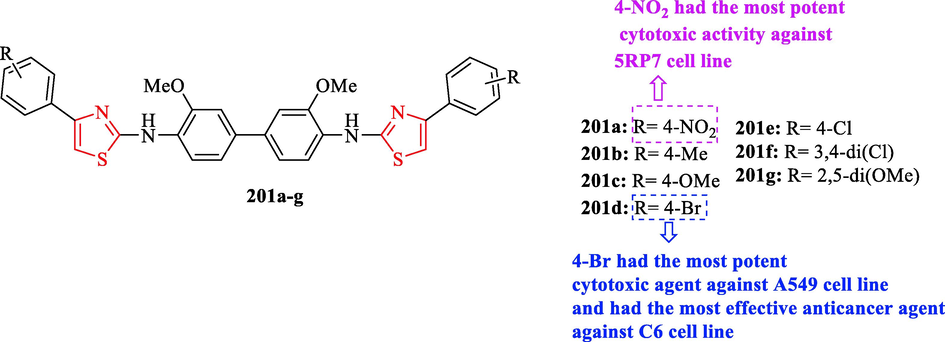
Chemical structures 201a-g (Turan-Zitouni et al., 2016)
Cpd. no.
IC50 (μg/mL)
A549 cell lines
C6 cell lines
5RP7 cell lines
201a
82.3 ± 2.5
33.3 ± 5.8
5.83 ± 1.04
201b
98.3 ± 7.6
21.0 ± 1.7
201c
58.3 ± 7.6
28.7 ± 1.2
13.33 ± 1.53
201d
37.3 ± 6.8
11.3 ± 1.2
68.33 ± 22.55
201e
146.7 ± 5.8
201f
146.7 ± 20.8
201 g
12.33 ± 0.58
Mitoxantrone
15.7 ± 4.0
11.0 ± 1.7
0.73 ± 0.06
The anticancer activity of eleven derivatives containing thiazole scaffold was evaluated against 3 cancer cell lines with respect to foretinib as a reference drug by Lei and co-workers. Structures 202a, 202b, 202e, 202f, and 202 h against the HT-29 cell line exhibited remarkable activity (Fig. 65 and Table 54). Structures 202a, 202b, 202d, 202e, 202f, and 202 h displayed substantial anticancer activity against the MKN-45 cells (Fig. 65 and Table 54). Structures 202a, 202b, 202c, 202d, 202e, 202f, 202 g, 202 h, 202i, 202j, and 202 k against H460 showed superior activity (Fig. 65 and Table 54). It seems that the presence of R2 groups is a substantial effect on anticancer activity, but R1 groups had little effect on cytotoxicity (Lei et al., 2016)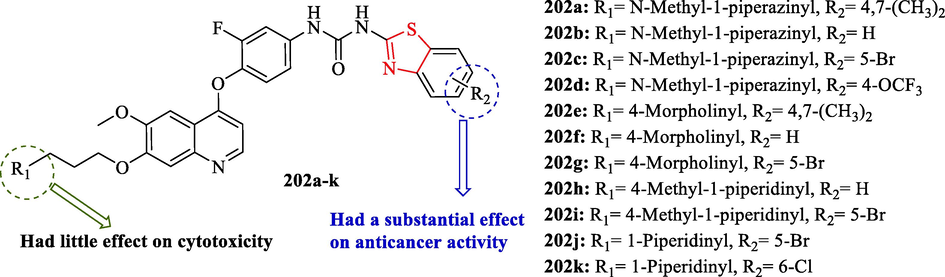
Chemical structures 202a-k (Lei et al., 2016)
Cpd. no.
IC50 (μM)
HT-29
MKN-45
H460
202a
0.18 ± 0.02
0.06 ± 0.01
0.01 ± 0.003
202b
0.26 ± 0.03
0.02 ± 0.005
0.05 ± 0.007
202c
0.08 ± 0.006
202d
0.22 ± 0.02
0.15 ± 0.02
202e
0.21 ± 0.03
0.03 ± 0.005
0.09 ± 0.02
202f
0.70 ± 0.05
0.04 ± 0.005
0.14 ± 0.03
202 g
0.09 ± 0.02
202 h
0.46 ± 0.04
0.04 ± 0.004
0.06 ± 0.02
202i
0.08 ± 0.02
202j
0.12 ± 0.03
202 k
0.10 ± 0.02
Foretinib
0.18 ± 0.02
0.031 ± 0.004
0.18 ± 0.04
The anticancer activity of eleven derivatives containing thiazole scaffold was evaluated against 3 cancer cell lines with respect to 5-Fluorouracil as a reference drug by Cai et al. At a concentration of 5 μg/mL, some of the derivatives such as structures 203a and 203b displayed good anticancer activity against A-549 compared to the reference drug (Fig. 66 and Table 55). Among all the mentioned derivatives, just structure 204 showed moderate activity against HCT-8, while other derivatives showed low inhibitor or no inhibition impacts against HCT-8 and Bel7402 (Fig. 66 and Table 55) (Cai et al., 2016)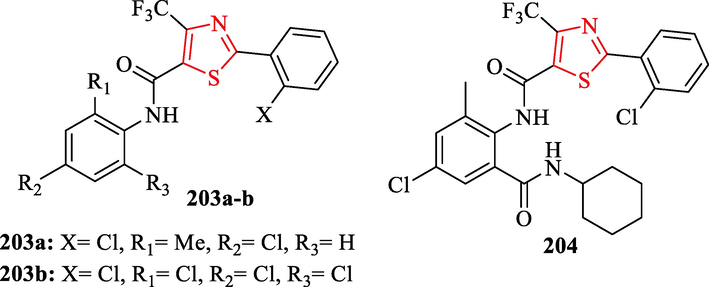
Chemical structures 203a-b and 204 (Cai et al., 2016)
Cpd. no.
%, inhibitory
A-549
HCT-8
203a
48
203b
40
204
40
5-Fluorouracil
57
79
The anticancer activity of 21 derivatives containing thiazole scaffold was evaluated against 3 cancer cell lines with respect to cisplatin as a reference drug by Shi et al. Most of the mentioned derivatives against some or all of MCF-7, SGC-7901 and H446 exhibited moderate antiproliferative activity (Fig. 67 and Table 56). Structure 205e against MCF-7 showed the most potent anticancer activity. Structures 205d, 205e, and 205f which contained a chloro group displayed better antitumor activity against MCF-7, SGC-7901, and H446 compared to structures 205a, 205b, and 205c which contained a methyl group (Fig. 67 and Table 56). In addition, structure 205e against H446 showed the best antitumor activity. Structures 206a, 206b, 206c, 206d, 206e, 206f, and 206 g were only active toward SGC-7901 cells (Fig. 67 and Table 56). Structures 207a, 207b, 207c, 207d, 207e, 207f, and 207 g against all cancer cell lines exhibited more potent activity compared to structures unsubstituted at the same position (Fig. 67 and Table 56) (Shi et al., 2016)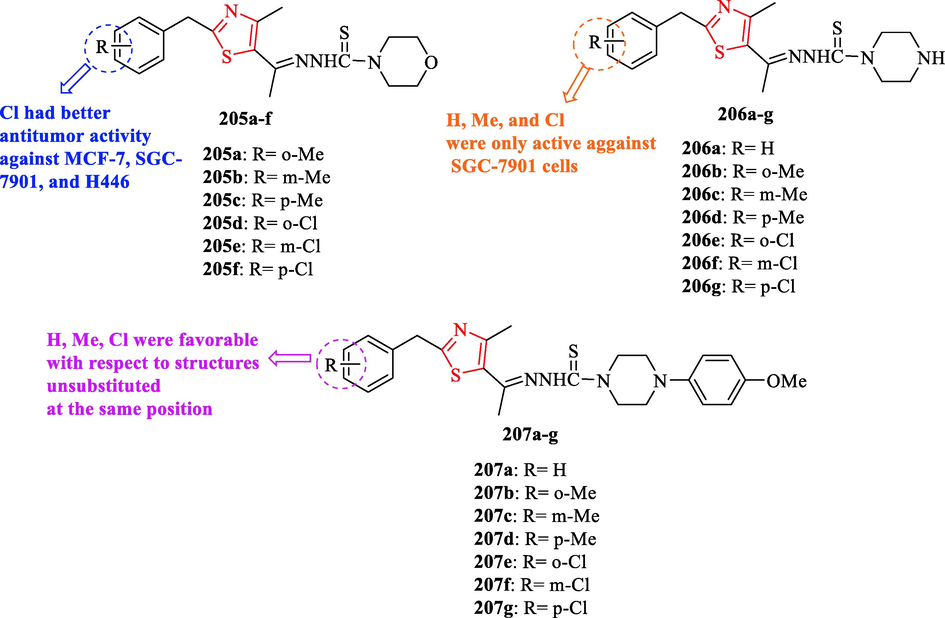
Chemical structures 205a-f, 206a-g, and 207a-g (Shi et al., 2016)
Cpd. no.
IC50 (μM)
SGC-7901
H446
MCF-7
205a
45.91
48.37
20.52
205b
64.06
>100
57.07
205c
33.29
40.32
53.97
205d
25.09
21.13
17.74
205e
16.55
10.33
5.49
205f
26.47
34.05
11.11
206a
>100
206b
18.25
206c
33.04
206d
71.99
206e
>100
206f
31.03
206 g
>100
207a
65.95
>100
38.28
207b
18.54
56.13
15.04
207c
30.21
71.19
39.51
207d
21.35
81.79
49.61
207e
16.50
>100
34.65
207f
38.49
83.95
45.43
207 g
11.58
35.03
23.29
Cisplatin
1.74
2.23
6.53
In 2017, the anticancer activity of ten derivatives containing thiazole scaffold was evaluated by Gomha et al against 1 cancer cell line with respect to cisplatin as a reference drug. Among the ten structures tested, structures 208 and 209 showed the best anticancer activity compared to the reference drug (Fig. 68). It seems that the number of thiazole rings is effective on the activity, especially the fewer number of them which causes a dramatic drop in activity. Moreover, the cytotoxic activity depends on the 1,3,4-Thiadiazole ring (Gomha et al., 2017)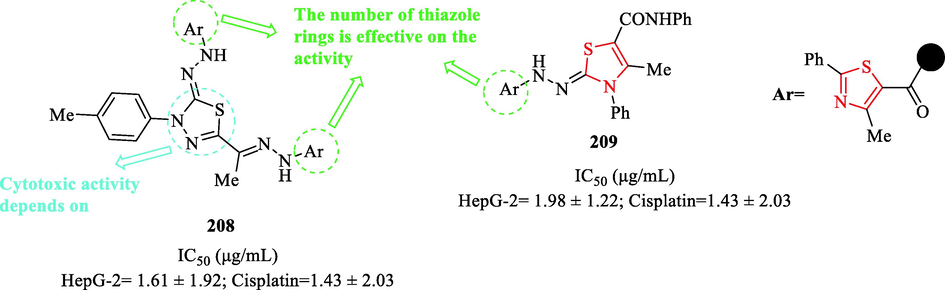
Chemical structures 208–209 and their IC50 values (Gomha et al., 2017)
Pansare and co-workers evaluated the anticancer activity of 14 derivatives containing thiazole scaffold against 2 human breast cancer cells line with respect to adriamycin as a reference drug. Among fourteen derivatives, structures 210a, 210b, 210c, 210d, and 210e against both cancer cell lines displayed the most activity (Fig. 69 and Table 57) (Pansare et al., 2017)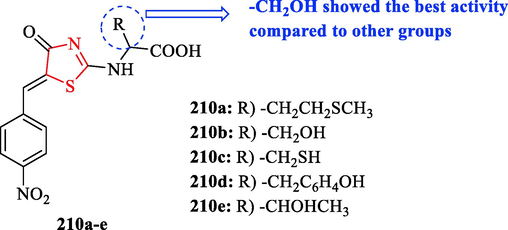
Chemical structures 210a-e (Pansare et al., 2017)
Cpd. no.
IC50 (μM)
MCF-7
BT-474
210a
7.5
8.6
210b
1.4
0.6
210c
7.2
6.1
210d
4.1
10.1
210e
1.6
1.2
Adriamycin
0.9
0.5
The anticancer activity of a series of derivatives containing thiazole scaffold against 4 cancer cell lines with respect to doxorubicin as a reference drug was evaluated by Mirza et al. Structures 211a, 211b, 211c, 211d, 211e, 211f, 212a, and 212b against MCF-7 were active (Fig. 70 and Table 58). Structures 211a, 211b, 211c, 211d, 211e, and 211f against HCT-116 exhibited anticancer activity (Fig. 70 and Table 58). In addition, structures 211a, 211b, 211c, 211d, 211e, 211f, and 212a against HeLa showed activity. It seems that structure 211a is the best structure compared to other structures (Fig. 70 and Table 58) (Mirza et al., 2017)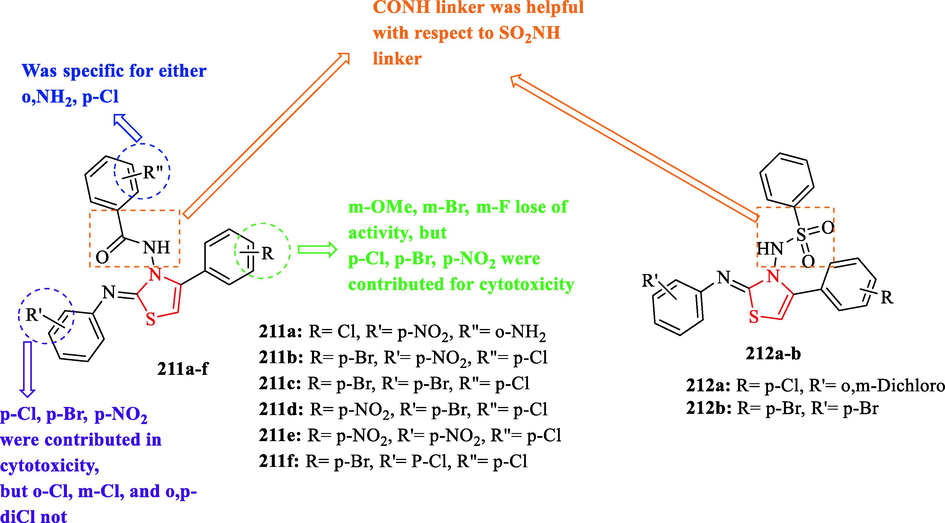
Chemical structures 211a-f and 212a-b (Mirza et al., 2017)
Cpd. no.
(IC50 ± SEM)1 (μM)
MCF-7
HCT-116
HeLa
211a
5.37 ± 0.56
16.99 ± 0.94
48.02 ± 0.98
211b
10.96 ± 0.33
41.48 ± 1.08
8.28 ± 0.21
211c
13.23 ± 1.6
40 0.3 ± 2.09
7.8 ± 0.07
211d
11.79 ± 0.58
34.17 ± 0.87
7.82 ± 0.12
211e
26.47 ± 1.09
40.51 ± 0.60
7.95 ± 0.10
211f
14.53 ± 0.23
40.06 ± 0.32
8.77 ± 0.27
212a
46.72 ± 1.80
19.86 ± 0.11
212b
40.21 ± 4.15
Doxorubicin
1.56 ± 0.05
1.09 ± 0.1
0.7 ± 0.08
In 2018, the in vitro anticancer activity of a series of pyrimidine-thiazole derivatives was evaluated against 3 cancer cell lines with respect to erlotinib as a reference drug by Sharma et al. Among all derivatives that were tested, structured 213b, 213c, 213d, 213e, and 213f against all cancer cell lines at 1 μM concentration showed high activity (Fig. 71 and Table 59). Structure 213d against MCF-7 and A375 had remarkable cytotoxic activity. In addition, structures 213b, 213c, 213d, 213e, and 213f exhibited good activity against A375 (Fig. 71 and Table 59). Two structures (213a and 213f) against MCF-7 and HeLa showed good activity. The activity of structures 213b, 213c, 213d, 213e, and 213f against MCF-7 and A375 had comparable to the reference drug (Fig. 71 and Table 59). Also, the same structures had comparable inhibitory activities against MCF-7. Structure 213f against MCF-7, A375, and HeLa showed better anticancer activity (Sharma et al., 2018) NA: Compound showing IC50 value greater than 50 μM/mL.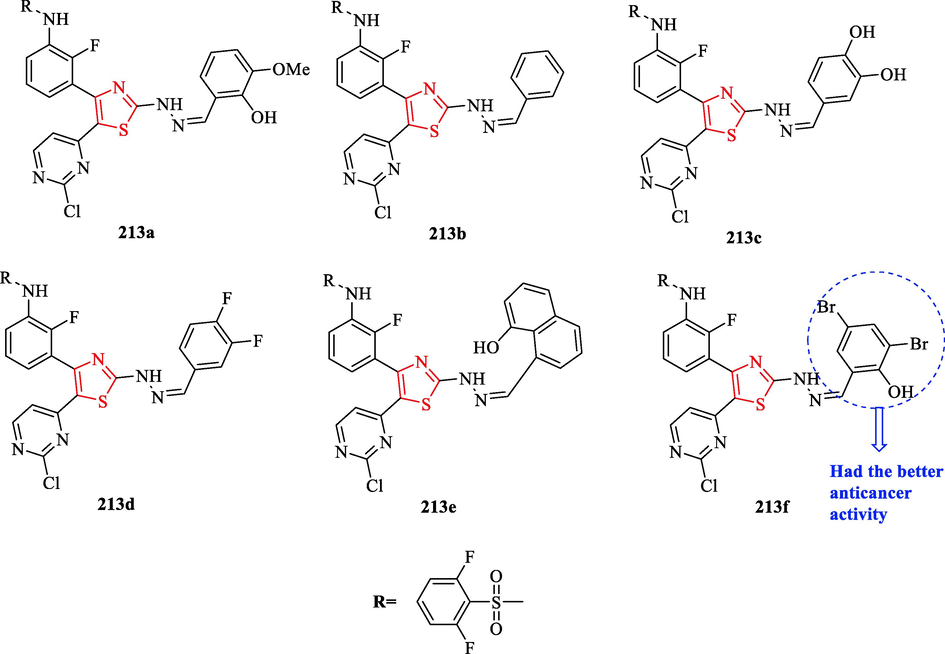
Chemical structures 213a-f (Sharma et al., 2018)
Cpd. no.
IC50 Values [μM]
A375
HeLa
MCF-7
213a
26.32 ± 1.95
8.562 ± 1.19
213b
1.768 ± 0.12
NA
7.781 ± 2.36
213c
2.532 ± 0.31
NA
8.674 ± 0.91
213d
1.456 ± 0.21
NA
6.328 ± 1.15
213e
1.208 ± 0.04
NA
5.632 ± 0.67
213f
1.819 ± 0.17
24.6 ± 2.21
6.478 ± 0.96
Erlotinib
3.60
41.80
15.55
Sateesh Kumar and Umadevi evaluated in vitro anticancer activity of a series of derivatives containing thiazole scaffold against 4 cancer cells with respect to combretastatin-A4 as a reference drug. Most of the structures against the 4 cell lines showed moderate to excellent activity. Structure 214 g against HT-29, A549, and MCF-7 showed strong activity, and structure 214d against MCF-7 displayed high activity (Fig. 72 and Table 60). In contrast, structure 214f containing nitro group at the same position showed low activity. Moreover, structures 214b and 214c against MCF-7 and A549 had more potent activity. Also, structure 214e against MCF-7 and A549 exhibited comparable activity (Fig. 72 and Table 60). Generally, structures 214a, 214b, 214c, 214d, 214f, and 214 g demonstrated more potent activity (Sateesh Kumar and Umadevi, 2018)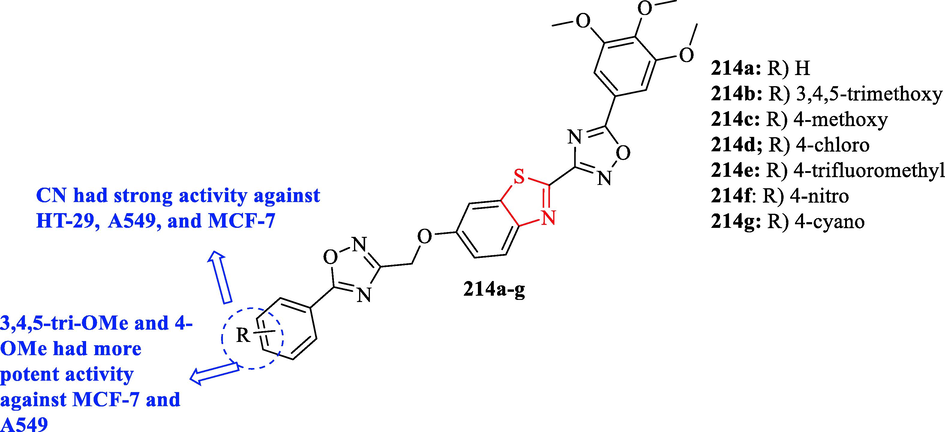
Chemical structures 214a-g (Sateesh Kumar and Umadevi, 2018)
Cpd. no.
IC50 μM1
A549
MCF-7
HT-29
214a
0.23 ± 0.013
1.67 ± 0.17
214b
0.11 ± 0.01
0.78 ± 0.024
214c
0.13 ± 0.01
0.70 ± 0.038
214d
0.90 ± 0.022
214e
1.56 ± 0.17
1.95 ± 0.18
214f
14.6 ± 3.89
214 g
1.89 ± 0.19
0.20 ± 0.029
0.76 ± 0.022
Combretastatin-A4
0.11 ± 0.02
0.18 ± 0.021
0.93 ± 0.034
The antitumor activity of a series of pyrazolines containing thiazole scaffold was investigated against 1 cancer cell line with respect to cisplatin as a reference drug by Edrees et al. According to the cytotoxic activity results, structure 215b had the highest cytotoxic activity. After that, structures 215a and 216 displayed excellent activity (Fig. 73 and Table 61) (Edrees et al., 2018)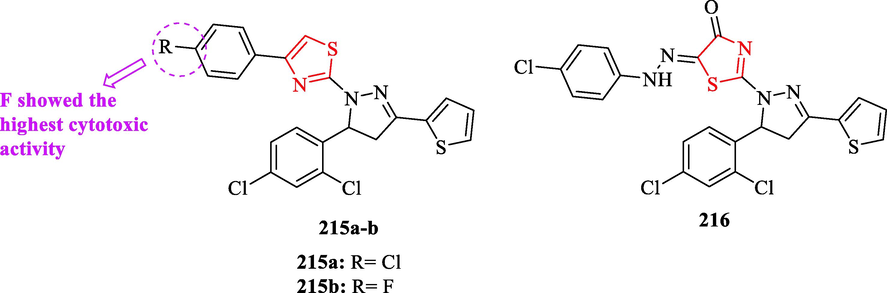
Chemical structures 215a-b and 216(Edrees et al., 2018)
Cpd. no.
IC50 (μM)
HepG2
215a
2.98 ± 1.8
215b
1.70 ± 8.2
216
3.54 ± 1.8
Cisplatin
0.90 ± 1.1
In 2019, the anticancer activity of a series of derivatives containing thiazole scaffold against 4 cancer cell lines with respect to etoposide as a reference drug was evaluated by Yakantham and co-workers. Four structures (217a, 217d, 217e, and 217f) displayed higher anticancer activity against A549, MCF-7, A2780, and Colo-205 (Fig. 74 and Table 62). Notably, structure 217f had the most promising activity. Structure 217b (with 3,5-dimethoxyphenyl substituent) showed low activity against A549, A2780, and Colo-205 compared to structure 217a with 3,4,5-trimethoxyphenyl substituent (Fig. 74 and Table 62). Moving along the same line, structure 217c (4-methoxy) showed lower activity than structure 217b against A549, MCF-7, A2780, and Colo-205 (Fig. 74 and Table 62). Finally, structure 217f against four cell lines displayed the highest activity (Fig. 74 and Table 62) (Yakantham et al., 2019)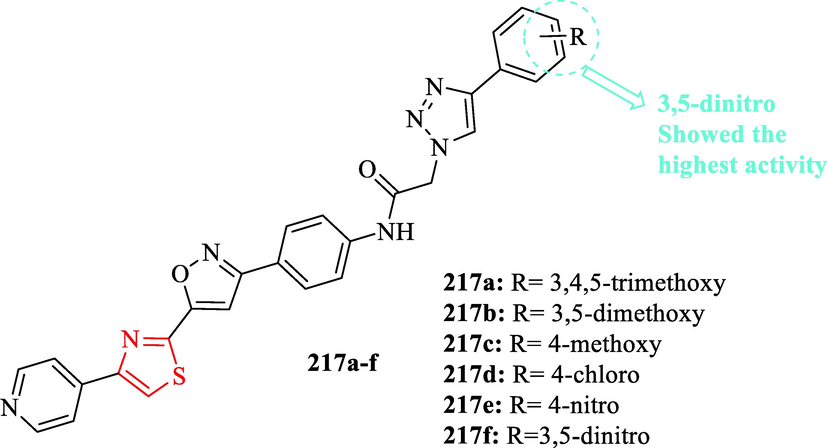
Chemical structures 217a-f (Yakantham et al., 2019)
Cpd. no.
IC50 (μM)
MCF-7
A549
Colo-205
A2780
217a
1.55 ± 0.83
1.95 ± 0.32
0.38 ± 0.063
1.10 ± 0.72
217b
3.17 ± 2.13
2.43 ± 1.64
2.11 ± 1.22
Not determined
217c
4.56 ± 2.88
10.3 ± 6.45
12.9 ± 6.11
7.82 ± 4.55
217d
1.66 ± 0.19
1.87 ± 0.22
0.93 ± 0.061
1.34 ± 0.89
217e
0.83 ± 0.018
0.19 ± 0.093
0.11 ± 0.045
1.23 ± 0.77
217f
0.083 ± 0.005
0.01 ± 0.009
0.014 ± 0.002
0.77 ± 0.017
Etoposide
2.11 ± 0.024
3.08 ± 0.135
0.13 ± 0.017
1.31 ± 0.27
Sayed et al. evaluated the anticancer activity of a series of derivatives against 1 cancer cell line with respect to doxorubicin as a reference drug. Five structures (218, 219, 220, 221, and 222) were tested against HepG-2 (Fig. 75 and Table 63). Structure 220 against HepG-2 had higher antitumor inhibitory activity (Fig. 75 and Table 63). Structure 222 had higher antitumor activity compared to structure 237 (Fig. 75 and Table 63) (Sayed et al., 2019)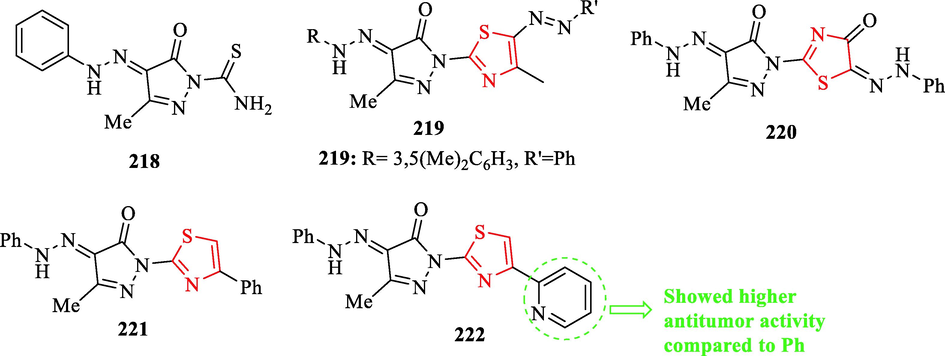
Chemical structures 218–222 (Sayed et al., 2019)
Cpd. no.
IC50 (μg/mL)
HepG2
218
4.39 ± 0.47
219
3.90 ± 0.41
220
2.20 ± 0.13
221
12.5 ± 0.97
222
4.80 ± 0.56
Doxorubicin
3.07 ± 0.27
The antiproliferative activity of 15 derivatives containing thiazole scaffold was investigated against 4 cancer cell lines with respect to crizotinib as a reference drug by Zhang et al. Structure 223a against A549, Hela, HT29, and Karpas299 had the best anticancer activity compared to other structures (Fig. 76 and Table 64). Structures 223b, 223c and 223d against A549, Hela, HT29, and Karpas299 demonstrated good activity (Fig. 76 and Table 64). Two structures (223e and 223f) were perfectly inactive on all cells (Zhang et al., 2019)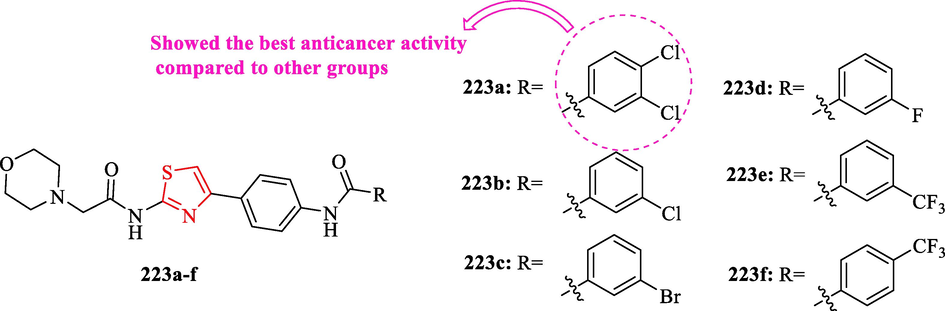
Chemical structures 223a-f (Zhang et al., 2019)
Cpd. no.
IC50 (μM)
A549
Hela
HT29
Karpas299
223a
8.46
6.05
0.63
13.87
223b
13.24
8.81
6.33
17.45
223c
18.83
17.25
14.15
34.52
223d
14.66
13.75
10.36
28.06
223e
> 40
> 40
> 40
> 40
223f
> 40
> 40
> 40
> 40
Etoposide
2.39
1.10
1.09
0.03
Abu-Melha et al. investigated the anticancer activity of 14 derivatives containing thiazole scaffold against 3 cancer cell lines with respect to doxorubicin as a reference drug. Most derivatives showed antitumor activity, especially structures 224 and 225 which had the most potent activity against HepG-2, HCT-116, and MCF-7 (Fig. 77 and Table 65) (Abu-Melha et al., 2019)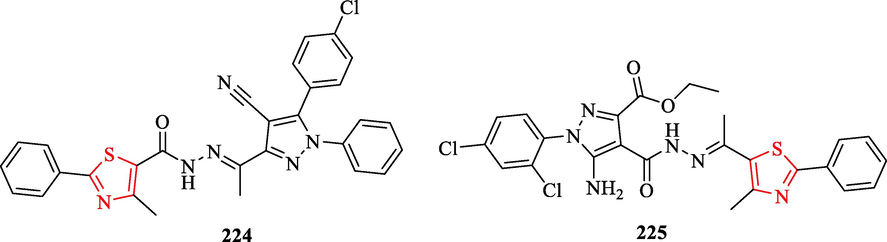
Chemical structures of 224 and 225 (Abu-Melha et al., 2019)
Cpd. no.
IC50 (g/mL)
HepG-2
HCT-116
MCF-7
224
4.24 ± 0.3
7.35 ± 0.4
2.99 ± 0.2
225
7.4 ± 0.2
11.8 ± 0.5
3.77 ± 0.2
Doxorubicin
0.36 ± 0.04
0.49 ± 0.07
0.35 ± 0.03
The in vitro anticancer activity of 18 derivatives against 5 cancer cell lines with respect to 5-FU as a reference drug was investigated by Afifi et al. Structures 226 and 228 against 5 cell lines had broad spectrum activity (Fig. 78 and Table 66). Structure 228 against A549 had the most potent activity, followed by structures 226 and 227. Further, structure 228 against MCF-7 showed significant activity, while structure 226 displayed moderate activity (Fig. 78 and Table 66). Structures 228 and 226 showed high activity against HepG-2. Moreover, higher cytotoxic potency against Caco-2 compared to the reference drug was shown by structure 228, while structures 226 and 227 showed moderate activity (Fig. 78 and Table 66). Structure 226 exhibited higher anticancer activity compared to the reference drug against PC3. Pyrazole carboxaldehydes (231a, 231b, and 231c) and their rhodanine condensation products (229a, 229b, 229c, 230a, 230b, and 230c) did not illustrate pronounced anticancer activity (Fig. 78) (Afifi et al., 2019)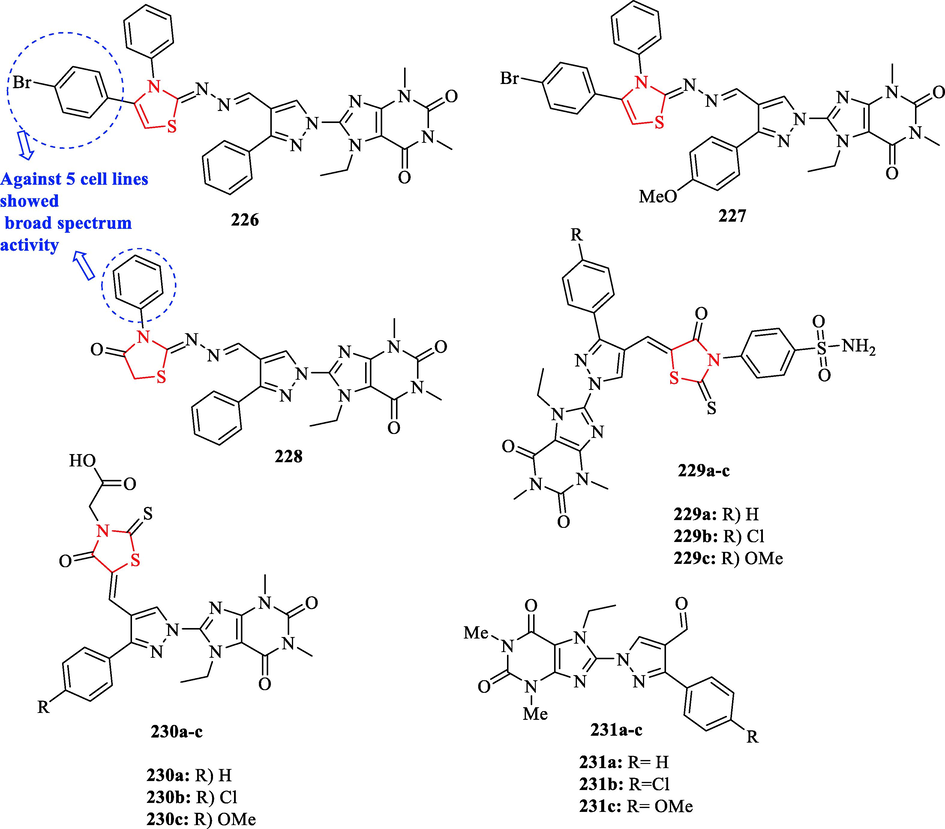
Chemical structures 226–228, 229a-c, 230a-c, and 231a-c (Afifi et al., 2019)
Cpd. no.
IC50 (μM)
A549
MCF-7
HepG-2
Caco-2
PC3
226
36.51
95.39
74.72
94.25
44.30
227
64.48
109.28
228
18.85
23.43
23.08
23.08
18.50
5-FU
83.03
93.79
96.86
112.24
82.26
Sultanova et al. investigated the anticancer activity of 15 derivatives against 4 cancer cell lines with respect to doxorubicin as a reference drug. Structures 232c and 232d showed pronounced cytotoxic activities, especially towards the Jurkat and HEK293 cell lines (Fig. 79 and Table 67). In addition, structures containing 2-allyl- (232b), 2-acetyl- (232a), and 2-methyl- (232e) aminothiazoles showed moderate cytotoxic activity (Fig. 79 and Table 67) (Sultanova et al., 2021)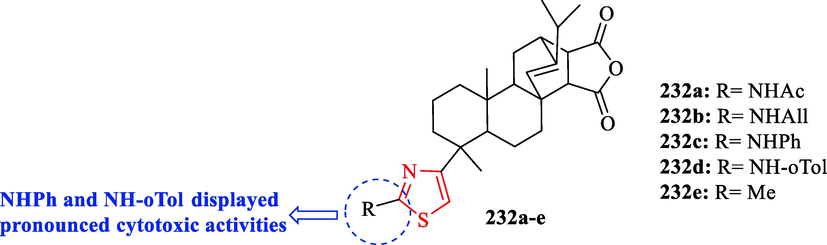
Chemical structures 232a-e (Sultanova et al., 2021)
Cpd. no.
IC50 (μM)
HEK293
Jurkat
HepG2
SH-SY5Y
232a
25.14 ± 0.72
27.65 ± 1.47
47.19 ± 2.63
(p = 0.00002)1
17.77 ± 1.94
(p = 0.003)1
232b
20.30 ± 0.08
25.26 ± 2.49
(p = 0.01)1
37.97 ± 1.00
(p = 0.00003)1
23.29 ± 5.61
232c
15.13 ± 3.15
22.21 ± 5.61
57,04 ± 1.40
(p = 0.00001)1
9.73 ± 1.55
232d
5.36 ± 2.10
9.60 ± 0.52
41.20 ± 5.93
(p = 0.00002)1
11.67 ± 0.10
232e
22.76 ± 7.61
14.65 ± 1.76
(p = 0.05)1
76.37 ± 1.63
(p = 0.00001)1
26.75 ± 3.49
Doxorubicin
1.90 ± 0.04
5.42 ± 0.38
7.21 ± 0.16
0.22 ± 0.005
Mahmoud and co-workers investigated the in vitro anticancer activity of 22 derivatives against 2 cancer cells with respect to doxorubicin as a reference drug. Among all derivatives, Structures 237a, 237d, 237c, 237b, 234a, 237e, 236a, 236c, and 236e had much better cytotoxic activities than the reference drug against HCT-116 (Fig. 80 and Table 68). Structure 233 in comparison with derivatives 234 and 235, there was only one structure (234a) that was higher activity than it against the HCT-116 and MCF-7 (Fig. 80 and Table 68). Nineteen structures (233, 234a-d, 235a-e, 236a-e, and 237a-d) exhibited higher activity compared to the reference drug against MCF-7 (Mahmoud et al., 2021)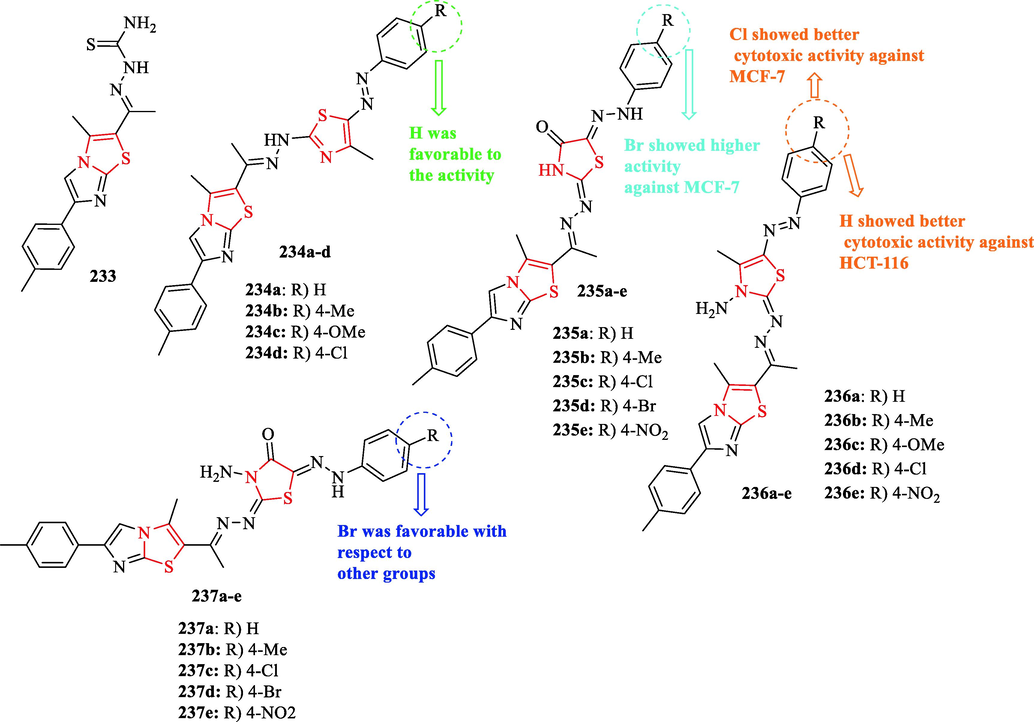
Chemical structures 233, 234a-d, 235a-e, 236a-e, and 237a-e (Mahmoud et al., 2021)
Cpd. no.
IC50 (μM) ± SD
HCT-116
MCF-7
233
8.8 ± 3.9
4.7 ± 2.7
234a
6.2 ± 2.7
2.4 ± 1.5
234b
4.5 ± 2.1
234c
3.9 ± 2.1
234d
3.8 ± 1.9
235a
3.4 ± 1.9
235b
3.2 ± 3.1
235c
3.2 ± 2.1
235d
2.4 ± 1.8
235e
5.3 ± 2.5
236a
8.0 ± 2.9
2.2 ± 1.7
236b
3.6 ± 1.5
236c
8.5 ± 3.1
2.3 ± 1.9
236d
1.4 ± 0.4
236e
8.9 ± 2.5
4.2 ± 2.1
237a
1.1 ± 1.5
6.3 ± 3.6
237b
5.6 ± 2.5
6.6 ± 3.1
237c
1.8 ± 1.9
6.2 ± 2.9
237d
1.1 ± 1.3
1.9 ± 0.3
237e
7.3 ± 3.6
Doxorubicin
9.4 ± 3.9
6.7 ± 2.1
The anticancer activity of some derivatives against 2 cancer cells with respect to cisplatin as a reference drug was investigated by Farghaly et al. Structures 238, 239, 240, and 241 against HCT-116 and HepG2 exhibited excellent antitumor activity (Fig. 81 and Table 69). Structure 239 showed good activity against HCT-116 and HepG2 compared to structures 240 and 241 (Fig. 81 and Table 69) (Farghaly et al., 2021)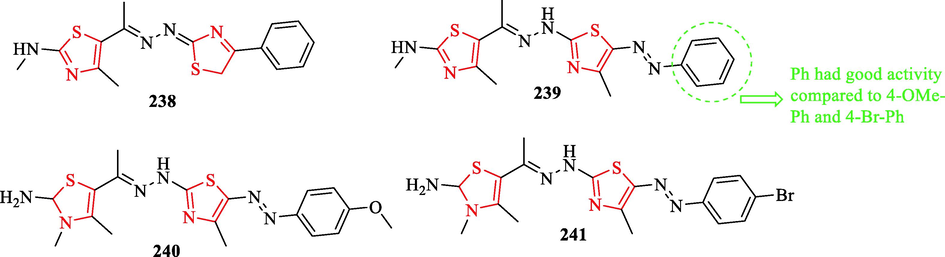
Chemical structures 238–241 (Farghaly et al., 2021)
Cpd. no.
IC50 (μg/mL)
HCT-116
HepG2
238
15
25
239
6.7
5
240
18
30
241
12
23
Cisplatin
2
3.7
In 2020, the anticancer activity of novel bithiazole derivatives against 3 cancer cells with respect to doxorubicin as a reference drug was evaluated by Latif et al. Structures 249c and 249a showed more potent anticancer activity against HCT-116 (Fig. 82 and Table 70). Structures 249a, 242b, and 242a had remarkably more potent activity against MCF-7 (Fig. 82 and Table 70). In addition, structures 246, 245a, 247b, 249c, 249b, 245b, 248, 243, and 249a showed higher activity toward the reference drug against HepG2 human liver cancer cells (Fig. 82 and Table 70). Structures 246, 245a, 249b, 245b, and 248 did not active against human breast cancer and colon but were active against human liver cancer (Fig. 82 and Table 70). further, structures 247a and 247b against MCF-7 and HCT-116 had significant cytotoxic effects (Fig. 82 and Table 70). In the case of 249a–c derivatives, structures 249a and 249c against the 3 tested cells displayed the most activity (Fig. 82 and Table 70) (Latif et al., 2020)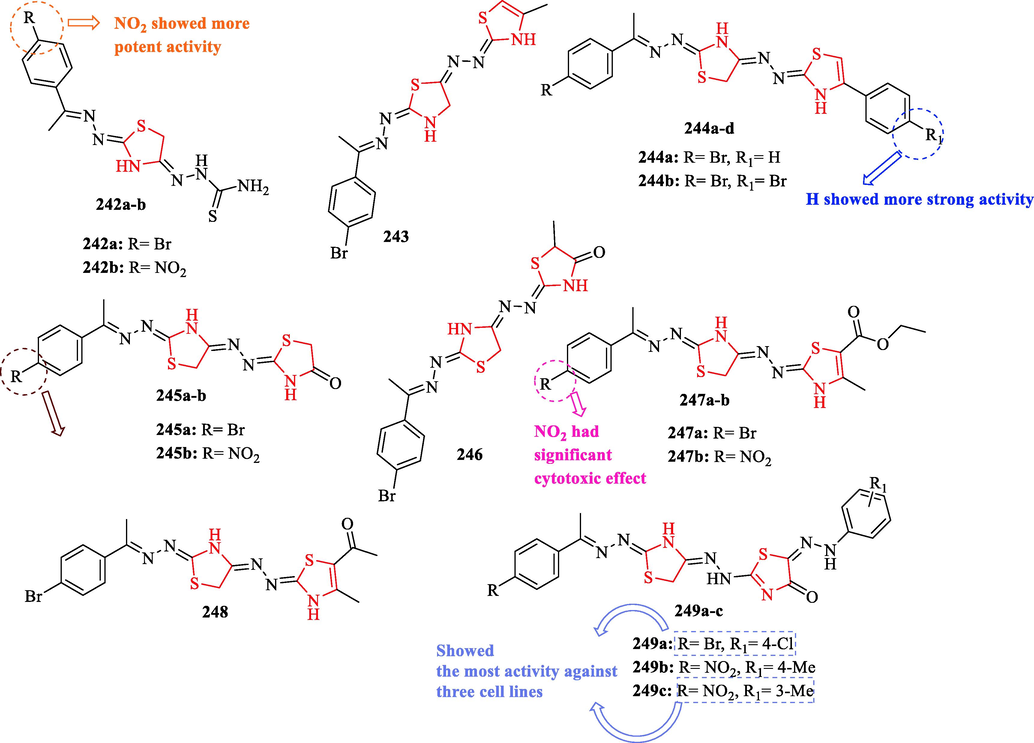
Chemical structures 242a-b, 243, 244a-b, 245a-b, 246, 247a-b, 248, and 249a-c (Latif et al., 2020)
Cpd. no.
IC50 (μM)
HCT-116
MCF-7
HepG2
242a
19.9 ± 2.3
242b
16.3 ± 2.1
243
30.0 ± 3.2
244a
16.3 ± 3.1
244b
17.1 ± 2.5
245a
28.0 ± 3.5
245b
29.5 ± 3.6
246
23.5 ± 2.3
247a
13.5 ± 1.8
24.7 ± 3.8
247b
13.2 ± 2.1
21.4 ± 3.3
28.8 ± 3.5
248
29.9 ± 3.5
249a
11.5 ± 1.5
14.4 ± 1.8
30.8 ± 3.3
249b
29.5 ± 3.9
249c
11.4 ± 1.7
20.7 ± 3.2
29.4 ± 3.5
Doxorubicin
12.0 ± 2.1
20.5 ± 2.1
32.7 ± 3.2
Bayazeed and Alnoman investigated in vitro anticancer activity of novel thiazole derivatives against 4 cell lines with respect to 5-Fluorouracil as a reference drug by the MTT assay. Structure 250c against breast cancer cell lines showed higher activity (Fig. 83 and Table 71). Structures 250a, 250b, and 251c had the most potent activity against 4 cell lines (Fig. 83 and Table 71). Structures 251a, 251b, and 251c had less activity than structures 250a, 250b, and 250c, but had strong activity against breast cancer cell lines (Fig. 83 and Table 71) (Bayazeed and Alnoman, 2020)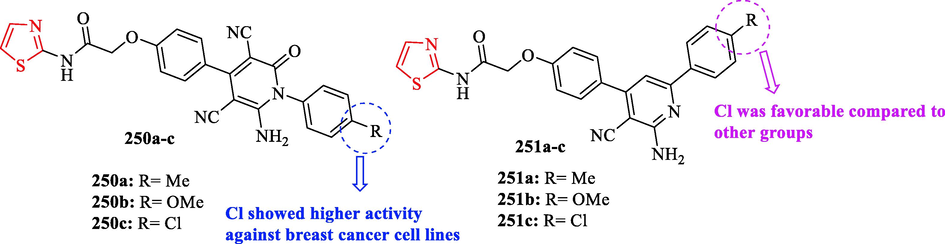
Chemical structures 250a-c and 251a-c (Bayazeed and Alnoman, 2020)
Cpd. no.
IC50 (μM)
PC3
HepG2
Hep-2
MCF-7
250a
32.46 ± 2.15
20.34 ± 0.15
18.37 ± 0.13
11.03 ± 0.23
250b
30.57 ± 0.11
16.20 ± 0.03
13.52 ± 0.10
8.40 ± 0.16
250c
18.10 ± 0.01
13.18 ± 0.02
11.27 ± 0.05
5.71 ± 0.15
251a
37.48 ± 0.63
32.27 ± 0.44
27.13 ± 0.11
21.09 ± 0.27
251b
38.56 ± 0.81
29.32 ± 0.66
24.72 ± 0.73
17.41 ± 0.62
251c
24.82 ± 0.03
17.19 ± 0.07
18.23 ± 0.27
12.57 ± 0.49
5-Fu
8.30 ± 0.23
7.19 ± 0.45
5.22 ± 0.82
6.14 ± 0.31
Suma et al. investigated the anticancer activity of some chalcone-linked thiazole-imidazopyridine derivatives against 4 cancer cells with respect to etoposide as a reference drug. Among all derivatives, structure 252a against 4 cell lines exhibited more potent activity (Fig. 84 and Table 72). In addition, structures 252b, 252c, 252d, and 252e showed significant activity against all cell lines (Fig. 84 and Table 72). In the case of 252a–e derivatives, Structure 252b (3,5-dimethoxy) displayed just lower activity toward structure 252a (3,4,5-trimethoxy) (Fig. 84 and Table 72). Also, Structure 252c (4-methoxy) had decreased activity on all cell lines toward structure 252a (3,4,5-trimethoxy) and 252b (3,5-dimethoxy) (Fig. 84 and Table 72) (Suma et al., 2020)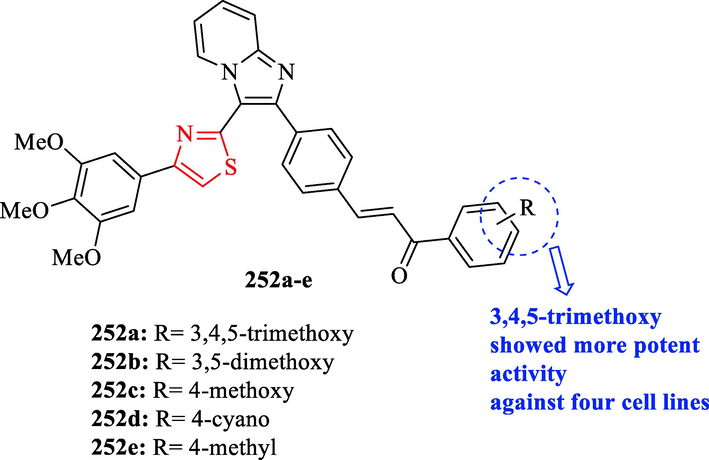
Chemical structures 252a-e (Suma et al., 2020)
Cpd. no.
IC50 (μM)
MCF-7
A549
DU-145
MDA MB-231
252a
0.18 ± 0.094
0.66 ± 0.071
1.03 ± 0.45
0.065 ± 0.0082
252b
0.44 ± 0.018
1.23 ± 0.37
1.12 ± 0.25
0.95 ± 0.066
252c
1.98 ± 0.52
2.10 ± 1.66
2.44 ± 1.32
1.81 ± 0.84
252d
2.33 ± 1.73
2.17 ± 1.50
1.90 ± 0.88
2.02 ± 0.99
252e
1.73 ± 0.11
1.69 ± 0.32
1.33 ± 0.45
3.22 ± 2.48
Etoposide
2.11 ± 0.024
3.08 ± 0.135
1.97 ± 0.45
1.91 ± 0.84
Ansari et al. investigated the anticancer activity of some derivatives containing thiazole scaffold against 3 cell lines with respect to etoposide as a reference drug by the MTT assay. Structures 254a and 254b against MCF-7 demonstrated the best inhibitory (Fig. 85 and Table 73). The activity of structure 254a was better than the reference drug against MCF-7. Also, structure 254b against A549 had the most potent activity. Structures 253a, 253b, 253c, and 253d against SKOV3 had the most activity (Fig. 85 and Table 73). Moreover, by comparing structures 254b and 255 can be understood that the insertion of methylene between 4-chlorophenyl moiety and thiazole-2(3H)-thione ring is effective for making the potent activity against MCF-7 and A549 (Fig. 85 and Table 73) (Ansari et al., 2020)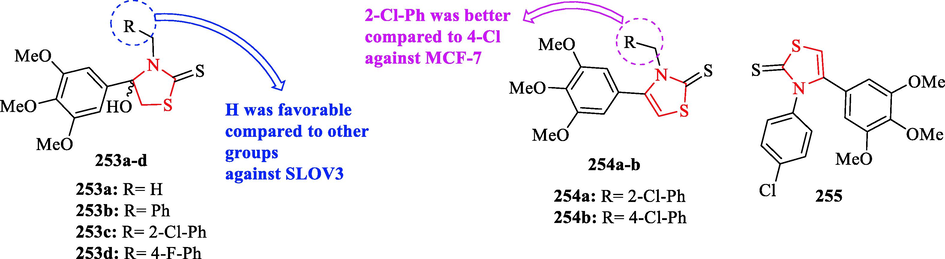
Chemical structures 253a-d, 254a-b, and 255 (Ansari et al., 2020)
Cpd. no.
IC50 (μg/mL)1
A549
MCF-7
SKOV3
253a
4.23 ± 0.14 (13.4)
253b
5.38 ± 0.16 (13.8)
253c
5.13 ± 0.18 (12.1)
253d
5.05 ± 0.22 (12.4)
254a
1.14 ± 0.18 (2.8)
254b
2.72 ± 0.15 (6.7)
2.41 ± 0.11 (5.9)
255
6.04 ± 0.23 (15.4)
6.63 ± 0.08 (16.9)
Etoposide
3.52 ± 0.23 (6.0)
3.37 ± 0.24 (5.7)
5.41 ± 0.06 (9.2)
Farghaly et al. investigated in vitro antitumor activity of some thiazole derivatives against 3 cell lines with respect to doxorubicin as a reference drug. Structures 257c, 257d, and 256 had potent activity and showed less toxicity against WI-38 with high selectivity index (Fig. 86 and Table 74). Structure 257a against 3 cells exhibited the best activity. In addition, structure 257d had more activity against 3 cells (Fig. 86 and Table 74). Also, two structures (257b and 257c) had comparable antitumor activity against 3 cells (Fig. 86 and Table 74) (Farghaly et al., 2020)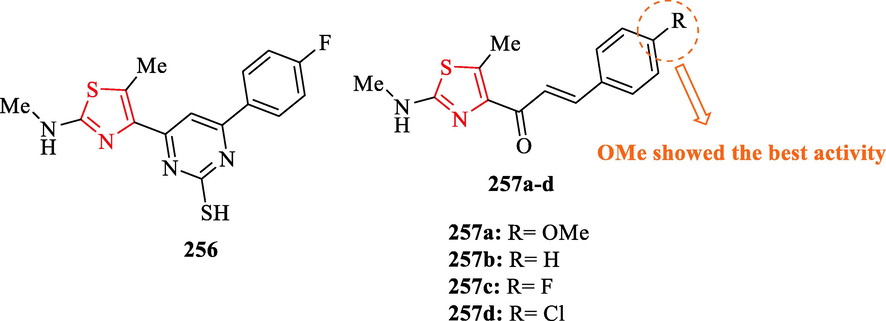
Chemical structures 256 and 257a-d (Farghaly et al., 2020)
Cpd. no.
IC50 (μM)
HepG-2
A549
MCF-7
WI-381
Selectivity index2
HepG-2
A549
MCF-7
256
3.5 ± 0.19
2.37 ± 0.12
4.11 ± 0.3
107.66 ± 1.7
35.88
45.42
26.19
257a
1.56 ± 0.07
1.39 ± 0.061
1.97 ± 0.09
137.35 ± 1.05
88.04
98.8
69.7
257b
4.53 ± 0.35
3.78 ± 0.2
4.9 ± 0.39
257c
3.79 ± 0.21
3.17 ± 0.15
4.14 ± 0.35
93.44 ± 1.49
24.65
29.47
22.57
257d
2.29 ± 0.11
2.01 ± 0.1
2.38 ± 0.14
121.29 ± 2.3
52.96
60.34
50.96
Doxorubicin
3.54 ± 0.2
3.19 ± 0.14
4.39 ± 0.31
The in vitro anticancer activity of some novel thiazole derivatives against 3 cell lines with respect to doxorubicin as a reference drug was investigated by Ghoneim and Ali Hassan, using the MTT assay (Fig. 87 and Table 75). Structures 258, 259, 260, and 261 showed better activity against HCT-116 toward the reference drug (Fig. 87 and Table 75). In the case of PC-3, structures 260 and 261 showed lower IC50 than the reference drug (Fig. 87 and Table 75). Also, all structures had high IC50 against HepG-2 (Ghoneim and Ali Hassan, 2022)
Chemical structures 258–261 (Ghoneim and Ali Hassan, 2022)
Cpd. no.
IC50 (nM) ± SD
HCT-116
PC-3
HepG-2
258
119 ± 4.1
133.5 ± 4.8
259
94.8 ± 3.6
284.1 ± 3.8
260
91.2 ± 1.7
119.8 ± 3.8
175.9 ± 2.8
261
106.2 ± 2.8
111.5 ± 3.7
333.9 ± 9.8
Doxorubicin
126.8 ± 8.8
129.6 ± 2.3
116.9 ± 5.7
Sayed et al. investigated in vitro anticancer activity of some novel structures containing thiazole scaffold against 3 cancer cells with respect to harmine and cisplatin as reference drugs which was synthesized via the one-pot reaction, using the MTT colorimetric assay. The results revealed that structures 262a, 262b, and 263 against HCT-116 had growth inhibition activity toward reference drugs (Fig. 88 and Table 76). In addition, three structures (263, 262b, and 262a) displayed significant activity against HT-29 toward both reference drugs (Fig. 88 and Table 76). In the case of HepG2 cell lines, structures 262b, 262a, 263, and 264 showed the most activity toward both reference drugs (Sayed et al., 2020)
Chemical structures 262a-b, and 263–264 (Sayed et al., 2020)
Cpd. no.
IC50 (μM)
HCT-116
HepG2
HT-29
262a
3.80 ± 0.80
2.94 ± 0.62
7.24 ± 0.62
262b
3.65 ± 0.90
2.31 ± 0.43
4.13 ± 0.51
263
3.16 ± 0.90
4.57 ± 0.85
3.47 ± 0.79
264
9.86 ± 0.78
Harmine
2.40 ± 0.12
2.54 ± 0.82
4.59 ± 0.67
Cisplatin
5.18 ± 0.94
9.41 ± 0.63
11.68 ± 1.54
The anticancer activity of a series of amide-based thiazole–pyrimidines against 4 cancer cells with respect to etoposide as a reference drug was evaluated by Bandaru et al, using the MTT method. According to the structure–activity relationship studies, structure 265a against 4 cells exhibited remarkable activity (Fig. 89 and Table 77). Structure 265b showed potent activity against 4 cells. Structure 265c in comparison to structure 265b showed slightly decreased activity against 4 cells (Fig. 89 and Table 77). Also, structure 265d against 4 cells displayed lower activity compared to structures 265b and 265c. Structure 265f against A2780, MCF-7, and A549 demonstrated moderate activity (Fig. 89 and Table 77). In addition, Structure 265 g in comparison to Structure 265f exhibited potentially improved activity against 4 cells (Fig. 89 and Table 77). Structure 265e against 4 cell lines had slightly improved activity. Generally, six structures (265a, 265b, 265c, 265d, 265e, and 265 g) had very promising activity (Fig. 89 and Table 77) (Bandaru et al., 2022)
Chemical structures 265a-g (Bandaru et al., 2022)
Cpd. no.
IC50 (μM)
A549
MCF-7
Colo-205
A-2780
265a
1.46 ± 0.17
0.74 ± 0.054
2.35 ± 1.34
2.01 ± 0.35
265b
0.043 ± 0.006
0.87 ± 0.045
0.12 ± 0.038
0.33 ± 0.071
265c
0.24 ± 0.043
1.28 ± 0.27
2.66 ± 1.58
2.14 ± 1.32
265d
2.77 ± 1.98
3.44 ± 2.31
2.93 ± 2.11
2.35 ± 1.77
265e
0.52 ± 0.019
0.39 ± 0.056
0.16 ± 0.044
0.79 ± 0.037
265f
4.56 ± 2.53
7.38 ± 3.72
Not active
10.52 ± 5.98
265 g
0.96 ± 0.074
0.28 ± 0.058
1.55 ± 0.64
2.67 ± 1.40
Etoposide
3.25 ± 0.132
2.38 ± 0.027
0.12 ± 0.015
1.36 ± 0.28
Gomha and co-workers investigated the anticancer activity of a series of novel thiazole–thiophene derivatives against the MCF-7 tumor cells and the LLC-Mk2 normal cell line with respect to cisplatin as a reference drug, using the MTT method. According to the results, most of the derivatives had variable activity. Structures 268a and 266 against MCF-7 demonstrated significant activity (Fig. 90 and Table 78). Structure 268a with an acetyl group (Ac) showed higher activity compared to structure 268b with an ester group (CO2Et) (Fig. 90 and Table 78). Also, Structures 267a and 267b showed poor antitumor activity (Gomha et al., 2022)
Chemical structures 266, 267a-b, and 268a-b (Gomha et al., 2022)
Cpd. no.
IC50 (μM)
MCF-7
266
10.2 ± 0.8
267a
38.2 ± 1.4
267b
54.8 ± 1.6
268a
11.5 ± 0.7
268b
16.3 ± 1.4
Cisplatin
13.3 ± 0.61
Liu et al. evaluated the anticancer activity of a series of thiazole-based stilbene derivatives against 3 cancer cell lines with respect to etoposide and CPT as reference drugs, using the MTT method. The results revealed that structures 269a, 269b, 269c, 269d, 269f, and 269 g had potent cytotoxicity against MCF-7 toward both reference drugs (Fig. 91 and Table 79). Structure 269a against MCF-7 demonstrated the highest cytotoxicity activity. In addition, structures 269a, 269b, 269c, 269e, 269f, 269 g, 269 k and 269 m showed high cytotoxicity against HCT116 (Fig. 91 and Table 79). Also, structure 269b exhibited the highest cytotoxicity against HCT116. Structures 269a, 269b, 269c, 269e, and 269 g were tested against HEK293T and the results showed all structures had possess a degree of cytotoxicity, while CPT had potent cytotoxicity (Fig. 91 and Table 79). It seems that derivatives containing fluorine-substituted (269a-d) displayed higher cytotoxicity than derivatives containing chlorinated and brominated against MCF-7 and HCT116 (Fig. 91 and Table 79). Structures 269 k, 269 l, and 269 m containing a hydroxy group had raised cytotoxicity against MCF-7 and HCT116 than structures 269 h, 269i, and 269j containing a methoxy group, which revealed that a hydroxy group was effective for improving the cytotoxicity (Fig. 91 and Table 79) (Liu et al., 2022)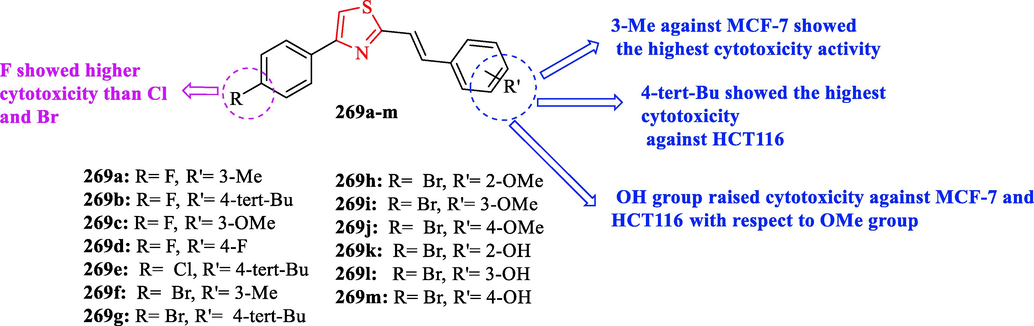
Chemical structures 269a-m (Liu et al., 2022)
Cpd. no.
Cytotoxicity (IC50, μM)
MCF-7
HCT116
HEK293T
269a
0.78 ± 0.12
1.48 ± 0.16
10.15 ± 0.68
269b
6.31 ± 0.51
0.62 ± 0.09
12.02 ± 2.45
269c
9.85 ± 0.47
4.99 ± 0.80
25.16 ± 1.52
269d
4.14 ± 0.40
269e
7.22 ± 0.40
2.87 ± 0.26
18.67 ± 1.37
269f
2.09 ± 0.15
269 g
3.78 ± 1.66
1.39 ± 0.16
21.78 ± 3.80
269 h
>100
>100
269i
45.82 ± 4.47
40.30 ± 7.80
269j
>100
>100
269 k
39.18 ± 1.79
4.15 ± 0.76
269 l
22.79 ± 1.40
9.99 ± 0.19
269 m
38.01 ± 5.82
2.86 ± 0.46
CPT
0.34 ± 0.026
0.012 ± 0.001
0.10 ± 0.003
Etoposide
24.68 ± 3.12
18.95 ± 1.35
Al-Warhi et al. evaluated the in vitro antiproliferative activity of a series of new thiazolyl-pyrazoline derivatives against 2 cancer cells with respect to staurosporine and erlotinib as reference drugs. In the case of A549, structures 270f and 270b exhibited the most potent activity toward both reference drugs (Fig. 92 and Table 80). In addition, Structures 270j and 270i demonstrated moderate in vitro anti-proliferative activities toward the reference drugs. In the case of the T-47D, Structures 270j, 270i, 270f, and 270b displayed more potent antiproliferative activity toward the reference drugs (Fig. 92 and Table 80). Moreover, structures 270c and 270d had moderate cytotoxic activity. Structures 270c, 270 g, and 270j which contain p-methoxyphenyl on the phenyl ring showed potent to moderate antiproliferative activity against A549 and T-47D (Fig. 92 and Table 80). structures 270b with hydroxyl substituent, 270c with methoxy substituent, and 270d with chloro substituent showed better anti-proliferative activity against A549 and T-47D than structure 270a with hydrogen substituent (Fig. 92 and Table 80). Also, structures 270f with hydroxyl substituent, 270 g with methoxy substituent, and 270 h with chloro substituent showed better anti-proliferative activity against T-47D than Structure 270e with hydrogen substituent (Fig. 92 and Table 80). In the case of the MCF-10A, the cytotoxic activity of structures 270b, 270f, 270i, and 270j was evaluated to explore their selectivity (Fig. 92 and Table 80). The results revealed that the structures had non-significant toxicity toward the MCF-10A (Al-Warhi et al., 2022)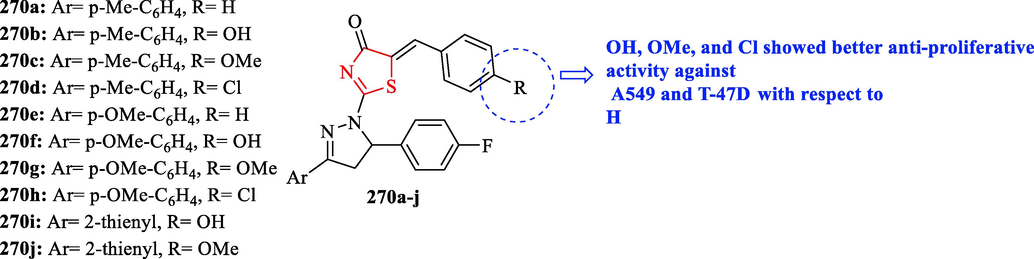
Chemical structurs 270a-j (Al-Warhi et al., 2022)
Cpd. no.
IC50 (μM)
A549
T-47D
270a
66.50 ± 3.38
17.10 ± 1.03
270b
4.41 ± 0.59
1.15 ± 0.56
270c
11.72 ± 1.06
9.30 ± 0.07
270d
43.11 ± 3.70
7.06 ± 0.43
270e
77.10 ± 4.68
270f
3.92 ± 0.18
0.88 ± 0.05
270g
21.73 ± 1.96
14.4 ± 0.87
270h
55.10 ± 3.34
270i
8.10 ± 0.37
1.66 ± 0.1
270j
6.53 ± 0.23
0.75 ± 0.05
Staurosporine
4.29 ± 0.72
6.83 ± 1.03
Erlotinib
5.73 ± 0.69
8.14 ± 0.97
The anticancer activity of a new series of nano-sized fluorinated thiazoles against 1 cancer cell with respect to cisplatin as a reference drug was investigated by Alsaedi et al. Structures 272 and 271c showed the most anticancer activity, while structures 271a and 271e exhibited the least activity in this series (Fig. 93 and Table 81). In addition, structure 271d displayed moderate activity. In the case of 270a-d, structures 270a and 270c showed moderate activity, while structure 270d had weak activity (Fig. 93 and Table 81). In general, structure 271c was more strong than the reference drug, and structures 270b and 271b had good activity (Fig. 93 and Table 81) ((Alsaedi et al., 2022)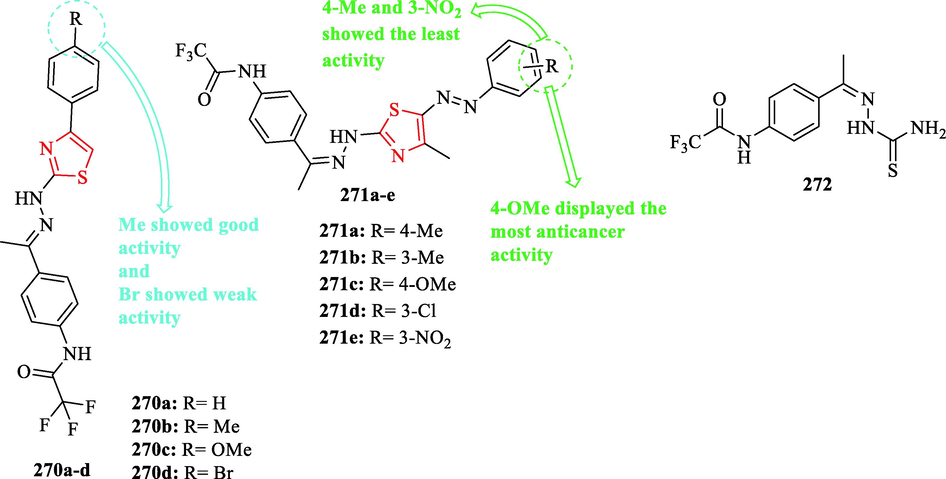
Chemical structures 270a-d, 271a-e, and 272 (Alsaedi et al., 2022)
Cpd. no.
IC50 values (μg/ml)
MDA-MB-231
270a
28.8 ± 1.9
270b
13.4 ± 0.85
270c
55.8 ± 3.8
270d
408 ± 19.8
271a
97.1 ± 4.9
271b
14.9 ± 0.97
271c
2.97 ± 0.32
271d
27.2 ± 1.7
271e
366 ± 21.4
272
7.7 ± 0.41
Cisplatin
4.33 ± 0.12
El-Naggar et al. investigated the anticancer activity of a series of hydrazinyl-thiazole derivatives against 3 cancer cells with respect to roscovitine as a reference drug. Structures 273b and 273a showed the most cytotoxic activity against three cells (Fig. 94 and Table 82). In addition, structure 273d against HCT-116 and MCF-7 was more selective, while structure 273e was more selective against the three cells, and structure 273f was more selective against HCT-116 than HePG-2 and MCF-7 (Fig. 94 and Table 82). It seems that the presence of the methoxy (273a) and methyl (273c) groups as electron donating group and chlorine (273d) group as electron withdrawing group at position 4 of the phenyl ring enhance cytotoxic activity against 3 cell lines (Fig. 94 and Table 82). Some structures were chosen for in vitro inhibition of epidermal growth factor receptor (EGFR) and aromatase (ARO) enzymes with respect to erlotinib and letrozole as reference drugs. According to the results, structure 273d was the most active EGFR inhibitor toward erlotinib (Fig. 94 and Table 82). Also, the same structure had the most potent ARO inhibitory action but had less potent than letrozole (El-Naggar et al., 2022)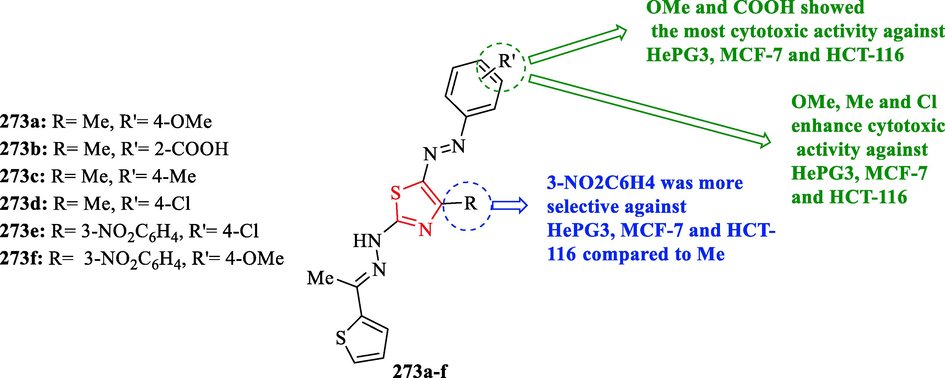
Chemical structures 273a-f (El-Naggar et al., 2022)
Cpd. no.
IC50 (μM)1
EGFR inhibition
IC50 (nM)1
ARO inhibition
IC50 (nM)1
HePG2
MCF-7
HCT-116
273a
3.81 ± 0.2
7.19 ± 0.6
8.22 ± 0.7
273b
11.34 ± 0.9
7.29 ± 0.5
6.87 ± 0.3
273c
33.57 ± 2.3
21.68 ± 1.9
26.85 ± 2.1
273d
6.73 ± 0.6
10.87 ± 0.9
13.79 ± 1.2
82.8 ± 0.004
98.6 ± 0.006
273e
9.29 ± 0.8
18.38 ± 1.4
22.17 ± 1.8
273f
17.91 ± 1.5
10.33 ± 0.8
8.93 ± 0.6
Roscovitine
13.82 ± 1.15
9.32 ± 0.49
12.24 ± 1.17
Erlotinib
62.4 ± 0.005
NT2
Letrozole
NT2
79 ± 0.005
The in vitro anticancer activity and cytotoxic activity of 13 derivatives containing thizole scaffold against 5 cancer cells and 1 human normal cell with respect to doxorubicin and cisplatin as reference drugs was evaluated by Bhandare et al. Structure 274 with three heterocyclic rings exhibited anticancer activity against HeLa, MCF-7, A2780, and BGC-823 (Fig. 95 and Table 83). However, The conversion of structure 274 into structures 275 and 276 remarkably improved the anticancer activity (Fig. 95 and Table 83). Structure 276 had the highest activity against BGC-823. Comparison between structures of 277a-e and 278a-e exhibited structures of 278a-e series had modest activity compared to cisplatin. In the case of 277a-e, structure 277e had excellent activity against MCF-7 (Fig. 95 and Table 83). In the case of 278a-e, the additional carbonyl group dwindled the important interactions, one of which was electronic and another reason was steric. These reasons caused low activity in the structures 278a-e series (Fig. 95 and Table 83). In the case of the results of the L02, the results showed that all structures were non-toxic ((Bhandare et al., 2022)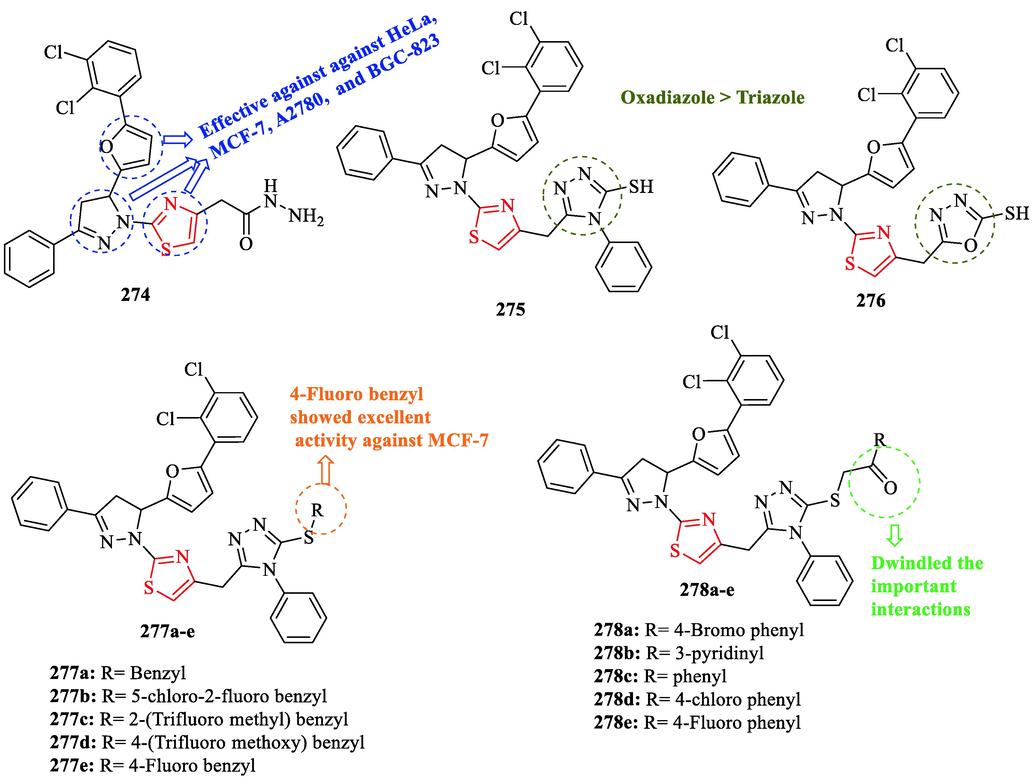
Chemical structures 274–276, 277a-e, and 278a-e ((Bhandare et al., 2022)
Cpd. no.
IC50 ± SD, μM
HeLa
A549
MCF-7
A2780
BGC-823
274
4.23 ± 0.23
2.18 ± 0.45
1.65 ± 0.44
2.12 ± 0.32
1.34 ± 0.11
275
4.86 ± 0.49
1.21 ± 0.92
2.50 ± 0.42
3.03 ± 1.54
1.20 ± 0.56
276
2.24 ± 0.48
1.39 ± 0.33
3.76 ± 0.54
1.56 ± 0.34
0.49 ± 1.45
277a
5.02 ± 0.34
3.98 ± 0.32
3.43 ± 0.33
2.88 ± 1.55
2.87 ± 0.55
277b
5.11 ± 0.55
2.87 ± 0.44
3.12 ± 0.65
3.12 ± 0.55
2.08 ± 0.55
277c
3.98 ± 0.54
2.09 ± 0.54
4.14 ± 0.58
5.76 ± 0.87
1.66 ± 0.54
277d
5.23 ± 0.67
3.84 ± 0.48
2.74 ± 0.32
1.57 ± 0.54
2.54 ± 0.65
277e
1.43 ± 0.77
1.22 ± 0.45
0.65 ± 0.53
2.12 ± 0.44
1.45 ± 0.28
278a
7.23 ± 0.47
4.48 ± 0.84
2.34 ± 0.54
2.78 ± 0.56
1.22 ± 0.37
278b
6.09 ± 0.33
1.59 ± 0.42
2.60 ± 0.48
3.93 ± 0.57
1.28 ± 0.30
278c
6.45 ± 1.84
4.58 ± 0.11
2.94 ± 0.21
6.32 ± 1.25
3.35 ± 0.16
278d
5.13 ± 0.35
1.25 ± 0.87
2.89 ± 0.35
5.51 ± 2.32
3.31 ± 0.54
278e
4.71 ± 0.24
3.11 ± 0.64
1.72 ± 1.98
4.33 ± 1.59
1.97 ± 0.91
Doxorubicin
1.03 ± 0.22
0.67 ± 0.13
0.73 ± 0.25
0.95 ± 0.31
1.08 ± 0.15
Cisplatin
5.65 ± 0.21
1.83 ± 0.62
1.85 ± 0.46
2.39 ± 0.47
0.98 ± 0.25
According to the analyzes carried out on structures with antibacterial and antifungal properties, depending on the type of structure, electron-withdrawing or electron-donating groups are effective in improving their properties, for example, in structures 20a-f and 21a-f, the presence of electron groups Killing agents such as nitro increase the activity of imam, while electron-donating groups such as methyl and methoxy decrease it. Also, in the structures of 36a-j, this is the opposite, and the electron-donating groups such as methoxy are preferred over the electron-withdrawing groups of chlorine, which increases the activity of these structures. However, these electron-killing groups increase antifungal and antibacterial activity. The position of the groups has also been effective in increasing and decreasing the activity, for example, the combination of 40d with the placement of the flora group in position three has shown more suitable activity. Even increasing the number of thiazole rings improves the antibacterial and antifungal activity, like the structure of 68d.
In terms of anti-inflammatory activity, the structures hybridized with thiazole showed remarkable characteristics, such as structure 113, which has a coumarin appendage, and increased the anti-inflammatory properties of this compound. Also, the presence of an ester group attached to the thiazole ring is preferable to an acidic group and increases the anti-inflammatory properties of the structures (structures 120a-h, 125b, and 126d).
Regarding the anticancer activity of different structures, the number of thiazole rings is effective, and by increasing the number of these rings, the anticancer activity improves, such as the structures of 208 and 209. The type of linker is also very important, for example, linker CONH is more effective than linker SO2NH (Structures 211a-f and 212a-b, respectively). Therefore, special attention should be paid to the type of linker and the groups connected to it in the design of the structures. Also, electron-withdrawing groups are preferred over electron-donating groups and are more effective on the anti-cancer property of the structures (for example 217f and 201 a). Also, the existence of hybridized structures with thiazole is also effective on the anticancer property and increases the anticancer property of the desired compounds, similar to the structures 274 and 275.
5 Future remarks
According to the contents mentioned above, we suggest that you focus on the structures containing the thiazole ring hybridized with other structures such as pyrimidine, imidazole, pyrazole, triazole, piperazine, pyrrole, pyridine, furan, coumarin, and etc. Examine the electron-donating and electron-withdrawing groups at the same time to determine the greater influence of each group. Also, research should move towards clinicalization, and only laboratory results do not help the development of structures, because the interaction of structures in the body environment is different from the laboratory, and its results help the development of these types of structures for better design of advanced drugs. Linkers are very important in interactions because they can help in structure interaction if they are designed and studied correctly. Therefore, we suggest that in the synthesis and design of structures with anti-microbial, anti-inflammatory and anti-cancer properties, attention should be paid to the type of linkers and groups used, and a lot of research is still needed in this field to improve the conditions of the structures.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel diphenylthiazole derivatives with multi-target mechanism: Synthesis, docking study, anticancer and anti-inflammatory activities. Bioorg. Chem.. 2017;75:127-138.
- [CrossRef] [Google Scholar]
- New pyrimidine/thiazole hybrids endowed with analgesic, anti-inflammatory, and lower cardiotoxic activities: Design, synthesis, and COX-2/sEH dual inhibition. Arch. Pharm.. 2022;355(7):2200024.
- [CrossRef] [Google Scholar]
- Design and Synthesis of Novel 6-(5-Methyl-1 H-1, 2, 3-triazol-4-yl)-5-[(2-(thiazol-2-yl) hydrazono) methyl] imidazo [2, 1-b] thiazoles as Antimicrobial Agents. J. Heterocycl. Chem.. 2017;54(1):489-494.
- [CrossRef] [Google Scholar]
- Synthesis of novel benzodifuranyl; 1, 3, 5-triazines; 1, 3, 5-oxadiazepines; and thiazolopyrimidines derived from visnaginone and khellinone as anti-inflammatory and analgesic agents. Molecules. 2020;25(1):220.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of some novel thiazole-based heterocycles as potential anticancer and antimicrobial agents. Molecules. 2019;24(3):539.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of purine-pyrazole hybrids incorporating thiazole, thiazolidinone or rhodanine moiety as 15-LOX inhibitors endowed with anticancer and antioxidant potential. Bioorg. Chem.. 2019;87:821-837.
- [CrossRef] [Google Scholar]
- Synthesis, in vitro anticancer evaluation and in silico studies of novel imidazo [2, 1-b] thiazole derivatives bearing pyrazole moieties. Eur. J. Med. Chem.. 2014;75:492-500.
- [CrossRef] [Google Scholar]
- Review of the synthesis and biological activity of thiazoles. Synth. Commun.. 2021;51(5):670-700.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial, anticancer and anti-oxidant activities of novel 2, 3-dihydropyrido [2, 3-d] pyrimidine-4-one and pyrrolo [2, 1-b][1, 3] benzothiazole derivatives via microwave-assisted synthesis. Molecules. 2022;27(4):1246.
- [CrossRef] [Google Scholar]
- Fluorinated azole anticancer drugs: Synthesis, elaborated structure elucidation and docking studies. Arab. J. Chem.. 2022;15(5):103782
- [CrossRef] [Google Scholar]
- New series of thiazole derivatives: synthesis, structural elucidation, antimicrobial activity, molecular modeling and MOE docking. Molecules. 2019;24(9):1741.
- [CrossRef] [Google Scholar]
- Synthesis and in vitro evaluation of new nitro-substituted thiazolyl hydrazone derivatives as anticandidal and anticancer agents. Molecules. 2014;19(9):14809-14820.
- [CrossRef] [Google Scholar]
- Novel 2-(5-Aryl-4, 5-dihydropyrazol-1-yl) thiazol-4-one as EGFR inhibitors: synthesis, biological assessment and molecular docking insights. Drug Des. Devel. Ther. 2022:1457-1471.
- [CrossRef] [Google Scholar]
- Synthesis and molecular docking of novel 1, 3-thiazole derived 1, 2, 3-triazoles and in vivo biological evaluation for their anti anxiety and anti inflammatory activity. J. Mol. Struct.. 2021;1236:130357
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of some novel quinoline-pyrazoline-based coumarinyl thiazole derivatives. Med. Chem. Res.. 2017;26:1481-1496.
- [CrossRef] [Google Scholar]
- New thiazole-2 (3H)-thiones containing 4-(3, 4, 5-trimethoxyphenyl) moiety as anticancer agents. Eur. J. Med. Chem.. 2020;185:111784
- [CrossRef] [Google Scholar]
- Thiazole in the targeted anticancer drug discovery. Future Med. Chem.. 2019;11(16):1929-1952.
- [CrossRef] [Google Scholar]
- Design, synthesis, anticancer evaluation, and molecular docking studies of thiazole–pyrimidine linked amide derivatives. Polycycl. Aromat. Compd.. 2022;42(8):5368-5384.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of 3-aroyl-4-heteroaryl pyrroles and pyrazoles. Med. Chem. Res.. 2015;24:954-964.
- [CrossRef] [Google Scholar]
- Synthesis of New Thiazole-Pyridine Hybrids and Their Anticancer Activity. Russ. J. Gen. Chem.. 2020;90:2004-2011.
- [CrossRef] [Google Scholar]
- Multistep synthesis and screening of heterocyclic tetrads containing furan, pyrazoline, thiazole and triazole (or oxadiazole) as antimicrobial and anticancer agents. J. Saudi Chem. Soc.. 2022;26(3):101447
- [CrossRef] [Google Scholar]
- Design, synthesis and antimalarial evaluation of novel thiazole derivatives. Bioorg. Med. Chem. Lett.. 2016;26(16):3938-3944.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of novel thiazole-5-carboxamide derivatives. Appl. Sci.. 2016;6(1):8.
- [CrossRef] [Google Scholar]
- Synthesis, anti-inflammatory evaluation, and docking studies of some new thiazole derivatives. Med. Chem. Res.. 2014;23:2780-2792.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and antimicrobial activity of some novel thiazole clubbed 1, 3, 4-oxadiazoles. Med. Chem. Res.. 2015;24:258-266.
- [CrossRef] [Google Scholar]
- One-pot multi-component synthesis of novel ethyl-2-(3-((2-(4-(4-aryl) thiazol-2-yl) hydrazono) methyl)-4-hydroxy/isobutoxyphenyl)-4-methylthiazole-5-carboxylate derivatives and evaluation of their in vitro antimicrobial activity. J. Heterocycl. Chem.. 2020;57(3):1361-1367.
- [CrossRef] [Google Scholar]
- Eco-friendly synthesis, characterization and biological evaluation of some novel pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules. 2018;23(11):2970.
- [CrossRef] [Google Scholar]
- Synthesis and efficiency of new pyridine, chromene and thiazole containing compounds as antimicrobial and antioxidant agents. Bull. Chem. Soc. Ethiop.. 2022;36(1):137-148.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial evaluation of novel 1, 3-thiazoles and unsymmetrical azines. Res. Chem. Intermed.. 2016;42:3333-3349.
- [CrossRef] [Google Scholar]
- Design, synthesis and docking studies of new hydrazinyl-thiazole derivatives as anticancer and antimicrobial agents. J. Saudi Chem. Soc.. 2022;26(4):101488
- [CrossRef] [Google Scholar]
- Discovery of thiazole-based-chalcones and 4-hetarylthiazoles as potent anticancer agents: Synthesis, docking study and anticancer activity. Bioorg. Chem.. 2020;98:103761
- [CrossRef] [Google Scholar]
- Antimicrobial properties of some new synthesized benzothiazole linked carboxamide, acetohydrazide, and sulfonamide systems. Journal of Heterocyclic Chemistry. 2019;56(9):2369-2378.
- [Google Scholar]
- Synthesis, molecular docking and antitumor activity of new dithiazoles. Polycycl. Aromat. Compd.. 2021;41(8):1591-1607.
- [CrossRef] [Google Scholar]
- Pharmacological evaluation of some novel synthesized compounds derived from spiro (cyclohexane-1, 2′-thiazolidines) Med. Chem. Res.. 2014;23:2515-2527.
- [CrossRef] [Google Scholar]
- Synthesis and in vitro cytotoxic activity of novel coumarinylimidazo [2, 1-b] thiazole derivatives. RSC Adv.. 2014;4(96):53812-53819.
- [CrossRef] [Google Scholar]
- An Efficient Procedure of Synthesis Acyclic C-Glycosides of Thiazolo [4, 5-b] Pyrazine and Imidazo [4, 5-d] Thiazole with Expected Anti-Cancer Activities. Polycycl. Aromat. Compd.. 2022;42(6):3328-3338.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of arylazothiazoles and 1, 3, 4-thiadiazoles using chitosan-grafted-poly (4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd.. 2015;51:1030-1038.
- [CrossRef] [Google Scholar]
- A facile access and evaluation of some novel thiazole and 1, 3, 4-thiadiazole derivatives incorporating thiazole moiety as potent anticancer agents. Chem. Cent. J.. 2017;11(1):1-8.
- [CrossRef] [Google Scholar]
- Synthesis, molecular docking study, and cytotoxic activity against MCF cells of new thiazole-thiophene scaffolds. Molecules. 2022;27(14):4639.
- [CrossRef] [Google Scholar]
- Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1 H-indol-5yl) methylene)-hydrazinyl)-thiazole Derivatives. Molecules. 2017;22(2):260.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of anti-inflammatory and analgesic activity of some substituted thiazolyl and thaizolidinonyl tetrahydronapthalene derivatives. Med. Chem. Res.. 2014;23:3418-3435.
- [CrossRef] [Google Scholar]
- Ligand-based optimization to identify novel 2-aminobenzo [d] thiazole derivatives as potent sEH inhibitors with anti-inflammatory effects. Eur. J. Med. Chem.. 2021;212:113028
- [CrossRef] [Google Scholar]
- Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176-187.
- [CrossRef] [Google Scholar]
- J. Chem. Ed.. 2010;87:1348.
- Synthesis and antimicrobial evaluation of new thiazolyl-1, 2, 3-triazolyl-alcohol derivatives. Med. Chem. Res.. 2020;29:989-999.
- [CrossRef] [Google Scholar]
- Anticancer potential of thiazole derivatives: a retrospective review. Mini Rev. Med. Chem.. 2018;18(8):640-655.
- [CrossRef] [Google Scholar]
- Pyridine-and thiazole-based hydrazides with promising anti-inflammatory and antimicrobial activities along with their in silico studies. ACS Omega. 2020;5(39):25228-25239.
- [CrossRef] [Google Scholar]
- Synthesis and in silico investigation of thiazoles bearing pyrazoles derivatives as anti-inflammatory agents. Comput. Biol. Chem.. 2016;61:86-96.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of new imidazo [2, 1-b] thiazole derivatives as anticancer agents. Med. Chem. Res.. 2016;25:2471-2484.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of new heteroaryl (aryl) thiazole derivatives molecular docking studies. Antibiotics. 2022;11(10):1337.
- [CrossRef] [Google Scholar]
- Solid-phase synthesis of sulfur containing heterocycles. J. Sulfur Chem.. 2018;39(5):544-577.
- [CrossRef] [Google Scholar]
- Photochemical mediated reactions in five-membered O-heterocycles synthesis. Synth. Commun.. 2018;48(17):2119-2149.
- [CrossRef] [Google Scholar]
- Silver-assisted Syntheses of Fused Five-membered N-heterocycles. Curr. Org. Chem.. 2021;25(19):2232-2256.
- [CrossRef] [Google Scholar]
- Nitrogen-containing six-membered heterocycles: solid-phase synthesis. Synth. Commun.. 2014;44(9):1173-1211.
- [CrossRef] [Google Scholar]
- Design, synthesis and docking studies of novel thiazole derivatives incorporating pyridine moiety and assessment as antimicrobial agents. Sci. Rep.. 2021;11(1):7846.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of pyrazolylthiazole carboxylic acids as potent anti-inflammatory–antimicrobial agents. Bioorg. Med. Chem. Lett.. 2015;25(6):1177-1181.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer evaluation of 3-aryl-6-phenylimidazo [2, 1-b] thiazoles. Bioorg. Med. Chem. Lett.. 2014;24(23):5428-5431.
- [CrossRef] [Google Scholar]
- Solvent-free synthesis of bacillamide analogues as novel cytotoxic and anti-inflammatory agents. Eur. J. Med. Chem.. 2016;123:718-726.
- [CrossRef] [Google Scholar]
- Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives. Bioorg. Chem.. 2021;107:104608
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and anticancer screening of some new bithiazole derivatives. Russ. J. Org. Chem.. 2020;56:1096-1107.
- [CrossRef] [Google Scholar]
- Design, Synthesis, and Biological Evaluation of 4-Phenoxyquinoline Derivatives Containing Benzo [d] thiazole-2-yl Urea as c-Met Kinase Inhibitors. Arch. Pharm.. 2016;349(8):651-661.
- [CrossRef] [Google Scholar]
- Design and synthesis of thiazole derivatives as potent FabH inhibitors with antibacterial activity. Eur. J. Med. Chem.. 2014;75:438-447.
- [CrossRef] [Google Scholar]
- Synthesis and Antimicrobial Activity of Novel 2-Substituted Phenoxy-N-(4-substituted Phenyl-5-(1H–1, 2, 4-triazol-1-yl) thiazol-2-yl) acetamide Derivatives. J. Heterocycl. Chem.. 2017;54(2):1506-1513.
- [CrossRef] [Google Scholar]
- Thiazole-based aminopyrimidines and N-phenylpyrazolines as potent antimicrobial agents: synthesis and biological evaluation. MedChemComm. 2014;5(7):915-922.
- [CrossRef] [Google Scholar]
- Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem.. 2018;151:248-260.
- [CrossRef] [Google Scholar]
- Design, synthesis and cytotoxicity of thiazole-based stilbene analogs as novel DNA topoisomerase IB inhibitors. Molecules. 2022;27(3):1009.
- [CrossRef] [Google Scholar]
- Novel class of benzimidazole-thiazole hybrids: The privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes. Bioorg. Med. Chem.. 2020;28(7):115403
- [CrossRef] [Google Scholar]
- Synthesis of thiazole linked imidazo [2, 1-b] thiazoles as anticancer agents. Polycycl. Aromat. Compd.. 2021;41(8):1608-1622.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of some new thiopyrano [2, 3-d] thiazoles incorporating pyrazole moiety. Chem. Pharm. Bull.. 2015;63(7):495-503.
- [CrossRef] [Google Scholar]
- Facile synthesis of novel substituted aryl-thiazole (SAT) analogs via one-pot multi-component reaction as potent cytotoxic agents against cancer cell lines. Bioorg. Chem.. 2017;70:133-143.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and Anti-ulcer Activities of New Fused Thiazole Derivatives Derived from 2-(2-Oxo-2H-chromen-3-yl) thiazol-4 (5H)-one. Acta Chim. Slov.. 2017;64(2):349-364.
- [Google Scholar]
- Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole. Eur. J. Med. Chem.. 2011;46(2):526-534.
- [CrossRef] [Google Scholar]
- Synthesis of some new benzimidazole–thiazole derivatives as anticancer agents. J. Heterocycl. Chem.. 2014;51(6):1797-1806.
- [CrossRef] [Google Scholar]
- Chemical synthesis and molecular docking study of new thiazole, thiophene, and thieno [2, 3-d] pyrimidine derivatives as potential antiproliferative and antimicrobial agents. J. Mol. Struct.. 2022;1270:133926
- [CrossRef] [Google Scholar]
- A Facial Synthesis and Anticancer Activity of (Z)-2-((5-(4-nitrobenzylidene)-4-oxo-4, 5-dihydrothiazol-2-yl) amino)-substituted Acid. J. Heterocycl. Chem.. 2017;54(6):3077-3086.
- [CrossRef] [Google Scholar]
- An efficient synthesis of designed 4-thiazolidinone fused pyrimidine derivatives as potent antimicrobial agents. J. Heterocycl. Chem.. 2020;57(10):3531-3543.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial evaluation of novel ethyl 2-(2-(4-substituted) acetamido)-4-subtituted-thiazole-5-carboxylate derivatives. Bioorg. Med. Chem. Lett.. 2016;26(15):3525-3528.
- [CrossRef] [Google Scholar]
- Thiazole ring—a biologically active scaffold. Molecules. 2021;26(11):3166.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer evaluation of 2-phenyl thiaolidinone substituted 2-phenyl benzothiazole-6-carboxylic acid derivatives. J. Saudi Chem. Soc.. 2015;19(2):181-185.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of amine linked bis-and tris-heterocycles. Eur. J. Med. Chem.. 2014;82:347-354.
- [CrossRef] [Google Scholar]
- 2-(Benzo [d] thiazol-2-yl) phenyl-4-nitrophenyl alkyl/aryl substituted phosphoramidates: synthesis, characterization and antimicrobial activity evaluation. Phosphorus Sulfur Silicon Relat. Elem.. 2018;193(3):136-140.
- [CrossRef] [Google Scholar]
- Bifunctional ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives: synthesis and in vitro biological evaluation as antimicrobial and anticancer agents. Eur. J. Med. Chem.. 2014;76:170-181.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of antimicrobial and anti-inflammatory activity of 6-aryliden-2-methyl-2, 3-dihydroimidazo [2, 1-b][1, 3] thiazoles. Appl. Chem. 2021;12:292-303.
- [Google Scholar]
- Recent advances in the synthesis and medicinal chemistry of SF5 and SF4Cl compounds. Synthesis. 2022;54(2022):4184-4209.
- [CrossRef] [Google Scholar]
- Novel bis (1, 2, 4-oxadiazolyl) Fused thiazole derivatives: synthesis and anticancer activity. Russ. J. Gen. Chem.. 2018;88:2611-2615.
- [CrossRef] [Google Scholar]
- Design, efficient synthesis and molecular docking of some novel thiazolyl-pyrazole derivatives as anticancer agents. BMC chemistry. 2019;13(1):1-13.
- [CrossRef] [Google Scholar]
- One-pot synthesis of novel thiazoles as potential anti-cancer agents. Drug Des. Devel. Ther. 2020:1363-1375.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted green synthesis and antimicrobial assessment of 1, 3-thiazoles and 1, 3, 4-thiadiazines. Green Chem. Lett. Rev.. 2021;14(4):679-688.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of some imidazothiazole derivatives of benzofuran. Arab. J. Chem.. 2017;10:S554-S558.
- [CrossRef] [Google Scholar]
- On‐Water NiFe2O4 nanoparticle‐catalyzed one‐pot synthesis of biofunctionalized pyrimidine‐thiazole derivatives: in silico binding affinity and in vitro anticancer activity studies. ChemistrySelect. 2018;3(39):11012-11019.
- [CrossRef] [Google Scholar]
- Synthesis, molecular modelling and biological significance of N-(4-(4-bromophenyl) thiazol-2-yl)-2-chloroacetamide derivatives as prospective antimicrobial and antiproliferative agents. BMC Chem.. 2019;13:1-14.
- [CrossRef] [Google Scholar]
- 4-(4-Bromophenyl)-thiazol-2-amine derivatives: synthesis, biological activity and molecular docking study with ADME profile. BMC Chem.. 2019;13(1):1-16.
- [CrossRef] [Google Scholar]
- Synthesis of 5-acetyl-2-arylamino-4-methylthiazole thiosemicarbazones under microwave irradiation and their in vitro anticancer activity. J. Chem. Res.. 2016;40(2):67-72.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of 2-(4-(benzo [d] thiazol-5-ylsulfonyl) piperazine-1-yl)-N-substituted acetamide derivatives. J. Heterocycl. Chem.. 2020;57(11):3907-3917.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of new 2-piperazin-1-yl-N-1, 3-thiazol-2-ylacetamides of cyclopenta [c] pyridines and pyrano [3, 4-c] pyridines. Arch. Pharm.. 2021;354(1):2000208.
- [CrossRef] [Google Scholar]
- Molecular properties prediction, synthesis, and antimicrobial activity of bis (azolyl) sulfonamidoacetamides. Arch. Pharm.. 2021;354(8):e2000483-e.
- [CrossRef] [Google Scholar]
- Efficient L-Proline catalyzed synthesis of some new (4-substituted-phenyl)-1, 5-dihydro-2H-pyrimido [4, 5-d][1, 3] thiazolo [3, 2a]-pyrimidine-2, 4 (3H)-diones bearing thiazolopyrimidine derivatives and evaluation of their pharmacological activities. J. Mol. Struct.. 2022;1247:131324
- [CrossRef] [Google Scholar]
- Synthesis of new 1, 3-thiazol derivatives of maleopimaric acid as anticancer, antibacterial and antifungal agents. Nat. Prod. Res.. 2021;35(8):1340-1348.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of chalcone-linked thiazole-imidazopyridine derivatives as anticancer agents. Med. Chem. Res.. 2020;29:1643-1654.
- [CrossRef] [Google Scholar]
- 2-(3′-Indolyl)-N-arylthiazole-4-carboxamides: synthesis and evaluation of antibacterial and anticancer activities. Bioorg. Med. Chem. Lett.. 2015;25(19):4225-4231.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of novel 4-(6-substituted quinolin-4-yl)-N-aryl thiazol-2-amine derivatives as potential antimicrobial agents. J. Heterocycl. Chem.. 2021;58(9):1867-1877.
- [CrossRef] [Google Scholar]
- Heterocycles 39. Synthesis, characterization and evaluation of the anti-inflammatory activity of thiazolo [3, 2-b][1, 2, 4] triazole derivatives bearing pyridin-3/4-yl moiety. Med. Chem. Res.. 2017;26:2602-2613.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of bis-thiazole derivatives as new anticancer agents. Eur. J. Med. Chem.. 2016;107:288-294.
- [CrossRef] [Google Scholar]
- One-pot synthesis and in-vitro anticancer evaluation of 5-(2′-indolyl) thiazoles. Sci. Rep.. 2016;6(1):23401.
- [CrossRef] [Google Scholar]
- Synthesis of benzo [d] thiazole-hydrazone analogues: molecular docking and SAR studies of potential H+/K+ ATPase inhibitors and anti-inflammatory agents. MedChemComm. 2017;8(6):1173-1189.
- [CrossRef] [Google Scholar]
- Origins of Organic Chemistry and Organic Synthesis. Eur. J. Org. Chem.. 2022;e202101492
- [CrossRef] [Google Scholar]
- Design, Synthesis, and Anticancer Activity of 1, 2, 3-triazole Likned Thiazole-1, 2-Isoxazole Derivatives. Russ. J. Gen. Chem.. 2019;89:2522-2527.
- [CrossRef] [Google Scholar]
- Analgesic and anti-inflammatory activities of novel heterocyclic substituted thiazole derivatives. Rasayan J. Chem.. 2022;15(1)
- [Google Scholar]
- Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bioorg. Chem.. 2022;124:105855
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of 2, 4-disubstituted thiazole amide derivatives as anticancer agent. Chem. Pap.. 2019;73:355-364.
- [CrossRef] [Google Scholar]







