Translate this page into:
Treatment of nerve cancer with the green synthesis of CuO NPs
⁎Corresponding author. gyf8869@126.com (Yafei Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The green-synthesized copper nanoparticles were characterized using different techniques such as XRD, EDX, FT-IR, TEM, FE-SEM, and UV–Vis. The FE-SEM results confirm spherical morphology for the nanoparticles with size of 14.1 to 37.9 nm. The IC50 of the copper nanoparticles was 163 and 245 against S462 Human Peripheral Nerve Sheath Tumor and BL1391 cancer cells, respectively.
Abstract
With technological advances in molecular techniques and bioinformatics, many information has been gained that will help in the cancer early diagnosis. Studying and investigating agents of natural origin, such as plants nanocompounds is one of the most important research goals in the cancer treatment field. In the recent study, copper nanoparticles were synthesized according to green chemistry rules using the Boswellia thurifera aqueous extract. After doing the clinical trial studies, the recent nanoparticles may be used as an anti-carcinoma supplement in humans. The properties of copper nanoparticles against common human nerve carcinoma cell lines i.e. were determined. The green-synthesized copper nanoparticles were characterized using different techniques such as X-ray diffraction (XRD), energy dispersive X-ray (EDX), fourier-transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), and ultraviolet–visible spectroscopy (UV–Vis). The FE-SEM results confirm spherical morphology for the nanoparticles with size of 14.1 to 37.9 nm. The IC50 of the copper nanoparticles was 163 and 245 against S462 Human Peripheral Nerve Sheath Tumor and BL1391 cancer cells, respectively. Copper nanoparticles induce cell apoptosis, accompanied by the pro-apoptotic markers upregulation (cleaved caspase-8 and Bax) and anti-apoptotic marker downregulation, Bcl-2. Also, copper nanoparticles prevent colony formation compared to their matched control. More significantly, the molecular pathway analysis of copper nanoparticles-treated cells indicated that copper nanoparticles raises p53 expression, while preventing the expression of total and phosphorylated Signal Transducer and Activator Of Transcription 3 (STAT3) in cell lines, offering p53 and STAT3 are the main key players behind the biological events provoked by the extract in human cancer cells.
Keywords
Cytotoxicity
Cancer
Copper nanoparticles
Boswellia thurifera leaf
1 Introduction
Cancer is a main death cause in the whole world. So, more than 20% of deaths in the world are related to cancer (Agarwal et al., 2012). The number of people with cancer increases annually; According to the statistics provided by the World Health Organization in 2014, about 14 million new cases of cancer were registered (Bray et al., 2014). Cancer treatment solutions in classical medicine include chemotherapy and radiotherapy, which can improve the patient's condition in some cases, but are always associated with significant side effects. Medicinal plants have compounds with anti-tumor properties, such as taxol, which is a known anti-cancer compound, of plant origin (Singh et al., 2002 May 1; Esmaeilbeig et al., 2015 Oct; Motavalizadeh et al., 2012; Hatami et al., 2008). The knowledge of using plant resources has been compiled by the predecessors in the form of valuable books over hundreds of years of experience; so, the large number of works written in the field of traditional medicine is one of the strengths of traditional medicine. Investigating the knowledge of traditional medicine, especially in cases such as cancer treatment, can make research more targeted and achieve results in a shorter time (Soltani, 1983; Ebn Rabban eTabari, 2003; Akhawayni, 1992; Chaghmini, 2010).
Today, with the progress of human societies, diseases have also progressed a lot. One of the ways to save people from various diseases is to fully understand nature and get to know the properties of plants (Assimopoulou et al., 2005; Kulkarni et al., 1991; Abdelwahab et al., 1987; Behnamrasuli et al., 2001; Shen and Lou, 2008; Ghasemidehkordi et al., 2002). Boswellia thurifera is also one of those plants that has extraordinary and incredible properties. Boswellia thurifera is obtained from the sap of a native tree called Baswalia tree. To obtain this sap, farmers make cuts in the trunk of the tree, then a milky liquid flows from the tree, which is known as resin. This viscous substance hardens on exposure to air; the nature of this plant is hot and dry and it has useful and therapeutic properties (Krohn et al., 2001; Shailesh et al., 2007; Poeckel and Werz, 2006; Rall et al., 1996; Gupta et al., 2001; Ammon et al., 1991). Boswellia thurifera is found in countries such as India, Pakistan, Oman, Yemen and Somalia. The sap obtained from this tree is used as herbal medicines and making perfumes. The smell of this gum (juice) is very popular and makes people interested in it after every smell. This plant is seen in very pale yellow, brown and orange colors (Singh et al., 1996; Hostanska et al., 2002; Sharma et al., 1996; Safayhi et al., 1992). This plant works well to strengthen the brain and intelligence and helps you to concentrate. Boswellia thurifera consumption has properties such as: preventing Alzheimer's, strengthening the nervous system and Parkinson's (impairing the body's motor system) and is also used as an antidepressant. One of the useful properties of Boswellia thurifera is the anti-inflammatory and antibacterial property of this plant, which helps to reduce the infection in the lungs and helps to breathe more easily (Safayhi et al., 1997; Reddy et al., 1989; Gupta et al., 1997; Sailer et al., 1996; Gerhardt et al., 2001; Krieglstein et al., 2001). In addition to that, it helps to expel phlegm from the lungs, opening blood vessels for easy breathing. People can use Boswellia thurifera tea to cure lung infections. The use and consumption of Boswellia thurifera plant helps reduce pain during periods and reduces heavy bleeding, and helps to regulate: balance hormones, stimulate blood flow in the uterus, regularize irregular menstruation and strengthen the power of pregnancy (Borrelli et al., 2006; Kimmatkar et al., 2003; Kulkarni et al., 1991; Gupta et al., 1998). Using soaked Boswellia thurifera, in addition to reducing gum infection, eliminates bad breath and relieves your toothache. Another feature of Boswellia thurifera is anti-inflammatory and helps reduce arthritis. The extract of this plant has a soothing effect and is useful for treating tingling in body parts, reducing pain, and preventing arthritis. This plant is a strong antioxidant and has anti-aging properties. Antioxidants slow down and stop free radicals, which helps to rejuvenate the skin (Sailer et al., 1996; Gerhardt et al., 2001; Krieglstein et al., 2001; Borrelli et al., 2006; Kimmatkar et al., 2003). The recent plant has many polyphenol and phenolic compounds. The effects of polyphenol compounds have been proven in various cell and animal studies in the cancer treatment or prevention. Also, phenolic compounds can regulate the expression of genes that play a major role in the apoptosis and cell cycle process in cancer cells. Based on epidemiological and clinical studies, polyphenol compounds can prevent cancer (Tsukada et al., 1986; Huang et al., 2000; Sharma et al., 2009; Singh and Atal, 1986).
The use of chemotherapy drugs and synthetic additives has disrupted the functioning of healthy cells. Studies conducted in cell toxicity have shown that these synthetic substances cause severe apoptosis and DNA breakage. Due to the increasing concerns about the safety of chemical drugs and additives, the demand for the use of bioactive has attracted the most attention in recent years (Rashid et al., 2019; Ramesh, 2019; Saleem et al., 2020; Alkasir et al., 2020). The search for natural biological compounds led to the introduction of plant nanoparticles as a bioactive compound in the pharmaceutical and food industries (Saleem et al., 2020; Hoque et al., 2020; Goudenhooft et al., 2019; Zhou et al., 2020; Chhillar et al., 2021; Suri et al., 2020). Today, cancer means the growth, proliferation and sometimes abnormal proliferation of body cells. Cancer can be induced through various agricultural toxins, environmental pollutants, chemotherapy drugs and food additives (Saleem et al., 2020; De Jong and Borm, 2008; Shaabani et al., 2008; Budarin et al., 2008). In general, many chemical drugs and food additives cause changes in the process of cell division and disrupt the reproduction and differentiation of healthy cells (Shaabani et al., 2008; Li et al., 2023). The main drawback of chemotherapy drugs and synthetic food additives is that they harm the healthy cells of a person. Therefore, scientists are trying to use natural bioactives with low toxic effects to replace these chemical drugs and synthetic additives (De Jong and Borm, 2008; Shaabani et al., 2008; Budarin et al., 2008; Li et al., 2023).
Today, the use of nanomaterials in combination with anticancer drugs is increasing widely, and the use of these compounds has many applications in medicine. One of the practical aspects of nanomaterials is to combine them with anticancer drugs so that the drug can be delivered to the cell with a higher efficiency, also, its side effects and toxicity on healthy cells can be decreased (Shaabani et al., 2008; Budarin et al., 2008; Li et al., 2023; Veisi et al., 2021). Due to the rising death prevalence caused by cancers and defects in chemotherapy and radiotherapy methods in cancer advanced stages, it is necessary to find modern techniques to control and treat cancer. The nanoparticles use with a diameter of 100 nm or less in combination with anticancer drugs to target cancer cells in cancer medical projects is so developed (Elkhenany et al., 2020; Borm et al., 2006; Stapleton and Nurkiewicz, 2014; Patra et al., 2018). A significant relationship between nanoparticle size and cytotoxic effects has been reported. One of the most common nanoparticles in this field is Cu nanoparticles, which are considered one of the potential anticancer agents. Studies show that nanoparticles cause the death of cancer cells by two mechanisms (Patra et al., 2018; Itani and Al Faraj, 2019; Trojer et al., 2013; Liu et al., 2014). One is its direct effect or interaction with cell compounds (RNA, DNA and protein and cell structures (organelles and cell membranes), which leads to the induction of cell death. Second, their indirect effect creates toxic oxygen radicals, which ultimately promote cell death (Trojer et al., 2013; Liu et al., 2014; Koyyati et al., 2016). The studies conducted indicate that 56% of the share of nanoparticles in the world is allocated to nanoparticles (Patra et al., 2018; Itani and Al Faraj, 2019). Available information shows that some insoluble nanoparticles can pass through protective barriers and distribute in the body and accumulate in different organs (Itani and Al Faraj, 2019; Trojer et al., 2013). There are many methods for preparing nanoparticles, notable examples of which include biological production, microwave processing, photochemical method, chemical method, electron radiation, gamma radiation, and laser radiation methods (Patra et al., 2018; Itani and Al Faraj, 2019; Trojer et al., 2013). The chemical method is the most common way to synthesize nanoparticles, which leads to reduction by inorganic and organic agents. In a biological way, microorganisms, bacteria and fungi can produce metal nanoparticles with their metabolic system (Trojer et al., 2013; Liu et al., 2014; Koyyati et al., 2016). In the recent study, the properties of nanoparticles against common human nerve carcinoma cell lines were evaluated.
2 Experimental
2.1 Preparation of nanoparticles
First, the Boswellia thurifera leaves, after being dried in the air, were powdered using an electric mill (Moulinex, model AR1066Q). 30 g of it was soaked by maceration method and with 96% ethanol solvent and was kept at room temperature for a period of at least three days with frequent stirring to dissolve the soluble substance. After 4 days, the water and extract mixture was filtered. Finally, using a rotary evaporator (Laborota 4000-efficient, Heidolph, Germany), the excess solvent was concentrated and evaporated. It was completely dried using a freeze dryer (LTE Scientific Ltd, UK) and turned into a powder.
The CuNPs were biosynthesized based on the following protocol. A 15 ml of the aqueous solution of plant extract (30 mg/mL) was added into 10 ml of 0.4 M Cu(NO3)2·3H2O (in all stages deionized water was used). After that, the mixture was refluxed at 75 °C for 12 h. The obtained precipitate was rinsed three times and centrifuged at 13000 rpm for 10 min subsequently. Then, to dry the residuum, an oven was used at a temperature of 60 °C.
In this research, to record the UV–Vis spectra, a Shimadzu UV spectrophotometer was used. Also, X-ray diffraction (XRD) analyses (Philips PW1730) were carried out for studying of the crystalline structure of metallic silver nanoparticles. To investigate the size and morphology of NPs, Philips EM208S was employed to record transmission electron microscopy (TEM) images. Also, JASCO (FT/IR-6200) spectrophotometer was utilized to record the FT-IR spectra in this research. To evaluate the different morphological characteristic of NPs such as size distribution, surface morphology and the particle shape, MIRA3TESCAN-XMU FESEM was used to record Field Emission Scanning Electron Microscopy (FESEM) images.
2.2 Investigating the antioxidant effects
After that, the amount of antioxidant property of Cu nanoparticles was investigated using the DPPH test. To perform the DPPH test, DPPH powder which is purple is used, which is first made into a free radical. This powder turns into its radical color in ethanol and has the highest value at 517 nm. DPPH can reach its stable form by receiving an electron or hydrogen atom. When this radical is exposed to an antioxidant substance, it is removed during the reaction with it, and then its absorption at 517 nm decreases. To do the test, 2 mg DPPH was mixed in 33.8 ml of 96% ethanol. The solution was placed in the dark at 37 °C for 30 min, then the dilations of 1–1000 μg/ml NPs were treated with DPPH solution. It was left in the dark for 1 h and then the absorbance was read with a spectrophotometer. The standard compound, butyl hydroxytoluene (BHT) was used as a standard antioxidant. The inhibition percentage was computed from the following formula.
3 3. Anti-carcinoma effects
The cytotoxic effects of formulated nanoparticles on common human nerve carcinoma cell lines i.e. S462 Human Peripheral Nerve Sheath Tumor and BL1391 cells were assessed using MTT colorimetric method.
10% RPMI culture medium was used for the cultivation of cancer cells. To prepare the complete culture medium, 10% RPMI was added to 90 cc of prepared RPMI culture medium in the amount of 10 ml of FBS and 1 ml of streptomycin and penicillin. The vial containing cancer cells was removed from the incubator and then transferred to Bain-Marie. Then the contents of the vial were mixed with about 5 cc of the culture medium in the Falcon and the contents of the Falcon were centrifuged at 2700 rpm for 5 min. The supernatant and 1 ml culture medium were added to the cell plate. Then, the cells were transferred to a flask containing 5 cc of culture medium, and the flasks were placed in a CO2 incubator with appropriate humidity and temperature. At regular intervals, the cells were passaged and transferred to a fresh culture medium. An inverted microscope was applied to estimate the amount of live cells and check any type of contamination. Then the liquid solution in the flasks was removed. After that, the bottom of the flask was washed with PBS. To separate the cells from the plate bottom, trypsin enzyme was added. The cells were counted with trypan blue dye and finally the cells were transferred to a 96-well plate to perform the MTT test and placed in an incubator. The basis of the work is that living cells have the succinate dehydrogenase enzyme in their mitochondrial membrane, which reacts with tetrazolium and creates purple crystals, and since dead cells do not have this enzyme, curmazan is not formed and remains colorless. The working method is that cancer cells prepared from the cell bank were cultured in a 96-well plate in an RPMI culture medium. After 24 h, treatment with nanoparticles synthesized in several dilations (1–1000 µg/ml) was done. Finally, 20 µl MTT solution was added to wells and the plates were placed in an incubator at a temperature of 37 °C for about 4 h (Koyyati et al., 2016):
To determine the cell cycle, 106 cells were put in 200 mm Petri dishes for 48 h. G0 phase (Synchronized cells) were treated with Cu nanoparticles for 60 h. Then, the DNA was then stained by the FXCycle PI/RNase staining solution at 60 mg/ml. Cells in the S, G2/M and G0/G1 phases were quantified by the Flow Jo software. Cellular apoptosis was evaluated by the ApoScreen Annexin V-fluorescein isothiocyanate (FITC)/7-amino-actinomycin D (7-AAD) Apoptosis Kit (Koyyati et al., 2016).
Soft agar colony formation assay was done for assessing the cells ability to grow in an anchorage-independent manner. In immunoblotting analysis, 106 cells were put in Petri dishes and treated with the Cu nanoparticles for 60 h. Protein lysates equal amounts were run on 10% polyacrylamide gels and transferred onto PVDF membranes. The membranes were incubated with the following primary antibodies overnight; mouse anti-Bax, mouse anti-Bcl-2, mouse anti-cleaved caspase-8, rabbit anti-p53, mouse anti-STAT3, and rabbit anti-phosphorylated-STAT3. PVDF membranes were re-probed with rabbit anti-GAPDH to confirm the proteins equal loading. Quantification was performed by the ImageJ software and the bands’ intensities was normalized to GAPDH to evaluate the relative protein expression (Koyyati et al., 2016).
SPSS version 22 software and one-way ANOVA were used to calculate the HUVEC and cancer cells viability percentage of the synthesized nanoparticles and to check for significant differences. The confidence level of 0.01 was considered for calculations.
4 Results and discussion
The copper oxide nanoparticles formation is certified by the peaks presence at 533 and 624 cm−1, which belongs to the bending vibration of Cu—O (Lu et al., 2021; Shahzadi et al., 2019; Mahdavi et al., 2021; Ahmeda et al., 2020; Mahdavi et al., 2019). Take peaks in 3421 and 2938 cm−1 as examples, which are related to O—H and aliphatic C—H, in order (Fig. 1). Likewise, the peaks extending from 1627 to 1405 cm−1 correspond to C⚌O and C⚌C stretching, and the peaks related to —C—O and C—N stretching are detectable at 1244 and 1077 cm−1, respectively. The presence of various compounds like carbohydrates, alkaloids, flavonoids, phenolic compounds, terpenoids, glycosides, which have been reported previously, are certified by these peaks (Mahdavi et al., 2019; Baghayeri et al., 2018; Matinise et al., 2018; Gingasu, 2016).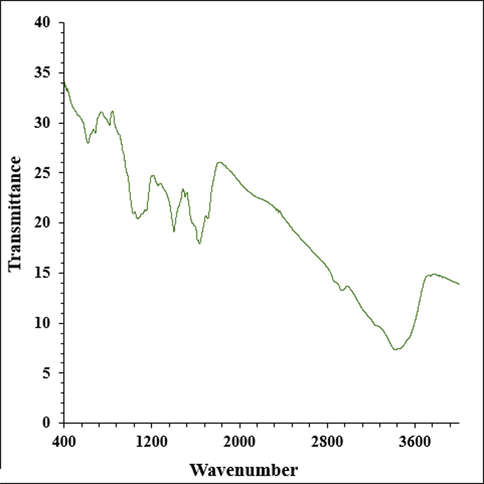
The FT-IR pattern of Cu nanoparticles.
In the recent study, Fig. 2 exhibits the UV–Vis. spectrum of Cu nanoparticles. The band at 334 nm approves the formation of the Cu nanoparticles.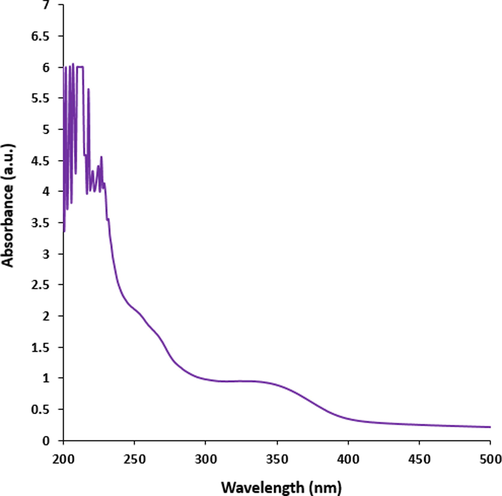
The UV–Visible spectrum of Cu nanoparticles.
Figs. 3 and 4 exhibit the TEM and FE-SEM images of Cu nanoparticles. The spherical morphology with a range size of 14.1 to 37.9 nm is obtained for synthesized nanoparticles. The images show an aggregation for nanoparticles that is one of common properties of green synthesized for the metallic nanoparticles (Lu et al., 2021; Shahzadi et al., 2019; Mahdavi et al., 2021; Ahmeda et al., 2020; Mahdavi et al., 2019). So far, nanoparticles, which have green synthesized using plants extracts, are formed in a wide range size of 10 to 100 nm. This reveals a resemblance with particle size of nanoparticles (Baghayeri et al., 2018; Matinise et al., 2018; Gingasu, 2016; Ahmed et al., 2016; Koyyati et al., 2016).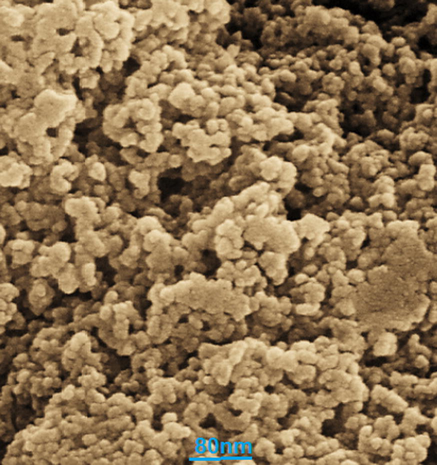
FE-SEM images of Cu nanoparticles.
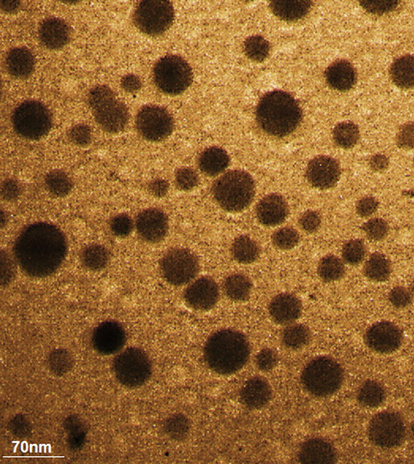
TEM image of Cu nanoparticles.
The peaks at 2θ values of 32.7, 35.5, 38.8, 48.8, 58.5, 61.6, and 68.3 are indexed as (1 1 0), (11–1), (1 1 1), (20–2), (2 0 2), (−1 1 3), and (2 2 0) planes (Fig. 5). These peaks are in good agreement with standard database ICDD PDF card no. 96–901-6327. The average crystal size of Cu nanoparticles was 27.2 nm that was calculated using X‐Ray diffraction based on Scherrer equation
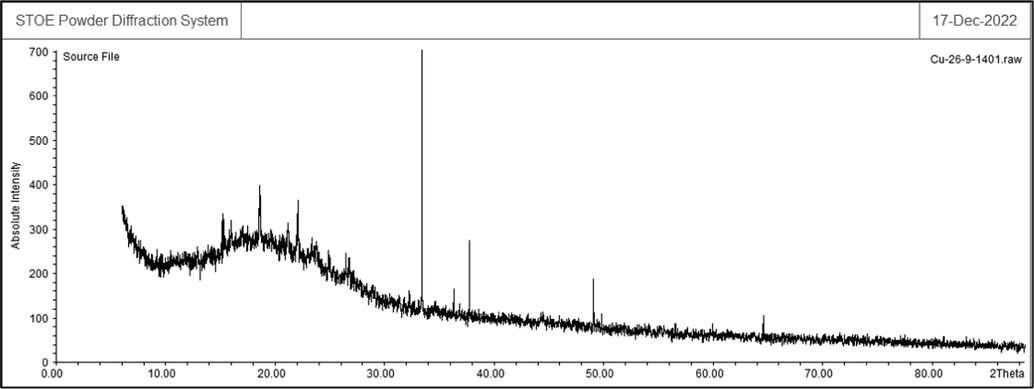
XRD analysis of Cu nanoparticles.
In the EDS analysis, the signals which are at 0.9 Kev (for CuLα), and at 8 Kev (for Cu Kα) approve the presences of copper, matchable to previous biosynthesized CuNPs which were reported (Lu et al., 2021; Shahzadi et al., 2019; Mahdavi et al., 2021; Ahmeda et al., 2020; Mahdavi et al., 2019). The oxygen in copper oxide nanoparticles and some organic molecules existing in extract that linked to the CuNPs surface result in the formation of a signal around 0.5 Kev. A signal around 0.3 Kev certifies the presence of carbon on the CuNPs surface (Fig. 6).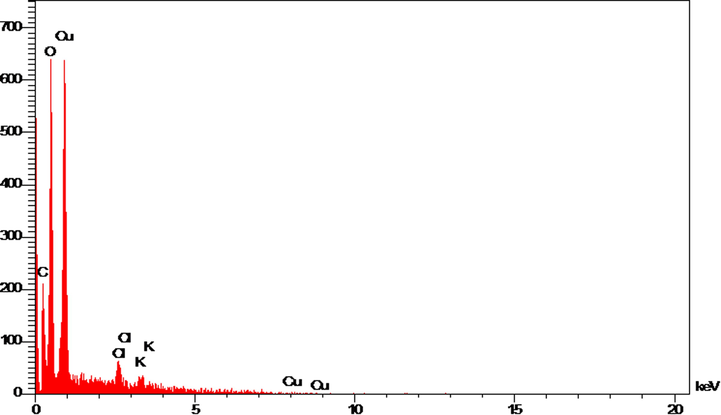
EDX analysis of Cu nanoparticles.
Nanoparticles enter the body in different ways and can be transferred to the vital organs of the body through the bloodstream and cause cell damage (De Jong and Borm, 2008; Shaabani et al., 2008; Budarin et al., 2008; Li et al., 2023). In general, the size of nanoparticles is between 1 and 100 mm, and these metal nanoparticles have various physiochemical properties than the metal itself (Li et al., 2023; Veisi et al., 2021; Elkhenany et al., 2020). Nanoparticles are applied in all life dimensions such as the fight against microbes, electrical systems, diagnosis and treatment of diseases (Borm et al., 2006; Stapleton and Nurkiewicz, 2014; Patra et al., 2018; Itani and Al Faraj, 2019).
Nanotechnology is one of the basic and emerging fields of research in modern sciences, which have many applications in different sciences and have a great impact on different areas of human life. Nanoparticles are simple particles with dimensions of 1–100 nm that provide unique activities such as an extraordinary surface/volume ratio and high surface energy (Suri et al., 2020; Saleem et al., 2020; De Jong and Borm, 2008). Recently, the synthesis of NPs has gained special importance due to its many applications in the packaging industry to preserve the health of food and as permitted food additives. Among the metal oxides, copper nanoparticles have attracted the interest of many researchers due to their antibacterial, antifungal, UV filter and antioxidant properties. Several chemical ways have been proposed for the CuONPs synthesis, which include the copper reaction with transfer alcohol, steam, precipitation method, hydrothermal synthesis, etc (De Jong and Borm, 2008; Shaabani et al., 2008; Budarin et al., 2008; Li et al., 2023). Recently, the concentration of nanoparticles using plants or the green synthesis of nanoparticles has been proposed as a new method that can be an alternative to the synthesis of nanoparticles by physical and chemical methods (Li et al., 2023; Veisi et al., 2021; Elkhenany et al., 2020; Borm et al., 2006; Stapleton and Nurkiewicz, 2014). This method is cost-effective and environmentally friendly and is easily performed without the use of toxic chemicals or the need for high-pressure energy and temperature. The use of this method is cost-effective due to the variety of climates and vegetation. By adding copper nanooxide particles to the polymers used in the packaging industry, the thermomechanical properties and the amount of water and gas penetration from the packaging surfaces can be changed to an optimal value. Also, because of the antibacterial effects of these nanoparticles, according to the recommendations of the Food and Drug Organization, they can be used in milk packaging and improve its lifespan and health (Trojer et al., 2013; Liu et al., 2014; Koyyati et al., 2016).
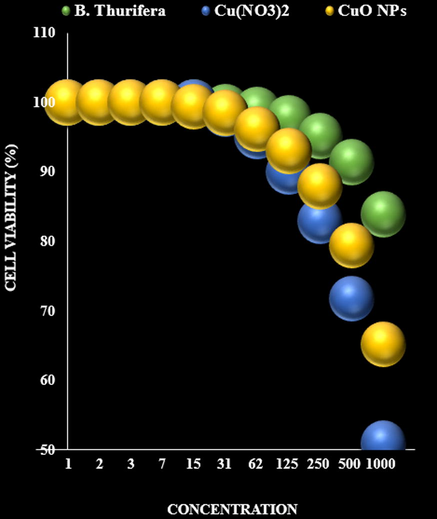
It appears that the anti-human nerve carcinoma effect of Cu nanoparticles against common human carcinoma cell lines is due to their antioxidant effects. The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Cu nanoparticles. The viability of human nerve carcinoma cell lines (S462 Human Peripheral Nerve Sheath Tumor and BL1391) reduced in the presence of Cu nanoparticles. The IC50 of Cu nanoparticles were 163 and 245 µg/mL against S462 Human Peripheral Nerve Sheath Tumor and BL1391 cell lines, respectively (Fig. 7, Fig. 8, Table 1).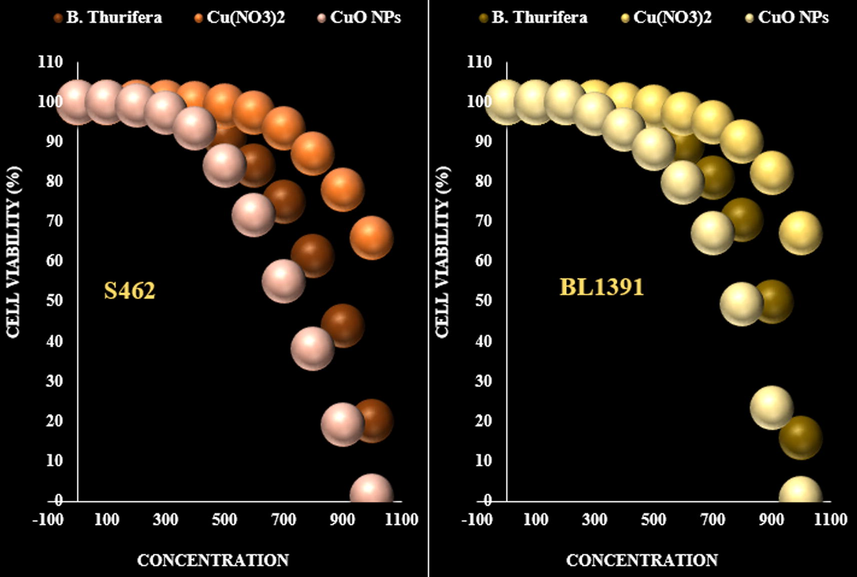
The anti-nerve carcinoma properties (Cell viability (%)) of B. Thurifera, Cu(NO3)2, and CuO NPs (Concentrations of 0–1000 µg/mL) against S462 Human Peripheral Nerve Sheath Tumor and BL1391.
The cytotoxicity effects of B. Thurifera, Cu(NO3)2, and CuO NPs against normal (HUVEC) cell line.
B. Thurifera (µg/mL)
Cu(NO3)2 (µg/mL)
CuO NPs (µg/mL)
IC50 against HUVEC
–
–
–
IC50 against S462
413 ± 7b
–
163 ± 3a
IC50 against BL1391
500 ± 6b
–
245 ± 3a
In the antioxidant test, the IC50 of B. Thurifera, CuO NPs, and BHT against DPPH free radicals were 334, 143, and 127 µg/mL, respectively (Fig. 9, Table 2).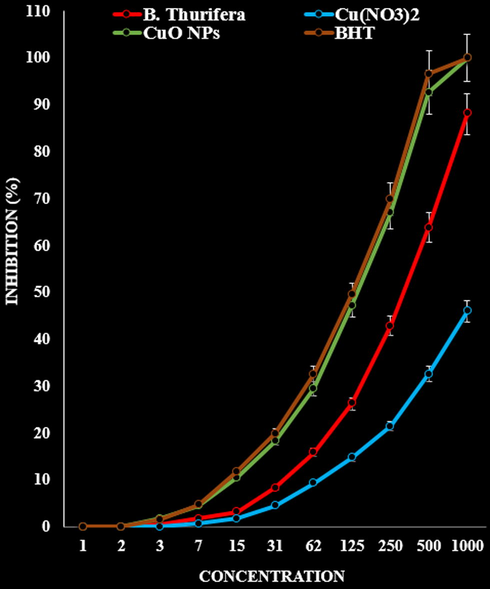
The antioxidant properties of B. Thurifera, Cu(NO3)2, CuO NPs, and BHT against DPPH.
B. Thurifera (µg/mL)
Cu(NO3)2 (µg/mL)
CuO NPs (µg/mL)
BHT (µg/mL)
IC50 against DPPH
334 ± 6b
–
143 ± 3a
127 ± 4a
Oxidation is a destructive process that causes nutritional value loss and chemical composition changes. Oils and fats are very prone to oxidation. Oxidation in fats and oils makes them spicy. In addition, the products obtained from the oxidation of lipids can hurt other components in the food (Onwudiwe et al., 2020; Radini et al., 2018; Beheshtkhoo et al., 2018). So that in addition to adverse food products organoleptic effects, by destroying essential vitamins and fatty acids of the body and creating toxic compounds, they can cause various side effects. The toxic effects of artificial antioxidants on the one hand and consumers' acceptance of natural additives on the other have increased the desire to use natural antioxidants. These antioxidants are polyphenolic compounds found in all plants and all their parts such as leaves, stems, fruits, roots, seeds, etc (Beheshtkhoo et al., 2018; Xinli, 2012). The protective effects of fruits and vegetables against chronic diseases are partly attributed to the antioxidants present in this category of food. Today, natural antioxidants obtained from plants and spices are widely evaluated for their antioxidant properties (Koyyati et al., 2016; Beheshtkhoo et al., 2018). Recently, the medicinal plants have been used for synthesizing the metallic nanoparticles due to their high therapeutic effects. The pollution created during the NPs biosynthesis by plant extract is almost zero. As a result, the herbal nanoparticles has very low environmental effects. But, the production speed, quality and other properties of metallic NPs depend on many factors such as the extract concentration, metal salt concentration, pH, temperature, reaction time, and plant extract nature (Radini et al., 2018; Beheshtkhoo et al., 2018; Xinli, 2012).
Nanotechnology is a branch of material science, recognized as one of the key technologies of the future. Nanomaterials are used in many different parts of human life and can provide solutions for technological and environmental challenges. These materials have unique properties due to their particle size used in many fields of science such as medicine, technology and engineering (including electronics, environmental measurement, Raman spectroscopy of bacterial surface, antibacterial agent, drug delivery and water purification) (Onwudiwe et al., 2020; Radini et al., 2018; Beheshtkhoo et al., 2018; Xinli, 2012). The application of nanomaterials in medicine, especially in drug delivery, has increased in the last decade. However, some nanomaterials have harmful effects, the most important of which is the poisoning of humans and animals (Xinli, 2012; Li and G f., 2014; Mohammed et al., 2016; Gao et al., 2015; Flavin, 2007). For example, zinc oxide nanoparticles are liver function disorder and silver nanotoxicity in zebrafish embryos. In addition, this toxicity can directly harm human health, which hurts reproduction and fetal development. These factors have done a research to develop non-toxic nanomaterial production methods (Mohammed et al., 2016; Gao et al., 2015; Flavin, 2007). Therefore, to minimize waste products use and maximize the chemical processes efficiency, the basic principles of green chemistry should be adopted. Therefore, any chemical process or synthetic pathway must meet these principles with environmentally non-toxic chemicals and benign solvents (Beheshtkhoo et al., 2018; Xinli, 2012; Li and G f., 2014; Mohammed et al., 2016).
Boswellia thurifera may help fight certain cancers. Bosolic acids in Boswellia thurifera prevent the spread of cancer cells. Boswellia thurifera oil can also distinguish cancer cells from normal cells and destroy only cancer cells. Boswellia thurifera essential oil contains a chemical compound called AKBA, which is successful in killing cancer cells that eventually become resistant to chemotherapy. This could be a major breakthrough in the long search for a cancer cure (Kulkarni et al., 1991; Gupta et al., 1998; Tsukada et al., 1986; Huang et al., 2000; Sharma et al., 2009). According to studies, Boswellia thurifera can fight pancreatic, prostate, breast, colon and skin cancer cells, meaning it can prevent the spread of cancerous masses. It can also help decrease the cancer treatment side effects. 60% of people who are treated for brain tumors consume 4.2 g of Boswellia thurifera daily, and Boswellia thurifera in these cases helps reduce brain swelling and fluid accumulation in the brain. Boswellia thurifera can boost thyroid function, and at the same time, its gogelsterones can help with weight loss (Tsukada et al., 1986; Huang et al., 2000; Sharma et al., 2009; Singh and Atal, 1986). These goglesterones help control cholesterol levels, reduce arthritis symptoms, and remove toxins from the body. Boswellia thurifera also has anti-inflammatory properties and can be effective for people with certain diseases who have to use hormonal drugs such as Corton, Leothroxine, etc (Borrelli et al., 2006; Kimmatkar et al., 2003; Kulkarni et al., 1991; Gupta et al., 1998). In general, it can be said that the consumption of oral Boswellia thurifera balances hormones, especially thyroid hormones. It increases the body's metabolism, helps to detoxify and cleanse the body, especially the intestines, and by increasing fat burning, it causes slimming and weight loss (Sailer et al., 1996; Gerhardt et al., 2001; Krieglstein et al., 2001).
In various clinical and cellular studies, a powerful anticancer effect of Boswellia thurifera resin has been observed. Bosolic acids prevent the glioma cells proliferation by inducing apoptosis pathways in these cancer cells and show an anti-edema effect in glioblastoma patients (Tsukada et al., 1986). In a study, Boswellia thurifera resin alcohol extract inhibited tumor growth in mice by disrupting the biosynthesis of RNA, DNA and protein (Huang et al., 2000). Also, in another study, the effect of Boswellia thurifera on the breast cancer cells metastasis and tumor formation in the brain was investigated. Since the arachidonite lipoxygenase pathway is in the growth of brain tumors and Boswellia thurifera is a lipoxygenase inhibitor, brain metastases that did not respond to common treatments were cured by using Boswellia thurifera (Gao et al., 2015). In another study, it has been found that AKBA exerts its anticancer effect by inhibiting the genes expression regulated by the transcription factor NF-kappaB (Flavin, 2007). Research shows that bosolic acids and triterpenoids of Bosulia serrata have apoptotic and inhibitory effects against the growth of 60-HL human leukemia cells (Takada et al., 2006; Shao et al., 1998). Bosolic acids induce apoptosis by caspase 8-dependent pathway in 29HT-colon cancer cells and have cytotoxic properties against malignant glioma (Bhushan et al., 2007). Among the derivatives of bosolic acid, acetyl 11-keto-beta bosolic acid (AKBA) has the property of inhibiting tumor growth (Park et al., 2002) and induces apoptosis in meningioma cells (Hoernlein et al., 1999). AKBA consumption inhibits prostate tumor growth in mice by preventing angiogenesis induced by vascular endothelial growth factor (Liu et al., 2002). AKBA has been shown to inhibit STAT3 activity. Activation of STAT3 pathway plays a role in survival, proliferation of drug resistance and angiogenesis of cancer cells, and the inhibitor of this pathway can be used as a cancer therapy (Pang et al., 2009).
As revealed in Fig. 10, our findings presents that Cu nanoparticles induce a notable cell cycle arrest at the S phase, with a significant decrease in G0/G1 and G2/M cancer cell lines phases, indicating cell-cycle progression inhibition under the effect of Cu nanoparticles.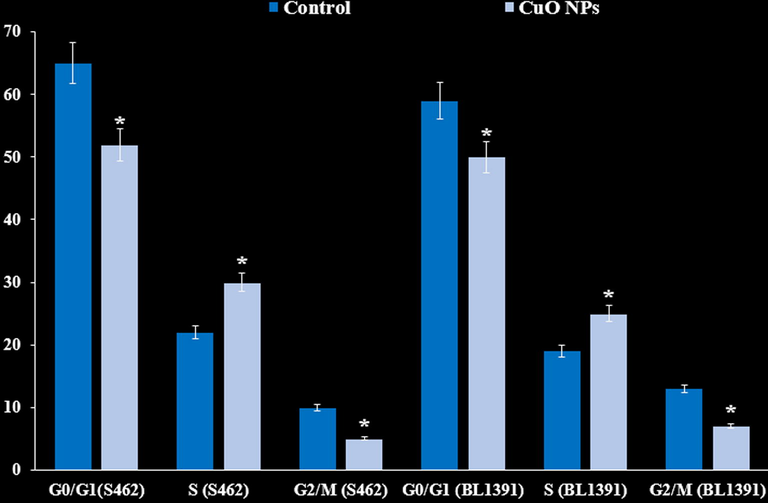
Cell cycle flow cytometry analysis (%) of the cancer cell lines.
Our cell apoptosis findings reveals that Cu nanoparticles remarkably induces early and late apoptosis in nerve cancer cell lines (Fig. 11).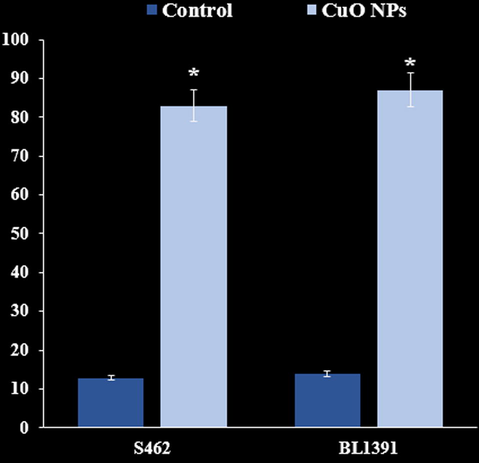
Induction of apoptosis (%).
As presented in Fig. 12, our colony formation findings reveal a notable reduce in the colonies number for cancer cell lines under the colony formation effect. These data confirm colony-forming ability loss in cancer cells upon treatment with Cu nanoparticles, which may influence the ability to inhibit tumorigenesis in vivo.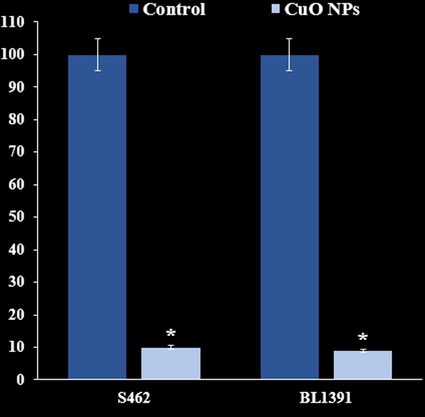
The effect of CuO NPs on colony formation (%).
Western blot analysis results revealed a notable increase in the pro-apoptotic markers expression (Bax and Cleaved caspase-8) in Cu nanoparticles-treated cells (Fig. 13). Also, the Bcl-2 (anti-apoptotic protein) expression was reduced in cancer cell lines. Bax/Bcl-2 ratio was enhanced profoundly in cells treated with Cu nanoparticles.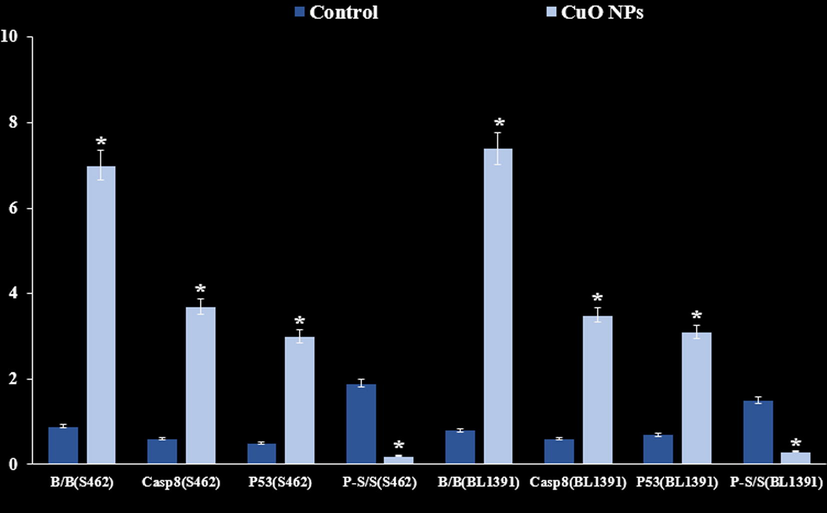
Outcome of CuO NPs on the expression patterns of Bax, BCL-2, Caspase-8, P53 and STAT3.
Also, we evaluated the STAT3, p53 and p-STAT-3 expression pattern in nerve cancer cells exposed to Cu nanoparticles. We found that treatment with Cu nanoparticles stimulates the p53 expression pattern whereas, Cu nanoparticles prevents the STAT3 expression and phosphorylation in cancer cells.
5 Conclusion
In conclusion, the Cu nanoparticles green-synthesis were carried out using the Boswellia thurifera extract. The Cu nanoparticles was assessed in biological applications like cytotoxicity and anticancer activities against common human nerve carcinoma cells. The carcinoma cell lines viability decreased in the Cu nanoparticles presence. The nanoparticles were characterized using XRD, FT-IR, EDX, UV–Visible, TEM, FE-SEM techniques. The outputs of the present study confirm the formation of Cu nanoparticles as Cu with spherical morphology. To confirm the cytotoxic power of the effective substance of this plant and the metal nanoparticles obtained from it and possibly their medicinal use, more and additional research is needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis of cobalt nanoparticles by using methanol extract of plant leaf as reducing agent. Pure Appl. Biol.. 2016;5:453-457.
- [Google Scholar]
- Chemical characterization and anti-hemolytic anemia potentials of tin nanoparticles synthesized by a green approach for bioremediation applications. Appl. Organomet. Chem.. 2020;34(3):e5433.
- [Google Scholar]
- Evaluation cytotoxicity effects of biosynthesized zinc oxide nanoparticles using aqueous Linum Usitatissimum extract and investigation of their photocatalytic activityackn. Inorg. Chem. Commun.. 2020;119:108066
- [Google Scholar]
- Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta Med.. 1991;57:203-207.
- [Google Scholar]
- Antioxidant activity of natural resins and bioactive triterpenes in oil substrate. Food Chem.. 2005;92:721-727.
- [Google Scholar]
- Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem.. 2018;32:e4057.
- [Google Scholar]
- Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl. Phys. A. 2018;124:363-369.
- [Google Scholar]
- Empowering effect of Frankincense extract on memory. Tarbiat Moalem University J. of Sci. 2001:1-14.
- [Google Scholar]
- A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis. 2007;12:1911-1926.
- [Google Scholar]
- The potential risks of nanomaterials: a review carried out for ECETOC. Particle Fibre Toxicol.. 2006;3(1):11.
- [Google Scholar]
- Effect of Boswellia serrata on intestinal motility in rodents: inhibition of diarrhea without constipation. Br. J. Pharmacol.. 2006;148:553-560.
- [Google Scholar]
- Planning and developing population-based cancer registration in low-and middle-income settings. IARC (International Agency for Research on Cancer) 2014
- [Google Scholar]
- Green Chem.. 2008;10:382-387.
- Chaghmini, M., Qanunche fI al-Tibb, Tehran: Iran University of Medical Sciences; 2010. (in Persian).
- Lignans from linseed (Linum usitatissimum L.) and its allied species: retrospect, introspect and prospect. Critical Rev. Food Sci. Nutr.. 2021;61(16):2719-2741.
- [Google Scholar]
- Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomed.. 2008;3(2):133-149.
- [Google Scholar]
- Ebn Rabban eTabari. Ferdos-al-Hekmah (Paradise of wisdom), 2003. Beirut: Dar-al-kotob-al-Ilmyah.
- Int. J. Biol. Macromol.. 2020;143:763-774.
- An investigation of the growth inhibitory capacity of several medicinal plants from Iran on tumor cell lines. Iranian Journal of. Cancer Prev.. 2015 Oct;8(5)
- [Google Scholar]
- A lipoxygenase inhibitor in breast cancer brain metastases. J. Neurooncol. 2007;82:91-93.
- [Google Scholar]
- Liposome encapsulated of temozolomide for the treatment of glioma tumor: preparation, characterization and evaluation. Drug Discov. Ther.. 2015;9(3):205-212.
- [Google Scholar]
- Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Gastroenterol.. 2001;39:11-17.
- [Google Scholar]
- Ghasemidehkordi, N., 2002. Iranian Herbal Pharmacopoeia. First edition. Ministry of health and medical education, Tehran, pp: 647 - 54.
- Synthesis of nanocrystalline cobalt ferrite through soft chemistry methods: a green chemistry approach using sesame seed extract. Mater. Chem. Phys.. 2016;182:219-230.
- [Google Scholar]
- Flax (Linum usitatissimum L.) fibers for composite reinforcement: exploring the link between plant growth, cell walls development, and fiber properties. Frontiers. Plant Sci.. 2019;10:411.
- [Google Scholar]
- Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res.. 1997;2:37-43.
- [Google Scholar]
- Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res.. 1998;3:511-514.
- [Google Scholar]
- Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med.. 2001;67:391-395.
- [Google Scholar]
- Cancer word concepts in medical resources of ancestors. Med Refinement.. 2008;69:51-62.
- [Google Scholar]
- Mashhad: Mashhad University of Medical Sciences; 1992. p. :602.
- Acetyl-11-keto-beta-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther.. 1999;288:613-619.
- [Google Scholar]
- Genetic diversity analysis of a flax (Linum usitatissimum L.) global collection. BMC Genomics. 2020;21(1):1-13.
- [Google Scholar]
- Cytostatic and apoptotsis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res.. 2002;22:2853-2862.
- [Google Scholar]
- Anti-tumor and anti-carcinogenic activities of triterpenoid, betaboswellic acid. Biofactors. 2000;13:225-230.
- [Google Scholar]
- siRNA Conjugated Nanoparticles-A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci.. 2019;20(23):6088.
- [Google Scholar]
- Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee – a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3-7.
- [Google Scholar]
- Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int. J. PharmTech. Res.. 2016;9:466-472.
- [Google Scholar]
- Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int. J. PharmTech Res.. 2016;9(3):466-472.
- [Google Scholar]
- Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal Dis.. 2001;16:88-95.
- [Google Scholar]
- HPTLC analysis of anti-inflammatory triterpenoids from Boswellia serrata Roxb. Phytochem. Anal. 2001;12:374-376.
- [Google Scholar]
- Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J. Ethnopharmacol.. 1991;33:91-95.
- [Google Scholar]
- Treatment of osteoarthritis with a herbomineral formulation:a double- blind, placebo-controlled, crossover study. J. Ethnopharmacol.. 1991;33:91-99.
- [Google Scholar]
- Recent progress in doxorubicin nano-drug delivery systems for reserving multidrug resisitance. Anti-infection Pharm.. 2014;11(3):177-181.
- [Google Scholar]
- A new formulation of Ni/Zn bi-metallic nanocomposite and evaluation of its applications for pollution removal, photocatalytic, electrochemical sensing, and anti-breast cancer. Environ. Res.. 2023;233:116462
- [Google Scholar]
- Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J. Liposome Res.. 2014;24(1):17-26.
- [Google Scholar]
- Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23:2087-2093.
- [Google Scholar]
- Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4- nitrophenol and performance as anti-colorectal carcinoma. J. Mater. Res. Technol.. 2021;12:1832-1843.
- [Google Scholar]
- Ziziphora clinopodioides Lam leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl. Organomet. Chem.. 2019;33:e5164.
- [Google Scholar]
- Green synthesis of NiONPs using Trigonella subenervis extract and its applications as a highly efficient electrochemical sensor, catalyst, and antibacterial agent. Appl. Organomet. Chem.. 2021;36:e6264.
- [Google Scholar]
- Green synthesis of cobalt (II, III) oxide nanoparticles using Moringa oleifera natural extract as high electrochemical electrode for supercapacitors, içinde. AIP Conf. Proc. 2018
- [Google Scholar]
- Transdermal delivery of vancomycin hydrochloride using combination of nano-ethosomes and iontophoresis: in vitro and in vivo study. Drug Deliv.. 2016;23(5):1558-1564.
- [Google Scholar]
- Medical treatment of cancer in traditional Iranian medicine. J. Islamic Iranian Traditional Med.. 2012;1:3-18.
- [Google Scholar]
- Eco-friendly synthesis, structural properties and morphology of cobalt hydroxide and cobalt oxide nanoparticles using extract of Litchi chinensis. Nano-Struct. Nano-Objects. 2020;23:100470
- [Google Scholar]
- Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res.. 2009;69:5893-5900.
- [Google Scholar]
- Cytotoxic action of acetyl-acetyl 11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta Med.. 2002;68:397-401.
- [Google Scholar]
- Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol.. 2018;16:71.
- [Google Scholar]
- Boswellic acids: biological actions and molecular targets. Curr. Med. Chem.. 2006;13:3359-3369.
- [Google Scholar]
- Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol. B. 2018;183:154-163.
- [Google Scholar]
- Flax (Linum usitatissimum L.) fibre reinforced polymer composite materials: a review on preparation, properties and prospects. Prog. Mater Sci.. 2019;102:109-166.
- [Google Scholar]
- Linum usitatissimum L. seeds: flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym.. 2019;215:29-38.
- [Google Scholar]
- Studies on the metabolism of glycosaminoglycans under the influence of new herbal anti-inflammatory agents. Biochem. Pharmacol.. 1989;38:3527-3534.
- [Google Scholar]
- Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther.. 1992;261:1143-1146.
- [Google Scholar]
- Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther.. 1997;281:460-463.
- [Google Scholar]
- Acetyl-11-keto–boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Br. J. Pharmacol.. 1996;117:615-618.
- [Google Scholar]
- Flax (Linum usitatissimum L.): A potential candidate for phytoremediation? Biological and economical points of view. Plants. 2020;9(4):496.
- [Google Scholar]
- Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manage.. 2020;257:109994
- [Google Scholar]
- Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res.. 2020;27(5):5211-5221.
- [Google Scholar]
- Catal. Commun.. 2008;9:13-16.
- Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arab. J. Sci. Eng.. 2019;44(7):6435-6444.
- [Google Scholar]
- Estimation of boswellic acids from market formulations of Boswellia serrata extract and 11- keto -boswellic acid in human plasma by high-performance thin-layer chromatography. J. of Chromatography B. 2007;848:232-238.
- [Google Scholar]
- Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med.. 1998;64:328-331.
- [Google Scholar]
- Immunomodulatory activity of boswellic acids (Pentacyclic triterpene acids) from Boswellia serrata. Phytotherapy Res.. 1996;10:107-112.
- [Google Scholar]
- In vivo genotoxicity evaluation of a plantbased antiarthritic and anticancer therapeutic agent Boswelic acids in rodents. Phytomedicine 2009
- [Google Scholar]
- Bioactive constituents of myrrh and frankincense, two simultaneously prescribed gum resins in Chinese traditional medicine. Chem. Biodivers.. 2008;5:540-553.
- [Google Scholar]
- Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new nonsteroidal anti-inflammatory agent. Agents Actions. 1986;18:407-412.
- [Google Scholar]
- Phytochemicals as cell cycle modulators a less toxic approach in halting human cancers. Cell Cycle. 2002;1(3):155-160.
- [Google Scholar]
- history and treatment of cancer in traditional medicine. [Collection of articles about Persian medicine] Proc. Traditional Med. J. 1983
- [Google Scholar]
- Vascular distribution of nanomaterials. Wiley Interdisc. Rev. Nanomed Nanobiotechnol.. 2014;6(4):338-348.
- [Google Scholar]
- Influence of microwave roasting on chemical composition, oxidative stability and fatty acid composition of flaxseed (Linum usitatissimum L.) oil. Food Chem.. 2020;326:126974
- [Google Scholar]
- Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF- kappa B and NF-kappa B-regulated gene expression. J. Immunol.. 2006;176:3127-3140.
- [Google Scholar]
- Charged microcapsules for controlled release of hydrophobic actives Part II: surface modification by Lbl adsorption and lipid bilayer formation on properly anchored dispersant layers. J. Colloid Interface Sci.. 2013;409:8-17.
- [Google Scholar]
- Archidonate 5-lipoxygenase inhibitors show potent antiproliferative effects on human leukemia cells. Biochem. Biophys. Res. Commun.. 1986;140:812-816.
- [Google Scholar]
- Int. J. Biol. Macromol.. 2021;172:104-113.
- Applications of nanocarriers with tumor molecular targeted in chemotherapy. Chemistry. 2012;75(7):621-627.
- [Google Scholar]
- HPLC phenolic profile and induction of apoptosis by Linum usitatissimum extract in LNCaP cells by caspase3 and Bax pathways. AMB Express. 2020;10(1):1-11.
- [Google Scholar]







