Translate this page into:
Study of the chemical composition and evaluation of the antioxidant and antimicrobial activity of Taxus baccata L.
⁎Corresponding author. ouassimariffi95@gmail.com (Ouassima Riffi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This work focuses on the search for natural molecules with biological activity that can be used to find alternatives to synthetic products by studying the chemical composition and evaluating the antioxidant and antibacterial activity of the extract of Taxus baccata (L.). HPLC-UV chromatography was employed to determine its chemical composition, while the antioxidant activity was determined using DPPH radical scavenging method and cyclic voltammetry. The broth dilution method was used to achieve antibacterial activity against in total 14 different foodborne pathogenic bacteria, with 4 being Gram-positive and 10 being Gram-negative.
The results of the analysis of the extract studied by HPLC-UV made it possible to identify about ten phenolic compounds. Salicylic acid (18.8 µg/mg), gallic acid (5.45 µg/mg), quercitin (4.8 µg/mg), rutin (3.98 µg/mg), hydroxybenzoic acid (3.34 µg/ mg), caffeine (1 µg/mg), caffeic acid (0.267 µg/mg) and vanillin (0.258 µg/mg). the antioxidant activity showed an interesting antiradical activity, the voltammogram also shows an oxidation peak relating to the oxidation of the phenolic compounds contained in Taxus baccata (L.). The antibacterial activity results show significant antibacterial properties against Gram-positive and Gram-negative strains.
Keywords
Taxus baccata (L.)
HPLC-UV
Antioxidant activity
Cyclic voltammetry
Pathogenic bacteria
1 Introduction
The correlation that exists, from an epidemiological point of view, between high concentrations of naturally occurring antioxidants and the prevention of degenerative illnesses can be explained by the free radical scavenging capacity of these compounds (Ruiz-Terán et al., 2008). Among natural antioxidants, plants, foods, and medicinal plants are the most well-known. In developing countries, healthcare mainly primarily on medicinal plants and their commercial value is known to be prolific (Fioccardi et al., 2022; Chaachouay et al., 2019; Zubay et al., 2021). The drug producing companies have placed a higher value on medicinal plants in the quest for natural remedies and they are mainly used in the treat of various diseases (Ivanz, 2018; p B. Abubakar et al., 2022). The WHO has advised Folk Medicine as a reliable treatment for microbial and non-microbial illnesses and because of the wide variety of chemical compounds in medicinal plants, it has gained more attention in the last two decades (Atanasov, 2021). Furthermore, phenols, flavonoids, tannins, alkaloids, steroids and saponins are the major bio-active components of the plants and such metabolites differ in their structures as well as their properties (Nithya et al., 2018; Assaf et al., 2022).
The bioactive substances in plants are responsible for their various activities, and phytotherapy is considered to be the oldest health approach available to mankind (Asumang, 2021; Negm et al., 2022; Ralte et al., 2021). The quality of nutraceuticals and herbal medicines is usually related to its antioxidant capacity, which can be determined with spectrophotometric or electrochemical techniques (Chevion et al., Mar. 2000; Huang et al., Mar. 2005). In recent years, plant-based antimicrobials have received increasing attention as alternatives to antibiotics, due to their potency and their low propensity to develop resistance in bacteria. In addition, some natural products are reported to reinforce the action of common antibiotics with a synergistic action (Górniak et al., 2019).
Taxus baccata (L.) is a conifer species belonging to the family Taxaceae, because of its other substances with high physiological properties, it is also well known in pharmacology as well as in medicine. In the past, leaves have been used in the form of juice and tea for various medical conditions like asthma, bronchitis, rheumatism, and epilepsy. The seeds are surrounded by a red flashy structure called arils, which are mostly eaten by birds (Tabaszewska, 2021). The young shoots and berries are used to make a homoeopathic remedy. Cystitis, eruptions, headaches, heart and kidney disorders, rheumatism, and other infections are all treated with it.
The constituents of the Taxus genus, such as lignans, steroids and flavonoids and other phenolic constituents, have antibacterial and antifungal properties (Sharma et al., 2021; Lange and Conner, 2021; Maroso, 2021), enzyme inhibitory and antioxidant activity (Kucukboyacı et al., 2010); antiulcerogenic (Erdemoglu et al., 2004) and anticancer activity (Hamedi et al., 2022; Hao, 2021). Taxus baccata (L.) as a source of the anticancer agent paclitaxel has attracted considerable interest and has been extensively researched. Currently, it has been used to manufacture drugs to treat several types of cancer. Paclitaxel is also being clinically investigated for the treatment of a variety of other types of cancer in conjunction with other chemotherapeutic products. (Pullaiah et al., 2022; Saif, 2013).
In 2003, Küpeli et al. (Küpeli et al., 2003) used ethanol to extract toxoids (taxusin, baccatin VI, baccatin III, and 1-hydroxybaccatin I) and lignin (lariciresinol, taxiresinol, 30-demethylisolaricire sinol-90-hy droxyisopropylether, isolariciresinol, and 3-demethylisolariciresinol). T. baccata contain the majority of the alkaloids and can be isolated in large quantities, Taxine A and B make up the majority of the alkaloid. This species is also enriched with flavonoids, an essential component of medical, the composition of flavonols and biflavones vary with species to species. The examples of some of the flavonoids are the analogs of Quercetin, Myricetin, and Kaempferol (Sharma et al., 2021).
The objective of this study was therefore the valorisation of the plant Taxus baccata (L.), on one hand, study of the chemical composition and evaluation of the antioxidant power by the DPPH radical scavenging method and the electrochemical method. On the other hand, the evaluation of the antimicrobial activity.
2 Materials and methods
2.1 Plant collection
The leaves of Taxus baccata (L.) was collected in the IFRANE forest (33° 32′ North, 5° 06′ West) in Morocco.
This is a continuous solid–liquid extraction. 50 g of powder are introduced into a cellulose cartridge, which is placed in a soxhlet, topped by a refrigerator, which is connected to a 500 ml flask containing a sufficient quantity of organic solvent (Ethanol). With the help of a flask heater, the solvent is boiled for 3 h allowing the continuous extraction of the constituents contained in the powder.
2.2 Analysis by HPLC-UV chromatography
Analyses were performed on a Jasco LC-Net II/ ADC instrument, consisting of a PU- 2089 plus 2695, a Jasco UV-2070 plus detector, and a manual injector. An Agilent Zorbax Extend-C18 column (5 μm, 4.6 mm × 250 mm × 4.6 Col) was used. The extract at a concentration of 10 mg/ml was dissolved in ethanol and then filtered. Mobile phase consisted of two eluents, Formic acid-Ultra pure water (0.1%) (A) and Acetonitrile (B), elution started with 7% B and using a graduate to get 18% in 10 min, 20% at 25 min, 30% at 35 min, 50% at 50 min, 80% at 65 min and 100% at 80 min, at a rate of 0.7 ml/min. UV detection was performed at λ = 280 nm and the volume of injection was 20 μL. After each injection the analytical system was rinsed 30 min with a MeOH/ Acetonitrile /H2O (20/40/40) mixture. The HPLC system is controlled by the HPLC software ChromNAV 2.0 - JASCO.
Ten phenolic compounds were tested, including 7 phenolic acids (gallic acid, hydroxybenzoic acid, vanillic acid, caffeic acid, acetylsalicylic acid, P-coumaric acid and salicylic acid) and 4 flavonoids (rutin, quercetin, caffeine, vanillin).
2.3 Antioxidant activities
2.3.1 The DPPH method
To 0.1 ml of the extract 3.9 ml of DPPH (2.4 mg in 100 ml of methanol) was added, the solutions were incubated in the dark for 30 min. the reading was taken at 515 nm (Benzidia, 2019).
The DPPH inhibition percentage is calculated from the following equation:
Acontrol: Absorption of the solution containing only the DPPH solution.
Asimple: Absorption of the test sample solution in the presence of DPPH.
2.3.2 Electrochemical method: Cyclic voltametry
Cyclic voltammetry was performed using a potentiostat–galvanostat-OGF01A controlled by Origamaster 5 software. A three-electrode thermostatised electrolysis cell was used, with a platinum electrode as the working electrode, a Hg|Hg2Cl2|KClsat electrode as the reference electrode and a Pt electrode as the counter electrode. The potential was scanned from 200 to + 1000 mV with a sweep time of 500 mV/s. Electrolyte solution consisted of phosphate ions (KH2PO4 and K2HPO4 0.1 M, pH = 7). Before each test, the solutions were deaerated by bubbling nitrogen for a few minutes. The voltammograms presented are the average of 4 to 5 measurements.
The antioxidant capacity of the Taxus baccata (L.) fractions studied was determined from the current density of the anodic voltammogram graph. The calibration curve is obtained by plotting the current density of the anodic voltammogram curve of ascorbic acid against its concentration (0,05–0,6 g/L).The total antioxidant activity AAT is calculated from the following relationship:
C: Concentration of the extract in the electrochemical cell,
Ceq: Equivalent concentration of ascorbic acid (Benchikha et al., 2014; Rebiai and Lanez, 2014).
2.4 Antibacterial activity
The antimicrobial activity of Taxus baccata (L.) leaves was carried out at the Laboratory of Food Microbiology of the Department of Veterinary Sciences of the University of Messina in Italy on a total of 14 different foodborne pathogenic bacteria (4 Gram-positive and 10 Gram-negative).
All cultures used were prepared by inoculating a bead of frozen cryovial stock (−80 °C) in Brain Heart Infusion broth (BHIb; Biolife, Milan, Italy) and kept at 37 °C for 20 h, except for Aeromonas strains that were incubated at 30 °C for 20 h (See Table 1).
Gram
Strain
Abbreviation
Positive
Listeria monocytogenes ATCC 13932
L.monocytogenes 13,932
Listeria monocytogenes ATCC 7644
L. monocytogenes 7644
Staphylococcus aureus ATCC 6538
S. aureus 6538
Staphylococcus aureus ATCC 25923
S.aureus 25,923
Negative
Salmonella enterica subsp. enterica serovar Enteritidis
ATCC 13076
S. enteritidis 13,076
Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028
S. serovar 14,028
Escherichia coliATCC 8739
E. coli 8739
Escherichia coliATCC 35218
E. coli 35,218
Pseudomonas aeruginosa ATCC 27853
P. aeruginosa 27,853
Vibrio parahaemolyticus ATCC 17802
V. parahaemolyticus 17,802
Yersinia enterocolitica subsp. enterocolitica
ATCC 23715
Y. enterocolitica 23,715
Yersinia enterocolitica subsp. enterocolitica
ATCC 9610
Y. enterocolitica 9610
Aeromonas hydrophilaATCC 7966
A. hydrophila 7966
Aeromonas hydrophilaATCC 35654
A. hydrophila 35,654
2.4.1 Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) (microdilution method)
The determination of MIC and BMC was performed according to the microdilution method in liquid Mueller-Hinton medium (MH) using microtubes (Bouhdid and Abrini, 2009).
Indeed, from a stock solution of methanolic extract (10 mg/ml solubilized in DMSO) a series of dilutions was prepared to obtain the following concentrations (0.0001 mg/ml, 0.0005 mg/ml, 0.001 mg/ml, 0.005 mg/ml, 0.01 mg/ml, 0. 05 mg/ml, 0.1 mg/ml, 0.5 mg/ml, 1 mg/ml, 1.5 mg/ml, and 2 mg/ml), these were added aseptically to a series of microtubes containing 140 µL of BHI and 20 µL of 0.5 Mc farland bacterial suspension broth (2.106 CFU/ml). For each bacterium and extract tested, a positive control (without plant extract) and a blank (without bacteria) were prepared. The inoculated microtubes were closed and kept at 37 °C during 24 h for all bacteria tested, except for Aeromonas strains which were incubated at 30 °C for 24 h.
Afterwards, 40 µL of triphenyl-2,3,5-tetrazolium chloride (tetrazolium red) of concentration 0.2 g/mL was inserted. Subsequently, the microtubes were incubated again at 37 °C for 30 min (for Aeromonas strains, the incubation was performed at 30 °C). Four suggestions can be obtained:
-
Clear solution without pink coloration: The bacteria could not multiply and died.
-
Clear solution with pink coloration: the bacteria could not multiply but are alive.
-
Cloudy solution without pink coloration: The bacteria could have multiplied but died.
-
Cloudy solution with pink coloration: Bacteria could have multiplied and are alive.
3 Results and discussion
3.1 Analysis of the ethanolic extract of Taxus baccata (L.) by (HPLC-UV)
The chromatographic profiles of the ethanolic extract of Taxus baccata (L.) analysed is shown in Fig. 1, while the results of analyses are given in Table 2.
Chromatographic of Taxus baccata (L.) extract 1: Gallic acid; 2: Hydroxybenzoic acid; 3: Caffeic acid; 6: Acetylsalicylic acid; 8: Rutin; 9: Quercetin; 10: caffeine; 11: Vanillin.
Phenolic compounds
RT (min)
Taxus baccata (L.) (µg/mg)
1
Gallic acid
8.992
5.45
2
Hydroxybenzoic acide
14.8
3.34
3
Caféic acide
20.625
0.267
4
Vanillic acide
19.467
–
5
P-coumaric acide
26.458
–
6
Acétylsalicylic acide
30.7
18.8
7
Salicylique acide
34.858
–
8
Rutin
31.975
3.98
9
Quercétin
49.383
4.8
10
Caféine
20.233
1
11
Vanillin
25.442
0.258
Analysis of the spectrum of the Taxus baccata (L.) extract with those of the different standards made it possible to suspect the presence of 8 phenolic compounds (Table 2).
The analyses showed the existence of gallic acid (5.45 µg/mg), hydroxybenzoic acid (3.34 µg/mg), acid caffeic acid (0.267 µg/mg), acetylsalicylic acid (18.8 µg/mg), rutin (3.98 µg/mg), quercetin (4.8 µg/mg), caffeine (1 µg/mg) and vanillin (0.258 µg/mg), while vanillic acid, P-coumaric acid and salicylic acid are not detected. Studies have been done on the chemical composition of Taxus canadensis by LC/MSD have identified six compounds as p-hydroxybenzoic acid (1), putraflavone (2), sequoiavone (3), 7, 13-dideacetyl-9, 10-debenzoyltaxchinin C (4), baccatin VI (5) and taxumairone A (6) (Park et al., Jun. 2000).
3.2 Antioxidant activity
3.2.1 The DPPH method
Measurements of the inhibition of absorbance of the DPPH• radical caused by the presence of ascorbic acid (as a powerful natural antioxidant) or by the presence of Taxus baccata (L.) made it possible to plot the curves shown in Fig. 2. Table 3 groups the values of the IC50 indices for ascorbic acid and the extract studied.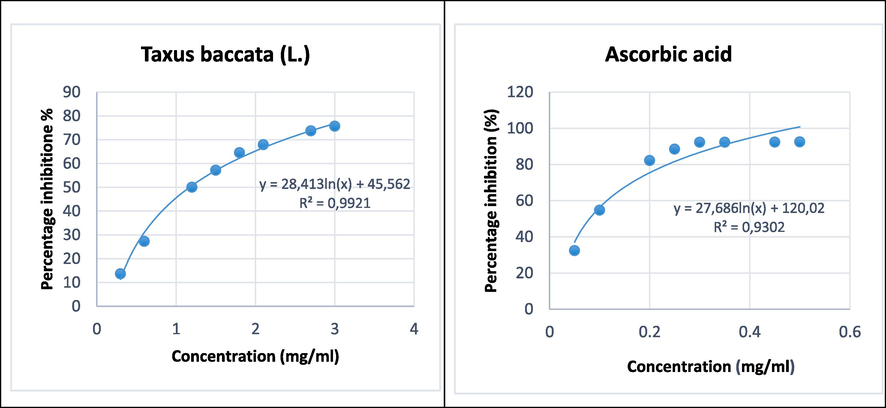
Percentage inhibition of ascorbic acid and Taxus baccata (L.).
IC50 (mg/ml)
Taxus baccata (L.)
1,17
Ascorbic acid
0,08
The values of the IC50 inhibitory concentrations obtained (Table 3) make it possible to classify the DPPH• radical scavenging capacity of the extract tested in relation to ascorbic acid. We found that extract of Taxus baccata (L.) exhibits significant antioxidant activity (IC50: 1.17 mg/mL).
The antioxidant power being inversely proportional to the value of the IC50 inhibitory concentration. Ascorbic acid having an inhibitory concentration IC50 (0.08 mg/mL), then has the greatest antiradical activity by comparison with Taxus baccata (L.).
The antioxidant activity of Taxus baccata (L.) has been studied in a number of works, the results obtained show that they have a significant antioxidant potential and can replace synthetic antioxidants.
Extracts of Taxus baccata (L.) (methanol, acetone and aqueous) were assayed for their capacity to scavenge free radicals DPPH test. They observed that all extracts have a strong capacity. In particular, the methanolic leaf extract showed the greatest DPPH scavenging activity (78.40%) at 100 µg/mL (Prakash et al., 2018).
A study done on the antioxidant activity of methanolic extracts (80%) of 26 medicinal plants from northwestern Himalayas. The results showed that the scavenging efficiency measured by DPPH- radical scavenging test varied from 0.18 to 7.69 and the average was 0.56. The greatest free radical scavenging efficiency was found in Taxus baccata (L.) (IC50 = 0.18 mg/mL) (Guleria et al., 2013).
There is not a large difference between the IC50 found experimentally and that found in the literature, and this can be explained by the sampling site, the extraction method, the solvent and the harvesting period.
3.2.2 Cyclic voltammetry method
In order to express the antioxidant capacity of of Taxus bacccata (L.) in equivalent terms of antioxidant capacity equivalent to that of ascorbic acid. The calibration curve was drawn and presented in supplemental.
Under the same previous conditions (pH = 7 and V = 500 mV/s) applied to ascorbic acid, extract of our plant was treated. The cyclic voltammogram obtained is shown in Fig. 3.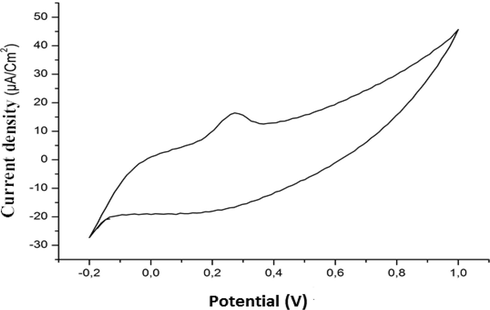
Cyclic voltammograms of Taxus baccata (L.).
The total antioxidant activity (AAT) is expressed in (mg/g), this parameter is used to present the results. It defines the concentration of phenolic compounds contained in mg in 1 g of dry extract equivalent as standard (ascorbic acid). Table 4 represents the values TAA of Taxus baccata (L.).
Taxus baccata (L.)
TAA (mg Eq AAs/g)
197.45
The results obtained show that the extract of Taxus baccata (L.) behaves like a reducer (a single “anodic” going peak and absence of a “cathodic” return peak), and the oxidation of this extract in these conditions is completely irreversible.
It is clearly noted that the ethanolic extracts of Taxus baccata (L.) have significant antioxidant activity. The explanation that can be given in this case is that the extract of Taxus baccata (L.) contains molecules with the highest concentrations of polyphenols and flavonoids.
Analysisof the antioxidant capacity of Origanum majorana and Origanum vulgare was made from electrochemical experiments (cyclic voltammetry), the results obtained showed that the extract of O. majorana has the greater antioxidant capacity (292.97 mg/g) than O. vulgare (163.64 mg/g) (Benchikha et al., 2014).
TAA of Limoniastrum feei by electrochemical method was studied, the results showed an irreversible oxidation peak at about 300–320 mV/(Ag/AgCl) and its equivalent capacity of 112.4 ± 1.97 μg per 1 mg expressed as ascorbic acid equivalent (Keffous et al., 2016).
3.3 Antibacterial activity of Taxus baccata (L.)
The antimicrobial activity of extract of the leaves of Taxus baccata (L.) was evaluated against Gram positive (L.monocytogenes 13932, L. monocytogenes 7644, S. aureus 6538, S.aureus 25923) and Gram negative strains. (S. enteritidis 13076, S. serovar 14028, E. coli 8739, E. coli 35218, P. aeruginosa 27853, V. parahaemolyticus 17802, Y. enterocolitica 23715, Y. enterocolitica 9610, A. hydrophila 7966, A. hydrophila 35654). The results obtained for the antibacterial activity are grouped in Tables 5 and 6.
Bacterial strains
Gram positiveTaxus baccata (L.)
MIC (mg/mL)
MBC
(mg/mL)MBC/MIC
L.monocytogenes 13,932
0.01
0.01
1
L.monocytogenes 7644
0.01
0.01
1
S. aureus 6538
0.01
0.05
5
S.aureus 25,923
0.01
0.05
5
Bacterial strains
Gram négativeTaxus baccata (L.)
MIC (mg/mL)
MBC (mg/mL)
MBC/CIMI
S. enteritidis 13,076
0.01
0.1
10
S. serovar 14,028
0.05
0.1
2
E. coli 8739
0.05
0.05
1
E. coli 35,218
0.05
0.05
1
P. aeruginosa 27,853
0.5
1
2
V. parahaemolyticus 17,802
0.5
1
2
Y. enterocolitica 23,715
0.01
0.01
1
Y. enterocolitica 9610
0.01
0.01
1
A. hydrophila 7966
0.1
0.5
5
A. hydrophila 35,654
0.1
0.5
5
The results presented in Tables 5 and 6 clearly showed that all the strains tested are sensitive to extract, the minimum inhibitory concentrations obtained vary according to the bacterium treated. They are around 0.01 mg/mL for gram-positive bacterial strains and between 0.01 and 0.5 mg/mL for gram-negative bacterial strains. Moreover, these bacteria were killed at concentrations ranging from 0.01 and 0.05 mg/mL for gram-positive bacteria, from 0.01 and 1 mg/mL for gram-negative ones.
With regard to the MBC/MIC activity ratio, the extract of Taxus baccata (L.) tested shows a bactericidal effect against all the bacterial strains except for the strains of S. aureus, Y. enterocolitica and A. hydrophila which have a bacteriostatic effect.
Researchers worked on ethanolic extracts of several plants, including Taxus baccata (L.). They showed that Taxus baccata (L.) was the plant with the best efficacy in inhibiting Candida albicans, Bacillus cereus and Pseudomonas aeruginosa (Fazeli-Nasab et al., 2021).
Another study that was conducted by (Zazharskyi et al., 2019) on 50 species of plants (seeds, grass, shoots, leaves, compound fruit, bark). This study analysed the efficacy of these plants on six bacteria and a fungus. Taxus baccata (L.) showed sensitivity against E. coli, C. xerosis, C. albicans strains with a wide zone of inhibition varying between 1.3 and 3.1 mm.
A study carried out on the evaluation of the antimicrobial activity of the heartwood of Taxus baccata (L.). This activity was tested against eight bacteria. Taxus baccata (L.) showed significant activity at a concentration of 2 mg/mL against certain bacteria compared to ampicillin and tobramycin, determining their zones of inhibition at 7, 5.5 and 8.5 mm respectively, while it showed no activity against the Gram-positive bacteria tested (Kucukboyaci and Sener, 2010).
4 Conclusion
The results of the qualitative and quantitative analysis of Taxus baccata (L.) by HPLC-UV made it possible to identify ten phenolic compounds. Salicylic acid (18.8 µg/ml), gallic acid (5.45 µg/ml), quercitin (4.8 µg/ml), rutin (3.98 µg/ml), hydroxybenzoic acid (3.34 µg/ml), caffeine (1 µg/ml), caffeic acid (0.267 µg/ml) and vanillin (0.258 µg/ml). The antioxidant activity revealed that the extract of Taxus baccata (L.) can be used as an antioxidant and in the fields: agri-food and pharmaco-medical. The antimicrobial activity of Taxus baccata (L.) has been proven to be rich in flavonoids and lignans, suggesting its use as an antibiotic or an alternative to synthetic molecules.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Traditional medicinal plants used for treating emerging and re-emerging viral diseases in northern Nigeria. Eur. J. Integr. Med.. 2022;49
- [CrossRef] [Google Scholar]
- Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. South African J. Bot.. 2022;144:480-493.
- [CrossRef] [Google Scholar]
- Antimicrobial, antioxidant and wound healing activities of methanol leaf extract of Bridelia micrantha (Hochst.) Baill. Sci. African. 2021;14:e00980.
- [Google Scholar]
- Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov.. 2021;20(3):200-216.
- [CrossRef] [Google Scholar]
- Innovare academic sciences in vitro evaluation of antioxidant capacity of algerian origanum plant by spectrophotometrical and electrochemical assays. Int. J. Pharm. Sci 2014:62-65.
- [Google Scholar]
- Chemical composition and antioxidant activity of tannins extract from green rind of Aloe vera (L.) Burm. F. J. King Saud Univ. - Sci.. 2019;31(4):1175-1181.
- [CrossRef] [Google Scholar]
- Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol.. 2009;106(5):1558-1568.
- [CrossRef] [Google Scholar]
- Ethnobotanical and ethnomedicinal study of medicinal and aromatic plants used against dermatological diseases by the people of Rif, Morocco. J. Herb. Med.. 2022;32(November 2019):100542
- [CrossRef] [Google Scholar]
- The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radical Biol. Med.. 2000;28(6):860-870.
- [CrossRef] [Google Scholar]
- Bioactivity of Lignans from Taxus baccata. Zeitschrift für Naturforschung. Section C. 2004;59(7–8):494-498.
- [CrossRef] [Google Scholar]
- Evaluation of antimicrobial activity of some medicinal plants on human standard bacteria and Candida albicans. Gene, Cell Tissue. 2021;8(3):e113092.
- [Google Scholar]
- First phytochemical study of six tree and shrub species with high health-promoting potential from Madagascar: Innovative uses for food and medicinal applications. Sci. Hortic. (Amsterdam). 2022;299:111010
- [CrossRef] [Google Scholar]
- Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev.. 2019;18(1):241-272.
- [CrossRef] [Google Scholar]
- In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. J. Plant Biochem. Biotechnol.. 2013;22(1):9-15.
- [CrossRef] [Google Scholar]
- A review of potential anti-cancer properties of some selected medicinal plants grown in Iran. J. Herb. Med.. 2022;33:100557
- [CrossRef] [Google Scholar]
- Hao, D.-C., 2021. Drug metabolism and pharmacokinetic diversity of Taxus medicinal compounds, D.-C. B. T.-T. and C. Hao, Ed. Academic Press, 2021, pp. 123–189 (Chapter 4).
- The chemistry behind antioxidant capacity assays. J. Agric. Food Chem.. 2005;53(6):1841-1856.
- [CrossRef] [Google Scholar]
- Recommended medicinal plants as source of natural products: a review. Digit. Chinese Med.. 2018;1(2):131-142.
- [CrossRef] [Google Scholar]
- Determination of the antioxidant activity of Limoniastrum feei aqueous extract by chemical and electrochemical methods. Cogent Chem.. 2016;2(1):1186141
- [CrossRef] [Google Scholar]
- Assessment of enzyme inhibitory and antioxidant activities of lignans from Taxus baccata L. Zeitschrift für Naturforsch. C. 2010;65(3–4):187-194.
- [CrossRef] [Google Scholar]
- Biological activities of lignans from Taxus baccata L. growing in Turkey. Plants Res.. 2010;4(12):1136-1140.
- [Google Scholar]
- Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J. Ethnopharmacol.. 2003;89(2):265-270.
- [CrossRef] [Google Scholar]
- Taxanes and taxoids of the genus Taxus – A comprehensive inventory of chemical diversity. Phytochemistry. 2021;190:112829
- [CrossRef] [Google Scholar]
- Genetic diversity and structure of Taxus baccata from the Cantabrian-Atlantic area in northern Spain: a guide for conservation and management actions. Forest Ecol. Manage.. 2021;482:118844
- [CrossRef] [Google Scholar]
- Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat. Prod. Res. 2022
- [CrossRef] [Google Scholar]
- “Immunomodulatory activity of Salvinia molesta D.S. Mitchell in fresh water crab Oziotelphusa senex senex bacterially challenged with Pseudomonas aeruginosa. J. King Saud Univ.–Sci. 2018
- [Google Scholar]
- Adsorption characteristics and separation of taxol from yew tree by NP-HPLC. Sep. Purif. Technol.. 2000;19(1–2):27-37.
- [CrossRef] [Google Scholar]
- Analysis of antibacterial and antioxidant activity of Taxus baccata Linn. J. Med. Plants Stud.. 2018;6(5):40-44.
- [Google Scholar]
- Pullaiah, T., Karuppusamy, S., Swamy, M.K., 2022. “7 - Propagation of paclitaxel biosynthesizing plants. In: Swamy, M.K., Pullaiah, T., Chen, Z.-S. B. T.-P. (Eds.) Academic Press, pp. 171–202.
- Traditionally used edible Solanaceae plants of Mizoram, India have high antioxidant and antimicrobial potential for effective phytopharmaceutical and nutraceutical formulations. Heliyon. 2021;7(9):e07907.
- [Google Scholar]
- In vitro evaluation of antioxidant capacity of algerian propolis by spectrophotometrical and electrochemical assays. Int. J. Pharmacol. 2014
- [Google Scholar]
- Antioxidant and free radical scavenging activities of plant extracts used in traditional medicine in Mexico. African J. Biotechnol.. 2008;7(12):1886-1893.
- [Google Scholar]
- U.S. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane ®) in combination with gemcitabine as first-line treatment of patients with metastatic cancer. Pancreas News. 2013;14(6):686-688.
- [CrossRef] [Google Scholar]
- A brief review on phytochemistry and pharmacology of Taxus baccata L. Mater. Today: Proc. 2021
- [CrossRef] [Google Scholar]
- Red arils of taxus baccata l.—a new source of valuable fatty acids and nutrients. Molecules. 2021;26(3):1-17.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of 50 plant extracts. Biosyst. Divers.. 2019;27(2):163-169.
- [CrossRef] [Google Scholar]
- In the shade – Screening of medicinal and aromatic plants for temperate zone agroforestry cultivation. Ind. Crop Product.. 2021;170:113764
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105334.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







