Translate this page into:
A simple and efficient catalyst for Suzuki reaction based on ultra-low palladium chloride supported on ZnO nanowires
⁎Corresponding authors at: School of Chemistry, Tiangong University, No.399 BinShuiXi Road, XiQing District, Tianjin 300387, PR China (Jianzhou Gui). liudan@tiangong.edu.cn (Dan Liu), guijianzhou@tiangong.edu.cn (Jianzhou Gui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A simple and facile synthesis of Pd-based heterogeneous catalyst was developed for the Suzuki-Miyaura reaction; The catalytic activity of the synthesized catalyst is significantly influenced by the morphology and crystal structure of zinc oxide. Pd-based heterogenous catalyst with highly catalytic efficiency (7.6–76 mol ppm Pd species) and high recyclability.
Abstract
Zinc oxide nanowires were synthesized and impregnated with trace amounts of palladium ions by adsorption, which was then employed as a heterogeneous catalyst in the Suzuki reaction. To obtain an in-depth understanding of the structure and properties of the ZnO nanowires and the resultant catalyst, different analysis techniques were performed. The as-synthesized catalyst demonstrated exceptional efficiency in promoting the reaction between aryl halides and arylboronic acids, enabling the achievement of biphenyl derivatives in high yields ranging between 82% and 99%. The analysis conducted using transmission electron microscopy demonstrated the formation of palladium nanoparticles during the reaction, confirming their role as the active species driving the catalytic transformation. Further investigation was carried out to examine the effect of the support on the catalytic activity of the catalyst. The results indicated that the morphology and crystallographic structure of zinc oxide had a significant impact on the catalytic activity of the prepared catalyst. The catalytic performance of PdCl2/ZnONWs, where palladium chloride immobilized on ZnO nanowires, was found to be exceptional. The catalyst demonstrated the ability to be recovered and reused up to three times without a noticeable decline in its catalytic activity. Additionally, the loading of palladium species could be reduced to 7.6 mol part per million. Remarkably, the catalyst achieved a total turnover number of 130,000 and a turnover frequency of 0.75 s−1.
Keywords
Catalyst Design
Catalyst Recycling
Palladium chloride
Zinc Oxides
Heterogeneous Catalysis
1 Introduction
The Suzuki-Miyaura reaction, one of the most important methods for the construction of carbon–carbon bonds, has been widely used in organic synthesis as one of the most important discoveries in the field of synthetic chemistry (Miyaura and Suzuki, 1995; Hassan et al., 2002; Yin and Liebscher, 2007). Despite the impressive catalytic performance of palladium in organic reactions, the use of palladium as a catalyst is hindered by two prominent drawbacks: its cost and toxicity (Greenwood, and Earnshaw, 1997; Abernethy et al., 2010; Kushwaha, 2021; Kovaleva et al., 2018). The expense of utilizing palladium can make up a significant portion of the overall cost of a reaction, while its toxic nature can cause contamination issues in the final product (Abernethy et al., 2010; Kushwaha, 2021; Kovaleva et al., 2018). Hence, cross-coupling reactions are commonly excluded in the late stages of drug synthesis due to the conventional use of catalyst loadings that are impractical for industrial application, requiring multiple mole percentages (Farina, 2004). By reducing the amount of catalyst used, however, these reactions can be made more economically feasible and safer. A catalyst loading of 100 mol ppm (ppm = parts per million) can completely eliminate the catalyst cost in the reaction, while a loading level of 10 mol ppm would address any concerns regarding toxicity. Notably, this loading level meets the requirement for palladium impurities in pharmaceutical drugs.
The utilization of heterogeneous switching in homogeneous transition-metal catalysis has garnered increasing attention in the realm of organic synthetic chemistry. This approach offers significant advantages, including the ability to recover and reuse catalysts, as well as reducing the presence of metal contaminants in the final products. These benefits contribute to the realization of environmentally friendly and high-throughput molecular transformations (Fechete et al., 2012; Gholinejad et al., 2017; Gholinejad et al., 2018; Vásquez-Céspedes et al., 2021; Deepika and Sethuraman, 2022; Gholinejad et al., 2023; Sheldon and van Bekkum, 2001; Barbaro and Liguori, 2010). To date, numerous heterogeneous catalytic systems based on palladium (Pd) have been devised for the Suzuki-Miyaura reaction (Hassan et al., 2002; Yin and Liebscher, 2007). However, there is a limited number of reports on heterogeneous catalysts featuring trace amounts of palladium loading that exhibit both high catalytic activity and high recyclability (Roy and Uozumi, 2018). Uozumi and co-workers reported a metalloenzyme-inspired polymeric imidazole palladium catalyst and its application in Suzuki-Miyaura reaction (Yamada et al., 2012). The reaction was conducted with a 40 mol ppm palladium species. Additionally, they also developed a convoluted polyvinylpyridine-palladium catalyst for Suzuki-Miyaura reaction with the same loading of palladium species (Ohno et al., 2020). A new biarylamine-based substituted palladacycle was developed in Lipshuz group developed and was applied to the Suzuki-Miyaura reaction. The reaction was carried out in water under mild conditions and at a 300 mol ppm level of palladium species high recyclability (Takale et al., 2019). Meanwhile, Zhang group has developed a palladium single-atom catalyst, formed from bimetal oxides via an in situ co-precipitation method, for use in Suzuki-Miyaura reaction (Ding et al., 2020). This catalyst exhibits high catalytic activity (37 mol ppm Pd) and high recyclability. However, the availability of heterogeneous catalysts that exhibit both high activity and high recyclability, while also being simple and low-cost to prepare, is scarce. Therefore, designing a heterogeneous catalyst with excellent catalytic activity and high recyclability, while being synthesized through a cost-effective and facile method, is highly desired.
On the other hand, adsorption is a technique widely used for removing heavy metal ions due to its simple design as well as its economical and eco-friendly nature (Renu et al., 2017). Last decades, nanoadsorbents have become increasingly popular for mitigating heavy metal pollution in water sources due to their high surface area-to-volume ratios, which provide numerous sites for heavy metal adhesion (Kyzas and Matis, 2015; Ali et al., 2020). Among the various nanoadsorbent materials, zinc oxide (ZnO) exhibits significant potential for heavy metal remediation as it displays exceptional biocompatibility and low toxicity (Mahdavi et al., 2012; Al-Mur, 2023; Le et al., 2019; Dhiman and Kondal, 2021; Deepika and Sethuraman, 2023). Despite several reports on the application of palladium catalysts supported on zinc oxide for the Suzuki-Miyaura reaction (Del Zotto and Zuccaccia, 2017; Kim and Choi, 2009; Hosseini-Sarvari and Razmi, 2015; Bao et al., 2017; Hu et al., 2017; Hosseini-Sarvari and Bazyar, 2018; Rezaei et al., 2020), using zinc oxide to adsorb trace amounts of palladium ions and employing them as heterogeneous catalysts for organic synthesis remains a challenging task.
Recently, our group has successfully synthesized a highly efficient hierarchical TiO2 microsphere-supported palladium catalyst and applied it in the Suzuki-Miyaura reaction (Pan et al., 2023). The catalyst exhibited exceptional qualities in promoting the reaction, utilizing a 10–200 mol ppm loading of palladium species. As a continuation of our efforts in the development of a greener synthetic pathway for organic transformation, in the present work, a simple and facile synthesis of Pd-based heterogeneous catalyst was developed for the Suzuki-Miyaura reaction. The catalyst PdCl2/ZnONWs with highly catalytic efficiency (7.6–76 mol ppm Pd species) and high recyclability was achieved. The catalyst exhibited impressive performance with a total turnover number reaching as high as 130,000 and a turnover frequency of 0.75 s−1, respectivelyDuring the reaction, the generation of palladium nanoparticles was observed through the use of transmission electron microscopy (TEM) analyses. These nanoparticles were found to be the active species responsible for catalyzing the transformation. Further investigation was carried out to examine the effect of the support on the catalytic activity of the catalyst. The results indicated that the morphology and crystallographic structure of zinc oxide had a significant impact on the catalytic activity of the synthesized catalyst.
2 Experimental
2.1 Preparation procedure for ZnO nanowires
Zinc chloride (0.2 g), sodium dodecyl sulfate (SDS, 1.5 g) and sodium carbonate (20 g) were dissolved in 40 mL deionized water, and stirred for 30 min. Then the solution was transferred into a 50 mL Teflon-lined autoclave and kept at 140 °C for 12 h. After cooled to the room temperature, the precipitate was collected by filtration, rinsed with deionized water and absolute ethanol for three times, and finally dried at 80 °C for 12 h under vacuum, to give the desired ZnO nanowires.
2.2 Preparation procedure for PdCl2/ZnO catalyst
The catalyst PdCl2/ZnONWs was prepared by adsorption method: Firstly, take 1.8 mg of palladium chloride and dissolve it in a small amount of concentrated hydrochloric acid, and then dilute it to a 20 mL solution. Secondly, weigh 0.1 g of zinc oxide nanowires and disperse them in 1 mL of water. Then add 2 mL of the above prepared palladium chloride solvent and stir at room temperature for 3 h. The precipitate was separated by centrifugation, washed three times with water and ethanol separately, and dried under vacuum to give the desired PdCl2/ZnONWs catalyst. The catalysts, PdCl2/ZnONPs and PdCl2/ZnOCA, were prepared with the same procedure.
2.3 General procedure for the Suzuki-Miyaura reaction
A mixture of aryl halide (0.25 mmol), arylboronic acid (0.375 mmol), PdCl2/ZnONWs (1.0 mg, 76 mol ppm Pd species), and Na2CO3 (0.5 mmol) in EtOH/H2O (1.0 mL/1.0 mL) were stirred at 80 °C for 12 h (24 h for aryl bromides). After completion of the reaction, the reaction mixture was cooled to room temperature, the mixture was extracted with ethyl acetate (3 mL x3). The combined organic phases were concentrated in vacuo and the crude products were purified by column chromatography (hexane/AcOEt) to give the corresponding products.
2.4 Recycling experiment for the Suzuki-Miyaura reaction
After the Suzuki-Miyaura reaction discussed previously, the supported catalyst was recovered by centrifugation and washed twice with EtOAc and water. The recovered catalyst was dried in vacuo and reused in a subsequent reaction without an additional charge of palladium.
3 Results and discussion
The catalyst of ZnO nanowires-supported palladium chloride (PdCl2/ZnONWs) was prepared as shown in Scheme 1. First, zinc oxide nanowires (ZnONWs) were synthesized by a one-step reaction using a facile hydrothermal method. A mixture containing zinc chloride, sodium dodecyl sulfate (SDS), sodium carbonate, and water was stirred at room temperature for 30 min. The resulting mixture was then transferred to a Teflon autoclave and subjected to an oven temperature of 140 °C for a duration of 12 h. This process ultimately yielded the desired ZnO nanowires. Subsequently, the PdCl2/ZnONWs catalyst was prepared by employing the adsorption method to support palladium chloride onto the as-synthesized ZnO nanowires. The mixture of as-synthesized ZnO nanowires and palladium chloride was stirred at room temperature for a duration of 2 h, resulting in the desired catalyst denoted as PdCl2/ZnONWs.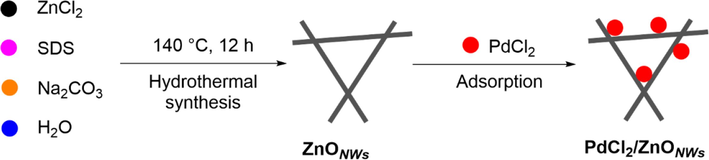
Schematic illustration for the synthesis process of PdCl2/ZnONWs.
The ZnO nanowires and the catalyst were characterized by different technologies. In Fig. 1a and 1b, it can be clearly seen that the as-synthesized ZnO nanowires have a typical linear structure with uniform size and smooth surface. After loading of palladium species, the morphology and structure of the resulting PdCl2/ZnONWs catalyst remain essentially unchanged. Fig. 2 displays the results of an Energy-dispersive X-ray (EDX) analysis conducted on the PdCl2/ZnONWs catalyst, confirming the presence of palladium within the catalyst. This analysis provides strong evidence that palladium species have been successfully immobilized onto the ZnO nanowires. Through an ICP-OES analysis of PdCl2/ZnONWs, it has been further verified that the immobilization of Pd onto ZnO nanowires was successful. The analysis determined a palladium loading of 0.019 mmol g−1, providing quantitative confirmation of the amount of palladium present on the nanowires.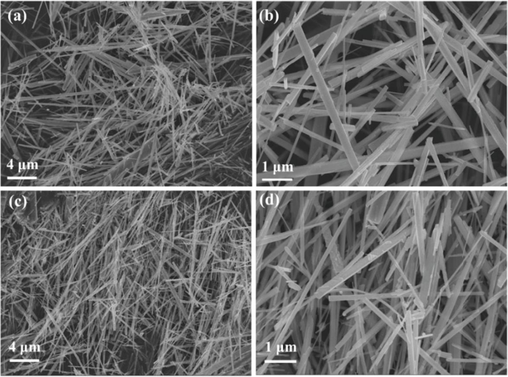
SEM images of ZnONWs (a and b) and PdCl2/ZnONWs (c and d).
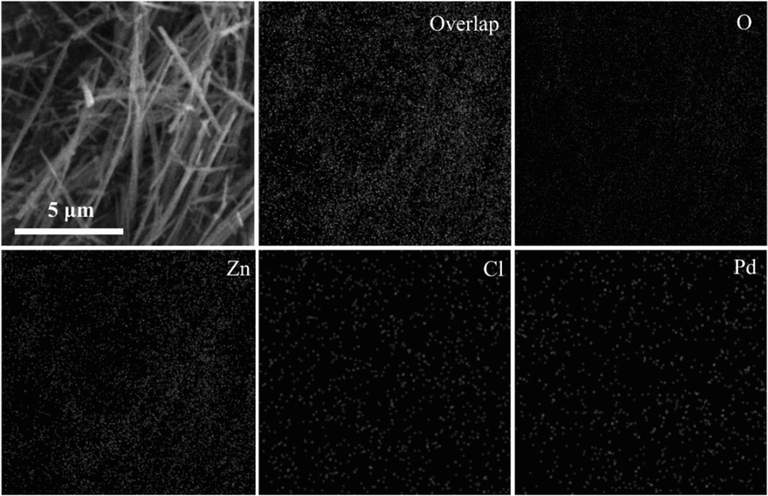
EDX mappings of PdCl2/ZnONWs.
The XRD technique was employed to characterize the structure of the crystals. The as-synthesized ZnO nanowires exhibited characteristic peaks in their X-ray diffraction pattern, indicating the presence of the crystalline phase of ZnO (JCPDS 36-1451). These peaks were observed at angles of 31.52°, 34.42°, 36.24°, 47.52°, and so on. It can be seen that the XRD pattern of the as-synthesized PdCl2/ZnO NWs have the same characteristic peaks with ZnO nanowires, indicating that the crystal structure of ZnO nanowire has no change after loading of palladium species. In the XRD patterns of the as-synthesized catalyst, no presence of the Pd(0) phase was detected. This result was corroborated by TEM analysis, which revealed the absence of any palladium nanoparticles. To gain further insights into the state of palladium in the as-synthesized catalyst, X-ray photoelectron spectroscopy (XPS) analyses of ZnO nanowires and PdCl2/ZnONWs were performed (Fig. 3b-3e). Fig. 3e showed binding energies (BEs) of Pd 3d5/2 and 3d3/2 at 337.6 and 343.2 eV, respectively, indicating the presence of Pd(II) species in the catalyst (Shin et al., 2019) These results indicated that the adsorption of palladium species onto the surface of ZnO nanowires occurred through physical adsorption. This can be attributed to the negatively charged surface, which was generated by the presence of OH– groups during the growth process of ZnO nanowires (Renu et al., 2017).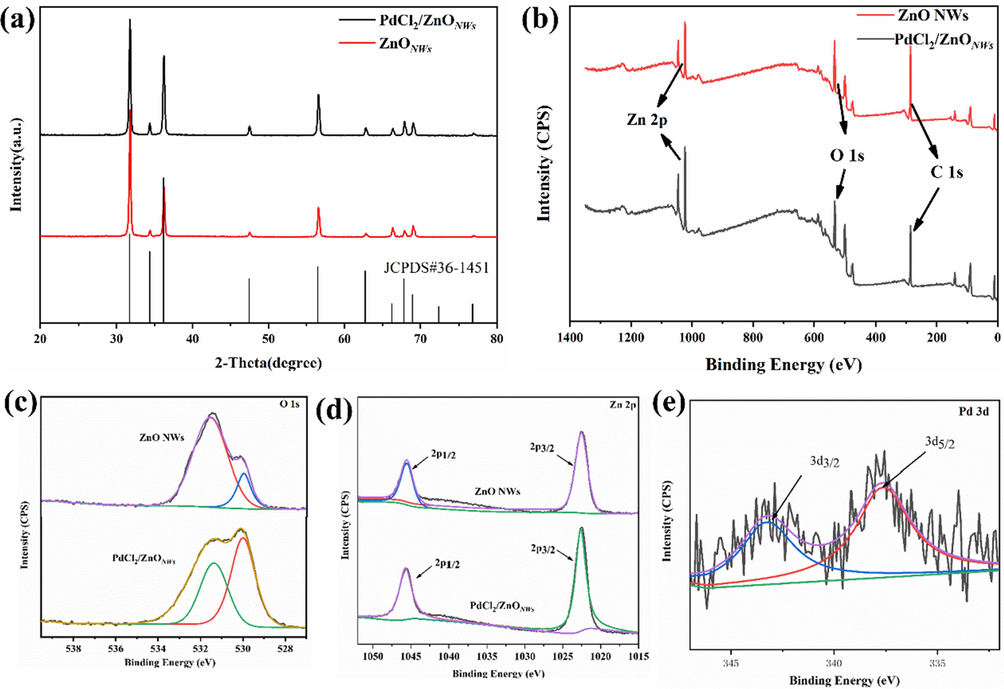
(a) XRD study of PdCl2/ZnONWs catalyst, and (b-e) XPS spectra of ZnO NWs and PdCl2/ZnONWs catalyst.
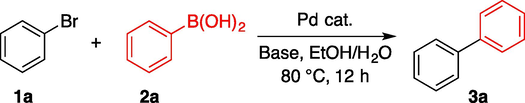
After obtaining the as-synthesized catalyst, bromobenzene (1a) and phenylboronic acid (2a) were chosen as model substrates to optimize the reaction conditions, and the results were summarized in Table 1. Initially, several carbonates were evaluated in this transformation, and the use of Na2CO3 resulted in a 99% GC yield of biphenyl 3a (Entries 1–4). Although other bases, such as potassium phosphate and triethylamine were also investigated, their yields were lower than Na2CO3 (Entries 5 and 6). Under the same reaction conditions, iodobenzene reacted with 2a yielded the desired biphenyl with outstanding GC yield (entry 7). When neither a catalyst nor a base was present, the desired product 3a did not form (Entries 8 and 9). To verify that a trace amount of palladium species effectively facilitated this conversion, the same amount of the as-synthesized ZnO nanowires was used only in the reaction. However, none of 3a was observed (Entry 10).
Entry
Pd cat.
Base
GC Yield (%)b
1
PdCl2/ZnONWs
Li2CO3
71
2
PdCl2/ZnONWs
Na2CO3
99
3
PdCl2/ZnONWs
K2CO3
95
4
PdCl2/ZnONWs
Cs2CO3
94
5
PdCl2/ZnONWs
K3PO4
76
6
PdCl2/ZnONWs
NEt3
22
7c
PdCl2/ZnONWs
Na2CO3
99
8
–
Na2CO3
0
9
PdCl2/ZnONWs
–
0
10
ZnONWs
Na2CO3
0
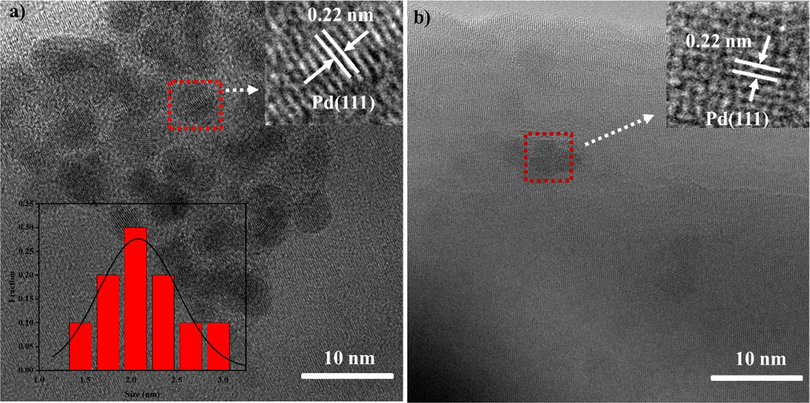
A comparative study was conducted to assess the catalytic activity of homogenous and heterogeneous PdCl2 catalysts (Scheme 2). When the reaction performed with 76 mol ppm of PdCl2, the product 3a was only obtained in 64% yield. These results indicated that the catalyst of PdCl2 immobilized on ZnO showed higher catalytic activity than that of a homogeneous PdCl2 catalyst. In order to determine the active species involved in this conversion process, we conducted a transmission electron microscopy (TEM) analysis on the catalyst. This catalyst was obtained through the treatment of PdCl2/ZnONWs with phenylboronic acid, in the presence of Na2CO3, using a mixture of ethanol and water (v/v = 1:1) at a temperature of 80 °C for a duration of 30 min. The TEM image revealed that palladium nanoparticles were generated and dispersed on ZnO nanowires (Fig. 4a). The size of the particles was distributed in the range 1.5–3.0 nm, and the calculated lattice distance of 0.225 nm corresponds to the (1 1 1) planes of Pd(0) species. The generation of palladium nanoparticles is likely attributed to the oxidation of two molecules of arylboronic acid, resulting in the formation of self-coupling products through the loss of two electrons. In contrast, the palladium(II) species acquire two electrons and undergo reduction to palladium(0) (Moreno-Mañas et al., 1996). Furthermore, we conducted a transmission electron microscopy (TEM) analysis on the reaction mixture following the completion of the reaction. In Fig. 4b, we observed the presence of palladium nanoparticles in the selected region. These experimental findings confirm that the palladium nanoparticles indeed serve as the active catalytic species in this reaction.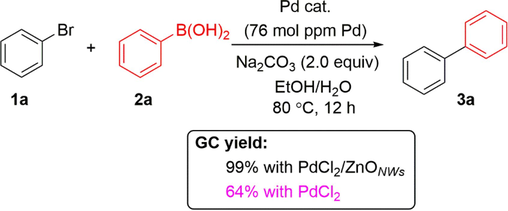
A comparative study between homogenous and heterogeneous PdCl2 catalysts.
Next, a comparative study was conducted on the catalytic activity and recyclability of palladium chloride supported on various ZnO supports. Using the same method as the one employed for palladium chloride immobilization on zinc oxide nanowires, we prepared catalysts by loading palladium chloride onto zinc oxide nanoparticles (sizes: 20–40 nm) and commercially available zinc oxide. The catalytic performance of these catalysts was then compared. However, the catalysts prepared with zinc oxide nanoparticles and commercially available zinc oxide yielded lower results (90% and 75% respectively). These findings clearly demonstrate that the ZnO nanowires synthesized in this study outperforms both the ZnO nanoparticles and commercial ZnO as a support. Furthermore, the reusability of various PdCl2/ZnO catalysts was evaluated for synthesizing of biphenyl. After the reaction, the catalyst was recovered by centrifugation, washed three times with EtOAc and H2O, dried in a vacuum oven and placed in the next use. ICP analysis shown that 5.1% of palladium species was leached to the solution. As shown in Fig. 5, the catalyst PdCl2/ZnONWs exhibited the capability of being recovered and reused for up to three cycles with only a minor decrease in catalytic activity, while the catalysts of PdCl2/ZnONPs and PdCl2/ZnOCA showed a poor reusability (According to the ICP analyses). These results further indicated that the as-synthesized ZnO nanowire sample was superior to commercial ZnO as the support.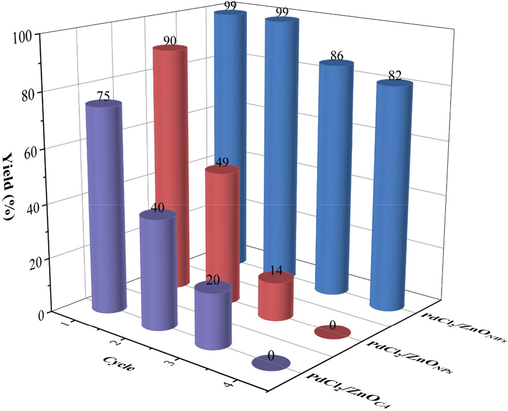
Recycle and reuse of various PdCl2/ZnO catalysts.
Finally, the optimized conditions specified in entry 2 of Table 1 were utilized to explore the range of substrates, and the results were consolidated and presented in Scheme 3. The products 3a-3d obtained from the reaction of iodobenzene with different arylboronic acids were all in 99% yield. Iodobenzenes containing a 4-position methoxy, methyl, or acetyl group underwent reactions with various arylboronic acids to yield the desired products 3e-3i and 3f’ with high yields ranging from 82% to 99%. By expanding the range of aryl halides, the applicability can also be extended to include aryl bromides (The range of aryl halides). For instance, the reaction of bromobenzene with phenylboronic acid resulted in the formation of 3a in an impressive yield of 99%. Phenylboronic acid bearing an electron-denoting group (OCH3) in the 4-position facilitated this transformation, and the desired product 3b derived from bromobenzene was obtained in 99% yield. No electronic effect was observed for aryl bromides, as bromobenzene bearing a methoxy, methyl, or acetyl group in the 4-position gave the products (3h-3k, 3b’, 3d’ and 3f’) in 83–99% yields. Under the catalytic conditions, functional groups, including halo, cyano, and acetyl groups, demonstrated good compatibility and stability.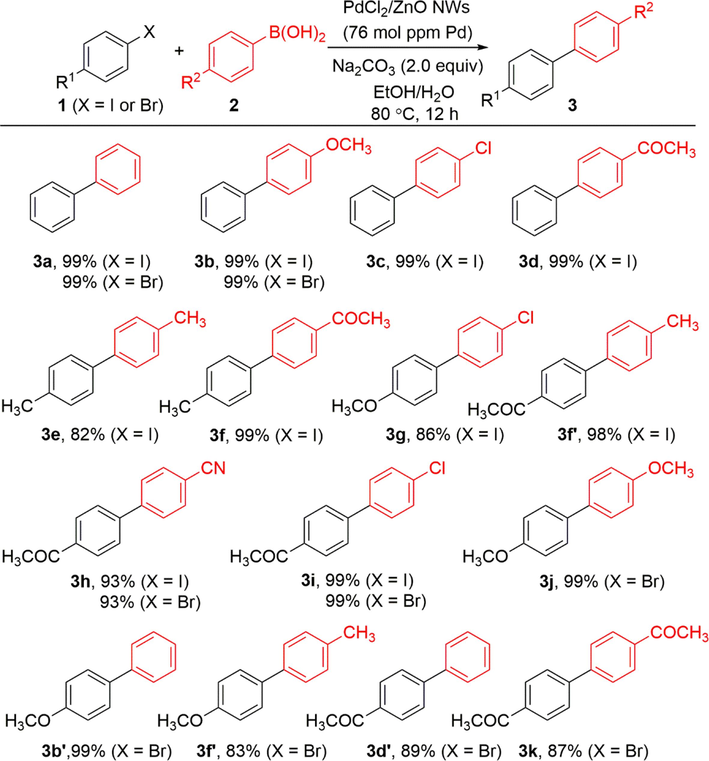
Scope of substrates. Reaction conditions: Ar-X (X = I or Br, 0.25 mmol), Ar-B(OH)2 (0.375 mmol), PdCl2/ZnO NWs (1.0 mg, 76 mol ppm active metal), Na2CO3 (0.5 mmol), EtOH/H2O (1.0 mL/1.0 mL), 80 °C for 12 h. Isolated yields are reported.
Lowering the catalyst loading makes the reactions safer and cost-efficient for synthetic chemistry. The loading of palladium species in this transformation can be further reduced to a level as low as 10 mol ppm. In scheme 4a, the reaction between iodobenzene and phenylboronic acid was carried out using a mere 7.6 mol ppm of Pd species, resulting in the formation of product 3a with an exceptional yield of 99% within a reaction time of 48 h. Similarly, product 3b was obtained in a 99% yield using the same reaction conditions, as depicted in Scheme 4b. As high as 130,000 turnover number (TON) and 0.75 s−1 turnover frequency (TOF) were achieved in this transformation. In Scheme 4c, when bromobenzene was subjected to this transformation, the reaction necessitated an extended reaction time, resulting in the isolation of biphenyl 3a’ in a yield of 92%.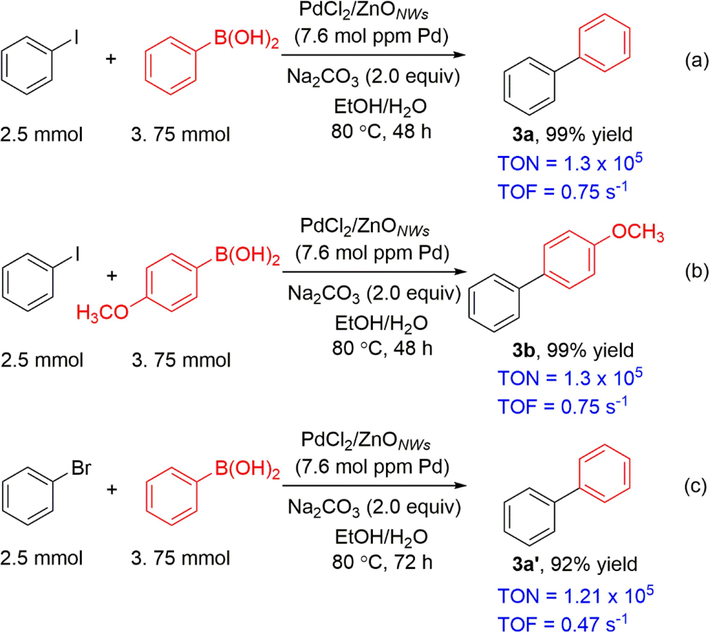
Synthesis of biphenyl derivatives with 7.6 mol ppm palldium species.
Furthermore, in Table 2, a comparison is presented to evaluate the catalytic efficiency of PdCl2/ZnONWs in comparison to other palladium catalysts supported on ZnO for Suzuki coupling reactions documented in the literature. The comparison results indicate that PdCl2/ZnONWs exhibited remarkable turnover number (TON) and turnover frequency (TOF) values.
Entry
Catalyst
(mol% Pd)Conditions
Cycle
(times)Ref.
1
Pd-Ag/ZnO(13.3)
EtOH, K3PO4, 78 ˚C
6
25
2
Pd/ZnO NPs(0.093)
H2O, K2CO3, 100 ˚C
7
26
3
Pd/ZnO(0.08)
EtOH/H2O, K3PO4, reflux
3
27
4
Pd NP/ZnO@Zn(0.01)
DMF, K2CO3, 100 ˚C
3
28
5
Pd/ZnO(15)
EtOH/H2O, K2CO3, 70 ˚C
5
29
6
Pd-PdO/ZnO(3.24)
EtOH/H2O, K2CO3, 25 ˚C
5
30
7
PdCl2/ZnONWs (0.00076–0.0076)
EtOH/H2O, Na2CO3, 80 ˚C
4
this work
4 Conclusions
In summary, we have developed a catalyst composed of palladium chloride immobilized on ZnO nanowires and successfully applied it in the Suzuki reaction. This catalyst efficiently promoted the reaction of aryl halides with arylboronic acids, resulting in the synthesis of biphenyl derivatives with high yields ranging from 82% to 99%. TEM analyses showed that palladium nanoparticles were generated during the reaction and were the actual catalytically active species in this transformation. The impact of the support on catalytic activity was thoroughly studied, and the results revealed that the supported catalyst’s performance is predominantly influenced by the crystalloid structure and morphology of zinc oxide. The catalyst of palladium chloride immobilized on ZnO nanowires (PdCl2/ZnONWs) emonstrated remarkable catalytic activity. Furthermore, it exhibited the ability to be recovered and reused up to three times with minimal loss in catalytic efficiency. Additionally, the loading of palladium species has been successfully reduced to 7.6 mol part per million, resulting in an impressive total turnover number of 130,000 and a turnover frequency of 0.75 s−1 for the PdCl2/ZnONWs catalyst. Furthermore, our laboratory is currently exploring the potential of expanding the applications of this catalyst to other transformations.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21576211), Tianjin Science and Technology Program (No. 22YDTPJC00920), Program for Tianjin Innovative Research Team in Universities (No. TD13-5031), and Tianjin 131 Research Team of Innovative Talents.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pharm. Res.. 2010;27:750-755.
- [CrossRef]
- According to the ICP analyses, the first cycle resulted in the leaching of 13.0% and 27.8% of palladium species from PdCl2/ZnONPs and PdCl2/ZnOCA, respectively.
- Int. J. Environ. Sci. Technol.. 2020;17:4095-4132.
- [CrossRef]
- Water. 2023;15:455.
- [CrossRef]
- ACS Appl. Mater. Interfaces. 2017;9:16417-16425.
- [CrossRef]
- Barbaro P., Liguori F., eds. Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes. New York: Springer, Dordrecht; 2010.
- Res. Chem. Intermed.. 2022;48:1111-1128.
- [CrossRef]
- J. Mol. Struct.. 2023;1278:134910
- [CrossRef]
- Catal. Sci. Technol.. 2017;7:3934-3951.
- [CrossRef]
- Colloid Interface Sci. Commun.. 2021;41:100380
- [CrossRef]
- Commun. Chem.. 2020;3:43.
- [CrossRef]
- Adv. Synth. Catal.. 2004;346:1553-1582.
- [CrossRef]
- Catal. Today.. 2012;189(2012):2-27.
- [CrossRef]
- Mol. Catal.. 2017;433:12-19.
- [CrossRef]
- J. Catal.. 2018;363:81-91.
- [CrossRef]
- J. Organomet. Chem.. 2023;987–988:122636.
- [CrossRef]
- Chemistry of the Elements (second ed.). Boston: Butterworth-Heinemann, Oxford; 1997.
- Chem. Rev.. 2002;102:1359-1470.
- [CrossRef]
- ChemistrySelect. 2018;3:1898-1907.
- [CrossRef]
- Helv. Chim. Acta.. 2015;98:805-818.
- [CrossRef]
- RSC Adv.. 2017;7:7964-7972.
- [CrossRef]
- J. Nanomater.. 2009;2009:1-7.
- [CrossRef]
- Pharm. Chem. J.. 2018;52:84-89.
- [CrossRef]
- Curr. Pharm. Anal.. 2021;17:960-968.
- [CrossRef]
- J. Mol. Liq.. 2015;203:159-168.
- [CrossRef]
- Heliyon. 2019;5:e01440.
- [CrossRef]
- J. Nanoparticle Res.. 2012;14:846.
- [CrossRef]
- Chem. Rev.. 1995;95:2457-2483.
- [CrossRef]
- J. Org. Chem.. 1996;61:2346-2351.
- [CrossRef]
- Adv. Synth. Catal.. 2020;362:4687-4698.
- [CrossRef]
- Arab. J. Chem.. 2023;16:104410
- [CrossRef]
- J. Water Reuse Desalination.. 2017;7:387-419.
- [CrossRef]
- Mater. Today Chem.. 2020;18:100353
- [CrossRef]
- Adv. Synth. Catal.. 2018;360:602-625.
- [CrossRef]
- Sheldon R.A., van Bekkum H., eds. Fine Chemicals Through Heterogenous Catalysis. New York: Wiley-VCH, Weinheim; 2001.
- Carbon. 2019;143:362-370.
- [CrossRef]
- Chem. Sci.. 2019;10:8825-8831.
- [CrossRef]
- The range of aryl halides was also extended to aryl chloride, however, trace amount (<5%) of desired product was obtained under the similar conditions.
- Org. Process Res. Dev.. 2021;25:740-753.
- [CrossRef]
- J. Am. Chem. Soc.. 2012;134:3190-3198.
- [CrossRef]
- Chem. Rev.. 2007;107:133-173.
- [CrossRef]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105343.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1