Translate this page into:
Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonated soft drinks using magnetic SPE-GC/MS method
⁎Corresponding authors. snazmara@razi.tums.ac.ir (Shahrokh Nazmara), ghjahed@sina.tums.ac.ir (GholamReza Jahed Khaniki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The synthesis of compounds with an excellent adsorption capability plays an essential role to remove contaminants such as phthalic acid esters (PAEs) with potential carcinogenic characteristics from different food products. In this context, for the first time, a novel adsorbent (MWCNT-Fe3O4/Ag) was synthesized by using iron (magnetic agent), and silver (catalytic and surface enhancer agent) to further approach in a magnetic SPE-GC/MS method for determining of PAEs in carbonated soft drink samples. The limit of detection (LOD) and limit of quantification (LOQ) values of MSPE-GC/MS were determined in six PAEs as a range of 10.8–22.5 and 36–75 ng/L, respectively. Also, the calibration curves of PAEs were linear (R2 = 0.9981–0.9995) over the concentration level of 10.000 ng/L and the recoveries of the six PAEs were ranging from 96.60% to 109.22% with the RSDs less than 8%. Moreover, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX), X-ray diffraction (XRD), vibrating sample magnetometry (VSM) and transmission electron microscopy analyses (TEM) were utilized to characterize the produced MWCNT-Fe3O4/Ag. Based on the findings, the surface of MWCNT is relatively uniform, which became coarser after loading with Fe3O4/Ag particles. Also, EDX spectrum showed the carbon (C), iron (Fe), oxygen (O), Ag and copper (Cu) are the main components of synthesized MWCNTs-Fe3O4/Ag. The successful adhesion of Fe3O4/Ag on the texture of MWCNTs using a co-precipitation method was confirmed by XRD and FT-IR assays. Additionally, excellent crystallinity and clear lattice nanocrystals fringes of prepared MWCNT-Fe3O4/Ag was demonstrated by TEM analysis. Based on the obtained VSM images, the prepared sorbent (MWCNT-Fe3O4/Ag) has the good magnetic performance for magnetic separation and extraction processes. It was concluded that the synthesized MWCNT-Fe3O4/Ag could be used as an efficient adsorbent for determining contaminants such as PAEs in different beverage samples.
Keywords
Magnetic solid phase extraction (MSPE)
Multi-walled carbon nanotubes (MWCNTs)
Iron oxide (Fe3O4)
Silver (Ag)
Phthalic acid esters (PAEs)
1 Introduction
Presence of organic compounds in environment and water is an important concern (Dobaradaran et al., 2010). The extraction of organic compounds such as phthalate esters (PAEs), due to their high matrix complexity and low concentration levels, is a matter of concern due to their carcinogenic and hazardous properties. Furthermore, sample preparation has always been one of the most challenging processes, delicate and intricate exertion methods in the laboratory research projects (Jeddi et al., 2014). Binding process of PAEs to polymers occurs through feeble secondary molecular interactions with polymer chains. They could bound physically and chemically to polymers insomuch that once they are released from a polymer, they can infiltrate products, and consequently contaminate food, beverage and other environments (Wu et al., 2012).
To detect and measure a trace concentration of pollutants such as PAEs in a sample prior any instrumental analysis, the pre-concentration is required. Different pre-treatment techniques such as liquid-liquid extraction (LLE), solid-phase extraction (SPE), accelerated solvent extraction (ASE), single-drop microextraction (SDME), liquid-phase microextraction (LPME), solid-phase microextraction (SPME), LPME method based on the solidification of a floating organic micro drop (LPME-SFO), dispersive liquid–liquid microextraction (DLLME), hollow fiber-based liquid-phase microextraction (HF-LPME), stir-bar sorptive extraction (SBSE), polymer monolith microextraction (PMME), supercritical fluid extraction (SFE), and ultrasound-assisted emulsification–microextraction (USAEME) have been used to extract phthalate esters from different kinds of samples (Wu et al., 2012; Amiridou and Voutsa, 2011; Mol et al., 2000; Chang et al., 2011; Jiao et al., 2012).
However, these techniques might present some disadvantages such as low efficiency, high cost, excessive use of energy, requiring special types of chemical agents, and sludge disposal problems. Amongst cited techniques, SPE and SPME are the most popular techniques which have been widely used for sample preparation. Nevertheless, SPE technique is difficult and laborious (Mol et al., 2000), and SPME is time-consuming, and its fibers are quite costly, and the material used to coat polymers is sensitive to mechanical stress.
Lately, a new type of magnetic SPE (MSPE), has been introduced and developed using magnetically modified adsorbents (Kouhpayeh et al., 2017; Rastkari and Ahmadkhaniha, 2013; Kiani et al., 2018). Magnetic adsorbents simplify the separation process, make it stronger and faster without the necessity of any additional filtration or centrifugation techniques, which resulted in the elimination of time-consuming process in the preparation of traditional adsorbents (Kouhpayeh et al., 2017; Rastkari and Ahmadkhaniha, 2013). Magnetic adsorbents have been widely used so far in various applications, such as biotechnology, medicine and analytical chemistry (Wu et al., 2012; Mol et al., 2000).
In recent investigations, various adsorbents like powder activated carbon, granular activated carbon, single/multi-walled CNTs, mineral cartridge and biological/agricultural waste have been utilized to extract organic and inorganic compounds (Ahn et al., 2009; Aliabadi et al., 2012; Kakavandi et al., 2014; Narayanan and Ganesan, 2009). Amongst cited adsorbents, multi-walled CNTs (MWCNTs) have gained more interests than the others based on its high porosity, efficiency and wide surface area (Kakavandi et al., 2014; Kakavandi et al., 2013). However, the most important problem concerning MWCNTs lies within its separation from aqueous solution (Kakavandi et al., 2014; Baghani et al., 2017; Rostamia et al., 2017). Thus, it is essential to establish optimal conditions in order to facilitate MWCNTs separation from a solution after adsorption process. Employing an induced magnetic compound such as iron oxide (Fe3O4) in order to physically collect and separate adsorbent using an external magnet can be proposed as a particular aspect for achieving this purpose (Baghani et al., 2017; Gorji et al., 2016; Moazzen and Ahmadkhaniha, 2013; Baghani et al., 2016). For the past few years, this technique has been widely applied due to its simplicity, low cost and rapid performance (Kakavandi et al., 2014; Kakavandi et al., 2013; Lv et al., 2011; Rocher et al., 2008).
Employing highly active metals such as silver (Ag), nickel (Ni), copper (Cu) and lead (Pb), increases the catalytic capability, adsorbent rate, increased surface area, improved ordered structure with considerable mechanical and thermal strength, and provided a maximum amount of empty reactive surface sites that show metallic/semi-metallic behavior (Kakavandi et al., 2014; Luo et al., 2012). Considering all these advantages, they significantly accelerate extraction, absorption, and desorption of organic compounds by protecting Fe3O4 particles from surface oxidation (Lv et al., 2011). High specific area of silver nanoparticles, make them unique adsorbent for extraction of organic and inorganic compounds (Tuan et al., 2011). Silver nanoparticles have been used on the adsorbents like chitosan, activated carbon and MWCNT to remove heavy metals, contaminant, dyes and other components from aqueous solutions (Ghaedi et al., 2012; Ramana et al., 2013). To the best of our knowledge, the extraction of organic compounds such as PAEs using MWCNT-Fe3O4/Ag, as an adsorbent has not been investigated.
Due to high electrochemical potentiality (E0 = 0.8) of silver nanoparticles, they are mostly used to enhance both catalytic ability and surface area of MWCNT-Fe3O4 (Kakavandi et al., 2014; Ghaedi et al., 2012; Kakavandi et al., 2014; Selvakumar et al., 2011; Ali et al., 2017). In the current study, the MWCNT with hydroxyl and carboxyl group were prepared and then magnetized MWCNTs with Fe3O4, and in the last step gave catalytic properties to MWCNT-Fe3O4 using silver nanoparticles which led to the production of MWCNT-Fe3O4/Ag. Also, an MSPE-GC/MS method with further benefits for silver nanoparticles (giving catalytic property and enhancing surface area), and iron oxide (for increasing separation ability) was used to improve adsorption, and desorption of PAEs in carbonated soft drinks by using MWCNT.
2 Materials and methods
2.1 Chemicals, reagents, and instruments
PAEs compounds include Diethyl phthalate (DEP), Dimethyl phthalate (DMP), Butyl benzyl phthalate (BBP), Dibutyl phthalate (DBP), Bis (2-ethyl hexyl) phthalate (DEHP), Din-octyl phthalate (DNOP), and other chemicals such as n-hexane and methanol in analytical grade were purchased from Sigma–Aldrich (St. Louis, MO, USA). MWCNTs (length 5.0–30 mm, diameter 30–60 nm, Panchkula, India), FeCl3·6H2O, NaBH4, AgNO3, ethanol, and benzyl benzoate (internal standard, I.S.), also in analytical grade were obtained from Sigma-Aldrich and Merck.
Morphological analysis was performed utilizing scanning electron microscope (SEM, PHILIPS, and S360 Mv2300), transmission electron microscopy (TEM, PHILIPS, EM 208S 100 KV) and Vibrating sample magnetometer (VSM, Lakeshore 7307), respectively. Elemental characterization was conducted by energy dispersive X-ray (EDX, PHILIPS, S360, and Mv2300) and phase identification of the samples was conducted by X-ray Diffraction (XRD, Philips, X'PertPro 2002) and Fourier Transform Infrared (FT-IR, Nicolet model 510).
The phthalates stock solution (100 µg/mL) in methanol was prepared. Afterward, the working standard solutions for PAEs were prepared by consecutive dilutions of the stock solution with an equal concentration of methanol-water at 50:50 v/v. Ten µg benzyl benzoate was dissolved in 10 mL ethanol, as internal standard and then 100 µL of this solution was added to each sample. The samples for quality control (QC) were made from diluted stock standard solutions (on the same day of the analysis). The solutions were kept in the dark at 4 °C until further analysis. In this study, all the laboratory glassware was washed with a solution of aluminum oxide for a few minutes before usage, and then were immersed in acetone for 40 min, rinsed with n-hexane and after that dried at 150 °C for 5 h.
2.2 Adsorbent and MSPE procedure
MWCNT-Fe3O4/Ag composite was prepared in three phases.
2.2.1 Functionalizing MWCNTs with hydroxyl and carboxyl groups
In the first step, 2 g of MWCNTs was added to a mixture of 20 mL of commercial nitric acid and 60 mL of sulfuric acid. Treatment of MWCNT using H2SO4/HNO3 resulted in negatively charged functional groups (Yamamoto et al., 2008). Then, the mixture was put in ultrasonic for 6 h to add groups of hydroxyl and carboxyl onto MWCNTs surfaces. Next, 100 mL of deionized water was added to the solution. In the end, this mixture was washed and filtered for several times with deionized water and dried at 60 °C for 12 h.
2.2.2 Magnetization MWCNTs with Fe3O4
In this step, 6 g of FeCl3·6H2O, 1 g of the functionalized MWCNTs and 7 g of sodium acetate were added to 100 mL ethylene glycol and was shaken for 30 min. The prepared solution was refluxed for 16 h, and after cooling (at room temperature), 100 mL ethanol was added and then was shaken for 10 min. The solution was filtered and washed with deionized water. Finally, the synthesized magnetic MWCNTs (MWCNT-Fe3O4) was dried at 50 °C for 24 h.
2.2.3 Enhancing catalytic properties and surface area of MWCNT-Fe3O4 with nanoparticles of silver
In order to synthesize nano-composites with Ag, 2 g of MWCNT-Fe3O4 were poured into a beaker containing 50 mL of distilled water and mixed for 10 min on a hotplate magnetic stirrer (120–150 °C). Then, 0.05 g of AgNO3 was dissolved in 25 mL of distilled water and then added to the first solution and mixed at the same temperature for 10 min. Afterward, 0.1 g of NaBH4 was dissolved in 25 mL of distilled water and slowly added to the mixed solution. All previous mixing steps were repeated. After cooling and sedimentation of the solution, the settled particles were kept down by a magnet, and the upper solution was discarded. The retained particles were washed for three times with distilled water, and previous steps were repeated. Finally, MWCNT modified with catalytic nanoparticles of iron oxide and silver (MWCNT-Fe3O4/Ag) were dried in an oven at 60–70 °C for 24 h, and kept in containers with lids. A scheme for MWCNT-Fe3O4/Ag preparation and determination of PAEs by GC–MS are shown in Fig. 1.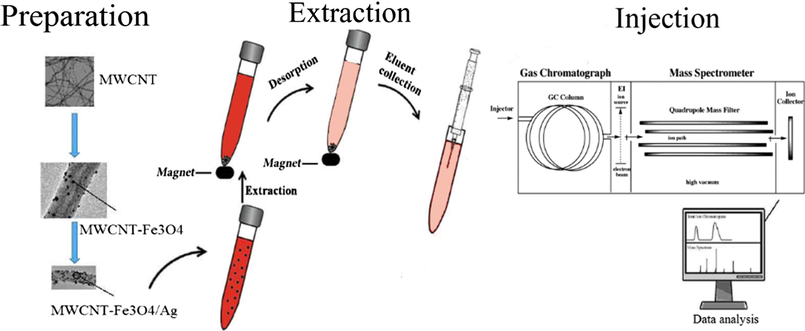
A scheme of MWCNT-Fe3O4/Ag preparation and determination of PAEs.
2.3 Sampling, sample preparation, and instrumentation
At first, eight random carbonated soft drink samples were purchased from a supermarket in Tehran, Iran and flatted in an ultrasonic bath at room temperature for 20 min. Then, 10 mg of MWCNT-Fe3O4/Ag particles were weighed and activated with water and methanol. The activated MWCNT-Fe3O4/Ag, 2 g of NaCl, and 100 µL of benzyl benzoate solution (0.1% in ethanol) as internal standard were added to the 10 mL of decarbonized soft drink. To extract the analytes (PAEs), the mixture was shaken vigorously for 4. 0 min, then adsorbent was collected to the side of the beaker (within 90 s) with the aid of an external magnet, and the supernatant was removed. Afterward, the remaining material at the bottom of the container was discarded followed by adding 2 mL of n-hexane along with 2.0 min of the vigorous vortex to elute analytes (PAEs) from the adsorbent (MWCNT-Fe3O4/Ag). Next, the adsorbent was collected to the side of the vial using an external magnet, and the supernatant was transferred to a vial. Desorption solvent dried at room temperature under a gentle flow of nitrogen gas and kept in the refrigerator. The dried content of the vial was dissolved in 1.0 mL n-hexane, and 1 µL of the solution was injected into the GC–MS. The samples were assessed and analyzed by using a gas chromatograph (GC) (Agilent 7890 N) with a mass selective detector (5975). The chromatographic column was DB-5–J&W Scientific (30 m, 250 μm, 0.5 μm), and the carrier gas was helium at 1 mL/min with a split ratio of 50:1. The injection mode was splitless with an inlet temperature of 290 °C. The temperature program was: 80 °C, held for 2 min, 80–285 °C at 7 °C min−1, held for 10 min. Selective ion monitoring mode was used for the quantitative determination of analytes. The GC–MS analysis (the retention times, qualitative and quantitative ions) of 6 PAEs and benzyl benzoate (internal standard, IS) are shown in Table 1.
Ion group
Compound
Retention time (min)
Quantitative ion (abundance) (m/z)
Qualitative ion (abundance) (m/z)
1
DMP
13.0–13.4
163
77, 135, 163, 194
2
DEP
15.4–15.7
149
121, 149, 177, 222
3
DBP
21.0–21.3
149
121, 149, 205, 223
4
BBP
26.0–26.3
149
91, 149, 206, 238
5
Benzyl benzoate (IS)
27.2–27.5
105
212 (40), 194 (35)
6
DEHP
28.2–28.5
149
113, 149, 167, 279
7
DnOP
30.2–30.6
149
149, 179, 261, 279
2.4 Method optimization of the extraction process
The method optimization was accomplished according to the technique of one factor at a time. In this procedure, all of the practical and efficient factors are kept constant except one, which is systematically changed to find the optimum value. By optimizing each factor, the experiment was repeated for another factor to find the optimum value, while studied factors are regulated to their determined optimum value. Ultimately, the overall procedure is repeated whereas all of the factors are adjusted to their determined optimum value. To achieve the optimal method conditions, the value of the determinative parameter in the last experiment should be the best between acquired values (Rastkari and Ahmadkhaniha, 2013).
2.5 Method validation
The method validation was accomplished based on the currently accepted USA Food and Drug Administration (FDA) Guideline for industries (Food and Drug Administration, 2001).
3 Result and discussion
3.1 Morphological and structural characterization of MWCNT-Fe3O4/Ag
3.1.1 BET analyze, total pore volume and average pore diameter of the MWCNT-Fe3O4/Ag
The specific surface area, average pore diameter, and average pore volume of MWCNT-Fe3O4/Ag, MWCNT, Fe3O4, and Ag were analyzed and summarized in Table 2. As included in Table 2, the specific surface area of MWCNT-Fe3O4/Ag with Fe3O4: Ag ratio of 2:1 was higher than other samples, which can be correlated to the formation of new pores and the extra surface on MWCNT. In simple term, reducing particle size and shaping of the binary nano-oxide (ball-like nanoparticle) could be the main reason for the increase in the surface area. Also, according to Azari et al. the replacement of Fe2+ with Ag+ to shape Fe–Ag solid solutions due to further inducing crystal defects might cause the promotion of their specific surface areas (Azari et al., 2015). Moreover, these samples were not simple and easy mixtures of nanoparticles (FeO and Ag2O), and a remarkable synergistic effect existed in this binary oxide system. Increasing in Ag content (Fe3O4: Ag ratio more than 2:1) have a negative effect on the specific surface area of adsorbent which led to decrease.
Sorbent
BET area (m2/g)
Total pore volume (Cm3/g)
Average pore diameter (Å)
MWCNT
79.4
0.18
2.16
Fe3O4
41.96
0.252
5.84
2:1*
143.34
0.743
8.31
3:1
124.24
0.678
5.91
1:1
119.57
0.591
5.31
1:3
86.74
0.409
3.21
1:2
95.74
0.456
4.21
Ag
23.81
0.224
3.45
3.1.2 SEM images and EDX analysis
The SEM/EDX images of the MWCNT-Fe3O4/Ag were presented in Figs. 2 and 3. It can be seen that the distribution of Fe3O4/Ag on the surface of MWCNT is relatively uniform, and the surface of the MWCNT became coarser after loading with Fe3O4/Ag particles Fig. 2a–c). As shown in Fig. 2(c), no significant change in the surface structure of the MWCNT-Fe3O4/Ag was observed after the extraction process. Only an increase in sample diameter is evident. Also, the sample showed a chain-like morphology without apparent aggregation. Therefore, the MWCNT as the supporting material is available for good dispersion of the Fe3O4/Ag particles. This leads to high adsorption ability of sorbent.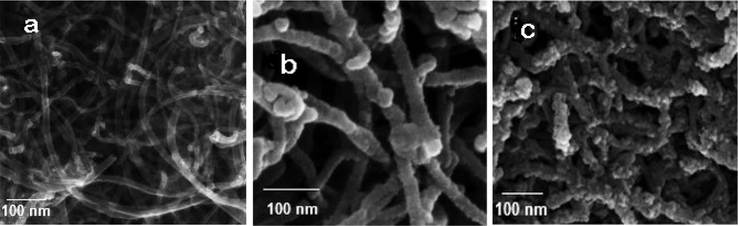
SEM image of MWCNT (a), SEM image of MWCNTs-Fe3O4/Ag (b) and SEM image of MWCNTs-Fe3O4/Ag after extraction process (c).
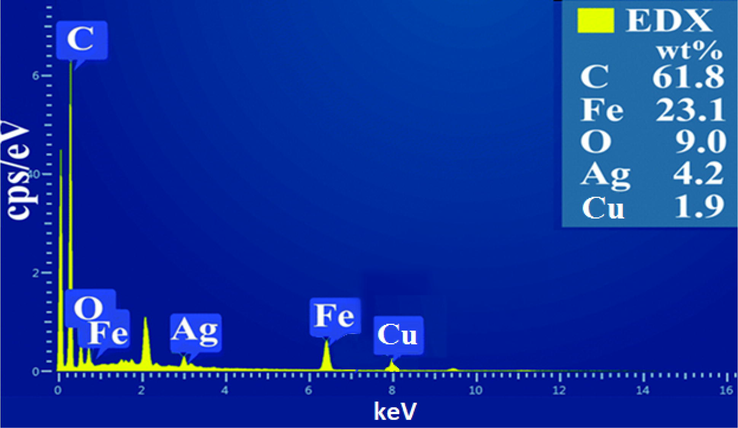
EDX spectrum of the synthesized MWCNTs-Fe3O4/Ag.
The chemical composition of the synthesized nanoparticles was analyzed by EDX. The EDX spectrum presents carbon (C), iron (Fe), oxygen (O), Ag and Copper (Cu). The atomic C, Fe, O, and Ag ratio (61.8, 23.1, 9.0, and 4.2%, respectively) as the primary elements in the MWCNT-Fe3O4/Ag structure confirmed the quantitative representation of the presence of Fe3O4 and Ag nanoparticles on the MWCNT surface Fig. 3. The peak obtained from Cu (1.9% wt) was caused by the copper grid utilized to fasten nanoparticles while the peak of C was obtained from carbon nanotubes.
3.1.3 XRD images
The structure of MWCNT and MWCNT-Fe3O4/Ag composites were further evaluated by using XRD analysis. The XRD patterns of samples were displayed in Fig. 4a, b. The strong diffraction peaks at 2θ = 26.2° correspond to reflections of (0 0 2) crystallographic planes of MWCNT (JCPDS No. 01-0646). After the deposition of iron oxide and silver nanoparticles, well-resolved diffraction peaks revealed the good crystallinity of the Fe3O4 specimens, with different defection peak located at 2θ of 35.96°, 44.17°, 54.45°, and 62.39°, respectively Fig. 4b). These diffraction peaks corresponding to planes (3 1 1), (4 0 0), (4 2 2) and (4 4 0) provide clear evidence for the formation of spinel structure of the ferrite. Meanwhile, it is observable from XRD diffraction that reflection arising from Ag exhibits at 2θ = 39.2°, 46.4°, and 65.8° correspond to the (1 1 1), (2 0 0), and (2 2 0) crystalline planes of metallic Ag (JCPDS No. 04-0783), while planes corresponding to MWCNT are remained at (0 0 2). The obtained XRD results confirmed that the Fe3O4/Ag were successfully coated on the texture of MWCNTs using a co-precipitation method.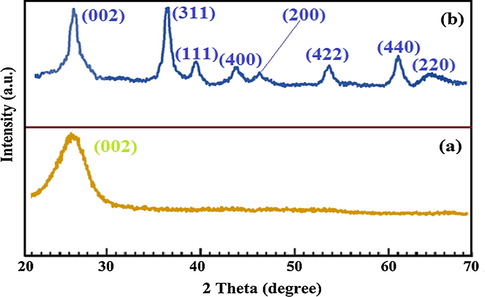
XRD pattern of MWCNTs (a) and MWCNT-Fe3O4/Ag (b).
3.1.4 TEM image
Representative TEM images of MWCNT-Fe3O4/Ag composites prepared by deposition-precipitation methods clearly showed that MWCNT were coated with large aggregates of bimetallic particles with a spherical shape Fig. 5). In another side, the composites composed of bimetallic particles (Fe3O4/Ag) demonstrated a homogenous distribution of silver particles on the iron oxide surface. Beside TEM image of the sample, considering Fig. 5, the MWCNT surface was loaded with nanoclusters, and each nanocluster was composed of several nanocrystals. The good crystallinity and clear lattice nanocrystals fringes were confirmed by TEM analyses. Fe3O4/Ag structure and shape were analyzed by TEM micrographs at 90 keV Fig. 5. According to this analysis, synthesized absorbent (MWCNT-Fe3O4/Ag) was sphere-shaped structure, almost uniform and intertwined. The results of TEM image indicate, small Ag NPs (∼5 nm) were distributed on the Fe (∼10 nm) surface.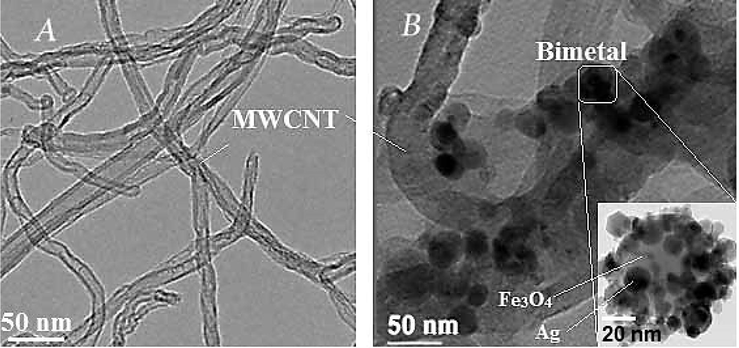
TEM image of MWCNTs (A), and modified MWCNTs with iron oxide and silver (MWCNT-Fe3O4/Ag) (B).
3.1.5 VSM image
The magnetic properties of the MWCNT-Fe3O4 and MWCNT-Fe3O4/Ag at room temperature were measured using a vibration sample magnetometer (VSM), and results were demonstrated in Fig. 6. It can be seen that the magnetic properties of MWCNT-Fe3O4 and MWCNT-Fe3O4/Ag were measured as 77.94 emu g−1 and 61.18 emu g−1, respectively. The smaller analyzed nanoparticles could offer superparamagnetic characteristics. In our study, the decrease in the values of saturation magnetization (Ms) of the MWCNT-Fe3O4/Ag in comparison with MWCNT/Fe3O4 can be attributed to the formation of an imprinting layer on the MWCNT-Fe3O4 surface, which makes the former particles occupy more space comparing to latter one and consequently decrease their superparamagnetic properties which are in good agreement with the previously conducted study (Qu et al., 2008). The Fig. 6 indicates that MWCNT-Fe3O4/Ag can be separated from the solution rapidly with no secondary pollution under an external magnet at ∼15 s. It can be observed from Fig. 7 that the prepared sorbent (MWCNT-Fe3O4/Ag) has the good magnetic performance for magnetic separation and extraction processes.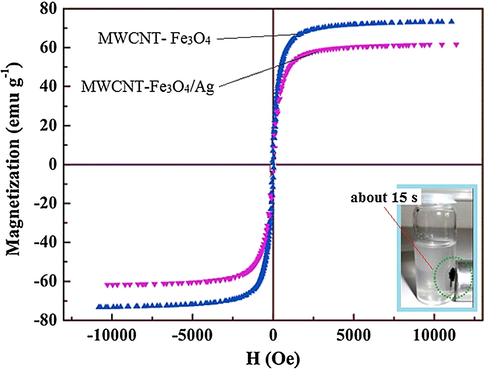
Magnetization curve of magnetic MWCNT-Fe3O4 and MWCNT-Fe3O4/Ag.
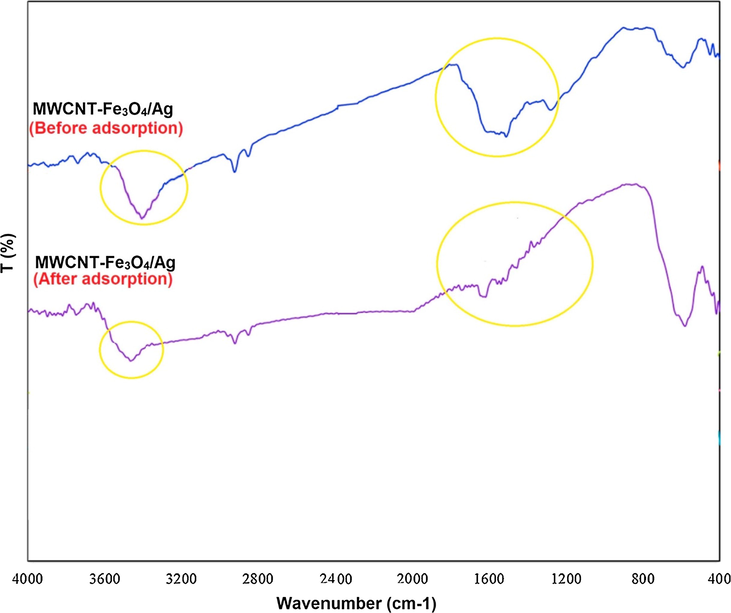
FT-IR spectra MWCNT-Fe3O4/Ag before and after extraction.
3.1.6 Fourier Transform Infrared (FT-IR) analysis
FT-IR spectra of the spectra MWCNT-Fe3O4/Ag before and after extraction was shown in Fig. 7. The typical broad peak at 3000–3500 cm−1, denotes to the O—H stretching frequencies of the hydroxyl group. The peak at 586 cm−1 attributed to the stretching vibration of Fe-O-Fe in Fe3O4. The peak at 1610 cm−1 is for the C⚌C stretching mod and N—H bending vibrations. A big broad peak in the range of 3000–3500 cm−1 is consistent with the presence of OH groups become shifts and narrower after PAEs extraction slightly, indicating that the population of OH-containing groups decreased during the extraction process. Beside, Vibration peaks near 1180–1460 cm−1 (Metal-OH) show a decrease in intensity that implied the loss and decrease of surface hydroxyl groups during the PAEs extraction. In another word, surface hydroxyl groups (M−OH) were replaced by the adsorbed PAEs and can play an important role in the extraction of PAEs. Some researchers believed different possible mechanisms which could be considered: (i) hydrophobic interactions between PAEs and adsorbent; (ii) π-π electron donor-acceptor (EDA) interactions; (iii) hydrogen bonding between the PAEs molecule and the adsorbent O-containing functional groups; (iv) replacement between the —OH groups on adsorbent surface and PAEs aromatic surfaces. Based on FTIR after PAEs extraction —OH groups replacement between PAEs molecules and MWCNT-Fe3O4/Ag surface could play a key role in adsorption and was considered to be a primary mechanism (Azari et al., 2015; Ghaffar and Abbas, 2016).
3.2 Extraction procedure optimization
A certain quantity of MWCNT-Fe3O4/Ag as the adsorbent is required for recovery of PAEs. Concerning this matter, some tests were performed by adding 0.5–2.5 mg/mL of adsorbent to every sample in order to find the optimum dose. As shown in Fig. 8A, a dose level of 10 mg sorbent was adequate to extract the PAEs from 10 mL of carbonated soft drink. The recoveries PAEs analytes were reported as higher than 90%.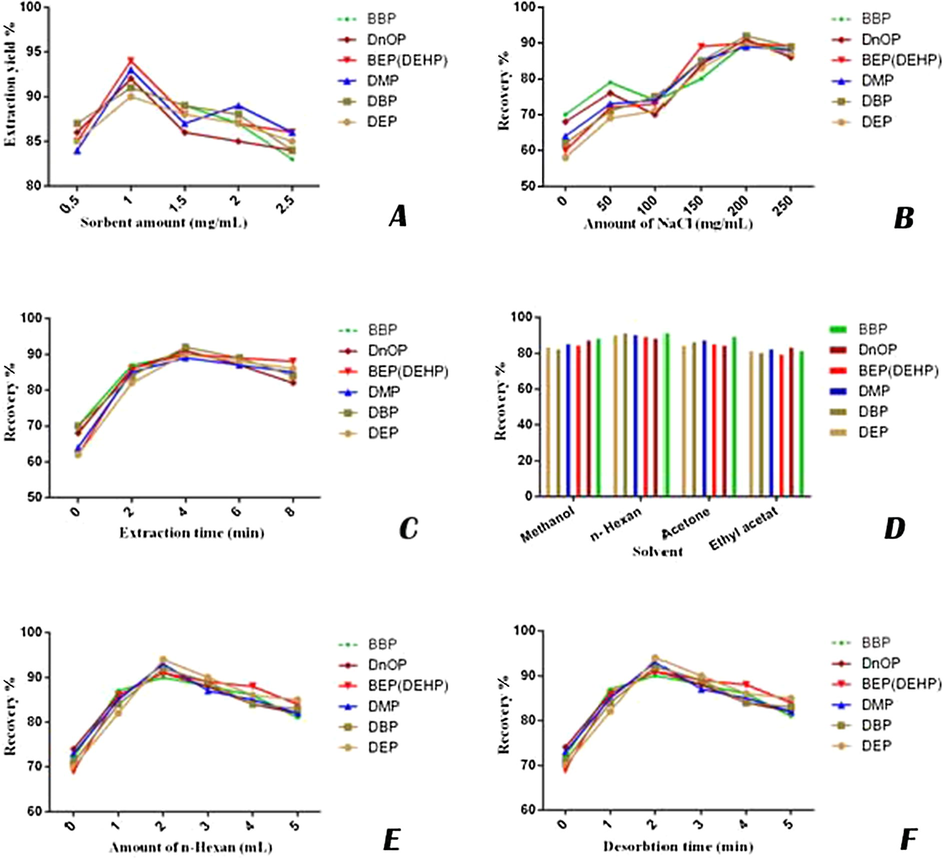
The effect of different factors on extraction efficiency in sample solution: effect of sorbent amount (A), the effect of salt concentration (B), investigating the extraction time (C), optimization of desorption solvent (D), amount of n-hexane (E), and investigating the desorption time (F).
Furthermore, the effect of salting out on the efficiency of PAEs extraction was assessed. Based on the previous investigations, adding salt can have positive effects on MSPE procedure by reducing the solubility of organic analytes such as PAE and increasing their distribution constant. Hence, the concentration of remaining analyte can be determined rapidly. However, the addition of salt above a specific level might increase the viscosity of organic analyte, consequently decrease extraction capability (Wang et al., 2014). Thus, various amounts of NaCl (0–2.5 g) was added to each sample in order to evaluate the effect of salt on the extraction efficiency. The maximum extraction efficiency was achieved when adding 2 g of NaCl Fig. 8B). The effect of salt addition can significantly vary depending on the targeted compound. Therefore, the maximum concentration should be determined for each analyte in order to obtain the best extraction efficiency. Liang et al. (2008) investigated ionic strength on three different PAEs and found out that addition of NaCl (0–5% (w/v)) decreased the solubility of extraction solvent, while it had no significant effect on the extraction recovery of PAEs using dispersive liquid-liquid microextraction (DLLME) (Liang et al., 2008). The similar results were obtained by Meng et al. (2011). However, they investigated PAEs in water samples. The type of sample that contains PAEs to be examined will dramatically alter the effect of ionic strength.
Extraction times, for all the analytes, were in the range of 0–8 min Fig. 8C). After 4 min of the extraction process, almost it was possible to recover over 90% of 6 types of PAEs. Four solvents including; methanol, n-hexane, acetone and ethyl acetate were used for desorption. The results showed that the best extraction was obtained by using n-hexane Fig. 8D). As shown in Fig. 8E, 1–5 mL n-hexane was used for desorption. The results showed that 2 mL had the highest efficiency for desorption between all examined analytes. Also as shown in Fig. 8F, desorption time of 2 min was the best time between all examined desorption times (from 0.5 to 5 min).
3.3 Quantitative analysis
Six different PAEs (DBP, BBP, DEP, DMP, DEHP, and DNOP), at concentrations ranging from LOQ to 10,000 ng/L, were detected. The calibration curves were plotted, and the linearity was determined at the mentioned concentration ranges. The obtained limit of quantitation (LOQ) was considered as the lower range of linear range and 10,000 ng/L as the higher limit of linearity. The coefficient of correlation in the obtained calibration curves was excellent, ranging from 0.9981 to 0.9995 Table 3). The LODs (Limit of Detections) in our study was defined based on 3σ criteria and theoretically determined at three times the standard deviation (SD) of the baseline noise (n = 6), which was experimentally evaluated by serially spiking diluted analyte standards of phthalate esters into the control carbonated soft drink sample. The LOQ was also determined based on 10σ criteria. The results of our study exhibited that the limit of detections (LODs) and limit of quantitations (LOQs) for the target analyte ranged from 10.8 to 22.5 ng/L and 36 to 75 ng/L, respectively Table 3. Three levels of low, medium and high concentrations of prepared mix standard samples (between calibration range) were analyzed and duplicated after extraction with MWCNT-Fe3O4/Ag after several days in order to control the quality of developed calibration models. The intra- and inter-day precision was assessed for 3 consecutive days in triplicate analyzes which were less than 8% and 7.8%, respectively Table 4). The relative error of the analytes was studied at the same concentration levels of QC samples, and the results are shown in Table 4. According to the intra-day and inter-day RSDs%, the developed magnetic SPE method coupled with GC–MS showed perfect precision. On the other hand, relative error (RE %) with the RSDs less than 8%, indicated the very good accuracy of the suggested method. It is worthy to mention that no interfering peak in the region of the phthalate esters analytes and internal standard had been seen. The batch-to-batch synthesis of MWCNTs and MWCNT/Fe3O4/Ag were evaluated during the repetition of experiments in the calculation of estimated recoveries for QC samples. In another word, due to high amounts of samples which were evaluated in the current work, synthesis of MWCNT and MWCNT/Fe3O4/Ag were done in different batches, and no significant lack in recoveries was observed. In the present study, the recovery (%) of the six target analyte (PAEs) that spiking in real samples (100 ng/L) was determined at a range of 96.60–109.22 % Table 5). Table 6 summarizes the reported recoveries of other absorbents on PEAs in literature, compared with prepared MWCNT-Fe3O4/Ag in our study. It was found that the recovery (%) of MWCNT-Fe3O4/Ag was premiered to many other adsorbents mainly due to an increase of the porous structure in the adsorbents. Therefore, synthesized adsorbent could be considered as a new adsorbent for determination of PAEs. NA: not applicable because 50 ng/L is lower than LOQ.
Compound
Upper limit of linearity (ng/L)
LOD (ng/L)
LOQ (ng/L)
Coefficient of estimation (r2)
DMP
10,000
22.5
75
0.9981
DEP
10,000
16.2
54
0.9985
DBP
10,000
10.8
36
0.9995
BBP
10,000
18
60
0.9992
DNOP
10,000
13.5
45
0.9990
DEHP
10,000
14.4
48
0.9987
Compound
Sample
Nominal concentration (ng/L)
Mean of calculated concentration (ng/L)
RSD (%) of calculated concentration (intra-day)
RSD (%) of calculated concentration (inter-day)
Relative error (%)
DMP
QC1
50
NA
–
–
–
QC2
500
503
6.6
7.1
0.6
QC3
5000
5050
7.9
7.8
1.0
DEP
QC1
50
NA
–
–
–
QC2
500
510
8.0
6.4
2.0
QC3
5000
5100
5.8
6.2
2.0
DBP
QC1
50
53
7.2
6.3
6.0
QC2
500
505
6.7
6.8
1.0
QC3
5000
5045
6.9
7.0
0.9
BBP
QC1
50
56
5.9
5.6
12.0
QC2
500
512
7.7
6.4
2.4
QC3
5000
5039
7.0
6.5
0.8
DNOP
QC1
50
56
6.3
5.2
12.0
QC2
500
504
7.2
7.3
0.8
QC3
5000
5105
5.9
6.6
2.1
DEHP
QC1
50
49
7.8
6.9
−2.0
QC2
500
515
6.7
6.0
3.0
QC3
5000
5070
7.3
6.8
1.4
Real and spike samples
Recoveries (%)
Sample/target compound
Amount of spike
DMP
DEP
DBP
BBP
DEHP
DNOP
DMP
DEP
DBP
BBP
DEHP
DNOP
1
0
ND
ND
55.35 ± 3.1
ND
4008.26 ± 5.7
54.23 ± 2.4
–
–
–
–
–
–
1
100
102
98
159.22
97
4110
149
102
98
102.49
97
100.04
96.60
2
0
ND
ND
73.43 ± 2.5
ND
2105.60 ± 6.3
49.43 ± 2.7
–
–
–
–
–
–
2
100
105
106
172
102
2210
160
105
106
99.17
102
100.22
107.07
3
0
ND
ND
42.89 ± 3
ND
4448.37 ± 3.6
117.78 ± 4.5
–
–
–
–
–
–
3
100
97
101
150
99
4600
220
97
101
104.97
99
101.13
101.01
4
0
ND
ND
ND
ND
3420.71 ± 7.5
250.81 ± 3
–
–
–
–
–
–
4
100
102
98
99
103
3600
348
102
98
99
103
102.25
99.19
5
0
ND
ND
67.33 ± 2.5
ND
1669.04 ± 5
86.86 ± 3.5
–
–
–
–
–
–
5
100
98
101
170
99
1810
190
98
101
101.59
99
102.31
101.68
6
0
ND
ND
ND
ND
1762.17 ± 4.8
112.84 ± 3.2
–
–
–
–
–
–
6
100
99
103
101
97
1900
222
99
103
101
97
102.03
104.30
7
0
ND
ND
102.27 ± 2.1
ND
2680.53 ± 2
155.87 ± 1.3
–
–
–
–
–
–
7
100
103
98
212
97
2800
271
103
98
104.81
97
100.70
105.91
8
0
ND
ND
128.89 ± 2.2
ND
9301.6 ± 5.3
665.94 ± 4.2
–
–
–
–
–
–
8
100
98
102
250
97
9450
770
98
102
109.22
97
100.52
100.5
Phthalate
Sorbent/time
Matrix
R2
Repeatability
LOD/LOQ
Recovery
Refs
DMP, DEP, DBP, BBP, DAP, DNOP
Molecularly imprinted polymer/30 min
Bottled water, tap water
0.9898–0.9993
3.08–7.81
3–21/20–34
94.54–105.34
He et al. (2010)
DPP, DBP, DiBP, DPP, BBP
MWCNTs-PPy/25 min
Mineral water, tap water
0.9891–0.9959
8.4–10.5
50–100/–
96–105
Asadollahzadeh et al. (2010)
DBP, BBP
Polyacrylate fiber/30 min
Food simulant
0.9854–0.9905
11.7–16.2
80–310/200–500
–
Moreira et al. (2013)
DMP, DEP, DiBP, DBP, BBP, DEHP, DNOP
Polypyrrole-coated Fe3O4/15 min
Tap water
0.9985–0.9910
3.4–11.7
6–68/20–30
91.1–113.4
Meng et al. (2011)
DMP, DiPP, DEP, DPP, DiBP, DBP, DiAP, DEHP, DOP, BBP
PA + PDMS/20 min
Bottled water, tap water
0.9613–0.9987
11.3–18.5
10–60/40–190
73.9–100.7
Santana et al. (2014)
DMP, DEP, DBP, BBP, DEHP, DNOP
CTFs-Ni/5 min
in plastic packaging materials/
0.9989–0.9993
–
0.024–0.085/0.08–0.32
70.6–119
Yan et al. (2017)
DEP, DPP, DBP, DIPP, DNPP, BBP, DCHP, DEHP, DNOP, DINP, DIDP, DEHA
Fe3O4@pDA NPs/5 min
water samples
0.9911–1.0000
–
–/9–20
71–120
González-Sálamo et al. (2017)
DEP, DAP, DPRP
MSC/2 min
lake water and milk samples
0.9947–0.9999
2.1–8.6
0.05–0.08 and 0.1–0.2/–
80.1–112.6
Wu et al. (2017)
DEP, DMP, DBP, BBP, DNOP, DEHP
MWCNT-Fe3O4/Ag/2 min
Soft drinks
0.9981–0.9995
–
10.8–22.5/36–75
96.60–109.22
This study
Moreover, the MWCNT-Fe3O4/Ag was dried in an oven (at 50 °C) among regeneration. In this study, adsorption/desorption of PAEs by MWCNTs-Fe3O4/Ag was performed in batch condition and shown in Fig. 9. This Figure shows that ability of reuse of MWCNTs-Fe3O4/Ag slightly reduced after the 6 successive sorption-desorption cycles. Therefore, we recommended that MWCNTs-Fe3O4/Ag can repeatedly be used for PAEs sorption without many losses in initial sorption efficiency. Furthermore, >63.65% adsorbed PAEs could be desorbed/recovered in the presence of soft drinks in the sixth cycle.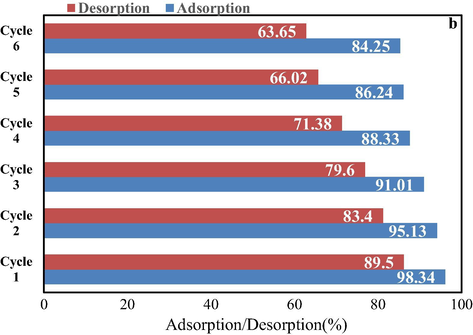
The adsorption/desorption performance of prepared MWCNTs-Fe3O4/Ag.
3.4 Determining PAEs in carbonated soft drink samples
In order to verify the efficiency of new adsorbent (MWCNT-Fe3O4/Ag), the extraction of 6 different PAEs was investigated in real samples. For this, eight random carbonated soft drink samples were collected, prepared and analyzed in triplicates with the method mentioned above in further part to determine the concentrations of PAEs. The results showed that the highest measured concentration among PAEs belonged to DEHP Table 5, which was similar to the previously reported results regarding the concentration of PAEs in carbonated soft drinks (Wu et al., 2012; Rocher et al., 2008) and mineral water (Jeddi et al., 2014). There is no Maximum Contamination Level (MCL) for PAEs concentration level in soft drinks. However, in the case of DEHP, United States Environmental Protection Agency (EPA) established a limit of 6 ppb (μg/L) in drinking water (Jeddi et al., 2014). The results showed that the concentration of DEHP in sample #8 (9301.6 ± 5.3 ng/L) was higher than the recommended level by EPA (Jeddi et al., 2014; Kouhpayeh et al., 2017). To check the selectivity of the method, several spike studies in mentioned carbonated soft drink samples were conducted. Furthermore, the good recovery was obtained without any interfering of analyte signals by other compounds in real samples Table 5. The chromatograph of GC–MS for a real carbonated soft drink sample was shown in Fig. 10.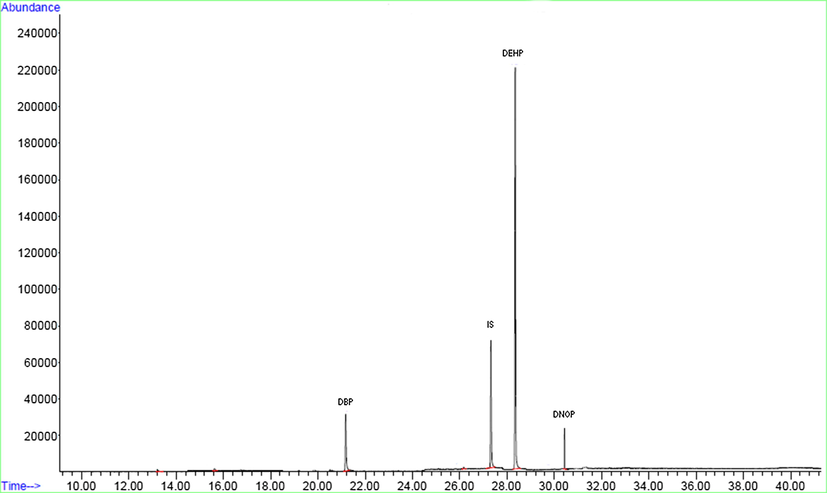
A typical chromatogram of GC–MS for real sample.
4 Conclusion
In the current study, MWCNT-Fe3O4/Ag as a novel adsorbent was synthesized using iron (magnetic agent), and silver (catalytic and surface enhancer agents) to determine the concentration of PAEs in carbonated soft drinks. The high efficiency and sensitivity of prepared adsorbent (MWCNT-Fe3O4/Ag) were confirmed by MSPE-GC/MS. LOD and LOQ values were in the range of 10.8–22.5 and 36–75 ng/L, respectively. The XRD pattern, FT-IR, EDX analysis, SEM, TEM and VSM images showed that the MWCNT-Fe3O4/Ag had been successfully synthesized and the efficiency and adsorbent power of MWCNT-Fe3O4/Ag has confirmed accordingly. It should be noted that both preparation and construction methods of adsorbent described in this study are novel, and the prepared adsorbent has high capability to adsorb and desorb PAEs as organic compounds in complex environments such as food and beverage.
Conflict of interest
Authors declare that they have no conflict of interest.
Acknowledgment
This work was funded by the Department of Public Health, and Medical Sciences of Tehran University (Grant no. 93-02-27-25826) and at this moment authors are grateful for their financial support. Also, the collaboration of Iran Nanotechnology Initiative Council and the Research Consultation Center (RCC) at Shiraz University of Medical Sciences is greatly appreciated. I. Eş gratefully acknowledges the financial support of the São Paulo Research Foundation (FAPESP) (Grant # 2015/14468-0). Amin Mousavi Khaneghah likes to thank the support of CNPq-TWAS Postgraduate Fellowship (Grant #3240274290).
References
- Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J. Hazard. Mater.. 2009;164(2):1130-1136.
- [Google Scholar]
- Novel multifunctional carbon nanotube containing silver and iron oxide nanoparticles for antimicrobial applications in water treatment. Mater. Today:. Proc.. 2017;4(1):57-64.
- [Google Scholar]
- Hexavalent chromium removal from aqueous solutions by using low-cost biological wastes: equilibrium and kinetic studies. Int. J. Environ. Sci. Technol.. 2012;9(2):319-326.
- [Google Scholar]
- Alkylphenols and phthalates in bottled waters. J. Hazard. Mater.. 2011;185(1):281-286.
- [Google Scholar]
- Solid-phase microextraction of phthalate esters from aqueous media by electrochemically deposited carbon nanotube/polypyrrole composite on a stainless steel fiber. Anal. Chim. Acta. 2010;669(1–2):32-38.
- [Google Scholar]
- Iron–silver oxide nanoadsorbent synthesized by co-precipitation process for fluoride removal from aqueous solution and its adsorption mechanism. RSC Adv.. 2015;5(106):87377-87391.
- [Google Scholar]
- One-pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr (VI) and Ni (II) ions from aqueous solution: kinetic, isotherm and thermodynamic studies. J. Environ. Health Sci. Eng.. 2016;14(1):11.
- [Google Scholar]
- Synthesis and characterization of amino-functionalized magnetic nanocomposite (Fe3O4-NH2) for fluoride removal from aqueous solution. Desalin. Water Treat.. 2017;65:367-374.
- [Google Scholar]
- Subcritical water extraction for the remediation of phthalate ester-contaminated soil. J. Hazard. Mater.. 2011;192(3):1203-1209.
- [Google Scholar]
- Hazardous organic compounds in groundwater near Tehran automobile industry. Bull. Environ. Contam. Toxicol.. 2010;85(5):530-533.
- [Google Scholar]
- U. Food, Drug Administration FDA, Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human, Services Food and Drug Administration, and Center for Drug Evaluation and Research, 2001.
- Kinetics, thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chem. Eng. J.. 2012;187:133-141.
- [Google Scholar]
- Adsorption of phthalic acid esters (PAEs) on chemically aged biochars. Green Process. Synth,. 2016;5(4):407-417.
- [Google Scholar]
- Determination of phthalic acid esters in water samples using core-shell poly (dopamine) magnetic nanoparticles and gas chromatography tandem mass spectrometry. J. Chromatogr. A. 2017;1530:35-44.
- [Google Scholar]
- Gorji, M.E.H., Ahmadkhaniha, R., Moazzen, M., Yunesian, M., Azari, A., Rastkari, N., 2016. Polycyclic aromatic hydrocarbons in Iranian Kebabs. Food Control 60, 57–63.
- Preparation and evaluation of molecularly imprinted solid-phase micro-extraction fibers for selective extraction of phthalates in an aqueous sample. Anal. Chim. Acta. 2010;674(1):53-58.
- [Google Scholar]
- Jeddi, M.Z., Ahmadkhaniha, R., Yunesian, M., Rastkari, N., 2014. Magnetic solid-phase extraction based on modified magnetic nanoparticles for the determination of phthalate diesters in water samples. J. Chromatogr. Sci. bmu058.
- Determination of trace leaching phthalate esters in water by magnetic solid phase extraction based on magnetic multi-walled carbon nanotubes followed by GC-MS/MS. Anal. Methods. 2012;4(9):2729-2734.
- [Google Scholar]
- Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: equilibrium, kinetic and thermodynamic studies. Iran J Environ Health Sci Eng. 2013;10(1):10-19.
- [Google Scholar]
- Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng. 2014;12:115.
- [Google Scholar]
- Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng.. 2014;12(1):115.
- [Google Scholar]
- Method development for determination of migrated phthalate acid esters from polyethylene terephthalate (PET) packaging into traditional Iranian drinking beverage (Doogh) samples: a novel approach of MSPE-GC/MS technique. Environ. Sci. Pollut. Res. 2018
- [Google Scholar]
- Extraction and determination of phthalate esters (PAEs) in Doogh. J. Mazandaran Univ. Med. Sci.. 2017;26(145):257-267.
- [Google Scholar]
- Application of dispersive liquid–liquid microextraction and high-performance liquid chromatography for the determination of three phthalate esters in water samples. Anal. Chim. Acta. 2008;609(1):53-58.
- [Google Scholar]
- Improved debromination of polybrominated diphenyl ethers by bimetallic iron–silver nanoparticles coupled with microwave energy. Sci. Total Environ.. 2012;429:300-308.
- [Google Scholar]
- Removal of chromium (VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere. 2011;85(7):1204-1209.
- [Google Scholar]
- Preparation of polypyrrole-coated magnetic particles for micro solid-phase extraction of phthalates in water by gas chromatography–mass spectrometry analysis. J. Chromatogr. A. 2011;1218(12):1585-1591.
- [Google Scholar]
- Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in grilled meat samples. Talanta. 2013;115:957-965.
- [Google Scholar]
- Determination of endocrine disruptors in water after derivatization with N-methyl-N-(tert.-butyldimethyltrifluoroacetamide) using gas chromatography with mass spectrometric detection. J. Chromatogr. A. 2000;879(1):97-112.
- [Google Scholar]
- Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int. J. Env. Res. Pub. He.. 2013;11(1):507-526.
- [Google Scholar]
- Use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. J. Hazard. Mater.. 2009;161(1):575-580.
- [Google Scholar]
- Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard. Mater.. 2008;160(2):643-647.
- [Google Scholar]
- Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu (II) and Cd (II) from water: surface, kinetic, equilibrium, and thermal adsorption properties. Chem. Eng. J.. 2013;223:806-815.
- [Google Scholar]
- Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of phthalate monoesters in urine samples. J. Chromatogr. A. 2013;1286:22-28.
- [Google Scholar]
- Removal of organic dyes by magnetic alginate beads. Water Res.. 2008;42(4):1290-1298.
- [Google Scholar]
- Application of nano aluminum oxide and multi-walled carbon nanotube in fluoride removal. Desalination. 2017;1:6.
- [Google Scholar]
- Preliminary toxicological assessment of phthalate esters from drinking water consumed in Portugal. Environ. Sci. Pollut. R.. 2014;21(2):1380-1390.
- [Google Scholar]
- As (V) removal using carbonized yeast cells containing silver nanoparticles. Water Res.. 2011;45(2):583-592.
- [Google Scholar]
- Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J. Hazard. Mater.. 2011;192(3):1321-1329.
- [Google Scholar]
- A graphene-coated magnetic nanocomposite for the enrichment of fourteen pesticides in tomato and rape samples prior to their determination by gas chromatography-mass spectrometry. Anal. Methods. 2014;6(1):253-260.
- [Google Scholar]
- Extraction of phthalate esters from water and beverages using a graphene-based magnetic nanocomposite prior to their determination by HPLC. Microchim. Acta. 2012;177(1–2):23-30.
- [Google Scholar]
- Magnetic spherical carbon as an efficient adsorbent for the magnetic extraction of phthalate esters from lake water and milk samples. J. Sep. Sci.. 2017;40(10):2207-2213.
- [Google Scholar]
- Magnetic covalent triazine framework for rapid extraction of phthalate esters in plastic packaging materials followed by gas chromatography-flame ionization detection. J. Chromatogr. A. 2017;1525:32-41.
- [Google Scholar]
- A novel structure for carbon nanotube reinforced alumina composites with improved mechanical properties. Nanotechnology. 2008;19(31):315708.
- [Google Scholar]







