Translate this page into:
Effects of different extraction methods on physicochemical characteristics and bioactivities of fig (Ficus carica L.) leaves polysaccharides
⁎Corresponding authors at: School of Biology and Food Engineering, Changshu Institute of Technology, Suzhou 266003, Jiangsu Province, PR China(Y.-T. Xue), Department of Cardiology, XinHua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200093, PR China(Q. Wang). xueyiting@cslg.edu.cn (Yi-Ting Xue), wangqian8464@xinhuamed.com.cn (Qian Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
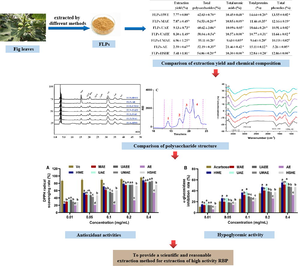
Abstract
Fig leaves polysaccharides obtained by ultrasound assisted extraction method (FLPs-UAE) showed the strongest antioxidant and hypoglycemic activities. FLPs-UAE possessed higher Mw, total polysaccharide content (60.62 ± 3.06 %) and Glucosamine content (7.68 %). UAE was a good way to extract FLPs with high antioxidant and anti-diabetic activities.
Abstract
The tea of fig (Ficus carica L.) has long been used as a medicine in China due to its excellent effects on hemorrhoids, damp heat diarrhea and other diseases. The present study was designed to evaluate the impacts of different extraction methods, including hot water extraction (HWE), microwave assisted extraction (MAE), ultrasound assisted extraction (UAE), ultrasound assisted enzymatic extraction (UAEE), ultrasound-microwave assisted extraction (UMAE), alkali extraction (AE) and high-speed shearing homogeneous extraction (HSHE), on physicochemical characteristics and bioactivities of polysaccharide from fig leaves (FLPs). Results indicated that there were great differences between the structure and activity of FLPs prepared by seven methods. Among them, FLPs extracted by UAE (FLPs-UAE) showed the strongest antioxidant and hypoglycemic activities due to its higher Mw, total polysaccharide content (60.62 ± 3.06 %) and glucosamine (GlcN) content (7.68 %). In addition, FLPs extracted by HWE possessed more total polysaccharide content (62.03 ± 0.70 %), total phenolic compounds content (13.55 ± 0.02 %) and GlcN content (5.78 %) contributed to its strong antioxidant activity and the inhibition on α-amylase and α-glucosidase, which was second to FLPs-UAE. These finding suggested that UAE and HWE, especially UAE, were good ways to extract FLPs with high antioxidant and anti-diabetic activities, which provide foundations for choosing the suitable extraction strategy to prepare FLPs with desired bioactivities and promoting the application of FLPs in function food industries.
Keywords
Fig leaves
Polysaccharide
Extraction method
Antioxidant activity
α-glucosidase
1 Introduction
Fig (Ficus carica L.), a common deciduous fruit tree distributed in warm temperate zones, is classified as the Moraceae family (Khadivi et al., 2018; Kislev et al., 2006; Ercisli et al., 2012). In China, fig fruit and leaves are highly requested as a general tea raw material and an important Chinese medicine to treat hemorrhoids, damp heat diarrhea and other diseases (Ercisli et al., 2012). Modern medical research had proved that fig leaves contain a large number of components that are beneficial to the human body, including polysaccharides, flavonoids, polyphenols and coumarin compounds (Sedaghat and Rahemi, 2018; Yang et al., 2009; Li et al., 2021; Zou et al., 2020). The rich polysaccharides in fig leaves, as the main functional factor, had antioxidant, anti-inflammatory, anti-diabetics, anti-atheroscloresis, immune regulation, and anti-tumor effects (Yang et al., 2009; Du et al., 2018; Li et al., 2021; Zou et al., 2020). So, polysaccharides extracted from fig leaves (FLPs) have a potential application value in the field of medicine and functional food.
There are many extraction techniques for polysaccharides with its own advantages and disadvantages. As the most commonly used method, hot water extraction (HWE) has the characteristics of simple operation and low instrument requirements (Yang et al., 2017; Chen et al., 2016). However, its disadvantage is time consuming, energy consuming, and low yield (He et al., 2016). With the continuous promotion and application of modern technology, new extraction methods for polysaccharides have emerged to improve extraction efficiency, including microwave assisted extraction (MAE) (Chen et al., 2017), high-speed shearing homogenization extraction (HSHE) (Dias et al., 2020), ultrasound assisted extraction (UAE) (Lin et al., 2018), ultrasound assisted enzymatic extraction (UAEE) (Wu et al., 2019; Li et al., 2016), ultrasound-microwave assisted extraction (UMAE) (Chen et al., 2010; Sun et al., 2019) and chemical solvent extraction (Jiang et al., 2019). Among them, MAE, UAE, UMAE and UAEE are environmentally friendly methods due to the savings in energy and solvent by accelerating the mass transfer of target compounds, which had high requirements on equipment. HSHE and chemical solvent extraction are efficient extraction techniques with low extraction temperature, low energy consumption, and short extraction time. Studies have shown that extraction technology is crucial for the utilization of natural polysaccharides to alter the yields, physicochemical properties and biological activities of polysaccharides (Meng et al., 2022; Chen et al., 2018). Fu et al. found that selecting appropriate extraction methods can obtain polysaccharides with desired bioactivities for applications (Fu et al., 2020). For example, loquat leaves polysaccharides (LLPs) extracted by UMAE possessed a strong prebiotic effect. The strong antioxidant activities and inhibitory effects on α-amylase and α-glucosidase were appeared in LLPs extracted by MAE and UAE. However, so far, there was no in-depth study available about the relationship between extraction techniques and physicochemical property or bioactivity of FLPs.
In this paper, in order to evaluate the effects of different extraction techniques on the physicochemical properties and biological activities of FLPs, we firstly prepared FLPs from fig leaves by seven extraction methods, including HWE, MAE, UAE, UAEE, UMAE, HSHE, and alkali extraction (AE). After that, the physicochemical properties, structure and bioactivities of FLPs obtained by seven methods were determined, analyzed and compared. The results will provide a scientific basis for selecting a suitable extraction method to obtain FLPs with relatively high biological activity, and also provide a reference for realizing the high-value utilization of fig leaves and other agricultural wastes.
2 Experimental
2.1 Materials
Fig leaves were obtained from Jiujiang (Jiangxi, China). Glucose (Glc), galactose (Gal), fucose (Fuc), rhamnose (Rha), glucuronic acid (GlcA), glucosamine (GlcN), galactosamine (GalN), phenyl-3-methyl-5-pyrazolone (PMP), α-amylase (1000 U/mg), α-glucosidase (10 U/mg) were purchased from Sigma Aldrich (Sigma, St Louis, MO, USA). Mannose (Man) and xylose (Xyl) were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Galacturonic acid (GalA) was provided from Honeywell FlukaTM (Seelze, Germany). Arabinose (Ara) was obtained from VetecTM (Sigma-Aldrich, USA). The dextran standards (180, 2700, 5250, 9750, 13050, 36800, 64650, 135350, 2000000 Da) were provided from the National Institute for Food and Drug Control (Beijing, China). 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). All of the other reagents used were of analytical grade.
2.2 Preparation of FLPs by different extraction methods
Fig leaves were dried, pulverized and passed through a 60-mesh sieve. Then, the powders were degreased with petroleum ether for 12 h at a ratio of 1:2 (m/v). After filtration and drying, 10 g of degreased FLPs was mixed with water at a ratio of 1:20 (m/v), and extracted using seven techniques including HWE, MAE, UAE, UAEE, UMAE, HSHE and AE, according to previous researches (Fu et al., 2020; Meng et al., 2022). Each method was performed in triplicate.
HWE was performed in a water-bath at 80 °C for 3 h. MAE was performed in a microwave oven at 500 W for 6.5 min. UAE was performed in an ultrasonic homogenizer with 400 W for 60 min. For UAEE, 100 mg of cellulase was added and reacted for 1 h, and then performed in an ultrasonic homogenizer at 400 W for 60 min. UMAE was performed in an ultrasound-microwave collaborative extraction device at 100 W for 60 min. For AE, sodium carbonate powder was slowly added to a concentration of 12 % and reacted in a constant temperature water bath at 70 °C for 1.5 h. HSHE was performed in a high-speed shearing homogenizer at a speed of 7500 rpm for 60 min.
After centrifugation, the protein of crude extract in each supernatant was removed with papain (pH 6.2, 0.07 mg/mL) at 55 °C for 1 h. Then, the enzyme was inactivated at 90 °C for 15 min, and supernatant was condensed to 1/3 of its original volume. Three volumes of ethanol were added to precipitate crude polysaccharides. Furthermore, crude polysaccharides were redissolved and dialyzed (Mw cut of: 1000 Da) against distilled water for 48 h. Finally, FLPs extracted by seven methods were obtained, freeze-dried and numbered as FLPs-HWE, FLPs-MAE, FLPs-UAE, FLPs-UAEE, FLPs-UMAE, FLPs-HSHE, and FLPs-AE, respectively.
The FLPs yield of seven extraction techniques was calculated using the following equation:
Where, mFLPs and msample were the quality in grams of FLPs and fig leaves powder, respectively.
2.3 Chemical components analysis of FLPs
The protein content in FLPs was measured by the Bradford's method (Bradford, 1976; Blumenkrantz and Asboe-Hansen, 1973) with bovine serum albumin (BSA) as the standard. The content of uronic acids in FLPs was determined by the sulfuric acid-carbazole method (Blumenkrantz and Asboe-Hansen, 1973) with GalA as the standard. Total polysaccharides content was tested by the sulfuric acid-phenol method (Dubois et al., 1956) using Glc as the standard. Total phenolics content was detected by the Folin-Ciocalteu method (Chen et al., 2015) using gallic acid as the standard.
2.4 Structural characterization of FLPs
2.4.1 Monosaccharide composition analysis
Monosaccharide composition of FLPs was detected by pre-derivatization high performance liquid chromatography (HPLC) as previously described (Meng et al., 2022; Chai and Zhao, 2016). Briefly, 20 mg of FLPs was degraded in a sealed glass ampoule with 1 mL trifluoroacetic acid (2 M) at 115 ℃ for 4 h. 200 µL of hydrolysate solution was dried with a nitrogen blower, redissolved in equal amounts of water, and then mixed with 200 µL NaOH solution (0.3 mol/L) and 244 µL PMP solution (0.5 mol/L). After incubating at 70 ℃ for 1 h, the mixture was neutralized with 220 µL HCl solution (0.3 mol/L), and removed excess PMP with 1 mL chloroform for three times. Mixed monosaccharide standard, including Man, GlcN, Rha, GlcA, GalA, GalN, Glc, Gal, Xyl, Ara and Fuc, was derivatized following the above method without degradation.
The aqueous layer was filtered through a 0.22 μm membrane and analyzed by a Agilent 1260 system equipped with an UV detector (245 nm). A Thermo C18 column (4.6 × 250 mm, 5 µm, USA) was used for separation. The mobile phase consisted 0.1 mol/L phosphate buffer (pH 7.0) and acetonitrile in a ratio of 83:17 (v/v, %) was used to elute at 0.8 mL/min.
2.4.2 Mw analysis
The relative Mw of FLPs was detected by high performance gel permeation chromatography (HPGPC) as previously described (Xue et al., 2016). 5 mg of FLPs or dextran standards were dissolved with 1.0 mL Na2SO4 (0.1 mol/L), filtered through a 0.22 μm membrane, and then analyzed by a Agilent 1260 system equipped with a refractive index detector (RID). A Shodex OHpak SB-803 HQ column (300 × 8.0 mm i.d.) was used to separated, and 0.1 mol/L Na2SO4 solution was used to elute with at a flow rate of 0.5 mL/min.
2.4.3 Fourier transform infrared spectroscopy (FT-IR) analysis
The FT-IR of FLPs was tested by a Nicolet Nexus 470 infrared spectrometer in the range of 400 to 4000 cm−1 with a KBr pellet as previously described (Xin et al., 2018). The data were processed by OMMIC software.
2.5 Antioxidant activity assay in vitro
The scavenging activities of FLPs-HWE, FLPs-MAE, FLPs-UAE, FLPs-UAEE, FLPs-UMAE, FLPs-AE and FLPs-HSHE on DPPH radical, hydroxyl radical and superoxide radical were measured as previously described (Meng et al., 2022; Xue et al., 2021). Ascorbic acid (Vc) was used as a positive control.
2.5.1 DPPH radical scavenging activity
FLPs solution with different concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.4 mg/mL) was mixed with three volumes of DPPH-ethanol solution (0.1 mmol/L). After incubation at room temperature and away from light for 15 min, the absorbance was detected at 517 nm.
2.5.2 Hydroxyl radical scavenging activity
4 mL of FLPs solution with different concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.4 mg/mL) was mixed with 2.0 mL FeSO4 solution (6 mmol/L), 2.0 mL of salicylic acid–ethanol solution (6 mmol/L) and 2.0 mL of H2O2 solution (7 mmol/L). After incubation at 35 ℃ for 25 min, the absorbance was detected at 536 nm.
2.5.3 Superoxide radical scavenging activity
2.4 mL of FLPs solution with different concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.4 mg/mL) was mixed with 0.9 mL of Tris-HCl buffer (50 mmol/L, pH 8.2), 0.08 mL of gallic acid solution (25 mmol/L). After incubation at 25 ℃ for 5 min, 0.2 mL of HCl solution (8 mmol/L) was added to terminate the reaction. Then, the absorbance was detected at 325 nm.
The scavenging activities of FLPs against three radicals were calculated using the following equation:
Where, A0, A1 and A2 were the absorbance values of blank control (distilled water), FLPs samples, sample background (the mixture without FLPs samples, DPPH or FeSO4 or gallic acid).
2.6 Hypoglycemic activity assay in vitro
α-amylase and α-glucosidase inhibitory effects of FLPs-HWE, FLPs-MAE, FLPs-UAE, FLPs-UAEE, FLPs-UMAE, FLPs-AE and FLPs-HSHE were measured as previously described (Meng et al., 2022; Fu et al., 2020). Acarbose was used as the positive control.
2.6.1 Determination of α-amylase activity
0.5 mL of FLPs solution with different concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.4 mg/mL) was mixed with 900 µL of α-amylase solution (10 U/mL) and incubated at 37 ℃ for 30 min. Next, 1.0 mL of soluble starch (0.5 %) was added and continue to incubate at the same temperature for 10 min. Subsequently, the reaction was terminated with 0.6 mL of HCl solution (0.1 mol/L). In addition, 2.0 mL of DNS regent was added for coloration at 100 ℃ for 5 min. The absorbance value was detected at 540 nm.
2.6.2 Determination of α-glucosidase activity
100 µL of FLPs solution with different concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.4 mg/mL) were mixed with 600 µL of PBS solution (0.1 mol/L, pH 6.8) and 300 µL of α-glucosidase solution (2 mg/mL). After incubation at 37 ℃ for 15 min, 100 µL of PNPG regent (2.74 mg/mL) was added and continue to incubate at the same temperature for 20 min. Then, 3.9 mL of Na2CO3 solution (0.5 mol/L) was added to terminate the reaction. And the absorbance value was detected at 405 nm.
The inhibitory activities of FLPs on α-amylase and α-glucosidase were calculated using the following equation:
Where, A0, A1 and A2 were the absorbance values of blank control (PBS), FLPs samples, sample background (the mixture without FLPs samples, α-amylase or α-glucosidase).
2.7 Statistical analysis
The experimental data were displayed as the means ± standard deviation or percentage. Data and significant differences were analyzed and evaluated with ANOVA and Tukey's test, with p < 0.05 defined significant. The graphs were drawn using Graph Pad prism 8.0 software or OriginPro software.
3 Results and discussions
3.1 Yield and chemical composition analysis of FLPs extracted by different methods
Seven extraction methods (HWE, MAE, UAE, UAEE, UMAE, AE, HSHE) were used to prepare FLPs. The yields and chemical compositions of seven FLPs were shown in Table 1. The yields of FLPs extracted by seven methods ranged from 2.59 % to 9.13 %, and the yields of FLPs-UAE (9.13 %) and FLPs-UAEE (8.30 %) were significantly higher than other methods (p < 0.05), suggesting that the behavior of cavitation bubbles during ultrasonic mass transfer maybe enhance the rate of solvent diffusion and the solubility of target components, thereby improve the extraction rate (Li et al., 2016; Wu et al., 2019). This result was close to the study of polysaccharides from loquat (Eriobotrya japonica) leaves (Fu et al., 2020). The total polysaccharide contents of FLPs extracted by seven methods were different following the order of FLPs-AE (52.19 %) < FLPs-HSHE (54.06 %) < FLPs-MAE (54.53 %) < FLPs-UMAE (55.11 %) < FLPs-UAEE (58.04 %) < FLPs-UAE (60.62 %) < FLPs-HWE (62.03 %). The contents of uronic acid and total phenolic compounds in FLPs obtained by HWE, MAE, UAE, UAEE, UMAE, HSHE were 9.63 %∼10.85 % and 10.19 %∼13.55 %, respectively. This phenomenon was also observed in some previous studies (Fu et al., 2020; Meng et al., 2022). However, both contents in FLPs-AE were 21.46 % and 5.26 %, because alkali solution was easier to extract acid related substances. In addition, the protein contents in FLPs-HWE, FLPs-MAE, FLPs-UAE, FLPs-UAEE, FLPs-UMAE, FLPs-AE and FLPs-HSHE was determined to be 14.64 %, 11.44 %, 18.64 %, 10.77 %, 9.64 %, 15.11 % and 12.84 %, respectively. The results indicated that polysaccharides were the main bioactive compounds in fig leaves, and the content of uronic acid in FLPs was relatively high, which proved that pectin-like acidic polysaccharides existed (Han et al., 2019). Moreover, FLPs might contain natural phenolic-polysaccharide conjugates due to the presence of phenolic compounds (Nie et al., 2019). Among seven extraction methods, UAE obtained polysaccharides exhibiting the highest extraction yield, total proteins and total polysaccharides (there was no obvious difference with 62.03 % in HWE).
Extraction yield (%)
Total polysaccharides (%)
Total uronic acids (%)
Total proteins (%)
Total phenolics (%)
FLPs-HWE
7.77 ± 0.80b
62.03 ± 0.70 a
10.45 ± 0.68b
14.64 ± 0.20b
13.55 ± 0.02 a
FLPs-MAE
7.87 ± 0.49b
54.53 ± 0.20 cd
10.85 ± 0.09b
11.44 ± 0.35 d
12.16 ± 0.19c
FLPs-UAE
9.13 ± 0.73 a
60.62 ± 3.06 a
10.09 ± 0.05c
18.64 ± 0.20 a
10.91 ± 0.02 e
FLPs-UAEE
8.30 ± 1.49 a
58.04 ± 0.54b
10.57 ± 0.00b
10.77 ± 0.31 e
11.64 ± 0.02 d
FLPs-UMAE
6.96 ± 1.25b
55.11 ± 0.20c
9.63 ± 0.05 d
9.64 ± 0.20f
10.19 ± 0.02f
FLPs-AE
2.59 ± 0.67 d
52.19 ± 0.35 d
21.46 ± 0.42 a
15.11 ± 0.12b
5.26 ± 0.05 g
FLPs-HSHE
5.41 ± 1.81c
54.06 ± 0.20 cd
10.30 ± 0.00b
12.84 ± 0.20c
12.86 ± 0.00b
The results were displayed as the mean ± sd (n = 3), and significant difference was denoted by superscripts a-g.
3.2 Structural characterization of FLPs by different extraction methods
The bioactivity of polysaccharide is closely related to its structure, including monosaccharide composition, Mw and substituent group (Xue et al., 2016; Xin et al., 2016). In this paper, we firstly used the pre-column derivatization HPLC method to determine the monosaccharide composition of FLPs extracted by seven methods, shown in Fig. 1. Results (Table 2) showed that FLPs was a heteropolysaccharide. FLPs extracted by HWE, MAE and UAE had the most complex and diverse structure with seven monosaccharides, including Man, GlcN, Rha, GalA, Glc, Gal, and Ara. In contrast, six monosaccharides were measured in FLPs-UAEE and FLPs-HSHE, except for GalA. Five monosaccharides were measured in FLPs-UMAE, except for GlcN and GalA. For AE, FLPs was composed of Man, GlcN, Rha, Glc, and Gal, the percentages were 37.12 %, 3.70 %, 4.26 %, 49.49 %, 5.44 %. Among them, Glc possessed the highest proportion, accounting for 38.63 %, 37.25 %, 37.40 %, 35.33 %, 39.05 %, 49.49 %, and 37.41 %, respectively. Interestingly, compared with other six techniques, AE could extract more Man and Glc, but no GlcA and GalA, which might contain some other uronic acid. In addition, highest GalA content (6.18 %) and GlcN content (7.68 %) were found in FLPs-UAE. Literatures reported that high Xyl and GlcN contents were beneficial to the play of antioxidant activity (Shen et al., 2019; Sheng et al., 2014). In addition, the structure characteristics with unsaturated cyclohexene ring and glycosidic nitrogen linkage are necessary to hypoglycemic activity (Kim et al., 1999). We do not know whether the difference in polysaccharide content caused by different extraction processes will lead to changes in activities.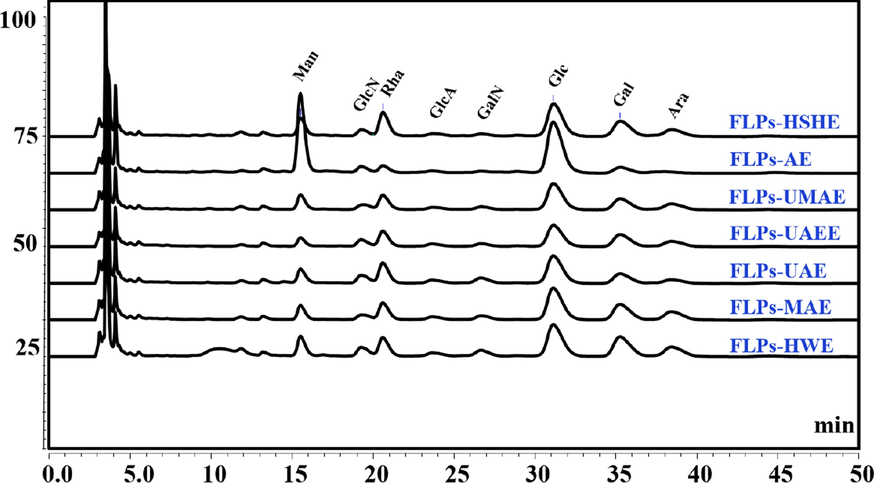
HPLC chromatograms of FLPs obtained by different extraction methods.
Man
GlcN
Rha
GalA
Glc
Gal
Ara
FLPs-HWE
8.79
5.78
11.05
4.70
38.63
19.98
11.24
FLPs-MAE
8.10
4.49
12.96
3.96
37.25
20.49
12.75
FLPs-UAE
9.29
7.68
17.83
6.18
37.40
14.62
6.99
FLPs-UAEE
6.93
7.25
16.46
–
35.33
22.59
11.44
FLPs-UMAE
10.94
–
14.31
–
39.05
18.38
11.58
FLPs-AE
37.12
3.70
4.26
–
49.49
5.44
–
FLPs-HSHE
10.10
4.83
17.64
–
37.41
19.68
10.34
Next, HPGPC method was used to determine the effects of different extraction processes on the Mws of FLPs. As shown in Fig. 2, different extraction methods had significant effects on the HPGPC-RID chromatograms of FLPs. Three polysaccharide fractions (fraction 2–4) were measured in seven FLPs. While only fraction 1 was detected in FLPs-UAE and FLPs-HSHE, and their Mws were 2.25 × 106 Da and 0.95 × 106 Da, respectively. Mws of fractions 2, 3 and 4 in FLPs prepared by seven methods ranged from 3.80 × 104 to 9.29 × 104 Da, 2.82 × 103 to 8.69 × 103 Da, 500 to 700 Da, respectively (Table 3). In general, the average Mw order of seven tested samples was FLPs-UAE > FLPs-HSHE > FLPs-MAE≈FLPs-UAEE≈FLPs-UMAE > FLPs-HWE > FLPs-AE. In all tested samples, the Mw of FLPs-UAE was significantly larger than other FLPs. Studies have shown that a certain ultrasonic power during the extraction process can increase the Mw of natural polysaccharides (Pu et al., 2015), while high temperature and organic solvents can damage the polysaccharide glycosidic bonds to result in a decrease in Mw (Constant et al., 2016), which was consistent with our experimental results. In addition, we also found that microwave would damage long chain structure of polysaccharides, and the Mws of FLPs extracted by MAE and UMAE were lower than FLPs-UAE. The results showed that MAE can be used as an effective technique for the preparation of more homogeneous polysaccharides.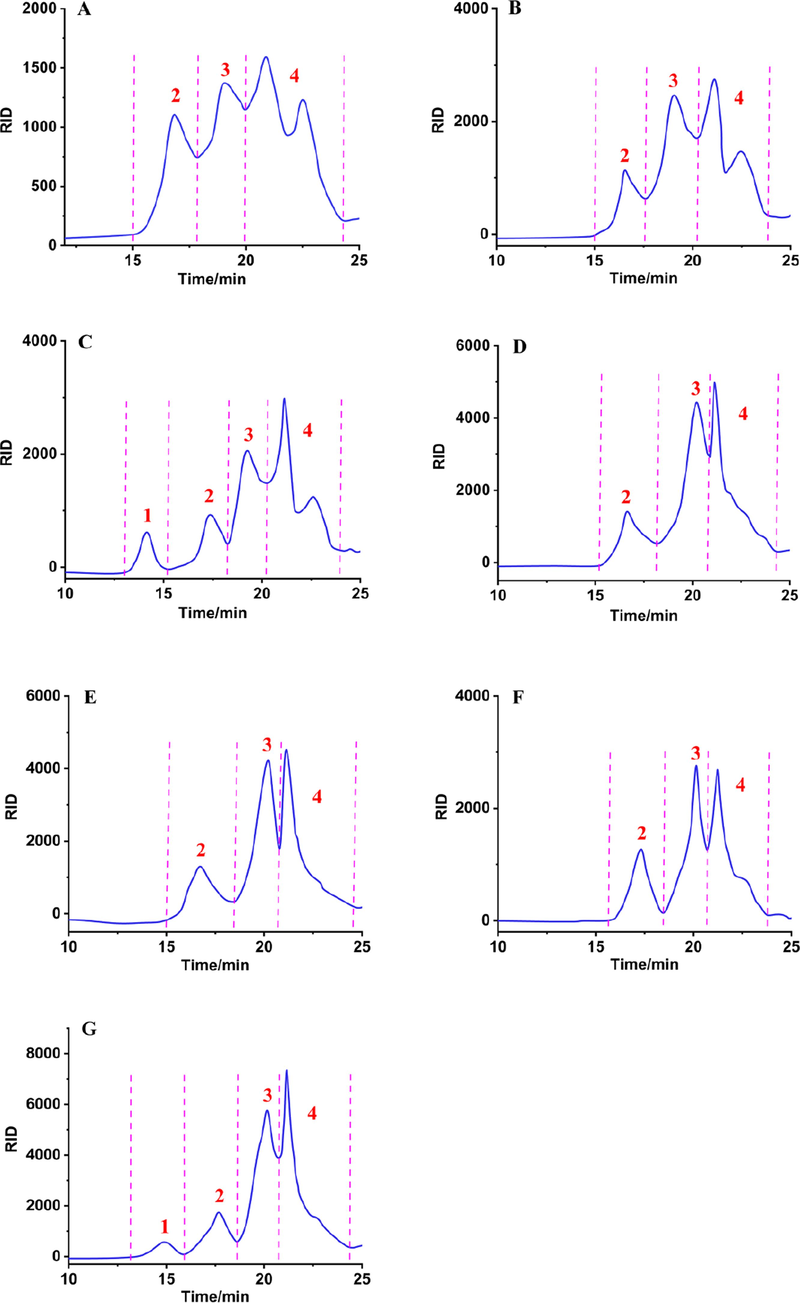
HPGPC chromatograms of FLPs-HWE (A), FLPs-MAE (B), FLPs-UAE (C), FLPs-UAEE (D), FLPs-UMAE (E), FLPs-AE (F) and FLPs-HSHE (G) prepared by different extraction methods.
Fraction1 (Da)
Fraction 2 (Da)
Fraction 3 (Da)
Fraction 4 (Da)
FLPs-HWE
-
83794
7028
512
FLPs-MAE
-
91668
8691
507
FLPs-UAE
2254416
59975
6790
519
FLPs-UAEE
-
92853
3117
703
FLPs-UMAE
-
91743
3559
674
FLPs-AE
-
38025
3025
697
FLPs-HSHE
945282
39422
2818
652
Furthermore, FT-IR spectra of FLPs-HWE, FLPs-MAE, FLPs-UAE, FLPs-UAE, FLPs-UMAE, FLPs-AE and FLPs-HSHE were detected to identify the functional groups in polysaccharides. As shown in Fig. 3, the FT-IR spectra of seven tested samples were similar. The strong absorption peak at 3418.12 cm−1 was the characteristic stretching vibration of O–H from hydroxyl and carboxyl groups, and the signal at 2918.64 cm−1 could be attributed to the stretching vibration peak of C–H (Qiu et al., 2016). The band at 1631.99 cm−1 corresponded to esterified carboxyl groups, which proved that FLPs contain uronic acid (Yuan et al., 2019; Wu et al., 2019). In addition, the absorption band at 1096.48 cm−1 was the C-O-C stretching vibration peak (Fu et al., 2020). The absorption peaks in the 800 ∼ 1200 cm−1 named fingerprint region were very similar, which indicated that the structures of seven tasted FLPs had no obvious difference (Gao et al., 2018). Moreover, there was no signals at 1651 cm−1 and 1555 cm−1, which were called typical protein bands. It was demonstrated that only a small amount of protein was present in FLPs, which was consistent with the results of protein content in Table 3.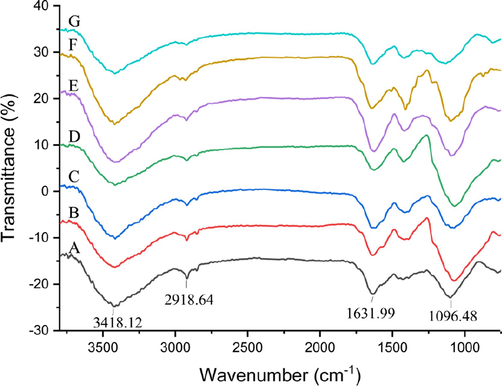
FT-IR spectra of FLPs-HWE (A), FLPs-MAE (B), FLPs-UAE (C), FLPs-UAEE (D), FLPs-UMAE (E), FLPs-AE (F) and FLPs-HSHE (G) prepared by different extraction methods.
3.3 Antioxidant activities in vitro
There is substantial evidence that persistent production of reactive oxygen species (ROS) can account for a variety of diseases, including cancer, diabetes, hyperlipidemia, cirrhosis, and cardiovascular disease (Valko et al., 2007; Yang et al., 2013). It is believed that the use of antioxidants can help to reduce oxidative damage to the body. Water extract and crude polysaccharide from fig fruit have been proved to possess excellent antioxidant activities (Yang et al., 2009). We need to consider how to obtain the most active FLPs as an antioxidant against oxidative damage. In this paper, impacts of different extraction techniques on the antioxidant activities of FLPs were evaluated and compared. As displayed in Fig. 4, seven FLPs exerted the reducing powers on DPPH radical, hydroxyl radical and superoxide radical with a concentration-dependent manner in the concentration from 0.01 to 0.4 mg/mL. For DPPH radical, FLPs-HWE had a strongest scavenging ability, the second was FLPs-UAE, and FLPs-AE was the weakest (Fig. 4A). It should be related to the total polysaccharides content of FLPs obtained by different extraction method. Total polysaccharides contents of FLPs-HWE (62.03 ± 0.70 %) and FLPs-UAE (60.62 ± 3.06 %) were significantly higher than other methods. For hydroxyl radical, FLPs-UAE with largest Mw showed an optimal reducing power (Fig. 4B), because macromolecules reduced the number of Fe2+ exposed to weaken their binding to the hydroxyl radical. Besides, the best scavenging capacity on superoxide radical appeared in FLPs-UAE and FLPs-MAE. And FLPs-HWE and FLPs-HSHE were slightly less effective than FLPs-UAE and FLPs-MAE (Fig. 4B). We speculate that higher total phenolic compounds of FLPs-MAE (12.16 ± 0.19 %), FLPs-HWE (13.55 ± 0.02 %) and FLPs-HSHE (12.86 ± 0.00 %) maybe contribute to their stronger reducing effects on superoxide radical, because phenolic compounds were reported to be used as efficient antioxidants (Shahidi and Wanasundara, 1992). The highest GlcN content (7.68 %) among seven tested samples was beneficial to the ability of FLPs-UAE on scavenging superoxide radical, since GlcN was found to have an antioxidant activity in animal models of arthritis by inhibiting the production of superoxide radicals (Gottardi et al., 2014; Liu et al., 2022). On the whole, FLPs extracted by UAE showed the best antioxidant activity, which attributed to its higher Mw, total polysaccharide content, GlcN content. From this, it can be inferred that UAE was a potential extraction method to preparing FLPs with a high antioxidant activity.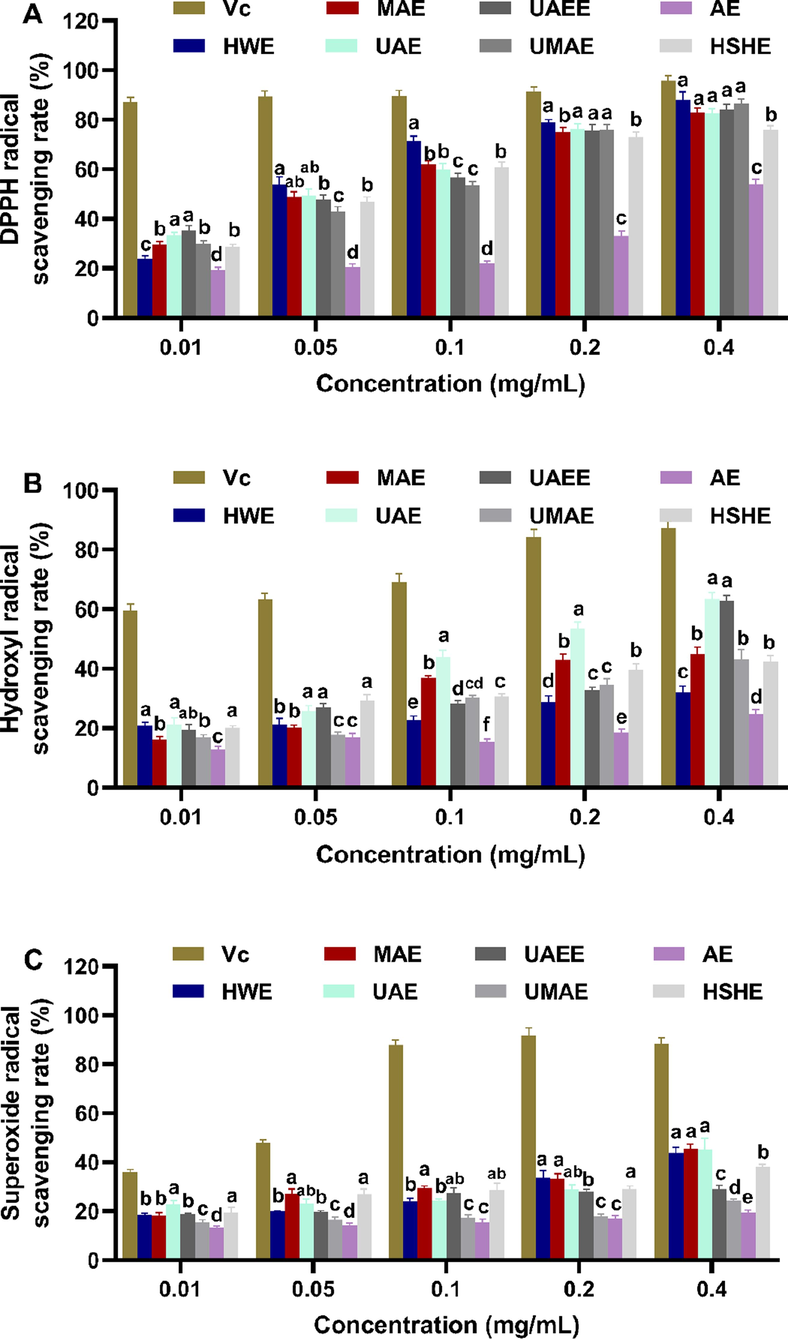
The scavenging activities of FLPs obtained by seven extraction methods against DPPH radical (A), hydroxyl radical (B), superoxide radical (C). The results were displayed as the mean ± sd (n = 3), and significant difference was denoted by superscripts a-f.
3.4 Hypoglycemic activity in vitro
For the people with type II diabetes mellitus, the rapid rise of postprandial blood glucose is closely related to the hydrolysis of glucose by starch (Chen et al., 2018). α-amylase and α-glucosidase inhibitors became the major strategy to treat metabolic alterations related with type II diabetes mellitus (Gao et al., 2018). It has been confirmed that plant polysaccharides exhibit excellently inhibitory effects on α-amylase and α-glucosidase (Fu et al., 2020). As presented in Fig. 5, effects of seven FLPs were tested and compared on α-amylase and α-glucosidase, due to the difference of FLPs structure extracted by different extraction techniques. The inhibitory impacts of seven FLPs on α-amylase and α-glucosidase showed a concentration-dependent pattern in the concentration from 0.01 to 0.4 mg/mL. Among them, the inhibitory activity of FLPs-UAE was the strongest on α-amylase. And FLPs-UAE and FLPs-HWE possessed the greatest reduction effects on α-glucosidase. It was reported that the unsaturated cyclohexene ring and glycosidic nitrogen linkage are necessary structure characteristics to exert anti-amylase activity (Kim et al., 1999). Hypoglycemic activity of FLPs-UAE may be benefited from its highest GlcN content (7.68 %), and GlcN content of FLPs-HWE was slightly lower (5.78 %). In addition, prominently anti-diabetic activities of FLPs-UAE and FLPs-HWE were related with their total polysaccharide content (62.03 ± 0.70 % and 60.62 ± 3.06 %), larger Mw of FLPs-UAE, total phenolic compounds of FLPs-HWE (13.55 ± 0.02 %). Overall, it was confirmed that UAE and HWE, especially UAE, could be potential extraction methods to preparing FLPs with a high hypoglycemic activity.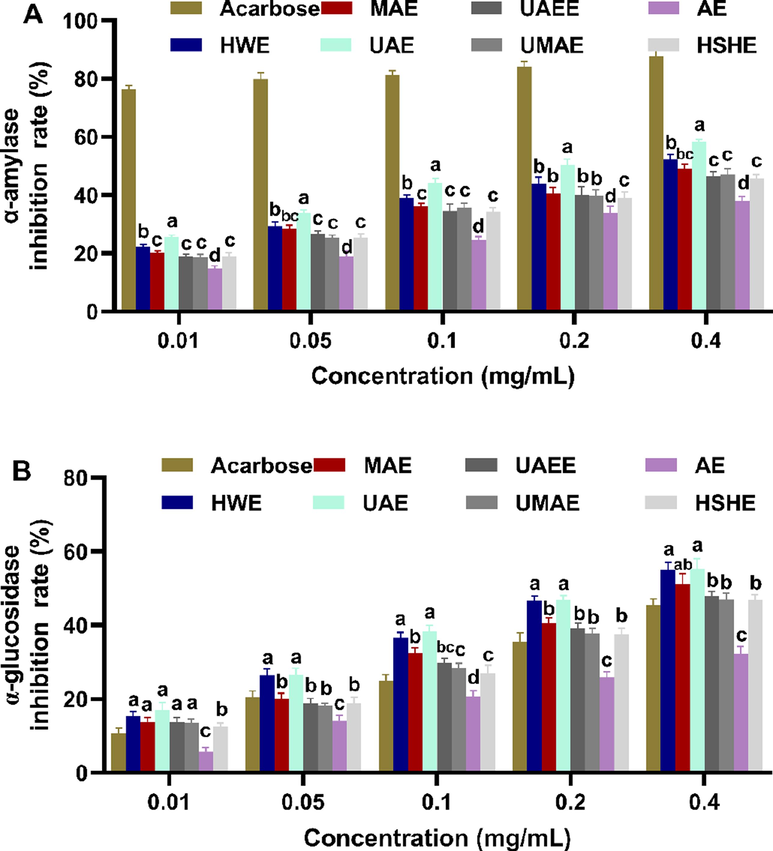
Inhibitory effects of FLPs obtained by seven extraction methods on α-amylase (A) and α-glucosidase (B). The results were displayed as the mean ± sd (n = 3), and significant difference was denoted by superscripts a-d.
4 Conclusion
In this paper, seven extraction methods possessed noticeably influence on bioactivities and physicochemical characteristics of FLPs, including proteins, uronic acids, total polysaccharides, total phenolics, Mw and monosaccharide composition. The strongest antioxidant activity and anti-diabetic activity were observed in FLPs-UAE with highest GlcN content and Mw. The second was found in FLP-HWE with highest total polysaccharide content and total phenolic compounds content. While FLPs-AE was the weakest. These findings indicated that UAE and HWE, especially UAE, may be potential extraction methods to obtain FLPs with good bioactivities, which provided a scientific basis for selecting an appropriate extraction technique to obtain FLPs with relatively high biological activity.
CRediT authorship contribution statement
Xin-Yang Jiang: Conceptualization, Project administration, Methodology, Resources, Writing – original draft. Cai-Wei Wang: Methodology, Validation. Jun Zhang: Software, Data curation. Pei-Pei Xu: Investigation, Supervision. Yi-Ting Xue: Project administration, Writing – review & editing. Qian Wang: Supervision, Project administration, Writing – review & editing.
Acknowledgement
This study was sponsored by the University Basic Science Research Project of Jiangsu Province (21KJB350022, China), Changshu Agricultural Projects (CN202114, China), the Fundamental Research Funds for the Central Universities (YG2023QNB12, China).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New method for quantitative determination of uronic acids. Anal. Biochem.. 1973;54(2):484-489.
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Purification, characterization and anti-proliferation activities of polysaccharides extracted from Viscum coloratum (Kom.) Nakai. Carbohydr. Polym.. 2016;149:121-130.
- [CrossRef] [Google Scholar]
- Effect of esterification condensation on the Folin-Ciocalteu method for the quantitative measurement of total phenols. Food Chem.. 2015;170:10-15.
- [CrossRef] [Google Scholar]
- Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol.. 2010;46(4):429-435.
- [CrossRef] [Google Scholar]
- Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crop Prod.. 2018;121:18-25.
- [CrossRef] [Google Scholar]
- Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym.. 2016;153:663-678.
- [CrossRef] [Google Scholar]
- Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crop Prod.. 2017;100:1-11.
- [CrossRef] [Google Scholar]
- Composition, texture and methane potential of cellulosic residues from Lewis acids organosolv pulping of wheat straw. Bioresour. Technol.. 2016;216:737-743.
- [CrossRef] [Google Scholar]
- Effects of pressurized hot water extraction on the yield and chemical characterization of pectins from Campomanesia xanthocarpa Berg fruits. Int. J. Biol. Macromol.. 2020;146:431-443.
- [CrossRef] [Google Scholar]
- Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct.. 2018;9:3930-3943.
- [CrossRef] [Google Scholar]
- Colorimetric method for determination of sugars and related substances. Anal. Chem.. 1956;28:350-356.
- [CrossRef] [Google Scholar]
- Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from northeastern Turkey. Plant Foods Hum. Nutr.. 2012;67(3):271-276.
- [CrossRef] [Google Scholar]
- Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol.. 2020;146:508-517.
- [CrossRef] [Google Scholar]
- Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. Carbohydr. Polym.. 2018;201:624-633.
- [CrossRef] [Google Scholar]
- Conjugation of gluten hydrolysates with glucosamine at mild temperatures enhances antioxidant and antimicrobial properties. LWT-Food Sci. Technol.. 2014;57:181-187.
- [CrossRef] [Google Scholar]
- Extraction optimization, physicochemical characteristics, and antioxidant activities of polysaccharides from kiwifruit (Actinidia chinensis Planch.) Molecules. 2019;24(3):461.
- [CrossRef] [Google Scholar]
- Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox) Carbohydr. Polym.. 2016;151:295-304.
- [CrossRef] [Google Scholar]
- Optimization of ultrasonic-microwave assisted alkali extraction of arabinoxylan from the corn bran using response surface methodology. Int. J. Biol. Macromol.. 2019;128:452-458.
- [CrossRef] [Google Scholar]
- Morphological and pomological characterization of edible fig (Ficus carica L.) to select the superior trees. Sci. Hortic.. 2018;238:66-74.
- [CrossRef] [Google Scholar]
- Comparative study of the inhibition of alpha-glucosidase, alpha-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch. Biochem. Biophys.. 1999;371(2):277-283.
- [CrossRef] [Google Scholar]
- Early domesticated fig in the Jordan Valley. Science. 2006;312(5778):1372-1374.
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted enzymatic extraction and antioxidant activity of polysaccharides from Setaria viridis. Sepr. Sci. Technol.. 2016;51:1798-1805.
- [CrossRef] [Google Scholar]
- A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves. Biomed. Pharmacother.. 2021;137:111393
- [CrossRef] [Google Scholar]
- The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind. Crop Prod.. 2018;125:150-219.
- [CrossRef] [Google Scholar]
- Conjugation of the glutelin hydrolysates-glucosamine by transglutaminase and functional properties and antioxidant activity of the products. Food Chem.. 2022;380:132210
- [CrossRef] [Google Scholar]
- Impacts of extraction methods on physicochemical characteristics and bioactivities of polysaccharides from rice bran. J. Food Meas. Charact.. 2022;16:1137-1145.
- [CrossRef] [Google Scholar]
- Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol.. 2019;139:459-467.
- [CrossRef] [Google Scholar]
- Multi-optimization of ultrasonic-assisted enzymatic extraction of Atratylodes macrocephala polysaccharides and antioxidants using response surface methodology and desirability function approach. Molecules. 2015;20(12):22220-22235.
- [CrossRef] [Google Scholar]
- Response surface methodology for the synthesis of an Auricularia auriculajudae polysaccharides-CDDP complex. Int. J. Biol. Macromol.. 2016;93(Pt A):333-343.
- [CrossRef] [Google Scholar]
- Effects of physio-chemical changes during fruit development on nutritional quality of fig (Ficus carica L. var. ‘Sabz’) under rain-fed condition. Sci. Hortic.. 2018;237:44-50.
- [CrossRef] [Google Scholar]
- Physicochemical properties, phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of okra (Abelmoschus esculentus) fruit at different maturation stages. J. Food Sci. Tech.. 2019;56(3):1275-1286.
- [CrossRef] [Google Scholar]
- Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym.. 2019;206:557-564.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39(1):44-84.
- [CrossRef] [Google Scholar]
- Polyethylene glycol-based ultrasonic-assisted enzymatic extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Lonicerae japonica leaves. Food Sci. Nutr.. 2019;7(11):3452-3462.
- [CrossRef] [Google Scholar]
- Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol.. 2019;136:891-900.
- [CrossRef] [Google Scholar]
- Anticoagulant and antithrombotic activities of low-molecular-weight propylene glycol alginate sodium sulfate (PSS) Eur. J. Med. Chem.. 2016;114:33-40.
- [CrossRef] [Google Scholar]
- Propylene glycol guluronate sulfate (PGGS) reduces lipid accumulation via AMP-activated kinase activation in palmitate-induced HepG2 cells. Int. J. Biol. Macromol.. 2018;114:26-34.
- [CrossRef] [Google Scholar]
- Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS) Carbohydr. Polym.. 2016;144:330-337.
- [CrossRef] [Google Scholar]
- Optimization of preparation process and antioxidant activity of the chelate of a Hericium erinaceus polysaccharide with zinc. J. Food Meas. Charact.. 2021;15:2039-2048.
- [CrossRef] [Google Scholar]
- The synergy of Box-Behnken designs on the optimization of polysaccharide extraction from mulberry leaves. Ind. Crop Prod.. 2017;99:70-78.
- [CrossRef] [Google Scholar]
- Compositional characterisation of soluble apple polysaccharides, and their antioxidant and hepatoprotective effects on acute CCl4-caused liver damage in mice. Food Chem.. 2013;138(2–3):1256-1264.
- [CrossRef] [Google Scholar]
- Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Foods Hum. Nutr.. 2009;64(2):167-173.
- [CrossRef] [Google Scholar]
- Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus) Int. J. Biol. Macromol.. 2019;127:178-186.
- [CrossRef] [Google Scholar]
- Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct.. 2020;11(7):6666-6679.
- [CrossRef] [Google Scholar]







