Translate this page into:
Angelica sinensis polysaccharide inhibits inflammation of collagen-induced arthritis rat fibroblast-like synoviocytes by inhibiting JAK2/STAT3 and MAPK signaling
⁎Corresponding authors. xzmulwx@sina.com (Weixi Liu), liuruiping216@yahoo.com (Ruiping Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A. sinensis polysaccharide (ASP), one of the effective components of A. sinensis, has been used to treat inflammatory diseases in China. However, its effect on rheumatoid arthritis (RA) is unknown. The purpose of this study was to explore the anti-inflammatory effects and mechanisms of ASP on RA using a rat model of collagen-induced arthritis (CIA). For evaluation of the therapeutic effects of ASP in vitro, the CIA model was used. Our study showed that ASP (100 and 200 μg/mL) dose-dependently inhibited tumor necrosis factor (TNF)-α-induced (10 ng/mL) proliferation, migration, and invasion of fibroblast-like synovial (FLS) cells, promoted apoptosis, and arrested the cell cycle in G0/G1 phase (P < 0.05). In vivo, ASP increased body weight in CIA rats while decreasing paw swelling, arthritis score, and synovial tissue proliferation (P < 0.05). ASP also reduced the expression of pro-inflammatory cytokines (interleukin (IL) −6, IL-1β, inducible nitric oxide synthetase (iNOS), matrix metalloproteinase (MMP)-1 and MMP-3) (P < 0.05). ASP inhibited the phosphorylation of JAK2/STAT3 and MAPK signaling pathway components (P < 0.05) induced by TNF-α in CIA-FLS cells. Using JAK and p38 inhibitors, we found that JAK2/STAT3 might be an upstream pathway of MAPK. In conclusion, our study investigated the therapeutic effects of ASP in RA in vitro and in vivo. Our research suggests that ASP can inhibit the invasiveness and secretion of inflammatory cytokines of FLS cells in CIA rats via JAK2/STAT3 and MAPK signaling. Elucidation of the underlying mechanism will provide a theoretical basis for clinical application of ASP.
Keywords
ASP
FLS
JAK2/STAT3
MAPK
TNF-α
1 Introduction
Approximately 0.5–1.0 % of the global population suffers from rheumatoid arthritis (RA), a chronic autoimmune disease (Huang et al., 2021). RA is distinguished by persistent synovial inflammation, pannus hyperplasia, cartilage and subchondral bone degradation, joint space stenosis, and joint deformity. (Yi et al., 2022). Although there have been many studies, the specific pathogenesis of RA is still unknown (Fearon et al., 2022, Petrelli et al., 2022).
Disease-modifying anti rheumatic drugs (DMARDs) and glucocorticoids (GCs) are commonly used to treat RA. Most of these drugs only improve clinical symptoms and do not prevent disease progression. New biological agents and biologically-targeted drugs have also begun to be used in clinical practice. However, they are expensive, may only benefit some patients, and have side effects. Therefore, finding new drugs that are clinically effective, low cost, and have few side effects has become an important goal (Prasad et al., 2022).
Traditional Chinese medicine has been used in the treatment of RA for a long time. Many Chinese medicine monomers, such as Tripterygium wilfordii polyglycoside and total glycoside of paeony, have achieved some clinical effects (Luo et al., 2017, Jiang et al., 2020, Zhang et al., 2021, Wang et al., 2022). Previous studies have shown that Lycium barbarum polysaccharide, Astragalus polysaccharide, and ASP can alleviate RA (Wang et al., 2020). At present, traditional Chinese medicine formulas with Angelica sinensis as the main drug have been used to treat RA, with higher research value compared to Goji berries and Astragalus membranaceus. However, the mechanism of action remains unclear. Therefore, we chose to study Angelica polysaccharides to explore their role in treatment of RA. A. sinensis, a perennial herb of the Umbelliferae, promotes the blood circulation, regulates menstruation, and can treat wind cold dampness (Yang et al., 2013). Researchers in Taiwan have found that one of the effective components of A. sinensis, pistil isoflavone, may affect the IL-6 receptor and RA (Lee et al., 2014). ASP is a water-soluble extract that is mostly in the form of acidic and neutral polysaccharides. In addition to some monosaccharides, the acidic polysaccharide contains uronic acid and hemiuronic acid (Cao 2019). ASP is extracted from the dried root of A. sinensis and has immunoregulatory, anti-inflammatory, and antitumor activities. We recently used ASP to investigate its antioxidative role in rat chondrocytes. We found that ASP protected chondrocytes from H2O2-induced oxidative stress and subsequent cell damage by inhibiting the caspase pathway (Zhuang et al., 2020). Following oral administration of ASP to rats with complete Freund's adjuvant (CFA) arthritis for 4 weeks, the inflammatory factors IL-6 and TNF-α were significantly reduced, resulting in a therapeutic effect (Li et al., 2020). In rats with collagen-induced arthritis (CIA), ASP reduced the methylation status of nucleotide sites in the promoter region of exons of two genes, γ-glutamyltransferase 7 (Ggt7) and angiotensin-I-converting enzyme (Ace), and led to remission of RA (Wang et al., 2020). These studies indicate that ASP has a therapeutic effect on RA, but the specific mechanism remains unclear. However, ASP has been studied and applied in the treatment of RA. If the effects of ASP are studied in fibroblast-like synovial (FLS) cells of RA patients, its clinical value can be reflected, which will be significant for the future application of ASP in FLS cell-targeted treatment of RA patients.
The occurrence and progression of RA is characterized by abnormal signal transduction pathway regulation, leading to overexpression of proinflammatory mediators and inflammatory factors, and excessive proliferation of FLS cells (Ridgley et al., 2018, Liu et al., 2021). Multiple signaling pathways are involved in the pathogenesis of RA. Some research has found that the MAPK and JAK2/STAT3 pathways are important in the development of RA (Schett et al., 2008, Chauhan et al., 2021, Ibrahim and Huttunen 2021).
Therefore, we designed this study to investigate the following: (1) whether ASP had a therapeutic effect on FLS cells in CIA rats; (2) whether ASP exerted therapeutic effects via JAK2/STAT3 and MAPK signaling; and (3) how ASP exerted a therapeutic effect through these two signaling pathways.
2 Materials and methods
2.1 Cell isolation and identification
To obtain a relatively pure FLS cells, we surgically isolated rat synovium, cultured synovial cells, and identified cells via immunohistochemistry. The following is the process of CIA rat modeling, synovial membrane isolation, and cell identification. The CIA rat model was established in Sprague-Dawley rats aged 7 weeks (Cavens, Changzhou, China). The Experimental Animal Welfare Ethics Committee of Nanjing Medical University approved the animal experiment (No. IACUC-2101040). Injections of 0.6 % pentobarbital sodium (40 mg/kg) were used to anesthetize the rats, and 75 % alcohol was used to disinfect their tails. We subcutaneously injected 100 mL of bovine type II collagen emulsion with complete Freund's adjuvant (Chondrex, Redmond, WA, USA) 10 mm from the tail on both sides of the midline. One week later, bovine type II collagen and incomplete Freund's adjuvant was injected in the same manner. About 2 weeks after the second immunization, model CIA rats with arthritis scores greater than four had synovial tissue removed from their knee joints, which was digested with 0.2 % type II collagenase for 2–3 h. Isolated FLS cells were cultured at 37 °C in 5 % CO2 with Dulbecco's modified Eagle's medium containing high glucose (containing 10 % fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin) (Gibco, Gaithersburg, MD, USA). FLS cells from normal rat knee joints were isolated and cultured using the same method.
FLS cells were identified by immunochemistry after digestion with trypsin 0.25 % and overnight incubation in 6-well plates. Fixation of cells with 4 % paraformaldehyde was followed by permeabilization with 0.1 % Triton X-100. Following that, cells were sealed with 5 % bovine serum albumin (BSA) for 1 h at room temperature. The primary antibody (CD68, vimentin) (ABclone, Wuhan, China) was incubated overnight at 4 °C, followed by 1 h at room temperature with the corresponding secondary antibody. DAB was used to stain cells and hematoxylin was used to stain the nucleus.
2.2 ASP cytotoxicity
Different ASP concentrations were tested for cytotoxicity effects. In 96-well plates, 5 × 103 cells per well were seeded with normal rat knee joints (NC-FLS) and incubated overnight. CIA-FLS cells were treated with ASP (12.5, 25, 50, 100, 200, or 400 μg/mL) (Shanxi Ciyuan Bio-Technology Co. Ltd., Xi’an, Shanxi, China) for 24 or 48 h. Then, 10 μL CCK-8 solution (Beyotime, Shanghai, China) was added to each well and cells were incubated at 37 °C for 3 h. The absorbance was measured at 450 nm with a microplate reader (BioTek Epoch, USA).
2.3 Selection of TNF-α stimulation concentration
To understand the role and mechanism of ASP in RA treatment, it is necessary to verify the inhibitory effects of ASP on inflammatory factor-induced FLS activation, cell proliferation, etc. Based on previous research, we used TNF-α stimulated FLS activation as a positive control group. CIA-FLS cells were grown at a density of 5 × 103 per well in 96-well plates. TNF-α (Sigma-Aldrich, St. Louis, MO, USA) was added following a 24-h incubation period. After 12 or 24 h of intervention, 10 μL of CCK-8 solution was added to each well. After 3 h of incubation, absorbance was measured at 450 nm.
2.4 Scratch test
The main cause of RA joint damage is related to the enhanced migration ability of FLS cells. The scratch test can be used to assess reflect the migration ability of cells. A 6-well plate was seeded with CIA-FLS cells. At > 90 % confluency, the bottom of the plate was scraped with a 200-μL pipette tip. To remove separated cells, 1 mL of PBS was added to each well and imaged were taken with a general optical microscope (OLYMPUS, IX71).
2.5 Invasion test
The invasiveness of FLS cells also affects the occurrence and development of RA; this can be assessed using the invasion test. At 4 °C, Matrigel (Corning, NY, USA) was diluted with serum-free media before being added to the upper chamber of the Transwell (Corning, NY, USA) and incubated for 2 h at 37 °C. In the experimental and control groups, to generate a cell suspension of 3 × 104/mL, FLS cells were digested with 0.25 % trypsin and placed in serum-free media. Then, a 300 μL suspension of cells was added to the upper chamber and the lower chamber was filled with 10 % fetal bovine serum (FBS) (600 μL) and incubated at 37 °C for 24 h. Cells were washed with PBS and treated for 2 min with 4 % paraformaldehyde and 30 min with 100 % methanol after discarding the Transwell upper chamber culture medium. Cells were washed again two or three times with PBS before staining with crystal violet for 2 h. The Transwell upper chamber was wiped off with a cotton swab after the cells had been washed twice with PBS and the Transwell was placed in a fumehood for 30 min. The Transwell bottom was photographed using a microscope (OLYMPUS IX71), and the average cell count was determined.
2.6 Apoptosis test
One characteristic of RA is the abnormal survival of activated FLS cells caused by immune system imbalance, which may be caused by “cell apoptosis resistance”. We therefore studied the effect of ASP on FLS apoptosis. Six-well plates were seeded with 2.5 × 105 CIA-FLS cells per well and cultured for 12 h. Cells were digested after intervention (group 1: CIA-FLS; group 2: CIA-FLS + TNF-α; group 3: CIA-FLS + TNF-α + ASP) and centrifuged at 800 × g for 5 min. After collection, cells were washed with pre-cooled PBS and resuspensed with 100 μL binding buffer. Cells were then incubated with an Annexin V-FITC/PI apoptosis detection reagent (Vazyme, Nanjing, China) for 10 min in the dark at room temperature with 5 μL FITC and 5 μL PI. Using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), apoptotic cells were detected.
2.7 Cell cycle assay
The excessive growth of RA synovial cells is also affected by the cell cycle. Cells were fixed overnight at −20 °C with 75 % ethanol after collection. The following day, cells were washed with PBS. Staining solution containing 25 μL PI and 10 μL RNase A was prepared according to the instructions of the cell cycle detection kit (ShareBio, Shanghai, China). Each container received 0.5 mL of staining solution and was incubated at room temperature for 30 min in a darkened room and FACSCalibur flow cytometry was performed.
2.8 ELISA
According to previous studies, regulating the secretion of inflammatory factors in FLS cells may be an important target for the treatment of RA. Treated cell supernatants were collected, and the levels of IL-1β, IL-6, iNOS, MMP-1, and MMP-3 in the supernatant were detected by ELISA (MULTISCIENCES, Zhejiang, China). Using ELISA, plasma concentrations of inflammatory factors were determined in rat plasma.
2.9 Western blotting
After treating cells with RIPA buffer (containing protease and phosphatase inhibitors), protein concentration was determined using the BCA Protein Assay Kit (Beyotime, Shanghai, China). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5 % BSA for 1 h, the primary antibody (MAPK p38, p-p38, JNK, p-JNK, ERK, p-ERK, JAK2, p-JAK2, STAT3, or p-STAT3) (ABclonal, Wuhan, China) was added and membranes were incubated at 4 °C. The next day, the membranes were washed three times with Tris-buffered saline with Tween-20 (TBST) followed by incubation with the corresponding secondary antibody for 1 h at room temperature. Membranes were then rinsed three times with TBST and the reaction was developed with ECL chemiluminescence (NCM Biotech, Suzhou, China). GAPDH was used as an internal reference.
2.10 Grouping, drug treatment, measurement of weight, paw swelling and arthritis score of CIA rats
The therapeutic effects of ASP were evaluated by establishing a rat model of CIA. Twenty rats were split into four groups (n = 5) at random: control group (normal rats), CIA model group, and ASP treatment groups (400 and 800 mg/kg) (Hu et al., 2022). ASP was dissolved in saline and administered by gavage for 21 days. Both the control and CIA model groups were administered saline. From the first day of intragastric administration, the following were measured every 3 days: (1) rat body weight; (2) water volume method to measure the volume below and including the ankle joint of rats; and (3) arthritis index. CIA rats were scored according to their arthritis score. The scoring criteria were as follows: 0, no erythema or swelling; 1, toe erythema; 2, swelling and redness of the paw; 3, severe swelling and redness of the ankle; and 4, severe swelling and redness of the whole leg and all digits.
2.11 Hematoxylin-eosin staining of synovial tissue of experimental rat knee joints
The morphology and structure of synovial pathological tissues were observed by hematoxylin-eosin (HE) staining to investigate the therapeutic effects of ASP on CIA rats. At 24 h after the final oral administration, synovial tissue was separated from the knee joints of rats, fixed in 10 % neutral formalin, and dehydrated in ethanol. Slices were then cut, embedded in paraffin, stained with HE, and observed under a light microscope.
2.12 Statistical analysis
GraphPad Prism version 8.0.2 (GraphPad Software, Inc., San Diego, CA, USA) was used to perform statistical analysis, and means and standard deviations were determined. Using a single factor analysis of variance and a Student's t-test, P < 0.05 was determined to be statistically significant.
3 Results
3.1 Selection of concentrations for ASP intervention and TNF-α stimulation
The synovium of the knee joint was obtained after the CIA rat model was established. CIA-FLS cells were obtained using collagenase digestion. The cells were spindle shaped (Fig. 1A). When the culture reached the third generation, immunocytochemical identification was carried out. Anti-vimentin antibodies (Fig. 1B), but not anti-CD68 antibodies (Fig. 1C), were observed, consistent with CIA-FLS cells.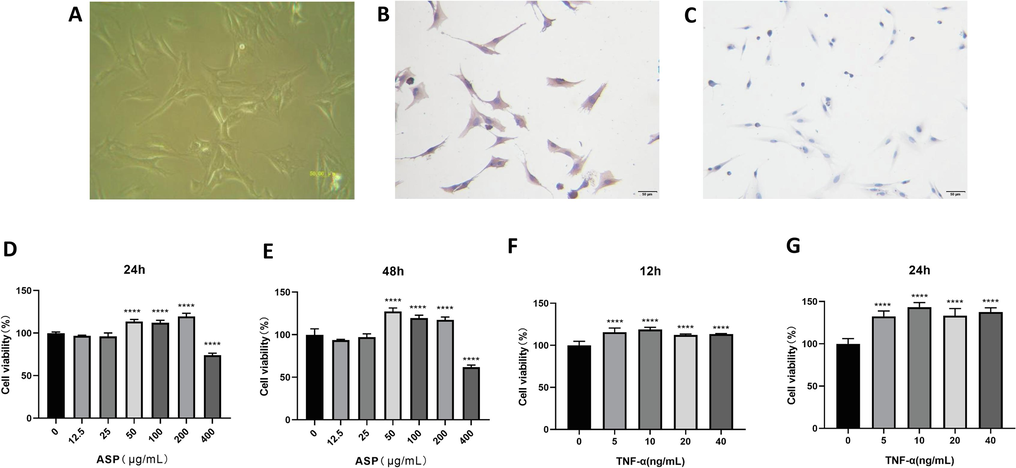
CIA rat knee joint fibroblast-like synoviocytes (FLS) were identified by immunocytochemistry. (A) FLSs digested from CIA rat knee joint (×200); (B) Anti-Vimentin antibody test was positive (×200); (C) Anti-CD68 antibody test was negative (×200); (D, E) Viability of NC-FLS was observed at 24 h and 48 h after intervention of ASP (1.25–400 μg/mL); (F, G) Viability of CIA-FLS was observed at 12 h and 24 h after TNF-α stimulated with the concentration of 5–40 ng/mL. ****P < 0.0001.
ASP became significantly toxic at concentrations of 400 μg/mL and greater (Fig. 1D, E). The viability of NC-FLS cells was highest when the ASP content was 200 µg/mL for 24 h. Therefore, these were chosen as the intervention conditions.
Optimal TNF-α concentrations were 5–20 ng/mL, and treatment duration was determined to be 12/24 h (Fig. 1F, G). CIA-FLS cell viability was optimal at 10 ng/mL and 24 h. Therefore, these conditions were chosen.
3.2 ASP inhibited migration and invasion of CIA-FLS cells
The TNF-α stimulated group showed significantly faster wound healing than the CIA-FLS group (P < 0.05) (Fig. 2A). Wound healing in the ASP intervention group, on the other hand, was significantly slower than the TNF-α stimulated group (P < 0.05) (Fig. 2B). The ASP 200 µg/mL group showed better inhibition than the ASP 100 µg/mL group. Thus, ASP inhibited CIA-FLS cell migration.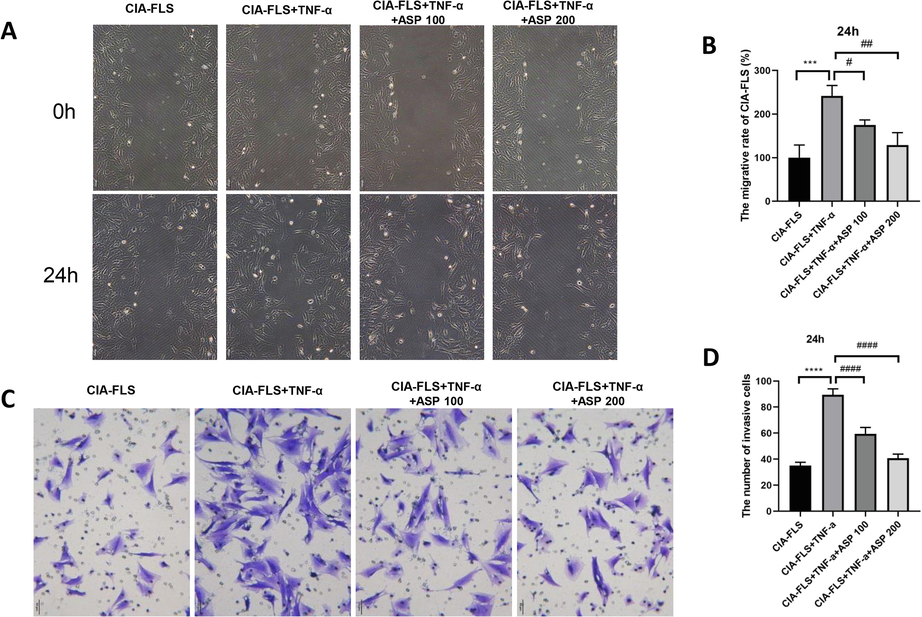
ASP inhibited migration and invasion of CIA-FLS. (A, B) ASP inhibited migration. (C, D) ASP inhibited invasion. ASP 100: ASP 100 μg/mL; ASP 200: ASP 200 μg/mL.***P < 0.001, ****P < 0.0001; #P < 0.05, ##P < 0.01, ####P < 0.0001.
The TNF-α stimulation group displayed significantly higher rates of cell invasion than the CIA-FLS group (P < 0.05) (Fig. 2C), while the ASP intervention group showed significantly lower rates of invasion than the TNF-α stimulation groups (P < 0.05) (Fig. 2D). ASP at 200 µg/mL inhibited cell invasion to a greater extent than 100 µg/mL ASP. These results suggest ASP inhibits CIA-FLS cell invasion.
3.3 Effects of ASP on apoptosis and the cell cycle
Flow cytometry revealed that apoptosis was substantially reduced in the TNF-α group compared to the CIA-FLS group (P < 0.05) (Fig. 3A). Apoptosis was significantly higher in the ASP 200 µg/mL group than in the TNF-α group. (P < 0.05) (Fig. 3B), suggesting apoptosis was promoted by ASP in CIA-FLS cells.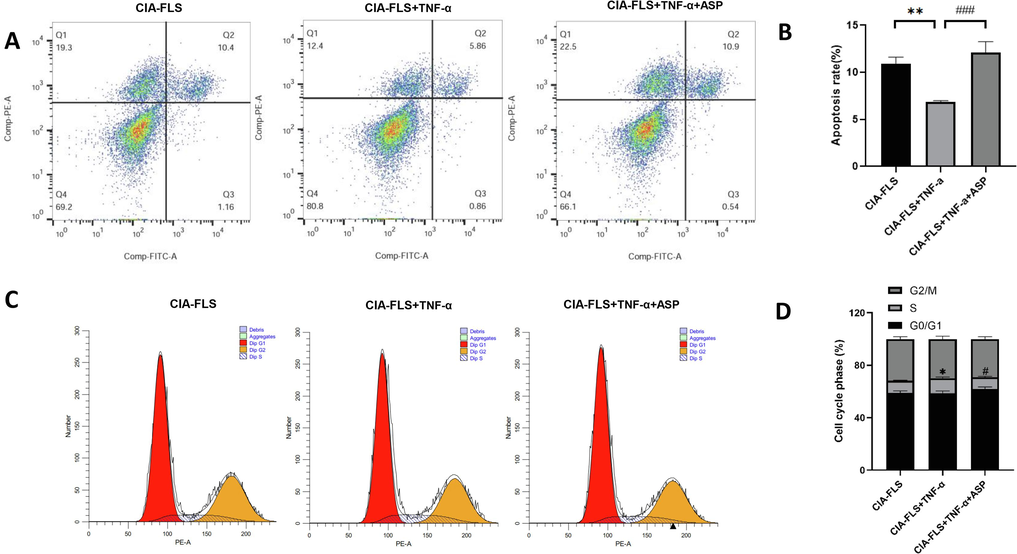
ASP influenced apoptosis and cell cycle phase of CIA-FLS. (A, B) The apoptosis rate of CIA-FLS was detected by flow cytometry. (C, D) The cell cycle phase of CIA-FLS was detected by flow cytometry. *: CIA-FLS vs. CIA-FLS + TNF-α; #: CIA-FLS + TNF-α vs. CIA-FLS + TNF-α + ASP. *P < 0.05, **P < 0.01; #P < 0.05, ###P < 0.001.
The proportion of S phase cells was considerably higher in the TNF-α group than in the CIA-FLS group (P < 0.05) (Fig. 3C). The ASP 200 µg/mL group exhibited a considerably lower proportion of S phase cells compared with the TNF-α group (P < 0.05) (Fig. 3D). According to these results, ASP inhibited the transition of CIA-FLS cells from G0/G1 to S phase.
3.4 Effects of ASP on JAK2/STAT3 and MAPK pathways
The concentration of inflammatory factors in the TNF-α group was substantially higher than in the CIA-FLS group, according to ELISAs of the culture medium supernatant (P < 0.05) (Fig. 4A). There was a significant reduction in the concentration of inflammatory factors in the ASP 200 µg/mL group compared with the TNF-α group (P < 0.05), indicating ASP significantly inhibited the inflammatory response of CIA-FLS cells.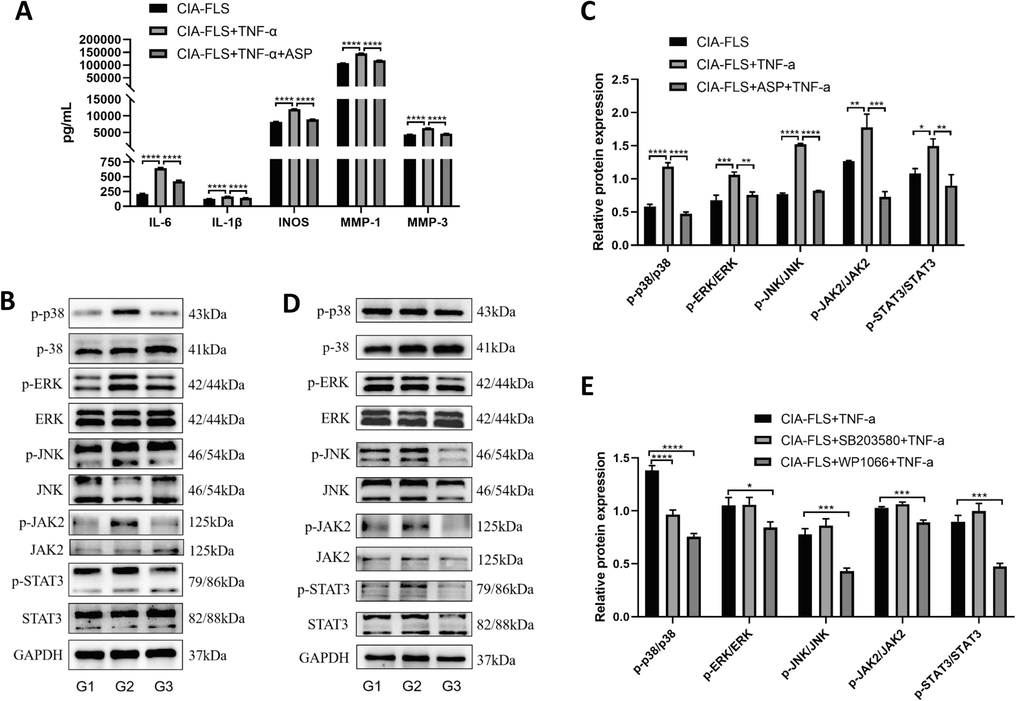
(A) The concentrations of inflammatory factors in the supernatant of cell culture in the CIA-FLS group, the CIA-FLS + TNF-α group and the CIA-FLS + TNF-α + ASP group were detected by ELISA (IL-6, IL-1β, iNOS, MMP-1, MMP-3); (B, C) The phosphorylation levels of key proteins of MAPK and JAK2/STAT3 in G1: CIA-FLS group, G2: CIA-FLS + TNF-α group and G3: CIA-FLS + ASP + TNF-α group were detected by Western Blotting (p38, ERK, JNK, JAK2 and STAT3); (D, E)The phosphorylation of key proteins of MAPK and JAK2/STAT3 pathway components in G1: CIA-FLS + TNF-α group, G2: CIA-FLS + SB203580 + TNF-α group and G3: CIA-FLS + WP1066 + TNF-α group were detected by Western Blotting (p38, ERK, JNK, JAK2 and STAT3).
We then treated CIA-FLS cells with ASP, and then treated the CIA-FLS + ASP group with TNF-α to evaluate JAK2/STAT3 and MAPK signaling (Fig. 4B, C). Phosphorylation of JAK2/STAT3 and MAPK pathway components in the TNF-α group was significantly higher than that in the CIA-FLS group(P < 0.05). Compared with the CIA-FLS + TNF-α group, the phosphorylation of the JAK2/STAT3 and MAPK pathway components in the CIA-FLS + ASP group decreased significantly (P < 0.05). According to these results, by inhibiting JAK2/STAT3 and MAPK pathways, ASP exerted an anti-inflammatory effect.
The p38 inhibitor, SB203580, significantly decreased the phosphorylation of p38 (Fig. 4D, E). However, the phosphorylation levels of other key proteins of the MAPK and JAK2/STAT3 pathways were not affected. The JAK2 inhibitor WP1066 significantly decreased the phosphorylation of JAK2. Phosphorylation levels of key proteins of the MAPK pathway were also significantly decreased. These results suggest the JAK2 pathway is upstream of MAPK.
3.5 ASP inhibits the inflammatory response in CIA rats
As shown by ELISA, the CIA group had significantly higher levels of inflammatory factors (IL-6, IL-1β, iNOS, MMP-1, and MMP-3) than the control group (P < 0.05) (Fig. 5A). ASP treatment groups (low and high dose) had significantly lower plasma inflammatory factor concentrations than the CIA group (P < 0.05). These results suggest ASP significantly reduced the inflammatory response in CIA rats.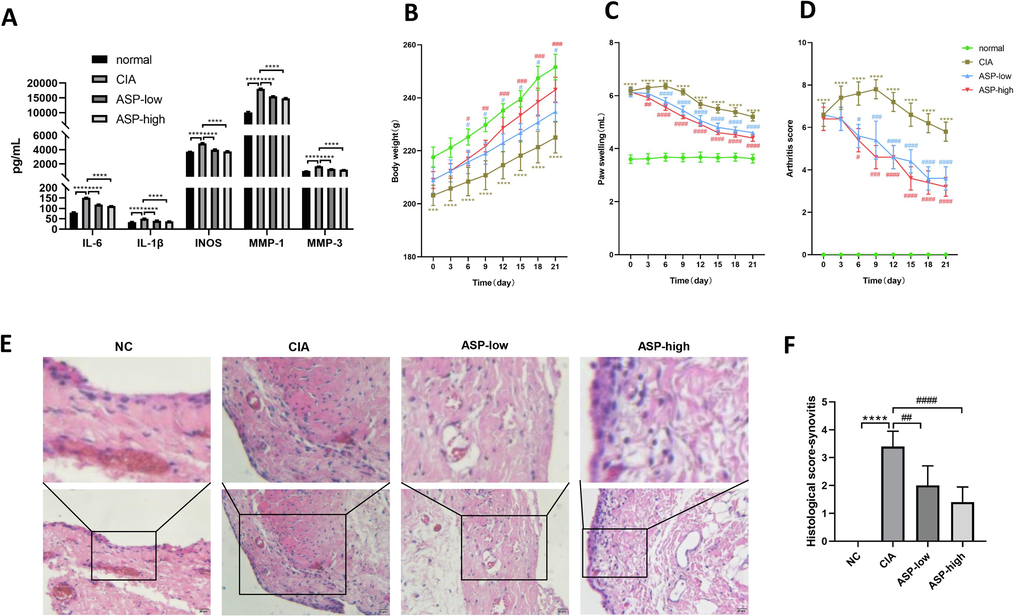
(A) The plasma concentrations of inflammatory factors in rats of the normal group, the CIA group, the ASP-low group and the ASP-high group were detected by ELISA (IL-6, IL-1β, iNOS, MMP-1, MMP-3). Effects of ASP on the body weight (B), paw swelling (C), arthritis score (D) of CIA rats in different time point, and Histological analysis of knee synovitis in CIA rats(E, F). Histological analysis using HE (scale bar 20 μm) labelled sections of rat hind paw knee synovium for NC group, CIA group, ASP-low (ASP 400 mg/kg) group, ASP-high (ASP 800 mg/kg) group. Values were expressed as mean ± SD (n = 5). *: CIA group compared with normal group; #: ASP group compared with CIA group. Values were expressed as mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
We established a CIA rat model to assess the efficacy of ASP (Fig. 6). Rat body weight, paw swelling and arthritis scores were recorded from the day before gavage (day 0). From day 0 of gavage, body weights of the CIA model group were considerably lower than that of the normal rats. (Fig. 5B). However, the low and high doses of ASP reversed the weight loss in CIA rats from day 6 of gavage. Rats in the CIA group had considerably more paw swelling than the normal rats (Fig. 5C). Beginning on day 3, ASP dramatically and dose-dependently decreased paw swelling. As of day 6, ASP significantly and dose-dependently decreased the arthritis Index score (Fig. 5D).
ASP inhibits arthritis development in CIA rats. (A) Graphic solution for CIA induction and ASP administration. SD rats were given two injections of the emulsion separated by one week as described in methods. From 7 days following the second injection, rats (n = 5 in each group) were treated with ASP every day for 21 days. During this period, the rats' body weight, paw swelling and arthritis scores were measured every 3 days. After gavage, synovial tissue was isolated from the knee joint of rats and blood was collected from the heart. (B) Images of rat paws in different groups on the last day of intragastric administration.
Synovial histological, established by HE staining, was used to analyze the synovitis of hind paw knee joint in rats. Thickness of the synovium is used to diagnose synovitis. Synovitis scores were as follows: 0, less than 3 cells thick; 1, 3–5 cells thick; 2, 6–10 cells thick; 3, 10–20 cells thick; 4, 20–30 cells thick; and 5, more than 30 cells thick. The synovitis score in the ASP treatment group was significantly less than that in the CIA group, especially in the high-dose ASP treatment group (P < 0.05) (Fig. 5E, F).
4 Discussion
RA is a chronic inflammatory disease characterized by synovial cell growth and inflammatory infiltration, which gradually results in joint degeneration. FLS cells in the lining of the synovial membrane of RA can produce cytokines that sustain inflammation and lead to the destruction of articular cartilage. FLS cells also have a unique aggressive phenotype that can aggravate articular cartilage damage. Elucidating the intracellular signaling of FLS cells could provide a basis for studying new methods of targeting these cells (Bartok and Firestein 2010). We constructed the CIA rat model and obtained FLS cells from the knee joint. TNF-α is a pro-inflammatory factor that triggers inflammation and causes joint damage, and can be used to measure the inflammatory activity of RA. Based on previous studies (Ho et al., 1987, Zhan et al., 2021, Zhang et al., 2021, Zhai et al., 2022), we chose an inflammation model stimulated by 10 ng/mL TNF-α for 24 h.
Zhang et al.(Zhang et al., 2022) found, via molecular pharmacology tracking of its anti-rheumatoid activity, that the oral bioavailability of the main components of A. sinensis was 57.12 % and the drug sensitivity was 0.69 %. Thus, A. sinensis could be used to treat RA. Some researchers believe that A. sinensis could be used with other traditional Chinese medicines with anti-inflammatory effects or DMARDs to treat RA (Feng et al., 2022, Han et al., 2022). There are many active ingredients in A. sinensis, such as nonstarch polysaccharides, brass, columbianadin, and angelol A (Lee et al., 2014, Chen et al., 2022, Wang et al., 2022). We chose to use ASP to interfere with CIA-FLS cells and study its potential mechanism. To observe the anti-inflammatory effect of ASP on CIA-FLS cells, we conducted a grouped intervention experiment. We conducted CCK-8 assays and tested the effects of 200 mg/mL ASP for 24 h on FLS cells. The migration ability of FLS cells was closely related to the improved invasiveness of these cells on cartilage and bone surfaces. Studies have found that ASP inhibits the migration and invasiveness of CIA-FLS cells. Through apoptosis experiments, we found that ASP promoted FLS apoptosis, suggesting that the therapeutic effects of ASP may be related to cell apoptosis. The cell cycle controls the potential for cell growth or apoptosis; the excessive growth of RA synovial cells is also affected by the cell cycle. In this study, ASP inhibited progression of FLS cells from G0/G1 phase to S phase, thereby inhibiting cell proliferation and differentiation. Activated FLS cells secrete inflammatory and chemotactic factors, which erode and destroy cartilage and bone. ELISAs showed that ASP inhibited the production of inflammatory factors such as IL-6, IL-1β, iNOS, MMP-1, and MMP-3 in CIA-FLS cells. Our study confirmed that ASP had significant anti-invasive, anti-proliferative, and anti-inflammatory effects on CIA-FLS cells.
The anti-inflammatory mechanism of ASP will require further investigation. Although there are many signaling pathways that affect RA, we selected the JAK2/STAT3 and MAPK pathways (Morel and Berenbaum 2004, Jose Alcaraz 2021, Wu et al., 2022, Yang et al., 2022). We investigated the effect of ASP on key proteins of the JAK2/STAT3 and MAPK pathways. ASP significantly decreased protein phosphorylation levels of components of these pathways, indicating ASP has an anti-inflammatory role by downregulating the JAK2/STAT3 and MAPK signaling.
How do the JAK2/STAT3 and MAPK pathways work? To further investigate these pathways, we used inhibitors of JAK2 (WP1066) and p38 (SB203580). Key proteins of the JAK2/STAT3 pathway were not significantly affected by the p38 inhibitor. However, the JAK2 inhibitor significantly inhibited key proteins of the MAPK pathway. This shows that JAK2/STAT3 may be the upstream pathway of MAPK.
In the CIA rat model, downregulation of the colon gene Ggt7 and upregulation of the Ace gene was observed following treatment with ASP, which reduced the methylation status of nucleotide sites in the exon/promoter regions of these two genes (Wang et al., 2020). Another study based on the CIA rat model showed that ASP oral administration improved RA by regulating intestinal microbiota and gene expression. In this study, analysis of the colon transcriptome revealed that ASP restored RA-induced intestinal dysfunction by upregulating Cldn5 (Hu et al., 2022). We generated a CIA rat model and administered ASP to rats by gavage. We found that the toe volume and arthritis score of CIA rats were significantly improved following intragastric administration of ASP. The plasma concentration of inflammatory factors was also decreased significantly. Inflammation score was calculated after HE staining of knee joint synovium in rats. These results confirmed that knee synovitis in CIA rats was significantly reduced after oral administration of ASP. The CIA rat model confirmed the significant therapeutic effects of ASP.
However, this study currently only found that ASP may function via JAK2/STAT3 and MAPK signaling, and no rescue experiments have been conducted to further demonstrate the inhibitory mechanism of ASP. More specific mechanisms still need to be explored. In addition, our research had only made progress in animal experiments. If ASP can be studied on FLS cells of RA patients, it can reflect the clinical value of ASP and will be significant for the future application of ASP in targeted treatment of FLS cells in RA.
5 Conclusion
Our study investigated the therapeutic effects of ASP in RA in vitro and in vivo. Our research suggested that ASP inhibited the invasiveness and secretion of inflammatory cytokines of FLS cells in CIA rats via JAK2/STAT3 and MAPK signaling. Elucidation of this mechanism will provide a theoretical basis for the clinical application of ASP (Fig. 7).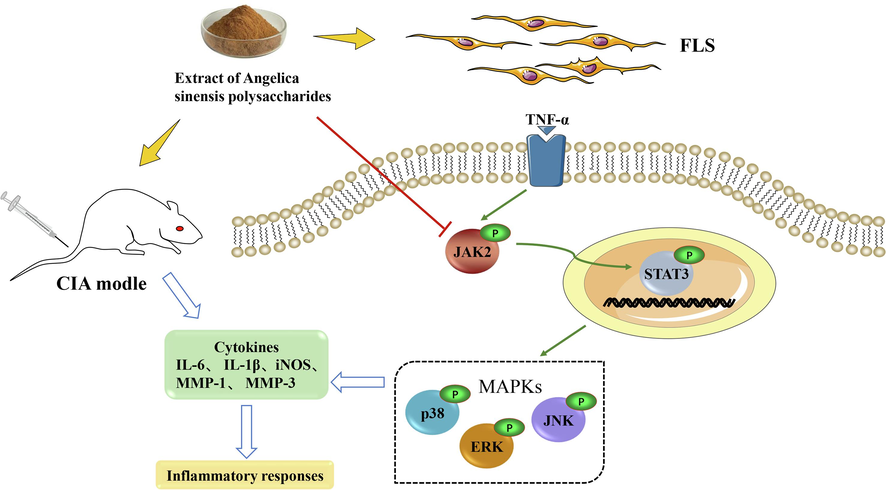
Schematic diagram of the mechanism by which ASP inhibits inflammation of rat CIA-FLS through the JAK2/STAT3 and MAPK signaling pathways.
Funding
This work was supported by the Changzhou Sci&Tech Program (CJ20210064), the Changzhou High-Level Medical Talents Training Project (2016CZLJ011) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_1603).
CRediT authorship contribution statement
Yujing Xue: Investigation, Data curation, Formal analysis, Software, Writing – original draft. Sheng Zhou: Investigation, Visualization, Writing – original draft. Zhicheng Yang: Investigation. Pengyan Hao: Investigation. Liqun Wang: Data curation. Weiding Cui: Investigation. Weixi Liu: Methodology, Validation, Funding acquisition. Ruiping Liu: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev.. 2010;233:233-255.
- [CrossRef] [Google Scholar]
- Analysis of chemical constituents and pharmacological effects of Angelica Sinensis. World Latest Med. Inform.. 2019;19:93+95.
- [CrossRef] [Google Scholar]
- The role of signaling pathway in the biological cause of rheumatoid arthritis. Curr. Drug Res. Rev.. 2021;13:130-139.
- [CrossRef] [Google Scholar]
- Immunosuppressive effect of Columbianadin on maturation, migration, allogenic T cell stimulation and phagocytosis capacity of TNF-alpha induced dendritic cells. J. Ethnopharmacol.. 2022;285:114918
- [CrossRef] [Google Scholar]
- Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implications. Nat. Rev. Rheumatol.. 2022;18:398-414.
- [CrossRef] [Google Scholar]
- The Herbal Combination of Radix astragali, Radix angelicae sinensis, and Caulis lonicerae Regulates the Functions of Type 2 Innate Lymphocytes and Macrophages Contributing to the Resolution of Collagen-Induced Arthritis. Front. Pharmacol.. 2022;13:964559
- [CrossRef] [Google Scholar]
- Conventional disease-modifying anti-rheumatic drugs combined with Chinese Herbal Medicines for rheumatoid arthritis: A systematic review and meta-analysis. J. Tradit. Complement. Med.. 2022;12:437-446.
- [CrossRef] [Google Scholar]
- Unilateral hypertrophic osteoarthropathy in a patient with an infected axillary-axillary bypass graft. Radiology. 1987;162:573-574.
- [CrossRef] [Google Scholar]
- Angelica sinensis polysaccharide improves rheumatoid arthritis by modifying the expression of intestinal Cldn5, Slit3 and Rgs18 through gut microbiota. Int. J. Biol. Macromol.. 2022;209:153-161.
- [CrossRef] [Google Scholar]
- Promising therapeutic targets for treatment of rheumatoid arthritis. Front. Immunol.. 2021;12:686155
- [CrossRef] [Google Scholar]
- Orchestrated modulation of rheumatoid arthritis via crosstalking intracellular signaling pathways. Inflammopharmacology. 2021;29:965-974.
- [CrossRef] [Google Scholar]
- Total glucosides of paeony: A review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J. Ethnopharmacol.. 2020;258:112913
- [CrossRef] [Google Scholar]
- New potential therapeutic approaches targeting synovial fibroblasts in rheumatoid arthritis. Biochem. Pharmacol.. 2021;194:114815
- [CrossRef] [Google Scholar]
- Treatment of rheumatoid arthritis with traditional chinese medicine. Biomed Res. Int.. 2014;2014:528018
- [CrossRef] [Google Scholar]
- Polysaccharide from Angelica Sinensis Suppresses Inflammation and Reverses Anemia in Complete Freund's Adjuvant-induced Rats. Curr. Med. Sci.. 2020;40:265-274.
- [CrossRef] [Google Scholar]
- Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol.. 2021;230:108793
- [CrossRef] [Google Scholar]
- Total glucosides of paeony for rheumatoid arthritis: A systematic review of randomized controlled trials. Complement. Ther. Med.. 2017;34:46-56.
- [CrossRef] [Google Scholar]
- Signal transduction pathways: new targets for treating rheumatoid arthritis. Jt., Bone, Spine.. 2004;71:503-510.
- [CrossRef] [Google Scholar]
- Pathogenesis of rheumatoid arthritis: one year in review 2022. Clin. Exp. Rheumatol.. 2022;40:475-482.
- [CrossRef] [Google Scholar]
- Rheumatoid arthritis: advances in treatment strategies. Mol. Cell. Biochem. 2022
- [CrossRef] [Google Scholar]
- What are the dominant cytokines in early rheumatoid arthritis? Curr. Opin. Rheumatol.. 2018;30:207-214.
- [CrossRef] [Google Scholar]
- The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis.. 2008;67:909-916.
- [CrossRef] [Google Scholar]
- Recovery of Ggt7 and ace expressions in the colon alleviates collagen-induced arthritis in rats by specific bioactive polysaccharide intervention. J. Agric. Food Chem.. 2020;68:14531-14539.
- [CrossRef] [Google Scholar]
- A novel method for investigating the mechanism of the anti-rheumatoid arthritis activity of Angelicae pubescentis radix by integrating UHPLC-QTOF/MS and network pharmacology. Biomed. Chromatogr.. 2022;36:e5389.
- [Google Scholar]
- A systematic review and network meta-analysis about the efficacy and safety of Tripterygium wilfordii Hook F in rheumatoid arthritis. Evid. Based Complement. Alternat. Med.. 2022;2022:3181427.
- [CrossRef] [Google Scholar]
- System pharmacology analysis to decipher the effect and mechanism of active ingredients combination from herb couple on rheumatoid arthritis in rats. J. Ethnopharmacol.. 2022;288:114969
- [CrossRef] [Google Scholar]
- Sesquiterpenes from Kadsura coccinea attenuate rheumatoid arthritis-related inflammation by inhibiting the NF-kappaB and JAK2/STAT3 signal pathways. Phytochemistry. 2022;194:113018
- [CrossRef] [Google Scholar]
- Scientific basis of botanical medicine as alternative remedies for rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2013;44:284-300.
- [CrossRef] [Google Scholar]
- Lactate metabolism in rheumatoid arthritis: Pathogenic mechanisms and therapeutic intervention with natural compounds. Phytomedicine. 2022;100:154048
- [CrossRef] [Google Scholar]
- Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-induced caspase 3/gasdermin E-mediated pyroptosis. Arthritis Rheumatol.. 2022;74:427-440.
- [CrossRef] [Google Scholar]
- Internalization of neutrophil-derived microvesicles modulates TNFalpha-stimulated proinflammatory cytokine production in human fibroblast-like synoviocytes. Int. J. Mol. Sci.. 2021;22
- [CrossRef] [Google Scholar]
- Glaucocalyxin A suppresses inflammatory responses and induces apoptosis in TNF-a-induced human rheumatoid arthritis via modulation of the STAT3 pathway. Chem. Biol. Interact.. 2021;341:109451
- [CrossRef] [Google Scholar]
- Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev.. 2021;41:1337-1374.
- [CrossRef] [Google Scholar]
- Computation and molecular pharmacology to trace the anti-rheumatoid activity of Angelicae Pubescentis Radix. BMC Complement. Med. Ther.. 2022;22:312.
- [CrossRef] [Google Scholar]
- Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of Angelica Sinensis polysaccharide. Oxid. Med. Cell. Longev.. 2020;2020:3240820.
- [CrossRef] [Google Scholar]







