Translate this page into:
HPLC–DAD–MS identification of polyphenols from Passiflora leschenaultii and determination of their antioxidant, analgesic, anti-inflammatory and antipyretic properties
⁎Corresponding author at: Bioprospecting Laboratory, Department of Botany, School of Life Sciences, Bharathiar University, Coimbatore 641 046, Tamil Nadu, India. Tel.: +91 0422 2428305. drparimel@gmail.com (Parimelazhagan Thangaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Passion fruit (Passiflora leschenaultii DC), an endemic species to peninsular India, is traditionally used to treat various ailments such as dysentery, urinary stone disease and wounds. The present study aimed to investigate the antioxidant, analgesic, anti-inflammatory, antipyretic activities and chemical composition of leaf extracts of P. leschenaultii. Bioactive secondary metabolites such as total phenolics, tannins and flavonoids were quantified. Antioxidant activities were determined by DPPH•, ABTS+•, FRAP, metal chelating and phosphomolybdenum assays. Hot plate, acetic acid and formalin induced pain models were used to evaluate the analgesic activity. In order to study the acute and chronic anti-inflammatory activities, carrageenan and cotton pellet induced models were used in rats. Brewer’s yeast induced pyrexia method was applied for the antipyretic test. Functional compounds from the plant were identified and quantified through HPLC–DAD–MS analysis. The obtained results revealed that the acetone extract of leaves exhibited higher phenolic (440.24 mg GAE/g extract) and flavonoid (253.33 mg RE/g extract) contents and scavenged the DPPH• (IC50 29.14 μg/mL), ABTS+• (10509.69 μM TEAC/g extract) effectively. On investigating the analgesic, anti-inflammatory and antipyretic activities, the acetone extracts of leaves, at a dose of 400 mg/kg (p.o.) reduced significantly (p < 0.001) the pain, inflammation and fever responses in vivo. Bioactive compounds such as hyperin, chlorogenic acid, rutin and caffeic acids were identified in the leaves of P. leschenaultii employing HPLC–DAD–MS analysis. These findings illustrate the excellent potential of this species as valuable source of natural phytochemicals with pharmacological properties.
Keywords
P. leschenaultii
Free radicals
Chlorogenic acid
Hyperin
Cotton pellet
1 Introduction
The use of medicinal plants is one of the most important human concerns in the modern world. Various traditional and ethnomedicine systems essentially based on using medicinal plants are currently available. These systems encompass a variety of health benefits, awareness, religious therapies, handling techniques, and beliefs on plants to treat, identify or prevent diseases. Recent report from the World Health Organization reveals that more than 60% of the world’s population in developing countries depends on medicinal plants (MPs) for the treatment of various human ailments such as cancer, diabetic mellitus, cardiovascular and neurodegenerative diseases (WHO, 2010). Currently, a wide variety of plant based drugs are available in the form of crude extracts, powder, mixture or parts in herbal markets and these derived from MPs are gaining economic importance (Saqib et al., 2014).
Inflammation, pain and fever are the initial symptoms of all major human and animal diseases. Inflammation is a multifarious pathophysiological process intermediated by variety of signaling molecules produced by leukocytes and macrophages which includes phagocytic uptake and the production of inflammatory mediators such as nitric oxide (NO), prostaglandin enzyme (PGE2) and tumor necrosis factor alpha (TNF-α) (Sengar et al., 2015). In the meantime, the production of cytokines either from immune or from central nervous system cells might directly sensitize the peripheral nociceptors, the prostaglandins (PGs) induce hyperalgesia by affecting the transducing property of free nerve endings, bradykinins, TNF-α, and interleukins (ILs) and induce pain (Obreja et al., 2002). Fever or pyrexia is a result of secondary impact of inflammation (Shukla et al., 2010). During the course of fever, the generation of pyrogens including ILs, TNF-α and interferon induces PGE2 production in hypothalamus which subsequently increases the body temperature (Sengar et al., 2015). Pain, inflammation and fever are all closely related to and are associated with enhanced production of prostaglandins (Shukla et al., 2010).
In the modern system of medicine, non-steroidal anti-inflammatory drugs (NSAIDs) are mostly used as anti-inflammatory agents which also possess analgesic and antipyretic activities (Sengar et al., 2015). Owing to adverse effects of NSAIDs, there is a high demand for the search of new drugs without any adversative effects. Recent research has focused on medicinal plants because of their availability, accessibility and being devoid of harmful effects (Ibrahim et al., 2012). Polyphenolic compounds such as phenolics, flavonoids, and carotenoids are secondary metabolites synthesized by many plants and these exhibit proven biological activities. Intake of more polyphenolics from fruits and vegetables has greatly been associated with reduced risk of inflammatory disorders, cardiovascular, diabetic and other developing degenerative diseases (Ibrahim et al., 2013).
Passiflora leschenaultii DC belonging to the family of Passifloraceae is an endemic species to peninsular India, distributed in Tamil Nadu, Karnataka, Kerala and Maharashtra states (Prabhu Kumar et al., 2012). The Paliyar community tribals of Theni district in Tamil Nadu use the leaf juice of P. leschenaultii to treat dysentery and other ailments (Ignacimuthu et al., 2008). Mohamed Farook et al. (2009) reported that the fruit juice and seeds of the plant are used to inhibit the formation of calcium oxalate, calcium phosphate and calcium carbonate so as to cure the urinary stone disease. The roots of the plant are grounded with little quantity of water and the poultice is applied topically on the affected body once a day for 6–8 days to cure wounds (Punjaji, 2012). Besides the traditional usage reported, there exists no report on pharmacological and functional properties of the plant species. Thus the present study was aimed to focus on the antioxidant, anti-inflammatory, analgesic and antipyretic properties while identifying the functional compounds present in leaves of P. leschenaultii. The results of the present study could be anticipated to promote P. leschenaultii as a promising source of therapeutic potential to develop new drugs for pharmaceutical industries.
2 Materials and methods
2.1 Chemicals
Acetic acid, butylated hydroxytoluene (BHT), 2,2′-azinobis (3-ethylbenzothiozoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picryl hydrazyl (DPPH•), 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride (FeCl3), ethylenediaminetetraacetic acid (EDTA), ferrozine, ferrous chloride (FeCl2) and 5-5′-thiobis-2-nitrobenzoic acid (DTNB) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Aluminum chloride, ammonium molybdate, Folin–Ciocalteu’s phenol reagent, hydrochloric acid, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium hydroxide, sodium nitrite, sodium phosphate and α-tocopherol were obtained from Himedia (Mumbai, India). Solvents such as acetone, chloroform, methanol and petroleum ether used were of the highest purity (>99.5%) and analytical grade (Merck) made in India.
2.2 Plant collection and extract preparation
The fresh leaves of P. leschenaultii were collected from Kotagiri hills during the month of September 2012. The collected plant material was identified and their authentication was confirmed from Botanical Survey of India, Southern Regional Centre, Coimbatore, Tamil Nadu (No. BSI/SRC/5/23/2013-14/Tech/532). The voucher specimen (BU/HB/006168) of the plant was deposited at the herbarium in the Department of Botany, Bharathiar University, Coimbatore. Fresh plant material was cleaned to remove adhering dust and then dried under shade. The dried sample was powdered and used for further studies. The powdered leaf material (100 g) was subjected to soxhlet extraction using petroleum ether to remove the lipophilic substances. The residues were dried in a hot air oven (30 °C) and 98.25 g of this dried matter was further extracted using 300 mL of each of the several solvents such as chloroform, acetone, methanol and water. The extracts obtained were concentrated in a rotary vacuum-evaporator (Yamato B0410, Japan) at 40 °C and lyophilized (Vir Tis Benchtop 4K, USA). The freeze dried extracts were then stored at −20 °C and used for further antioxidant analysis and pharmacological investigation.
2.3 Quantification of bioactive secondary metabolites
The quantification of bioactive secondary metabolites in the plant extracts was performed by the standard UV spectrophotometric methods. The total phenolic and tannin contents of different solvent extracts of P. leschenaultii leaves were determined according to the method described by Siddhuraju and Becker (2003) and the results were calculated as gallic acid equivalent (GAE). The method of Zhishen et al. (1999) was followed to quantify the total flavonoid content and the amounts are expressed in terms of rutin equivalent (RE).
2.4 In vitro antioxidant activity
2.4.1 DPPH• scavenging assay
Radical scavenging activity in bleaching of the purple, stable DPPH radical by the various solvent extracts of leaves of P. leschenaultii was measured according to the method described by Blois (1958). IC50 values of the extract i.e., concentration of extract necessary to decrease the initial concentration of DPPH by 50% were calculated. The lower value of the IC50 indicates the higher activity.
2.4.2 ABTS+• scavenging assay
The total antioxidant activity of different solvent extracts of the P. leschenaultii leaves was measured by ABTS radical cation decolorization assay according to the method described by Re et al. (1999). The units of total antioxidant activity (TAA) are expressed as the concentration of trolox equivalent antioxidant activity in terms of μM/g extract.
2.4.3 Ferric reducing/antioxidant power (FRAP) assay
The ferric reducing antioxidant capacity of P. leschenaultii leaves extract was estimated by the method described by Pulido et al. (2000). The results of FRAP values are expressed as mM Fe(II) equivalent/g extract.
2.4.4 Metal chelating activity
The chelating of ferrous ions by P. leschenaultii leaves extract was estimated by the method of Dinis et al. (1994). The chelating activity of the extracts was evaluated using EDTA as standard. The results are expressed as mg EDTA equivalent/g extract.
2.4.5 Phosphomolybdenum assay
The antioxidant activity of extracts was evaluated by the green phosphomolybdenum complex formation following the method of Prieto et al. (1999). The results are reported as ascorbic acid equivalents/g extract.
2.5 HPLC–DAD–MS analysis
The HPLC analysis was performed on a Shimadzu system consisting of a degasser DGU-20A3, a SIL-20A autosampler, two LC-20AD pumps, and a SPDM20Avp photodiode array detector (DAD), coupled with a CBM 20A interface. The chromatographic separation was performed using a Phenomenex Luna C18 analytical column (250 mm × 4.6 mm × 5 μm particle size). The flow rate was 0.6 mL/min and the injection volume was 20 μL. The analytical method employed a linear gradient system which consisted of (solvent A) 1% (v/v) acetic acid in water and (solvent B) methanol. The gradient elution started with 35–40% B for 5 min, 40–50% B to 15 min, 50–60% B to 20 min, 60–70% B to 25 min, 70–78% to 30 min, and 78–80% to 36 min (end analysis). Photodiode array detector was set at 340 nm for acquiring chromatograms.
Mass spectrometric analysis was performed on a Bruker mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) fitted with ion trap analyzer. Mass spectra data were recorded on a ionization mode for a mass range of m/z 100–1000. Other mass spectrometer conditions were as follows: nebulizing gas pressure: 40 psi; drying gas flow: 9 L/min; drying gas temperature: 300 °C; and flow rate: 200 μL/min. For MRM, the specific negative ionization modes (m/z [M−H]−) were used to analyze the compounds.
2.6 In vivo studies
2.6.1 Animals
Swiss albino male mice (25–30 g) and Wistar rats (150–200 g) were used for this study. The animals were housed under standard conditions of temperature (23 ± 1 °C), relative humidity (55 ± 1%), 12/12 h light/dark cycles and fed with standard pellet and water ad libitum. The animals were allowed to acclimatize to the environment for 7 days before the commencement of experiments. All animal experiments were conducted with the permission from Institutional Ethical Committee (KMCRET/Ph.D/12/2012-13), KMCH College of Pharmacy, Coimbatore, Tamil Nadu, India.
2.6.2 Acute toxicity study
Oral acute toxicity studies were performed according to Organization for Economic Co-operation and Development (OECD) guidelines (Ecobichon, 1997). Swiss albino male mice (n = 6) were selected by random sampling technique in this study. The animals were fasted for 12 h with free access to water only. The acetone extract of P. leschenaultii leaves (suspended in distilled water with 0.1% of carboxymethyl cellulose) was administered orally at a dose of 5 mg/kg initially to mice and mortality was observed for 24 h. If there was no mortality observed then the extract treatment was repeated with higher doses such as 50, 300, 500, 1000 and 2000 mg/kg and mortality observed. Further, the animals were also observed individually for 24 h for any behavioral and neurological changes such as tremors, convulsions, salivation, diarrhea, sleep, lacrimation and feeding behavior as a sign of acute toxicity for 24 h.
2.7 Evaluation of analgesic activity
2.7.1 Acetic acid induced writhing test
The test was carried out according to the method described by Siegmund et al. (1957). Four groups of Swiss albino male mice, each consisting of 6 animals (n = 6) were fasted overnight prior to the start of experiment with water ad libitum. The peripheral analgesic drug, morphine was used as positive control. Group 1 received the vehicle distilled water with 10 mL/kg per oral (p.o.), and group 2 was treated with indomethacin (5 mg/kg, p.o.). Groups 3 and 4 were orally administered with acetone extracts of leaves of P. leschenaultii at doses of 200 and 400 mg/kg p.o., respectively. Thirty minutes after treatment, the mice were injected (intraperitoneal) with 0.1 mL of 1% acetic acid solution to induce the characteristic writhing. After the administration of acetic acid the mice were placed in an observation box, and the number of writhings occurring between 5 and 30 min was recorded. The percentage inhibition was calculated using the following formula:
2.7.2 Formalin induced paw licking method
The method of Hunskaar and Hole (1987) was employed to carry out the formalin induced paw licking test. 25 μl of 1% formalin was injected subcutaneously into the right hind paw of mice. The time (in seconds) spent in licking and biting responses of the injected paw was observed as an indication of nociception response. Responses were measured until 5th min (first phase) and 30th min (second phase) after formalin injection. Swiss albino male mice were divided into four groups of six animals (n = 6) each. Group one received water and it was considered as control, group two received morphine at a dose of 10 mg/kg intra peritoneal (i.p.) and it was considered as standard. Groups three and four, received 200 and 400 mg/kg, respectively of P. leschenaultii leaves acetone extract orally. The percentage inhibition was calculated using the following formula:
2.7.3 Hot plate assay
The hot-plate test measured response latencies according to the method described by Eddy and Leimback (1953). Swiss albino male mice were divided into four groups of six animals each. Group one, considered as control received water, and group two received morphine (10 mg/kg i.p.) and it was considered as standard. Groups three and four, received 200 and 400 mg/kg, respectively of P. leschenaultii leaves acetone extract orally. Animals were placed on an Eddy’s insight hot-plate maintained at 55 ± 1.0 °C and the time between placement of the animal on the hot-plate and the occurrence of either the licking of the hind paws, shaking or jump off from the surface was recorded as response latency at the time interval of 0, 30, 60 and 120 min after administration of the test substances. Post-treatment cut-off time of 15 s was used. The percentage protection was calculated using the following formula:
2.8 Evaluation of anti-inflammatory activity
2.8.1 Carrageenan induced paw edema
The anti-inflammatory property of acetone extract of P. leschenaultii leaves was studied against carrageenan-induced acute paw edema in rats, according to the methodology described by Kulkarni et al. (1986). For the experiment, the Wistar male rats (150–200 g) were divided into four groups each consisting of 6 animals (n = 6). The first group received distilled water (10 mL/kg p.o.), while the second group was treated with the standard drug indomethacin (10 mg/kg p.o.). The third and fourth groups were administered with the acetone extracts of P. leschenaultii leaves, being 200 and 400 mg/kg p.o., respectively. Acute inflammation was produced by the subplantar administration of 0.1 mL of 1% carrageenan (in 1% carboxymethylcellulose, w/v) in the right hind paw of the rats. The animals were pre-treated with the drug 1 h before the administration of carrageenan. The volume of the paw was measured immediately by plethysmographically at 60, 120, 180, 240 and 300 min time intervals after the carrageenan injection. The percentage inhibition was calculated using the following formula:
2.8.2 Cotton pellet-implanted granuloma
For the experiment, the Wistar male rats (150–200 g) were divided into four groups, each consisting of six animals each (n = 6). The animals were fasted overnight prior to the start of the experiment, with water ad libitum. Cotton pellets, weighing 5 mg each, were sterilized. Using Ketamine and Xylazine anesthesia, the pellets were introduced subcutaneously through a skin incision in the back of the animals. Group one, considered as control received distilled water (10 mL/kg). Group two received indomethacin (10 mg/kg) and it was considered as standard. Groups three and four, received 200 and 400 mg/kg, respectively of acetone extracts of P. leschenaultii leaves. All the groups were treated orally for 5 days which started 30 min after cotton pellet implantation. On the fifth day, the animals were sacrificed with ether, the granulomas were removed and dried for 24 h at 60 °C and the dry weights were determined. The difference between the initial and final dry weights was considered to be the weight of the granulomatous tissue produced (Winter et al., 1962).
2.9 Antipyretic activity
2.9.1 Brewer’s yeast-induced pyrexia
Wistar male rats were divided into four groups, each consisting of six animals (n = 6) each. The normal body temperature of each rat was measured rectally at predetermined intervals and recorded. The procedure of Adams et al. (1968) was followed for the antipyretic studies. Pyrexia was induced by subcutaneously injecting 15% w/v brewer’s yeast suspension (10 mL/kg) into the animal’s dorsum region. Eighteen hours after the injection, the rectal temperature of each rat was measured using a digital thermometer (BD, model no: 524928). Only rats that showed an increase in temperature at 0.5–1 °C were used for experiments. The first group received distilled water and was considered as control while the second group received paracetamol (150 mg/kg) and was considered as standard. The third and fourth groups received 200 and 400 mg/kg, respectively of acetone extracts of P. leschenaultii leaves. The rectal temperatures of all the rats were measured at 1, 2, 3, 4 and 5 h after drug administration and were compared with the control group.
2.10 Statistical analysis
All the in vitro results were expressed as means ± standard deviation values with triplicate determinations (n = 3). Analysis of variance (ANOVA) and significant (p < 0.05) difference between means were determined by Duncan’s multiple range test. For in vivo studies, the results were expressed as mean ± standard error means (S.E.M.) values. Statistical analysis was carried out by ANOVA followed by Dunnett test wherein p < 0.01 and p < 0.05 probability levels were considered as indication of significance, as compared to the control group. All calculations were performed using SPSS, version 17.0 (Stat Soft Inc., Tulsa, OK, USA).
3 Results and discussion
3.1 Quantification of bioactive secondary metabolites
Secondary metabolites especially polyphenolic compounds in plants or plant products form the main source of natural antioxidants. The extract yield and quantifications of such biologically important natural compounds namely total phenolics, tannins and total flavonoid contents in different solvent extracts of leaves of P. leschenaultii are summarized in Table 1a. Among the various solvent extracts analyzed, the acetone extract revealed the highest total phenolic, tannin and total flavonoid contents (440.24 mg GAE/g extract, 229.29 mg GAE/g extract and 253.33 mg RE/g extract, respectively) followed by the methanol extract of leaves. Medicinal plants comprising a variety of natural secondary metabolites such as phenolics, tannins and flavonoids, are closely associated with the antioxidant activity. These compounds are reported to greatly affect the course of disease processes by counteracting oxidative stress and maintaining the levels of reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive sulfur species (RSS) and develop the antioxidant defense mechanism by playing an important role in maintaining proper health status in preventing and treatment of many diseases (Fernandes et al., 2014). These results prove that the extracts of P. leschenaultii leaves can act as rich source of phenolics, tannins and flavonoids contents which may be used to cure various human diseases. Values are means of triplicate determination of each sample ± standard deviation (n = 3). Mean values followed by different superscript letters in a column indicate significant statistical difference (p < 0.05). GAE – gallic acid equivalent; RE – rutin equivalent.
Solvents
Extract yield (%)
Total phenolics (mg GAE/g extract)
Tannin (mg GAE/g extract)
Flavonoids (mg RE/g extract)
Petroleum ether
1.75
125.24 ± 1.80e
20.24 ± 2.97d
141.67 ± 11.55c
Chloroform
2.3
141.67 ± 1.49d
22.38 ± 0.82d
90.00 ± 5.00e
Acetone
9.4
440.24 ± 0.82a
229.29 ± 1.24a
253.33 ± 7.64a
Methanol
17.3
290.00 ± 0.71b
128.81 ± 0.41b
210.00 ± 5.00b
Hot water
8.3
187.21 ± 0.65c
32.43 ± 0.23c
123.76 ± 7.33d
3.2 In vitro antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrazyl), the stable free radical has its maximum absorption at 517 nm. When the absorption level is decreased the radicals loose their stability and get reduced to hydrazine derivative. This mechanism happens due to the antioxidants which donate electrons/hydrogen atoms to the stable radicals (Koşar et al., 2011). Fig. 1 shows the results of DPPH radical scavenging activity of different solvent extracts of P. leschenaultii leaves. The antioxidant activity of the extracts was evaluated from determination of IC50 values. The results revealed that the acetone extracts display a maximum radical scavenging activity with the IC50 value of 29.14 μg/mL. However, the standards rutin (5.79 μg/mL) and BHT (6.30 μg/mL) revealed higher activity. The results of ABTS+•, FRAP, metal chelation and phosphomolybdenum activity of different solvent extracts of P. leschenaultii leaves are shown in Table 1b. These results clearly imply that all sample extracts inhibit or scavenge ABTS radical cation in a concentration depended manner and also produced the maximum ferric reducing, chelating the metal ions and phosphomolybdenum reduction capability significantly (p < 0.05). Particularly, the acetone extracts of P. leschenaultii leaves showed a higher ABTS+• scavenging (10509.69 μM TEAC/g extract), FRAP (1511.74 ± 20.25 mM Fe (II)/g extract), metal chelating (279.41 ± 0.60 mg EDTAE/g extract) and phosphomolybdenum (250.27 ± 1.80 mg AAE/g extract) activity. The mechanism including hydrogen atoms donation ability and electron transfer governs the scavenging of radicals, whereby exhibiting antioxidant ability. The results indicate that the acetone extracts of P. leschenaultii leaves which exhibited higher antioxidant capacity also contained higher amount of polyphenolic compounds such as total phenolics, tannins and total flavonoids which could involve in the radical scavenging mechanism of the extract. Recently in our previous study we reported that the leaves of species of P. subpeltata contain high quantities of phenolic and flavonoid contents along with higher antioxidant capability (Saravanan et al., 2014). Furthermore, the related species such as P. edulis, P. incarnata, P. foetida, P. alata, P. actinia and P manicata are known to possess the antioxidant capacity especially in their leaves (Dhawan et al., 2004; Sasikala et al., 2011; Montefusco-Pereira et al., 2013). Values are means ± standard deviation of triplicate determination of each sample (n = 3). Mean values followed by different superscript letters in a column indicate significant statistical difference (p < 0.05). TEAC: trolox equivalent antioxidant capacity; Fe (II) E: Fe (II) equivalent. EDTAE: ethylene diamine tetra acetic acid equivalent; AAE: ascorbic acid equivalent.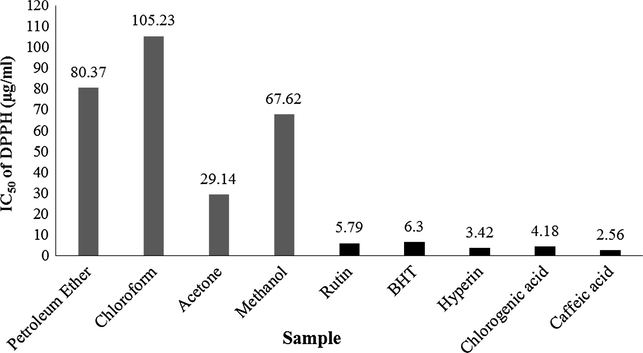
DPPH radical scavenging activity of extracts obtained from P. leschenaultii leaves. Footnote: values are expressed as mean ± standard deviation of three different aliquot determinations (n = 3). Mean values followed by different superscript letters indicate significant statistical difference (p < 0.05).
Solvents
ABTS+• (μM TEAC/g extract)
FRAP (mM Fe (II) E/g extract)
Metal chelating activity (mg EDTAE/g extract)
Phosphomolybdenum assay (mg AAE/g extract)
Petroleum ether
3054.36 ± 4.59c
528.77 ± 15.47c
156.31 ± 2.07c
32.93 ± 0.61d
Chloroform
2973.36 ± 5.27d
587.17 ± 5.85c
175.05 ± 0.72b
48.00 ± 1.44c
Acetone
10509.69 ± 3.80a
1511.74 ± 20.25a
279.41 ± 0.60a
250.27 ± 1.80a
Methanol
6349.46 ± 8.23b
1107.24 ± 20.62b
234.13 ± 0.20a
123.33 ± 5.00b
Hot water
4321.12 ± 6.58b
654.32 ± 7.89c
179.56 ± 1.24b
112.34 ± 3.32b
3.3 HPLC–DAD–MS analysis
The HPLC–DAD analysis of the acetone extract of P. leschenaultii leaves revealed the presence of four major peaks P1, P2, P3 and P4 which showed good chromatography separation and resolution. The retention time of the peaks was as follows: P1 = 15.6 min, P2 = 16.7 min P3 = 27.7 and P4 = 30.1 (Fig. 2). The separated peaks were identified according to retention time in comparison with standard compounds and were quantified as hyperin (quercetin-3-O-galactoside) (P1 – 2.7 mg/g extract), chlorogenic acid (P2 – 1.9 mg/g extract), caffeic acid (P3 – 4.6 mg/g extract) and rutin (P4 – 1.8 mg/g extract). Additionally, the identity of the peaks was also confirmed with the mass spectrometric (HPLC–MS) analysis and the results are shown in Table 2.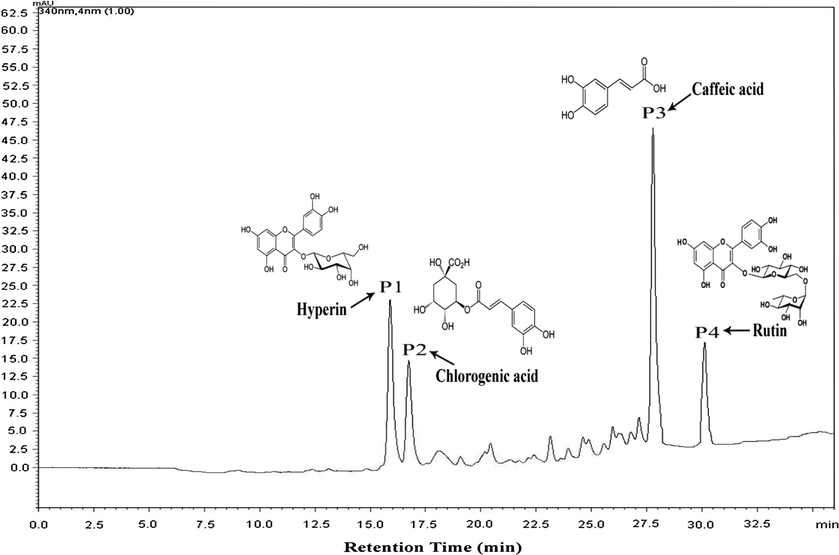
HPLC–DAD profile obtained from the acetone extract of P. leschenaultii leaves.
Peak
Retention time (min)
MS [M−H]− m/z
MS2 [M−H]− m/z
Compounds
Quantity in mg/g extract
P1
15.6
463.00
300.90
Hyperin
2.7
P2
16.7
353.03
190.94
Chlorogenic acid
1.9
P3
30.1
178.93
134.95
Caffeic acid
4.6
P4
27.7
609.01
300.97
Rutin
1.8
The possible mechanism of reduction of the DPPH• and ABTS+• can be correlated to the available hydroxyl groups in the reported caffeic acid and chlorogenic acid of phenolic derivatives. Recent studies have demonstrated that the radical scavenging activity of phenolic compounds is largely influenced by the number of hydroxyl groups on the aromatic ring, i.e. the higher the number of hydroxyl groups, the higher the radical scavenging activity (Lazarova et al., 2014). Transition metals are vital elements in human body and these are required for the activity of many enzymes. However, they contain unpaired electrons which react very quickly with peroxides, resulting in the formation of alkoxyl radicals. The chelation of transition metals by antioxidants can be considered an important mechanism in oxidation processes (Lazarova et al., 2014). It is well known that the flavonoids such as quercetin and rutin derivatives efficiently chelate metal ions and these also possess the ferric reducing antioxidant power. The earlier work also proved that the quercetin-3-O-galactoside and rutin rich fruits and vegetables have more antioxidant capacity when compared to ascorbic acid (Azuma et al., 1999). Furthermore, our previous study has also shown that the flavonoids and phenolics found in P. subpeltata possess antinociceptive, anti-inflammatory and anti-pyretic effects (Saravanan et al., 2014). Kim et al. (2004) reported that certain flavonoids, especially flavone derivatives of quercetin and rutin express their anti-inflammatory activity by modulation of pro-inflammatory gene expression such as cyclooxygenase-2 and inducible nitric oxide synthase, and by several pivotal cytokines. Due to these unique action mechanisms and significant in vivo activity, flavonoids are considered to be reasonable substances for new anti-nociceptive, anti-inflammatory and antipyretic drugs. The epidemiological reports revealed that the more intake of polyphenol rich foods reduces the chronic diseases such as cancer, coronary heart disease and inflammation related disorders (Ibrahim et al., 2013).
Chlorogenic acid is one of the most abundant polyphenol compounds in human diet which also presents antioxidant and anti-carcinogenic activities (Santos et al., 2006). The acetone extract of P. leschenaultii leaves contained 1.9 mg/g extract of chlorogenic acid. Thus this research finding revealed that the leaves of P. leschenaultii are good source of phenolic compounds, which possess strong antioxidants, anti-nociceptive, anti-inflammatory and antipyretic properties.
3.4 In vivo studies
3.4.1 Acute toxicity study
From the quantification of bioactive secondary metabolites, in vitro antioxidant activity and HPLC results, the acetone extracts possess higher amounts of polyphenolic compounds and radical scavenging activity. Thus based on these results, the acetone extracts of P. leschenaultii leaves were selected to carry out the pharmacological investigations. The acetone extracts of P. leschenaultii leaves were initially subjected for acute toxicity testing in Swiss albino mice. The animals were monitored for 24 h after administration of acetone extracts at various doses of 50, 500, 1000 and 2000 mg/kg. The plant extracts did not cause any mortality and did not alter the general behavior up to 2000 mg/kg. Thus the acute toxicity study of acetone extract of plant leaves revealed that these extracts are safe and non-toxic.
3.5 Evaluation of analgesic activity
The oral administration of acetone extracts of P. leschenaultii showed a dose dependent analgesic activity and the values are shown in Table 3. Intraperitoneal administration of acetic acid to the control mice resulted in 17.25 writhings. Pre-treatment with acetone extract of P. leschenaultii leaves at 200 and 400 mg/kg doses reduced the writhings to 9.50 and 3.25 (44.75% and 81.43% inhibition), respectively. The extracts at a dose of 400 mg/kg registered a significantly (p < 0.001) higher level (81.43%) of analgesic activity when compared to control (Table 3a). Regarding formalin test, the results obtained from the acute phase (10 min) showed that morphine and the acetone extracts possessed only mild inhibitory effects. During the delayed phase (30 min), morphine and acetone extracts exhibited significant (p < 0.001) analgesic effect. Morphine showed 72.19% of analgesic activity while the acetone extracts showed an analgesic effect of 57.26% and 70.80% at the doses of 200 mg/kg and 400 mg/kg p.o, respectively (Table 3b). In the case of hot plate test, results showed that in early phase period of 30 min, 400 mg/kg of leaves extract and 10 mg/kg of morphine showed a significant (p < 0.05) analgesic activity. However, in the late phase period of 120 min, 400 mg/kg of leaves extract (81.96%) and 10 mg/kg of morphine (85.92%), showed very high analgesic activity and increased the reaction time when compared with the control group at significant level (p < 0.001) (Table 3c). Values are expressed as mean ± SEM (n = 6). Values are expressed as mean ± SEM (n = 6). Values are expressed as mean ± SEM (n = 6).
Groups and dose
Number of writhings measured at different time intervals
% Inhibition of writhings (30 min)
5 min
10 min
15 min
20 min
25 min
30 min
Acetic acid induced control (10 mL/kg)
26.25±0.85
24.75±0.48
20.75±16.21
18.75±0.48
18.75±0. 48
17.25±0.48
–
Morphine (mg/kg)
10
23.53±0.65⁎
21.75±0.63⁎
16.12±0.82⁎⁎
9.25±0.48⁎⁎⁎
7.25±0.38⁎⁎⁎
3.12±1.47⁎⁎⁎
83.17
P. leschenaultii (mg/kg)
200
31.50±0.65
23.75±0.63⁎
18.50±0.65⁎⁎
14.75±0.25⁎⁎⁎
11.50±0.63⁎⁎⁎
9.50±0.29⁎⁎⁎
44.75
400
25.25±1.03
17.25±0.75⁎⁎
13.25±0.48⁎⁎⁎
10.75±0.41⁎⁎⁎
6.50±0.29⁎⁎⁎
3.25±0.75⁎⁎⁎
81.43
Groups and doses
Number of lickings measured at different time intervals
% Inhibition of lickings (30 min)
5 min
10 min
15 min
20 min
25 min
30 min
Control (formalin) 1%
54.51 ± 0.65
56.53 ± 1.55
60.21 ± 0.91
62.10 ± 0.41
58.25 ± 0.85
54.52 ± 1.04
–
Morphine (mg/kg)
10
54.02 ± 1.41
53.75 ± 1.26
50.25 ± 0.50⁎
49.20 ± 4.55⁎⁎
39.12 ± 2.94⁎⁎⁎
15.25 ± 6.65⁎⁎⁎
72.19
P. leschenaultii (mg/kg)
200
52.25 ± 0.48
52.25 ± 0.95
51.75 ± 1.11
50.00 ± 3.11
43.25 ± 0.85
23.25 ± 0.85
57.26
400
52.00 ± 0.91
50.50 ± 0.29
50.25 ± 2.06⁎
49.75 ± 1.03⁎⁎
39.00 ± 1.35⁎⁎⁎
16.00 ± 1.83⁎⁎⁎
70.80
Groups and doses (mg/kg)
Reaction time in seconds
Latency (%)
0 min
30 min
60 min
120 min
Control
–
1.25 ± 0.25
2.3 ± 0.25
2.3 ± 0.25
2.5 ± 0.29
–
Morphine
5
2.00 ± 0.29
6.0 ± 0.71⁎
11.8 ± 0.95⁎⁎⁎
17.8 ± 0.48⁎⁎⁎
85.92
P. leschenaultii
200
2.50 ± 0.29
3.75 ± 0.25
6.75 ± 0.48⁎⁎
8.75 ± 0.48⁎⁎⁎
71.66
400
2.25 ± 0.25
6.00 ± 0.41⁎
9.75 ± 0.63⁎⁎⁎
14.00 ± 0.41⁎⁎⁎
81.96
Acetic acid induced writhing, formalin induced paw licking and hot plate tests are widely used models to study the peripheral and central analgesic effects of drugs. Acetic acid can indirectly induce the significant release of endogenous mediators such as bradykinins, prostaglandins and pro-inflammatory cytokines that stimulate the splanchnosomatic pain nociceptive to neurons, which are sensitive to NSAIDs (Koster et al., 1959).
Formalin test and hot plate tests are very useful methods for not only assessing the anti-nociceptive drugs but also helping in the elucidation of the action mechanism. Shibata et al. (1989) reported that the centrally acting drugs such as morphine inhibited both phases (first neurogenic pain and second inflammatory nociception), while peripheral acting drugs, such as the NSAIDs and corticoids, inhibited mainly the second phase. In the present study, oral pre-treatment with acetone extract of P. leschenaultii leaves strongly inhibited both phases especially at the 400 mg/kg dose which reduced significantly (p > 0.001) the formalin-induced licking or biting responses in mice. Moreover the plant extract also increases the latency time of the animals on the hot plate test by reducing the licking and jumping responses which prove that the plant extracts stimulate the thermal stability of the animals. The results also suggest the plant extract counteracts both peripheral neurogenic pain and central inflammatory nociception responses.
3.6 Evaluation of anti-inflammatory activity
The results of the anti-inflammatory effect of the acetone extract of P. leschenaultii leaves on carrageenan-induced edema in hind paws and cotton pellet-implanted chronic inflammatory response of the experimental rats are presented in Table 4. There was a gradual increase in paw edema volume of rats in the control (carrageenan treated group). However at the same time, the standard drug and plant extracts treated groups showed a significant reduction in the paw edema volume but these were not significantly different (p < 0.001). The results also revealed that the acetone extract of P. leschenaultii leaves expressed inhibitory activity at the doses of 200 mg/kg and 400 mg/kg p.o, being 42.07% and 81.37%, respectively. However, standard indomethacin showed highest activity (89.16%) (Table 4a). Meanwhile, in the cotton pellet-implanted chronic inflammatory response the plant extract exhibited a significant (p < 0.001) anti-inflammatory effect in a dose dependent manner and these results were comparable to that of standard drug indomethacin. The doses of 200 mg/kg and 400 mg/kg of acetone extracts of P. leschenaultii leaves exhibited 56% and 79.04% granuloma inhibition, respectively which is equal to the standard indomethacin (80.31%) at a dose of 10 mg/kg (Table 4b). Values are expressed as mean ± SEM (n = 6). Values are expressed as mean ± SEM (n = 6).
Groups and doses
Edema induced by carrageenan/paw volume (mL) at different time intervals
% Inhibition of paw edema (5 h)
0 h
1 h
2 h
3 h
4 h
5 h
Carrageenan induced control (10 mL/kg)
1.01 ± 0.02
1.40 ± 0.14
1.47 ± 0.10
1.89 ± 0.21
1.94 ± 0.17
2.03 ± 0.13
–
Indomethacin (mg/kg)
10
1.00 ± 0.01
1.39 ± 0.03
0.86 ± 0.07⁎⁎⁎
1.98 ± 0.17
1.68 ± 0.14⁎
1.09 ± 0.05⁎⁎⁎
89.16
P. leschenaultii (mg/kg)
200
0.99 ± 0.02
1.34 ± 0.23
1.42 ± 0.19⁎⁎
1.49 ± 0.21⁎⁎
1.42 ± 0.08⁎⁎
1.44 ± 0.05⁎⁎
42.07
400
1.32 ± 0.17
1.53 ± 0.10
1.59 ± 0.29
1.44 ± 0.06⁎⁎
1.41 ± 0.20⁎⁎
1.14 ± 0.09⁎⁎⁎
81.37
Group and dose
Weight of cotton pellet (mg)
Inhibition (%) of granuloma formation
Induced control (cotton pellet) (5 mg/kg)
81.25 ± 6.29
–
Indomethacin
10 mg/kg
16.00 ± 1.41⁎⁎⁎
80.31
P. leschenaultii
200 mg/kg
35.75 ± 2.22⁎⁎⁎
56.00
400 mg/kg
17.03 ± 1.18⁎⁎⁎
79.04
Carrageenan induced acute peritonitis is a renowned and universally accepted model for assessing the acute inflammatory process. The mechanism and pathophysiological phenomenon of inflammation is a biphasic (early phase and late phase) process regulated through the several inflammatory mediators, including interleukin-1 beta (IL-1b), IL-6, TNF-α, NO, PGE2, bradykinin, serotonin, histamine, leukotriene B4, and other chemotactic agents (Prasad et al., 2014). The result of pre-treatment with the acetone extract of P. leschenaultii leaves demonstrated that the doses of 200 and 400 mg/kg p.o. effectively reduce the edema formation in the early phase (1st h) of inflammation which is due to release of histamine and serotonin primarily. Further, the anti-inflammatory effect of the extract significantly (p < 0.001) remains up to the late phase (5th h) of the experiment because the extract may possess the capability to reduce the over production of the late phase mediators such as PGE2, protease, lysosomes and chemotactic agents.
Cotton pellet induced granuloma formation is a classic model to investigate the chronic inflammatory test. The transudative and proliferative components of chronic inflammation were assessed by this method. Difference between the fluid adsorbed by the pellet and dry weight pellet indicates the amount of granulomatous tissue formed in the inflammation center. Increasing level of fibroblasts and synthesis of collagen with mucopolysaccharide are natural proliferative events of granulation tissue formation (Sengar et al., 2015). The acetone extract of P. leschenaultii leaves showed a decrease in granuloma formation at a significant level (p < 0.001) which may also reflect its efficacy in reducing the increased level of fibroblasts and synthesis of collagen with mucopolysaccharide. Naturally occurring phenolic and flavonoid compounds exert a wide range of pharmacological activities such as antioxidant, anti-inflammatory, analgesic, and anti-carcinogenic actions with different mechanism (Liu and Lin, 2012). Thus the quantitative results of acetone extract of P. leschenaultii leaves encompass higher amount of phenolics and flavonoid compounds which may be correlated to the antioxidant and anti-inflammatory properties.
3.7 Antipyretic activity
The data on antipyretic activity of acetone extracts of P. leschenaultii leaves are presented in Table 4c. The results showed that acetone extracts of leaves at doses of 200 and 400 mg/kg p.o. cause significant (p < 0.001) lowering of the body temperature up to 5 h following the plant extract administration in a dose dependent manner. The maximum lowering of body temperature was noticed at dose of 400 mg/kg p.o. where the mean temperature of 37.92 °C was reduced to 35.50 °C within 5 h period of pyrexia induced by yeast. The animals treated with acetone extracts of P. leschenaultii leaves at 200 and 400 mg/kg doses showed a decrease in the rectal temperature by 0.14 and 0.34 °C, respectively within 1 h while after 5 h, the temperature was reduced by 1.05 and 2.42 °C, respectively. Values are expressed as mean ± SEM (n = 6).
Groups and dose
Rectal temperature in °C at different time intervals
−18 h
0 h
1 h
2 h
3 h
4 h
5 h
Yeast induced control 10 mL/kg
35.93 ± 0.39
36.93 ± 0.39
36.27 ± 0.54
36.84 ± 0.32
36.93 ± 0.33
36.97 ± 0.34
36.87 ± 0.31
Paracetamol 150 mg/kg
35.00 ± 0.60
36.82 ± 0.35
36.33 ± 0.30
36.06 ± 0.40⁎
35.69 ± 0.33⁎⁎
35.26 ± 0.31⁎⁎⁎
35.14 ± 0.24⁎⁎⁎
P. leschenaultii 200 mg/kg
35.78 ± 0.48
38.40 ± 0.26
37.82 ± 0.43
38.08 ± 0.33
37.82 ± 0.36
36.97 ± 0.08⁎
36.50 ± 0.12⁎⁎
P. leschenaultii 400 mg/kg
35.23 ± 0.26
37.92 ± 0.22
37.61 ± 0.24
37.98 ± 0.49
36.57 ± 0.24⁎⁎
36.23 ± 0.27⁎⁎
35.50 ± 0.07⁎⁎⁎
Subcutaneous injection of Brewer’s yeast induced pyrexia is considered as a useful test for the screening of plants materials as well as synthetic drugs for their antipyretic effect. Yeast induced pyrexia is also called pathogenic fever and its etiology could be the production of higher level of PGs synthesis. The inhibition of PGs synthesis could be the possible mechanism of antipyretic action, as it can be achieved by blocking the cyclo-oxygenase enzyme activity. There are several mediators for pyrexia and the inhibition of these mediators is responsible for the antipyretic effect (Muhammad et al., 2012). The results of antipyretic activity revealed that the acetone extract of P. leschenaultii leaves possesses a significant (p < 0.001) difference when compared with paracetamol antipyretic effect in maintaining normal body temperature and reducing yeast-induced elevated body temperature in rats and their effects are comparable to those of the standard antipyretic drug paracetamol. The result obtained could be attributed to the fact that the plant extract may possess certain influence on the inhibition of PGs development because the prostaglandins are well-thought-out to stand a foremost controller of fever.
The results of secondary metabolite quantifications, antioxidant and in vivo animal studies concluded that the acetone extract of P. leschenaultii leaves possesses the ability to quench the radicals in vitro and reduce the responses of pain, inflammation and fever in vivo. This study is further supported by identifying the possible major compounds present in the leaves extract by HPLC analysis. The results obtained from this analysis revealed that the presence of flavonoids hyperin (P1), and phenolics compounds such as chlorogenic acid (P2), caffeic acid (P3) and rutin (P4) in acetone extracts from P. leschenaultii leaves is reported for the first time. All the reported compounds are suggested to possess a natural antioxidant capacity and pharmacological properties.
4 Conclusion
The research findings proved that the acetone extracts obtained from P. leschenaultii leaves revealed significant antioxidant, anti-nociceptive, anti-inflammatory and antipyretic activities in all the animal models tested. This study serves as a first report on the pharmacological properties of P. leschenaultii. Additionally, the relatively high levels of identified phenolic and flavonoid compounds in the leaves significantly increased their pharmacological benefits. However, further studies are required to elucidate exact mechanism of action and to characterize the other chemical compounds responsible for the pharmacological properties of P. leschenaultii as a new promising source of health products for food and pharmaceutical industries.
Acknowledgment
The authors acknowledge the support of Programa Nacional de Pós-doutorado/CAPES (PNPD/CAPES - 2013), Brazil, for Post-Doctoral fellowship to author, S. Saravana and for providing financial assistance.
References
- Some aspects of the pharmacology of ibufenac, a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol.. 1968;20:305-312.
- [Google Scholar]
- Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem.. 1999;47:3963-3966.
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200.
- [Google Scholar]
- Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archieves Biochem. Biophys.. 1994;315:161-169.
- [Google Scholar]
- The Basis of Toxicology Testing. New York: CRC Press; 1997. p. :43-86.
- Synthetic analgesics: II. Dithyienylbutenyl-amines and dithyienylbutylamines. J. Pharmacol. Exp. Ther.. 1953;3:554-557.
- [Google Scholar]
- The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103-114.
- [Google Scholar]
- Antiinflammatory, analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae) J. Ethnopharmacol.. 2012;141:282-289.
- [Google Scholar]
- Effects of mangifera pajang kostermans juice on plasma antioxidant status and liver and kidney function in normocholesterolemic subjects. J. Funct. Foods. 2013;5:1900-1908.
- [Google Scholar]
- Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia. 2008;79:562-568.
- [Google Scholar]
- Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci.. 2004;96:229-245.
- [Google Scholar]
- In vitro antioxidant properties and phenolic composition of Salvia halophila Hedge from Turkey. Food Chem.. 2011;129:374-379.
- [Google Scholar]
- Anti-inflammatory actions of clonidine, guanfacine and B-HT 920 against various inflammation-induced acute paw edemas in rats. Archives Internationales de Pharmacodynamie et de Therapie. 1986;279:324-334.
- [Google Scholar]
- HPLC–DAD analysis of phenolic compounds and antioxidant properties of Asphodeline lutea roots from Bulgaria and Turkey. Ind. Crops Prod.. 2014;61:438-441.
- [Google Scholar]
- Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio. Food Chem. Toxicol.. 2012;50:3032-3039.
- [Google Scholar]
- Inhibition of mineralization of urinary stone forming minerals by medicinal plants. E-J. Chem.. 2009;6:938-942.
- [Google Scholar]
- Antioxidant, anti-inflammatory, and hypoglycemic effects of the leaf extract from Passiflora nitida Kunth. Appl. Biochem. Biotechnol.. 2013;170:1367-1378.
- [Google Scholar]
- Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complementory Altern. Med.. 2012;12:59.
- [Google Scholar]
- IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J.: Official Publ. Fed. Am. Soc. Exp. Biol.. 2002;16:1497-1503.
- [Google Scholar]
- Validation and documentation of rare endemic and threatened (RET) plants from Nilgiri, Kanuvai and Madukkarai forests of southern Western Ghats, India. J. Threatened Taxa. 2012;4:3436-3442.
- [Google Scholar]
- Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol. Adv.. 2014;32:1053-1064.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem.. 2000;48:3396-3402.
- [Google Scholar]
- Some less known herbal remedies against cut and wounds from Ahmednagar areas in Maharashtra, India. Int. J. Basic Appl. Sci.. 2012;1:184-197.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Indigenous knowledge of medicinal plants in Kotli Sattian, Rawalpindi district, Pakistan. J. Ethnopharmacol.. 2014;151:820-828.
- [Google Scholar]
- Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull.. 2006;29:2236-2240.
- [Google Scholar]
- Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves – a promising species of Passiflora. Ind. Crops Prod.. 2014;54:272-280.
- [Google Scholar]
- Evaluation of antioxidant potential of different parts of wild edible plant Passiflora foetida L. J. Appl. Pharm. Sci.. 2011;01(04):89-96.
- [Google Scholar]
- Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol.. 2015;160:140-148.
- [Google Scholar]
- Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347-352.
- [Google Scholar]
- Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem. Toxicol.. 2010;48:61-64.
- [Google Scholar]
- Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem.. 2003;51:2144-2155.
- [Google Scholar]
- Siegmund, E., Cadmus, R., Lu, G., 1957. A method for evaluating both non-narcotic and narcotic analgesics. Proceedings of the Society for Experimental Biology and Medicine. Soc. Exp. Biol. Med (New York, N.Y.) 95, 729–731.
- (WHO), W.H.O., 2010. Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS).
- Carrageenan induced edema in hind paw of rats as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med.. 1962;11:544-547.
- [Google Scholar]
- The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.. 1999;64:555-559.
- [Google Scholar]







