Translate this page into:
Evaluation of novel solvent extraction systems for Zr/Hf separation with MIBK homologues
⁎Corresponding author. wangjunlian306@163.com (Junlian Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Zr/Hf separation has always been a great challenge. Although the methyl isobutyl ketone (MIBK)-thiocyanic acid (HSCN) extraction system selectively extracts Hf over Zr and can realize Zr/Hf separation, it has the following disadvantages: it has a strong smell; it is easy to burn; and there is large loss of extractant during extraction due to the low boiling point (115.7 °C), low flashing point (13 °C), and high solubility of MIBK in water (1.7 % at 25 °C). In this study, Zr/Hf separation performance by MIBK homologues — of which one side of C = O is isopentyl, isohexyl, n-pentyl, n-hexyl or n-heptyl, while the other side are all methyl — were studied and compared with MIBK. Effects of HSCN pre-extraction, HCl concentration, NH4SCN concentration on Zr, Hf extraction and separation performance were investigated. Relationships between methyl alkyl ketone structure and their Zr/Hf separation performance were revealed. A new Zr/Hf separation system MNPK (methyl n-pentyl ketone)-HSCN was found. MNPK has a higher boiling point (151 °C), a higher flashing point (47 °C) and a lower solubility in water (0.43 % at 20 °C) than MIBK. Compared to MIBK, it is safer, more friendly operation environments and less extractant loss during extraction. MNPK has as fast extraction kinetics as MIBK. It also has good anti-emulsification performance. Its maximum βHf/Zr can reach to 12.61. The loaded Zr and Hf in MNPK have very similar stripping behaviors with those loaded in MIBK by HCl and H2SO4 solutions, respectively. MNPK is the most possible candidate to replace MIBK from HSCN media in Zr/Hf separation industry.
Keywords
Zirconium
Hafnium
Separation
HSCN
MIBK homologues
1 Introduction
Zirconium (Zr) and hafnium (Hf) are all-important strategic metals, as ∼ 90 % of global Zr and Hf output are consumed in nuclear industry(Wang and Lee, 2016). Zr and Hf have totally different ability to capture thermal neutrons. Zr has the thermal neutron adsorption cross-section as small as 0.18b, while that of Hf is as large as 120b(Xiong et al., 2002). This causes their totally different applications in nuclear industry. Therefore, Zr and Hf have to be deeply separated from each other before their utilization in nuclear industry. The content of Hf in nuclear grade Zr has to be less than 0.01 %, while the purity of nuclear grade Hf has to be more than 98 %(Li et al., 2014). However, Zr and Hf are both the IVB group elements with adjacent periods on the periodic table, which leads to their very similar physical and chemical properties. Therefore, deep separation of Zr and Hf has always been a great challenge.
Three Zr/Hf separation technologies have been established: multiple crystallization, extractive distillation, and solvent extraction (Poriel et al., 2006; Sathiyamoorthy, 1999), among which solvent extraction is the main method (Xu et al., 2014; Xu et al., 2015; Wang and Lee 2016). Zircon (ZrSiO4) is the main commercial source of Zr, which also contains 1 ∼ 3 % of Hf(Vermaak et al., 2018). As Hf is the minor element, the Hf selective extraction over Zr is very important. The MIBK-HSCN separation system has been commercialized since 1950 for nuclear grade Zr and Hf production(Reinhardt, 1966). This method shares two thirds of the worldwide nuclear grade Zr production(Wang et al., 2020). MIBK selectively extracts Hf over Zr in HSCN media. There are also two other commercial separation systems TBP(tributyl phosphate)–HNO3-HCl and TOA(tri-n-octylamine)-H2SO4, which both extract Zr over Hf. They only share a small portion of the global nuclear grade Zr production.
MIBK-HSCN separation system has the advantages of extracting Hf over Zr, simple Zr/Hf separation process and high Zr/Hf separation efficiency(Reinhardt, 1966). However, MIBK has a low boiling point (115.7 °C), a low flashing point (13 °C) and a high solubility in water (1.7 % at 25 °C), which leads to the drawbacks of ease to burn and large extractant loss during Zr/Hf separation (Gokel, 2004). Therefore, researchers have been exploring new Zr/Hf separation systems using various commercial extractants (TBP(Amaral and Morais, 2020; Amaral et al., 2020; Kanth et al., 2020), PC-88A(2-ethylhexylphosphonic acid mono-2-ethylhexyl ester)(Min and Lee, 2015), Cyanex 272 (di-(2,4,4′-trimethylpentyl)phosphinic acid) (Banda et al., 2014; Lee et al., 2015), D2EHPA(di-(2-ethylhexyl)phosphoric acid) (Yang et al., 2019; Zhao et al., 2019), Cyanex 572(He et al., 2021), LIX 63(5,8-diethyl-7-hydroxy-6-dodecanone oxime)(Wang and Lee 2014), TOPO(tri-n-octylphosphine oxide)(Banda et al., 2013; Wu et al., 2022) and amines(Wang and Lee, 2015; Conradie et al., 2016) etc.) and novel kinds of compounds (BEAP (bis(2-ethylhexyl)-1-(2-ethylhexylamino) propylphosphonate) (Chen et al., 2017), diglycolamide derivatives(Saleh, 2012), diglycolamic acid derivatives(Ma et al., 2021), INET-1((n-octyl)(2,4,4′-trimethylpentyl) phosphinic acid)(Wang et al., 2022; Wang et al., 2023), OADMPPA (N,N-n-octylamine di(methylene phenylphosphinic acid))(Zhao et al., 2020), phosphonic acid modified calix (Banda et al., 2014)arene(Morohashi et al., 2019), ionic liquids(Pathak et al., 2021), etc.). Few of these separation systems can replace MIBK-HSCN method in nuclear grade Zr/Hf separation industry.
Since MIBK-HSCN separation system produces two thirds of the worldwide nuclear grade Zr sponge, ketone extractants maybe a good direction of exploring new Zr/Hf separation systems which have the merits similar to MIBK-HSCN system. Xu et al(Xu et al., 2012) focused their Zr/Hf separation study on diisobutyl ketone (DIBK). Compared to MIBK-HSCN system, they found that DIBK-HSCN system has far weaker extraction ability for Hf, which means low Zr/Hf separation efficiency. Furthermore, they developed a series of DIBK based synergistic Zr/Hf separation systems, like DIBK-P350(Wu et al., 2019), DIBK-P204(Xu et al., 2016), DIBK-TBP(Xu et al., 2012). They even used DIBK as a diluent in Zr/Hf separation with Cyanex 572(He et al., 2021). These mixed systems make Zr/Hf separation more complicated than MIBK-HSCN system.
As we compare the structures of MIBK and DIBK, we find that MIBK has one methyl and one isobutyl that connect to the carbonyl (C = O), while DIBK has two isobutyls connecting to the C = O (See Fig. 1). Hence, DIBK has a higher boiling point (168.1 °C), a higher flashing point (48 °C), and a lower solubility in water (0.06 % at 25℃) (Gokel, 2004). It has been reported that Hf is extracted by MIBK from HSCN media through the following two equations(Xiong et al., 2002):
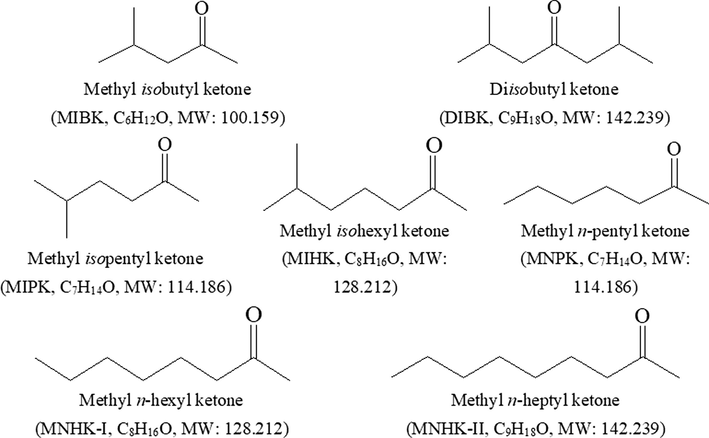
Structures of MIBK and its homologues.
Anyway, C = O is the active group, which interacts with the central ion in Hf extraction. Large steric hindrance on both sides of C = O makes this interaction harder, which leads to weak extraction ability of DIBK(Xu et al., 2012). If we only increase the carbon atoms on one side of C = O — the other side is still methyl — what would happen during Zr/Hf separation? Till now, there are no reports on this study.
As the number of carbon atoms is increased, the corresponding ketones have higher boiling point, higher flashing point, and lower solubility in water than MIBK (Oshima and Miyake, 2021). Meanwhile, as the other side of C = O is still methyl, there is still enough space to make C = O interact with Hf ions. They may have better comprehensive Zr/Hf separation performance in HSCN media than MIBK. In this paper, we studied five MIBK homologues — of which one side of C = O is isopentyl, isohexyl, n-pentyl, n-hexyl or n-heptyl, while the other side are all methyl — on Zr/Hf separation from HSCN media and compared with that of MIBK. We tried to find the effect of the alkyl structure of methyl alkyl ketones on Zr/Hf separation, and also tried to find better ketone extractant for Zr/Hf separation than MIBK. Structures of MIBK, DIBK and the studied MIBK homologues are shown in Fig. 1. The main physical parameters of these ketones are listed in Table 1.
Boiling point / ℃
Flashing point / ℃
Solubility in water at 25 °C / %
Density ratio at 20 °C
Vapor pressure at 25 °C / mmHg
MIBK
115.7a
13a
1.7a
0.8006a
18.2
DIBK
168.1a
48a
0.06a
0.806a
1.5
MIPK
141a
41a
0.5a
0.888a
5.2
MIHK
167
44.6
–
0.8151
–
MNPK
151a
47a
0.43
0.8197a
4.7
MNHK-I
173a
62a
0.09
0.819a
1.7
MNHK-II
195.3
64
0.05
0.8208
0.6
2 Experimental
2.1 Reagents
Zirconium tetrachloride (ZrCl4) and hafnium tetrachloride (HfCl4) were both bought from Aladdin Biochemical Technology Co., Ltd (Shanghai, China), and their purities were ≥ 99.9 % and ≥ 99.5 %, respectively. Concentrated hydrochloric acid (HCl) was guaranteed reagent (G.R.), and provided by Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Ammonium thiocyanate (NH4SCN) was analytical reagent (A.R.), which was bought from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). NaOH standard solution was manufactured by Yida Technology (Quanzhou) Co., Ltd (Fujian China).
Extractants methyl isobutyl ketone (MIBK, purity: >99.5 %), methyl isopentyl ketone (MIPK, purity: 99 %), methyl n-pentyl ketone (MNPK, purity: 98 %) and methyl n-hexyl ketone (MNHK-I, purity: 98 %) were bought from Aladdin Biochemical Technology Co., Ltd (Shanghai, China), while methyl isohexyl ketone (MIHK, purity: 98 %) and methyl n-heptyl ketone (MNHK-II, purity: 99 %) were provided by Macklin Biochemical Co., Ltd (Shanghai, China). All these ketone extractants were not diluted in all the extraction experiments.
2.2 Apparatus
All the extraction experiments were performed on a multi-tube Vortexer UMV-2 (Usun Technologies Co., Ltd, Beijing, China), except the oscillating time experiments which were performed on a KB-5010 Test Tube Shaker (Kylin-Bell Lab Instruments Co., Ltd, Haimen, China). Phase separation was accelerated by a Guanghe TD4C Low Speed Tabletop Centrifuge (Changzhou, China). The Zr, Hf concentration in the aqueous phase was determined by an iCAP 7400 ICP-OES (Thermo Fisher Scientific, USA).
2.3 Extraction procedure
2.3.1 Preparation of HSCN solutions
A 100 mL of 2.0 mol/L HSCN solution was prepared by dissolving 15.224 g NH4SCN with 20 mL deionized water, then adding 16.7 mL concentrated HCl and diluting to desired volume in a 100 mL volumetric flask. The HSCN solutions with concentration of 0.5 mol/L, 1.0 mol/L and 1.5 mol/L were prepared by diluting the 2.0 mol/L HSCN solution.
2.3.2 Extraction procedure
The extraction procedures were generally as follows: Certain volumes of aqueous feed solution and the extractant were sealed in centrifugal tubes, and vibrated on the Multi-tube Vortexer at 2500 rpm for 30 min. The vibration time at such high speed is sufficient to reach extraction equilibrium. After that, the mixture was centrifugated on the Tabletop Centrifuge at 3000 rpm for 5 min. The aqueous phase was separated for Zr, Hf measurement by ICP-OES or HSCN determination by titration with standard NaOH solution. The corresponding Zr, Hf and HSCN concentration in the organic phase at equilibrium was calculated according to Eq. (3). The Zr, Hf extraction percentage (E, %), distribution ratio (D) and their separation factor (βHf/Zr) were calculated according to Eq. (4) ∼ (6), respectively.
2.3.3 Loading capacity experiments
The loading capacity experiments were similar to the above-described extraction procedure. Differently, the aqueous phase was individual Hf solution with Hf, NH4SCN and HCl concentrations of 65.36 mg/L, 2.0 mol/L and 1.25 mol/L, respectively. The organic phase was repeatedly mixed with fresh aqueous feed solution with A/O of 1:1 until the Hf concentration in the aqueous phase remained unchanged before and after extraction. The amount of Hf loaded in the organic phase was calculated by summing up the amount of Hf that transferred into the organic phase in each extraction (see Eq.7).
2.3.4 Stripping
The Zr and Hf loaded organic phases were prepared using separating funnels. Equal volumes of extractant and aqueous feed solution, which contains 2.18 g/L Zr (or 56.92 mg/L Hf), 2.0 mol/L NH4SCN and 1.25 mol/L HCl, were measured, put into a separating funnel and mixed by hand-shaking the separating funnel. After standing for a period of time, the organic phase was separated for stripping.
The stripping experiments were carried out with centrifugal tubes, of which procedures were similar to the extraction procedure described in Section 2.3.2. The stripping percentage (S, %) was calculated according to Eq. (8).
All the aqueous feed solutions that contained Zr and/or Hf were aged for more than 24 h before extraction. All the extraction and stripping experiments were repeated twice, and all the samples were measured three times. Their average was taken as the final result. All the experiments were performed at room temperature.
3 Results and discussion
3.1 Extraction ability for HSCN
In the Zr/Hf separation industry with MIBK-HSCN system, the organic phase usually pre-extracts HSCN before contacting with aqueous feed solution(Xiong et al., 2002; Lin, 2007). It is reported that the pre-extraction of HSCN with MIBK enlarges the Zr/Hf separation factor (βHf/Zr)(Lv et al., 2008). It seems the HSCN pre-loading is important to Zr/Hf separation with ketone extractants. Hence, we investigated the extraction ability of MIBK and its homologues for HSCN at different initial HSCN concentrations of 0.5 mol/L, 1.0 mol/L, 1.5 mol/L and 2.0 mol/L at A/O of 1:1. The results are shown in Fig. 2. The molar ratio of the extractant to HSCN in the organic phase at equilibrium (RL/HSCN) was calculated according to Eq. (9):
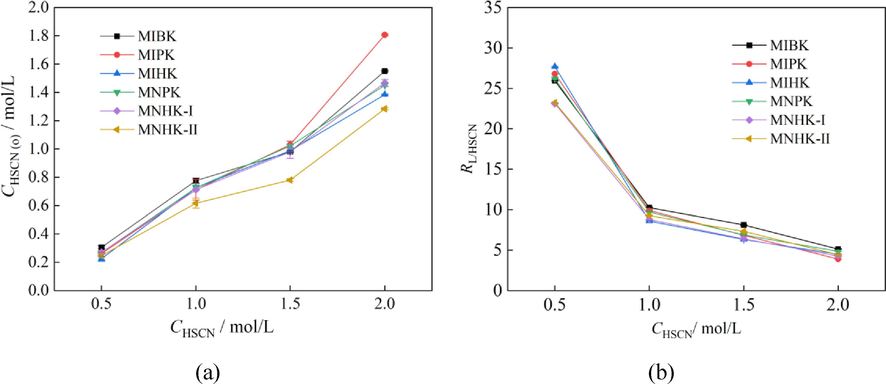
Pre-extraction of HSCN at different initial HSCN concentrations (A/O = 1:1).
where ρ is the extractant density, g/cm3; MW is the extractant molecular weight, g/mol; Coe(HSCN) represents the HSCN concentration in the organic phase at equilibrium, mol/L.
At initial HSCN concentration of 0.5 mol/L in the aqueous feed solution, the HSCN that extracted into the organic phases of MIBK and its homologues are similar (Fig. 2(a)). As the initial HSCN concentration is increased, the amounts of HSCN that entered into the organic phases all increase, and their differences begin to appear and become apparently — especially at initial HSCN concentration of 2.0 mol/L. As the MIBK and its homologues have different molecular weight (MW) and similar density (Fig. 1, Table 1) and volumes of the organic phases are the same, the molar quantities of the extractants that participated in HSCN extraction are different. It is more reasonable to compare the extraction ability of MIBK and its homologues for HSCN according to the differences of RL/HSCN values (Fig. 2(b)). The RL/HSCN values of all the investigated ketone extractants are similar at initial HSCN concentrations of 1.0 ∼ 2.0 mol/L, indicating the studied methyl alkyl ketones have nearly equivalent extraction ability for HSCN.
3.2 HSCN pre-extraction
To investigate the effect of HSCN pre-extraction on Zr, Hf extraction and separation behaviors, extraction experiments were carried out using the HSCN pre-loading organic phases that obtained in Section 3.1. The aqueous feed solution contained 2.02 g/L Zr, 85 mg/L Hf, 2.0 mol/L NH4SCN and 1.0 mol/L HCl. The phase ratio A/O was set at 2:1. The results are shown in Fig. 3.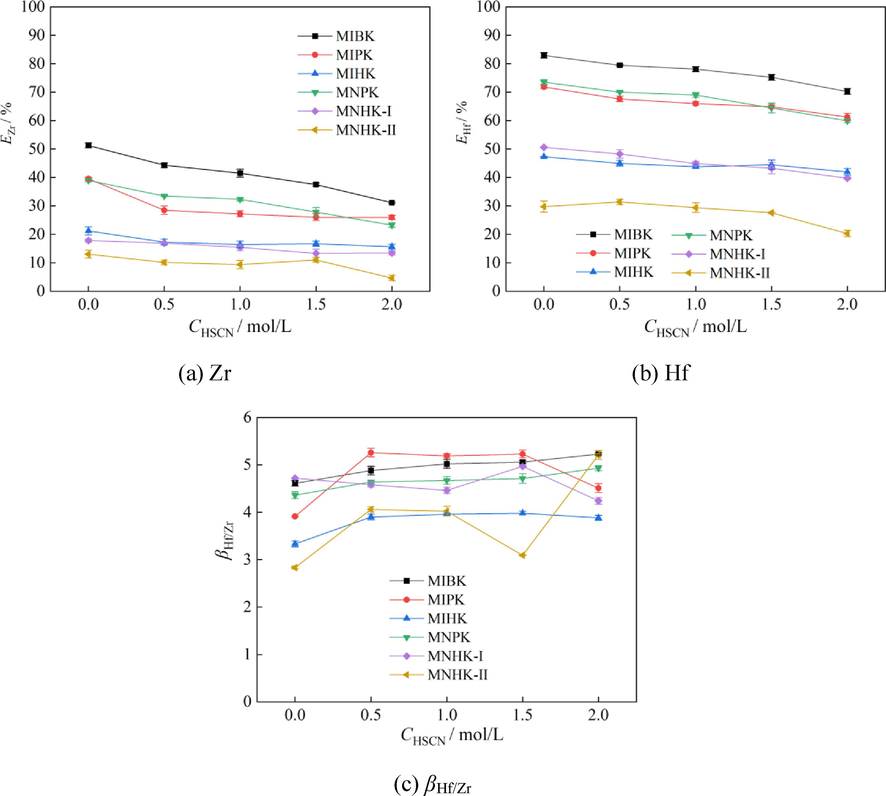
Effect of HSCN pre-extraction on Zr, Hf extraction and separation (aqueous phase: CZr = 2.02 g/L, CHf = 85 mg/L, CNH4SCN = 2.0 mol/L, CHCl = 1.0 mol/L; A/O = 2:1).
From Fig. 2, we know that the larger initial HSCN concentration in the aqueous feed solution the more HSCN are extracted into the organic phases. Fig. 3(a) and Fig. 3(b) indicate that both the extraction percentages of Zr and Hf basically decrease for all the investigated ketone extractants as more and more HSCN are pre-loaded by the extractants. The extraction ability for both Zr and Hf are in the orders: MIBK > MIPK > MIHK; MNPK > MNHK-I > MNHK-II. For both methyl isoalkyl ketones and methyl n-alkyl ketones, their extraction ability for Zr and Hf both reduce as carbon number of the alkyl is increased. MIPK and MNPK have little difference in their extraction ability for Zr and Hf (Fig. 3(a)(b)). The same phenomenon occurs to MIHK and MNHK-I. This indicates the alkyl isomeric difference — a linear chain and a branched chain with a methyl locating at the penultimate carbon at the end — has little effect on the extraction ability of methyl alkyl ketones for Zr and Hf. As it is known, Zr and Hf are extracted by MIBK from HSCN media in the form of M(OH)2(SCN)2·2MIBK (M = Zr or Hf) (Xiong et al., 2002; Lin, 2007). It is most likely that MIBK homologues extract Zr and Hf in the same way. Carbonyl of the ketone extractants is the active group that interacts with Zr(IV) and Hf(IV). Steric hindrance is probably the main factor that influences the extraction ability of ketones for Zr and Hf from HSCN media. Hence, increasing the length of alkyl results in reduction of their extraction ability for Zr and Hf. As the methyl branched chain is far from carbonyl, it has little effect on the extraction ability of ketones for Zr and Hf. The ketone structure also determines its polarizability. The polarizabilities of MIBK, MIPK, MNPK, MIHK, MNHK-I, and MNHK-II are 11.82, 13.66, 13.68, 15.50, 15.51 and 17.35, respectively (https://www.chemsrc.com/). It is notably that the extraction ability order of these ketones for Zr and Hf is MIBK > MIPK ≈ MNPK > MIHK ≈ MNHK-I > MNHK-II, which is also consistent with their extraction ability for Zr and Hf.
As to the Zr/Hf separation behaviors, pre-loading HSCN in the organic phases is really beneficial to Zr/Hf separation (Fig. 3(c)). As βHf/Zr depends on Zr and Hf distribution ratios, which directly relates to their extraction percentages, it is hard to find some definite laws between the ketone structures and their selectivity for Zr/Hf separation. Their Zr/Hf separation performance is basically in the orders of MIPK > MIBK > MIHK and MNPK ≈ MNHK-I > MNHK-II at initial HSCN concentration in the range of 0.5 ∼ 1.5 mol/L.
3.3 HCl concentration
The aqueous acidity is an important factor to determine the Zr, Hf extraction and separation behaviors. Hence extraction experiments were performed by mixing MIBK or its homologues with a series of Zr-Hf mixed solutions with HCl concentration of 0.25 mol/L, 0.5 mol/L, 0.75 mol/L, 1.0 mol/L and 1.5 mol/L. The concentrations of Zr, Hf and NH4SCN in the aqueous feed solutions were 1.98 ± 0.02 g/L, 74 ± 3 mg/L and 2.0 mol/L, respectively. The phase ratio A/O was still fixed at 2:1. The results are shown in Fig. 4, and the phenomena after centrifugation are shown in Fig. 5.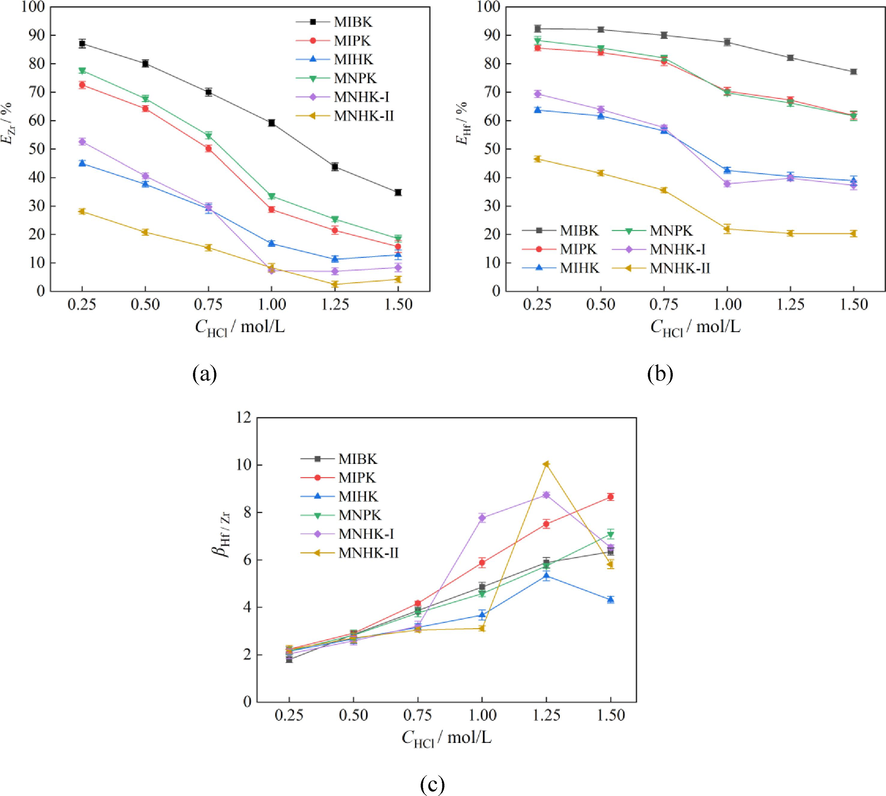
Effect of HCl concentration on Zr, Hf extraction and separation (aqueous phase: CZr = 1.98 ± 0.02 g/L, CHf = 74 ± 3 mg/L, CNH4SCN = 2.0 mol/L; A/O = 2:1).
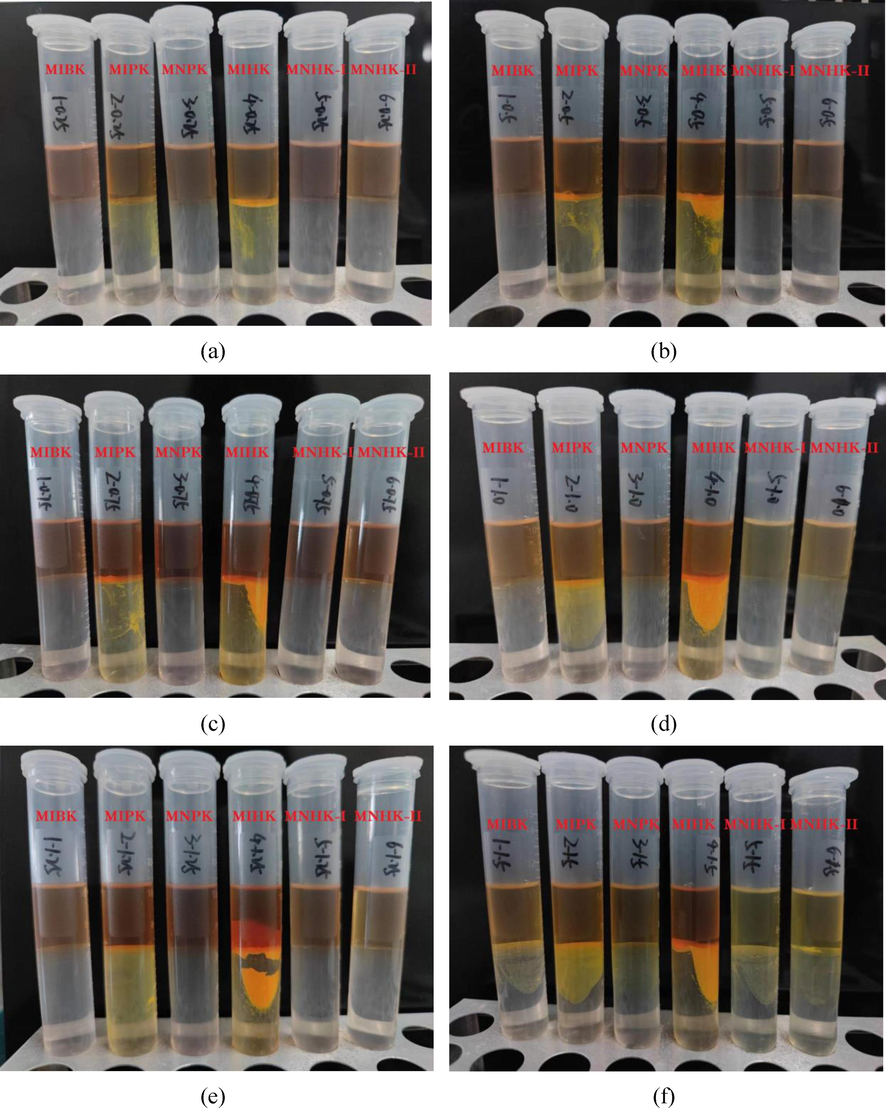
Extraction phenomena at different HCl concentrations (a) 0.25 mol/L (b) 0.5 mol/L (c) 0.75 mol/L (d) 1.0 mol/L (e) 1.25 mol/L (f) 1.5 mol/L.
In the range of investigated HCl concentrations, Hf are still always selectively extracted over Zr. Both extraction efficiencies of Zr and Hf monotonically decrease as the HCl concentration is increased (Fig. 4(a)(b)). The standard dissociation constant ( ) of HSCN at 298.15 K is 0.141. As the NH4SCN concentration was set at 2.0 mol/L, the amount of free SCN- decreases as the HCl concentration is increased. According to equations (1) ∼ (2), the SCN- ions participate in the formation of extraction complexes, which means lower SCN- concentration goes against Zr and Hf extraction. Therefore, it is easy to understand the Zr and Hf extraction behaviors when fixing the NH4SCN concentration at 2.0 mol/L while increasing the HCl concentration from 0.25 to 1.50 mol/L.
As SCN- has a stronger affinity for Hf than Zr(Xiong et al., 2002), the extraction efficiency of Zr is more influenced than Hf as the amount of SCN- decreases in the aqueous feed solutions. Hence, EZr goes down more quickly than EHf with increasing the HCl concentration, namely higher acidity is beneficial to Zr/Hf separation (as shown in Fig. 4(c)). The highest Zr/Hf separation factors of MIHK, MNHK-I and MNHK-II occur at 1.25 mol/L HCl, while those of MIBK, MIPK and MNPK occur at 1.50 mol/L HCl. Considering low EHf means low Hf separation efficiency from Zr-Hf mixed solutions, 1.25 mol/L HCl is chosen for Zr/Hf separation in the following experiments.
The effects of alkyl carbon number and the methyl branched chain locating at end of the alkyl on Zr and Hf extraction efficiencies are once again proved (Fig. 4(a)(b)). Nearly at any HCl concentrations the Zr and Hf extraction percentages are in the orders: MIBK > MIPK > MIHK; MNPK > MNHK-I > MNHK-II; MIPK ≈ MNPK; MIHK ≈ MNHK-1 (similar to the results in section 3.2).
The methyl n-alkyl ketone extractants MNPK, MNHK-I and MNHK-II all show good anti-emulsification performance at any HCl concentrations in the range of 0.25 ∼ 1.25 mol/L (Fig. 5). Only at 1.5 mol/L HCl, slight emulsification phenomena were observed. MIBK also did not show any emulsification at HCl concentrations of 0.25 ∼ 1.25 mol/L. As to the methyl iso-alkyl ketones MIPK and MIHK, emulsification phenomena occurred at any of the investigated HCl concentrations, and the higher HCl concentration the more serious emulsification. MIHK has poorer anti-emulsification performance than MIPK. All these indicate that (i) methyl n-alkyl ketones have good anti-emulsification performance, (ii) methyl iso-alkyl ketones have poorer anti-emulsification performance than methyl n-alkyl ketones, and (iii) for methyl iso-alkyl ketones, the more carbon number of alkyl the poorer anti-emulsification performance.
3.4 NH4SCN concentration
The effect of NH4SCN concentration on Zr, Hf extraction and separation behaviors were studied. All the aqueous feed solutions had been aged for > 20 h before extraction, and there were orange crystals when the NH4SCN concentration exceeded 2.5 mol/L. The more aging time the more crystals generated. Hence the NH4SCN concentration in the range of 0.5 ∼ 2.5 mol/L were investigated. The Zr, Hf and HCl concentrations in the aqueous feed solutions were 2.10 ± 0.05 g/L, 98 ± 2 mg/L and 1.25 mol/L, respectively. The phase ratio A/O was also 2:1. The results are shown in Fig. 6, and the corresponding Zr/Hf separation factors (βHf/Zr) are listed in Table 2.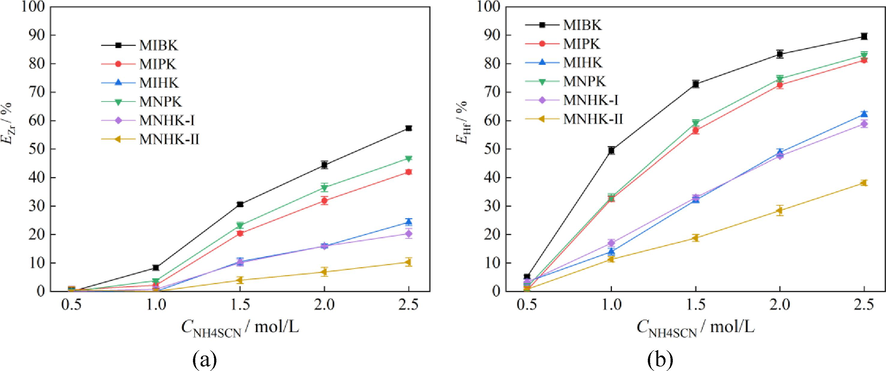
Effect of NH4SCN concentration on Zr, Hf extraction and separation (aqueous phase: CZr = 2.10 ± 0.05 g/L, CHf = 98 ± 2 mg/L, CHCl = 1.25 mol/L; A/O = 2:1).
CNH4SCN / mol/L
MIBK
MIPK
MIHK
MNPK
MNHK-I
MNHK-II
1.0
10.83
21.36
–
12.61
–
–
1.5
6.07
5.07
4.01
4.80
4.39
5.61
2.0
6.27
5.63
5.03
5.14
4.80
5.41
2.5
6.37
5.98
5.12
5.54
5.61
5.36
At the NH4SCN concentration as low as 0.5 mol/L, Zr are hardly extracted, and EHf are all less than 5.13 %. With increasing NH4SCN concentration, both EZr and EHf increase steadily. These results are consistent with equations (1) ∼ (2), which means higher SCN- concentration is in favor of Zr and Hf extraction. At NH4SCN concentration of 1.0 mol/L, the βHf/Zr of MIBK, MIPK and MNPK are the highest (see Table 2), but the EHf are all less than 50 %. As to MIHK, MNHK-I and MNHK-II, they hardly extract Zr, and the EHf are as small as less than 20 %. These indicate poor Hf separation efficiency from Zr-Hf mixed solutions at NH4SCN concentration of 1.0 mol/L. With increasing NH4SCN concentration in the range of 1.5 ∼ 2.5 mol/L, most of the extractants (MIBK, MIPK, MIHK, MNPK, MNHK-I) have increasing βHf/Zr (Table 2). In light of the risk of crystal generation during aging of aqueous feed solution in industrial production, 2.0 mol/L NH4SCN is optimal.
3.5 Equilibrium time comparison of MIBK and MNPK
Based on the above results, MIHK, MNHK-I and MNHK-II have much weaker extraction abilities than MIBK, which will result in poor Zr/Hf separation efficiency. MIPK and MNPK have a little bit weaker extraction ability than MIBK. Their boiling points and flashing points are all higher than MIBK, which means safer and more friendly operation environments. Besides, their solubilities in water are much lower than MIBK, which means less extractant loss during extraction. MIPK is much more expensive than MNPK, and its anti-emulsification performance is poor. MIBK is produced via a three-step process using acetone as the starting material (condensation, dehydration, and hydrogenation), while MNPK is produced also via a three-step process using ethyl acetoacetate as the starting material (condensation, hydrolyzation, and decarboxylation). Besides, MNPK has a pleasant banana aroma with a slight medicinal note (Chen et al., 2003). Therefore, it seems MNPK is the most possible candidate to replace MIBK in Zr/Hf separation industry.
To evaluate and compare the extraction speeds of MIBK and MNPK for Zr and Hf, extraction experiments were performed on a KB-5010 Test Tube Shaker at a very slow oscillating speed of 50 rpm, at which a clear two-phase interface was seen shaking. The concentrations of Zr, Hf, HCl and NH4SCN in the aqueous feed solution were 2.16 g/L, 106.16 mg/L, 1.25 mol/L and 2.0 mol/L, respectively. The phase ratio was also fixed at 2:1. The results are shown in Fig. 7. The EZr-t curve of MNPK is nearly parallel with that of MIBK (Fig. 7(a)), while the EHf-t of MNPK only has a slightly bigger gradient than that of MIBK (Fig. 7(b)). These indicate MNPK also has fast extraction kinetics for Zr and Hf.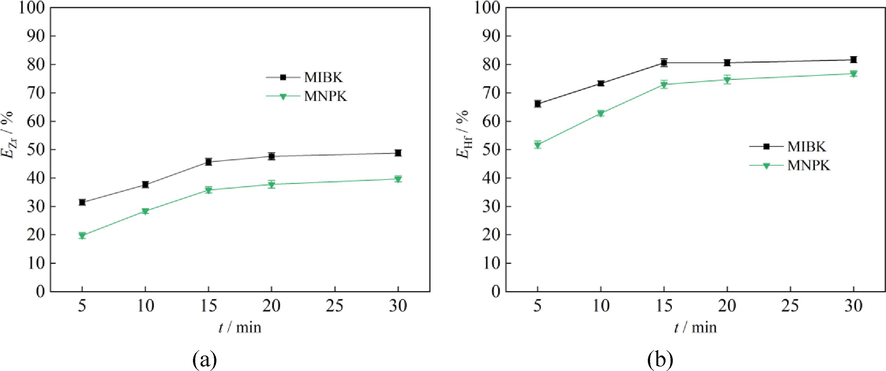
Effect of oscillating time on Zr and Hf extraction (aqueous phase: CZr = 2.16 g/L, CHf = 106.16 mg/L, CHCl = 1.25 mol/L, CNH4SCN = 2.0 mol/L; A/O = 2:1).
3.6 Saturated loading capacity comparison of MIBK and MNPK
As Hf is preferentially extracted over Zr, the key of Zr/Hf separation with ketone extractants is to remove Hf from Zr-Hf mixed solutions. Hence, the maximum loading capacity of ketone extractants for Hf is important. The results are shown in Fig. 8. After the 4th extraction stage, the amount of Hf loaded in MNPK basically levelled off. Its maximum loading capacity for Hf is 131.97 mg/L at the given conditions. As to MIBK, its maximum loading capacity for Hf is 189.07 mg/L. MIBK has higher loading capacity than MNPK. This result is consistent with their extraction ability for Zr and Hf.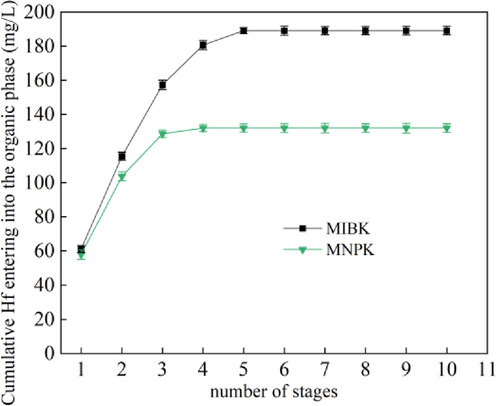
Saturated loading capacity of MIBK and MNPK for Hf (aqueous phase: CHf = 65.36 mg/L, CHCl = 1.25 mol/L, CNH4SCN = 2.0 mol/L; A/O = 2:1).
3.7 Stripping
In Zr/Hf separation industry by solvent extraction of MIBK-HSCN system, the co-extracted Zr are usually scrubbed by HCl solutions, and after that the loaded Hf are stripped by H2SO4 solutions. Therefore, stripping behaviors of Zr and Hf by HCl and H2SO4 solutions respectively were studied. The amounts of Zr loaded in MIBK and MNPK were 1.57 g/L and 1.43 g/L, respectively, while those of Hf loaded in MIBK and MNPK were 53.75 mg/L and 51.80 mg/L, respectively. The phase ratios A/O were all 1:1. The results are shown in Fig. 9.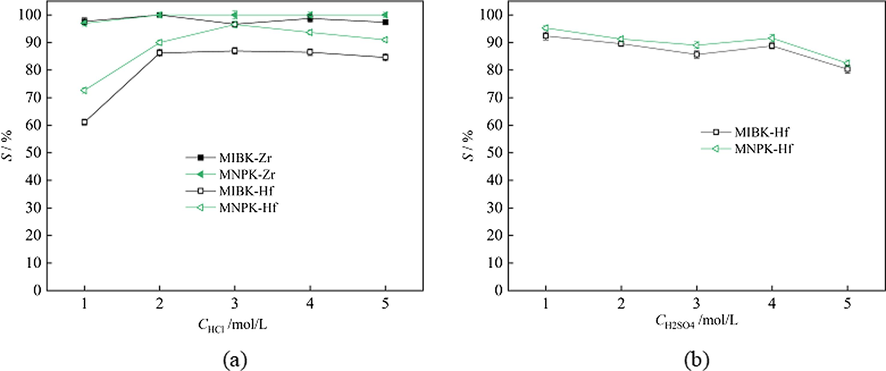
Stripping behaviors of Zr and Hf by HCl and H2SO4 solutions respectively (a) Zr (b) Hf.
The Zr and Hf loaded in MNPK are easier to be stripped by HCl solutions than corresponding those loaded in MIBK (Fig. 9(a)). The difference of Zr and Hf stripping percentages by 1 mol/L HCl is the highest. Hence 1 mol/L HCl is the best for Zr scrubbing. After scrubbing of Zr, only Hf is left in the organic phase. Fig. 9(b) verifies that the stripping behaviors of Hf loaded in MNPK are very similar to those of Hf loaded in MIBK by H2SO4 solutions. The stripping percentages of Hf loaded in MNPK are a little bit higher than corresponding those of Hf loaded in MIBK (Fig. 9(b)), indicating the Hf in MNPK are a little bit easier to be stripped than those in MIBK by H2SO4 solutions. The optimal H2SO4 concentration for Hf stripping is 1 mol/L.
4 Conclusion
The Zr, Hf extraction and separation performance of MIBK and its five homologues (MIPK, MIHK, MNPK, MNHK-I, MNHK-II) from HSCN media were studied and compared. (1) Their extraction ability for Zr and Hf are both in the orders of MIBK > MIPK > MIHK (for methyl isoalkyl ketones) and MNPK > MNHK-I > MNHK-II (for methyl n-alkyl ketones). The increase of carbon number reduces extraction ability of methyl alkyl ketones for Zr and Hf. Methyl isoalkyl ketone and its isomeric methyl n-alkyl ketone have nearly equivalent extraction ability for Zr and Hf. (2) Methyl n-alkyl ketones MNPK, MNHK-I and MNHK-II all have good anti-emulsification performance, while methyl iso-alkyl ketones MIPK and MIHK will emulsify during Zr and Hf extraction. (3) MNPK is the most possible candidate to replace MIBK in Zr/Hf separation industry. Its boiling point, flashing point and solubility in water at 20 °C are 151 °C, 47 °C and 0.43 %, respectively. Compared to MIBK, it is safer, more friendly operation environments and less extractant loss during extraction. It has as fast extraction kinetics as MIBK. It has good anti-emulsification performance. Its maximum βHf/Zr can reach to 12.61. The loaded Zr and Hf have very similar stripping behaviors with those loaded in MIBK by HCl and H2SO4 solutions, respectively.
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (51974026). Special thanks to Peilong Wang and Chengyan Xu for metal ion determination with ICP-OES.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Equilibrium of zirconium and hafnium in the process of extraction with TBP in nitric medium - Influence in the Zr/Hf separation. Miner. Eng.. 2020;146
- [CrossRef] [Google Scholar]
- Liquid-liquid separation of zirconium and hafnium from nitric liquor in order to obtain nuclear zirconium oxide using TBP as extractant. Chem. Eng. Commun.. 2020;207:73-83.
- [CrossRef] [Google Scholar]
- Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants. J. Radioanal. Nucl. Ch.. 2013;298:259-264.
- [CrossRef] [Google Scholar]
- Selective extraction of Hf(IV) over Zr(IV) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorous based extractants. J. Chem. Technol. Biot.. 2014;89:1712-1719.
- [CrossRef] [Google Scholar]
- The synthesis and application of 2-heptanone. J. Jiangxi Normal Univ. (Nat. Sci. Edit.). 2003;27:298-300.
- [CrossRef] [Google Scholar]
- Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP. Hydrometall.. 2017;169:607-611.
- [CrossRef] [Google Scholar]
- The separation of zirconium and hafnium from (NH4)3Zr(Hf)F7 using amine-based extractants. J. S. Afr. i. Min. Metall.. 2016;116:915-920.
- [CrossRef] [Google Scholar]
- Dean’s Handbook of Organic Chemistry (2nd Edition). McGraw-Hill Companies Inc.; 2004.
- He, H. Y., Xu, F., Li, Q. L., Dong, P. F., Zheng, j., Wu, C. J., He, Z. Y., Qu, J., Xu, Z. G., Chi, R., 2021. Separation of hafnium from zirconium in HNO3 solution by solvent extraction with Cyanex572. Hydrometallurgy. 202. Doi: 10.1016/j.hydromet.2021.105600 105600.
- A thermodynamic model for reactive extraction of macro amounts of zirconium and hafnium with TBP. Sep. Purif. Technol.. 2020;240
- [CrossRef] [Google Scholar]
- Separation of Hf(IV)-Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy. 2015;152:84-90.
- [CrossRef] [Google Scholar]
- Progress in extraction and separation of zirconium and hafnium with organic phosphorus and nitrogen extractors. Chin. J. Rare Met.. 2014;45:749-757.
- [CrossRef] [Google Scholar]
- Extraction separation of zirconium and hafnium with methyl isobutyl ketone (MIBK) Chin. J. Rare Met. Letters.. 2007;26:93-96.
- [Google Scholar]
- Infuence parameters of separation of zirconium and hafnium in MIBK-HSCN systems. Chin. J. Rare Met.. 2008;32:754-757.
- [CrossRef] [Google Scholar]
- Highly efficient and selective solvent extraction of zirconium and hafnium from chloride acid solution including amic acid extractant. Sep. Purif. Technol.. 2021;279
- [CrossRef] [Google Scholar]
- Selective separation of hafnium from sulfuric acid solution containing Zr by solvent extraction with PC88A. Korean J. Met. Maters.. 2015;53:51-58.
- [CrossRef] [Google Scholar]
- Selective extraction of Zr(IV) over Hf(IV) from aqueous hydrochloric acid with p-tert-butylcalix[4]arenediphosphonic acid. B. Chem. Soc. Jap.. 2019;92:967-972.
- [CrossRef] [Google Scholar]
- Au(III) extraction using ketone compounds with physical properties superior to current commercial extractants. AIChE J.. 2021;67:e17214.
- [Google Scholar]
- Application of task specific ionic liquid for the extraction of Zirconium and Hafnium. J. Mol. Liq.. 2021;338:116616
- [CrossRef] [Google Scholar]
- Zirconium and hafnium separation, Part 1. Liquid/liquid extraction in hydrochloric acid aqueous solution with Aliquat 336. Sep. Sci. Technol.. 2006;41:1927-1940.
- [CrossRef] [Google Scholar]
- Solvent extraction of Zr(IV) and Hf(IV) with N, N, N ', N '-tetraoctyldiglycolamide. J. Radioanal. Nucl. Ch.. 2012;292:1109-1114.
- [CrossRef] [Google Scholar]
- Pyrochemical separation of zirconium and hafnium tetrachlorides using fused salt extractive distillation process. High Temp. Mater. Process.. 1999;18:213-226.
- [CrossRef] [Google Scholar]
- Mechanistic study of hafnium and zirconium extraction with organophosphorus extractants. Solvent Extr. Ion Exc.. 2018;36:150-161.
- [CrossRef] [Google Scholar]
- Wang, J. L., Liu, H., Zhao, H. R., Wang, H. J., 2022. Selective extraction of Hf over Zr by a novel extractant (n-octyl)(2,4,4 '-trimethylpentyl)phosphinic acid (INET-1) from sulfuric acid media. J. Radioanal. Nucl. Ch. 332, 843-843. Doi: 10.1007/s10967-022-08463-2.
- Performance of various extractants for zirconium and hafnium separation by solvent extraction. Chin. J. Rare Met.. 2020;44:658-667.
- [CrossRef] [Google Scholar]
- Separation of zirconium and hafnium from nitric acid solutions with LIX 63, PC 88A and their mixture by solvent extraction. Hydrometall.. 2014;150:153-160.
- [CrossRef] [Google Scholar]
- Separation of Zr and Hf from sulfuric acid solutions with amine-based extractants by solvent extraction. Sep. Purif. Technol.. 2015;142:83-89.
- [CrossRef] [Google Scholar]
- A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem.. 2016;39:1-9.
- [CrossRef] [Google Scholar]
- Selective extraction of hafnium over zirconium with dialkylphosphinic acids from H2SO4 media. J. Brazil. Chem. Soc.. 2023;34:1003-1012.
- [CrossRef] [Google Scholar]
- High-efficient and selective extraction of Hf over Zr with DIBK-P350 synergistic extraction system. Sep. Purif. Technol.. 2019;212:255-261.
- [CrossRef] [Google Scholar]
- Process for synergistic extraction of Hf(IV) over Zr(IV) from thiocyanic acid solution with TOPO and N1923. Chem. Eng. Process.. 2022;170
- [CrossRef] [Google Scholar]
- Xiong, B. K., Wen, W. G., Yang, X. M., Li, H. Y., Luo, F. C., Zhang, W., Guo, J. M., 2002. Zirconium and hafnium metallurgy, first ed. Metallurgical Industry Press, Beijing.
- Xu, Z. G., Wang, L. J., Chi, R. A., Zhang, L., 2012. Solvent extraction and separation of hafnium from zirconium with DIBK. Nonferr. Metal. (Extr. M.). 3, 35-38. Doi: 10.3969/j.issn.1007-7545.2012.03.010.
- Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system. T. Nonferr. Metal. Soc.. 2012;22:1760-1765.
- [CrossRef] [Google Scholar]
- Separation of zirconium and hafnium by solvent extraction using mixture of DIBK and P204. Hydrometall.. 2016;165:275-281.
- [CrossRef] [Google Scholar]
- Separation of zirconium and hafnium: A review. Energy Mater.. 2014;2014:451-457.
- [CrossRef] [Google Scholar]
- Production of nuclear grade zirconium: A review. J. Nucl. Mater.. 2015;466:21-28.
- [CrossRef] [Google Scholar]
- Separation of hafnium from zirconium in hydrochloric acid solution with di(2-ethylhexyl)phosphoric acid by solvent extraction. Nucl. Sci. Tech.. 2019;30:22.
- [CrossRef] [Google Scholar]
- Preferential extraction of hafnium over zirconium with D2EHPA through selective complexation of organic acids. J. Radioanal. Nucl. Ch.. 2019;321:333-339.
- [CrossRef] [Google Scholar]
- A new diphosphonic acid extractant N, N-n-octylamine di(methylene phenylphosphinic acid) for extraction and separation of zirconium and hafnium in hydrochloric acid. J. Radioanal. Nucl. Ch.. 2020;324:339-348.
- [CrossRef] [Google Scholar]







