Translate this page into:
Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain
⁎Corresponding author. bbabalol@purdue.edu (Benjamin Ayodipupo Babalola)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carica papaya, commonly referred to as papaya, is a member of the Caricaceae family, boasting a diverse array of compounds and biomolecules, notably papain, with significant industrial and medicinal value. This comprehensive review meticulously examines the properties, extraction techniques, and pharmacological importance of papain, coupled with an exploration of the pharmacological activities inherent in C. papaya. Extensive research data from reputable sources such as Google Scholar, PubMed, Scopus, and Web of Science were scrutinized to collate pertinent information on the plant and its enzyme. Within C. papaya, a spectrum of bioactive compounds were identified, encompassing alkaloids, tannins, phenolics, flavonoids, saponins, terpenoids, sugars, glycosides, amino acids, steroids, and the pivotal enzyme papain. Papain extraction methods and their activity modulation in response to pH, temperature, and other physicochemical parameters were examined in detail. This review provides a nuanced analysis of papain, addressing both its pharmacological implications and potential side effects. Its far-reaching applications in the medical, industrial, and pharmaceutical sectors were meticulously discussed, unveiling its market potential. Furthermore, C. papaya emerged as a reservoir of pharmacological activities, exhibiting antioxidant properties, anti-cancer potential, wound healing capabilities, digestive disorder management, and anxiolytic effects. The research underscores the promising potential of C. papaya and papain for diverse biological applications, prompting a call for further studies to unlock their therapeutic benefits, particularly in the realm of public health. Looking ahead, continued advancements in drug development hold the key to unraveling the intricate bioactive components of papaya and its papain cysteine proteases. These insights are poised to catalyze their development and application, promising substantial contributions to the betterment of humanity.
Keywords
Carica papaya
Papain
Extraction methods
Characterization
Drug development
Therapeutic benefits
1 Introduction
The Carica papaya plant belongs to the Caricaceae family and is commonly called papaya. It is also known as papaw, paw-paw, kapaya, lapaya, tapaya, papayao, papaya, papaia, papita, lechosa, fruta bomba, mamon, mamona, mamao and tree melon in different parts of the world. Interestingly, a separate plant Asimina triloba of the Annonaceae family is also known as pawpaw. Papaya is native to the Caribbean Coast of Central America and is grown in tropical and subtropical parts of the world (Babalola, 2019). Among the 31 species that make up the botanical family Caricaceae and the genus Carica, the papaya species is the most economically significant and frequently grown species (Biswal et al., 2022).
During the early years of growth, papaya develops a single stem, which can develop into heavy lateral branches in highly fertile soil, promoting favorable growth conditions (Sagonoy, 2022). The leaves of a mature papaya plant are palmate with deep lobes and are supported by smooth, hollow petioles (Babalola, 2019). The stems, fruits, and leaves of the papaya plant contain a copious amount of latex, although the source is unclear (Babalola et al., 2023b).
Ripe papaya fruits have a slight resemblance to melons, and they are rich in retinol and ascorbic acid. Every part of the plant has been found to be important (Fig. 1). They have been reported to be used in the treatment of diseases just like every other plant (Adetobi et al., 2022; Otunba et al., 2022; Otunba, 2021). Every part of the papaya plant, including its leaves, fruits, seeds, bark, stem, and root, can be processed for nutritional and pharmaceutical purposes (Fig. 1). They are rich sources of antioxidants and mineral ions, and they also contain phytochemicals and B vitamins (Santana et al., 2019). Papaya enzymes have been reported to treat arthritis and colon cancer (Babalola, 2019). C. papaya contains biologically active compounds and enzymes (Babalola, 2019).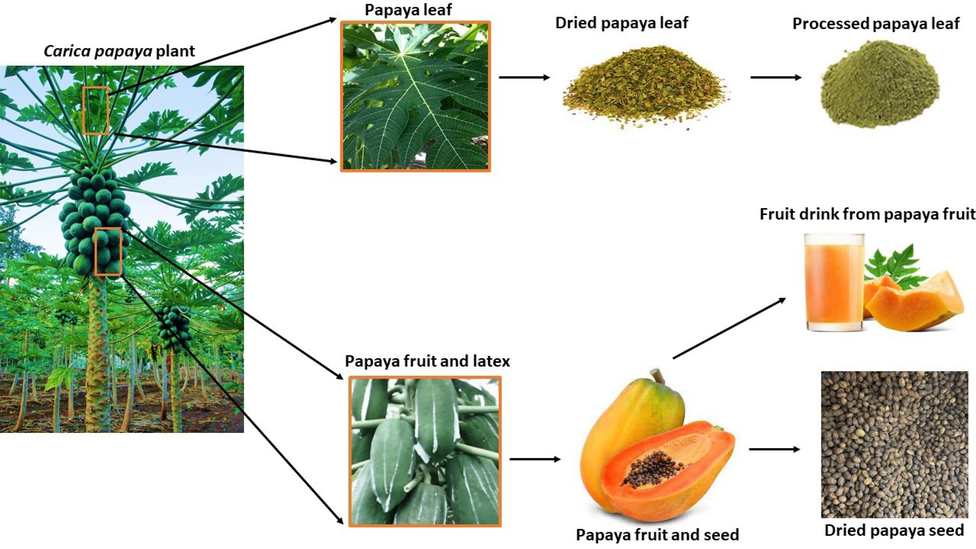
Papaya plant and its processing. Different parts of the plant including the leaves, fruits, latex, and seed can be processed for different use. This may be for medicinal, nutraceutical or industrial purposes.
Papaya latex is rich in hydrolases, including tributyroylglycerol hydrolase, GDSL-motif carboxylester hydrolase, Carica papaya lipase, and glycosyl hydrolase, as documented in studies by Giordani et al. (1991), Abdelkafi et al. (2009), de Maria et al. (2006), and Huet et al. (2008). Additionally, papaya latex contains the cysteine protease enzyme papain, as identified in research by Haber et al. (2022). The latex of the papaya contains a sulfhydryl protease, papain, and chymopapain. Papain is of vital importance because of its inherent proteolytic properties (Thangavel and Albratty, 2023). It is primarily produced by creating cuts in the unripe papaya fruit's epicarp, gathering, and drying the latex that leaks out. Greener fruit produces more active papain (Amri and Mmaboya, 2012). The functionality of papain is due to its unique structure, which helps in the comprehension of the mechanism of the proteolytic enzyme and its usefulness for a variety of purposes (Zlobin et al., 2022). According to Chandan et al. (2022), papain’s catalysis involves arginine, lysine, and cysteine primarily. Papain is soluble in water and has optimum temperature stability, with high dependence on pH. They are also stable between pH 4.5 – 6.7 (Babalola, 2019).
In our previous study, crude extracts containing enzymes underwent a three-step purification process involving 70 % NH4(SO4)2, DEAE-Cellulose column chromatography, and Sephadex G-25 column chromatography, resulting in a purification fold of 1.6424, signifying significantly enhanced enzyme purity (Babalola et al., 2023b). Furthermore, our research revealed the presence of five distinct cysteine-proteases in papaya leaves, each exhibiting varying binding affinities and catalytic efficiencies towards casein-substrate. These proteases showcased optimal activity within the temperature range of 50 °C − 59 °C and a pH range of 4.5 – 6.7, with augmented activity observed in the presence of Mg2 +. The enzyme's kinetics, represented by Km and Vmax values, spanned from 1.47 mg/ml − 8.70 mg/ml and 0.42 µmol/ml/min − 0.4167 µmol/ml/min, demonstrating their diverse characteristics. Notably, our findings highlighted papain E's superior performance, with optimal activity at pH 5.5 and 50 °C in the presence of Mg2+, boasting the best catalytic efficiency (Kcat/Km 59.776 - pmL/mg.min) and binding affinity (Km − 0.83 mg/mL) compared to isolated enzymes.
The protease is utilized as a commercial meat tenderizer because it can disintegrate dead tissue without harming living cells. We reported its usage for wound care (Babalola et al., 2023b). Papain is used in the management and treatment of allergies and injuries (Babalola, 2019). According to Babalola (2019), some studies indicate that the enzyme helps in the prevention of diabetic-dependent-heart-disease. Papain is known for its efficiency and less destructive nature compared to other proteases, making them commonly used in cell isolation procedures on certain tissues (Aminlari, 2022). It is a constituent of contact lens solution and is used in clarifying beer, and in the pharmaceutical industry. In a study by Soleimani et al. (2022), traditional medicine through the administration of papain extract from papaya leaves is shown to be effective against the nematodes as papain causes damage to the cuticles of the nematodes.
Papain is generally a sulfhydryl protease obtained from C. papaya. Several studies have focused on the extraction and application of papaya plant enzymes (Babalola et al., 2023b; Jain et al., 2022; Oliveira et al., 2022). The enzyme is known for its ability to tenderize flesh and enhance wound healing. This is particularly useful for people who experience poor wound healing, as they can avoid expensive and complex materials that may not be readily available.
This report presents an in-depth analysis of papain, focusing on its properties, extraction methods, and pharmacological significance. Additionally, it explores the pharmacological activities associated with Carica papaya. In this review, the pharmacological side-effect of papain, as well as its medical, industrial, and pharmaceutical importance while exploring its market potential. Hence, knowledge from this review offers intelligence into the pharmacological potential of C. papaya and may find its application in alternative therapetics towards the treatment of infectious as well as terminal diseases (Babalola et al., 2023a). More industrial and research effort and investment on the plant subject is able to contribute immensely towards areas of agri-business, health, and science. In addition, the relevance of this study gives a perspective toward the achievement of Sustainable Development Goal 3. The knowledge and application of the papain enzyme and other bioactive components of papaya in this study will guide the pharmaceutical and research industries to ensure healthy lives and promote the well-being of all at all ages. Hence, this present study will also be important to pharmaceutical industries, medical industries, researchers, policymakers, governments of nations, and students.
2 Constituents of papaya
Papaya leaves have been found to contain significant amounts of protein, alkaloids, amino acids, carbohydrates, steroids, and glycosides, which can be easily detected (Table 1). Additionally, significant amounts of alkaloids, tannins, phenolics, and flavonoids have been found in papaya leaves. The presence of sugars, glycosides, saponins, proteins, amino acids, steroids, and terpenoids in measurable amounts has also been reported (Babalola, 2019). Computational studies aimed at understanding the interaction and binding affinity of specific chemical compounds towards drug targets of pharmacological importance can be conducted using papaya leaves (Babalola et al., 2021b; Adetobi et al., 2022).
NO.
Categories
Phytoconstituents
Plant Part(s)
1
Enzymes
Papain, chymopapain A and B, endopeptidase papain III and IV glutamine cyclotransferase, peptidase A and B, and lysozymes.
Unripe fruit (Latex)
2
Carotenoids
β carotene, crytoxanthin, violaxanthin, zeaxanthin.
Fruits
3
Alkaloid & Enzyme
Carposide, and an enzyme myrosin.
Roots
4
Glucosinolates
Benzyl isothiocyanate, benzylthiourea, βsitosterol, papaya oil, caricin, and myrosin.
Seeds
5
Minerals
Calcium, potassium, magnesium, iron, copper, zinc.
Shoots and Leaves
6
Monoterpenoids
4-terpineol, linalool, linalool oxide.
Fruits
7
Flavoniods
Quercetin, myricetin, kaempferol.
Shoots
8
Alkaloids
Carpinine, carpaine, pseudocarpine, vitamin C and E, choline, carposide.
Leaves and Heartwood
9
Vitamins
Thiamine, riboflavin, niacin, ascorbic acid, αtocopherol.
Shoots and Leaves
10
Carbohydrates
Glucose, sucrose, and fructose.
Fruits
3 Papain overview
Papain, a pivotal cysteine-protease, has a complex biochemical profile encompassing various forms and functionalities. Here, we dissect its structure, properties, and mechanisms, shedding light on its versatile applications.
3.1 Papain precursor
From cells infected with recombinant baculovirus, propapain, which is the precursor of papain, has been expressed in insects. Propapain is a glycosylated protein with a molecular weight of 39 kDa, which is processed into the active enzyme papain with a molecular weight of 24.5 kDa (Thangaraju et al., 2023). Propapain can be stabilized by Hg2+. A synthetic propapain gene has been reported to be expressed in the yeast- Saccharomyces cerevisiae- under the control of the α-factor promoter (Babalola, 2019). In vitro, the intracellular zymogen propapain can be fully transformed into active mature papain. Propapain is purified using affinity, size-exclusion, and ion-exchange chromatography methods. The papain precursor is capable of undergoing autoproteolytic cleavage (Zhao et al., 2022).
3.2 Papain: Structure and features
Papain is a globular cysteine-protease family consisting of a single polypeptide chain with three disulfide bridges and a sulfhydryl group necessary for proteolytic activity. Its application is extensive to the fields of medicine and food. Papain has high proteolytic activity and in the 1980 s, its structure was solved to a 1.65 Å resolution. The functionality of papain is due to its unique structure which helps in the comprehension of the mechanism of the proteolytic enzyme and its usefulness for a variety of purposes (Zlobin and Golovin, 2022).
3.2.1 Properties, structure and features of papain
The papain enzyme is a protein with a molecular weight of 24,500 Da and a polypeptide containing three disulfide bridges and a sulfuhydyl group that are required for the enzyme's activity (Fig. 2) (Thangaraju et al., 2023; Amri and Mamboya, 2012). The papain enzyme has two clearly defined domains that makes it a superb system for comprehending the folding-unfolding behavior of proteins (Yadav and Venkatesu, 2022).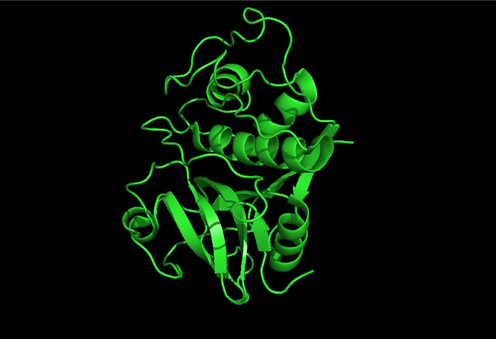
Pymol quaternary protein structure of the papain (PDB ID: 9PAP). Source: Protein, Data Bank, https://www.rcsb.org/structure/9PAP.
Under a variety of circumstances, papain is stable and functional. Papain is said to be stable at room temperatures, according to Wongphan et al. (2022). Babalola (2019) reported that a pH of 4.5 to 6.7 is ideal for the papain enzyme to function at its highest level. Papain, unlike most enzymes, is refractory to high concentrations of denaturing chemicals.
The papain enzyme is folded along three disulfide bridges, and this results in strong interactions between the side chains, which also helps to maintain the stability of the enzyme (Danait-Nabar and Singhal, 2023; Edwin and Jagannadham, 2000). The active site of the enzyme is located in a cleft between two unique structural domains in the three-dimensional structure. The papain molecule has an anti-parallel-sheet domain (Samad et al., 2023). Papain's conformational behavior in aqueous solution was observed in the presence of SDS, and it was shown to have a large concentration of helices and an unfolded structure because of intense electrostatic attraction (Guo et al., 1996). Further studies towards understanding the properties have been conducted- studies on selectivity, the effect of adding inhibitors, pH, temperature, metal ions, and fluorinated alcohols (Babalola et al., 2023b; Valipour, 2022; Zhao et al., 2022).
3.2.2 Hydrophobicity of papain
The hydrophobic interactions have an influence on the conformation of the protein, hence its functionality. The hydrophobic amino acids alanine, leucine, methionine, valine, and Isoleucine are responsible for the hydrophobicity of papain enzyme. The relative hydrophobicity of the amino acids in a protein's secondary sequence may be a good indicator of the protein's overall folding pattern (Kim et al., 2022). The major thermodynamic force that drives protein folding is the interaction between the hydrophobic and hydrophilic amino acids of the papain molecule. According to Dragomirescu (2022), papain and chymopapain are more stable in hydrophobic solvents and can catalyze a variety of reactions with their substrate in organic solvents. While the outer polar surfaces interact with the surrounding aqueous media, the hydrophobic core of the enzyme improves the stability of the tertiary structure through hydrophobic contact (Kikani et al., 2023).
3.3 Mechanism of papain functions
The Cys-25 region of the active site, which attacks the carbonyl carbon in the peptide chain's backbone to release the amino terminal region and cause the protein to disassemble, is what enables papain's method of action. Asparagine-175 aids in the reorientation of His-159′s imidazole ring, which enables Cys-25 to be deprotonated by His-159, causing the peptide bonds to be broken (Amri and Mambayo, 2012). These three amino acids are near one another because of the folding structure even though they are widely apart inside the chain. Cys-25 engages in a nucleophilic assault on the carbonyl carbon of a peptide backbone (Babalola, 2019). Non-peptidyl and peptidyl N-nitrosoanilines might effectively decrease papain activity (Babalola, 2019). Papain becomes inactive when a strong S-NO (Snitroso-Cys25) link forms in the active site (Wang et al., 2006).
3.4 Stability of papain
The stability of papain is affected by its environment. Papain can dissolve in water, but for use, it can be diluted in solutions containing cysteine. Papain can be stabilized by using certain chemicals, such as EDTA, cysteine, and dimercaptopropanol (Babalola, 2019). Papain is also resistant to some denaturing agents. Even after recrystallization in a solution containing 70 % methanol and 8 M urea, its full activity can be retained (Babalola, 2019).
The stability and activity of papain solution depends greatly on its pH and temperature. Papain is optimally active from 50 °C − 59 °C and pH 4.5 – 6.7. Results from our recent study show the effect of temperature on the papain cysteine-proteases activity in different pools (Fig. 3) (Babalola et al., 2023b). The activities in pools A, B, C, D, and E ranged from 0.437 to 0.392 µmol/min/mL, 0.464–0.319 µmol/min/mL, 0.418–0.340 µmol/min/mL, 0.296–0.398 µmol/min/mL, 0.135–0.376 µmol/min/mL, respectively and their optimum activity was obtained at 59 °C, 58 °C, 59 °C, 59 °C, and 50 °C, respectively.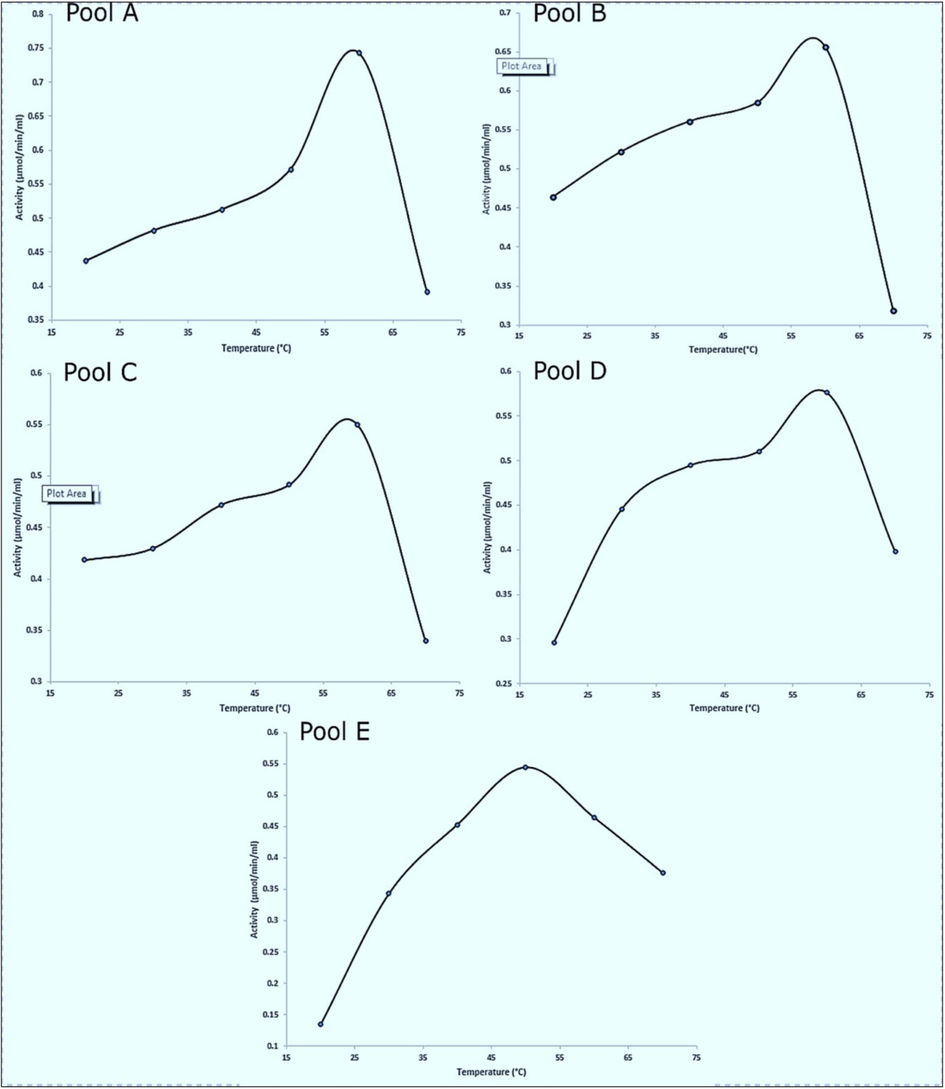
Effect of temperature on papain activity. In Fig. 3, we illustrate the impact of temperature on papain activity in pools A, B, C, D, and E. The enzyme's activity in each pool was measured at varying temperatures, ranging from 20 °C to 70 °C. As indicated in the figure, the enzyme's optimal activity was observed at 59 °C for pools A, C, and D, at 58 °C for pool B, and at 50 °C for pool E. These temperature ranges reflect the conditions under which papain exhibited its highest catalytic efficiency for each specific pool ().
Adapted from: Babalola et al. 2023b
We also reported the effect of pH on papain activity in different enzymatic pools A, B, C, D and E as shown in Fig. 4. The activities in pool A, B, C, D and E ranged from 0.037 to 0.024 µmol/min/mL, 0.280 – 0.389 µmol/min/mL, 0.042 – 0.034 µmol/min/mL, 0.226 – 0.169 µmol/min/mL, 0.182–––0.150 µmol/min/mL, respectively, and their optimum activity was obtained at 5.5, 6.7, 4.5, 4.5, and 5.5, respectively.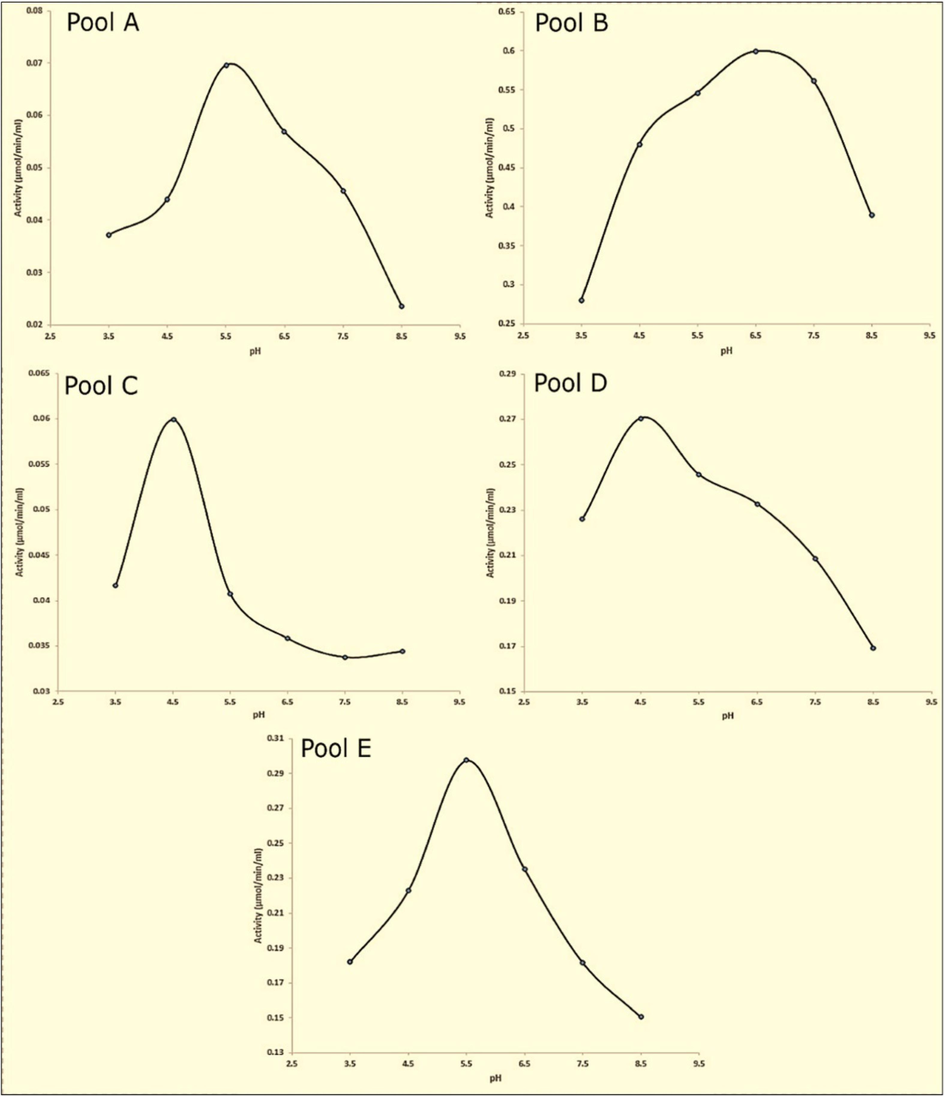
Effect of pH on Papain Activity. Fig. 4 demonstrates the influence of pH on papain activity in the same enzymatic pools. The enzyme's activity was assessed across a pH range from 3.5 to 8.5. Optimal activity was observed at pH 5.5 for pool A, pH 6.7 for pool B, pH 4.5 for pools C and D, and pH 5.5 for pool E. These pH values represent the specific acidic or alkaline conditions at which papain in each pool demonstrated its peak catalytic efficiency. ().
Adapted from: Babalola et al. 2023b
By analyzing the enzyme's behavior in different pools under varying pH and temperature conditions, our study provides valuable insights into the nuanced activity of papain, enhancing our understanding of its functionality in diverse environments.
3.5 Methods of extraction of papain
3.5.1 Papain from papaya latex
Previously, commercial products containing papain were isolated from the unripe latex of the papaya fruit and were further purified through drying and salt precipitation (Di Giacomo et al., 2022; Esti et al., 2013; Elsson et al., 2019). However, natural inhibitors present in the latex can now be utilized to purify papain without the need for thiol-containing chemicals or high salt concentrations (Babalola, 2019; Baeyens et al., 2015; Monti et al., 2000). A higher level of purity can be achieved in less time when papain is purified from fresh latex using an aqueous two-phase extraction (Yu and Zhang, 2020; Zhu and Zhang, 2019). The conventional papain extraction process from papaya includes making incisions in the unripe fruit to gather latex, which is subsequently processed and dried to obtain papain powder. However, this method can diminish the market value of the unripe green fruit due to the cuts made during the process.
3.5.2 Papain from papaya leaf
The utilization of papaya leaves and other parts of the papaya tree has led to the maximization of the benefits and usefulness of the plant, and several studies have been carried out on papain extraction from papaya leaves. One common requirement in these studies is the drying of the leaves for about 5–7 days. The extraction of papain from papaya leaves is chemically possible, and the proteolytic assay of papain is commonly done using casein as a substrate (Babalola, 2019). Various methods have been utilized for the extraction of papain from papaya leaves, including ultrasonication, enzyme-assisted, and blending homogenization methods (Babalola, 2019). It has been reported that sonicated papain extract at 60 °C for 60 mins showed maximum absorbance compared to the ground papain sample (Babalola, 2019). Two-step salt precipitation is another method used for the purification of papain extracted from fresh papaya leaves (Babalola, 2019). However, it should be noted that high papain purity cannot be obtained through the two-step salt precipitation method.
3.6 Techniques for extracting papain enzyme from leaves and preparing biologically active compounds
To extract papain enzyme from leaves and prepare other biologically active compounds, various techniques can be employed. The first step weighing the papaya leaves into ice-cold phosphate buffer pH 5.5 (Babalola et al., 2023b). In our previous study, we homogenized the mixture with the aid of warring blender (Thomas Scientific 3390D40). The homogenate was pooled and filtered; the residue was discarded. The filtrate obtained was then centrifuged at 3000 rpm for 10 mins at 4 °C using the cold-refrigerated centrifuge (Lacter-21). The supernatant was discarded and the sediment obtained was rinsed with 1 mL of cold 0.1 M phosphate buffer, pH 5.5. The crude extract then containing the enzyme was kept at 4 °C for 10 mins.
Blending or crushing the leaves using a blender to release the intracellular product. Mechanical, enzymatic, or a combination of both methods can break down the cell wall and speed up mass transfer and extraction kinetics. Additionally, hot water extraction, subcritical water extraction, supercritical fluid extraction, and solvent extraction can be used to remove plant material (Nur, 2010). To reduce the use of chemical compounds, blending with distilled water, ultrasonication, and enzyme-assisted extraction are employed. The efficiency of the extraction process depends on factors such as enzyme susceptibility to denaturation, high-temperature working conditions, or excessive usage of organic solvents (Nur, 2010). Papaya leaves that are ground up contain more papain enzyme than sonicated papaya, and enzyme purity is relatively increased in sonicated leaf samples. Temperature, pH, substrate concentration, metal ions, and cofactors are some of the conditions that affect papain's activity, with the ideal temperature for isolated papain enzyme being 60 °C. Ultrasound technology is also used to break apart prokaryotic and eukaryotic cells mechanically (Singh et al., 2022). However, according to a study, ultrasound can exert negative effects on biomolecules and cell walls of leaf tissue (Jiao et al., 2022). Enzyme extraction is more affordable and environmentally friendly compared to other extraction techniques, such as mechanical action. Moreover, enzyme-assisted extraction is used in the industry for oil extraction from seeds and fruits.
3.7 Activation and inhibition of papain
3.7.1 Activation of papain
In a previous study, it was observed that all concentrations of Mg2+ ion significantly increased the activity of the papain enzyme, indicating that Mg2+ ion serves as a good activator for the papain enzyme (Babalola et al., 2023b). Similarly, Kaul et al. (2002) reported a 24 and 18 % increase in papain activities when Mg2+ and Ca2+, respectively, were used as activators of papain at a concentration of 1.0 × 10−3 M. Other reported papain enzyme activators include cysteine, cyanide, sulfide, reduced glutathione, and thiosulfonate (Kimmel and Smith, 1954; Jaffe, 1945). Mg2+ has a wide range of activity in enzymes, serving as a co-factor in various metabolic pathways. In another study, it was reported that a low-dose of Hg2+ at concentrations ≥ 10−4 mol/L stimulated the activity of papain (Liu et al., 2015). It was noted that the changes observed in the activities of papain due to the addition of metal ions might be due to changes in the secondary structure of papain, which can be determined through infrared and fluorescence spectroscopy.
Jaffe (1945) examined the thiosulfate activation of milk clotting activity of six ferments from the papain group in a study. The outcomes are shown in the table below. Papain's milk clotting action uses cysteine, KCN, and Na2S as activators. The stability and activity of immobilized papain are activated by cysteine (Oliveira et al., 2022). Each of the six papainase formulations used in Jaffe's investigation had its gelatin, peptone, and milk clotting activities activated by sodium thiosulfate. Tables 2 shows a list of activators of papain and their percentage activities.
Ferment
Activator
Clotting Time (minutes)
Papain
1.25
Papain
Thiosulfate
0.57
Chympopapain
12.00
Chymopapain
Thiosulfate
0.83
Ficin
2.75
Ficin
Thiosulfate
0.42
Ficin
Cysteine
0.46
Bromelin
15.00
Bromelin
Thiosulfate
6.30
Tabernamontanain
2.33
Tabernamontanain
Thiosulfate
0.42
Calatropain
1.75
Calatropain
Cysteine
1.68
3.7.2 Inhibition of papain
The activity of papain can be diminished by exposure to urea and guanidine hydrochloride. However, optical rotation measurements suggest that this reduction in activity is not due to a change in the protein's secondary structure, and thus, does not constitute true inactivation. Despite a slight change in papain's specific rotation at 6 M urea, the protein is almost completely inactivated at this concentration, yet remains reversibly inactivated. On the other hand, exposure to 5 M guanidine hydrochloride results in irreversible inactivation of papain, leading to complete unfolding of the protein, as demonstrated by optical rotation tests (Hill et al., 1959).
Aldehydes are highly effective competitive inhibitors of papain, which are structurally linked to the acyl part of substrates. Acetyl-L-phenylalanylaminoacetaldehyde binds one mole of papain at a pH of 5.5 and a temperature of 25 °C with a Ki value of 4.6 × 10-8 M. Furthermore, aldehydes prevent the enzyme from being inactivated by ethylmaleimide. A distinct mechanism inactivates papain with the diazomethyl ketone of z-Phe and z-Phe-Phe, which differs from chloromethyl ketone. A stoichiometric reaction at the active center thiol of papain causes inactivation. Additionally, the rate of inactivation increases with decreasing pH (Leary et al., 1977).
Babalola (2019) reported on Fossum and Whitaker's investigation published in 1968 regarding the effects of papain and ficin on a protein inhibitor extracted from chicken egg white. Regardless of the substrate used (casein or p-nitrophenyl benzyloxycarbonylglycinate), one mole of either ficin or papain reacts with one mole of the protein inhibitor. The purified inhibitor is equally effective, and it only slightly inhibits bromelain. Moreover, it does not inhibit proteolytic enzymes from bacteria, trypsin, or chymotrypsin. The inhibitor's effect on pH between pH 4 and pH 9 was minimal when combined with ficin. The dissociation constant of the ficin-inhibitor complex was approximately 1–109 M. After boiling at pH values of 4 and 9 for 30 min, 10 % and 60 % of the inhibitory activity, respectively, were lost. According to gel filtration, the inhibitor's molecular weight was 12.7 kDa.
Carino and Whitaker researched the characteristics of the ficin-papain inhibitor extracted from avian egg white (Babalola, 2019). Electrophoresis on polyacrylamide disc gel made it possible to purify the inhibitor from chicken egg white. The dissociation constant of the ficin-inhibitor complex was 1.47, and hydrophobic forces appeared to have played a significant role in creating the complex. The extent of complex function was unaffected by variations in pH, ionic strength, or temperature. Babalola (2019) also reported on how methyl jasmonate induces papain inhibitors in Lycopersicon esculentum leaves. Lycopersicon esculentum leaves exposed to gaseous methyl jasmonate for 24 h at 30 °C and under continuous lighting conditions contained significant levels of soluble protein that inhibited papain. Chromatographic analysis was used to determine the protein's molecular mass, which ranged from 80,000D to 90,000D. The plant treated for less than 18 h showed an increase in inhibitory activity over time, which remained stable for up to four days before declining. Furthermore, the younger leaf lost activity more quickly than the older leaf. Methyl jasmonate therapy resulted in much less inhibitor action in the dark. Injured tomato leaves' extracts did not show any papain inhibitory activity or immunological response with antibodies produced to potato tuber skin cysteine protein.
Rhodes and Hoff also reported that the inhibitor from potato tubers is a non-competitive inhibitor of papain (Babalola, 2019). The inhibition is caused by the formation of a reversible complex between the inhibitor and papain, with a dissociation constant of approximately 10-10 M. The complex is not affected by pH or ionic strength. Furthermore, the inhibitor is not a substrate of papain, as shown by the absence of hydrolysis of the inhibitor by papain.
The inhibition of papain by potato tuber inhibitor is related to the presence of an arginine residue in the inhibitor molecule, which interacts with the glutamic acid residue in the papain molecule (Babalola, 2023). In contrast, the inhibition of papain by the avocado proteinase inhibitor involves a covalent bond between the inhibitor and the active center thiol of papain (Babalola, 2019). In summary, various inhibitors of papain have been discovered, each with different mechanisms of inhibition. Aldehydes and some plant-derived inhibitors are competitive inhibitors, shielding the enzyme from other inactivating agents. On the other hand, some inhibitors form covalent bonds with the enzyme's active site, rendering it inactive. The effectiveness of these inhibitors varies, depending on the substrate used and the pH and temperature conditions. It is worth noting that the discovery and characterization of inhibitors of papain have significant implications for the development of therapeutic agents for diseases associated with uncontrolled proteolysis, such as cancer and arthritis.
4 Pharmacological and nutraceutical properties of papaya
4.1 As a nutraceutical for health benefits
It is believed that all parts of the papaya plant, including the fruit that is commonly consumed, possess medicinal properties. Nutraceuticals are known to enhance the immune system and promote overall well-being. Papaya can be considered a therapeutic food, and its use as a nutraceutical plant has been found to aid in the management of neurological illnesses, specifically (Jain et al., 2022). Consumption of papaya may also help to strengthen the immune system and reduce the viral load in HIV patients, making it a viable treatment for the AIDS virus (Jadaun et al., 2023). Reactive oxygen species can cause oxidative stress, which may result in cell damage, diseases such as Alzheimer's and cancer, and accelerated aging. Fermented papaya preparation is believed to have potential benefits as a nutraceutical and food supplement, displaying antioxidant, anti-inflammatory, and immunostimulatory activities (Koul et al., 2022).
4.2 Treatment of digestive system disorders, anxiolytic and antioxidant activity
It's interesting to note that papaya has a long history of traditional use in treating digestive and gastrointestinal diseases. The papain enzyme found in papaya has been shown to aid in the digestion of protein and has been used in traditional medicine to treat indigestion (Babalola, 2019). The use of Caricol®, a papaya extract, in preserving a healthy digestive system is a promising finding (Babalola, 2019). Furthermore, green papaya leaf tea has also been used in traditional medicine for its various health benefits, including aiding in digestion (Ansari et al., 2022).
Another interesting finding is the sedative and anxiolytic effects of papaya reported by Mariano et al. (2022) and Bartuzi et al. (2022). These effects may be due to papaya's antioxidant properties. Jiao et al. (2022) reported that papain, the enzyme extracted from unripe papaya, has potential antioxidant properties. Antioxidants help to reduce oxidative stress, which is a major contributor to the development of various diseases, including anxiety and depression.
Overall, these findings suggest that papaya may have potential therapeutic benefits for a range of health conditions, including digestive disorders, cardiovascular diseases, and mental health disorders. However, more research is needed to better understand the mechanisms of action and potential side effects of using papaya for these purposes.
4.3 Anticancer property
It is well established that papaya contains natural compounds with anti-tumor properties (Babalola, 2019). In fact, papaya extract has been found to have activity towards tumor destruction, and components of papaya extract, such as tocopherol, lycopene, and flavonoids, have been shown to stimulate anti-tumor effects and inhibit tumor cell growth (Rameshthangam et al., 2022; Babalola, 2019). Papaya has been found to have a direct antitumor effect on cancer cells and can be useful in cancer therapy (Donadio et al., 2022).
Further research conducted by Otsuki and his colleagues revealed that papaya extract significantly inhibited the proliferation of tumor cell lines (Babalola, 2019). The addition of the extract decreased the production of IL-2 and IL-4 in PBMC, while increasing the production of IL-12p40, IL-12p70, IFN-gamma, and TNF-alpha without inhibiting growth. Microarray investigations also demonstrated that the addition of papaya extract improved the expression of 23 immunomodulatory genes that were categorized by gene ontology analysis, including CCL2, CCL7, CCL8, and SERPINB2, which may act as indices for the immunomodulatory effects. Thus, it can be inferred that papaya contains active components that inhibit tumor cell development.
Li and her colleagues also reported that bio-conjugation of 5-FluoroUracil and PpGNPs was more effective in fighting lung cancer due to its synergistic effects (Babalola, 2019). When the conjugate was compared to pure 5-FU, the efficacy of 5F-PpGNPs against human lung cancer increased significantly. Further studies on the effect of papaya on cancer immune evasion can be conducted to understand the mechanism of action (Babalola et al., 2021a) and other hallmarks of cancer.
4.4 Antimalaria and anti-dengue fever
The virus that causes dengue fever, also known as “breakbone fever” (Flaviviridae), is transmitted by the Aedes albopictus and Aedes aegypti mosquitoes, which have contagious bites. Dengue fever is controlled using drugs, personal safety measures, and vector management. The mosquito-borne illness has become a significant global public health problem, with tropical and subtropical cities and semi-urban areas being the most commonly affected (Mishra et al., 2022).
Thrombocytopenia is one of the defining symptoms of dengue fever. A study conducted on mice with cyclophosphamide-induced thrombocytopenia to assess the platelet-augmentation potential of Carica papaya leaf aqueous extract revealed a significant increase in platelet count and decrease in clotting time (Babalola, 2019). Active papaya extract components inhibit the immune-mediated destruction of platelets, suppress the bone marrow caused by the virus, and stabilize the membrane of infected cells. This speed up the healing process naturally, increasing platelet count and preventing complications.
Papaya leaf juice as a cure for the dengue virus is possible. Ahmad et al. (2011) conducted a study to investigate the effectiveness of papaya leaf extracts against the Dengue Fever Virus (DFV) in a 45-year-old patient. A 25 mL aliquot of the aqueous extract was administered twice daily for five consecutive days. The study found a significant increase in platelet count, white blood cells, and neutrophils (NEUT). Blood sample analyses revealed that papaya leaf aqueous extract has a potent anti-DFV capability.
In addition, malaria is treated using pharmaceutical combinations like Arthemeter and Lumenfanthrine. Many people use mature leaves and fruits of papaya to treat malaria (Munir et al., 2022). Additionally, papaya seeds exhibit antimalarial properties (Jiao et al., 2022; Bhat and Surolia, 2001). Munir et al. (2022) documented the antiplasmodial action of the leaves and seeds of Carica papaya, demonstrating the plant's ability to prevent malaria.
4.5 Proteolytic properties (Wound healing and meat tenderization)
Wound healing involves the regeneration of the dermis, which is aided by blood clotting factors promoted by Vitamin K. Papaya epicarp extracts have been shown to be beneficial for wound treatment. Papain and chymopapain are useful for treating pediatric infected burns topically to prevent infection and create a granulating wound (Leitao et al., 2022).
In terms of its tenderization property, rural villages in Africa, particularly Nigeria, have demonstrated the use of Papaya leaves to tenderize meat. It was once believed that papain is the enzyme responsible for catalyzing the tenderization of meat, and that the iron present in the wire gauze on which the meat is being smoked serves as a co-factor activator of the enzyme. Purified papaya enzymes are meat tenderizers, with chymopapain being the primary contributor to tenderization as it constitutes the major protease in the mixture (Aminlari, 2022).
5 Toxicity
Papain, an enzyme found in papaya, plays a vital role in protecting papaya trees from lepidopteran larvae, such as Samia ricini, Mamestra brassicae, and Spodoptera litura (Konno et al., 2004). The leaves of several laticiferous plants, including papaya and Ficus virgata, have been found to exhibit high toxicity and growth inhibition against lepidopteran larvae, despite the absence of any known toxic components from these species (Konno et al., 2004). However, the lethal potency of these plants' leaves is lost after the removal of latex. Papain, ficin, and bromelain have been attributed to provide papaya with the defense system against herbivorous insects (Konno et al., 2004).
Due to the widespread practice of avoiding papaya consumption during pregnancy in several Asian regions, Adebiyi et al. (2002) conducted a study to evaluate the safety of papaya consumption during pregnancy. They used conventional isolated-organ-bath techniques to evaluate the effects of ripe papaya juice and crude papaya latex on the uteri of pregnant and non-pregnant rats. The study revealed that regular consumption of ripe papaya during pregnancy may not pose significant risks. However, unripe or semi-ripe papaya, which contains a high proportion of latex that causes noticeable uterine contractions, may be unsafe during pregnancy. It is worth noting that the papain enzyme is found in papaya latex. Although animal experiments are commonly used to predict the risk of potentially harmful substances in humans, additional research is necessary to determine the actual risk associated with consuming unripe-to-semi-ripe papaya and papain during pregnancy (Adebiyi et al., 2002).
6 Medical, industrial, and pharmaceutical importance of papain
Papaya leaves have traditionally been used to tenderize meat by wrapping it in them. Among several proteases, papain is the most commonly used enzyme in cell isolation methods due to its efficiency and less damaging nature (Babalola, 2019). Papain is a component of contact lens solution, used in the pharmaceutical industry and to clarify beer. The traditional technique has also been demonstrated to be effective against nematodes (Soleimani et al., 2022). Papain is present in tissues lacking Alpha-1-antitrypsin (AAT) plasmatic antiprotease, which prevents proteolysis (Flindt, 1979). This is achieved by the hydrolysis of collagen cross-links or the cleavage of polypeptide chains. These cross-linkages stabilize collagen fibrils, preventing them from weakening when exposed to papain gel. Papain enzyme has a long history of use in treating allergies, various types of trauma, and sports injuries (Tao, 2022). Minor wounds heal more quickly with papain protease than with placebos. According to reports, papain may also be effective in dentin biochemical excavation methods (Elgewazi, 2022). Singh et al. (2022) reported that papain significantly reduced acute allergic sinusitis symptoms, such as headache and toothache pain, without causing any negative side effects.
As mentioned earlier, papain is a meat tenderizing agent. Protease enzymes are used to modify the proteins responsible for tenderness, such as myofibrillar proteins and connective tissue proteins; papain is a common ingredient in brewery products and the meat-processing sector (Abeyrathne et al., 2022). As a proteolytic enzyme, papain is used to treat dyspepsia and other gastrointestinal tract digestion issues (Kostiuchenko et al., 2022). The pharmaceutical industry produces papain in the form of gel doses because of its antifungal, antibacterial, and anti-inflammatory characteristics (Babalola, 2019).
7 Market opportunity and commercialization of papain
Papain is a highly beneficial product for various industries. It is derived from papaya, a tropical, herbaceous, succulent plant with self-supporting stems that thrives in all tropical nations and many sub-tropical regions of the world (Babalola, 2019; Jaime et al., 2007). Papain is available all year round, and there are no limitations on its production (Amri and Mamboya, 2012). However, in some countries, such as Malaysia, papain cannot be manufactured due to unsuitable climate conditions for papaya growth (Nur, 2010). Papain is used as a food ingredient and is manufactured, distributed, and sold worldwide due to its high export demand. A well-managed papaya production can result in a high yield of papain (Kamalkumar et al., 2007; Reddy et al., 2012). Papain is used in various industrial processes due to its numerous benefits. The major raw material of papain is latex, while the leaves of papaya are also of significance as they are used in the treatment of asthma, hypertension, and malaria. The good prospects for papain in the market have brought about an income source for farmers and manufacturers, which also contributes to the economic growth of a country.
8 Conclusion
In conclusion, the multifaceted applications of Carica papaya and its enzyme, papain, in traditional medicine and modern healthcare are both intriguing and promising. Our study delved into the extensive use of every part of the papaya plant within traditional medicine, demonstrating the plant's versatility in therapeutic applications. Notably, the pharmacological properties of papain, such as its remarkable potential in the treatment of allergies, sports injuries, and its utility in the food and health sectors, underscore its significance in diverse fields. This research sheds light on papain's pivotal role as nutraceuticals, specifically in the management of digestive diseases. With its proven antioxidant properties, papain emerges as a valuable candidate for combating oxidative stress-related ailments. Moreover, the observed anxiolytic qualities of papain open new avenues for research in mental health treatments, signifying its relevance beyond physical health concerns.
One of the most compelling aspects explored in this study is papain's potential in cancer treatment and wound healing. The promising results suggest a targeted approach in cancer therapy and a novel method for accelerating the wound healing process, offering hope for patients and healthcare professionals alike. These findings not only contribute significantly to the existing body of knowledge but also hint at papain's transformative impact on future medical practices. Furthermore, the commercialization and market possibilities of papain cannot be overstated. Its diverse applications in the medical, industrial, and pharmaceutical sectors signify a booming industry with extensive opportunities. As we navigate the landscape of medical advancements, understanding papain's pharmacological properties and its natural source, papaya, is fundamental for harnessing its full potential.
In essence, our study serves as a gateway to further exploration and innovation in the realm of natural medicine. While we have made significant strides in unraveling the mysteries of papain and Carica papaya, there is much more to discover. Future research endeavors could focus on in-depth clinical trials, molecular studies, and exploring synergistic effects with other compounds, offering a comprehensive understanding of papain's therapeutic mechanisms.
In summary, the findings presented in this study underscore the importance of Carica papaya and its enzyme papain in both traditional and modern medicine. By unlocking the secrets of this remarkable plant, we pave the way for a future where natural remedies play a central role in healthcare, offering effective, safe, and accessible solutions to a myriad of health challenges.
CRediT authorship contribution statement
Benjamin Ayodipupo Babalola: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Amos Ifeoluwa Akinwande: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Adebisi A. Otunba: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Writing – review & editing. Gboyega Ebenezer Adebami: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Writing – review & editing. Oluwabukunmi Babalola: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Writing – review & editing. Chekwube Nwufo: Conceptualization, Methodology, Resources, Project administration, Validation, Supervision, Software, Writing – review & editing.
Acknowledgements
This project did not receive any funding. However, we are grateful to Prof Amos Akinwande for his supervision and thorough review the draft of this manuscript. Also, we acknowledge Mr. & Mrs. Wole Babalola, and the D.K.O. Foundation for their immense support during the course of Benjamin Babalola's undergraduate study whose bachelor's degree project has led to this publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification and biochemical characterization of a GDSL-motif carboxylester hydrolase from Carica papaya latex. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2009;1791(11):1048-1056.
- [Google Scholar]
- Plant-and animal-based antioxidants’ structure, efficacy, mechanisms, and applications: a review. Antioxidants. 2022;11:1025.
- [Google Scholar]
- Papaya (Carica papaya) consumption is unsafe in pregnancy: fact or fable? Scientific evaluation of a common belief in some parts of Asia using a rat model. Br. J. Nutr.. 2002;88(2):199-203.
- [Google Scholar]
- In silico evaluation of the inhibitory potential of cymbopogonol from cymbopogon citratus towards falcipain-2 (FP2) cysteine protease of plasmodium falciparum. Trop. J. Nat. Prod.. 2022;6
- [Google Scholar]
- Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop. Biomed.. 2011;1(4):330-333.
- [Google Scholar]
- Plants-and animal-derived enzymes and their potential application in food processing and preservation. In: Novel Food Grade Enzymes. Singapore: Springer; 2022. p. :17-63.
- [Google Scholar]
- Papain, a plant enzyme of Biological importance: A review. Am. J. Biochem. Biotechnol.. 2012;6:99-104.
- [Google Scholar]
- Hyperglycaemia-linked diabetic foot complications and their management using conventional and alternative therapies. Appli. Sci.. 2022;12:11777.
- [Google Scholar]
- Extraction, purification and characterization of papain enzyme (EC 3.4.22.2) from the leaves of Carica papaya. Ogun state, Nigeria: Mountain Top University; 2019. BSc Thesis
- Mechanistic basis for cancer immune evasion and role of immune checkpoint blockades in immuno-oncology. Glob. J. Cancer Ther.. 2021;7:35-42.
- [CrossRef] [Google Scholar]
- Computational study of the therapeutic potential of novel heterocyclic derivatives against SARS-CoV-2. COVID. 2021;1(4):757-774.
- [CrossRef] [Google Scholar]
- Exploring the future of SARS-CoV-2 treatment after the first two years of the pandemic: A comparative study of alternative therapeutics. Biomed. Pharmacother. 2023115099
- [Google Scholar]
- Extraction, Purification and Characterization of Papain Cysteine-Proteases from the Leaves of Carica papaya. Sci. Afr.. 2023;5:e01538.
- [Google Scholar]
- A novel form of ficin from Ficus carica latex: Purification and characterization. Phytochemistry. 2015;117:154-167.
- [Google Scholar]
- Funnel metadynamics and behavioral studies reveal complex effect of D2AAK1 ligand on anxiety-like processes. Sci. Rep.. 2022;12:1-14.
- [Google Scholar]
- In vitro antimalarial activity of extracts of three plants used in the traditional medicine of India. Ame. J. Trop. Med. Hyg.. 2001;65:304-308.
- [Google Scholar]
- Maize Lethal Necrosis disease: review of molecular and genetic resistance mechanisms, socio-economic impacts, and mitigation strategies in sub-Saharan Africa. BMC Plant Bio. 2022;22:1-21.
- [Google Scholar]
- Systematic approach & application in relevance to economic & therapeutic actions of proteolytic enzyme: a review. J. Posi. Sch. Psyc. 2022:10476-10486.
- [Google Scholar]
- Investigation into the chemical modification of α-amylase using octenyl succinic anhydride: enzyme characterisation and stability studies. Biopro. Biosys. Engin.. 2023;24:1-20.
- [Google Scholar]
- Carica papaya lipase (CPL): An emerging and versatile biocatalyst. Biotechnol. Adv.. 2006;24(5):493-499.
- [Google Scholar]
- Cloud point extraction based on non-ionic surfactants: An ecofriendly tool for recovering papain from papaya latex. J. Environ. Chem. Eng.. 2022;10(6):108762
- [Google Scholar]
- Effects of pectins on colorectal cancer: targeting hallmarks as a support for future clinical trials. Food Funct. 2022
- [Google Scholar]
- Stabilization of enzymes for biotechnological applications. J. Pharm. Negat. Res. 2022:125-134.
- [Google Scholar]
- Single disulfide bond reduced papain exists in a compact intermediate state. Biochem. Biophys. Acta. 2000;1479:69-82.
- [Google Scholar]
- Current strategies to control recurrent and residual caries with resin composite restorations: operator-and material-related factors. J. Clin. Med.. 2022;11:6591.
- [Google Scholar]
- Elsson, M., Wijanarko, A., Hermansyah, H. and Sahlan, M., 2019. Michaelis-menten parameters characterization of commercial papain enzyme “paya”. In IOP Conference Series: Earth and Environmental Science (Vol. 217, No. 1, p. 012037). IOP Publishing.
- Papain from papaya (Carica papaya L.) fruit and latex: Preliminary characterization in alcoholic–acidic buffer for wine application. Food Bioprod. Process.. 2013;91(4):595-598.
- [Google Scholar]
- Tributyroylglycerol hydrolase activity in Carica papaya and other latices. Phytochemistry. 1991;30(4):1069-1072.
- [Google Scholar]
- ChemInform abstract: S-Nitrosation of proteins by N-methyl-N-nitrosoanilines. ChemInformation. 1996;70:205-212.
- [Google Scholar]
- Papaya (Carica papaya L.) for cancer prevention: Progress and promise. Crit. Rev. Food Sci. Nutr. 2022:1-21.
- [Google Scholar]
- The effect of urea and guanidine hydrochloride on activity and optical rotation of crystalline papain. J. Bio Chem.. 1959;234:572-576.
- [Google Scholar]
- Crystallization and preliminary X-ray analysis of a family 19 glycosyl hydrolase from Carica papaya latex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun.. 2008;64(5):371-374.
- [Google Scholar]
- Antiviral and ROS scavenging potential of Carica papaya Linn and Psidium guajava leaves extract against HIV-1 infection. BMC Complement. Med. Ther.. 2023;23(1):1-14.
- [Google Scholar]
- The activation of papain and related plant enzymes with sodium thiosulfate. Arch. Biochem.. 1945;8(3):385-393.
- [Google Scholar]
- A brief review on nutraceuticals and its application. Asian J. Dent. Heal. Sci.. 2022;2:7-13.
- [Google Scholar]
- Biological functions and utilization of different part of the papaya: a review. Food Rev. Int. 2022:1-24.
- [Google Scholar]
- Screening of dioecious papaya hybrids for papain yield and enzyme activity. J. Agric. Bio Sci.. 2007;3:447-449.
- [Google Scholar]
- Effect of metal ions on structure and activity of papain from Carica papaya. Food Nahrung. 2002;46(1):2-6.
- [Google Scholar]
- Solvent tolerant enzymes in extremophiles: Adaptations and applications. Int. J. Biol. Macro. 2023;16:124051
- [Google Scholar]
- Kim TE, Tsuboyama K, Houliston S et al. (2022) Dissecting the stability determinants of a challenging de novo protein fold using massively parallel design and experimentation. Proceed Nat Acad Sci 119:e2122676119.
- Kimmel JR, Smith EL (1954) Crystalline papain (Doctoral dissertation, Dept. of Biochemistry, University of Utah).
- Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J.. 2004;37:370-378.
- [Google Scholar]
- Effects of proteases from pineapple and papaya on protein digestive capacity and gut microbiota in healthy C57BL/6 mice and dose-manner response on mucosal permeability in human reconstructed intestinal 3D tissue model. Metabolites. 2022;12:1027.
- [Google Scholar]
- Carica papaya L.:a tropical fruit with benefits beyond the tropics. Diversity. 2022;14:683.
- [Google Scholar]
- Diazomethyl ketone substrates derivatives as active-site-directed inhibitors of thiol proteases, papain. ACS Publications. 1977;16:5857-5861.
- [Google Scholar]
- Determination of the kinetics and influence of the mercury ion on papain catalytic activity. RSC Adv.. 2015;5(84):68906-68913.
- [Google Scholar]
- Exotic medicinal plants used in Brazil with diuretic properties: a review. Chem. Biodiv.. 2022;19:e202200258.
- [Google Scholar]
- A comparative analysis of effectiveness of recombinant interleukin-11 versus papaya leaf extract for treatment of thrombocytopenia: a review. Ind. J. Clini. Biochem. 2022:1-8.
- [Google Scholar]
- Purification of papain from fresh latex of Carica papaya. Braz. Arch. Biol. Technol.. 2000;43:501-507.
- [Google Scholar]
- delving into the therapeutic potential of Carica papaya leaf against thrombocytopenia. Molecules. 2022;27:2760.
- [Google Scholar]
- The extraction of papain from papaya leaves. Thesis submitted in fulfillment of the requirements for the award of the degree of Bachelor of Chemical Engineering (Biotechnology). Malaysia Pahang: Faculty of Chemical & Natural Resources Engineering University; 2010.
- Immobilization and characterization of latex cysteine peptidases on different supports and application for cow’s milk protein hydrolysis. Process Biochem.. 2022;117:180-190.
- [Google Scholar]
- Genomic, biochemical and microbial evaluation of probiotic potentials of bacterial isolates from fermented sorghum products. Heliyon. 2021;7(12):e08536.
- [Google Scholar]
- Characterization of novel bacteriocin PB2 and comprehensive detection of the pediocin gene ped-A1 from Pediococcus pentosaceus PB2 strain isolated from a sorghum-based fermented beverage in Nigeria. Biotechnol. Rep. (Amst.). 2022;1(36):e00772.
- [Google Scholar]
- Anti-cancer and bactericidal activity of electrospun chitosan/poly (ethylene oxide)/papaya nanofibers. App. Nano 2022:1-9.
- [Google Scholar]
- Sex determination of Papaya (Carica papaya) at seedling stage through RAPD markers. Res. Biotechnol.. 2012;3:21-28.
- [Google Scholar]
- Plants utilized for folk medicine in Pambujan, Northern Samar, Philippines. Asian Plant Res. J.. 2022;10:47-83.
- [Google Scholar]
- Identification of novel inhibitors for SARS-CoV-2 as therapeutic options using machine learning-based virtual screening, molecular docking and MD simulation. Front. Molec. Biosci.. 2023;10
- [Google Scholar]
- Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients. 2019;11(7):1608.
- [Google Scholar]
- Phytochemistry and nutraceutical properties of Carica papaya (Linn.): a review. Diet. Suppl. Nutra.. 2022;1:1-15.
- [Google Scholar]
- Soleimani A, Keivani N, Lotfipour S, Abolhasani S, Valizadeh S, Behniafar H (2022) Promising effects of herbal compounds against strongyloidiasis: a systematic review. J Paras Disea 1-12.
- Allergenicity alleviation of bee pollen by enzymatic hydrolysis: regulation in mice allergic mediators, metabolism, and gut microbiota. Foods. 2022;11:3454.
- [Google Scholar]
- Effect of processing on the functional potential of bioactive components. In: Bioactive Components. Singapore: Springer; 2023. p. :183-207.
- [Google Scholar]
- Benchmarked molecular docking integrated molecular dynamics stability analysis for prediction of SARS-CoV-2 papain-like protease inhibition by olive secoiridoids. J. King Saud Univer. Sci.. 2023;35:102402
- [Google Scholar]
- Valipour M (2022) Chalcone-amide, a privileged backbone for the design and development of selective SARS-CoV/SARS-CoV-2 papain-like protease inhibitors. European Jou.
- A histidine/tryptophan π-stacking interaction stabilizes the heme-independent folding core of microsomal apocytochrome b5 relative to that of mitochondrial apocytochrome b5. Biochemistry. 2006;45:13750-13759.
- [Google Scholar]
- Novel edible starch films incorporating papain for meat tenderization. Food Pack. Shelf Life. 2022;31:100787
- [Google Scholar]
- Current understanding and insights towards protein stabilization and activation in deep eutectic solvents as sustainable solvent media. PCCP. 2022;24(22):13474-13509.
- [Google Scholar]
- Separation and purification of papain crude extract from papaya latex using quaternary ammonium ionic liquids as adjuvants in PEG-based aqueous two-phase systems. Food Anal. Methods. 2020;13:1462-1474.
- [Google Scholar]
- Study on activation mechanism and cleavage sites of recombinant butelase-1 zymogen derived from Clitoria ternatea. Biochimie. 2022;199:12-22.
- [Google Scholar]
- Optimization of [CnPy] Cl (n= 2, 4, 6) ionic liquid aqueous two-phase system extraction of papain using response surface methodology with Box-Behnken design. Process Biochem.. 2019;77:113-121.
- [Google Scholar]
- Between protein fold and nucleophile identity: multiscale modeling of the tev protease enzyme–substrate complex. ACS Omega. 2022;7:40279-40292.
- [Google Scholar]







