Translate this page into:
Geraniol inhibits cell growth and promotes caspase-dependent apoptosis in nasopharyngeal cancer C666-1 cells via inhibiting PI3K/Akt/mTOR signaling pathway
⁎Corresponding author at: Department of Otolaryngology-Head and Neck Surgery, Central Hospital Affiliated to Shandong First Medical University, Jinan 250013, China. hanxiaopan12@outlook.com (Xiaopan Han)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Background
Nasopharyngeal carcinoma (NPC) is a major type of head and neck cancer that develops from the nasopharynx with a highly distorted cultural and regional prevalence.
Objective
The present work was aimed at discovering the anticancer activity of geraniol on C666-1 cells by inhibiting PI3K/Akt/mTOR signaling and promoting apoptosis.
Methodology
The viability of geraniol (5–50 µM)-treated C666-1 cells was investigated using an MTT assay. The MMP levels were evaluated using JC-1 staining. The apoptosis level was studied by dual staining, DAPI staining, and flow cytometry techniques. The oxidative stress markers (TBARS, SOD, and GSH) and apoptotic biomarkers (Bcl-2, Bax, caspase-3, and −9) in the geraniol-treated C666-1 cells were analyzed using respective kits. The RT-PCR technique was done to evaluate the inhibition of PI3K/Akt/mTOR signaling in the geraniol-treated C666-1 cells.
Results
The outcomes of the MTT assay demonstrated that geraniol decreased C666-1 cell viability. The geraniol treatment reduced MMP and promoted apoptosis in C666-1 cells, which is evidenced by the fluorescent staining assay results. The flow cytometry assay results also confirmed the occurrence of apoptosis in the geraniol-treated C666-1 cells. The levels of TBARS were increased, while decreased GSH and SOD levels were observed in the geraniol-treated C666-1 cells. The geraniol also promoted pro-apoptotic gene expressions, including Bax, caspase-3, and -9, and additionally inhibited PI3K/Akt/mTOR signaling in the C666-1 cells.
Conclusion
The present results elucidate that geraniol is an effective anticancer agent for NPC. It can effectively inhibit cell growth, promote apoptosis, and inhibit the PI3K/Akt/mTOR pathway. Therefore, it can be an effective antitumor drug for NPC treatment.
Keywords
Caspases
Pro-apoptotic genes
Glutathione
Geraniol
C666-1 cells

1 Introduction
Nasopharyngeal cancer (NPC) is one of the most frequent head and neck cancers, with a highly distorted cultural and regional prevalence. NPC develops from the uppermost epithelial layer of the nasopharynx. NPC is considered a rare cancer globally, though it is common in Southeast Asian countries, particularly China (Chen et al., 2019). The onset of NPC is closely linked to Epstein-Barr virus (EBV) infection; however, there are multiple risk factors associated with NPC progression, such as drinking, smoking, and other external causes. The incidence of NPC ranges from 10 to 50 per 100,000 people worldwide (Bray et al., 2018). Chemotherapy and radiotherapy are the two main therapeutic options for people with NPC. Despite the fact that both treatment modalities have produced positive results, a significant number of patients with NPC do not respond to therapies aimed at averting the recurrence and die due to metastasis and drug resistance (Glastonbury et al., 2020). Hence, developing effective therapies for NPC remains a critical priority.
PI3K/AKT/mTOR signaling is vital for several hallmarks of cancer, including growth, metabolism, and genetic instability, angiogenesis, and can function independently or in conjunction with numerous other crucial signaling pathways (Miricescu et al., 2020). It was already highlighted that NPC pathogenesis involves several genetic and epigenetic alterations that lead to the aberrant activation of numerous signaling cascades, including PI3K/AKT/mTOR signaling (Kang et al., 2020). Furthermore, abnormal activation of AKT/mTOR signaling is associated with a poor prognosis of NPC and also participates in the growth, survival regulation, metastasis, and development of therapeutic resistance (Bamodu et al., 2020).
The vast majority of patients with NPC can be successfully treated with radiotherapy, chemotherapy, and surgery, resulting in prolonged local control and an improved survival rate. If patients with NPC are diagnosed and treated at an early stage, they have a higher chance of a complete recovery (Suarez et al., 2010). However, patients with advanced NPC still have a poor prognosis, with a decreased 5-year survival rate despite recent rapid advancements in treatment modalities (Zhou et al., 2016). Many tumors, including NPC, have poor prognoses, which makes treatment difficult. It was already highlighted that the PI3K/AKT/mTOR pathway has a tight connection with the prognosis of patients with NPC (Wang et al., 2014). The majority of NPC patients have a chance of metastasis to the cervical lymph node and far other tissues, which significantly increases the difficulty of successful treatment of NPC (Zhang et al., 2019). Unfortunately, radiotherapy may also damage normal tissues and the epithelium of the nasopharynx (Sidaway, 2019). For this reason, the effective management of NPC required the rapid development of novel therapeutic approaches to treat and protect against the metastasis and recurrence of NPC and to extend the survival period of patients.
Geraniol is an acyclic monoterpenoid that is extensively found in various essential oils, including orange, lavender, lemon, and rose (Lei et al., 2019). Geraniol has been linked to anti-inflammatory (Wang et al., 2016), anti-tumor (Qi et al., 2018), neuroprotective (Kandeil et al., 2019), nephroprotective (El-Said et al., 2020), hepatoprotective (El-Emam et al., 2020), cardioprotective (Crespo et al., 2017), and anti-colitic (Soubh et al., 2015) properties. However, its anticancer properties against NPC have yet to be investigated. Furthermore, the anticancer properties of geraniol against several cancer cell lines, such as lung cancer (Abo El-Ella, 2022), colon cancer (Qi et al., 2018), and prostate cancer (Kim et al., 2012), were already well reported. Hence, the current work was aimed at discovering the anticancer property of geraniol on NPC C666-1 cells by inhibiting PI3K/Akt/mTOR signaling and promoting apoptosis.
2 Materials and methods
2.1 Chemicals
Geraniol, DMEM medium, fetal bovine serum (FBS), and other chemicals and reagents were acquired from Sigma-Aldrich, USA. To carry out the biochemical assessments, the ELISA-based assay kits were obtained from ThermoFisher Scientific, USA.
2.2 Collection and maintenance of cell cultures
The NPC C666-1 cells were acquired from ATCC, USA, and then DMEM medium with heat-inactivated FBS (10 %) was used to grow the cells at 37 °C in a humidified CO2 (5 %) incubator. Cells were trypsinized and used for additional assays once they reached 80 % confluency.
2.3 MTT assay
The impacts of geraniol at different concentrations (5–50 μM) on the growth of NPC C666-1 cells were studied by MTT assay. Both cells were seeded separately on the 96-well plate and grown for 24 h. After that, C666-1 cells were treated with varying concentrations (5, 10, 15, 20, 25, & 50 μM) of the geraniol and further incubated for 24 h. Later to incubation, 20 μl MTT along with 100 µl DMEM was loaded to each well and incubated for an additional 4 h. The DMSO (100 μl) was mixed into each well to liquefy the formazan crystal formations. Finally, the absorbance was measured by using the microplate reader at 570 nm.
2.4 Quantification of oxidative stress biomarker levels
The control and geraniol-treated C666-1 cells were separated and lysed using lysis buffer. Then the suspension was centrifuged to obtain the cell lysate, and the same was utilized for the biochemical assessments. The glutathione (GSH) level, SOD activity, and TBARS level in the cell lysates were analyzed using respective ELISA assay kits according to the described instructions of the kit's manufacturer (ThermoFisher Scientific, USA).
2.5 Detection of caspases and Bax/Bcl-2 expressions
The expressions of Bcl-2, Bax, caspase-3, and -9 in the cell lysates of the control and geraniol-treated C666-1 cells were investigated using the commercially purchased assay kits using the instructions provided by the manufacturer (ThermoFisher, USA).
2.6 Dual staining
The dual staining (AO/EB) assay technique was done to examine the impact of geraniol on the induction of apoptosis in the C666-1 cells. Briefly, the 24-well plate was used to cultivate the C666-1 cells for 24 hr and then treated with 20 μM of geraniol and the standard drug DOX (2 μg) for 24 h. Later, for incubation, the AO/EB (100 μg/ml) fluorescent dye mixture at a 1:1 ratio was added to each well for 5 min. Finally, to detect the apoptotic incidences, the intensity of the fluorescence in the wells was evaluated using a fluorescent microscope.
2.7 JC-1 staining
The changes in the MMP status of geraniol-treated C666-1 cells were observed by using the JC-1 fluorescent staining technique. Briefly, a 6-well plate was used to grow the C666-1 cells at a population of 2 × 105 per well for 24 h. After that, 20 μM of geraniol was treated to the C666-1 cells for 24 hr. Then 1 µg/ml of JC-1 fluorescent stain was loaded for 20 min and finally, the cells were rinsed using PBS and investigated under a fluorescent microscope.
2.8 DAPI staining
The proportion of apoptotic cells in the geraniol-treated C666-1 cells was investigated using DAPI staining. The cells were seeded on a 24-wellplate and 20 μM of geraniol and/or 2 μg of DOX were treated for 24 hr. Later, control and geraniol-treated C666-1 cells were fixed using paraformaldehyde (4 %) for 30 min. After that, 200 µg/mL of DAPI fluorescent stain was loaded into each well for 15 min. Finally, the fluorescent microscope was used to investigate the cells to detect the alterations in the chromatin caused by geraniol treatment.
2.9 Analysis of apoptosis by flow cytometry
To analyze the impact of geraniol on the apoptosis level in the C666-1 cells, flow cytometry was performed. The C666-1 were cultured on the 6-well plate for 24 hr and then exposed to 20 μM of geraniol and 2 μg of the DOX for 24 hr. After the treatment period, the proportion of apoptotic incidences was investigated by the Annexin V-FITC/PI-Apoptosis Detection Kit. The collected cells were immediately investigated using flow cytometry. All assays were done in triplicate, which was carried out using the instructions provided by the kit's manufacturer (Abcam, USA).
2.10 RT-PCR analysis
The effect of geraniol on the inhibition of the PI3K/Akt/mTOR signaling pathway in the C666-1 cells was assessed by the RT-PCR technique. Briefly, the total RNA was isolated from control and geraniol-treated C666-1 cells using TRIzol reagent (ThermoFisher Scientific, USA). Then purified RNA was reverse-transcribed into cDNA, and gene expressions were studied using an RT-PCR kit in accordance with the recommended guidelines of the manufacturer (Takara, Japan). The primers for AKT forward: 5′- TTCTGCAGCTATGCGCAATGTG-3′, reverse: 5′- TGGCCAGCATACCATAGTGAGGTT-3′; mTOR forward: 5′- GCTTGATTTGGTTCCCAGGACAGT-3′, reverse: 5′- TGCTGAGTTTGCTGTACCCATGT-3′; and PI3K forward: 5′- GGTTGTCTGTCAATCGGTGACTGT-3′, reverse: 5′- GAACTGCAGTGCACCTTTCAAGC −3′ were used. The expression of genes was studied by the 2 − ΔΔCT method, and GADPH was used to standardize the results.
2.11 Statistical analysis
The values are analyzed using GraphPad Prism software. Tukey's post hoc assay and one-way ANOVA were done to measure the values. Results were illustrated as the mean ± SD of triplicate measurements, and p < 0.05 was fixed as significant.
3 Results
3.1 Geraniol inhibits the viability of C666-1 cells
The impact of geraniol on the growth of C666-1 cells was investigated by an MTT assay (Fig. 1). The treatment of geraniol at various doses (5–50 µM) substantially reduced the C666-1 cell growth. This study demonstrates the cytotoxicity of geraniol on C666-1 cell growth (Fig. 1). The IC50 of geraniol for C666-1 cells was determined to be 20 µM, which was chosen for further fluorescent staining and biochemical assays.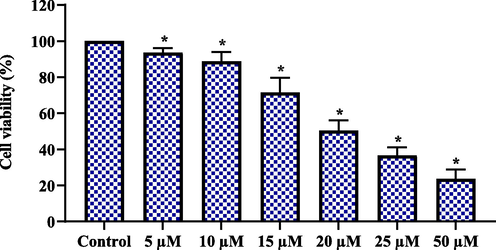
Effect of geraniol on the viability of C666-1 cells. Results were represented as the mean ± SD of three independent experiments. GraphPad Prism software was used to analyze the final data using one-way ANOVA and Tukey's post hoc analysis. Note: An asterisk (*) indicates that the results are significantly differed from the control at p < 0.05.
3.2 Geraniol decreases the MMP level in the C666-1 cells
The influence of geraniol on the MMP level of C666-1 cells was assessed using JC-1 staining. As illustrated in Fig. 2, the JC-1 produces red fluorescence in the control, which indicates normal, intact MMP. Contrastingly, the C666-1 cells, after being exposed to 20 μM of geraniol and/or 2 µg of DOX for 24 hr, produce green fluorescence with mild orange/red spots. The change of fluorescence from red to green indicates that geraniol can decrease the MMP status in the C666-1 cells (Fig. 2).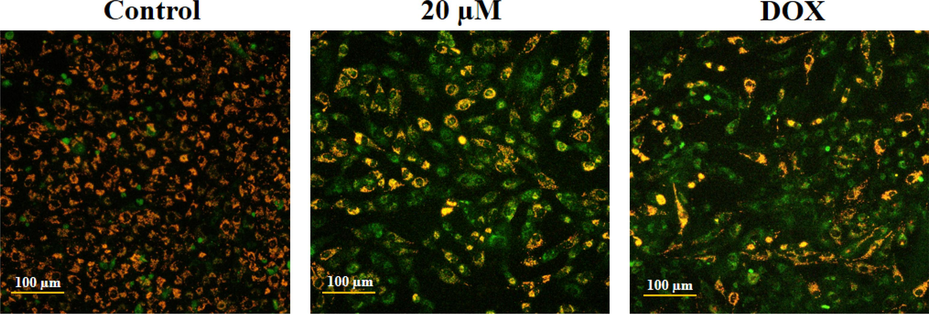
Effect of geraniol on the MMP level in the C666-1 cells. The geraniol-treated C666-1 cells showed increased green fluorescence when compared to the control cells, which proves the reduction of MMP levels.
3.3 Geraniol induces apoptosis in the C666-1 cells
The impact of geraniol treatment on apoptotic incidence in C666-1 cells is determined by dual staining (Fig. 3). The control cells demonstrated green fluorescent viable cells, while 20 µM geraniol-treated C666-1 cells displayed more cell structural damages and apoptotic events, as verified by the EB-stained bright red fluorescence compared to the control. DOX treatment (positive control) also showed stronger EB-stained red fluorescence, indicating increased apoptotic events, supporting geraniol's apoptotic-inducing potential in C666-1 cells.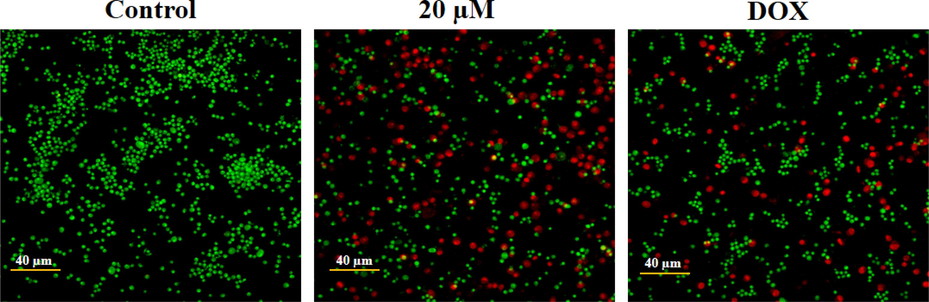
Effect of geraniol on the apoptosis in the C666-1 cells. The microphotographs of geraniol-treated C666-1 cells, which are analyzed by dual staining, revealed intense red fluorescence that demonstrates the occurrence of apoptotic incidences.
3.4 Geraniol causes apoptotic cell nuclear damage in C666-1 cells
The level of apoptotic cell nuclear damage in the geraniol-treated C666-1 cells was evaluated using DAPI staining (Fig. 4). The findings reveal that the 20 µM geraniol treatment substantially increased the cell structural changes, nuclear damage, and apoptotic body developments in the C666-1 cells. The same observations were also seen in the DOX-exposed C666-1 cells. Therefore, it was clear that geraniol could increase nuclear damage and facilitate apoptosis in C666-1 cells.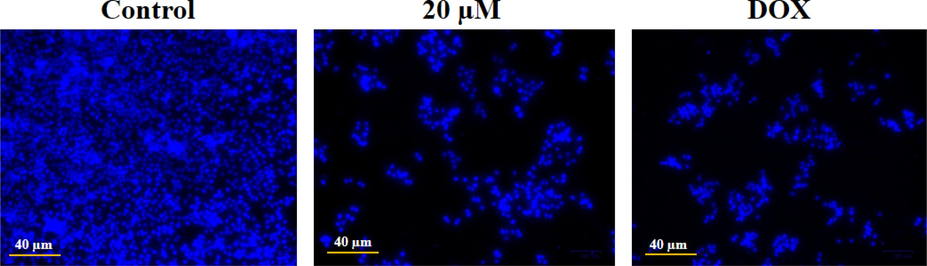
Effect of geraniol on the apoptotic cell nuclear damage in the C666-1 cells. The DAPI staining revealed that the geraniol treatment caused more cellular damage and the development of apoptotic bodies when compared to the control.
3.5 Geraniol increases apoptosis in C666-1 cells
The proportion of apoptotic cell death in both control and geraniol-treated C666-1 cells was studied by flow cytometry (Fig. 5). The outcomes proved that 20 µM of geraniol treatment substantially increased the percentage of apoptosis in the C666-1 cells. Additionally, DOX also increased the proportion of apoptosis, which supports the activity of geraniol (Fig. 5).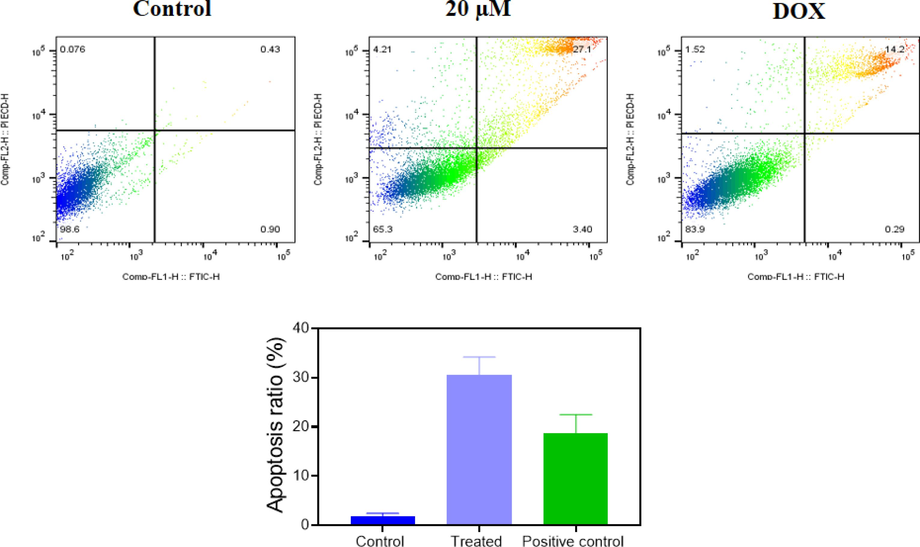
Effect of geraniol on the apoptosis in C666-1 cells. The proportion of apoptotic cells in the control and geraniol-treated C666-1 cells was analyzed using the flow cytometry method. The findings demonstrated that geraniol treatment effectively augmented the percentage of apoptotic incidences in the C666-1 cells. Treated: 20 µM geraniol-treated cells; positive control: standard drug DOX-treated cells.
3.6 Geraniol decreases antioxidants while increasing oxidative stress in C666-1 cells
The proportion of TBARS and antioxidants in geraniol-treated C666-1 cells was determined, and the outcomes are shown in Fig. 6. The concentration of TBARS in 20 µM geraniol-treated C666-1 cells was significantly increased. Furthermore, the same concentration of geraniol effectively reduced the SOD and GSH levels. These outcomes show that geraniol efficiently reduces antioxidants while improving oxidative stress in C666-1 cells (Fig. 6). The DOX treatment (positive control) also significantly increased TBARS levels while decreasing antioxidants in the C666-1 cells, which supports the activity of geraniol.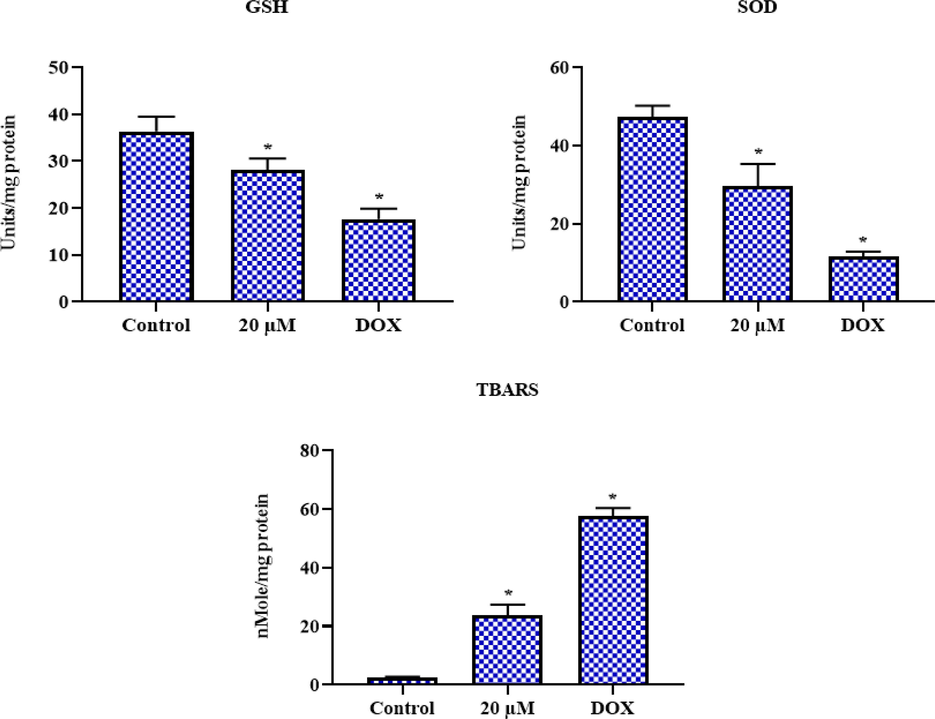
Effect of geraniol on the oxidative stress marker levels in C666-1 cells. Results were represented as the mean ± SD of three independent experiments. GraphPad Prism software was used to analyze the final data using one-way ANOVA and Tukey's post hoc analysis. Note: An asterisk (*) indicates that the results are significantly differed from the control at p < 0.05.
3.7 Geraniol increases pro-apoptotic proteins while decreasing anti-apoptotic protein expression in C666-1 cells
The impact of geraniol on the pro- and anti-apoptotic marker expressions such as Bcl-2, Bax, caspase-3, and −9 in the C666-1 was examined, and the outcomes are shown in Fig. 7. The C666-1 cells treated with 20 µM geraniol showed elevated expressions of Bax, caspase-3, and −9. Whereas, geraniol lowered the Bcl-2 expression in the C666-1 cells. The standard drug DOX also improved Bax, caspase-3, and −9 while decreasing Bcl-2 expression, as seen in the geraniol treatment (Fig. 7).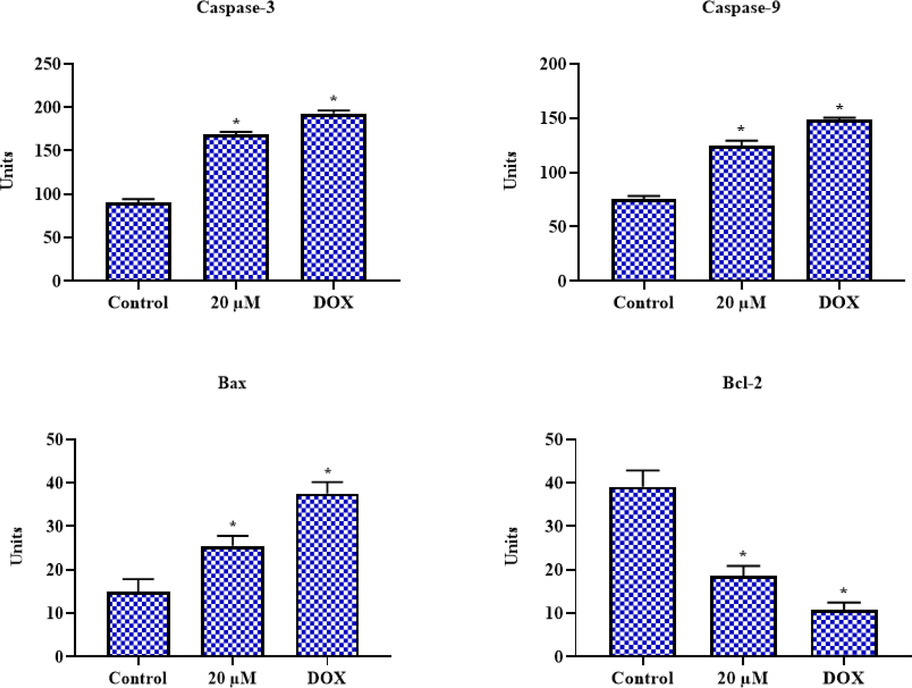
Effect of geraniol on the apoptotic protein expressions in C666-1 cells. Results were represented as the mean ± SD of three independent experiments. GraphPad Prism software was used to analyze the final data using one-way ANOVA and Tukey's post hoc analysis. Note: An asterisk (*) indicates that the results are significantly differed from the control at p < 0.05.
3.8 Geraniol inhibits the PI3K/Akt/mTOR signaling in the C666-1 cells
The effect of geraniol on the PI3K/Akt/mTOR signaling axis in the C666-1 cells was scrutinized by RT-PCR, and results are shown in Fig. 8. After 20 µM geraniol treatment, the expressions of Akt, mTOR, and PI3K were significantly abridged in C666-1 cells. The decreased expression of PI3K/Akt/mTOR genes was also observed in the DOX (positive control)-treated C666-1 cells (Fig. 8). This finding confirmed that geraniol inhibited the PI3K/Akt/mTOR pathway in the C666-1 cells.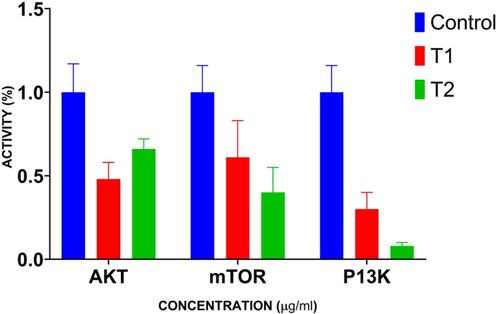
Effect of geraniol on the PI3K/Akt/mTOR signaling pathway in the C666-1 cells. Results were represented as the mean ± SD of three independent experiments. GraphPad Prism software was used to analyze the final data using one-way ANOVA and Tukey's post hoc analysis. Note: T1: 20 µM geraniol treatment; T2: 2 µg Doxorubicin treatment (positive control). The values of treatment groups are significantly differed from the control at p < 0.05.
4 Discussion
NPC usually develops from the nasopharyngeal epithelium. NPC is probably undiagnosed in its early stages because it is asymptomatic or exhibits very minimal clinical symptoms (Voon et al., 2015). The distant metastases are present in the majority of NPC patients. The effective treatment of individuals with advanced NPC, particularly those who have metastasized, recurred, or are resistant to therapy, also poses a significant difficulty (Karam et al., 2016). The recovery of primary NPC patients has considerably improved because of recent improvements in chemotherapy and radiotherapy. Despite this, the average survival time for NPC patients with metastasis or recurrence is only about 20 months (Miao et al., 2021). Hence, the search for novel anti-nasopharyngeal cancer medications that are more effective and less harmful is urgently needed. Therefore, the present work aimed to reveal the beneficial properties of geraniol in inducing oxidative stress, apoptosis, and inhibiting the PI3K/Akt/mTOR pathway in NPC C666-1 cells. The cytotoxic effect of geraniol was assessed using NPC C666-1 cells. Our results proved that geraniol remarkably inhibited C666-1 cell growth, which indicates its cytotoxicity to NPC cells. The current findings were in agreement with the earlier study by Dong et al. (Dong et al., 2021), who found a considerable decrease in the growth of opiopogonin B-treated C666-1 cells.
The MMP level is a biomarker of the permeability of the mitochondrial membrane. MMP levels have been shown to decrease in the early stages of apoptosis (Bate et al., 2010). In addition to ROS accumulation, the influence of geraniol on the alterations in the MMP level of C666-1 cells was evaluated using JC-1 staining. Aggregated JC-1 gives red fluorescence at intact MMP, while monomeric JC-1 at depleted MMP produces green fluorescence (Li et al., 2010). As seen in Fig. 2, JC-1 fluoresces red in control cells, whereas green fluorescence with little red fluorescent spots was seen in C666-1 cells after treatment with 20 µM of geraniol and/or 2 µg of DOX (positive control) for 24 h. As the fluorescence changes from red to green, it is clear that geraniol can cause the MMP to dissipate in the C666-1 cells.
The unbalance between free radicals and their detoxifying antioxidants causes oxidative stress in the cells, which thereby leads to cell death (Illan-Cabeza et al., 2013). When the balance between oxidants and antioxidants is disturbed, free radicals cause lipid peroxidation, genetic mutation, oxidation of proteins and DNA, and alter chromosome structure in the cells. Nonetheless, considerable focus has been given to the essential role of oxidative stress-mediated cell death in tumors (Hung et al., 2016). Usually, the amount of TBARS is proportional to the degree of lipid peroxidation. The excess oxidative stress is believed to cause cell death in tumor cells (Zhang et al., 2002). GSH is the most prevalent endogenous nonenzymatic antioxidant that protects cells from oxidative damage and participates in numerous detoxifying activities. It was already known that increased cellular GSH levels correlate with resistance to apoptosis (Guha et al., 2011). On the other hand, depletion of endogenous GSH and increased oxidative stress are known to stimulate cell death. This highlights the role of antioxidants in promoting the proliferation and survival of tumor cells (Harris et al., 2015). Additionally, SOD deficiency contributes to oxidative DNA damage in the cells (Oberley and Buettner, 1979). In the current study, the geraniol treatment causes depletion in the GSH and SOD levels while improving the TBARS level. Therefore, it was clear that geraniol increased oxidative stress and thus promoted cell death in C666-1 cells.
Apoptosis induction is a critical mechanism in the treatment of cancer with antitumor medicines. Inducing apoptosis in cancer cells is a talented approach to treating NPC and other types of malignancies (Steinbach and Weller, 2004). The relative expression of pro- and anti-apoptotic genes can influence cancer cell fate and response to chemotherapy. A mechanism of resistance to chemotherapy-triggered apoptosis in tumor cells involves increased Bcl-2 expression and inhibition of Bax expression (Song et al., 2017). Chemotherapeutic drugs often inhibit tumor cell growth by preventing Bcl-2 and elevating the expressions of caspase-9 and −3 (Malla et al., 2010). Caspase-3 is an essential regulator of apoptosis, while caspase-9 is a vital player in mitochondria-mediated apoptosis (Yaacoub et al., 2016). The strategy of inhibiting Bcl-2 and promoting caspase-9 and −3 is followed in the treatment of several tumors (Tomicic et al., 2010). The current work found that geraniol significantly increased the Bax, caspase-3, and −9 expressions while inhibiting the Bcl-2 expression. Hence, it was clear that geraniol could promote apoptosis in C666-1 cells by encouraging pro-apoptotic gene expression while decreasing the anti-apoptotic gene Bcl-2. In line with this, the results of the fluorescent staining assays and flow cytometry assays also demonstrated the stimulation of apoptosis in the C666-1 cells treated with geraniol. Therefore, it can be concluded that geraniol is effective in promoting apoptosis in NPC cells.
Several malignancies have been linked to aberrant activation of the PI3K/Akt/mTOR signaling pathway, which facilitates their resistance to therapies (Brennan et al., 2013). There are many pro-tumorigenic effects that are regulated by this pathway, including cell proliferation, survival, protein translation, invasion, and metastasis (Touat et al., 2017). The activated PI3K/Akt/mTOR signaling axis has been proven to develop and maintain oncogenic phenotypes. As an additional function, the activation of this pathway is essential for tumor cell resistance to standard therapy (Wei et al., 2020; Zhu et al., 2020). As a result, PI3K/Akt/mTOR signaling inhibitors are currently being developed for the possible treatment of malignancies, especially NPC (Liu et al., 2019; Wang et al., 2021). Interestingly, the current findings demonstrated that geraniol significantly blocked PI3K/Akt/mTOR signaling in C666-1 cells. Therefore, it can inhibit NPC growth and promote apoptosis by inhibiting the PI3K/Akt/mTOR signaling axis.
5 Conclusion
The current study elucidates that geraniol is an effective anticancer agent for NPC cells. It can inhibit cell growth by inducing oxidative stress and promoting caspase-mediated apoptosis in C666-1 cells. Regarding its apoptosis-inducing mechanism, depletion of MMP levels, pro-apoptotic gene expressions, and inhibition of the PI3K/Akt/mTOR signaling axis also play major roles. Furthermore, our study lacks in-depth molecular assays to study its role in other molecular pathways. In the future, these limitations will be further explored in order to promote geraniol as an effective antitumor drug candidate for the treatment of NPC.
References
- Autophagy/apoptosis induced by geraniol through HIF-1α/BNIP3/Beclin-1 signaling pathway in A549 CoCl2 treated cells. Adv. Pharm. Bull.. 2022;12(1):155-162.
- [Google Scholar]
- Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells. 2020;9:746.
- [Google Scholar]
- A glycosylphosphatidylinositol analogue reduced prion-derived peptide mediated activation of cytoplasmic phospholipase A2, synapse degeneration and neuronal death. Neuropharmacology. 2010;59:93-99.
- [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68:394-424.
- [Google Scholar]
- Effect of geraniol on rat cardiomyocytes and its potential use as a cardioprotective natural compound. Life Sci.. 2017;172:8-12.
- [Google Scholar]
- Ophiopogonin B induces reactive oxygen species-dependent apoptosis through the Hippo pathway in nasopharyngeal carcinoma. Mol. Med. Rep.. 2021;24(1):534.
- [Google Scholar]
- Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2020;393:1849-1858.
- [Google Scholar]
- Geraniol ameliorates diabetic nephropathy via interference with miRNA-21/PTEN/Akt/mTORC1 pathway in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2020;393:2325-2337.
- [Google Scholar]
- Head and neck squamous cell cancer: Approach to staging and surveillance. In: Hodler J., Kubik-Huch R.J., von Schulthess G.K., eds. Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [internet]. Cham (CH): Springer; 2020. Chapter 17
- [Google Scholar]
- Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J. Pharmacol. Exp. Ther.. 2011;336(1):206-214.
- [Google Scholar]
- Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27(2):211-222.
- [Google Scholar]
- Roles of reactive oxygen species in anticancer therapy with Salvia miltiorrhiza Bunge. Oxid. Med. Cell. Longev.. 2016;2016:5293284.
- [Google Scholar]
- A potential antitumor agent, (6-amino-1-methyl-5-nitrosouracilato-N3)-triphenylphosphine-gold(I): Structural studies and in vivo biological effects against experimental glioma. Eur. J. Med. Chem.. 2013;64:260-272.
- [Google Scholar]
- Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci.. 2019;228:145-151.
- [Google Scholar]
- Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct. Target. Ther.. 2020;5:245.
- [Google Scholar]
- Outcomes after reirradiation for recurrent nasopharyngeal carcinoma: North American experience. Head Neck. 2016;38(Suppl 1):E1102-E1109.
- [Google Scholar]
- Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int. J. Oncol.. 2012;40(5):1683-1690.
- [Google Scholar]
- Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett.. 2010;298(2):222-230.
- [Google Scholar]
- Casticin inhibits nasopharyngeal carcinoma growth by targeting phosphoinositide 3-kinase. Cancer Cell Int.. 2019;21(19):348.
- [Google Scholar]
- Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/AKT pathway in gliomas. PLoS One. 2010;5:e13731.
- [Google Scholar]
- IAP-1 promoted cisplatin resistance in nasopharyngeal carcinoma via inhibition of caspase-3-mediated apoptosis. Am. J. Cancer Res.. 2021;11(3):640-667.
- [Google Scholar]
- PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int. J. Mol. Sci.. 2020;22:173.
- [Google Scholar]
- Role of superoxide dismutase in cancer: a review. Cancer Res.. 1979;39(4):1141-1149.
- [Google Scholar]
- Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. Balk. Union Oncol.. 2018;23:346-352.
- [Google Scholar]
- Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. B.U.ON.. 2018;23(2):346-352.
- [Google Scholar]
- Escape of U251 glioma cells from temozolomide-induced senescence was modulated by CDK1/survivin signaling. Am. J. Transl. Res.. 2017;9:2163-2180.
- [Google Scholar]
- Geraniol ameliorates TNBS-induced colitis: involvement of Wnt/-catenin, p38MAPK, NF-κB, and PPAR signaling pathways. Life Sci.. 2015;136:142-150.
- [Google Scholar]
- Apoptosis in gliomas: molecular mechanisms and therapeutic implications. J. Neurooncol. 2004;70:245-254.
- [Google Scholar]
- Current treatment options for recurrent nasopharyngeal cancer. Eur. Arch. Otorhinolaryngol.. 2010;267:1811-1824.
- [Google Scholar]
- Topotecan triggers apoptosis in p53-deficient cells by forcing degradation of XIAP and survivin thereby activating caspase-3-mediated Bid cleavage. J. Pharmacol. Exp. Ther.. 2010;332:316-325.
- [Google Scholar]
- Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann. Oncol.. 2017;28:1457-1472.
- [Google Scholar]
- Nutlin-3 sensitizes nasopharyngeal carcinoma cells to cisplatin-induced cytotoxicity. Oncol. Rep.. 2015;34:1692-1700.
- [Google Scholar]
- Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp. Ther. Med.. 2016;12:3607-3613.
- [Google Scholar]
- Correlation of the AKT/mTOR signaling pathway with the clinicopathological features and prognosis of nasopharyngeal carcinoma. Eur. J. Histochem.. 2021;65(4):3304.
- [Google Scholar]
- Activation of Akt/mTOR pathway is associated with poor prognosis of nasopharyngeal carcinoma. PLoS One. 2014;9:e106098.
- [Google Scholar]
- Genipin induces autophagy and suppresses cell growth of oral squamous cell carcinoma via PI3K/ AKT/MTOR pathway. Drug Des. Devel. Ther.. 2020;14:395-405.
- [Google Scholar]
- Role of the tumor microenvironment in regulating apoptosis and cancer progression. Cancer Lett.. 2016;378:150-159.
- [Google Scholar]
- Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogen.. 2002;23(1):207-211.
- [Google Scholar]
- Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med.. 2019;381:1124-1135.
- [Google Scholar]
- Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget. 2016;7:13634-13650.
- [Google Scholar]
- Celastrol suppresses glioma vasculogenic mimicry formation and angiogenesis by blocking the PI3K/Akt/mTOR signaling pathway. Front. Pharmacol.. 2020;11:25.
- [Google Scholar]






